Abstract
This research investigated the effects of dietary rutin supplementation on the intestinal morphology, antioxidant capacity, immunity, and microbiota of aged laying hens. The results showed that 500 mg/kg rutin supplementation increased the villus height of jejunum (P < 0.05). Rutin affected the immune system of the ileum and the jejunum. In the jejunum, a diet with 500 mg/kg rutin supplementation enhanced secretory immunoglobulin A (sIgA) and reduced tumor necrosis factor- (TNF-) levels (P < 0.05). A diet with 1000 mg/kg rutin supplementation increased jejunal sIgA, immunologlobulin M (IgM), and interleukin-4 (IL-4) levels while decreasing interleukin-1 (IL-1), TNF-, and interferon- (IFN-) levels (P < 0.05). Meanwhile, a diet with 500 mg/kg rutin increased sIgA, immunologlobulin G (IgG), IgM, and interleukin-10 (IL-10) levels and reduced TNF- and IFN- levels in the ileum (P < 0.05). In the ileum, a diet with 1000 mg/kg rutin supplementation raised sIgA, IgG, IgM, IL-4, and IL-10 levels while decreasing IL-1, TNF-, and IFN- levels (P < 0.05). At the family level, a diet with 500 mg/kg rutin supplementation raised the relative abundance of Monoglobaceae and decreased the relative abundance of Eubacteriaceae (P < 0.05) compared to the control group. In the 1000 mg/kg rutin group, the relative abundance of Lactobacillaceae and Unclassified Coriobacteriale was considerably lower and the relative abundance of Monoglobaceae was higher than the control group (P < 0.05). This study showed that a diet with rutin supplementation can improve the intestinal health of aged laying hens, and the mechanism is related to improving the intestinal morphology and intestinal immune status, and regulating the intestinal microbes.
Keywords: rutin, aged laying hens, intestinal morphology, antioxidant capacity, immunity, microbiota
1. Introduction
Aging is a complicated process that involves morphological and metabolic changes in individual cells as well as the whole organism. The oxidative stress theory is one of the most prevalent ideas for how aging happens at the molecular level [1]. During the aging process in laying hens, harmful reactive oxygen species (ROS) are gradually produced, resulting in oxidative stress damage, including a decrease in liver antioxidant capacity [2,3], changes in intestinal oxidative state [4], and the aging of reproductive organs [5,6], which lead to a decline in egg production performance. Oxidative stress reaches its peak when the hen is about 65 to 70 weeks old [7]. After 75 weeks of age, laying hens’ performance often declines significantly [8], which is connected with oxidative damage and lower immunity [9,10,11,12,13]. The gut, as a key organ of diet digestion, absorption, and transformation, is easily impacted by oxidants and antioxidants in diet, resulting in redox balance shifts. Oxidative stress is a major contributor to the onset of inflammatory disorders [14]. Furthermore, intestinal inflammation decreases nutritional absorption and egg production, and inflammatory cytokines in turn can cause oxidative stress [15]. Changes in intestinal morphology, antioxidant status, and inflammation lead to poor intestinal health, which is one of the key causes of poor laying performance [16]. Changing components of the diet can quickly affect the makeup and activity of the gut microbiota [17], which plays a significant role in the development of the immune system in mammals and poultry, as well as maintaining immune system homeostasis [18,19].
Polyphenols, which are critical micronutrients in the diet, are one of the most abundant and widespread groups in the plant world, with over 8000 polyphenolic chemicals discovered. Polyphenols (phenolic acids, flavonoids, stilbenes, and lignans) have been extensively explored in recent decades due to their numerous health advantages [20,21]. Polyphenols have anti-inflammatory, antibacterial, and antioxidant activities that can affect egg production and health in older laying hens by improving gut integrity and function, lowering inflammation, or modifying microbial communities [22,23]. In older laying hens, resveratrol administration improved performance, lipid-related markers, and antioxidant activity [24]. Tea polyphenols can improve serum and liver antioxidant capability while increasing egg production [25,26,27]. Flavonoids have been used as feed additive to promote laying hens health and productive performance [28]. Rutin is a flavonoid that is found in abundance in plants. Rutin has a wide range of pharmacological effects on biological systems, including analgesic, anti-inflammatory, and anti-arthritis properties [29,30,31]. Rutin supplementation at doses of 0.50 and 1.00 g/kg substantially increased superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) activity and decreased malondialdehyde (MDA) concentrations in serum, showing rutin’s potential to transport electrons, activate antioxidant enzymes, and thereby reduce oxidative stress [32]. Among the immunological parameters studied, lysozyme activity, nitric oxide concentrations, and IgM synthesis were considerably greater in rutin-fed birds than in control birds; however, rutin at any quantity had no influence on IgG and IgA concentrations or lymphoid organ weight [33]. Previous studies suggested that rutin is a promising feed additive for broilers. Considering that aged laying hens suffer from significant oxidative damage and lower immunity, the use of antioxidants is crucial; however, there is limited study on the effects of antioxidant rutin on aging laying hens. The purpose of this research was to investigate rutin’s antioxidant capacity, immunomodulatory effects, and gut microbiome impacts in an aged laying hen.
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
All procedures were conducted under the guidelines of Nanjing Agricultural University Institutional Animal Care and Use Committee (Certification No.: SYXK(Su)2017-0007).
A total of 216 Hyline Brown aged laying hens (560 days of age) were obtained from a local commercial laying hens farm in this study. Laying hens were randomly divided into three treatments with six replicates per group. After a two-week adjustment, laying hens were separately fed with one of three kind of diets: basal diet; basal diet +500 mg/kg rutin; basal diet +1000 mg/kg rutin, and the trial lasted eight weeks. Rutin was provided by Jiangsu Bison Biotechnology Co., Ltd. (Taizhou, Jiangsu, China). The content of rutin was 96%, determined by high-performance liquid chromatography (HPLC) diffraction. These diets were formulated to meet or exceed the National Research Council (NRC, 1994) recommendation, and the composition and nutrient levels are shown in Table 1. Hens were free to drink water throughout the experiment and were exposed to a 16-h/8-h light/dark cycle.
