Abstract
Poor water solubility and low chemical stability, seriously limit the efficient bioavailability of resveratrol. Here, we propose encapsulating resveratrol in lecithin-polysaccharide self-assembled microspheres (LPSM). An LPSM was designed with a lecithin core, and alginate-carboxymethyl chitosan biolayer shell. The LPSM had a spherical shape with 12.171 ± 0.960 μm of particle size and −30.86 ± 1.37 mV of zeta potential. The introduce of lecithin remarkably increased the encapsulation efficiency of resveratrol to 92.78 ± 0.82%. The LPSM elevated the antioxidant capacity and ultraviolet resistance of resveratrol. Moreover, LPSM inhibited release in a simulated gastric environment, promoted sustained release in simulated intestinal environment, and elevated the bioavailability of resveratrol during in vitro simulated digestion. Results indicate that LPSM is promising as a carrier for resveratrol delivery to enhance stability and bioaccessibility.
Keywords: resveratrol, encapsulation, soybean lecithin, polysaccharide, microspheres, stability
1. Introduction
Resveratrol (trans-3,5,4′-trihydroxy-stilbene) is a polyphenolic compound present in mulberries, peanuts, and grapes, among others [1,2,3]. The potential benefits of resveratrol for health have been widely proven by numerous studies based on in vitro and in vivo investigations, including anti-obesity [2], anti-inflammatory [3], anti-carcinogenic [4], heart-protective [5] and brain benefit effects [6]. It is regarded as a multifunctional bioactive agent in food and pharmaceuticals with considerable interest. Nevertheless, its poor water solubility, low chemical stability and bioavailability, seriously limit the application of resveratrol. The intrinsic resveratrol compound is slightly soluble in water, only 0.021–0.030 mg mL−1, impeding the incorporation of resveratrol into aqueous-based food [7]. More importantly, resveratrol is prone to chemical degradation due to exogenous environment stress stimuli, including temperature, ultraviolet light, pH, and enzymes [8,9]. Resveratrol as food antioxidant or food additive also tends to undergo rapid and extensive degradation during ingestion, reducing its bioavailability and bioaccessibility [10].
Encapsulation of food-grade resveratrol has gained increasing attention to overcome the challenges mentioned above [11]. Application of bioactive compound loading with encapsulating agents allows the formation of a capsule that protects the core from degradation induced by external environment stimuli [12]. Biological molecules, including proteins, polysaccharides, and lipids, are used as food-grade carriers for the fabrication of capsules [13]. Leena et al. [14] developed electro-spinning zein nanofibers with an encapsulation efficiency for resveratrol up to 96.9%, effectively protecting resveratrol when exposed to simulated gastric fluid. Jayan et al. [15] used an electro-spraying technique to encapsulate resveratrol in zein for the improvement of bioavailability. Sanna et al. [16] prepared novel cationic chitosan-coated and anionic alginate-coated poly (D,L-latctide-co-glycolide) nanoparticles loaded with resveratrol, which provided significant protection against degradation and transisoformation caused by light exposure. The electro-spinning encapsulation of resveratrol into fibers provided localized delivery and improved the apoptotic effect on K562 cancer cells [17].
Lipid-based encapsulation, including emulsion, solid lipid nanoparticles, and liposomes has unique advantages compared to the protein-based and polysaccharide-based encapsulation, resulted from higher solubilities of resveratrol. Bryla et al. [18] reported soybean lecithin exhibited great stability for the formation of liposomes. Huang et al. [19] used liposome encapsulation as a strategy for the delivery of resveratrol with improved encapsulation efficiency. A lipid-core nanocapsule containing resveratrol was developed by Coradini et al. [20] for elevated photostability and controlled release. Balanc et al. [21] developed a novel resveratrol delivery system based on alginate-sucrose and alginate-chitosan microbeads containing liposomes, focusing on the composition of the polysaccharide layer. However, it is still unknown how the existence of the lipid core influenced the delivery characteristics of polysaccharide-encapsulated resveratrol.
In the present study, a lipid core was introduced into polysaccharide self-assembled microspheres (PSM) to develop lecithin-polysaccharide self-assembled microspheres (LPSM, Figure 1) for improvement of resveratrol delivery properties. Subsequently, scanning electron microscopy and Fourier infrared spectroscopy were used to analyze the structure of microspheres. Then, the particle size, zeta potential, and encapsulation efficiency (EE) were measured. Antioxidant capability, ultraviolet resistance, and in vitro release were determined to evaluate the effectiveness of the two encapsulation methods. The purpose of the present study was to develop a promising method to enhance the stability and bioaccessibility of resveratrol.
