Abstract
Opioid use disorder (OUD) has been associated with the emergence of sleep disturbances. Although effective treatments for OUD exist, evidence suggests that these treatments also may be associated with sleep impairment. The extent to which these effects are an effect of OUD treatment or a result of chronic opioid use remains unknown. We investigated the acute effects of methadone, buprenorphine, and naltrexone on actigraphy-based sleep-like parameters in non-opioid-dependent male rhesus monkeys (Macaca mulatta, n=5). Subjects were fitted with actigraphy monitors attached to primate collars to measure sleep-like parameters. Actigraphy recordings were conducted under baseline conditions, or following acute injections of vehicle, methadone (0.03–1.0 mg/kg, i.m.), buprenorphine (0.01–1.0 mg/kg, i.m.), or naltrexone (0.03–1.0 mg/kg, i.m.) in the morning (4h after “lights on”) or in the evening (1.5h before “lights off”). Morning and evening treatments with methadone or buprenorphine significantly increased sleep latency and decreased sleep efficiency. The effects of buprenorphine on sleep-like measures resulted in a biphasic dose-response function, with the highest doses not disrupting actigraphy-based sleep. Buprenorphine induced a much more robust increase in sleep latency and decrease in sleep efficiency compared to methadone, particularly with evening administration, and detrimental effects of buprenorphine on sleep-like measures were observed up to 25.5h after drug injection. Treatment with naltrexone, on the other hand, significantly improved sleep-like measures, with evening treatments improving both sleep latency and sleep efficiency. The currently available pharmacotherapies for OUD significantly alter sleep-like parameters in non-opioid-dependent monkeys, and opioid-dependent mechanisms may play a significant role in sleep-wake regulation.
Keywords: actigraphy, opioids, nonhuman primates, sleep
1. Introduction
Opioid use disorder (OUD) is a chronic, relapsing disorder that currently represents a global public health crisis (Strang et al., 2020). According to the 2016 Global Burden of Disease study, 26.8 million people were estimated to be living with OUD worldwide (GBD 2016 Disease and Injury Incidence and Prevalence Collaborators, 2017). In the United States, which leads the world in opioid consumption, drug overdose fatalities exceeded 90,000 in the 12-month period ending in February 2021 (Ahmad et al., 2021), and over 70% are attributable to opioids (Mattson et al., 2021). Several treatments are available for OUD, including FDA-approved medication-assisted therapies (MAT) using the mu-opioid receptor agonist, methadone, the partial mu-opioid agonist, buprenorphine, or the opioid receptor antagonist naltrexone, all of which have been shown to facilitate recovery (Kampman and Jarvis, 2015). However, relapse rates remain high and adherence to treatment continues to be a challenge (Hoffman et al., 2019).
Recently, treatment with methadone and buprenorphine has been associated increasingly with the emergence of sleep disturbances (Le et al., 2019; Tripathi et al., 2020). Chronic use of opioids in general has been shown to induce sleep impairment (Cutrufello et al., 2020), and OUD patients on MAT medications often complain about their sleep quality (Dunn et al., 2018). Importantly, sleep disruption may alter the course of opioid addiction, with individuals having a history of sleep problems being more likely to relapse during and after treatment (Eacret et al., 2020; Fathi et al., 2020). Therefore, understanding the extent to which the impairing effects of MAT medications on sleep are a direct result of their action or an indirect effect of having a substance use disorder is extremely important.
The aim of the present study was to investigate the acute effects of methadone, buprenorphine, or naltrexone on actigraphy-based sleep-like parameters in adult naïve male rhesus monkeys. Previous studies from our laboratory have shown that experimenter-administered psychoactive drugs may have sleep-disrupting effects even when administered several hours before “lights off” (Berro et al., 2021). Therefore, to investigate the effect of time of administration on the sleep-related effects of these drugs, methadone, buprenorphine, and naltrexone were administered in the morning (4h after “lights on”) or in the evening (1.5h before “lights off”).
2. Material and Methods
2.1. Subjects
Five adult, non-opioid-dependent, male rhesus monkeys (Macaca mulatta) ranging from 11–18 kg were chosen as subjects for the studies. Subjects were housed individually with access to toys and a mirror in their cage, and had visual, auditory and olfactory contact with other monkeys throughout the duration of the study. A 12h light/12h dark cycle (lights on at 0600 h), at a temperature of 21±1°C, was maintained with water available ad libitum and monkey diet available once/day, supplemented by freshly cut fruit and forage (seeds and dry fruit). All subjects were weighed monthly. Prior to the study, subjects were fitted with collars (Primate Products). All of the procedures and animal maintenance were in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), with review and approval via the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
2.2. Drugs
(±)-Methadone HCl and (±)-Buprenorphine HCl were supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC, USA). Naltrexone HCl was purchased commercially (Sigma-Aldrich®, St. Louis, MO, USA). Methadone and naltrexone were dissolved in 0.9% sterile physiological saline, and buprenorphine was dissolved in sterile water. Doses were expressed as the salt form of the drug. All drugs were administered intramuscularly (i.m.) at a volume of 0.1 ml/kg.
2.3. Actigraphy-based sleep-like parameters
Actiwatch sensors (Mini Mitter, Bend, OR, USA) were used to assess nighttime activity, as described previously (Berro et al., 2021). Briefly, nighttime activity data generated the following sleep-like behavior parameters: sleep efficiency (i.e., the percentage of the 12 h dark phase – 1800h to 0600h – spent sleeping) and sleep latency (i.e., the number of minutes from “lights off” at 1800h until the first sleep bout). All parameters were calculated using the Actiware Sleep 3.4 software program (Mini-Mitter, Bend, OR, USA).
2.4. Study design
At the start of each drug condition associated with each experiment (see below), sleep-like behaviors were measured for 1 week to serve as the sleep-like baseline measures for the drug condition, and recordings continued throughout the duration of treatments. During Experiment 1, each subject received methadone (0.1–1.0 mg/kg, i.m.), buprenorphine (0.03–1.0 mg/kg, i.m.), or naltrexone (0.03–1.0 mg/kg, i.m.) at 1000 h (4h after “lights on”). Vehicle tests (saline for methadone and naltrexone, sterile water for buprenorphine) were included for each drug, so that each drug condition consisted of a 1-week baseline period, a vehicle test and drug doses. To minimize any potential influences of one treatment over another on sleep-like parameters, all doses within a drug condition were counterbalanced across each drug treatment. During Experiment 2, the same procedures were used, except subjects received an injection of methadone (0.03–0.3 mg/kg, i.m.), buprenorphine (0.01–1.0 mg/kg i.m.), or naltrexone (0.03–1.0 mg/kg, i.m.) at 1630 h (1.5h before “lights off”). Vehicle tests (saline for methadone and naltrexone, sterile water for buprenorphine) were included for each drug, so that each drug condition consisted of a 1-week baseline period, a vehicle test and drug doses. Following a within-subject design, every monkey received all doses of each drug. Within each experiment, each drug or vehicle condition was administered once for each animal. A 2-week drug-free baseline period was given between each experiment during which sleep-like measures were recorded. During each set of treatments, at least 2 days were given between each treatment. All subjects were housed in the same colony room, but individually housed. All experimental treatments were performed contemporaneously, and baseline sleep-like data were collected during the same week for all animals.
