Abstract
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases regulate production of reactive oxygen species (ROS) that cause oxidative damage to cellular components but also regulate redox signaling in many cell types with essential functions in the cardiovascular system. Research over the past couple of decades has uncovered mechanisms by which NADPH oxidase (NOX) enzymes regulate oxidative stress and compartmentalize intracellular signaling in endothelial cells, smooth muscle cells, macrophages, cardiomyocytes, fibroblasts, and other cell types. NOX2 and NOX4, for example, regulate distinct redox signaling mechanisms in cardiac myocytes pertinent to the onset and progression of cardiac hypertrophy and heart failure. Heart failure with preserved ejection fraction (HFpEF), which accounts for at least half of all heart failure cases and has few effective treatments to date, is classically associated with ventricular diastolic dysfunction, i.e., defects in ventricular relaxation and/or filling. However, HFpEF afflicts multiple organ systems and is associated with systemic pathologies including inflammation, oxidative stress, arterial stiffening, cardiac fibrosis, and renal, adipose tissue, and skeletal muscle dysfunction. Basic science studies and clinical data suggest a role for systemic and myocardial oxidative stress in HFpEF, and evidence from animal models demonstrates the critical functions of NOX enzymes in diastolic function and several HFpEF-associated comorbidities. Here, we discuss the roles of NOX enzymes in cardiovascular cells that are pertinent to the development and progression of diastolic dysfunction and HFpEF and outline potential clinical implications.
Keywords: HFpEF, heart failure, diastolic dysfunction, ROS, oxidative stress, redox signaling, NADPH oxidases, NOX2, NOX4, Rac1, nitrosative stress, S-nitrosylation, cardiomyopathy, cardiac hypertrophy, angiotensin, RAAS
1. Introduction
1.1. Heart Failure with Preserved Ejection Fraction (HFpEF)
Heart failure with preserved ejection fraction (HFpEF) is a complex, heterogenous, and highly prevalent multiorgan syndrome commonly associated with advanced age, obesity, and/or hypertension [1,2]. There are more than 3 million patients with HFpEF in the United States alone [3] and the prevalence is increasing dramatically due to associated cardiovascular and noncardiovascular comorbidities, the advanced age of the population, and increased recognition and diagnosis [1,4,5]. HFpEF results in classical clinical symptoms of congestive heart failure, including fatigue, exercise intolerance, and peripheral and pulmonary edema (i.e., congestion), but without a reduction in ejection fraction, although more subtle systolic impairment can often be detected [1,6,7]. The prognosis for HFpEF remains poor, with a 5-year mortality rate in excess of 50%, similar to heart failure with reduced ejection fraction (HFrEF) [3,4,8]. A number of cardiovascular insults and chronic diseases, including hypertension, arrhythmia, chronic kidney disease, and various metabolic conditions such as obesity and diabetes mellitus are common comorbidities and predispose patients to HFpEF. Consequently, there has been a dramatic increase in cases worldwide [1,3].
Diastolic dysfunction, or the inability of the ventricles to effectively relax and fill with blood, is a key cause of heart failure in patients with HFpEF and results in abnormally high left ventricular filling pressures, despite the maintenance of adequate left ventricular ejection fraction [9,10,11,12,13,14]. Concentric cardiac remodeling and left ventricular hypertrophy are frequently but not always present in HFpEF and can contribute to cardiac maladaptation [1,15]. Cardiac fibrosis, the deposition of excess extracellular matrix components in the cardiac interstitium, often occurs in HFpEF, reduces the compliance of the myocardium, and is a major contributor to the deterioration of diastolic function [1,10,12,13,16]. Systemic inflammation [1,3,9,10,17,18] and oxidative stress [1,10,17,18,19,20] are common components of the molecular etiology of HFpEF. Although the majority of reactive oxygen species (ROS) produced results from the inflammation of the endothelium [17,21,22], elevated myocardial oxidative stress also occurs in animal models [17,23,24,25,26] and patients with HFpEF [17,19,25] (Table 1). Moreover, elevated levels of reactive oxidative metabolites in the circulation correlate with rehospitalization or death due to a heart failure-related event in HFpEF [27,28], suggesting roles for oxidative stress in HFpEF disease severity and outcomes (Table 1).
Table 1.
Reported evidence of oxidative stress and dysregulation of NOX enzymes in patients and animal models with HFpEF.
| Patients with HFpEF | Effect |
|---|---|
| Myocardium | • Trend towards increased NOX2 expression in cardiac macrophages [17] • Increased myocardial H2O2 levels [17,25] • Increased myocardial lipid peroxidation [25] |
| Serum | • Elevated levels of derivatives of reactive oxidative metabolites (DROMs) in patients with HFpEF with HF-related events [27,28] • Increased thiobarbituric acid reactive substances (TBARS, biomarker of lipid peroxidation) [19] |
| Peripheral Blood Monocytes | • Increased NOX1 and NOX4 mRNA levels correlate with diastolic dysfunction in patients with HFpEF [29] |
| Pre-clinical HFpEF Models | Effect |
| HFD †/L-NAME (mice) | • Increased myocardial Nox4 protein expression [30] |
| Unilateral nephrectomy and aldosterone infusion (mice) |
• Increased myocardial oxidative stress (DHE fluorescence) [23,24] |
| Obese ZSF1/ZDF rats | • Increased cardiac macrophage Nox2 [17] • Increased myocardial H2O2 levels [17,25] • Increased myocardial lipid peroxidation [25] |
| DOCA/Western diet †† (Göttingen miniswine) |
• Increased 8-isoprostane levels in plasma [20] |
| Streptozotocin, HFD †††, and renal artery embolization (Yorkshire x Landrace swine) |
• Increased myocardial NADPH-stimulated superoxide production [26] |
DHE, dihydroethidium (ROS sensor); DOCA, 11-deoxycorticosterone acetate (mineralocorticoid/glucocorticoid); DROMs, derivatives of reactive oxidative metabolites; HFD, high-fat diet; HFpEF, heart failure with preserved ejection fraction; L-NAME, Nω-nitro-L-arginine (NOS inhibitor); NADPH, nicotinamide adenine dinucleotide phosphate; TBARS, thiobarbituric acid reactive substances. Diet information: † 60% of calories from lard; †† 1% cholesterol, 20% fat, 8.9% fructose, 2% salt; ††† 10% sucrose, 15% fructose, 25% saturated fats, 1% cholesterol.
1.2. NADPH Oxidase (NOX) Enzymes
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) enzymes control the regulated cellular production of ROS and have indispensable functions in cardiovascular physiology and disease [31,32,33,34,35,36,37,38,39] (Table 2). ROS are short-lived, diffusible gaseous molecules that, in addition to oxidatively damaging cellular constituents, can locally oxidize cysteine or methionine residues on proteins to modify activity, function, oligomerization, and/or localization that can have profound impacts on intracellular signaling (i.e., redox signaling), including pathways that are instrumental to the onset and progression of cardiac hypertrophy and failure [31,32,33,40,41,42,43,44]. Although substantial ROS production occurs as a byproduct of oxidative metabolism in mitochondria [45,46] and can also be generated by monoamine oxidases [47,48,49], xanthine oxidase [50], or the uncoupling of the catalytic cycle of cytochrome P450s [51] and nitric oxide synthases (NOS) [52,53] (Section 4.1), the dedicated function of NOX enzymes is ROS production [37]. Thus, NOXs are the primary source of regulated, enzymatic ROS production and are responsible for a significant portion of ROS generated in the cardiovascular system, inducing oxidative stress and redox signaling central to the progression of cardiovascular disease [36,37].
Table 2.
Cell type distribution of NOX isoforms and functions in cardiac and vascular pathologies. “+” indicates promotion of the designated cardiovascular disease phenotype, whereas “−” indicates repression of the disease phenotype. CMs, cardiomyocytes; ECs, endothelial cells; VSMCs, vascular smooth muscle cells. See Section 2.
| NOX Isoform | Cell Types | Cardiac | Vascular | |||
|---|---|---|---|---|---|---|
| Hypertrophy | Fibrosis | Inflammation | Hypertension | Atherosclerosis | ||
| NOX1 | CMs, ECs, VSMCs, Macrophages |
+ | + | + | + | + |
| NOX2 | CMs, ECs, VSMCs, Macrophages, Fibroblasts |
+ | + | + | + | + |
| NOX4 | CMs, VSMCs, Macrophages, Fibroblasts |
+ | + | + | + | |
| ECs | − | − | − | − | − | |
| NOX5 | CMs, ECs, VSMCs | + | + | + | ||
There are seven NOX family enzymes (NOX1-5 and DUOX1-2), which are multi-pass transmembrane proteins that enzymatically generate superoxide via the transfer of electrons to molecular oxygen [36,37,38]. NOX1-5 have six transmembrane domains, whereas DUOX1 and 2 have seven transmembrane domains and are not expressed in cardiovascular cells [36,37]. NOX5 is expressed in human cardiomyocytes and vascular cells but not in rodents [36,38,54,55] and is regulated by calcium, similar to DUOX1 and 2 [36,37,38]. NOX3 expression is largely restricted to embryonic development and the inner ear [36]. NOX isoforms 1–4 require association with the membrane-embedded p22phox regulatory subunit for oxidase activity and protein stability [36,37,38]. NOX1 and NOX2 (also known as gp91phox) require the translocation of additional cytosolic subunits to the oxidase complex for activation: NOX1 requires association with the p47phox or NOXO1 organizer subunit, p67phox or NOXA1 activator subunit, and the active (GTP-bound) Rho family small GTPase, Ras-related C3 botulinum toxin substrate 1 (Rac1), whereas NOX2 necessitates the interaction of p47phox, p67phox, p40phox, and active Rac1 or Rac2 with the NOX2:p22phox complex at the membrane to evoke superoxide production [36,37,38,56,57].
With regards to the cardiovascular system, NOX1, 2, 4, and 5 are expressed in cardiomyocytes, endothelial cells, and vascular smooth muscle cells, and NOX2 and NOX4 in fibroblasts (Table 2, Figure 1). NOX2 is also robustly expressed in phagocytes, including neutrophils and macrophages [36,37,38,39,56,58,59], and NOX1 and NOX4 also function in monocytes and monocyte-derived macrophages [29,60]. These tissue- and cell type-specific expression patterns (Table 2, Figure 1), as well as distinct regulatory mechanisms governing NOX isoform enzyme activity and abundance, contribute to nonoverlapping redox-sensitive signaling circuitry and cellular functions that are regulated by different NOX isoforms (Figure 2).
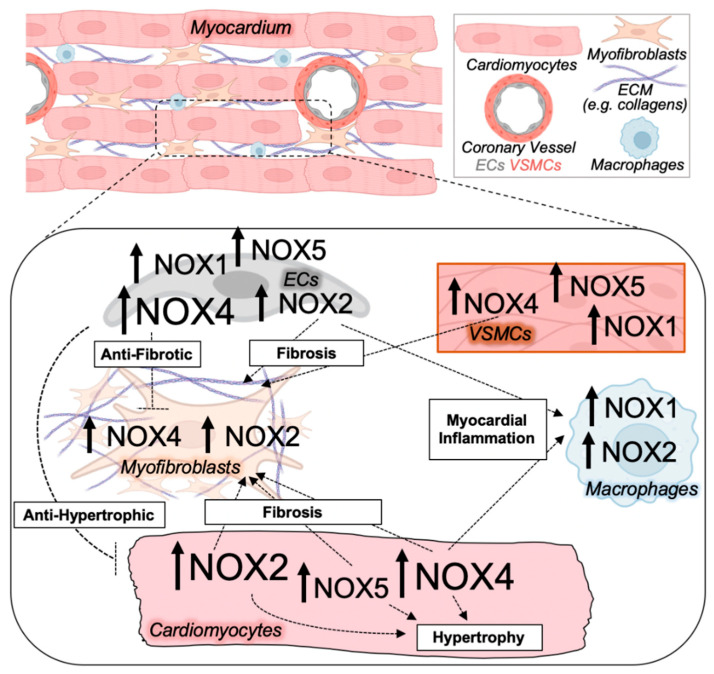
Figure 1.
NOX isoform upregulation and crosstalk between cell types in pathological cardiac remodeling. The diseased myocardium is composed of several cell types all expressing NOX enzymes, including cardiomyocytes, activated myofibroblasts that deposit collagen and extracellular matrix (ECM) components, macrophages, and endothelial cells (ECs), as well as vascular smooth muscle cells (VSMCs) that make up the coronary vessels. NOX isoform activity and ROS production in various cell types of the heart mediate paracrine effects on other cardiac cell types to impact myocardial growth, inflammation, microvascular dysfunction, and fibrosis central to adverse cardiac remodeling. Magnitude and directionality of alterations in NOX isoform activity and/or expression in the indicated cardiac cell types are depicted by the font size and arrow, respectively.
