Abstract
Introduction
Coronavirus disease 2019 (COVID‐19) has caused >3.5 million deaths worldwide and affected >160 million people. At least twice as many have been infected but remained asymptomatic or minimally symptomatic. COVID‐19 includes central nervous system manifestations mediated by inflammation and cerebrovascular, anoxic, and/or viral neurotoxicity mechanisms. More than one third of patients with COVID‐19 develop neurologic problems during the acute phase of the illness, including loss of sense of smell or taste, seizures, and stroke. Damage or functional changes to the brain may result in chronic sequelae. The risk of incident cognitive and neuropsychiatric complications appears independent from the severity of the original pulmonary illness. It behooves the scientific and medical community to attempt to understand the molecular and/or systemic factors linking COVID‐19 to neurologic illness, both short and long term.
Methods
This article describes what is known so far in terms of links among COVID‐19, the brain, neurological symptoms, and Alzheimer's disease (AD) and related dementias. We focus on risk factors and possible molecular, inflammatory, and viral mechanisms underlying neurological injury. We also provide a comprehensive description of the Alzheimer's Association Consortium on Chronic Neuropsychiatric Sequelae of SARS‐CoV‐2 infection (CNS SC2) harmonized methodology to address these questions using a worldwide network of researchers and institutions.
Results
Successful harmonization of designs and methods was achieved through a consensus process initially fragmented by specific interest groups (epidemiology, clinical assessments, cognitive evaluation, biomarkers, and neuroimaging). Conclusions from subcommittees were presented to the whole group and discussed extensively. Presently data collection is ongoing at 19 sites in 12 countries representing Asia, Africa, the Americas, and Europe.
Discussion
The Alzheimer's Association Global Consortium harmonized methodology is proposed as a model to study long‐term neurocognitive sequelae of SARS‐CoV‐2 infection.
Key Points
The following review describes what is known so far in terms of molecular and epidemiological links among COVID‐19, the brain, neurological symptoms, and AD and related dementias (ADRD)
The primary objective of this large‐scale collaboration is to clarify the pathogenesis of ADRD and to advance our understanding of the impact of a neurotropic virus on the long‐term risk of cognitive decline and other CNS sequelae. No available evidence supports the notion that cognitive impairment after SARS‐CoV‐2 infection is a form of dementia (ADRD or otherwise). The longitudinal methodologies espoused by the consortium are intended to provide data to answer this question as clearly as possible controlling for possible confounders. Our specific hypothesis is that SARS‐CoV‐2 triggers ADRD‐like pathology following the extended olfactory cortical network (EOCN) in older individuals with specific genetic susceptibility.
The proposed harmonization strategies and flexible study designs offer the possibility to include large samples of under‐represented racial and ethnic groups, creating a rich set of harmonized cohorts for future studies of the pathophysiology, determinants, long‐term consequences, and trends in cognitive aging, ADRD, and vascular disease.
We provide a framework for current and future studies to be carried out within the Consortium. and offers a “green paper” to the research community with a very broad, global base of support, on tools suitable for low‐ and middle‐income countries aimed to compare and combine future longitudinal data on the topic.
The Consortium proposes a combination of design and statistical methods as a means of approaching causal inference of the COVID‐19 neuropsychiatric sequelae. We expect that deep phenotyping of neuropsychiatric sequelae may provide a series of candidate syndromes with phenomenological and biological characterization that can be further explored. By generating high‐quality harmonized data across sites we aim to capture both descriptive and, where possible, causal associations.
Keywords: cognitive impairment, dementia, neuropsychiatric sequelae, predictors, SARS‐CoV‐2
1. INTRODUCTION
1.1. SARS‐CoV‐2 and the brain
Coronavirus disease 2019 (COVID‐19) has caused >5.6 million deaths worldwide and affected >450 million people. At least twice as many have been infected but remained asymptomatic or minimally symptomatic. Though initially understood as a respiratory illness, COVID‐19 includes central nervous system (CNS) manifestations mediated by inflammation, and cerebrovascular, anoxic, and/or viral neurotoxicity mechanisms. 1 More than one third of patients with COVID‐19 experience neurologic complications during the acute illness, including loss of sense of smell or taste, seizures, and stroke. Following the acute illness, the risk of incident neurological or psychiatric disorder remains elevated for at least 6 months. 2 , 3 , 4 , 5 Mounting evidence indicates that the risk of late cognitive and neuropsychiatric complications may be independent of the severity of the original pulmonary and systemic illness. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16
One possible account of the neurological complication of COVID‐19 is that the causative virus, severe acute respiratory syndrome coronavirus disease 2 (SARS‐CoV‐2), may directly invade the brain. Both SARS‐CoV‐2 and SARS‐CoV use human angiotensin‐converting enzyme 2 (ACE2) 17 receptors as the molecular mechanism for invading cells. These receptors are richly expressed in the brain and olfactory bulb. 18 , 19 It is reasonable then to consider whether SARS‐CoV‐2's effects on the olfactory bulb (resulting in anosmia) may extend into the olfactory cortical network. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 Experimental work in non‐human primates 28 and rodents 26 has provided evidence in support of this mechanism. Neuroimaging in sub‐acute COVID‐19 patients also provided evidence of regional involvement of the olfactory bulb and its first‐ and second‐order projections. 29 , 30 , 31 , 32 , 33 , 34 We note too that involvement of the olfactory cortical network in early Alzheimer's disease (AD) is well established, and olfactory dysfunction is a strong clinical correlate of mild cognitive impairment (MCI) in AD and other forms of dementia. 35 , 36 , 37
Other possible (or additive) pathological mechanisms that may underlie chronic neurological consequences of SARS‐CoV‐2 infection include persistent cytokine‐mediated inflammation, antibody‐mediated autoimmunity, and cerebrovascular pathology. These all contribute to the acute neurological complications of COVID‐19 and may act as predisposing factors or ongoing insults for chronic or progressive neurological impairment.
Given these concerning findings, it behooves the scientific and medical community to attempt to understand the molecular and/or systemic factors linking COVID‐19 to neurologic illness, both short and long term. The following review describes what is known so far in terms of links among COVID‐19, the brain, neurological symptoms, and AD and related dementias (ADRD), with a focus on risk factors and possible molecular, inflammatory, and viral pathways. We conclude with a description of the Alzheimer's Association Consortium on Chronic Neuropsychiatric Sequelae of SARS‐CoV‐2 infection (CNS SC2), which seeks to address these and other questions through an international consortium.
1.2. Evidence of lingering cognitive impairment after SARS‐CoV‐2 infection
There is significant evidence supporting a connection between cognitive impairment and coronavirus infection. After the coronavirus pandemics of 2002 and 2012, 20% of recovered individuals reported memory impairment. 38 An early report during the ongoing pandemic found that one third of individuals with COVID‐19 had dysexecutive syndrome at the time of hospital discharge. 38 Impaired cognitive abilities lead to poor occupational and functional outcomes, but the extent to which cognitive deficits contribute to long‐term disability in COVID‐19 survivors remains unknown. Cognitive impairment can precipitate or exacerbate existing mental health disorders, which in turn worsen cognitive dysfunction. 39 , 40 In a recent meta‐analysis and systematic review, the most common post‐COVID‐19 neurological symptoms were headache, nausea, vomiting, muscular pain, anosmia, and ageusia. 12 The same study reported that SARS‐CoV‐2 infection may result in cognitive impairment even after mild or asymptomatic infection. 12 , 13 , 14 , 15 Concerningly, even asymptomatic COVID‐19 individuals had lowered scores in visuoperception, naming, and fluency; those older than 60 years old fared the worst 16 whereas young, previously healthy individuals recovered within 4 months after infection. 41 In a sample of COVID‐19 patients discharged from critical care to rehabilitation, 80% had working memory, set‐shifting, attention, and processing speed deficits. 42 In two independent studies of patients discharged to home, clinically significant cognitive impairment persisted in 60% to 70% of patients 3 to 4 months after discharge, with verbal learning, psychomotor speed, and executive function most affected. 11 , 13 Finally, in two independent studies of patients assessed 6 months after hospital admission for mild to moderate COVID‐19, olfactory dysfunction and cognitive impairment were linearly predicted by older age but not disease severity. 43 , 44 Thus, there is a connection between COVID‐19 and lingering cognitive impairment. The data also suggest that even asymptomatic infections can result in cognitive dysfunction.
1.3. SARS‐CoV‐2 and the risk of early AD
The idea that infectious agents may contribute to the risk of AD was recently reaffirmed. 45 , 46 , 47 , 48 , 49 , 50 A meta‐analysis of >100,000 participants found that several viruses associated with a higher risk of AD, 51 and bacteria have also been implicated. 47 , 51 The presence of immunity to herpes simplex virus 1 (HSV‐1), the best studied pathogen, correlates with greater cognitive impairment 52 and increased neuropathological biomarkers of AD in humans. 53 , 54 In mice models, HSV‐1 infection increases the expression of amyloid precursor protein, 55 triggers the accumulation of amyloid beta (Aβ) and hyperphosphorylated tau, 54 , 56 , 57 and impairs adult hippocampal neurogenesis. 52 , 56 , 58
Of note, susceptibility to COVID‐19 is driven in part by risk factors that overlap with those of ADRD, including older age 60 , 61 and apolipoprotein E (APOE) ε4 status. 62 , 63 , 64 In regard to the latter, in vitro experiments show that human neurons derived from induced pluripotent stem cells (iPSCs) are more susceptible to SARS‐CoV‐2 infection and neurodegenerative changes if they carry APOE ε4/ε4 genotypes. 65 Given that ethnic minorities in both the United States and UK, 66 , 67 as well as in individuals globally who have blood type A, 67 , 68 are at higher risk of COVID‐19 complications and death, it appears that ancestry interacts (whether directly or through health disparities) with environmental factors to contribute to SARS‐CoV‐2–related disease susceptibility, and therefore potentially also COVID‐19–related ADRD risk. In short, after the acute pandemic recedes, its sequelae are likely to impact dementia research for years to come. 1 The possibility that such sequelae may deepen existing health inequities heightens the need to focus future research on the long‐term impact of SARS‐CoV‐2 infections on minoritized and unrepresented populations.
1.4. The complexity of AD causation
The total number of people living with dementia worldwide approaches 50 million and is projected to surpass 130 million by 2050, 69 the majority of whom have AD. Despite massive investments, no effective treatments are available. 70 , 71 , 72 , 73 Slow progress in understanding and treating AD may be due in large part to disease heterogeneity and the multiplicity of causal contributions. 69 , 74 However, dementia syndromes continue to be refined with contributions from neuropathology, longitudinal clinical assessments, advanced neuroimaging, and molecular markers. 69 , 73 , 75 The emerging view is that dementias comprise overlapping phenotypes linked to multiple biological substrates. 76 , 77 Studies of causation reveal contributions from genetic variations, lifestyle choices, and environmental risk factors, including infections, plus highlighted interactions among them. 69 , 74 , 78 , 79
Identification of causal genetic variation was expected to guide development of disease‐modifying treatments for dementia, yet at the time of this submission, the majority of heritability remains unexplained despite large lists of disease‐associated genetic variants. 69 Risk prediction improves when large numbers of genetic variants are combined into polygenic risk scores (PRS), but these successes are largely limited to populations of European ancestry. 80 , 81 , 82 When applied across ancestry groups, or even across different segments of the same ancestry, PRS performance deteriorates. 83 Therefore, the genetics of under‐represented minorities in genetic studies of ADRD represent a severe knowledge gap that increasingly may result in greater health disparities as precision medicine becomes the prevalent paradigm. 84
It is our firm belief that untangling the complexity of ADRD will require novel, data‐driven strategies that take advantage of complex datasets (neuropsychological, environmental, neuroimaging, genomics, blood‐based biomarkers), 85 , 86 , 87 deep learning and explanatory artificial intelligence, 88 and the inclusion of ancestral populations 89 to uncover naturally occurring data structures or architectures. Such an approach is discovery based and agnostic, allowing diagnostic heterogeneity and overlap to assist in the uncovering of specific biological mechanisms. A promising environmental factor that could be used in such an effort is SARS—CoV‐2 exposure, 18 , 20 , 28 , 90 , 91 , 92 , 93
1.5. Epidemiological factors predictive of cognitive impairment
Though sedentary lifestyle, smoking, and obesity (but not heavy alcohol consumption) are related to increased rates of COVID‐19 hospital admissions, 94 specific risks for transient or persistent cognitive impairment after SARS‐CoV‐2 infection have not yet been identified. Diabetes mellitus increases the risk for dementia and other severe outcomes after SARS‐CoV‐2 infection. 40 Diabetes is highly prevalent in certain demographics, such as Blacks and Latinos, and these groups also appear to be at higher risk for the neurological complications of COVID‐19. 95 Disparities in COVID‐19 hospitalizations and mortality according to ethnicity remain even after correcting for neighborhood, household crowding, smoking, body size, diabetes, and mental illness. 96
Age also appears to be a factor, as COVID‐19 patients who are ≥65 years of age have more severe systemic disease and higher rates of neurologic complications. It is already well known that COVID‐19 morbidity and mortality are very high in the elderly population, with 6 to 930 times higher likelihood of death compared to younger cohorts. The highest risks are among the most elderly (≥85 years) and older persons with medical comorbidities such as hypertension, diabetes, heart disease, and underlying respiratory illness. 60 Elderly patients with preexisting neurologic diseases are both more susceptible to severe COVID‐19 infection and show higher rates of mortality than their neurologically healthy counterparts. 60 , 61 Most intriguingly, in a very large study of the UK Biobank, the APOE ε4/ε4 genotype was associated with COVID‐19 test positivity at genome‐wide significance in individuals of European ancestry, and the APOE ε4/ε4 genotype was also associated with a 4‐fold increase in mortality after testing positive for COVID‐19. 33 This finding was replicated in an independent community sample in Spain. 64
1.6. Clinical factors predictive of cognitive impairment
Variations in host immune responses to SARS‐CoV‐2 infection may partially explain age and sex differences in disease severity, 97 , 98 and possibly also the frequency and severity of chronic sequelae. 1 Levels of inflammatory markers, such as C‐reactive protein, 99 ferritin, 100 and D‐dimer 101 were associated with elevated risk of poor outcomes of COVID‐19 in a dose‐dependent manner, and a marker of heart failure was associated with increased mortality in COVID‐19 pneumonia. 102 Delirium in hospitalized COVID‐19 patients also correlates with elevated inflammatory markers. 10 However, in community cases, COVID‐induced impairments in short‐term memory, attention, and concentration did not correlate with hospitalization, treatment, viremia, or acute inflammation. 41 Likewise, in patients discharged from critical care to rehabilitation, persistent executive dysfunction was not associated with mechanical ventilation or preexisting cardiovascular or metabolic disease. 13 On the other hand, in a small sample of community cases with mild symptoms, hyposmia was correlated with cognitive impairment. 103 Although younger patients (<60) may frequently complain of cognitive dysfunction, objective changes in performance are mild or absent in this group. The best predictors of subjective cognitive impairment in younger adults include psychiatric complaints and physical symptoms (headache, diarrhea), with the only common risk factor across age groups being olfactory dysfunction. 6 , 14 , 103 , 104 Taken together, these findings hint at a role for inflammation in disease severity generally, but not necessarily for cognitive sequelae, and reinforce concerns about olfactory involvement in relation to cognitive impairment.