Table 1.
Composition and nutrient level of basal diet (g/kg, as fed basis unless otherwise stated).
| Item | Content |
|---|---|
| Ingredient | |
| Corn grain | 640 |
| Soybean meal | 240 |
| Limestone | 90 |
| Premix * | 30 |
| Calculated nutrient levels | |
| Apparent metabolizable energy (MJ/kg) | 11.04 |
| Crude protein | 161.6 |
| Lysine | 8.0 |
| Methionine + cystine | 6.2 |
| Calcium | 36 |
| Total phosphorus | 5.6 |
* Supplied per kg of diet: Vitamin A, 10,000 IU; vitamin D3, 3000 IU; vitamin E, 20 IU; vitamin K3, 1.5 mg; vitamin B1, 2 mg; vitamin B2, 6 mg; vitamin B6, 3 mg; pantothenic acid, 10 mg; vitamin B12, 0.02 mg; nicotinamide, 40 mg; folic acid, 1 mg; biotin, 0.24 mg; choline chloride, 350 mg; Fe (ferrous sulfate), 80 mg; Cu (copper sulfate), 8 mg; Mn (manganese sulfate), 100 mg; Zn (zinc oxide), 65 mg; I (calcium iodate), 0.8 mg; Se (sodium selenite), 0.3 mg; calcium, 3.6 g; phosphorus, 2.4 g; methionine, 1 g; sodium chloride, 3 g.
2.2. Sample Collection
The small intestine was dissected and sampled on a refrigerated stainless-steel tray, clear of the mesentery. Middle sections of the jejunum and ileum from all hens were harvested, flushed several times with ice-cold phosphate-buffered saline (pH 7.4), fixed with 4% paraformaldehyde-PBS, and kept at 4 C for evaluation of the mucosal morphology. The jejunum and ileum mucosas were quickly scraped and fully washed with ice-cold phosphatebuffered saline before being frozen in liquid nitrogen and stored at −80 C, for intestinal antioxidant capacity and immune parameters, as well as mRNA expression analysis. Meanwhile, the contents of the cecum were collected aseptically, put in a centrifugal tube, quickly frozen in liquid nitrogen, and kept at −80 C for microbiota analysis.
2.3. Intestinal Morphological Analysis
The middle sections of ileum and jejunum from all laying hens were removed from 4% paraformaldehyde-PBS and rinsed with water. Then, they were dehydrated with a series of different ethanol concentrations, cleared with xylene until saturation, and embedded with paraffin. A section of 5 was cut for histological analysis. The sections were deparaffinized and hydrated, and then stained with H&E. Villus height and crypt depth were measured.
2.4. Intestinal Antioxidant Capacity
The SOD activity and MDA, total antioxidant capacity (T-AOC) levels in jejunal mucosa and ileal mucosa were measured in accordance with the manufacturer’s instructions using a commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Intestinal Immune Parameters
Protein concentration was tested in the supernatant of the jejunum mucosa and ileum mucosa. Meanwhile, sIgA, IgG, IgM, IL-1, TNF-, IFN-, IL-4, IL-6, and IL-10 were tested by using ELISA kits in accordance with the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.6. Real-Time Quantitative (RT-PCR)
For total RNA extraction, a sample of 50.0–60.0 mg of tissue (intestinal mucosa) was homogenized in 1.00 mL of RNAiso Plus (TaKaRa, Dalian, China). The RNA quality and quantity were then assessed using a Nano Drop ND-1000 (Nano Drop Technologies, Wilmington, DE, USA) of optical density at 260 and 280 nm. The concentration of RNA samples was diluted with diethyl pyrocarbonate-treated water to 0.5 g/L, then reverse-transcribed into cDNA using the Prime Script RT Master Mix reagentkit in accordance with the manufacturer’s instructions (TaKaRa, Dalian, China) immediately. The total RNA of the samples was processed to synthesize complementary DNA with a PrimeScriptTM RT reagent kit according to the manufacturer’s instructions (TaKaRa, Dalian, China). The reverse transcription was conducted at 37 C for 15 min, 85 C for 5 s. The relative mRNA expression of target genes was assessed using quantitative real-time PCR. The primer sequences, including IL-1, TNF-, IFN-, IL-4, IL-10, Occludin, Claudin-1, Claudin-2, ZO-1, -actin, and their gene bank ID numbers are presented in Table 2.
Table 2.
Sequences for real-time PCR primers.
| Genes | Gene Bank ID | Primer Sequence (5-3) | Product Length (bp) | |
|---|---|---|---|---|
| IL-1 | NM_204524.1 | F | TGCCTGCAGAAGAAGCCTCG | 204 |
| R | GACGGGCTCAAAAACCTCCT | |||
| TNF- | NM204267 | F | TGTGTATGTGCAGCAACCCGTAGT | 229 |
| R | GGCATTGCAATTTGGACAGAAGT | |||
| IFN- | NM_205149.1 | F | ATGTAGCTGACGGTGGACCT | 196 |
| R | TTCACGCCATCAGGAAGGTT | |||
| IL-4 | NM_001007079.1 | F | GTGCCCACGCTGTGCTTAC | 82 |
| R | AGGAAACCTCTCCCTGGATGTC | |||
| IL-10 | NM_001004414.2 | F | GGAGCTGAGGGTGAAGTTTGA | 129 |
| R | GACACAGACTGGCAGCCAAA | |||
| Occludin | NM_205128.1 | F | CCGTAACCCCGAGTTGGAT | 214 |
| R | ATTGAGGCGGTCGTTGATG | |||
| Claudin-1 | NM_001013611.2 | F | AGGTGTACGACTCGCTGCTT | 242 |
| R | AGCCACTCTGTTGCCATACC | |||
| Claudin-2 | NM_001277622.1 | F | CCTGCTCACCCTCATTGGAG | 144 |
| R | GCTGAACTCACTCTTGGGCT | |||
| ZO-1 | XM_015278975.2 | F | TTTACATGATGACCGCCTGT | 213 |
| R | GAATCCTCCCTAACGGGTTC | |||
| -actin | NM_205518 | F | TGCTGTGTTCCCATCTATCG | 150 |
| R | TTGGTGACAATACCGTGTTCA |
2.7. Gut Microbiota Sequencing
Total genome DNA was isolated from the cecum using the QIAamp DNA Stool Mini Kit (QIAGEN, Dusseldorf, Germany), and DNA concentration and purity were measured on 1% agarose gels. The v3-v4 region of the 16S rDNA gene sequences were amplified by using 338F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT).Then, the elution with Tris-HCl buffer and detection by 2% agarose electrophoresis were done, and all amplicons were purified using an AxyPrep DNA gel extraction kit (Axygen Biosciences, Union City, CA, USA). After being quantified and purified, amplicons were sequenced on an Illumina MiSeq Sequencer. The data were all analyzed using the free online platform of Majorbio Cloud Platform (www.majorbio.com). The sequences were analyzed and assigned to operational taxonomic units (OTUs; 97% identity). Alpha diversity included calculation of ACE, Chao1, Shannon, and Simpson indices, beta diversity using principal coordinate analysis (PCoA) and unweighted Unifrac cluster tree and environmental factor correlation analysis.
