Abstract
Our previous study found that dietary nucleotide supplementation, including adenosine 5ʹ-monophosphate (AMP), could increase AMP content in sow milk and promote piglet growth, but its effects on placental efficiency and piglet vitality remain unknown. This experiment aimed to investigate the effects of dietary AMP or its metabolite adenosine (ADO) supplementation on sow reproductive performance and placental angiogenesis. A total of 135 sows with a similar farrowing time were blocked by backfat and body weight (BW) at day 65 of gestation and assigned to one of three dietary treatment groups (n = 45 per treatment): basal diet, basal diet supplemented with 0.1% AMP or 0.1% ADO, respectively. Placental analysis and the characteristics of sows and piglets unveiled that compared with control (CON) group, AMP or ADO supplementation could improve sow placental efficiency (P < 0.05) and newborn piglet vitality (P < 0.05), increase piglet birth weight (P < 0.05), and reduce stillbirth rate (P < 0.05). More importantly, AMP or ADO supplementation could increase the contents of AMP, ADO, and their metabolites in placentae (P < 0.05). Meanwhile, AMP or ADO supplementation could also increase placental vascular density (P < 0.05) and the expression of vascular endothelial growth factor A (P < 0.05), as well as promote the migration and tube formation of porcine iliac artery endothelial cells (P < 0.05). Overall, maternal dietary AMP or ADO supplementation could increase their contents in the placenta, thereby improving placental angiogenesis and neonatal piglet vitality.
Keywords: adenosine 5ʹ-monophosphate, adenosine, angiogenesis, piglet vitality, placenta
The present findings may offer insights into new strategies for regulating sow reproductive performance and piglet growth, highlighting the potential application of AMP in sow production.
Introduction
The increase in litter size leads to a decrease in piglet vitality and growth (Rooney et al., 2020), and low piglet vitality and birth weight can have adverse effects on piglet growth and development, including postnatal survival, weaning weight, and reproductive performance (Magnabosco et al., 2016; Vanden Hole et al., 2018; Hu et al., 2020), which was shown to be associated with competition for nutrients in the limited space of the womb (Town et al., 2005; Foxcroft et al., 2006). All the nutrients and energy needed for fetal growth and development before birth come from the mother, and the placenta is the only medium for the fetus to absorb nutrients from the mother and release metabolic waste (Macpherson et al., 2017; Hu et al., 2019). Therefore, placental vascular development is a key factor determining the reproductive performance of piglets, such as piglet vitality and placental efficiency.
Studies have shown that adenosine (ADO) could significantly promote biological and pathological angiogenesis (Troncoso et al., 2020; Valls et al., 2021), and ADO is mainly generated by phosphorylation of adenosine 5ʹ-Monophosphate (AMP) in response to 5ʹ-nucleotide enzymes (Chen et al., 2013). In our previous study, AMP supplementation was found to increase AMP content in sow milk and promote suckling piglet growth, but its effect on piglet vitality and placental efficiency remains unclear (Tan et al., 2021). In addition, in late gestation, maternal metabolism can be disturbed to varying degrees, thereby affecting the intake of nutrients by the fetus. Uric acid is an end product of purine metabolism that mediates a variety of severe pregnancy-related diseases and poor pregnancy outcomes (Paula et al., 2019), and elevated uric acid levels are considered as the key diagnostic criterion for preeclampsia (Martin and Brown, 2010).
In this study, the decomposition of AMP into ADO was assumed to promote placental angiogenesis and thus improve pregnancy outcomes. Therefore, this study aimed to test this hypothesis by analyzing the effects of AMP or ADO supplementation on the expression of their related metabolites and placental angiogenesis in the placenta.
Materials and Methods
Animal ethics
All procedures involving animals are carried out in accordance with the protocol approved by the Animal Ethics Committee of South China Agricultural University.
Animals, diets, and housing
This experiment was conducted in Xinxing Agriculture and Animal Husbandry Co., LTD., Wannian County, Shangrao City (Jiangxi, China). A total of 135 sows with a similar farrowing time were blocked by backfat and body weight (BW) at day 65 of gestation, and assigned to one of three dietary treatment groups (n = 45 per treatment): basal diet, basal diet supplemented with 0.1% AMP or 0.1% ADO, respectively. All diets were formulated in accordance with the National Research Council nutritional standards for gestating sows (NRC, 2012). The composition and nutrient levels of the experimental diet are shown in Table 1.
Table 1.
Ingredients and nutrient composition of experimental gestation diets (as-fed basis)
| Item | CON | ADO | AMP |
|---|---|---|---|
| Ingredient, % | |||
| Corn | 34.78 | 34.68 | 34.68 |
| Rice bran meal | 10.00 | 10.00 | 10.00 |
| Broken rice | 16.67 | 16.67 | 16.67 |
| Soybean meal (43% CP) | 14.50 | 14.50 | 14.50 |
| Triticale | 20.00 | 20.00 | 20.00 |
| adenosine (ADO) | — | 0.10 | — |
| adenosine 5ʹ-monophosphate (AMP) | — | — | 0.10 |
| Limestone | 1.10 | 1.10 | 1.10 |
| Dicalcium phosphate | 0.96 | 0.96 | 0.96 |
| Sodium chloride | 0.40 | 0.40 | 0.40 |
| Lysinesulfate (70%) | 0.10 | 0.10 | 0.10 |
| Choline chloride | 0.13 | 0.13 | 0.13 |
| Threonine | 0.05 | 0.05 | 0.05 |
| Mildewcide1 | 0.05 | 0.05 | 0.05 |
| Antiseptic2 | 0.08 | 0.08 | 0.08 |
| Premix3 | 1.18 | 1.18 | 1.18 |
| Calculated composition4, % | |||
| NE, mcal/kg | 2.39 | 2.39 | 2.39 |
| CP | 13.87 | 13.86 | 13.86 |
| EE | 2.32 | 2.32 | 2.32 |
| CF | 3.22 | 3.22 | 3.22 |
| Ca | 0.72 | 0.72 | 0.72 |
| Total P | 0.63 | 0.63 | 0.63 |
| Lys | 0.76 | 0.76 | 0.76 |
| Met | 0.24 | 0.24 | 0.24 |
| Thr | 0.56 | 0.56 | 0.56 |
| Trp | 0.16 | 0.16 | 0.16 |
Mildewcide contains 99.5% potassium propionate.
