Abstract
The emergence of multidrug-resistant bacteria and fungi requires the development of antibiotics and antifungal agents. This review identified natural products isolated from Asian angiosperms with antibacterial and/or antifungal activities and analyzed their distribution, molecular weights, solubility, and modes of action. All data in this review were compiled from Google Scholar, PubMed, Science Direct, Web of Science, ChemSpider, PubChem, and a library search from 1979 to 2022. One hundred and forty-one antibacterial and/or antifungal alkaloids were identified during this period, mainly from basal angiosperms. The most active alkaloids are mainly planar, amphiphilic, with a molecular mass between 200 and 400 g/mol, and a polar surface area of about 50 Å2, and target DNA and/or topoisomerase as well as the cytoplasmic membrane. 8-Acetylnorchelerythrine, cryptolepine, 8-hydroxydihydrochelerythrine, 6-methoxydihydrosanguinarine, 2′-nortiliacorinine, pendulamine A and B, rhetsisine, sampangine, tiliacorine, tryptanthrin, tylophorinine, vallesamine, and viroallosecurinine yielded MIC ≤ 1 µg/mL and are candidates for the development of lead molecules.
Keywords: medicinal plants, antibacterial, antifungal, alkaloids, Asia
1. Introduction
The resistance of bacteria and fungi to antimicrobial agents necessitates the continuous development of antibiotics and antifungal agents with original chemical frameworks that may come from flowering plants. The flowering plants also termed angiosperms comprise 11 major taxa or clades grouped into three groups: (i) basal angiosperms including Protomagnoliids, Magnoliids, Monocots, Eudicots; (ii) core angiosperms including Core Eudicots, Rosids, Fabids, Malvids; and (iii) upper angiosperms including Asterids, Lamiids, and Campanulids [1]. Within each clade, plants yield both non-specific and specific secondary metabolites such as alkaloids to control the growth of phytopathogenic bacteria and fungi. These antimicrobial principles fall into two main groups: phytoanticipins and phytoalexin. Phytoanticipins are antimicrobial compounds in plants that are present before phytopathogenic microorganism challenge or inactive immediate precursors stored in healthy tissues that are converted into antimicrobial metabolites known as phytoalexins [2]. Phytoanticipins and phytoalexins containing primary, secondary, tertiary, or quaternary amines are called alkaloids, which belong to various chemical classes, principally amides, indoles, piperidines, quinolines, isoquinolines, pyrrolidines, imidazoles, diterpenes, sesquiterpeness, and steroidal alkaloids [3].
Phytopathogenic Gram-negative bacteria are more resistant to alkaloids than Gram-positive bacteria due, at least in part, to an outer hydrophilic and negatively charged layer of lipopolysaccharides [4]. In Gram-negative bacteria, porins allow for the entry of water and nutrients through the outer layer and hydrophilic and amphiphilic xenobiotics with a molecular mass below 600 g/mol xenobiotics without nutritional or physiological benefit or toxins are actively cleared from the cytoplasm by efflux pumps [5]. The assessment of the antibacterial and antifungal strength of isolated secondary metabolites in vitro is qualitatively appreciated by the measurement of the diameter of an inhibition zone and quantitively based on the minimum inhibiting concentration (MIC), and several thresholds of activity have been proposed [6,7,8,9]. Angiosperms produce alkaloids that inhibit the growth of both Gram-positive, Gram-negative bacteria, yeasts, and filamentous fungi and these principles are of potential therapeutic value. Among the factors that influence the antibacterial or antibacterial strength, and the targets of alkaloids are the molecular mass and water solubility [10].
Over the last 80 years, enormous research efforts have been devoted to the aim of identifying antibiotic or antifungal lead molecules in flowering plants globally, but to date, none of these have been developed as drugs. The present work attempts to provide a comprehensive review of the main findings regarding the antibacterial and antifungal alkaloids from Asian angiosperms. All data in this review were compiled from Google Scholar, PubMed, Science Direct, Web of Science, ChemSpider, PubChem, and a library search from 1979 to 2022 and were analyzed to address the following points: (i) distribution; (ii) strongest principles identified; (iii) spectrum of activity; (iv) influence of molecular mass, water solubility, and polar surface; (v) the mode of action; (vi) structure–activity; and (vii) efflux pump inhibition.
2. Distribution
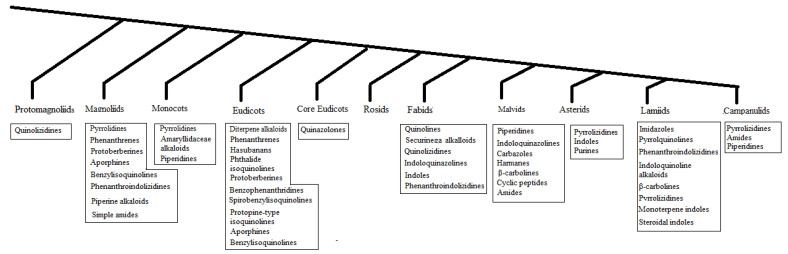
Regarding the distribution of antibacterial and antifungal alkaloids from Asian angiosperms (Figure 1), the following could be observed:
All clades yielded antibacterial and/or antifungal alkaloids, except for the Rosids.
Most antibacterial and/or antifungal alkaloids can be found in basal angiosperms, which are isoquinolines.
Some clades yielded a specific class of antibacterial and/or antifungal alkaloids such as the Amaryllidaceae alkaloids in the Monocots, phenanthrene alkaloid in the Magnoliids, Securinega alkaloids in Fabids, carbazoles in the Malvids, and monoterpene indole alkaloids in the Lamiids.
Core angiosperms and upper angiosperms use various classes of alkaloids and phytoalexins.
Most antibacterial and/or antifungal alkaloids have been isolated from medicinal plants (Table 1, Table S1).
Tripathi et al. (1994) observed changes in the antibacterial alkaloid concentrations in plants over time [11].
Figure 1.
The phylogenetic distribution of antibacterial and/or antifungal alkaloids in angiosperms.
Table 1.
The chemical structures of antibacterial and/or antifungal alkaloids and their botanical origin.
| CLASS Compound |
Chemical Structure | Genus, Species | Family |
|---|---|---|---|
| ACRIDANONES | |||
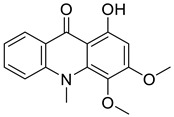
| 1-Hydroxy-3,4-dimethoxy-10-methylacridan-9-one |
|
Limonia acidissima L. | Rutaceae |
| 1-Hydroxy-3-methoxy-10-methylacridan-9-one |
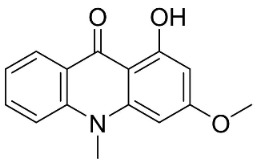
|
Limonia acidissima L. | Rutaceae |
| AMARYLLIDACEAE ALKALOIDS | |||
| Crinamine |
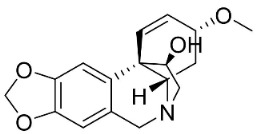
|
Crinum asiaticum L. | Amaryllidaceae |
| Lycoricidine |
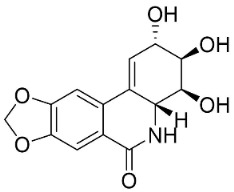
|
Lycoris radiata (L’Hér.) Herb. | Amaryllidaceae |
| Lycorine |
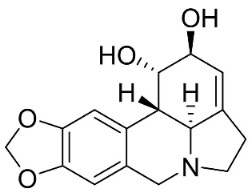
|
Lycoris radiata (L’Hér.) Herb. | Amaryllidaceae |
| Narciclasine |
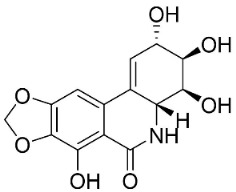
|
Lycoris radiata (L’Hér.) Herb. | Amaryllidaceae |
| Tazettine |
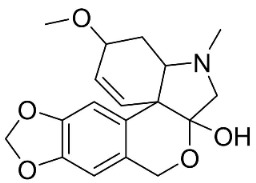
|
Narcissus tazetta L. | Amaryllidaceae |
| APORPHINES | |||
| Anonaine |
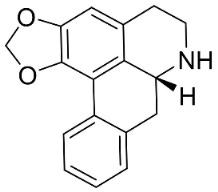
|
Michelia alba DC. | Magnolicaceae |
| Artabotrine |
|
Artabotrys suaveolens (Bl.) Bl. | Annonaceae |
| Bulbocapnine |
|
Corydalis bulbosa DC. | Fumariaceae |
| Dicentrinone |
|
Phoebe lanceolata (Nees) Nees | Lauraceae |
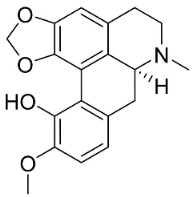
| Isoboldine |
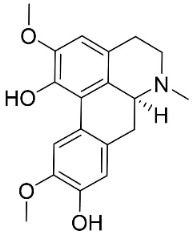
|
Corydalis bulbosa DC. | Fumariaceae |
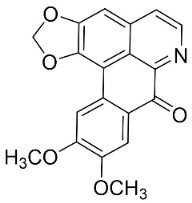
| Lanuginosine |
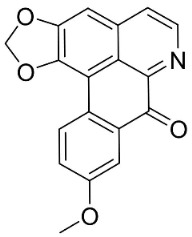
|
Eupomatia laurina R. Br. | Eupomatiaceae |
| Liriodenine |
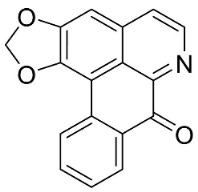
|
Cananga odorata Hook. F. & Thomson | Annonaceae |
| Lysicamine |
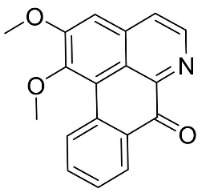
|
Phoebe grandis (Nees) Merr. | Lauraceae |
| Magnoflorine |
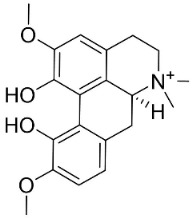
|
Mahonia bealei (Fortune) Carrière | Papaveraceae |
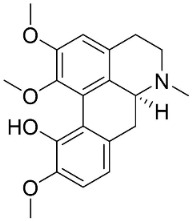
| Nordicentrine |
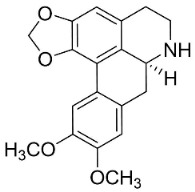
|
Phoebe lanceolata (Nees) Nees | Lauraceae |
| O-Methylmoschatoline |
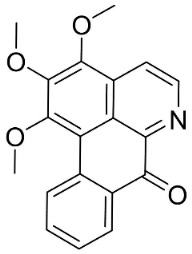
|
Cananga odorata (Lam.) Hook. F. & Thomson | Annonaceae |
| Roemerine |
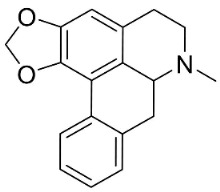
|
Papaver rhoeas L. | Papaveraceae |
| Sampangine |
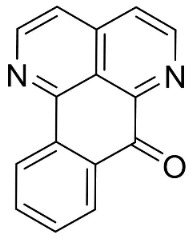
|
Eupomatia laurina R. Br. | Eupomatiaceae |
| Thailandine |
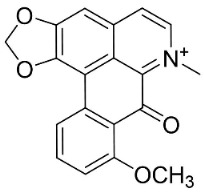
|
Stephania venosa (Bl.) Spreng | Menispermaceae |
| Xylopine |
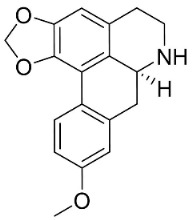
|
Artabotrys suaveolens (Bl.) Bl. | Annonaceae |
| BENZOPHENANTHRIDINES | |||
| 8-Acetylnorchelerythrine |
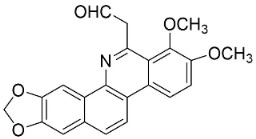
|
Toddalia asiatica (L.) Lam. | Rutaceae |
| Avicine |
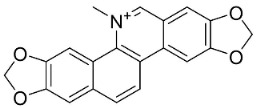
|
Toddalia asiatica (L.) Lam. | Rutaceae |
| Chelerythrine |
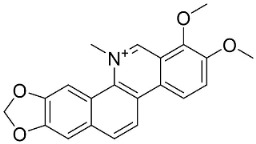
|
Macleaya cordata (Willd.) R. Br. | Papaveraceae |
| Corynoline |
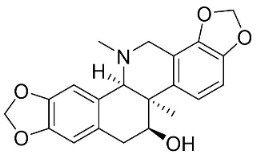
|
Corydalis incisa (Thunb.) Pers. | Fumariaceae |
| Dihydrochelerythrine |
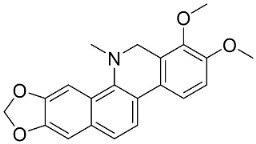
|
Macleaya cordata (Willd.) R. Br. | Papaveraceae |
| Dihydrosanguinarine |
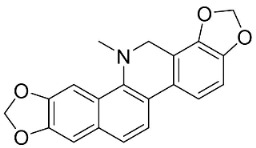
|
Macleaya cordata (Willd.) R. Br | Papaveraceae |
| 8-Hydroxydihydrochelerythrine |
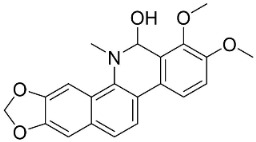
|
Chelidonium majus L. | Fumariaceae |
| 8-Hydroxydihydrosanguinarine |
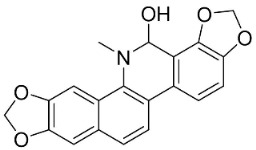
|
Chelidonium majus L. | Fumariaceae |
| 6-Methoxydihydrosanguinarine |
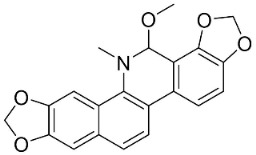
|
Chelidonium japonicum Thunb | Fumariaceae |
| Nitidine |
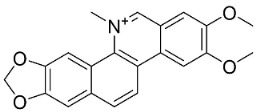
|
Zanthoxylum L. | Rutaceae |
| Norchelerythrine |
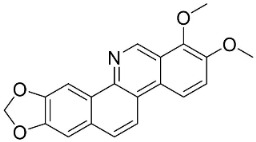
|
Toddalia asiatica (L.) Lam. | Rutaceae |
| Norsanguinarine |
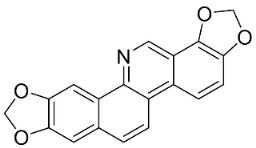
|
Fumaria indica Pugsley | Fumariaceae |
| Rhoifoline B |
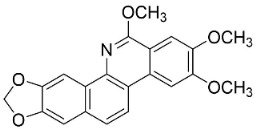
|
Toddalia asiatica (L.) Lam. | Rutaceae |
| Sanguinarine |
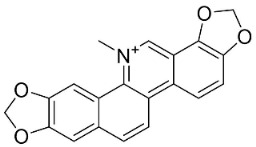
|
Fumaria officinalis L. | Fumariaceae |
| Stylopine |
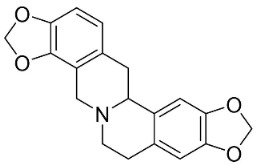
|
Fumaria officinalis L. | Fumariaceae |
| CARBAZOLES | |||
| 3,3′-[Oxybis(methylene)]bis(9-methoxy-9H-carbazole) |
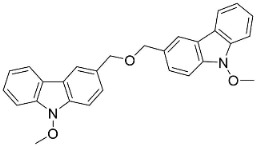
|
Murraya koenigii (L.) Spreng. | Rutaceae |
| Clausamine A |
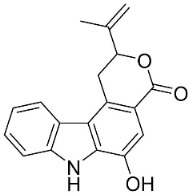
|
Clausena harmandiana (Pierre) Guillaumin | Rutaceae |
| Clausamine B |
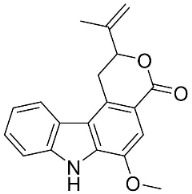
|
Clausena harmandiana (Pierre) Guillaumin | Rutaceae |
| Clausine F |
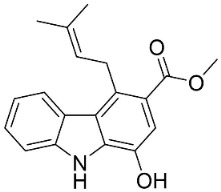
|
Clausena harmandiana (Pierre) Guillaumin | Rutaceae |
| Clauszoline N |
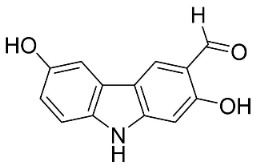
|
Clausena harmandiana (Pierre) Guillaumin | Rutaceae |
| 3-Formylcarbazole |
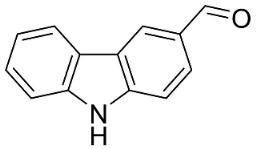
|
Clausena mexicana Burm.f. | Rutaceae |
