Summary
Background
Long COVID in children and adolescents remains poorly understood due to a lack of well-controlled studies with long-term follow-up. In particular, the impact of the family context on persistent symptoms following SARS-CoV-2 infection remains unknown. We examined long COVID symptoms in a cohort of infected children, adolescents, and adults and their exposed but non-infected household members approximately 1 year after infection and investigated clustering of persistent symptoms within households.
Methods
1267 members of 341 households (404 children aged <14 years, 140 adolescents aged 14-18 years and 723 adults) were categorized as having had either a SARS-CoV-2 infection or household exposure to SARS-CoV-2 without infection, based on three serological assays and history of laboratory-confirmed infection. Participants completed questionnaires assessing the presence of long COVID symptoms 11-12 months after infection in the household using online questionnaires.
Findings
The prevalence of moderate or severe persistent symptoms was statistically significantly higher in infected than in exposed women (36.4% [95% CI: 30.7–42.4%] vs 14.2% [95% CI: 8.7–21.5%]), infected men (22.9% [95% CI: 17.9–28.5%] vs 10.3% [95% CI: 5.8–16.9%]) and infected adolescent girls (32.1% 95% CI: 17.2–50.5%] vs 8.9% [95%CI: 3.1–19.8%]). However, moderate or severe persistent symptoms were not statistically more common in infected adolescent boys aged 14–18 (9.7% [95% CI: 2.8–23.6%] or in infected children <14 years (girls: 4.3% [95% CI: 1.2–11.0%]; boys: 3.7% [95% CI: 1.1–9.6%]) than in their exposed counterparts (adolescent boys: 0.0% [95% CI: 0.0–6.7%]; girls < 14 years: 2.3% [95% CI: 0·7–6·1%]; boys < 14 years: 0.0% [95% CI: 0.0–2.0%]). The number of persistent symptoms reported by individuals was associated with the number of persistent symptoms reported by their household members (IRR=1·11, p=·005, 95% CI [1.03–1.20]).
Interpretation
In this controlled, multi-centre study, infected men, women and adolescent girls were at increased risk of negative outcomes 11-12 months after SARS-CoV-2 infection. Amongst non-infected adults, prevalence of negative outcomes was also high. Prolonged symptoms tended to cluster within families, suggesting family-level interventions for long COVID could prove useful.
Funding
Ministry of Science, Research and the Arts, Baden-Württemberg, Germany.
Keywords: COVID-19, Long COVID, Post-COVID syndrome, Children and young people, Paediatric, Adolescents, Families, SARS-CoV-2
Research in context.
Evidence before this study
Children infected with SARS-CoV-2 usually have mild or even no symptoms. However, concerns have been raised that children may develop long-term symptoms (i.e., long COVID) following such infections. A Pubmed and BioRxiv search was performed before the study design in 2020 using the search terms “long COVID children”, “long COVID kids”, “post-COVID children” and “post-COVID kids” but could not identify suitable pediatric studies at this time. The first larger study including a control group was published after1 enrolment for this study started in December, 2020. To date, studies have focussed on individual risk factors, yet evidence from other pediatric illnesses indicates that symptoms in family members influence children's and adolescent's symptoms. A recent meta-analysis of long COVID in children found small but statistically significant pooled risk differences (2-8%) for a small number of symptoms following SARS-CoV-2 infection. However, only five studies with control groups were identified and none of these had follow-up data beyond six months. Moreover, none addressed whether persistent symptoms might cluster in families.
Added value of this study
Our study provides evidence on persistent symptoms in children, adolescents and adults approximately one year after SARS-CoV-2 infection. Because we included household members of infected participants, we had well-matched exposed but non-infected control participants. We found no evidence that children under 14 experience more moderate or severe persistent symptoms one year after SARS-CoV-2 infection than exposed children. In contrast, around a third of infected female adolescents aged 14-18 experienced one or more moderate or severe persistent symptoms – statistically significantly more than exposed uninfected female adolescents (9%). Moderate or severe persistent symptoms were also more common in infected adults than in their exposed counterparts (36% of infected women and 23% of infected men vs. 14% of exposed women and 10% of exposed men). Moreover, our results indicate that prolonged symptoms in individuals are associated with prolonged symptoms in their household members.
Implications of all the available evidence
Our study extends the existing evidence by showing that, one year after SARS-CoV-2 infection, persistent symptoms continue to be uncommon in children. However, there is accumulating evidence that adolescent girls are at particular risk of prolonged symptoms. A particular focus on preventative and treatment possibilities for this group seems justified. Our findings suggest that research on family-level mechanisms and treatments for long COVID is likely to be especially valuable.
Alt-text: Unlabelled box
Introduction
SARS-CoV-2 infections in children and adolescents typically produce mild or asymptomatic disease and so acute infection in this age group is considered to be low risk.2 A concerning outcome however is the possibility of “post-COVID syndrome”, “long COVID”, or “post-acute sequelae of COVID-19 (PASC)”. These terms describe heterogeneous medical entities, ranging from non-specific symptoms such as fatigue to specific organ dysfunction,3 that persist or emerge after the acute phase of a SARS-CoV-2 infection. Despite ongoing ambiguity about the definition and epidemiology of long COVID,4,5 large studies suggest that around a third of infected adults have symptoms 3-6 months after acute illness.6
The pandemic has resulted in reduced social contacts, school and nursery closures, and decreased access to leisure activities, which have had a substantial impact on children and adolescents.7, 8, 9 Given that the pandemic and infection control measures have increased the prevalence of nonspecific and psychological symptoms amongst never-infected individuals,10,11 distinguishing between long-term infection sequelae and general consequences of the pandemic is crucial. However, studies that distinguish between these widespread pandemic effects and possible long COVID are especially challenging in children and adolescents and are therefore scarce. Several studies have found no or only small differences in the prevalence of symptoms amongst infected children compared to controls.1,12,13 However, other data suggest that prolonged symptoms may be relatively common in children and adolescents.14,15 A recent meta-analysis concluded that studies with a suitable control group were less likely than uncontrolled studies to find elevated prevalence of prolonged symptoms following SARS-CoV-2 infection in children and adolescents.16
Studies of long COVID to date have considered individual-level risk factors, yet the family context can be an exceptionally important contributor to young people's health and wellbeing. In various pediatric conditions, including chronic pain,17 fatigue,18 and other chronic conditions,19, 20, 21, 22, 23 symptom measures in children/adolescents are associated with parents’ symptoms, stress and/or parenting behaviour. Families could therefore also play a role in long COVID symptomatology.
Here we examined symptoms 11-12 months after an acute SARS-CoV-2 infection, confirmed by reverse transcription-polymerase chain reaction (RT-PCR) and/or serology data, amongst families who participated in a study of SARS-CoV-2 transmission within households with children.24,25 Since family context may be important not only in SARS-CoV-2 transmission, but also for long COVID, we took advantage of the opportunity to compare infected with exposed, but non-infected family members to examine whether prevalence of reported symptoms was associated with reported symptoms amongst family members.