Figure 2.
Pathogenic NOX-regulated redox signaling circuitry in cardiac myocytes. Depiction of major pathological redox-sensitive signaling effectors and pathways regulated by NOX2 and NOX4 in cardiomyocytes. CaMKII, calcium/calmodulin-dependent protein kinase II; HDAC4, histone deacetylase 4; MEF2, myocyte enhancer factor 2; NFAT, nuclear factor of activated T cells; NFκB, nuclear factor kappa B; ROS, reactive oxygen species.
NOX2 and NOX4 are the best-studied NOX enzymes in the cardiovascular system and cardiomyocytes more specifically. While the generation of ROS by NOX2 is inducible by Rac1/2 GTPase activity such as downstream of angiotensin receptor (AT1R) activation by angiotensin-II (AngII) [38,57,61], NOX4 exhibits constitutive activity but its levels are upregulated in response to pathological stimulation [38,62,63,64,65]. NOX4 is found at intracellular membrane organelles such as the endoplasmic reticulum [66,67], nuclear envelope [62], and mitochondria [35,63,65,67,68], whereas NOX2 is predominantly found at plasma membrane microdomains such as the T-tubule, lateral membrane, and intercalated disc in cardiomyocytes [69,70]. This results in the regulated compartmentalization of ROS production through the oxidase activity of distinct NOX isoforms. Cell type-specific expression patterns (Figure 1), the intracellular localization of NOX enzymes at distinct cell and organellar membranes (Figure 2), and unique regulatory mechanisms controlling NOX activity and expression enable the intricate spatiotemporal regulation of redox signaling through the dynamic modulation of NOX activity. Perhaps not surprisingly, NOXs have established roles in cardiac aging [32,35,68], cardiac hypertrophy [61,70,71], hypertension [72], atherosclerosis [34,38], diabetic cardiomyopathy [73], and myocardial infarction [40,58,74] (Table 2), so a significant role in HFpEF seems probable.
2. NADPH Oxidases in Cardiovascular Disease
2.1. NOX Isoforms in Cardiac Hypertrophy, Fibrosis, and Diastolic Dysfunction
Given the paucity of animal models that faithfully recapitulate the multi-organ pathophysiology of HFpEF [1,3] and the lack of highly selective isoform-specific NOX inhibitors [37], there is limited knowledge on specific NOX enzymes in HFpEF. However, several studies have uncovered roles for NOX enzymes in diastolic dysfunction and the promotion of cardiac fibrosis in models of cardiac hypertrophy, injury, and failure (Table 2). NOX enzyme expression levels and/or oxidase activity are induced in cardiovascular cells in response to neurohumoral stimulation such as the activation of the renin-angiotensin-aldosterone system (RAAS) [36,39] that occurs in both HFrEF, as well as HFpEF [10,75,76,77,78], and thus, NOX-mediated oxidative stress and redox signaling in the heart and vasculature are likely to occur in HFpEF. Elevated levels of aldosterone or AngII in the circulation correlate with left ventricular hypertrophy and an increased risk of rehospitalization and mortality in patients with HFpEF [78,79,80,81], suggesting RAAS activation as a key pathomechanism in HFpEF. Notably, the pharmacological inhibition of NOX enzymes with apocynin reduces myocardial ROS production and diastolic dysfunction in rodent models of diabetes mellitus [82,83] and AngII infusion [84], as well as attenuating cardiac hypertrophy, fibrotic remodeling, and oxidative stress in response to AngII or aldosterone [84,85] and myocardial infarction injury [86], underscoring the centrality of NOXs to diastolic impairment and the deterioration of cardiac function in response to bolstered RAAS activity and comorbid cardiovascular disease.
Basic science studies implicate NOX2, NOX4, and NOX5 in the deterioration of diastolic function and adverse cardiac remodeling in response to pathological stimuli (Table 2). A global loss of Nox2 limits myocardial ROS production and preserves diastolic function in response to diet-induced obesity [87], doxorubicin treatment [88], and myocardial infarction [89], and mitigates cardiac fibrosis in response to AngII, aldosterone, or pressure overload [41,90,91,92], whereas Nox2 overexpression in endothelial cells promotes cardiac fibrosis, inflammation, and diastolic dysfunction in response to AngII [93]. Inducible NOX2 activity in the heart in response to AngII appears to be particularly important for adverse cardiac remodeling and oxidative stress, as the loss of Nox2 protects from AngII-induced myocardial oxidative stress, hypertrophy, and fibrosis without altering AngII-induced hypertension [41]. Similarly, the cardiomyocyte-specific loss of Rac1, which is required for the induction of NOX2 oxidase activity, rescues diastolic dysfunction in response to pressure overload [94] and suppresses cardiac hypertrophy, fibrosis, and oxidative stress in response to AngII [61] and streptozotocin-induced diabetes [83]. Moreover, statin treatment, which represses small GTPase signaling by inhibiting the biosynthesis of lipid precursors needed for protein prenylation [95], impairs myocardial Rac1 activation and oxidative stress in rats in response to AngII or pressure overload [96] and in human heart failure [97]. Statin treatment is also associated with reduced cardiomyocyte hypertrophy and resting tension in myocardium from patients with HFpEF [22], suggesting that an effective in vivo strategy to target NOX2 could alleviate cardiac maladaptation in HFpEF.
Many studies have also revealed indispensable functions of NOX4 in cardiac biology and disease. The transgenic overexpression of Nox4 at modest levels in cardiomyocytes elicits elevated myocardial ROS production, fibrosis, and cardiomyocyte hypertrophy and apoptosis with advanced age [63]. The cardiomyocyte-specific overexpression of human NOX4, at higher levels that recapitulate upregulation in response to AngII, was sufficient to induce myocardial ROS and fibrosis comparable to AngII treatment, but in the absence of cardiac hypertrophy [98]. Moreover, NOX4 overexpression further promoted cardiac hypertrophy, fibrosis, and oxidative stress in response to AngII [98]. The role of NOX4 in cardiac pressure overload is somewhat unclear, with one study reporting protection by the cardiomyocyte-specific deletion of Nox4, while another study found that the deletion of Nox4 in cardiomyocytes or endothelial cells exacerbated pressure overload-induced cardiac hypertrophy, dysfunction, and fibrosis [99,100]. Consistent with a role for cardiomyocyte NOX4 in the maladaptation of the heart to RAAS hyperactivation, the treatment of mice with a mitochondrial-targeted antioxidant peptide prevented the upregulation of Nox4 and mitochondrial oxidative stress, as well as ameliorated cardiac hypertrophy, fibrosis, and diastolic dysfunction in response to AngII [64].
NOX4 also has critical roles in the inflammatory and wound healing response of the heart to ischemic injury. Although the transgenic overexpression of Nox4 in cardiac myocytes was sufficient to promote macrophage recruitment to the heart and polarization towards the M2 phenotype that was associated with a modest reduction in cardiac hypertrophy, fibrosis, and mortality following myocardial infarction [101], endogenous Nox4 plays an indispensable role in cardiac inflammation and tissue damage in the infarcted heart, as mice with a global loss of Nox4 exhibited less myocardial infarction-induced macrophage infiltration, ischemic injury, cardiomyocyte hypertrophy, and oxidative stress [74]. Moreover, the overexpression of a dominant-negative Nox4 mutant resulted in reductive stress and increased mitochondrial ROS, infarction size, and myocardial energetic deficits in response to ischemia-reperfusion injury [102], suggesting that NOX4 also has adaptive functions in modulating inflammation and injury resolution in the ischemic myocardium. Since advanced age is a major risk factor for the development of HFpEF [2,103] and oxidative stress is a major contributor to the aging of the heart and other organs [32,68,104], it is notable that Nox4 protein levels increased with age in the heart [63] and treatment with a pharmacological NOX1/NOX4 inhibitor preserved cardiac function in aged mice [68]. More importantly perhaps, cardiac Nox4 protein levels were upregulated in a mouse model of HFpEF [30]. It is also noteworthy that NOX2 and NOX4 contribute to atrial oxidative stress and susceptibility to atrial fibrillation [54,105,106,107,108,109,110,111,112], a comorbidity that is present in roughly one third of patients with HFpEF that can result in chronic arrhythmogenicity that precipitates the progression of cardiac failure [113,114,115,116,117].
Although not expressed in rodents, gain-of-function studies in mice suggest a role for the calcium-sensitive NOX5 enzyme in the adverse remodeling of the heart in response to RAAS activation or mechanical stress. Indeed, the cardiomyocyte-specific overexpression of human NOX5 in mice exacerbated cardiac hypertrophy, fibrosis, and oxidative stress in response to AngII infusion and cardiac pressure overload [55]. Thus, NOX enzymes have well-established physiologic functions in various cell types of the cardiovascular system [31,36,37], many of which are likely important in the context of HFpEF. Here, we discuss evidence for regulated ROS production by NOX enzymes in diastolic dysfunction and HFpEF and the NOX-regulated intracellular hypertrophic signaling mechanisms in cardiomyocytes controlled by NOX2 and NOX4.
2.2. NADPH Oxidases in Non-Myocyte Cardiac Cell Types
NOX enzymes are expressed in all cell types of the vasculature including the coronary vessels supplying blood to the heart [36,37,38]. Vascular NOX-generated ROS play essential roles in cardiovascular oxidative stress, aging, atherosclerosis, angiogenesis, inflammation, and blood pressure homeostasis [36,37,118,119,120,121]. Crosstalk between NOX enzymes and redox signaling pathways in the coronary vasculature, fibroblasts, and immune cells of the heart are likely involved in coronary microvascular dysfunction, inflammation, and myocardial relaxation defects in HFpEF (Figure 1).
2.2.1. NOX Enzymes in the Vasculature
NOX4 is the most abundant NOX isoform in endothelial cells and a major source of ROS production in the vasculature and heart [37,122]. In contrast to the maladaptive roles of most ROS generated by NOX enzymes in the cardiovascular system, endothelial NOX4 is protective (Table 2). The overexpression of Nox4 in endothelial cells reduces hypertension, myocardial inflammation, and fibrosis in response to AngII [118,123], whereas the endothelial cell-specific loss of Nox4 exacerbates cardiac hypertrophy, dysfunction, and fibrosis in response to pressure overload [100]. Similarly, the endothelial-specific overexpression of Nox4 attenuates atherosclerotic remodeling and vascular inflammation in ApoE−/− mice [124], while the loss of Nox4 promotes the formation of atherosclerotic lesions and vascular inflammation [125].
Conversely, endothelial NOX1, NOX2, and NOX5 contribute to impaired vascular function, endothelial nitric oxide synthase (eNOS) uncoupling (Figure 3), and adverse vascular remodeling and hypertension [38,126]. Indeed, endothelial cell Nox1 is required for hypertension in response to AngII [127], while NOX2 overexpression in endothelial cells further promotes blood pressure elevation in response to AngII [128] and stimulates macrophage recruitment and the initiation of atherosclerotic plaque formation in ApoE−/− mice [129]. Most notable with regards to HFpEF pathophysiology, endothelial cell NOX2 is instrumental to inflammatory cell recruitment, myocardial fibrosis, and diastolic dysfunction in response to AngII [93]. The expression of NOX5 is increased in the coronary arteries of atherosclerosis patients [130], and the endothelial cell-specific expression of human NOX5 in mice results in hypertension with advanced age via the uncoupling of eNOS (Figure 3) and the repression of cyclic GMP (cGMP) signaling [131], indicating maladaptive roles for endothelial NOX5 in cardiovascular disease.
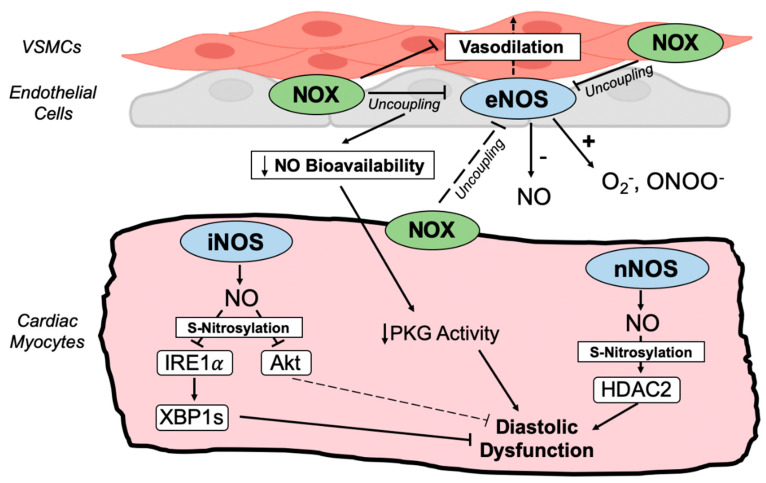
Figure 3.