3. Statistical Analysis
All data were normalized using SPSS 19.0 software and then subjected to statistical analysis using one-way analysis of variance (ANOVA). The replicate was defined as an experimental unit for the trail. The Tukey’s multiple comparison test was used to compare the differences among different groups. Data are expressed as the means ± standard error of the mean (SEM). The difference was considered to be significant at P < 0.05. An ANOVA test with P between 0.05 and 0.10 was considered as a trend toward significance.
4. Results
4.1. Intestinal Morphological Analysis
Table 3 showed that the diet with 500 mg/kg rutin significantly increased the villus height of jejunum (P < 0.05). Meanwhile, the diet with 500 mg/kg and 1000 mg/kg rutin significantly increase the ratio of villus height to crypt compared with the control group (P < 0.05). However, crypt depth of jejunum, as well as villus height, crypt depth, and the ratio of villus height to crypt depth of ileum were not altered in response to rutin treatments (P > 0.05).
Table 3.
Effect of rutin on the intestinal morphology of aged laying hens.
| Items | Rutin, mg/kg | SEM | P-Value | ||
|---|---|---|---|---|---|
| CON | 500 | 1000 | |||
| Jejunum | |||||
| Villus height (m) | 935 | 1100 | 981 | 29 | 0.044 |
| Crypt depth (m) | 142 | 132 | 119 | 5 | 0.175 |
| Villus height: crypt depth | 6.7 | 8.6 | 8.5 | 0.3 | 0.003 |
| Ileum | |||||
| Villus height (m) | 675 | 791 | 808 | 36 | 0.271 |
| Crypt depth (m) | 150 | 151 | 170 | 5 | 0.278 |
| Villus height: crypt depth | 4.7 | 5.5 | 5.0 | 0.2 | 0.259 |
a,b Means with different superscripts within a row differ significantly (P < 0.05). One-way ANOVA was used to analyze the data (means ± SEM).
4.2. Intestinal Barrier-Associated Gene Expression
As shown in Table 4, in the jejunal mucosa, a diet with rutin at 500 mg/kg enhanced claudin-2 expression (P < 0.05), but there was no difference in gene abundance of ZO-1, occludin, and claudin-1 among three groups (P > 0.05). In addition, a diet with 1000 mg/kg rutin had no effect on the expression of ZO-1, occludin, claudin-1, and claudin-2 in the jejunal mucosa (P > 0.05). Compared with the control group, dietary supplementation of rutin at 500 mg/kg and 1000 mg/kg had no significant effect on the expression of tight barrier genes (ZO-1, occludin, claudin-1 and claudin-2) in ileal mucosa (P > 0.05).
Table 4.
Effects of rutin supplementation on the relative mRNA abundances of tight junction in the intestine of aged laying hens.
| Items | Rutin, mg/kg | SEM | P-Value | ||
|---|---|---|---|---|---|
| CON | 500 | 1000 | |||
| Jejunum | |||||
| ZO-1 | 1.00 | 0.91 | 1.04 | 0.09 | 0.832 |
| Occludin | 1.00 | 0.80 | 0.81 | 0.06 | 0.321 |
| Claudin-1 | 1.00 | 1.49 | 1.38 | 0.18 | 0.513 |
| Claudin-2 | 1.00 | 2.06 | 1.04 | 0.16 | 0.002 |
| Ileum | |||||
| ZO-1 | 1.00 | 1.27 | 1.28 | 0.09 | 0.346 |
| Occludin | 1.00 | 1.70 | 1.52 | 0.12 | 0.050 |
| Claudin-1 | 1.00 | 0.74 | 1.16 | 0.11 | 0.313 |
| Claudin-2 | 1.00 | 1.46 | 1.63 | 0.20 | 0.458 |
a,b Means with different superscripts within a row differ significantly (P < 0.05). One-way ANOVA was used to analyze the data (means ± SEM).
4.3. Intestinal Antioxidant Capacity
Table 5 showed that the two rutin supplemented diets had no significant influence on antioxidant parameters including MDA, SOD, and T-AOC (P > 0.05) compared with the control group in laying hens.
Table 5.
Effect of rutin on the intestinal antioxidant status of aged laying hens.
| Items | Rutin, mg/kg | SEM | P-Value | ||
|---|---|---|---|---|---|
| CON | 500 | 1000 | |||
| Jejunum | |||||
| MDA (nmol/mg protein) | 0.49 | 0.45 | 0.39 | 0.02 | 0.194 |
| SOD (U/mg protein) | 180.03 | 181.26 | 175.11 | 4.59 | 0.861 |
| T-AOC (U/mg protein) | 1.37 | 1.37 | 1.30 | 0.06 | 0.889 |
| Ileum | |||||
| MDA (nmol/mg protein) | 0.38 | 0.41 | 0.38 | 0.08 | 0.736 |
| SOD (U/mg protein) | 176.10 | 173.84 | 186.30 | 4.70 | 0.543 |
| T-AOC (U/mg protein) | 1.20 | 1.29 | 1.43 | 0.05 | 0.116 |
MDA: malondialdehyde; SOD: superoxide dismutase; T-AOC: total antioxidant capacity. One-way ANOVA was used to analyze the data (means ± SEM).