Method
Cohort and participants
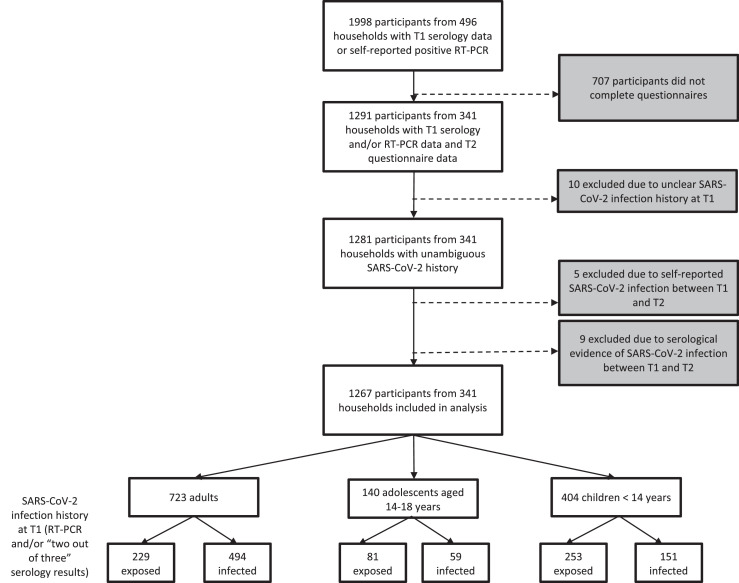
This study forms part of a prospective observational multi-centre cohort investigation of 341 households each with at least one individual with an RT-PCR-proven SARS-CoV-2 infection and/or a symptomatic and later serologically proven infection.24,25 Participants were recruited in May-August 2020 (T1, 9-17 weeks after household infection/exposure) via local health authorities, through traditional and social media information and an in-hospital database of households with at least one laboratory-confirmed SARS-CoV-2 infection. The sample size at T1 was based on feasibility and availability of subjects. The time interval for the initial recruitment period was limited due to visiting restrictions from the involved University Hospitals. Inclusion criteria were:(i) Children (male or female) aged 1–18 years.(ii) Parents and other adults (male or female) living in the same household with the investigated children (without age limit).(iii) Residency in the state of Baden-Württemberg.(iv) Written consent to the study. Key exclusion criteria were:(i) Severe congenital diseases (e.g. infantile cerebral palsy, severe congenital malformations).(ii) Congenital or acquired immunodeficiencies.(iii) Insufficient comprehension of German language. At three study centers (Freiburg, Tübingen and Ulm), all households who consented to be re-contacted were invited to participate at a second timepoint (T2). At the fourth study center (Heidelberg), ongoing visiting restrictions and personnel shortages meant that a more limited number of households could be invited to take part at T2; recruitment here focussed on families with younger children, since at the time, data from this group was scarcest. T2 participation occurred approximately 11-12 months after the earliest SARS-CoV-2 infection in the households (median [IQR] delay between earliest household infection and T2=335 days [312·5; 363]). Results of T2 serological analyses have been reported separately25; the current investigation involved online questionnaires. Of 1998 participants who had taken part in T1 serology testing and/or reported having had a positive PCR-confirmed SARS-CoV-2 infection prior to T1, 1291 completed T2 questionnaires, representing 64.6% of participants. 72.1% of those defined as infected (see below for definition) completed the questionnaire, whereas only 57.1% of those defined as exposed did so. Questionnaire completion was highest for adolescents aged 14-18 years (70.1%) and slightly lower for children under 14 years (62.9%) and adults (64.5%). Of the 1291 individuals who completed the questionnaires. (Figure 1), 10 were excluded because of unclear SARS-CoV-2 infection history at T1 (based on the criteria described below) and 14 participants were excluded because of a self-reported PCR-confirmed SARS-CoV-2 infection or asymptomatic seroconversion without vaccination between T1 and T2. There were thus 1267 participants in the final sample.
Figure 1.
Flowchart of participant enrollment in study of long COVID symptoms in households with children. T1, timepoint 1; T2, timepoint 2; RT-PCR, reverse transcription PCR; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Ethics
The study was conducted by the University Children's Hospitals in Freiburg, Heidelberg, Tübingen and Ulm, Germany), all located in the state of Baden-Württemberg in southwest Germany. Ethics approval was obtained from the Medical Faculties’ independent ethics committees (Freiburg: 256/20_201553; Heidelberg: S-294/2020; Tübingen: 293/2020BO2; Ulm: 152/20). Written informed consent was obtained from all participating adults and from parents of children at both time points at the in-person visit or by post. Children gave written assent where age-appropriate and their preferences on whether to provide blood samples were respected. The study was registered at the German Clinical Trials Register (DRKS), study ID 00021521, conducted according to the Declaration of Helsinki, and designed, analysed and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Serology and infection history
Blood for serological investigations at both T1 and T2 was drawn from all family members for whom we all obtained appropriate informed consent (and for children, assent). The following serological assays were used at both time points: (1) EuroImmun-Anti-SARS-CoV-2 ELISA IgG (S1), (2) Siemens Healthineers SARS-CoV-2 IgG (RBD) and (3) Roche Elecsys Ig (N). Participants were defined as seropositive if at least two of these three assays were positive and as seronegative if at least two out of three were negative. If neither of these criteria were met due to borderline or missing results, the serology result was considered unclear. For 8 participants, results from the Siemens and Roche assays were missing (due to insufficient sample volume) but a result from a recomWell SARS-CoV-2 IgG ELISA assay (Mikrogen Diagnostik) was available. In all 8 cases, the result from the Mikrogen assay was concordant with the EuroImmun assay and these results were therefore included.
Participants were defined as “infected” if they either (1) reported having had a positive RT-PCR test for SARS-CoV-2 prior to T1 or (2) were seropositive at T1 in at least two out of three commercial antibody tests, as defined above. Participants were defined as “exposed” controls if they both (1) did not report having had a positive SARS-CoV-2 RT-PCR test prior to T1 and (2) were seronegative at T1 as defined above. Amongst adults, 124 infected cases were identified by serology only (at least 2 of 3 antibody tests seropositive); 44 infected cases were identified by self-reported RT-PCR only; and 317 infected cases identified via both methods. Amongst adolescents, 35 infected cases were identified by serology only; no infected cases were identified by self-reported PCR only; and 24 infected cases identified by both methods. Amongst children, 114 infected cases were identified by serology only; 6 infected cases were identified by self-reported PCR only; and 30 infected cases identified by both methods. Thus in total 50 participants (6 children, 44 adults) reported having had a positive RT-PCR test for SARS-CoV-2 prior to T1 but were seronegative at T1 (“non-seroconverters”); these individuals were defined as infected, since they met the first criterion for the “infected” definition.