Antiseptic contains 99% sodium diacetate.
Provided the following per kilogram of diet: VA, 12000 IU; VD3, 4800 IU; VC, 200 mg; VE, 205 mg; VK, 3.6 mg; VB1, 3.6 mg; VB2, 12 mg; VB 6, 7.2 mg; VB12, 0.048 mg; pantothenic acid, 30.0 mg; nicotinic acid, 48.0 mg; folic acid, 8.6 mg; biotin, 0.6 mg; Cu, 10.0 mg; Fe, 130 mg; Zn, 60 mg; Mn, 45 mg; I, 0.3 mg; Co, 0.1 mg.Premix is provided by Xinxing Agriculture and Animal Husbandry Co., LTD., Wannian County, Shangrao City (Jiangxi, China).
Calculated chemical concentrations using values for feed ingredients from (NRC, 2012). Amino acid levels in diets are expressed as totals.
Sows were housed in individual stalls and fed once (05:30) per day in late gestation at constant feeding 2.0 kg. At day 15 before parturition, the feeding amount was gradually increased to 2.5 kg. At day 7 before parturition, sows were moved from the gestation compartment to the farrowing room. The ventilation system kept the temperature at 20–25 °C in the birthing room and the sows had free access to water throughout the experiment.
Reproductive performance measurement and sample collection
After delivery, the number and weight of newborn piglets, live piglets, stillborn piglets, and mummified fetuses were recorded. Meanwhile, the piglet vitality was recorded as previously reported (König et al., 2021), i.e., piglets were scored according to their respiratory status and motor ability within 15 s after birth. Placental efficiency was calculated by dividing the birth weight of piglets by placental weight. Piglets with birth weight <800 g were defined or recorded as born weak piglets. Uniformity of newborns was determined by intralitter coefficient of variation, which was calculated by dividing average birth weight by standard deviation within the litter. Based on a previously reported method (Huang et al., 2021a), during sow delivery, the umbilical cord of newborn piglets was tied with a short cotton thread and tagged with a numbered label to ensure the matching of each piglet with the corresponding placenta. After the placenta was discharged and weighed, part of the placenta (n = 6 per group) was collected and put into liquid nitrogen for quick freezing, and the remaining fresh placental tissue was immediately fixed with 4% paraformaldehyde. The blood samples of sows (n = 6 per group) were collected in 10-mL centrifuge tubes from the ear vein of the fasted sows at farrowing, followed by centrifugation at 3,000 × g and 4 °C for 15 min to recover the serum, and then storing the samples at −80 °C until further analysis.
Placental vascular density
The placental tissue embedded in paraffin was cut into 5-μm thick sections, followed by hematoxylin-eosin staining. Areas covering the placental tissue and the placental vessels were tracked using a projection microscope (Olympus CX41, Japan), followed by quantification of the placental blood vessel area through Image J software (National Institutes of Health, Bethesda, MD) to determine the relative number of placental blood vessels per unit tissue area.
Wound healing assay
Briefly, Porcine iliac artery endothelial cells (PIECs) were seeded onto a 6-well plate and cultured overnight until the formation of a confluent monolayer, followed by making a scratch wound with a 200-μL pipette tip, and measuring the effects of AMP and ADO on scratch healing at 24 h post the scratch wound treatment. The images of wound surface were collected with an Olympus inverted microscope at 0 and 24 h and quantified by Image J software. In Supplementary Figure S1, 10 μM AMP was seen to significantly improve PIECs activity, so this concentration was selected for the test. Meanwhile,100 μM ADO was also selected for the test based on the significant effect of this concentration on PIECs viability (unpublished data in our laboratory).
Tube formation assay
A total of 1.5 × 104 PIECs were inoculated into a 96-well plate coated with 50 μL Matrigel (Becton, Dickinson and Company, USA) and cultured in serum-free medium or conditioned medium at 37 °C for 6 h, followed by collecting the images with an Olympus inverted microscope (100×) and analyzing the tube formation results by Image J software.
Quantitative real-time PCR
Total RNA from placental tissues were extracted with the reagent box of Tissue RNA Purification Kit (EZBioscience, Suzhou, China) following the manufacturer’s instructions. The concentration of RNA was quantified using a NanoDrop-2000 (Thermo Fisher, USA). After reverse transcription using Color Reverse Transcription Kit (EZBioscience, Suzhou, China), qRT-PCR was conducted using SYBR Green on a QuantStudio 6 RealTime PCR System (Thermo Fisher, USA) under the conditions of denaturation at 95 °C for 10 min, amplification at 95 °C for 15 s and 60 °C for 1 min for 40 cycles. Using 18S ribosomal RNA as the control gene, the relative mRNA abundances were expressed as the value of ∆Ct (Hu et al., 2017), which was estimated by the equation: ∆Ct = 2−∆∆Ct (sample − control), where ∆∆Ct (sample − control) = (Ct target gene − Ct reference genes) treated − (Ct target gene − Ct reference genes) control. Primers used in this study are shown in Supplementary Table S1.