4.4. Intestinal Immunity
The results in Table 6 indicated that a diet with 500 mg/kg rutin supplementation raised the sIgA content in the jejunum mucosa (P < 0.05), Meanwhile, adding 1000 mg/kg rutin to the diet significantly increased the sIgA and IgM contents (P < 0.05). However, dietary supplementation of rutin had no significant effect on jejunal IgG content (P > 0.05). In the ileum mucosa, the contents of sIgA, IgG, and IgM significantly increased in response to a diet with 500 mg/kg rutin supplementation (P < 0.05), and a diet with 1000 mg/kg rutin supplementation significantly increased the contents of sIgA, IgG, and IgM compared with the control group, but had no difference on the contents of IgG compared with the 500 mg/kg supplementation group (P > 0.05).
Table 6.
Effect of rutin supplementation on the intestinal immunoglobulins levels of aged laying hens.
| Items | Rutin, mg/kg | SEM | P-Value | ||
|---|---|---|---|---|---|
| CON | 500 | 1000 | |||
| Jejunum | |||||
| sIgA (g/mg) | 1.11 | 1.41 | 1.60 | 0.06 | 0.000 |
| IgG (g/mg) | 167.06 | 196.45 | 198.78 | 6.28 | 0.062 |
| IgM (g/mg) | 1.98 | 2.38 | 2.82 | 0.11 | 0.003 |
| Ileum | |||||
| sIgA (g/mg) | 1.10 | 1.48 | 1.97 | 0.09 | 0.000 |
| IgG (g/mg) | 169.59 | 208.81 | 229.10 | 8.67 | 0.008 |
| IgM (g/mg) | 1.79 | 2.37 | 3.02 | 0.15 | 0.000 |
a,b,c Means with different superscripts within a row differ significantly (P < 0.05). One-way ANOVA was used to analyze the data (means ± SEM).
In comparison to the control group, dietary supplementation of 500 mg/kg rutin significantly reduced the TNF- content in the jejunal mucosa (P < 0.05), but had no significant effect on other cytokines (IL-1, IFN-, IL-4 and IL-10) (P < 0.05). In comparison to the control group, dietary supplementation with 1000 mg/kg rutin lowered the level of IL-1, TNF- and IFN- in the jejunal mucosa (P < 0.05), while increasing the amount of IL-4 (P < 0.05), and there was no significant impact on IL-10 (P > 0.05). Dietary supplementation of 500 mg/kg rutin reduced the contents of TNF- and IFN- in the ileal mucosa (P < 0.05) and raised the contents of IL-10 (P < 0.05), while there was no significant difference on IL-1 and IL-4 (P > 0.05). Diet treatment with 1000 mg/kg rutin considerably reduced the contents of IL-1, TNF- and IFN- in the ileal mucosa (P < 0.05), while significantly increasing the contents of IL-4 and IL-10 (P < 0.05). When compared to a diet with 500 mg/kg rutin supplementation, adding 1000 mg/kg rutin reduced the level of IL-1 and TNF- significantly (P < 0.05), as shown in Table 7.
Table 7.
Effect of rutin supplementation on the levels of intestinal cytokines of aged laying hens.
| Items | Rutin, mg/kg | SEM | P-Value | ||
|---|---|---|---|---|---|
| CON | 500 | 1000 | |||
| Jejunum | |||||
| IL-1 (ng/g) | 61.60 | 55.81 | 46.41 | 1.95 | 0.001 |
| TNF- (ng/g) | 24.93 | 20.00 | 15.13 | 1.15 | 0.000 |
| IFN- (pg/g) | 298.34 | 256.01 | 225.25 | 10.20 | 0.005 |
| IL-4 (pg/g) | 188.97 | 224.3 | 258.12 | 10.41 | 0.014 |
| IL-10 (ng/g) | 12.29 | 17.89 | 17.57 | 1.27 | 0.127 |
| Ileum | |||||
| IL-1 (ng/g) | 58.04 | 53.29 | 47.53 | 1.34 | 0.001 |
| TNF- (ng/g) | 24.37 | 18.01 | 13.50 | 1.25 | 0.000 |
| IFN- (pg/g) | 294.07 | 241.02 | 208.40 | 10.88 | 0.001 |
| IL-4 (pg/g) | 185.27 | 231.52 | 233.51 | 8.90 | 0.032 |
| IL-10 (ng/g) | 11.72 | 18.41 | 23.10 | 1.35 | 0.000 |
a,b,c Means with different superscripts within a row differ significantly (P < 0.05). One-way ANOVA was used for analysing the data (means ± SEM).
4.5. Intestinal Gene Expression of Cytokines
The results in Table 8 demonstrate that dietary supplementation of 500 mg/kg and 1000 mg/kg rutin significantly reduced the mRNA expression of IL-1, IFN-, and IL-4 in the jejunal mucosa when compared to the control group (P < 0.05), whereas TNF- and IL-10 mRNA expression levels were unaffected (P > 0.05). Both groups treated with rutin considerably lowered TNF- mRNA expression while significantly increasing IFN-, IL-4 and IL-10 mRNA expression compared to the control group in ileal mucosa (P < 0.05) and the levels of IL-1 mRNA expression were unaltered (P > 0.05).
Table 8.