Amongst the 430 participants categorized as exposed (non-infected) at T1 for whom we had T2 serological data, we found evidence of seroconversion, implying a intervening silent SARS-CoV-2 infection, in only 6 participants (1.4%). Evidence of seroconversion was also present for 3 participants who were categorized as infected at T1 based on self-reported positive RT-PCR. These 9 participants were excluded for all analyses.
T2 serological data were missing from 89 adults, 22 adolescents, and 81 children. Moreover, T2 serological data were incomplete or ambiguous for 34 adults, 1 adolescent, and 6 children, typically because of waning antibody levels (e.g., seropositive status at T1 but unclear or seronegative at T2). Specifically within the group of those categorized as exposed (non-infected) at T1, serological data were missing from 41/229 adults (17.9%), 21/81 adolescents (26%) and 65/253 children (25.7%); we therefore had T2 serological data from 77.4% of participants categorized as exposed (non-infected) at T1. Assuming a seroconversion rate of 1.4% amongst exposed (non-infected) participants with missing T2 serological data, we would have excluded <1 participant from each age group (1.4% of 41 adults with missing T2 serological data = 0.57; 1.4% of 21 adolescents with missing T2 serological data = 0.29; 1.4% of 65 children with missing serological data = 0.91). We therefore do not believe that intervening silent infections could have substantially biased our results. Moreover, we repeated key analyses both including and excluding those with missing, incomplete or ambiguous T2 serological data. Since the pattern of findings remained the same, analyses are reported including these individuals.
Underlying health conditions were comparable between exposed and infected adults, and largely absent in adolescents and children (Table S1).
Questionnaires
At T1, participants provided basic demographic information as well as data on presence/absence of core COVID-19 symptoms in the time period around the first household SARS-CoV-2 infection (fever, cough, diarrhea, dysgeusia; for further details, see24,25). A subset of adults also completed a single-item self-report measure of health status at T1 (further details in the Supplementary Materials) comparing the current health status with the time before the pandemic. Answers were on a 5 point Likert scale (somewhat worse, much worse, same, somewhat better or much better). These scores were dichotomized (somewhat worse or much worse vs same, somewhat better or much better) and used to examine whether T1 health status change of the parent who completed the child's questionnaire was related to the child's prolonged symptoms. Scores on this measure were available for 161 adults (22.3%) who subsequently completed T2 questionnaires for their children.
T2 questionnaire data were collected and managed using Research Electronic Data Capture (REDCap) tools26,27 hosted at the respective study locations. REDCap is a secure, web-based software platform designed to support data capture for research studies, providing 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources.
At T2, adults completed German-language versions of the RAND 36-Item Health Survey 1.0,28, 29, 30, 31 a visual analogue scale (VAS) for current health status and the Fatigue Assessment Scale (FAS),32 as well as questions on long COVID symptoms designed specifically for this study. Questionnaires for adolescents and children were identical to those for adults, except that items from the RAND 36 were only completed by adults, since the activities described are mostly not applicable to children.
The RAND 36-Item Health Survey 1.028, 29, 30, 31 comprises 36 items that assess eight health concepts; for the current study, only the physical functioning scale (PF, 10 items), the role limitations caused by physical health scale (RL, 4 items) and the health change scale (1 item) were used. For the health change scale, instead of “Compared to one year ago”, the wording was changed to “Compared to the time before [month]”, where [month] was the month in which the earliest infection in the household occurred. Scores on all scales range from 0 to 100, with higher scores representing better outcomes. Due to wide variability in reported duration of reduced physical functioning and role limitations in the time period since the first household infection, PF scores and RL scores were dichotomized. One group comprised participants with a score of 100 (corresponding to no reduction in physical functioning/no role limitations) and participants with scores <100 who reported that the reduced physical functioning/role limitations were no longer present at T2. The other group comprised participants with reduced physical functioning/role limitations (scores < 100) still present at T2.
The Fatigue Assessment Scale (FAS, ©ild care foundation, www.ildcare.nl)32 is a ten-item questionnaire assessing self-reported frequency of a range of fatigue symptoms. It is widely used to assess fatigue in a range of diseases.32, 33, 34, 35 Scores can range from 10 to 50; scores between 10 and 21 indicate absence of fatigue (normal range); scores from 22-34 indicate fatigue and scores above 34 indicate severe fatigue. Questions were rephrased into the past tense and the instructions referred to the time period since the earliest confirmed SARS-CoV-2 infection in their household. However, participants’ indicated wide variability in fatigue duration across this time period, so FAS scores were also dichotomized. One group comprised participants with scores ≥ 21 (reflecting fatigue or severe fatigue) who additionally reported that this fatigue was still present at T2. All remaining participants were considered non-fatigued at T2 on this measure.
The visual analogue scale (VAS) was based on the EQ-VAS.36 Participants were asked “How would you describe your current health status?” (“Wie würden Sie Ihren heutigen Gesundheitszustand beschreiben?”) and provided answers on a visual analogue scale ranging from 0 (worst imaginable health state) to 100 (best imaginable health state).
Additional items were specifically developed for this study, based on discussion between participating clinicians and psychologists and drawing on existing literature on long COVID at the time of study design. These addressed:
-
(1)
changes in physical resilience in the timeperiod since the earliest confirmed SARS-CoV-2 infection in their household (7 point Likert scale with the anchor points -3=“very clear worsening”, 0=“no change” and +3=“very clear improvement”);
-
(2)
persistent pain (lasting three or more days and requiring medicinal treatment);
-
(3)
presence of the following symptoms in the entire time period since the earliest confirmed SARS-CoV-2 infection in their household: fatigue/tiredness/exhaustion; reduced physical resilience; dyspnoea; chest pressure; dysgeusia/dysosmia; low mood/anxiety; hair loss; disturbed sleep; concentration problems and memory problems. If any of these symptoms were present, participants reported the symptom's duration, severity (e.g., how much it limited daily activities: not at all, mildly, moderately or severely).
T2 questionnaires for adolescents and children were identical to those for adults, except that items from the RAND 36 were only completed by adults, since the activities described are mostly not applicable to children. Adults and adolescents aged 14-18 completed the questionnaires themselves; a parent (77·5% mothers, 22·5% fathers) completed the questionnaires for children <14 years.
