Abstract
Introduction
To determine whether slowed gait and weakened grip strength independently, or together, better identify risk of cognitive decline or dementia.
Methods
Time to walk 3 meters and grip strength were measured in a randomized placebo‐controlled clinical trial involving community‐dwelling, initially cognitively healthy older adults (N = 19,114).
Results
Over a median 4.7 years follow‐up, slow gait and weak grip strength at baseline were independently associated with risk of incident dementia (hazard ratio [HR] = 1.44, 95% confidence interval [CI]: 1.19–1.73; and 1.24, 95% CI: 1.04–1.50, respectively) and cognitive decline (HR = 1.38, 95% CI: 1.26–1.51; and 1.04, 95% CI: 0.95–1.14, respectively) and when combined, were associated with 79% and 43% increase in risk of dementia and cognitive decline, respectively. Annual declines in gait and in grip over time showed similar results.
Discussion
Gait speed and grip strength are low‐cost markers that may be useful in the clinical setting to help identify and manage individuals at greater risk, or with early signs, of dementia, particularly when measured together.
Highlights
Grip strength and gait speed are effective predictors and markers of dementia.
Dementia risk is greater than cognitive decline risk with declines in gait or grip.
Decline in gait speed, more so than in grip strength, predicts greater dementia risk.
Greater risk prediction results from combining grip strength and gait speed.
Keywords: cognitive decline, dementia, gait speed, grip strength, motor performance, physical performance
1. BACKGROUND
Aging increases the risk of significant declines in cognitive (a precursor to dementia) and physical health, and can ultimately lead to loss of independence. 1 Global dementia numbers are estimated to rise from 57.4 million cases in 2019 to 152.8 million in 2050. 2 Numerous studies have reported a relationship between physical and cognitive function, such that those with poor physical performance exhibit low cognition. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 This suggests that age‐related cognitive and motor decline may share a common causation, or one may drive the other. Physical function impairment can precede cognitive decline in later life, 13 , 14 , 15 suggesting that declining physical function may predict future cognitive decline and dementia risk. 8 Better understanding of this association could enhance early detection of disease onset or higher risk individuals, resulting in improved prevention or delaying strategies.
The relationship between attenuated physical function and cognitive performance remains unclear, as illustrated by the full spectrum of physical impairment (none to severe) seen in those with and without dementia. Two commonly used physical function measures are grip strength and gait speed, which are measured relatively easily, without invasive procedures. Although studies have looked at these measures individually and found them to be associated with cognitive impairment, 4 , 13 , 16 , 17 , 18 , 19 none has reported whether prediction is improved when considered together.
This study aims to determine whether habitual gait speed and hand grip strength, assessed individually and in combination, are associated with the risk of cognitive decline or incident dementia. We hypothesize that both physical measures will be independent predictors of cognitive decline and dementia risk, and when considered together, will enhance risk prediction compared to either alone.
2. METHODS
2.1. Participants
ASPREE was a randomized placebo‐controlled trial of daily 100 mg aspirin in older adults. 20 The design, recruitment, and baseline characteristics of the ASPREE study have been previously reported. 21 , 22 Community‐dwelling individuals aged 70+ years (65+ if U.S. Blacks and Hispanics), were recruited from 2010 to 2014 through general practitioners (Australia) and clinic‐based mailing lists (United States), and followed until June 2017 (median 4.7 years follow‐up). Participants were required to be free from evidence of cardiovascular disease and major physical disability (ascertained by the ability to perform all six of the basic activities of daily living 23 without assistance or a lot of difficulty) and expected to survive for at least 5 years. Individuals with a self‐report or physician diagnosis of dementia, or a Modified Mini‐Mental State Examination (3MS) 24 score of < 78/100, were ineligible. A study population flow diagram is shown in Figure S1 in supporting information; 1052 participants died during follow‐up. Participants provided informed consent. Local ethics committees approved the study.
2.2. Gait speed and grip strength
Gait time and grip strength were measured at face‐to‐face visits at enrollment (i.e., baseline), years 2, 4, 6 and/or close‐out (2017) visits, by trained staff adhering to strict protocols. Gait speed was assessed as the time to walk 3 meters at habitual pace, performed twice, indoors on a flat surface from a standing start, with at least 1 meter spare at the end to ensure usual pace was maintained over the full course. The average time (total seconds for 3 meters) was used for analysis. Participants who used assistive devices, for example walking sticks or frames, were asked if they could walk the course unaided, but if not, were not excluded for doing so.
RESEARCH IN CONTEXT
Systematic Review: The relationship between age‐related declines in cognition and motor function is unclear. Literature evaluating the association between physical/motor function and cognitive function was reviewed using traditional sources (e.g., PubMed and preprint servers). We did not identify any studies examining the combined effect of multiple physical measures in relation to cognitive impairment risk.
Interpretation: We show that both gait time and grip strength are strong predictors of incident dementia and cognitive decline, but the novel combination of upper and lower limb functional assessments resulted in greater risk prediction and may identify earlier, individuals at risk or in early stage disease. This allows implementation of preventative or delaying interventions and thus, should be incorporated into routine health assessments.
Future directions: More research is needed to disentangle the potential contribution of conditions directly affecting physical functions (e.g., musculoskeletal disease) from the impacts of neurocognitive disease on physical performance.
Hand grip strength, in kilograms of force (kgf), was measured using spring‐type or hydraulic hand‐held dynamometers (Jamar; Lafayette Instruments). Staff checked dynamometers weekly for consistency and any devices with a high degree of variation were replaced. Calibration was performed annually either by a validated servicing agent (Australia), or through weighing a 5‐pound weight bag (United States). If a contraindication to performing the test on either hand (e.g., recent surgery, inflammation, severe pain or injury) existed, that hand was not included. Tests were conducted as per the American Society of Hand Therapists (ASHT) protocol; 25 seated, with the arm at the side of the body, wrist neutral and thumb facing up, elbow flexed to 90° ensuring neither the arm nor hand was resting on any support during the test. Dynamometer grip length was adjusted to fit the participant's hand size and a semi‐maximal practice trial performed. Participants completed a maximum of three trials per hand, received encouragement from staff for each trial, and completed one hand at a time with a 15‐ to 20‐second rest between each trial. Grip strength measures are reported as per the ASHT; 25 the mean left and right grip strength measurements were calculated, and the strongest of the two hands’ grip used in this analysis.
Population distributions for gait time and grip strength are shown in Figure S2 in supporting information.
