Introduction
Powassan virus is a tick-borne flavivirus that is endemic to the United States, causes severe encephalitis, and is diagnosed with increasing frequency. Since its discovery in 1958, approximately 270 cases have been reported in the United States and Canada; however multiple lines of epidemiological evidence suggest that it may be more prevalent than is currently appreciated. Here, we will briefly summarize the virology, ecology, and epidemiology of Powassan virus and review clinical features of infection, with a focus on brain imaging, diagnostic testing, and neuropathology.
Powassan virus belongs to the Flaviviridae family, and is a small enveloped virus with an approximately 10.8 kb positive-sense RNA genome that encodes a single open reading frame1. It is a member of the flavivirus genus, which is primarily comprised of arthropod-borne viruses, and it is closely related to tick-borne encephalitis virus (TBEV). TBEV is a relatively well-studied cause of encephalitis in Europe and Asia, and several of its features are worth considering as they may parallel Powassan virus. First, TBEV causes a substantial burden of disease, with more than 10,000 cases per year despite the availability of a vaccine2. Many TBEV infections are asymptomatic3, and its seroprevalence ranges from 2–5% or higher in high-risk populations4–6. Finally, within TBEV, there are three viral subtypes that differ by about 5% at the amino acid level, have different (but overlapping) geographic distributions, and cause different frequencies of neuroinvasive disease and fatality7.
Compared to TBEV, relatively less is known about Powassan virus. It was discovered in 1958 in Ontario, Canada8, and only about 25 cases were reported over the subsequent 40 years9. In part, this may have been due to under-recognition and limited testing. However, infection may truly have been infrequent because the canonical Powassan virus lineage I is transmitted by tick species rarely encountered by humans, Ixodes cookei and Ixodes marxi. In 1996, a second lineage of Powassan virus was discovered and termed deer tick virus (DTV), as it is transmitted by the common vector of human disease, Ixodes scapularis10. Unlike other Ixodes-borne infections, Powassan virus can be transmitted within a very short period of tick attachment11,12. To date, more than 240 cases have been reported. The two Powassan virus lineages differ by around 15% at the nucleotide level and 5% at the amino acid level13 and thus can be distinguished by molecular testing such as polymerase chain reaction (PCR) and sequencing. All recent human infections for which molecular characterization was performed have been due to lineage II / DTV14–21. However, because most Powassan virus infections are diagnosed by serology, and lineages I and II are serologically indistinguishable, they are clinically diagnosed together as “Powassan virus encephalitis.”
Ecology
Powassan virus is maintained in an enzootic cycle that is comprised of small mammal reservoir hosts and tick vectors. Ticks can become infected by feeding on a viremic host or co-feeding with an infected tick, even on a non-viremic host. The virus persists through later stages of the tick life-cycle, e.g. an infected larval tick will remain infected as a nymph and adult (through transstadial transmission), and ticks can pass the virus on to progeny (through transovarial transmission)22. Large mammals, including humans, become infected as incidental, dead-end hosts. In addition to being transmitted by different vectors, Powassan virus lineages I and II are maintained by different small mammal reservoir species in different geographic regions. Lineage I has been detected primarily in woodchucks and squirrels in regions of Canada and the U.S. surrounding the Great Lakes. By contrast, Powassan virus lineage II / DTV is found in the northeast and north central U.S. Shrews have recently been implicated as a primary reservoir23, and there is little evidence that it is maintained in white-footed mice, which are the primary reservoir for other Ixodes scapularis-borne pathogens.
Powassan virus has a prevalence of around 1–3% in Ixodes scapularis ticks in endemic regions of the northeast and north central U.S. and has higher prevalence in adult ticks24–29. By comparison to other tick-borne pathogens, a study of Ixodes scapularis from New York found a prevalence of 3.4% for Powassan virus, 3.2% for Babesia microti, 18% for Anaplasma phagocytophilum, and 55% for Borrelia burgdorferi29. Experimental work has demonstrated that other tick species, Amblyomma americanum and Dermacentor variabilis, are competent vectors for Powassan virus30. However, relatively little is known about how frequently these vectors carry Powassan virus in the wild31, and to what extent Powassan virus is present in parts of the U.S. with lower prevalence of Ixodes scapularis. Interestingly, Powassan virus is also found in Ixodes ticks and humans in Far Eastern Russia, and is proposed to have been introduced from Canada through fur trade in the twentieth century32.
Multiple wild mammals have high seroprevalence for Powassan virus. A study in Ontario identified positive sera in 50% of coyotes, 47% of skunks, 26% of foxes, and 10% of raccoons33, although another study identified only one positive elk from 217 total cervid samples34. In the U.S., Powassan virus seroprevalence increased in white-tailed deer between 1979 and 2009, reaching over 80% in Connecticut in 2005, 2006, and 200935. In nearby Vermont and Maine, 11% and 13% of deer were seropositive, respectively, in 201035. In New York, serologic evidence of Powassan virus infection was detected in woodchucks (4/6), an opossum (1/6), and birds (4/727)28. The relatively widespread seropositivity in mammals, combined with the prevalence in ticks and the expansion of the tick vector itself36, suggest increasing risk of human exposure to Powassan virus, similar to what has been observed with other Ixodes-borne infections.
Epidemiology
The high prevalence of Powassan virus in ticks and wild mammals raises the question of whether human infection may be more common than is currently appreciated. Powassan virus neuroinvasive disease was formally added to the list of notifiable diseases in the U.S. in 2001, and non-neuroinvasive infection was added in 2004, while Canada has no national reporting requirements. Diagnosis of Powassan virus infection has increased from around 1 case/year between 1958–2005, to 7.7 cases/year from 2006–201513, to 21–43 cases/year from 2016–201937,38. However, the true incidence of Powassan virus infections in North America is unknown, and likely well above the number of reported cases. Because there is limited to no testing in patients without neuroinvasive symptoms, very little is known about subclinical or mild infection.
The few recent human seroprevalence studies that have been conducted to date have yielded a range of results13. Positive serology was detected in 9/95 (9.5%) of patients with suspected tick exposure and 2/50 (4%, p=0.33) of patients without suspected tick exposure in Wisconsin in 201539. Similarly, seroprevalence was significantly higher in samples submitted for Lyme disease testing in Wisconsin (10/106, 9.4%) than in samples from patients in the northeastern United States without tick exposure or symptoms of tickborne disease (2/100, 2%, p=0.034)40. A seroprevalence study identified Powassan virus neutralizing antibodies in 1% of individuals in a general population in Sussex County, New Jersey, where a cluster of recent encephalitis cases was described41. Only a single equivocal result was detected among 230 serum samples from individuals bitten by Ixodes scapularis or I. cookei ticks in Maine from 2009201342. Among samples from 52 patients with confirmed Lyme disease in New York between 2011–2015, only one was positive for IgM, and this sample was negative by confirmatory testing for neutralizing antibodies43. Given the variability in reported seroprevalence rates, it would be of value to conduct larger studies across the broader geographic area from which Powassan virus has been reported.