Studies investigating the antinociceptive and response rate-altering effects of methadone and buprenorphine in rhesus monkeys were our starting point for dose selection (Walker et al., 1995; Banks et al., 2010; Schwienteck et al., 2019). Once a dose of methadone or buprenorphine was determined to induce impairments in actigraphy-based sleep-like measures following morning treatments (i.e. increase in sleep latency with 1.0 mg/kg), it was expected that the same dose also would induce sleep impairment when given closer to “lights off”. Therefore, we chose to cap the evening dose of opioid agonists to one half-log dose below the dose that was determined to induce sleep impairment when given in the morning due to concerns over potential respiratory suppressant effects overnight, when animals were not under constant monitoring by an experimenter. For methadone, given that a significant effect was observed for both sleep latency and sleep efficiency with the dose of 0.3 mg/kg for evening treatments, we chose not to pursue further studies with higher doses due to concerns over the animals’ safety. For buprenorphine, once we reached a dose that had no effects on sleep measures (i.e. an antagonist dose), we did not pursue further studies with higher doses. Doses of naltrexone were chosen based on previous studies in rhesus monkeys, with the highest dose being selected based on its ability to block opioid self-administration (Rowlett et al., 1998; Maguire et al., 2020).
2.5. Data analysis
Table 1 shows individual-subject baseline actigraphy-based sleep-like parameters. As can be seen in Table 1, there was a high degree of variability between subjects regarding their normal, baseline sleep (i.e. subjects 205–2002 vs 98–003). Therefore, baseline data were averaged across the 7-day period immediately preceding each experiment and all drug/vehicle tests were normalized to baseline sleep-like parameters (i.e., sleep latency and efficiency) by computing the percentage of baseline (i.e. sleep data from treatment night / sleep data from averaged baseline night * 100). Percent sleep latency (i.e., the percent increase or decrease in time to first sleep bout) and efficiency (i.e., the percentage increase or decrease in percent time spent on sleep-like behavior during the dark phase) were each analyzed using separate one-way repeated-measures (RM) analysis of variance (ANOVA) with the within-subject factor of treatment (vehicle or methadone, buprenorphine, naltrexone doses). Because vehicle testing allowed for the control over the effects of an injection on baseline sleep parameters, which is particularly important for evening treatments given that an injection alone might affect measures such as sleep latency, drug treatment tests were compared to vehicle. Because of buprenorphine’s unique and long-lasting effects (see Results section), we also conducted a time-course analysis of the effects of buprenorphine on actigraphy-based sleep-like parameters. Percent sleep latency and efficiency were each analyzed using a separate two-way RM ANOVA with the within-subject factors of treatment (vehicle, buprenorphine doses) and time (1.5, 8, 25.5 or 32h before “lights off). For all RM ANOVAs, corrections for violations of sphericity were performed using the Geisser-Greenhouse method. For multiple comparisons, Bonferroni tests were used to compare each dose within a drug condition to the baseline sleep-like parameters. All graphical data presentations and statistical tests were performed using GraphPad Prism 9 (GraphPad Software). Family-wise error was constrained to an alpha value of p≤0.05, except for tests using a correction procedure.
Table 1.
Individual-subject baseline actigraphy-based sleep parameters
| Subject | Sleep Latency (min) | Sleep Efficiency (%) |
|---|---|---|
| 205–2002 | 13.5 ± 1.3 | 84.5 ± 2.9 |
| 98–003 | 30.5 ± 2.1 | 66.5 ± 1.5 |
| 164–2002 | 24.9 ± 4.6 | 82.3 ± 1.1 |
| 184–2007 | 7.1 ± 1.6 | 68.5 ± 1.1 |
| RQ-6133 | 10.9 ± 2.4 | 85.7 ± 1.5 |
| Mean ± SEM | 17.4 ± 4.4 | 80.3 ± 3.6 |
Individual data are expressed as mean ± SEM for an average of baseline periods (7 days each) across the experimental protocol (6 blocks of 7 days total).
3. Results
Table 1 shows individual-subject baseline actigraphy-based sleep-like parameters. No significant differences were observed between the 7-day baseline periods preceding each experimental treatment for each monkey. Vehicle averages were not significantly different from average baseline values for any measure.
3.1. Effects of acute morning treatment with methadone, buprenorphine or naltrexone on actigraphy-based sleep-like parameters in male rhesus monkeys
The percent baseline sleep latency (panel A) and efficiency (panel B) for methadone, buprenorphine, and naltrexone on the night of morning treatments are illustrated in Figure 1. One-way RM ANOVA showed a main effect for methadone on sleep latency (F [3, 12] = 9.134; p=0.002). At the highest dose tested (1.0 mg/kg), methadone significantly increased percent of time to sleep onset compared to vehicle (Bonferroni t-test, p=0.0025). However, morning treatment with methadone had no effects on sleep efficiency (F [3, 12] = 1.472; p=0.2716). A significant effect was also observed for buprenorphine for both percent sleep latency (F [4, 16] = 3.571; p=0.029) and percent sleep efficiency (F [4, 16] = 4.603; p=0.01). Specifically, the dose of 0.3 mg/kg significantly increased percent of time to sleep onset (Bonferroni t-tests, p=0.037) and decreased percent sleep efficiency (Bonferroni t-tests, p=0.0027) compared to vehicle. Interestingly, the effects of buprenorphine on actigraphy-based sleep-like parameters resulted in a biphasic function, with the highest dose not significantly disrupting any sleep-like measure (Bonferroni t-test, p>0.05). Finally, a significant effect was observed for naltrexone for percent sleep latency (F [4, 16] = 5.896; p=0.004), but not percent sleep efficiency (F [4, 16] = 0.7347; p=0.58). At the highest dose tested (1.0 mg/kg), naltrexone significantly decreased percent of time to sleep onset compared with vehicle (Bonferroni t-tests, p=0.009).