2.3. Cognitive function
Cognitive assessments were administered by accredited staff at enrollment (i.e., baseline) and years 1, 3, 5, and 7, and/or close‐out (2017) visit, and included the 3MS 24 for global cognition, the Hopkins Verbal Learning Test–Revised (HVLT‐R) Delayed Recall task 26 for episodic memory, the single letter (F) Controlled Oral Word Association Test (COWAT) 27 for language and executive function, and the Symbol Digit Modalities Test (SDMT) 28 for psychomotor speed (Appendix S1 in supporting information has references to ASPREE normative data).
All reported cases (“triggers”) of suspected dementia were referred for further standardized cognitive and functional assessments. Triggers were defined as a 3MS score < 78/100, 29 a drop of > 10.15 points from the predicted score calculated by baseline 3MS adjusted for age and education, 30 a report of cognitive concerns to a specialist, clinician diagnosis of dementia, or prescription of cholinesterase inhibitors. An expert panel, blinded to treatment allocation and physical performance measures, adjudicated dementia cases according to Diagnostic and Statistical Manual of Mental Disorders 4th edition criteria. 31 This required evidence of memory impairment plus at least one of the following: aphasia, apraxia, agnosia, or executive dysfunction. Cognitive impairments must have caused significant impairment in social or occupational functioning and represented significant decline from a previous level of functioning.
Cognitive decline was defined as > 1.5 standard deviation (SD) decline in cognitive scores from baseline on the 3MS, HVLT‐R Delayed Recall, SDMT, or COWAT. This definition did not include participants with evidence of only a transient decline (e.g., > 1.5 SD drop at one follow‐up, but scoring above this threshold at a subsequent follow‐up).
2.4. Other participant characteristics
At enrollment, participants reported their age, sex, race, ethnicity, level of education, smoking status, and alcohol use. Measures included weight, height, body mass index (BMI; kg/m2), blood pressure, quality of life assessments, 32 and basic activities of daily living. 23 Morbidity or chronic condition was collected by self‐report, direct measure (e.g., blood pressure, blood pathology), or medical record review, and included hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, and emotional/psychiatric problems. 22 A score of 8+/30 on the 10‐item Center for Epidemiologic Studies Depression scale (CES‐D 10) was used for indicating depressive symptoms. 20 Mortality was collected via annual review of clinical records, and at trial end, via linkage with the National Death Indices in each country. 20
2.5. Statistical analysis
Participants were defined as having “slow” gait if they were in the slowest quintile (i.e., longest time to walk 3 meters) of the study population for their sex and height (Table S1 in supporting information). 13 Similarly, participants with “weak” grip strength were in the lowest quintile of grip strength of the study population within sex‐ and BMI‐specific categories (Table S2 in supporting information). Finally, participants were grouped into one of four categories based on the combination of their baseline grip strength and gait time (not weak and not slow, slow but not weak, weak but not slow, weak and slow). The overlap between baseline weak grip, and/or slow gait and the study population, is shown in Figure S3 in supporting information.
Cox proportional hazards (PH) regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) to examine the association between gait speed or grip strength at enrollment (either as continuous measures or categories), and dementia or cognitive decline. Age, sex, education, race/ethnicity, baseline cognitive status (3MS), living situation, smoking, alcohol, diabetes, and depression were included as covariates in Cox PH regression models. BMI and height were additional covariates in the analyses with the continuous measures of grip strength and gait speed. Aspirin was not included as our previous analyses showed no effect on dementia, 33 cognitive decline, 33 or physical disability 23 and subgroup analyses in each of these studies did not reveal any effect of aspirin across the subgroups. A non‐parametric cumulative incidence of dementia and cognitive decline, stratified by grip and gait groups, was calculated accounting for the competing risk of death. We tested and confirmed that the combination of both grip and gait in the model provides incremental improvement to the model fit compared to only gait, only grip, or no grip and no gait (Table S3 and Figure S4 in supporting information).
We investigated potential effect modification by grip on gait (and vice versa) and by sex, by including an interaction term in the models (Tables S4 and S5 in supporting information). The highest and lowest 2.5 percentiles of gait and grip (Figure S2) were excluded in the analysis with the continuous gait and grip, to maintain an assumption of linearity according to Martingale residuals. Proportionality assumptions were checked using a test based on Schoenfeld residuals and significance level of 0.05, and were all found to be satisfactory.
To investigate the association between the longitudinal changes in gait or grip with dementia or cognitive decline, a two‐stage approach was used (Figure S5 in supporting information). Baseline (enrollment) and year 2 grip and gait measurements from participants without dementia or cognitive decline in this time frame, were used in a mixed effects linear regression model to estimate participant‐specific intercept and slope (yearly change over time) for both measures, which were used in the second stage to determine associations with subsequent dementia and cognitive decline in Cox PH regression (Table S6 in supporting information).
3. RESULTS
Participants with incident dementia had a slower baseline gait (mean = 3.5 seconds/3 meter, 0.86 meters/second, 36% slow) and weaker baseline grip strength (mean = 26 kgf, 35% weak) compared to dementia‐free participants (gait: mean = 3.1 seconds/3 meter, 0.97 meters/second, 19% slow; grip: mean = 28 kgf, 20% weak; Table 1). A similar trend was observed for those who developed cognitive decline compared to those who did not. Participants diagnosed with dementia were older than those without cognitive decline. Participants without dementia and no cognitive decline had higher education level, were more likely to live with someone than alone, and not consume alcohol. There were no notable differences in other characteristics such as smoking, diabetes, depression, race/ethnicity, or sex.
TABLE 1.