Western blotting
Total proteins were extracted from placental tissues following the manufacturer’s guide for the protein extraction kit (Beyotime, Beijing, China). Briefly, an amount of 10 μg protein was loaded and separated by SDS-PAGE gel electrophoresis, followed by transferring the proteins onto the polyvinylidene fluoride membranes (Merck Millipore). After blocking with tris buffered saline with tween 20 (TBST) containing 5% milk, the membranes were incubated with the primary antibodies against vascular endothelial growth factor A (VEGF-A) (19003-1-AP, Proteintech, USA, 1:1000) and β-actin (4970, CST, USA, 1:1000). Subsequently, the membranes were incubated with appropriate HRP-conjugated anti-rabbit IgG secondary antibody (AS014, Abclonal, China, 1:5000). Images were captured using the ChemiDoc MP system (Bio-Rad, Hercules, CA), and band densities were quantified using Image Lab soft-ware (Bio-Rad, Hercules, CA) and then normalized to β-actin content.
Analysis of AMP metabolites in placenta by liquid chromatography mass spectrometry (LC–MS)
After thawing, the samples were homogenized by vortexing for 2 min, followed by mixing 200 μL of each sample with 800 μL 50% methanol in a centrifuge tube under vortexing for 2 min, and then centrifugation for 15 min to collect the supernatant. Next, 1000-μL supernatant was blown dry with a nitrogen blower, followed by mixing the sample with 200-μL 50% methanol in a centrifuge tube under vortexing for 2 min, centrifugation for 15 min, filtrating the supernatant through a 0.22-μm organic filter membrane and placing the filtrate in a vial with lined cap for LC-MS analysis. Meanwhile, the standard was diluted to different gradients and placed separately in the sample bottle. Standard substances are AMP (Solarbio, SA8060), ADO (Solarbio, SA9610), adenine (Sigma, PHR1383-2G), SAH (Sigma, A9384-25MG), xanthine (Sigma, X0626-5G), hypoxanthine (Sigma, H9377-1G), inosine (Sigma, I4125-1G), and uric acid (Sigma, U0881-10G). The metabolite levels in the standard and samples were detected by LC-MS (Thermo Fisher, USA), and then the metabolite content in the samples was calculated quantitatively by Xcalibur (Thermo Fisher, USA).
Statistical analyses
Except for rates of stillbirth, mummified fetuses, weak piglets, and piglet vitality, analysis of farrowing performance was performed using the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC). For sow and litter data, the sow or the litter represented the experimental unit. For farrowing performance, the model included treatment, days of pregnancy, and BW of sows at day 109 of gestation. The PDIFF option of SAS adjusted to TUKEY comparisons test was used to detect significant differences between groups when necessary. The rates of stillbirth, mummified fetuses, and weak piglets were calculated using chi square. Data from six duplicate placental samples and four duplicate cell samples from each treatment group were analyzed using SAS for one-way ANOVA. Results are presented with bar charts using the GraphPad Prism (GraphPad Software Inc, San Diego, CA) and each bar represents mean ± SEM. Differences between mean values were considered statistically significant at P < 0.05, with a trend toward significance at 0.05 ≤ P ≤ 0.10.
Results
Effects of ADO and AMP supplementation on sow reproductive performance and piglet growth
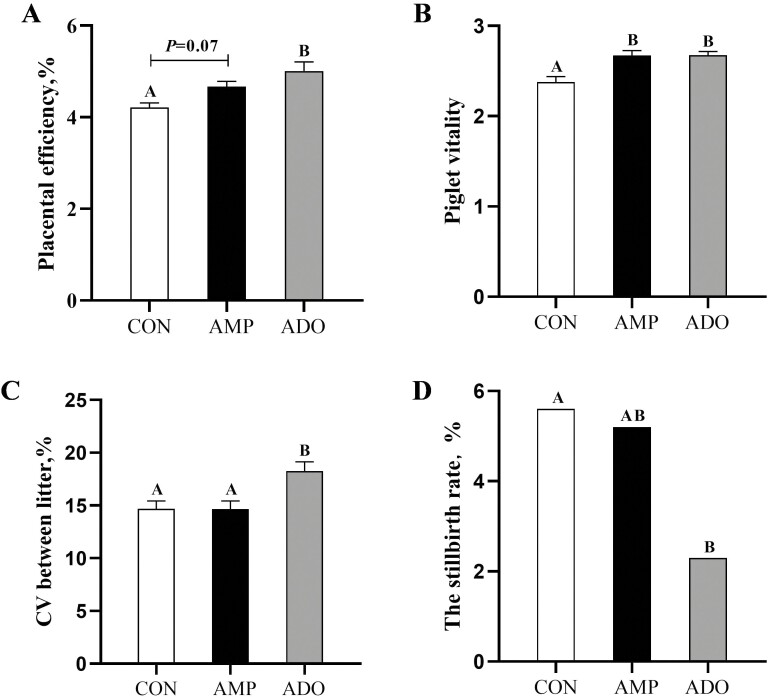
In Figure 1A and B, compared with CON group, AMP group was seen to have an uptrend in placental efficiency of sows (P = 0.07) and was significantly higher in newborn piglet vitality (P < 0.05), whereas ADO group was significantly higher in both sow placental efficiency and piglet vitality (P < 0.05). Additionally, compared with CON group, ADO group showed a significant increase in piglet birth weight (P < 0.05) (Table 2), coupled with an increase in the coefficient of variation between litters (P < 0.05) (Figure 1C) and a decrease in total stillbirth rate (P < 0.05) (Figure 1D).