There are overlapping risk factors between COVID‐19–induced cognitive impairment and progressive cognitive decline and AD. Both ADRD and COVID‐19 are age‐dependent disorders, becoming much more frequent and severe with advancing age. 47 Morbidity and mortality of COVID‐19 are also elevated in AD, and individuals with AD are more likely to develop COVID‐19 and to die as a consequence of the illness. 47 Other risk factors potentially linking SARS‐CoV‐2 infection with progressive cognitive decline and ADRD include molecular pathway abnormalities, clinical profiles, and partially overlapping neuroimaging signatures. The ACE2 receptor acts as the ligand for the spike protein of SARS‐CoV‐2 mediating cell entry. 105 ACE2 expression declines with age, resulting in a pro‐inflammatory state that may explain the increased severity and comorbid diabetic and hypertensive complications observed in older adults. 1 SARS‐CoV‐2 specifically infects endothelial cells expressing ACE2, potentially leading to the observed deterioration of vascular architecture. 1 This could lead to brain hypoperfusion and accelerate cognitive decline in the elderly. 1 , 40 , 90 As a result of ACE2 downregulation, SARS‐CoV‐2 infection in older adults induces aggressive secretion of pro‐inflammatory cytokines. 1 Indeed, COVID‐19 results in high levels of proinflammatory cytokines, acute respiratory distress, and hypoxia, each of which may contribute to cognitive decline in healthy and in already predisposed individuals. 1 , 40 , 106 , 107 Pro‐inflammatory cytokines increase oxidative stress, resulting in downregulation of excitatory amino acid transporters and elevated glutamate levels, which may, in turn, cause excitotoxicity. This pathway is already postulated to play a role in several neurodegenerative diseases, including ADRD. The olfactory bulb has one of the highest levels of ACE2 expression in the brain, and direct viral entry into neurons may create an additional cytotoxic insult. 107 Even a transient presence of the virus in the olfactory bulb may precipitate an underlying proteinopathy associated with age‐related neurodegenerative disorders. 1 , 108 , 109 , 110 , 111 The neuroinvasive potential of SARS‐CoV‐2 may result in senescence of several different CNS cell types, including oligodendrocytes, astrocytes, and neural stem cells that can differentiate into neurons that integrate into the granule layer. 1 , 112 Viral aggravation of underlying AD neuropathology has the potential to hasten the onset of, or further deteriorate, motor and cognitive deficits. 20 , 40 , 112
In silico network‐based relationships have been reported as pathways and processes that are implicated in ADRD, and they have been confirmed in transcription studies. 113 In addition, abnormal expression of AD biomarkers was found in the cerebrospinal fluid and blood of patients with COVID‐19. 113 As already mentioned, APOE ε4, a strong genetic risk factor for ADRD, has been associated with increased risk for severe COVID‐19. Notably, the neurotropism and neurotoxicity of SARS‐CoV‐2 in human iPSC‐derived neuron‐astrocyte co‐cultures and brain organoids were found to be much higher in APOE ε4/ε4 neurons and astrocytes. 114 Systems biology approaches have predicted the interaction between prohibitins, a class of mitochondrial proteins, and SARS‐CoV‐2. 115 The same prohibitins have been shown to mediate altered mitochondrial bioenergetics in olfactory bulb neurons donated from AD patients, 116 possibly representing a common underlying molecular mechanism. A broader picture of overlapping mechanisms in the olfactory bulb includes equivocal disruption of mitogen‐activated protein kinase cascades, which has been detected specifically in the olfactory bulb in AD 116 and is a hallmark of SARS‐CoV‐2 infection. 117 Furthermore, cases of persistent anosmia and parosmia may in fact reveal pre‐existing neurogenesis defects, unmasked by SARS‐CoV‐2 infection and providing the niche for the onset of neurodegenerative disease. 21 Along with neuropathological evidence of SARS‐CoV‐2's intraneuronal entry, its neuroinvasive potential may be defined by immune fitness on the cellular level.
Neuroimaging studies also provide possible links between COVID‐19 and brain changes. A defined profile of brain positron emission tomography hypometabolism in long COVID patients with biologically confirmed SARS‐CoV‐2 and persistent memory impairment was shown more than 3 weeks after the initial infection symptoms. Alterations involved the olfactory gyrus and connected limbic/paralimbic regions, extending to the brainstem and cerebellum, and were associated with symptoms. 118 In older adults (average age 66), a significant reduction of frontoparietal and temporal glucose metabolism was related to cognitive impairment.8 These reductions persisted with some improvement 6 months after COVID‐19 diagnosis. 119 Conversely, in younger individuals, persistent impairment in executive function, attention, and less frequently in memory are far more common in women and associated with hypometabolism in the cingulate cortex, precuneus, and brain stem. 120 , 121 , 122
The reviewed literature does not, however, prove a link between SARS‐CoV‐2 infection and ADRD. Most specifically, no available evidence supports the notion that cognitive impairment after SARS‐CoV‐2 infection is a form of dementia (ADRD or otherwise), because no data regarding the progression of neuropathological disease are available. Even though COVID‐19 has significant, attendant lethality in the acute phase, death is not a result of an extended, progressive neuropathological disease. Therefore, until and unless a clear progressive pattern of disease is demonstrated in at least some individuals as a direct sequela of infection with SARS‐CoV‐2, this will remain an open question. The longitudinal methodologies espoused by the consortium are intended to provide data to answer it as clearly as possible controlling for possible confounders.
1.7. The Alzheimer's Association Consortium on Chronic Neuropsychiatric Sequelae of SARS‐CoV‐2 infection (CNS SC2)
Collectively, the information reviewed here provides important clues and evidence to support a hypothesis that cognitive impairment after SARS‐CoV‐2 infection in older adults may be progressive in nature and associated with epidemiological risk factors (including genetic ancestry), biomarkers, and neurosignatures that are overlapping with, or identical to, those of ADRD. To test this hypothesis, the Consortium has embarked on a large‐scale, international collaboration to provide a harmonized set of tools and protocols to probe the association of SARS‐CoV‐2 infection with neurological, psychiatric, and cognitive outcomes in a variety of settings covering ethnically and geographically diverse populations. The underlying hypothesis is that the COVID‐19 pandemic will increase rates of cognitive decline and dementia in older adults worldwide, presenting a very unwelcome but unique opportunity to understand interactions between the genomic risk of ADRD and relevant environmental factors, including viral exposure to SARS‐CoV‐2. 18 , 20 , 90 , 91 , 92 , 93 , 123 The primary objective of this large‐scale collaboration is to clarify the pathogenesis of ADRD and to advance our understanding of the impact of a neurotropic virus on the long‐term risk of cognitive decline and other CNS sequelae. The proposed harmonization strategies and flexible study designs offer the possibility to include large samples of under‐represented racial and ethnic groups, creating a rich set of harmonized cohorts for future studies of the pathophysiology, determinants, long‐term consequences, and trends in cognitive aging, ADRD, and vascular disease. Thus, the present proposal provides a framework for current and future studies to be carried out within the Consortium. and offers a “green paper” to the research community with a very broad, global base of support, on tools suitable for low‐ and middle‐income countries aimed to compare and combine future longitudinal data on the topic. Our specific hypothesis is that SARS‐CoV‐2 triggers ADRD‐like pathology following the extended olfactory cortical network (EOCN) in older individuals with specific genetic susceptibility. Of specific interest is the consequence that cognitive complaints in younger adult individuals may be of a different nature than those observed in older adults and obey different molecular mechanisms, clinical course, and outcomes. The proposed methods will allow us to address this and other questions.
2. METHODS
2.1. Enrollment countries
Member countries include (see Figure 1): Argentina, Australia, Austria, Bolivia, Brazil, Canada, Chile, China, Colombia, Cuba, Denmark, Dominican Republic, Ethiopia, Finland, France, Germany, Greece, Haiti, Honduras, Iceland, India, Israel, Kenya, Mexico, the Netherlands, Nigeria, Peru, the Philippines, Qatar, South Africa, Spain, Sweden, Tanzania, Thailand, Uganda and the UK (England, Wales, and Scotland). Given the variety of countries involved, cohorts will allow inclusion of all major genetic backgrounds found in low, lower–middle, upper–middle, and high‐income countries. Data collection is already ongoing using components of the presented methodology in Argentina, Canada, Greece, India, Israel, and the United States and will commence soon in several other of the member countries.
FIGURE 1.
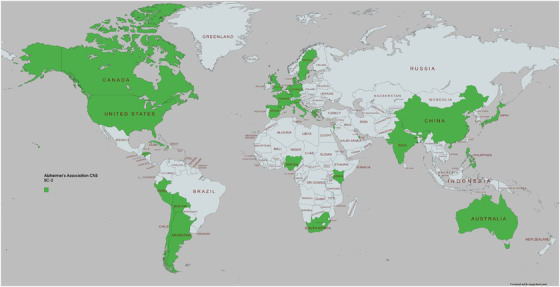
Map of countries of origin of Consortium members
2.2. Enrollment criteria
New cohorts will recruit participants of age ≥50 years, with the cut‐off being different in some locations. About half of COVID‐10 hospitalized patients are >55 years, making them a good population for investigating interactions between viral infections and the risk of cognitive decline and dementia.1,18 Both males and females will be recruited. Existing cohorts will not include new recruitment for the purpose of harmonization with the Consortium, but instead will collect a minimum harmonized data set (see below).
2.3. Recruitment and sampling procedures
The principal objective of the CNS‐SC2 protocol is to provide sufficient flexibility of recruitment methods and data collection to member countries to maximize sampling opportunities, while at the same time harmonizing procedures and methods sufficiently to allow for cross‐site comparisons, meta‐analytic approaches, and other forms of appropriate data collation. Thus, participant recruitment processes are permitted to vary as described below, depending on the site and study sample. Screening questionnaires will be used to determine eligibility and recruit participants via either telephonic and video interviews or during clinic and hospital visits. When possible, one informant (family member or close friend) will be enrolled per participant. A description of ongoing recruitment efforts is provided in Table 1. We plan to use several complementary recruitment frameworks:
Hospital‐based samples: We propose to derive these from sampling frames constructed using current lists of hospital admissions for COVID‐19 in academic centers. Participating academic groups with immediate access to hospital admissions data for patients who tested positive for COVID‐19 allow recruitment of persons at relatively high risk of neurological complications, given that severity of infection warranted hospitalization. That is, while the relationship between acute severity and neurological complications does not hold as well for individuals with less severe disease, it is well established for cases that required hospitalization. 5 Such patients will be contacted and offered enrollment in a cohort with a minimal longitudinal follow‐up of between 12 and 24 months of the initial assessment. Representativeness of the sample will be determined by comparing characteristics of the full list of hospital admissions against those who enroll. Wherever possible, individuals discharged from the hospital but negative for COVID‐19 infection (and matched for age range) will be recruited to represent the background risk of cognitive decline and neuropsychiatric pathology and act as a control group. For instance, the ongoing recruitment in Greece uses patients with chronic obstructive pulmonary disease admitted with complications as the comparison group. Further, there are extended comparison groups at some sites, such as a cohort of Early Onset Alzheimer's Disease with deep clinical and biogenetic phenotyping in England (NCT04992975; NCT03861884). Most ongoing data collection (for instance in Canada, Greece, England, South Africa, and in San Antonio, Texas, USA) follow this model.
Population registry samples: Wherever they are available, we will establish new cohorts by sampling from existing national, regional, or local (e.g., city) population registries that include SARS‐CoV‐2 testing data (regardless of hospitalization or the result of the testing) as part of the pandemic response. Such samples will include a wide range of acute COVID‐19 outcomes, including respiratory or general symptoms severe enough to warrant hospitalization (with and without intensive care admission), mild symptoms (managed in ambulatory settings), asymptomatic positive individuals, and those who tested negative. The latter will act as controls for infected participants. From these lists, we will randomly invite participants stratified by testing status and regardless of symptom severity. This approach will make it possible to estimate population‐level effect sizes, including error estimates that take account of, and are corrected for, each sampling fraction and the numbers successfully obtained, leading to greater external validity. Cohorts collected with this methodology are being recruited in Argentina and the United States (Laredo, Texas). To trim the samples, scores from semi‐structured interviews may be used to determine the clinical severity of the COVID‐19 and to populate the stratified sample from the cohort.
Pre‐existing population‐based cohort samples of aging individuals: Wherever there are surviving participants of ongoing, longitudinal, community‐based cohort studies already collecting biosamples, and cognitive, behavioral, and neuroimaging data in populations that fit our age criteria, we will attempt to include them in CNS‐SC2. COVID‐19 status will be determined using both standardized case report forms (CRFs) developed by the NeuroCOVID Forum of the World Health Organization (WHO) and via antibody titer for SARS‐CoV‐2 exposure. Participants in these cohorts already have pre‐existing extensive baseline phenotyping, and in many cases have been extensively genotyped as well, allowing direct assessment of predictors of the short‐ and long‐term effects of exposure to COVID‐19 infection and complications from SARS‐CoV‐2. Because follow‐up data collection in surviving participants of such historic samples are less likely to be representative of the original populations they were sampled from, analyses will check for lost to follow‐up (non‐participation) bias. However, as with comparisons with pre‐COVID‐19 samples (below) that are unlikely to have adopted the same measurement methods used in this protocol, synthetic data analysis methods will be required to combine findings from newly enrolled samples (e.g., recruitment frameworks 1 and 2 above). In this case, cohort participants who remained free of infection during the pandemic will act as ideal controls, because they have extensive pre‐pandemic phenotyping. Pre‐existing cohorts of these characteristics are available in, for instance, Australia, Denmark, England, India, and New York City in the United States, among several others.
Population‐based pre and post COVID‐19 multiple, cross‐sectionally representative (probabilistic) samples: Where available, these may also be included to compare pre and post COVID‐19 individuals. Such designs provide extensive pre COVID‐19 population data for comparison with a new sample to be collected post COVID‐19 in the same individuals. Whereas participant data in the pre COVID‐19 samples can be presumed to be COVID‐19 negative, it will be necessary in the post COVID‐19 samples to determine their case status by questionnaire or COVID‐19 test results. Such a design will be able to disaggregate the effect of viral infection from the social, economic, and psychological effects of living through the pandemic period. Samples obtained with this methodology will be available in England and Pakistan.
TABLE 1.