Crosstalk between NADPH oxidases and nitric oxide synthases. Schematic depiction of redox-nitroso crosstalk between coronary vasculature and cardiac myocytes in HFpEF. Endothelial NOS (eNOS) promotes vasodilation that is impaired when oxidative stress uncouples eNOS, diminishing NO bioavailability, cGMP levels, and protein kinase G (PKG) activity. Decreased myocardial PKG activity facilitates diastolic dysfunction by reducing titin phosphorylation. Uncoupled NOS enzymes generate damaging superoxide and peroxynitrite that further promote oxidative stress. Nitrosative stress in the HFpEF myocardium, on the other hand, is provoked by the upregulation of inducible NOS (iNOS) and neuronal NOS (nNOS) in cardiac myocytes. nNOS-dependent S-nitrosylation of histone deacetylase 2 (HDAC2) and defective XBP1 splicing (XBP1s) due to iNOS-dependent S-nitrosylation of IRE1𝛼 promote diastolic dysfunction and heart failure in HFpEF animal models. iNOS also mediates Akt S-nitrosylation. NOS uncoupling and nitrosative stress-responsive signaling contribute to oxidative stress, coronary microvascular endothelial dysfunction, and impaired myocardial relaxation in HFpEF.
NOX1, NOX4, and NOX5, and to a lesser extent NOX2, are expressed in vascular smooth muscle cells [39,126,132]. Nox1 overexpression in vascular smooth muscle cells increases vascular ROS production, blood pressure, aortic medial thickness, and the uncoupling of eNOS in response to AngII [133,134] while a deficiency of NOX1 limits neointima formation in response to wire injury [135] and vascular smooth muscle cell migration in response to AngII or platelet-derived growth factor (PDGF) [135,136]. Moreover, Noxa1, the activator subunit for Nox1, in vascular smooth muscle cells is indispensable for fulminant atherosclerotic remodeling and oxidative stress in multiple models of dyslipidemia [34].
NOX4 in vascular smooth muscle cells plays critical roles in vascular aging, inflammation, and atherogenesis. NOX4 expression is upregulated in the atherosclerotic lesions of mouse models and humans [137,138] and is required for ROS generation and the induction of an inflammatory gene expression program in vascular smooth muscle cells in response to transforming growth factor-β (TGF-β) [137]. NOX4 in the mitochondria of vascular smooth muscle cells is particularly important in cardiovascular disease. The overexpression of mitochondrial-targeted Nox4 elicited a vascular aging phenotype including aortic stiffening, fibrosis, elevated mitochondrial ROS production, and the impairment of aortic smooth muscle cell contractility and mitochondrial respiration [35]. In contrast, the knockdown of Nox4 in aortic smooth muscle cells from aged mice reduced mitochondrial ROS generation and improved mitochondrial function [68].
The calcium-dependent NOX5 enzyme is upregulated in the vascular smooth muscle cells of hypertensive patients and is required for AngII- and PDGF-induced ROS production [139,140]. NOX5 promotes vascular contractility, as the transgenic expression of human NOX5 in mouse vascular smooth muscle cells inhibits endothelium-dependent relaxation and increases vasoconstriction in isolated mesenteric arteries [139,141]. NOX5 also incites vascular calcification in response to calcium through its oxidase activity in vascular smooth muscle cells [142]. NOX enzymes in the coronary and peripheral vasculature contribute to systemic oxidative stress, inflammation, and vascular remodeling, all of which facilitate the development of HFpEF. It is plausible that the well-established functions of NOX-generated ROS in vascular oxidative stress, remodeling, and redox signaling also contribute to myocardial intercellular communication and microvascular inflammation in HFpEF.
2.2.2. NOX Enzymes in Fibroblasts
Cardiac fibrosis is a major driver of cardiac pathology and diastolic dysfunction in HFpEF [10,12]. Resident cardiac fibroblasts in the injured heart transform into myofibroblasts that synthesize and secrete extracellular matrix proteins such as collagens, thereby promoting wound healing but impinging upon myocardial elasticity [3,10,143]. Unfortunately, studies of NOX enzymes specifically in cardiac fibroblasts remain limited. NOX enzymes in vascular adventitial fibroblasts play vital roles in vascular inflammation and signaling to other vascular cell types [144]. Fibroblast Nox2 is required for AngII-induced hypertension and vascular remodeling and mediates paracrine signaling through the secretion of growth differentiation factor 6 (GDF6), which promotes vascular smooth muscle cell growth [59].
NOX4 is the best-studied NOX isoform in cardiac fibroblasts and has important roles in the fibrotic remodeling of the heart. NOX4 is upregulated in cardiac fibroblasts in response to TGF-β and in heart failure and is required for TGF-β-induced superoxide production, Smad2/3 phosphorylation, myofibroblast transformation, and collagen production [145,146]. Future studies using Cre lines to temporally control the deletion of NOX isoforms in cardiac fibroblast lineages will help to delineate the functions of NOX enzymes in cardiac fibroblasts, fibrotic remodeling, and the stiffening of the ventricular myocardium.
2.2.3. NOX Enzymes in Immune Cells
NADPH-dependent oxidase activity was originally discovered in neutrophils and was later found to be mediated by NOX2 [56,147,148,149]. Indeed, NOX2 is robustly expressed in phagocytes, including not only neutrophils, but also macrophages [150] that infiltrate the stressed heart to promote wound healing and injury resolution, particularly in response to myocardial infarction [151,152]. The recruitment of monocyte-derived macrophages in nonischemic heart failure is also a critical component of disease progression, fibrotic remodeling, and decompensation [153,154]. NOX2 levels are increased in cardiac macrophages and peripheral monocytes in human patients with HFpEF and the obese ZSF1 rat model of HFpEF [17,29]. Nonetheless, a direct role for NOX2 in resident cardiac macrophages or monocyte-derived macrophages recruited to the injured heart has not been directly examined.
NOX4 generates ROS in peripheral monocytes and macrophages where it is upregulated in response to oxidized low-density lipoprotein to promote macrophage cytotoxicity [60], suggesting roles for monocyte NOX4 in atherosclerosis; yet similarly, no studies have interrogated NOX4 in cardiac macrophages to date. It is noteworthy, however, that NOX4 in cardiomyocytes has crucial roles in the recruitment of macrophages to the heart in response to ischemic injury [101]. Perhaps most germane to the molecular etiology of HFpEF, the global loss of Nox1 mitigates cardiac hypertrophy, coronary endothelial cell activation, and macrophage recruitment in a mouse model of metabolic cardiomyopathy [29]. Intriguingly, the expression levels of NOX1 in peripheral monocytes correlate with the degree of diastolic dysfunction in patients with HFpEF [29], indicating roles for monocyte NOX1 in inflammation and defective myocardial relaxation in HFpEF.
NOX-dependent superoxide production by peripheral blood mononuclear cells is increased in patients with hypertension compared to normotensive controls and even further enhanced among patients with hypertension and left ventricular hypertrophy [155], suggesting critical roles for NOX enzymes in lymphocytes and monocytes in hypertension and cardiac hypertrophy. Recent studies elucidated required functions for NOX2 in regulatory T cells (Tregs) that suppress inflammatory responses in cardiac and vascular remodeling. The Treg-specific deletion of Nox2 or the adoptive transfer of Tregs lacking Nox2 reduced blood pressure, cardiac hypertrophy, and cardiac fibrosis in response to AngII through a mechanism that involved increasing the number of cardiac resident Tregs and promoting their immune suppressive activity [156]. Thus, NOXs in immune cells likely contribute directly to both systemic and local inflammatory responses in the heart in HFpEF and other forms of heart disease. Analyses of NOX activity in peripheral blood cells in human patients with HFpEF coupled with studies using HFpEF animal models and the genetic manipulation of NOX expression will help to reveal the cell type-specific functions of NOX isoforms in diastolic dysfunction and cardiac failure.
3. NOX-Regulated Pathogenic Redox Signaling in Cardiomyocytes
NOX2 and NOX4 have emerged as central regulators of the intracellular redox signaling landscape in cardiomyocytes (Figure 2). NOX2 is activated by pathological stimuli such as AngII, aldosterone, and pressure overload [37,57,71], enabling the rapid inducibility of superoxide production [37,57,71,91]. In contrast, NOX4 has constitutive basal oxidase activity but its expression levels are upregulated in the heart by cardiovascular stress, such as in response to AngII, adrenergic stimulation, pressure overload [38,62,63,64,65], or the high-fat diet and Nω-nitro-L-arginine (HFD/L-NAME) model of HFpEF [30]. NOX4 is predominantly localized at intracellular organelle membranes, including at mitochondria, the endoplasmic reticulum, and nuclear envelope [62,63,67,157]. Thus, the positioning and activity of NOX2 at sarcolemmal membrane domains and NOX4 at intracellular membranes dictate the topography of redox signaling that can have instrumental roles in cardiac hypertrophy and diastolic dysfunction (Figure 2).
3.1. Redox Regulation of Hypertrophic and Maladaptive Gene Expression Programs
Notably, both NOX2 and NOX4 activity promote the activation of hypertrophic gene expression in cardiomyocytes through distinct mechanisms that provoke oxidation-dependent nuclear export of histone deacetylase 4 (HDAC4), thereby derepressing the transcriptional activity of the hypertrophic transcription factors, myocyte enhancer factor 2 (MEF2) and nuclear factor of activated T cells (NFAT) [40,42,44,62]. The NOX2-dependent oxidation of calcium/calmodulin-dependent protein kinase II (CaMKII) in response to AngII or aldosterone results in the noncanonical activation of CaMKII and the consequent phosphorylation and nuclear export of HDAC4 that induces MEF2-dependent transcription and cardiomyocyte hypertrophy [40,44] (Figure 2). In contrast, NOX4 at the nuclear envelope induces the direct oxidation of HDAC4 in response to phenylephrine that similarly promotes the nuclear-to-cytoplasmic shuttling of HDAC4, thereby activating pro-hypertrophic NFAT-dependent transcription [42,62] (Figure 2).
Nuclear factor kappa B (NFκB) transcriptional activity regulates inflammatory gene expression programs and is enhanced in a NOX2-dependent manner in response to AngII [61,158]. Indeed, Rac1 in cardiomyocytes is required for oxidative stress and NFκB activation in response to AngII [61], while Nox4 overexpression in cardiomyocytes is also sufficient to induce NFκB activation [98], suggesting roles for NOX2 and NOX4 in the regulation of NFκB signaling and inflammatory gene expression that may contribute to myocardial remodeling in HFpEF (Figure 2). Thus, NOX2 and NOX4 control unique redox-sensitive hypertrophic signal transduction circuitry that can integrate multiple inputs and converge on the regulation of maladaptive gene expression programs.
3.2. Sarcoplasmic Reticulum Calcium Handling and Myocardial Contractility
NOX2 activity in cardiomyocytes promotes sarcoplasmic reticulum calcium cycling and contractility in response to AngII in part through the hyperphosphorylation of phospholamban [70], while mechanical stretch evokes the NOX2-dependent sensitization of ryanodine receptors and arrhythmogenic calcium sparks [69], both of which could also be enhanced by the NOX2-mediated oxidation of CaMKII.
3.3. Mitochondrial ROS and Cardiomyocyte Death
NOX4-generated ROS at cardiac mitochondria can influence redox signaling and promote mitochondrial oxidative damage and cardiomyocyte death [63,64] (Figure 2). Nox4 overexpression is sufficient to induce mitochondrial dysfunction and cardiomyocyte apoptosis with aging [63] and Nox4 in cardiomyocytes is required for the pressure overload-induced production of mitochondrial ROS and cardiomyocyte apoptosis [99]. Moreover, AngII infusion in mice promotes Nox4 expression in conjunction with mitochondrial oxidative damage, diastolic dysfunction, fibrosis, and apoptosis, all of which are normalized by the administration of a mitochondrial-targeted antioxidant peptide [64]. Thus, the targeted inhibition of mitochondrial NOX4 could potentially exert therapeutic benefits in patients with diastolic dysfunction and/or HFpEF by limiting excessive mitochondrial ROS production (Figure 2). Notably, however, the broad inhibition of NOX4 could repress its adaptive functions in response to cardiomyocyte stress, including at mitochondria-associated membranes and the endoplasmic reticulum, where it inhibits mitochondrial calcium overload [67] and induces autophagy in response to nutrient deprivation [66], respectively.
