Abstract
Pulmonary fibrosis is a chronic, progressive, incurable interstitial lung disease with high mortality after diagnosis and remains a global public health problem. Despite advances and breakthroughs in understanding the pathogenesis of pulmonary fibrosis, there are still no effective methods for the prevention and treatment of pulmonary fibrosis. The existing treatment options are imperfect, expensive, and have considerable limitations in effectiveness and safety. Hence, there is an urgent need to find novel therapeutic targets. The nuclear factor erythroid 2-related factor 2 (Nrf2) is a central regulator of cellular antioxidative responses, inflammation, and restoration of redox balance. Accumulating reports reveal that Nrf2 activators exhibit potent antifibrosis effects and significantly attenuate pulmonary fibrosis in vivo and in vitro. This review summarizes the current Nrf2-related knowledge about the regulatory mechanism and potential therapies in the process of pulmonary fibrosis. Nrf2 orchestrates the activation of multiple protective genes that target inflammation, oxidative stress, fibroblast–myofibroblast differentiation (FMD), and epithelial–mesenchymal transition (EMT), and the mechanisms involve Nrf2 and its downstream antioxidant, Nrf2/HO−1/NQO1, Nrf2/NOX4, and Nrf2/GSH signaling pathway. We hope to indicate potential for Nrf2 system as a therapeutic target for pulmonary fibrosis.
Keywords: nuclear factor erythroid 2-related factor 2, pulmonary fibrosis, inflammation, oxidative stress, signaling pathways
1. Introduction
Pulmonary fibrosis (PF) describes a heterogeneous group of chronic, progressive, and incurable interstitial lung disorders characterized by induced scar formation and irreversible destruction of the lung parenchyma [1,2,3,4]. Based on etiological factors, fibrotic lung diseases are mainly classified into idiopathic pulmonary fibrosis (IPF), allergic asthma, cystic lung disease, scleroderma, granulomatous lung disease, sarcoidosis, and chronic obstructive pulmonary disease (COPD) [5]. Among these, IPF is the most notable and common type of idiopathic interstitial pneumonia, lacking an identifiable etiology and with high mortality [1,6]. It is characterized by aberrantly activated lung epithelial cells, inflammatory infiltrate, activation of lung fibroblasts, and excessive accumulation of extracellular matrix (ECM) in lung tissues that ultimately lead to respiratory failure, and eventually death if left untreated [7,8]. An array of triggers, including environmental pollutants, herbicides, drug side effects, particles, genetic abnormalities, autoimmune disorders, chronic infection, and cigarette smoking may cause IPF [9,10,11,12]. Nearly 200,000 people in the United States and over 5 million people worldwide are affected by IPF, and approximately 82–83% of deaths, incident cases, and prevalent cases occur in patients over 70 years old, imposing a great economic burden on the country as well as individuals [13,14,15]. A recent study from Germany showed that most patients with IPF did not receive medication [13], but previous research has demonstrated that patients who develop IPF without being treated have a median survival of only 3–5 years after diagnosis [3]. Consequently, there remains a major medical need for effective, safe, and well-tolerated treatments for IPF.
Unfortunately, there is currently no effective treatment for curing or reversing the progression of PF. Immunosuppressants (e.g., cyclophosphamide) and corticosteroids (e.g., dexamethasone) have been used to treat acute exacerbation of IPF, aiming at reducing symptoms and the underlying inflammation, but limited efficacy and potential side effects have restricted their application [16,17]. The FDA approved the anti-fibrotic drugs nintedanib and pirfenidone for the treatment of PF in 2014 because they were shown to delay the progression of PF [18,19]. However, while they show a clinical benefit, they cannot improve survival [20]. Currently, lung transplantation is the only life-sustaining intervention for end-stage IPF [21], but chronic lung transplant dysfunction, infection, and extrapulmonary complications lead to a poor postoperative long-term survival rate [22]. The high cost of operation and the scarcity of lung donors are two specific challenges that require consideration. Even though the pathogenic mechanisms of IPF having been studied extensively (Figure 1), few therapeutics have been successfully used in the clinic, and potential treatment methods to improve patients’ quality of life are lacking [23].
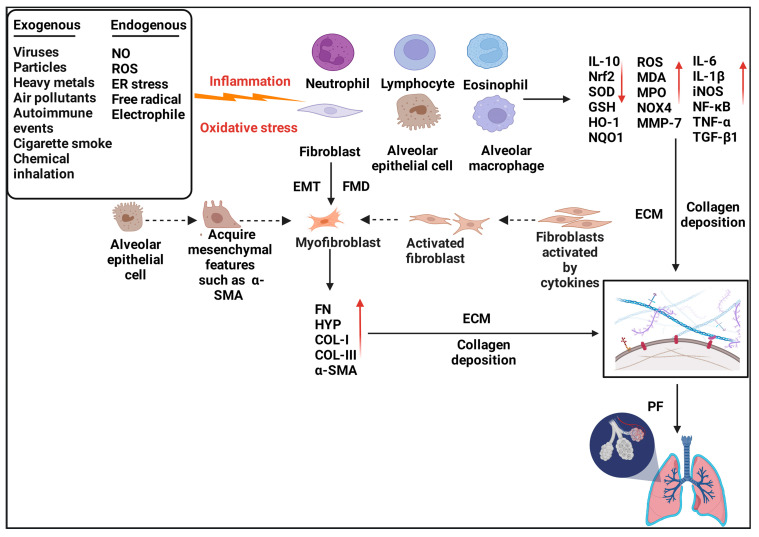
Figure 1.
Pathogenesis of pulmonary fibrosis. Various exogenous and endogenous factors, such as autoimmune diseases, viral infections, cigarette smoke, inhalation of toxic substances, and free radicals, can lead to damage to the alveolar epithelial cells (AECs), aberrant activation of immune cells (neutrophils, alveolar macrophages, lymphocytes, and eosinophils), and fibroblasts. These lung cells secrete multiple pro-inflammatory and pro-fibrotic factors which can accelerate the EMT process, induce the transition of AECs to lung fibroblasts, trigger the activation of quiescent fibroblasts, and promote the differentiation of fibroblasts to myofibroblasts. Myofibroblasts can further release a large amount of collagen and ECM, resulting in hyperproliferation of fibroblasts and accelerates the progression of pulmonary fibrosis.
Therefore, clarifying the underlying molecular mechanism in PF and discovering efficient prevention and treatment approaches that could increase life expectancy are urgent future research directions.
2. Nrf2
Extensive studies have demonstrated that the nuclear factor erythroid 2-related factor 2 (Nrf2) is a critical transcription factor that coordinates the expression of more than 500 cytoprotective and metabolic genes [24], particularly classic antioxidant and detoxification enzymes, to restore internal cellular homeostasis, redox balance, and the response to diverse stresses [25,26,27]. It is now widely recognized that Nrf2 plays a protective role in many diseases in multiple organ systems, such as osteoporosis [28], Alzheimer’s disease [29], lung fibrosis [30], kidney [31], and cardiovascular system disease [32], in which oxidative stress and inflammation are thought to participate in the underlying pathological mechanisms.
2.1. Structure of Nrf2 and Keap1
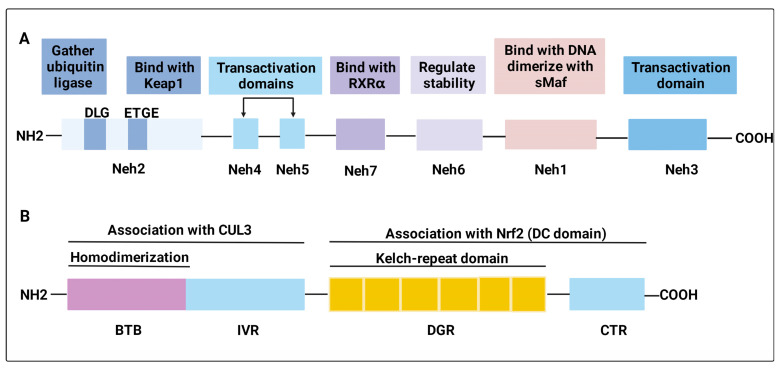
Nrf2, first isolated and characterized by Moi et al. in 1994, is encoded by the Nuclear Factor, Erythroid 2 Like 2 (NFE2) gene and belongs to the Cap“n” Collar (CNC) subfamily of basic leucine zipper (bZIP) transcription factors [33]. Nrf2 contains seven highly conserved Nrf2 ECH homology (Neh) domains, each with a distinct function, known as Neh1–Neh7 (Figure 2A) [34]. Among these, the Neh2 domain, which is located in the N-terminus of Nrf2, plays a major regulatory role. Neh2 harbors two separate sequences, the DLG element and the ETGE tetrapeptide, mediating the process of gathering a ubiquitin ligase to the fusion protein and the redox-sensitive recruitment of Nrf2 to Kelch-like ECH-associated protein 1 (Keap1), respectively [35,36,37]. Notably, the ETGE motif is the Keap1-binding site. Neh1 contains the DNA binding motif and the Cap“n” collar basic leucine zipper domain that dimerizes with small Maf proteins on the promoters of target genes. It has been reported to regulate the stability of Nrf2 by forming a nuclear complex with UbcM2, a ubiquitin-conjugating enzyme [38]. Neh3, Neh4, and Neh5 are transactivation domains that interact with coactivators [39,40]. Neh6 is a Keap1-independent degron of Nrf2. It harbors a group of serine residues phosphorylated by glycogen synthase kinase 3 (GSK-3), resulting in the facilitation of Nrf2 degradation and regulating the stability of Nrf2 [41,42]. Previous evidence has shown that Neh7 binds with retinoic X receptor alpha (RXRα) to weaken the cytoprotective effect of Nrf2 [43].
Figure 2.
Structures of Nrf2 and Keap1. (A) The Nrf2 protein comprises seven Neh domains, known as Neh1–Neh7. The Neh1 domain is responsible for DNA binding and dimerization with the sMaf proteins; the Neh2 domain mediates the interaction with Keap1 through the ETGE motifs and gathers a ubiquitin ligase to the fusion protein through the DLG element; the Neh3, Neh4, and Neh5 domains are transactivation domains; the Neh6 domain regulates Nrf2 stability; and the Neh7 domain binds with RXRα to weaken the cytoprotective effect of Nrf2. (B) Keap1 possesses three functional domains. The BTB domain mediates Keap1 homodimerization and associates with Cul3; the DC directly associates with Nrf2 Neh2 domain; IVR domain contains critical cysteine residues and connects the BTB domain with DC domain.