Current active cohorts in the Consortium
| Argentina | Ministry of Health – Jujuy | Dr. Agustin Yecora | ISAVRAD | 865 |
| Australia | Centre for Healthy Brain Ageing (CHeBA) – University of New South Wales | Dr. Katya Numbers | MAS | 173 |
| Australia | Centre for Healthy Ageing | Prof. Hamid Sohrabi | Western Australia Memory Study | Projectd: 200 |
| Cuba | Universidad de Ciencias Medicas de la Habana | Prof. Antonio Caballero | Longitudinal study of convalescent COVID‐19 patients | ∼400 |
| France | Clinique de la Mémoire. Université de Paris | Prof. Jacques Hugon | Longitudinal follow up post‐COVID‐19 with PET imaging | 100 |
| Greece | University of Thessaly | Dr. George Vavougios, Prof. Konstantinos I. Gourgoulianis | COVALENT Tier 1 and Tier 2 Cohorts | 250 and 200, respectively |
| India | Iqra International Hospital and Research Center – Calicut, India | Dr. Uvais Arakkal | CNS SARS CoV‐2: Prospective Cohort | Pilot initiated |
| India | Center for Brain Research, IISc – Bangalore, India | Dr. Vijay Ravindranath, Jonas Sundarakumar | SANSCOG and TLSA studies | 3170 and 583, respectively |
| Israel | University of Haifa | Dr. Galit Weinstein | Pilot initiated | |
| South Africa | University of Cape Town | Prof. Dan Stein | Collaborative study | ∼200 |
| UK | University of Leicester | Dr. Elizabetta Mukaetova‐Ladinska | ||
| UK | University of Nottingham | Dr. Akram Hosseini | 7T MRI COVID Project | |
| USA | University of Mississippi | Prof. Thomas Mosley | Member of ARIC | 5046 |
| USA | Pacific Neuroscience Institute | Dr. David Merrill, Dr. Stella Panos | Pacific Brain Health Center Clinic | Pilot initiated |
| USA | UT Health San Antonio, University of Pittsburgh, Houston Methodist, Massachusetts General Hospital | Drs. Sudha Seshadri, Gabriel de Erausquin | 7T MRI COVID Project | ≈240 |
| USA | UT Health San Antonio/Laredo | Drs. Sudha Seshadri, Gabriel de Erausquin | ISAVRAD | 250 |
| USA | Albert Einstein Medical College | Mindy Katz | Bronx Study of Aging | 250 |
| Venezuela | University of Zulia, University of Texas Rio Grande Valley | Drs. Gladys Maestre, Carlos A. Chavez | Maracaibo Aging Study | Pilot initiated |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; COVALENT, A COVID‐19 Clinical, Research and Phenotyping Network; COVID, coronavirus disease; ISAVRAD, Interaction between SARS‐CoV‐2 Infection and Ancestral genomic Variations in the Risk of Alzheimer's Disease and Related Disorders; MAS, Memory and Aging Studies; MRI, magnetic resonance imaging; PET, positron emission tomography; SANSCOG, Srinivaspura Aging, Neuro Senescence and Cognition; TLSA, Tata Longitudinal Study on Aging; UT, University of Texas.
2.4. Identification of SARS‐CoV‐2 exposure
COVID‐19 positivity will be categorized as definite, probable, and possible based on testing, documentation, and symptomatology (see Table 2). However, the only criterion for inclusion in prospective cohorts will be a positive result on either a polymerase chain reaction (PCR) test or, where available, a positive antigen test at the time of detection. A positive PCR occurring within 3 months of enrollment will be exclusionary, as it could indicate current infection. Because the pandemic is still ongoing, seroconversion of participants in the uninfected comparison group is a potentially serious concern. Seroconversion could occur after the documented initial negative PCR but before the initial assessment or after the initial assessment but prior to the 24‐month follow‐up visit. The primary method for confirming seronegativity will be lack of detection of circulating antibodies against SARS‐CoV‐2 nucleocapside protein. 124 We will also strive to collect clinical history documentation and monitoring of the registry for repeated PCR tests documenting active infection at a later time. Even with careful monitoring of both, we may fail to identify asymptomatic infections in some individuals. However, this limitation may improve the robustness of any findings of cognitive decline in the targeted population (i.e., participants with documented positive infection), because undetected asymptomatic infections would have the effect of increasing cognitive decline in the comparison group, reducing any potential group differences.
TABLE 2.
Case definitions
| Definite case definition variants | |
| Positive infection test (PCR or rapid test) | |
| Positive infection test with a later positive antibody test | |
| Positive infection test with at least 2 core symptoms a | |
| Positive infection test with at least 1 core symptom ^ and 2 supportive symptoms b | |
| Positive infection test, core symptoms, and hospitalization as an index of severity | |
| Probable case definition variants | |
| Antibody test positive on two occasions (without vaccination) | |
| Positive antibody test (without vaccination) with at least 2 core symptoms a or 1 core + 2 supportive symptoms | |
| Negative infection test with at least 2 core symptoms a or 1 core + 2 supportive symptoms and typical chest CT | |
| Possible case definitions (e.g., drawn from survey questionnaires or interview findings) | |
| Single core symptoms | |
| Self‐reported without laboratory testing confirmation | |
| Positive antibody test on just one occasion (without vaccination) |
aCore symptoms: fever, chills, cough, sore throat, anosmia, dyspnea, hypoxia, muscle pain, fatigue, altered mental status, or delirium.
bSupportive symptoms: diarrhea, headache, skin rash.
Abbreviations: CT, computed tomography; PCR, polymerase chain reaction.
2.5. Stratification of COVID‐19 symptom severity
For symptom severity, baseline evaluations of all enrollees will include (as part of the minimum data set for all cohorts) detailed CRFs for COVID‐19 developed by the WHO's NeuroCOVID‐19 Work Group (several Consortium investigators including the lead author are members of this group). The protocol proposed in this article has been presented and discussed at several stages of development to the WHO Work Group, whose members have provided feedback and technical advice. The WHO CRF will be used to stratify COVID‐19 severity according to a four‐level scale: Care level 0: no treatment required; Care level 1: ambulatory treatment; Care level 2: hospital admission without or with oxygen supplementation; and Care level 3: intensive care unit admission with or without mechanical ventilation.
2.6. Data collection time points
The minimum expectation calls for three data points (i.e., baseline and 12 and 24 months). A schematic description of the planned data collection is provided in Figure 2. Because cohorts such as those proposed here are highly valuable assets, longer follow‐up is desirable and will be sought.
FIGURE 2.
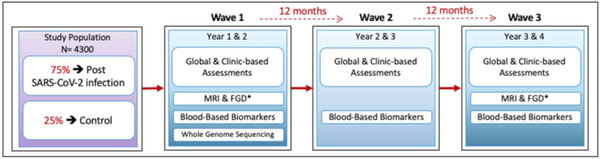
Proposed longitudinal schedule for assessment of cohort members. FDG, fluorodeoxyglucose; MRI, magnetic resonance imaging; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
2.7. Core outcome measures (minimum data set)
Since the first cases of human infection by SARS‐CoV‐2 were detected just over 2 years ago, it is impossible to predict the range of neuropsychiatric sequelae that may ensue. On the other hand, as reviewed above, acute and post‐acute manifestations of COVID‐19 disease commonly include cognitive impairment, and less frequently, overt psychiatric symptoms including mood abnormalities and psychosis. Therefore, we have chosen assessment instruments that allow an exhaustive assessment of neurological and psychiatric symptoms. Given the multinational nature of the consortium, we have also chosen instruments that are available and validated in as many languages as possible or, as is the case for cognitive assessment tests, are as unbiased as possible when used in individuals with varying mother tongues, literacy levels, and cultural contexts. The following specific tools were selected (see Table 3):
-
Phenomenological description: To be able to capture novel patient descriptions and clinical signs, our assessment approach is flexible and semi‐structured. Specifically, the WHO semi‐structured interview Schedules for Clinical Assessment in Neuropsychiatry (WHO SCAN) will be used to ascertain psychopathology and neurological symptoms. 125 Version 3 of WHO SCAN contains detailed semiquantitative (dimensional) assessments of the subject's report of behavioral neurology (cognitive efficiency, memory for recent events, executive function, language, etc.), and psychiatry (anxiety, mood, hallucinations, delusions) phenomenology, as well as the interviewer's observations of interviewee behaviors. WHO SCAN also provides automatized algorithms for all of the clinical diagnosis contained in Section F of the International Classification of Diseases revisions 10 (ICD‐10) and 11 (ICD‐11). When possible, and as provided for in WHO SCAN, informants will be interviewed for their impressions of the subject's cognition and to confirm the accuracy of the subject's responses. Pre COVID‐19 data on historical clinical phenomena and the previous life course, both of which are important in modeling future outcomes, are also assessed in SCAN and the SCAN 2.1 Clinical History Schedule, which takes account of externally provided data.
With the exception of personality disorders, the WHO SCAN covers all forms of neuropsychiatric outcomes, including somatic complaints; anxiety and mood disorders; obsessional phenomena; neurodevelopmental phenomena (autism, attention deficit hyperactivity disorder); psychosis; drug, alcohol, gambling, and eating problems; and an assessment of cognitive decline. Outputs include pre‐specified symptoms (e.g., delusion, panic, elation), dimensional symptom scores, and the determination of published diagnostic criteria. Experienced psychopathologists can be trained by means of a 3‐day online course that includes role play and interview rating sessions to ensure concordance and reliability. WHO SCAN is coordinated by a WHO advisory group that can advise on training, translation, and research protocol specifications with the support of centers throughout the world. If WHO SCAN is not available in the local language, the semi‐structured interview for the Clinical Dementia Rating (CDR) scale and the WHO Mental Health Gap Action Programme interview may be used as suitable replacements. 120
Neurological examination: The neurological evaluation at each site is conducted and supervised by trained clinicians who are blind to neuropsychological tests and PCR results and to SARS‐CoV‐2 testing status and history. The evaluation includes semiquantitative assessments of smell, visual, and auditory perception; muscle strength and tone; eye and facial movements; coordination, gait, and balance; and muscular fatigue (after 2 minutes of walking). With participant consent, neurological exams will be videotaped. Diagnoses of parkinsonism and focality due to completed stroke will be noted, as will incidental diagnoses of non‐cognitive neurological disorders (e.g., seizure neuropathy, headache). Finally, the WHO SCAN interview permits the collection of the information needed to score the CDR 126 and will be used for this purpose.
Cognitive assessment battery: A customized neurocognitive assessment was developed by a subcommittee of the Consortium members with technical expertise and extensive representation of low‐ and middle‐income countries (Argentina, China, Haiti, India) to meet three criteria: (1) adapted to multiple cultural settings and languages, and therefore minimally biased by formal education and native tongue; (2) robust to low levels of formal education or literacy; and (3) reasonably brief (it takes less than 60 minutes to complete). Details are provided in Table 3. The neuropsychiatric manifestations of SARS‐CoV‐2 infection have been characterized in the acute phase of the disease, but less is known about long‐term sequelae. Given this uncertainty, it is reasonable to include tests that probe multiple cognitive domains. Because both cortical and subcortical circuits may be affected, a broad cognitive assessment is warranted. Pictorial versions of most tests are proposed to minimize biases imposed by language of origin and literacy levels. The two tests that may need to be completed in a face‐to‐face format, as they require understanding of natural language, are the Addenbrooke's Cognitive Examination III (a short but comprehensive cognitive battery), and the shortened Boston Naming Test. All the other tests can be computerized easily. To our knowledge, there is currently no Visual Paired Associates Test that clearly mirrors the verbal version. Orbito‐frontal functions will be assessed using the Iowa Gambling Task and the Probabilistic Reversal Learning Task. The classic test for psycho‐motor speed is the Digit Symbol Substitution Test. The battery is completed by test for neglect, high order visual perception, and social cognition. We expect some degree of variation across sites on the specific tests used as a consequence of, among other things, the availability of local norms and validation, but every site will collect data on the same cognitive domains using analogous tests when the exact versions are not available. For meta‐analytic assessments, we will use normalized z‐scores of the performance for each domain.
TABLE 3.
Summary of data to be collected
| Domain | Measures | |
|---|---|---|
| Clinical, cognitive, and psychosocial assessments | ||
| Cognitive domains | Orientation & language* | ACE III and Shortened Boston Naming Test |
| Memory | Episodic: Visual Paired Associates | |
| Working: Corsi Block‐Tapping Test | ||
| Semantic: Cactus & Camel Test | ||
| Executive function | Inhibition (& psycho‐motor speed): Color (or Size) Stroop | |
| Planning – Problem solving: Tower of Hanoi | ||
| Decision making – Impulsivity: Iowa, Gambling task | ||
| Psychomotor speed | Symbol Substitution Test | |
| Attention & visuo‐spatial abilities | Search Neglect: Bell cancellation | |
| Perception Apperceptive Agnosia: Poppelreuter‐Ghent's Overlapping Figures Test | ||
| Social cognition | Theory of Mind: Frith‐Happé animations | |
| Neuropsychiatry and behavioral neurology | World Health Organization Schedules for Clinical Assessment in Neuropsychiatry (WHO SCAN) | |
| Clinical evaluation of neurodegenerative disorders | The National Alzheimer's Coordinating Center Uniform Dataset (NACC UDS) | |
| Emotional reactivity assessment | The Perth Emotional Reactivity Scale (PERS) 81 | |
| Clinical cognitive diagnosis | Mild cognitive impairment (amnestic or non‐amnestic), and dementia | |
| Psychosocial measures | Quality of life measures; stressful life events; poverty and financial hardship | |
| Semiquantitative clinical variables | Anosmia/hyposmia smell recognition test; 2‐minute walk test of fatigability | |
| Neuroimaging | ||
| Structural MRI | Region specific volumetric, cortical surface White matter hyperintensities as a proxy for vascular disease Vascular lesion burden: Infarcts, microbleeds | |
| Diffusion tensor imaging | Tract‐specific fractional anisotropy (FA) and mean diffusivity (MA) | |
| BOLD fMRI | Data from functional connectivity (FC) analyses BOLD‐derived voxel‐based physiological (VBP) indices of neurovascular coupling | |
| 18F‐DG PET (only at UTHSA site) | Region‐specific glucose uptake as markers of tissue metabolism and synaptic integrity | |
| Blood‐based biomarkers | ||
| AD‐specific biomarkers | Aβ42, Aβ40, p‐tau181, p‐tau217 | |
| Neurodegeneration and neuronal activity/injury | NfL, GFAP, sTREM‐2 | |
| Inflammatory biomarkers | Bio‐Plex Pro Human Cytokine panel: FGF basic, Eotaxin, G‐CSF, GM‐CSF, IFN‐γ, IL‐1β, IL‐1ra, IL‐1α, IL‐2Rα, IL‐3, IL‐12 (p40), IL‐16, IL‐2, IL‐4, IL‐5, IL‐6, IL‐7, IL‐8, IL‐9, GRO‐α, HGF, IFN‐α2, LIF, MCP‐3, IL‐10, IL‐12 (p70), IL‐13, IL‐15, IL‐17A, IP‐10, MCP‐1 (MCAF), MIG. β‐NGF, SCF, SCGF‐β, SDF‐1α, MIP‐1α, MIP‐1β, PDGF‐BB, RANTES, TNF‐α, VEGF, CTACK, MIF, TRAIL, IL‐18, M‐CSF, TNF‐β | |
| Genetics | ||
| DNA collection for GWAS or Whole Genome Sequencing | ||
Note: Harmonized measures will not be able to be collected as suggested at all sites. The intent of the list of measures is to secure harmonization of those measures that are locally available, to ensure maximum and optimum data shareability.
Abbreviations: Aβ, amyloid beta; ACE III, Addenbrooke's Cognitive Examination III; AD, Alzheimer's disease; BOLD, blood oxygen level dependent; 18F‐DG; fluorodeoxyglucose; fMRI, functional magnetic resonance imaging; GFAP, glial fibrillary acidic protein; GWAS, genome‐wide association studies; MRI, magnetic resonance imaging; NfL, neurofilament light chain; PET, positron emission tomography; p‐tau, phosphorylated tau; sTREM‐2, soluble triggering receptor expressed on myeloid cells 2; UTHSA, University of Texas Health San Antonio.