Effects of rutin supplementation on the relative mRNA abundances of the intestinal cytokines of aged laying hens.
| Items | Rutin, mg/kg | SEM | P-Value | ||
|---|---|---|---|---|---|
| CON | 500 | 1000 | |||
| Jejunum | |||||
| IL-1 | 1.00 | 0.32 | 0.43 | 0.10 | 0.004 |
| TNF- | 1.00 | 0.85 | 0.87 | 0.08 | 0.741 |
| IFN- | 1.00 | 0.29 | 0.41 | 0.09 | 0.000 |
| IL-4 | 1.00 | 0.25 | 0.33 | 0.12 | 0.015 |
| IL-10 | 1.00 | 1.02 | 1.06 | 0.03 | 0.785 |
| Ileum | |||||
| IL-1 | 1.00 | 0.93 | 1.12 | 0.05 | 0.312 |
| TNF- | 1.00 | 0.66 | 0.70 | 0.05 | 0.005 |
| IFN- | 1.00 | 1.46 | 1.42 | 0.07 | 0.006 |
| IL-4 | 1.00 | 1.88 | 1.55 | 0.11 | 0.000 |
| IL-10 | 1.00 | 1.32 | 1.27 | 0.05 | 0.004 |
a,b Means with different superscripts within a row differ significantly (P < 0.05). One-way ANOVA was used to analyze the data (means ± SEM).
4.6. Cecal Microbiota
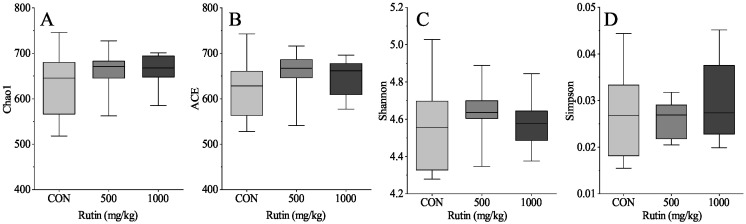
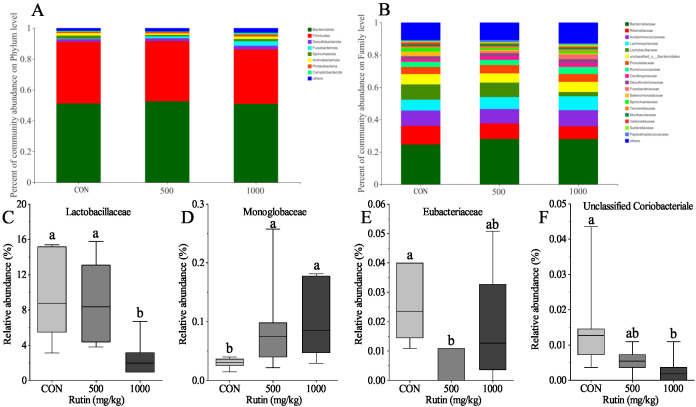
The results demonstrated that dietary rutin had no influence on the community richness (Chao1 and ACE) and the community diversity (Shannon and Simpson) indices in this research (P > 0.05) (Figure 1). Furthermore, PCoA demonstrated that a diet with rutin supplementation had no effect on the cecal microbiota composition of aged laying hens (P > 0.05) (Figure 2). Rutin supplementation had no effect on the relative abundances at the phylum level (Figure 3A). At the family level, when compared to control group, a diet with 500 mg/kg rutin supplementation raised the relative abundance of Monoglobaceae and decreased the relative abundance of Eubacteriaceae (P < 0.05) (Figure 3D,E). In the 1000 mg/kg rutin group, the relative abundance of Lactobacillaceae and Unclassified Coriobacteriale was considerably lower and the relative abundance of Monoglobaceae was higher than in the control group (P > 0.05) (Figure 3C,F).
Figure 1.
Alpha diversity of caecal microflora in aged laying hens. Chao1 (A), ACE (B), Shannon (C), and Simpson (D). Data are expressed as the mean ± SEM. Different lower-case letters indicate a significant difference among different groups (P < 0.05).
Figure 2.
PCoA analysis of caecal microflora in aged laying hens.
Figure 3.
Beta diversity of caecal microflora composition in aged laying hens. (A,B) bar plots of microbial composition at phylum (A) and family (B) levels; (C–F), significantly differential bacteria taxa at family levels. Data are presented as mean ± SEM and analyzed by Kruskal-Wallis H test. Different lower-case letters indicate a significant difference among different groups (P < 0.05).
5. Discussion
Intestinal integrity and intestinal morphology play a crucial role in intestinal function, such as the absorption of nutrient, and form a major physical barrier to protect against pathogens and toxic compounds [23,34]. An increase in the height or the width of the villus indicates an increase in the surface area, which can better absorb the available nutrients, thereby regulating the nutritional status and improving the development and health of the poultry [35]. Fideles et al. [36] pointed out that pretreatment with rutin (100 and 200 mg/kg) showed a marked reversal in villi shortening in the duodenum and the jejunum in mice of 5-Fluorouracil (FU) induced experimental intestinal mucositis; however, all rutin doses failed to reverse the villi shortening caused by 5-FU in the ileum. Additionally, Zhang et al. [37] reported that dietary rutin (250 mg/kg) and rutin (1000 mg/kg) in weaned mice for 21 days increased jejunum villus height, indicating that rutin improved intestinal morphology. The current study found that the villus height and villus-crypt ratio were longer in a rutin-supplemented diet in jejunal compared with the control group. Rutin had no influence on ileal villus height, crypt depth, and the villi-crypt ratio. Rutin has a greater impact on gut morphology in the jejunum than in the ileum. Previous research has suggested that rutin may reduce inflammation and oxidative stress via the cyclooxygenase 2 (COX-2) pathway, while also improving intestinal morphology [36]. However, the detailed mechanism of rutin on intestinal morphology needs further evaluation. The diet with 500 mg/kg rutin dramatically improved claudin-2 expression in the jejunum. Claudin-2 is expressed in the tight junctions of leaky epithelia, where it forms cation-selective and water permeable paracellular channels [38]. The results suggested that rutin is beneficial for improving intestinal integrity.
In vitro, phenolic compounds have ideal structural chemistry for free radical-scavenging activities, high levels of 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity, and have been shown in vitro to be more effective antioxidants than vitamins E and C on a molar basis [39,40]. In the animal antioxidant system, SOD removes superoxide radicals by accelerating their conversion to hydrogen peroxide [41]. MDA is a byproduct of polyunsaturated fatty acid peroxidation in cells, and MDA levels are routinely used to assess oxidative stress and antioxidant status [42]. T-AOC is usually applied as a comprehensive measure to assess an animal’s antioxidant capability [43]. A previous study have indicated that dietary rutin supplementation enhanced the serum antioxidant capacity by increasing SOD, CAT, and GSH-Px activity while decreasing MDA levels in serum of broilers [32]. In this study, rutin supplementation in the diet had no effect on the levels of MDA, and T-AOC, or SOD activity in the jejunum and ileum. This suggests that the antioxidant effect of rutin differs significantly in vivo and in vitro, and that differences in antioxidant ability of rutin in vivo studies may be related to animal species, age, tissue, and other factors.