Figure 1.
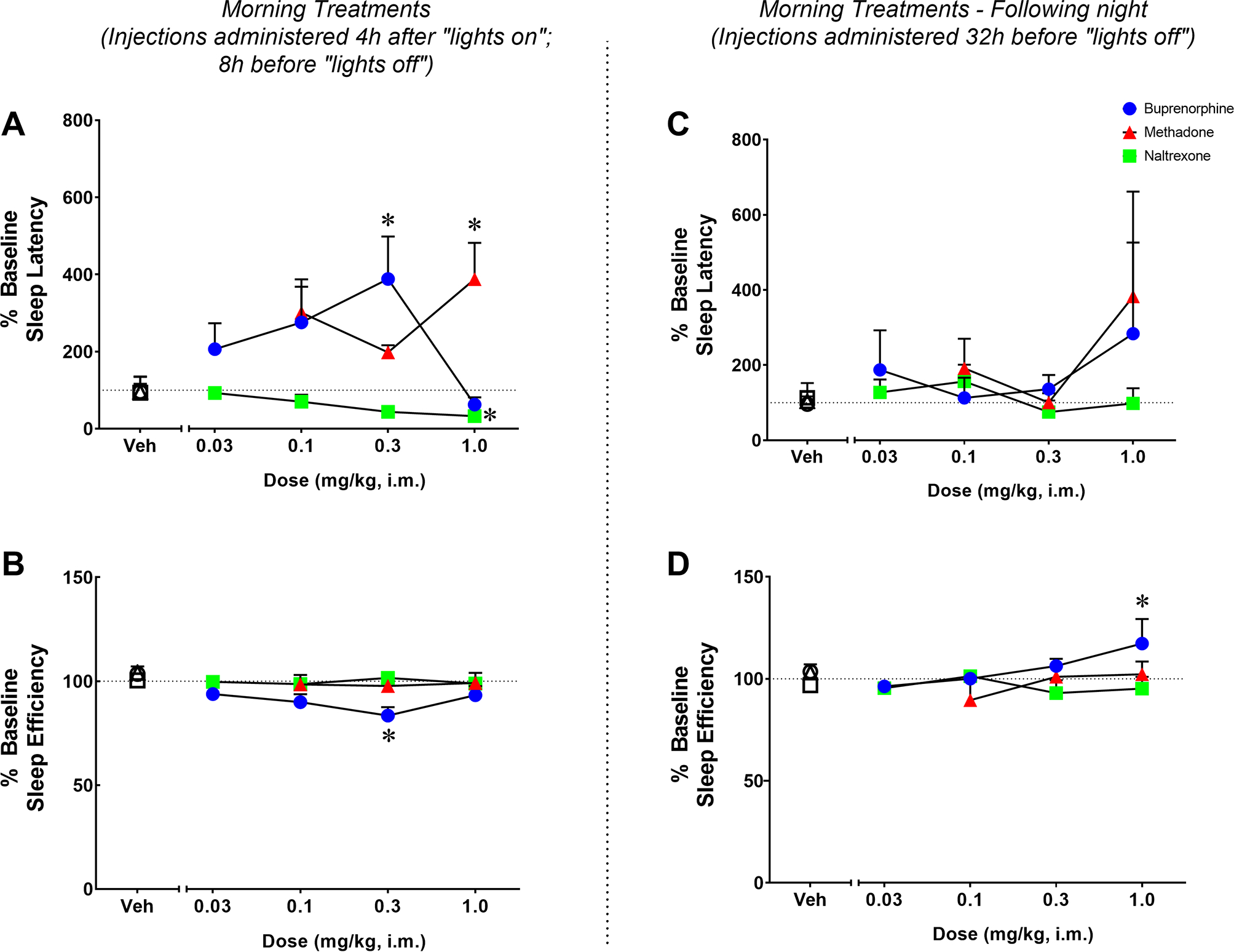
Effects of acute morning treatment with methadone, buprenorphine or naltrexone on actigraphy-based sleep parameters in naïve monkeys. Sleep latency (A) and sleep efficiency (B) in the nights after morning (10:00h) administration of vehicle (Veh), methadone (0.1–1.0 mg/kg, i.m.), buprenorphine (0.03–1.0 mg/kg, i.m.) or naltrexone (0.03–1.0 mg/kg, i.m.) or during the following evening (right panel, C and D) in male rhesus monkeys (N=5). Actigraphy-based sleep-like measures are presented as normalized data (percentage of baseline) averaged for the 5 subjects, representing mean ± SEM. Actigraphy-based sleep parameters were evaluated over the 12-hour dark phase (1800 h to 0600 h). Dotted lines represent baseline sleep parameters (100%). *p<0.05 compared to Veh (Bonferroni t-tests, p<0.05).
The percent baseline sleep latency (panel C) and efficiency (panel D) for methadone, buprenorphine, and naltrexone during the following night (32 h-post injection) are illustrated in Figure 1. During the following night, neither methadone (percent sleep latency: F [3, 12] = 1.283; p=0.3249; percent sleep efficiency: F [3, 12] = 1.157; p=0.3662) nor naltrexone (percent sleep latency: F [4,16] = 1.560; p=0.2329; percent sleep efficiency: F [4, 16] = 0.9438; p=0.4641) had any impact on actigraphy-based sleep-like parameters. A main effect was observed for buprenorphine on percent sleep efficiency (F [4, 16] = 3.062; p=0.047), but not percent sleep latency (F [4, 16] = 0.5214; p=0.7214), during the following night. At the dose of 1.0 mg/kg, buprenorphine significantly increased percent sleep efficiency compared to vehicle (Bonferroni t-tests, p=0.027).
