Abstract
Objective
Partial-thickness rotator cuff tears have a high prevalence in older people. Treatment for such tears remains controversial. Platelet-rich plasma has recently attracted attention for treating partial-thickness rotator cuff tears, due to its regenerative characteristics. However, the results of application of platelet-rich plasma in non-operative treatments are unclear. The aim of this review is to evaluate the effects on shoulder function improvement and pain relief of platelet-rich plasma injection in partial-thickness rotator cuff tears, at different follow-up times (3–6 weeks, 8–12 weeks, and more than 24 weeks after treatment) compared with placebo or corticosteroids.
Design
A systematic review and meta-analysis.
Methods
Several databases, including PubMed, EMBASE, and Cochrane, were searched. Eleven studies met the inclusion criteria for the meta-analysis. The quality of research was evaluated using the Cochrane risk-of-bias tool. The effectiveness of platelet-rich plasma was calculated as the difference between baseline measurements and post-injection outcomes. The standardized mean difference was used to compare different outcome scales or questionnaire measurements. Statistical analysis was performed using Stata 15.0.
Results
The analysis included 11 studies, with a total of 641 patients (318 treated with platelet-rich plasma and 323 controls). Compared with placebo, platelet-rich plasma exhibited significantly better effects on shoulder function improvement and pain relief at all 3 follow-up times. Compared with other conservative treatments, platelet-rich plasma exhibited significantly better effects on shoulder function and pain relief at 8–12 weeks and at more than 24 weeks after treatment.
Conclusion
This review showed positive effects on shoulder function improvement and pain relief of the use of platelet-rich plasma in treating partial-thickness rotator cuff tears, especially in relatively late stages of follow-up (more than 8 weeks) after treatment.
LAY ABSTRACT
Partial-thickness rotator cuff tears are common, especially in older people, and can cause shoulder pain and movement limitation. Platelet-rich plasma is a platelet concentrate made from autologous blood, which may have anti-inflammatory and healing effects. This systematic review and meta-analysis collected data from existing studies to determine the specific effects of platelet-rich plasma injection on partial-thickness rotator cuff tears. The results showed that, compared with both no treatment and other conservative treatments, platelet-rich plasma reduced pain and improved shoulder function when used to treat partial-thickness rotator cuff tears. Meanwhile, the effects were most significant at 8–12 weeks and at more than 24 weeks after treatment.
Key words: partial-thickness rotator cuff tear, platelet-rich plasma, shoulder function, shoulder pain
A partial-thickness rotator cuff tear (PTRCT) is defined as the disruption of tendon fibres and is characterized by pain and activity limitation, occurring due to degenerative factors and overhead movements. A study by Milgrom et al. reported that the prevalence of PTRCTs in patients over the age of 60 years was 26%, compared with 4% under the age of 40 years (1). Another study showed that the prevalence of PTRCT in overhead athletes (such as baseball athletes, volleyball athletes, etc.) was 40% in the dominant shoulder (2). There are various classification systems for PTRCTs, but there is, as yet, no consensus on the optimal system according to clinical studies (3–6). Studies using various different classification systems have shown that there is no simple treatment strategy for PTRCTs, and the treatment options are controversial. Histological studies have shown that PTRCTs cannot heal themselves over time (7, 8), and open or arthroscopic surgical treatments to repair the damage are effective for many patients. Non-operative management is usually the first option for treating PTRCTs, and these methods mainly include rest, activity modification, pain medication, anti-inflammatory drugs, corticosteroid injections, and physical therapy. However, such treatments are limited, since they do not impact the progression of PTRCTs. Recent reports have also questioned their efficacy and suggested that they merely relieve clinical symptoms, but do not enhance tendon healing in the injured rotator cuff (9, 10). Therefore, further studies are needed to search for an effective treatment for tendon healing in PTRCTs.
Platelet-rich plasma (PRP) is a platelet concentrate derived from autologous whole blood with a high concentration of platelets; PRP contains 3- to 6-fold more platelets than whole blood (11–13). The granules in platelets can release high levels of growth factors, including vascular endothelial growth factor (VEGF), transforming growth factor β (TGF-β), and platelet-derived growth factor (PDGF) (14); as such, PRP has the potential to enhance the healing of injured or degenerated tendons and is currently being tested widely in different fields of medicine (15). In the last few years, an increasing number of studies has focused on the use of PRP injection in treatment aiming to repair PTRCTs. Clinical studies on the use of PRP in conventional non-operative treatments are controversial, and most studies have focused on full-thickness rotator cuff tears. While the use of PRP in the repair of PTRCTs is still in the exploratory stage, it has already shown positive results in improvement of shoulder function and pain relief, with a potential effect of tendon healing (16–18).
The aim of this review is to evaluate data from randomized controlled studies of the effects on shoulder function improvement and pain relief of PRP injection for treatment of PTRCT, measured at different follow-up times (3–6, 12, and 24 weeks after treatment), compared with placebo or corticosteroids.
