Abstract
Sarcopenia often affects patients with various types of cancer, and has been reported to affect patient prognosis and therapeutic effects. However, to the best of our knowledge, there are no reports on the relationship between gemcitabine plus nab-paclitaxel combination therapy (GnP) and sarcopenia in patients with unresectable pancreatic cancer. The present study analyzed the relationship between overall survival (OS), progression-free survival (PFS), response rate, disease control rate, adverse events (AEs) and sarcopenia in patients with pancreatic cancer treated with GnP. A total of 121 consecutive patients with advanced pancreatic cancer who received GnP as first-line chemotherapy between January 2015 and December 2017 were retrospectively analyzed. GnP consisted of 1,000 mg/m2 gemcitabine and 125 mg/m2 nab-paclitaxel, which were administered on days 1, 8 and 15 every 4 weeks. The skeletal muscle index (SMI) was calculated using bioimpedance analysis (BIA) as an index of sarcopenia prior to GnP. The patients were divided into sarcopenia (n=41) and non-sarcopenia (n=80) groups using cutoff values of 8.87 and 6.42 kg/m2 for male and female patients, respectively. The sarcopenia and non-sarcopenia groups had a median OS of 8.1 and 13.9 months, respectively [hazard ratio (HR) 0.79; 95% confidence interval (CI) 0.53-1.20], and a median PFS of 4.3 and 6.3 months, respectively (HR 0.63; 95% CI 0.42-0.95). The response and disease controls rate were not statistically different between the groups (20 vs. 32%, P=0.20; 81 vs. 80%, P=1.0). In addition, comparison of common grade 3 and 4 AEs between the two groups revealed no statistically significant differences. In conclusion, the results of the present study indicated that SMI obtained by BIA may be a predictor of treatment response and prognosis in patients with advanced pancreatic cancer who undergo GnP.
Keywords: pancreatic cancer, sarcopenia, chemotherapy, bioimpedance method, GnP, survival, AEs, efficacy, treatment response
Introduction
An estimated 460,000 new pancreatic cancer cases occur worldwide each year, resulting in 430,000 annual deaths (1). Pancreatic cancer has several risk factors, including smoking, age, alcohol abuse, obesity, genetic factors, diabetes, diet, and lack of exercise, and it is one of the most intractable carcinomas, with a 5-year survival rate of approximately 9% (2,3). In Japan, the number of pancreatic cancer patients has been increasing in recent years; it is responsible for more than 30,000 deaths every year, making it the fourth leading cause of cancer death following lung cancer, colon cancer, and stomach cancer (4).
Gemcitabine plus nab-paclitaxel combination therapy (GnP) was shown to be superior to gemcitabine monotherapy in terms of overall survival (OS) as a first-line treatment for patients with metastatic pancreatic cancer in a phase III trial (5). The treatment guideline of the National Comprehensive Cancer Network and Japanese Pancreas Society recommends GnP as a standard treatment for pancreatic cancer in combination with a FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin) regimen (6,7). GnP and FOLFIRINOX have become the standard treatment for unresectable pancreatic cancer not only in Japan but also worldwide.
Sarcopenia is a condition in which muscle mass decreases with age (8). Sarcopenia can be divided into primary or secondary sarcopenia according to its origin (i.e., malignant tumors, chronic heart failure, or chronic kidney disease) (9,10). In recent years, global interest in sarcopenia has increased, and diagnostic algorithms were proposed by the European Working Group on Sarcopenia in Older People (EWGSOP) in 2010 and the Asian Working Group for Sarcopenia in 2014, respectively (11,12). In both sets of criteria, sarcopenia is defined as a morbid status involving loss of muscle mass or loss of physical function.
Because it is defined by a decrease in skeletal muscle mass, the skeletal muscle index (SMI) is useful for the evaluation of sarcopenia (13). The methods of measuring the SMI include measuring the cross-sectional area of the muscle by examining images, such as computed tomography (CT) and magnetic resonance imaging (MRI); bioelectrical impedance analysis (BIA); and dual-energy X-ray absorptiometry (DEXA) (14,15).
Sarcopenia has been reported to be a prognostic factor in various cancers, including pancreatic cancer (16–19). Moreover, in patients receiving cytotoxic chemotherapy, sarcopenia has been reported to be a predictor of response and to contribute to increased toxicity (20–22). Although GnP is the standard chemotherapy for pancreatic cancer and has been administered to numerous patients, there are no reports on the relationship between GnP treatment outcomes and sarcopenia in patients with advanced pancreatic cancer. As the relationship between sarcopenia and prognosis becomes clear, early nutritional and exercise interventions may improve the prognosis. Therefore, we retrospectively analyzed the impact of sarcopenia on OS, progression-free survival (PFS), response rate, disease control rate, and adverse events (AEs) by assessing SMI via BIA.
Materials and methods
Ethics
The present study was approved by the Institutional Review Board of Kanagawa Cancer Center. Informed consent was obtained in the form of an opt-out form on the website.
Patients
We retrospectively analyzed 121 consecutive patients with advanced pancreatic cancer who received GnP as the first-line treatment from January 2015 to December 2017. GnP treatment included 1,000 mg/m2 of gemcitabine and 125 mg/m2 of nab-paclitaxel administered on days 1, 8, and 15 at four-week-intervals. The dose was reduced or postponed depending on general condition, laboratory data, and AEs.
The inclusion criteria were as follows: patients with unresectable pancreatic cancer diagnosed as stage III or IV according to the 7th edition of the Union for International Cancer Control TNM classification and an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1 (23,24). Adenocarcinoma was diagnosed by tissue biopsy or cytology. These patients received GnP as first-line chemotherapy. Any patient unsuitable for InBody 720 measurement due to massive ascites and pleural effusion was excluded from the study. We also excluded patients with clinically symptomatic cholangitis or pancreatitis.