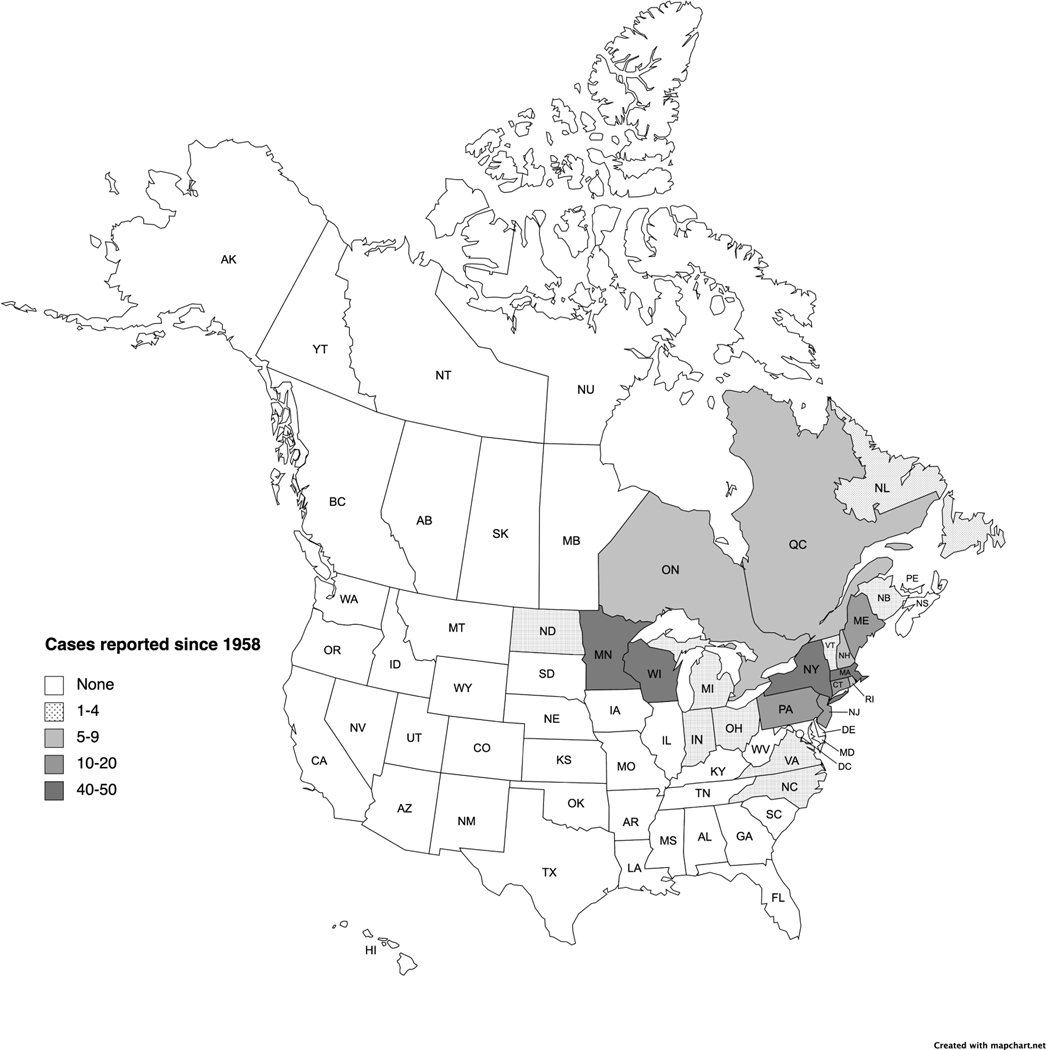
With the important caveat that most available information is from patients with neuroinvasive disease, epidemiological information can be derived from approximately 270 cases described in case reports and case series (Table 1) and reported by the CDC National Arbovirus Surveillance System (ArboNET)44. The geographic distribution of Powassan virus cases in North America centers around the Great Lakes region and east coast (Figure 1). The first case was identified in Ontario in 19588, and the majority of subsequent cases from Canada have been from Ontario (approximately 8) and Quebec (approximately 5). Single cases have also been reported from Newfoundland and New Brunswick45,46. The first case in the U.S. was identified in New Jersey in 197047, and an additional 10 cases were described in New York in the 1970s48–50. Subsequently, Powassan virus was not reported until 1994 in Massachusetts44, 1999 in Vermont51, and 2000 in Maine51,52 coinciding with the recognition of DTV (lineage II). The first cases reported in the Midwest include Michigan in 2002, Wisconsin in 2003, and Minnesota in 200851,53,44. Altogether in the U.S., more than 250 cases have been reported from 16 states. The greatest number of cases of have been reported from New York (n=50), Wisconsin (n=46), Minnesota (n=45), and Massachusetts (n=44). Fewer cases have been reported from Maine (n=16), New Jersey (n=14), Connecticut (n=13), and Pennsylvania (n=10), with a handful from Rhode Island (n=5), New Hampshire (n=5), and North Dakota (n=2), and one case each in Indiana, Michigan, North Carolina, Ohio, Vermont, and Virginia.
Table 1.
Published cases of Powassan virus infection
| Case number | Age/Sex | Year/Location | Fatal (Y/N) | Immunocompromised (Y/N) | PMID(s) |
|---|---|---|---|---|---|
| 1 | 5 M | 1958 Canada (Ontario) | Y | N | 13652010 |
| 2 | 57 F | 1970 New Jersey | N | N | 4684890 |
| 3 | 7 M | 1971 New York | N | N | 222159; 4856896 |
| 4 | 5 F | 1972 New York | N | N | 4856896 |
| 5 | 12 M | 1972 New York | N | N | 222159; 4856896 |
| 6 | 14m M | 1972 New York | N | N | 222159; 4856896 |
| 7 | 8 M | 1972 Canada (Quebec) | N | N | 4829843 |
| 8 | 6–15 M | 1974 New York | N | N | 222159; 1059007 |
| 9 | 3 M | 1975 Canada (Quebec) | N | N | N/A: Can Dis Wkly Rep 1976;2:85–7. |
| 10 | 6–15 M | 1975 New York | N | N | 222159; 267829 |
| 11 | 6–15 M | 1975 New York | N | N | 222159; 267829 |
| 12 | 82 M | 1975 New York | Y | N | 222159; 267829 |
| 13 | 15 F | 1976 Canada (Ontario) | N | N | N/A: Can Dis Wkly Rep 1976;2:202–3 |
| 14 | 13m F | 1977 Canada (Ontario) | N | N | 223757 |
| 15 | 0–5 F | 1977 New York | N | N | 222159 |
| 16 | 8 M | 1978 New York (Canada, Nova Scotia) | N | N | 6297420 |
| 17 | 19 F | 1979 Canada (Ontario) | N | N | N/A: Can Dis Wkly Rep 1979;5:129–30. |
| 18 | 7 M | 1979 Canada (Ontario) | N | N | 6254625 |
| 19 | 9 F | 1980 Canada (Quebec) | N | N/A: Can Dis Wkly Rep 1982; 8:185–91. | |
| 20 | 62 M | 1987 Canada (Ontario) | N | N | 2547526 |
| 21 | 76 M | 1988 Canada (New Brunswick) | N | N | 21233909 |
| 22 | 49 F | 1994 Massachusetts | N | N | 7643850 |
| 23 | 4m ? | 1994–1995 Canada | N | N | 9502462 |
| 24 | 64 M | 1997 Canada (Ontario) | Y | N | 10906899 |
| 25 | 66 M | 1999 Vermont | N | N | 18959500; 11693145 |
| 26 | 25 M | 2000 Maine | N | N | 18959500; 11693145 |
| 27 | 53 F | 2000 Maine | N | N | 18959500; 12771287; 11693145 |
| 28 | 70 M | 2001 Maine | N | N | 18959500; 11693145 |
| 29 | 60 F | 2002 Michigan | N | N | 18959500 |
| 30 | 69 M | 2003 Wisconsin | N | N | 20443328; 18959500 |
| 31 | 74 F | 2004 Maine | N | N | 18959500 |
| 32 | 91 F | 2004 New York | N | N | 24274334; 18959500 |
| 33 | 83 M | 2005 New York | N | N | 24274334; 18959500 |
| 34 | 49 M | 2006 Wisconsin | N | N | 20443328 |
| 35 | 47 F | 2007 Wisconsin | N | N | 20443328 |
| 36 | 81 ? | 2007 New York | Y | ? | 24274334 |
| 37 | 81 ? | 2007 New York | N | ? | 24274334 |
| 38 | 77 ? | 2007 New York | Y | ? | 24274334 |
| 39 | 62 M | 2007 New York | Y | Y | 24274334; 19439744 |
| 40 | 5 ? | 2007 New York | N | ? | 24274334 |
| 41 | 70 ? | 2007 New York | Y | ? | 24274334 |
| 42 | 61 M | 2008 Canada (Quebec) | N | N | 20929710 |
| 43 | 9 F | 2008 New York | N | N | 24274334; 20736878 |
| 44 | 22 M | 2009 New York | N | N | 23969017 |
| 45 | 73 ? | 2009 New York | N | ? | 24274334 |
| 46 | 76 ? | 2009 New York | N | ? | 24274334 |
| 47 | 4 ? | 2009 New York | N | ? | 24274334 |
| 48 | 77 M | 2010 New York | Y | N | 24274334; 23166187 |
| 49 | 67 F | 2011 Minnesota | Y | Y | 23017222 |
| 50 | 69 M | 2011 Minnesota North Dakota | N | N | 23111001 |
| 51 | 61 M | 2011 Minnesota | N | Y | 23111001 |
| 52 | 60 M | 2011 Minnesota North Dakota | N | N | 23111001 |
| 53 | 18 M | 2011 Minnesota | N | N | 23111001 |
| 54 | 43 M | ? Minnesota | N | N | 22040725 |
| 55 | 32/34 M | 2012 New York | N | N | 24274334; 23969017 |
| 56 | 44 M | 2013 New Hampshire | N | N | 26668338 |
| 57 | 35 F | 2013 Wisconsin | N | N | 29431163 |
| 58 | 49 M | 2014 Massachusetts | Y | N | 26668338 |
| 59 | 52 M | 2014 Massachusetts | N | N | 26668338 |
| 60 | 65 F | 2014 Massachusetts | N | N | 26668338 |
| 61 | 67M | 2014 Massachusetts | N | N | 26668338 |
| 62 | 21 M | 2014 Massachusetts | N | N | 26668338 |
| 63 | 74 M | 2015 Massachusetts | N | Y | 26668338 |
| 64 | 82 M | 2015 Massachusetts | Y | N | 26668338 |
| 65 | 81 F | 2016 Rhode Island | N | N | 29942757 |
| 66 | 61 M | 2016 Massachusetts | N | Y | 29020227 |
| 67 | 63 M | 2016 Massachusetts | Y | Y | 33094116; 29554185 |
| 68 | 5m M | 2016 Connecticut | N | N | 33111953, 28426641 |
| 69 | 72 F | ? Maine | Y | N | 28903511 |