| 3-Formyl-1-methoxycarbazole |
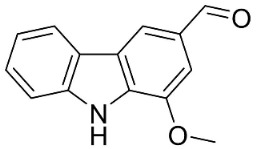
|
Murraya koenigii (L.) Spreng. | Rutaceae |
| Girinimbine |
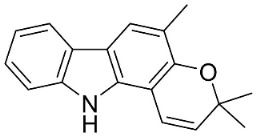
|
Murraya koenigii (L.) Spreng. | Rutaceae |
| Glycozolidol |
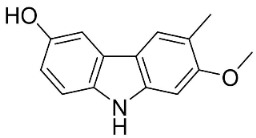
|
Glycosmis pentaphylla (Retz.) DC. | Rutaceae |
| Harmane |
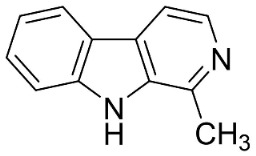
|
Murraya mexicana (L.) Jack | Rutaceae |
| Koenimbine |
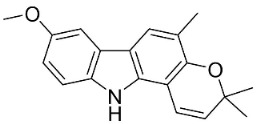
|
Murraya koenigii (L.) Spreng. | Rutaceae |
| Koenigine |
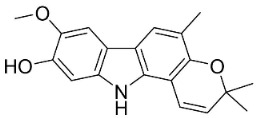
|
Murraya koenigii (L.) Spreng. | Rutaceae |
| Lansine |
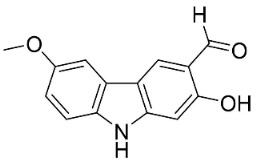
|
Micromelum pubescens Bl. | Rutaceae |
| Murrayamine J |
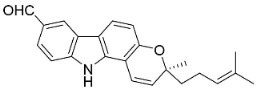
|
Murraya mexicana (L.) Jack | Rutaceae |
| BENZYLISOQUINOLINES | |||
| Reticuline |
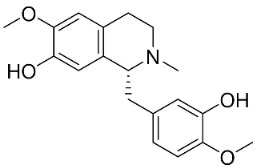
|
Annona squamosa L. | Annonaceae |
| Fuyuziphine |
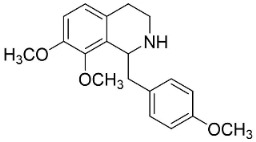
|
Fumaria indica Pugsley | Fumariaceae |
| BISBENZYLISOQUINOLINES | |||
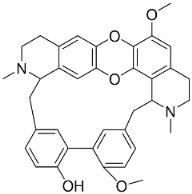
| 2′-Nortiliacorinine |
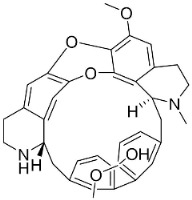
|
Tiliacora triandra Diels | Menispermaceae |
| Tetrandrine |
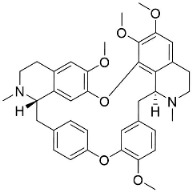
|
Cyclea barbata Miers | Menispermaceae |
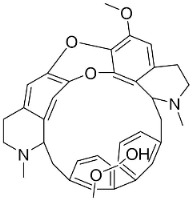
| Tiliacorine |
|
Tiliacora triandra Diels | Menispermaceae |
| Tiliacorinine |
|
Tiliacora triandra Diels | Menispermaceae |
| DITERPENE ALKALOIDS | |||
| 8-Acetylheterophyllisine |
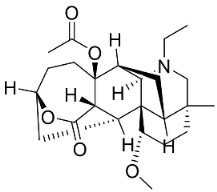
|
Delphinium denudatum Wall. Ex Hook. F. & Thomson yields | Ranunculaceae |
| Panicutine |
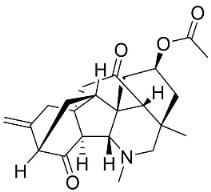
|
Delphinium denudatum Wall. Ex Hook. F. & Thomson yields | Ranunculaceae |
| Vilmorrianone |
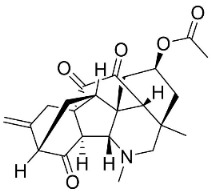
|
Delphinium denudatum Wall. Ex Hook. F. & Thomson yields | Ranunculaceae |
| HASUBANANS | |||
| Glabradine |
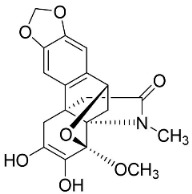
|
Stephania glabra (Roxb.) Miers | Menispermaceae |
| IMIDAZOLES | |||
| Allantoin |
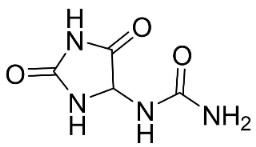
|
Tournefortia sarmentosa Lam | Borraginaceae |
| INDOLOQUINAZOLINES | |||
| Dehydroevodiamine |
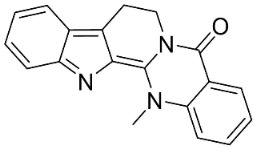
|
Euodia rutaecarpa Benth | Rutaceae |
| Evodiamine |
|
Euodia rutaecarpa Benth | Rutaceae |
| Tryptanthrin |
|
Indigofera tinctoria L. | Fabaceae |
| INDOLOQUINOLINES | |||
| Cryptolepine |
|
Cryptolepis sanguinolenta (Lindl.) Schltr. | Apocynaceae |
| MONOTERPENE INDOLE ALKALOIDS | |||
| Alstoniascholarine A |
|
Alstonia scholaris (L.) R.Br. | Apocynaceae |
| Alstoniascholarine E |
|
Alstonia scholaris (L.) R.Br. | Apocynaceae |
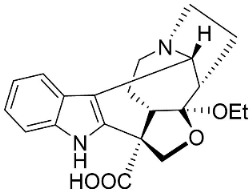
| Alstoniascholarine J |
|
Alstonia scholaris (L.) R.Br. | Apocynaceae |
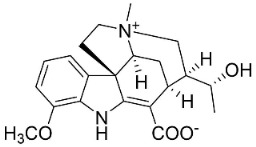
| Cadambine |
|
Neolamarckia cadamba (Roxb.) Bosser | Rubiaceae |
| Ibogaine |
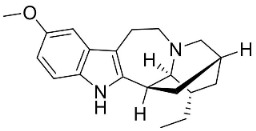
|
Ervatamia mexicana (L.) Burkill | Apocynaceae |
| 3-Oxocoronaridine |
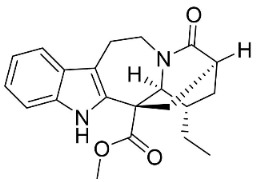
|
Ervatamia mexicana (L.) Burkill | Apocynaceae |
| 5-Oxocoronaridine |
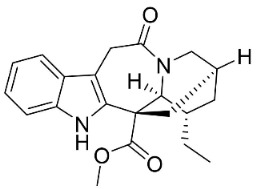
|
Ervatamia mexicana (L.) Burkill | Apocynaceae |
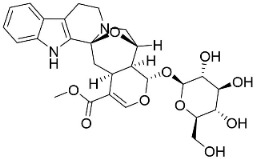
| Strictosidine |
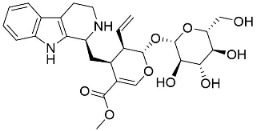
|
Guettarda speciosa L. | Rubiaceae |
| Vallesamine |
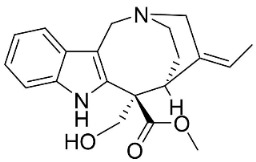
|
Ervatamia mexicana (L.) Burkill | Apocynaceae |
| Voacamine |
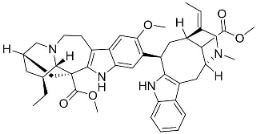
|
Ervatamia mexicana (L.) Burkill | Apocynaceae |
| Voacangine |
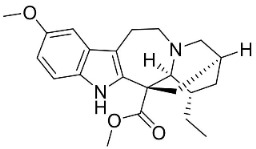
|
Ervatamia exicana (L.) Burkill | Apocynaceae |
| Vobasine |
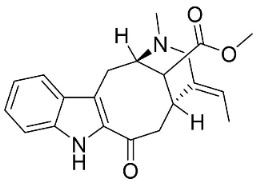
|
Ervatamia mexicana (L.) Burkill | Apocynaceae |
| Tubotaiwine |
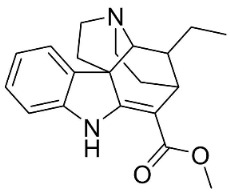
|
Alstonia scholaris (L.) R.Br. | Apocynaceae |
| PHENANTHRENE ALKALOIDS | |||
| Aristolochic acid |
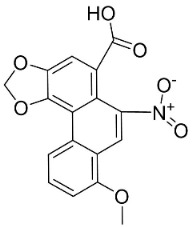
|
Aristolochia L. | Aristolochiaceae |
| Aristolactam N-(6′-trans-p-coumaroyl)-β-d-glucopyranoside |
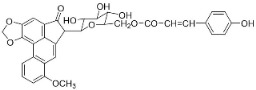
|
Aristolochia L. | Aristolochiaceae |
| 1-N-monomethylcarbamate-argentinine-3-O-β-d-glucoside |
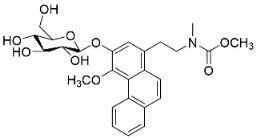
|
Stephania succifera H.S. Lo & Y. Tsoong | Meninspermaceae |
| PHENANTHROINDOLIZIDINE ALKALOIDS | |||
| 7-Demethoxytylophorine |
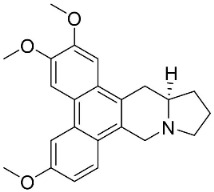
|
Cynanchum atratum Bunge | Asclepiadaceae |
| Tylophorinine |
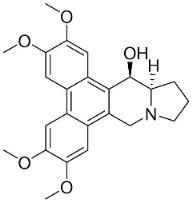
|
Tylophora indica (Burm.f.) Merr. | Asclepiadaceae |
| Tylophorinidine |
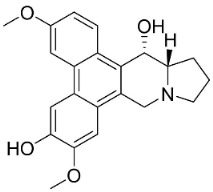
|
Tylophora indica (Burm.f.) Merr | Asclepiadaceae |
| PIPERINE ALKALOIDS | |||
| Piperine |
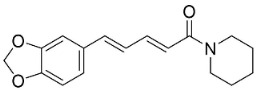
|
Piper nigrum L. | Piperaceae |
| Piperlongumine |
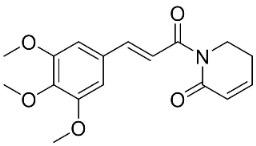
|
Piper longum L. | Piperaceae |
| PHTHALIDES | |||
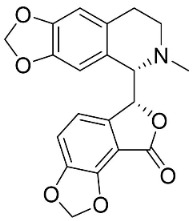
| Adlumidine |
|
Fumaria officinalis L. t | Fumariaceae |
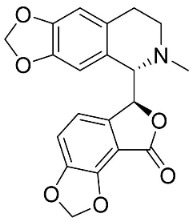
| Bicuculline |
|
Corydalis bulbosa DC. | Fumariaceae |
| PROTOBERBERINES | |||
| Berberine |
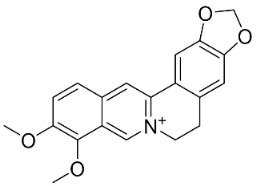
|
Coptis chinensis Franch. | Ranunculaceae |
| Jatrorrhizine |
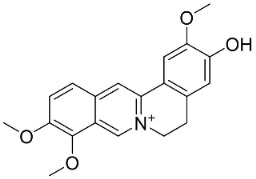
|
Coptis chinensis Franch. | |
| Palmatine |
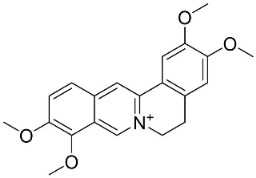
|
Corydalis exicana (Thunb.) Pers. | Fumariaceae |
| Sinactine |
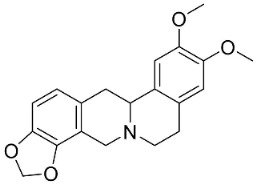
|
Fumaria officinalis L. | Fumariaceae |
| PYRROLIDINES | |||
| Brachyamide B |
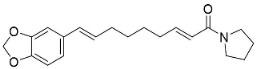
|
Piper nigrum L. | Piperaceae |
| Isopiperolein B |
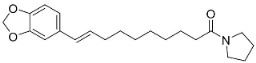
|
Piper nigrum L. | Piperaceae |
| N-[9-(3,4-Methylenedioxyphenyl)-2E,4E,8E-nonatrienoyl]pyrrolidine |
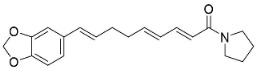
|
Piper nigrum L. | Piperaceae |
| Pandamarilactonine A |
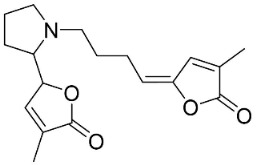
|
Pandanus odorus Ridl. | Pandanaceae |
| Trachyone |
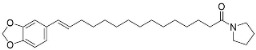
|
Piper nigrum L. | Piperaceae |
| QUINOLINONES | |||
| Antidesmone |
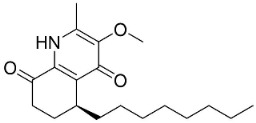
|
Waltheria indica L. | Malvaceae |
| Evocarpine |
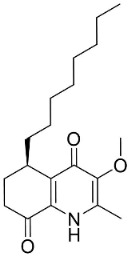
|
Euodia rutaecarpa Benth | Rutaceae |
| 1-Methyl-2-nonyl-4(1H)-quinolone |
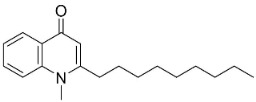
|
Euodia rutaecarpa Benth | Rutaceae |
| 1-Methyl-2-[(Z)-5′-pentadecenyl]-4(1H)-quinolone |
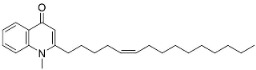
|
Euodia rutaecarpa Benth | Rutaceae |
| Waltherione C |
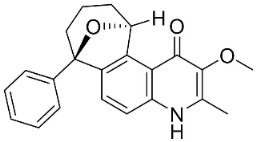
|
Waltheria indica L. | Malvaceae |
| QUINOLIZIDINE ALKALOIDS | |||
| 6,6′-Dihydroxythiobinupharidine |
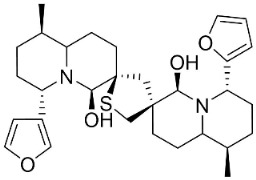
|
Nuphar japonica DC. | Nymphaeaceae |
| 7-Hydroxylupanine |
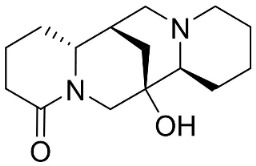
|
Sophora flavescens Aiton | Fabaceae |
| N-Butylcytisine |
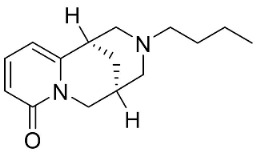
|
Sophora flavescens Aiton | Fabaceae |
| SECURINEGA ALKALOIDS | |||
| Securinine |
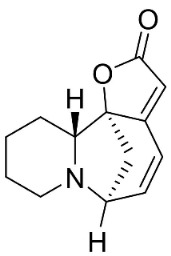
|
Flueggea virosa (Roxb. Ex Willd.) Royle | Phyllanthaceae |
| Viroallosecurinine |
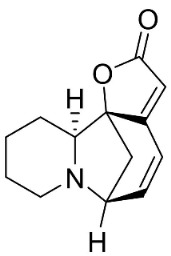
|
Flueggea virosa (Roxb. Ex Willd.) Royle | Phyllanthaceae |
| MISCELLANEOUS PIPERINE ALKALOIDS | |||
| Dihydrodioscorine |
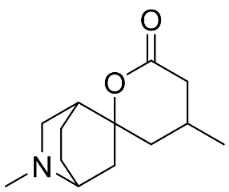
|
Dioscorea bulbifera L. | Dioscoreaceae |
| Haloxyline B |

|
Haloxylon salicornicum (Moq.) Bunge ex Boiss. | Chenopodiaceae |
| Pandamarilactone-1 |
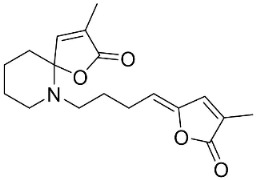
|
Pandanus odorus Ridl. | Pandanaceae |
| SIMPLE QUINOLINE ALKALOIDS | |||
| Camptothecin |
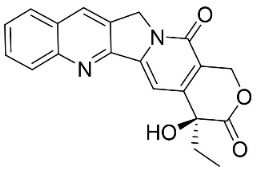
|
Gomphandra Wall. Ex Lindl. | Icacinaceae |
| Dictamine |
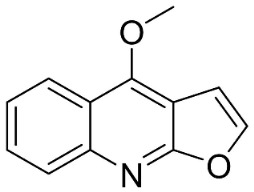
|
Dictamnus albus L. | Rutaceae |
| γ-Fagarine |
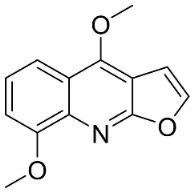
|
Dictamnus albus L. | Rutaceae |
| 4-Methoxy-2-phenylquinoline |
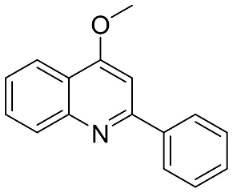
|
Lunasia amara Blanco | Rutaceae |
| 4-Methylquinoline |
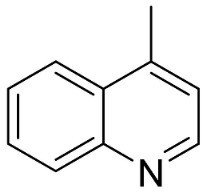
|
Citrullus colocynthis (L.) Schrad. | Cucurbitaceae |
| Robustine |
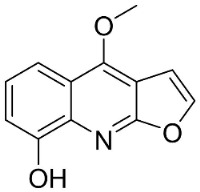
|
Dictamnus albus L. | Rutaceae |
| PROTOBERBERINES | |||
| Pendulamine A |
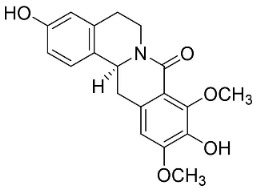
|
Polyalthia longifolia (Sonn.) | Annonaceae |
| Pendulamine B |
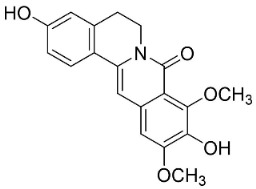
|
Polyalthia longifolia (Sonn.) | Annonaceae |
| PROTOPINES | |||
| Allocryptopine |
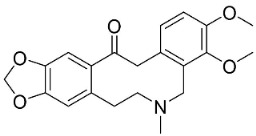
|
Macleaya cordata (Willd.) R. Br | Papaveraceae |
| Protopine |
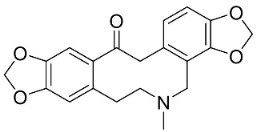
|
Argemone mexicana L. | |
| SPIROBENZYLISOQUINOLINES | |||
| (+)-Fumariline |
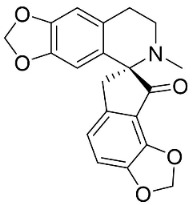
|
Fumaria officinalis L. | Fumariaceae |
| Fumarophycine |
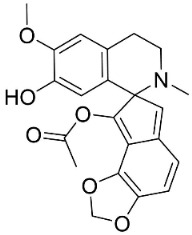
|
Fumaria officinalis L. yields | Fumariaceae |
| (+)-Parfumine |
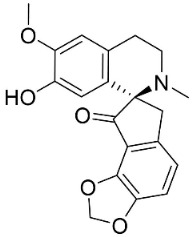
|
Fumaria indica Pugsley | Fumariaceae |
| STEROIDAL ALKALOIDS | |||
| Conimine |
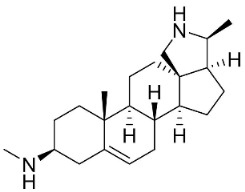
|
Holarrhena pubescens Wall. Ex G. Don | Apocynaceae |
| N-Formylconessimine |
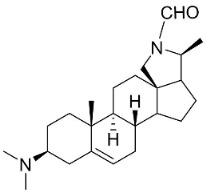
|
Holarrhena pubescens Wall. Ex G. Don | Apocynaceae |
3. The Strongest Antibacterial and/or Antifungal Alkaloids Identified and Spectrum of Activity
Rios and Recio defined a crude extract with MIC greater than 1000 µg/mL as inactive and suggested interesting antibacterial activity for MICs of 100 µg/mL or lower [6]. Previously, Fabry et al. (1998) defined crude active extracts as having MIC values below 8000 µg/mL [7], while more recently, Kuete (2010) defined crude extracts with MIC values less than 100 µg/mL as active and MICs above 625 µg/mL as weakly active [9]. Here, a compound was very strongly antibacterial or antifungal for a MIC value below or equal to 1 µg/mL; strongly antibacterial (or antifungal for a MIC value above 1 and below or equal to 50 µg/mL; moderately antibacterial or antifungal for a MIC from 50 and below 100 µg/mL; weakly antibacterial (or antifungal) for a MIC from 100 and below 500 µg/mL; very weakly antibacterial or antifungal for a MIC ranging from 500 to below 2500 µg/mL; and inactive for a MIC value above 2500 µg/mL. Following this classification, 8-acetylnorchelerythrine, cryptolepine, 8-hydroxydihydrochelerythrine, 6-methoxydihydrosanguinarine, 2′-nortiliacorinine, pendulamine A and B, rhetsisine, sampangine, tiliacorine, tryptanthrin, tylophorinine, vallesamine, and viroallosecurinine showed very strong activities (Table S2).