Predictors
Body composition was evaluated using an InBody 720® (InBody, Tokyo, Japan) prior to the initiation of GnP. SMI was calculated by dividing the skeletal muscle mass of the extremities by the square of the height. The cutoff values were based on values measured in healthy adults in Taiwan using BIA. The SMI cutoff values were below the mean adult skeletal muscle mass indicated by the EWGSOP, with reference to the value determined as −2 standard deviations. In this study, the cutoff values were 8.87 kg/m2 for males and 6.42 kg/m2 for females (25). Cases with an SMI higher than the cutoff values were allocated to the non-sarcopenia group and those with an SMI lower than the cutoff values were allocated to the sarcopenia group. In both groups, sex age, PS, body mass index (BMI), clinical stage, the modified Glasgow prognostic score (mGPS), tumor localization (pancreatic head or body tail), and tumor marker [serum carbohydrate antigen 19-9 (CA19-9)] were analyzed.
CA19-9 was measured at the start of GnP. We measured CA19-9 by chemiluminescent immunoassay using an ARCHITECTi2000SR® (Abbott Japan, Tokyo, Japan). The BMI was calculated by dividing the weight at the start of GnP by the square of the height. The standard value was set at 22 (26). The adopted mGPS was based on both the C-reactive protein (CRP) and albumin levels as sarcopenia indicators (27). Patients with both elevated CRP (>1.0 mg/l) and hypoalbuminemia (<3.5 g/l) were assigned a score of 2; those patients with either elevated CRP or hypoalbuminemia were assigned a score of 1; and those with neither elevated CRP nor hypoalbuminemia were assigned a score of 0. The relative dose intensity (RDI) for each chemotherapy regimen was calculated by dividing the actual dose by the planned dose. We analyzed the RDI up to 12 weeks after the start of GnP because it is expected to decrease with long-term treatment, mainly due to peripheral neuropathy caused by nab-paclitaxel (5). Second-line treatment after GnP failure was initiated when patients had adequate organ function and a good PS; otherwise, they received best supportive care.
Follow-up
The follow-up period was until December 2019. The response and disease control rates were evaluated based on 5-mm slice CT scans every 6–10 weeks and the (Response Evaluation Criteria in Solid Tumors version 1.1 (28). GnP was continued until the appearance of unacceptable AEs or disease progression.
Outcomes
In this study, the primary outcomes were OS, PFS, disease control rate, and response rate. OS was calculated from the date of GnP initiation to the date of death by any cause. PFS was calculated from the date of GnP initiation to the date of disease progression or death by any cause. Patients lost to follow-up were treated as censored cases.
The secondary outcomes were AEs. Hematologic toxicities, such as leukopenia, neutropenia, thrombocytopenia, and anemia, and non-hematologic toxicities, such as nausea, anorexia, diarrhea, constipation, fatigue, peripheral neuropathy, and anorexia were listed as retrospectively evaluable AEs. AEs were classified into grades 1–5 according to the Common Terminology Criteria for Adverse Events version 5 (29).
Statistical method
According to previous reports (30), the proportion of sarcopenia was estimated as 30–65%, and its impact on hazard ratio (HR) of survival was at least 0.5. We set the duration of accrual and follow-up as 2 years and 1 year, respectively, and considered that a sample size of 109 patients was required to detect the impact of sarcopenia on the survival, with an alpha error of 0.05 and a power of 90%. JMPPro15.0 (JMP Japan, Toyo, Japan) was used for the statistical analysis. The unpaired Student's t-test and Fisher's exact test were used for comparisons of patient backgrounds and AEs. The comparison of RDI was evaluated by unpaired Student's t-test for each drug.
PFS and OS were analyzed using the Kaplan-Meier method and the HR and corresponding 95% confidence interval (CI) were estimated using the log-rank test and Cox regression analysis. Multivariate analysis for prognostic factors was performed using the Cox Proportional Hazards model with the backward selection method. P<0.05 was considered to indicate a statistically significant difference.
Results
Patients
Table I shows the patient backgrounds in each group. There were no differences in age, tumor location, clinical stage, mGPS, PS, or CA19-9 between the two groups. There were significantly more males than females in the sarcopenia group.
Table I.
Patient backgrounds of the sarcopenia and non-sarcopenia groups.