Figure 1.
Effects of AMP and ADO supplementation on reproductive performance of sows. (A) Placental efficiency. (B) Piglet vitality. (C) Coefficient of variation between litters. (D) Stillbirth rate. All data are presented as the mean ± SEM. Each sow or litter was regarded as an experimental unit (n = 44, 45, 43). A–B, columns with different large letters mean significant differences at P < 0.05.
Table 2.
Effects of dietary AMP or ADO on reproductive performance of sows1
| Item | CON | ADO | AMP | P |
|---|---|---|---|---|
| No. of sows2 | 44 | 45 | 43 | |
| No. of total born piglets/litter | 10.8 ± 3.2 | 9.9 ± 1.7 | 10.8 ± 2.1 | 0.10 |
| No. of live-born piglets/litter | 10.0 ± 2.7 | 9.6 ± 1.8 | 10.2 ± 1.9 | 0.39 |
| Birth weight of litter, kg | 13.8 ± 3.7 | 14.3 ± 3.1 | 14.5 ± 2.3 | 0.51 |
| Birth weight of piglets, kg | 1.4 ± 0.2A | 1.5 ± 0.2B | 1.4 ± 0.2AB | <0.05 |
| Placental weight, kg | 3.2 ± 0.7 | 3.0 ± 0.8 | 3.2 ± 0.5 | 0.21 |
| Rate of mummified fetus3, % | 1.3 (6) | 0.5 (2) | 0.7 (3) | 0.32 |
| Rate of weak piglets3, % | 0.7 (3) | 0.9 (4) | 2.3 (10) | 0.08 |
| No. of IUGR piglets/litter4 | 1.3 ± 1.2 | 0.6 ± 1.5 | 1.0 ± 1.6 | 0.09 |
Data are presented as means ± SD. Statistical significance was determined by MIXED procedure of SAS followed by TUKEY comparisons test.
During the experimental period, we eliminated the sows with serious lameness or reproductive failure such as non-pregnant in late gestation.
Rates of mummified fetuses, and weak piglets were calculated using chi square. Piglets with birth weight < 800 g were defined or recorded as born weak piglets.
Intrauterine growth retardation (IUGR) was defined as birth weight below 2 standard deviations of average birthweight.
A–B, mean values with different large letters in the same row differ significantly at P < 0.05.
Effects of AMP and ADO supplementation on contents of AMP, ADO, and their metabolites in placental tissue
In Figure 2, AMP group was seen to be significantly higher than CON group in the contents of AMP, adenine, hypoxanthine, and inosine (P <0.05). Meanwhile, ADO group was significantly higher than CON group in the levels of AMP, ADO, adenine, S-Adenosine-L-Homocysteine (SAH), hypoxanthine, and inosine (P < 0.05). In contrast, CON group was significantly higher than ADO and AMP groups in the contents of uric acid and xanthine metabolites (P < 0.05).
Figure 2.
Effects of AMP and ADO supplementation on concentrations of AMP, ADO, and their downstream metabolites in placental tissue (A–H): ADO (A), Adenine (B), AMP (C), SAH (D), Xanthine (E), Hypoxanthine (F), Inosine (G), and Uric Acid (H). All data are presented the mean ± SEM. Each placenta tissue was regarded as an experimental unit (n = 6 per group) A–B, columns with different large letters mean significant differences at P < 0.05.
Effects of ADO and AMP supplementation on blood vessel density and expression of mRNA and protein of angiogenesis-related factors in placentae
In Figure 3A and B, H&E staining results showed that the density of placental vessels was significantly higher in AMP and ADO groups than in CON group (P < 0.05). In Figure 3C, q-PCR results showed that placental growth factor (PIGF) mRNA expression was significantly higher in AMP group than in CON group (P < 0.05), and the VEGF-A mRNA expression was significantly higher in ADO group than in CON group (P < 0.05). Meanwhile, ADO and AMP groups were significantly higher than CON group in the mRNA expression of vascular endothelial growth factor D (VEGF-D) (P < 0.05) (Figure 3C). In Figure 3D, Western blot results indicated that VEGF-A protein expression was significantly higher in AMP and ADO groups than in CON group (P < 0.05).
Figure 3.
Effects of AMP and ADO supplementation on placental angiogenesis. Each placenta tissue was regarded as an experimental unit (n = 6 per group). (A and B) Hematoxylin and eosin staining analysis of blood vessel density in CON, ADO, and AMP placental tissues, with black arrows indicating placental blood vessels. (C–E) Real-time PCR and Western blot analysis of mRNA and protein expression of angiogenesis-related factors in placentae. All data are presented as mean ± SEM. A–B, columns with different large letters mean significant differences at P < 0.05.