Looking at the spectrum of activity, most alkaloids showed activity against Gram-positive bacteria, followed by Gram-negative bacteria, yeasts, filamentous fungi, and mycobacteria (Table S2).
4. Influence of Molecular Mass
The molecular mass of natural products dictates their ability to fit in the catalytic pockets of enzymes and to cross biological membranes. Here, low molecular mass molecules were defined with a molecular mass below 200 g/mol; medium molecular mass molecules were defined with a molecular mass from 200 to 400 g/mol; and high molecular mass molecules were defined for a molecular mass above 400 g/mol. Following this classification, we noted that principles with very strong activity against bacteria and fungi had a molecular mass mainly from 200 to 400 g/mol whereas very strong repressors of mycobacteria mainly had a mass above 400 g/mol (Table S2).
5. Influence of Solubility and Polar Surface
Because solubility is a fundamental criterion in which to consider the efficacy of alkaloids, there is need to use mathematical values. Log P is equal to the ratio of concentrations of a compound between octanol and water. Hydrophilic compounds (hydrophilic) have low or negative values (about −3) (compounds are mainly found in the water phase). Mid-hydrophilic compounds have a Log P close to 0 (the compound is equally partitioned between the octanol and water layers). Non-hydrophilic (hydrophobic, liposoluble) compounds have a high Log P (up to about 7) (note that lipophilic alkaloids tend to remain in and destabilize the cytoplasmic membrane of bacteria and fungi). However, Log P is only relevant for non-ionizable principles and for an ionized substance, a Log D is preferable (in terms of ADME), but the pH must be fixed. In some databases, one can find a Log P of 3 for the ionic alkaloid berberine, suggesting a lipophilic substance, which is not sensible. Since compounds destined for pharmaceutical development will mainly be exposed to physiological pH and often a weak base or a weak acid, we defined, at pH 7.4, lipophilic compounds for a negative Log D value of 4, amphiphilic (mid-polar) compounds for a Log D up to about 4.5, and lipophilic for a Log D above 4.5. Note that the Log D values given here are predicted values. Following this classification, we noted that principles with very strong activity against bacteria and fungi were mainly amphiphilic, whereas very strong antimycobacterial agents were mainly lipophilic (Table S2). Most antibiotics with very strong antibacterial and/or antifungal effects have a polar surface area around 50 Å (Table S2).
6. Mechanisms of Action and Structure-Activity Relationships
Most antibacterial and/or antifungal alkaloids from Asian angiosperms either bear a quinoline or an indole framework and the main mechanisms of action involve the targeting of the DNA, topoisomerases, and cytoplasmic membrane.
6.1. Alkaloids Targeting DNA and/or Topoisomerase
Some alkaloids including aristolochic acids bind to DNA or pyrrolizidine alkaloids, which form DNA cross-linking and DNA-protein cross-linking, leading to mutations [12,13]. Planar alkaloids tend to intercalate with bacterial DNA such as sanguinarine DNA [14], canthin-6-one [15], carbazoles [16], and tryptanthrin [17]. Cryptolepine intercalates into DNA and stimulates DNA cleavage by II [18] and was bacteriolytic for Staphylococcus aureus (NCTC 10788) [19]. Evodiamine inhibits topoisomerase I by stabilizing the enzyme-DNA covalent complex [20].
The mode of antibacterial and antifungal action of quinoline alkaloids mainly evokes interaction with DNA. Dictamnine binds to DNA under UV light [21]. Camptothecin stabilizes the topoisomerase I–DNA complex [22]. Liriodenine blocks topoisomerase II [23]. Berberine was active against Actinobacillus pleuropneumoniae and Streptococcus agalactiae (CVCC 1886) via DNA synthesis inhibition and the blockage of synthesis [24,25]. Anonaine induces DNA damage [26] as well as magnoflorine [27]. Aporphine alkaloids are planar and intercalate DNA and inhibit topoisomerase [28] as well as Amaryllidaceae alkaloids [29]. The quaternary ammonium ion of protoberberine alkaloids and their heterocyclic planar framework account for topoisomerase I inhibition [30].
During bacterial division, topoisomerase IV catalyzes the relaxation of the DNA chain and 6,6′-dihydroxythiobinupharidine blocks this enzyme in S. aureus [31]. Phenanthroindolizidine alkaloids interact with DNA [32]. In Gram-positive bacteria, topoisomerase IV is a target for bactericidal quinolone antibiotics [33]. Asian angiosperms produce a vast array of quinoline, isoquinoline, piperidine, and quinolizidine alkaloids, representing a fascinating reservoir of topoisomerase IV inhibitors.
In summary, heterocyclic alkaloids with low to medium molecular mass, close to planar or planar, with the presence of a few hydroxy or ketone groups, almost always target DNA and/or RNA in bacteria and fungi. From this perspective, quinazolone alkaloids in the family Hydrangeaceae (order Saxifragales; clade Core Eudicots) could be examined for their antibacterial and or antifungal properties.
6.2. Alkaloids Targeting the Cytoplasmic Membrane
Phenanthroindolizidine alkaloids disturb the cytoplasmic membrane integrity [34]. The long alkyl chain of piperine and piperlongumine could penetrate the membrane of bacteria and fungi. In Candida albicans, piperine affects the membrane integrity, leading to oxidative stress followed by cell cycle arrest and apoptosis [35]. Marques et al. (2010) presented evidence that amides were more active against Cladosporium cladosporoides when the non-substituted aromatic ring, single double bonds, and substitution of nitrogen with alkyl groups were present [36]. In fungi, berberine targets the mitochondrial membrane [24]. Liriodenine in Paracoccidioides brasiliensis evoked cytoplasmic alterations and damage to the cell wall [37] while berberine evoked cytoplasmic insults in Streptococcus agalactiae (CVCC 1886) [34] and targeted the mitochondrial membrane of fungi [25].
6.3. Miscellaneous Targets
Aristolochic acids inhibited the H+-ATPase-mediated proton pump in E. coli [12]. Securinine induces mitotic block in cancer cells by binding to tubulin and inhibits microtubule assembly [38], therefore, microtubules could be involved in the antibacterial and/or antifungal properties of cytotoxic monoterpenoid indole alkaloids.
7. Efflux Pumps Inhibitors
P. aeruginosa and Gram-negative bacteria can resist a broad spectrum of natural products because they have extra classes of efflux pumps such as ABC (ATP binding cassette), RND (resistance nodulation cell-division), MF (major facilitator), SMR (small multidrug resistance) and MATE (multidrug and toxic compound extrusion) pumps [39]. ABC efflux pumps located in the cytoplasmic membrane of both Gram-positive and Gram-negative bacteria that use the energy derived from ATP hydrolysis to expel xenobiotics. RND efflux pumps located in the cytoplasmic and outer membrane of the Gram-negative bacteria (specific to Gram-negative bacteria) that expel xenobiotics using the H+ gradient (antiporters). MF efflux pumps located in the cytoplasmic membrane of both Gram-positive and Gram-negative bacteria that expel xenobiotics using the H+ gradient (antiporters). SMR efflux pumps located in the cytoplasmic membrane of both Gram-positive and Gram-negative bacteria that expel xenobiotics using the H+ gradient (antiporters). MATE efflux pumps located in the cytoplasmic membrane and are antiporters (the exit of the xenobiotic coincides with the entry of a Na+).
Tryptanthrin inhibits efflux P-glycoprotein in Caco-2 cells [16] and as such may inhibit bacteria and or fungal efflux pumps. Apocynaceous monoterpene indole alkaloids are often vasorelaxant [40], and therefore, with some inhibition levels of bacterial and or fungal efflux-pumps. For instance, the reserpine from Rauvolfia serpentina (L.) Benth. ex Kurz decreased the resistance of S. aureus (1199B, NorA hyperproducer) to ciprofloxacin and norfloxacin [10]. Reserpine is a calcium channel antagonist and an inhibitor of efflux pumps in Gram-negative bacteria and mycobateria [41]. From Rauvolfia serpentina (L.) Benth. ex Kurz, ajmaline and yohimbine are neuroactive and efflux pump inhibitors in Gram-negative bacteria [42]. Verapamil is another example of a calcium channel antagonist that inhibits the efflux pump in bacteria [42]. The reason why calcium channel antagonists have the tendency to inhibit the bacterial efflux pump could be because of the correlations between the bacterial efflux pumps and bacterial calcium transport [43]. Tetrandrine, which is a calcium channel antagonist in mammalian cells, inhibited the efflux pumps in S. aureus Rv2459 (jefA), Rv3728, and Rv3065 (mmr) efflux pumps in Mycobacterium species [44]. Therefore, natural products known for being calcium channel inhibitors should be screened as antibiotic potentiators. 4′-O-Methyldopamine inhibits NorA [45], showing that N-caffeoylphenalkylamide with the strongest efflux pump inhibitor activities presented hydroxyl substitution on the aromatic rings of the caffeic acid part and methoxy substitution on the aromatic ring of the dopamine moiety, which led to an increase in activity. Dopamine is a neurotransmitter, and it could be argued that neuroactive principles are a first line candidate for the development of efflux pump inhibitors. L-dopa increased the resistance of C. neoformans toward amphotericin B [46]. In line, erythrinan-type alkaloids and amide alkaloids in the family Piperaceae interact with GABAergic receptors and as such may be able to inhibit bacterial efflux pumps as in pellitorine, which at 16 µg/mL increased the sensitivity of S. aureus (RN4220) to erythromycin at 16 µg/mL via inhibition of the efflux pumps [5]. Canthin-6-one has a chemical structure with some similarity with serotonin and thus might be able to inhibit bacteria and/or fungal efflux pumps. In mammalian cells, tryptanthrin inhibits the expression of P-glycoprotein efflux pumps [46] and one could investigate its effect on the expression of efflux pumps in bacteria and fungi.
8. Amide Alkaloids
8.1. Simple Amide Alkaloids
Plants in the order Piperales (clade Magnoliids) yield antibacterial amide alkaloids with strong activity against Gram-positive bacteria (Figure 1). Pellitorine inhibited the growth of Bacillus sphaericus (ATCC 14577), Bacillus subtilis (ATCC 6051), Staphylococcus aureus (ATCC 9144), Escherichia coli (ATCC 25922), Pseudomonas syringae (ATCC 13457), and S. typhimurium (ATCC 23564) with the MIC values of 25, 12.5, 20, 150, 75, and 200 µg/mL, respectively [47]. Pellitorine (50 µg/disk) inhibited the growth of Listeria monocytogenes with an inhibition zone diameter of 9 mm and the MIC value of 500 µg/mL [48]. Pellitorine (20 µL of a 2 µg/mL solution/6 mm well) inhibited the growth of Aspergillus flavus, Aspergillus fumigatus, Coniophora puteana, Fibrophoria vaillentii, Fusarium proliferatum, and Rhisopus sp. with the inhibition zone diameters of 27, 29, 26, 28, 29, and 27 mm, respectively [49]. Piperlonguminine suppressed B. sphaericus (ATCC 14577), B. subtilis (ATCC 6051), S. aureus (ATCC 9144), E. coli (ATCC 25922), P. syringae (ATCC 13457), and S. typhimurium (ATCC 23564) with the MIC values of 20, 9, 12.5, 150, 75, and 175 µg/mL, respectively [47]. Piperlonguminine inhibited Mycobacterium tuberculosis with a MIC value of 50 µg/mL [50]. 8Z-N-isobutyleicosatrienamide and pellitorine had moderate potencies with S. aureus (MIC: 34 µM) [51]. Pellitorine restrained M. tuberculosis (H37Ra) with the MIC value of 25 µg/mL [52]. di-p-Coumaroyl-caffeoylspermidine weakly inhibited the mycelial growth of Pyrenophora avenae and Blumeria graminis [53]. In the clade Clampanulids, Spilanthes paniculata Wall. ex DC yielded N-Isobutyl-2 (E), 6 (Z), 8 (E)-decatrienamide (also known as spilanthol), which was bactericidal for Streptococcus mutans with MIC/MBC values of 125/125 µg/mL and weakly repressed C. albicans (ATCC 10231) [44]. The condensation of ferulic acid and dopamine yielded N-trans-feruloyl-4-methyldopamine 200 µg/disk that developed halos with a broad-spectrum of bacteria [54] and increased the susceptibility of multidrug-resistant S. aureus (overexpressing the multidrug efflux transporter NorA) to norfloxacin at 100 μg/mL [44].
8.2. Cyclopeptides
Plants in the Fabids and Malvids produce broad-spectrum antibacterial cyclic peptides such as frangulanine, which inhibited the growth of S. aureus, B. subtilis, E. faecium, E. coli, E. cloacae, S. typhimurium, and P. aeruginosa with the MIC values of 50, 50, 25, 6.2, 50, 50, 0.7, and 25 µg/mL, respectively [55]. Frangulanine inhibited the growth of C. albicans and Saccharomyces cerevisae with the minimum amounts of 25 and 50 µM, respectively [56]. Nummularine H isolated from the roots of Ziziphus mauritiana Lam. (family Celastraceae; order Celastrales; clade Fabids) inhibited the growth of M. tuberculosis (H37Ra) with the MIC of 4.5 μM [57].
9. Indole Alkaloids
9.1. Simple Indoles
9.1.1. Brassicaceous Indoles
Plants in the family Brassicaceae yield antifungal sulfurated indole alkaloids such as caulilexin A from Brassica oleracea L. and at the concentration of 5 × 10−4 M, inhibited the growth of Leptosphaeria maculans, Sclerotinia sclerotiorum, and Rhizoctonia solani by 55, 100, and 100%, respectively [58]. The thiazoyl-substituted indole camalexin was active against Alternaria brassicae (MIC: 80 µg/mL) [59].
9.1.2. β-Carbolines
The condensation of tryptophan and oxaloacetaldehyde yields strong broad-spectrum antibacterial and antimycobacterial β-carboline alkaloids in the clades Malvids and Lamiids. Canthin-6-one restrained the growth of S. aureus (1199B), S. aureus (XU212), and S. aureus (ENRSA-15) with the MIC values of 8, 8, and 32 µg/mL, respectively [60] as well as Mycobacterium fortuitum (ATCC 6841), Mycobacterium smegmatis (ATCC 14468), M. smegmatis (mc222700), Mycobacterium phlei (ATCC 111758), and Mycobacterium abcessus (ATCC 19977) with the MIC values of 16, 8, 8, 8, and 16 µg/mL, respectively [60]. Rhetsinine very strongly suppressed Xanthomonas oryxae pv oryzae, Xanthomonas oryxae pv oryzicola with the EC50 values of 1 and 4.5 µg/mL, respectively [61]. The condensation of 2 β-carboline yielded borrerine yields in Borreria verticillata (L.) G. Mey. (clade Lamiids), which at the concentration of 50 µg/mL inhibited the growth of S. aureus and at 6 µg/mL restrained Vibrio cholerae [62].