Characteristics of study participants at enrollment (i.e., baseline) into the ASPREE trial
| ASPREE | ASPREE participants with known grip and gait at baseline | ASPREE participants with known grip and gait at baseline and defined cognitive decline | |||||
|---|---|---|---|---|---|---|---|
| Overall | All | Dementia a | Cognitive decline a | ||||
| No | Yes | All | No | Yes | |||
| (N = 19,114) | (N = 18,729) | (N = 18,171) | (N = 558) | (N = 17,668) | (N = 14,895) | (N = 2773) | |
| Gait time over 3 meters (seconds) | |||||||
| Mean (SD) | 3.2 (± 0.95) | 3.1 (± 0.94) | 3.1 (± 0.93) | 3.5 (± 1.0) | 3.1 (± 0.91) | 3.1 (± 0.86) | 3.4 (± 1.1) |
| Slow gait b | |||||||
| No | 15198 (80 %) | 15024 (80 %) | 14666 (81 %) | 358 (64 %) | 14306 (81 %) | 12298 (83 %) | 2008 (72 %) |
| Yes | 3783 (20 %) | 3705 (20 %) | 3505 (19 %) | 200 (36 %) | 3362 (19 %) | 2597 (17 %) | 765 (28 %) |
| Grip strongest hand (kgf) | |||||||
| Mean (SD) | 28 (± 10) | 28 (± 10) | 28 (± 10) | 26 (± 11) | 28 (± 10) | 28 (± 10) | 27 (± 10) |
| Weak grip b | |||||||
| No | 14922 (78 %) | 14862 (79 %) | 14501 (80 %) | 361 (65 %) | 14076 (80 %) | 12022 (81 %) | 2054 (74 %) |
| Yes | 3889 (20 %) | 3867 (21 %) | 3670 (20 %) | 197 (35 %) | 3592 (20 %) | 2873 (19 %) | 719 (26 %) |
| Age (years) | |||||||
| Mean (SD) | 75 (± 4.5) | 75 (± 4.5) | 75 (± 4.5) | 78 (± 5.1) | 75 (± 4.5) | 75 (± 4.3) | 76 (± 5.0) |
| Sex | |||||||
| Men | 8332 (44 %) | 8196 (44 %) | 7931 (44 %) | 265 (47 %) | 7768 (44 %) | 6511 (44 %) | 1257 (45 %) |
| Women | 10782 (56 %) | 10533 (56 %) | 10240 (56 %) | 293 (53 %) | 9900 (56 %) | 8384 (56 %) | 1516 (55 %) |
| Race/ethnicity | |||||||
| White AUS | 16361 (86 %) | 16052 (86 %) | 15594 (86 %) | 458 (82 %) | 15229 (86 %) | 12872 (86 %) | 2357 (85 %) |
| White US | 1088 (6 %) | 1068 (6 %) | 1014 (6 %) | 54 (10 %) | 1016 (6 %) | 866 (6 %) | 150 (5 %) |
| Black | 901 (5 %) | 866 (5 %) | 839 (5 %) | 27 (5 %) | 744 (4 %) | 579 (4 %) | 165 (6 %) |
| Hispanic | 488 (3 %) | 475 (3 %) | 466 (3 %) | 9 (2 %) | 423 (2 %) | 358 (2 %) | 65 (2 %) |
| Asian | 164 (1 %) | 160 (1 %) | 155 (1 %) | 5 (1 %) | 151 (1 %) | 134 (1 %) | 17 (1 %) |
| Education c | |||||||
| <12 years | 8636 (45 %) | 8467 (45 %) | 8209 (45 %) | 258 (46 %) | 7965 (45 %) | 6652 (45 %) | 1313 (47 %) |
| 12–15 years | 5574 (29 %) | 5455 (29 %) | 5276 (29 %) | 179 (32 %) | 5116 (29 %) | 4292 (29 %) | 824 (30 %) |
| 16+ years | 4903 (26 %) | 4806 (26 %) | 4685 (26 %) | 121 (22 %) | 4587 (26 %) | 3951 (27 %) | 636 (23 %) |
| Living situation | |||||||
| Alone | 6251 (33 %) | 6093 (33 %) | 5870 (32 %) | 223 (40 %) | 5681 (32 %) | 4657 (31 %) | 1024 (37 %) |
| With someone | 12780 (67 %) | 12556 (67 %) | 12223 (67 %) | 333 (60 %) | 11917 (67 %) | 10184 (68 %) | 1733 (62 %) |
| Smoking | |||||||
| Never | 10580 (55 %) | 10374 (55 %) | 10057 (55 %) | 317 (57 %) | 9847 (56 %) | 8333 (56 %) | 1514 (55 %) |
| Former | 7799 (41 %) | 7637 (41 %) | 7411 (41 %) | 226 (41 %) | 7193 (41 %) | 6053 (41 %) | 1140 (41 %) |
| Current | 735 (4 %) | 718 (4 %) | 703 (4 %) | 15 (3 %) | 628 (4 %) | 509 (3 %) | 119 (4 %) |
| Alcohol | |||||||
| Never | 3336 (17 %) | 3258 (17 %) | 3139 (17 %) | 119 (21 %) | 3025 (17 %) | 2506 (17 %) | 519 (19 %) |
| Former | 1136 (6 %) | 1110 (6 %) | 1064 (6 %) | 46 (8 %) | 1011 (6 %) | 823 (6 %) | 188 (7 %) |
| Current | 14642 (77 %) | 14361 (77 %) | 13968 (77 %) | 393 (70 %) | 13632 (77 %) | 11566 (78 %) | 2066 (75 %) |
| Height (m) | |||||||
| Mean (SD) | 1.7 (± 0.09) | 1.7 (± 0.09) | 1.7 (± 0.09) | 1.6 (± 0.09) | 1.7 (± 0.09) | 1.7 (± 0.09) | 1.7 (± 0.09) |
| BMI (kg/m 2) | |||||||
| Mean (SD) | 28 (± 4.7) | 28 (± 4.7) | 28 (± 4.7) | 27 (± 4.7) | 28 (± 4.7) | 28 (± 4.6) | 28 (± 5.0) |
| Diabetes d | |||||||
| No | 17069 (89 %) | 16720 (89 %) | 16227 (89 %) | 493 (88 %) | 15795 (89 %) | 13355 (90 %) | 2440 (88 %) |
| Yes | 2045 (11 %) | 2009 (11 %) | 1944 (11 %) | 65 (12 %) | 1873 (11 %) | 1540 (10 %) | 333 (12 %) |
| Depression c , d | |||||||
| No | 17231 (90 %) | 16886 (90 %) | 16397 (90 %) | 489 (88 %) | 15972 (90 %) | 13517 (91 %) | 2455 (89 %) |
| Yes | 1879 (10 %) | 1839 (10 %) | 1770 (10 %) | 69 (12 %) | 1693 (10 %) | 1375 (9 %) | 318 (11 %) |
Abbreviations: BMI, body mass index; kgf, kilograms of force; SD, standard deviation.
Dementia and cognitive decline categories are based on events occurring after a median of 4.7 years follow‐up.
Slow gait is defined as the highest quintile of gait (i.e., longest time to walk 3 meters [in seconds]), adjusted for sex and height. Weak grip strength is defined as the lowest quintile of grip strength on the strongest hand (kgf), adjusted for sex and BMI.
One participant has a missing education field and four participants have missing depression fields.