Figure 1.
Preparation of polysaccharide self-assembled microspheres (PSM) and lecithin-polysaccharide self-assembled microspheres (LPSM). Alg, alginate; SL, soybean lecithin; CMCS, carboxymethyl chitosan.
2. Materials and Methods
Preparation of microspheres. All experimental operations were performed in dark conditions and carried out on three independent batches as three biological replications. Resveratrol (0.05 g, 99%, bought from Aladdin, pre-dissolved in 1 mL ethanol) was dissolved in deionized water to 100 mL. An equal volume of 3% sodium alginate (analytical reagent, bought from Aladdin) solution was added, and stirred for 15 min, then solid microspheres were collected in the collection solution, containing 0.5% carboxymethyl chitosan (CMCS, biochemical regent, bought from Aladdin) and 2% calcium chloride (analytical reagent, bought from Aladdin) for 10 min. Polysaccharide self-assembled microspheres (PSM) were obtained after freezing under vacuum for 24 h. Soybean lecithin (2 g, 98%, bought from Aladdin) and 0.05 g of resveratrol (pre-dissolved in 1 mL ethanol) were dissolved in deionized water to 100 mL and stirred at the speed of 800 rpm for 50 min. Then, the liposome suspension was added into an equal volume of 3% sodium alginate solution and stirred for 15 min. Solid microspheres were collected in a collection solution containing 0.5% CMCS and 2% calcium chloride, for 10 min. Lecithin-polysaccharide self-assembled microspheres (LPSM) were obtained after freezing under vacuum for 24 h.
Fourier transform infrared spectroscopy (FTIR) analysis. FTIR was used to measure structural changes of PSM and LPSM. FTIR spectra were recorded using an FT-IR spectrophotometer (FTS 135, Bio-Rad, Hercules, CA, USA) at a resolution of 2 cm−1 with a scanning wavelength of 4000–400 cm−1.
Scanning electron microscope (SEM) images. The morphology of the PSM and LPSM were examined by SEM (SEM, S-4800, Hitachi, Tokyo, Japan). Freeze-dried powders glued in two squares on round metal. Vacuum coating was carried out in a vacuum instrument with 2 kV to form a gold film of about 10 nm, then the samples were examined by SEM.
Particle size and potential of microspheres. According to the methods of Chaudhari et al. [22], the freeze-dried microsphere samples were diluted with water to a certain concentration and a multi-angle particle size analyzer was used to determine nanoparticle size and zeta potential.
Encapsulation efficiency (EE). Encapsulation efficiency was determined by the extraction of total resveratrol and free resveratrol. A quantity of 10 mg of nanoparticles loaded with resveratrol was dissolved in 10 mL of 0.01 mol L−1 sodium citrate solution and then vortexed vigorously for 10 min at ambient temperature. After centrifugation at 10,000 rpm for 10 min, the supernatant was taken to measure the content of total resveratrol. The resveratrol was determined by high-performance liquid chromatography (HPLC, Agilent technologies 1290 infinity) using a Welch ultimate LP-C18 (4.6 × 250 mm). A 1% formic acid was used as mobile phase A and acetonitrile was used as mobile phase B for gradient elution. The linear gradient program was: 0–56 min, 3–30% B in A; 56–79 min, 24–67% B in A; 79–81 min, 67–100% B in A; 81–85 min, 100–0% B in A. Resveratrol was quantified based on standard curves (y = 17.628x − 5.7081, R2 = 0.9999) at 280 nm. Each experiment was carried out in triplicate. The encapsulation efficiency of resveratrol was calculated as follows:
| EE = (Total amount of resveratrol-free resveratrol)/(Total amount of resveratrol) × 100% | (1) |
2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH) radical scavenging activity. DPPH radical scavenging activities of free resveratrol (FR), PSM, and LPSM were measured according to Huang et al. [23] with a slight modification. To determine the antioxidant activity of DPPH of embedded resveratrol microspheres, three solutions were prepared. A volume of 2 mL of 0.2 mmol L−1 DPPH (dissolved in ethanol) was mixed with a 2 mL sample and then placed in the dark at 37 ± 0.5 °C for 30 min to obtain a sample at 517 nm. The control was prepared by replacing the sample with 2 mL of anhydrous ethanol. A blank sample was prepared by replacing the DPPH with 2 mL anhydrous ethanol. Each experiment was carried out in triplicate. DPPH scavenging was calculated as follows:
| DPPH scavenging = (1 − (Asample − Acontrol)/Ablank) × 100% | (2) |
where Asample stands for the absorbance of the sample at 517 nm, Acontrol stands for the absorbance of sample at 517 nm, and Ablank stands for the absorbance of sample at 517 nm
Ultraviolet (UV) resistance. The retention rates of resveratrol in different forms were determined to evaluate UV resistance. The FR, PSM, and LPSM were placed under UV light for 48 h. The resveratrol content was determined every day. Each experiment was carried out in triplicate. The retention rate was calculated as follows:
| Retention rate = Ct/C0 × 100% | (3) |
where C0 stands for the initial content of resveratrol, and Ct is defined as the content of resveratrol at time t.