9.1.3. Carbazoles
The condensation of anthranilic acid and malonyl-CoA yields quinolinones that after prenylation and cyclization yield carbazole alkaloid phytoalexins in the family Nitrariaceae and Rutaceae in the order Sapindales (clade Malvids) (Figure 1). One such carbazole is glycozolidol (also known as 6-hydroxy-2-methoxy-3-methylcarbazole; 200 µg/mL/well), which restrained S. aureus, Bacillus firmis, S. lutea, Agrobacterium tumefaciens, and Proteus vulgaris [63]. 3-Formylcarbazole moderately inhibited the growth of Mycobacterium sp. [64]. Clausamine A (Log D = 3.9 at pH 7.4; molecular mass = 293.3 g/mol) inhibited the growth of MRSA (SK1), S. aureus (TISTR 1466), E. coli (TISTR 780), and S. typhimurium (TISTR 292) with the MIC values of 8, 32, 128, and 128 µg/mL, respectively [65]. Clausamine B very strongly inhibited the growth of MRSA (SK1) (MIC: 0.2 µg/mL) [65]. Clauszoline N inhibited the growth of MRSA (SK1), E. coli (TISTR 780), and S. typhimurium (TISTR 292) with the MIC values of 16, 64, and 128 µg/mL, respectively [65]. The prenylated carbazole clausine F very strongly inhibited MRSA (SK1) and S. aureus (TISTR 1466) with the MIC values of 4 µg/mL, respectively, and was moderately active for E. coli (TISTR 780) and S. typhimurium (TISTR 292) [65]. In the Rutaceae, lansine yielded a MIC of 14.3 µg/mL against M. tuberculosis (H37Rv) [66]. Murrayamine J restrained S. aureus (ATCC 29213), Bacillus cereus (IIIM 25) with the IC50 values of 11.7 and 23.2 µM [67]. The prenylated carbazole girinimbine was active against B. cereus (IIIM 25) with the IC50 value of 3.4 µM as well as S. aureus and Aspergillus niger with the MIC values of 3.1 and µg/mL, respectively [68]. Koenimbine repressed S. aureus (ATCC 29213) and B. cereus (IIIM 25) with IC50 values of 17 and 22.5 µM [67] and koenigine was active against a variety of Candida sp. (MIC90: 12.5–100 μg/mL) [68]. 3-Formyl-1-1methoxycarbazole moderately repressed S. aureus, B. subtilis, E. coli, P. vulgaris, A. niger, and C. [68]. 3,3′-[Oxybis(methylene)]bis(9-methoxy-9H-carbazole) inhibited the growth of Proteus vulgaris and C. albicans with the MIC values of 6.2 and 25 µg/mL, respectively [69].In the Nitrariaceae, harmane is very strongly active against Vibrio anguillarum (MIC: 3.1 µg/mL), [70]. In a subsequent study, harman weakly inhibited the growth of A. niger, C. albicans (Neenah, 2010), Cryptococcus gattii, and Cryptococcus neoformans [71].
9.1.4. Monoterpene Indole Alkaloids
Plants in the order Gentianales produce monoterpene indole alkaloids with moderate broad-spectrum antibacterial properties (Figure 1).
Strictosidine-typeindole alkaloid glycosides: The condensation of tryptophan and iridoid glycoside secologanin yields strictosidine, the precursor of 5α-carboxystrictosidine (Log D-3.1 at pH 7.4; molecular mass = 574.5 g/mol), which inhibited the growth of M. tuberculosis (H37Rv) with the MIC value of 26.3 µg/mL [72].
Iboga indole alkaloids: Iboga indole alkaloids are derived from strictosidine. Voacangine and ibogaine moderately inhibited the growth of M. tuberculosis and Mycobacterium kansasi [73]. 5-Oxocoronaridine and coronaridine were moderately active against repressed E. coli, B. subtilis, A. flavus, A. niger, and Rhizoctonia phaseoli [74]. The growth E. coli, K. pneumoniae, S. aureus, S. pneumoniae [74], A. flavus, C. albicans, and R. phaseoli was moderately inhibited by ibogamine [74]. Penicillium chrysogenum was moderately inhibited by 5-oxocoronaridine, 3-oxocoronaridine, and tabernamontamine [74].
Vobasine-type indole alkaloids: The growth of A. niger and A. flavus was moderately suppressed by vobasine [74]. Voacamine repressed R. phaseoli, P. chrysogenum, and C. albicans [74]. The growth was moderately hampered by vobasine [74].
Isomalindan-type indole alkaloids: Cadambine from Neolamarckia cadamba (Roxb.) Bosser (clade Lamiids) was weakly active toward Staphylococcus epidermidis, S. aureus, B. cereus, and B. subtilis, and C. albicans [75].
Corynanthe indole alkaloids: Strictosidine is the precursor to corynanthe indole alkaloids such as alstoniascholarine A, which hindered K. pneumoniae, Providencia smaitii, and E. coli with the MIC values of 12.5, 25, and 50 µg/mL, respectively [76] while alstoniascholarine E yielded the MIC values of 25, 25, and 25 µg/mL, against K. pneumoniae, P. smaitii, and E. coli with the MIC values of 100, 50, 50, respectively. Alstoniascholarine A and E moderately restrained M. gypseum, E. floccosum, and T. mentagrophytes [76]. Alstoniascholarine J repressed E. floccosum with the MIC of 31.2 µg/mL, and yielded the MICs of 3.1, 12.5, 12.5, 1.5, and 25 µg/mL with P. aeruginosa, E. faecalis, K. pneumoniae, P. smaitii, and E. coli, respectively [76]. Vallesamine and 5-hydroxy-19,20-Z-alschomine very strongly inhibited the growth of E. faecalis (ATCC 10541) and P. aeruginosa (ATCC 27853) with the MIC values of 1.5 and 0.7 µg/mL, respectively [77,78].
Strychnos-type indole alkaloids: Strictosidine is the precursor of Strychnos-type indole alkaloids such as tubotaiwine that moderately inhibited the growth of M. tuberculosis H37Rv (MIC: 100 µg/mL) [79].
9.1.5. Miscellaneous
The condensation of anthranilic acid with isatin yielded the indoloquinazoline alkaloid tryptanthrin, which very strongly inhibited MRSA (MIC: 0.5 μg/mL) and Malassezia furfur (MIC: 4 μg/mL) [34,80]. Tryptanthrin is a broad-spectrum antifungal alkaloid that yielded the MIC values of 3.1, 3.1, 3.1, 3.1, 6.3, and 3.1 µg/mL, against T. mentagrophytes, Trichophyton rubrum, Trichophyton tonsurans, Microsporum canis, M. gypseum, and E. floccosum respectively [81,82]. Tryptanthrin was strongly fungistatic with C. neoformans, and Cryptococcus deuterogattii (MIC/MFC: 2/>64 and 8/32 μg/mL) [83].
Plants in the genus Euodia from tryptophan via the condensation of dihydronorharman and N-methylanthranilic acid yield evodiamine with weak potencies against K. pneumoniae (MDR, clinical strain) [84]. Dehydroevodiamine (molecular mass = 301.3 g/mol) from the fruits of Euodia rutaecarpa Benth very strongly restrained X. oryxae pv oryzae (EC50: 1.4 µg/mL) [61]
In the family Apocynaceae (clade Lamiids), the condensation of anthranilic acid with indoxyl yielded the indoloquinoline alkaloid cryptolepine, which repressed S. aureus (NCTC 10788), S. dysenteriae (ATCC 13313), V. cholerae (ATCC 11623), S. sclerotiorum, and Botrytis cinerea with the MIC values of 5, 6.2, <1.5, 5.5, and 0.05 µg/mL, respectively [7,18,85,86].
10. Piperidine Alkaloids
10.1. Piperine Alkaloids
Plants in the family Piperaceae (clade Magnoliids) convert L-lysine into piperidine, which combines with piperoyl-CoA to yield the strong broad-spectrum antibacterial piperine alkaloids (Figure 1). Piperine moderately impeded B. sphaericus (ATCC 14577), B. subtilis (ATCC 6051), E. coli (ATCC 25922), P. syringae (ATCC 13457), and S. typhimurium (ATCC 23564) and yielded a MIC of 3.9 µg/mL with S. aureus [47,87]. Piperine (200 µL of the 10 mg/mL/11 mm well) muzzled S. aureus, P. aeruginosa, and C. albicans [87]. Piperine is an antibiotic potentiator that inhibits mycobacterial efflux pumps [50,87]. Piperine very strongly constrained albicans [87] and at the concentration of 100 µg/mL suppressed R. solani, Fusarium gramineum, Alternaria tenuissima, Gloeosporium theae-sinensis, Phytophthora capsici, and Phomopsis adianticola by 63.1, 53, 66.1, 76.9, 41.8, and 29.3%, respectively [88]. Piperlongumine (200 µL of the 10 mg/mL/11 mm well) from Piper longum L. is active against S. aureus and P. aeruginosa increased the susceptibility of S. aureus to rifampicin [87] and very strongly inhibited C. albicans (MIC: 3.9 µg/mL) [87].
10.2. Quinolizidine Alkaloids
From L-lysine are produced quinolizidine alkaloids [89] in the Nymphaeaceae and Fabaceae (Figure 1). 6,6′-Dihydroxythiobinupharidine very strongly suppressed E. faecalis and Enterococcus faecium with the MIC ranging from 2 to 4 µg/mL and MRSA with a MIC of 2 µg/mL [90]. 7-Hydroxylupanineand N-butylcytisine from Sophora flavescens Aiton (clade Fabids) hindered S. aureus with the MIC values of 16 µg/mL [90] and N-butylcytisine yielded the MIC of 8 µg/mL with E. coli [90].
10.3. Phenanthroindolizidine Alkaloids
Plants in the family Lauraceae (clade Magnoliids), Moraceae (clade Fabids, and Asclepiadaceae (clade Lamiids) combine cinnamic acid and ornithine to yield strong broad-spectrum antifungal phenanthroindolizidine alkaloids [91] (Figure 1). One such alkaloid is tylophorinine, which suppressed C. albicans (14503), Candida krusei, Candida glabrata, and A. fumigatus with MIC values of 0.6, 0.6, 2.5, and 5 µg/mL, respectively [92]. Tylophorinidine yielded MIC values of 2, 4, 8, and 8 µg/mL with C. albicans (14503), C. krusei, C. glabrata, and A. fumigatus, respectively [92] (Table 1). 7-Demethoxytylophorine from Cynanchum atratum Bunge was very strongly antifungal with Penicillium italicum (MIC/MFC: 1.5/6.2 µg/mL) [25] and Penicillium digitatum (MIC/MFC: 1.5/12.5 µg/mL) [92].
10.4. Securinega Alkaloids
Plants in the family Phyllanthaceae (clade Fabids) produce Securinega alkaloids (Figure 1) such as viroallosecurinine, which is very active against P. aeruginosa and S. aureus (MIC: 0.4 μg/mL) [93]. Securinine weakly restrained P. aeruginosa, S. aureus, and M. smegmatis [93]. Allosecurinine and ent-seco norsecurinine inhibited the growth of a broad-spectrum of filamentous fungi [94,95].
10.5. Miscellaneous
Dihydrodioscorine (0.1% of agar) from Dioscorea bulbifera L. inhibited the mycelial growth and spore production of Sclerotium rolfsii, C. lunata, F. moniliforme, Botryodiplodia theobromae, and Macrophomina phaseolina [8]. Pandamarilactone-1 weakly restrained E. coli, P. aeruginosa, and S. aureus [96]. Among the Chenopodiaceae, haloxyline B moderately restrained M. tuberculosis H37Rv with the MIC of 50 µg/mL [97].
11. Quinoline Alkaloids
11.1. Simple Quinolines
From tryptophan via anthranilic acid with condensation with malonyl CoA plants in the clade Fabids yielded simple quinolines (Figure 1). 4-Methylquinoline hindered S. aureus (KCCM 11335) with the MIC/MBC values of 12.2/50 µg/mL [98]. Lunasia amara Blanco yielded 4-methoxy-2-phenylquinoline with MIC values of 16 µg/mL against M. tuberculosis H37Rv [98].
In the family Rutaceae (clade Malvids), the condensation of anthranilic acid with malonyl CoA followed by prenylation yielded antibacterial furanoquinolines such as dictamine from Dictamnus albus L. hindered Micrococcus luteus (TISTR 884) and B. cereus (TISTR 688) with the MIC values of 26 and 64 µg/mL, respectively [99,100] and was active against M. tuberculosis H37Rv [101]. γ-Fagarine and robustine are moderate broad-spectrum antibacterial furanoquinolines [100,101].
Members of the genus Gomphandra Wall. ex Lindl. are produced from the condensation of tryptamine and secologanin yields via strictodamide camptothecin, which is a strong broad-spectrum antifungal pyrrolquinoline alkaloid [102,103].
11.2. Benzylisoquinolines
In basal angiosperms (clade Magnoliids, Eudicots), the condensation of dopamine and p-hydroxyphenylacetaldehyde yielded strong broad-spectrum antibacterial and antifungal benzylisoquinoline alkaloids (Figure 1). One such alkaloid is reticuline [104,105]. From Fumaria indica Pugsley, fuyuziphine at a concentration of 500 ppm hindered the germination of spores of Alternaria brassicicola, A. solani, Alternaria melongenae, C. maculans, Erysiphe cichoracearum, and Helminthosporium pennisetti by more than 80% [106].
11.3. Bisbenzylisoquinolines
The radical coupling of benzylisoquinolines gives birth to antibacterial and antifungal bisbenzylisoquinoline alkaloids in plants in the clade Eudicots (Figure 1). For instance, the coupling of N-methyl coclaurine yields tetrandine, which is weakly bactericidal for S. aureus (ATCC 25923) and MRSA (ATCC 33591) [107] and is a bacterial efflux pump inhibitor [90,107].
Tiliacora triandra Diels produces tiliacorinine, 2′-nortiliacorinine, and tiliacorine, which achieved MIC values of 6.2, 3.1, and 3.1 µg/mL, respectively, toward M. tuberculosis (H37Rv) and MIC values ranging from 0.7 to 6.2 µg/mL against several clinical isolates of multidrug-resistant M. tuberculosis [108]. Tiliacorine inhibited the germination of the conidia of A. tenuissima by more than 60% at 100 µg/mL [109].
11.4. Aporphines
The cyclization by oxidative coupling of benzylisoquinolines yields aporphines in basal angiosperms. For instance, in Figure 1, liriodenine [110] inhibited a very broad spectrum of bacteria and phytopathogenic fungi [111] as well as Histoplasma capsulatum (MIC: 1.9 μg/mL) [28].
Anonaine developed halos against B. cereus (ATCC-14.579), E. coli (ATCC-11.105), S. aureus (ATCC-6538), and S. epidermidis (ATCC-12.228) with diameters of 20, 8, 14, and 12 mm, respectively (1 mg/mL, 70 µL/well) [84]. In a subsequent study, anonaine weakly inhibited the growth of S. mutans (ATCC 25175) (Lall et al., 2017), T. rubrum, and M. gypseum [85].
Lysicamine was moderately active with S. epidermidis (MIC: 50 µg/mL) [112], and very strongly repressed L. monocytogenes, MSSA, S. pneumoniae, and Actinobacillus sp. with the MIC values of 2.5, 1, 4, and 2.5 µg/mL, respectively, and K. pneumoniae (ESBL)with the MIC of 20 µg/mL [113]. Lysicamine (10 µg/6 mm disk) developed inhibition zone diameters of 12, 13, and 15.5 mm toward S. epidermidis, S. aureus, and B. subtilis, respectively [113], and had meek effects with Candida dubliniensis (ATCC 777) [33].
O-methylmoschatoline (200 µg/disk) inhibited the growth of S. aureus, E. coli, P. aeruginosa, S. typhi, and K. pneumoniae with zone diameters of 20, 12, 12, 20, and 22 mm, respectively [114]. Xylopine hindered B. cereus, Micrococcus sp., and S. aureus [115] as well as V. cholerae, E. coli, and S. dysenteriae [116]. Artabotrine was very strongly bactericidal against K. pneumoniae (ESBL) (MIC/MBC: 2.5/2.5 μg/mL) [113]. The azaoxoaporphine sampangine (Log D 2.7 at pH 7.4; molecular weight 232.2 g/mol) was very strongly active with C. albicans (ATCC 90028), C. glabrata (ATCC 90030), C. kruseii (ATCC 6258), A. fumigatus (ATCC 90906), and C. neoformans (ATCC 90113) with MIC values of 3.1, 3.1, 6.2, 6.2, and 0.05 µg/mL, respectively [117] and at 10 µg/disk developed halos with B. cereus, S. aureus, E. coli, and S. typhi [118]. Lanuginosine (10 µg/disk) invoked inhibition zone diameters of 12, 14, 10, 14, and 12 mm, with halos with B. cereus, S. aureus, E. coli, K. pneumoniae, and P. aeruginosa, respectively [118].
Nordicentrine (Log D 3.0 at pH 7.4; molecular weight = 325.3 g/mol) suppressed M. tuberculosis with a MIC of 12.5 µg/mL [119] as well as Cladosporium clodosporioides (6 µg/spot) [120]. Dicentrinone was moderately antimycobacterial [121].
The oxoaporphine thailandine restrained S. pneumoniae, S. aureus, and E. faecalis with MIC values of 30, 30, and 60 µg/mL, respectively, and yielded an IC50 value of 6.2 µg/mL with M. tuberculosis (H37Ra) [122].
Isoboldine was moderately active with E. coli, P. aeruginosa, P. mirabilis, K. pneumoniae, A. baumanii, S. aureus, B. subtilis [104], and C. albicans [123]. Bulbocapnine restrained K. pneumoniae and A. baumanii with the MIC values of 32, 64, 32, 8, 8, 64, and 128 µg/mL respectively [104].
Roemerine is a moderate inhibitor of MRSA (135), S. aureus (ATCC25913) C. albicans (SC 5314), C. glabrata (8535), C. krusei (4996), Candida tropicalis (8915), Candida parapsilosis (90018), and A. fumigatus (7544), respectively [124] and yielded a MIC value of 10 μg/mL with C. albicans (ATCC 90028) [125].
Magnoflorine moderately repressed C. albicans (KCTC7965), C. albicans (KACC30071), C. parapsilosis var. parapsilosis (KACC45480), T. rubrum, and T. mentagrophytes [126,127].
11.5. Protopines
Benzylisoquinolines are precursors of protopine that restrained E. coli, P. aeruginosa, P. mirabilis, K. pneumoniae, A. baumanii, S. aureus, and B. subtilis with the MIC values of 32, 64, 32, 8, 8, 64, and 128 µg/mL, respectively [104] as well as Streptococcus agalactiae [128]. Protopine inhibited the growth of C. albicans with the MIC value of 4 µg/mL [104]. Allocryptopine was weakly active with S. aureus, P. aeruginosa, E. coli, and S. agalactiae [128].