2.2. Nrf2 Activation
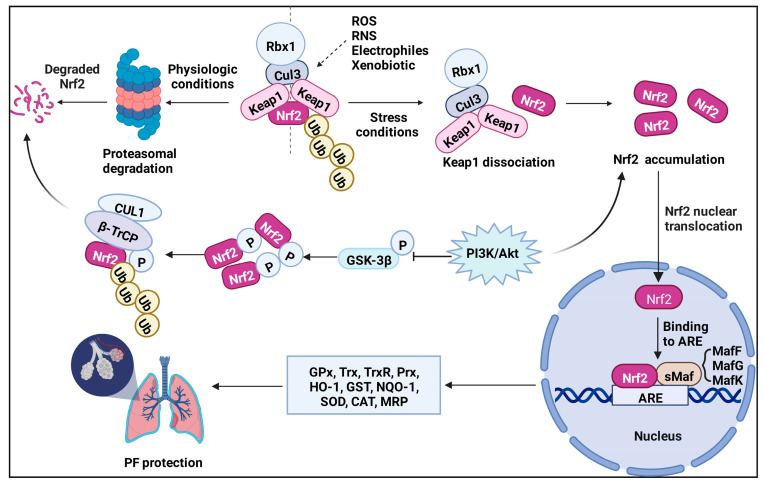
Under a normal physiologic state, Nrf2 is anchored in the cytoplasm by binding to the E3 ubiquitin ligase Keap1 or phosphorylated by glycogen synthase kinase 3β (GSK-3β), targeting Nrf2 for its ubiquitination and proteasomal degradation [44,45]. Keap1 is a component of a ubiquitin E3 ligase [46], acting as a cysteine thiol-rich sensor of redox insults [47]. In 1999, Keap1 was identified by analyzing differential Nrf2 activity manifested in a transfected cell line [36]. Keap1 possesses three functional domains, a broad complex/tram track/bric-a-brac (BTB), an intervening region (IVR), and a double glycine repeat (DGR) and COOH-terminal region (CTR) domain (DC domain) (Figure 2B). Keap1 associates with CUL3 through its BTB domain, which is required for Keap1 dimerization [48], and part of its intervening region (IVR) domain [49,50]. The Nrf2 Neh2 domain directly interacts with the Keap1 DC domain [51]. Keap1, Cul3, and Rbx1 assemble into an efficient E3 ubiquitin ligase, which negatively regulates Nrf2 protein levels [52]. Nrf2 is ubiquitously expressed in all cell types, but its expression is usually maintained at a low level under basal conditions because the Nrf2 protein is synthesized but constantly degraded. However, upon exposure to oxidative and xenobiotic stresses, cysteine residues on Keap1, which have high redox sensitivity, can easily be covalently modified or oxidized, resulting in a conformational change and the dissociation of Nrf2 [49]. Another Keap1-independent mechanism is to escape GSK-3β-mediated degradation [44,53]. Nrf2 is phosphorylated by GSK-3β, making it identifiable by β-transducin repeat-containing protein (β-TRCP), which later marks Nrf2 for ubiquitination and degradation through the proteasome [54,55]. Meanwhile, several investigators have found that Nrf2 signaling can be activated through the PI3K-AKT signaling pathway [56,57], because GSK-3β can be inhibited by AKT-mediated phosphorylation. Subsequently, Nrf2 degradation is stagnated, leading to a rapid accumulation of Nrf2 in the cytoplasm and translocation to the nucleus. In the nucleus, Nrf2 heterodimerizes with small v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog (sMaf) proteins to activate stress-dependent expression of a cluster of cytoprotective genes involved in the regulation of metabolism, detoxication, and redox balance via cis-acting antioxidant/electrophile response elements (AREs) (Figure 3) [58,59,60]. Rapamycin1 has been reported to attenuate the paraquat (PQ)-induced PF by promoting Nrf2 translocation to the nucleus and enhancing the expression of Nrf2 [61]. Vitamin U ameliorates lung toxicity by binding to the ETGE motif to promote Nrf2 dissociation from the Nrf2/Keap1 complex and translocation to the nucleus [62]. Notably, some small molecules can interfere with Keap1-mediated Nrf2 ubiquitination and degradation, causing the accumulation of Nrf2. For example, in the process of inhibiting PF, tanshinone IIA2 [63] and pterostilbene3 [64] regulate redox homeostasis by impairing the binding of Keap1 with Nrf2 and maintaining Nrf2 stability.
Figure 3.
Mechanism of Nrf2 activation. Under normal physiological conditions, Nrf2 is sequestered in the cytoplasm by its physical interaction with Keap1 and is degraded by the ubiquitin-proteasome in the Keap1-dependent manner or the Keap1-independent manner (GSK-3β pathway). In the stress conditions, Keap1 is inactivated while Nrf2 is stabilized. The stabilized Nrf2 performs nuclear translocation and heterodimerizes with sMaf to activate target genes for cell protection through ARE. Nrf2 target genes include GPx, Trx, TrxR, Prx, HO−1, GST, NQO1, SOD, and CAT which defend lungs from further damages. Furthermore, Nrf2 also can be directly phosphorylated by GSK-3β, enabling its recognition by β-TRCP, which later marks Nrf2 for ubiquitination and degradation through the proteasome. However, PI3K/AKT signaling pathway can suppress GSK-3β by AKT-mediated phosphorylation and causing Nrf2 accumulation in the cytoplasm. The accumulation of Nrf2 further activates transcription of a number of cytoprotective genes and protection against pulmonary fibrosis.
Currently, the downstream genes of Nrf2 can be divided into three categories according to their functions: cellular antioxidants, such as glutathione peroxidase (GPx), thioredoxin (Trx), thioredoxin reductase (TrxR), peroxiredoxin (Prx), and heme oxygenase-1 (HO−1); phase II detoxifying enzymes, including glutathione S-transferase (GST), NAD(P)H quinone oxidoreductase 1 (NQO1), superoxide dismutase (SOD), and CAT [65,66]; and transporters, such as multidrug resistance-associated protein (MRP) [67]. The above gene expression products can effectively regulate intracellular levels of reactive oxygen species (ROS), protect macromolecules from oxidative and xenobiotic damage, and reduce the toxicity of exogenous substances. Hence, the critical role of Nrf2 manifests in regulating oxidative stress and suppressing the inflammatory response. Researchers have therefore set their sights on Nrf2 and attempted to unravel its complex role in PF.
3. Nrf2 and Inflammation in Pulmonary Fibrosis
Endogenous and exogenous poisons, such as free radicals, air pollutants, and chemicals, attack the lungs, causing multiple inflammatory events due to their unique anatomical location. There is interplay between various cell types, including alveolar epithelial cells, fibroblasts, endothelial cells, and inflammatory cells (e.g., neutrophils, macrophages, lymphocytes, and eosinophils), whose injury recruits a fibrotic response, implicating that the occurrence and development of PF is a dynamic process [4]. Neutrophils and eosinophils are mainly involved in the acute phase of lung injury, whereas lymphocytes and macrophages govern chronic inflammation [68,69]. In the early stage of PF, many inflammatory cells infiltrate in the alveoli, releasing pro-inflammatory proteins and pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, which are considered the primary cause of lung tissue scarring [70,71]. Etiologically, sustained inflammatory responses produce several significant ROS, resulting in direct or indirect injury to alveolar epithelial cells [72]. Damaged bronchial and alveolar epithelial cells and other resident cells further exacerbate various inflammatory mediators, proteases, and ROS release, which boost the recruitment of inflammatory cells and aggravate collagen accumulation in the lung tissue [73]. Alveolar epithelial cells are composed of alveolar type I (AT1), through which gas exchange takes place, and AT2 cells, which generate a large amount of surfactant. AT2 cells, which are considered as alveolar stem cells and can differentiate into AT1 cells [74], represent underlying initiating mechanisms responsible for PF in response to repetitive lung damage [75]. The functional impairment of AT2 cells and the development of a pro-fibrotic phenotype play critical roles in driving IPF. A recent study showed that loss of Cdc42 function in AT2 cells exposed to elevated mechanical tension promoted periphery-to-center progressive lung fibrosis [76], providing support for this hypothesis. EMT in alveolar epithelial cells, a key step in PF, will be discussed in detail later.
Indeed, innate immune cells, alveolar macrophages, and principally monocyte-derived alveolar macrophages play an important role in the formation of PF. When harmful agents attack the lungs, alveolar macrophages are the first line of defense to initiate inflammatory reactions and boost the infiltration of neutrophils [77,78]. Studies have shown that two distinct subsets of macrophages can be found in the lungs, tissue-resident alveolar macrophages and monocyte-derived alveolar macrophages, in a mouse model of bleomycin (BLM)-induced PF [79]. They also causally implicated that specific deletion of monocyte-derived alveolar macrophages reduced asbestos-induced fibrosis severity [80].
The transcription factor Nrf2 is known to attenuate inflammatory reactions [81,82]. Cho et al. reported that Nrf2−/− mice were more vulnerable to BLM-induced inflammation and PF than wild-type mice and ascertained the protective effects of Nrf2 [83]. In mice lacking Nrf2, the resolution of lung injury and inflammation is compromised [84]. In irradiated Nrf2 null mice, AT2 cell loss and the corresponding development of PF were potentiated [85]. Nrf2 is believed to be required for alveolar macrophage-mediated apoptotic neutrophil clearance [86]. A single exposure of mouse lungs to zinc oxide nanoparticles increased the number of total cells, including macrophages, lymphocytes, neutrophils, and eosinophils, in bronchoalveolar lavage fluid (BALF) both in wild-type mice and Nrf2−/− mice, but Nrf2−/− mice expressed a greater increase [87]. In addition, when exposed to multiwalled carbon nanotubes, Nrf2 knockout (KO) mice displayed apparent pulmonary infiltration of granulocytes, macrophages, and B and T lymphocytes [88].
Several cellular and molecular mechanisms related to Nrf2 have been proven to be involved in the advancement and resolution of lung inflammation (Figure 4).
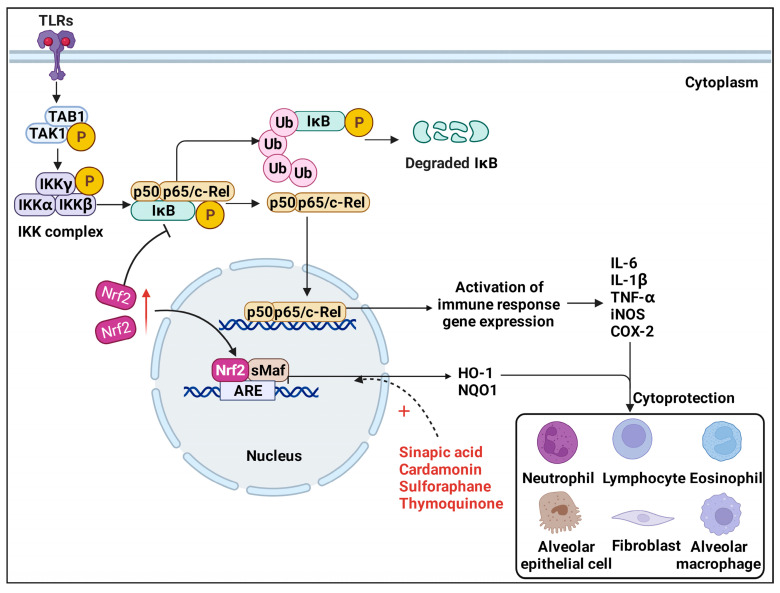
Figure 4.
Nrf2/HO−1 pathway and TLRs/NF-κB pathway. In canonical TLRs/NF-κB signaling, TLRs activate TAK1-TAB1 kinase complex, resulting in IKK complex-meditated release of NF-κB dimers and phosphorylation of IκB. Phosphorylated IκB frees NF-κB dimers and permits NF-κB dimers translocated into the nucleus. However, activation of Nrf2 can inhibit IκB phosphorylation in the canonical NF-κB pathway, thereby reducing the nuclear accumulation of NF-κB dimers and inhibiting its downstream immune response genes expression, such as IL-6, TNF-α, IL-1β, iNOS, and COX-2. Moreover, elevated expression of HO−1 mediated by Nrf2 also demonstrated significant anti-inflammatory and inhibition of apoptosis effects in the progression of PF.
3.1. TLRs/NF-κB Pathway
Nuclear factor-κB (NF-κB) is a dimeric multifunctional nuclear transcription factor composed of p50 and p65 subunits, which are transferred from the cytoplasm to the nucleus when activated. Extensive biological activities, including the transcription of various cytokines (IL-6, TNF-α, and iNOS), adhesion molecules (COX-2), and chemokines, can be promoted by NF-κB, which is considered a prototypical pro-inflammatory signaling pathway [89]. It has been reported that Nrf2-Keap1 attenuates IκBα phosphorylation in the canonical NF-κB activation pathway, thereby reducing the nuclear accumulation of NF-κB [90]. During canonical NF-κB signaling, the release of canonical NF-κB dimers is controlled by the inhibitor of kappa B kinase (IKK) complex, which consists of IKK1 (IKK α), IKK2 (IKK β), and NEMO (IKK γ) [91]. Transcriptional activation of NF-κB occurs when the IKK complex is activated by transforming growth factor (TGF)-β1-activated kinase 1 and the TAK1-binding protein 1 (TAK1-TAB1) kinase complex, resulting in phosphorylated IkB and its later degradation [92,93]. Phosphorylation of IκBα frees NF-κB and permits NF-kB dimers to enter the nucleus [94]. Hence, Nrf2-mediated inhibition of IκBα phosphorylation and NF-κB translocation to the nucleus might be effective treatment options. Treatment with pirfenidone suppressed chronic intermittent hypoxia-augmented lung fibrosis in BLM-treated mice by upregulating Nrf2 and downregulating NF-κB [95]. Another study showed that, through inhibition of apoptosis and induction of Nrf2/HO−1-mediated antioxidant enzymes by means of suppressing NF-κB signaling, sinapic acid4 ameliorates BLM-induced lung fibrosis in rats [96].