4. NADPH Oxidases and Nitric Oxide (NO) Signaling
4.1. Nitric Oxide Synthase (NOS) Uncoupling and cGMP/Protein Kinase G (PKG) Signaling
Nitric oxide (NO), which is predominantly generated in the endothelium by eNOS, has potent vasodilatory activity in the coronary and peripheral vasculature via the activation of soluble guanylate cyclase (sGC) in vascular smooth muscle cells [159,160] (Figure 3). NOX-generated ROS interfere with the catalytic cycle of NOS enzymes, resulting in the production of superoxide rather than NO that reduces NO bioavailability, impairing cGMP-dependent vascular smooth muscle relaxation, protein kinase G (PKG) signaling, and further contributing to oxidative stress [21,36,161]. Additionally, the uncoupling of NOS enzymes impairs myofilament relaxation and diastolic function by dampening the PKG-mediated phosphorylation of titin [21,36,162,163]. Peroxynitrite is also generated from NO and superoxide by uncoupled NOS enzymes, further reducing NO bioavailability and resulting in protein nitration by the reaction of peroxynitrite with tyrosine residues [164] (Figure 3). Indeed, myocardial cGMP levels and PKG activity are substantially reduced in the myocardium of patients with HFpEF [165], as are the serum levels of NO-derived metabolites [166,167], whereas myocardial nitrotyrosine levels are elevated [165], suggesting that NOS uncoupling and oxidative stress contribute markedly to HFpEF pathophysiology. Thus, NOX-generated ROS can facilitate NOS uncoupling that both attenuates the cardiovascular protection afforded by NO and further promotes the generation of damaging ROS and reactive nitrogen species [21,36].
4.2. Nitrosative Stress and Protein S-Nitrosylation
Despite the elevation of ROS and the dampening of signaling downstream of NO (i.e., cGMP levels, PKG activity) in HFpEF, paradoxically, NO and nitrosative stress seem to be nodal drivers of cardiac disease in HFpEF (Figure 3). NO can be covalently attached to protein cysteines, termed S-nitrosylation, which serves as a post-translational mechanism that can modulate protein activity and function [159,168]. Indeed, multiple studies have uncovered pathogenic roles for NO in HFpEF, mediated at least in part through protein S-nitrosylation that is upregulated in the myocardium of mouse models and human patients with HFpEF [7,169]. Most notably, inducible NOS (iNOS) is upregulated in cardiomyocytes and is required for cardiac hypertrophy, fibrosis, diastolic dysfunction, edema, mitochondrial abnormalities, and exercise intolerance in the HFD/L-NAME mouse model of HFpEF [7,30]. S-nitrosylation of the endoplasmic reticulum stress transducing protein, inositol-requiring enzyme 1𝛼 (IRE1𝛼), is induced in HFpEF and prevents the IRE1𝛼 -mediated mRNA splicing of its effector, X-box-binding protein 1 (Xbp1), that is required for the ER stress response and cardiac adaptation [7,170]. Importantly, the loss of iNOS or cardiomyocyte-specific overexpression of spliced Xbp1 rescues cardiac and systemic HFpEF phenotypes in the HFD/L-NAME model [7], suggesting essential roles for the iNOS-dependent S-nitrosylation of IRE1𝛼 in HFpEF. Akt S-nitrosylation at Cys-224 is also induced by iNOS in response to HFD/L-NAME, resulting in the inhibition of cardioprotective Akt signaling by reducing Ser-473 phosphorylation [30]. Thus, the upregulation of iNOS in HFpEF cardiomyocytes initiates pathologic S-nitrosylation-regulated signaling.
S-nitrosylation of histone deacetylase 2 (HDAC2) by neuronal NOS (nNOS) also plays a key role in HFpEF pathophysiology [169]. nNOS expression and HDAC2 S-nitrosylation are elevated in the hearts of patients with HFpEF and mice subjected to the salty drinking water/unilateral nephrectomy/aldosterone (SAUNA) model of HFpEF [169]. The genetic ablation of nNOS or knock-in mutation of the HDAC2 S-nitrosylation sites (Cys262/274) mitigates SAUNA-induced HDAC2 S-nitrosylation, diastolic dysfunction, and exercise exhaustion [169]. Thus, the upregulation of iNOS and nNOS in HFpEF facilitates the activation of nitrosative stress-responsive signaling pathways that are critical for the development of cardiac and systemic pathologies in HFpEF animal models and are a distinguishing feature of HFpEF versus HFrEF (Figure 3).
The interplay between oxidative stress and NO production is complex but basic science studies and clinical data suggest that both NOX and NOS enzymes and oxidative and nitrosative stress play important roles in HFpEF. The NOX-mediated uncoupling of NOS enzymes in the vasculature could limit NO production and further promote the generation of ROS and reactive nitrogen species, oxidative tissue damage, and pathogenic redox signaling [21,36,161]. However, enhanced nitrosative stress is emerging as a hallmark and defining feature of HFpEF myocardium that occurs largely as a consequence of the induction of iNOS and nNOS levels and activity in cardiac myocytes [1,7,169]. There is also evidence that nitrosative stress can promote NOX activity and redox signaling in HFpEF, as iNOS was required for the upregulation of cardiac Nox4 levels and mitochondrial oxidative stress in response to HFD/L-NAME [30]. Further investigation of NOX and NOS isoforms in diastolic dysfunction will shed light on nitroso-redox crosstalk and redox/nitrosative signaling mechanisms that participate in the onset and progression of HFpEF.
5. Clinical Implications and Future Perspectives
5.1. Renin-Angiotensin-Aldosterone System (RAAS) Antagonists
There is interest in targeting oxidative stress, and NOX isoforms in particular, as therapeutic avenues to delay adverse outcomes in HFpEF. As discussed above, NOXs play essential roles in cardiovascular oxidative stress and redox signaling in response to RAAS activation. NOX2- and NOX4-dependent ROS generation are specifically induced in the heart in response to elevated AngII, aldosterone, and catecholamines in the circulation in cardiovascular disease and can contribute to the deterioration of diastolic function. Unfortunately, for the most part, pharmacological strategies used to treat HFrEF have not been effective at reducing mortality or cardiovascular events in patients with HFpEF [1,2,171]. Although serum aldosterone levels correlate with the risk of rehospitalization and death in HFpEF [79], mineralocorticoid receptor antagonist (MRA) treatment did not reduce the risk of death or heart failure rehospitalization in HFpEF [172]. These data suggest that the inhibition of NOX2 activity downstream of the mineralocorticoid receptor with MRA therapy is insufficient to substantially alter outcomes in patients with HFpEF. Similarly, angiotensin receptor blocker (ARB) [173], angiotensin converting enzyme (ACE) inhibitor [174,175], or ARB/neprilysin inhibitor (sacubitril) [176] therapy have had insufficient efficacy in the treatment of HFpEF, indicating that antagonism of the RAAS system alone, at least with existing pharmacologic agents and the timing of therapeutic intervention, does not significantly impede HFpEF disease progression. It is possible that some NOX activation mechanisms in HFpEF (e.g., the upregulation of NOX4 expression levels [30,62,63]) could evade RAAS inhibition, or that NOX-regulated oxidative damage and/or activation of pathogenic redox signaling prior to treatment cause enduring adverse effects on cardiac remodeling, such as through the activation of prohypertrophic transcriptional pathways (Figure 2, Section 3.1). Further exploration of strategies targeting RAAS activity, their antioxidant properties, and efficacy in combination therapies in HFpEF is warranted.
5.2. Nitric Oxide Donors and cGMP/Protein Kinase G Signaling
Similar to commonly prescribed heart failure therapies targeting the RAAS pathway, nitric oxide donor therapies have failed to improve HFpEF outcomes [177,178]. Neither isosorbide mononitrate [177] nor inorganic nitrite [178] had an impact on diastolic function, exercise capacity, or the risk of adverse cardiovascular events in patients with HFpEF. Nitrates may circumvent some pathogenic consequences of reduced NO bioavailability in HfpEF, but the induction of pathogenic NOX-regulated redox signaling mechanisms may not be perturbed. Nitrates could promote the elevation of myocardial cGMP levels and PKG activity that are reduced in HFpEF [17,165], but signaling activated by NOX enzymes in the heart (Figure 2) in response to increased circulating aldosterone, AngII, and enhanced sympathetic activity in HFpEF may remain unchanged. Targeting cGMP/PKG signaling with a soluble guanylate cyclase activator (vericiguat) [179], phosphodiesterase-5 inhibitor (sildenafil) [180], or neprilysin inhibitor (sacubitril) [176] was similarly not effective in HFpEF clinical trials.
Another important caveat to NO donor therapy is that it could even promote pathogenic nitrosative stress in the heart and the upregulation of S-nitrosylation-dependent signaling mechanisms in cardiomyocytes that are central to HFpEF disease progression [7,169] (Figure 3, Section 4.2). Indeed, IRE1α and HDAC2 S-nitrosylation are required for diastolic dysfunction in pre-clinical models of HFpEF [7,169], and these pathways may be further activated by nitrates.
5.3. Sodium-Glucose Cotransporter 2 Inhibitors
Excitingly, recent clinical trials have demonstrated that sodium-glucose cotransporter 2 (SGLT2) inhibitors, which lower blood glucose by repressing glucose resorption in the proximal tubule of the kidney [181,182], reduce the risk of death or hospitalization and improve exercise capacity and heart failure symptoms in patients with HFpEF [183,184]. The success of SGLT2 inhibitors in the treatment of HFpEF is a breakthrough in heart failure therapeutics that underscores the systemic and cardiometabolic nature of HFpEF. Although originally designed for the treatment of diabetes, SGLT2 inhibitors have proven effective in treating HFrEF [185,186] and HFpEF [183,184] in patients both with and without diabetes. SGLT2 inhibitors have obvious benefits on blood glucose homeostasis and cardiac bioenergetics [181,182], but their marked success independent of diabetes as a comorbidity has pointed to additional mechanisms of cardioprotection, including antioxidant and anti-inflammatory effects on the heart [25,187]. Most notably, empagliflozin attenuates myocardial oxidative stress in human HFpEF [188], the ZDF obese rat HFpEF model [25], and multiple mouse models of diabetic cardiomyopathy [189,190]. The amelioration of myocardial redox status in response to empagliflozin is associated with the restoration of NOS coupling, PKG-mediated titin phosphorylation, and diastolic function [25]. Intriguingly, cardiac Nox4 protein levels are elevated in the diabetic rodent heart, an effect that is normalized by empagliflozin treatment along with myocardial oxidative stress, fibrosis, and cGMP levels [189,190]. Canagliflozin similarly blunted the upregulation of Nox4, as well as the induction of iNOS protein levels in the mouse heart in response to β-adrenergic stimulation [191], collectively suggesting that a reduction in NOX4-generated ROS and nitrosative stress may contribute to the efficacy of SGLT2 inhibitors in the treatment of HFpEF. Canagliflozin also reduced Rac1 activation, membrane translocation, NOX activity, and ROS levels in cultured ex vivo atrial myocardium [192], suggesting that SGLT2 inhibitors may also disrupt NOX2-dependent oxidative stress and/or redox signaling. Thus, impacts on NOX2, NOX4, and myocardial nitroso-redox status may be indirect beneficial effects of SGLT2 inhibitor therapy in HFpEF.
5.4. Future Perspectives
Future studies interrogating the regulation of NOX-mediated oxidative stress and redox signaling by distinct NOX isoforms in various cell types of the heart using pre-clinical models of HFpEF and diastolic dysfunction will facilitate the identification of novel targets to repress oxidant stress and pathogenic redox signaling. An examination of the phenotypic response, myocardial redox status, and redox signaling landscape of NOX isoform-specific conditional deletion mice in models of HFpEF (e.g., HFD/L-NAME, SAUNA) will help to uncover the mechanistic contribution of NOXs to HFpEF. Evidence from humans and animal models suggests that NOX-regulated oxidative stress and redox signaling mechanisms likely participate in HFpEF pathogenesis. Further investigation could suggest the development of isoform-specific NOX inhibitors, the targeting of regulators of NOX oxidase activity (e.g., Rac1), or strategies to intervene with specific NOX-regulated redox signaling effectors as therapeutic targets for the treatment of diastolic dysfunction and HFpEF.