11.6. Protoberberines
The cyclization of benzylisoquinolines yielded very strong antibacterial protoberberines such as in pendulamine A from Polyalthia longifolia (Sonn.) Thwaites that yielded the MIC of 2, 0.02, 0.2, 0.02, 2, 2, 0.2, and 0.02 µg/mL against B. subtilis, Corynebacterium hoffmanii, S. aureus, Micrococcus lysodickycus, K. pneumoniae, P. aeruginosa, S. typhi, and S. paratyphi A, respectively [129]. Pendulamine B restrained Corynebacterium hoffmanii, S. aureus, S. faecalis, S. viridans, M. lysodickycus, K. pneumoniae, P. aeruginosa, S. typhi, and S. paratyphi A with the MIC values of 0.02, 0.2, 2, 0.02, 0.02, 2, 0.2, and 0.2 µg/mL, respectively [129].
11.7. Spirobenzylisoquinolines
Spirobenzylisoquinolines are derived from benzylisoquinolines via protoberberine. and include (+)-parfumine, fumarophycine and (+)-fumariline (that moderately restrained E. coli, P. aeruginosa, P. mirabilis, K. pneumoniae, A. baumanii, S. aureus, and B. subtilis [104].
11.8. Benzophenanthridines
Plants in the clade Eudicots use reticuline as a precursor of benzophenanthridines such as stylopine or sanguinarine L. that moderately repressed E. coli, P. aeruginosa, P. mirabilis, K. pneumoniae, A. baumanii, S. aureus, and B. Subtilis [104]. Dihydrosanguinarine was a weak repressor of E. coli [128]. Sanguinarine impeded S. mutans (ATCC 25175) with the MIC value of 32 µg/mL [130] as well as S. aureus, P. aeruginosa, E. coli, and S. agalactiae with MIC values of 31.3, 250, 62.5, 15.6 µg/mL, respectively [128]. 6-Methoxydihydrosanguinarine (10 µg/disk) developed an inhibition zone diameter of about 17 mm with S. aureus and MRSA [131]. 8-Hydroxydihydrosanguinarine (molecular weight = 349.3 g/mol) inhibited the growth of clinical strains of MRSA with MIC ranging from 0.4 to 7.8 µg/mL and MBC ranging from 1.9 to 31.2 µg/mL, ESBL strains of E. coli with MIC ranging from 15.6 to 250 µg/mL and MBC ranging from 62.5 to 500 µg/mL, and E. coli with MIC of 15.6 µg/mL and MBC of 31.2 µg/mL [109]. 8-Hydroxydihydrosanguinarine was moderately active against C. parapsilosis, C. tropicalis, C. krusei, C. glabrata, and C. neoformans [132].
6-Methoxydihydrosanguinarine is a very strong inhibitor of E. faecalis and S. aureus (ATCC 25925) with the MIC/MBC of 5/10, 2.5/5 µg/mL, respectively, and evoked milder potencies with E. coli (ATCC 25922) [133] and C. albicans (CMCC 85021) [133]. Norsanguinarine is moderately antibacterial [104].
Allocryptopine is the precursor of dihydrochelerythrine that strongly hindered S. epidermidis (ATCC 12228), S. aureus (ATCC 6538), S. pyogenes (ATCC 19615), B. subtilis (ATCC 6633), K. pneumoniae (ATCC 13883), and E. coli (ATCC 25922) with MIC/MBC values of 6.2/12.5, 12.5/50, 12.5/50, 25/50, 12.5/25, 25/25 µg/mL, respectively [134]. 8-Hydroxydihydrochelerythrine (molecular weight = 365.4 g/mol) inhibited the growth of 20 strains of MRSA clinical isolated with MIC ranging from 0.9 to 15.6 µg/mL and MBC ranging from 7.8 to 62.5 µg/mL [135] and displayed meeker effects with E. coli and K. pneumoniae (ESBL) [109,135]. Dihydrochelerythrine suppressed MRSA (SK1) and E. coli (TISTR 780) with the MIC values of 8 and 16, and 128 µg/mL [100]. Dihydrochelerythrine is the precursor of chelerythrine, which very strongly hindered S. epidermidis (ATCC 12228), S. aureus (ATCC 6538), S. pyogenes (ATCC 19615), B. subtilis (ATCC 6633), K. pneumoniae (ATCC 13883), and E. coli (ATCC 25922) with MIC/MBC values of 1.5/12.5, 1.5/3.1, 1.5/6.2, 1.5/50, 1.5/50, and 1.5/25 µg/mL, respectively [128,134]. Chelerythrine was also very strongly antifungal with C. albicans (ATCC 10231), S. cerevisae (ATCC 2601), and C. neoformans (ATCC 28952) with MIC/MBC values of 3.1/3.1, 6.2/6.2, and 3.1/6.2 µg/mL, respectively [134,136]. Corynoline and acetylcorynoline from Corydalis incisa (Thunb.) Pers. evoked inhibitory halos with Cladosporium herbarum (3 µg/spot) [137].
Norchelerythrine inhibited the growth of M. tuberculosis with the MIC value of 25 µg/mL [138]. Avicine very strongly suppressed S. epidermidis (ATCC 12228), S. aureus (ATCC 6538), S. pyogenes (ATCC 19615), B. subtilis (ATCC 6633), K. pneumoniae (ATCC 13883), and E. coli (ATCC 25922) with the MIC/MBC values of 3.1/12.5, 1.5/25, 1.5/12.5, 1.5/6.2, and 6.2/12.5 µg/mL, respectively [134]. 8-Acetylnorchelerythrine yielded the MIC values of 1 µg/mL with S. epidermidis, E. coli, E. cloacae, K. pneumoniae, and P. aeruginosa [139]. Rhoifoline B moderately inhibited the growth of S. aureus, S. epidermidis, E. coli, E. cloacae, K. pneumoniae, P. aeruginosa, and S. dysenteriae [139]. Nitidine, from a member of the genus Zanthoxylum L. (clade Malvids), weakly inhibited the growth of M. luteus, S. aureus, and M. smegmatis [140].
11.9. Protoberberines
Reticuline is precursor of the broad-spectrum antibacterial berberine [29,128,141,142,143,144]. Of note, berberine curbed the growth of K. pneumonia and A. baumanii with the MIC value of 8 µg/mL [104] and Neisseria gonorrhea with the MIC of 13.5 µg/mL [145]. Berberine decreased the MICs of ampicillin and oxacillin against MRSA and evoked a synergistic effect [146] and impeded the MexAB antibiotic efflux pump in P. aeruginosa [147]. Berberine suppressed fluconazole-resistant clinical strains of C. tropicalis, C. albicans, C. parapsilosis, and C. neoformans as well as C. krusei (ATCC 6258) and C. parapsilosis (ATCC 22019) with the MIC values of 8, 8, 8, 8, 8, 8, 8, 16, 16, 16, 16, 4, and 16 µg/mL, respectively [148] and at a concentration of 500 ppm blocked the germination of spores of Curvularia lunata, Erysiphe cichoracearum, F. udum, and Penicillium sp. by more than 80% [106]. Berberine at the concentration of 1.9 µg/mL decreased the MIC of fluconazole from 1.9 to 0.4 µg/mL [149] and inhibited the growth of M. smegmatis (ATCC 607) with the MIC value of 25 µg/mL [150].
Berberine is a precursor of jatrorrhizine that curbed strains of P. acnes with MIC between 5 and 50 µg/mL, [143]. In a subsequent study, jatrorrhizine weakly inhibited S. aureus, B. subtilis, E. coli, and S. dysenteriae [151] as well as the clinical isolates of E. floccosum, T. rubrum, Trichophyton interdigitale, Trichophyton violaceum, T. mentagrophytes, T. equinum, M. canis, M. gypseum, C. albicans, and C. tropicalis [152]. Palmatine t is moderately antibacterial as well as a sinactine [104] and MexAB antibiotic efflux pump blocker in P. aeruginosa [147].
11.10. Phthalides
Protoberberine alkaloids are precursors of phthalides with moderate antibacterial potencies including adlumidine or bicuculline [104]. Bicuculline at 200 ppm restrained the spore germination of A. brassicae, Curvularia lanata, and F. udum [104,153].
11.11. Hasubanans
Within the family Menispermaceae, intramolecular coupling of benzylisoquinolines form hasubanan alkaloids. One such alkaloid is glabradine, isolated from the tubers of Stephania glabra (Roxb.) Miers, which suppressed S. aureus and S. mutans with the MIC value of 50 µg/mL as well as M. gypseum, M. canis, and T. rubrum with the MIC values of 25, 25, and 50 µg/mL, respectively [154].
11.12. Amaryllidaceae Alkaloids
Plants in the family Amaryllidaceae (clade Monocots) condensate protocatechuic aldehyde and tyramine to yield antibacterial Amaryllidaceae alkaloids such as crinamine [9]. Other examples are lycorine and lycoricidine (1 mg/mL/8 mm disk), which developed an inhibition zone of 13 and 12 mm with E. coli, respectively [106]. Lycorine at the concentration of 100 µg/mL hindered Alternaria oleracea, C. gloeosporioides, F. graminearum, Colletotrichum ophiopogonis, and Pleospora lycopersici by 61.9, 57.2, 63.7, 63.2, and 52.6%, respectively [155]. Lycorine yielded the MIC values of 39, 32, 512, 64, and 97.3 µg/mL with C. albicans, Candida dubium, C. glabrata, Lodderomyces elongisporus, and S. cerevisae, respectively [156]. Narciclasine inhibited the growth of Corynebacterium fascians and C. neoformans [157] and hippeastrine moderately curbed C. albicans [156]. Tazettine from Narcissus tazetta L. weakly restrained C. dubliniensis and L. elongisporus [156].
11.13. Miscellaneous
11.13.1. Quinolinones
Plants in the clade Malvids yield antibacterial and antifungal quinolinones. Antidesmone at the concentration of 50 µg/mL restrained Botryosphaeria dothidea, Colletotrichum musae, Pestalotipsis guepinii, Colletotrichum orbiculare, Phylophthora nicotianae, Pestalotiopsis longiseta, Carbendazim-sensitive strains of S. sclerotiorum, and Carbendazim-resistant strains of S. sclerotiorum by 71.9, 84.6, 86.7, 75.5, 78.8, 83.4, 56.5, and 100%, respectively [158]. Waltherione C at the concentration of 50 µg/mL inhibited B. dothidea, Colletotrichum musae, Pestalotipsis guepinii, Colletotrichum orbiculare, Phylophthora nicotianae, Pestalotiopsis longiseta, carbendazim-sensitive strains of S. sclerotiorum, and carbendazim-resistant strains of S. sclerotiorum by 60, 43, 71.4, 60.9, 41.6, 3.6, 79.8, and 81.8%, respectively [158].
Members of the genus Euodia J.R. Forst. & G. Forst. bring to being series of antibacterial (Gram-positive) long chain quinolinones born of the condensation of anthranilic acid, malonyl-CoA and fatty acyl-CoA (also produced by Gram-negative bacteria). 1-Methyl-2-nonyl-4(1H)-quinolone very strongly repressed S. aureus (ATCC 25393), S. epidermidis (ATCC 12228), and B. subtilis (ATCC 6633) with the MIC values of 4, 4, and 8 µg/mL, respectively [159]. 1-Methyl-2-[(Z)-5′-pentadecenyl]-4(1H)-quinolone isolated from the fruits hindered S. aureus (ATCC 25393), S. epidermidis (ATCC 12228), and B. subtilis (ATCC 6633) with the MIC values of 16, 4, and 16 µg/mL, respectively [159]. In a subsequent study, the lipophilic long chain quinolinone alkaloid evocarpine inhibited the growth of MRSA (ATCC 33591) and S. aureus (ATCC 25923) with the MIC values of 8 and 8 µg/mL, respectively [160] and very strongly suppressed M. fortuitum (ATCC 6841), M. smegmatis (ATCC 19429), and M. phlei (ATCC 19249) with the MIC values of 2, 2, and 2 µg/mL, respectively [161].
11.13.2. Acridanones
In the family Rutaceae (clade Malvids), the condensation of anthranilate with 3 malonyl CoA yielded acridanones such as 1-hydroxy-3,4-dimethoxy-10-methylacridan-9-one [162] or 1-hydroxy-3-methoxy-10-methyl-acridan-9-one that repressed E. coli [69] (Table 1).
11.13.3. Phenanthrene Alkaloids
Benzylisoquinolines are precursors of antibacterial phenanthrene in basal clades as aristolochic acid, which inhibited the growth of Moraxella catarrhalis (GTC 01544) with MIC/MBC values of 25/50 µg/mL [163] as well as B. subtilis and E. coli [164], P. aeruginosa, S. faecalis, S. aureus, and Staphylococcus epidermidis [165]. Another example is aristolactam N-(6′-trans-p-coumaroyl)-β-d-glucopuyranoside, which restrained B. subtilis and S. lutea with the MIC values of 43.8 and 175 µg/mL, respectively [166]. These phenanthrene alkaloids bind covalently with DNA [167,168]. 1-N-monomethylcarbamate-argentinine-3-O-β-d-glucoside at 500 µg/disk hindered MRSA with an inhibition zone diameter of 8 mm [169].
12. Pyrrolidines and Imidazole Alkaloids
In the family Piperaceae (clade Magnoliids), the pyrrolidines brachyamide B yielded the IC50 of 41.8 µg/mL against a clinical isolate of C. albicans [170] and suppressed C. neoformans (ATCC 90 113) with an IC50 of 7.1 µg/mL [171]. Other instances are trachyone and isopiperolein B with S. aureus (MIC of 30 and 36 µM, respectively) [51]. The pyrrolidine alkaloid lactone pandamarilactonine A moderately inhibited the growth of E. coli, P. aeruginosa, and S. aureus [96]. The pyrrolidine amide N-[9-(3,4-Methylenedioxyphenyl)-2E,4E,8E-nonatrienoyl] pyrrolidine was active toward M. tuberculosis (H37Ra MIC: 25 µg/mL) [170].
The imidazole alkaloid allantoin very strongly hindered B. subtilis with the MIC value of 4 µg/mL as well as S. aureus, E. coli, and K. pneumoniae with the MIC value of 8, 8, and 8 µg/mL, respectively [172] (Table 1).
13. Diterpene Alkaloids
Plants in the family Ranunculaceae (clade Eudicots) produce a unique type of moderately antifungal diterpene alkaloids. For instance, 8-acetylheterophyllisine, vilmorrianone, and panicutine inhibited the growth of Allescheria boydii, A. niger, E. floccosum, and Pleurotus ostreatus [173] (Table 1).
14. Steroidal Alkaloids
N-formylconessimine and conimine suppressed MSSA and MRSA with MIC values of 32 and 128 μg/mL, respectively, and conimine increased the sensitivity of MSSA to vancomycin [174].
15. Concluding Remarks
Weinstein and Albersheim (1983) presented evidence that phytoalexins, especially flavonoids from angiosperms, act as nonspecific membrane antimicrobials that alter the structural integrity of the cytoplasmic membrane, thereby causing the membrane to be a less efficient matrix for membrane-dependent processes [175]. They also argue that phytoalexins with non-specific antimicrobial targets targeting the cytoplasm and proteins also makes it difficult for bacteria or fungi to develop resistance. Furthermore, phytoalexins are often toxic to herbivorous predators as well as repellent and therefrom exhibit low therapeutic indices. In the case of alkaloids, none have been known act on specific antibacterial or antifungal targets [176]. It is for this reason that antibiotic or antifungal alkaloids for systemic use in humans working at micromolar plasmatic concentrations have not been found yet. Research efforts, however, need to continue with the determination of the selectivity indices. Alkaloids from Asian angiosperms represent yet another mind-blowing source of original chemical frameworks that can be used for the hemisynthesis of clinical antibiotics, antimycobacterial agents, and antifungal drugs as well as efflux pump inhibitors of clinical value. 8-Acetylnorchelerythrine, cryptolepine, 8-hydroxydihydrochelerythrine, 6-methoxydihydrosanguinarine, 2′-nortiliacorinine, pendulamine A and B, rhetsisine, sampangine, tiliacorine, tryptanthrin, tylophorinine, vallesamine, and viroallosecurinine with a MIC ≤1 µg/mL are first line candidates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11091146/s1, Table S1: Medicinal Plants of Asia and the Pacific yielding antibacterial and/or antifungal alkaloids; Table S2: Antibacterial and/or antifungal alkaloid in vitro and from Asian Angiosperms with MIC ≤ 5 µg/mL.