METHODS
Inclusion and exclusion criteria
Inclusion criteria were: (i) PTRCT; (ii) diagnosis based on magnetic resonance imaging (MRI) or sonography; (iii) application of PRP; (iv) reported outcomes, including function improvement and pain relief; (v) randomized controlled design.
Exclusion criteria were: (i) the use of surgery or other treatment combined with PRP; (ii) diagnosis not based on MRI or sonography findings; (iii) full-thickness tear of the rotator cuff; (iv) unavailable mean and standard deviation (SD) of outcomes.
Search strategy
The authors independently screened the literature, extracted data, and performed crosschecks following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Electronic databases, including PubMed, EMBASE, and Cochrane, were searched using the following medical subject heading (MeSH) terms: “Platelet-Rich Plasma” (MeSH) AND “Rotator Cuff” (MeSH) as keywords. Articles were identified through a manual search of the reference lists of the relevant articles. The literature search spanned the date of database inception to 31 December 2021. Two reviewers independently reviewed the full texts of all potentially relevant articles to identify articles meeting the eligibility criteria. The individually recorded decisions of the 2 reviewers were then compared, and a third reviewer resolved dissimilarities in the decisions.
Data items
The following information was obtained from each article identified: characteristics of rotator cuff tears, follow-up duration, outcome measurements, and PRP preparation and application schemes.
Risk-of-bias assessment
The quality of RCTs was evaluated using the Cochrane risk-of-bias tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (19). Possible biases are divided into 5 domains (selection bias, performance bias, detection bias, attrition bias, and reporting bias) and a generalized category of other biases. All items were independently assessed by 2 authors. Each outcome within a study across domains and each outcome across the studies was rated as having “Low risk”, “Unclear risk”, or “High risk” of bias. The kappa statistic determined inter-rater reliability to evaluate the strength for the risk-of-bias assessments. Discussions with the corresponding author were made to solve the disputes.
Statistical analysis
The effectiveness of PRP was calculated as the difference between baseline measurement and post-injection outcome. The standardized mean difference (SMD) was adopted to properly compare different outcome scales or questionnaire measurements. A random-effects model was used to calculate the pooled SMD with a 95% confidence interval (95% CI). Heterogeneity was assessed by I-square. An I-square > 50% was recognized as significant heterogeneity. The analysis was performed using Stata 15.0 (StataCorp LP, College Station, TX, USA). All p-values were 2-sided, and the significance level was set at 5% except for the test of between-study heterogeneity. Sensitivity analysis was performed by removing the relative low-quality studies and re-performing the meta-analysis. Publication bias was not analysed because of the limited number (< 10) of studies included in each analysis.
RESULTS
Search results
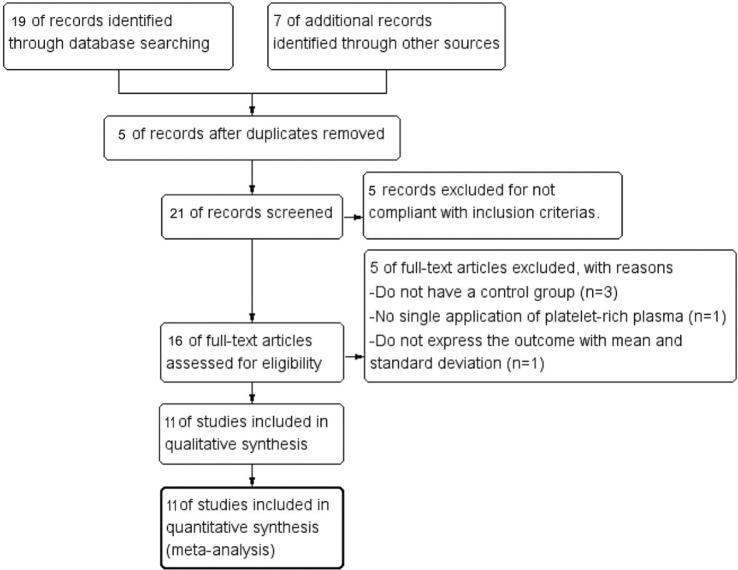
With the search terms listed above, 26 articles were initially retrieved. Five duplicates were excluded using EndNote X9. 3 Thomson ResearchSoft. Citations that were non-compliant with the inclusion criteria were excluded after screening their title and abstract. The full text of the remaining 16 citations was screened, revealing 3 citations did not have a control group, 1 citation did not apply singly, and 1 citation did not express the outcome with mean and SD. A final total of 11 articles were selected for this systematic review and meta-analysis (Fig. 1).
Fig. 1.
Flow diagram of study inclusion.