| 70 | 55 M | 2017 Massachusetts | N | N | 31538917 |
| 71 | 56 M | 2017 New York | N | N | 32007325 |
| 72 | 8 F | 2017 Wisconsin | N | N | 32060042 |
| 73 | 51 M | 2017 New Jersey | N | Y | 34283672 |
| 74 | 70 M | ? Pennsylvania | N | Y | 30104234 |
| 75 | 68 F | ? Canada (Quebec Ontario) | N | N | 30559280 |
| 76 | 79 M | 2018 Massachusetts | Y | N | 33094116 |
| 77 | 30 F | 2018 Indiana | N | Y | 32539111 |
| 78 | 62 M | ? Canada (Newfoundland Ontario) | N | N | 31158072 |
| 79 | 88 M | 2018 Wisconsin | Y | N | 33020816 |
| 80 | 87 M | ? New York | N | N | 31712240 |
| 81 | 75 F | 2019 Massachusetts | Y | N | 33094116 |
| 82 | 2m M | 2019 Connecticut | N | N | 33111953 |
| 83 | 42 M | 2019 Wisconsin | N | N | 33227515 |
| 84 | 76 M | ? Wisconsin | N | N | 33758161 |
| 85 | 62 M | ? Connecticut | N | Y | 34430178 |
| 86 | 82 F | ? New York | Y | Y | 34596868 |
Figure 1:
Geographic distribution of 273 human Powassan virus infections reported between 1958–2021. (Created with mapchart.net)
Detailed clinical descriptions are available for 86 cases (Table 1), and general information from annual or aggregated summaries is available for another 147 cases38,54–56. Of these 233 cases, the majority were males (n=167; 72%) and over age 60 (n=135, 58%), with 59 (25%) cases ages 18–59, and 39 (17%) cases under age 18. Illness onset most often occurred from April to June (n=104, 45%), followed by July to September (n=71, 30%) and October to December (n=51, 22%), with only rare cases in January to March (n=3, 1%). The vast majority of reported cases were from hospitalized patients (n=217, 93%) and patients with neuroinvasive disease (n=218, 94%), likely reflecting a bias in testing. The overall number of deaths was 28 (12%), including deaths from sequelae of hospitalization for Powassan virus disease.
Tick identification and skin pathology
Patients may present at the time of, or soon after, a known tick bite, and ticks may be submitted for laboratory identification57. I. scapularis females are characterized by: a red abdomen; a black, ovoid scutum covering up to half of the dorsum; black capitulum and legs; narrow and elongated, straight club-like palps; no eye spots or festoons; and an inverted U-shaped anal groove (Figure 2A). I. scapularis nymphs appear increasingly black during feeding, with an unchanged black ovoid scutum. The length of feeding can be estimated by the scutal index, the ratio of tick body length to scutum width58, although the relevance to POWV disease is negligible due to the short time required to transmit infection11,12.
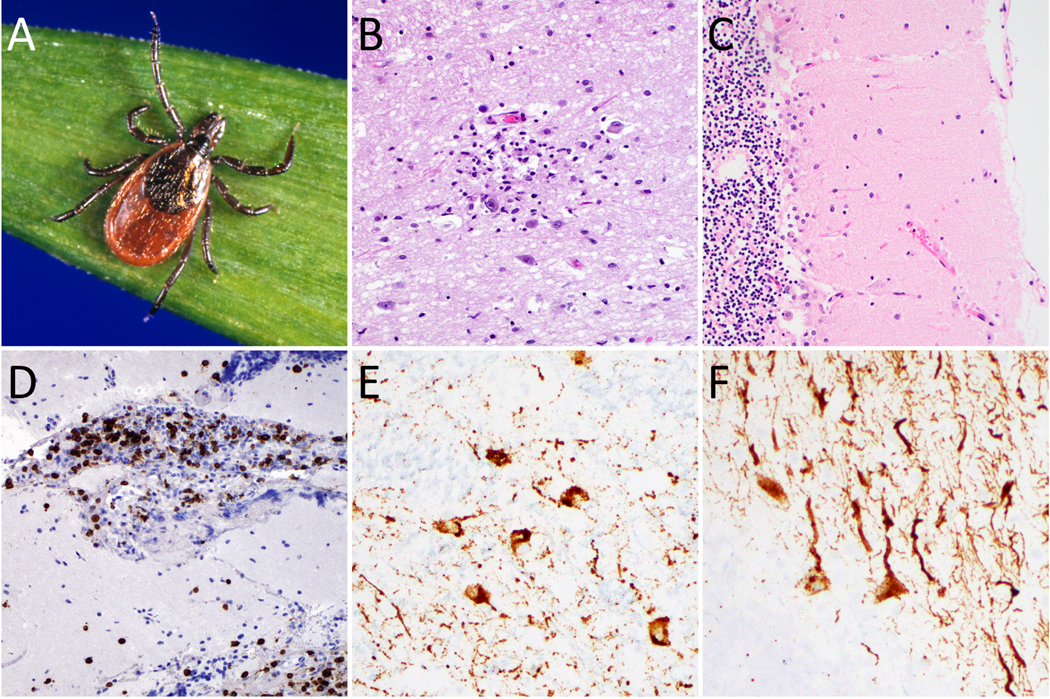
Figure 2: Powassan virus neuropathological features.
Female Ixodes scapularis (deer tick, black-legged tick) can be grossly identified by characteristic U-shaped anal groove (A). Sections from fatal Powassan encephalitis cases show a microglial nodule with neuronophagia in the thalamus (B), marked Purkinje cell loss and Bergmann gliosis in the cerebellum (C), and CD3-positive T cell infiltrate in the leptomeninges (D). Powassan virus RNA in situ hybridization highlights infected neurons in the thalamus (E) and cerebellum (F). (Image A courtesy of Michael L. Levin, PhD via the CDC Public Health Image Library)
Skin at the site of a tick bite can appear normal to markedly erythematous while the tick is attached, and papular and nodular rashes may develop once removed59. Acute lesions may show histological evidence of tick mouthparts attached to the epidermis, with an intradermal cavity containing red blood cells and a predominantly neutrophilic inflammatory infiltrate, which transitions to eosinophilic over time. Chronic lesions contain a dense inflammatory infiltrate of lymphocytes and eosinophils in a wedge shape, with overlying acanthotic and pseudoepitheliomatous epidermis, and associated with granulomata. While the features of Powassan virus infection in skin have not been well described, mouse models do provide some insight into the distribution of virus and inflammatory response60,61.
Clinical Evaluation
Powassan virus has an incubation period of approximately two weeks, though this can range up to a month or longer31. Patients may initially experience nonspecific symptoms such as fever and fatigue. Mild disease is occasionally identified (Box 1). However, neuroinvasive disease occurs in about 95% of recognized cases37,38 and patients often develop confusion and focal neurological deficits requiring hospitalization19,62. Powassan virus has also rarely been described to cause chororetinitis63 (which is also seen in West Nile virus), ophthalmoplegia64, and spinal cord (anterior horn) involvement leading to flaccid weakness45.
Box 1. Case Title: 67-year old woman with systemic illness.
Case Presentation:
A 67-year-old woman presented to an outpatient clinic with a subacute systemic illness. She initially had a week of fever, headache, and profound fatigue. Following resolution of her fevers, she felt tremulous with dysregulated temperature, had difficulty concentrating, and noted decreased quality in handwriting; these symptoms were gradually improving. She had a persistently poor appetite and mild nausea without vomiting. Her gastrointestinal symptoms worsened with doxycycline. Over the last 2 days, she developed a pruritic rash on the extensor surface of her hands, elbows, and knees. Notable exposures include ticks, mosquitoes, and her baby grandchild. On physical exam, she had an erythematous papular rash most notable on her knees and extensor surfaces of her hands. Neurologic exam was unremarkable, including cranial nerves, motor strength, sensation, fine motor coordination, balance, and gait.