The gut is the biggest immunological organ [44]. Secretory immunoglobulin A (sIgA) is a crucial line of defense on the mucosal surface of the intestine that protects the intestinal epithelium from toxins and pathogenic bacteria [45]. Our findings revealed that a diet with 500 mg/kg rutin increased the sIgA content but had no effect on the IgG and IgM content. Furthermore, a diet with 1000 mg/kg rutin can enhance sIgA and IgM concentrations. Ileal immunoglobulins were more responsive to rutin addition than those in the jejunum. The addition of two doses of rutin, 500 mg/kg and 1000 mg/kg, increased the concentrations of sIgA, IgM and IgG in the ileal mucosa. The results showed that rutin increases the function of the intestinal immune barrier by promoting the synthesis and release of immune globulin in the intestinal mucosa of aged laying hens. Previous investigations have reported that rutin inhibits the production of proinflammatory cytokines in microglia by lowering TNF- and IL-1 levels in human neuroblastoma ( SH-SY5Y ) cells [46]. Furthermore, rutin supplementation 10 or 20 days after trimethyltin (TMT) injection reduced the TMT-induced elevation of mRNA expression levels of reactive microglia marker and pro-inflammatory cytokines in rats [47]. In this study, we discovered that supplementing with rutin reduces TNF- in jejunal and ileal mucosa, the effect on IFN- in the ileum was greater than that in the jejunum, and high-dose rutin supplementation can significantly decrease the IL-1, TNF-, and IFN- and increase IL-10 content. The research showed that dietary rutin supplementation had an influence on intestinal cytokines, which was dose-dependent. The effect on the ileum is stronger than that on the jejunum, which may be associated with the intestinal metabolism mechanism of rutin. When compared to the control group, dietary supplementation of rutin substantially reduced IL-1, IFN-, and IL-4 mRNA expression in the jejunal mucosa. In the ileal mucosa, both groups treated with rutin dramatically reduced TNF- mRNA expression while significantly boosting IFN-, IL-4, and IL-10 mRNA expression. In view of the above data, we speculated that rutin can regulate cytokine content via modulating cytokine gene expression, and it can also function as an immunomodulator to reduce inflammation.
Gut bacteria play an important role in rutin function [48]. The majority of dietary rutin passes through the large intestine, where the colonic bacteria breaks down rutin into quercetin aglycone. Enterobacteria can then absorb or further degrade the quercetin aglycone to form different ring-fission products [49]. Rutin treatments all modulated the gut microbiota in rats during high diet fat intake [50]. The influence of rutin on the alpha and beta diversity of the gut microbiota in laying hens was not seen in this investigation. Rutin, on the other hand, increased the relative abundance of Lactobacillaceae. Lactobacillus has been demonstrated in several studies to increase the amount of Treg cells, which are key in controlling inflammation [51,52]. Meanwhile, rutin treatment reduced the relative abundance of Monoglobaceae, Eubacteriaceae, and Unclassified Coriobacteriale in the current research. Based on these findings, we infer that rutin may alter gut microbial composition and thereby change the intestinal immune system function in aged laying hens.
6. Conclusions
This study indicated that rutin supplementation for aged laying hens can modify the intestinal morphology and intestinal immune status, and modulate intestinal microbes in aged laying hens. The diet with 500 mg/kg of rutin showed an obvious effect on aged laying hens. Thus, a rutin supplementation of 500 mg/kg can act as a functional feed additive in aged laying hens to improve gut health and reduce inflammation.
Acknowledgments
The authors thank the Nutrition Regulation Innovation Team of Jiangsu Modern Agricultural Industry (Laying Hens) Technology System (61KA210022) for funding this research work.
Author Contributions
Conceptualization, Y.Z.; methodology, H.L., Y.Z., R.J. and Q.H.; validation, H.L., Y.G. and R.J.; investigation, H.L., Q.H. and Y.Z.; writing—original draft preparation, H.L.; writing—review and editing, H.L. and Y.Z.; visualization, H.L. and R.J.; supervision, Q.H. and Y.Z.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of Nanjing Agricultural University Institutional Animal Care and Use Committee (Certification No.: SYXK(Su)2017-0007).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Nutrition Regulation Innovation Team of Jiangsu Modern Agricultural Industry (Laying Hens) Technology System (61KA210022).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Olinski R., Siomek A., Rozalski R., Gackowski D., Foksinski M., Guz J., Dziaman T., Szpila A., Tudek B. Oxidative damage to DNA and antioxidant status in aging and age-related diseases. Curr. Acta Biochim. Pol. 2007;54:11–26. doi: 10.18388/abp.2007_3265. [DOI] [PubMed] [Google Scholar]
- 2.Liu X., Lin X., Mi Y., Zeng W., Zhang C. Age-related changes of yolk precursor formation in the liver of laying hens. Curr. J. Zhejiang Univ.-Sci. B. 2018;19:390–399. doi: 10.1631/jzus.B1700054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu Y.F., Chen Y.P., Jin R., Wang C., Wen C., Zhou Y.M. Age-related changes in liver metabolism and antioxidant capacity of laying hens. Poult. Sci. 2021;100:101478. doi: 10.1016/j.psj.2021.101478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang D., Wu J., Jiao H., Wang X., Zhao J., Lin H. The development of antioxidant system in the intestinal tract of broiler chickens. Poult. Sci. 2019;98:664–678. doi: 10.3382/ps/pey415. [DOI] [PubMed] [Google Scholar]