| Characteristic | All patients (n=121) | Non-sarcopenia (n=81) | Sarcopenia (n=40) | P-value |
|---|---|---|---|---|
| Median age, years (range) | 69 (43–80) | 67 (43–80) | 70 (44–78) | 0.05 |
| Sex, n | <0.01 | |||
| Male | 71 | 36 | 35 | |
| Female | 50 | 45 | 5 | |
| Performance status, n | 0.84 | |||
| 0 | 43 | 28 | 15 | |
| 1 | 78 | 53 | 25 | |
| Mean BMI, kg/m2 (SD) | 20.6 (3.0) | 21.5 (2.67) | 18.5 (2.45) | <0.01 |
| Mean SMI, kg/m2 (SD) | ||||
| All | 8.4 (1.15) | 8.7 (1.1) | 7.8 (0.98) | <0.01 |
| Male | 8.9 (1.04) | 9.7 (0.61) | 8.1 (0.7) | <0.01 |
| Female | 7.7 (0.94) | 7.9 (0.79) | 5.9 (0.98) | <0.01 |
| UICC clinical stage, n | 0.83 | |||
| III | 33 | 23 | 10 | |
| IV | 88 | 58 | 30 | |
| Mean albumin, g/dl (SD) | 3.7 (0.54) | 3.7 (0.53) | 3.5 (0.50) | 0.07 |
| Median CRP, mg/dl (IQR) | 1.56 (0.12-1.29) | 0.2 (0.10-1.02) | 0.54 (0.14-2.26) | 0.08 |
| mGPS, n | 0.18 | |||
| 0 | 59 | 44 | 15 | |
| 1 | 30 | 19 | 11 | |
| 2 | 32 | 18 | 14 | |
| Localization of tumor, n | 0.44 | |||
| Head | 47 | 36 | 11 | |
| Body or tail | 74 | 45 | 29 | |
| Median CA19-9, U/ml (IQR) | 628.2 (99.4-17,966) | 564.7 (70.0-20,349) | 1,477 (332–16,147) | 0.37 |
BMI, body mass index; SMI, skeletal muscle index; UICC, Union for International Cancer Control; CRP, C-reactive protein; mGPS, modified Glasgow prognostic score; SD, standard deviation; IQR, inter-quartile range. The unpaired Student's t-test and Fisher's exact test were used for comparisons of patient backgrounds.
Treatment course
In the non-sarcopenia and sarcopenia groups, the median RDIs at 12 weeks after the start of GnP were 0.84 (range: 0.11-1.0) and 0.73 (range: 0.22-1.0) for gemcitabine, and 0.80 (range: 0.11-1.0) and 0.67 (range: 0.11-1.0) for nab-paclitaxel, respectively, with corresponding P-values of 0.32 and 0.26 for gemcitabine and nab-paclitaxel, respectively (Table II). The number of patients with dose reduction at GnP initiation was significantly higher in the sarcopenia group (P=0.03, Table II) than in the non-sarcopenia group. The reasons for discontinuing chemotherapy were not different between the two groups.
Table II.
Treatment courses of the sarcopenia and non-sarcopenia groups.
| Variable | Non-sarcopenia (n=81) | Sarcopenia (n=40) | P-value |
|---|---|---|---|
| Median relative dose intensity at 12 weeks (range) | |||
| Gemcitabine | 0.84 (0.11-1.0) | 0.73 (0.22-1.0) | 0.32 |
| Nab-paclitaxel | 0.80 (0.11-1.0) | 0.67 (0.11-1.0) | 0.26 |
| Dose reduction at GnP initiation, n (%) | 5 (6) | 4 (10) | 0.03 |
| Reason for GnP discontinuation, n (%) | |||
| Disease progression | 68 (84) | 30 (75) | 0.28 |
| Adverse events | 5 (6) | 5 (12.5) | 0.26 |
| Poor performance status | 2 (2) | 3 (7) | 0.20 |
| Patient's request | 3 (4) | 2 (5) | 0.77 |
| Conversion surgery | 3 (4) | 0 (0) | 0.20 |
| Secondary treatment, n (%) | 60 (74) | 29 (73) | 0.85 |
| Chemotherapy, n (%) | 54 (67) | 27 (68) | |
| Chemoradiotherapy, n (%) | 3 (4) | 1 (3) | |
| Conversion surgery, n (%) | 3 (4) | 0 (0) | |
| Others, n (%) | 0 (0) | 1 (3) |
The unpaired Student's t-test and Fisher's exact test were used for comparisons of treatment courses.
A total of 60 patients (74%) in the non-sarcopenia group and 29 patients (73%) in the sarcopenia group received second-line chemotherapy (P=0.85, Table II). The regimen for the second-line treatment was at the physician's discretion: FOLFIRINOX, S-1, or other investigational drugs. Three patients in the non-sarcopenia group who showed remarkable tumor shrinkage underwent surgical resection as a conversion surgery.
Efficacy
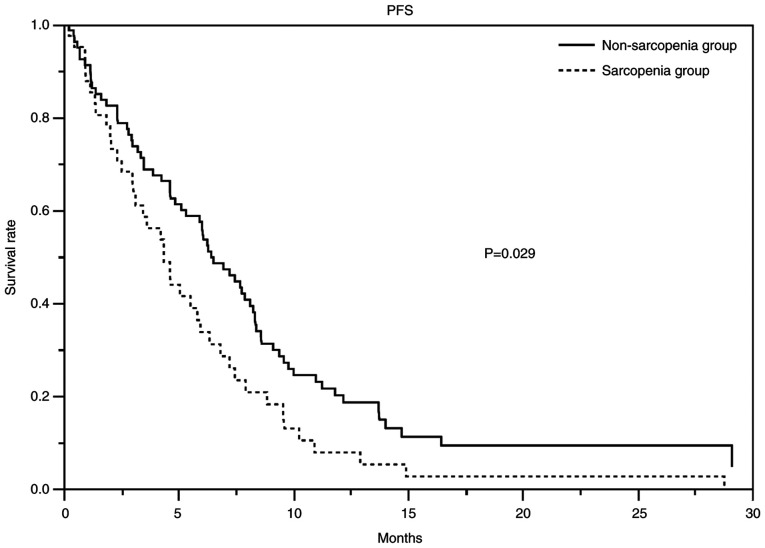
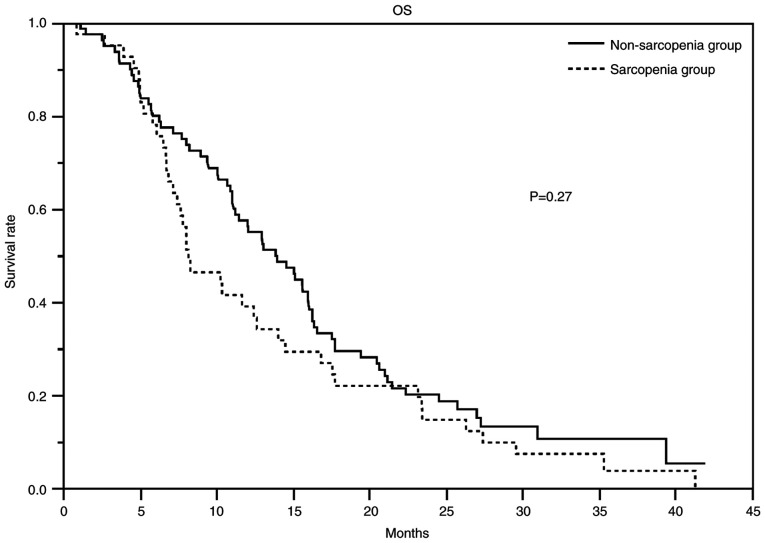