In vitro release studies. In vitro release studies of PSM and LPSM were carried out to simulate digestion and absorption in the stomach and intestine according to Huang et al. [18] with slight modifications. To prepare the simulated gastric fluid (SGF), 3.2 g L−1 of pepsin was prepared in physiological saline, and the pH of the solution was adjusted to 1.2 using hydrochloric acid. The PSM and LPSM samples in SGF were placed in a thermostatic shaker at 37 ± 0.5 °C, with a continuous shaking at 100 rpm for 2 h. Then, the samples were transferred into simulated intestinal fluid (SIF), which contained 10 g L−1 trypsin in phosphate buffer solution (pH 7.4) at 37 ± 0.5 °C, with continuous shaking at 100 rpm for 3 h. At predetermined time intervals (1, 2, 3, 4 and 5 h), the suspensions were centrifuged, and the supernatant was taken to determine the resveratrol concentration by HPLC. The resveratrol was quantified by the areas of peaks based on standard curves. Each experiment was carried out in triplicate. The release rate was calculated as follows.
| (4) |
where RRt stands for the release rate of resveratrol from microspheres, Ct is the concentration of resveratrol at time t, Ct−1 is the concentration of resveratrol at time t − 1, V0 is the total volume of buffer solution, and V is the volume of supernatant taken out.
Bioaccessibility was recorded as the final release rate in SIF.
Statistics analysis. The determinations of particle size, Zeta potential, encapsulation efficiency, antioxidant capability, retention rate in response to ultraviolet, and release rate of resveratrol in vitro were carried out in three technical replications. Analysis of variance was conducted using the Duncan test with SPSS software (Version 19.0). All results are presented as means ± standard deviations with significant difference denoted by letters.
3. Results and Discussion
3.1. Morphology of Resveratrol Microspheres
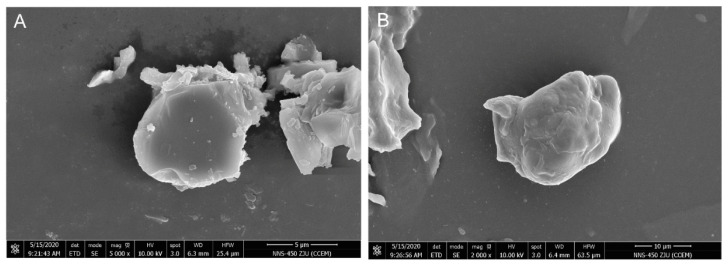
Structures of encapsulated resveratrol microspheres are shown in Figure 2. Both PSM and LPSM encapsulations exhibited spherical shapes, which were well suited for embedding resveratrol. Ahmad et al. [24] reported that resveratrol micrographs showed a needle-like structure. In the present study, spherical structures, instead of a resveratrol-like structure, indicated the successful preparation of the two kinds of self-assembled microspheres.
Figure 2.
Scanning electron microscopy (SEM) images of polysaccharide self-assembled microspheres (PSM, (A)), and lecithin-polysaccharide self-assembled microspheres (LPSM, (B)).
3.2. FTIR Analysis
The FTIR spectra of PSM and LPSM loaded resveratrol were shown in Figure 3, and the FTIR spectra of microspheres without resveratrol were shown in Figures S1 and S2. O-H stretching at wavenumbers ranging from 3300 to 3500 cm −1 probably represent the hydroxyl group of polysaccharides and the phenolic hydroxyl group of resveratrol. The peaks at 1587.02 cm−1, 1512.37 cm−1, and 1443.64 cm−1 in PSM, 1597.40 cm−1, 1517.77 cm−1, and 1417.62 cm−1 are the characteristic absorption peaks of the aromatic skeletons in resveratrol, indicating the successful encapsulation of resveratrol. The peaks at 1153.57 cm−1 of PSM and 1144.06 cm−1 represent the C-O-C stretching of polysaccharides.