Study characteristics
All 11 included studies were published between 2013 and 2021. A total of 641 patients were included (318 patients in the PRP group and 323 in the control group). The control groups in the included studies were as follows: 3 studies used placebo (saline or dry needle) (20–22); and 8 used conservative treatments (corticosteroids injection or physical therapy) (23–30). Ten studies prepared PRP via centrifugation. Three studies applied multiple injections at different intervals, while another 8 studies applied injections only once. The main characteristics of the 11 included studies are summarized in Tables I and II.
Table I.
Research on the non-operative treatment of partial-thickness rotator cuff tears (PTRCTs) with platelet-rich plasma (PRP)
| Study | Patients(PRP/control) | Control | Inclusion criteria | Follow-up duration | Outcome measurement | ||
|---|---|---|---|---|---|---|---|
| Tear time, months | Size | Shoulder function | Pain | ||||
| Rha et al. 2013 (17) | 16/14 | Placebo (dry needles) | > 6 | < 1.0 cm | 6 months | SPADI | Pain in SPADI |
| Ilhanli et al. 2015 (26) | 30/32 | Physical therapy | Not mentioned | Not mentioned | 12 months | DASH score | VAS score |
| von Wehren et al. 2016 (22) | 25/25 | Corticosteroids | > 2 | Not mentioned | 6 months | SST score, CS, ASES score | VAS score (specific data were not given) |
| Shams et al. 2016 (21) | 20/20 | Corticosteroids | > 3 | Not mentioned | 6 months | SST score, CS, ASES score | VAS score (specific data were not given) |
| Cai et al. 2019 (16) | 45/47 | Placebo (saline) | < 6 | < 1.0 cm | 1 year | CS, ASES score | VAS score |
| Schwitzguebel et al. 2019 (18) | 41/39 | Placebo (saline) | > 6 | Not mentioned | > 12 months | ASES score | VAS score |
| Ibrahim et al. 2019 (25) | 15/15 | Corticosteroids | Not mentioned | Not mentioned | 7 months | SDQ score | VAS score |
| Cory et al. 2020 (19) | 52/47 | Corticosteroids | > 3 | Not mentioned | 1 year | ASES score, WORC score | VAS score |
| Sari et al. 2020 (20) | 30/30 | Corticosteroids | > 3 | Grade 1 | 24 weeks | ASES score, WORC score | VAS score |
| Dadgostar et al. 2021 (23) | 30/28 | Corticosteroids | > 3 | Not mentioned | 3 months | WORC score, DASH score | VAS score |
| Thepsoparn et al. 2021 (24) | 15/16 | Corticosteroids | > 3 | Not mentioned | 6 months | OSS | VAS score |
SPADI: Shoulder Pain and Disability Index; SST: simple shoulder test; CS: Constant (Murley) score; ASES: American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form; WORC: Western Ontario Rotator Cuff; SDQ: Strength and Difficulties Questionnaire; DASH: Disabilities of the Arm, Shoulder and Hand; OSS: Oxford Shoulder Score; VAS: visual analogue scale.
Table II.
Platelet-rich plasma (PRP) preparation and application schemes in studies included in the review