Effects of ADO and AMP supplementation on PIECs tube formation and migration in vitro
The effect of AMP and ADO on angiogenesis was evaluated by in vitro PIECs culture. In Figure 4A–D, AMP and ADO treatments were shown to significantly improve PIECs migration and pipe-forming capacity when compared with CON group (P < 0.05). In Figure 4E–G, Western blot results revealed that ADO and AMP groups were significantly higher than CON group in the protein expression levels of VEGF-A (P < 0.05).
Figure 4.
Effects of AMP and ADO supplementation on PIECs tube formation and migration. (A and B) Representative images of tube formation by PIECs. (C and D) Wound healing assay in each group. (E–G) Western blot analysis of VEGF-A protein expression in PIECs. All data are presented as mean ± SEM. Each Petri dish with cells is considered as one experimental unit (n = 4 per group). A–B, columns with different large letters mean significant differences at P < 0.05.
Discussion
Studies have shown that late trimester is a critical window for fetal growth and development (Huang et al., 2021a), and placental angiogenesis plays a supporting role in fetal growth and development (Hu et al., 2021; Huang et al., 2021b). AMP dephosphorylation was reported to produce ADO (Chen et al., 2013), which can promote blood vessels in tumors and retinal tissues, but whether it plays the same role in placental angiogenesis remains unclear. In pigs, the incidence of low piglet birth weight and stillbirth has been reported to be very high (Kraeling and Webel, 2015). In this study, we investigated whether dietary AMP or ADO supplementation can improve pregnancy outcomes by detecting the levels of AMP metabolites and placental angiogenic factors in sows during late pregnancy. Results showed that AMP or ADO supplementation could significantly increase placental efficiency and piglet vitality, suggesting the great potential of AMP or ADO supplementation to improve pregnancy outcomes, including increasing piglet birth weight and reducing stillbirth rate, by upregulating specific AMP metabolites, down-regulating uric acid levels, and promoting placental angiogenesis.
The placenta provides the interface between maternal and fetal circulations, allowing the efficient delivery of nutrients and oxygen from the mother to the fetus to ensure normal fetal growth (Sun et al., 2020). Therefore, placental vascular development is a key factor influencing the reproductive performance, such as placental efficiency and piglet birth weight (Gualdoni et al., 2022; Tan et al., 2022). To investigate whether AMP or ADO supplementation affects placental development during pregnancy, we examined the levels of AMP metabolites in the placentae of different treatment groups. Our results showed that AMP or ADO supplementation could significantly increase several AMP metabolites in placentae when compared with CON group. Note that after AMP supplementation, high levels of ADO could be detected in the placenta, confirming that AMP plays a role in the placenta after decomposition into ADO. Meanwhile, our results showed that CON group showed higher levels in uric acid and xanthine, which was consistent with a previous report that elevated uric acid levels are established markers of the pathological mechanisms of many gestation diseases (Maiuolo et al., 2016). Uric acid is formed from xanthine catalyzed by xanthine oxidase (Costa et al., 2002; Hayden and Tyagi, 2004; Sui et al., 2008), and it was reported as a common symptom of pre-eclamptic pregnancy (Powers et al., 2006). Normal placental vascular structure and transport function are responsible for transporting oxygen and nutrients to the developing fetus. However, uric acid can damage the vascular system of adults (Johnson et al., 2003) and produce a profound negative effect on the proliferation and migration of endothelial cells, inhibiting the proliferation of serum-induced human umbilical vein endothelial cells (HUVEC) by 50% and blocking the migration of HUVEC by 75% (Kang et al., 2005).
In this study, we examined placental angiogenesis in different treatment groups. Compared to CON group, AMP and ADO groups had a significant increase in placental vascular density, which is consistent with previous reports that ADO plays an important role in the in vivo model of angiogenesis, and in some cases, ADO can contribute as much as 50% to 70% of angiogenic response (Adair, 2005; Desai et al., 2005). In early studies, ADO was found to be produced in hypoxic tissues, thereby causing blood vessels to dilate and increase oxygen supply (Berne, 1963; Berne et al., 1983). In our study, AMP and ADO treatments were found to increase the expression level of vascular endothelial growth factor (VEGF), resulting a significant increase in the level of VEGF-A. This was consistent with several previous studies. Specifically, ADO could stimulate endothelial cells (ECs) to release basic fibroblast growth factor and VEGF, while inhibiting the production of thrombospondin-1, an anti-angiogenic factor (Feoktistov et al., 2002; Adair, 2005). Additionally, ADO could also stimulate mast cells to produce VEGF, interleukin-8, and angiopoietin-1 (Feoktistov et al., 2003).
ADO has a direct mitogenic effect on vascular cells, contributing to angiogenesis (Auchampach, 2007), with ECs proliferation and migration as key steps in angiogenesis (Adolfsson, 1986; Cronstein, 2004). Consistent with previous studies, the present study found that AMP and ADO treatments could significantly enhance the proliferation and migration ability of PIECs and increase the expression level of VEGF-A in PIECs. Extracellular ADO was reported to stimulate parenchymal cells to release VEGF-A by binding VEGF-A to its receptor to stimulate the proliferation and migration of vascular endothelial cells (Lutty et al., 1998; Grant et al., 2001; Dubey et al., 2002). ADO was also reported to stimulate endothelial cell proliferation independently of VEGF-A, which may involve the regulation of other pro-angiogenic and anti-angiogenic growth factors, as well as intracellular mechanisms (Meininger et al., 1988; Sexl et al., 1997). Overall, our results indicated that AMP or ADO supplementation can promote placental angiogenesis during pregnancy and improve adverse pregnancy outcomes.