Figure 3.
Fourier Transform Infrared (FTIR) spectra of resveratrol loaded in polysaccharide self-assembled microspheres (PSM, (A)) and lecithin-polysaccharide self-assembled microspheres (LPSM, (B)).
Compared to PSM, the peaks at 3010.34 cm−1, 2923.08 cm−1 and 2852.87 cm−1 of LPSM reflect CH3 anti-symmetric stretching, CH3 symmetrical stretching, and CH2 symmetrical stretching, respectively, indicating the presence of soy lecithin (Figure 3B). Due to the addition of soy lecithin, resveratrol was isolated from alginate and carboxymethyl chitosan. The peaks of PSM at the wavenumbers from 1250 cm−1 to 1400 cm−1 might result from the C-N stretching and CH2 swing influenced by encapsulated resveratrol (Figure 3A).
3.3. Particle Size and Zeta Potential
The stability of PSM and LPSM microspheres was tested by assessing particle size and zeta potential. As shown in Table 1, the particle sizes of PSM and LPSM microspheres were 6.422 ± 0.721 μm and 12.171 ± 0.960 μm, respectively. This result is in line with that shown in the SEM image. Ahmad et al. [24] prepared nano-encapsulated resveratrol particles with starch from horse chestnut, lotus stem, and water chestnut, and the particle sizes were found to be 419, 797, and 691 nm, respectively. The increase from PSM to LPSM in particle size may be due to the layer-by-layer assembly interaction of polysaccharides and lecithin. The greater size of microspheres might add to the existing soybean lecithin layer, thickening the microspheres.
Table 1.
Particle size and Zeta potential of microspheres.
| Sample | Particle Size (μm) | Zeta Potential (mV) |
Encapsulation Efficiency (%) |
|---|---|---|---|
| PSM | 6.422 ± 0.721 b | −21.18 ± 1.05 b | 65.45 ± 0.43 b |
| LPSM | 12.171 ± 0.960 a | −30.86 ± 1.37 a | 92.78 ± 0.82 a |
PSM, polysaccharide self-assembled microspheres; LPSM, lecithin-polysaccharide self-assembled microspheres. The different letters indicate significant differences.
In the present study, the Zeta potentials of PSM and LPSM, indicating particle stability, were found to be negative, −21.18 ± 1.05 mV and −30.86 ± 1.37 mV, respectively. The negative charge of microspheres could be due to the presence of the carboxyl group of carboxymethyl chitosan. The electrostatic repulsion forces created by the surface charge could decrease the Van der Waals force between particles, avoiding the agglomeration of particles to form bigger particles [11,25]. The higher the zeta potential value, the more difficult for the particles to aggregate [25]. In the present study, it was evident from the higher zeta potential that the stable properties and resistance to aggregation were better maintained by the LPSM compared to PSM. This result may be due to the negative charge of soybean lecithin [18].
3.4. Encapsulation Efficiency
EE has been widely used to characterize the encapsulation efficiency of the guest molecule. As shown in Table 1, the EE values for PSM and LPSM were 65.45 ± 0.43% and 92.78 ± 0.82%, respectively, and LPSM exhibited significantly greater encapsulation efficiency than PSM (The HPLC chromatograms were shown in Figures S2–S7). The difference of EE between LPSM and PSM was probably attributed to the lipophicity of resveratrol. The addition of lecithin promoted the formulation of liposomes, enhanced the solubility of resveratrol, and promoted its encapsulation [23].