| Study | PRP volume, ml | PRP preparation method | Preparation kit | Application scheme | Injection method |
|---|---|---|---|---|---|
| Rha et al. 2013 (17) | 3 | Centrifugation | Prosys PRP Platelet Concentration System (Tozaiholdings) | 2 injections, 4-week interval | Ultrasound-guided injection |
| Ilhanli et al. 2015 (26) | 6 | Centrifugation | Not mentioned | 1 injection | Not mentioned |
| von Wehren et al. 2016 (22) | 5 | Centrifugation | Arthrex Centrifuge (Rotofix) | 3 injections, 7-day interval | Subacromial injection |
| Shams et al. 2016 (21) | 2–2.5 | Centrifugation | MyCells Autologous Platelet Preparation System (ProTech) | 1 injection | Subacromial injection |
| Cai et al. 2019 (16) | 5–6 | Centrifugation | Labofuge 400R (Heraeus) | 4 injections, 7-day interval | Ultrasound-guided subacromial injection |
| Schwitzguebel et al. 2019 (18) | 2 | Centrifugation | RegenKit BCT (Regen Lab) | 1 injection | Ultrasound-guided subacromial injection |
| Ibrahim et al. 2019 (25) | 2 | Centrifugation | Not mentioned | 1 injection | Not mentioned |
| Cory et al. 2020 (19) | 3–5 | Centrifugation | RegenLab (Lausanne) | 1 injection | Ultrasound-guided subacromial injection |
| Sari et al. 2020 (20) | 5 | Centrifugation | Not mentioned | 1 injection | Ultrasound-guided subacromial injection |
| Dadgostar et al. 2021 (23) | 3 | Not mentioned | ROOYA GEN (Arya Mabna Tashkhis Co.) | 1 injection | Ultrasound-guided subacromial injection |
| Thepsoparn et al. 2021 (24) | 5 | Centrifugation | ACP Double Syringe System (Arthrex) | 1 injection | Ultrasound-guided subacromial injection |
Risk-of-bias assessment
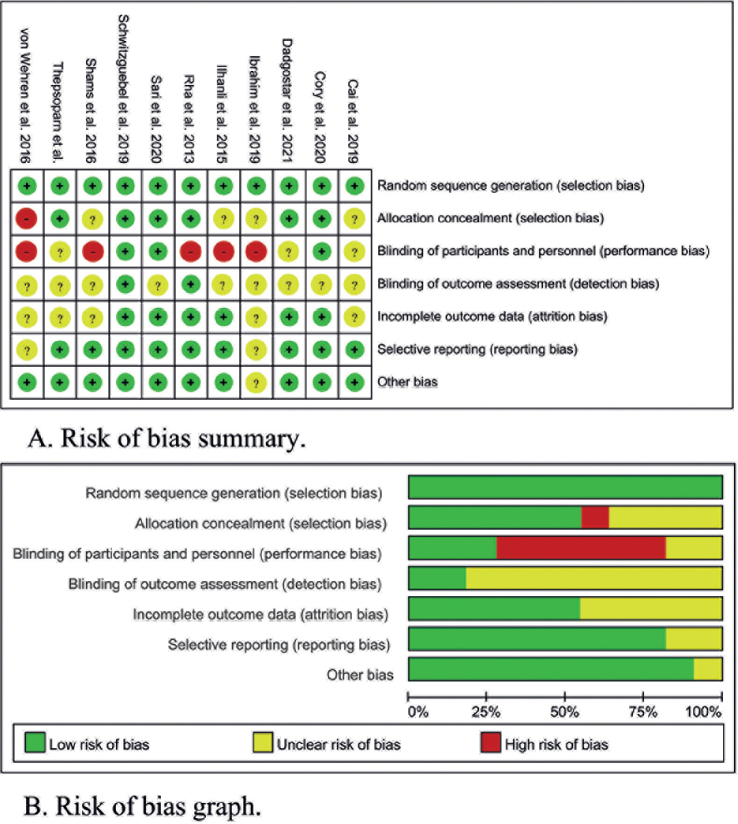
The graph and summary of the risk-of-bias are shown in Fig. 2. Most of the higher or unclear risks of bias were generated in the allocation concealment, blinding of participants and personnel, and blinding of outcome assessment. A possible reason is that it is difficult to blind patients and operators to drawing blood for PRP preparation.
Fig. 2.
Risk-of-bias (A) summary and (B) graph. Green: low risk-of-bias; red: high risk-of-bias; yellow: unclear risk-of-bias.
Shoulder function
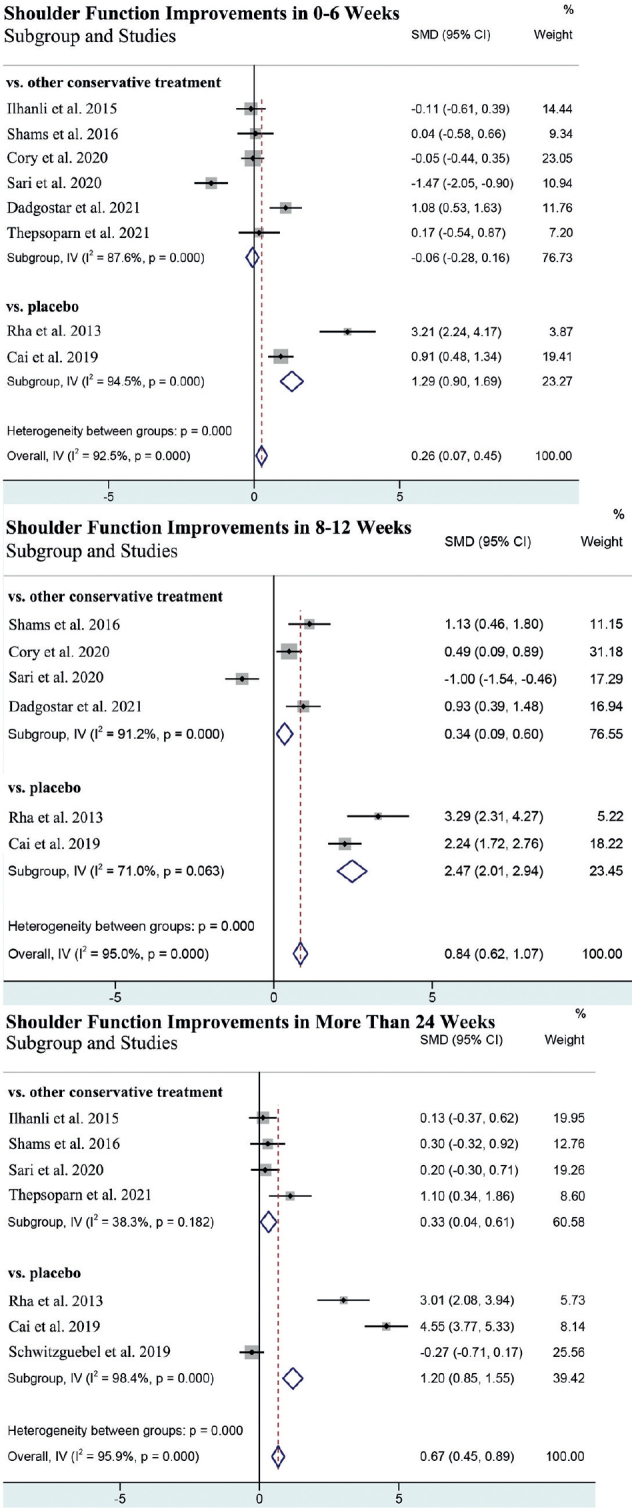
Shoulder function was reported in all 11 studies. The shoulder function was significantly improved in the PRP group compared with in the control group in all 3 stages (Fig. 3A). Subgroup analysis revealed that PRP was more effective in shoulder function improvement than placebo in all 3 stages (Fig. 3A). However, the PRP group exhibited significant improvements in shoulder function compared with other conservative treatments in 8–12 weeks (SMD = 0.40, 95% CI (0.17, 0.62)) and more than 24 weeks (SMD = 0.31, 95% CI (0.05, 0.56)) (Fig. 3A).