3.2. Effects of acute evening treatment with methadone, buprenorphine or naltrexone on actigraphy-based sleep-like parameters in male rhesus monkeys
The percent baseline sleep latency (panel A) and efficiency (panel B) for methadone, buprenorphine, and naltrexone following evening treatment administration are illustrated in Figure 2. One-way RM ANOVA showed an effect for methadone on both percent sleep latency (F [3, 12] = 6.518; p=0.007) and percent sleep efficiency (F [3, 12] = 19.96; p<0.0001). Specifically, the dose of 0.3 mg/kg significantly increased percent of time to sleep onset (Bonferroni t-tests, p=0.0083) and decreased percent sleep efficiency (Bonferroni t-tests, p=0.0004) compared to vehicle. A significant effect was also observed for buprenorphine on percent sleep latency (F [4, 16] = 3.391; p=0.034) and percent sleep efficiency (F [4, 16] = 5.175; p=0.0072). Specifically, the doses of 0.03 and 0.1 mg/kg significantly increased percent of time to sleep onset (Bonferroni t-tests, p=0.047 and p=0.031, respectively) and decreased percent sleep efficiency (Bonferroni t-tests, p=0.036 and p=0.002, respectively) compared to vehicle. Similar to the morning treatments, the effects of buprenorphine on actigraphy-based sleep-like parameters resulted in a biphasic function, with the highest doses not significantly disrupting either sleep-like parameter. Finally, naltrexone also had a significant effect on percent sleep latency (F [4, 16] = 9.036; p=0.0005) and percent sleep efficiency (F [4, 16] = 4.946; p=0.0087). Naltrexone significantly decreased percent of time to sleep onset compared to vehicle at the doses of 0.1, 0.3 and 1.0 mg/kg (Bonferroni t-tests, p=0.008, p=0.001 and p=0.001, respectively) and increased percent sleep efficiency compared with vehicle at the doses of 0.3 and 1.0 mg/kg (Bonferroni t-tests, p=0.049 and p=0.025, respectively).
Figure 2.
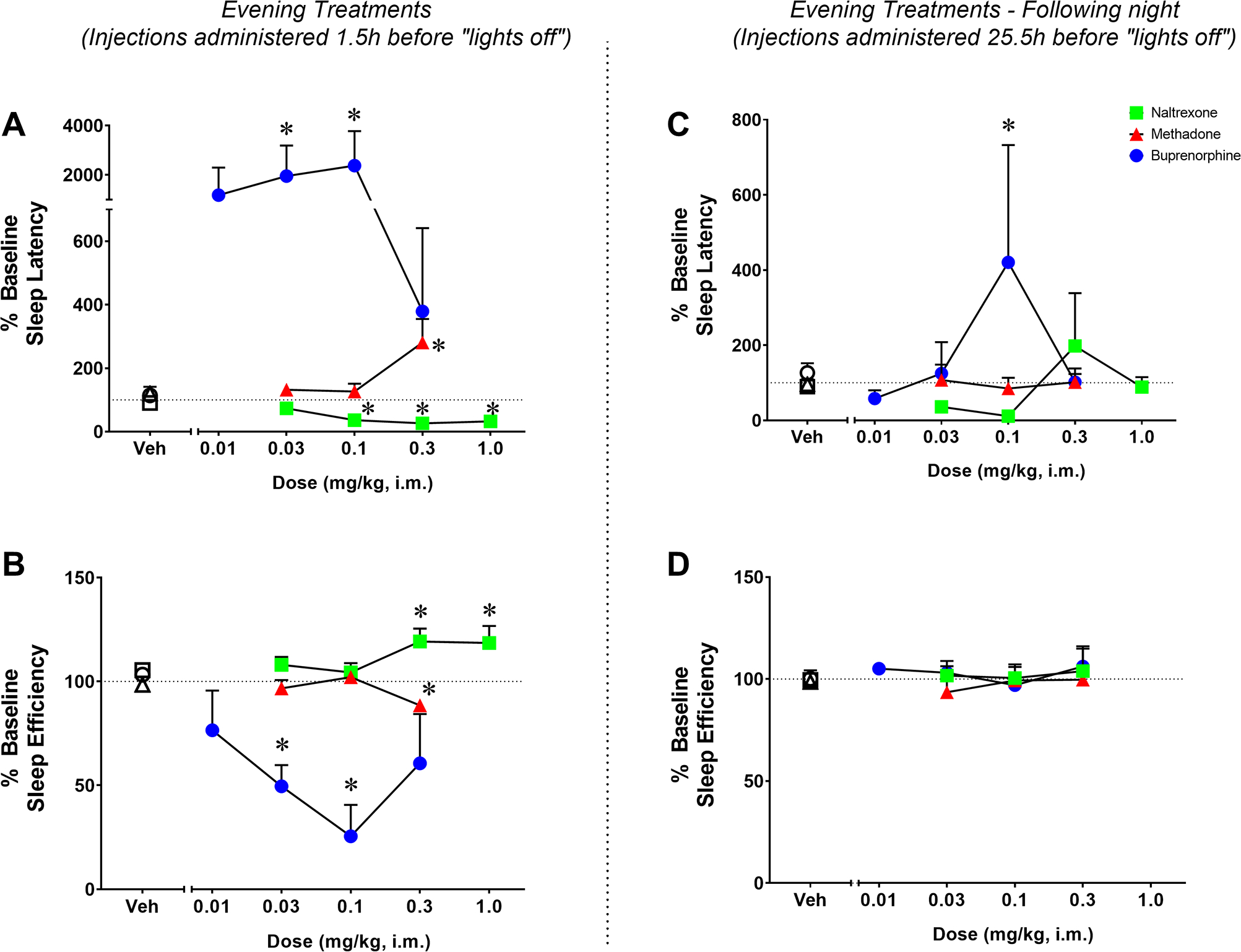
Effects of acute evening treatment with methadone, buprenorphine or naltrexone on actigraphy-based sleep parameters in naïve monkeys. Sleep latency (A) and sleep efficiency (B) in the nights after evening (16:30h) administration of vehicle (Veh), methadone (0.03–0.3 mg/kg, i.m.), buprenorphine (0.01–0.3 mg/kg, i.m.) or naltrexone (0.03–1.0 mg/kg, i.m.) or during the following evening (right panel, C and D) in male rhesus monkeys (N=5). Actigraphy-based sleep-like measures are presented as normalized data (percentage of baseline) averaged for the 5 subjects, representing mean ± SEM. Actigraphy-based sleep parameters were evaluated over the 12-hour dark phase (1800 h to 0600 h). Dotted lines represent baseline sleep parameters (100%). *p<0.05 compared to Veh (Bonferroni t-tests, p<0.05).
The percent sleep latency (panel C) and efficiency (panel D) for methadone, buprenorphine, and naltrexone during the following night (25.5 h-post drug administration) are illustrated in Figure 2. Neither methadone (percent sleep latency: F [3, 12] = 0.1949; p=0.8978; percent sleep efficiency: F [3, 12] = 0.8018; p=0.5165) nor naltrexone (percent sleep latency: F [4, 16] = 1.374; p=0.2869; percent sleep efficiency: F [4, 16] = 0.05009; p=0.9948) had significant effects on actigraphy-based sleep-like parameters during the following night. Buprenorphine dose-dependently increased percent of time to sleep onset (F [4, 16] = 5.130; p=0.0075), but not percent sleep efficiency (F [4, 16] = 0.3469; p=0.8423), during the following night. Specifically, the 0.1 mg/kg dose of buprenorphine significantly increased percent of time to sleep onset compared to vehicle (Bonferroni t-tests, p=0.017).