3.2. Nrf2/HO−1 Pathway
Heme oxygenase-1 (HO−1) is the inducible, rate-limiting enzyme in the catabolism of heme, catalyzing the breakdown of heme into iron, biliverdin, and carbon monoxide [97]. HO−1 is one of the classic genes controlled by Nrf2 [98]. Elevated expression of HO−1 mediated by Nrf2 demonstrated significant anti-inflammatory and inhibition of apoptosis effects in the progression of PF. The latest research shows that cardamonin provokes the Nrf2/HO−1 axis in alveolar macrophages and exhibits anti-inflammatory and antioxidative effects on phorbol 12-myristate 13-acetate-induced pulmonary inflammation [71]. Thymoquinone5 targeting the Nrf2/HO−1 signaling pathway abrogates the inflammatory response in BLM-induced PF in rats [99]. Additionally, dihydroartemisinin6 regulated the oxidative stress process through the Nrf2/HO−1 signaling pathway in BLM-induced PF model [100]. Atractylenolide III7 attenuates BLM-induced experimental PF through the Nrf2/NQO1/HO−1 pathway [101]. The polysaccharide FMP-1 has been noted to attenuate cellular oxidative stress and protect alveolar epithelial cells through the PI3K/AKT/Nrf2/HO−1 pathway [102]. These observations indicate that Nrf2 is essential for the control of inflammation. Previously, it was generally believed that Nrf2 suppresses inflammation by controlling redox levels. More recent research has shown that Nrf2 also plays a part in controlling the expression of inflammatory cytokines. Nrf2 disturbs LPS-induced transcriptional upregulation of pro-inflammatory cytokines, such as IL-6 and IL-1β [103]. Nrf2 has also been reported to attenuate inflammation by suppressing Toll-like receptor (TLR)4 and Akt signaling [104].
4. Nrf2 and Oxidative Stress in Pulmonary Fibrosis
The triggering process of PF is multifactorial, and accumulating evidence indicates that oxidative stress is still a key player in the pathogenesis of PF [105,106]. Given the high level of oxygen to which the lungs are exposed, the lungs are more sensitive to oxidative stress than other tissues [107]. Oxidative stress stimulates an imbalance between oxidants/antioxidants, which significantly contributes to lung fibrosis [108]. Exogenous (air pollution, cigarette smoke, silica particles) and endogenous oxidants (mitochondrial ROS, hydrogen peroxide, superoxide anions, and NO) attack alveolar epithelial cells, pulmonary vascular endothelial cells and lung macrophages and induce the formation of ROS and reactive nitrogen species (RNS) [10,12,61,109]. ROS are important oxidative stress markers, and oxidative stress usually arises from the overproduction of ROS. ROS may damage cellular macromolecules, including DNA, lipids, and lesioned proteins, which perturb normal cell signaling pathways and cause irreversible dysfunction and apoptosis [110]. It is well known that silica can effectively increase the production of ROS in airway epithelial cells and lead to PF [111]. In recent years, a link between the inhalation of crystal-line silica and PF has been reported [112]. Tanshinone IIA has been reported attenuate silica-induced PF via activation of the Nrf2/Trx/TrxR axis and Nrf2-mediated inhibition of NOX4 expression, EMT, and TGF-β1/Smad signaling [113,114,115]. These findings indicate that Nrf2 plays an important role in protecting against silica-mediated oxidative stress and PF. Moreover, the inflammatory state is possibly elevated by oxidative stress via the activation of NF-kB, subsequently activating and recruiting immune cells (macrophages and T-cells) [116]. These inflammatory cells further irritate free radical production, including hydroxyl radicals and superoxide radicals [117], resulting in a decrease in classic antioxidant enzymes, including superoxide dismutase (SOD), catalase, and glutathione.
4.1. Nrf2 Downstream Antioxidant Products
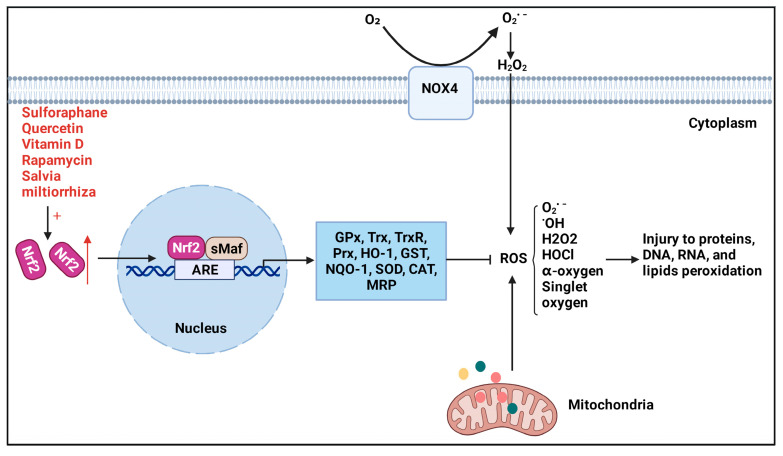
Previous evidence has shown that the Keap1-Nrf2 pathway regulates ROS production through mitochondria and NADPH oxidase [118]. Nrf2 and its target genes (e.g., HO−1 and NQO1) can protect normal cells from oxidative stress and readily eliminate ROS. Melatonin has been reported to activate the Nrf2 signal transduction key antioxidant target genes HO−1 and NQO1 by increasing Sirtuin1 (SIRT1) expression and peroxisome proliferator-activated receptor coactivator-1α (Pgc-1α) deacetylation, defending against Cr(VI)-induced pulmonary injury [119]. Nrf2 regulates classical antioxidant enzymes, including SODs, catalase, GPx, and GSH reductase, directly inactivating ROS and preventing ROS-initiated reactions, while the phase 2 detoxifying enzymes GSTs and NQO1 play an indirect role by promoting the excretion of oxidative and active secondary metabolites and the biosynthesis/cycling of thiols [120]. For instance, Nrf2 directly enhanced the expression of the antioxidant proteins Trx and TrxR, promoted by sodium tanshinone IIA sulfonate (STS), and has been proven to attenuate silica-induced PF [113]. Rosavin8 activates the Nrf2 pathway to inhibit the occurrence of oxidative stress and enhance MDA, SOD, and GSH-Px expression [121]. The therapeutic roles of vitamin D39 in the treatment of particle-associated PF also by activating Nrf2 signaling and promoting the expression of its downstream antioxidant products [122].
4.2. Nrf2/NOX4 Pathway
Mitochondria are suspected to be the main site of intracellular ROS generation, and NADPH oxidase (NOX), especially NADPH oxidase-4 (NOX4)-mediated superoxide production, is the main nonmitochondrial source of ROS accumulation [123,124,125]. In the NOX family (NOX1, NOX3, NOX4, NOX5, DUOX1, and DUOX2) [126], most NOX enzymes can catalyze the reduction of molecular oxygen to superoxide (O2−), but NOX4 catalyzes the reduction of molecular oxygen to H2O2 [127]. NOX4 also potentiates myofibroblast activation in response to TGF-β1, which seems to be a key factor in promoting fibrosis [128,129]. Therefore, therapeutic targeting of the Nrf2/NOX4 pathway to alleviate oxidative stress appears to be an effective option. Research has shown that polydatin protects against ROS-induced PF and reverses TGF-β1-induced pulmonary epithelial cell EMT in asthma by promoting Nrf2-mediated expression of HO−1 and NQO1 and inhibiting NOX1 and NOX4 expression [130]. S-Allylmercaptocysteine10 [131] and a gallic acid derivative11 [132] exhibited antioxidative capacity by influencing the TGF–β1/Smad and NOX4/Nrf2 pathways and increasing the expression of antioxidants, such as HO−1, GSH, and SOD. Ethyl acetate extract of salvia miltiorrhiza12 (EASM) [133] and tanshinone IIA [115] have been reported to upregulate Nrf2, and downregulating NOX4 positively alleviated oxidative stress in mice.
In addition, it has been reported that the intrinsic mechanism by which itaconate facilitates the transition of the macrophage phenotype from M1 to M2 presumably covers the activation of the Nrf2-Keap1 pathway and the deterrent of ROS [134]. In general, due to the central role Nrf2 plays in ROS detoxification (Figure 5), Nrf2 is an attractive therapeutic candidate for the pharmacological protection of PF.
Figure 5.
Nrf2 downstream antioxidant products and Nrf2/NOX4 pathway. Various Nrf2 activators can effectively promote Nrf2 entry into the nucleus and lead to increased expression of its downstream antioxidant products, such asHO−1and SOD, which can further eliminate potential damage to DNA, RNA, proteins, and lipid peroxidation caused by mitochondrial-derived and NOX4-derived ROS.
5. Nrf2 and Fibroblasts in Pulmonary Fibrosis—TGF-β1/Smad Pathway
Aberrant activation and proliferation of myofibroblasts appear to be positively related to the pathogenesis of PF (Figure 6) [135,136], which can synthesize pro-fibrotic proteins including α-smooth muscle actin (α-SMA), collagen I and III, and fibronectin, finally resulting in excessive secretion and accumulation of ECM [137,138].
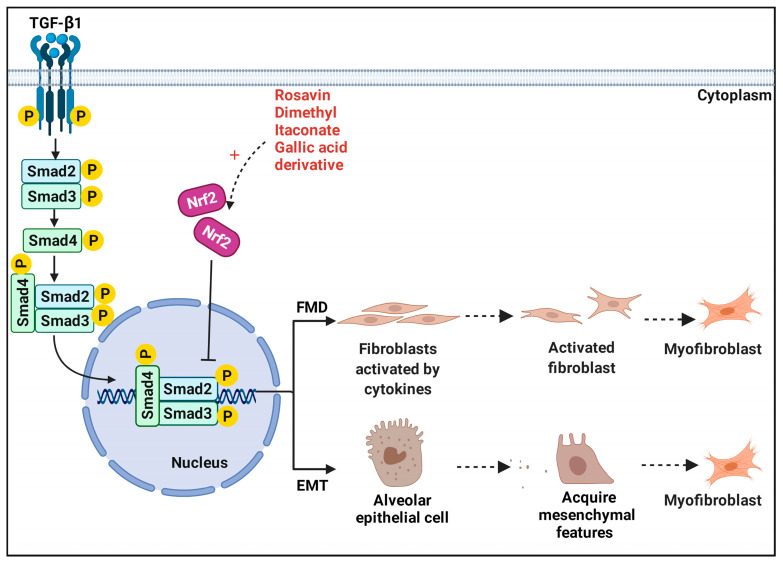
Figure 6.
Nrf2 and TGF-β1/Smad pathway. Under pathological conditions, TGF-β1 can be produced by a wide variety of cell types, including alveolar macrophages, neutrophils, alveolar epithelial cells, endothelial cells, fibroblasts, and myofibroblasts. Abnormally activated TGF-β1/Smad pathway induced disturbances of the homeostatic microenvironment are critical to promote FMD and EMT processes. However, some compounds, such as rosavin and itaconate can specifically block these two processes by activating Nrf2 and provide protection against pulmonary fibrosis.
5.1. Fibroblast–Myofibroblast Differentiation
TGF-β1 has been identified as a major pro-fibrogenic cytokine in IPF patients and can be produced by a variety of cells, such as alveolar macrophages, alveolar epithelial cells, and myofibroblasts [139,140]. Recently, a growing number of studies have suggested that fibroblast–myofibroblast differentiation (FMD) is a critical cellular phenotype during the occurrence and deterioration of PF [141,142,143]. FMD can increase with an elevated level of ROS in fibroblasts under oxidative stress. Moreover, FMD is known as the primary source of myofibroblast accumulation [144,145]. Under pathological conditions, lung fibroblasts are irritated by TGF-β1 and stress, triggering differentiation into myofibroblasts and eventually leading to PF [146]. Consequently, FMD is an important therapeutic target for PF. Studies have shown that Nrf2 has a protective effect during PF and can inhibit the FMD process. Compared with Nrf2 knockdown, Nrf2 activation increased antioxidant capacity and myofibroblast dedifferentiation in IPF fibroblasts [147]. The activation of Nrf2 by dimethyl itaconate13 can protect against TGF-β1-induced FMD via the ROS/TXNIP signaling pathway and inhibit TXNIP-mediated FMD in PF [141]. Tanshinone IIA inhibited myofibroblast activation, rebalanced Nrf2-NOX4, and limited glutaminolysis in myofibroblast proliferation by activating the Nrf2/GSH signaling pathway [63].
5.2. Epithelial–Mesenchymal Transition
The antifibrotic function of Nrf2 is also embodied in the suppression of EMT and TGF-β1/Smad signaling [109,130,148]. EMT is considered to be a convertible process in the evolution of PF, during which epithelial cells gradually acquire mesenchymal features, such as the mesenchymal marker α-smooth muscle actin (α-SMA), and lose the epithelial adhesion protein E-cadherin (E-cad) [30,149]. The dysregulation between the alveolar epithelium and its associated mesenchyme lead to unchecked proliferation of extracellular matrix-producing cells [150]. It is widely recognized that EMT is another major source of myofibroblasts [151]. The TGF-β1/Smad signaling pathway promotes EMT and regulates the expression and irreversible deposition of ECM proteins, such as collagen I, fibronectin, and α-SMA [152,153]. Previous evidence has shown that Nrf2 alleviates PF by blocking EMT progression [154]. In addition, Nrf2 attenuated TGF-β1-induced EMT with downregulation of high-mobility group box 1 (HMGB1), a transcription factor-like protein and novel mediator of EMT [30]. Nrf2-mediated Tan IIA copes with silica-induced oxidative stress, EMT, and TGF-β1/Smad pathway inhibition [114]. Through the phosphoinositide 3-kinase (PI3K)/GSK-3β axis activating the Nrf2-dependent antioxidant pathway, melatonin attenuated LPS-induced EMT [155]. Similarly, activating the Nrf2-dependent antioxidant pathway successfully alleviated the evolution of EMT by regulating the aberrant expression of Numb, a phosphotyrosine-binding domain (PTB) protein, implicated in EMT [148].