Conclusion
Dietary supplementation of sows with 0.1% AMP or ADO during pregnancy can promote placental angiogenesis, thereby improving pregnancy outcomes, with an increase in piglet vitality and birth weight and a decrease in stillbirth rate.
Supplementary Material
Acknowledgments
The present work was supported by the National Key R&D Program of China (2021YFD1300700), Natural Science Foundation of Guangdong Province (2021A1515012116), and Natural Science Foundation of Guangzhou City (202102080090).
Glossary
Abbreviations
- ADO
adenosine
- AMP
adenosine 5ʹ-monophosphate
- BW
body weight
- CON
control
- ECs
endothelial cells
- HUVEC
human umbilical vein endothelial cells
- IUGR
intrauterine growth retardation
- LC-MS
liquid chromatography mass spectrometry
- NRC
National Research Council
- PIECs
porcine iliac artery endothelial cells
- PIGF
placental growth factor
- SAH
S-adenosine-L-Homocysteine
- TBST
tris buffered saline with tween 20
- VEGF
vascular endothelial growth factor
- VEGF-A
vascular endothelial growth factor A
- VEGF-D
vascular endothelial growth factor D
Contributor Information
Deyuan Wu, Guangdong Provincial Key Laboratory of Animal Nutrition Control, National Engineering Research Center for Breeding Swine Industry, Institute of Subtropical Animal Nutrition and Feed, College of Animal Science, South China Agricultural University, Guangzhou, Guangdong 510642, China.
Li Feng, Guangdong Provincial Key Laboratory of Animal Nutrition Control, National Engineering Research Center for Breeding Swine Industry, Institute of Subtropical Animal Nutrition and Feed, College of Animal Science, South China Agricultural University, Guangzhou, Guangdong 510642, China.
Xiangyu Hao, Guangdong Provincial Key Laboratory of Animal Nutrition Control, National Engineering Research Center for Breeding Swine Industry, Institute of Subtropical Animal Nutrition and Feed, College of Animal Science, South China Agricultural University, Guangzhou, Guangdong 510642, China.
Shuangbo Huang, Guangdong Provincial Key Laboratory of Animal Nutrition Control, National Engineering Research Center for Breeding Swine Industry, Institute of Subtropical Animal Nutrition and Feed, College of Animal Science, South China Agricultural University, Guangzhou, Guangdong 510642, China.
Zifang Wu, Guangdong Provincial Key Laboratory of Animal Nutrition Control, National Engineering Research Center for Breeding Swine Industry, Institute of Subtropical Animal Nutrition and Feed, College of Animal Science, South China Agricultural University, Guangzhou, Guangdong 510642, China.
Shuo Ma, Guangdong Provincial Key Laboratory of Animal Nutrition Control, National Engineering Research Center for Breeding Swine Industry, Institute of Subtropical Animal Nutrition and Feed, College of Animal Science, South China Agricultural University, Guangzhou, Guangdong 510642, China.
Yulong Yin, Guangdong Provincial Key Laboratory of Animal Nutrition Control, National Engineering Research Center for Breeding Swine Industry, Institute of Subtropical Animal Nutrition and Feed, College of Animal Science, South China Agricultural University, Guangzhou, Guangdong 510642, China; National Engineering Laboratory for Pollution Control and Waste Utilization in Livestock and Poultry Production, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, Hunan 410125, China.
Chengquan Tan, Guangdong Provincial Key Laboratory of Animal Nutrition Control, National Engineering Research Center for Breeding Swine Industry, Institute of Subtropical Animal Nutrition and Feed, College of Animal Science, South China Agricultural University, Guangzhou, Guangdong 510642, China.
Conflict of Interest Statement
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Literature Cited
- Adair, T. H. 2005. Growth regulation of the vascular system: an emerging role for adenosine. Am. J. Physiol-Reg. I. 289:R283–R296. doi: 10.1152/ajpregu.00840.2004. [DOI] [PubMed] [Google Scholar]
- Adolfsson, J. 1986. The time dependence of training-induced increase in skeletal muscle capillarization and the spatial capillary to fibre relationship in normal and neovascularized skeletal muscle of rats. Acta Physiol. Scand. 128:259–266. doi: 10.1111/j.1748-1716.1986.tb07974.x. [DOI] [PubMed] [Google Scholar]
- Auchampach, J. A. 2007. Adenosine receptors and angiogenesis. Circ. Res. 101:1075–1077. doi: 10.1161/CIRCRESAHA.107.165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne, R. M. 1963. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am. J. Physiol. 204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Berne, R. M., Knabb R. M., Ely S. W., and Rubio R.. . 1983. Adenosine in the local regulation of blood flow: a brief overview. Fed. Proc. 42:3136–3142. https://www.webofscience.com/wos/alldb/full-record/MEDLINE:6641949. [PubMed] [Google Scholar]
- Chen, J. F., Eltzschig H. K., and Fredholm B. B.. . 2013. Adenosine receptors as drug targets—what are the challenges? Nat. Rev. Drug Discov. 12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, A., Igualá I., Bedini J., Quintó L., and Conget I.. . 2002. Uric acid concentration in subjects at risk of type 2 diabetes mellitus: relationship to components of the metabolic syndrome. Metabolism 51:372–375. doi: 10.1053/meta.2002.30523. [DOI] [PubMed] [Google Scholar]