Clinical Questions:
What are potential causes of this patient’s improving systemic illness? What additional testing could be ordered to confirm the diagnosis?
Discussion:
Recommended tests included Lyme test with Western blot, Anaplasma/Ehrlichia PCR, Powassan virus antibody, West Nile virus antibody, and CMV IgM and IgG. This patient’s serum Powassan virus IgM was positive with PRNT of 1:20 indicating recent infection.
Learning Point:
Powassan virus may present with mild symptoms and minimal neurological involvement.
Although thrombocytopenia has occasionally been reported62,65, there are no peripheral laboratory abnormalities classically associated with Powassan virus. Cerebrospinal fluid (CSF) analysis generally demonstrates low-to-moderate elevation in protein, normal glucose, and pleocytosis in the range of hundreds of white blood cells (WBC)/microliter, which can be either lymphocyte or polymorphonuclear predominant62. It is worth considering that, like other tick-borne pathogens, Powassan virus may occur in co-infection66, so compatible clinical findings or laboratory abnormalities should prompt testing for Lyme disease, Borrelia miyamotoi, anaplasmosis, or babesiosis. Finally, although primarily associated with tick exposure, Powassan virus can be transmitted by blood transfusion67 and is worth considering in the differential diagnosis for patients who receive transfusion in endemic areas.
Imaging
Results of neuroradiological studies from head CT or brain MRI have been reported for approximately 60 cases of Powassan virus encephalitis, and the wide range of findings is consistent with a similarly wide spectrum of clinical symptoms. Approximately 25% of published cases reported no acute abnormalities or nonspecific findings, including in two fatal cases9,68. The most common abnormalities reported were T2/FLAIR hyperintensities (suggestive of edema and inflammation) in basal ganglia (50%), cerebellum (40%), thalamus (30%), brainstem (30%), cerebral cortex (30%), and temporal lobes (10%). Occasional diffusion restriction, suggesting cytotoxic edema, and non-specific white matter hyperintensities have also been noted. Leptomeningeal contrast enhancement was present in 15% of cases, and parenchymal enhancement was rare. Cerebellar involvement has previously been suggested to be a poor prognostic factor for POWV disease62, which is supported here by cerebellar abnormalities in approximately 70% of fatal cases compared to less than 20% of non-fatal cases (p= 0.0004). Overall, imaging findings range from mild to diffuse meningoencephalitis/rhombencephalitis and do not have distinguishing features compared to other viruses infecting the central nervous system.
Diagnostic Approach
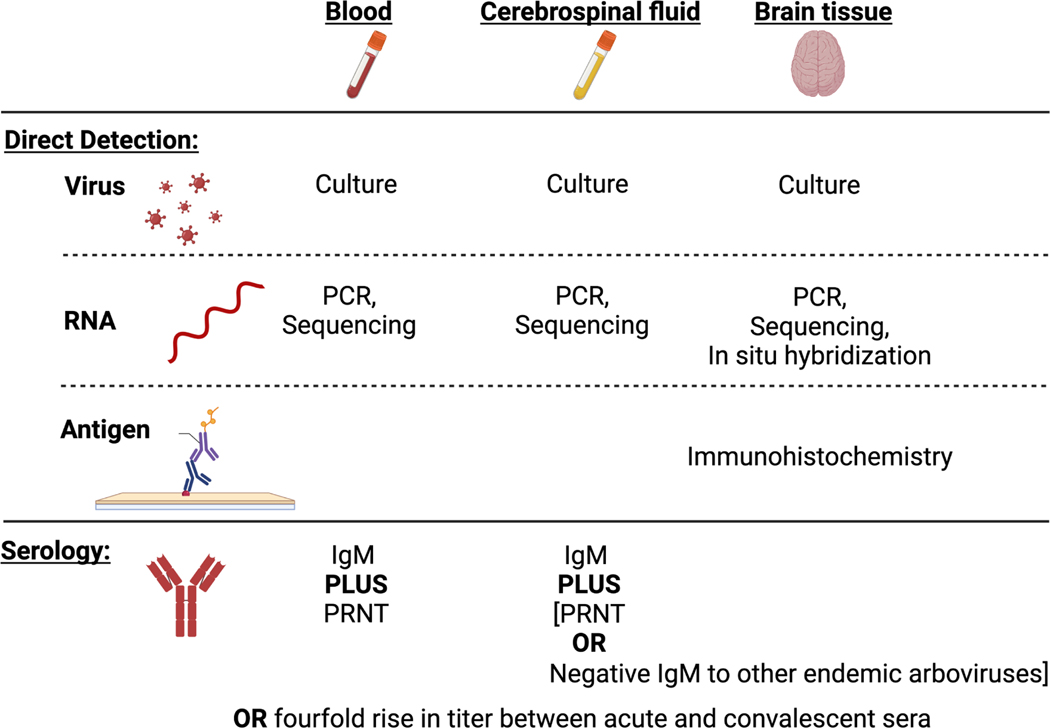
The nonspecific nature of the symptoms, laboratory, and imaging findings associated with Powassan virus makes it incumbent upon physicians to consider it as a potential cause of encephalitis and pursue dedicated diagnostic testing. Diagnostic criteria have been outlined by the Centers for Disease Control and Prevention (CDC) for Powassan virus and other arboviruses68 (Figure 3). Diagnosis can be made by direct detection, and although Powassan virus is rarely recovered in culture, molecular tests frequently detect its RNA, and this may be particularly useful in immunocompromised patients who do not mount sufficient antibody responses14,16. Several studies have demonstrated the use of sequencing-based assays to detect Powassan virus RNA in CSF and brain tissue14–18. It can also be identified by PCR20,21, and a multiplex PCR for Powassan virus and other Ixodes-borne infections has been used in nonclinical applications69. While these reports suggest that molecular diagnostics are generally higher-yield for Powassan virus than other arboviral infections such as WNV, they are rarely available outside of a research setting. Direct detection in brain tissue samples can also be useful, as discussed in the subsequent section.
Figure 3: Diagnostic Approaches for Powassan virus.
Testing modalities are listed for each specimen type (blood, cerebrospinal fluid, and brain tissue) and target (virus, RNA, antigen, antibody). Abbreviations: PCR, polymerase chain reaction; PRNT, plaque reduction neutralization test. (Created with BioRender.com)
However, serology is the most common method used to diagnose Powassan virus infection. The diagnosis can be made by observing a four-fold rise in antibody titers between acute and convalescent sera (including seroconversion), or by detecting both virus-specific IgM and neutralizing antibodies in serum or CSF. Confirmation of neutralizing antibodies via a plaque reduction neutralization test (PRNT) is necessary due to the high overall antibody cross-reactivity between flaviviruses. According to CDC guidelines, neuroinvasive disease can also be diagnosed by detecting Powassan virus IgM in CSF and not detecting IgM to other endemic viruses, though in practice a positive IgM from CSF is generally followed up with confirmation by PRNT.
Neuropathology
Information regarding the neuropathology of Powassan virus encephalitis has been derived from both biopsy and autopsy reports. Surgical brain biopsies may be collected for diagnostic purposes after less invasive testing options have been exhausted, or in conjunction with procedures to relieve intracranial pressure, while autopsies are performed to establish or confirm a suspected cause of death and to document the extent of the disease process. Neuropathological findings have been published for 10 cases of Powassan virus encephalitis including 2 non-fatal cases. Four cerebellar biopsies showed mild to severe T-cell predominant lymphocytic inflammation involving leptomeninges and cerebellar cortex, microgliosis with occasional microglial nodules, Purkinje cell loss with Bergmann gliosis, and thickened dura14,16,19,21. A thalamic biopsy showed hemorrhagic infarction with lymphoplasmacytic infiltrate70. These histological features, including the lack of significant neutrophilic or granulomatous inflammation, are characteristic of arboviral encephalitis, but require correlation with other laboratory and clinical findings to rule out other infectious and non-infectious causes of encephalitis.