6. Potential Therapies and Nrf2 Activators
In general, recurrent damage to susceptible individuals’ lungs will promote pro-oxidant, pro-inflammatory, and pro-fibrotic process microenvironments [156]; these regulatory processes do not operate independently but commonly interfere with each other. Oxidative stress and ROS production are related to the activation and production of many of inflammatory cytokines and pro-fibrotic growth factors [157], such as TGF-β1, in turn, promote ROS formation primarily by inducing the expression and activity of NOX4 in various cell types [158]. Evidence has clearly shown that oxidative stress and inflammation are the driving forces of myofibroblast activation [159]. Mechanistically, the Nrf2 signaling pathway plays a crucial role in these processes; thus, Nrf2-dependent antifibrosis therapies are vital for the treatment of PF. Three different mechanisms, as mentioned above, have been demonstrated to account for the activation of Nrf2: Keap1-dependent, Keap1-independent, and other regulators. Hence, targeting Nrf2 activators to find feasible future treatment options for PF patients has been a proven perspective in recent studies. Scientists have discovered many natural or synthetic Nrf2 agonists that may be effective in treating PF (Table 1).
Table 1.
Potential therapies and Nrf2 activators for the treatment of pulmonary fibrosis.
| Compound | Model | Target | Function and Detection Index | Refs |
|---|---|---|---|---|
| Rapamycin1 | PQ-treated male rats and LFs | Nrf2 activating | Suppressed PQ-induced oxidant stress, cell death and apoptosis, fibrosis-related factors, reversed PQ-induced FMD and PF induced by PQ. | [61] |
| Tanshinone IIA2 | Silica-treated silicosis rat and NIH-3T3 cells | TGFβ1/Smad Nrf2/ NOX4 Nrf2/GSH |
Reduced the levels of collagen deposition, TGF-β1, α-SMA, Col-I, Col-III, NOX4, ROS; increased the levels of Nrf2, HO−1, NQO1, Gclc, Gclm, and GSH; regulated myofibroblast activation, protected Nrf2 from protein ubiquitination, promoted Keap1 degradation. | [113,114,115] |
| Pterostilbene3 | Lps-treated female BALB/C mice | Nrf2 activating | Decreased lung injury and fibrosis scores; reduced levels of Col-I, TGF-β1, HYP, IL-1β, IL-6, TNF-α; increased the levels of Nrf2, HO−1, NQO1, GSH, SOD. | [64] |
| Sinapic acid4 | BLM-treated SD rats | Nrf2/HO−1 NF-κB |
Increased the levels of Nrf2, inflammatory cell population, GPx, CAT, Bcl-2; reduced the levels of MDA, TNF-α, IL-1β, MPO, MMP-7, HYP, TGF-β1, NF-κB; restore the antioxidant system, inflammatory or fibrotic alterations. | [96] |
| Thymoquinone5 | BLM-treated Wistar rats | Nrf2/HO−1 | Decreased levels of HYP, LDH, total and differential leukocytes, MDA, TNF-α, IL-1β, MPO, MMP-7, caspase-3, Bax, NF-κB; upregulate Nrf2, HO−1, Bcl2; ameliorated severe hemorrhage, thickening of alveolar septa, emphysema, infiltration of leukocytes in walls alveoli and fibroplasia, inflammation, and PF. | [99] |
| Dihydroartemisinin6 | BLM-treated SD rats and AECs | Nrf2/HO−1 | Reduced the levels of α-SMA, MDA; increased the levels of E-cadherin, Nrf2, HO−1, SOD, and GSH; mitigated alveolitis severity, relieved fibrosis scores, inhibited the increase in the myofibroblasts–like processes of the AECs. | [100] |
| Atractylenolide III7 | BLM-treated SD rats | Nrf2/NQO1/HO−1 | Reduced the expression of Caspase-3 and Caspase-9, IL-6, iNOS, TNF-α, MDA, LDH; upregulated the levels of Nrf2, NQO1, HO−1, SOD, GSH, IL-10; improved lung function alleviated PF and oxidative stress. | [101] |
| Rosavin8 | BLM-treated Kunming mice | Nrf2/NF-κB TGF-β1 |
Inhibited inflammatory cells, MDA, HYP, NF-κB-p65, α-SMA TGF-β1 levels; improved Nrf2, SOD, GSH-Px levels; ameliorated PF, alveolar inflammatory cell contents. | [121] |
| Vitamin D39 | Particles-treated Nrf2+/+ and Nrf2−/− C57BL/6 mice, HFLIII cells | Nrf2 activating | Reduced the levels of α-SMA, FN, E-cadherin; increased the levels of N-cadherin, Nrf2, VDR; limited fibroblast cells’ migration, FDM, ECM. | [122] |
| S-Allylmercaptocysteine10 | BLM-treated C57/BL6 mice | Nrf2/NOX4 TGF-β1/Smad |
Increased antioxidants such as HO−1, GSH, and SOD; decreased HYP, SMA; ameliorated the pathological structure, and decrease inflammatory cell infiltration and pro-inflammatory cytokines in BALF. | [131] |
| Gallic acid derivative (GAD)11 | BLM-treated C57/BL6 mice | Nrf2/NOX4 TGF-β1/Smad |
Reduced the levels of α-SMA, HYP, collagen type I/III, IL-6, TGF-β1, NOX4; increase the levels of SOD and GSH; increased body weight, survival rate, and alleviated alveolar structure, alveolar inflammation, and the degree of PF. | [132] |
| Salvia miltiorrhiza12 | BLM-treated C57/BL6 mice and NIH-3T3 cells | Nrf2/GSH Nrf2/Keap1 Nrf2/Nox4 |
Reduced the levels of TGF-β1, α-SMA, ECM, COL-1, NOX4, ROS, PKC-δ, Smad3; increase the levels of Nrf2, NQO1, HO−1; protected Nrf2 from protein ubiquitination, PF; regulated myofibroblasts activation, Increased the sensitivity of fibroblasts to the loss of GSH. | [133] |
| Dimethyl itaconate13 | TGF-β1-induced FMD in vitro and BLM-treated mouse | Nrf2 activating | Nrf2 decreased TXNIP expression and alleviated FMD in PF; Nrf2 inhibited TGF-β1-induced FMD and the increase of ROS. | [141] |
| Sulforaphane14 | BLM-treated C57/BL6 mice | Nrf2 activating | Reduced the levels of caspase-3, IL-1β, TNF-α, TGF-β, HYP, 3-NT, and 4-HNE; increased the levels of Nrf2, HO−1, NQO1, SOD1, and CAT; alleviated BLM-induced alveolar epithelial cell apoptosis, alveolitis, collagen accumulation, lung oxidative stress, and lung fibrosis. | [168] |
| Bletilla striata15 | SiO2-treated C57BL/6 mice and A549 cells line | Nrf2/HO−1/γ-GCSc | Reduced the levels of MDA, ROS; increased the levels of γ-GCSc, Nrf2, SOD, HO−1; protective effect of lung injury, lung cell viability, apoptosis, and ROS accumulation. | [10] |
| Sarcodon aspratus16 | BLM-treated Kunming mice and A549 cells | MAPK/Nrf2/HO−1 TGF-β1/MAPK TLR4/NF-κB |
Reduced the levels of ROS, MDA, TNF-α, IL-1β, IL-6, CTGF, MMP-2, HYP, α-SMA, ECM, TLR4, MyD88, NF-κB-p65; increased the levels of GSH-Px, SOD, Nrf2, HO−1, CAT, Smad7; inhibited H2O2-induced cell apoptosis, oxidative stress, fibrosis, phosphorylation of JNK, ERK and P38, weight loss. | [169] |
| Arenaria kansuensis17 | PQ-treated C57BL mice | TGF-β1/Smad NF-κB-p65 Nrf2/NOX4 |
Downregulated α-SMA, TGF-β1, TNF-α, IL-6, IL-1β1, HYP, ROS, collagen deposition, NOX4; upregulate Nrf2, SOD, and GSH; improved mice survival rate, body weight, lung pathological lesion, and the lung index. | [170] |
| Quercetin18 | BLM-treated BEAS-2B cells | Nrf2 activating | Reduced the expression levels of ROS, TNF-α, and IL-8; increased Nrf2-ARE binding, HO−1, and γ-GCS; restored the disturbed redox balance and reduce inflammation. | [171] |
| Chelerythrine19 | BLM-treated C57/BL6 mice | Nrf2/ARE | Reduced the expression levels of fibronectin, α-SMA, TGF-β, 4-HNE, and HYP; upregulated the levels of SOD, GSH, Nrf2, HO−1, and NQO1; alleviates collagen deposition, oxidative stress, and PF. | [172] |
| Bergenin20 | BLM-treated C57/BL6 mice and NIH3T3 cells | p62/Nrf2 | Decreased content of α-SMA, COL-1, HYP, ROS, MDA; increased the levels and activity of Nrf2, GSH, SOD, HO−1, NQO1; inhibited the TGF-β1 induced FDM, oxidative stress, and PF. | [173] |
| Jinshui Huanxian formula21 | BLM-treated SD rats, MRC-5 cells and NIH-3T3 cells | Nrf2/NOX4 TGF-β1 |
Reduced the levels of TGF-β1, collagen deposition, HYP, α-SMA, COL-I, COL-III, MDA, MPO, NOX4, FN1; increased the levels of Nrf2, GSH, SOD, CAT, NQO1, HO−1; suppressed the increases of lung coefficient, TGF-β1-induced FDM, ROS production | [174] |
| Dimethyl fumarate22 | BLM-treated C57/BL6 mice; RAW264.7 and NIH-3T3 cells coculture | Nrf2 activating | Attenuated macrophage activity and fibrosis in mice; promoted Nrf2 and HO−1 expression and suppress TGF-β and ROS production; reduced fibroblast-to-myofibroblast transition and collagen production by NIH-3T3 cells. | [175] |
| Chloroquine23 | PQ-treated male C57BL/6 mice | Nrf2/NQO1/HO−1 TGF-β |
Reduced the levels of TNF-α, IL-1β, IL-6, NO, iNOS, MDA, α-SMA, TGF-β; increased the levels of SOD, NQO1, Nrf2, HO−1; attenuated lung injury, oxidative stress, decreases protein, inflammatory cells. | [176] |
| Esomeprazole24 | BLM- or TGF-β-treated PHLE cells and fibroblasts | MAPK/Nrf2/HO−1 DDAH/iNOS | Reduced the levels of DDAH, iNOS, IL-1β, IL-6, TNF-α, COL-I, COL-III, COL-V; increased the levels of HO−1, NQO1, Nrf2; downregulates pro-inflammatory and profibrotic molecules, collagen expression; activates MAPK via phosphorylation. | [156] |
The superscript number in the upper right corner of the compounds is numbered according to the order in which the compounds first appeared in the main text.
Itaconate, an endogenous metabolite from the tricarboxylic acid cycle, was recently reported to have notable anti-inflammatory and immune-regulated effects [160]. It has been demonstrated that itaconate directly modifies 151, 257, 288, 273, and 297 on the protein Keap1 via alkylation of cysteine residues enabling Nrf2 to increase the expression of downstream antioxidant and anti-inflammatory genes [161]. Dimethyl itaconate (DMI), a cell-permeable itaconate derivative synthesized in vitro, was reported to protect against PF via activating Nrf2 and inhibiting thioredoxin-interacting protein (TXNIP) expression, thereby restraining TXNIP-mediated FMD [141]. Collectively, itaconate is a crucial anti-inflammatory metabolite that acts via Nrf2 to limit inflammation and control the severity of PF [161,162,163].