Diabetes was based on participants’ report of diabetes mellitus or a fasting glucose level of at least 126 mg per deciliter (≥7 mmol per liter) or receipt of treatment for diabetes. Depression is defined as a self‐report of depression, or a baseline Center for Epidemiological Studies 10 item (CES‐D 10) score of 8 or more.
Cumulative incidence and event rates of dementia and cognitive decline are shown in Figure 1 (A and B, respectively). The incidence rates of dementia for those with only weak grip (group 2) or only slow gait (group 3) were similar, and were higher than when neither gait nor grip were slow or weak (group 1), while the incidence rates for those with both weak grip and slow gait (group 4) were the highest of all groups. Similarly, the incidence rates of cognitive decline for those with both weak grip and slow gait were the highest of all groups examined.
FIGURE 1.
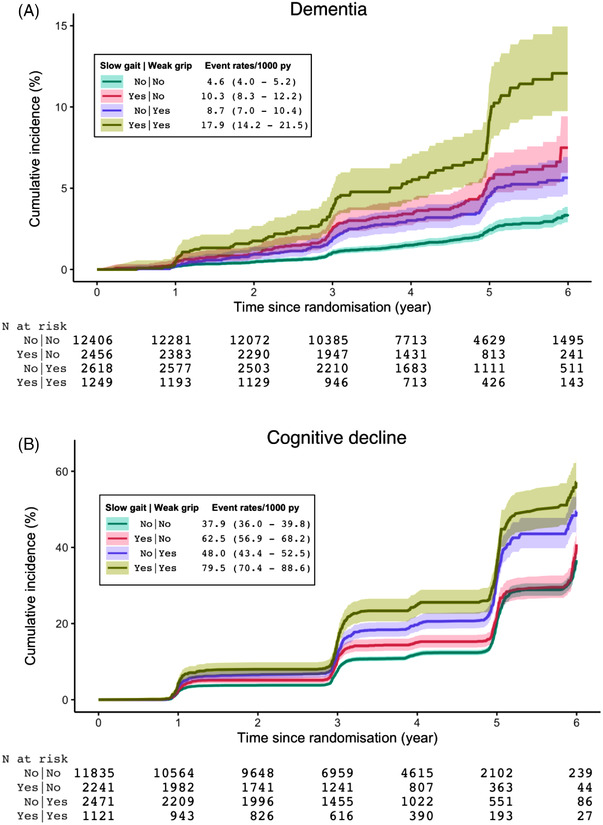
Cumulative incidence and event rates of dementia (A) and cognitive decline (B) by baseline gait and grip groups
The relationships between grip and gait and risk of dementia or cognitive decline were modelled using Cox PH regression models as continuous measures (Table 2) and as categorical measures (Table 3). Here, slower gait at baseline, by 1.5 seconds, was associated with 49% increased risk of dementia and 45% increased risk of cognitive decline, and this association was also observed for weaker grip at baseline, by 10 kgf, with dementia (28%), and a less pronounced risk of cognitive decline (6%; Table 2). Having both slower baseline gait (by 1.5 seconds) and weaker baseline grip (by 10 kgf) was associated with the highest increased risk of dementia (91%) or cognitive decline (54%). There was no evident effect modification of grip by gait, and vice versa (Table S4), or by sex (Table S5).
TABLE 2.
Risk of incident dementia and cognitive decline by baseline gait and grip continuous measures with effects estimated for specific between‐participant differentials
| Gait at baseline slower by a | Grip at baseline weaker by a | Risk of dementia (n = 17,072) | Risk of cognitive decline (n = 16,144) | ||
|---|---|---|---|---|---|
| HR (95% CI) b | P‐value | HR (95% CI) b | P‐value | ||
| 0 seconds | 0 kgf | 1.00 (reference) | 1.00 (reference) | ||
| 0.5 seconds | 0 kgf | 1.14 (1.07 to 1.22) | <.001 | 1.13 (1.10 to 1.17) | <.001 |
| 1 second | 0 kgf | 1.31 (1.14 to 1.50) | 1.28 (1.20 to 1.36) | ||
| 1.5 seconds | 0 kgf | 1.49 (1.22 to 1.83) | 1.45 (1.32 to 1.59) | ||
| 0 seconds | 0 kgf | 1.00 (reference) | 1.00 (reference) | ||
| 0 seconds | 5 kgf | 1.13 (1.05 to 1.22) | .002 | 1.03 (0.99 to 1.07) | .11 |
| 0 seconds | 10 kgf | 1.28 (1.10 to 1.50) | 1.06 (0.99 to 1.14) | ||
| 0 seconds | 0 kgf | 1.00 (reference) | 1.00 (reference) | ||
| 0.5 seconds | 5 kgf | 1.29 (1.17 to 1.43) | <.001 | 1.16 (1.11 to 1.22) | <.001 |
| 1 second | 10 kgf | 1.67 (1.38 to 2.03) | 1.36 (1.24 to 1.48) | ||
| 1.5 seconds | 10 kgf | 1.91 (1.50 to 2.43) | 1.54 (1.37 to 1.72) | ||
Note: The 2.5 percentiles of extreme low and high gait measurements (less than 2.04 seconds and more than 5.5 seconds, respectively, out of the whole range from 1 to 21.95 seconds of all gait time measurements) and grip strength measurements (less than 11.33 and more than 48.67 kgf, out of the whole range from 0 to 98 kgf) at baseline observations (Figure S1A,B in supporting information) were excluded from the analysis to maintain a reasonable linearity assumption and to exclude influential outliers.
Abbreviations: HR (95% CI), hazard ratio (95% confidence interval); kgf, kilograms of force.
The gait and grip variables were included as continuous covariates in the model and hazard ratios are presented for specific combinations of grip strength and gait speed differentials, for example, compared to a reference group of participants with specific gait speed and grip strength, a group of participants who take 0.5 seconds longer and have 5 kgf weaker grip have 39% increased risk of incident dementia.
Adjusted for age, sex, education, race/ethnicity, baseline cognition (Modified Mini‐Mental State Examination), diabetes, living situation, depression, smoking, and alcohol.
TABLE 3.
Risk of incident dementia and cognitive decline by baseline gait and grip categories a
| Risk of dementia (n = 18,724) | Cognitive decline (n = 17,665) | ||||
|---|---|---|---|---|---|
| Baseline variable | HR (95% CI) b | P‐value | HR (95% CI) b | P‐value | |
| Slow gait c | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 1.44 (1.19 to 1.73) | <.001 | 1.38 (1.26 to 1.51) | <.001 | |
| Weak grip c | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 1.24 (1.04 to 1.50) | .02 | 1.04 (0.95 to 1.14) | .38 | |
| Weak grip and slow gait | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 1.79 (1.40 to 2.29) | <.001 | 1.43 (1.27 to 1.62) | <.001 | |
Abbreviations: HR (95% CI), hazard ratio (95% confidence interval); kgf, kilograms of force.