Seven autopsies have been reported, with gross brain findings ranging from unremarkable8, to mild diffuse swelling of cerebral hemispheres9, to marked edema resulting in herniation and Duret hemorrhages14,19,21. The degree of involvement of different brain regions varied between cases, but included cerebellum, thalamus, basal ganglia, cerebral cortex, deep white matter, hippocampus, midbrain, medulla, pons, and spinal cord. Histological findings at autopsy included leptomeningeal, perivascular, and parenchymal inflammation consistent with meningoencephalitis (Figure 2B–C)8,9,19,21,68. Mild to severe T lymphocyte-predominant inflammatory infiltrates were present in the meninges and Virchow-Robin spaces (Figure 2D). Focal to dense perivascular T-cell predominant inflammation was observed surrounding parenchymal blood vessels without true vasculitis. Parenchymal inflammation (greater in grey matter than white matter) consisted of diffuse microgliosis with focal to dense microglial nodules, neuronophagia, and focal areas of tissue necrosis without true infarction. Severe loss of neurons was present including cerebellar Purkinje cell and spinal cord anterior horn cells. These histological findings likely reflect viral infection and death of neurons with associated host inflammatory responses. General autopsy findings have not suggested infection outside of the central nervous system, except in one patient who presented with orchitis and was found to have chronic inflammation and positive Powassan virus immunohistochemistry (IHC) in testicle sections14,16.
In contrast to many other causes of CNS viral infections, including herpesviruses, polyomaviruses, and rabies virus, no viral inclusions have been seen in biopsy or autopsy sections for any of the reported cases of Powassan virus, which is typical for arboviral encephalitis. Powassan virus immunohistochemistry (IHC), available at reference laboratories including the CDC Infectious Diseases Pathology Branch, has identified viral antigen in neurons and macrophages, likely representing the primary cellular targets in the CNS and subsequent clearance of infected cells14,16,19,21,68. Overall, the gross and histopathological findings of Powassan virus infection are consistent with other causes of viral meningoencephalitis, thus definitive diagnosis requires detection of viral antigen by IHC or viral RNA by in situ hybridization (Figure 2E–F) or molecular studies.
Therapeutic Options and Clinical Outcomes
Treatment for Powassan virus is largely supportive, and no antiviral therapies have been tested to date. Because much of the disease is believed to be due to neuro-inflammation, some patients have been treated with steroids, sometimes with apparent benefit19,66 and other times without16,71. Given the small number of cases and the difficulty in assessing confounders such as age and comorbidity, there is too little data to support or refute the use of steroids. Similarly, intravenous immunoglobulin (IVIG) has been used in some cases66, based on reports of success in WNV72, but its effectiveness is unclear62. The overall mortality for Powassan virus is around 10%, as discussed above and in prior reviews73. Many patients have substantial long-term morbidity, including persistent neurological symptoms in approximately a third of patients who survive31.
Discussion
Given the substantial morbidity and mortality associated with known cases of Powassan virus encephalitis, as well as the likelihood that exposure and infection are more common than appreciated, there is substantial need to better understand the pathogenesis of this emerging virus and develop preventative and therapeutic strategies.
In vitro and animal studies of Powassan virus and the closely related TBEV suggest several salient features of pathogenesis. First, tick saliva enhances transmission and neuroinvasion74,75, likely by stimulation of an inflammatory response and recruitment of target cells. Thus, interventions targeting tick saliva represent an intriguing strategy to prevent this and other tick-borne infections36. Following transmission, dendritic cells and macrophages are thought to be among the first cells infected, allowing transport to lymph nodes, virus replication, and viremia76. Mechanisms of neuroinvasion are poorly understood, though a recent study suggests possible crossing of the blood-brain barrier through vascular endothelial cells77. Neuropathogenesis occurs through direct infection of neurons, and astrocytes restrict replication78. Interestingly, while laboratory mice (BALB/c and C57BL/6) experience lethal infection with Powassan virus, the white-footed mouse Peromyscus leucopus is refractory to intraperitoneal infection and experiences only mild brain inflammation after intracranial infection79. This suggests the presence of one or more restriction factors10,79, which RNAseq analysis suggests may be an interferon stimulated gene80. As an interesting parallel, TRIM79alpha was shown to restrict TBEV by degrading the NS5 polymerase81. Improved understanding of Powassan virus pathogenesis and host-virus interactions is needed to guide intervention strategies.
Currently, no antiviral medications are available to treat Powassan virus, though preclinical studies have identified several possibilities82,83. Vaccination is another compelling avenue of future research, especially given the successful implementation of vaccines against TBEV. Unfortunately, TBEV vaccines themselves do not provide cross-protection against POWV, as they do for other tick-borne flaviviruses84. Recently, a lipid nanoparticle mRNA vaccine targeting the Powassan virus premembrane and envelope genes was shown to protect mice85. As further research into pathogenesis, treatment and prevention continues, minimizing the effects of Powassan virus infection will rely on ecological approaches to decrease tick population, as well as individual practices for tick avoidance and removal36.
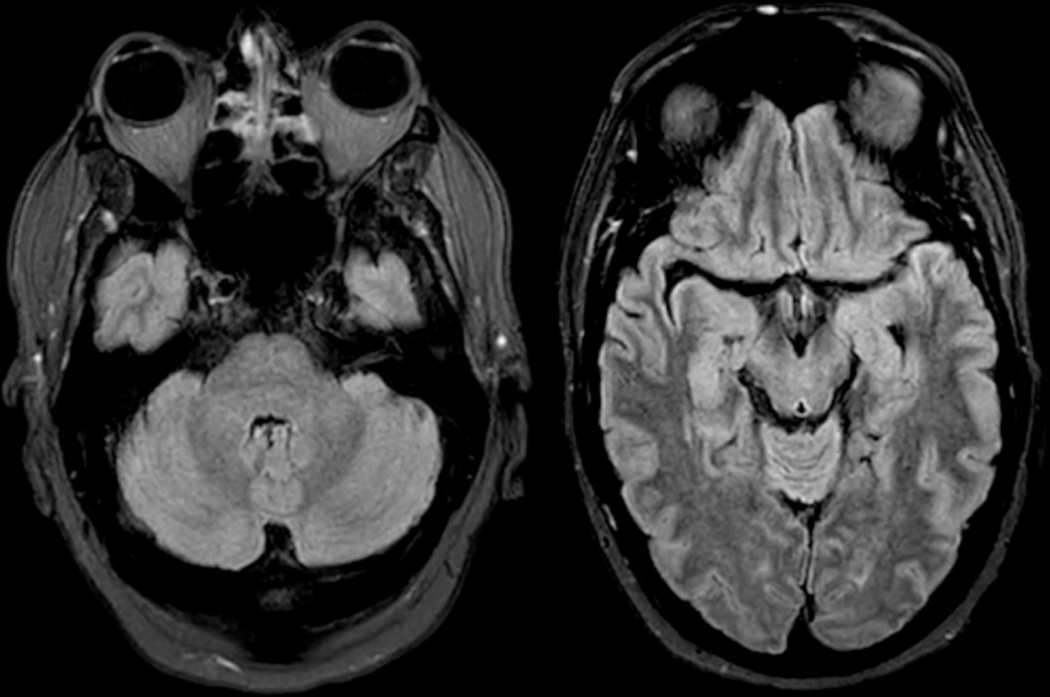
Figure 4: Brain MRI -.
MRI from patient in Box 2 at four days from initial symptom onset shows mild T2/FLAIR hyperintensity in the cerebellar vermis, pons, midbrain, and medial temporal lobes. No significant contrast enhancement or diffusion restriction was identified.
Box 2. Case Title: 58-year-old immunocompromised man with subacute neurological symptoms.
Case Presentation:
A 58-year-old man with a history of hypertension, hyperlipidemia, hypothyroidism, non-alcoholic fatty liver disease, remote Hodgkin’s lymphoma treated with bone marrow transplant and rituximab, and hypogammaglobulinemia requiring IVIG infusions every 6 weeks, presented with 1 to 2 days of gradual onset vertical diplopia, ataxia, dysarthria, and short-term memory loss. He reported a subjective fever. On exam, although alert and oriented, he had profoundly impaired short-term memory and an odd affect with mildly elevated mood and inappropriate laughter at times. MRI showed mild T2/FLAIR hyperintensity in the cerebellar vermis, pons, midbrain, and medial temporal lobes (left greater than right), with no significant contrast enhancement or diffusion restriction (Figure 4). A lumbar puncture was performed at day 6 after symptom onset, with an opening pressure of 22 cm H2O, and cerebrospinal fluid analysis was notable for protein=84.7 mg/dL (normal 10–44 mg/dL), glucose=56 mg/dL (normal 40–70 mg/dL), and cell counts with 1 RBC/μL and 92 WBCs/μL (89% lymphocytes, 11% monocytes, 0% neutrophils). Testing for specific pathogens was negative, including Powassan virus IgM from CSF.