Rapamycin (sirolimus) is a potent inhibitor of the mammalian target of rapamycin (mTOR) which has been increasingly used to help organ transplant recipients prevent graft rejection [164]. The potential value of rapamycin in PQ-induced and TGF-α–induced PF has been proved [165,166]. Studies have shown that rapamycin protects against PQ-induced PF by activating the Nrf2 signaling pathway and inhibiting the EMT process [167]. A recent report also supports this notion by showing that rapamycin could inhibit PQ-induced oxidant stress and enhance the expression of Nrf2 [61]. However, the exact mechanism of rapamycin regulating the expression of Nrf2 needs further study.
Sulforaphane14 (SFN), a Brassica oleracea extract, is a well-studied potent activator of Nrf2 which has been previously reported to prevent BLM-induced PF in mice via Nrf2 activation [168]. The anti-fibrotic function of SFN was largely dependent on LOC344887, a long noncoding RNA, which presents a novel therapeutic axis for the prevention and intervention of PF [8]. Other natural products, such as Bletilla Striata15 [10], Sarcodon aspratus16 [169], Arenaria kansuensis17 [170], quercetin18 [171], chelerythrine19 [172], and bergenin20 [173] can all protect against PF through the Nrf2-dependent mechanism. Except for a single botanical drug, Chinese herbal formulas including Jinshui Huanxian formula21 (JHF) which is composed of 11 medicinal herbs also exhibit protective effects on PF [174].
In addition, dimethyl fumarate22 which has been approved for the first-line treatment of relapsing-remitting multiple sclerosis [175]; dihydroartemisinin which is traditionally used to treat malaria [100]; chloroquine23 which has been used for malaria treatment [176]; and proton pump inhibitors such as esomeprazole24 [156] have all been shown to inhibit the progression of PF by Nrf2 signaling. Thus, there is great potential for translating these drugs rapidly into clinical practice for treatment of PF.
In order to overcome the physiological barriers and prolong the treatment time of drug in the lungs, local drug administration, such as inhalation, can provide drugs directly to lung lesions and reduce the accumulation of drugs in other organs and improve therapeutic efficacy. However, inhaled particles can be swept out of the lung by cilia in the trachea and bronchi or phagocytosed by tissue-resident alveolar macrophages, which significantly affects their therapeutic effect. Recent evidence suggests that the application of nanotechnology is expected to solve these problems. Liu et al. designed a dimethyl fumarate-loaded ROS responsive liposome as an inhaled drug which presented enhanced antifibrotic effect, compared with direct dimethyl fumarate instillation [175]. This ROS-responsoftenive liposome is clinically promising as an ideal delivery system for inhaled drug delivery.
7. Conclusions and Perspectives
PF is an intractable disease that has long plagued humans. Research continues to face challenges, as the exact mechanistic aspects of PF are not well understood. The pathogenesis and molecular mechanisms associated with PF include pathological inflammation, imbalanced oxidative stress responses, and abnormal activation of myofibroblasts. The key pathological processes include FMD, EMT, and ECM deposition. Alveolar epithelial cells, fibroblasts, endothelial cells, neutrophils, macrophages, lymphocytes, and eosinophils are involved in this process. Given the complex biological pathogenesis of PF, which is regulated by multiple signaling pathways and cytokines, targeting a single mechanism to address unmet clinical needs in PF seems unlikely to reverse the disease. The transcription factor Nrf2 coordinates the expression of more than 500 cytoprotective and metabolic genes in response to various stresses to restore cellular homeostasis [25]. Alterations in Nrf2 regulatory genes play fundamental roles in the pathogenesis of PF, and the signaling pathways include TLRs/NF-κB, MAPK/Nrf2/HO−1/NQO1, p62/Nrf2, Nrf2/NOX4, Nrf2/GSH, and TGF-β1/Smad. Due to this wide range of functions, it may be effective to consider Nrf2 activators in combination with currently available treatment options in the clinic. These natural products come from a wide range of sources and act on multiple pathways to exert their pathological effects, which are appropriate for the multisystem, multitarget pathogenesis of PF [177]. Moreover, most Nrf2 activators are natural products with extraordinary therapeutic effects and can be easily applied to the daily diet, reducing the high physical and psychological burden on patients and enhancing quality of life.
Recently, considering the challenges associated with conventional oral and intravenous routes of drug administration, local delivery of drugs via nanoparticle carriers to the lungs is an emerging area of interest. Nanotechnology-based inhalation drug delivery methods possess numerous advantages including (1) uniform distribution of the inhaled drug among the alveoli, (2) better solubilization of the drug, (3) reduced drug accumulation in other organs, (4) long-term drug release, (5) lesser side effects, and (6) improved drug internalization to the lung cells [178,179,180]. In addition, some natural products, such as bergenin [173] and tanshinone IIA [63] whose anti-fibrotic effects have been reported by using human lung fibroblasts (HFL-1) cells and human fetal lung fibroblast (MRC-5) cells, respectively. These natural products may have significant potential for clinical translation, but the preclinical studies or tests are needed.
PF is known to result in irreversible loss of lung function, and future research should focus on prevention rather than cure. Continued investigations of Nrf2-mediated cellular defense mechanisms and preventive effects may provide insights to cure PF.
Acknowledgments
Thanks to all the teachers and students in the lab for their contributions to this study. We are also grateful to https://biorender.com/ (accessed on 25 March 2022). for helping creating figures.
Author Contributions
Conceptualization, H.Y. and X.L.; writing—original draft preparation, Y.W. and J.W.; writing—review and editing, Y.W., J.W., H.D., and L.Z.; supervision, H.Y. and X.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 81871601, and No. 82000085); Natural Science Foundation of Shanghai (No. 22ZR1452200); Program of Shanghai Academic Research Leader (No. 21XD1402800); Shanghai “Rising Stars of Medical Talent” Youth Development Program: Outstanding Youth Medical Talents; and the Young Elite Scientist Sponsorship Program by CAST (2018QNRC001).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richeldi L., Collard H.R., Jones M.G. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 2.Martinez F., Collard H., Pardo A., Raghu G., Richeldi L., Selman M., Swigris J., Taniguchi H., Wells A. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 3.Wolters P.J., Blackwell T.S., Eickelberg O., Loyd J.E., Kaminski N., Jenkins G., Maher T.M., Molina-Molina M., Noble P.W., Raghu G., et al. Time for a change: Is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir. Med. 2018;6:154–160. doi: 10.1016/S2213-2600(18)30007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson M.S., Wynn T.A. Pulmonary fibrosis: Pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rackow A.R., Nagel D.J., McCarthy C., Judge J., Lacy S., Freeberg M.A.T., Thatcher T.H., Kottmann R.M., Sime P.J. The self-fulfilling prophecy of pulmonary fibrosis: A selective inspection of pathological signalling loops. Eur. Respir. J. 2020;56:2000075. doi: 10.1183/13993003.00075-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore B.B., Fry C., Zhou Y., Murray S., Han M.K., Martinez F.J., Flaherty K.R., The C.I. Inflammatory leukocyte phenotypes correlate with disease progression in idiopathic pulmonary fibrosis. Front. Med. 2014;1:56. doi: 10.3389/fmed.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu P., Luo G., Dodson M., Schmidlin C., Wei Y., Kerimoglu B., Ooi A., Chapman E., Garcia J., Zhang D. The NRF2-LOC344887 signaling axis suppresses pulmonary fibrosis. Redox Biol. 2021;38:101766. doi: 10.1016/j.redox.2020.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng G., Li L., Ouyang Y.C. Elegans Modeling paraquat-induced lung fibrosis in reveals KRIT1 as a key regulator of collagen gene transcription. Aging. 2021;13:4452–4467. doi: 10.18632/aging.202406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G., Chang W., Li X., Han L., Zhou D., Feng Y., Li B., Zhu F., Li N. n-BuOH extract of Bletilla striata exerts chemopreventive effects on lung against SiO nanoparticles through activation of Nrf2 pathway. Phytomedicine. 2021;82:153445. doi: 10.1016/j.phymed.2020.153445. [DOI] [PubMed] [Google Scholar]
- 11.Yu G., Tzouvelekis A., Wang R., Herazo-Maya J.D., Ibarra G.H., Srivastava A., de Castro J.P.W., DeIuliis G., Ahangari F., Woolard T., et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med. 2018;24:39–49. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Amico R., Monaco F., Fusco R., Siracusa R., Impellizzeri D., Peritore A., Crupi R., Gugliandolo E., Cuzzocrea S., Di Paola R., et al. Atrazine Inhalation Worsen Pulmonary Fibrosis Regulating the Nuclear Factor-Erythroid 2-Related Factor (Nrf2) Pathways Inducing Brain Comorbidities. Cell Physiol. Biochem. 2021;55:704–725. doi: 10.33594/000000471. [DOI] [PubMed] [Google Scholar]
- 13.Kreuter M., Picker N., Schwarzkopf L., Baumann S., Cerani A., Postema R., Maywald U., Dittmar A., Langley J., Patel H. Epidemiology, healthcare utilization, and related costs among patients with IPF: Results from a German claims database analysis. Respir. Res. 2022;23:62. doi: 10.1186/s12931-022-01976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher T., Bendstrup E., Dron L., Langley J., Smith G., Khalid J., Patel H., Kreuter M. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir. Res. 2021;22:197. doi: 10.1186/s12931-021-01791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox I., Otahal P., de Graaff B., Corte T., Moodley Y., Zappala C., Glaspole I., Hopkins P., Macansh S., Walters E., et al. Incidence, prevalence and mortality of idiopathic pulmonary fibrosis in Australia. Respirology. 2021;27:209–216. doi: 10.1111/resp.14194. [DOI] [PubMed] [Google Scholar]
- 16.Novelli L., Ruggiero R., De Giacomi F., Biffi A., Faverio P., Bilucaglia L., Gamberini S., Messinesi G., Pesci A. Corticosteroid and cyclophosphamide in acute exacerbation of idiopathic pulmonary fibrosis: A single center experience and literature review. Sarcoidosis Vasc. Diffus. Lung Dis. 2016;33:385–391. [PubMed] [Google Scholar]
- 17.Luppi F., Cerri S., Beghè B., Fabbri L.M., Richeldi L. Corticosteroid and immunomodulatory agents in idiopathic pulmonary fibrosis. Respir. Med. 2004;98:1035–1044. doi: 10.1016/j.rmed.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Richeldi L., du Bois R.M., Raghu G., Azuma A., Brown K.K., Costabel U., Cottin V., Flaherty K.R., Hansell D.M., Inoue Y., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 19.King T.E., Jr., Bradford W.Z., Castro-Bernardini S., Fagan E.A., Glaspole I., Glassberg M.K., Gorina E., Hopkins P.M., Kardatzke D., Lancaster L., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 20.Woodcock H.V., Maher T.M. The treatment of idiopathic pulmonary fibrosis. F1000Prime Rep. 2014;6:16. doi: 10.12703/P6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George P., Patterson C., Reed A., Thillai M. Lung transplantation for idiopathic pulmonary fibrosis. Lancet Respir. Med. 2019;7:271–282. doi: 10.1016/s2213-2600(18)30502-2. [DOI] [PubMed] [Google Scholar]