3.3. Time-course analysis of buprenorphine’s effects on actigraphy-based sleep-like parameters
Because of buprenorphine’s long half-life (Kelly et al., 2014), unique receptor binding profile (Lewis, 1985) and long-lasting effects that were observed even during the following night after morning and evening treatments, we also conducted a time-course analysis of the effects of buprenorphine on actigraphy-based sleep-like parameters (Figure 3). The doses of 0.01 and 1.0 mg/kg were excluded from this analysis due to missing data-points. Two-way RM ANOVAs with treatment (vehicle vs buprenorphine) and injection time (1.5, 8, 25.5 or 32h before “lights off”) showed a significant interaction between the two factors for both sleep latency (F [9, 36] = 2.01; p<0.05) and sleep efficiency (F [9, 36] = 5.914; p<0.0001). Detrimental effects of buprenorphine on actigraphy-based sleep-like measures were observed up to 25.5h after drug injection (significant increase in sleep latency at the dose of 0.1 mg/kg compared to vehicle, Bonferroni t-test, p<0.05).
Figure 3.
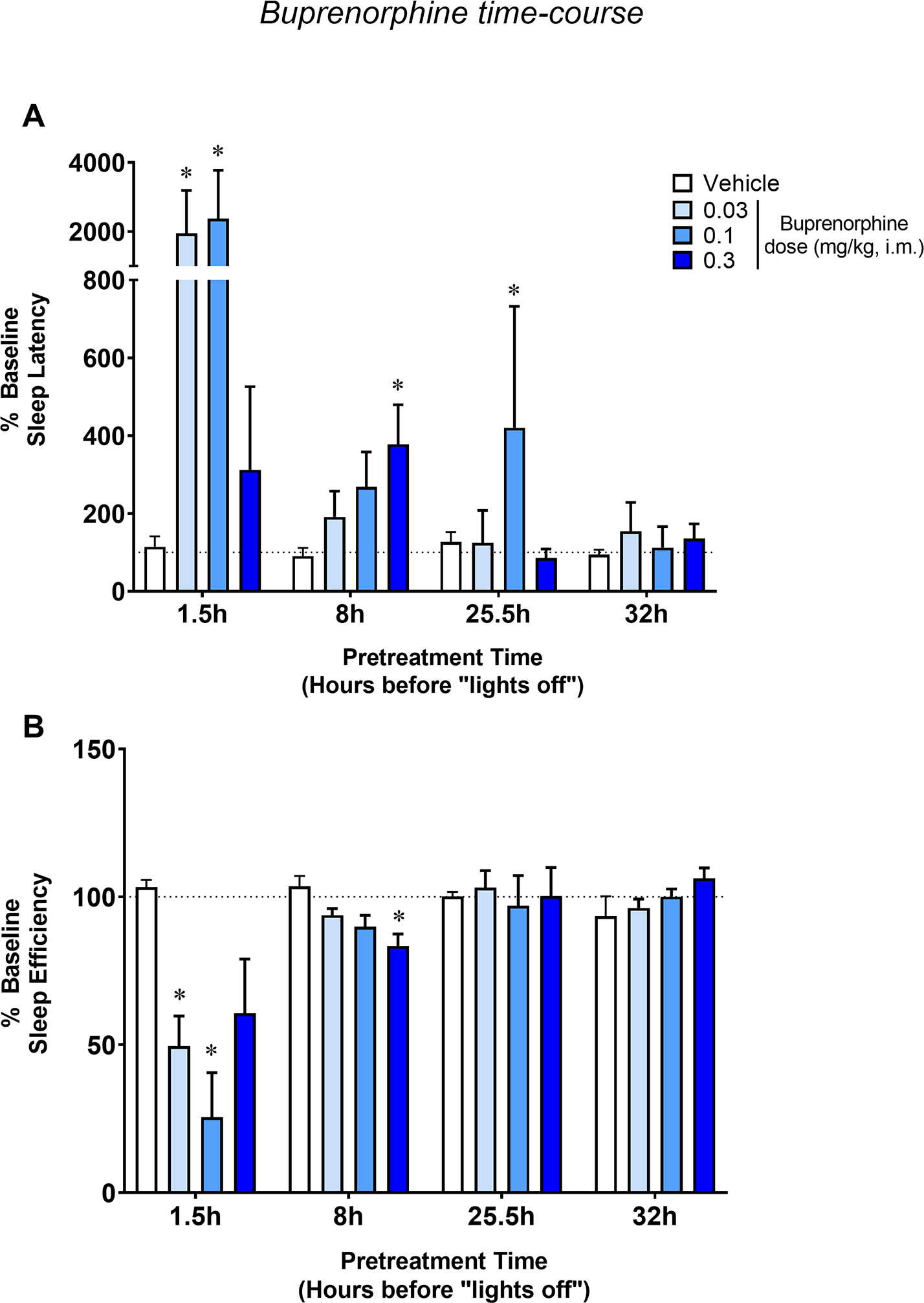
Time-course of the effects of buprenorphine on actigraphy-based sleep parameters in naïve monkeys. Sleep latency (A) and sleep efficiency (B) as a function of pretreatment time (hours before “lights off”) for vehicle or buprenorphine (0.03–0.3 mg/kg, i.m.) treatments in male rhesus monkeys (N=5). Actigraphy-based sleep-like measures are presented as normalized data (percentage of baseline) averaged for the 5 subjects, representing mean ± SEM. Actigraphy-based sleep parameters were evaluated over the 12-hour dark phase (1800 h to 0600 h). Dotted lines represent baseline sleep parameters (100%). *p<0.05 compared to Veh (Bonferroni t-tests, p<0.05).