The following supplemental measures are proposed to facilitate comparisons and data collation across studies affiliated with the consortium.
To facilitate data sharing with ongoing studies, wherever feasible sites will collect information to fill the National Alzheimer's Coordinating Center Uniform Data Set (NACC UDS). 127 , 128 , 129 , 130 The NACC established the UDS for longitudinal data by means of a standardized clinical evaluation. 127 , 128 , 129 , 130 NACC is responsible for developing and maintaining a database of participant information collected from all of the Alzheimer's Disease Centers (ADCs) funded by the National Institute on Aging (NIA). UDS defines an expanded, standardized clinical data set to provide ADC researchers a standard set of assessment procedures, collected longitudinally, to better characterize ADC participants with mild AD and MCI compared to non‐demented controls. The UDS has data collection forms for initial and follow‐up visits based on NACC definitions, a relational database, and a data submission system enhanced to provide efficient and secure access data submission and retrieval systems (https://www.alz.washington.edu). 128 The NACC UDS is validated for international AD cohorts and is available in English, Spanish, and Chinese (Mandarin). Psychosocial measures, including quality of life, stressful life events, and poverty and financial hardship will also be collected where possible. Admittedly, this information is partially duplicative with other components of the proposed assessment. Local decisions over use will be driven by the availability of locally validated and culturally adapted assessment tools, as well as participant burden.
Emotional reactivity assessment: The Perth Emotional Reactivity Scale (PERS), a self‐report measure of trait levels of emotional reactivity, assesses the typical ease of activation, intensity, and duration of individual positive and negative emotional responses. 127 Concurrent validity has been demonstrated via congruent correlations with other emotion measures. 131
- Neuroimaging will be performed with two types of scans.
- 1.5 and 3 Tesla scanners: To promote consistency in data analysis, we will follow standardized magnetic resonance imaging (MRI) imaging datasets developed for the acquired 1.5T and 3T scans by the Alzheimer's Disease Neuroimaging Initiative Study 3 (ADNI‐3). We are using ADNI phantoms to ensure adequate harmonization of data collection across sites. By so doing, we will optimize direct comparisons of various analysis methods, particularly given large variations among older MRI systems and the state‐of‐the‐art systems available at high‐end academic centers. ADNI‐3 provides a two‐tiered approach to accommodate the range of variability in scanners, including ADNI‐3 Basic and ADNI‐3 Advanced. The latter include structural T1‐weighted, 3D fluid‐attenuated inversion recovery, T2* gradient recalled echo, arterial spin labeling, and high‐resolution images of the hippocampus. The advanced diffusion MRI and resting state functional MRI scans take advantage of simultaneous multi‐slice acceleration for echo‐planar images. For longitudinal consistency, advanced sequences can be downsampled post‐scan to match the basic sequences. The standard ADNI‐3 sequence acquisitions are listed in Table 3. We will collect region‐specific volumetric and cortical surface measures; white matter hyperintensities as a proxy for vascular disease; vascular lesion burden (including infarcts and microbleeds); tract‐specific fractional anisotropy and mean diffusivity; BOLD‐derived voxel‐based physiological indices of neurovascular coupling; and, if PET is available, region‐specific glucose uptake as markers of tissue metabolism and synaptic integrity.
- 7 Tesla ultrahigh field scanners: The higher contrast and spatial resolution of 7T MRI provides submillimeter measurements, allowing study of small cortical and subcortical structures of the brain and providing superior detail to 3T. Enhanced anatomical detail at 7T allows higher sensitivity in measuring sub‐structural volume loss, including hippocampal subfields 132 and the earlier detection of neurodegeneration implicated in ADRD. The UK 7T Network (which includes members of the CNS SARS‐CoV‐2 consortium) have previously tested and proved the reproducibility of 7T scanners (Siemens and Phillips) across various sites. 133 , 134 , 135 The harmonized sequences, listed in Table 4, are designed to study volumetric assessment of: cortex, hippocampal subfields, and thalamus; quantitative cerebral white matter changes and inflammation; iron content from blood breakdown (cerebral microbleeds, microthrombi); markers of endothelial injury; and volume and injury to the sub‐structures of the brain stem, including locus coeruleus. Comparative control groups with identical 7T MRI images include age‐, sex‐, and ethnicity‐matched participants who are both healthy or have an illness of similar severity (intensive care unit [ICU] admission, hospitalization without ICU, or no hospitalization). The study of 7T imaging precursors of AD will further benefit from an independent 7T MRI study of patients with early onset AD as an additional comparison group (clinicaltrials.org Identifier: NCT04992975). We will analyze the acquired data through data sharing agreements based on the local expertise of each site within the 7T MRI COVID Consortium.
Biomarkers: Collection methods for whole bloods, plasma, serum, anucleated blood cells, mouth swab for epigenomics, and cerebrospinal fluid are detailed in Tables S1 and S2 in supporting information. Blood spot is recommended for all sites, and blood or salivary swab is recommended for DNA (genome‐wide association studies). Participating sites will collect, store, use, share, and dispose of human biospecimens in accordance with the informed consent signed by the subject, or under a waiver of informed consent granted by an independent ethical review body at each institution. When specimens are collected from humans for the study purposes, the collection and storage process should aim to adhere, as closely as possible, to harmonized study protocols and procedures appropriate for the type of biospecimen being collected and its intended uses. We will establish biorepositories within global regions where biospecimens will be collected. Raw data will be analyzed locally, such that only metadata will be shared across the consortium. Specific agreements between each repository and collection site will be (or have already been) established. All biorepositories, whether large or represented by individual freezers in laboratories, will follow best practices using effective facility environments that include ambient temperature controls, good air circulation, lighting, and security. Systems will be in place to allow for local and remote temperature monitoring of freezers, refrigerators, and other temperature‐controlled environments. Biorepositories will have emergency preparedness plans that cover equipment failures and power interruption that include back‐up storage capacity and back‐up power supplies such as generators (https://oir.nih.gov/sites/default/files/uploads/sourcebook/documents/ethical_conduct/guidelines‐biospecimen.pdf). Special attention will be paid to the appropriate packaging and shipping of human biospecimens between the collection site and the biorepository. This includes conforming to all applicable regulations and standards, including, but not limited to, those of the US Department of Transportation (DOT; DOT PHMSA PHH50‐0079, 2006) and the International Air Transport Association (IATA; IATA Dangerous Goods Regulations, 2019; IATA Infectious Substances Shipping Guidelines, 2019). All personnel involved in shipping biological materials should be trained properly for both air and ground shipments. A full list of the proposed biomarkers is included in Table 3.
Genotyping: Specific approaches will vary across sites, but at a minimum, each dataset will contain genome‐wide genotypes from cohort individuals to address the role of ancestry and genetic variation on susceptibility to neuropsychiatric sequelae. When available, sites will obtain whole‐genome sequencing data. Our consortium is in a unique position to address the interaction between genetics (including ancestral DNA) and viral strain variation on CNS sequelae of SARS‐CoV‐2. If available, genotyping will be carried out using Illumina GSA (or equivalent chip) and imputation to the best available panel for persons of specified ancestry.
TABLE 4.
Description of 7 Tesla high field MRI sequences proposed
| Sequence | Acquisition parameters | Measures assessed | Time (min) |
|---|---|---|---|
| Set up / localizer | GRE | Positioning; shimming | 5.5 |
| 3DT1 MP2RAGE | 348 slices (0.55 iso); TR∼6000; TE∼22.54; TI1/2∼800/2500; AF=2 | Morphometry; registration; hippocampus segmentation | 12.5 |
| 3D SWI | 208 slices (0.375x0.375x0.75); TR∼24; TE1/2∼8.16/18.35; AF=2 | Small vessel analysis; T2* mapping; QSM | 9 |
| T2 TSE | 36 slices (0.375x0.375x1.5); TR∼10060; TE∼61; AF = 2 | Hippocampus segmentation | 4 |
| T2 FLAIR | 80 slices (0.75x0.75x1.5); TR∼14000; TE∼99; TI∼2900; AF = 2 | White matter hyperintensities | 11 |
| 3D T2 Space | 256 slices (0.6 iso); TR∼3400; TE∼367; AF = 3 | Morphometry; hippocampus segmentation; perivascular spaces | 9.5 |
| MT & non MT | 60 slices (0.4 iso); TR=548; TE=4.08; AF = 8 | Locus coeruleus intensity; contrast' MT | 8 |
| TOF (4 slabs) | 192 slices (0.375 iso); TR∼14; TE∼4.5; AF = 3 | Angiography; arteriolar analysis | 6.5 |
Abbreviations: FLAIR, fluid‐attenuated inversion recovery; GRE, gradient echo; MT, magnetization transfer; MP2RAGE, magnetization‐prepared 2 rapid acquisition gradient echo; QSM, quantitative susceptibility mapping; SWI, susceptibility weighted imaging; TE, echo time; TOF, time of flight; TR, repetition time; TSE, turbo spin echo.
2.8. Data analysis
Longitudinal data analysis approaches, including generalized linear models, will be used, depending on the outcome of our interests and data distribution. Time‐to‐event models will also be considered if there are a sufficient number of data points. There will also be ample scope for nested case‐control design approaches to analyze within selected subsets of the cohort data. These could be led by individual investigators, for example, in small substudies for which limited numbers of patients have undergone particular laboratory tests. When estimating the size of effects at the population level, and in particular for probabilistic cohorts, error estimates will take account of and be corrected for each sampling fraction, leading to greater generalizability and external validity. Where individual level data sharing is not possible, we will use meta‐analytic approaches to compare findings across countries.
Despite the great effort made by the Consortium to harmonize methods and assessment tools, the approach proposed here has limitations due to the likely heterogeneity of the samples that will be collected across several important dimensions. Ethnic and genetic heterogeneity is a desirable feature of the approach and will increase generalizability of the findings across populations. On the other hand, multiplicity of virus variants as the pandemic has progressed is a likely source of variance that will need to be addressed. Voluntary reporting of viral genomes to the Global Initiative on Sharing All Influenza Data (GISAID) website is the primary source for phylodynamics of SARS‐CoV‐2 in participant countries (https://www.gisaid.org/phylodynamics/) including most of the Consortium members. Because individual‐level data of viral genomes are unlikely to be available for most sites, prevailing variants at the time of infection for cohort participants will be used as a covariate for all analyses and meta‐analyses. Similarly, type and dose number of vaccines will be used as a covariate. Another possible source of heterogeneity is the precision of COVID‐19 diagnosis across sites, particularly when comparing academic centers in developed countries to community samples in developing countries. We will minimize the impact of such differences by adhering to WHO diagnostic criteria across the board, and by using the CRF developed by the WHO workgroup on NeuroCOVID to collect clinical information.
Data heterogeneity is an inescapable aspect of large‐scale, multinational studies, but previous experience shows that it can be addressed with appropriate harmonization. 136 , 137 , 138 , 139 The Consortium has additionally adopted flexibility of study designs, which will increase the richness of the datasets but further increase variability. The intent of the Consortium members is that by harmonizing data collection and measurements we will increase the likelihood of cross‐comparisons and the interpretability of meta‐analytic approaches. Neuropsychiatric sequelae of COVID‐19 obey multifactorial causation and may potentially emerge by the interactions of multiple, rather than a singular factor, including baseline lifestyle differences, impact of social restrictions and other measures, type and timing of COVID‐19 treatment (including vaccines), genomic viral variants, and possibly others. Observational studies combining an in‐depth analytical design and multiple measurements have been proposed as a means to address such a complex interaction problem. 140 Also, integrative analytical approaches have been shown to perform well in observational studies in infectious diseases. 141 Furthermore, more extensive measurements may more accurately capture and parametrize the biological question at hand, as has been shown by, for example, the inclusion of genetic data in observational studies toward establishing inference. 142 Causality inference can be approached in a stepwise, tiered fashion that will be possible by the scope of the Consortium. For instance, estimands of causal relationships identified among recorded variables may be identified from prospective evaluation of COVID‐19 survivors and the refinement of associations. 143 Confounders and mimics will be addressed where possible via supplementary assessments where available (such as psychosocial measures, including quality of life, stressful life events, poverty, and financial hardship). Biomarkers and neuroimaging indices may also be used to improve causal associations.
In summary, the Consortium proposes a combination of design and statistical methods as a means of approaching causal inference 144 , 145 of the COVID‐19 neuropsychiatric sequelae. We expect that deep phenotyping of neuropsychiatric sequelae may provide a series of candidate syndromes with phenomenological and biological characterization that can be further explored. 146 By generating high‐quality harmonized data across sites we aim to capture both descriptive and, where possible, causal associations. Notably, even descriptive (rather than causal) associations will advance our knowledge of post‐COVID‐19 neuropsychiatric manifestations.
2.9. Stay‐in‐touch strategy
To maintain contact with participants after the initial assessment, we will use a cell phone–based technology developed by Prof. Sriram Iyengar, termed Txt2Info, which provides precision bidirectional mass communications during pandemics. Txt2Info combines judicious use of text messaging and an easy‐to‐use REDCap survey instrument in a simple, lightweight manner. English and Spanish are currently supported, but other languages can be easily and quickly added. Txt2Info is designed to be rapidly customized and deployed for any scenario that requires real‐time dissemination of information and community‐sourced data collection.
2.10. Determining pre‐exposure cognitive status
A key consideration in the recruitment of new cohorts is the assessment of pre‐exposure cognitive status because pre‐exposure decline (even in the absence of a clinical diagnosis of cognitive impairment or dementia) will result in exclusion from analyses. Because we will be collecting new cohorts, we will not have pre‐exposure assessments and will have to rely on indirect strategies to establish pre‐morbid level of function. First, we will gather information about the pre‐baseline functional ability of the participant through the WHO SCAN interview. Second, where available we will interview a caregiver/informant using the CDR scale or the corresponding section of WHO SCAN. Finally, we will develop cognitive estimates of pre‐morbid abilities. 147 , 148 , 149 , 150 , 151 These combination methods are necessary because measures typically used in the United States (e.g., the National Adult Reading Test or the Weschler vocabulary subtest) are very limited in high illiteracy contexts such as Argentina. Most cognitive tests have robust norms established in our study population. In making diagnoses, we will incorporate clinical judgment of cognitive decline, particularly with respect to pre‐morbid and baseline levels of cognition. Local norms that include age and education will also be routinely considered, both in making consensus diagnoses and in formal statistical analysis.
2.11. Mortality endpoints
Efforts will be made to ascertain death certificates, contact significant others, or to search the National Death Index (https://www.cdc.gov/nchs/ndi/index.htm) to track participants who are lost to follow‐up. In Argentina, we will track deaths in the registry of the provincial Emergency Operations Committee (http://coe.jujuy.gob.ar/noticias/). Other locations will track as available.
2.12. Consortium agreement and data sharing procedures
The Consortium is led by a steering committee. Multiple subcommittees address specific areas of focus, including clinical definitions, epidemiological designs, clinical evaluation, cognitive assessments, biomarkers, and neuroimaging. Subcommittees meet ad hoc based on specific needs. The entire Consortium meets every fortnight via remote conferencing. Funding opportunities and publication proposals are discussed in the open meeting, including invitations to collaborate, and interested parties can continue to meet at their discretion. All protocols, publication drafts, and minutes from subcommittee meetings are made available to all members through a digital board. Each local site will be led by one to two principal investigators (neurologists, psychiatrists, or epidemiologists) and a team of trained clinical research associates. A data sharing agreement regulates (and allows collation of) deidentified results using meta‐analytic approaches.