Fig. 3.
Forest plot of meta-analysis. (A) Shoulder function improvements. (B) Pain relief.
Pain relief
Pain relief was reported in all 11 studies, while only 9 showed a specific mean and SD. One of them reported in Pain score of Shoulder Pain and Disability Index (SPADI) (21), and another 8 reported in visual analogue scale (VAS) score. The pain was significantly released in the PRP group than in the control group in 8–12 weeks and more than 24 weeks (Fig. 3B). Subgroup analysis revealed that PRP was more effective in pain relief than placebo in all 3 stages (Fig. 3B). Meanwhile, compared with other conservative treatment, PRP exhibited better effects in pain relief in 8–12 weeks (SMD = – 0.36, 95% CI (– 0.62, – 0.11)) and more than 24 weeks (SMD = – 0.62, 95% CI (– 0.95, – 0.30)) after treatment (Fig. 3B).
Sensitivity analysis
Because of the large heterogeneity between studies, the sensitivity analysis was performed by removing the relatively low-quality studies and re-performing the meta-analysis. As shown in Fig. 2, the risk-of-bias in the included studies exists mainly in allocation concealment, blinding of participants and personnel, and blinding of outcome assessment, which is because PRP injection is an invasive treatment and PRP needs to be prepared with autologous blood. Thus, we selected the relatively low-quality studies ignoring the 3 items, and 2 studies (von Wehren et al. 2016 and Ibrahim et al. 2019 et al.) (22, 25) were selected. Meanwhile, the 2 studies contribute little to the analysis of pain relief, sensitivity analysis was performed only on shoulder function improvements analysis in all 3 stages. As shown in Fig. 4, all results are consistent with Fig. 3, indicating that the analysis results are stable.
Fig. 4.
The sensitivity analysis by removing relatively low-quality studies and re-performing meta-analysis on shoulder function improvements.
DISCUSSION
This systematic review and meta-analysis focused on the effect of PRP on the non-operative treatment of PTRCT. The results of the analysis revealed statistically significant differences in the following aspects:
PRP exhibited significantly better improvement in shoulder function and pain relief despite the subgroup analysis.
Compared with placebo, PRP exhibited significantly better effects on shoulder function improvement and pain relief in all 3 stages.
Compared with other conservative treatment, PRP exhibited significantly better effects on shoulder function and pain relief at follow-up at 8–12 weeks and more than 24 weeks after treatment.
The application of PRP for tendon-to-bone healing has already been widely adopted, due to acquisition of PRP being convenient and its ability to promote regenerative bioactivity (16). Given these regenerative properties, the application of PRP has attracted the interest of clinicians, especially specialists in osteoarthropathy. In the treatment of tendinous injuries, both fundamental experiments and clinical studies have exhibited the great value of PRP (16–18).
PRP can be classified into leukocyte-rich PRP (Lr-PRP) and leukocyte-poor PRP (Lp-PRP) based on the platelet concentration. Animal experiments have shown that high leukocyte levels in PRP could increase the levels of proinflammatory cytokines and weaken the beneficial healing effects of PRP on chronic tendinopathy (31). In addition, Lp-PRP exhibited more significant effects on promoting normal collagen matrix synthesis and decreasing the levels of cytokines associated with matrix degradation than Lr-PRP in degenerative tendons (32–34). Two main methods are generally applied to obtain PRP: 1 is apheresis, which produces purer PRP with higher platelet and lower leukocyte levels (35, 36); the other is centrifugation, which is more widely used, as in all of the studies reviewed in this article, and has the advantages of a lower cost and requiring simpler materials (37). Regarding the reparative effects, apheresis is better, due to the lower leukocyte levels. However, when including consideration of cost, the method of choice should be further assessed.