At the time of data cutoff, the median duration of follow-up for censored cases was 27.4 months. Table III showed the response and disease control rates, PFS, and OS. The response rates in the non-sarcopenia and sarcopenia groups were 32 and 20% (P=0.20), respectively, and the disease control rates were 81 and 80% (P=1.00), respectively. The non-sarcopenia and sarcopenia groups had a median PFS of 6.4 months (95% CI 4.9-8.1) and 4.4 months (range: 3–5.9), respectively, and a median OS of 13.9 months (95% CI 11.0-16.1) and 8.2 months (95% CI 6.9-12.7), respectively. Figs. 1 and 2 show the PFS and Kaplan-Meier curves for OS, respectively. The HR for PFS in the non-sarcopenia group compared with the sarcopenia group was 0.63 (95% CI 0.42-0.95; Table IV), whereas that for OS was 0.79 (95% CI 0.53-1.20; Table V). We could not reject the null hypothesis for the OS.
Table III.
Treatment efficacy in the sarcopenia and non-sarcopenia groups.
| Variable | Non-sarcopenia (n=81) | Sarcopenia (n=40) | P-value |
|---|---|---|---|
| Objective response, n (%) | 0.30 | ||
| CR | 0 (0) | 1 (3) | |
| PR | 26 (32) | 7 (18) | |
| SD | 40 (49) | 24 (60) | |
| PD | 15 (19) | 8 (20) | |
| Response rate, % | 32 | 20 | 0.20 |
| Disease control rate, % | 81 | 80 | >0.99 |
| Median PFS, months (95% CI) | 6.4 (95% CI 4.9-8.1) | 4.3 (95% CI 3.0-5.9) | 0.02 |
| Median OS, months (95% CI) | 13.9 (95% CI 11.0-16.1) | 8.3 (95% CI 6.9-12.7) | 0.18 |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PFS, progression-free survival; OS, overall survival; CI, confidence interval. The Fisher's exact test was used to assess objective response, response rate and disease control rate. PFS and OS were analyzed using the Kaplan-Meier method and the 95% CI was estimated using the log-rank test.
Figure 1.
PFS curves of the patients who received gemcitabine and nab-paclitaxel. The PFS of the non-sarcopenia group (solid line) was significantly longer than that of the sarcopenia group (dotted line). The median PFS was 6.4 months (95% CI 4.9-8.1) in the non-sarcopenia group and 4.3 months (95% CI 3.0-5.9) in the sarcopenia group. The hazard ratio was 0.64 (95% CI 0.41-0.99). PFS, progression-free survival; CI, confidence interval.
Figure 2.
OS of the patients who received gemcitabine and nab-paclitaxel. The median OS was 13.9 months (95% CI 11.0-16.1) in the non-sarcopenia group (solid line) and 8.2 months (95% CI 6.9-12.7) in the sarcopenia group (dotted line). The hazard ratio was 0.90 (95% CI 0.58-1.40). OS, overall survival; CI, confidence interval.
Table IV.
Factors related to progression-free survival.
| Univariate analysis | Multivariate analysisa | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Group | 0.02 | 0.03 | ||||
| Non-sarcopenia | 0.62 | 0.42-0.92 | 0.63 | 0.42-0.95 | ||
| Sarcopenia | 1 | 1 | ||||
| Sex | 0.80 | |||||
| Male | 1.05 | 0.71-1.55 | ||||
| Female | 1 | |||||
| Performance status | 0.45 | |||||
| 0 | 0.85 | 0.57-1.28 | ||||
| 1 | 1 | |||||
| Clinical stage | 0.02 | 0.03 | ||||
| III | 0.59 | 0.38-0.91 | 0.62 | 0.39-0.97 | ||
| IV | 1 | 1 | ||||
| Age, years | 0.88 | |||||
| ≤75 | 1.03 | 0.66-1.61 | ||||
| >75 | 1 | |||||
| BMI, kg/m2 | 0.60 | |||||
| >22 | 1.11 | 0.74-1.69 | ||||
| ≤22 | 1 | |||||
| mGPS | 0.08 | 0.56 | ||||
| 0 or 1 | 0.69 | 0.45-1.05 | 0.88 | 0.56-1.37 | ||
| 2 | 1 | 1 | ||||
| Tumor localization | 0.99 | |||||
| Head | 0.99 | 0.68-1.46 | ||||
| Body or tail | 1 | |||||
| CA19-9, U/ml | 0.30 | |||||
| <628 | 0.82 | 0.56-1.20 | ||||
| ≥628 | 1 | |||||
Multivariate analysis was conducted using the Cox regression hazard model with the backward selection method. HR, hazard ratio; CI, confidence interval; BMI, body mass index; mGPS, modified Glasgow prognostic score.