Author Contributions
Conceptualization, C.W.; Methodology, M.S. (Mazdida Sulaiman) and J.-F.W.; Formal analysis, M.S.B. and M.K.B.B.; Investigation, C.W.; Resources, C.W.; Data curation, A.K.P.; Writing—original draft preparation, C.W.; Writing—review and editing, K.J., V.N., M.R. (Mogana Rajagopal), A.K.P., M.d.L.P., M.S. (Mazdida Sulaiman), M.S. (Monica Suleiman) and M.R. (Mohammed Rahmatullah); Visualization, P.W.; Supervision, C.W.; Project administration, C.W.; Funding acquisition, C.W. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project was funded by a grant from the Malaysian Ministry of Education (FRGS/1/2018/ WAB07/UNIM/02/1, Malaysian Ministry of Higher Education, Malaysia) and Project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Angiosperm Phylogeny Group An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016;181:105–121. [Google Scholar]
- 2.Tiku A.R. Co-Evolution of Secondary Metabolites. Springer; Cham, Switzerland: 2020. Antimicrobial compounds (phytoanticipins and phytoalexins) and their role in plant defense; pp. 845–868. [DOI] [Google Scholar]
- 3.Hashimoto T., Yamada Y. Alkaloid biogenesis: Molecular aspects. Annu. Rev. Plant Biol. 1994;45:257–285. doi: 10.1146/annurev.pp.45.060194.001353. [DOI] [Google Scholar]
- 4.Denyer S.P., Maillard J.Y. Cellular impermeability and uptake of biocides and antibiotics in Gram-negative bacteria. J. Appl. Microbiol. 2002;92:35S–45S. doi: 10.1046/j.1365-2672.92.5s1.19.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahirrao P., Tambat R., Chandal N., Mahey N., Kamboj A., Jain U.K., Singh I.P., Jachak S.M., Nandanwar H.S. MsrA efflux pump inhibitory activity of Piper cubeba lf and its phytoconstituents against S. aureus RN4220. Chem. Biodivers. 2020;17:e2000144. doi: 10.1002/cbdv.202000144. [DOI] [PubMed] [Google Scholar]
- 6.Rios J.L., Recio M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Ablordeppey S.Y., Fan P., Li S., Clark A.M., Hufford C.D. Substituted indoloquinolines as new antifungal agents. Bioorg. Med. Chem. 2002;10:1337–1346. doi: 10.1016/S0968-0896(01)00401-1. [DOI] [PubMed] [Google Scholar]
- 8.Adeleye A., Ikotun T. Antifungal activity of dihydrodioscorine extracted from a wild variety of Dioscorea bulbifera L. J. Basic Microbiol. 1989;29:265–267. doi: 10.1002/jobm.3620290504. [DOI] [Google Scholar]
- 9.Adesanya S.A., Nia R., Martin M.T., Boukamcha N., Montagnac A., Païs M. Stilbene derivatives from Cissus quadrangularis. J. Nat. Prod. 1999;62:1694–1695. doi: 10.1021/np9902744. [DOI] [Google Scholar]
- 10.Aeschlimann J.R., Dresser L.D., Kaatz G.W., Rybak M.J. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of S. aureus. Antimicrob. Agents Chemother. 1999;43:335–340. doi: 10.1128/AAC.43.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tripathi Y.C., Rathore M., Kumar H. On the variation of alkaloidal contents of Fumaria indica at different stages of life span. Anc. Sci. Life. 1994;13:271. [PMC free article] [PubMed] [Google Scholar]
- 12.Kuete V., Poumale H.P., Guedem A.N., Shiono Y., Randrianasolo R., Ngadjui B.T. Antimycobacterial, antibacterial and antifungal activities of the methanol extract and compounds from Thecacoris annobonae (Euphorbiaceae) S. Afr. J. Bot. 2010;76:536–542. doi: 10.1016/j.sajb.2010.04.003. [DOI] [Google Scholar]
- 13.Joosten L., van Veen J.A. Defensive properties of pyrrolizidine alkaloids against microorganisms. Phytochem. Rev. 2011;10:127–136. doi: 10.1007/s11101-010-9204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maiti M., Nandi R., Chaudhuri K. Sanguinarine: A monofunctional intercalating alkaloid. FEBS Lett. 1982;142:280–284. doi: 10.1016/0014-5793(82)80152-X. [DOI] [PubMed] [Google Scholar]
- 15.Cao R., Peng W., Wang Z., Xu A. β-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007;14:479–500. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- 16.Tanious F.A., Ding D., Patrick D.A., Tidwell R.R., Wilson W.D. A new type of DNA minor-groove complex: Carbazole dication−DNA interactions. Biochemistry. 1997;36:15315–15325. doi: 10.1021/bi971599r. [DOI] [PubMed] [Google Scholar]
- 17.Bandekar P.P., Roopnarine K.A., Parekh V.J., Mitchell T.R., Novak M.J., Sinden R.R. Antimicrobial activity of tryptanthrins in E. coli. J. Med. Chem. 2010;53:3558–3565. doi: 10.1021/jm901847f. [DOI] [PubMed] [Google Scholar]
- 18.Bailly C., Laine W., Baldeyrou B., Pauw-Gillet D., Colson P., Houssier C., Cimanga K., Van Miert S., Vlietinck A.J., Pieters L. DNA intercalation, topoisomerase II inhibition and cytotoxic activity of the plant alkaloid neocryptolepine. Anti-Cancer Drug Des. 2000;15:191–201. [PubMed] [Google Scholar]
- 19.Sawer I.K., Berry M.I., Ford J.L. The killing effect of cryptolepine on Staphylococcus aureus. Lett. Appl. Microbiol. 2005;40:24–29. doi: 10.1111/j.1472-765X.2004.01625.x. [DOI] [PubMed] [Google Scholar]
- 20.Chan A.L.F., Chang W.S., Chen L.M., Lee C.M., Chen C.E., Lin C.M., Hwang J.L. Evodiamine stabilizes topoisomerase I-DNA cleavable complex to inhibit topoisomerase I activity. Molecules. 2009;14:1342–1352. doi: 10.3390/molecules14041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Towers G.H.N., Whitehead F.W., Abramowski Z.A., Mitchell J.C. Dictamnine, an alkaloid which crosslinks DNA in the presence of ultraviolet light. Biochem. Biophy. Res. Comm. 1980;95:603–607. doi: 10.1016/0006-291X(80)90827-X. [DOI] [PubMed] [Google Scholar]
- 22.Pommier Y., Leo E., Zhang H., Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo S.H., Reynolds M.C., Sun N.J., Cassady J.M., Snapka R.M. Inhibition of topoisomerase II by liriodenine. Biochem. Pharmacol. 1997;54:467–473. doi: 10.1016/S0006-2952(97)00198-6. [DOI] [PubMed] [Google Scholar]
- 24.Kang S., Li Z., Yin Z., Jia R., Song X., Li L., Chen Z., Peng L., Qu J., Hu Z., et al. The antibacterial mechanism of berberine against Actinobacillus pleuropneumoniae. Nat. Prod. Res. 2015;29:2203–2206. doi: 10.1080/14786419.2014.1001388. [DOI] [PubMed] [Google Scholar]
- 25.Peng L., Kang S., Yin Z., Jia R., Song X., Li L., Li Z., Zou Y., Liang X., Li L., et al. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Pathol. 2015;8:5217. [PMC free article] [PubMed] [Google Scholar]
- 26.Chen B.H., Chang H.W., Huang H.M., Chong I.W., Chen J.S., Chen C.Y., Wang H.M. (−)-Anonaine induces DNA damage and inhibits growth and migration of human lung carcinoma h1299 cells. J. Agric. Food Chem. 2011;59:2284–2290. doi: 10.1021/jf103488j. [DOI] [PubMed] [Google Scholar]
- 27.Okon E., Kukula-Koch W., Jarzab A., Halasa M., Stepulak A., Wawruszak A. Advances in chemistry and bioactivity of magnoflorine and magnoflorine-containing extracts. Int. J. Mol. Sci. 2020;21:1330. doi: 10.3390/ijms21041330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo S.H., Sun N.J., Cassady J.M., Snapka R.M. Topoisomerase II inhibition by aporphine alkaloids. Biochem. Pharmacol. 1999;57:1141–1145. doi: 10.1016/S0006-2952(99)00018-0. [DOI] [PubMed] [Google Scholar]
- 29.Chen G.L., Tian Y.Q., Wu J.L., Li N., Guo M.Q. Antiproliferative activities of Amaryllidaceae alkaloids from Lycoris radiata targeting DNA topoisomerase I. Sci. Rep. 2016;6:38284. doi: 10.1038/srep38284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X., Wang D., Jiang L., Yang D. DNA topoisomerase I inhibitory alkaloids from Corydalis saxicola. Chem. Biodivers. 2008;5:1335–1344. doi: 10.1002/cbdv.200890121. [DOI] [PubMed] [Google Scholar]
- 31.Okamura S., Nishiyama E., Yamazaki T., Otsuka N., Taniguchi S., Ogawa W., Hatano T., Tsuchiya T., Kuroda T. Action mechanism of 6, 6′-dihydroxythiobinupharidine from Nuphar japonicum, which showed anti-MRSA and anti-VRE activities. Biochim. Biophys. Acta Gene. Subj. 2015;1850:1245–1252. doi: 10.1016/j.bbagen.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z., Lv H., Li H., Zhang Y., Zhang H., Su F., Xu S., Li Y., Si Y., Yu S., et al. Interaction studies of an anticancer alkaloid, (+)-(13aS)-deoxytylophorinine, with calf thymus DNA and four repeated double-helical DNAs. Chemotherapy. 2011;57:310–320. doi: 10.1159/000329506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitton-Fry M.J., Brickner S.J., Hamel J.C., Barham R., Brennan L., Casavant J.M., Ding X., Finegan S., Hardink J., Hoang T., et al. Novel 3-fluoro-6-methoxyquinoline derivatives as inhibitors of bacterial DNA gyrase and topoisomerase IV. Bioorganic Med. Chem. Lett. 2017;27:3353–3358. doi: 10.1016/j.bmcl.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Chen C., Qi W., Peng X., Chen J., Wan C. Inhibitory effect of 7-demethoxytylophorine on Penicillium italicum and its possible mechanism. Microorganisms. 2019;7:36. doi: 10.3390/microorganisms7020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thakre A., Jadhav V., Kazi R., Shelar A., Patil R., Kharat K., Zore G., Karuppayil S.M. Oxidative stress induced by piperine leads to apoptosis in Candida albicans. Med. Myc. J. 2021;59:366–378. doi: 10.1093/mmy/myaa058. [DOI] [PubMed] [Google Scholar]
- 36.Marques J.V., Oliveira A.D., Raggi L., Young M., Kato M.J. Antifungal activity of natural and synthetic amides from Piper species. J. Braz. Chem. Soc. 2010;21:1807–1813. doi: 10.1590/S0103-50532010001000003. [DOI] [Google Scholar]
- 37.Vinche A.D.L., de-La-Cruz-Chacón I., González-Esquinca A.R., Silva J.D.F.D., Ferreira G., Santos D.C.D., Garces H.G., Oliveira D.V.M.D., Marçon C., Cavalcante R.D.S., et al. Antifungal activity of liriodenine on agents of systemic mycoses, with emphasis on the genus Paracoccidioides. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020;26:e20200023. doi: 10.1590/1678-9199-jvatitd-2020-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashraf S.M., Mahanty S., Rathinasamy K. Securinine induces mitotic block in cancer cells by binding to tubulin and inhibiting microtubule assembly: A possible mechanistic basis for its anticancer activity. Life Sci. 2021;287:120105. doi: 10.1016/j.lfs.2021.120105. [DOI] [PubMed] [Google Scholar]
- 39.Nishino K., Yamaguchi A. Role of xenobiotic transporters in bacterial drug resistance and virulence. IUBMB Life. 2008;60:569–574. doi: 10.1002/iub.90. [DOI] [PubMed] [Google Scholar]
- 40.Hirasawa Y., Dai X., Deguchi J., Hatano S., Sasaki T., Ohtsuka R., Nugroho A.E., Kaneda T., Morita H. New vasorelaxant indole alkaloids, taberniacins A and B, from Tabernaemontana divaricata. J. Nat. Med. 2019;73:627–632. doi: 10.1007/s11418-019-01293-9. [DOI] [PubMed] [Google Scholar]
- 41.Gupta S., Tyagi S., Almeida D.V., Maiga M.C., Ammerman N.C., Bishai W.R. Acceleration of tuberculosis treatment by adjunctive therapy with verapamil as an efflux inhibitor. Am. J. Respir. Crit. Care Med. 2013;188:600–607. doi: 10.1164/rccm.201304-0650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dwivedi G.R., Singh D.P., Sharma S.A., Darokar M.P. Efflux Pumps: Warheads of Gram-Negative Bacteria and Efflux Pump Inhibitors. In: Sinha R.P., Richa, editors. New Approaches in Biological Research. Nova Science Publishers; New York, NY, USA: 2017. pp. 35–72. Chapter 2. [Google Scholar]
- 43.Jones H.E., Holland I.B., Jacq A., Wall T., Campbell A.K. Escherichiacoli lacking the AcrAB multidrug efflux pump also lacks nonproteinaceous, PHB–polyphosphate Ca2+ channels in the membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 2003;1612:90–97. doi: 10.1016/S0005-2736(03)00082-8. [DOI] [PubMed] [Google Scholar]
- 44.Sharma A., Gupta V.K., Pathania R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 2019;149:129. doi: 10.4103/ijmr.IJMR_2079_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michalet S., Cartier G., David B., Mariotte A.M., Dijoux-Franca M.G., Kaatz G.W., Stavri M., Gibbons S. N-caffeoylphenalkylamide derivatives as bacterial efflux pump inhibitors. Bioorganic Med. Chem. Lett. 2007;17:1755–1758. doi: 10.1016/j.bmcl.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Casadevall A. Growth of Cryptococcus neoformans in presence of L-dopa decreases its susceptibility to amphotericin B. Antimicrob. Agents Chemother. 1994;38:2648–2650. doi: 10.1128/AAC.38.11.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivasa Reddy P., Jamil K., Madhusudhan P., Anjani G., Das B. Antibacterial activity of isolates from Piper longum and Taxus baccata. Pharm. Biol. 2001;39:236–238. doi: 10.1076/phbi.39.3.236.5926. [DOI] [Google Scholar]
- 48.Oh J., Hwang I.H., Kim D.C., Kang S.C., Jang T.S., Lee S.H., Na M. Anti-listerial compounds from Asari radix. Arch. Pharmacal Res. 2010;33:1339–1345. doi: 10.1007/s12272-010-0907-9. [DOI] [PubMed] [Google Scholar]
- 49.Nna P.J., Tor-Anyiin T.A., Igoli J.O. Fagaramide and pellitorine from the stem bark of Zanthoxylum zanthoxyloides and their antimicrobial activities. S. Asian Res. J. Nat. Prod. 2019;2:1–8. [Google Scholar]
- 50.Saranya A.M.A.D., Yuenyongsawad S., Wattanapiromsakul C. Investigation of antitubercular and cytotoxic activities of fruit extract and isolated compounds from Piper retrofractum Vahl. Walailak J. Sci. Technl. 2017;14:731–739. [Google Scholar]
- 51.Reddy S.V., Srinivas P.V., Praveen B., Kishore K.H., Raju B.C., Murthy U.S., Rao J.M. Antibacterial constituents from the berries of Piper nigrum. Phytomedicine. 2004;11:697–700. doi: 10.1016/j.phymed.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Rukachaisirikul T., Siriwattanakit P., Sukcharoenphol K., Wongvein C., Ruttanaweang P., Wongwattanavuch P., Suksamrarn A. Chemical constituents and bioactivity of Piper sarmentosum. J. Ethnopharmacol. 2004;93:173–176. doi: 10.1016/j.jep.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 53.Walters D., Meurer-Grimes B., Rovira I. Antifungal activity of three spermidine conjugates. FEMS Microbiol. Lett. 2001;201:255–258. doi: 10.1111/j.1574-6968.2001.tb10765.x. [DOI] [PubMed] [Google Scholar]
- 54.Rahman M.M., Alam A.K., Sadik G., Islam M.R., Khondkar P., Hossain M.A., Rashid M.A. Antimicrobial and cytotoxic activities of Achyranthes ferruginea. Fitoterapia. 2007;78:260–262. doi: 10.1016/j.fitote.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Dahmer J., do Carmo G., Mostardeiro M.A., Neto A.T., da Silva U.F., Dalcol I.I., Morel A.F. Antibacterial activity of Discaria americana Gillies ex Hook (Rhamnaceae) J. Ethnopharmacol. 2019;239:111635. doi: 10.1016/j.jep.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 56.Emile A., Waikedre J., Herrenknecht C., Fourneau C., Gantier J.C., Hnawia E., Cabalion P., Hocquemiller R., Fournet A. Bioassay-guided isolation of antifungal alkaloids from Melochia odorata. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007;21:398–400. doi: 10.1002/ptr.2078. [DOI] [PubMed] [Google Scholar]
- 57.Panseeta P., Lomchoey K., Prabpai S., Kongsaeree P., Suksamrarn A., Ruchirawat S., Suksamrarn S. Antiplasmodial and antimycobacterial cyclopeptide alkaloids from the root of Ziziphus mauritiana. Phytochemistry. 2011;72:909–915. doi: 10.1016/j.phytochem.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Pedras M.S.C., Sarwar M.G., Suchy M., Adio A.M. The phytoalexins from cauliflower, caulilexins A, B and C: Isolation, structure determination, syntheses and antifungal activity. Phytochemistry. 2006;67:1503–1509. doi: 10.1016/j.phytochem.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 59.Jimenez L.D., Ayer W.A., Tewari J.P. Phytoalexins produced in the leaves of Capsella bursa-pastoris (shepherd’s purse) Phytoprotection. 1997;78:99–103. doi: 10.7202/706124ar. [DOI] [Google Scholar]
- 60.O’Donnell G., Gibbons S. Antibacterial activity of two canthin-6-one alkaloids from Allium neapolitanum. Phytother. Res. 2007;21:653–657. doi: 10.1002/ptr.2136. [DOI] [PubMed] [Google Scholar]