Regardless of the type of method, PRP is prepared from autologous blood; thus it has lower immunogenicity and is safer than traditional drug injections (e.g. corticosteroids) without specific adverse effects (15–18). Most studies have shown no adverse events during treatment with PRP (15–18). Regarding the injection method, although injection under ultrasound guidance is highly recommended and exhibits good efficacy and safety (38), subacromial injection without guidance also shows significant improvements in shoulder function and pain relief (25, 26, 39). However, ultrasound-guided injections might be more precise, safer and more accessible for young physicians to less-experienced practitioners. Both ultrasound-guided and freehand injections are safe and effective, with no evidence that one is better than the other (15–18); hence personal preference and experience should guide the choice of delivery method.
PTRCTs can be classified as articular-side, bursal-side, and intra-tendinous tears, based on the anatomical location of the tear. In general, it is believed that treatments for tears at different locations exhibit different effects and prognoses. More positive results are found for tears on the bursal side, because they can easily be reached by subacromial injection. Kim et al. also found that the treatment of bursal-side tears results in comparable or superior postoperative functional outcomes compared with the treatment of articular-side tears after arthroscopic surgery (40). However, none of the studies in this field have analysed the results of different tear types or included only bursal-side tears. As a result, whether PRP exhibits considerable clinical efficacy at all 3 locations is unclear, and more studies with further analysis based on the tear type are required.
PTRCTs are complex injuries influenced by multiple factors, and the natural history of PTRCTs remains unclear. As a result, studies on the single use of PRP cannot draw reliable conclusions, and the potential of combined uses of PRP might be ignored. In the treatment of PTRCTs, PRP combined with marrow aspirate concentrate (BMAC) and (or) sodium hyaluronate (SH) has been proven effective (20, 41). A recent in vitro study found that PRP combined with insulin could promote human adipose-derived stem cells’ chondrogenic and osteogenic differentiation (42). Intra-articular injection of PRP combined with mesenchymal stem cells for treating patellofemoral osteoarthrosis also resulted in improved joint function (43). Although the feasibility and usage of combined applications should be evaluated in detail, and there are still possibilities for the combined use of PRP, not only in the treatment of rotator cuff tears, but also in other fields of sports medicine.
PRP is prepared from autologous blood, which means it has relatively low immunogenicity. However, there are situations in which it is not suitable to draw autologous blood, such as in patients with anaemia or platelet dysfunction. Jo et al. compared the application of allogeneic and autologous PRP obtained from patients undergoing arthroscopic rotator cuff repair and prepared using a plateletpheresis system with leukocyte removal (44). They showed that allogeneic and autologous PRP had comparable effects with no adverse events (44), which further broadens the use of PRP and makes it possible for PRP to be commercialized.
Due to individual differences, the space around the injured tendons is different. And in some cases, this space could contain exudates that could affect the PRP concentration. However, studies have found that the proper platelet concentration is 3–6 times above the baseline, and higher levels could exhibit inhibitory effects (11, 12, 36). As a result, increasing the PRP concentration or the number of platelets might not be feasible. Most studies included in this review applied PRP only once. Further research is needed to determine whether repeated application of PRP at suitable intervals could lead to improved effects.
Study limitations and strengths
This review is limited by the relatively few studies comparing PRP with traditional or placebo treatments. Thus it is not possible to draw a robust conclusion regarding the superiority of PRP. Meanwhile, few clinical studies focus on the effects of PRP on rotator cuff healing. Further research into the possible effects is needed.
Previous reviews or meta-analyses of PRP for treatment of rotator cuff tears have not focused solely on PTRCTs; hence the value of PRP in treating PTRCTs has not been analysed in detail. Although some studies have posited that PTRCTs cannot heal themselves over time (7, 8), it is thought that they could heal with proper conservative treatment (38). A meta-analysis published in December 2021 also focused on the application of PRP for PTRCT. It did subgroup analysis of PRP preparation by centrifugation times. However, PRP in their included studies was prepared using kits from different manufacturers (45). Bias due to different centrifugation times appears to be less important than bias due to different manufacturers. Meanwhile, the main objective of analysing the application of PRP is to find a better treatment to replace traditional conservative treatments. This is the first review to focus mainly on the clinical effects of PRP on the non-operative treatment of PTRCTs in the near, medium, and long term after treatment compared with other conservative treatments.
CONCLUSION
This review of studies into the use of PRP injections in non-operative treatments for treatment of PTRCTs, showed improvements in both shoulder function and pain relief, especially at a relatively late stage (12 weeks or more) after treatment. However, due to the limitations mentioned above, this conclusion is not sufficiently robust and further research is needed.