Table V.
Factors related to overall survival.
| Univariate analysis | Multivariate analysisa | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Group | 0.17 | 0.27 | ||||
| Non-sarcopenia | 0.76 | 0.51-1.13 | 0.79 | 0.53-1.20 | ||
| Sarcopenia | 1 | 1 | ||||
| Sex | 0.43 | |||||
| Male | 1.16 | 0.79-1.72 | ||||
| Female | 1 | |||||
| Performance status | 0.02 | 0.04 | ||||
| 0 | 0.60 | 0.40-0.91 | 0.64 | 0.42-0.98 | ||
| 1 | 1 | 1 | ||||
| Clinical stage | <0.01 | 0.01 | ||||
| III | 0.50 | 0.32-0.78 | 0.53 | 0.33-0.83 | ||
| IV | 1 | 1 | ||||
| Treatment after GnP | 0.05 | |||||
| Secondary treatment | 0.65 | 0.43-1.01 | ||||
| BSC | 1 | |||||
| Age, years | 0.94 | |||||
| ≤75 | 1.02 | 0.65-1.59 | ||||
| >75 | 1 | |||||
| BMI, kg/m2 | 0.24 | |||||
| <22 | 1.29 | 0.85-1.95 | ||||
| ≥22 | 1 | |||||
| mGPS | <0.01 | 0.13 | ||||
| 0 or 1 | 0.49 | 0.32-0.76 | 0.69 | 0.43-1.11 | ||
| 2 | 1 | 1 | ||||
| Tumor localization | 0.69 | |||||
| Head | 0.92 | 0.64-1.35 | ||||
| Body or tail | 1 | |||||
| CA19-9, U/ml | 0.19 | |||||
| <628 | 0.78 | 0.53-1.13 | ||||
| ≥628 | 1 | |||||
Multivariate analysis was conducted using the Cox regression hazard model with the backward selection method. HR, hazard ratio; CI, confidence interval; BMI, body mass index; mGPS, modified Glasgow prognostic score; BSC, best supportive care.
AEs
The incidences of AEs are shown in Table VI. The incidences of all grade AEs and grade 3–4 AEs of hematologic toxicities were not significantly different between the non-sarcopenia and sarcopenia groups.
Table VI.
Adverse events in the non-sarcopenia and sarcopenia groups.
| Non-sarcopenia (n=81) | Sarcopenia (n=40) | P-value | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Event | All grades | Grade 3/4 | All grades | Grade 3/4 | All grades | Grade 3/4 |
| Leukopenia, n (%) | 73 (90) | 36 (44) | 36 (90) | 13 (33) | 1.0 | 0.24 |
| Neutropenia, n (%) | 66 (81) | 50 (62) | 29 (73) | 19 (48) | 0.34 | 0.17 |
| Thrombocytopenia, n (%) | 68 (84) | 5 (6) | 33 (83) | 0 (0) | 1.0 | 0.17 |
| Anemia, n (%) | 78 (96) | 21 (26) | 40 (100) | 5 (12.5) | 0.55 | 0.10 |
| Nausea, n (%) | 11 (14) | 1 (1) | 6 (15) | 0 (0) | >0.99 | >0.99 |
| Diarrhea, n (%) | 10 (12) | 0 (0) | 6 (15) | 0 (0) | 0.78 | - |
| Constipation, n (%) | 20 (25) | 0 (0) | 11 (28) | 0 (0) | 0.83 | - |
| Peripheral neuropathy, n (%) | 55 (68) | 9 (11) | 26 (65) | 6 (15) | 0.84 | 0.57 |
| Anorexia, n (%) | 22 (27) | 1 (1) | 15 (38) | 0 (0) | 0.30 | 1.0 |
| Malaise, n (%) | 25 (31) | 1 (1) | 11 (28) | 0 (0) | 0.83 | 1.0 |
The Fisher's exact test was used for comparisons of adverse events.
Discussion
In this study, it was suggested that the evaluation of SMI by BIA may be an indicator of the prognosis and therapeutic effect of GnP. It has been reported that sarcopenia and chemotherapeutic outcomes have relationships in various cancers, including pancreatic cancer, and various evaluation methods have been utilized for sarcopenia (30–34). However, there have been no reports regarding the association of sarcopenia on the outcomes of GnP for advanced pancreatic cancer patients.
We noted a statistically significant difference in the PFS between advanced pancreatic cancer patients with and without sarcopenia, and a tendency toward a slight prolongation of OS in the non-sarcopenia group. OS was affected not only by the first-line treatment but also by the second-line treatment. In this study, we observed a trend toward longer OS in patients who were able to receive secondary treatment, although the difference was not significant (HR 0.65; 95% CI 0.43-1.01). Approximately 74 and 73% of the patients in the sarcopenia and non-sarcopenia groups received second-line treatment between, which was not significantly different. Since secondary treatment was not limited, a wide variety of treatment methods may have affected the OS. In addition, only the SMI values before the first administration of the chemotherapy regimen were available, so it was not possible to evaluate changes in SMI values during the treatment course, which may impact the prognosis (35).
Patients with advanced pancreatic cancer often have a poor nutritional status due to cachexia (36). The relationship between cachexia, malnutrition, and reduced SMI has been reported (37). Approximately one-third of cancer-related deaths are reportedly due to malnutrition rather than cancer (38). Cachexia and malnutrition associated with cancer would reduce SMI and affect secondary sarcopenia. Recently, a clinical trial focusing on multimodal exercise, nutrition, and anti-inflammatory medication for cachexia confirmed the importance of exercise and nutrition therapy (39). Maintaining or increasing skeletal muscle mass through nutritional or exercise interventions may prevent sarcopenia and improve prognosis as well as quality of life.