For slow gait, the reference includes those with weak grip; for weak grip, the reference includes those with slow gait; but for weak grip and slow gait, the reference is those with neither weak grip nor slow gait.
HRs are adjusted for age, sex, education, race/ethnicity, baseline cognition (Modified Mini‐Mental State Examination), diabetes, living situation, depression, smoking and alcohol.
The slow gait category is those participants in the slowest quintile (i.e., the longest time to walk 3 meters), for their sex and height. The weak grip category is those participants in the lowest quintile of grip strength (kgf) for their sex and body mass index.
The categorical analysis of the association between the risk of dementia or cognitive decline with slow gait or weak grip at baseline, adjusting for one another, showed similar associations: slow gait independent of grip, or weak grip independent of gait, were associated with increased risk of dementia (44% and 24%, respectively) or cognitive decline (38% and 4%, respectively; Table 3). Similarly, there was no effect modification of gait by grip, or vice versa (Table S4), nor by sex (Table S5). For participants with both slow gait and weak grip, the risk was estimated to be highest (by 79% and 43% for dementia and cognitive decline, respectively) compared to participants with neither slow gait nor weak grip.
Annual declines in either gait or grip were associated with increased risk of dementia and cognitive decline (Table 4 and Figure 2). Risk was greatest when both gait and grip changed simultaneously (increased risk of 89% for dementia and 55% for cognitive decline). Smaller annual changes in combined grip and gait were associated with the same risk of dementia or cognitive decline as the larger changes in only grip, or gait, alone (Figure 2).
TABLE 4.
The association between yearly decline in grip and/or gait and time to incident dementia and cognitive decline
| Gait time increase per year a | Grip strength decrease per year a | Risk of dementia | Risk of cognitive decline | ||
|---|---|---|---|---|---|
| (N = 16,375, 86% of ASPREE) | (N = 13,914, 73% of ASPREE) | ||||
| HR (95% CI) b | P‐value | HR (95% CI) b | P‐value | ||
| 0 seconds | 0 kgf | 1.00 (reference) | 1.00 (reference) | ||
| 0.5 seconds | 0 kgf | 1.21 (1.09 to 1.34) | <.001 | 1.18 (1.11 to 1.24) | <.001 |
| 0 seconds | 0 kgf | 1.00 (reference) | 1.00 (reference) | ||
| 0 seconds | 5 kgf | 1.56 (1.22 to 2.00) | <.001 | 1.32 (1.18 to 1.47) | <.001 |
| 0 seconds | 0 kgf | 1.00 (reference) | 1.00 (reference) | ||
| 0.5 seconds | 5 kgf | 1.89 (1.47 to 2.43) | <.001 | 1.55 (1.38 to 1.74) | <.001 |
Abbreviations: HR (95% CI), hazard ratio (95% confidence interval); kgf, kilograms of force.
Individual yearly change: baseline and year 2 grip and gait measurements were used to estimate the individual annual change. Participants with a diagnosis of dementia or cognitive decline in the first 2 years were excluded, as were participants whose follow‐up time was under 2 years and who did not have gait and grip measured at baseline and 2‐year follow‐up. Cox proportional hazards regression models were time to event starting at year 2.
HRs are adjusted for age, sex, education, race/ethnicity, baseline cognition (Modified Mini‐Mental State Examination), depression, smoking, alcohol, living situation, diabetes, and gait and grip intercepts (from the mixed effects model).
FIGURE 2.
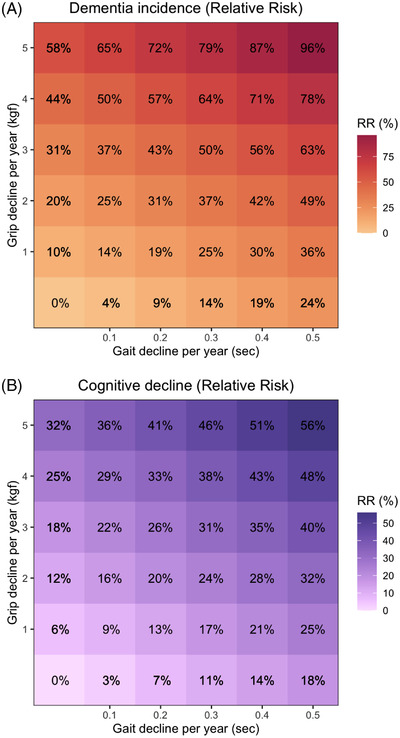
The percent risk of incident dementia (A) and cognitive decline (B) associated with gait and grip decline per year. Abbreviations: kgf, kilograms of force; RR, relative risk.
4. DISCUSSION
In this cohort of community‐dwelling adults, aged predominantly 70+ years, slow gait or weak grip strength at baseline, and declines in performance over time, were independently associated with cognitive decline or incident dementia. Risk was greatest for individuals with both slow gait and weak grip strength compared to those with declines in either measure alone, highlighting the benefit of including both measures in assessments of older individuals to identify those at risk of cognitive impairment. Timely identification of high‐risk or early‐stage individuals enables earlier assessment of cognition, appropriate planning, and implementation of interventions aimed at preventing or treating dementia.
Our data show that readily administered physical assessments of gait speed/time and grip strength can predict the development of cognitive decline or dementia. It is well established that slow gait speed predicts dementia and cognitive decline, 34 even in the “very old”, 35 but the link with grip strength is not as clear. While studies have shown weak grip strength, at baseline and longitudinally, is associated with cognitive dysfunction, 5 , 6 , 7 , 14 , 15 , 36 , 37 , 38 other studies have failed to find a relationship. 39 , 40 , 41 However, these latter studies had various limitations: most involved small sample sizes (< 600), or had few participants over 80 years, poorly documented physical capacity at enrollment, and used varying modes of dementia ascertainment.