ACKNOWLEDGEMENTS
Ethical approval
All applicable international, national, and/or institutional guidelines were followed.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br 1995; 77: 296–298. [PubMed] [Google Scholar]
- 2.Connor PM, Banks DM, Tyson AB, Coumas JS, D’Alessandro DF. Magnetic resonance imaging of the asymptomatic shoulder of overhead athletes: a 5-year follow-up study. Am J Sports Med 2003; 31: 724–727. [DOI] [PubMed] [Google Scholar]
- 3.Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res 1990: 64–74. [PubMed] [Google Scholar]
- 4.Snyder SJ, Pachelli AF, Del Pizzo W, Friedman MJ, Ferkel RD, Pattee G. Partial thickness rotator cuff tears: results of arthroscopic treatment. Arthroscopy 1991; 7: 1–7. [DOI] [PubMed] [Google Scholar]
- 5.Conway JE. Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am 2001; 32: 443–456. [DOI] [PubMed] [Google Scholar]
- 6.Bauer S, Wang A, Butler R, Fallon M, Nairn R, Budgeon C, et al. Reliability of a 3 T MRI protocol for objective grading of supraspinatus tendonosis and partial thickness tears. J Orthop Surg Res 2014; 9: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda H, Hamada K, Nakajima T, Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res 1994: 60–67. [PubMed] [Google Scholar]
- 8.Fukuda H, Mikasa M, Yamanaka K. Incomplete thickness rotator cuff tears diagnosed by subacromial bursography. Clin Orthop Relat Res 1987: 51–58. [PubMed] [Google Scholar]
- 9.Alvarez CM, Litchfield R, Jackowski D, Griffin S, Kirkley A. A prospective, double-blind, randomized clinical trial comparing subacromial injection of betamethasone and xylocaine to xylocaine alone in chronic rotator cuff tendinosis. Am J Sports Med 2005; 33: 255–262. [DOI] [PubMed] [Google Scholar]
- 10.Lee WH, Do HK, Lee JH, Kim BR, Noh JH, Choi SH, et al. Clinical outcomes of conservative treatment and arthroscopic repair of rotator cuff tears: a retrospective observational study. Ann Rehabil Med 2016; 40: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anitua E, Sanchez M, Nurden AT, Nurden P, Orive G, Andia I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol 2006; 24: 227–234. [DOI] [PubMed] [Google Scholar]
- 12.Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res 2006; 17: 212–219. [DOI] [PubMed] [Google Scholar]
- 13.Weibrich G, Kleis WK, Hafner G, Hitzler WE, Wagner W. Comparison of platelet, leukocyte, and growth factor levels in point-of-care platelet-enriched plasma, prepared using a modified Curasan kit, with preparations received from a local blood bank. Clin Oral Implants Res 2003; 14: 357–362. [DOI] [PubMed] [Google Scholar]
- 14.Harrison P, Cramer EM. Platelet alpha-granules. Blood Rev 1993; 7: 52–62. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez M, Anitua E, Orive G, Mujika I, Andia I. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med 2009; 39: 345–354. [DOI] [PubMed] [Google Scholar]
- 16.Kia C, Baldino J, Bell R, Ramji A, Uyeki C, Mazzocca A. Platelet-rich plasma: review of current literature on its use for tendon and ligament pathology. Curr Rev Musculoskelet Med 2018; 11: 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sánchez M, Jorquera C, Sánchez P, Beitia M, García-Cano B, Guadilla J, et al. Platelet-rich plasma injections delay the need for knee arthroplasty: a retrospective study and survival analysis. Int Orthop 2021; 45: 401–410. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Wang JH. PRP treatment efficacy for tendinopathy: a review of basic science studies. Biomed Res Int 2016; 2016: 9103792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019; 10: Ed000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai YU, Sun Z, Liao B, Song Z, Xiao T, Zhu P. Sodium hyaluronate and platelet-rich plasma for partial-thickness rotator cuff tears. Med Sci Sports Exerc 2019; 51: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rha DW, Park GY, Kim YK, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil 2013; 27: 113–122. [DOI] [PubMed] [Google Scholar]
- 22.Schwitzguebel AJ, Kolo FC, Tirefort J, Kourhani A, Nowak A, Gremeaux V, et al. Efficacy of platelet-rich plasma for the treatment of interstitial supraspinatus tears: a double-blinded, randomized controlled trial. Am J Sports Med 2019; 47: 1885–1892. [DOI] [PubMed] [Google Scholar]
- 23.Kwong CA, Woodmass JM, Gusnowski EM, Bois AJ, Leblanc J, More KD, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy 2021; 37: 510–517. [DOI] [PubMed] [Google Scholar]
- 24.Sari A, Eroglu A. Comparison of ultrasound-guided platelet-rich plasma, prolotherapy, and corticosteroid injections in rotator cuff lesions. J Back Musculoskelet Rehabil 2020; 33: 387–396. [DOI] [PubMed] [Google Scholar]