Abbreviations
| ACE | angiotensin converting enzyme |
| AngII | angiotensin II |
| ARB | angiotensin receptor blocker |
| AT1R | angiotensin receptor type 1 |
| CaMKII | calcium/calmodulin-dependent protein kinase II |
| cGMP | cyclic GMP |
| eNOS | endothelial nitric oxide synthase |
| GDF6 | growth differentiation factor 6 |
| HDAC2, 4 | histone deacetylase 2, 4 |
| HFD | high-fat diet |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| iNOS | inducible nitric oxide synthase |
| IRE1𝛼 | inositol-requiring enzyme 1𝛼 |
| L-NAME | Nω-nitro-L-arginine |
| MEF2 | myocyte enhancer factor 2 |
| MRA | mineralocorticoid receptor antagonist |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NFAT | nuclear factor of activated T cells |
| NFκB | nuclear factor kappa B |
| nNOS | neuronal nitric oxide synthase |
| NOS | nitric oxide synthase |
| NOX | NADPH oxidase |
| PDGF | platelet-derived growth factor |
| PKG | protein kinase G |
| RAAS | renin-angiotensin-aldosterone system |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| ROS | reactive oxygen species |
| SAUNA | salty drinking water/unilateral nephrectomy/aldosterone |
| sGC | soluble guanylate cyclase |
| SGLT2 | sodium-glucose cotransporter 2 |
| TGF-β | transforming growth factor-beta |
| Treg | regulatory T cell |
| VSMC | vascular smooth muscle cell |
| Xbp1(s) | (spliced) X-box-binding protein 1. |
Author Contributions
Writing—original draft preparation, J.P.T., K.E., S.L.H. and M.J.B. Writing—review and editing, J.P.T., N.R.M., K.E., S.L.H. and M.J.B. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grants from the National Institutes of Health (R00HL136695 to M.J. Brody, R01HL139813 to S.L. Hummel, and R01HL139842 to N.R. Madamanchi), the American Heart Association (827440 to K. Essandoh and 898429 to J.P. Teuber), and the U.S. Department of Veterans Affairs (CARA-009-16F9050 to S.L. Hummel).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mishra S., Kass D.A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2021;18:400–423. doi: 10.1038/s41569-020-00480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlay S.M., Roger V.L., Redfield M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 3.Shah S.J., Borlaug B.A., Kitzman D.W., McCulloch A.D., Blaxall B.C., Agarwal R., Chirinos J.A., Collins S., Deo R.C., Gladwin M.T., et al. Research Priorities for Heart Failure With Preserved Ejection Fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation. 2020;141:1001–1026. doi: 10.1161/CIRCULATIONAHA.119.041886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah K.S., Xu H., Matsouaka R.A., Bhatt D.L., Heidenreich P.A., Hernandez A.F., Devore A.D., Yancy C.W., Fonarow G.C. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017;70:2476–2486. doi: 10.1016/j.jacc.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 5.Clark K.A.A., Velazquez E.J. Heart Failure With Preserved Ejection Fraction: Time for a Reset. JAMA. 2020;324:1506–1508. doi: 10.1001/jama.2020.15566. [DOI] [PubMed] [Google Scholar]
- 6.Kjeldsen S.E., von Lueder T.G., Smiseth O.A., Wachtell K., Mistry N., Westheim A.S., Hopper I., Julius S., Pitt B., Reid C.M., et al. Medical Therapies for Heart Failure With Preserved Ejection Fraction. Hypertension. 2020;75:23–32. doi: 10.1161/HYPERTENSIONAHA.119.14057. [DOI] [PubMed] [Google Scholar]
- 7.Schiattarella G.G., Altamirano F., Tong D., French K.M., Villalobos E., Kim S.Y., Luo X., Jiang N., May H.I., Wang Z.V., et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019;568:351–356. doi: 10.1038/s41586-019-1100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tribouilloy C., Rusinaru D., Mahjoub H., Souliere V., Levy F., Peltier M., Slama M., Massy Z. Prognosis of heart failure with preserved ejection fraction: A 5 year prospective population-based study. Eur. Heart J. 2008;29:339–347. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 9.Pfeffer M.A., Shah A.M., Borlaug B.A. Heart Failure With Preserved Ejection Fraction In Perspective. Circ. Res. 2019;124:1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweeney M., Corden B., Cook S.A. Targeting cardiac fibrosis in heart failure with preserved ejection fraction: Mirage or miracle? EMBO Mol. Med. 2020;12:e10865. doi: 10.15252/emmm.201910865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeWinter M.M., Meyer M. Mechanisms of diastolic dysfunction in heart failure with a preserved ejection fraction: If it's not one thing it's another. Circ. Heart Fail. 2013;6:1112–1115. doi: 10.1161/CIRCHEARTFAILURE.113.000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Travers J.G., Tharp C.A., Rubino M., McKinsey T.A. Therapeutic targets for cardiac fibrosis: From old school to next-gen. J. Clin. Investig. 2022;132:e148554. doi: 10.1172/JCI148554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zile M.R., Baicu C.F., Ikonomidis J.S., Stroud R.E., Nietert P.J., Bradshaw A.D., Slater R., Palmer B.M., Van Buren P., Meyer M., et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: Contributions of collagen and titin. Circulation. 2015;131:1247–1259. doi: 10.1161/CIRCULATIONAHA.114.013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane G.C., Karon B.L., Mahoney D.W., Redfield M.M., Roger V.L., Burnett J.C., Jr., Jacobsen S.J., Rodeheffer R.J. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zile M.R., Gottdiener J.S., Hetzel S.J., McMurray J.J., Komajda M., McKelvie R., Baicu C.F., Massie B.M., Carson P.E., Investigators I.P. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed S.F., Hussain S., Mirzoyev S.A., Edwards W.D., Maleszewski J.J., Redfield M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franssen C., Chen S., Unger A., Korkmaz H.I., De Keulenaer G.W., Tschope C., Leite-Moreira A.F., Musters R., Niessen H.W., Linke W.A., et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:312–324. doi: 10.1016/j.jchf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Shah S.J., Kitzman D.W., Borlaug B.A., van Heerebeek L., Zile M.R., Kass D.A., Paulus W.J. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitiello D., Harel F., Touyz R.M., Sirois M.G., Lavoie J., Myers J., Ducharme A., Racine N., O'Meara E., Gayda M., et al. Changes in cardiopulmonary reserve and peripheral arterial function concomitantly with subclinical inflammation and oxidative stress in patients with heart failure with preserved ejection fraction. Int. J. Vasc. Med. 2014;2014:917271. doi: 10.1155/2014/917271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharp T.E., 3rd, Scarborough A.L., Li Z., Polhemus D.J., Hidalgo H.A., Schumacher J.D., Matsuura T.R., Jenkins J.S., Kelly D.P., Goodchild T.T., et al. Novel Gottingen Miniswine Model of Heart Failure With Preserved Ejection Fraction Integrating Multiple Comorbidities. JACC Basic Transl. Sci. 2021;6:154–170. doi: 10.1016/j.jacbts.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo L., Chuang C.C., Hemmelgarn B.T., Best T.M. Heart failure with preserved ejection fraction: Defining the function of ROS and NO. J. Appl. Physiol. 2015;119:944–951. doi: 10.1152/japplphysiol.01149.2014. [DOI] [PubMed] [Google Scholar]
- 22.Paulus W.J., Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L., Chen J., Yan L., He Q., Xie H., Chen M. Resveratrol Ameliorates Cardiac Remodeling in a Murine Model of Heart Failure With Preserved Ejection Fraction. Front. Pharmacol. 2021;12:646240. doi: 10.3389/fphar.2021.646240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H.J., Kong B., Shuai W., Zhang J.J., Huang H. MD1 deletion exaggerates cardiomyocyte autophagy induced by heart failure with preserved ejection fraction through ROS/MAPK signalling pathway. J. Cell Mol. Med. 2020;24:9300–9312. doi: 10.1111/jcmm.15579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolijn D., Pabel S., Tian Y., Lodi M., Herwig M., Carrizzo A., Zhazykbayeva S., Kovacs A., Fulop G.A., Falcao-Pires I., et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Galpha oxidation. Cardiovasc. Res. 2021;117:495–507. doi: 10.1093/cvr/cvaa123. [DOI] [PubMed] [Google Scholar]
- 26.Sorop O., Heinonen I., van Kranenburg M., van de Wouw J., de Beer V.J., Nguyen I.T.N., Octavia Y., van Duin R.W.B., Stam K., van Geuns R.J., et al. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc. Res. 2018;114:954–964. doi: 10.1093/cvr/cvy038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishihara T., Yamamoto E., Sueta D., Fujisue K., Usuku H., Oike F., Takae M., Tabata N., Ito M., Yamanaga K., et al. Impact of Reactive Oxidative Metabolites Among New Categories of Nonischemic Heart Failure. J. Am. Heart Assoc. 2021;10:e016765. doi: 10.1161/JAHA.120.016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirata Y., Yamamoto E., Tokitsu T., Kusaka H., Fujisue K., Kurokawa H., Sugamura K., Maeda H., Tsujita K., Yamamuro M., et al. Reactive oxidative metabolites are associated with the severity of heart failure and predict future cardiovascular events in heart failure with preserved left ventricular ejection fraction. Int. J. Cardiol. 2015;179:305–308. doi: 10.1016/j.ijcard.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Xu L., Balzarolo M., Robinson E.L., Lorenz V., Verde G.D., Joray L., Mochizuki M., Kaufmann B.A., Valstar G., de Jager S.C.A., et al. NOX1 mediates metabolic heart disease in mice and is upregulated in monocytes of humans with diastolic dysfunction. Cardiovasc. Res. 2021:cvab349. doi: 10.1093/cvr/cvab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y., Wen J., He A., Qu C., Peng Y., Luo S., Wang X. iNOS contributes to heart failure with preserved ejection fraction through mitochondrial dysfunction and Akt S-nitrosylation. J. Advanced. Res. 2022 doi: 10.1016/j.jare.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M., Perino A., Ghigo A., Hirsch E., Shah A.M. NADPH oxidases in heart failure: Poachers or gamekeepers? Antioxid. Redox Signal. 2013;18:1024–1041. doi: 10.1089/ars.2012.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ago T., Matsushima S., Kuroda J., Zablocki D., Kitazono T., Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging. 2010;2:1012–1016. doi: 10.18632/aging.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zablocki D., Sadoshima J. Angiotensin II and oxidative stress in the failing heart. Antioxid. Redox Signal. 2013;19:1095–1109. doi: 10.1089/ars.2012.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vendrov A.E., Sumida A., Canugovi C., Lozhkin A., Hayami T., Madamanchi N.R., Runge M.S. NOXA1-dependent NADPH oxidase regulates redox signaling and phenotype of vascular smooth muscle cell during atherogenesis. Redox Biol. 2019;21:101063. doi: 10.1016/j.redox.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canugovi C., Stevenson M.D., Vendrov A.E., Hayami T., Robidoux J., Xiao H., Zhang Y.Y., Eitzman D.T., Runge M.S., Madamanchi N.R. Increased mitochondrial NADPH oxidase 4 (NOX4) expression in aging is a causative factor in aortic stiffening. Redox Biol. 2019;26:101288. doi: 10.1016/j.redox.2019.101288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Murugesan P., Huang K., Cai H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020;17:170–194. doi: 10.1038/s41569-019-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 38.Sirker A., Zhang M., Shah A.M. NADPH oxidases in cardiovascular disease: Insights from in vivo models and clinical studies. Basic Res. Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sirker A., Zhang M., Murdoch C., Shah A.M. Involvement of NADPH oxidases in cardiac remodelling and heart failure. Am. J. Nephrol. 2007;27:649–660. doi: 10.1159/000109148. [DOI] [PubMed] [Google Scholar]