Clinical Questions:
What are potential causes of this patient’s illness? What additional testing could be ordered to confirm the diagnosis? What treatment should the patient receive?
Discussion:
The patient was empirically treated with acyclovir, which was stopped when testing for herpes simplex virus was negative. All targeted viral studies were negative. The patient was treated empirically with 1g methylprednisolone daily for 3 days and received scheduled intravenous immunoglobulin during admission. His symptoms improved during his hospital stay, with better speech, and regained ability to walk. He was discharged to a subacute care facility on hospital day 7 (approximately 13 days after symptom onset) with mild residual ataxia and had no residual side effects at one year follow up. Metagenomic next-generation sequencing performed on CSF was positive for Powassan virus.
Learning Point:
Powassan virus serology may be negative in patients with immunodeficiency, and molecular testing should be considered. In all patients with suspicion for Powassan virus infection and negative initial serology, convalescent serology testing should be pursued.
Key Points:
Powassan virus is an Ixodes-borne flavivirus that can cause severe encephalitis.
Prevalence studies in ticks, and seroprevalence studies in wild mammals and humans suggest that human exposure to Powassan virus is more common than currently appreciated, and non-neuroinvasive disease is likely under-recognized.
Diagnosis is typically established by serological testing of blood or cerebrospinal fluid, but can also be confirmed by RT-PCR or sequencing from cerebrospinal fluid or immunohistochemistry, in situ hybridization, or molecular testing of brain tissue.
Synopsis:
Powassan virus is an increasingly-recognized cause of severe encephalitis that is transmitted by Ixodes ticks. Given the nonspecific clinical, laboratory, and imaging features of Powassan virus disease, providers should consider it in patients with compatible exposures and request appropriate testing.
Clinics Care Points:
Testing for Powassan virus infection should be considered in Ixodes-endemic regions in patients presenting in the spring, summer, and fall. A high index of suspicion is needed, as symptoms and clinical evaluation can be nonspecific.
Diagnosis of Powassan virus infection is generally made by serology testing of blood or cerebrospinal fluid. In immunocompromised patients who may not mount sufficient antibody response, molecular testing can be helpful.
Treatment for Powassan virus is supportive. There is too little evidence to support or refute the use of high-dose steroids or intravenous immunoglobulin.
Acknowledgments
Disclosure Statement
The authors have no financial or commercial conflicts of interest to report. AP is funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K08AI139348. AP and IHS are funded by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R21NS119660.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anne Piantadosi, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta GA USA; Department of Medicine, Division of Infectious Diseases, Emory University School of Medicine, Atlanta GA USA.
Isaac H. Solomon, Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston MA USA.
References
- 1.Mandl CW, Holzmann H, Kunz C, Heinz FX. Complete genomic sequence of Powassan virus: evaluation of genetic elements in tick-borne versus mosquito-borne flaviviruses. Virology. May 1993;194(1):173–84. doi: 10.1006/viro.1993.1247 [DOI] [PubMed] [Google Scholar]
- 2.Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet. May 31 2008;371(9627):1861–71. doi: 10.1016/S0140-6736(08)60800-4 [DOI] [PubMed] [Google Scholar]
- 3.Gritsun TS, Nuttall PA, Gould EA. Tick-borne flaviviruses. Adv Virus Res. 2003;61:317–71. doi: 10.1016/s0065-3527(03)61008-0 [DOI] [PubMed] [Google Scholar]
- 4.Rigaud E, Jaulhac B, Garcia-Bonnet N, et al. Seroprevalence of seven pathogens transmitted by the Ixodes ricinus tick in forestry workers in France. Clin Microbiol Infect. Aug 2016;22(8):735 e1–9. doi: 10.1016/j.cmi.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 5.Thortveit ET, Aase A, Petersen LB, Lorentzen AR, Mygland A, Ljostad U. Human seroprevalence of antibodies to tick-borne microbes in southern Norway. Ticks Tick Borne Dis. Jul 2020;11(4):101410. doi: 10.1016/j.ttbdis.2020.101410 [DOI] [PubMed] [Google Scholar]
- 6.Svensson J, Christiansen CB, Persson KEM. A Serosurvey of Tick-Borne Encephalitis Virus in Sweden: Different Populations and Geographical Locations. Vector Borne Zoonotic Dis. Aug 2021;21(8):614–619. doi: 10.1089/vbz.2020.2763 [DOI] [PubMed] [Google Scholar]
- 7.Ruzek D, Avsic Zupanc T, Borde J, et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Res. Apr 2019;164:23–51. doi: 10.1016/j.antiviral.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 8.McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J. May 1 1959;80(9):708–11. [PMC free article] [PubMed] [Google Scholar]
- 9.Gholam BI, Puksa S, Provias JP. Powassan encephalitis: a case report with neuropathology and literature review. CMAJ. Nov 30 1999;161(11):1419–22. [PMC free article] [PubMed] [Google Scholar]
- 10.Telford SR 3rd, Armstrong PM, Katavolos P, et al. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis. Apr-Jun 1997;3(2):165–70. doi: 10.3201/eid0302.970209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of powassan virus by deer ticks. Am J Trop Med Hyg. Sep 2004;71(3):268–71. [PubMed] [Google Scholar]
- 12.Feder HM, Telford S, Goethert HK, Wormser GP. Powassan Virus Encephalitis Following Brief Attachment of Connecticut Deer Ticks. Clin Infect Dis. Oct 5 2021;73(7):e2350–e2354. doi: 10.1093/cid/ciaa1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell O, Krause PJ. The emergence of human Powassan virus infection in North America. Ticks Tick Borne Dis. Nov 2020;11(6):101540. doi: 10.1016/j.ttbdis.2020.101540 [DOI] [PubMed] [Google Scholar]
- 14.Normandin E, Solomon IH, Zamirpour S, et al. Powassan Virus Neuropathology and Genomic Diversity in Patients With Fatal Encephalitis. Open Forum Infect Dis. Oct 2020;7(10):ofaa392. doi: 10.1093/ofid/ofaa392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piantadosi A, Kanjilal S, Ganesh V, et al. Rapid Detection of Powassan Virus in a Patient With Encephalitis by Metagenomic Sequencing. Clin Infect Dis. Feb 10 2018;66(5):789–792. doi: 10.1093/cid/cix792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon IH, Spera KM, Ryan SL, et al. Fatal Powassan Encephalitis (Deer Tick Virus, Lineage II) in a Patient With Fever and Orchitis Receiving Rituximab. JAMA Neurol. Jun 1 2018;75(6):746–750. doi: 10.1001/jamaneurol.2018.0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng X, Achari A, Federman S, et al. Metagenomic sequencing with spiked primer enrichment for viral diagnostics and genomic surveillance. Nat Microbiol. Mar 2020;5(3):443–454. doi: 10.1038/s41564-019-0637-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavanaugh CE, Muscat PL, Telford SR, 3rd, et al. Fatal Deer Tick Virus Infection in Maine. Clin Infect Dis. Sep 15 2017;65(6):1043–1046. doi: 10.1093/cid/cix435 [DOI] [PubMed] [Google Scholar]
- 19.El Khoury MY, Camargo JF, White JL, et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, U.S.A. Emerg Infect Dis. Dec 2013;19(12):1926–33. doi: 10.3201/eid1912.130903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Khoury MY, Hull RC, Bryant PW, et al. Diagnosis of acute deer tick virus encephalitis. Clin Infect Dis. Feb 2013;56(4):e40–7. doi: 10.1093/cid/cis938 [DOI] [PubMed] [Google Scholar]
- 21.Tavakoli NP, Wang H, Dupuis M, et al. Fatal case of deer tick virus encephalitis. N Engl J Med. May 14 2009;360(20):2099–107. doi: 10.1056/NEJMoa0806326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costero A, Grayson MA. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari:Ixodidae). Am J Trop Med Hyg. Nov 1996;55(5):536–46. doi: 10.4269/ajtmh.1996.55.536 [DOI] [PubMed] [Google Scholar]