- 22.Christie J.D., Edwards L.B., Aurora P., Dobbels F., Kirk R., Rahmel A.O., Stehlik J., Taylor D.O., Kucheryavaya A.Y., Hertz M.I. The Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Lung and Heart-Lung Transplantation Report-2009. J. Heart Lung Transplant. 2009;28:1031–1049. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Sontake V., Gajjala P., Kasam R., Madala S. New therapeutics based on emerging concepts in pulmonary fibrosis. Expert Opin. Ther. Targets. 2019;23:69–81. doi: 10.1080/14728222.2019.1552262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Audousset C., McGovern T., Martin J. Role of Nrf2 in Disease: Novel Molecular Mechanisms and Therapeutic Approaches—Pulmonary Disease/Asthma. Front. Physiol. 2021;12:727806. doi: 10.3389/fphys.2021.727806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torrente L., DeNicola G. Targeting NRF2 and Its Downstream Processes: Opportunities and Challenges. Annu. Rev. Pharmacol. Toxicol. 2022;62:279–300. doi: 10.1146/annurev-pharmtox-052220-104025. [DOI] [PubMed] [Google Scholar]
- 26.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Cuadrado A., Rojo A.I., Wells G., Hayes J.D., Cousin S.P., Rumsey W.L., Attucks O.C., Franklin S., Levonen A.L., Kensler T.W., et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019;18:295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 28.Chen X., Zhu X., Wei A., Chen F., Gao Q., Lu K., Jiang Q., Cao W. Nrf2 epigenetic derepression induced by running exercise protects against osteoporosis. Bone Res. 2021;9:15. doi: 10.1038/s41413-020-00128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W., Feng C., Jiang H. Novel target for treating Alzheimer’s Diseases: Crosstalk between the Nrf2 pathway and autophagy. Ageing Res. Rev. 2021;65:101207. doi: 10.1016/j.arr.2020.101207. [DOI] [PubMed] [Google Scholar]
- 30.Qu J., Zhang Z., Zhang P., Zheng C., Zhou W., Cui W., Xu L., Gao J. Downregulation of HMGB1 is required for the protective role of Nrf2 in EMT-mediated PF. J. Cell Physiol. 2019;234:8862–8872. doi: 10.1002/jcp.27548. [DOI] [PubMed] [Google Scholar]
- 31.Gu X., Liu Y., Wang N., Zhen J., Zhang B., Hou S., Cui Z., Wan Q., Feng H. Transcription of MRPL12 regulated by Nrf2 contributes to the mitochondrial dysfunction in diabetic kidney disease. Free Radic. Biol. Med. 2021;164:329–340. doi: 10.1016/j.freeradbiomed.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Sun W., Wang Z., Sun M., Huang W., Wang Y., Wang Y. Aloin antagonizes stimulated ischemia/reperfusion-induced damage and inflammatory response in cardiomyocytes by activating the Nrf2/HO-1 defense pathway. Cell Tissue Res. 2021;384:735–744. doi: 10.1007/s00441-020-03345-z. [DOI] [PubMed] [Google Scholar]
- 33.Moi P., Chan K., Asunis I., Cao A., Kan Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaramillo M.C., Zhang D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahon M., Thomas N., Itoh K., Yamamoto M., Hayes J.D. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J. Biol. Chem. 2004;279:31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- 36.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong K., Padmanabhan B., Kobayashi A., Shang C., Hirotsu Y., Yokoyama S., Yamamoto M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol. Cell. Biol. 2007;27:7511–7521. doi: 10.1128/mcb.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plafker K.S., Nguyen L., Barneche M., Mirza S., Crawford D., Plafker S.M. The ubiquitin-conjugating enzyme UbcM2 can regulate the stability and activity of the antioxidant transcription factor Nrf2. J. Biol. Chem. 2010;285:23064–23074. doi: 10.1074/jbc.M110.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh Y., Itoh K., Yoshida E., Miyagishi M., Fukamizu A., Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–868. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 40.Nioi P., Nguyen T., Sherratt P.J., Pickett C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell. Biol. 2005;25:10895–10906. doi: 10.1128/MCB.25.24.10895-10906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chowdhry S., Zhang Y., McMahon M., Sutherland C., Cuadrado A., Hayes J.D. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Q., Gao Y., Ci X. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxidative Med. Cell. Longev. 2019;2019:7090534. doi: 10.1155/2019/7090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H., Liu K., Geng M., Gao P., Wu X., Hai Y., Li Y., Li Y., Luo L., Hayes J.D., et al. RXRalpha inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013;73:3097–3108. doi: 10.1158/0008-5472.CAN-12-3386. [DOI] [PubMed] [Google Scholar]
- 44.Hayes J.D., Chowdhry S., Dinkova-Kostova A.T., Sutherland C. Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of beta-TrCP and GSK-3. Biochem. Soc. Trans. 2015;43:611–620. doi: 10.1042/BST20150011. [DOI] [PubMed] [Google Scholar]
- 45.Sotolongo K., Ghiso J., Rostagno A. Nrf2 activation through the PI3K/GSK-3 axis protects neuronal cells from Abeta-mediated oxidative and metabolic damage. Alzheimers Res. Ther. 2020;12:13. doi: 10.1186/s13195-019-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zipper L.M., Mulcahy R.T. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J. Biol. Chem. 2002;277:36544–36552. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi A., Kang M.I., Watai Y., Tong K.I., Shibata T., Uchida K., Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furukawa M., Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol. Cell. Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adams J., Kelso R., Cooley L. The kelch repeat superfamily of proteins: Propellers of cell function. Trends Cell Biol. 2000;10:17–24. doi: 10.1016/S0962-8924(99)01673-6. [DOI] [PubMed] [Google Scholar]
- 52.Zhang D.D., Lo S.C., Cross J.V., Templeton D.J., Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cuadrado A., Kügler S., Lastres-Becker I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018;14:522–534. doi: 10.1016/j.redox.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/beta-TrCP. Free Radic. Biol. Med. 2015;88:147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 55.Rada P., Rojo A.I., Chowdhry S., McMahon M., Hayes J.D., Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li K., Cao Z., Guo Y., Tong C., Yang S., Long M., Li P., He J. Selenium Yeast Alleviates Ochratoxin A-Induced Apoptosis and Oxidative Stress via Modulation of the PI3K/AKT and Nrf2/Keap1 Signaling Pathways in the Kidneys of Chickens. Oxidative Med. Cell. Longev. 2020;2020:4048706. doi: 10.1155/2020/4048706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao Q., Piao R., Wang H., Li C., Song L. Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced oxidative damage via induction of JNK and PI3K/AKT activation. Int. J. Biol. Macromol. 2018;118:747–755. doi: 10.1016/j.ijbiomac.2018.06.130. [DOI] [PubMed] [Google Scholar]
- 58.Motohashi H., Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen T., Sherratt P.J., Pickett C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 60.Rushmore T.H., Morton M.R., Pickett C.B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991;266:11632–11639. doi: 10.1016/S0021-9258(18)99004-6. [DOI] [PubMed] [Google Scholar]
- 61.Tai W., Deng S., Wu W., Li Z., Lei W., Wang Y., Vongphouttha C., Zhang T., Dong Z. Rapamycin attenuates the paraquat-induced pulmonary fibrosis through activating Nrf2 pathway. J. Cell. Physiol. 2020;235:1759–1768. doi: 10.1002/jcp.29094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oztay F., Tunali S., Kayalar O., Yanardag R. The protective effect of vitamin U on valproic acid-induced lung toxicity in rats via amelioration of oxidative stress. J. Biochem. Mol. Toxicol. 2020;34:e22602. doi: 10.1002/jbt.22602. [DOI] [PubMed] [Google Scholar]
- 63.An L., Peng L., Sun N., Yang Y., Zhang X., Li B., Liu B., Li P., Chen J. Tanshinone IIA Activates Nuclear Factor-Erythroid 2-Related Factor 2 to Restrain Pulmonary Fibrosis via Regulation of Redox Homeostasis and Glutaminolysis. Antioxid. Redox Signal. 2019;30:1831–1848. doi: 10.1089/ars.2018.7569. [DOI] [PubMed] [Google Scholar]
- 64.Yang H., Hua C., Yang X., Fan X., Song H., Peng L., Ci X. Pterostilbene prevents LPS-induced early pulmonary fibrosis by suppressing oxidative stress, inflammation and apoptosis in vivo. Food Funct. 2020;11:4471–4484. doi: 10.1039/C9FO02521A. [DOI] [PubMed] [Google Scholar]
- 65.Kikuchi N., Ishii Y., Morishima Y., Yageta Y., Haraguchi N., Itoh K., Yamamoto M., Hizawa N. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir. Res. 2010;11:31. doi: 10.1186/1465-9921-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maher J., Dieter M., Aleksunes L., Slitt A., Guo G., Tanaka Y., Scheffer G., Chan J., Manautou J., Chen Y., et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- 68.Moldoveanu B., Otmishi P., Jani P., Walker J., Sarmiento X., Guardiola J., Saad M., Yu J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009;2 [PMC free article] [PubMed] [Google Scholar]
- 69.Robb C.T., Regan K.H., Dorward D.A., Rossi A.G. Key mechanisms governing resolution of lung inflammation. Semin. Immunopathol. 2016;38:425–448. doi: 10.1007/s00281-016-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harari S., Caminati A. IPF: New insight on pathogenesis and treatment. Allergy. 2010;65:537–553. doi: 10.1111/j.1398-9995.2009.02305.x. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y., Cheng Y., Chen J., Tsai C., Wang T., Wu C., Chang P., Yeh W. Cardamonin attenuates phorbol 12-myristate 13-acetate-induced pulmonary inflammation in alveolar macrophages. Food Chem. Toxicol. 2021;159:112761. doi: 10.1016/j.fct.2021.112761. [DOI] [PubMed] [Google Scholar]
- 72.Sauler M., Bazan I.S., Lee P.J. Cell Death in the Lung: The Apoptosis-Necroptosis Axis. Annu. Rev. Physiol. 2019;81:375–402. doi: 10.1146/annurev-physiol-020518-114320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu L., Luo Z., Zheng J., Yao P., Yuan Z., Lv X., Zhao J., Wang M. IL-33 Can Promote the Process of Pulmonary Fibrosis by Inducing the Imbalance Between MMP-9 and TIMP-1. Inflammation. 2018;41:878–885. doi: 10.1007/s10753-018-0742-6. [DOI] [PubMed] [Google Scholar]
- 74.Barkauskas C.E., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R., Randell S.H., Noble P.W., Hogan B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parimon T., Yao C., Stripp B.R., Noble P.W., Chen P. Alveolar Epithelial Type II Cells as Drivers of Lung Fibrosis in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2020;21:2269. doi: 10.3390/ijms21072269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu H., Yu Y., Huang H., Hu Y., Fu S., Wang Z., Shi M., Zhao X., Yuan J., Li J., et al. Progressive Pulmonary Fibrosis Is Caused by Elevated Mechanical Tension on Alveolar Stem Cells. Cell. 2020;180:107–121.e17. doi: 10.1016/j.cell.2019.11.027. [DOI] [PubMed] [Google Scholar]
- 77.Hu G., Christman J.W. Editorial: Alveolar Macrophages in Lung Inflammation and Resolution. Front. Immunol. 2019;10:2275. doi: 10.3389/fimmu.2019.02275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu J., Yan Z., Schwartz D.E., Yu J., Malik A.B., Hu G. Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J. Immunol. 2013;190:3590–3599. doi: 10.4049/jimmunol.1200860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Misharin A.V., Morales-Nebreda L., Reyfman P.A., Cuda C.M., Walter J.M., McQuattie-Pimentel A.C., Chen C.I., Anekalla K.R., Joshi N., Williams K.J.N., et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joshi N., Watanabe S., Verma R., Jablonski R.P., Chen C.I., Cheresh P., Markov N.S., Reyfman P.A., McQuattie-Pimentel A.C., Sichizya L., et al. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. Eur. Respir. J. 2020;55:1900646. doi: 10.1183/13993003.00646-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobayashi E., Suzuki T., Yamamoto M. Roles nrf2 plays in myeloid cells and related disorders. Oxidative Med. Cell. Longev. 2013;2013:529219. doi: 10.1155/2013/529219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qin S., Du R., Yin S., Liu X., Xu G., Cao W. Nrf2 is essential for the anti-inflammatory effect of carbon monoxide in LPS-induced inflammation. Inflamm. Res. 2015;64:537–548. doi: 10.1007/s00011-015-0834-9. [DOI] [PubMed] [Google Scholar]