- 40.He B.J., Joiner M.L., Singh M.V., Luczak E.D., Swaminathan P.D., Koval O.M., Kutschke W., Allamargot C., Yang J., Guan X., et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat. Med. 2011;17:1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bendall J.K., Cave A.C., Heymes C., Gall N., Shah A.M. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 42.Ago T., Liu T., Zhai P., Chen W., Li H., Molkentin J.D., Vatner S.F., Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 43.Takimoto E., Kass D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 44.Erickson J.R., Joiner M.L., Guan X., Kutschke W., Yang J., Oddis C.V., Bartlett R.K., Lowe J.S., O'Donnell S.E., Aykin-Burns N., et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai D.F., Rabinovitch P.S., Ungvari Z. Mitochondria and cardiovascular aging. Circ. Res. 2012;110:1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Kaludercic N., Takimoto E., Nagayama T., Feng N., Lai E.W., Bedja D., Chen K., Gabrielson K.L., Blakely R.D., Shih J.C., et al. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ. Res. 2010;106:193–202. doi: 10.1161/CIRCRESAHA.109.198366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaludercic N., Carpi A., Menabo R., Di Lisa F., Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim. Biophys. Acta. 2011;1813:1323–1332. doi: 10.1016/j.bbamcr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maggiorani D., Manzella N., Edmondson D.E., Mattevi A., Parini A., Binda C., Mialet-Perez J. Monoamine Oxidases, Oxidative Stress, and Altered Mitochondrial Dynamics in Cardiac Ageing. Oxid. Med. Cell Longev. 2017;2017:3017947. doi: 10.1155/2017/3017947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cantu-Medellin N., Kelley E.E. Xanthine oxidoreductase-catalyzed reactive species generation: A process in critical need of reevaluation. Redox Biol. 2013;1:353–358. doi: 10.1016/j.redox.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veith A., Moorthy B. Role of Cytochrome P450s in the Generation and Metabolism of Reactive Oxygen Species. Curr. Opin. Toxicol. 2018;7:44–51. doi: 10.1016/j.cotox.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takimoto E., Champion H.C., Li M., Ren S., Rodriguez E.R., Tavazzi B., Lazzarino G., Paolocci N., Gabrielson K.L., Wang Y., et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Investig. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okazaki T., Otani H., Shimazu T., Yoshioka K., Fujita M., Katano T., Ito S., Iwasaka T. Reversal of inducible nitric oxide synthase uncoupling unmasks tolerance to ischemia/reperfusion injury in the diabetic rat heart. J. Mol. Cell Cardiol. 2011;50:534–544. doi: 10.1016/j.yjmcc.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Youn J.Y., Zhang J., Zhang Y., Chen H., Liu D., Ping P., Weiss J.N., Cai H. Oxidative stress in atrial fibrillation: An emerging role of NADPH oxidase. J. Mol. Cell Cardiol. 2013;62:72–79. doi: 10.1016/j.yjmcc.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao G.J., Zhao C.L., Ouyang S., Deng K.Q., Zhu L., Montezano A.C., Zhang C., Hu F., Zhu X.Y., Tian S., et al. Ca(2+)-Dependent NOX5 (NADPH Oxidase 5) Exaggerates Cardiac Hypertrophy Through Reactive Oxygen Species Production. Hypertension. 2020;76:827–838. doi: 10.1161/HYPERTENSIONAHA.120.15558. [DOI] [PubMed] [Google Scholar]
- 56.Knaus U.G., Heyworth P.G., Evans T., Curnutte J.T., Bokoch G.M. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 57.Hordijk P.L. Regulation of NADPH oxidases: The role of Rac proteins. Circ. Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 58.Hahn N.E., Meischl C., Kawahara T., Musters R.J., Verhoef V.M., van der Velden J., Vonk A.B., Paulus W.J., van Rossum A.C., Niessen H.W., et al. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am. J. Pathol. 2012;180:2222–2229. doi: 10.1016/j.ajpath.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 59.Harrison C.B., Trevelin S.C., Richards D.A., Santos C.X.C., Sawyer G., Markovinovic A., Zhang X., Zhang M., Brewer A.C., Yin X., et al. Fibroblast Nox2 (NADPH Oxidase-2) Regulates ANG II (Angiotensin II)-Induced Vascular Remodeling and Hypertension via Paracrine Signaling to Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2021;41:698–710. doi: 10.1161/ATVBAHA.120.315322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee C.F., Qiao M., Schroder K., Zhao Q., Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ. Res. 2010;106:1489–1497. doi: 10.1161/CIRCRESAHA.109.215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Satoh M., Ogita H., Takeshita K., Mukai Y., Kwiatkowski D.J., Liao J.K. Requirement of Rac1 in the development of cardiac hypertrophy. Proc. Natl. Acad. Sci. USA. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsushima S., Kuroda J., Ago T., Zhai P., Park J.Y., Xie L.H., Tian B., Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ. Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ago T., Kuroda J., Pain J., Fu C., Li H., Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dai D.F., Chen T., Szeto H., Nieves-Cintron M., Kutyavin V., Santana L.F., Rabinovitch P.S. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J. Am. Coll. Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsushima S., Kuroda J., Zhai P., Liu T., Ikeda S., Nagarajan N., Oka S., Yokota T., Kinugawa S., Hsu C.P., et al. Tyrosine kinase FYN negatively regulates NOX4 in cardiac remodeling. J. Clin. Investig. 2016;126:3403–3416. doi: 10.1172/JCI85624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sciarretta S., Zhai P., Shao D., Zablocki D., Nagarajan N., Terada L.S., Volpe M., Sadoshima J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circ. Res. 2013;113:1253–1264. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beretta M., Santos C.X., Molenaar C., Hafstad A.D., Miller C.C., Revazian A., Betteridge K., Schroder K., Streckfuss-Bomeke K., Doroshow J.H., et al. Nox4 regulates InsP3 receptor-dependent Ca(2+) release into mitochondria to promote cell survival. EMBO J. 2020;39:e103530. doi: 10.15252/embj.2019103530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vendrov A.E., Vendrov K.C., Smith A., Yuan J., Sumida A., Robidoux J., Runge M.S., Madamanchi N.R. NOX4 NADPH Oxidase-Dependent Mitochondrial Oxidative Stress in Aging-Associated Cardiovascular Disease. Antioxid. Redox Signal. 2015;23:1389–1409. doi: 10.1089/ars.2014.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prosser B.L., Ward C.W., Lederer W.J. X-ROS signaling: Rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 70.Zhang M., Prosser B.L., Bamboye M.A., Gondim A.N.S., Santos C.X., Martin D., Ghigo A., Perino A., Brewer A.C., Ward C.W., et al. Contractile Function During Angiotensin-II Activation: Increased Nox2 Activity Modulates Cardiac Calcium Handling via Phospholamban Phosphorylation. J. Am. Coll. Cardiol. 2015;66:261–272. doi: 10.1016/j.jacc.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hingtgen S.D., Tian X., Yang J., Dunlay S.M., Peek A.S., Wu Y., Sharma R.V., Engelhardt J.F., Davisson R.L. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol. Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 72.Forte M., Nocella C., De Falco E., Palmerio S., Schirone L., Valenti V., Frati G., Carnevale R., Sciarretta S. The Pathophysiological Role of NOX2 in Hypertension and Organ Damage. High Blood Press. Cardiovasc. Prev. 2016;23:355–364. doi: 10.1007/s40292-016-0175-y. [DOI] [PubMed] [Google Scholar]
- 73.Hansen S.S., Aasum E., Hafstad A.D. The role of NADPH oxidases in diabetic cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:1908–1913. doi: 10.1016/j.bbadis.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 74.Stevenson M.D., Canugovi C., Vendrov A.E., Hayami T., Bowles D.E., Krause K.H., Madamanchi N.R., Runge M.S. NADPH Oxidase 4 Regulates Inflammation in Ischemic Heart Failure: Role of Soluble Epoxide Hydrolase. Antioxid. Redox Signal. 2019;31:39–58. doi: 10.1089/ars.2018.7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Berlo J.H., Maillet M., Molkentin J.D. Signaling effectors underlying pathologic growth and remodeling of the heart. J. Clin. Investig. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bishu K., Deswal A., Chen H.H., LeWinter M.M., Lewis G.D., Semigran M.J., Borlaug B.A., McNulty S., Hernandez A.F., Braunwald E., et al. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am. Heart J. 2012;164:763–770.e3. doi: 10.1016/j.ahj.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoon S., Eom G.H. Heart failure with preserved ejection fraction: Present status and future directions. Exp. Mol. Med. 2019;51:1–9. doi: 10.1038/s12276-019-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Binder C., Poglitsch M., Duca F., Rettl R., Dachs T.M., Dalos D., Schrutka L., Seirer B., Ligios L.C., Capelle C., et al. Renin Feedback Is an Independent Predictor of Outcome in HFpEF. J. Pers. Med. 2021;11:370. doi: 10.3390/jpm11050370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ke B., Tan X., Ren L., Fan Y., Zhang Y., Li F., Sun Q., Liu T., Jia L., Wang Y., et al. Aldosterone dysregulation predicts the risk of mortality and rehospitalization in heart failure with a preserved ejection fraction. Sci. China Life Sci. 2022;65:631–642. doi: 10.1007/s11427-021-1945-6. [DOI] [PubMed] [Google Scholar]
- 80.Pfeffer M.A., Braunwald E. Treatment of Heart Failure With Preserved Ejection Fraction: Reflections on Its Treatment With an Aldosterone Antagonist. JAMA Cardiol. 2016;1:7–8. doi: 10.1001/jamacardio.2015.0356. [DOI] [PubMed] [Google Scholar]
- 81.Catena C., Verheyen N., Pilz S., Kraigher-Krainer E., Tomaschitz A., Sechi L.A., Pieske B. Plasma aldosterone and left ventricular diastolic function in treatment-naive patients with hypertension: Tissue-Doppler imaging study. Hypertension. 2015;65:1231–1237. doi: 10.1161/HYPERTENSIONAHA.115.05285. [DOI] [PubMed] [Google Scholar]
- 82.Gimenes R., Gimenes C., Rosa C.M., Xavier N.P., Campos D.H.S., Fernandes A.A.H., Cezar M.D.M., Guirado G.N., Pagan L.U., Chaer I.D., et al. Influence of apocynin on cardiac remodeling in rats with streptozotocin-induced diabetes mellitus. Cardiovasc. Diabetol. 2018;17:15. doi: 10.1186/s12933-017-0657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J., Zhu H., Shen E., Wan L., Arnold J.M., Peng T. Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes. 2010;59:2033–2042. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y.Q., Li X.B., Guo S.J., Chu S.L., Gao P.J., Zhu D.L., Niu W.Q., Jia N. Apocynin attenuates oxidative stress and cardiac fibrosis in angiotensin II-induced cardiac diastolic dysfunction in mice. Acta Pharmacol. Sin. 2013;34:352–359. doi: 10.1038/aps.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park Y.M., Park M.Y., Suh Y.L., Park J.B. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem. Biophys. Res. Commun. 2004;313:812–817. doi: 10.1016/j.bbrc.2003.11.173. [DOI] [PubMed] [Google Scholar]
- 86.Bidwell P.A., Liu G.S., Nagarajan N., Lam C.K., Haghighi K., Gardner G., Cai W.F., Zhao W., Mugge L., Vafiadaki E., et al. HAX-1 regulates SERCA2a oxidation and degradation. J. Mol. Cell. Cardiol. 2018;114:220–233. doi: 10.1016/j.yjmcc.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hafstad A.D., Hansen S.S., Lund J., Santos C.X.C., Boardman N.T., Shah A.M., Aasum E. NADPH Oxidase 2 Mediates Myocardial Oxygen Wasting in Obesity. Antioxidants. 2020;9:171. doi: 10.3390/antiox9020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao Y., McLaughlin D., Robinson E., Harvey A.P., Hookham M.B., Shah A.M., McDermott B.J., Grieve D.J. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Cancer Res. 2010;70:9287–9297. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Looi Y.H., Grieve D.J., Siva A., Walker S.J., Anilkumar N., Cave A.C., Marber M., Monaghan M.J., Shah A.M. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–325. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 90.Johar S., Cave A.C., Narayanapanicker A., Grieve D.J., Shah A.M. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20:1546–1548. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 91.Grieve D.J., Byrne J.A., Siva A., Layland J., Johar S., Cave A.C., Shah A.M. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J. Am. Coll. Cardiol. 2006;47:817–826. doi: 10.1016/j.jacc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 92.Parajuli N., Patel V.B., Wang W., Basu R., Oudit G.Y. Loss of NOX2 (gp91phox) prevents oxidative stress and progression to advanced heart failure. Clin. Sci. 2014;127:331–340. doi: 10.1042/CS20130787. [DOI] [PubMed] [Google Scholar]
- 93.Murdoch C.E., Chaubey S., Zeng L., Yu B., Ivetic A., Walker S.J., Vanhoutte D., Heymans S., Grieve D.J., Cave A.C., et al. Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. J. Am. Coll. Cardiol. 2014;63:2734–2741. doi: 10.1016/j.jacc.2014.02.572. [DOI] [PubMed] [Google Scholar]
- 94.Ayuzawa N., Nagase M., Ueda K., Nishimoto M., Kawarazaki W., Marumo T., Aiba A., Sakurai T., Shindo T., Fujita T. Rac1-Mediated Activation of Mineralocorticoid Receptor in Pressure Overload-Induced Cardiac Injury. Hypertension. 2016;67:99–106. doi: 10.1161/HYPERTENSIONAHA.115.06054. [DOI] [PubMed] [Google Scholar]