- Cronstein, B. N. 2004. Adenosine receptors and wound healing. Sci. World J. 4:1–8. doi: 10.1100/tsw.2004.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, A., Victor-Vega C., Gadangi S., Montesinos M. C., Chu C. C., and Cronstein B. N.. . 2005. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol. Pharmacol. 67:1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- Dubey, R. K., Gillespie D. G., and Jackson E. K.. . 2002. A(2B) adenosine receptors stimulate growth of porcine and rat arterial endothelial cells. Hypertension 39:530–535. doi: 10.1161/hy0202.103075. [DOI] [PubMed] [Google Scholar]
- Feoktistov, I., Goldstein A. E., Ryzhov S., Zeng D., Belardinelli L., Voyno-Yasenetskaya T., and Biaggioni I.. . 2002. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ. Res. 90:531–538. doi: 10.1161/01.res.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- Feoktistov, I., Ryzhov S., Goldstein A. E., and Biaggioni I.. . 2003. Mast cell-mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ. Res. 92:485–492. doi: 10.1161/01.Res.0000061572.10929.2d. [DOI] [PubMed] [Google Scholar]
- Foxcroft, G. R., Dixon W. T., Novak S., Putman C. T., Town S. C., and Vinsky M. D.. . 2006. The biological basis for prenatal programming of postnatal performance in pigs. J. Anim. Sci. 84(Suppl_13): E105–E112. doi: 10.2527/2006.8413_supple105x. [DOI] [PubMed] [Google Scholar]
- Grant, M. B., Davis M. I., Caballero S., Feoktistov I., Biaggioni I., and Belardinelli L.. . 2001. Proliferation, migration, and ERK activation in human retinal endothelial cells through A(2B) adenosine receptor stimulation. Invest. Ophthalmol. Vis. Sci. 42:2068–2073. https://www.webofscience.com/wos/alldb/full-record/MEDLINE:11481274. [PubMed] [Google Scholar]
- Gualdoni, G. S., Jacobo P. V., Barril C., Ventureira M. R., and Cebral E.. . 2022. Early abnormal placentation and evidence of vascular endothelial growth factor system dysregulation at the feto-maternal interface after periconceptional alcohol consumption. Front. Physiol. 12:815760. doi: 10.3389/fphys.2021.815760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden, M. R., and Tyagi S. C.. . 2004. Uric acid: a new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: the urate redox shuttle. Nutr. Metab. (Lond). 1:10. doi: 10.1186/1743-7075-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, C. J., Jiang Q. Y., Zhang T., Yin Y. L., Li F. N., Su J. Y., Wu G. Y., and Kong X. F.. . 2017. Dietary supplementation with arginine and glutamic acid enhances key lipogenic gene expression in growing pigs. J. Anim. Sci. 95:5507–5515. doi: 10.2527/jas2017.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, C., Wu Z., Huang Z., Hao X., Wang S., Deng J., Yin Y., and Tan C.. . 2021. Nox2 impairs VEGF-A-induced angiogenesis in placenta via mitochondrial ROS-STAT3 pathway. Redox Biol. 45:102051. doi: 10.1016/j.redox.2021.102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, C., Yang Y., Deng M., Yang L., Shu G., Jiang Q., Zhang S., Li X., Yin Y., Tan C., . et al. 2020. Placentae for low birth weight piglets are vulnerable to oxidative stress, mitochondrial dysfunction, and impaired angiogenesis. Oxid. Med. Cell Longev. 2020:8715412. doi: 10.1155/2020/8715412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, C., Yang Y., Li J., Wang H., Cheng C., Yang L., Li Q., Deng J., Liang Z., Yin Y., . et al. 2019. Maternal diet-induced obesity compromises oxidative stress status and angiogenesis in the porcine placenta by upregulating nox2 expression. Oxid. Med. Cell Longev. 2019:2481592. doi: 10.1155/2019/2481592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Z., Huang S., Song T., Yin Y., and Tan C.. . 2021b. Placental angiogenesis in mammals: a review of the regulatory effects of signaling pathways and functional nutrients. Adv. Nutr. 12:2415–2434. doi: 10.1093/advances/nmab070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S., Wu Z., Huang Z., Hao X., Zhang L., Hu C., Wei J., Deng J., and Tan C.. . 2021a. Maternal supply of cysteamine alleviates oxidative stress and enhances angiogenesis in porcine placenta. J. Anim. Sci. Biotechnol. 12:91. doi: 10.1186/s40104-021-00609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. J., Kang D. H., Feig D., Kivlighn S., Kanellis J., Watanabe S., Tuttle K. R., Rodriguez-Iturbe B., Herrera-Acosta J., and Mazzali M.. . 2003. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 41:1183–1190. doi: 10.1161/01.Hyp.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- Kang, D. H., Park S. K., Lee I. K., and Johnson R. J.. . 2005. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 16:3553–3562. doi: 10.1681/asn.2005050572. [DOI] [PubMed] [Google Scholar]
- König, N. L., Wähner M., Seeger J., Sigmarsson H. L., and Kauffold J.. . 2021. An investigation into uterine capacity based on litter and placental characteristics in two sow lines with different prolificacy (Danish Landrace x Danish Yorkshire versus German Saddleback). Reprod. Domest. Anim. 56:34–45. doi: 10.1111/rda.13847. [DOI] [PubMed] [Google Scholar]