- 23.Goethert HK, Mather TN, Johnson RW, Telford SR 3rd., Incrimination of shrews as a reservoir for Powassan virus. Commun Biol. Nov 22 2021;4(1):1319. doi: 10.1038/s42003-021-02828-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebel GD, Campbell EN, Goethert HK, Spielman A, Telford SR, 3rd. Enzootic transmission of deer tick virus in New England and Wisconsin sites. Am J Trop Med Hyg. Jul-Aug 2000;63(1–2):36–42. doi: 10.4269/ajtmh.2000.63.36 [DOI] [PubMed] [Google Scholar]
- 25.Brackney DE, Nofchissey RA, Fitzpatrick KA, Brown IK, Ebel GD. Stable prevalence of Powassan virus in Ixodes scapularis in a northern Wisconsin focus. Am J Trop Med Hyg. Dec 2008;79(6):971–3. [PMC free article] [PubMed] [Google Scholar]
- 26.Knox KK, Thomm AM, Harrington YA, Ketter E, Patitucci JM, Carrigan DR. Powassan/Deer Tick Virus and Borrelia Burgdorferi Infection in Wisconsin Tick Populations. Vector Borne Zoonotic Dis. Jul 2017;17(7):463–466. doi: 10.1089/vbz.2016.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson JF, Armstrong PM. Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. Am J Trop Med Hyg. Oct 2012;87(4):754–9. doi: 10.4269/ajtmh.2012.12-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupuis AP 2nd, Peters RJ, Prusinski MA, Falco RC, Ostfeld RS, Kramer LD Isolation of deer tick virus (Powassan virus, lineage II) from Ixodes scapularis and detection of antibody in vertebrate hosts sampled in the Hudson Valley, New York State. Parasit Vectors. Jul 15 2013;6:185. doi: 10.1186/17563305-6-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aliota MT, Dupuis AP 2nd, Wilczek MP, Peters RJ, Ostfeld RS, Kramer LD The prevalence of zoonotic tick-borne pathogens in Ixodes scapularis collected in the Hudson Valley, New York State. Vector Borne Zoonotic Dis. Apr 2014;14(4):245–50. doi: 10.1089/vbz.2013.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma R, Cozens DW, Armstrong PM, Brackney DE. Vector competence of human-biting ticks Ixodes scapularis, Amblyomma americanum and Dermacentor variabilis for Powassan virus. Parasit Vectors. Sep 9 2021;14(1):466. doi: 10.1186/s13071-021-04974-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corrin T, Greig J, Harding S, Young I, Mascarenhas M, Waddell LA. Powassan virus, a scoping review of the global evidence. Zoonoses Public Health. Sep 2018;65(6):595–624. doi: 10.1111/zph.12485 [DOI] [PubMed] [Google Scholar]
- 32.Leonova GN, Kondratov IG, Ternovoi VA, et al. Characterization of Powassan viruses from Far Eastern Russia. Arch Virol. 2009;154(5):811–20. doi: 10.1007/s00705-009-0376-y [DOI] [PubMed] [Google Scholar]
- 33.Artsob H, Spence L, Th’ng C, et al. Arbovirus infections in several Ontario mammals, 1975–1980. Can J Vet Res. Jan 1986;50(1):42–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Allen SE, Jardine CM, Hooper-McGrevy K, et al. Serologic Evidence of Arthropod-Borne Virus Infections in Wild and Captive Ruminants in Ontario, Canada. Am J Trop Med Hyg. Nov 2020;103(5):2100–2107. doi: 10.4269/ajtmh.20-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nofchissey RA, Deardorff ER, Blevins TM, et al. Seroprevalence of Powassan virus in New England deer, 1979–2010. Am J Trop Med Hyg. Jun 2013;88(6):1159–62. doi: 10.4269/ajtmh.12-058636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisen RJ, Eisen L. The Blacklegged Tick, Ixodes scapularis: An Increasing Public Health Concern. Trends Parasitol. Apr 2018;34(4):295–309. doi: 10.1016/j.pt.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piantadosi A, Kanjilal S. Diagnostic Approach for Arboviral Infections in the United States. J Clin Microbiol. Nov 18 2020;58(12)doi: 10.1128/JCM.01926-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vahey GM, Mathis S, Martin SW, Gould CV, Staples JE, Lindsey NP. West Nile Virus and Other Domestic Nationally Notifiable Arboviral Diseases - United States, 2019. MMWR Morb Mortal Wkly Rep. Aug 13 2021;70(32):1069–1074. doi: 10.15585/mmwr.mm7032a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frost HM, Schotthoefer AM, Thomm AM, et al. Serologic Evidence of Powassan Virus Infection in Patients with Suspected Lyme Disease(1). Emerg Infect Dis. Aug 2017;23(8):1384–1388. doi: 10.3201/eid2308.161971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomm AM, Schotthoefer AM, Dupuis AP 2nd, et al. Development and Validation of a Serologic Test Panel for Detection of Powassan Virus Infection in U.S. Patients Residing in Regions Where Lyme Disease Is Endemic. mSphere. Jan-Feb 2018;3(1)doi: 10.1128/mSphere.00467-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vahey GM, Wilson N, McDonald E, et al. Seroprevalence of Powassan Virus Infection in an Area Experiencing a Cluster of Disease Cases: Sussex County, New Jersey, 2019. Open Forum Infect Dis. Mar 2022;9(3):ofac023. doi: 10.1093/ofid/ofac023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith RP Jr., Elias SP, Cavanaugh CE, et al. Seroprevalence of Borrelia burgdorferi, B. miyamotoi, and Powassan Virus in Residents Bitten by Ixodes Ticks, Maine, USA. Emerg Infect Dis. Apr 2019;25(4):804–807. doi: 10.3201/eid2504.180202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wormser GP, McKenna D, Scavarda C, et al. Co-infections in Persons with Early Lyme Disease, New York, USA. Emerg Infect Dis. Apr 2019;25(4):748–752. doi: 10.3201/eid2504.181509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.and CfDC, Prevention. Arboviral disease--United States, 1994. MMWR Morb Mortal Wkly Rep. Sep 8 1995;44(35):641–4. [PubMed] [Google Scholar]
- 45.Picheca C, Yogendrakumar V, Brooks JI, Torres C, Pringle E, Zwicker J. Polio-Like Manifestation of Powassan Virus Infection with Anterior Horn Cell Involvement, Canada. Emerg Infect Dis. Aug 2019;25(8):1609–1611. doi: 10.3201/eid2508.190399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitch WM, Artsob H. Powassan encephalitis in new brunswick. Can Fam Physician. Jul 1990;36:1289–90. [PMC free article] [PubMed] [Google Scholar]
- 47.Goldfield M, Austin SM, Black HC, Taylor BF, Altman R. A non-fatal human case of Powassan virus encephalitis. Am J Trop Med Hyg. Jan 1973;22(1):78–81. doi: 10.4269/ajtmh.1973.22.78 [DOI] [PubMed] [Google Scholar]
- 48.Deibel R, Srihongse S, Woodall JP Arboviruses in New York State: an attempt to determine the role of arboviruses in patients with viral encephalitis and meningitis. Am J Trop Med Hyg. May 1979;28(3):577–82. [PubMed] [Google Scholar]
- 49.Smith R, Woodall JP, Whitney E, et al. Powassan virus infection. A report of three human cases of encephalitis. Am J Dis Child. May 1974;127(5):691–3. doi: 10.1001/archpedi.1974.0211024007701050. [DOI] [PubMed] [Google Scholar]
- 50.Embil JA, Camfield P, Artsob H, Chase DP. Powassan virus encephalitis resembling herpes simplex encephalitis. Arch Intern Med. Feb 1983;143(2):341–3. [PubMed] [Google Scholar]
- 51.Hinten SR, Beckett GA, Gensheimer KF, et al. Increased recognition of Powassan encephalitis in the United States, 1999–2005. Vector Borne Zoonotic Dis. Dec 2008;8(6):733–40. doi: 10.1089/vbz.2008.0022 [DOI] [PubMed] [Google Scholar]
- 52.From the Centers for Disease Control and Prevention. Outbreak of Powassan encephalitis-Maine and Vermont, 1999–2001. JAMA. Oct 24–31 2001;286(16):1962–3. [PubMed] [Google Scholar]
- 53.Johnson DK, Staples JE, Sotir MJ, Warshauer DM, Davis JP. Tickborne Powassan virus infections among Wisconsin residents. WMJ. Apr 2010;109(2):91–7. [PubMed] [Google Scholar]