4. Discussion
Chronic use of opioids is associated with sleep disturbances (Cutrufello et al., 2020), and OUD patients treated with methadone or buprenorphine often report sleep impairment (Dunn et al., 2018). A study by Peles et al. (2011) showed that while methadone maintenance does not alter sleep over a 12-month period, OUD patients in the study showed worse sleep parameters at baseline compared to healthy human volunteers (Boulos et al., 2019). In addition, a study by Frers and colleagues (2021) investigating sleep parameters in individuals with a history of chronic pain found that current or past OUD treatment with methadone or buprenorphine was associated with worse subject-rated sleep outcomes compared to chronic pain patients who were opioid-naïve. However, it is important to point out that chronic pain itself is associated with poor sleep quality (Frohnhofen et al., 2018). Therefore, these studies not only corroborate our findings, but also emphasize the importance of investigating the effects of opioid drugs under controlled laboratory conditions, such as in the present study, to rule out the influence of other factors that also can affect sleep (such as pain, chronic opioid exposure, and other health problems associated with chronic opioid use). In this context, the extent to which sleep impairment is due to the direct effect of the medications used for OUD treatment vs. an indirect effect of chronic drug use is unknown. In the present study, we show that acute administration of methadone and buprenorphine induced marked impairment in actigraphy-based sleep-like measures in non-opioid-dependent male rhesus monkeys, even when the drug treatments were administered in the morning. These findings indicate an effect of methadone and buprenorphine that may contribute to sleep impairment in OUD patients undergoing agonist replacement therapy.
Previous studies reported that acute administration of methadone in cats (De Andrés and Caballero, 1989) decreased total sleep time by suppressing both rapid eye movement (REM) and non-REM (NREM) sleep. Similarly, acute buprenorphine administration also disrupts sleep in rats, decreasing total sleep time and increasing latency to fall asleep (Gauthier et al., 2011). Our study extends these findings by comparing the major OUD medications in the same animals, and demonstrates that acute treatment with methadone and buprenorphine also impairs actigraphy-based sleep-like measures in a highly translational animal species that shows a striking similarity in sleep architecture and wake-sleep patterns with humans (Hsieh et al., 2008). Of note, the acute disrupting effects of buprenorphine on actigraphy-based sleep-like parameters in monkeys were more robust compared to methadone following evening treatments, and detrimental effects of buprenorphine on sleep-like measures were observed up to 25.5h after drug injection. Buprenorphine has a longer half-life compared to methadone (Ferrari et al., 2005; Kelly et al., 2014), which could explain its long-lasting effects. Furthermore, the pharmacological differences between methadone and buprenorphine could influence their effects on sleep-like measures. Specifically, methadone is a full agonist at the μ-opioid receptor, while buprenorphine is a partial μ-opioid receptor agonist and κ-opioid receptor antagonist (Ferrari et al., 2005; Lewis, 1985). While studies suggest that opioid-induced sleep impairment is primarily mediated by μ-opioid receptors, other receptor subtypes also have been implicated in opioid-induced sleep-wake regulation, including κ-opioid receptors (Eacret et al., 2020). Further studies are warranted to determine the specific role of opioid receptor subtypes in the effects of opioid drugs or sleep. Another difference between buprenorphine and methadone in the present study is the biphasic nature of the buprenorphine dose-response function (i.e., lack of effects at the highest buprenorphine dose tested) compared with the largely monotonic methadone dose-response functions. Biphasic dose-response functions for other effects of buprenorphine have been well-documented (e.g., antinociception; Walker et al., 1995) and may contribute to the overall low toxicity of this opioid partial agonist.
Surprisingly, we show in the present study that treatment with the opioid antagonist, naltrexone significantly improved actigraphy-based sleep-like measures in non-opioid-dependent male rhesus monkeys. The sleep-like promoting effects of naltrexone were most prominent when the drug was administered closer to “lights off”, although morning treatment with naltrexone also improved sleep latency. Opioids can affect the signaling mechanisms of several other neurotransmitters and neuromodulators involved in sleep-wake regulation, with wake-promoting systems being generally activated, and sleep-promoting systems inhibited, via activation of μ-opioid receptors (for review, see Eacret et al., 2020). Therefore, μ-opioid receptor antagonists might be expected to promote sleep under certain circumstances (e.g., if the antagonist blocked endogenous opioids at the μ receptor). Importantly, in a preliminary study investigating the effects of naltrexone on polysomnography-based sleep parameters in six healthy human volunteers, Sramek et al. (2014) showed that while total sleep time and sleep latency remained unchanged following naltrexone treatment compared to placebo, nighttime treatment with naltrexone altered sleep architecture. The authors report a significant increase in REM sleep latency and decrease in time spent in REM sleep (Sramek et al., 2014). These preliminary findings suggest the possibility of subclinical sleep disturbance associated with naltrexone use, and indicate that the increase in sleep efficiency and decrease in sleep latency observed in the present study may happen at the expense of changes in sleep architecture, as seen for other sleep aids, such as benzodiazepine-type drugs (Parrino and Terzano, 1996).
Few studies have investigated sleep in OUD patients maintained on naltrexone, with one study showing that difficulty sleeping was common among individuals dropping out of oral naltrexone treatment for OUD (Carroll et al., 2018). Studies also report that perioperative sedation management in patients receiving long-acting naltrexone can be a challenge (Petri and Richards, 2020). Yet another study showed that naltrexone induction may be associated with sleep impairment early in treatment in newly abstinent opioid-dependent individuals (Mysels et al., 2010). Importantly, many of these effects may be a result of opioid abstinence, which has been associated with sleep impairment (Cutrufello et al., 2020).
More recently, Soin and colleagues (2021) reviewed evidence suggesting that treatment with low-dose naltrexone in patients with chronic pain was positively associated with symptom relief, including pain and sleep disturbances. However, sleep impairment also is common among individuals with chronic pain (Frohnhofen et al., 2018), and sleep improvement may have been an indirect effect of chronic pain management. Therefore, further studies are needed to directly investigate the effects of naltrexone on sleep in OUD patients undergoing treatment, particularly the effects of sustained-release naltrexone on sleep architecture.
A final important consideration is the relevance of the dose ranges for the 3 opioid drugs that altered actigraphy-based sleep-like measures to doses typically used by human patients. Although differences in species and routes of administration clearly should be taken into account, in general it appears the dose ranges where we observed effects are relatively higher than those used to treat pain, and perhaps closer to the doses used to treat OUD. For example, recommended doses of buprenorphine used to treat OUD are 2–16 mg/70 kg (~0.03–0.23 mg/kg), which are comparable to our range of 0.1–1.0 mg/kg. However, it should be noted that the most commonly-used single dose formulations for buprenorphine (e.g., Suboxone®) use the sublingual route, and given species differences in body mass as well as our use of the i.m. route, comparisons of doses should be made with some caution. Regarding methadone, the initial recommended dose range is 20 or 30 mg/70 kg (~0.3, 0.4 mg/kg) which is similar to the doses tested in the present study (note that recommended analgesic doses for methadone are 10-fold lower). Again, species differences in exposure levels and route of administration (oral vs. i.m.) must be taken into consideration. However, this observation is supported indirectly by comparisons of our dose ranges to previous research on antinociception in rhesus monkeys, in which doses for buprenorphine and methadone, as well as naltrexone antagonism of mu opioid effects, are generally lower than tested here (Walker et al., 1995; Banks et al., 2010; Cremeans et al., 2012; Cornelissen et al., 2018).