3.5. Antioxidant Activity and UV Resistance
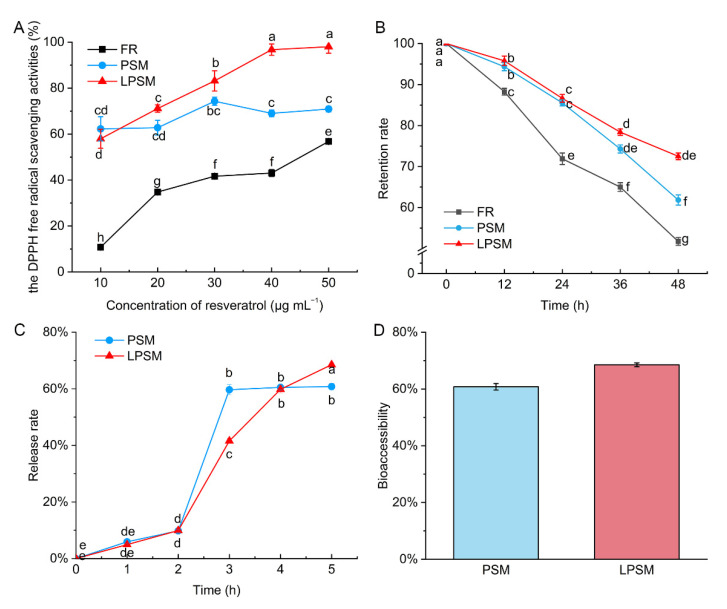
The DPPH radical scavenging method has been used to evaluate the antioxidant activity of various food systems [18]. To compare the difference between the antioxidant activity of free resveratrol and encapsulated resveratrol, DPPH free radical scavenging activities were determined among free resveratrol, PSM, and LPSM. Figure 4A shows an overview of the changes in antioxidant capability in the different forms. The DPPH free radical scavenging activities of free resveratrol increased with increased concentration. However, no good linear relationship was found between antioxidant capability and resveratrol concentration, which could result from the oxidation of free resveratrol. The DPPH free radical scavenging activities of PSM particles increased gradually with increased resveratrol concentration and rose to a maximum of 30 μg mL−1 and peaked at 74.27 ± 1.80%. The antioxidant capability of LPSM particles showed a steady increase with increased resveratrol concentration from 57.94 ± 4.19% at 10 μg mL−1 to 98.00 ± 2.82% at 50 μg mL−1 of resveratrol concentration. The resveratrol loaded in LPSM exhibited higher antioxidant activity compared to free resveratrol, indicating that the encapsulation of resveratrol by lecithin could enhance its stability. Similar results were found by Song et al. [26] in that chitosan coating elevated free radical scavenging activity. Increased antioxidant capacity by encapsulation may result from the many -OH and -NH2 groups in chitosan [27].
Figure 4.
The antioxidant capability (A), retention rate (B) in response to ultraviolet, release rate in vitro (C) and bioaccessibility (D) of resveratrol in different forms. The first two hours were in simulated in gastric fluid, and the latter three hours in simulated intestinal fluid.FR, free resveratrol; PSM, polysaccharide self-assembled microspheres; LPSM, lecithin-polysaccharide self-assembled microspheres. Different letters indicate significant difference.
It is well-known that resveratrol is prone to chemical degradation in response to UV radiation [8,9]. In the present study, we found the encapsulation of PSM and LPSM improved the UV resistance of resveratrol. After exposure to UV for 48 h, LPSM exhibited the highest retention rate, at 72.50 ± 0.82%, followed by PSM at 61.83 ± 1.24%, and free resveratrol at 51.73 ± 0.94% (Figure 4B). The introduction of a lipid core into polysaccharide self-assembled microspheres significantly improved the stability of resveratrol under UV light.
3.6. Release of Resveratrol In Vitro
Upon sequential exposure in SGF and SIF, an increasing amount of resveratrol was detected in both PSM and LPSM (Figure 4C). During the time exposed to SGF, the recovered resveratrol increased at a low speed in both microspheres. At the end of digestion in SGF, the release rate in vitro of resveratrol in PSM was 9.79 ± 0.53%, and that of LPSM was 9.92 ± 0.3636%. These results suggest that the PSM and LPSM were relatively stable in SGF. When it came to SIF, the release of resveratrol in vitro exhibited completely different behaviors in PSM and LPSM. The recovered resveratrol from PSM increased sharply and reached 59.67 ± 1.76% after digestion in SIF for an hour, and afterwards was steady. However, the release rate in vitro from LPSM increased gradually, indicating a sustained release of resveratrol from LPSM in SIF. Finally, the resveratrol loaded in LPSM exhibited a higher bioaccessibility at 68.52 ± 0.68%. The slower release rate could be due to the presence of soybean lecithin, which requires an emulsification process during the degradation of LPSM in SIF. Leena et al. [14] applied an electrospinning approach to prepare resveratrol-loaded zein nanofibers for improved bioaccessibility of resveratrol. Only 33.8% of encapsulated resveratrol from the zein nanofibers was released in gastric conditions. When introduced to intestinal conditions, the resveratrol was released rapidly (up to 56.1% within 0.3 h). This release profile is similar to that of PSM in the present study. Vankayala et al. [28] studied the in-vitro release of free resveratrol and resveratrol-niosomes with non-ionic surfactants and fatty alcohol as stabilizers. The results showed the release rate was slowed by nano-encapsulation, but different rates were no observed between the gastric and intestinal conditions. Release behavior of resveratrol from the genipin cross-linked chitosan microsphere was observed [29]. The release of resveratrol was faster in an acidic medium owing to the faster swelling of chitosan and the physicochemical properties of resveratrol. The difference of resveratrol release from encapsulation in the studies mentioned above and the present study was probably caused by the degradability of the wall materials. The lipid core introduced to the polysaccharide self-assembled microspheres resulted in the inhibited release in SGF and the sustainable release in SIF. This approach for delivery could be used in other lipophilic nutraceuticals in the food industry, and to promote absorption in the intestine.