A large meta‐analysis (37 studies) found three main motor domains, Parkinsonism and upper and lower limb function, were associated with increased incident dementia risk. However, results were again mixed for upper limb function and dementia. 4 Of the four studies describing grip strength, two found no association despite both involving > 2000 community‐dwelling older adults without dementia at baseline (although differing in dementia diagnosis criteria), one found a strong association (13% risk decrease with every quartile increase in grip strength, n = 2288), and another a weaker association (1% risk decrease with every one‐point increase in grip strength). However, this last study 36 analyzed the mean grip of both hands combined and involved older Catholic clergy members, whose physical fitness and cognitive function might be different from community‐dwelling adults. 4 Kueper et al. concluded that, while lower limb motor function was consistently associated with increased risk of dementia, grip strength associations were not as consistent, but may have been limited by involving populations with Parkinson's disease. 4 Another systematic review 42 showed that cognitive function and grip strength declined with aging, but with little to no evidence for longitudinal associations between rates of change of both, while a separate review 38 concluded that while grip strength and cognition are associated longitudinally, it is not clear “which variable at baseline affects the other in the long‐term.” Our data found strong correlations between the risk of dementia, and to a lesser extent, cognitive decline, with slow gait at baseline. However, associations with grip strength were not as pronounced.
Additionally, the literature describes variation in the association between weak grip and cognitive decline versus dementia. A recent meta‐analysis examining longitudinal relationships between baseline grip strength and risk of cognitive impairment found poorer grip strength was associated with a greater risk of cognitive decline (pooled HR = 1.99, 95% CI: 1.71–2.32, four studies) compared to risk of dementia (pooled HR = 1.54, 95% CI: 1.32–1.79, six studies), but included a number of middle‐aged population studies. 40 In contrast, our study found a strong association between weak grip and dementia (HR categorical = 1.24, 95% CI: 1.04–1.50, P = .02, HR continuous = 1.13–1.28 for 5 to 10 kgf loss in grip strength from baseline), but only a weak association with cognitive decline (HR categorical = 1.04, 95% CI: 0.95–1.14, P = .38; HR continuous = 1.03–1.06 for 5 to 10 kgf loss in grip strength). Thus, in our older cohort, weak grip strength was associated with a greater risk for dementia than for cognitive decline, and a similar trend was observed for slow gait.
A novel finding of our study was the impact of combined declines in grip strength and gait on the magnitude of risk for incident dementia or cognitive decline. To our knowledge, this combined effect has not been previously reported; although some studies report the co‐existence of declines in both, they were explored as adjusted variables in the same model, or in separate models. 4 , 13 , 16 , 17 , 18 , 19 One study reported that, when gait and grip were in the same model, one being adjusted for the other, only gait speed, independent of grip strength, was associated with processing speed, and no effect with either global cognition or processing speed was reported for grip, independent of gait. 13 Studies examining the association between cognition and the Fried frailty phenotype (two of the five components are grip and gait) have examined individual components separately or as a composite measure, but did not investigate combined grip and gait as we have done. 9 , 10 , 38
While both grip and gait require neuromuscular system input, our observed combined effect could reflect differences in complexity of the two tasks (grip strength is more a measure of the musculoskeletal system 17 , 36 whereas gait depends on a complex interplay of the nervous, musculoskeletal, and cardiorespiratory systems 11 ), or the differing regions of the brain that perform each action. Neuroimaging studies have shown variation in patterns of declines in the brain, possibly by dementia type; 12 for example, one study reported slower gait was related to poorer verbal memory, whereas weaker handgrip to poorer abstraction, although some overlap was observed. 37 The regions of the brain involved in performing gait and hand grip movements have some areas of overlap (e.g., cerebellum), 13 and areas that differ (hippocampus and basal ganglia for gait, and premotor cortex for grip). Simultaneous declines in both measures may reflect more widespread brain deterioration, making the combination a better predictor of dementia risk.
Several potential mechanisms to explain the relationship between gait and grip strength and cognition have been proposed. The accumulation of Alzheimer's disease (AD) pathology in motor‐related brain regions could contribute to motor function loss. 9 Many studies, some incorporating neuroimaging and post mortem analyses, have demonstrated the integrated biology of cognition (neural systems involved in planning, self‐motivating and monitoring goal‐directed effortful activities) and motor function, indicating common neuropathologies. 3 , 4 , 8 , 9 , 10 , 12 , 43 , 44 Cerebrovascular disease (e.g., infarcts, white matter hyperintensities [WMH], brain volume changes) may also play a role in cognitive dysfunction, 11 particularly because the co‐existence of AD and vascular disease is common; 43 however, longitudinal studies on WMH progression show mixed outcomes on motor function. 45 , 46 , 47
Other proposed mechanisms include shared pathogenic factors such as the increasing number of senescent cells during aging; secretion of pro‐inflammatory cytokines, growth factors, and proteases; 48 and low sex steroid levels leading to muscle loss. 49 Multiple health‐related conditions of aging, including multi‐morbidity and lifestyle factors, may affect systems that contribute to worsening of both cognitive and physical function. 50
Our study has several strengths. The large sample size of participants from metropolitan, regional city, and rural areas ensures population generalizability, with representatives from two countries (Australia and United States). All physical performance measures were administered by trained staff, participants were not cognitively or physically impaired at baseline, and were followed for a median of 4.7 years. Identification of dementia was performed by qualified clinicians using diagnostic criteria. This was a prospective, longitudinal study, in contrast to many other studies that are limited to cross‐sectional analyses.
Our study also has important limitations. It is a post hoc analysis examining associative findings, with the potential for reverse causation to play a role (cognitive dysfunction resulting in loss in motor function). The follow‐up period may be insufficient for dementia to manifest. The predictive ability of declines in gait and grip strength may need to be verified in an independent cohort, and ideally include neuroimaging or musculoskeletal studies to ascertain the potential contribution of cerebrovascular or muscular disease.
In this initially healthy older cohort, both gait time and grip strength are independent markers of incident dementia and of cognitive decline. Combining the annual change in both physical function measures yielded the greatest relative risks of cognitive decline and dementia. Thus, they can be regarded as early markers of cognitive decline and incident dementia and warrant consideration for inclusion in routine health assessments for older adults.