In summary, our findings show that acute administration of methadone or buprenorphine induced marked impairment in actigraphy-based sleep-like measures in rhesus monkeys, even when the drugs were administered in the morning, while naltrexone significantly improved sleep-like measures. These findings are in agreement with a study showing that insomnia scores were significantly lower in OUD patients treated with naltrexone compared to buprenorphine (Latif et al., 2018). It is important to note that a limitation of the present study is the investigation of acute treatments only. Therefore, future studies building upon our data are needed to investigate the acute and chronic effects of methadone, buprenorphine and naltrexone on sleep in opioid-dependent animals. Another limitation of our study is the use of actigraphy-based sleep-like measures (compared to other more direct measures, such as EEG-based sleep). While no studies to date have validated the use of actigraphy to investigate sleep in nonhuman primates using EEG-based sleep assessments, recent data from our laboratory show a close correspondence between actigraphy-based measures of sleep latency and efficiency and corresponding measures obtained from EEG-based telemetry studies (Berro et al., 2021, 2022). Furthermore, studies have validated the use of actigraphy for sleep studies in humans, showing a high agreement with polysomnography-based sleep (de Souza et al., 2003; Chinoy et al., 2021).
Nevertheless, the present study indicates that currently available pharmacotherapies for OUD significantly affect actigraphy-based sleep-like measures in non-opioid-dependent monkeys, and that opioid mechanisms play a significant role in sleep-wake regulation. Because sleep disruption may be a risk factor for relapse in OUD (Eacret et al., 2020; Fathi et al., 2020), sleep evaluations potentially would be important to include during OUD treatment, particularly for patients undergoing methadone and buprenorphine MAT. Considering that chronic use of opioids has been associated with sleep impairment (Cutrufello et al., 2020), further studies are needed to understand the interaction between opioid agonist-induced sleep impairment and sleep disruption associated with chronic opioid use in OUD patients.
Acknowledgments
The authors thank the Veterinary staff from the UMMC Center for Comparative Research for their exceptional care of our animals.
Footnotes
Declarations of Interest: None.
References
- Ahmad FB, Rossen LM, Sutton P (2021). Provisional drug overdose death counts. National Center for Health Statistics. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm [Google Scholar]
- Banks ML, Rice KC, Negus SS (2010). Antinociceptive interactions between Mu-opioid receptor agonists and the serotonin uptake inhibitor clomipramine in rhesus monkeys: role of Mu agonist efficacy. The Journal of Pharmacology and Experimental Therapeutics, 335, 497–505. doi: 10.1124/jpet.110.169276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berro LF, Moreira-Junior EDC, Rowlett JK (2021). The dual orexin receptor antagonist almorexant blocks the sleep-disrupting and daytime stimulant effects of methamphetamine in rhesus monkeys. Drug and Alcohol Dependence, 227, 108930. doi: 10.1016/j.drugalcdep.2021.108930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berro LF, Overton JS, Rowlett JK (2022). Methamphetamine-induced sleep impairments and subsequent slow-wave and REM sleep rebound in male rhesus monkeys. Frontiers in Neuroscience, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos MI, Jairam T, Kendzerska T, Im J, Mekhael A, Murray BJ (2019). Normal polysomnography parameters in healthy adults: a systematic review and meta-analysis. Lancet Respiratory Medicine, 7, 533–543. doi: 10.1016/S2213-2600(19)30057-8. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Frankforter TL, Yip SW, Kiluk BD, DeVito EE, Sofuoglu M (2018). Accounting for the uncounted: Physical and affective distress in individuals dropping out of oral naltrexone treatment for opioid use disorder. Drug and Alcohol Dependence, 192, 264–270. doi: 10.1016/j.drugalcdep.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinoy ED, Cuellar JA, Huwa KE, Jameson JT, Watson CH, Bessman SC, Hirsch DA, Cooper AD, Drummond SPA, Markwald RR (2021). Performance of seven consumer sleep-tracking devices compared with polysomnography. Sleep, 44:zsaa291. doi: 10.1093/sleep/zsaa291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen JC, Obeng S, Rice KC, Zhang Y, Negus SS, Banks ML (2018). Application of receptor theory to the design and use of fixed-proportion mu-opioid agonist and antagonist mixtures in rhesus monkeys. The Journal of Pharmacology and Experimental Therapeutics, 365, 37–47. doi: 10.1124/jpet.117.246439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremeans CM, Gruley E, Kyle DJ, Ko M-C. (2012). Roles of mu-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. The Journal of Pharmacology and Experimental Therapeutics, 343, 72–81. doi: 10.1124/jpet.112.194308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrufello NJ, Ianus VD, Rowley JA (2020). Opioids and sleep. Current Opinion in Pulmonary Medicine, 26, 634–641. doi: 10.1097/MCP.0000000000000733. [DOI] [PubMed] [Google Scholar]
- De Andrés I, Caballero A (1989). Chronic morphine administration in cats: effects on sleep and EEG. Pharmacology, Biochemistry and Behavior, 32, 519–26. doi: 10.1016/0091-3057(89)90191-3. [DOI] [PubMed] [Google Scholar]
- de Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM (2003). Further validation of actigraphy for sleep studies. Sleep, 26, 81–5. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- Dunn KE, Finan PH, Andrew Tompkins D, Strain EC (2018). Frequency and correlates of sleep disturbance in methadone and buprenorphine-maintained patients. Addictive Behavior, 76, 8–14. doi: 10.1016/j.addbeh.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacret D, Veasey SC, Blendy JA (2020). Bidirectional Relationship between Opioids and Disrupted Sleep: Putative Mechanisms. Molecular Pharmacology, 98, 445–453. doi: 10.1124/mol.119.119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi HR, Yoonessi A, Khatibi A, Rezaeitalab F, Rezaei-Ardani A (2020). Crosstalk between Sleep Disturbance and Opioid Use Disorder: A Narrative Review. Addiction & Health, 12, 140–158. doi: 10.22122/ahj.v12i2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A, Coccia CP, Bertolini A, Sternieri E (2004). Methadone--metabolism, pharmacokinetics and interactions. Pharmacological Research, 50, 551–9. doi: 10.1016/j.phrs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Frers A, Shaffer J, Edinger J, Wachholtz A (2021). The relationship between sleep and opioids in chronic pain patients. Journal of Behavioral Medicine, 44, 412–420. doi: 10.1007/s10865-021-00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnhofen H (2018). Pain and sleep : A bidirectional relationship. Zeitschrift für Gerontologie und Geriatrie, 51, 871–874. doi: 10.1007/s00391-018-01461-8. [DOI] [PubMed] [Google Scholar]