- 25.Shams A, El-Sayed M, Gamal O, Ewes W. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol 2016; 26: 837–842. [DOI] [PubMed] [Google Scholar]
- 26.von Wehren L, Blanke F, Todorov A, Heisterbach P, Sailer J, Majewski M. The effect of subacromial injections of autologous conditioned plasma versus cortisone for the treatment of symptomatic partial rotator cuff tears. Knee Surg Sports Traumatol Arthrosc 2016; 24: 3787–3792. [DOI] [PubMed] [Google Scholar]
- 27.Dadgostar H, Fahimipour F, Pahlevan Sabagh A, Arasteh P, Razi M. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res 2021; 16: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thepsoparn M, Thanphraisan P, Tanpowpong T, Itthipanichpong T. Comparison of a platelet-rich plasma injection and a conventional steroid injection for pain relief and functional improvement of partial supraspinatus tears. Orthop J Sports Med 2021; 9: 23259671211024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim DH, El-Gazzar NM, El-Saadany HM, El-Khouly RM. Ultrasound-guided injection of platelet rich plasma versus corticosteroid for treatment of rotator cuff tendinopathy: effect on shoulder pain, disability, range of motion and ultrasonographic findings. Egyptian Rheumatologist 2019; 41: 157–161. [Google Scholar]
- 30.Ilhanli I, Guder N, Gul M. Platelet-rich plasma treatment with physical therapy in chronic partial supraspinatus tears. Iran Red Crescent Med J 2015; 17: e23732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin WJ, Xu HT, Sheng JG, An ZQ, Guo SC, Xie XT, et al. Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in treating rabbit knee osteoarthritis. Med Sci Monit 2016; 22: 1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cross JA, Cole BJ, Spatny KP, Sundman E, Romeo AA, Nicholson GP, et al. Leukocyte-reduced platelet-rich plasma normalizes matrix metabolism in torn human rotator cuff tendons. Am J Sports Med 2015; 43: 2898–2906. [DOI] [PubMed] [Google Scholar]
- 33.Danieli MV, Guerreiro JPF, Queiroz AO, da Rosa Pereira H, Cataneo DC. Leucocyte-poor-platelet-rich plasma intra-operative injection in chondral knee injuries improve patients outcomes. A prospective randomized trial. Int Orthop 2021; 45: 463–471. [DOI] [PubMed] [Google Scholar]
- 34.Yan R, Gu Y, Ran J, Hu Y, Zheng Z, Zeng M, et al. Intratendon delivery of leukocyte-poor platelet-rich plasma improves healing compared with leukocyte-rich platelet-rich plasma in a rabbit achilles tendinopathy model. Am J Sports Med 2017; 45: 1909–1920. [DOI] [PubMed] [Google Scholar]
- 35.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol 2009; 27: 158–167. [DOI] [PubMed] [Google Scholar]
- 36.Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004; 34: 665–671. [DOI] [PubMed] [Google Scholar]
- 37.Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg 2000; 58: 297–300; discussion 300–291. [DOI] [PubMed] [Google Scholar]
- 38.Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc Rev 2011; 19: 244–250. [DOI] [PubMed] [Google Scholar]
- 39.Zafarani Z, Mirzaee F, Guity M, Aslani H. Clinical results of platelet-rich plasma for partial thickness rotator cuff tears: a case series. Arch Bone Jt Surg 2017; 5: 328–331. [PMC free article] [PubMed] [Google Scholar]
- 40.Kim KC, Shin HD, Cha SM, Park JY. Repair integrity and functional outcome after arthroscopic conversion to a full-thickness rotator cuff tear: articular- versus bursal-side partial tears. Am J Sports Med 2014; 42: 451–456. [DOI] [PubMed] [Google Scholar]
- 41.Kim SJ, Kim EK, Kim SJ, Song DH. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. J Orthop Surg Res 2018; 13: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scioli MG, Bielli A, Gentile P, Cervelli V, Orlandi A. Combined treatment with platelet-rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds. J Tissue Eng Regen Med 2017; 11: 2398–2410. [DOI] [PubMed] [Google Scholar]
- 43.Pintat J, Silvestre A, Magalon G, Gadeau AP, Pesquer L, Perozziello A, et al. Intra-articular injection of mesenchymal stem cells and platelet-rich plasma to treat patellofemoral osteoarthritis: preliminary results of a long-term pilot study. J Vasc Interv Radiol 2017; 28: 1708–1713. [DOI] [PubMed] [Google Scholar]
- 44.Jo CH, Shin JS, Lee SY, Shin S. Allogeneic platelet-rich plasma for rotator cuff repair. Acta Ortop Bras 2017; 25: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiang XN, Deng J, Liu Y, Yu X, Cheng B, He HC. Conservative treatment of partial-thickness rotator cuff tears and tendinopathy with platelet-rich plasma: a systematic review and meta-analysis. Clin Rehabil 2021; 35: 1661–1673. [DOI] [PubMed] [Google Scholar]