CONFLICTS OF INTEREST
Drs. Orchard, Polekhina, Ryan, Shah, Storey, Chong, Lockery, Wolfe, Reid, Newman, Espinoza, McNeil, Collyer, Callisaya, and Woods have nothing to disclose. Dr. Murray reports receiving consulting and travel fees from Bayer AG to present ASPREE primary results after their publication, and consulting fees from Alkahest, Inc. and reports grants from National Institute on Aging. Dr. Shah serves as a non‐compensated member of the Board of Directors of the Alzheimer's Association–Illinois Chapter. Dr. Shah's institution, Rush University Medical Center, receives research support for his role as a Site Principal Investigator or Site Sub‐Investigator for industry initiated clinical trials and research studies of Alzheimer's disease sponsored by Amylyx Pharmaceuticals, Inc., Athira Pharma, Eli Lilly & Co., Inc., Genentech, Inc., Merck & Co., Inc., Navidea Biopharmaceuticals, Novartis Pharmaceuticals, Inc., Roche Holdings AG, and Takeda Development Center Americas, Inc. Dr. Nelson has received consulting and travel fees from Bayer AG. Dr. Ward has received payment from Roche for participating in an advisory board meeting on Alzheimer's dementia. Author disclosures are available in the supporting information.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
Bayer AG provided aspirin and matching placebo for ASPREE. The authors acknowledge the dedicated and skilled staff in Australia and the United States for the conduct of the trial. The authors are also most grateful to the ASPREE participants, who so willingly volunteered for this study, and the general practitioners and medical clinics who supported the participants in the ASPREE study. Finally, the authors would like to thank Dr. Kathlyn Ronaldson for her valuable assistance in proof‐reading and editing the manuscript in preparation for submission. This work was supported by grants (U01AG029824 and U19AG062682) from the National Institute on Aging and the National Cancer Institute at the National Institutes of Health, by grants (334047 and 1127060) from the National Health and Medical Research Council of Australia, and by Monash University and the Victorian Cancer Agency. J.R. is funded by a NHMRC Dementia Research Leader Fellowship (APP1135727). C.M.R. is supported through a NHMRC Principal Research Fellowship (APP1136372). M.C. is supported by a NHMRC Dementia Research Leader Fellowship. The funders did not play a role in the study design, collection, analysis and interpretation of the data, nor in the writing of or decision to submit, this manuscript.
Orchard SG, Polekhina G, Ryan J, et al. Combination of gait speed and grip strength to predict cognitive decline and dementia. Alzheimer's Dement. 2022;14:e12353. 10.1002/dad2.12353
REFERENCES
- 1. Diem SJ, Lui L‐Y, Langsetmo L, et al. Effects of mobility and cognition on maintenance of independence and survival among women in late life. J Gerontol A Biol Sci Med Sci. 2017;73:1251‐1257. doi: 10.1093/gerona/glx209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022;7(2):e105‐e125. doi: 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT, Jr , Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age and Ageing. 2011;41:58‐64. doi: 10.1093/ageing/afr113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kueper JK, Speechley M, Lingum NR, Montero‐Odasso M. Motor function and incident dementia: a systematic review and meta‐analysis. Age and Ageing. 2017;46:729‐738. doi: 10.1093/ageing/afx084 [DOI] [PubMed] [Google Scholar]
- 5. Veronese N, Stubbs B, Trevisan C, et al. What physical performance measures predict incident cognitive decline among intact older adults? A 4.4 year follow up study. Exp Gerontol. 2016;81:110‐118. doi: 10.1016/j.exger.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 6. Wang T, Wu Y, Li W, et al. Weak grip strength and cognition predict functional limitation in older Europeans. J Am Geriatr Soc. 2019;67:93‐99. doi: 10.1111/jgs.15611 [DOI] [PubMed] [Google Scholar]
- 7. Jeong S, Kim J. Prospective association of handgrip strength with risk of new‐onset cognitive dysfunction in Korean adults: a 6‐year national cohort study. Tohoku J Exp Med. 2018;244:83‐91. doi: 10.1620/tjem.244.83 [DOI] [PubMed] [Google Scholar]
- 8. Bohannon RW. Grip strength: an indispensable biomarker for older adults. Clin Interv Aging. 2019;14:1681‐1691. doi: 10.2147/CIA.S194543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer's disease. Expert Rev Neurother. 2011;11:665‐676. doi: 10.1586/ern.11.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buchman AS, Yu L, Wilson RS, et al. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci. 2014;69:1536‐1544. doi: 10.1093/gerona/glu117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Callisaya ML, Beare R, Phan TG, Chen J, Srikanth VK. Global and regional associations of smaller cerebral gray and white matter volumes with gait in older people. PLoS One. 2014;9. doi: 10.1371/journal.pone.0084909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69:1375‐1388. doi: 10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chou M‐Y, Nishita Y, Nakagawa T, et al. Role of gait speed and grip strength in predicting 10‐year cognitive decline among community‐dwelling older people. BMC Geriatr. 2019;19:186. doi: 10.1186/s12877-019-1199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vancampfort D, Stubbs B, Firth J, Smith L, Swinnen N, Koyanagi A. Associations between handgrip strength and mild cognitive impairment in middle‐aged and older adults in six low‐and middle‐income countries. Int J Geriatr Psychiatr. 2019;34:609‐616. doi: 10.1002/gps.5061 [DOI] [PubMed] [Google Scholar]
- 15. Kim KH, Park SK, Lee DR, Lee J. The relationship between handgrip strength and cognitive function in Elderly Koreans over 8 years: a prospective population‐based study using Korean Longitudinal Study of Ageing. Korean J Fam Med. 2019;40:9‐15. doi: 10.4082/kjfm.17.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hooghiemstra AM, Ramakers IHGB, Sistermans N, et al. Gait speed and grip strength reflect cognitive impairment and are modestly related to incident cognitive decline in memory clinic patients with subjective cognitive decline and mild cognitive impairment: findings from the 4C study. J Gerontol A Biol Sci Med Sci. 2017;72(6):846‐854. doi: 10.1093/gerona/glx003 [DOI] [PubMed] [Google Scholar]
- 17. Cui M, Zhang S, Liu Y, Gang X, Wang G. Grip Strength and the risk of cognitive decline and dementia: a systematic review and meta‐analysis of longitudinal cohort studies. Front Aging Neurosci. 2021;13:625551. doi: 10.3389/fnagi.2021.625551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Forrest KY, Williams AM, Leeds MJ, Robare JF, Bechard TJ. Patterns and correlates of grip strength in older Americans. Curr Aging Sci. 2018;11:63‐70. doi: 10.2174/1874609810666171116164000 [DOI] [PubMed] [Google Scholar]
- 19. Zhang Q, Lu H, Pan S, Lin Y, Zhou K, Wang L. 6MWT performance and its correlations with VO2 and handgrip strength in home‐dwelling mid‐aged and older Chinese. Int J Environ Res Public Health. 2017;14:473. doi: 10.3390/ijerph14050473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability‐free survival in the healthy elderly. N Engl J Med. 2018;379:1499‐1508. doi: 10.1056/NEJMoa1800722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36:555‐564. doi: 10.1016/j.cct.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McNeil JJ, Woods RL, Nelson MR, et al. Baseline characteristics of participants in the ASPREE (ASPirin in reducing events in the elderly) study. J Gerontol A Biol Sci Med Sci. 2017;72:1586‐1593. doi: 10.1093/gerona/glw342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woods RL, Espinoza S, Thao LTP, et al. Effect of aspirin on activities of daily living disability in community‐dwelling older adults. J Gerontol A Biol Sci Med Sci. 2021;76:2007‐2014. doi: 10.1093/gerona/glaa316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teng EL, Chui HC. The Modified Mini‐Mental State (3MS) examination. J Clin Psychiatr. 1987;48:314‐318. [PubMed] [Google Scholar]
- 25. Fess E. Grip Strength. 2nd ed. American Society of Hand Therapists; 1992. [Google Scholar]
- 26. Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test–revised: normative data and analysis of inter‐form and test‐retest reliability. Clin Neuropsychol. 1998;12:43‐55. doi: 10.1076/clin.12.1.43.1726 [DOI] [Google Scholar]
- 27. Ross TP. The reliability of cluster and switch scores for the Controlled Oral Word Association Test. Arch Clin Neuropsychol. 2003;18:153‐164. doi: 10.1093/arclin/18.2.153 [DOI] [PubMed] [Google Scholar]
- 28. Smith A. Symbol digit modalities test (SDMT) manual (revised) Los Angeles: West Psychol Serv; 1982.