- 61.Su X.L., Xu S., Shan Y., Yin M., Chen Y., Feng X., Wang Q.Z. Three new quinazolines from Evodia rutaecarpa and their biological activity. Fitoterapia. 2018;127:186–192. doi: 10.1016/j.fitote.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Maynart G., Pousset J.L., Mboup S., Denis F. Antibacterial effect of borreverine, an alkaloid isolated from Borreria verticillata (Rubiaceae) Comptes Rendus Des Seances La Soc. Biol. Ses Fil. 1980;174:925–928. [PubMed] [Google Scholar]
- 63.Bhattacharyya P., Chakrabartty P.K., Chowdhury B.K. Glycozolidol, an antibacterial carbazole alkaloid from Glycosmis pentaphylla. Phytochemistry. 1985;24:882–883. doi: 10.1016/S0031-9422(00)84922-5. [DOI] [Google Scholar]
- 64.Sunthitikawinsakul A., Kongkathip N., Kongkathip B., Phonnakhu S., Daly J.W., Spande T.F., Nimit Y., Rochanaruangrai S. Coumarins and carbazoles from Clausena excavata exhibited antimycobacterial and antifungal activities. Planta Med. 2003;69:155–157. doi: 10.1055/s-2003-37716. [DOI] [PubMed] [Google Scholar]
- 65.Maneerat W., Phakhodee W., Ritthiwigrom T., Cheenpracha S., Promgool T., Yossathera K., Deachathai S., Laphookhieo S. Antibacterial carbazole alkaloids from Clausena harmandiana twigs. Fitoterapia. 2012;83:1110–1114. doi: 10.1016/j.fitote.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 66.Ma C., Case R.J., Wang Y., Zhang H.J., Tan G.T., Van Hung N., Cuong N.M., Franzblau S.G., Soejarto D.D., Fong H.H., et al. Anti-tuberculosis constituents from the stem bark of Micromelum hirsutum. Planta Med. 2005;71:261–267. doi: 10.1055/s-2005-837826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nalli Y., Khajuria V., Gupta S., Arora P., Riyaz-Ul-Hassan S., Ahmed Z., Ali A. Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities. Org. Biomol. Chem. 2016;14:3322–3332. doi: 10.1039/C6OB00267F. [DOI] [PubMed] [Google Scholar]
- 68.Joshi T., Jain T., Mahar R., Singh S.K., Srivastava P., Shukla S.K., Mishra D.K., Bhatta R.S., Banerjee D., Kanojiya S. Pyranocarbazoles from Murraya koenigii (L.) Spreng. as antimicrobial agents. Nat. Prod. Res. 2018;32:430–434. doi: 10.1080/14786419.2017.1308363. [DOI] [PubMed] [Google Scholar]
- 69.Rahman M.M., Gray A.I. A benzoisofuranone derivative and carbazole alkaloids from Murraya koenigii and their antimicrobial activity. Phytochemistry. 2005;66:1601–1606. doi: 10.1016/j.phytochem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Aassila H., Bourguet-Kondracki M.L., Rifai S., Fassouane A., Guyot M. Identification of harman as the antibiotic compound produced by a tunicate-associated bacterium. Mar. Biotechnol. 2003;5:163–166. doi: 10.1007/s10126-002-0060-7. [DOI] [PubMed] [Google Scholar]
- 71.Cruz K.S., Lima E.S., Silva M.D.J.A.D., Souza E.S.D., Montoia A., Pohlit A.M., Souza J.V.B.D. Screening and antifungal activity of a β-carboline derivative against Cryptococcus neoformans and C. gattii. Int. J. Microb. 2019;7157845:2019. doi: 10.1155/2019/7157845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Carvalho Junior A.R., Oliveira Ferreira R., de Souza Passos M., da Silva Boeno S.I., Glória das Virgens L.D.L., Ventura T.L.B., Calixto S.D., Lassounskaia E., de Carvalho M.G., Braz-Filho R., et al. Antimycobacterial and nitric oxide production inhibitory activities of triterpenes and alkaloids from Psychotria nuda (Cham. & Schltdl.) Wawra. Molecules. 2019;24:1026. doi: 10.3390/molecules24061026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rastogi N., Abaul J., Goh K.S., Devallois A., Philogène E., Bourgeois P. Antimycobacterial activity of chemically defined natural substances from the Caribbean flora in Guadeloupe. FEMS Immun. Med. Microbiol. 1998;20:267–273. doi: 10.1111/j.1574-695X.1998.tb01136.x. [DOI] [PubMed] [Google Scholar]
- 74.Singh B., A Sharma R., K Vyas G. Antimicrobial, antineoplastic and cytotoxic activities of indole alkaloids from Tabernaemontana divaricata (L.) R. Br. Curr. Pharm. Anal. 2011;7:125–132. doi: 10.2174/157341211795684844. [DOI] [Google Scholar]
- 75.Karaket N., Supaibulwatana K., Ounsuk S., Bultel-Poncé V., Pham V.C., Bodo B. Chemical and bioactivity evaluation of the bark of Neonauclea purpurea. Nat. Prod. Commun. 2012;7:169–170. doi: 10.1177/1934578X1200700208. [DOI] [PubMed] [Google Scholar]
- 76.Qin X.J., Zhao Y.L., Lunga P.K., Yang X.W., Song C.W., Cheng G.G., Liu L., Chen Y.Y., Liu Y.P., Luo X.D. Indole alkaloids with antibacterial activity from aqueous fraction of Alstonia scholaris. Tetrahedron. 2015;71:4372–4378. doi: 10.1016/j.tet.2015.04.046. [DOI] [Google Scholar]
- 77.Liu L., Chen Y.Y., Qin X.J., Wang B., Jin Q., Liu Y.P., Luo X.D. Antibacterial monoterpenoid indole alkaloids from Alstonia scholaris cultivated in temperate zone. Fitoterapia. 2015;105:160–164. doi: 10.1016/j.fitote.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 78.Macabeo A.P.G., Krohn K., Gehle D., Read R.W., Brophy J.J., Franzblau S.G., Aguinaldo M.A.M. Activity of the extracts and indole alkaloids from Alstonia scholaris against Mycobacterium tuberculosis H37Rv. Philip. Agric. Sci. 2008;91:348–351. [Google Scholar]
- 79.Kawakami J., Matsushima N., Ogawa Y., Kakinami H., Nakane A., Kitahara H., Nagaki M., Ito S. Antibacterial and antifungal activities of tryptanthrin derivatives. Trans. Mater. Res. Soc. Jpn. 2011;36:603–606. doi: 10.14723/tmrsj.36.603. [DOI] [Google Scholar]
- 80.Honda G., Tabata M. Isolation of antifungal principle tryptanthrin, from Strobilanthes cusia O. Kuntze. Planta Med. 1979;36:85–86. doi: 10.1055/s-0028-1097245. [DOI] [PubMed] [Google Scholar]
- 81.Hao Y., Guo J., Wang Z., Liu Y., Li Y., Ma D., Wang Q. Discovery of tryptanthrins as novel antiviral and anti-phytopathogenic-fungus agents. J. Agri. Food Chem. 2020;68:5586–5595. doi: 10.1021/acs.jafc.0c02101. [DOI] [PubMed] [Google Scholar]
- 82.Lin C.J., Chang Y.L., Yang Y.L., Chen Y.L. Natural alkaloid tryptanthrin exhibits novel anticryptococcal activity. Med. Myc. J. 2021;59:545–556. doi: 10.1093/mmy/myaa074. [DOI] [PubMed] [Google Scholar]
- 83.Wu J.Y., Chang M.C., Chen C.S., Lin H.C., Tsai H.P., Yang C.C., Yang C.H., Lin C.M. Topoisomerase I inhibitor evodiamine acts as an antibacterial agent against drug-resistant Klebsiella pneumoniae. Planta Med. 2013;79:27–29. doi: 10.1055/s-0032-1327925. [DOI] [PubMed] [Google Scholar]
- 84.Paulo M.D.Q., Barbosa-Filho J., Lima E.O., Maia R.F., de Cassia R., Barbosa B.B.C., Kaplan M.A.C. Antimicrobial activity of benzylisoquinoline alkaloids from Annona salzmanii DC. J. Ethnopharmacol. 1992;36:39–41. doi: 10.1016/0378-8741(92)90058-Y. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y.J., Liu H., Zhang S.Y., Li H., Ma K.Y., Liu Y.Q., Yin X.D., Zhou R., Yan Y.F., Wang R.X., et al. Design, synthesis, and antifungal evaluation of cryptolepine derivatives against phytopathogenic fungi. J. Agri. Food Chem. 2021;69:1259–1271. doi: 10.1021/acs.jafc.0c06480. [DOI] [PubMed] [Google Scholar]
- 86.Mgbeahuruike E.E., Stålnacke M., Vuorela H., Holm Y. Antimicrobial and synergistic effects of commercial piperine and piperlongumine in combination with conventional antimicrobials. Antibiotics. 2019;8:55. doi: 10.3390/antibiotics8020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J., Wang W., Xiong H., Song D., Cao X. Natural phenolic derivatives based on piperine scaffold as potential antifungal agents. BMC Chem. 2020;14:24. doi: 10.1186/s13065-020-00676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramírez-Betancourt A., Hernández-Sánchez A.M., Salcedo-Morales G., Ventura-Zapata E., Robledo N., Wink M., Bermúdez-Torres K. Unraveling the biosynthesis of quinolizidine alkaloids using the genetic and chemical diversity of Mexican lupins. Diversity. 2021;13:375. doi: 10.3390/d13080375. [DOI] [Google Scholar]
- 89.Zhang S.Y., Li W., Nie H., Liao M., Qiu B., Yang Y.L., Chen Y.F. Five new alkaloids from the roots of Sophora flavescens. Chem. Biodivers. 2018;15:e1700577. doi: 10.1002/cbdv.201700577. [DOI] [PubMed] [Google Scholar]
- 90.Teles M.M.R.S., Pinheiro A.A.V., Dias C.D.S., Tavares J.F., Barbosa Filho J.M., Da Cunha E.V.L. Alkaloids of the Lauraceae. Alkal. Chem. Biol. 2019;82:147–304. doi: 10.1016/bs.alkal.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Dhiman M., Parab R.R., Manju S.L., Desai D.C., Mahajan G.B. Antifungal activity of hydrochloride salts of tylophorinidine and tylophorinine. Nat. Prod. Commun. 2012;7:1171–1172. doi: 10.1177/1934578X1200700916. [DOI] [PubMed] [Google Scholar]
- 92.Xin Z., OuYang Q., Wan C., Che J., Li L., Chen J., Tao N. Isolation of antofine from Cynanchum atratum BUNGE (Asclepiadaceae) and its antifungal activity against Penicillium digitatum. Postharvest Biol. Technol. 2019;157:110961. doi: 10.1016/j.postharvbio.2019.110961. [DOI] [Google Scholar]
- 93.Mensah J.L., Lagarde I., Ceschin C., Michelb G., Gleye J., Fouraste I. Antibacterial activity of the leaves of Phyllanthus discoideus. J. Ethnopharmacol. 1990;28:129–133. doi: 10.1016/0378-8741(90)90069-6. [DOI] [PubMed] [Google Scholar]
- 94.Singh A.K., Pandey M.B., Singh U.P. Antifungal activity of an alkaloid allosecurinine against some fungi. Mycobiology. 2007;35:62–64. doi: 10.4489/MYCO.2007.35.2.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goel M., Maurya S., Pandey V.B., Singh V.P., Singh A.K., Singh U.P. Effect of Ent-norsecurinine, an alkaloid, on spore germination of some fungi. Mycobiology. 2002;30:225–227. doi: 10.4489/MYCO.2002.30.4.225. [DOI] [Google Scholar]
- 96.Laluces H.M.C., Nakayama A., Nonato M.G., dela Cruz T.E., Tan M.A. Antimicrobial alkaloids from the leaves of Pandanus amaryllifolius. J. Appl. Pharm. Sci. 2015;5:151–153. doi: 10.7324/JAPS.2015.501026. [DOI] [Google Scholar]
- 97.Bibi N., Tanoli S.A.K., Farheen S., Afza N., Siddiqi S., Zhang Y., Kazmi S.U., Malik A. In vitro antituberculosis activities of the constituents isolated from Haloxylon salicornicum. Bioorg. Med. Chem. Lett. 2010;20:4173–4176. doi: 10.1016/j.bmcl.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 98.Kim M.G., Lee S.E., Yang J.Y., Lee H.S. Antimicrobial potentials of active component isolated from Citrullus colocynthis fruits and structure–activity relationships of its analogues against foodborne bacteria. J. Sci. Food Agric. 2014;94:2529–2533. doi: 10.1002/jsfa.6590. [DOI] [PubMed] [Google Scholar]
- 99.Aguinaldo A.M., Dalangin-Mallari V.M., Macabeo A.P.G., Byrne L.T., Abe F., Yamauchi T., Franzblau S.G. Quinoline alkaloids from Lunasia amara inhibit Mycobacterium tuberculosis H37Rv in vitro. Int. J. Antimicrob. Agents. 2007;29:744–746. doi: 10.1016/j.ijantimicag.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 100.Tantapakul C., Sripisut T., Maneerat W., Ritthiwigrom T., Laphookhieo S. Antibacterial compounds from Glycosmis puberula Twigs. Nat. Prod. Commun. 2014;9:1705–1707. doi: 10.1177/1934578X1400901210. [DOI] [PubMed] [Google Scholar]
- 101.Huang H.Y., Ishikawa T., Peng C.F., Tsai I.L., Chen I.S. Constituents of the root wood of Zanthoxylum wutaiense with antitubercular activity. J. Nat. Prod. 2008;71:1146–1151. doi: 10.1021/np700719e. [DOI] [PubMed] [Google Scholar]
- 102.Ramesha B.T., Suma H.K., Senthilkumar U., Priti V., Ravikanth G., Vasudeva R., Kumar T.S., Ganeshaiah K.N., Shaanker R.U. New plant sources of the anti-cancer alkaloid, camptothecine from the Icacinaceae taxa, India. Phytomedicine. 2013;20:521–527. doi: 10.1016/j.phymed.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 103.Li S., Zhang Z., Cain A., Wang B., Long M., Taylor J. Antifungal activity of camptothecin, trifolin, and hyperoside isolated from Camptotheca acuminata. J. Agric. Food Chem. 2005;53:32–37. doi: 10.1021/jf0484780. [DOI] [PubMed] [Google Scholar]
- 104.Orhan I., Özçelik B., Karaoğlu T., Şener B. Antiviral and antimicrobial profiles of selected isoquinoline alkaloids from Fumaria and Corydalis species. Z. Naturforsch. 2007;62:19–26. doi: 10.1515/znc-2007-1-204. [DOI] [PubMed] [Google Scholar]
- 105.Ari S.N., Yuvita D.D., Phurpa W., Paul A.K. Anti-infective and anti-cancer properties of the Annona species: Their ethnomedicinal uses, alkaloid diversity, and pharmacological activities. Molecules. 2019;24:4419. doi: 10.3390/molecules24234419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pandey M.B., Singh A.K., Singh A.K., Singh U.P. Inhibitive effect of fuyuziphine isolated from plant (Pittapapra) (Fumaria indica) on spore germination of some fungi. Mycobiology. 2007;35:157–158. doi: 10.4489/MYCO.2007.35.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang H., Wang K., Zhang G., Ho H.I., Gao A. Synergistic anti-candidal activity of tetrandrine on ketoconazole: An experimental study. Planta Med. 2010;76:53–61. doi: 10.1055/s-0029-1185973. [DOI] [PubMed] [Google Scholar]
- 108.Sureram S., Senadeera S.P., Hongmanee P., Mahidol C., Ruchirawat S., Kittakoop P. Antimycobacterial activity of bisbenzylisoquinoline alkaloids from Tiliacora triandra against multidrug-resistant isolates of Mycobacterium tuberculosis. Bioorganic Med. Chem. Lett. 2012;22:2902–2905. doi: 10.1016/j.bmcl.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 109.Singh K.P., Pandey V.B., Tripathi Y.C., Singh U.P. Tiliacorinine, a new systemic fungicide effective against Alternaria blight of pigeon pea (Cajanus cajan)/Tiliacorinine, ein neues systemisches Fungizid mit Wirkung gegen die Alternaria-Blattfleckenkrankheit an Taubenerbsen (Cajanus cajan) J. Plant Dis. Protec. 1991;98:213–219. [Google Scholar]
- 110.Rahman M.M., Lopa S.S., Sadik G., Islam R., Khondkar P., Alam A.K., Rashid M.A. Antibacterial and cytotoxic compounds from the bark of Cananga odorata. Fitoterapia. 2005;76:758–761. doi: 10.1016/j.fitote.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 111.De la Cruz-Chacón I., González-Esquinca A.R., Fefer P.G., Garcia L.F.J. Liriodenine, early antimicrobial defence in Annona diversifolia. Z. Naturforsch. C. 2011;66:377–384. doi: 10.1515/znc-2011-7-809. [DOI] [PubMed] [Google Scholar]
- 112.Costa E.V., Pinheiro M.L.B., Barison A., Campos F.R., Salvador M.J., Maia B.H.L., Cabral E.C., Eberlin M.N. Alkaloids from the bark of Guatteria hispida and their evaluation as antioxidant and antimicrobial agents. J. Nat. Prod. 2010;73:1180–1183. doi: 10.1021/np100013r. [DOI] [PubMed] [Google Scholar]
- 113.Tan K.K., Khoo T.J., Rajagopal M., Wiart C. Antibacterial alkaloids from Artabotrys crassifolius Hook. f. & Thomson. Nat. Prod. Res. 2015;29:2346–2349. doi: 10.1080/14786419.2015.1013954. [DOI] [PubMed] [Google Scholar]
- 114.Omar H., Hashim N.M., Zajmi A., Nordin N., Abdelwahab S.I., Azizan A.H.S., Hadi A.H.A., Ali H.M. Aporphine alkaloids from the leaves of Phoebe grandis (Nees) Mer. (Lauraceae) and their cytotoxic and antibacterial activities. Molecules. 2013;18:8994–9009. doi: 10.3390/molecules18088994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsai I.L., Liou Y.F., Lu S.T. Screening of isoquinoline alkaloids and their derivatives for antibacterial and antifungal activities. Gaoxiong Yi Xue Ke Xue Za Zhi Kaohsiung J. Med. Sci. 1989;5:132–145. [PubMed] [Google Scholar]
- 116.Hossain M.S., Ferdous A.J., Hasan C.M. In vitro antimicrobial activities of alkaloids from the stem bark of Desmos longiflorus (Roxb.) Bangladesh J. Bot. 1993;22:37–40. [Google Scholar]
- 117.Agarwal A.K., Xu T., Jacob M.R., Feng Q., Lorenz M.C., Walker L.A., Clark A.M. Role of heme in the antifungal activity of the azaoxoaporphine alkaloid sampangine. Eukaryot. Cell. 2008;7:387–400. doi: 10.1128/EC.00323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khan M.R., Kihara M., Omoloso A.D. Antimicrobial activity of the alkaloidal constituents of the root bark of Eupomatia laurina. Pharm. Biol. 2003;41:277–280. doi: 10.1076/phbi.41.4.277.15671. [DOI] [Google Scholar]