The mGPS is widely used as an indicator of nutritional status (40). CRP and albumin, the acute proteins that constitute the mGPS, are sensitive and reliable markers that reflect the systemic inflammatory response of cancer patients. The mGPS 0 is defined as normal or symptomatically relieved hypocachexia, 1 as precachexia, and 2 as cachexia or irreversible cachexia, which may reflect tumor extension, necrosis, and undernutrition (41). A previous meta-analysis reported an association between mGPS and OS and PFS in pancreatic cancer patients (42). However, it was not a significant prognostic factor in this study. In pancreatic cancer, the mGPS may not necessarily reflect the disease state due to increases in CRP from cholangitis or associated pancreatitis. Hence, this study excluded patients with cholangitis or pancreatitis showing apparent clinical symptoms, but SMI may be useful in patients ineligible for evaluation by mGPS.
Regarding the relationship between sarcopenia and efficacy of chemotherapy for pancreatic cancer, a decrease in skeletal muscle mass, as measured by CT, was reported to be a prognostic predictor in patients who received FOLFIRINOX as second-line chemotherapy (43). Other studies showed that gemcitabine alone and gemcitabine plus erlotinib resulted in a significantly worse prognosis in patients with sarcopenia than in those without (44). The lack of difference in AEs between groups suggests that GnP was well tolerated and may be useful for patients with low SMI. GnP may be a tolerable treatment option for older patients with low SMI.
The optimal method for SMI measurement is controversial. DEXA has the advantage of being able to measure skeletal muscle mass throughout the body very accurately, but it requires special equipment and has the disadvantages of radiation exposure and excessive cost (45). Measuring the SMI via CT is commonly used and has been reported to be a useful method, although it is not completely consistent with the BIA method. However, we believe that BIA is more effective in terms of radiation exposure and cost (46,47). The measurement of skeletal muscle mass by MRI has also been reported as an effective method, but it has disadvantages such as excessive cost, limited availability of facilities, long examination time, and inability to be performed in patients with claustrophobia (48). We chose BIA because it is an inexpensive, simple, non-invasive measurement method with high reproducibility.
BIA can be measured repeatedly, and it is easy to check changes in SMI with active nutrition and exercise intervention support as needed. In the future, prevention and improvement of sarcopenia as well as chemotherapy are expected to improve the prognosis of patients with pancreatic cancer, and BIA may enable early detection of sarcopenia and early therapeutic intervention. Furthermore, chemotherapy may trigger immune responses mediated by tumor-specific T cells by stimulating immunogenic cell death, and novel minimal drug nanoplatforms that can stimulate the immunotherapeutic potential inherent in gemcitabine could be developed (49). In the future, we will study the association of SMI with other chemotherapy regimens and novel agents.
There were some limitations in this study. First it included only a small number of cases. Second, it was a retrospective study. In the future, verification in a prospective study with a larger sample size is desired. However, considering previous reports, our study tends to correlate sarcopenia with prognosis, which may be a reliable result. Third, we chose the cutoff values based on Taiwanese adults without cancer. The cutoff values were reliable because they study cohort also comprised East Asians (Japanese individuals) with similar physical characteristics. However, there may be more appropriate cutoff values for pancreatic cancer patients. Fourth, some patients received conversion surgery or radiotherapy as second-line treatment, so the OS may not reflect the effect of chemotherapy, and the cases were heterogeneous between the two groups. Fifth, the cutoff values in this study tended to be less for female patients with sarcopenia. The low number of females may have led to a lower estimate of toxicity. Thus, the setting of cutoff values may need to be examined.
In conclusion, SMI measured by BIA was indicative of the PFS of patients with advanced pancreatic cancer who received GnP as first-line treatment, suggesting its importance as a prognostic factor in these patients. Therefore, it may be one of the useful diagnostic methods of sarcopenia.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- GnP
gemcitabine plus nab-paclitaxel combination therapy
- OS
overall survival
- PFS
progression-free survival
- AEs
adverse events
- BIA
bioimpedance analysis
- HR
hazard ratio
- CI
confidence interval
- EWGSOP
European Working Group on Sarcopenia in Older People
- SMI
skeletal muscle index
- CT
computed tomography
- MRI
magnetic resonance imaging
- DEXA
dual-energy X-ray absorptiometry
- ECOG PS
Eastern Cooperative Oncology Group Performance Status
- BMI
body mass index
- mGPS
modified Glasgow prognostic score
- CA19-9
serum carbohydrate antigen 19-9
- CRP
C-reactive protein
- RDI
relative dose intensity
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YT, MU and MM conceptualized this study. YT, MU and SK analyzed the data. YT, MU and SK confirm the authenticity of all the raw data. YT, TF, YS, KK, AH, ST, HA, SaM, SoM, SO and ShM validated the results and investigated the data in the present study. All authors participated in the writing of the manuscript. All authors discussed the results and commented on the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of Kanagawa Cancer Center (approval no. 2021-152). Informed consent was obtained in the form of an opt-out form on the website.
Patient consent for publication
Patient consent for publication was obtained in the form of an opt-out form on the website.
Competing interests
Dr Ueno and Dr Kobayashi reports grants and personal fees from Taiho Pharmaceutical. All the other authors declare that they have no competing interests.