- Kraeling, R. R., and Webel S. K.. . 2015. Current strategies for reproductive management of gilts and sows in North America. J. Anim. Sci. Biotechnol. 6:3. doi: 10.1186/2049-1891-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutty, G. A., Mathews M. K., Merges C., and McLeod D. S.. . 1998. Adenosine stimulates canine retinal microvascular endothelial cell migration and tube formation. Curr. Eye Res. 17:594–607. doi: 10.1076/ceyr.17.6.594.5173. [DOI] [PubMed] [Google Scholar]
- Macpherson, A. J., de Aguero M. G., and Ganal-Vonarburg S. C.. . 2017. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 17:508–517. doi: 10.1038/nri.2017.58. [DOI] [PubMed] [Google Scholar]
- Magnabosco, D., Bernardi M. L., Wentz I., Cunha E. C. P., and Bortolozzo F. P.. . 2016. Low birth weight affects lifetime productive performance and longevity of female swine. Livest. Sci. 184:119–125. doi: 10.1016/j.livsci.2015.12.008. [DOI] [Google Scholar]
- Maiuolo, J., Oppedisano F., Gratteri S., Muscoli C., and Mollace V.. . 2016. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- Martin, A. C., and Brown M. A.. . 2010. Could uric acid have a pathogenic role in pre-eclampsia? Nat. Rev. Nephrol. 6:744–748. doi: 10.1038/nrneph.2010.125. [DOI] [PubMed] [Google Scholar]
- Meininger, C. J., Schelling M. E., and Granger H. J.. . 1988. Adenosine and hypoxia stimulate proliferation and migration of endothelial cells. Am. J. Physiol. 255:H554–H562. doi: 10.1152/ajpheart.1988.255.3.H554. [DOI] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. Washington (DC): National Academy Press. [Google Scholar]
- Paula, L. G., Pinheiro da Costa B. E., Hentschke M. R., Antonello I. C., Luz J. H., da Cunha Filho E. V., and Poli-de-Figueiredo C. E.. . 2019. Increased proteinuria and uric acid levels are associated with eclamptic crisis. Pregnancy Hypertens. 15:93–97. doi: 10.1016/j.preghy.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Powers, R. W., Bodnar L. M., Ness R. B., Cooper K. M., Gallaher M. J., Frank M. P., Daftary A. R., and Roberts J. M.. . 2006. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. Am. J. Obstet. Gynecol. 194:160. doi: 10.1016/j.ajog.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Rooney, H. B., O’Driscoll K., O’Doherty J V., and Lawlor P. G.. . 2020. Effect of increasing dietary energy density during late gestation and lactation on sow performance, piglet vitality, and lifetime growth of offspring. J. Anim. Sci. 98(1). doi: 10.1093/jas/skz379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexl, V., Mancusi G., Höller C., Gloria-Maercker E., Schütz W., and Freissmuth M.. . 1997. Stimulation of the mitogen-activated protein kinase via the A2A-adenosine receptor in primary human endothelial cells. J. Biol. Chem. 272:5792–5799. doi: 10.1074/jbc.272.9.5792. [DOI] [PubMed] [Google Scholar]
- Sui, X., Church T. S., Meriwether R. A., Lobelo F., and Blair S. N.. . 2008. Uric acid and the development of metabolic syndrome in women and men. Metabolism 57:845–852. doi: 10.1016/j.metabol.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, C., Groom K. M., Oyston C., Chamley L. W., Clark A. R., and James J. L.. . 2020. The placenta in fetal growth restriction: what is going wrong? Placenta 96:10–18. doi: 10.1016/j.placenta.2020.05.003. [DOI] [PubMed] [Google Scholar]
- Tan, C., Huang Z., Xiong W., Ye H., Deng J., and Yin Y.. . 2022. A review of the amino acid metabolism in placental function response to fetal loss and low birth weight in pigs. J. Anim. Sci. Biotechnol. 13:28. doi: 10.1186/s40104-022-00676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, C., Ji Y., Zhao X., Xin Z., Li J., Huang S., Cui Z., Wen L., Liu C., Kim S. W., . et al. 2021. Effects of dietary supplementation of nucleotides from late gestation to lactation on the performance and oxidative stress status of sows and their offspring. Anim. Nutr. 7:111–118. doi: 10.1016/j.aninu.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town, S. C., Patterson J. L., Pereira C. Z., Gourley G., and Foxcroft G. R.. . 2005. Embryonic and fetal development in a commercial dam-line genotype. Anim. Reprod. Sci. 85:301–316. doi: 10.1016/j.anireprosci.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Troncoso, F., Herlitz K., Acurio J., Aguayo C., Guevara K., Castro F. O., Godoy A. S., San Martin S., and Escudero C.. . 2020. Advantages in wound healing process in female mice require upregulation A2A-mediated angiogenesis under the stimulation of 17beta-estradiol. Int. J. Mol. Sci. 21(19). doi: 10.3390/ijms21197145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls, M. D., Soldado M., Arasa J., Perez-Aso M., Williams A. J., Cronstein B. N., Noguera M. A., Terencio M. C., and Montesinos M. C.. . 2021. Annexin A2-mediated plasminogen activation in endothelial cells contributes to the proangiogenic effect of adenosine A2A receptors. Front. Pharmacol. 12:654104. doi: 10.3389/fphar.2021.654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Hole, C., Aerts P., Prims S., Ayuso M., Van Cruchten S., and Van Ginneken C.. . 2018. Does intrauterine crowding affect locomotor development? A comparative study of motor performance, neuromotor maturation and gait variability among piglets that differ in birth weight and vitality. PLoS One 13:e0195961. doi: 10.1371/journal.pone.0195961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.