Statistics
Data were analysed using SPSS version 28 and Stata version 17. Continuous and ordinal variables are reported as median (IQR) and compared using Mann-Whitney U tests. Frequencies are presented as percentages, compared using Fisher's exact tests and visualized using 95% confidence intervals using the Wilson method. Number of persistent and moderate or severe persistent symptoms were analysed using univariable and multivariable Poisson regression models with the centres included as fixed effect and standard errors clustered at the household level. Results are shown as incidence rate ratios (IRR). Since analyses were exploratory, all tests were two-tailed and no attempt was made to adjust for multiple testing; p-values and confidence intervals are descriptive. Moreover, we did not attempt to replace missing values; rather, missing values were excluded from statistical analysis. For the core long COVID symptoms questions missing data were rare; exact numbers of missing data for each symptom and subgroup can be determined by comparing subgroup totals with individual symptom totals in Tables S1-S6. Scores on the RAND-36 Physical Functioning scale and Role Limitation scale were missing for 3 participants; due to missing duration data, dichotomized T2 scores could not be computed for a further 10 participants on the Physical Functioning scale and a further 3 participants on the Role Limitation scale. Similarly, Fatigue Assessment Scores were missing for 4 participants; dichotomized T2 scores on this scale could not be computed for a further 16 participants due to missing duration data. Figures were generated with GraphPad Prism 9, Affinity Designer and R studio (ggplot2 package).
Role of funders
The funder of the study had no role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Results
Current health
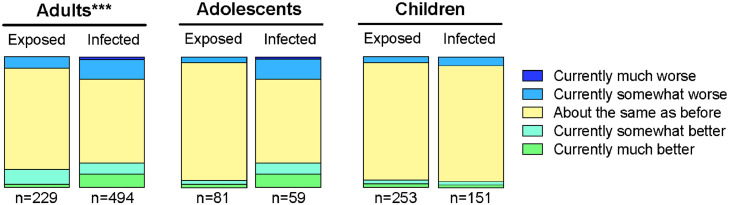
Overall, 723 adults, 140 adolescents (14-18 years) and 404 children (<14 years) were included in the study analysis (Table 1). The median household size was 4 persons, with 3 and 4 household members on the 25th and 75th percentile respectively (based on participants in the final data analysis). At T2, infected adults reported worse current health status on the VAS than exposed adults (Mann-Whitney U=43391.5, z=-5.072, p<·001, Table 1). Similarly, infected adolescents reported worse current health than their exposed peers (Mann-Whitney U=1842.0, z=-2.374, p<·018, Table 1). Current health status for infected and exposed children was not statistically significantly different (p = .601). The distribution of responses on the RAND-36 health change scale was significantly different for infected vs exposed adults (Mann-Whitney U=47990.0, z=-3.835, p<·001, Figure 2). For adolescents and children, distribution of scores on the RAND-36 health status scale did not differ statistically significantly between infected and exposed groups (p=.820 for adolescents, p=.336).
Table 1.
Demographic information for the participants included in the study. Current health at T2 compared to before the earliest infection in the household was lower in infected adults than exposed adults and in infected adolescents than exposed adolescents; there was no difference for children; see text for details.
| Adults |
Adolescents 14-18 |
Children <14 years |
|||||
|---|---|---|---|---|---|---|---|
| Exposed | Infected | Exposed | Infected | Exposed | Infected | ||
| N (% of age subgroup) | 229 (31.7%) | 494 (68.3%) | 81 (57.9%) | 59 (42.1%) | 253 (62.6%) | 151 (37.4%) | |
| Study centre, N (% within age and infection status subgroup) | Freiburg (145 households) | 103 (45.0%) | 208 (42.1%) | 45 (55.6%) | 19 (32.2%) | 111 (43.9%) | 58 (38.4%) |
| Heidelberg (49 households) | 23 (10.0%) | 73 (14.8%) | 7 (8.6%) | 13 (22.0%) | 26 (10.3%) | 22 (14.6%) | |
| Tübingen (99 households) | 68 (29.7%) | 150 (30.4%) | 19 (23.5%) | 14 (23.7%) | 87 (34.4) | 43 (28.5%) | |
| Ulm (48 households) | 35 (15.3%) | 63 (12.8%) | 10 (12.3%) | 13 (22.0%) | 29 (11.5%) | 28 (18.5%) | |
| Male, N (%) | 116 (50.7) | 236 (47.8) | 36 (44.4) | 31 (52.5) | 125 (49.4) | 81 (53.6) | |
| Age in years, median (Q1-Q3) | 43 (35-50) | 45 (39-50) | 16 (15-17) | 16 (14-17) | 7 (4-10) | 8 (5-11) | |
| Days between earliest household infection and T2, median (IQR) | 332 (315-366) | 332 (312-361) | 327 (314-350) | 350 (314-377) | 335 (313-358) | 340 (307-369) | |
| T2 Current health VAS score, median (IQR) | 92 (82-100) | 87 (80-95) | 96 (88-100) | 91 (80-100) | 100 (94-100) | 100 (94-100) | |
| Pre-existing conditions, N (% of age and infection status subgroup) | 85 (36.3%) | 181 (36.2%) | 13 (15.9%) | 9 (15.3%) | 15 (5.8%) | 21 (13.9%) | |
| Medication, N (% of age and infection status subgroup) | 86 (37.6%) | 168 (34.0%) | 4 (4.9%) | 6 (10.2%) | 8 (3.2%) | 8 (5.3%) | |
Figure 2.
Percentage of adults, adolescents (14-18 years) and children (<14 years) in each response category for the RAND-36 health change score, comparing current health to health before the earliest infection in their household. *** The Mann-Whitney U-test indicated that the distribution of responses for infected vs exposed adults was significantly different, p<.001. The distributions of responses for infected vs. exposed groups were not significantly different for adolescents (p=.820) or children (p=.336).
Moderate or severe persistent symptoms
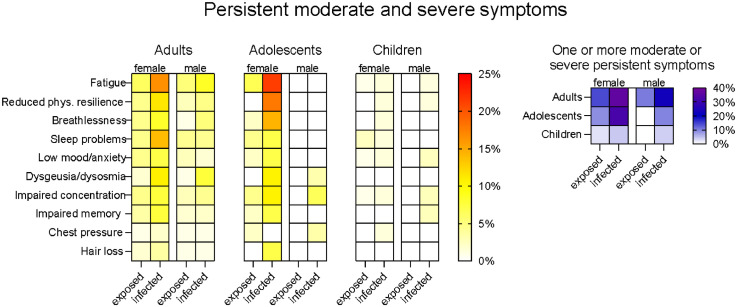
We investigated symptoms still present at T2 (11-12 months after infection/household exposure) that participants rated as moderate or severe (Figure 3); point estimates and their 95% confidence intervals are provided in Supplementary Tables S2–S4. Analyses of T2 symptoms of any severity are presented in the Supplementary Materials (Supplementary results, Table S5-S7 and Figure S1). At T2, infected women had higher prevalence than exposed women of moderate or severe fatigue (RR=2.66, 95% CI [1.23-5.74], p=·007), reduced physical resilience (RR=2.58, 95% CI [1.03–6.49], p=·033), dysgeusia/dysosmia (RR=6.08, 95% CI [1.47–25.12], p=·003) and sleep problems (RR=3.08, 95% CI [1.24–7.66], p=·010). Infected men had higher prevalence of moderate or severe dysgeusia/dysosmia (RR=8.85, 95% CI [1.20–65.45], p=·006) than their exposed counterparts. Moreover, infected adults showed an overall higher prevalence of moderate or severe breathlessness than exposed adults (RR=2.570, 95% CI [1.09–6.05], p=·022) but this did not reach significance in either gender subgroup (females: p=·270, males: p=·068).