2.13. Ethical considerations
The methods presented here have been approved by several institutional review boards and ethics committees affiliated with Consortium member institutions (University of Texas Health San Antonio, University of Haifa, University of Nottingham, Athens Naval Hospital, Ministry of Health of the Province of Jujuy, Laredo Health Department, among many others). The Consortium members will seek and obtain approval from the corresponding local regulatory institutional boards prior to enrolling participants in any cohort and will include specific informed consent forms for all supplementary data collection, particularly when risks of complications may be somewhat higher such as when collecting spinal fluid samples.
3. DISCUSSION
The research described here aims to provide harmonized methodologies to better understand whether and how the SARS‐CoV‐2 pandemic contributes to the risk (and mechanism[s]) of ADRD through a population‐based, quasi‐experimental model. Through a global network of study teams, we propose to provide a scientific framework to characterize the neurobehavioral and neuropsychiatric phenomenology associated with SARS‐CoV‐2 in harmonized, multinational, longitudinal cohorts of post SARS‐CoV‐2 infection patients. Recruitment is ongoing in several cohorts. We plan to obtain core initial data within 18 months of recovery from hospital discharge or documented infection by PCR. Longitudinal follow‐up is proposed at 12 and 24 months after the initial evaluation but with an expectation to extend the observation of cohorts as long as feasible. An mHealth keeping‐in‐touch process is planned to minimize attrition rates. High rates of mutation in SARS‐CoV‐2 (https://www.gisaid.org/phylodynamics/global/nextstrain/) strongly suggest that viral infectivity, including neurotropism, may not be uniform across countries impacted. However, regardless of the molecular mechanism(s) involved in chronic or progressive injury to the CNS, we assume that the fundamental biology driving disease development is largely the same across all human ancestries, even though redundant or parallel processes may result in diverse pathways leading to the same clinical phenotypes. Conversely, identical genetic variants may be associated with different phenotypes conditioned by the genomic context or ancestry, as well as by environmental influences. Therefore, variability, both in the effect of genomic variations and in the sources of risk for specific phenotypes, is expected to be inherently affected by contexts. 152 , 153 , 154 All members of the Consortium have agreed to share data for meta‐analytic and replication efforts in the future. Ongoing data collection efforts using CNS‐SC2 methodology in Argentina, Canada, Cuba, Denmark, England, Greece, India, Israel, Sweden, and the United States will provide multiple opportunities to attempt replication or expansion of the findings.
3.1. Detecting novel symptoms
A critical caveat of this proposal is that the cognitive impairment triggered by SARS‐CoV‐2 infection may resemble ADRD while differing from it in subtle but important ways. We therefore have chosen clinical assessment, imaging, and biomarker tools that will allow us to detect and describe even subtle differences. The semi‐structured interview WHO SCAN makes use of a conversational interviewing approach, helping patients to describe in their own words their feelings, thoughts, and perceptions. The WHO SCAN examiner is trained to determine which of these verbal and subjective descriptions represents abnormal psychopathological phenomena (predefined in a glossary of symptom definitions officially endorsed by WHO), 125 a technique that lends itself also to describing previously unrecognized phenomena or symptoms not catalogued as part of typical syndromes. Such novel emerging phenomena are often observed when the WHO SCAN is translated into indigenous culture first languages that not only do not share all of the Western conventional or universal experiences, but that also place importance on psychological experiences that are uncommon outside of that culture. 155 While there are useful structured (e.g., Composite International Diagnostic Interview, Clinical Interview Schedule–Revised) and semi‐structured (e.g., Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Diagnostic Interview for Genetic Studies) interviews and short checklists (e.g., General Anxiety Disorder‐7, Patient Health Questionnaire‐9, Edinburgh Postnatal Depression Schedule) in widespread use in neuropsychiatry, including clinical trial and epidemiological research, these more structured approaches are only capable of identifying established and recognized symptoms, syndromes, and predefined disorder categories. This is problematic because novel symptoms may prove crucial to tracking and predicting short‐ and longer‐term CNS effects of novel viruses, including COVID‐19 outcomes. Novel symptom discoveries could also lead to the development of new, more appropriate, brief structured assessments for wider use.
3.2. Minimizing cultural bias
Cultural variables can also exert a powerful effect on test performance through construct, method, and item biases, 156 but their impact is often underestimated. Indeed, the influence of culture on cognition poses great challenges to cognitive assessments in culturally diverse samples, not the least of which includes the difficulty of responding to the wide range of cultural contexts, conditions, and circumstances under which testing may occur around the world. 156 Thus, while a common neuropsychological assessment is an essential component of the longitudinal assessments planned by the Consortium, we recognized that harmonization of testing procedures across cultures, educational attainment levels, languages, and sociocultural environments is a very difficult task. Standard cognitive processes are biologically identical for all humans, but individual, social, and environmental differences may significantly change the way in which cognitive processes are engaged, resulting in different patterns of abilities across cultures. 157 , 158 For instance, studies in Aboriginal peoples show unique approaches to spatial relationships 159 and numerical and memory tasks. 160 , 161 , 162 , 163 To detect cognitive impairment and cognitive decline therefore requires a basic understanding of which skills are needed for normal function in a specific cultural context. 164 Culture‐informed adaptations are made to the content and administration of instruments to reflect the experiences of the population being assessed and to retain within‐population variance. 165
The basic idea behind cross‐cultural measurement is that the same aspect of cognitive abilities is assessed similarly in different cultural groups using tests selected, optimized, and normed for each individual group. In this case, absolute scores would not be directly comparable across groups, but deviance from norms would be comparable regardless of differences that may be present in a variety of important background characteristics that vary across and within cultures. To address these issues, a panel of experts from across the CNS‐SC2 Consortium (including key personnel from each continent and with expertise in Aboriginal cognitive assessments) worked on harmonization of culturally appropriate conceptual tasks (e.g., content, sensitivity, and face value of the tools) to minimize three key sources of bias: fairness, instrument, and administration. Fairness, understood as equitable treatment throughout the testing process, refers to the manner in which the tool is administered. Instrument bias refers to all the properties associated with an instrument that are not the target of study but nonetheless can result in group differences in test scores. For instance, if a computer is used to measure reaction times in individuals who have never used a device and others who have lifetime usage, differential familiarity with computers is expected to influence the obtained results regardless of the construct being investigated. Administration bias refers to group differences in test scores due to aspects of the interaction and communication between the examiner and examinee. Factors such as inappropriate testing conditions, unequal opportunity to familiarize oneself with the test format, unavailability of practice materials and unequal exposure to those materials, unequal performance feedback, and lack of standardized test administration can all lead to administration bias. We have created a standard operating procedure manual to ensure equitable treatment throughout the Consortium.
3.3. Focus on olfactory impairment
Last, our semi‐quantitative neurological examination is primarily focused on olfactory, motor, and cognitive function. Other aspects of the clinical examination (i.e., visual and auditory perception, muscle strength and tone, eye and facial movements, coordination, gait and balance, and muscular fatigue during 6 minutes of walking) are included to achieve broad characterization of concomitant complications. There are multiple sound reasons to pay particular attention to olfactory deficits in this context. First, increased Aβ burden is correlated with olfactory impairment in older adults with amnestic MCI, 166 , 167 and both factors may be predictive of ADRD. 166 , 167 Olfactory impairment is also correlated with tau pathology and neuroinflammation in patients with ADRD 168 and predictive of dementia diagnosis in several pathologies. 36 , 37 As mentioned in the introduction, SARS‐CoV‐2 invades the olfactory bulb, and this is the likely explanation for the prevalent anosmia in infected patients. 20 , 21 , 22 , 27 , 124 , 125 , 126 This mechanism has been well established in experimental animals 26 and is well supported by imaging studies of sub‐acute COVID‐19 patients. 29 , 30 , 31 , 32 , 33 , 34 Fruit and flower odor categories have a graded structure that is a universal property retained across categories, 169 such that they can be stably tested. Second, the amygdala is one of the primary connections of the olfactory bulb, 170 has among the highest levels of ACE2 expression in the brain, 171 is a preferential target of COVID‐19 in the post mortem tissue of patients, 172 and is affected in imaging studies of long COVID patients. 173 Most notably, a recent publication from the UK Biobank found reductions in gray matter thickness and tissue contrast in the orbitofrontal cortex and parahippocampal gyrus; changes in markers of tissue damage in regions functionally connected to the primary olfactory cortex, and reductions in global brain size of post‐COVID participants even after excluding those who had been hospitalized. 174 Moreover, infected participants had more cognitive decline than controls. 174 Likely as a consequence of this involvement, changes in emotional reactivity have been reported as a prominent behavioral change after SARS‐CoV‐2 infection.
CONFLICTS OF INTEREST
All authors state that they have no relationships/activities/interests to disclose related to the content of this submission. Author disclosures are available in the supporting information.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
The authors wish to thank the ongoing technical advice and expert consultations of Dr. Dévora Kestel, Director, Department of Mental Health and Substance Abuse and Dr. Tarun Dua, Director, Program for Neurological Diseases and Neuroscience, Management of Mental and Brain Disorders in the Department of Mental Health and Substance Abuse at the World Health Organization.
de Erausquin GA, Snyder H, Brugha TS, et al. Chronic neuropsychiatric sequelae of SARS‐CoV‐2: Protocol and methods from the Alzheimer's Association Global Consortium. Alzheimer's Dement. 2022;8:e12348. 10.1002/trc2.12348
REFERENCES
- 1. de Erausquin GA, Snyder H, Carrillo M, Hosseini AA, Brugha TS, Seshadri S. The chronic neuropsychiatric sequelae of COVID‐19: the need for a prospective study of viral impact on brain functioning. Alzheimers Dement. 2021;17(6):1056‐1065. doi: 10.1002/alz.12255 Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baig AM. Chronic COVID syndrome: need for an appropriate medical terminology for long‐COVID and COVID long‐haulers. J Med Virol. 2021;93(5):2555‐2556. doi: 10.1002/jmv.26624 Epub 2021 Mar 1 [DOI] [PubMed] [Google Scholar]
- 3. Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post‐acute covid‐19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 4. Korompoki E, Gavriatopoulou M, Hicklen RS, et al. Epidemiology and organ specific sequelae of post‐acute COVID19: a narrative review. J Infect. 2021:83(1):1‐16. doi: 10.1016/j.jinf.2021.05.004 Epub ahead of print. PMID: 33992686; PMCID: PMC8118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6‐month neurological and psychiatric outcomes in 236 379 survivors of COVID‐19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416‐427. doi: 10.1016/S2215-0366(21)00084-5 Epub 2021 Apr 6. PMID: 33836148; PMCID: PMC8023694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berlit P, Frölich L, Förstl H. Die „vierte Welle“? COVID‐19 und konsekutive kognitive Störungen [The “Fourth Wave”? COVID‐19 and consecutive cognitive impairment]. Dtsch Med Wochenschr. 2021;146(10):671‐676. German. Epub 2021 May 6. doi: 10.1055/a-1468-1529 PMID: 33957689 [DOI] [PubMed] [Google Scholar]
- 7. Mainali S, Darsie ME. Neurologic and neuroscientific evidence in aged COVID‐19 patients. Front Aging Neurosci. 2021;13:648662. doi: 10.3389/fnagi.2021.648662 PMID: 33833676; PMCID: PMC8021699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hosp JA, Dressing A, Blazhenets G, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID‐19. Brain. 2021;144(4):1263‐1276. doi: 10.1093/brain/awab009 PMID: 33822001; PMCID: PMC8083602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rizzo MR, Paolisso G. SC‐2 emergency and long‐term cognitive impairment in older people. Aging Dis. 2021;12(2):345‐352. doi: 10.14336/AD.2021.0109 PMID: 33815868; PMCID: PMC7990368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alnefeesi Y, Siegel A, Lui LMW, et al. Impact of SC‐2 infection on cognitive function: a systematic review. Front Psychiatry. 2021;11:621773. doi: 10.3389/fpsyt.2020.621773 PMID: 33643083; PMCID: PMC7902710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazza MG, Palladini M, De Lorenzo R, et al. Persistent psychopathology and neurocognitive impairment in COVID‐19 survivors: effect of inflammatory biomarkers at three‐month follow‐up. Brain Behav Immun. 2021;94:138‐147. doi: 10.1016/j.bbi.2021.02.021 Epub 2021 Feb 24. PMID: 33639239; PMCID: PMC7903920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Javed A. Neurological associations of SC‐2 infection: a systematic review. CNS Neurol Disord Drug Targets. 2022;21(2):246‐258. doi: 10.2174/1871527320666210216121211 Epub ahead of print. PMID: 33593267. (40) [DOI] [PubMed] [Google Scholar]