4. Conclusions
In the present study, lecithin-polysaccharide self-assembled microspheres (LPSM) were successfully developed for the encapsulation and delivery of resveratrol. Alginate and carboxymethyl chitosan were used in the formulation of multiple polysaccharide layer structures, and soybean lecithin was used as an inner layer of microspheres. Compared with PSM, the introduction of a lipid core in LPSM significantly improved the encapsulation efficiency of resveratrol and increased the zeta potential. The resveratrol loaded in LPSM exhibited elevated antioxidant capacity and improved stability under ultraviolet light. Both microspheres showed inhibited resveratrol release in simulated gastric digestion, while LPSM exhibited slower release of resveratrol in simulated intestinal fluid and had higher bioaccessibiliy. In conclusion, LPSM is a promising delivery system for resveratrol.
Acknowledgments
We would like to thank the Bio-ultrastructure Analysis Lab of Zhejiang University for their technical support with SEM.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11091666/s1, Figure S1: Fourier Transform Infrared (FTIR) spectra of polysaccharide self-assembled microspheres without resveratrol; Figure S2: Fourier Transform Infrared (FTIR) spectra of leci-thin-polysaccharide self-assembled microspheres without resveratrol; Figure S3: The High-performance liquid chromatography (HPLC) chromatogram of resveratrol standard; Figure S4: High-performance liquid chromatography (HPLC) chromatogram of polysaccharide self-assembled microspheres loaded resveratrol; Figure S5: High-performance liquid chroma-tography (HPLC) chromatogram of lecithin-polysaccharide self-assembled microspheres loaded resveratrol; Figure S6: High-performance liquid chromatography (HPLC) chromatogram of polysaccharide self-assembled microspheres without resveratrol; Figure S7: High-performance liquid chromatography (HPLC) chromatogram of lecithin-polysaccharide self-assembled mi-crospheres without resveratrol.
Author Contributions
L.W.: Conceptualization, formal analysis, writing—original draft. D.L.: data curation, formal analysis, writing—original draft. C.L.: formal analysis. Z.L.: conceptualization, supervision, funding acquisition. L.L. (Lingling Liu) and Y.J.: methodology, formal analysis. Y.J.: supervision, funding acquisition. L.L. (Li Li): conceptualization, writing—review & editing, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data are contained within this article.