- 83.Cho H.-Y., Reddy S.P.M., Yamamoto M., Kleeberger S.R. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 84.Reddy N.M., Kleeberger S.R., Kensler T.W., Yamamoto M., Hassoun P.M., Reddy S.P. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J. Immunol. 2009;182:7264–7271. doi: 10.4049/jimmunol.0804248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Traver G., Mont S., Gius D., Lawson W., Ding G., Sekhar K., Freeman M. Loss of Nrf2 promotes alveolar type 2 cell loss in irradiated, fibrotic lung. Free Radic. Biol. Med. 2017;112:578–586. doi: 10.1016/j.freeradbiomed.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reddy N., Tamatam C., Aparna A., Reddy S. Nrf2 Is Required for Optimal Alveolar-Macrophage-Mediated Apoptotic Neutrophil Clearance after Oxidant Injury. Antioxidants. 2022;11:212. doi: 10.3390/antiox11020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sehsah R., Wu W., Ichihara S., Hashimoto N., Hasegawa Y., Zong C., Itoh K., Yamamoto M., Elsayed A.A., El-Bestar S., et al. Role of Nrf2 in inflammatory response in lung of mice exposed to zinc oxide nanoparticles. Part Fibre Toxicol. 2019;16:47. doi: 10.1186/s12989-019-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dong J., Ma Q. Suppression of basal and carbon nanotube-induced oxidative stress, inflammation and fibrosis in mouse lungs by Nrf2. Nanotoxicology. 2016;10:699–709. doi: 10.3109/17435390.2015.1110758. [DOI] [PubMed] [Google Scholar]
- 89.Zhou E., Li Y., Wei Z., Fu Y., Lei H., Zhang N., Yang Z., Xie G. Schisantherin A protects lipopolysaccharide-induced acute respiratory distress syndrome in mice through inhibiting NF-kappaB and MAPKs signaling pathways. Int. Immunopharmacol. 2014;22:133–140. doi: 10.1016/j.intimp.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 90.Pedruzzi L.M., Stockler-Pinto M.B., Leite M., Jr., Mafra D. Nrf2-keap1 system versus NF-kappaB: The good and the evil in chronic kidney disease? Biochimie. 2012;94:2461–2466. doi: 10.1016/j.biochi.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 91.Liu S., Chen Z.J. Expanding role of ubiquitination in NF-kappaB signaling. Cell Res. 2011;21:6–21. doi: 10.1038/cr.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deng L., Wang C., Spencer E., Yang L., Braun A., You J., Slaughter C., Pickart C., Chen Z.J. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 93.Wang C., Deng L., Hong M., Akkaraju G.R., Inoue J., Chen Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 94.Chen Z.J., Parent L., Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 95.Kang H.H., Kim I.K., Yeo C.D., Kim S.W., Lee H.Y., Im J.H., Kwon H.Y., Lee S.H. The Effects of Chronic Intermittent Hypoxia in Bleomycin-Induced Lung Injury on Pulmonary Fibrosis via Regulating the NF-kappaB/Nrf2 Signaling Pathway. Tuberc. Respir. Dis. 2020;83:S63–S74. doi: 10.4046/trd.2020.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raish M., Ahmad A., Ahmad Ansari M., Ahad A., Al-Jenoobi F.I., Al-Mohizea A.M., Khan A., Ali N. Sinapic acid ameliorates bleomycin-induced lung fibrosis in rats. Biomed. Pharmacother. 2018;108:224–231. doi: 10.1016/j.biopha.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 97.Ryter S.W., Alam J., Choi A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 98.Alam J., Stewart D., Touchard C., Boinapally S., Choi A.M., Cook J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 99.Ahmad A., Alkharfy K.M., Jan B.L., Ahad A., Ansari M.A., Al-Jenoobi F.I., Raish M. Thymoquinone treatment modulates the Nrf2/HO-1 signaling pathway and abrogates the inflammatory response in an animal model of lung fibrosis. Exp. Lung Res. 2020;46:53–63. doi: 10.1080/01902148.2020.1726529. [DOI] [PubMed] [Google Scholar]
- 100.Yang D., Qiu J., Zhou H., Yu Y., Zhou D., Xu Y., Zhu M., Ge X., Li J., Lv C., et al. Dihydroartemisinin alleviates oxidative stress in bleomycin-induced pulmonary fibrosis. Life Sci. 2018;205:176–183. doi: 10.1016/j.lfs.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 101.Huai B., Ding J. Atractylenolide III attenuates bleomycin-induced experimental pulmonary fibrosis and oxidative stress in rat model via Nrf2/NQO1/HO-1 pathway activation. Immunopharmacol. Immunotoxicol. 2020;42:436–444. doi: 10.1080/08923973.2020.1806871. [DOI] [PubMed] [Google Scholar]
- 102.Li W., Cai Z., Mehmood S., Wang Y., Pan W., Zhang W., Lu Y., Chen Y. Polysaccharide FMP-1 from Morchella esculenta attenuates cellular oxidative damage in human alveolar epithelial A549 cells through PI3K/AKT/Nrf2/HO-1 pathway. Int. J. Biol. Macromol. 2018;120:865–875. doi: 10.1016/j.ijbiomac.2018.08.148. [DOI] [PubMed] [Google Scholar]
- 103.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan J., Li J., Zhang L., Sun Y., Jiang J., Huang Y., Xu H., Jiang H., Hu R. Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radic. Biol. Med. 2018;121:78–85. doi: 10.1016/j.freeradbiomed.2018.04.557. [DOI] [PubMed] [Google Scholar]
- 105.Cheresh P., Kim S.J., Tulasiram S., Kamp D.W. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta. 2013;1832:1028–1040. doi: 10.1016/j.bbadis.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kinnula V.L., Fattman C.L., Tan R.J., Oury T.D. Oxidative Stress in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kinnula V.L., Crapo J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 108.Walters D.M., Cho H.Y., Kleeberger S.R. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: A potential role for Nrf2. Antioxid. Redox Signal. 2008;10:321–332. doi: 10.1089/ars.2007.1901. [DOI] [PubMed] [Google Scholar]
- 109.Wang T., Dai F., Li G., Chen X., Li Y., Wang S., Ren D., Wang X., Lou H., Zhou B., et al. Trans-4,4’-dihydroxystilbene ameliorates cigarette smoke-induced progression of chronic obstructive pulmonary disease via inhibiting oxidative stress and inflammatory response. Free Radic. Biol. Med. 2020;152:525–539. doi: 10.1016/j.freeradbiomed.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 110.Wang S., Sarria B., Mateos R., Goya L., Bravo-Clemente L. TNF-alpha-induced oxidative stress and endothelial dysfunction in EA.hy926 cells is prevented by mate and green coffee extracts, 5-caffeoylquinic acid and its microbial metabolite, dihydrocaffeic acid. Int. J. Food Sci. Nutr. 2019;70:267–284. doi: 10.1080/09637486.2018.1505834. [DOI] [PubMed] [Google Scholar]
- 111.Leung C.C., Yu I.T.S., Chen W. Silicosis. Lancet. 2012;379:2008–2018. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 112.Koga Y., Satoh T., Kaira K., Hachisu Y., Ishii Y., Yajima T., Hisada T., Yokoo H., Dobashi K. Progression of Idiopathic Pulmonary Fibrosis Is Associated with Silica/Silicate Inhalation. Environ. Sci. Technol. Lett. 2021;8:903–910. doi: 10.1021/acs.estlett.1c00659. [DOI] [Google Scholar]
- 113.Zhu Z., Li Q., Xu C., Zhao J., Li S., Wang Y., Tian L. Sodium tanshinone IIA sulfonate attenuates silica-induced pulmonary fibrosis in rats via activation of the Nrf2 and thioredoxin system. Environ. Toxicol. Pharmacol. 2020;80:103461. doi: 10.1016/j.etap.2020.103461. [DOI] [PubMed] [Google Scholar]
- 114.Feng F., Cheng P., Xu S., Li N., Wang H., Zhang Y., Wang W. Tanshinone IIA attenuates silica-induced pulmonary fibrosis via Nrf2-mediated inhibition of EMT and TGF-β1/Smad signaling. Chem. Biol. Interact. 2020;319:109024. doi: 10.1016/j.cbi.2020.109024. [DOI] [PubMed] [Google Scholar]
- 115.Feng F., Cheng P., Zhang H., Li N., Qi Y., Wang H., Wang Y., Wang W. The Protective Role of Tanshinone IIA in Silicosis Rat Model via TGF-β1/Smad Signaling Suppression, NOX4 Inhibition and Nrf2/ARE Signaling Activation. Drug Des. Dev. Ther. 2019;13:4275–4290. doi: 10.2147/DDDT.S230572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paliogiannis P., Fois A.G., Collu C., Bandinu A., Zinellu E., Carru C., Pirina P., Mangoni A.A., Zinellu A. Oxidative stress-linked biomarkers in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Biomark. Med. 2018;12:1175–1184. doi: 10.2217/bmm-2018-0108. [DOI] [PubMed] [Google Scholar]
- 117.Fois A., Sotgiu E., Scano V., Negri S., Mellino S., Zinellu E., Pirina P., Pintus G., Carru C., Mangoni A., et al. Effects of Pirfenidone and Nintedanib on Markers of Systemic Oxidative Stress and Inflammation in Patients with Idiopathic Pulmonary Fibrosis: A Preliminary Report. Antioxidants. 2020;9:1064. doi: 10.3390/antiox9111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kovac S., Angelova P.R., Holmstrom K.M., Zhang Y., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta. 2015;1850:794–801. doi: 10.1016/j.bbagen.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han B., Li S., Lv Y., Yang D., Li J., Yang Q., Wu P., Lv Z., Zhang Z. Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 2019;10:5555–5565. doi: 10.1039/C9FO01152H. [DOI] [PubMed] [Google Scholar]
- 120.Zhao H., Eguchi S., Alam A., Ma D. The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;312:L155–L162. doi: 10.1152/ajplung.00449.2016. [DOI] [PubMed] [Google Scholar]
- 121.Xin X., Yao D., Zhang K., Han S., Liu D., Wang H., Liu X., Li G., Huang J., Wang J. Protective effects of Rosavin on bleomycin-induced pulmonary fibrosis via suppressing fibrotic and inflammatory signaling pathways in mice. Biomed. Pharmacother. 2019;115:108870. doi: 10.1016/j.biopha.2019.108870. [DOI] [PubMed] [Google Scholar]
- 122.Zhang H., Deng W., Yang Y., Wei S., Xue L., Tao S. Pharmaceutic application of vitamin D3 on particle-induced fibrotic effects through induction of Nrf2 signals. Toxicol. Res. 2020;9:55–66. doi: 10.1093/toxres/tfaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hecker L., Logsdon N.J., Kurundkar D., Kurundkar A., Bernard K., Hock T., Meldrum E., Sanders Y.Y., Thannickal V.J. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med. 2014;6:231ra247. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Veith C., Boots A.W., Idris M., van Schooten F.J., van der Vliet A. Redox Imbalance in Idiopathic Pulmonary Fibrosis: A Role for Oxidant Cross-Talk Between NADPH Oxidase Enzymes and Mitochondria. Antioxid. Redox Signal. 2019;31:1092–1115. doi: 10.1089/ars.2019.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chan E.C., Jiang F., Peshavariya H.M., Dusting G.J. Regulation of cell proliferation by NADPH oxidase-mediated signaling: Potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol. Ther. 2009;122:97–108. doi: 10.1016/j.pharmthera.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 126.Sirokmany G., Donko A., Geiszt M. Nox/Duox Family of NADPH Oxidases: Lessons from Knockout Mouse Models. Trends Pharmacol. Sci. 2016;37:318–327. doi: 10.1016/j.tips.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 127.Li Z.M., Xu S.Y., Feng Y.Z., Cheng Y.R., Xiong J.B., Zhou Y., Guan C.X. The role of NOX4 in pulmonary diseases. J. Cell. Physiol. 2021;236:1628–1637. doi: 10.1002/jcp.30005. [DOI] [PubMed] [Google Scholar]
- 128.Kawahara T., Quinn M.T., Lambeth J.D. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol. Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hecker L., Cheng J., Thannickal V.J. Targeting NOX enzymes in pulmonary fibrosis. Cell. Mol. Life Sci. 2012;69:2365–2371. doi: 10.1007/s00018-012-1012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zeng H., Wang Y., Gu Y., Wang J., Zhang H., Gao H., Jin Q., Zhao L. Polydatin attenuates reactive oxygen species-induced airway remodeling by promoting Nrf2-mediated antioxidant signaling in asthma mouse model. Life Sci. 2019;218:25–30. doi: 10.1016/j.lfs.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 131.Li C., Sun X., Li A., Mo M., Zhao Z. S-Allylmercaptocysteine attenuates Bleomycin-induced pulmonary fibrosis in mice via suppressing TGF-β1/Smad and oxidative stress pathways. Int. Immunopharmacol. 2020;79:106110. doi: 10.1016/j.intimp.2019.106110. [DOI] [PubMed] [Google Scholar]
- 132.Rong Y., Cao B., Liu B., Li W., Chen Y., Chen H., Liu Y., Liu T. A novel Gallic acid derivative attenuates BLM-induced pulmonary fibrosis in mice. Int. Immunopharmacol. 2018;64:183–191. doi: 10.1016/j.intimp.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 133.Peng L., An L., Sun N., Ma Y., Zhang X., Liu W., Liu B., Li P., Chen J. Salvia miltiorrhiza Restrains Reactive Oxygen Species-Associated Pulmonary Fibrosis via Targeting Nrf2-Nox4 Redox Balance. Am. J. Chin. Med. 2019;47:1113–1131. doi: 10.1142/S0192415X19500575. [DOI] [PubMed] [Google Scholar]
- 134.Wu Y., Zheng L., Yang H., Lyu X. Research progress of itaconate on the regulation of macrophage inflammation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33:1388–1392. doi: 10.3760/cma.j.cn121430-20201127-00735. [DOI] [PubMed] [Google Scholar]