Figure 3.
Moderate and severe persistent symptoms in adults, adolescents and children <14 years. Left panel: Percentage of participants in each age group who reported that each moderate or severe symptom was still present at T2, 11-12 months after the earliest household infection. Right panel: Percentage of participants in each group who reported one or more moderate and severe symptoms still present at T2. Overall participant numbers for each group were as follows: adults: 113 exposed females, 255 infected females, 116 exposed males, 236 infected males; adolescents 14-18 years: 45 exposed females, 28 infected females, 36 exposed males, 31 infected males; children <14 years: 128 exposed females, 70 infected females, 125 exposed males, 81 infected males. Numbers of participants for individual symptoms varied slightly due to missing data; precise values are given in the Supplementary Materials.
In infected adolescent girls, the prevalence of moderately or severely reduced physical resilience was higher than in exposed adolescent girls (p=·007). Although the prevalence of some other individual symptoms was markedly different between exposed and infected adolescent girls these differences did not reach significance, likely due to small subgroup size (e.g., fatigue: 3/45 amongst exposed and 6/28 amongst infected, RR=3.21, 95% CI [0.87-11.83], p=·078; see Table S5). In adolescent boys and in boys and girls <14 years, moderate and severe prolonged symptoms were uncommon or absent in both the exposed and infected groups (p values ≥ .148).
Compared with the respective exposed groups, more infected women (RR=2.57, 95% CI [1.59–4.17], p<·001), infected men (RR=2.21, 95% CI [1.23-3.97], p=·005) and infected adolescent girls (RR=3.62, 95% CI [1.23–10.64], p=·025) had one or more moderate or severe symptoms still present at T2. In other infected subgroups, the prevalence of one or more moderate or severe symptoms was not statistically significantly different to their exposed counterparts. However, the between-group difference amongst adolescent boys trended in the same direction as for adolescent girls (p=·094) and when the analysis was collapsed across gender, the prevalence was statistically significantly higher for infected than exposed adolescents (RR=4.12, 95% CI [1.40-12.14], p=·006).
Infected men more frequently experienced pain as compared to exposed men (RR=2.21, 95% CI [1.06–4.60], p=·026, Supplementary Table S7). This effect was not mirrored in other age or sex groups.
Physical functioning and fatigue
The distribution of reported changes in physical resilience was significantly different for infected vs exposed adults (Mann-Whitney U=49421, z=-2.882, p=·004, Supplementary Figure S2); the distributions were not statistically significantly different for adolescents and children.
125 (17·6%) participants had experienced ongoing reductions in physical functioning at T2 on the RAND-36 physical functioning scale, whereas the remaining 585 (82·4%) reported no or only temporarily reduced physical functioning. However, infected adults were statistically significantly more likely (21·6%) to have reduced physical function that persisted until T2 than exposed adults (8·9%, RR=2.44, 95% CI [1.55–3.83], p<·001). The difference was apparent in both men and women (Supplementary Figure S3).
On the RAND-36 role limitations scale, 83 (11·6%) participants reported ongoing role limitations due to physical functioning. The remaining 634 participants (88·4%) had no or only temporary role limitations. Again, infected adults were statistically significantly more likely (13·5%) to have role limitations that persisted until T2 than exposed adults (7·5%, RR=1.80, 95% CI [1.08-2.99], p=·023). The effect did not reach significance in either gender subgroup (Supplementary Figure S3).
Based on dichotomized Fatigue Assessment Scale scores, 132 adults, 20 adolescents and 10 children continued to have fatigue or severe fatigue at T2. All remaining participants were considered non-fatigued at T2 on this measure (578 adults, 119 adolescents, 388 children). 20·6% of infected adults were fatigued/severely fatigued at T2, compared to 14·3% of exposed adults (RR=1.44, 95% CI [1.00–2.08], p=·049). This association was present amongst men (RR=2.05, 95% CI[1.03-4.0], p=·044) but not women. For adolescents and children, there were no statistically significant differences between infected and exposed groups, including when split by gender (Supplementary Figure S4).
Influence of symptoms during the acute infection phase
At T1, 83.0% of infected adults, 48.5% of infected adolescents and 49.5% of infected children reported having had one or more of the core acute symptoms during the time period of the infections(s) in the household (data missing for 194 adults (26.8%), 43 adolescents (30.7%) and 105 children (26.0%)). 21 adults (4.3% of those infected), 1 child (0.7% of those infected) and no adolescents reported having been hospitalized due to COVID-19.
Amongst infected individuals, those with acute symptoms during the SARS-CoV-2 infection had more symptoms of any severity that persisted until T2 than those who were asymptomatic. This pattern was apparent in both men (IRR=2·11, p=·009, 95% CI [1.21–3.69]) and women (IRR=2·14, p=·037, 95% CI [1.05–4.39]) as well as in adolescent girls (IRR=11·49, p=·072, 95% CI [0.81–163.59]), but not in adolescent boys or children <14 years (p values >.37). In the multi-variable regression model using the individual acute symptoms as predictors, cough (IRR=1·39, p=·023, 95% CI [1.05–1.84]), diarrhoea (IRR=1·73, p<·001, 95% CI [1.27–2.36]) and dysgeusia (IRR=1·85, p<·001, 95% CI [1.39–2.47]), but not fever (p =.795) were statistically significant predictors of the number of persistent symptoms.
A similar pattern emerged for moderate or severe persistent symptoms: those with symptomatic acute infection had more moderate or severe persistent symptoms than those with an asymptomatic infection. When separated by age and gender, this pattern held for women (IRR=2·48, p=·024, 95% CI [1.13–5.44]) but not for other subgroups (p values > .39). The multi-variable regression model showed that diarrhoea (IRR=1·73, p=·010, 95% CI [1.14–2.61]) and dysgeusia (IRR=2·14, p=·001, 95% CI [1.38–3.30]), but not fever (p =.81) or cough (p = .29), during the acute phase statistically significantly predicted the number of moderate or severe persistent symptoms.
Symptom associations within families
We first examined the subset of children for whom we had data on the reporting parent's T1 health status (n=162). As in the whole cohort, persistent symptoms were uncommon in this subgroup (n=15, 9·2% across both exposed and infected children). However, parents who reported that their own health status at T1 was worse or much worse than before the pandemic (n=37) were around three times as likely to report that their child had symptoms that persisted until T2 compared with parents (n=125) who reported a T1 health status of same/better/much better (18·9% vs. 6·4%, p=·046).