Conflicts of Interest
Yunbin Jiang is the R&D director of Tianjin Gasin-DH Preservation Technologies Co., Ltd. The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundations of China (32072271) and the Key Research and Development Program of Zhejiang Province (2021C02015).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ramawat K.G., Merillon J.M., editors. Bioactive Molecules and Medicinal Plants. Springer; Berlin/Heidelberg, Germany: 2008. Biological Effects; pp. 25–54. [Google Scholar]
- 2.Fernandez A., Milton-Laskibar I., Gonzalez M., Portillo M.P. Antiobesity Effects of Resveratrol: Which Tissues Are Involved? Ann. N. Y. Acad. Sci. 2017;1403:118–131. doi: 10.1111/nyas.13413. [DOI] [PubMed] [Google Scholar]
- 3.Meng T., Xiao D., Muhammed A., Deng J., Chen L., He J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules. 2021;26:229. doi: 10.3390/molecules26010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu D., He B., Lin L., Malhotra A., Yuan N. Potential of Curcumin and Resveratrol as Biochemical and Biophysical Modulators during Lung Cancer in Rats. Drug Chem. Toxicol. 2019;42:328–334. doi: 10.1080/01480545.2018.1523921. [DOI] [PubMed] [Google Scholar]
- 5.Sun Z.-M., Guan P., Luo L.-F., Qin L.-Y., Wang N., Zhao Y.-S., Ji E.-S. Resveratrol Protects against CIH-Induced Myocardial Injury by Targeting Nrf2 and Blocking NLRP3 Inflammasome Activation. Life Sci. 2020;245:117362. doi: 10.1016/j.lfs.2020.117362. [DOI] [PubMed] [Google Scholar]
- 6.Villaflores O.B., Chen Y.-J., Chen C.-P., Yeh J.-M., Wu T.-Y. Curcuminoids and Resveratrol as Anti-Alzheimer Agents. Taiwan J. Obstet. Gynecol. 2012;51:515–525. doi: 10.1016/j.tjog.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Davidov-Pardo G., McClements D.J. Resveratrol Encapsulation: Designing Delivery Systems to Overcome Solubility, Stability and Bioavailability Issues. Trends Food Sci. Technol. 2014;38:88–103. doi: 10.1016/j.tifs.2014.05.003. [DOI] [Google Scholar]
- 8.Allan K.E., Lenehan C.E., Ellis A.V. UV Light Stability of Alpha-Cyclodextrin/Resveratrol Host-Guest Complexes and Isomer Stability at Varying PH. Aust. J. Chem. 2009;62:921–926. doi: 10.1071/CH08506. [DOI] [Google Scholar]
- 9.Pinto M.D., Garcia-Barrado J.A., Macias P. Oxidation of Resveratrol Catalyzed by Soybean Lipoxygenase. J. Agric. Food Chem. 2003;51:1653–1657. doi: 10.1021/jf025818d. [DOI] [PubMed] [Google Scholar]
- 10.Patel K.R., Scott E., Brown V.A., Gescher A.J., Steward W.P., Brown K. Clinical Trials of Resveratrol. Ann. N. Y. Acad. Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadi Z., Mohammadinejad R., Ashrafizadeh M. Drug Delivery Systems for Resveratrol, a Non-Flavonoid Polyphenol: Emerging Evidence in Last Decades. J. Drug Deliv. Sci. Technol. 2019;51:591–604. doi: 10.1016/j.jddst.2019.03.017. [DOI] [Google Scholar]
- 12.Carvalho I.T., Estevinho B.N., Santos L. Application of Microencapsulated Essential Oils in Cosmetic and Personal Healthcare—A Review. Int. J. Cosmetic Sci. 2016;38:109–119. doi: 10.1111/ics.12232. [DOI] [PubMed] [Google Scholar]
- 13.Özkan G., Bilek S.E. Microencapsulation of Natural Food Colourants. Int. J. Food Sci. Nutr. 2014;3:145. doi: 10.11648/j.ijnfs.20140303.13. [DOI] [Google Scholar]
- 14.Leena M.M., Yoha K.S., Moses J.A., Anandharamakrishnan C. Edible Coating with Resveratrol Loaded Electrospun Zein Nanofibers with Enhanced Bioaccessibility. Food Biosci. 2020;36:100669. doi: 10.1016/j.fbio.2020.100669. [DOI] [Google Scholar]
- 15.Jayan H., Leena M.M., Sundari S.K.S., Moses J.A., Anandharamakrishnan C. Improvement of Bioavailability for Resveratrol through Encapsulation in Zein Using Electrospraying Technique. J. Funct. Food. 2019;57:417–424. doi: 10.1016/j.jff.2019.04.007. [DOI] [Google Scholar]