- 95.Essandoh K., Auchus R.J., Brody M.J. Cardiac decompensation and promiscuous prenylation of small GTPases in cardiomyocytes in response to local mevalonate pathway disruption. J. Pathol. 2022;256:249–252. doi: 10.1002/path.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takemoto M., Node K., Nakagami H., Liao Y., Grimm M., Takemoto Y., Kitakaze M., Liao J.K. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J. Clin. Investig. 2001;108:1429–1437. doi: 10.1172/JCI13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maack C., Kartes T., Kilter H., Schafers H.J., Nickenig G., Bohm M., Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 98.Zhao Q.D., Viswanadhapalli S., Williams P., Shi Q., Tan C., Yi X., Bhandari B., Abboud H.E. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFkappaB signaling pathways. Circulation. 2015;131:643–655. doi: 10.1161/CIRCULATIONAHA.114.011079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuroda J., Ago T., Matsushima S., Zhai P., Schneider M.D., Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang M., Mongue-Din H., Martin D., Catibog N., Smyrnias I., Zhang X., Yu B., Wang M., Brandes R.P., Schroder K., et al. Both cardiomyocyte and endothelial cell Nox4 mediate protection against hemodynamic overload-induced remodelling. Cardiovasc. Res. 2018;114:401–408. doi: 10.1093/cvr/cvx204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mongue-Din H., Patel A.S., Looi Y.H., Grieve D.J., Anilkumar N., Sirker A., Dong X., Brewer A.C., Zhang M., Smith A., et al. NADPH Oxidase-4 Driven Cardiac Macrophage Polarization Protects Against Myocardial Infarction-Induced Remodeling. JACC Basic. Transl. Sci. 2017;2:688–698. doi: 10.1016/j.jacbts.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu Q., Lee C.F., Wang W., Karamanlidis G., Kuroda J., Matsushima S., Sadoshima J., Tian R. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. J. Am. Heart Assoc. 2014;3:e000555. doi: 10.1161/JAHA.113.000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eaton C.B., Pettinger M., Rossouw J., Martin L.W., Foraker R., Quddus A., Liu S., Wampler N.S., Hank Wu W.C., Manson J.E., et al. Risk Factors for Incident Hospitalized Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Postmenopausal Women. Circ. Heart Fail. 2016;9:e002883. doi: 10.1161/CIRCHEARTFAILURE.115.002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kregel K.C., Zhang H.J. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 105.Mighiu A.S., Recalde A., Ziberna K., Carnicer R., Tomek J., Bub G., Brewer A.C., Verheule S., Shah A.M., Simon J.N., et al. Inducibility, but not stability, of atrial fibrillation is increased by NOX2 overexpression in mice. Cardiovasc. Res. 2021;117:2354–2364. doi: 10.1093/cvr/cvab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim Y.M., Guzik T.J., Zhang Y.H., Zhang M.H., Kattach H., Ratnatunga C., Pillai R., Channon K.M., Casadei B. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ. Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 107.Purohit A., Rokita A.G., Guan X., Chen B., Koval O.M., Voigt N., Neef S., Sowa T., Gao Z., Luczak E.D., et al. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation. 2013;128:1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang J., Youn J.Y., Kim A.Y., Ramirez R.J., Gao L., Ngo D., Chen P., Scovotti J., Mahajan A., Cai H. NOX4-Dependent Hydrogen Peroxide Overproduction in Human Atrial Fibrillation and HL-1 Atrial Cells: Relationship to Hypertension. Front. Physiol. 2012;3:140. doi: 10.3389/fphys.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reilly S.N., Jayaram R., Nahar K., Antoniades C., Verheule S., Channon K.M., Alp N.J., Schotten U., Casadei B. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: Implications for the antiarrhythmic effect of statins. Circulation. 2011;124:1107–1117. doi: 10.1161/CIRCULATIONAHA.111.029223. [DOI] [PubMed] [Google Scholar]
- 110.Chen W.J., Chang S.H., Chan Y.H., Lee J.L., Lai Y.J., Chang G.J., Tsai F.C., Yeh Y.H. Tachycardia-induced CD44/NOX4 signaling is involved in the development of atrial remodeling. J. Mol. Cell Cardiol. 2019;135:67–78. doi: 10.1016/j.yjmcc.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 111.Sovari A.A., Dudley S.C., Jr. Reactive oxygen species-targeted therapeutic interventions for atrial fibrillation. Front. Physiol. 2012;3:311. doi: 10.3389/fphys.2012.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Adam O., Frost G., Custodis F., Sussman M.A., Schafers H.J., Bohm M., Laufs U. Role of Rac1 GTPase activation in atrial fibrillation. J. Am. Coll. Cardiol. 2007;50:359–367. doi: 10.1016/j.jacc.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 113.Batul S.A., Gopinathannair R. Atrial Fibrillation in Heart Failure: A Therapeutic Challenge of Our Times. Korean Circ. J. 2017;47:644–662. doi: 10.4070/kcj.2017.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zakeri R., Chamberlain A.M., Roger V.L., Redfield M.M. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: A community-based study. Circulation. 2013;128:1085–1093. doi: 10.1161/CIRCULATIONAHA.113.001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reddy Y.N.V., Obokata M., Verbrugge F.H., Lin G., Borlaug B.A. Atrial Dysfunction in Patients With Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation. J. Am. Coll. Cardiol. 2020;76:1051–1064. doi: 10.1016/j.jacc.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Packer M., Lam C.S.P., Lund L.H., Redfield M.M. Interdependence of Atrial Fibrillation and Heart Failure With a Preserved Ejection Fraction Reflects a Common Underlying Atrial and Ventricular Myopathy. Circulation. 2020;141:4–6. doi: 10.1161/CIRCULATIONAHA.119.042996. [DOI] [PubMed] [Google Scholar]
- 117.Ariyaratnam J.P., Elliott A.D., Mishima R.S., Gallagher C., Lau D.H., Sanders P. Heart failure with preserved ejection fraction: An alternative paradigm to explain the clinical implications of atrial fibrillation. Heart Rhythm. O2. 2021;2:771–783. doi: 10.1016/j.hroo.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ray R., Murdoch C.E., Wang M., Santos C.X., Zhang M., Alom-Ruiz S., Anilkumar N., Ouattara A., Cave A.C., Walker S.J., et al. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler. Thromb. Vasc. Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 119.Santillo M., Colantuoni A., Mondola P., Guida B., Damiano S. NOX signaling in molecular cardiovascular mechanisms involved in the blood pressure homeostasis. Front. Physiol. 2015;6:194. doi: 10.3389/fphys.2015.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vendrov A.E., Madamanchi N.R., Hakim Z.S., Rojas M., Runge M.S. Thrombin and NAD(P)H oxidase-mediated regulation of CD44 and BMP4-Id pathway in VSMC, restenosis, and atherosclerosis. Circ. Res. 2006;98:1254–1263. doi: 10.1161/01.RES.0000221214.37803.79. [DOI] [PubMed] [Google Scholar]
- 121.Li Y., Kracun D., Dustin C.M., El Massry M., Yuan S., Goossen C.J., DeVallance E.R., Sahoo S., St Hilaire C., Gurkar A.U., et al. Forestalling age-impaired angiogenesis and blood flow by targeting NOX: Interplay of NOX1, IL-6, and SASP in propagating cell senescence. Proc. Natl. Acad. Sci. USA. 2021;118:e2015666118. doi: 10.1073/pnas.2015666118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ago T., Kitazono T., Ooboshi H., Iyama T., Han Y.H., Takada J., Wakisaka M., Ibayashi S., Utsumi H., Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 123.Wang M., Murdoch C.E., Brewer A.C., Ivetic A., Evans P., Shah A.M., Zhang M. Endothelial NADPH oxidase 4 protects against angiotensin II-induced cardiac fibrosis and inflammation. ESC Heart Fail. 2021;8:1427–1437. doi: 10.1002/ehf2.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Craige S.M., Kant S., Reif M., Chen K., Pei Y., Angoff R., Sugamura K., Fitzgibbons T., Keaney J.F., Jr. Endothelial NADPH oxidase 4 protects ApoE-/- mice from atherosclerotic lesions. Free Radic. Biol. Med. 2015;89:1–7. doi: 10.1016/j.freeradbiomed.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schurmann C., Rezende F., Kruse C., Yasar Y., Lowe O., Fork C., van de Sluis B., Bremer R., Weissmann N., Shah A.M., et al. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur. Heart J. 2015;36:3447–3456. doi: 10.1093/eurheartj/ehv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Drummond G.R., Sobey C.G. Endothelial NADPH oxidases: Which NOX to target in vascular disease? Trends Endocrinol. Metab. 2014;25:452–463. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 127.Matsuno K., Yamada H., Iwata K., Jin D., Katsuyama M., Matsuki M., Takai S., Yamanishi K., Miyazaki M., Matsubara H., et al. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 128.Bendall J.K., Rinze R., Adlam D., Tatham A.L., de Bono J., Wilson N., Volpi E., Channon K.M. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: Studies in endothelial-targeted Nox2 transgenic mice. Circ. Res. 2007;100:1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 129.Douglas G., Bendall J.K., Crabtree M.J., Tatham A.L., Carter E.E., Hale A.B., Channon K.M. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE(-)/(-) mice. Cardiovasc Res. 2012;94:20–29. doi: 10.1093/cvr/cvs026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guzik T.J., Chen W., Gongora M.C., Guzik B., Lob H.E., Mangalat D., Hoch N., Dikalov S., Rudzinski P., Kapelak B., et al. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J. Am. Coll. Cardiol. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Elbatreek M.H., Sadegh S., Anastasi E., Guney E., Nogales C., Kacprowski T., Hassan A.A., Teubner A., Huang P.H., Hsu C.Y., et al. NOX5-induced uncoupling of endothelial NO synthase is a causal mechanism and theragnostic target of an age-related hypertension endotype. PLoS Biol. 2020;18:e3000885. doi: 10.1371/journal.pbio.3000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takaishi K., Kinoshita H., Kawashima S., Kawahito S. Human Vascular Smooth Muscle Function and Oxidative Stress Induced by NADPH Oxidase with the Clinical Implications. Cells. 2021;10:1947. doi: 10.3390/cells10081947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dikalova A., Clempus R., Lassegue B., Cheng G., McCoy J., Dikalov S., San Martin A., Lyle A., Weber D.S., Weiss D., et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 134.Dikalova A.E., Gongora M.C., Harrison D.G., Lambeth J.D., Dikalov S., Griendling K.K. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H673–H679. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee M.Y., San Martin A., Mehta P.K., Dikalova A.E., Garrido A.M., Datla S.R., Lyons E., Krause K.H., Banfi B., Lambeth J.D., et al. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler. Thromb. Vasc. Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bruder-Nascimento T., Chinnasamy P., Riascos-Bernal D.F., Cau S.B., Callera G.E., Touyz R.M., Tostes R.C., Sibinga N.E. Angiotensin II induces Fat1 expression/activation and vascular smooth muscle cell migration via Nox1-dependent reactive oxygen species generation. J. Mol. Cell Cardiol. 2014;66:18–26. doi: 10.1016/j.yjmcc.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lozhkin A., Vendrov A.E., Pan H., Wickline S.A., Madamanchi N.R., Runge M.S. NADPH oxidase 4 regulates vascular inflammation in aging and atherosclerosis. J. Mol. Cell Cardiol. 2017;102:10–21. doi: 10.1016/j.yjmcc.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu S., Chamseddine A.H., Carrell S., Miller F.J., Jr. Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol. 2014;2:642–650. doi: 10.1016/j.redox.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Camargo L.L., Montezano A.C., Hussain M., Wang Y., Zou Z., Rios F.J., Neves K.B., Alves-Lopes R., Awan F.R., Guzik T.J., et al. Central role of c-Src in NOX5- mediated redox signalling in vascular smooth muscle cells in human hypertension. Cardiovasc. Res. 2022;118:1359–1373. doi: 10.1093/cvr/cvab171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jay D.B., Papaharalambus C.A., Seidel-Rogol B., Dikalova A.E., Lassegue B., Griendling K.K. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic. Biol. Med. 2008;45:329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Montezano A.C., De Lucca Camargo L., Persson P., Rios F.J., Harvey A.P., Anagnostopoulou A., Palacios R., Gandara A.C.P., Alves-Lopes R., Neves K.B., et al. NADPH Oxidase 5 Is a Pro-Contractile Nox Isoform and a Point of Cross-Talk for Calcium and Redox Signaling-Implications in Vascular Function. J. Am. Heart Assoc. 2018;7:e009388. doi: 10.1161/JAHA.118.009388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Furmanik M., Chatrou M., van Gorp R., Akbulut A., Willems B., Schmidt H., van Eys G., Bochaton-Piallat M.L., Proudfoot D., Biessen E., et al. Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ. Res. 2020;127:911–927. doi: 10.1161/CIRCRESAHA.119.316159. [DOI] [PubMed] [Google Scholar]