References
- 1.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World J Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai HJ, Chang JS. Environmental risk factors of pancreatic cancer. J Clin Med. 2019;8:1427. doi: 10.3390/jcm8091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694–9705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Statistics, corp-author. Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan, Ministry of Health, Labour and Welfare) https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fganjoho.jp%2Freg_stat%2Fstatistics%2Fdata%2Fdl%2Fexcel%2Fcancer_mortality(1958–2020).xls&wdOrigin=BROWSELINK. [ May 9; 2022 ]; [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 7.Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka TE, Omuro Y, Nakajima T, Furuse J. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77:595–603. doi: 10.1007/s00280-016-2972-3. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg IH. Sarcopenia: Origins and clinical relevance. J Nutr. 1997;127((5 Suppl)):990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 9.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 10.Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz-Jentoft AJ, Dent E, Baracos VE, Crawford JA, Doehner W, Heymsfield SB, et al. Sarcopenia: A time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle. 2019;10:956–961. doi: 10.1002/jcsm.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Walowski CO, Braun W, Maisch MJ, Jensen B, Peine S, Norman K, Müller MJ, Bosy-Westphal A. Reference values for skeletal muscle mass-current concepts and methodological considerations. Nutrients. 2020;12:755. doi: 10.3390/nu12030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González Correa CH, Marulanda Mejía F, Castaño González PA, Vidarte Claros JA, Castiblanco Arroyabe HD. Bioelectrical impedance analysis and dual x-ray absorptiometry agreement for skeletal muscle mass index evaluation in sarcopenia diagnosis. Physiol Meas. 2020;41:064005. doi: 10.1088/1361-6579/ab8e5f. [DOI] [PubMed] [Google Scholar]
- 15.McSharry V, Glennon K, Mullee A, Brennan D. The impact of body composition on treatment in ovarian cancer: A current insight. Expert Rev Clin Pharmacol. 2021;14:1065–1074. doi: 10.1080/17512433.2021.1937125. [DOI] [PubMed] [Google Scholar]
- 16.McSharry V, Mullee A, McCann L, Rogers AC, McKiernan M, Brennan DJ. The impact of sarcopenia and low muscle attenuation on overall survival in epithelial ovarian cancer: A systematic review and meta-analysis. Ann Surg Oncol. 2020;27:3553–3564. doi: 10.1245/s10434-020-08382-0. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto T, Yagyu T, Uchinaka E, Miyatani K, Hanaki T, Kihara K, Matsunaga T, Yamamoto M, Tokuyasu N, Honjo S, Fujiwara Y. Sarcopenia as a prognostic factor in patients with recurrent pancreatic cancer: A retrospective study. World J Surg Oncol. 2020;18:221. doi: 10.1186/s12957-020-01981-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ubachs J, Ziemons J, Minis-Rutten IJG, Kruitwagen RFPM, Kleijnen J, Lambrechts S, Olde Damink SWM, Rensen SS, Van Gorp T. Sarcopenia and ovarian cancer survival: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10:1165–1174. doi: 10.1002/jcsm.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang M, Shen Y, Tan L, Li W. Prognostic value of sarcopenia in lung cancer: A systematic review and meta-analysis. Chest. 2019;156:101–111. doi: 10.1016/j.chest.2019.04.115. [DOI] [PubMed] [Google Scholar]
- 20.Ishida T, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, Kurokawa Y, Motoori M, Kimura Y, Nakajima K, et al. Impact of measurement of skeletal muscle mass on clinical outcomes in patients with esophageal cancer undergoing esophagectomy after neoadjuvant chemotherapy. Surgery. 2019;166:1041–1047. doi: 10.1016/j.surg.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 21.Kim IH, Choi MH, Lee IS, Hong TH, Lee MA. Clinical significance of skeletal muscle density and sarcopenia in patients with pancreatic cancer undergoing first-line chemotherapy: A retrospective observational study. BMC Cancer. 2021;21:77. doi: 10.1186/s12885-020-07753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, Benbow JM, Muss HB. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res. 2017;23:658–665. doi: 10.1158/1078-0432.CCR-16-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobin LH, Gospodarowicz MK, Wittekind C, editors. 7th edition. Chichester, West Sussex, UK: Wiley-Blackwell; 2010. TNM classification of malignant tumours. [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc. 2008;56:1710–1715. doi: 10.1111/j.1532-5415.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 26.Body mass index-BMI. World Health Organization, regional office for Europe, corp-author. https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index. [ February 13; 2022 ]; [Google Scholar]
- 27.Imaoka H, Mizuno N, Hara K, Hijioka S, Tajika M, Tanaka T, Ishihara M, Yogi T, Tsutsumi H, Fujiyoshi T, et al. Evaluation of modified Glasgow prognostic score for pancreatic cancer: A retrospective cohort study. Pancreas. 2016;45:211–217. doi: 10.1097/MPA.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et al. RECIST 1.1-update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Common Terminology Criteria for Adverse Events (CTCAE) Version 5, corp-author. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Published: November 27. [Google Scholar]