- 13. Woo MS, Malsy J, Pöttgen J, et al. Frequent neurocognitive deficits after recovery from mild COVID‐19. Brain Commun. 2020;2(2):fcaa205. doi: 10.1093/braincomms/fcaa205 PMID: 33376990; PMCID: PMC7717144. (41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubin R. As their numbers grow, COVID‐19 “Long Haulers” stump experts. JAMA. 2020;324(14):1381‐1383. doi: 10.1001/jama.2020.17709 PMID: 32965460. (42) [DOI] [PubMed] [Google Scholar]
- 15. Nakamura ZM, Nash RP, Laughon SL, Rosenstein DL. Neuropsychiatric complications of COVID‐19. Curr Psychiatry Rep. 2021;23(5):25. doi: 10.1007/s11920-021-01237-9 PMID: 33725218; PMCID: PMC7962429. (43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amalakanti S, Arepalli KVR, Jillella JP. Cognitive assessment in asymptomatic COVID‐19 subjects. Virusdisease. 2021;32(1):1‐4. doi: 10.1007/s13337-021-00663-w Epub ahead of print. PMID: 33614860; PMCID: PMC7883942. (44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan HW, Xu YM, Lau ATY. Angiotensin‐converting enzyme 2: the old door for new severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol. 2020;30(5):e2122. doi: 10.1002/rmv.2122 Epub 2020 Jun 30. PMID: 32602627; PMCID: PMC7361198. (29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bostanciklioğlu M. Severe acute respiratory syndrome coronavirus 2 is penetrating to dementia research. Curr Neurovasc Res. 2020;17(4):342‐343. doi: 10.2174/1567202617666200522220509 PMID: 32442082. (21) [DOI] [PubMed] [Google Scholar]
- 19. Singal CMS, Jaiswal P, Seth P. SC‐2, more than a respiratory virus: its potential role in neuropathogenesis. ACS Chem Neurosci. 2020;11(13):1887‐1899. doi: 10.1021/acschemneuro.0c00251 Epub 2020 Jun 18. PMID: 32491829. (45) [DOI] [PubMed] [Google Scholar]
- 20. Manzo C, Serra‐Mestres J, Isetta M, Castagna A. Could COVID‐19 anosmia and olfactory dysfunction trigger an increased risk of future dementia in patients with ApoE4? Med Hypotheses. 2021;147:110479. doi: 10.1016/j.mehy.2020.110479 Epub 2021 Jan 5. PMID: 33422806; PMCID: PMC7785277. (22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SC‐2 invasion as a port of central nervous system entry in individuals with COVID‐19. Nat Neurosci. 2021;24(2):168‐175. doi: 10.1038/s41593-020-00758-5 Epub 2020 Nov 30. PMID: 33257876. (46) [DOI] [PubMed] [Google Scholar]
- 22. Hopkins C, Lechien JR, Saussez S. More that ACE2? NRP1 may play a central role in the underlying pathophysiological mechanism of olfactory dysfunction in COVID‐19 and its association with enhanced survival. Med Hypotheses. 2021;146:110406. doi: 10.1016/j.mehy.2020.110406 Epub 2020 Nov 20. PMID: 33246692; PMCID: PMC7678428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Las Casas Lima MH, Cavalcante ALB, Leão SC. Pathophysiological relationship between COVID‐19 and olfactory dysfunction: a systematic review. Braz J Otorhinolaryngol. 2021:S1808‐8694(21)00073‐2. doi: 10.1016/j.bjorl.2021.04.001 Epub ahead of print. PMID: 33965353; PMCID: PMC8068782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Butowt R, von Bartheld CS. Anosmia in COVID‐19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. 2021:27(6):582‐603. doi: 10.1177/1073858420956905 Epub ahead of print. PMID: 32914699; PMCID: PMC7488171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiu A, Fischbein N, Wintermark M, Zaharchuk G, Yun PT, Zeineh M. COVID‐19‐induced anosmia associated with olfactory bulb atrophy. Neuroradiology. 2021;63(1):147‐148. doi: 10.1007/s00234-020-02554-1 Epub 2020 Sep 15. PMID: 32930820; PMCID: PMC7490479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Melo GD, Lazarini F, Levallois S, et al. COVID‐19‐related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021:eabf8396. doi: 10.1126/scitranslmed.abf8396 Epub ahead of print. PMID: 33941622; PMCID: PMC8158965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laurendon T, Radulesco T, Mugnier J, et al. Bilateral transient olfactory bulb edema during COVID‐19‐related anosmia. Neurology. 2020;95(5):224‐225. doi: 10.1212/WNL.0000000000009850 Epub 2020 May 22. PMID: 32444492. [DOI] [PubMed] [Google Scholar]
- 28. Jiao L, Yang Y, Yu W, et al. The olfactory route is a potential way for SC‐2 to invade the central nervous system of rhesus monkeys. Signal Transduct Target Ther. 2021;6(1):169. doi: 10.1038/s41392-021-00591-7 PMID: 33895780; PMCID: PMC8065334. (24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guedj E, Lazarini F, Morbelli S, et al. Long COVID and the brain network of Proust's madeleine: targeting the olfactory pathway. Clin Microbiol Infect. 2021:27(9)1196‐1198. doi: 10.1016/j.cmi.2021.05.015 Epub ahead of print. PMID: 34015528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bulfamante G, Chiumello D, Canevini MP, et al. First ultrastructural autoptic findings of SARS ‐Cov‐2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020;86(6):678‐679. doi: 10.23736/S0375-9393.20.14772-2 Epub 2020 May 13. PMID: 32401000. [DOI] [PubMed] [Google Scholar]
- 31. Aragão MFVV, Leal MC, Cartaxo Filho OQ, Fonseca TM, Valença MM. Anosmia in COVID‐19 associated with injury to the olfactory bulbs evident on MRI. AJNR Am J Neuroradiol. 2020;41(9):1703‐1706. doi: 10.3174/ajnr.A6675 Epub 2020 Jun 25. PMID: 32586960; PMCID: PMC7583088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donegani MI, Miceli A, Pardini M, et al. Brain metabolic correlates of persistent olfactory dysfunction after SARS‐Cov2 infection. Biomedicines. 2021;9(3):287. doi: 10.3390/biomedicines9030287 PMID: 33808956; PMCID: PMC7998481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kandemirli SG, Altundag A, Yildirim D, Tekcan Sanli DE, Saatci O. Olfactory Bulb MRI and Paranasal sinus CT findings in persistent COVID‐19 anosmia. Acad Radiol. 2021;28(1):28‐35. doi: 10.1016/j.acra.2020.10.006 Epub 2020 Oct 19. PMID: 33132007; PMCID: PMC7571972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shor N, Chougar L, Pyatigorskaya N. MR imaging of the olfactory bulbs in patients with COVID‐19 and anosmia: how to avoid misinterpretation. AJNR Am J Neuroradiol. 2021;42(3):E10‐E11. doi: 10.3174/ajnr.A6921 Epub 2020 Oct 29. PMID: 33122206; PMCID: PMC7959434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoo HS, Chung SJ, Lee YH, Ye BS, Sohn YH, Lee PH. Association between olfactory deficit and motor and cognitive function in Parkinson's disease. J Mov Disord. 2020;13(2):133‐141. doi: 10.14802/jmd.19082 Epub 2020 Apr 6. PMID: 32241078; PMCID: PMC7280943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Silva MME, Viveiros CP, Kotsifas NJE, et al. Olfactory impairment in frontotemporal dementia: a systematic review and meta‐analysis. Dement Neuropsychol. 2019;13(2):154‐161. doi: 10.1590/1980-57642018dn13-020003 PMID: 31285789; PMCID: PMC6601313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bathini P, Brai E, Auber LA. Olfactory dysfunction in the pathophysiological continuum of dementia. Ageing Res Rev. 2019;55:100956. doi: 10.1016/j.arr.2019.100956 Epub 2019 Aug 31. PMID: 31479764. [DOI] [PubMed] [Google Scholar]
- 38. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta‐analysis with comparison to the COVID‐19 pandemic. Lancet Psychiatry. 2020;7(7):611‐627. doi: 10.1016/S2215-0366(20)30203-0 Epub 2020 May 18. PMID: 32437679; PMCID: PMC7234781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cothran TP, Kellman S, Singh S, et al. A brewing storm: The neuropsychological sequelae of hyperinflammation due to COVID‐19. Brain Behav Immun. 2020;88:957‐958. doi: 10.1016/j.bbi.2020.06.008 Epub 2020 Jun 23. PMID: 32590055; PMCID: PMC7309913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Naughton SX, Raval U, Pasinetti GM. Potential novel role of COVID‐19 in Alzheimer's disease and preventative mitigation strategies. J Alzheimers Dis. 2020;76(1):21‐25. doi: 10.3233/JAD-200537 PMID: 32538855; PMCID: PMC8057202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mattioli F, Stampatori C, Righetti F, Sala E, Tomasi C, De Palma G. Neurological and cognitive sequelae of Covid‐19: a four month follow‐up. J Neurol. 2021:1‐7. doi: 10.1007/s00415-021-10579-6 Epub ahead of print. PMID: 33932157; PMCID: PMC8088203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jaywant A, Vanderlind WM, Alexopoulos GS, Fridman CB, Perlis RH, Gunning FM. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID‐19. Neuropsychopharmacology. 2021:(13):2235‐2240. doi: 10.1038/s41386-021-00978-8 Epub ahead of print. PMID: 33589778; PMCID: PMC7884062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cristillo V, Pilotto A, Cotti Piccinelli S, et al. Age and subtle cognitive impairment are associated with long‐term olfactory dysfunction after COVID‐19 infection. J Am Geriatr Soc. 2021;69(10):2778‐2780. doi: 10.1111/jgs.17296 Epub ahead of print. PMID: 34019707; PMCID: PMC8242714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pirker‐Kees A, Platho‐Elwischger K, Hafner S, Redlich K, Baumgartner C. Hyposmia is associated with reduced cognitive function in COVID‐19: first preliminary results. Dement Geriatr Cogn Disord. 2021;50(1):68‐73. doi: 10.1159/000515575 Epub 2021 Apr 14. PMID: 33853062; PMCID: PMC8089429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Readhead B, Haure‐Mirande JV, Ehrlich ME, Gandy S, Dudley JT. Clarifying the potential role of microbes in Alzheimer's disease. Neuron. 2019;104(6):1036‐1037. doi: 10.1016/j.neuron.2019.11.008 PMID: 31855627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Readhead B, Haure‐Mirande JV, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99(1):64‐82.e7. doi: 10.1016/j.neuron.2018.05.023 Epub 2018 Jun 21. PMID: 29937276; PMCID: PMC6551233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sadrameli M, Bathini P, Alberi L. Linking mechanisms of periodontitis to Alzheimer's disease. Curr Opin Neurol. 2020;33(2):230‐238. doi: 10.1097/WCO.0000000000000797 PMID: 32097126 [DOI] [PubMed] [Google Scholar]
- 48. Itzhaki RF. Corroboration of a major role for Herpes Simplex Virus Type 1 in Alzheimer's disease. Front Aging Neurosci. 2018;10:324. doi: 10.3389/fnagi.2018.00324 PMID: 30405395; PMCID: PMC6202583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rybakowski JK. Commentary: corroboration of a major role for Herpes Simplex Virus Type 1 in Alzheimer's disease. Front Aging Neurosci. 2019;10:433. doi: 10.3389/fnagi.2018.00433 PMID: 30687080; PMCID: PMC6335248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mancuso R, Sicurella M, Agostini S, Marconi P, Clerici M. Herpes Simplex Virus Type 1 and Alzheimer's disease: link and potential impact on treatment. Expert Rev Anti Infect Ther. 2019;17(9):715‐731. doi: 10.1080/14787210.2019.1656064 Epub 2019 Aug 23. PMID: 31414935. [DOI] [PubMed] [Google Scholar]
- 51. Ou YN, Zhu JX, Hou XH, et al. Associations of infectious agents with Alzheimer's disease: a systematic review and meta‐analysis. J Alzheimers Dis. 2020;75(1):299‐309. doi: 10.3233/JAD-191337 PMID: 32280095 [DOI] [PubMed] [Google Scholar]
- 52. Devanand DP, Andrews H, Kreisl WC, et al. Antiviral therapy: Valacyclovir Treatment of Alzheimer's Disease (VALAD) Trial: protocol for a randomised, double‐blind,placebo‐controlled, treatment trial. BMJ Open. 2020;10(2):e032112. doi: 10.1136/bmjopen-2019-032112 PMID: 32034019; PMCID: PMC7045215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marcocci ME, Napoletani G, Protto V, et al. Herpes Simplex Virus‐1 in the brain: the dark side of a sneaky infection. Trends Microbiol. 2020;28(10):808‐820. doi: 10.1016/j.tim.2020.03.003 Epub 2020 May 5. PMID: 32386801. [DOI] [PubMed] [Google Scholar]
- 54. Qin Q, Li Y. Herpesviral infections and antimicrobial protection for Alzheimer's disease: implications for prevention and treatment. J Med Virol. 2019;91(8):1368‐1377. doi: 10.1002/jmv.25481 Epub 2019 Apr 17. PMID: 30997676. [DOI] [PubMed] [Google Scholar]
- 55. Bearer EL, Wu C. Herpes Simplex Virus, Alzheimer's disease and a possible role for Rab GTPases. Front Cell Dev Biol. 2019;7:134. doi: 10.3389/fcell.2019.00134 PMID: 31448273; PMCID: PMC6692634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mangold CA, Szpara ML. Persistent infection with Herpes Simplex Virus 1 and Alzheimer's disease – A call to study how variability in both virus and host may impact disease. Viruses. 2019;11(10):966. doi: 10.3390/v11100966 PMID: 31635156; PMCID: PMC6833100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. De Chiara G, Piacentini R, Fabiani M, et al. Recurrent Herpes Simplex Virus‐1 infection induces hallmarks of neurodegeneration and cognitive deficits in mice. PLoS Pathog. 2019;15(3):e1007617. doi: 10.1371/journal.ppat.1007617 PMID: 30870531; PMCID: PMC6417650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. La Rosa F, Agostini S, Bianchi A, et al. Herpes Simplex Virus‐1 (HSV‐1) infection induces a potent but ineffective IFN‐λ production in immune cells of AD and PD patients. J Transl Med. 2019;17(1):286. doi: 10.1186/s12967-019-2034-9 PMID: 31455413; PMCID: PMC6712644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li Puma DD, Piacentini R, Leone L, et al. Herpes Simplex Virus type‐1 infection impairs adult hippocampal neurogenesis via amyloid‐β protein accumulation. Stem Cells. 2019;37(11):1467‐1480. doi: 10.1002/stem.3072 Epub 2019 Aug 22. PMID: 31381841. [DOI] [PubMed] [Google Scholar]