- 143.Khalil H., Kanisicak O., Vagnozzi R.J., Johansen A.K., Maliken B.D., Prasad V., Boyer J.G., Brody M.J., Schips T., Kilian K.K., et al. Cell-specific ablation of Hsp47 defines the collagen-producing cells in the injured heart. JCI Insight. 2019;4:e128722. doi: 10.1172/jci.insight.128722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Meijles D.N., Pagano P.J. Nox and Inflammation in the Vascular Adventitia. Hypertension. 2016;67:14–19. doi: 10.1161/HYPERTENSIONAHA.115.03622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Philip J.L., Razzaque M.A., Han M., Li J., Theccanat T., Xu X., Akhter S.A. Regulation of mitochondrial oxidative stress by beta-arrestins in cultured human cardiac fibroblasts. Dis. Model. Mech. 2015;8:1579–1589. doi: 10.1242/dmm.019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cucoranu I., Clempus R., Dikalova A., Phelan P.J., Ariyan S., Dikalov S., Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 147.Cross A.R., Jones O.T., Garcia R., Segal A.W. The association of FAD with the cytochrome b-245 of human neutrophils. Biochem. J. 1982;208:759–763. doi: 10.1042/bj2080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Volpp B.D., Nauseef W.M., Clark R.A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988;242:1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- 149.Nunoi H., Rotrosen D., Gallin J.I., Malech H.L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988;242:1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- 150.Xu Q., Choksi S., Qu J., Jang J., Choe M., Banfi B., Engelhardt J.F., Liu Z.G. NADPH Oxidases Are Essential for Macrophage Differentiation. J. Biol. Chem. 2016;291:20030–20041. doi: 10.1074/jbc.M116.731216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Adamo L., Rocha-Resende C., Prabhu S.D., Mann D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020;17:269–285. doi: 10.1038/s41569-019-0315-x. [DOI] [PubMed] [Google Scholar]
- 152.Prabhu S.D., Frangogiannis N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Patel B., Bansal S.S., Ismahil M.A., Hamid T., Rokosh G., Mack M., Prabhu S.D. CCR2(+) Monocyte-Derived Infiltrating Macrophages Are Required for Adverse Cardiac Remodeling During Pressure Overload. JACC Basic. Transl. Sci. 2018;3:230–244. doi: 10.1016/j.jacbts.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dick S.A., Epelman S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ. Res. 2016;119:159–176. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 155.Moreno M.U., San Jose G., Pejenaute A., Landecho M.F., Diez J., Beloqui O., Fortuno A., Zalba G. Association of phagocytic NADPH oxidase activity with hypertensive heart disease: A role for cardiotrophin-1? Hypertension. 2014;63:468–474. doi: 10.1161/HYPERTENSIONAHA.113.01470. [DOI] [PubMed] [Google Scholar]
- 156.Emmerson A., Trevelin S.C., Mongue-Din H., Becker P.D., Ortiz C., Smyth L.A., Peng Q., Elgueta R., Sawyer G., Ivetic A., et al. Nox2 in regulatory T cells promotes angiotensin II-induced cardiovascular remodeling. J. Clin. Investig. 2018;128:3088–3101. doi: 10.1172/JCI97490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Matsushima S., Sadoshima J. Yin and Yang of NADPH Oxidases in Myocardial Ischemia-Reperfusion. Antioxidants. 2022;11:1069. doi: 10.3390/antiox11061069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Higuchi Y., Otsu K., Nishida K., Hirotani S., Nakayama H., Yamaguchi O., Hikoso S., Kashiwase K., Takeda T., Watanabe T., et al. The small GTP-binding protein Rac1 induces cardiac myocyte hypertrophy through the activation of apoptosis signal-regulating kinase 1 and nuclear factor-kappa B. J. Biol. Chem. 2003;278:20770–20777. doi: 10.1074/jbc.M213203200. [DOI] [PubMed] [Google Scholar]
- 159.Chen K., Pittman R.N., Popel A.S. Nitric oxide in the vasculature: Where does it come from and where does it go? A quantitative perspective. Antioxid. Redox Signal. 2008;10:1185–1198. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Joyner M.J., Dietz N.M. Nitric oxide and vasodilation in human limbs. J. Appl. Physiol. 1997;83:1785–1796. doi: 10.1152/jappl.1997.83.6.1785. [DOI] [PubMed] [Google Scholar]
- 161.Mongirdiene A., Skrodenis L., Varoneckaite L., Mierkyte G., Gerulis J. Reactive Oxygen Species Induced Pathways in Heart Failure Pathogenesis and Potential Therapeutic Strategies. Biomedicines. 2022;10:602. doi: 10.3390/biomedicines10030602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Leggat J., Bidault G., Vidal-Puig A. Lipotoxicity: A driver of heart failure with preserved ejection fraction? Clin. Sci. 2021;135:2265–2283. doi: 10.1042/CS20210127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kruger M., Kotter S., Grutzner A., Lang P., Andresen C., Redfield M.M., Butt E., dos Remedios C.G., Linke W.A. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ. Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 164.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.van Heerebeek L., Hamdani N., Falcao-Pires I., Leite-Moreira A.F., Begieneman M.P., Bronzwaer J.G., van der Velden J., Stienen G.J., Laarman G.J., Somsen A., et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 166.Chirinos J.A., Akers S.R., Trieu L., Ischiropoulos H., Doulias P.T., Tariq A., Vasim I., Koppula M.R., Syed A.A., Soto-Calderon H., et al. Heart Failure, Left Ventricular Remodeling, and Circulating Nitric Oxide Metabolites. J. Am. Heart Assoc. 2016;5:e004133. doi: 10.1161/JAHA.116.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zamani P., French B., Brandimarto J.A., Doulias P.T., Javaheri A., Chirinos J.A., Margulies K.B., Townsend R.R., Sweitzer N.K., Fang J.C., et al. Effect of Heart Failure With Preserved Ejection Fraction on Nitric Oxide Metabolites. Am. J. Cardiol. 2016;118:1855–1860. doi: 10.1016/j.amjcard.2016.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hess D.T., Matsumoto A., Kim S.O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 169.Yoon S., Kim M., Lee H., Kang G., Bedi K., Margulies K.B., Jain R., Nam K.I., Kook H., Eom G.H. S-Nitrosylation of Histone Deacetylase 2 by Neuronal Nitric Oxide Synthase as a Mechanism of Diastolic Dysfunction. Circulation. 2021;143:1912–1925. doi: 10.1161/CIRCULATIONAHA.119.043578. [DOI] [PubMed] [Google Scholar]
- 170.Wang Z.V., Deng Y., Gao N., Pedrozo Z., Li D.L., Morales C.R., Criollo A., Luo X., Tan W., Jiang N., et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell. 2014;156:1179–1192. doi: 10.1016/j.cell.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Senni M., Greene S.J., Butler J., Fonarow G.C., Gheorghiade M. Drug Development for Heart Failure With Preserved Ejection Fraction: What Pieces Are Missing From the Puzzle? Can. J. Cardiol. 2017;33:768–776. doi: 10.1016/j.cjca.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 172.Pitt B., Pfeffer M.A., Assmann S.F., Boineau R., Anand I.S., Claggett B., Clausell N., Desai A.S., Diaz R., Fleg J.L., et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 173.Massie B.M., Carson P.E., McMurray J.J., Komajda M., McKelvie R., Zile M.R., Anderson S., Donovan M., Iverson E., Staiger C., et al. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 174.Kitzman D.W., Hundley W.G., Brubaker P.H., Morgan T.M., Moore J.B., Stewart K.P., Little W.C. A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: Effects on exercise tolerance and arterial distensibility. Circ. Heart Fail. 2010;3:477–485. doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cleland J.G., Tendera M., Adamus J., Freemantle N., Polonski L., Taylor J., Investigators P.-C. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur. Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 176.Solomon S.D., McMurray J.J.V., Anand I.S., Ge J., Lam C.S.P., Maggioni A.P., Martinez F., Packer M., Pfeffer M.A., Pieske B., et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 177.Redfield M.M., Anstrom K.J., Levine J.A., Koepp G.A., Borlaug B.A., Chen H.H., LeWinter M.M., Joseph S.M., Shah S.J., Semigran M.J., et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2015;373:2314–2324. doi: 10.1056/NEJMoa1510774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Borlaug B.A., Anstrom K.J., Lewis G.D., Shah S.J., Levine J.A., Koepp G.A., Givertz M.M., Felker G.M., LeWinter M.M., Mann D.L., et al. Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial. JAMA. 2018;320:1764–1773. doi: 10.1001/jama.2018.14852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Armstrong P.W., Lam C.S.P., Anstrom K.J., Ezekowitz J., Hernandez A.F., O'Connor C.M., Pieske B., Ponikowski P., Shah S.J., Solomon S.D., et al. Effect of Vericiguat vs Placebo on Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The VITALITY-HFpEF Randomized Clinical Trial. JAMA. 2020;324:1512–1521. doi: 10.1001/jama.2020.15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Redfield M.M., Chen H.H., Borlaug B.A., Semigran M.J., Lee K.L., Lewis G., LeWinter M.M., Rouleau J.L., Bull D.A., Mann D.L., et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kalra S. Sodium Glucose Co-Transporter-2 (SGLT2) Inhibitors: A Review of Their Basic and Clinical Pharmacology. Diabetes Ther. 2014;5:355–366. doi: 10.1007/s13300-014-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Joshi S.S., Singh T., Newby D.E., Singh J. Sodium-glucose co-transporter 2 inhibitor therapy: Mechanisms of action in heart failure. Heart. 2021;107:1032–1038. doi: 10.1136/heartjnl-2020-318060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nassif M.E., Windsor S.L., Borlaug B.A., Kitzman D.W., Shah S.J., Tang F., Khariton Y., Malik A.O., Khumri T., Umpierrez G., et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat. Med. 2021;27:1954–1960. doi: 10.1038/s41591-021-01536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Anker S.D., Butler J., Filippatos G., Ferreira J.P., Bocchi E., Bohm M., Brunner-La Rocca H.P., Choi D.J., Chopra V., Chuquiure-Valenzuela E., et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 185.Nassif M.E., Windsor S.L., Tang F., Khariton Y., Husain M., Inzucchi S.E., McGuire D.K., Pitt B., Scirica B.M., Austin B., et al. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients With Heart Failure With Reduced Ejection Fraction: The DEFINE-HF Trial. Circulation. 2019;140:1463–1476. doi: 10.1161/CIRCULATIONAHA.119.042929. [DOI] [PubMed] [Google Scholar]
- 186.McMurray J.J.V., Solomon S.D., Inzucchi S.E., Kober L., Kosiborod M.N., Martinez F.A., Ponikowski P., Sabatine M.S., Anand I.S., Belohlavek J., et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 187.Lopaschuk G.D., Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020;5:632–644. doi: 10.1016/j.jacbts.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Waddingham M.T., Sonobe T., Tsuchimochi H., Edgley A.J., Sukumaran V., Chen Y.C., Hansra S.S., Schwenke D.O., Umetani K., Aoyama K., et al. Diastolic dysfunction is initiated by cardiomyocyte impairment ahead of endothelial dysfunction due to increased oxidative stress and inflammation in an experimental prediabetes model. J. Mol. Cell Cardiol. 2019;137:119–131. doi: 10.1016/j.yjmcc.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 189.Li C., Zhang J., Xue M., Li X., Han F., Liu X., Xu L., Lu Y., Cheng Y., Li T., et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019;18:15. doi: 10.1186/s12933-019-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xue M., Li T., Wang Y., Chang Y., Cheng Y., Lu Y., Liu X., Xu L., Li X., Yu X., et al. Empagliflozin prevents cardiomyopathy via sGC-cGMP-PKG pathway in type 2 diabetes mice. Clin. Sci. 2019;133:1705–1720. doi: 10.1042/CS20190585. [DOI] [PubMed] [Google Scholar]
- 191.Hasan R., Lasker S., Hasan A., Zerin F., Zamila M., Chowdhury F.I., Nayan S.I., Rahman M.M., Khan F., Subhan N., et al. Canagliflozin attenuates isoprenaline-induced cardiac oxidative stress by stimulating multiple antioxidant and anti-inflammatory signaling pathways. Sci. Rep. 2020;10:14459. doi: 10.1038/s41598-020-71449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kondo H., Akoumianakis I., Badi I., Akawi N., Kotanidis C.P., Polkinghorne M., Stadiotti I., Sommariva E., Antonopoulos A.S., Carena M.C., et al. Effects of canagliflozin on human myocardial redox signalling: Clinical implications. Eur. Heart J. 2021;42:4947–4960. doi: 10.1093/eurheartj/ehab420. [DOI] [PMC free article] [PubMed] [Google Scholar]