- 30.Ozola Zalite I, Zykus R, Francisco Gonzalez M, Saygili F, Pukitis A, Gaujoux S, Charnley RM, Lyadov V. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: A systematic review. Pancreatology. 2015;15:19–24. doi: 10.1016/j.pan.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Begini P, Gigante E, Antonelli G, Carbonetti F, Iannicelli E, Anania G, Imperatrice B, Pellicelli AM, Fave GD, Marignani M. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann Hepatol. 2017;16:107–114. doi: 10.5604/16652681.1226821. [DOI] [PubMed] [Google Scholar]
- 32.Vergara-Fernandez O, Trejo-Avila M, Salgado-Nesme N. Sarcopenia in patients with colorectal cancer: A comprehensive review. World J Clin Cases. 2020;8:1188–1202. doi: 10.12998/wjcc.v8.i7.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang XM, Dou QL, Zeng Y, Yang Y, Cheng ASK, Zhang WW. Sarcopenia as a predictor of mortality in women with breast cancer: A meta-analysis and systematic review. BMC Cancer. 2020;20:172. doi: 10.1186/s12885-020-6645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bundred J, Kamarajah SK, Roberts KJ. Body composition assessment and sarcopenia in patients with pancreatic cancer: A systematic review and meta-analysis. HPB (Oxford) 2019;21:1603–1612. doi: 10.1016/j.hpb.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Choi Y, Oh DY, Kim TY, Lee KH, Han SW, Im SA, Kim TY, Bang YJ. Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLoS One. 2015;10:e0139749. doi: 10.1371/journal.pone.0139749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vashi P, Popiel B, Lammersfeld C, Gupta D. Outcomes of systematic nutritional assessment and medical nutrition therapy in pancreatic cancer. Pancreas. 2015;44:750–755. doi: 10.1097/MPA.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 37.Lv Y, Ji ML, Feng QY, Zhu DX, Lin SB, Mao YH, Xu YQ, Zheng P, He GD, Xu JM. Combined test of third lumbar skeletal muscle index and prognostic nutrition index improve prognosis prediction power in resected colorectal cancer liver metastasis. Aging (Albany NY) 2019;11:10301–10315. doi: 10.18632/aging.102457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Luna PP, Parejo Campos J, Pereira Cunill JL. Causes and impact of hyponutrition and cachexia in the oncologic patient. Nutr Hosp. 2006;21((Suppl 3)):S10–S16. (In Spanish) [PubMed] [Google Scholar]
- 39.Solheim TS, Laird BJA, Balstad TR, Stene GB, Bye A, Johns N, Pettersen CH, Fallon M, Fayers P, Fearon K, Kaasa S. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8:778–788. doi: 10.1002/jcsm.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proctor MJ, Morrison DS, Talwar D, Balmer SM, O'Reilly DS, Foulis AK, Horgan PG, McMillan DC. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: A Glasgow inflammation outcome study. Br J Cancer. 2011;104:726–734. doi: 10.1038/sj.bjc.6606087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Ren D, Jin X, Wu H. The prognostic value of modified Glasgow prognostic score in pancreatic cancer: A meta-analysis. Cancer Cell Int. 2020;20:462. doi: 10.1186/s12935-020-01558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu W, Wang K, Yan S, Wang X, Tang B, Chang J, Wang R, Wu T. Prognostic significance of the modified Glasgow prognostic score in patients with pancreatic cancer: A meta-analysis. Dose Response. 2020;18:1559325820942065. doi: 10.1177/1559325820942065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HS, Kim SY, Chung MJ, Park JY, Bang S, Park SW, Song SY. Skeletal muscle mass predicts poor prognosis in patients with advanced pancreatic cancer undergoing second-line FOLFIRINOX chemotherapy. Nutr Cancer. 2019;71:1100–1107. doi: 10.1080/01635581.2019.1597906. [DOI] [PubMed] [Google Scholar]
- 44.Park I, Choi SJ, Kim YS, Ahn HK, Hong J, Sym SJ, Park J, Cho EK, Lee JH, Shin YJ, Shin DB. Prognostic factors for risk stratification of patients with recurrent or metastatic pancreatic adenocarcinoma who were treated with gemcitabine-based chemotherapy. Cancer Res Treat. 2016;48:1264–1273. doi: 10.4143/crt.2015.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonio J, Kenyon M, Ellerbroek A, Carson C, Burgess V, Tyler-Palmer D, Mike J, Roberts J, Angeli G, Peacock C. Comparison of dual-energy X-ray absorptiometry (DXA) versus a multi-frequency bioelectrical impedance (InBody 770) device for body composition assessment after a 4-week hypoenergetic diet. J Funct Morphol Kinesiol. 2019;4:23. doi: 10.3390/jfmk4020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones DJ, Lal S, Strauss BJ, Todd C, Pilling M, Burden ST. Measurement of muscle mass and sarcopenia using anthropometry, bioelectrical impedance, and computed tomography in surgical patients with colorectal malignancy: Comparison of agreement between methods. Nutr Cancer. 2020;72:1074–1083. doi: 10.1080/01635581.2019.1659381. [DOI] [PubMed] [Google Scholar]
- 47.Looijaard WGPM, Stapel SN, Dekker IM, Rusticus H, Remmelzwaal S, Girbes ARJ, Weijs PJM, Oudemans-van Straaten HM. Identifying critically ill patients with low muscle mass: Agreement between bioelectrical impedance analysis and computed tomography. Clin Nutr. 2020;39:1809–1817. doi: 10.1016/j.clnu.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Chang MY, Park Y, Ha JW, Zhang HY, Lee SH, Hong TH, Lee SH. Paraspinal lean muscle mass measurement using spine MRI as a predictor of adjacent segment disease after lumbar fusion: A propensity score-matched case-control analysis. AJR Am J Roentgenol. 2019;212:1310–1317. doi: 10.2214/AJR.18.20441. [DOI] [PubMed] [Google Scholar]
- 49.Zhou S, Shang Q, Wang N, Li Q, Song A, Luan Y. Rational design of a minimalist nanoplatform to maximize immunotherapeutic efficacy: Four birds with one stone. J Control Release. 2020;328:617–630. doi: 10.1016/j.jconrel.2020.09.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.