- 29. Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini‐Mental State Examination (3MS) as a screen for dementia. Can J Psychiatr. 2001;46:506‐510. doi: 10.1177/070674370104600604 [DOI] [PubMed] [Google Scholar]
- 30. Tombaugh TN. Test‐retest reliable coefficients and 5‐year change scores for the MMSE and 3MS. Arch Clin Neuropsychol. 2005;20:485‐503. doi: 10.1016/j.acn.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 31. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV. 4th ed. American Psychiatric Association; 1994. [Google Scholar]
- 32. Lim L‐Y, Fisher J. Use of the 12‐item short‐form (SF‐12) Health Survey in an Australian heart and stroke population. Qual Life Res. 1999;8:1‐8. [DOI] [PubMed] [Google Scholar]
- 33. Ryan J, Storey E, Murray AM, et al. Randomized placebo‐controlled trial of the effects of aspirin on dementia and cognitive decline. Neurol. 2020;95:e320. doi: 10.1212/WNL.0000000000009277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grande G, Triolo F, Nuara A, Welmer A‐K, Fratiglioni L, Vetrano DL. Measuring gait speed to better identify prodromal dementia. Exp Gerontol. 2019;124:110625. doi: 10.1016/j.exger.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 35. Öhlin J, Gustafson Y, Littbrand H, Olofsson B, Toots A. Low or declining gait speed is associated with risk of developing dementia over 5 years among people aged 85 years and over. J Aging Phys Activity. 2021;29:678‐685. doi: 10.1123/japa.2020-0266 [DOI] [PubMed] [Google Scholar]
- 36. Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Grip strength and the risk of incident Alzheimer's disease. Neuroepidemiol. 2007;29:66‐73. doi: 10.1159/000109498 [DOI] [PubMed] [Google Scholar]
- 37. Camargo EC, Weinstein G, Beiser AS, et al. Association of physical function with clinical and subclinical brain disease: the Framingham Offspring Study. J Alzheimer's Dis. 2016;53:1597‐1608. doi: 10.3233/JAD-160229 [DOI] [PubMed] [Google Scholar]
- 38. Kobayashi‐Cuya KE, Sakurai R, Suzuki H, Ogawa S, Takebayashi T, Fujiwara Y. Observational evidence of the association between handgrip strength, hand dexterity, and cognitive performance in community‐dwelling older adults: a systematic review. J Epidemiol. 2018;28:373‐381. doi: 10.2188/jea.JE20170041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sibbett RA, Russ TC, Allerhand M, Deary IJ, Starr JM. Physical fitness and dementia risk in the very old: a study of the Lothian Birth Cohort 1921. BMC Psychiatr. 2018;18:285. doi: 10.1186/s12888-018-1851-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doi T, Tsutsumimoto K, Nakakubo S, et al. Physical performance predictors for incident dementia among Japanese community‐dwelling older adults. Phys Ther. 2019;99:1132‐1140. doi: 10.1093/ptj/pzz077 [DOI] [PubMed] [Google Scholar]
- 41. Ritchie SJ, Tucker‐Drob EM, Starr JM, Deary IJ. Do cognitive and physical functions age in concert from age 70 to 76? Evidence from the Lothian Birth Cohort 1936. Span J Psychol. 2016;19. doi: 10.1017/sjp.2016.85 [DOI] [PubMed] [Google Scholar]
- 42. Zammit AR, Robitaille A, Piccinin AM, Muniz‐Terrera G, Hofer SM. Associations between aging‐related changes in grip strength and cognitive function in older adults: a systematic review. J Gerontol: Ser A Biol Sci Med Sci. 2019;74:519‐527. doi: 10.1093/gerona/gly046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement. 2015;11:70‐98. doi: 10.1016/j.jalz.2014.04.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chong TTJ, Bonnelle V, Manohar S, et al. Dopamine enhances willingness to exert effort for reward in Parkinson's disease. Cortex. 2015;69:40‐46. doi: 10.1016/j.cortex.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurol. 2008;71:108‐113. doi: 10.1212/01.wnl.0000316799.86917.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moscufo N, Wolfson L, Meier D, et al. Mobility decline in the elderly relates to lesion accrual in the splenium of the corpus callosum. Age (Dordr). 2012;34:405‐414. doi: 10.1007/s11357-011-9242-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zheng JJ, Delbaere K, Close JC, et al. White matter hyperintensities are an independent predictor of physical decline in community‐dwelling older people. Gerontol. 2012;58:398‐406. doi: 10.1159/000337815 [DOI] [PubMed] [Google Scholar]
- 48. Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: a link between cancer and age‐related degenerative disease? Semin Cancer Biol. 2011;21:354‐359. doi: 10.1016/j.semcancer.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adriaensen W, Matheï C, van Pottelbergh G, et al. Significance of serum immune markers in identification of global functional impairment in the oldest old: cross‐sectional results from the BELFRAIL study. Age (Dordr). 2014;36:457‐467. doi: 10.1007/s11357-013-9558-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fabbri E, An Y, Zoli M, et al. Association between accelerated multimorbidity and age‐related cognitive decline in older Baltimore longitudinal study of aging participants without dementia. J Am Ger Soc. 2016;64:965‐972. doi: 10.1111/jgs.14092 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION