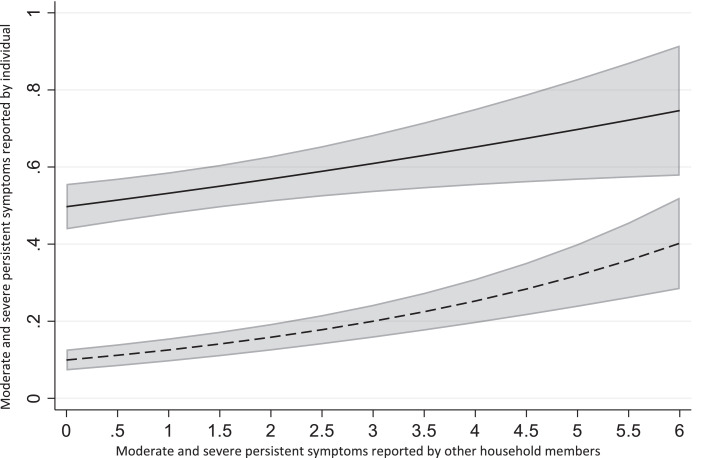
We then considered associations amongst numbers of symptoms within households. 41% of participants lived in households where none of the other household members reported moderate or severe persistent symptoms at T2. However, there was a statistically significant association between participants’ own moderate or severe persistent symptoms and the total number of moderate or severe persistent symptoms reported by other members of their household (IRR=1·11, p=·005, 95% CI [1.03–1.20], Figure 4), meaning that the risk that a participant experienced one additional moderate or severe persistent symptom increased by 12% for every additional moderate or severe persistent symptom amongst members of their household. When separated by age and gender (Supplementary Figure S5), the association was statistically significant for exposed women (IRR=1·17, p=·001, 95% CI [1.07–1.29]), exposed men (IRR=1·15, p<·001, 95% CI [1.07–1.24]), exposed men (IRR=1·41, p=·001, 95% CI [1.14–1.72]) and exposed girls <14 years (IRR=1·49, p<·001, 95% CI [1.28–1.73]) as well as for infected women (IRR=1·15, p=·006, 95% CI [1.04–1.27]) and infected girls <14 years (IRR=1·43, p=·065, 95% CI [0.98–2.10]).
Figure 4.
Associations between the total number of moderate and severe persistent symptoms at T2 reported by other household members and the number of moderate and severe symptoms reported by an individual, based on uni-variable Poisson regression model with the centre included as random intercepts, in exposed (dotted line) and infected (solid line) groups across all age and gender subgroups. The grey area shows 95% confidence intervals.
Discussion
Using a large prospective multi-centre cohort of families, we examined long COVID symptoms in families 11-12 months after mild/asymptomatic SARS-CoV-2 infection or household exposure. Our results indicate the critical role of family context on prolonged symptoms following SARS-CoV-2 infection.
Consistent with previous studies,3,5,6,37 infected adults were more likely than exposed adults to report worse health outcomes, including worse current health, more physical functioning difficulties, role limitations due to these difficulties, fatigue and a range of persistent physical and psychological symptoms. Notably, negative outcomes were also relatively common amongst the exposed non-infected adults, e.g., almost 1 in 4 exposed adults reported having at least one symptom still present around 11-12 months after SARS-CoV-2 exposure – despite subsequent negative SARS-CoV-2 serological results. This could reflect baseline prevalence of non-specific symptoms, elevated prevalence due to the pandemic and infection control measures, effects specific to exposed family members of infected individuals or – most likely – a mixture of these. Regardless of the reason, the high prevalence of such outcomes in exposed but non-infected individuals demonstrates the importance of including an appropriate comparator group in long COVID studies. In infected individuals, the prevalence of moderate or severe persistent symptoms 11-12 months after infection was elevated in men, women and adolescent girls. It is reassuring that younger children do not appear to be at statistically significant risk of developing prolonged moderate or severe symptoms, although our sample was not big enough to exclude the possibility of very low-prevalence sequelae.
We found evidence that individuals with symptomatic COVID-19 had more persistent symptoms than those with asymptomatic infection, consistent with previous studies.3,37,38 Our data suggest that acute diarrhoea and dysgeusia may be particularly associated with higher likelihood of persistent symptoms, which could help to identify individuals at risk of long COVID.
Consistent with previous data,37,39 we found gender differences in long COVID symptoms following SARS-CoV-2 infection. At T2, infected women had elevated prevalence of moderate or severe fatigue, sleep disruption and reduced physical resilience. Prevalence of moderate or severe dysguesia/dysosmia was elevated in both genders compared to exposed controls. Strikingly, over a third (36·4%) of infected women reported at least one moderate or severe symptom lasting until T2; this was less frequent in infected men (22·9%). Gender differences were also present in the exposed control group, though these were not so marked (e.g., 14·2% of exposed women vs 10·3% of exposed men reported at least one moderate or severe symptom). We also found evidence for gender differences in adolescents aged 14-18 - again consistent with previous studies.16 In particular, infected adolescent girls but not boys were more likely to have at least one moderate or severe symptom at T2.
The conspicuously high proportion of adolescent girls who reported ongoing symptoms 11-12 months after a SARS-CoV-2 infection warrants particular attention. Over half (15/28) of infected girls aged 14-18 reported at least one persistent symptom of any severity at T2. Moreover, almost a third (9/28) had at least one moderate or severe persistent symptom. Although the subgroup was small, this finding is consistent with previous pediatric data showing gender differences both in long COVID and fatigue/chronic fatigue syndrome,15,18,14,40 and is in keeping with the evidence for gender differences in adults. Our findings reinforce the need for further research focussing on the causes, risk factors and appropriate prevention and treatment strategies for long COVID in adolescent girls.
Another key finding of this study is that the number of moderate or severe persistent symptoms in both exposed and infected individuals was associated with the number of moderate or severe persistent symptoms in other household members. This finding was reinforced by the association between parents’ health status at T1 and reported symptoms in their children at T2. There are several plausible, non-mutually exclusive explanations for such associations. For example, shared genetic factors may predispose both parents and their children to develop prolonged symptoms following SARS-CoV-2 infection. Parents’ perceptions of their own symptoms may have influenced their perception or reporting of their children's symptoms. A significant role for the importance of symptom perceptions following COVID-19 is supported by a French study, where self-reported SARS-CoV-2 infection was more likely associated with persistent physical symptoms than laboratory confirmed disease.41 Another possible explanation is that family members’ symptoms or behaviour affected symptomatology in children. This would be consistent with previous studies showing that parents’ symptoms and behaviour can be associated with children's symptoms in a range of pediatric health conditions.17,18,23 It would also fit with evidence that negative mental health outcomes of lockdown in children may be mediated by parents’ stress and overreactivity.42 These findings have important implications, since support for parents or family-level therapeutic interventions could prove useful in preventing or treating long COVID in this age group.
Several strengths of the study should be emphasized. Our cohort comprised almost exclusively individuals with mild or asymptomatic disease, and thus is representative of the majority of SARS-CoV-2 infections.2 By using three serological assays, we could classify individuals as infected or exposed with high confidence, even following asymptomatic infection. Moreover, infected and exposed groups belonged to the same families and thus were exceptionally well matched for socio-demographic and environmental factors, including those related to the pandemic and associated public health measures. Importantly, both groups experienced household quarantine due to SARS-CoV-2 infection in the household. Thus between-group differences cannot be due to the pandemic in general, lockdown measures or household quarantine. Although having an infected family member and being in household quarantine could have caused psychosocial stress in the exposed group, with concomitant health impact,43 our study did not investigate families with no infected individuals, which precludes analysis of this issue.
The study also has several limitations. The sample size was relatively small, especially for adolescents, and thus was not able to detect very low prevalence symptoms. However, our findings are in keeping with a recent meta-analysis showing that most prolonged symptoms are uncommon in infected than non-infected children.16 The study was performed relatively early in the pandemic (infections in January-May 2020) in a specific region of Germany. It is possible that virus variants of concern, which have emerged since, show a different risk of long COVID, especially when causing a clinically milder disease. Also generalization to other regions should be done with caution. The data on children under 14 years old were reported by one of the parents, so the subjective interpretation of the child's health status as well as their own symptoms might have impacted the reporting of the child's symptoms and health status. Further, a selection bias may have been be generated through the retrospective identification of the participating families. Similarly, the retrospective collection of self-reported data might have introduced a recall bias, though we expect that the impact of this bias would be minimized by investigating all the family members irrespective of their serostatus. It is possible that a small number of individuals with long COVID symptoms may have received therapeutic interventions between T1 and T2; data on this were not collected. Although a possible intervention might have influenced the reported data, the absence of established effective treatments and the difficulties of accessing them during the pandemic means that we would assume that any effect of such interventions would be minimal. Finally, families were not blinded for their serological status, which was communicated after the T1 visit. Although this could represent a source of bias, long-term blinding of participants to their SARS-CoV-2 infection history would have been ethically and medically problematic. Additional sources of bias might have been introduced by participants not completing the T2 questionnaire (64.6% of T1 participants completed the T2 questionnaires) and the partial utilization of questions specifically developed for this study which were not specifically validated before.
In sum, our data from a controlled, multi-centre study suggests that infected men, women and adolescent girls are at increased risk of negative outcomes 11-12 months after SARS-CoV-2 infection compared to exposed household members. The prevalence of moderate to severe symptoms was not elevated in infected adolescent boys aged 14-18 or in children <14 years compared to controls. Moreover, we found some evidence that prolonged symptoms tend to cluster within families. Our findings highlight the need for caution in interpreting the causes of prolonged symptoms in SARS-CoV-2 infected individuals, especially children, since such symptoms almost certainly have multiple possible causes and are likely driven by both physiological and psychosocial mechanisms. Our results also emphasize the need for research on family-level mechanisms and interventions in long COVID.44,45
Contributors
A.H., P.F., P.H. and R.E. conceived the study. A.H., A.J., M.S., P.F. and R.E. designed the experiments. R.E., H.R., A.J., B.T., G.F.H., P.H., A.R.F. and K.M.D. procured funding. A.H., R.E., H.R., A.J., B.T., G.F.H., P.H., M.S., C.E., K.K., A.N., A.W., A.P., N.S.M., F.L., T.S., M.Z., D.F., B.M., D.H., A.D., E-M J.,A.F. and K.M.D. analyzed and curated data. A.H., A.J., M.S., R.E., P.H., S.B., H.R., B.H. J.R., T.H. and E-M.J. were involved in patient recruitment and sample or data collection. D.H., A.D., E-M J., D.F., T.S. H-G K., B.M. and T.G. performed and/or supervised experiments. A.H. and R.E. wrote the first draft of the manuscript. All authors approved the final version of the manuscript. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication.
Data sharing statement
A short version of the study protocol is available at the German Clinical Trials Register (DRKS, www.drks.de), study ID 00021521. The full study protocol is available from https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00021521. Anonymised participant data can be made available upon reasonable request with publication.
Declaration of interests
The authors declare that they have no relevant conflicts of interest.
Acknowledgements
We gratefully acknowledge the families who participated in this study and the physicians, nurses and laboratory staff at the four study centres.
We are grateful to the HILDA-Biobank and we thank Tessa Görne, Linus Fritsch, Bianca Rippberger, Simone Hock, Aileen Heselich, Alicia Zink, and Tara Marianna Ziegelbauer for organisational support and in conducting the study in Freiburg. We are also grateful to the HILDA biobank, in particular Ali Riz Kaya, Marco Teller and dirk Lebrecht at the FREEZE Biobank Freiburg. In addition, we would like to thank Julia Euler, Florian Gleich, Jürgen Grulich-Henn, Iris Schelletter, Heike Matzkuhn, Kristine Chobanyan-Jürgens, Michal Fischer, Christina Klose, Stefanie Wolf, Ira Pistorius-Knopf, Markus Zorn, Sylvia Parthé, and Maria Anders-Össwein (Heidelberg). We thank Andrea Evers-Bischoff, Andrea Bevot and the CPCS at the University Hospital Tübingen for organizational support in conducting the study. We are grateful to the REDCap Support Team at the University Hospital Tübingen, in particular Stephanie Biergans and Christian Erhardt for technical assistance and support in data management. We thank Carmen Blum, Sevil Essig, Ulrike Formentini, Gudrun Kirsch, Alexandra Niedermeyer, and Boram Song for assistance with sample processing and patient material storage. We thank Sandra Steinmann, Yvonne Müller, and Vanessa Missel for organizational support in conducting the study.
The COVID-19 BaWü study was funded by the Ministry of Science, Research and the Arts Baden-Württemberg, Germany, within the framework of the special funding line for COVID-19 research, part of the measures to combat Coronavirus SARS-CoV-2 pandemic in the field of medical research. Roland Elling was supported by the Berta Ottenstein Programme for Advanced Clinician scientists, Faculty of Medicine, University of Freiburg. The IMM-PACT Clinician Scientist Programme is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 413517907. The funders of the study had no role in design or conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104245.