- Gauthier EA, Guzick SE, Brummett CM, Baghdoyan HA, Lydic R (2011). Buprenorphine disrupts sleep and decreases adenosine concentrations in sleep-regulating brain regions of Sprague Dawley rat. Anesthesiology, 115, 743–53. doi: 10.1097/ALN.0b013e31822e9f85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet, 390, 1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KA, Ponce Terashima J, McCarty D (2019). Opioid use disorder and treatment: challenges and opportunities. BMC Health Services Research, 19, 884. doi: 10.1186/s12913-019-4751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh KC, Robinson EL, Fuller CA (2008). Sleep architecture in unrestrained rhesus monkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. Sleep, 31, 1239–50. [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Platt DM, Brasfield M, Follett ME, Prisinzano TE, Blough BE, Freeman KB (2020). Quantification of observable behaviors induced by typical and atypical kappa-opioid receptor agonists in male rhesus monkeys. Psychopharmacology (Berl), 237, 2075–2087. doi: 10.1007/s00213-020-05519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman K, Jarvis M (2015). American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Journal of Addiction Medicine, 9, 358–67. doi: 10.1097/ADM.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Pypendop BH, Christe KL (2014). Pharmacokinetics of buprenorphine following intravenous and intramuscular administration in male rhesus macaques (Macaca mulatta). Journal of Veterinary Pharmacology and Therapeutics, 37, 480–5. doi: 10.1111/jvp.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif ZE, Šaltyte Benth J, Solli KK, Opheim A, Kunoe N, Krajci P, Sharma-Haase K, Tanum L (2019). Anxiety, Depression, and Insomnia Among Adults With Opioid Dependence Treated With Extended-Release Naltrexone vs Buprenorphine-Naloxone: A Randomized Clinical Trial and Follow-up Study. JAMA Psychiatry, 76, 127–134. doi: 10.1001/jamapsychiatry.2018.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TA, Dang AD, Tran AHT, Nguyen LH, Nguyen THT, Phan HT, Latkin CA, Tran BX, Ho CSH, Ho RCM (2019). Factors Associated with Sleep Disorders among Methadone-Maintained Drug Users in Vietnam. International Journal of Environmental Research and Public Health, 16, 4315. doi: 10.3390/ijerph16224315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LW (1985). Buprenorphine. Drug and Alcohol Dependence, 14, 363–72. doi: 10.1016/0376-8716(85)90067-5. [DOI] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, Sanchez JJ, Javors MA, Disney A, Husbands SM, France CP (2020). Effects of acute and repeated treatment with methocinnamox, a mu opioid receptor antagonist, on fentanyl self-administration in rhesus monkeys. Neuropsychopharmacology, 45, 1986–1993. doi: 10.1038/s41386-020-0698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL (2021). Trends and Geographic Patterns in Drug and Synthetic Opioid Overdose Deaths — United States, 2013–2019. MMWR Morbidity and Mortality Weekly Report, 70, 202–207. doi: 10.15585/mmwr.mm7006a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrino L, Terzano MG (1996). Polysomnographic effects of hypnotic drugs. A review. Psychopharmacology (Berl), 126, 1–16. doi: 10.1007/BF02246405. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Hamburger RB, Adelson M (2011). No change of sleep after 6 and 12 months of methadone maintenance treatment. Journal of Addiction Medicine, 5, 141–7. doi: 10.1097/ADM.0b013e3181e8b6c4. [DOI] [PubMed] [Google Scholar]
- Petri CR, Richards JB (2020). Management of Sedation and Analgesia in Critically Ill Patients Receiving Long-Acting Naltrexone Therapy for Opioid Use Disorder. Annals of the American Thoracic Society, 17, 1352–1357. doi: 10.1513/AnnalsATS.202005-554CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece PA, Sedman AJ, Rose S, Wright S, Dawkins R, Rajagopalan R (1994). Diuretic effects, pharmacokinetics, and safety of a new centrally acting kappa-opioid agonist (CI-977) in humans. Journal of Clinical Pharmacology, 34, 1126–1132. doi: 10.1002/j.1552-4604.1994.tb01991.x. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Wilcox KM, Woolverton WL (1998). Self-administration of cocaine-heroin combinations by rhesus monkeys: antagonism by naltrexone. The Journal of Pharmacology and Experimental Therapeutics, 286, 61–9. [PubMed] [Google Scholar]
- Schwienteck KL, Negus SS, Banks ML (2019). Sex differences in the effectiveness of buprenorphine to decrease rates of responding in rhesus monkeys. Behavioural Pharmacology, 30, 358–362. doi: 10.1097/FBP.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soin A, Soin Y, Dann T, Buenaventura R, Ferguson K, Atluri S, Sachdeva H, Sudarshan G, Akbik H, Italiano J (2021). Low-Dose Naltrexone Use for Patients with Chronic Regional Pain Syndrome: A Systematic Literature Review. Pain Physician, 24, E393–E406. [PubMed] [Google Scholar]
- Sramek J, Andry JM, Ding H, Riordan HJ, Leibowitz M, Cutler NR (2014). The effect of naltrexone on sleep parameters in healthy male volunteers. Journal of Clinical Psychopharmacology, 34, 167–8. doi: 10.1097/JCP.0b013e3182a607ff. [DOI] [PubMed] [Google Scholar]
- Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, Marshall BDL, Tyndall M, Walsh SL (2020). Opioid use disorder. Nature Reviews. Disease Primers, 6, 3. doi: 10.1038/s41572-019-0137-5. [DOI] [PubMed] [Google Scholar]
- Tripathi R, Dhawan A, Rao R, Mishra AK, Jain R, Sinha S (2020). Assessment of Subjective Sleep Problems in Men With Opioid Dependence Maintained on Buprenorphine. Journal of Addiction Medicine, 14, 132–138. doi: 10.1097/ADM.0000000000000539. [DOI] [PubMed] [Google Scholar]
- Walker EA, Zernig G, Woods JH (1995). Buprenorphine antagonism of mu opioids in the rhesus monkey tail-withdrawal procedure. The Journal of Pharmacology and Experimental Therapeutics, 273, 1345–52. [PubMed] [Google Scholar]