- 5.Liu X., Lin X., Mi Y., Li J., Zhang C. Grape seed proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in the hens. Oxid. Med. Cell. Longev. 2018;2018:9390810. doi: 10.1155/2018/9390810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao M., Sun Q., Khogali M.K., Liu L., Geng T., Yu L., Gong D. Dietary selenized glucose increases selenium concentration and antioxidant capacity of the liver, oviduct, and spleen in laying hens. Biol. Trace. Elem. Res. 2021;199:4746–4752. doi: 10.1007/s12011-021-02603-7. [DOI] [PubMed] [Google Scholar]
- 7.Amevor F.K., Cui Z., Du X., Ning Z., Shu G., Jin N., Deng X., Tian Y., Zhang Z., Kang X., et al. Combination of Quercetin and Vitamin E Supplementation Promotes Yolk Precursor Synthesis and Follicle Development in Aging Breeder Hens via Liver–Blood–Ovary Signal Axis. Animals. 2021;11:1915. doi: 10.3390/ani11071915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gan L., Fan H., Nie W., Guo Y. Ascorbic acid synthesis and transportation capacity in old laying hens and the effects of dietary supplementation with ascorbic acid. J. Anim. Sci. Biotechnol. 2018;9:1–12. doi: 10.1186/s40104-018-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frisard M., Ravussin E. Energy metabolism and oxidative stress. Endocrine. 2006;29:27–32. doi: 10.1385/ENDO:29:1:27. [DOI] [PubMed] [Google Scholar]
- 10.Deng W., Dong X.F., Tong J.M., Zhang Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 2012;91:575–582. doi: 10.3382/ps.2010-01293. [DOI] [PubMed] [Google Scholar]
- 11.Jha R., Das R., Oak S., Mishra P. Probiotics (direct-fed microbials) in poultry nutrition and their effects on nutrient utilization, growth and laying performance, and gut health: A systematic review. Animals. 2020;10:1863. doi: 10.3390/ani10101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra B., Jha R. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019;6:60. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laptev G.Y., Yildirim E.A., Ilina L.A., Filippova V.A., Kochish I.I., Gorfunkel E.P., Dubrovin A.V., Brazhnik E.A., Narushin V.G., Novikova N.I., et al. Effects of essential oils-based supplement and salmonella infection on gene expression, blood parameters, cecal microbiome, and egg production in laying hens. Animals. 2021;11:360. doi: 10.3390/ani11020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li P., Chang M. Roles of PRR-mediated signaling pathways in the regulation of oxidative stress and inflammatory diseases. Int. J. Mol. Sci. 2021;22:7688. doi: 10.3390/ijms22147688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gessner D.K., Ringseis R., Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017;101:605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- 16.Jing M., Munyaka P.M., Tactacan G.B., Rodriguez-Lecompte J.C., House J.D. Performance, serum biochemical responses, and gene expression of intestinal folate transporters of young and older laying hens in response to dietary folic acid supplementation and challenge with Escherichia coli lipopolysaccharide. Poult. Sci. 2014;93:122–131. doi: 10.3382/ps.2013-03384. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y., Liu H., Qin N., Ren X., Zhu B., Xia X. Impact of food additives on the composition and function of gut microbiota: A review. Trends Food Sci. Technol. 2020;99:295–310. doi: 10.1016/j.tifs.2020.03.006. [DOI] [Google Scholar]
- 18.Diaz Carrasco J.M., Casanova N.A., Fernández Miyakawa M.E. Microbiota, gut health and chicken productivity: What is the connection? Microorganisms. 2019;7:374. doi: 10.3390/microorganisms7100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer E., Williams B.A., Smidt H., Verstegen M.W., Mosenthin R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr. Issues Intest. Microbiol. 2006;7:35–52. [PubMed] [Google Scholar]
- 20.Khalil A., Tazeddinova D. The upshot of Polyphenolic compounds on immunity amid COVID-19 pandemic and other emerging communicable diseases: An appraisal. Nat. Prod. Bioprospect. 2020;10:411–429. doi: 10.1007/s13659-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spencer J.P., Abd El Mohsen M.M., Minihane A.M., Mathers J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008;99:12–22. doi: 10.1017/S0007114507798938. [DOI] [PubMed] [Google Scholar]
- 22.Puupponen-Pimiä R.A.M.A., Aura A.-M., Oksman-Caldentey K.-M., Myllärinen P., Saarela M., Mattila-Sandholm T., Poutanen K. Development of functional ingredients for gut health. Trends Food Sci. Technol. 2002;13:3–11. doi: 10.1016/S0924-2244(02)00020-1. [DOI] [Google Scholar]
- 23.Patra A.K., Amasheh S., Aschenbach J.R. Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019;59:3237–3266. doi: 10.1080/10408398.2018.1486284. [DOI] [PubMed] [Google Scholar]
- 24.Feng Z.H., Gong J.G., Zhao G.X., Lin X., Liu Y.C., Ma K.W. Effects of dietary supplementation of resveratrol on performance, egg quality, yolk cholesterol and antioxidant enzyme activity of laying hens. Br. Poult. Sci. 2017;58:544–549. doi: 10.1080/00071668.2017.1349295. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L., Ding X., Wang J., Bai S., Zeng Q., Su Z., Xuan Y., Zhang K. Tea polyphenols increase the antioxidant status of laying hens fed diets with different levels of ageing corn. Anim. Nutr. 2021;7:650–660. doi: 10.1016/j.aninu.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan Z.H., Zhang K.Y., Ding X.M., Luo Y.H., Bai S.P., Zeng Q.F., Wang J.P. Effect of tea polyphenols on production performance, egg quality, and hepatic antioxidant status of laying hens in vanadium-containing diets. Poult. Sci. 2016;95:1709–1717. doi: 10.3382/ps/pew097. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., Yang Z., Celi P., Yan L., Ding X., Bai S., Zeng Q., Mao X., Feng B., Xu S., et al. Alteration of the antioxidant capacity and gut microbiota under high levels of molybdenum and green tea polyphenols in laying hens. Antioxidants. 2019;8:503. doi: 10.3390/antiox8100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iqbal Y., Cottrell J.J., Suleria H.A., Dunshea F.R. Gut microbiota-polyphenol interactions in chicken: A review. Animals. 2020;10:1391. doi: 10.3390/ani10081391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganeshpurkar A., Saluja A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017;25:149–164. doi: 10.1016/j.jsps.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selloum L., Bouriche H., Tigrine C., Boudoukha C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp. Toxicol. Pathol. 2003;54:313–318. doi: 10.1078/0940-2993-00260. [DOI] [PubMed] [Google Scholar]