- 60. Mainali S, Darsie ME. Neurologic and neuroscientific evidence in aged COVID‐19 patients. Front Aging Neurosci. 2021;13:648662. doi: 10.3389/fnagi.2021.648662 PMID: 33833676; PMCID: PMC8021699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rizzo MR, Paolisso G. SC‐2 emergency and long‐term cognitive impairment in older people. Aging Dis. 2021;12(2):345‐352. doi: 10.14336/AD.2021.0109. PMID: 33815868; PMCID: PMC7990368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kuo CL, Pilling LC, Atkins JL, et al. APOE e4 genotype predicts severe COVID‐19 in the UK Biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020;75(11):2231‐2232. doi: 10.1093/gerona/glaa131 PMID: 32451547; PMCID: PMC7314139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kuo CL, Pilling LC, Atkins JL, et al. ApoE e4e4 genotype and mortality with COVID‐19 in UK Biobank. J Gerontol A Biol Sci Med Sci. 2020;75(9):1801‐1803. doi: 10.1093/gerona/glaa169 PMID: 32623451; PMCID: PMC7337688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Del Ser T, Fernández‐Blázquez MA, Valentí M, et al. Residence clinical features, and genetic risk factors associated with symptoms of COVID‐19 in a cohort of older people in Madrid. Gerontology. 2021:67(3):281‐289. doi: 10.1159/000513182 Epub ahead of print. PMID: 33429394; PMCID: PMC7900450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang C, Zhang M, Garcia G Jr, et al. ApoE‐Isoform‐dependent SC‐2 neurotropism and cellular response. Cell Stem Cell. 2021;28(2):331‐342.e5. doi: 10.1016/j.stem.2020.12.018 Epub 2021 Jan 4. PMID: 33450186; PMCID: PMC7832490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meyerowitz EA, Kim AY, Ard KL, et al. Disproportionate burden of coronavirus disease 2019 among racial minorities and those in congregate settings among a large cohort of people with HIV. AIDS. 2020;34(12):1781‐1787. doi: 10.1097/QAD.0000000000002607 PMID: 32604138; PMCID: PMC7499878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID‐19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72(4):703‐706. doi: 10.1093/cid/ciaa815 PMID: 32562416; PMCID: PMC7337626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu Y, Feng Z, Li P, Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID‐19. Clin Chim Acta. 2020;509:220‐223. doi: 10.1016/j.cca.2020.06.026 Epub 2020 Jun 17. PMID: 32562665; PMCID: PMC7832938. (90) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer's disease. Lancet. 2021;397(10284):1577‐1590. doi: 10.1016/S0140-6736(20)32205-4 Epub 2021 Mar 2. PMID: 33667416. (1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Imbimbo BP, Ippati S, Watling M. Should drug discovery scientists still embrace the amyloid hypothesis for Alzheimer's disease or should they be looking elsewhere? Expert Opin Drug Discov. 2020;15(11):1241‐1251. doi: 10.1080/17460441.2020.1793755 Epub 2020 Jul 20. PMID: 32686970. (2) [DOI] [PubMed] [Google Scholar]
- 71. Imbimbo BP, Lozupone M, Watling M, Panza F. Discontinued disease‐modifying therapies for Alzheimer's disease: status and future perspectives. Expert Opin Investig Drugs. 2020;29(9):919‐933. doi: 10.1080/13543784.2020.1795127 Epub 2020 Jul 26. PMID: 32657175. (3) [DOI] [PubMed] [Google Scholar]
- 72. Yiannopoulou KG, Anastasiou AI, Zachariou V, Pelidou SH. Reasons for failed trials of disease‐modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines. 2019;7(4):97. doi: 10.3390/biomedicines7040097 PMID: 31835422; PMCID: PMC6966425. (4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhou J, Benoit M, Sharoar MG. Recent advances in pre‐clinical diagnosis of Alzheimer's disease. Metab Brain Dis. 2022;37(6):1703‐1725. doi: 10.1007/s11011-021-00733-4. Epub ahead of print. PMID: 33900524. (5) [DOI] [PubMed] [Google Scholar]
- 74. Badhwar A, McFall GP, Sapkota S, et al. A multiomics approach to heterogeneity in Alzheimer's disease: focused review and roadmap. Brain. 2020;143(5):1315‐1331. doi: 10.1093/brain/awz384 PMID: 31891371; PMCID: PMC7241959. (6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang J, Jia L, Li Y, Qiu Q, Quan M, Jia J. Fluid biomarkers in clinical trials for Alzheimer's disease: current and future application. J Alzheimers Dis. 2021;81(1):19‐32. doi: 10.3233/JAD-201068 PMID: 33749646. (7) [DOI] [PubMed] [Google Scholar]
- 76. Rabinovici GD, Carrillo MC, Forman M, et al. Multiple comorbid neuropathologies in the setting of Alzheimer's disease neuropathology and implications for drug development. Alzheimers Dement (N Y). 2016;3(1):83‐91. doi: 10.1016/j.trci.2016.09.002 PMID: 29067320; PMCID: PMC5651346. (8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age‐related and APOE4‐associated. Brain. 2018;141(7):2181‐2193. doi: 10.1093/brain/awy146 PMID: 29878075; PMCID: PMC6022546. (9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Floud S, Balkwill A, Sweetland S, et al. Cognitive and social activities and long‐term dementia risk: the prospective UK Million Women Study. Lancet Public Health. 2021;6(2):e116‐e123. doi: 10.1016/S2468-2667(20)30284-X PMID: 33516288; PMCID: PMC7848753. (10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. doi: 10.1016/S0140-6736(20)30367-6 Epub 2020 Jul 30. PMID: 32738937; PMCID: PMC7392084. (11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhou X, Li YYT, Fu AKY, Ip NY. Polygenic score models for Alzheimer's disease: from research to clinical applications. Front Neurosci. 2021;15:650220. doi: 10.3389/fnins.2021.650220 PMID: 33854414; PMCID: PMC8039467. (12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bellou E, Stevenson‐Hoare J, Escott‐Price V. Polygenic risk and pleiotropy in neurodegenerative diseases. Neurobiol Dis. 2020;142:104953. doi: 10.1016/j.nbd.2020.104953 Epub 2020 May 20. PMID: 32445791; PMCID: PMC7378564. (13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lambert SA, Abraham G, Inouye M. Towards clinical utility of polygenic risk scores. Hum Mol Genet. 2019;28(R2):R133‐R142. doi: 10.1093/hmg/ddz187 PMID: 31363735. (14) [DOI] [PubMed] [Google Scholar]
- 83. Feldman MW, Ramachandran S. Missing compared to what? Revisiting heritability, genes and culture. Philos Trans R Soc Lond B Biol Sci. 2018;373(1743):20170064. doi: 10.1098/rstb.2017.0064 PMID: 29440529; PMCID: PMC5812976. (15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Resende EPF, Llibre Guerra JJ, Miller BL. Health and socioeconomic inequities as contributors to brain health. JAMA Neurol. 2019;76(6):633‐634. doi: 10.1001/jamaneurol.2019.0362 PMID: 30907931; PMCID: PMC6682443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Oxtoby NP, Alexander DC. EuroPOND consortium . Imaging plus X: multimodal models of neurodegenerative disease. Curr Opin Neurol. 2017;30(4):371‐379. doi: 10.1097/WCO.0000000000000460 PMID: 28520598; PMCID: PMC5491241. (16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bellio M, Oxtoby NP, Walker Z, et al. Analyzing large Alzheimer's disease cognitive datasets: considerations and challenges. Alzheimers Dement (Amst). 2020;12(1):e12135. doi: 10.1002/dad2.12135 PMID: 33313379; PMCID: PMC7720865. (17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Thompson PM, Stein JL, Medland SE, et al. The ENIGMA consortium: large‐scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8(2):153‐82. doi: 10.1007/s11682-013-9269-5 PMID: 24399358; PMCID: PMC4008818. (18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. El‐Sappagh S, Alonso JM, Islam SMR, Sultan AM, Kwak KS. A multilayer multimodal detection and prediction model based on explainable artificial intelligence for Alzheimer's disease. Sci Rep. 2021;11(1):2660. doi: 10.1038/s41598-021-82098-3 PMID: 33514817; PMCID: PMC7846613. (19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Derex M, Perreault C, Boyd R. Divide and conquer: intermediate levels of population fragmentation maximize cultural accumulation. Philos Trans R Soc Lond B Biol Sci. 2018;373(1743):20170062. doi: 10.1098/rstb.2017.0062 PMID: 29440527; PMCID: PMC5812974. (20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Miners S, Kehoe PG, Love S. Cognitive impact of COVID‐19: looking beyond the short term. Alzheimers Res Ther. 2020;12(1):170. doi: 10.1186/s13195-020-00744-w PMID: 33380345; PMCID: PMC7772800. (25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. D'Arrigo JS. Nanotargeting of drug(s) for delaying dementia: relevance of covid‐19 impact on dementia. Am J Alzheimers Dis Other Demen. 2020;35:1533317520976761. doi: 10.1177/1533317520976761. PMID: 33307726. (26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Manca R, De Marco M, Venneri A. The impact of COVID‐19 infection and enforced prolonged social isolation on neuropsychiatric symptoms in older adults with and without dementia: a review. Front Psychiatry. 2020;11:585540. doi: 10.3389/fpsyt.2020.585540 PMID: 33192732; PMCID: PMC7649825. (27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhou J, Liu C, Sun Y, Huang W, Ye K. Cognitive disorders associated with hospitalization of COVID‐19: results from an observational cohort study. Brain Behav Immun. 2021;91:383‐392. doi: 10.1016/j.bbi.2020.10.019 Epub 2020 Oct 24. PMID: 33148439; PMCID: PMC7584518. (28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID‐19 hospitalization: a community‐based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184‐187. doi: 10.1016/j.bbi.2020.05.059 Epub 2020 May 23. PMID: 32454138; PMCID: PMC7245300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pinna P, Grewal P, Hall JP, et al. Neurological manifestations and COVID‐19: experiences from a tertiary care center at the Frontline. J Neurol Sci. 2020;415:116969. doi: 10.1016/j.jns.2020.116969 Epub 2020 Jun 3. PMID: 32570113; PMCID: PMC7832569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lassale C, Gaye B, Hamer M, Gale CR, Batty GD. Ethnic disparities in hospitalisation for COVID‐19 in England: the role of socioeconomic factors, mental health, and inflammatory and pro‐inflammatory factors in a community‐based cohort study. Brain Behav Immun. 2020;88:44‐49. doi: 10.1016/j.bbi.2020.05.074 Epub 2020 Jun 1. PMID: 32497776; PMCID: PMC7263214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Haitao T, Vermunt JV, Abeykoon J, et al. COVID‐19 and sex differences: mechanisms and biomarkers. Mayo Clin Proc. 2020;95(10):2189‐2203. doi: 10.1016/j.mayocp.2020.07.024 Epub 2020 Aug 4. PMID: 33012349; PMCID: PMC7402208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Brodin P. Immune determinants of COVID‐19 disease presentation and severity. Nat Med. 2021;27(1):28‐33. doi: 10.1038/s41591-020-01202-8 Epub 2021 Jan 13. PMID: 33442016 [DOI] [PubMed] [Google Scholar]
- 99. Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID‐19 hospitalization: a community‐based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184‐187. doi: 10.1016/j.bbi.2020.05.059 Epub 2020 May 23. PMID: 32454138; PMCID: PMC7245300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cheng L, Li H, Li L, Liu C, Yan S, Chen H, Li Y. Ferritin in the coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Clin Lab Anal. 2020;34(10):e23618. doi: 10.1002/jcla.23618 Epub 2020 Oct 19. PMID: 33078400; PMCID: PMC7595919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rostami M, Mansouritorghabeh H. D‐dimer level in COVID‐19 infection: a systematic review. Expert Rev Hematol. 2020;13(11):1265‐1275. doi: 10.1080/17474086.2020.1831383 Epub 2020 Oct 12. PMID: 32997543 [DOI] [PubMed] [Google Scholar]
- 102. Pranata R, Huang I, Lukito AA, Raharjo SB. Elevated N‐terminal pro‐brain natriuretic peptide is associated with increased mortality in patients with COVID‐19: systematic review and meta‐analysis. Postgrad Med J. 2020;96(1137):387‐391. doi: 10.1136/postgradmedj-2020-137884 Epub 2020 May 20. PMID: 32434874; PMCID: PMC7316121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pirker‐Kees A, Platho‐Elwischger K, Hafner S, Redlich K, Baumgartner C. Hyposmia is associated with reduced cognitive function in COVID‐19: first preliminary results. Dement Geriatr Cogn Disord. 2021;50(1):68‐73. doi: 10.1159/000515575 Epub 2021 Apr 14. PMID: 33853062; PMCID: PMC8089429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Almeria M, Cejudo JC, Sotoca J, Deus J, Krupinski J. Cognitive profile following COVID‐19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav Immun Health. 2020;9:100163. doi: 10.1016/j.bbih.2020.100163 Epub 2020 Oct 22. PMID: 33111132; PMCID: PMC7581383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Scialo F, Daniele A, Amato F, et al. ACE2: the major cell entry receptor for SARS‐CoV‐2. Lung. 2020;198(6):867‐877. doi: 10.1007/s00408-020-00408-4 Epub 2020 Nov 10. PMID: 33170317; PMCID: PMC7653219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Heneka MT, Golenbock D, Latz E, Morgan D, Brown R. Immediate and long‐term consequences of COVID‐19 infections for the development of neurological disease. Alzheimers Res Ther. 2020;12(1):69. doi: 10.1186/s13195-020-00640-3 PMID: 32498691; PMCID: PMC7271826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bougakov D, Podell K, Goldberg E. Multiple neuroinvasive pathways in COVID‐19. Mol Neurobiol. 2021;58(2):564‐575. doi: 10.1007/s12035-020-02152-5 Epub 2020 Sep 29. PMID: 32990925; PMCID: PMC7523266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Klingenstein M, Klingenstein S, Neckel PH, et al. Evidence of SARS‐CoV2 entry protein ACE2 in the human nose and olfactory bulb. Cells Tissues Organs. 2020;209(4‐6):155‐164. doi: 10.1159/000513040 Epub 2021 Jan 22. PMID: 33486479; PMCID: PMC7900466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chiappelli F. Towards neuro‐CoViD‐19. Bioinformation. 2020;16(4):288‐292. doi: 10.6026/97320630016288 PMID: 32773986; PMCID: PMC7392088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nagu P, Parashar A, Behl T, Mehta V. CNS implications of COVID‐19: a comprehensive review. Rev Neurosci. 2020;32(2):219‐234. doi: 10.1515/revneuro-2020-0070 PMID: 33550782 [DOI] [PubMed] [Google Scholar]
- 111. Brann DH, Tsukahara T, Weinreb C, et al. Non‐neuronal expression of SARS‐CoV‐2 entry genes in the olfactory system suggests mechanisms underlying COVID‐19‐associated anosmia. Sci Adv. 2020;6(31):eabc5801. doi: 10.1126/sciadv.abc5801 Epub 2020 Jul 24. PMID: 32937591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hascup ER, Hascup KN. Does SARS‐CoV‐2 infection cause chronic neurological complications? Geroscience. 2020;42(4):1083‐1087. doi: 10.1007/s11357-020-00207-y Epub 2020 May 25. PMID: 32451846; PMCID: PMC7247778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhou Y, Xu J, Hou Y, et al. Network medicine links SARS‐CoV‐2/COVID‐19 infection to brain microvascular injury and neuroinflammation in dementia‐like cognitive impairment. bioRxiv [Preprint]. 2021. doi: 10.1101/2021.03.15.435423. Update in: Alzheimers Res Ther. 2021;13(1):110. PMID: 33791705; PMCID: PMC8010732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang C, Zhang M, Garcia G Jr, et al. ApoE‐Isoform‐dependent SARS‐CoV‐2 neurotropism and cellular response. Cell Stem Cell. 2021;28(2):331‐342.e5. doi: 10.1016/j.stem.2020.12.018 Epub 2021 Jan 4. PMID: 33450186; PMCID: PMC7832490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kumar N, Mishra B, Mehmood A, Athar M, Shahid Mukhtar M. Integrative network biology framework elucidates molecular mechanisms of SARS‐CoV‐2 pathogenesis. iScience. 2020;23(9):101526. doi: 10.1016/j.isci.2020.101526 Epub 2020 Sep 3. PMID: 32895641; PMCID: PMC7468341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lachén‐Montes M, González‐Morales A, Zelaya MV, et al. Olfactory bulb neuroproteomics reveals a chronological perturbation of survival routes and a disruption of prohibitin complex during Alzheimer's disease progression. Sci Rep. 2017;7(1):9115. doi: 10.1038/s41598-017-09481-x PMID: 28831118; PMCID: PMC5567385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gordon DE, Hiatt J, Bouhaddou M, et al. Comparative host‐coronavirus protein interaction networks reveal pan‐viral disease mechanisms. Science. 2020;370(6521):eabe9403. doi: 10.1126/science.abe9403 Epub 2020 Oct 15. PMID: 33060197; PMCID: PMC7808408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Guedj E, Campion JY, Dudouet P, et al. 18F‐FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48(9):2823‐2833. doi: 10.1007/s00259-021-05215-4 Epub 2021 Jan 26. PMID: 33501506; PMCID: PMC7837643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Blazhenets G, Schroeter N, Bormann T, et al. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID‐19 patients. J Nucl Med. 2021;62(7):910‐915. doi: 10.2967/jnumed.121.262128 Epub 2021 Mar 31. PMID: 33789937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hugon J, Queneau M, Sanchez Ortiz M, Msika EF, Farid K, Paquet C. Cognitive decline and brainstem hypometabolism in long COVID: a case series. Brain Behav. 2022;12(4):e32513. doi: 10.1002/brb3.2513 Epub ahead of print. PMID: 35290729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hugon J, Msika EF, Queneau M, Farid K, Paquet C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol. 2022;269(1):44‐46. doi: 10.1007/s00415-021-10655-x Epub 2021 Jun 18. PMID: 34143277; PMCID: PMC8211714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hugon J. Long‐COVID: cognitive deficits (brain fog) and brain lesions in non‐hospitalized patients. Presse Med. 2021;51(2):104090. doi: 10.1016/j.lpm.2021.104090 Epub ahead of print. PMID: 34718113; PMCID: PMC8552626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Soldevila‐Domenech N, Forcano L, Boronat A, et al. Effects of COVID‐19 home confinement on mental health in individuals with increased risk of Alzheimer's disease. J Alzheimers Dis. 2021;79(3):1015‐1021. doi: 10.3233/JAD-201408 PMID: 33386809; PMCID: PMC7990405. (24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. mhGAP Training Manual for the mhGAP Intervention Guide for mental, neurological and substance use disorders in non‐specialized health settings – version 2.0. [PubMed]