- 16.Sanna V., Roggio A.M., Siliani S., Piccinini M., Marceddu S., Mariani A., Sechi M. Development of Novel Cationic Chitosan- and Anionic Alginate-Coated Poly(D,L-Lactide-Co-Glycolide) Nanoparticles for Controlled Release and Light Protection of Resveratrol. Int. J. Nanomed. 2012;7:5501–5516. doi: 10.2147/IJN.S36684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Attar T., Madihally S. Influence of Controlled Release of Resveratrol from Electrospun Fibers in Combination with SiRNA on Leukemia Cells. Eur. J. Pharm. Sci. 2018;123:173–183. doi: 10.1016/j.ejps.2018.07.043. [DOI] [PubMed] [Google Scholar]
- 18.Bryla A., Lewandowicz G., Juzwa W. Encapsulation of Elderberry Extract into Phospholipid Nanoparticles. J. Food Eng. 2015;167:189–195. doi: 10.1016/j.jfoodeng.2015.07.025. [DOI] [Google Scholar]
- 19.Huang H., Belwal T., Aalim H., Li L., Lin X., Liu S., Ma C., Li Q., Zou Y., Luo Z. Protein-Polysaccharide Complex Coated W/O/W Emulsion as Secondary Microcapsule for Hydrophilic Arbutin and Hydrophobic Coumaric Acid. Food Chem. 2019;300:125171. doi: 10.1016/j.foodchem.2019.125171. [DOI] [PubMed] [Google Scholar]
- 20.Coradini K., Lima F.O., Oliveira C.M., Chaves P.S., Athayde M.L., Carvalho L.M., Beck R.C.R. Co-Encapsulation of Resveratrol and Curcumin in Lipid-Core Nanocapsules Improves Their in Vitro Antioxidant Effects. Eur. J. Pharm. Biopharm. 2014;88:178–185. doi: 10.1016/j.ejpb.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Balanc B., Trifkovic K., Dordevic V., Markovic S., Pjanovic R., Nedovic V., Bugarski B. Novel Resveratrol Delivery Systems Based on Alginate-Sucrose and Alginate-Chitosan Microbeads Containing Liposomes. Food Hydrocoll. 2016;61:832–842. doi: 10.1016/j.foodhyd.2016.07.005. [DOI] [Google Scholar]
- 22.Chaudhari K.R., Ukawala M., Manjappa A.S., Kumar A., Mundada P.K., Mishra A.K., Mathur R., Monkkonen J., Murthy R.S.R. Opsonization, Biodistribution, Cellular Uptake and Apoptosis Study of PEGylated PBCA Nanoparticle as Potential Drug Delivery Carrier. Pharm. Res. 2012;29:53–68. doi: 10.1007/s11095-011-0510-x. [DOI] [PubMed] [Google Scholar]
- 23.Huang M., Liang C., Tan C., Huang S., Ying R., Wang Y., Wang Z., Zhang Y. Liposome Co-Encapsulation as a Strategy for the Delivery of Curcumin and Resveratrol. Food Funct. 2019;10:6447–6458. doi: 10.1039/C9FO01338E. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad M., Gani A. Ultrasonicated Resveratrol Loaded Starch Nanocapsules: Characterization, Bioactivity and Release Behaviour under in-Vitro Digestion. Carbohyd. Polym. 2021;251:117111. doi: 10.1016/j.carbpol.2020.117111. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer B., Hecht M., Harting J., Nirschl H. Agglomeration and Filtration of Colloidal Suspensions with DVLO Interactions in Simulation and Experiment. J. Colloid Interface Sci. 2010;349:186–195. doi: 10.1016/j.jcis.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 26.Song J., Zong J., Ma C., Chen S., Li H., Zhang D. Microparticle Prepared by Chitosan Coating on the Extruded Mixture of Corn Starch, Resveratrol, and Alpha-Amylase Controlled the Resveratrol Release. Int. J. Biol. Macromol. 2021;185:773–781. doi: 10.1016/j.ijbiomac.2021.06.154. [DOI] [PubMed] [Google Scholar]
- 27.Pu S., Li J., Sun L., Zhong L., Ma Q. An in Vitro Comparison of the Antioxidant Activities of Chitosan and Green Synthesized Gold Nanoparticles. Carbohyd.Polym. 2019;211:161–172. doi: 10.1016/j.carbpol.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Vankayala J.S., Battula S.N., Kandasamy R., Mariya G.A., Franklin M.E.E., Pushpadass H.A., Naik L.N. Surfactants and Fatty Alcohol Based Novel Nanovesicles for Resveratrol: Process Optimization, Characterization and Evaluation of Functional Properties in RAW 264.7 Macrophage Cells. J. Mol. Liq. 2018;261:387–396. doi: 10.1016/j.molliq.2018.04.058. [DOI] [Google Scholar]
- 29.Zhang Y., Yu Y., Shi X., Zhao S., Chen A., Huang D., Niu D., Qin Z. Study on the Preparation of Genipin Crosslinked Chitosan Microspheres of Resveratrol and in Vitro Release. J. Polym. Res. 2013;20:175. doi: 10.1007/s10965-013-0175-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are contained within this article.