- 31.Yoo H., Ku S., Baek Y., Bae J. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflamm. Res. 2014;63:197–206. doi: 10.1007/s00011-013-0689-x. [DOI] [PubMed] [Google Scholar]
- 32.Hassan F.A., Roushdy E.M., Kishawy A.T., Zaglool A.W., Tukur H.A., Saadeldin I.M. Growth performance, antioxidant capacity, lipid-related transcript expression and the economics of broiler chickens fed different levels of rutin. Animals. 2018;9:7. doi: 10.3390/ani9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Awad A., Zaglool A.W., Khalil S.R. Immunohaematological status and mRNA expression of the genes encoding interleukin-6, nuclear-factor kappa B, and tumor-necrosis factor-α in the spleen of broilers supplemented with dietary rutin. Anim. Prod. Sci. 2018;59:1454–1461. doi: 10.1071/AN18102. [DOI] [Google Scholar]
- 34.Heo J.M., Opapeju F.O., Pluske J.R., Kim J.C., Hampson D.J., Nyachoti C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013;97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- 35.Kamboh A.A., Arain M.A., Mughal M.J., Zaman A., Arain Z.M., Soomro A.H. Flavonoids: Health Promoting Phytochemicals for Animal Production—A Review. J. Anim. Health Prod. 2015;3:6–13. doi: 10.14737/journal.jahp/2015/3.1.6.13. [DOI] [Google Scholar]
- 36.Fideles L.D.S., de Miranda J.A.L., Martins C.D.S., Barbosa M.L.L., Pimenta H.B., Pimentel P.V.D.S., Teixeira C.S., Scafuri M.A.S., Façanha S., Barreto J.E.F., et al. Role of Rutin in 5-Fluorouracil-Induced Intestinal Mucositis: Prevention of Histological Damage and Reduction of Inflammation and Oxidative Stress. Molecules. 2020;25:2786. doi: 10.3390/molecules25122786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J.Q., Xiong W., Li Z.H., Liu H.J., Zhou B.B., Wang M.Y., Wang T., Wang C. Effects of rutin on growth, intestinal antioxidant capacity and lipid metabolism in weaned mice. J. Nanjing Agric. Univ. 2022;45:1–15. [Google Scholar]
- 38.Venugopal S., Anwer S., Szászi K. Claudin-2: Roles beyond permeability functions. Int. J. Mol. Sci. 2019;20:5655. doi: 10.3390/ijms20225655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rice-Evans C., Miller N., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- 40.Yang J., Guo J., Yuan J. In Vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008;41:1060–1066. doi: 10.1016/j.lwt.2007.06.010. [DOI] [Google Scholar]
- 41.Aruoma O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaweł S., Wardas M., Niedworok E., Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004;57:453–455. [PubMed] [Google Scholar]
- 43.Li L., Li M., Zhu R., Yu Z., Wang J., Duan J., Wang T., Wu L. Effects of β-conglycinin on growth performance, antioxidant capacity and intestinal health in juvenile golden crucian carp, Carassius auratus. Aquac. Res. 2019;50:3231–3241. doi: 10.1111/are.14278. [DOI] [Google Scholar]
- 44.Alverdy J.C. Effects of glutamine-supplemented diets on immunology of the gut. JPEN J. Parenter. Enteral Nutr. 1990;14:109S–113S. doi: 10.1177/014860719001400415. [DOI] [PubMed] [Google Scholar]
- 45.Winner L., Mack J., Weltzin R., Mekalanos J.J., Kraehenbuhl J.P., Neutra M.R. New model for analysis of mucosal immunity: Intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect. Immun. 1991;59:977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S.W., Wang Y.J., Su Y.J., Zhou W.W., Yang S.G., Zhang R., Zhao M., Li Y., Zhang Z., Zhan D., et al. Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. J. Neurotoxicol. 2012;33:482–490. doi: 10.1016/j.neuro.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Koda T., Kuroda Y., Imai H. Rutin supplementation in the diet has protective effects against toxicant-induced hippocampal injury by suppression of microglial activation and pro-inflammatory cytokines. Cell. Mol. Neurobiol. 2009;29:523–531. doi: 10.1007/s10571-008-9344-4. [DOI] [PubMed] [Google Scholar]
- 48.Kim H., Kong H., Choi B., Yang Y., Kim Y., Lim M.J., Neckers L., Jung Y. Metabolic and pharmacological properties of rutin, a dietary quercetin glycoside, for treatment of inflammatory bowel disease. Pharm. Res. 2005;22:1499–1509. doi: 10.1007/s11095-005-6250-z. [DOI] [PubMed] [Google Scholar]
- 49.Kawabata K., Yoshioka Y., Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. 2019;24:370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng L., Zhang Q., Zhang Y., Yao Z., Song P., Wei L., Zhao G., Yan Z. Effect of tartary buckwheat, rutin, and quercetin on lipid metabolism in rats during high dietary fat intake. Food Sci. Nutr. 2020;8:199–213. doi: 10.1002/fsn3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J., Bang J., Woo H.J. Effect of orally administered Lactobacillus brevis HY7401 in a food allergy mouse model. J. Microbiol. Biotechnol. 2013;23:1636–1640. doi: 10.4014/jmb.1306.06047. [DOI] [PubMed] [Google Scholar]
- 52.Shah M.M., Saio M., Yamashita H., Tanaka H., Takami T., Ezaki T., Inagaki N. Lactobacillus acidophilus strain L-92 induces CD4+ CD25+ Foxp3+ regulatory T cells and suppresses allergic contact dermatitis. Biol. Pharm. Bull. 2012;35:612–616. doi: 10.1248/bpb.35.612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.