- 119.Lekphrom R., Kanokmedhakul S., Kanokmedhakul K. Bioactive styryllactones and alkaloid from flowers of Goniothalamus laoticus. J. Ethnopharmacol. 2009;125:47–50. doi: 10.1016/j.jep.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 120.Puvanendran S., Wickramasinghe A., Karunaratne D.N., Carr G., Wijesundara D.S.A., Andersen R., Karunaratne V. Antioxidant constituents from Xylopia championii. Pharm. Biol. 2008;46:352–355. doi: 10.1080/13880200801887989. [DOI] [Google Scholar]
- 121.Camacho-Corona M.D.R., Favela-Hernández J.M.D.J., González-Santiago O., Garza-González E., Molina-Salinas G.M., Said-Fernández S., Delgado G., Luna-Herrera J. Evaluation of some plant-derived secondary metabolites against sensitive and multidrug-resistant Mycobacterium tuberculosis. J. Mex. Chem. Soc. 2009;53:71–75. doi: 10.29356/jmcs.v53i2.1008. [DOI] [Google Scholar]
- 122.Makarasen A., Sirithana W., Mogkhuntod S., Khunnawutmanotham N., Chimnoi N., Techasakul S. Cytotoxic and antimicrobial activities of aporphine alkaloids isolated from Stephania venosa (Blume) Spreng. Planta Med. 2011;77:1519–1524. doi: 10.1055/s-0030-1270743. [DOI] [PubMed] [Google Scholar]
- 123.Mollataghi A., Coudiere E., Hadi A.H.A., Mukhtar M.R., Awang K., Litaudon M., Ata A. Anti-acetylcholinesterase, anti-α-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species. Fitoterapia. 2012;83:298–302. doi: 10.1016/j.fitote.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 124.Ma C., Du F., Yan L., He G., He J., Wang C., Rao G., Jiang Y., Xu G. Potent activities of roemerine against Candida albicans and the underlying mechanisms. Molecules. 2015;20:17913–17928. doi: 10.3390/molecules201017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Agnihotri V.K., ElSohly H.N., Khan S.I., Jacob M.R., Joshi V.C., Smillie T., Khan I.A., Walker L.A. Constituents of Nelumbo nucifera leaves and their antimalarial and antifungal activity. Phytochem. Lett. 2008;1:89–93. doi: 10.1016/j.phytol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim J., Ha Quang Bao T., Shin Y.K., Kim K.Y. Antifungal activity of magnoflorine against Candida strains. World J. Microbiol. Biotechnol. 2018;34:167. doi: 10.1007/s11274-018-2549-x. [DOI] [PubMed] [Google Scholar]
- 127.Luo N., Jin L., Yang C., Zhu Y., Ye X., Li X., Zhang B. Antifungal activity and potential mechanism of magnoflorine against Trichophyton rubrum. J. Antibiot. 2021;74:206–214. doi: 10.1038/s41429-020-00380-4. [DOI] [PubMed] [Google Scholar]
- 128.Kosina P., Gregorova J., Gruz J., Vacek J., Kolar M., Vogel M., Roos W., Naumann K., Simanek V., Ulrichova J. Phytochemical and antimicrobial characterization of Macleaya cordata herb. Fitoterapia. 2010;81:1006–1012. doi: 10.1016/j.fitote.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 129.Faizi S., Khan R.A., Azher S., Khan S.A., Tauseef S., Ahmad A. New antimicrobial alkaloids from the roots of Polyalthia longifolia var. pendula. Planta Med. 2003;69:350–355. doi: 10.1055/s-2003-38883. [DOI] [PubMed] [Google Scholar]
- 130.Park K.M., You J.S., Lee H.Y., Baek N.I., Hwang J.K. Kuwanon G: An antibacterial agent from the root bark of Morus alba against oral pathogens. J. Ethnopharmacol. 2003;84:181–185. doi: 10.1016/S0378-8741(02)00318-5. [DOI] [PubMed] [Google Scholar]
- 131.Choi J.G., Kang O.H., Chae H.S., Obiang-Obounou B., Lee Y.S., Oh Y.C., Kim M.S., Shin D.W., Kim J.A., Kim Y.H., et al. Antibacterial activity of Hylomecon hylomeconoides against methicillin-resistant S. aureus. Appl. Biochem. Biotech. 2010;160:2467–2474. doi: 10.1007/s12010-009-8698-5. [DOI] [PubMed] [Google Scholar]
- 132.Meng F., Zuo G., Hao X., Wang G., Xiao H., Zhang J., Xu G. Antifungal activity of the benzo [c] phenanthridine alkaloids from Chelidonium majus Linn against resistant clinical yeast isolates. J. Ethnopharmacol. 2009;125:494–496. doi: 10.1016/j.jep.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 133.Xue X., Zhang H., Zhang X., Liu X., Xi K., Han Y., Guo Z. TLC bioautography-guided isolation and antimicrobial, antifungal effects of 12 alkaloids from Hylomecon japonica roots. Nat. Prod. Commun. 2017;12:1439–1442. doi: 10.1177/1934578X1701200914. [DOI] [Google Scholar]
- 134.Tavares L.D.C., Zanon G., Weber A.D., Neto A.T., Mostardeiro C.P., Da Cruz I.B., Oliveira R.M., Ilha V., Dalcol I.I., Morel A.F. Structure-activity relationship of benzophenanthridine alkaloids from Zanthoxylum rhoifolium having antimicrobial activity. PLoS ONE. 2014;9:e97000. doi: 10.1371/journal.pone.0097000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zuo G.Y., Li Y., Wang T., Han J., Wang G.C., Zhang Y.L., Pan W.D. Synergistic antibacterial and antibiotic effects of bisbenzylisoquinoline alkaloids on clinical isolates of methicillin-resistant S. aureus (MRSA) Molecules. 2018;16:9819–9826. doi: 10.3390/molecules16129819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miao F., Yang X.J., Zhou L., Hu H.J., Zheng F., Ding X.D., Sun D.M., Zhou C.D., Sun W. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat. Prod. Res. 2011;25:863–875. doi: 10.1080/14786419.2010.482055. [DOI] [PubMed] [Google Scholar]
- 137.Guang Ma W., Fukushi Y., Tahara S. Fungitoxic alkaloids from Hokkaido corydalis species. Fitoterapia. 1999;70:258–265. doi: 10.1016/s0367-326x(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 138.Phatchana R., Yenjai C. Cytotoxic coumarins from Toddalia asiatica. Planta Med. 2014;80:719–722. doi: 10.1055/s-0034-1368568. [DOI] [PubMed] [Google Scholar]
- 139.Hu J., Shi X., Chen J., Mao X., Zhu L., Yu L., Shi J. Alkaloids from Toddalia asiatica and their cytotoxic, antimicrobial and antifungal activities. Food Chem. 2014;148:437–444. doi: 10.1016/j.foodchem.2012.12.058. [DOI] [PubMed] [Google Scholar]
- 140.Gu J.Q., Graf T.N., Lee D., Chai H.B., Mi Q., Kardono L.B., Setyowati F.M., Ismail R., Riswan S., Farnsworth N.R., et al. Cytotoxic and antimicrobial constituents of the bark of Diospyros maritima collected in two geographical locations in Indonesia. J. Nat. Prod. 2004;67:1156–1161. doi: 10.1021/np040027m. [DOI] [PubMed] [Google Scholar]
- 141.Čerňáková M., Košťálová D. Antimicrobial activity of berberine—A constituent of Mahonia aquifolium. Folia Microbiol. 2002;47:375–378. doi: 10.1007/BF02818693. [DOI] [PubMed] [Google Scholar]
- 142.Villinski J., Dumas E., Chai H.B., Pezzuto J., Angerhofer C., Gafner S. Antibacterial activity and alkaloid content of Berberis thunbergii, Berberis vulgaris and Hydrastis canadensis. Pharm. Biol. 2003;41:551–557. doi: 10.1080/13880200390500768. [DOI] [Google Scholar]
- 143.Slobodníková L., KoSt’álová D., Labudová D., Kotulová D., Kettmann V. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother. Res. 2004;18:674–676. doi: 10.1002/ptr.1517. [DOI] [PubMed] [Google Scholar]
- 144.Yu H.H., Kim K.J., Cha J.D., Kim H.K., Lee Y.E., Choi N.Y., You Y.O. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant S. aureus. J. Med. Food. 2005;8:454–461. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- 145.Chomnawang M.T., Trinapakul C., Gritsanapan W. In vitro antigonococcal activity of Coscinium fenestratum stem extract. J. Ethnopharmacol. 2009;122:445–449. doi: 10.1016/j.jep.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 146.Wojtyczka R.D., Dziedzic A., Kępa M., Kubina R., Kabała-Dzik A., Mularz T., Idzik D. Berberine enhances the antibacterial activity of selected antibiotics against coagulase-negative Staphylococcus strains in vitro. Molecules. 2014;19:6583–6596. doi: 10.3390/molecules19056583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Blanco P., Hernando-Amado S., Reales-Calderon J.A., Corona F., Lira F., Alcalde-Rico M., Bernardini A., Sanchez M.B., Martinez J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms. 2016;4:14. doi: 10.3390/microorganisms4010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.da Silva A.R., de Andrade Neto J.B., da Silva C.R., de Sousa Campos R., Silva R.A.C., Freitas D.D., do Nascimento F.B.S.A., de Andrade L.N.D., Sampaio L.S., Grangeiro T.B., et al. Berberine antifungal activity in fluconazole-resistant pathogenic yeasts: Action mechanism evaluated by flow cytometry and biofilm growth inhibition in Candida spp. Antimicrob. Agents Chemother. 2016;60:3551–3557. doi: 10.1128/AAC.01846-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Iwazaki R.S., Endo E.H., Ueda-Nakamura T., Nakamura C.V., Garcia L.B., Dias Filho B.P. In vitro antifungal activity of the berberine and its synergism with fluconazole. Antonie Van Leeuwenhoek. 2010;97:201. doi: 10.1007/s10482-009-9394-8. [DOI] [PubMed] [Google Scholar]
- 150.Gentry E.J., Hanuman B., Keshavarz-Shokri J.A., Morton M.D., David V.V., Hanumaiah T., Lester A., Mitscher R.S., Debbi H., William B. Antitubercular natural products: Berberine from the roots of commercial Hydrastis c anadensis powder. Isolation of inactive 8-oxotetrahydrothalifendine, canadine, β-hydrastine, and two new quinic acid esters, hycandinic acid esters-1 and-2. J. Nat. Prod. 1998;61:1187–1193. doi: 10.1021/np9701889. [DOI] [PubMed] [Google Scholar]
- 151.Wang L.J., Ye X.L., Li X.G., Sun Q.L., Yu G., Cao X.G., Liang Y.T., Zhou J.Z. Synthesis and antimicrobial activity of 3-alkoxyjatrorrhizine derivatives. Planta Med. 2008;74:290–292. doi: 10.1055/s-2008-1034312. [DOI] [PubMed] [Google Scholar]
- 152.Volleková A., Košt’álová D., Kettmann V., Tóth J. Antifungal activity of Mahonia aquifolium extract and its major protoberberine alkaloids. Phytother. Res. 2003;17:834–837. doi: 10.1002/ptr.1256. [DOI] [PubMed] [Google Scholar]
- 153.Basha S.A., Mishra R.K., Jha R.N., Pandey V.B., Singh U.P. Effect of berberine and (±)-bicuculline isolated from Corydalis chaerophylla on spore germination of some fungi. Folia Microbiol. 2002;47:161–165. doi: 10.1007/BF02817675. [DOI] [PubMed] [Google Scholar]
- 154.Semwal D.K., Badoni R., Semwal R., Kothiyal S.K., Singh G.J.P., Rawat U. The genus Stephania (Menispermaceae): Chemical and pharmacological perspectives. J. Ethnopharmacol. 2010;132:369–383. doi: 10.1016/j.jep.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 155.Shen J.W., Ruan Y., Ren W., Ma B.J., Wang X.L., Zheng C.F. Lycorine: A potential broad-spectrum agent against crop pathogenic fungi. J. Microbiol. Biotechnol. 2014;24:354–358. doi: 10.4014/jmb.1310.10063. [DOI] [PubMed] [Google Scholar]
- 156.Nair J.J., van Staden J. Antifungal constituents of the plant family Amaryllidaceae. Phytother. Res. 2018;32:976–984. doi: 10.1002/ptr.6049. [DOI] [PubMed] [Google Scholar]
- 157.Pettit G.R., Melody N., Herald D.L. Antineoplastic agents. synthesis of (+)-pancratistatin from (+)-narciclasine as relay1a. J. Org. Chem. 2001;66:2583–2587. doi: 10.1021/jo000710n. [DOI] [PubMed] [Google Scholar]
- 158.Liang C., Yang L., Shao Y., Zhu X., Zhao H., Chen B., Song W., Song X., Ding X., Sun R. Broad-spectrum antifungal activity of dichloromethane extract of Waltheria indica stems and isolated compounds. Ind. Crops Prod. 2019;142:111855. doi: 10.1016/j.indcrop.2019.111855. [DOI] [Google Scholar]
- 159.Zhao Z., He X., Han W., Chen X., Liu P., Zhao X., Wang X., Zhang L., Wu S., Zheng X. Genus Tetradium L.: A comprehensive review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2019;231:337–354. doi: 10.1016/j.jep.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 160.Pan X., Bligh S.A., Smith E. Quinolone alkaloids from Fructus euodiae show activity against methicillin-resistant Staphylococcus aureus. Phytother. Res. 2014;28:305–307. doi: 10.1002/ptr.4987. [DOI] [PubMed] [Google Scholar]
- 161.Adams M., Mahringer A., Kunert O., Fricker G., Efferth T., Bauer R. Cytotoxicity and p-glycoprotein modulating effects of quinolones and indoloquinazolines from the Chinese herb Evodia rutaecarpa. Planta Med. 2007;73:1554–1557. doi: 10.1055/s-2007-993743. [DOI] [PubMed] [Google Scholar]
- 162.Sripisut T., Ritthiwigrom T., Promgool T., Yossathera K., Deachathai S., Phakhodee W., Cheenpracha S., Laphookhieo S. Glycopentaphyllone: The first isolation of hydroperoxyquinolone from the fruits of Glycosmis pentaphylla. Phytochem. Lett. 2012;5:379–381. doi: 10.1016/j.phytol.2012.03.007. [DOI] [Google Scholar]
- 163.Suliman Mohamed M., Timan Idriss M., Khedr A.I., Abd AlGadir H., Takeshita S., Shah M.M., Ichinose Y., Maki T. Activity of Aristolochia bracteolata against Moraxella catarrhalis. Int. J. Bacteriol. 2014;2014:481686. doi: 10.1155/2014/481686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Angalaparameswari S., Saleem T.M., Alagusundaram M., Ramkanth S., Thiruvengadarajan V., Gnanaprakash K., Chetty C.M., Pratheesh G. Anti-microbial activity of aristolochic acid from root of Aristolochia bracteata Retz. Int. J. Biomed. Life Sci. 2012;8:2–4. [Google Scholar]
- 165.Hinou J., Demetzos C., Harvala C., Roussakis C. Cytotoxic and antimicrobial principles from the roots of Aristolochia longa. Int. J. Crude Drug Res. 1990;28:149–151. doi: 10.3109/13880209009082801. [DOI] [Google Scholar]
- 166.Lee Y.S., Han S.H., Lee S.H., Kim Y.G., Park C.B., Kang O.H., Keum J.H., Kim S.B., Mun S.H., Seo Y.S., et al. The mechanism of antibacterial activity of tetrandrine against Staphylococcus aureus. Foodborne Pathog. Dis. 2012;9:686–691. doi: 10.1089/fpd.2011.1119. [DOI] [PubMed] [Google Scholar]
- 167.Attaluri S., Bonala R.R., Yang I.Y., Lukin M.A., Wen Y., Grollman A.P., Moriya M., Iden C.R., Johnson F. DNA adducts of aristolochic acid II: Total synthesis and site-specific mutagenesis studies in mammalian cells. Nucleic Acids Res. 2010;38:339–352. doi: 10.1093/nar/gkp815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pfau W., Schmeiser H.H., Wiessler M. Aristolochic acid binds covalently to the exocyclic amino group of purine nucleotides in DNA. Carcinogenesis. 1990;11:313–319. doi: 10.1093/carcin/11.2.313. [DOI] [PubMed] [Google Scholar]
- 169.Zeng Y.B., Wei D.J., Dong W.H., Cai C.H., Yang D.L., Zhong H.M., Mei W.L., Dai H.F. Antimicrobial glycoalkaloids from the tubers of Stephania succifera. Arch. Pharmacal Res. 2017;40:429–434. doi: 10.1007/s12272-014-0467-5. [DOI] [PubMed] [Google Scholar]
- 170.Tuntiwachwuttikul P., Phansa P., Pootaeng-on Y., Taylor W.C. Chemical constituents of the roots of Piper sarmentosum. Chem. Pharm. Bull. 2006;54:149–151. doi: 10.1248/cpb.54.149. [DOI] [PubMed] [Google Scholar]
- 171.Shi Y.N., Liu F.F., Jacob M.R., Li X.C., Zhu H.T., Wang D., Cheng R.R., Yang C.R., Xu M., Zhang Y.J. Antifungal amide alkaloids from the aerial parts of Piper flaviflorum and Piper sarmentosum. Planta Med. 2017;83:143–150. doi: 10.1055/s-0042-109778. [DOI] [PubMed] [Google Scholar]
- 172.Lakshmanan G., Sivaraj C., Ammar A., Gopinath S., Saravanan K., Gunasekaran K., Murugesan K. Isolation and structural elucidation of allantoin a bioactive compound from Cleome viscosa L.: A combined experimental and computational investigation. Pharmacogn. J. 2019;11:1391–1400. doi: 10.5530/pj.2019.11.215. [DOI] [Google Scholar]
- 173.Atta-ur-Rahman Nasreen A., Akhtar F., Shekhani M.S., Clardy J., Parvez M., Choudhary M.I. Antifungal diterpenoid alkaloids from Delphinium denudatum. J. Nat. Prod. 1997;60:472–474. doi: 10.1021/np960663n. [DOI] [PubMed] [Google Scholar]
- 174.Li-Na Z.H.O.U., Xiao-Lei G.E., Ting-Ting D.O.N.G., Hui-Yuan G.A.O., Bo-Hang S.U.N. Antibacterial steroidal alkaloids from Holarrhena antidysenteriaca. Chin. J. Nat. Med. 2017;15:540–545. doi: 10.1016/S1875-5364(17)30080-8. [DOI] [PubMed] [Google Scholar]
- 175.Weinstein L.I., Albersheim P. Host-pathogen interactions: XXIII. The mechanism of the antibacterial action of glycinol, a pterocarpan phytoalexin synthesized by soybeans. Plant Physiol. 1983;72:557–563. doi: 10.1104/pp.72.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lewis K., Ausubel F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006;24:1504–1507. doi: 10.1038/nbt1206-1504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.