- 54.Krow-Lucal ER, Lindsey NP, Fischer M, Hills SL. Powassan Virus Disease in the United States, 2006–2016. Vector Borne Zoonotic Dis. Jun 2018;18(6):286–290. doi: 10.1089/vbz.2017.2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curren EJ, Lehman J, Kolsin J, et al. West Nile Virus and Other Nationally Notifiable Arboviral Diseases - United States, 2017. MMWR Morb Mortal Wkly Rep. Oct 19 2018;67(41):1137–1142. doi: 10.15585/mmwr.mm6741a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDonald E, Martin SW, Landry K, et al. West Nile Virus and Other Domestic Nationally Notifiable Arboviral Diseases - United States, 2018. MMWR Morb Mortal Wkly Rep. Aug 9 2019;68(31):673–678. doi: 10.15585/mmwr.mm6831a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laga AC, Mather TN, Duhaime RJ, Granter SR. Identification of Hard Ticks in the United States: A Practical Guide for Clinicians and Pathologists. Am J Dermatopathol. Jun 15 2021;doi: 10.1097/DAD.0000000000002005 [DOI] [PubMed] [Google Scholar]
- 58.Pritt BS. Scutal Index and Its Role in Guiding Prophylaxis for Lyme Disease Following Tick Bite. Clin Infect Dis. Aug 1 2018;67(4):617–618. doi: 10.1093/cid/ciy222 [DOI] [PubMed] [Google Scholar]
- 59.Castelli E, Caputo V, Morello V, Tomasino RM. Local reactions to tick bites. Am J Dermatopathol. Jun 2008;30(3):241–8. doi: 10.1097/DAD.0b013e3181676b60 [DOI] [PubMed] [Google Scholar]
- 60.Hermance ME, Santos RI, Kelly BC, Valbuena G, Thangamani S. Immune Cell Targets of Infection at the Tick-Skin Interface during Powassan Virus Transmission. PLoS One. 2016;11(5):e0155889. doi: 10.1371/journal.pone.0155889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hermance ME, Thangamani S. Utilization of RNA in situ Hybridization to Understand the Cellular Localization of Powassan Virus RNA at the Tick-Virus-Host Interface. Front Cell Infect Microbiol. 2020;10:172. doi: 10.3389/fcimb.2020.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging Cases of Powassan Virus Encephalitis in New England: Clinical Presentation, Imaging, and Review of the Literature. Clin Infect Dis. Mar 15 2016;62(6):707–713. doi: 10.1093/cid/civ1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nord JM, Goldberg NR. Novel Case of Multifocal Choroiditis following Powassan Virus Infection. Ocul Immunol Inflamm. Jul 20 2021:1–3. doi: 10.1080/09273948.2021.1929335 [DOI] [PubMed] [Google Scholar]
- 64.Lessell S, Collins TE. Ophthalmoplegia in Powassan encephalitis. Neurology. May 27 2003;60(10):1726–7. doi: 10.1212/01.wnl.0000064167.16083.02 [DOI] [PubMed] [Google Scholar]
- 65.Raval M, Singhal M, Guerrero D, Alonto A. Powassan virus infection: case series and literature review from a single institution. BMC Res Notes. Oct 30 2012;5:594. doi: 10.1186/1756-0500-5-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dumic I, Glomski B, Patel J, et al. “Double Trouble”: Severe Meningoencephalitis Due to Borrelia burgdorferi and Powassan Virus Co-Infection Successfully Treated with Intravenous Immunoglobulin. Am J Case Rep. Mar 24 2021;22:e929952. doi: 10.12659/AJCR.929952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor L, Condon T, Destrampe EM, et al. Powassan Virus Infection Likely Acquired Through Blood Transfusion Presenting as Encephalitis in a Kidney Transplant Recipient. Clin Infect Dis. Mar 15 2021;72(6):1051–1054. doi: 10.1093/cid/ciaa738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu Q, Matkovic E, Reagan-Steiner S, Denison AM, Osborn R, Salamat SM. A Fatal Case of Powassan Virus Encephalitis. J Neuropathol Exp Neurol. Nov 1 2020;79(11):1239–1243. doi: 10.1093/jnen/nlaa094 [DOI] [PubMed] [Google Scholar]
- 69.Tokarz R, Tagliafierro T, Cucura DM, Rochlin I, Sameroff S, Lipkin WI. Detection of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi, Borrelia miyamotoi, and Powassan Virus in Ticks by a Multiplex Real-Time Reverse Transcription-PCR Assay. mSphere. Mar-Apr 2017;2(2)doi: 10.1128/mSphere.00151-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi EE, Taylor RA. A case of Powassan viral hemorrhagic encephalitis involving bilateral thalami. Clin Neurol Neurosurg. Feb 2012;114(2):172–5. doi: 10.1016/j.clineuro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 71.Kroopnick A, Jia DT, Rimmer K, et al. Clinical use of steroids in viral central nervous system (CNS) infections: three challenging cases. J Neurovirol. Oct 2021;27(5):727–734. doi: 10.1007/s13365-02101008-5 [DOI] [PubMed] [Google Scholar]
- 72.Haley M, Retter AS, Fowler D, Gea-Banacloche J, O’Grady NP. The role for intravenous immunoglobulin in the treatment of West Nile virus encephalitis. Clin Infect Dis. Sep 15 2003;37(6):e88–90. doi: 10.1086/377172 [DOI] [PubMed] [Google Scholar]
- 73.Hermance ME, Thangamani S. Powassan Virus: An Emerging Arbovirus of Public Health Concern in North America. Vector Borne Zoonotic Dis. Jul 2017;17(7):453–462. doi: 10.1089/vbz.2017.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hermance ME, Thangamani S. Tick Saliva Enhances Powassan Virus Transmission to the Host, Influencing Its Dissemination and the Course of Disease. J Virol. Aug 2015;89(15):7852–60. doi: 10.1128/JVI.01056-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santos RI, Hermance ME, Reynolds ES, Thangamani S. Salivary gland extract from the deer tick, Ixodes scapularis, facilitates neuroinvasion by Powassan virus in BALB/c mice. Sci Rep. Oct 22 2021;11(1):20873. doi: 10.1038/s41598-021-00021-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kemenesi G, Banyai K. Tick-Borne Flaviviruses, with a Focus on Powassan Virus. Clin Microbiol Rev. Jan 2019;32(1)doi: 10.1128/CMR.00106-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conde JN, Sanchez-Vicente S, Saladino N, et al. Powassan Viruses Spread Cell to Cell during Direct Isolation from Ixodes Ticks and Persistently Infect Human Brain Endothelial Cells and Pericytes. J Virol. Jan 12 2022;96(1):e0168221. doi: 10.1128/JVI.01682-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lindqvist R, Mundt F, Gilthorpe JD, et al. Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects. J Neuroinflammation. Oct 24 2016;13(1):277. doi: 10.1186/s12974-016-0748-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mlera L, Meade-White K, Saturday G, Scott D, Bloom ME. Modeling Powassan virus infection in Peromyscus leucopus, a natural host. PLoS Negl Trop Dis. Jan 2017;11(1):e0005346. doi: 10.1371/journal.pntd.0005346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mlera L, Meade-White K, Dahlstrom E, et al. Peromyscus leucopus mouse brain transcriptome response to Powassan virus infection. J Neurovirol. Feb 2018;24(1):75–87. doi: 10.1007/s13365-0170596-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor RT, Lubick KJ, Robertson SJ, et al. TRIM79alpha, an interferon-stimulated gene product, restricts tick-borne encephalitis virus replication by degrading the viral RNA polymerase. Cell Host Microbe. Sep 15 2011;10(3):185–96. doi: 10.1016/j.chom.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eyer L, Svoboda P, Balvan J, et al. Broad-Spectrum Antiviral Activity of 3’-Deoxy-3’-Fluoroadenosine against Emerging Flaviviruses. Antimicrob Agents Chemother. Jan 20 2021;65(2)doi: 10.1128/AAC.01522-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flint M McMullan LK, Dodd KA, et al. Inhibitors of the tick-borne, hemorrhagic fever-associated flaviviruses. Antimicrob Agents Chemother. Jun 2014;58(6):3206–16. doi: 10.1128/AAC.02393-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McAuley AJ, Sawatsky B, Ksiazek T, et al. Cross-neutralisation of viruses of the tick-borne encephalitis complex following tick-borne encephalitis vaccination and/or infection. NPJ Vaccines. 2017;2:5. doi: 10.1038/s41541-017-0009-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.VanBlargan LA, Himansu S, Foreman BM, Ebel GD, Pierson TC, Diamond MS. An mRNA Vaccine Protects Mice against Multiple Tick-Transmitted Flavivirus Infections. Cell Rep. Dec 18 2018;25(12):33823392 e3. doi: 10.1016/j.celrep.2018.11.082 [DOI] [PMC free article] [PubMed] [Google Scholar]