Appendix. Supplementary materials
References
- 1.Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA. 2021;326(9):869–871. doi: 10.1001/jama.2021.11880%JJAMA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yong SJ, Liu S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev Med Virol. 2022;32 doi: 10.1002/rmv.2315. [DOI] [PubMed] [Google Scholar]
- 5.Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5) doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9) doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravens-Sieberer U, Kaman A, Erhart M, Devine J, Schlack R, Otto C. Impact of the COVID-19 pandemic on quality of life and mental health in children and adolescents in Germany. Eur Child Adolesc Psychiatry. 2021;31:879–889. doi: 10.1007/s00787-021-01726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bussières EL, Malboeuf-Hurtubise C, Meilleur A, et al. Consequences of the COVID-19 pandemic on children's mental health: a meta-analysis. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.691659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Racine N, McArthur BA, Cooke JE, Eirich R, Zhu J, Madigan S. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA Pediatr. 2021;175(11):1142–1150. doi: 10.1001/jamapediatrics.2021.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarrington JS, Lasser J, Garcia D, et al. Impact of the COVID-19 pandemic on mental health among 157,213 Americans. J Affect Disord. 2021;286:64–70. doi: 10.1016/j.jad.2021.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19686. e2019686-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5(10):708–718. doi: 10.1016/S2352-4642(21)00198-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA. 2021;326(9):869–871. doi: 10.1001/jama.2021.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenson T, Pereira SP, Shafran R, et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Health. 2022;6(4):230–239. doi: 10.1016/S2352-4642(22)00022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roessler M, Tesch F, Batram M, et al. Post COVID-19 in children, adolescents, and adults: results of a matched cohort study including more than 150,000 individuals with COVID-19. MedRxiv. 2021 doi: 10.1101/2021.10.21.21265133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behnood SA, Shafran R, Bennett SD, et al. Persistent symptoms following SARS-CoV-2 infection among children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect. 2021;84(2):158–170. doi: 10.1016/j.jinf.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palermo TM, Valrie CR, Karlson CW. Family and parent influences on pediatric chronic pain: a developmental perspective. Am Psychol. 2014;69(2):142–152. doi: 10.1037/a0035216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lievesley K, Rimes KA, Chalder T. A review of the predisposing, precipitating and perpetuating factors in Chronic Fatigue Syndrome in children and adolescents. Clin Psychol Rev. 2014;34(3):233–248. doi: 10.1016/j.cpr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Anthony KK, Bromberg MH, Gil KM, Schanberg LE. Parental perceptions of child vulnerability and parent stress as predictors of pain and adjustment in children with chronic arthritis. Children’s Health Care. 2011;40:53–69. doi: 10.1080/02739615.2011.537938. [DOI] [Google Scholar]
- 20.Schanberg LE, Anthony KK, Gil KM, Lefebvre JC, Kredich DW, Macharoni LM. Family pain history predicts child health status in children with chronic rheumatic disease. Pediatrics. 2001;108(3):E47. doi: 10.1542/peds.108.3.e47. [DOI] [PubMed] [Google Scholar]
- 21.Spurrier NJ, Sawyer MG, Staugas R, Martin AJ, Kennedy D, Streiner DL. Association between parental perception of children's vulnerability to illness and management of children's asthma. Pediatr Pulmonol. 2000;29(2):88–93. doi: 10.1002/(sici)1099-0496(200002)29:2<88::aid-ppul2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 22.Law EF, Blume H, Palermo TM. Longitudinal impact of parent factors in adolescents with migraine and tension-type headache. Headache. 2020;60(8):1722–1733. doi: 10.1111/head.13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll S, Chalder T, Hemingway C, et al. Adolescent and parent factors related to fatigue in paediatric multiple sclerosis and chronic fatigue syndrome: a comparative study. Eur J Paediatr Neurol. 2019;23(1):70–80. doi: 10.1016/j.ejpn.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Stich M, Elling R, Renk H, et al. Transmission of severe acute respiratory syndrome coronavirus 2 in households with children, Southwest Germany, May–August 2020. Emerg Infect Dis. 2021;27(12):3009-–3019. doi: 10.3201/eid2712.210978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renk H, Dulovic A, Seidel A, et al. Robust and durable serological response following pediatric SARS-CoV-2 infection. Nat Commun. 2022;13(1):128. doi: 10.1038/s41467-021-27595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 29.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33(5):350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 30.Bullinger M. German translation and psychometric testing of the SF-36 health survey: preliminary results from the IQOLA project. Soc Sci Med. 1995;41(10):1359–1366. doi: 10.1016/0277-9536(95)00115-n. [DOI] [PubMed] [Google Scholar]
- 31.Bullinger M, Kirchberger I, Ware J. Der deutsche SF-36 health survey Übersetzung und psychometrische Testung eines krankheitsübergreifenden Instruments zur Erfassung der gesundheitsbezogenen Lebensqualität. Z Gesundh Wiss. 1995;3(1):21–36. [Google Scholar]
- 32.De Vries J, Michielsen H, Van Heck GL, Drent M. Measuring fatigue in sarcoidosis: the Fatigue Assessment Scale (FAS) Br J Health Psychol. 2004;9(Pt 3):279–291. doi: 10.1348/1359107041557048. [DOI] [PubMed] [Google Scholar]
- 33.Singh R, Kluding PM. Fatigue and related factors in people with type 2 diabetes. Diabetes Educ. 2013;39(3):320–326. doi: 10.1177/0145721713479144. [DOI] [PubMed] [Google Scholar]
- 34.Smith OR, van den Broek KC, Renkens M, Denollet J. Comparison of fatigue levels in patients with stroke and patients with end-stage heart failure: application of the fatigue assessment scale. J Am Geriatr Soc. 2008;56(10):1915–1919. doi: 10.1111/j.1532-5415.2008.01925.x. [DOI] [PubMed] [Google Scholar]
- 35.Schoormans D, Jansen M, Mols F, Oerlemans S. Negative illness perceptions are related to more fatigue among haematological cancer survivors: a PROFILES study. Acta Oncol. 2020;59(8):959–966. doi: 10.1080/0284186X.2020.1759823. [DOI] [PubMed] [Google Scholar]
- 36.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 37.Blomberg B, Mohn KG-I, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27(9):1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahanic S, Tymoszuk P, Ausserhofer D, et al. Phenotyping of acute and persistent COVID-19 features in the outpatient setting: exploratory analysis of an international cross-sectional online survey. Clin Infect Dis. 2022;75(1):e418–e431. doi: 10.1093/cid/ciab978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12(1):6571. doi: 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crawley E. The epidemiology of chronic fatigue syndrome/myalgic encephalitis in children. Arch Dis Child. 2014;99(2):171–174. doi: 10.1136/archdischild-2012-302156. [DOI] [PubMed] [Google Scholar]
- 41.Matta J, Wiernik E, Robineau O, et al. Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182(1):19–25. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Achterberg M, Dobbelaar S, Boer OD, Crone EA. Perceived stress as mediator for longitudinal effects of the COVID-19 lockdown on wellbeing of parents and children. Sci Rep. 2021;11(1):2971. doi: 10.1038/s41598-021-81720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet North Am Ed. 2020;395(10227):912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lloyd S, Chalder T, Rimes KA. Family-focused cognitive behaviour therapy versus psycho-education for adolescents with chronic fatigue syndrome: long-term follow-up of an RCT. Behav Res Ther. 2012;50(11):719–725. doi: 10.1016/j.brat.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Law E, Fisher E, Eccleston C, Palermo TM. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev. 2019;3(3) doi: 10.1002/14651858.CD009660.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.