- 135.Mora A.L., Rojas M., Pardo A., Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 2017;16:810. doi: 10.1038/nrd.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Selman M., Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. an integral model. Am. J. Respir. Crit. Care Med. 2014;189:1161–1172. doi: 10.1164/rccm.201312-2221PP. [DOI] [PubMed] [Google Scholar]
- 137.Loomis-King H., Flaherty K.R., Moore B.B. Pathogenesis, current treatments and future directions for idiopathic pulmonary fibrosis. Curr. Opin. Pharmacol. 2013;13:377–385. doi: 10.1016/j.coph.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saito S., Zhuang Y., Suzuki T., Ota Y., Bateman M.E., Alkhatib A.L., Morris G.F., Lasky J.A. HDAC8 inhibition ameliorates pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;316:L175–L186. doi: 10.1152/ajplung.00551.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aziz R.K., Kulkarni A.A., Thatcher T.H., Olsen K.C., Maggirwar S.B., Phipps R.P., Sime P.J. PPAR-γ Ligands Repress TGFβ-Induced Myofibroblast Differentiation by Targeting the PI3K/Akt Pathway: Implications for Therapy of Fibrosis. PLoS ONE. 2011;6:e15909. doi: 10.1371/journal.pone.0015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fernandez I.E., Eickelberg O. The impact of TGF-beta on lung fibrosis: From targeting to biomarkers. Proc. Am. Thorac. Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 141.Han Y., Gu X., Yang C., Ji H., Lan Y., Bi Y., Si R., Qu J., Cheng M., Gao J. Protective effect of dimethyl itaconate against fibroblast-myofibroblast differentiation during pulmonary fibrosis by inhibiting TXNIP. J. Cell. Physiol. 2021;236:7734–7744. doi: 10.1002/jcp.30456. [DOI] [PubMed] [Google Scholar]
- 142.Liu S.S., Liu C., Lv X.X., Cui B., Yan J., Li Y.X., Li K., Hua F., Zhang X.W., Yu J.J., et al. The chemokine CCL1 triggers an AMFR-SPRY1 pathway that promotes differentiation of lung fibroblasts into myofibroblasts and drives pulmonary fibrosis. Immunity. 2021;54:2042–2056.e48. doi: 10.1016/j.immuni.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 143.Wang Q., Liu J., Hu Y., Pan T., Xu Y., Yu J., Xiong W., Zhou Q., Wang Y. Local administration of liposomal-based Srpx2 gene therapy reverses pulmonary fibrosis by blockading fibroblast-to-myofibroblast transition. Theranostics. 2021;11:7110–7125. doi: 10.7150/thno.61085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hinz B., Phan S.H., Thannickal V.J., Prunotto M., Desmouliere A., Varga J., De Wever O., Mareel M., Gabbiani G. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am. J. Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tsubouchi K., Araya J., Minagawa S., Hara H., Ichikawa A., Saito N., Kadota T., Sato N., Yoshida M., Kurita Y., et al. Azithromycin attenuates myofibroblast differentiation and lung fibrosis development through proteasomal degradation of NOX4. Autophagy. 2017;13:1420–1434. doi: 10.1080/15548627.2017.1328348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.El Agha E., Kramann R., Schneider R.K., Li X., Seeger W., Humphreys B.D., Bellusci S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell. 2017;21:166–177. doi: 10.1016/j.stem.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 147.Artaud-Macari E., Goven D., Brayer S., Hamimi A., Besnard V., Marchal-Somme J., Ali Z.E., Crestani B., Kerdine-Romer S., Boutten A., et al. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid. Redox Signal. 2013;18:66–79. doi: 10.1089/ars.2011.4240. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Z., Qu J., Zheng C., Zhang P., Zhou W., Cui W., Mo X., Li L., Xu L., Gao J. Nrf2 antioxidant pathway suppresses Numb-mediated epithelial-mesenchymal transition during pulmonary fibrosis. Cell Death Dis. 2018;9:83. doi: 10.1038/s41419-017-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bartis D., Mise N., Mahida R.Y., Eickelberg O., Thickett D.R. Epithelial-mesenchymal transition in lung development and disease: Does it exist and is it important? Thorax. 2014;69:760–765. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 150.Barkauskas C.E., Noble P.W. Cellular mechanisms of tissue fibrosis. 7. New insights into the cellular mechanisms of pulmonary fibrosis. Am. J. Physiol. Cell Physiol. 2014;306:C987–C996. doi: 10.1152/ajpcell.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu F., Liu C., Zhou D., Zhang L. TGF-beta/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016;64:157–167. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lu Y., Zhang T., Shan S., Wang S., Bian W., Ren T., Yang D. MiR-124 regulates transforming growth factor-beta1 induced differentiation of lung resident mesenchymal stem cells to myofibroblast by repressing Wnt/beta-catenin signaling. Dev. Biol. 2019;449:115–121. doi: 10.1016/j.ydbio.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 154.Zhou W., Mo X., Cui W., Zhang Z., Li D., Li L., Xu L., Yao H., Gao J. Nrf2 inhibits epithelial-mesenchymal transition by suppressing snail expression during pulmonary fibrosis. Sci. Rep. 2016;6:38646. doi: 10.1038/srep38646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ding Z., Wu X., Wang Y., Ji S., Zhang W., Kang J., Li J., Fei G. Melatonin prevents LPS-induced epithelial-mesenchymal transition in human alveolar epithelial cells via the GSK-3β/Nrf2 pathway. Biomed. Pharmacother. 2020;132:110827. doi: 10.1016/j.biopha.2020.110827. [DOI] [PubMed] [Google Scholar]
- 156.Ebrahimpour A., Wang M., Li L., Jegga A.G., Bonnen M.D., Eissa N.T., Raghu G., Jyothula S., Kheradmand F., Hanania N.A., et al. Esomeprazole attenuates inflammatory and fibrotic response in lung cells through the MAPK/Nrf2/HO1 pathway. J. Inflamm. 2021;18:17. doi: 10.1186/s12950-021-00284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Richter K., Kietzmann T. Reactive oxygen species and fibrosis: Further evidence of a significant liaison. Cell Tissue Res. 2016;365:591–605. doi: 10.1007/s00441-016-2445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang L.S., Jiang P., Feghali-Bostwick C., Reddy S.P., Garcia J.G.N., Natarajan V. Lysocardiolipin acyltransferase regulates TGF-beta mediated lung fibroblast differentiation. Free Radic. Biol. Med. 2017;112:162–173. doi: 10.1016/j.freeradbiomed.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 159.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T.R., Horowitz J.C., Pennathur S., Martinez F.J., Thannickal V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mills E.L., Kelly B., Logan A., Costa A.S.H., Varma M., Bryant C.E., Tourlomousis P., Däbritz J.H.M., Gottlieb E., Latorre I., et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016;167:457.e13–470.e13. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mills E.L., Ryan D.G., Prag H.A., Dikovskaya D., Menon D., Zaslona Z., Jedrychowski M.P., Costa A.S.H., Higgins M., Hams E., et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Peace C.G., O’Neill L.A. The role of itaconate in host defense and inflammation. J. Clin. Investig. 2022;132:e148548. doi: 10.1172/JCI148548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ogger P.P., Albers G.J., Hewitt R.J., O’Sullivan B.J., Powell J.E., Calamita E., Ghai P., Walker S.A., McErlean P., Saunders P., et al. Itaconate controls the severity of pulmonary fibrosis. Sci. Immunol. 2020;5:eaan2946. doi: 10.1126/sciimmunol.abc1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sehgal S.N. Rapamune (RAPA, rapamycin, sirolimus): Mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin. Biochem. 1998;31:335–340. doi: 10.1016/S0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 165.Korfhagen T.R., Le Cras T.D., Davidson C.R., Schmidt S.M., Ikegami M., Whitsett J.A., Hardie W.D. Rapamycin Prevents Transforming Growth Factor-α–Induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2009;41:562–572. doi: 10.1165/rcmb.2008-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shao X., Li M., Luo C., Wang Y.-Y., Lu Y.-Y., Feng S., Li H., Lang X.-B., Wang Y.-C., Lin C., et al. Effects of rapamycin against paraquat-induced pulmonary fibrosis in mice. J. Zhejiang Univ. Sci. B. 2015;16:52–61. doi: 10.1631/jzus.B1400229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu Y., Tai W., Qu X., Wu W., Li Z., Deng S., Vongphouttha C., Dong Z. Rapamycin protects against paraquat-induced pulmonary fibrosis: Activation of Nrf2 signaling pathway. Biochem. Biophys. Res. Commun. 2017;490:535–540. doi: 10.1016/j.bbrc.2017.06.074. [DOI] [PubMed] [Google Scholar]
- 168.Yan B., Ma Z., Shi S., Hu Y., Ma T., Rong G., Yang J. Sulforaphane prevents bleomycininduced pulmonary fibrosis in mice by inhibiting oxidative stress via nuclear factor erythroid 2related factor2 activation. Mol. Med. Rep. 2017;15:4005–4014. doi: 10.3892/mmr.2017.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dong H., Yang J., Wang Y., Jiang Y., Chen J., Zhang W., Lu Y., Chen L., Chen Y. Polysaccharide SAFP from Sarcodon aspratus attenuates oxidative stress-induced cell damage and bleomycin-induced pulmonary fibrosis. Int. J. Biol. Macromol. 2020;164:1215–1236. doi: 10.1016/j.ijbiomac.2020.07.120. [DOI] [PubMed] [Google Scholar]
- 170.Cui Y., Xin H., Tao Y., Mei L., Wang Z. Arenaria kansuensis attenuates pulmonary fibrosis in mice via the activation of Nrf2 pathway and the inhibition of NF-kB/TGF-beta1/Smad2/3 pathway. Phytother. Res. 2021;35:974–986. doi: 10.1002/ptr.6857. [DOI] [PubMed] [Google Scholar]
- 171.Veith C., Drent M., Bast A., van Schooten F., Boots A. The disturbed redox-balance in pulmonary fibrosis is modulated by the plant flavonoid quercetin. Toxicol. Appl. Pharmacol. 2017;336:40–48. doi: 10.1016/j.taap.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 172.Peng L., Wen L., Shi Q., Gao F., Huang B., Wang C. Chelerythrine Ameliorates Pulmonary Fibrosis via Activating the Nrf2/ARE Signaling Pathway. Cell Biochem. Biophys. 2021;79:337–347. doi: 10.1007/s12013-021-00967-0. [DOI] [PubMed] [Google Scholar]
- 173.Zeng Q., Zhou T., Zhao F., Xiong D., He B., Hua Q., Lin M., Deng L., Sang X., Xie W., et al. p62-Nrf2 Regulatory Loop Mediates the Anti-Pulmonary Fibrosis Effect of Bergenin. Antioxidants. 2022;11:307. doi: 10.3390/antiox11020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bai Y., Li J., Zhao P., Li Y., Li M., Feng S., Qin Y., Tian Y., Zhou T. A Chinese Herbal Formula Ameliorates Pulmonary Fibrosis by Inhibiting Oxidative Stress via Upregulating Nrf2. Front. Pharmacol. 2018;9:628. doi: 10.3389/fphar.2018.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu J., Wu Z., Liu Y., Zhan Z., Yang L., Wang C., Jiang Q., Ran H., Li P., Wang Z. ROS-responsive liposomes as an inhaled drug delivery nanoplatform for idiopathic pulmonary fibrosis treatment via Nrf2 signaling. J. Nanobiotechnol. 2022;20:213. doi: 10.1186/s12951-022-01435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shen H., Wu N., Wang Y., Zhao H., Zhang L., Li T., Zhao M. Chloroquine attenuates paraquat-induced lung injury in mice by altering inflammation, oxidative stress and fibrosis. Int. Immunopharmacol. 2017;46:16–22. doi: 10.1016/j.intimp.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 177.Wang L., Li S., Yao Y., Yin W., Ye T. The role of natural products in the prevention and treatment of pulmonary fibrosis: A review. Food Funct. 2021;12:990–1007. doi: 10.1039/D0FO03001E. [DOI] [PubMed] [Google Scholar]
- 178.Mansour H.M., Rhee Y.-S., Wu X. Nanomedicine in pulmonary delivery. Int. J. Nanomed. 2009;4:299–319. doi: 10.2147/IJN.S4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Alipour S., Montaseri H., Tafaghodi M. Preparation and characterization of biodegradable paclitaxel loaded alginate microparticles for pulmonary delivery. Colloids Surf. B Biointerfaces. 2010;81:521–529. doi: 10.1016/j.colsurfb.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 180.Ahmad J., Akhter S., Rizwanullah M., Amin S., Rahman M., Ahmad M.Z., Rizvi M.A., Kamal M.A., Ahmad F.J. Nanotechnology-based inhalation treatments for lung cancer: State of the art. Nanotechnol. Sci. Appl. 2015;8:55–66. doi: 10.2147/NSA.S49052. [DOI] [PMC free article] [PubMed] [Google Scholar]