- 125. Wing JK, Babor T, Brugha T, et al. Schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry. 1990;47(6):589‐93. doi: 10.1001/archpsyc.1990.01810180089012 PMID: 2190539. (100) [DOI] [PubMed] [Google Scholar]
- 126. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412‐4. doi: 10.1212/wnl.43.11.2412-a PMID: 8232972 [DOI] [PubMed] [Google Scholar]
- 127. Preece D, Becerra R, Campitelli G. Assessing emotional reactivity: psychometric properties of the Perth Emotional Reactivity Scale and the development of a short form. J Pers Assess. 2019;101(6):589‐597. doi: 10.1080/00223891.2018.1465430 Epub 2018 May 15. PMID: 29764211 [DOI] [PubMed] [Google Scholar]
- 128. Besser L, Kukull W, Knopman DS, et al. Neuropsychology work group, directors, and clinical core leaders of the National Institute on Aging‐funded US Alzheimer's Disease Centers. Version 3 of the National Alzheimer's Coordinating Center's Uniform Data Set. Alzheimer Dis Assoc Disord. 2018;32(4):351‐358. doi: 10.1097/WAD.0000000000000279 PMID: 30376508; PMCID: PMC6249084. (103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210‐6. doi: 10.1097/01.wad.0000213865.09806.92 PMID: 17132964. (101) [DOI] [PubMed] [Google Scholar]
- 130. Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249‐58. doi: 10.1097/WAD.0b013e318142774e PMID: 17804958. (102) [DOI] [PubMed] [Google Scholar]
- 131. Burbelo PD, Riedo FX, Morishima C, et al. Detection of nucleocapsid antibody to SARS‐CoV‐2 is more sensitive than antibody to spike protein in COVID‐19 patients. medRxiv [Preprint]. 2020. doi: 10.1101/2020.04.20.20071423. Update in: J Infect Dis. 2020 May 19. PMID: 32511445; PMCID: PMC7239070 [DOI] [Google Scholar]
- 132. Miller TD, Chong TT, Aimola Davies AM, et al. Focal CA3 hippocampal subfield atrophy following LGI1 VGKC‐complex antibody limbic encephalitis. Brain. 2017;140(5):1212‐1219. doi: 10.1093/brain/awx070 PMID: 28369215; PMCID: PMC5405234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Rua C, Clarke WT, Driver ID, et al. Multi‐centre, multi‐vendor reproducibility of 7T QSM and R2* in the human brain: results from the UK7T study. Neuroimage. 2020;223:117358. doi: 10.1016/j.neuroimage.2020.117358 Epub 2020 Sep 9. PMID: 32916289; PMCID: PMC7480266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Clarke WT, Mougin O, Driver ID, et al. Multi‐site harmonization of 7 tesla MRI neuroimaging protocols. Neuroimage. 2020;206:116335. doi: 10.1016/j.neuroimage.2019.116335 Epub 2019 Nov 8. PMID: 31712167; PMCID: PMC7212005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Düzel E, Acosta‐Cabronero J, Berron D, et al. European Ultrahigh‐Field Imaging Network for Neurodegenerative Diseases (EUFIND). Alzheimers Dement (Amst). 2019;11:538‐549. doi: 10.1016/j.dadm.2019.04.010 PMID: 31388558; PMCID: PMC6675944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Campos S., Pizarro L., Valle C., Gray KR, Rueckert D, Allende H. Evaluating imputation techniques for missing data in ADNI: a patient classification study. In: Pardo A, Kittler J., eds. Progress in Pattern Recognition, Image Analysis, Computer Vision, and Applications. CIARP 2015. Lecture Notes in Computer Science, vol 9423. 2015; Springer, Cham. doi: 10.1007/978-3-319-25751-8_1 [DOI] [Google Scholar]
- 137. Lee KJ, Tilling KM, Cornish RP, et al. Framework for the treatment and reporting of missing data in observational studies: the treatment and reporting of missing data in observational studies framework. J Clin Epidemiol. 2021;134:79‐88. doi: 10.1016/j.jclinepi.2021.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Shishegar R, Cox T, Rolls D, et al. Using imputation to provide harmonized longitudinal measures of cognition across AIBL and ADNI. Scientific Reports. 2021;11(1):23788. doi: 10.1038/s41598-021-02827-6 PMID: 34893624; PMCID: PMC8664816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Goetz CG, Luo S, Wang L, Tilley BC, LaPelle NR, Stebbins GT. Handling missing values in the MDS‐UPDRS. Mov Disord. 2015;30(12):1632‐1638. doi: 10.1002/mds.26153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Baurley JW, Conti DV, Gauderman WJ, Thomas DC. Discovery of complex pathways from observational data. Stat Med. 2010;29(19):1998‐2011. doi: 10.1002/sim.3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Eckhardt M, Hultquist JF, Kaake RM. et al. A systems approach to infectious disease. Nat Rev Genet 2020;21:339‐354. doi: 10.1038/s41576-020-0212-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pingault JB, O'Reilly PF, Schoeler T. et al. Using genetic data to strengthen causal inference in observational research. Nat Rev Genet 2018;19:566‐580 [DOI] [PubMed] [Google Scholar]
- 143. Bowden J, Bornkamp B, Glimm E, Bretz F. Connecting instrumental variable methods for causal inference to the Estimand framework. Stat Med. 2021;40(25):5605‐5627. doi: 10.1002/sim.9143 [DOI] [PubMed] [Google Scholar]
- 144. Hammerton G, Munafò MR. Causal inference with observational data: the need for triangulation of evidence. Psycholo Med. 2021;51(4):563‐578. doi: 10.1017/S0033291720005127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Harper S. A future for observational epidemiology: clarity, credibility, transparency. Am J Epidemiol. 2019;188(5):840‐845. doi: 10.1093/aje/kwy280 [DOI] [PubMed] [Google Scholar]
- 146. Deer RR, Rock MA, Vasilevsky N, et al. Characterizing long COVID: deep phenotype of a complex condition. EBioMedicine. 2021;74:103722. doi: 10.1016/j.ebiom.2021.103722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Meyers JE, Miller RM, Rohling ML, Kalat SS. Premorbid estimates of neuropsychological functioning for diverse groups. Appl Neuropsychol Adult. 2020;27(4):364‐375. doi: 10.1080/23279095.2018.1550412 Epub 2019 Feb 18. PMID: 30773042. (92) [DOI] [PubMed] [Google Scholar]
- 148. Shura RD, Ord AS, Martindale SL, Miskey HM, Taber KH. Test of premorbid functioning: you're doing it wrong, but does it matter? Arch Clin Neuropsychol. 2020:acaa025. doi: 10.1093/arclin/acaa025 Epub ahead of print. PMID: 32407489. (93) [DOI] [PubMed] [Google Scholar]
- 149. Axelrod BN, Vanderploeg RD, Schinka JA. Comparing methods for estimating premorbid intellectual functioning. Arch Clin Neuropsychol. 1999;14(4):341‐346. PMID: 14590588. (94) [PubMed] [Google Scholar]
- 150. Berg JL, Durant J, Banks SJ, Miller JB. Estimates of premorbid ability in a neurodegenerative disease clinic population: comparing the Test of Premorbid Functioning and the Wide Range Achievement Test, 4th Edition. Clin Neuropsychol. 2016;30(4):547‐557. doi: 10.1080/13854046.2016.1186224 Epub 2016 May 17. PMID: 27187762. (95) [DOI] [PubMed] [Google Scholar]
- 151. Franzen MD, Burgess EJ, Smith‐Seemiller L. Methods of estimating premorbid functioning. Arch Clin Neuropsychol. 1997;12(8):711‐738. PMID: 14590649. (96) [PubMed] [Google Scholar]
- 152. Chasman DI, Giulianini F, Demler OV, Udler MS. Pleiotropy‐based decomposition of genetic risk scores: association and interaction analysis for type 2 diabetes and CAD. Am J Hum Genet. 2020;106(5):646‐658. doi: 10.1016/j.ajhg.2020.03.011 Epub 2020 Apr 16. PMID: 32302534; PMCID: PMC7212269. (63) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sims R, Hill M, Williams J. The multiplex model of the genetics of Alzheimer's disease. Nat Neurosci. 2020;23(3):311‐322. doi: 10.1038/s41593-020-0599-5 Epub 2020 Feb 28. PMID: 32112059. (64) [DOI] [PubMed] [Google Scholar]
- 154. Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111‐28. doi: 10.31887/DCNS.2009.11.2/cqiu PMID: 19585947; PMCID: PMC3181909. (65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Uscamayta Ayvar MJ, Sanchez Garrafa R, Escobar JI, de Erausquin GA. The concept of mania in traditional andean culture. Am J Psychiatry. 2019;176(5):338‐340. doi: 10.1176/appi.ajp.2018.18060681 PMID: 31039640; PMCID: PMC6499381. (99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Fernández, A.L. , Abe, J . Bias in cross‐cultural neuropsychological testing: problems and possible solutions. Cult. Brain 2018;6:1‐35. doi: 10.1007/s40167-017-0050-2 [DOI] [Google Scholar]
- 157. Ardila A. Towards a cross‐cultural neuropsychology. J Soc Evol Syst. 1996;19(3):237‐48. doi: 10.1016/S1061-7361(96)90034-X.23 [DOI] [Google Scholar]
- 158. Chiao JY, editor. Cultural neuroscience: Cultural influences on brain function. New York, NY: Elsevier; 2009. Levinson S. Studying spatial conceptualization across cultures: anthropology and cognitive science. Ethos. 1998;26(1):7‐24 Retrieved from: http://www.jstor.org/stable/640692 [Google Scholar]
- 159. Levinson S. Studying spatial conceptualization across cultures: anthropology and cognitive science. Ethos. 1998;26(1):7‐24 Retrieved from: http://www.jstor.org/stable/640692 [Google Scholar]
- 160. Butterworth B, Reeve R, Reynolds F. Using mental representations of space when words are unavailable: studies of enumeration and arithmetic in indigenous Australia. J Cross‐Cult Psychol. 2011;42(4):630‐8. doi: 10.1177/0022022111406020 [DOI] [Google Scholar]
- 161. Davidson GR. An ethnographic psychology of aboriginal cognitive ability. Oceania. 1979;49(4):270‐94. doi: 10.1002/j.1834-4461.1979.tb01917.x [DOI] [Google Scholar]
- 162. Kearins JM. Visual spatial memory in Australian aboriginal children of desert regions. Cogn Psychol. 1981;13(3):434‐60. doi: 10.1016/0010-0285(81)90017-7 [DOI] [PubMed] [Google Scholar]
- 163. Klich LZ, Davidson GR. A cultural difference in visual memory: On le voit, on ne le voit plus. Int J Psychol. 1983;18(3‐4):189‐201. doi: 10.1080/00207598308247473 [DOI] [Google Scholar]
- 164. Dingwall KM, Pinkerton J, Lindeman MA. “People like numbers”: a descriptive study of cognitive assessment methods in clinical practice for aboriginal Australians in the Northern Territory. BMC Psychiatry. 2013;13(42). doi: 10.1186/1471-244X-13-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Holding P, Anum A, van de Vijver FJR, et al. Can we measure cognitive constructs consistently within and across cultures? Evidence from a test battery in Bangladesh, Ghana, and Tanzania. Appl Neuropsychol Child. 2018;7(1):1‐13. doi: 10.1080/21622965.2016.1206823 Epub 2016 Jul 27. PMID: 27463827. (113) [DOI] [PubMed] [Google Scholar]
- 166. Kreisl WC, Jin P, Lee S, et al. Odor identification ability predicts PET amyloid status and memory decline in older adults. J Alzheimers Dis. 2018;62(4):1759‐1766. doi: 10.3233/JAD-170960 PMID: 29614678; PMCID: PMC6760657. (120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Klein J, Yan X, Johnson A, et al. Olfactory impairment is related to tau pathology and neuroinflammation in Alzheimer's disease. J Alzheimers Dis. 2021;80(3):1051‐1065. doi: 10.3233/JAD-201149 PMID: 33646153; PMCID: PMC8044007. (121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Chrea C, Valentin D, Abdi H. Graded structure in odour categories: a cross‐cultural case study. Perception. 2009;38(2):292‐309. doi: 10.1068/p5687 PMID: 19400437. (122) [DOI] [PubMed] [Google Scholar]
- 169. Patin A, Pause BM. Human amygdala activations during nasal chemoreception. Neuropsychologia. 2015;78:171‐94. doi: 10.1016/j.neuropsychologia.2015.10.009 Epub 2015 Oct 10. PMID: 26459095. (118) [DOI] [PubMed] [Google Scholar]
- 170. Lukiw WJ, Pogue A, Hill JM. SC‐2 Infectivity and Neurological Targets in the Brain. Cell Mol Neurobiol. 2022:42(1):217‐224. doi: 10.1007/s10571-020-00947-7 Epub ahead of print. PMID: 32840758; PMCID: PMC7445393. (117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Serrano GE, Walker JE, Arce R, et al. Mapping of SC‐2 Brain Invasion and Histopathology in COVID‐19 Disease. medRxiv [Preprint]. 2021. doi: 10.1101/2021.02.15.21251511 PMID: 33619496; PMCID: PMC7899461. (115) [DOI] [Google Scholar]
- 172. Guedj E, Million M, Dudouet P, Tissot‐Dupont H, Bregeon F, Cammilleri S, Raoult D. 18F‐FDG brain PET hypometabolism in post‐SC‐2 infection: substrate for persistent/delayed disorders? Eur J Nucl Med Mol Imaging. 2021;48(2):592‐595. doi: 10.1007/s00259-020-04973-x Epub 2020 Jul 30. PMID: 32728799; PMCID: PMC7391029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Douaud G, Lee S, Alfaro‐Almagro F. et al. SARS‐CoV‐2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697‐707. doi: 10.1038/s41586-022-04569-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Becerra R, Preece D, Campitelli G, Scott‐Pillow G. The assessment of emotional reactivity across negative and positive emotions: development and validation of the Perth Emotional Reactivity Scale (PERS). Assessment. 2019;26(5):867‐879. doi: 10.1177/1073191117694455 Epub 2017 Feb 1. PMID: 29214846. (114) [DOI] [PubMed] [Google Scholar]
- 175. Panza F, Lozupone M, Solfrizzi V, Watling M, Imbimbo BP. Time to test antibacterial therapy in Alzheimer's disease. Brain. 2019;142(10):2905‐2929. doi: 10.1093/brain/awz244 PMID: 31532495 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION


