Abstract
Background:
The impact of donor colonization or infection with multidrug-resistant organisms (MDROs) on solid organ transplant (SOT) recipient outcomes remains uncertain. We thus evaluated the association between donor MDROs and risk of posttransplant infection, graft failure, and mortality.
Methods:
A multicenter retrospective cohort study was performed. All SOT recipients with a local deceased donor were included. The cohort was divided into three exposure groups: recipients whose donors had (1) an MDRO, (2) a non-MDRO bacterial or candidal organism, or (3) no growth on cultures. The primary outcomes were (1) bacterial or invasive candidal infection within 3 months and (2) graft failure or death within 12 months posttransplant. Mixed effect multivariable frailty models were developed to evaluate each association.
Results:
Of 658 total SOT recipients, 93 (14%) had a donor with an MDRO, 477 (73%) had a donor with a non-MDRO organism, and 88 (13%) had a donor with no organisms on culture. On multivariable analyses, donor MDROs were associated with a significantly increased hazard of infection compared to those with negative donor cultures (adjust hazard ratio [aHR] 1.63, 95% CI 1.01–2.62, p = .04) but were not associated with graft failure or death (aHR 0.45, 95% CI 0.15–1.36, p = .16).
Conclusions:
MDROs on donor culture increase the risk of early posttransplant infection but do not appear to affect long-term graft or recipient survival, suggesting organ donors with MDROs on culture maybe safely utilized. Future studies aimed at reducing early posttransplant infections associated with donor MDROs are needed.
Keywords: bacteria, donor-derived infection, multidrug-resistant organisms
1 ∣. INTRODUCTION
There is significant concern about the use of donor organs that may be infected or colonized with multidrug-resistant organisms (MDROs), since such donors may transmit the MDRO to the solid organ transplant (SOT) recipient, causing a donor-derived infection (DDI).1 Prior case series have described poor outcomes associated with such MDRO DDIs, including recurrent posttransplant infections, graft loss, and even death.2-7
The hesitancy to use donor organs that may be infected or colonized with MDROs has significant ramifications for the organ pool. Prior work from our group has shown a notable reduction in organ utilization when MDR-Gram negative organisms are identified on donor culture.8 Further, MDROs are increasingly being observed among the deceased donor cohort. We have previously reported that approximately 15% of deceased organ donors have at least one MDRO identified on peri-procurement cultures.9 Moreover, with increasing rates of MDROs among hospitalized and critical care unit patients globally,10-12 and the ongoing opioid epidemic that has resulted in an increasing proportion of deceased organ donors having a history of injection drug use (an established risk factor for MDROs, particularly methicillin-resistant Staphylococcus aureus),13-16 it is likely that MDROs will become increasingly prevalent among deceased donors over time. It is thus imperative to determine the true risk of using organs from donors who are colonized or infected with MDROs, and if there is in fact a heightened risk, efforts to develop new strategies for mitigating this risk must be prioritized.
There are no prior studies, to our knowledge, that have systematically evaluated whether donor MDROs pose a risk for SOT recipient outcomes. Thus, in this study, we sought to determine the impact of donor MDROs on SOT recipient outcomes, including posttransplant infection, graft failure, and death.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Study design and setting
A multicenter retrospective cohort study was performed at three transplant centers in the Philadelphia region: the Hospital of the University of Pennsylvania (HUP), Temple University Hospital (TUH), and Hahnemann University Hospital (HUH).
2.2 ∣. Study population
The initial source population included all SOT recipients who received an organ from a deceased donor that was evaluated by the local organ procurement organization (OPO), the Gift of Life Donor Program (GLDP). SOT recipients were included from HUP, TUH, and HUH between January 1, 2015 and July 1, 2016. Eligible recipients were identified by the GLDP.
An SOT recipient was determined to be “exposed” if he/she had a donor who grew at least one bacterial or candidal organism on a culture taken during either the donor’s terminal hospitalization (“hospital cultures”) or at the time of organ procurement (“OPO cultures”). Donor hospital cultures included any clinical culture obtained from any anatomical site at any time point during the donor’s terminal hospitalization. Donor hospital cultures were not excluded based on timing relative to organ procurement, since 90% of the donors had a length of stay under 8 days (median length of stay was 4 days, interquartile range [IQR] 2–5 days).9 However, surveillance swab cultures (e.g., rectal, skin, nasal swabs) were excluded, since these were not uniformly performed or reported by donor hospitals. OPO cultures were obtained by the GLDP and were standardized; each donor had the same set of OPO cultures obtained regardless of which organs were procured (including blood, respiratory, urine, ureter, and perfusate fluid cultures). The only culture growth that was excluded from the exposure were those respiratory specimens that grew only routine mouth or respiratory flora, and those urinary specimens that grew only mixed urogenital flora as these were considered contaminants. Of note, we did not distinguish infection from colonization in donors with positive cultures, because (1) the clinical data in donor records are often insufficient for making this distinction accurately, (2) this distinction is not routinely made by transplant centers when evaluating an organ donor, and (3) both infection and colonization have been shown to affect recipient outcomes, including DDI.17
The exposed group was subsequently subdivided into two mutually exclusive exposure arms: (1) those whose donors had at least one MDRO on culture, and (2) those whose donors had at least one bacterial or candidal organism on culture but no MDROs. MDROs included methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus species (VRE), extended-spectrum cephalosporin-resistant (ESC-R) Enterobacterales, carbapenem-resistant Enterobacterales (CRE), MDR-Pseudomonas species, and MDR-Acinetobacter species. The Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network (NHSN) definitions for each MDRO were used (see Supporting Information Section A.).18 If an MDRO was isolated on multiple occasions in the same donor, only the first MDRO was considered.
An SOT recipient was determined to be “unexposed” if his/her donor did not grow any bacterial or candidal organisms, or grew only contaminants, on any hospital or OPO cultures.
This study was approved by the institutional review board at each of the participating transplant centers.
2.3 ∣. Outcomes
There were two primary outcomes evaluated among the SOT recipients. The first outcome was bacterial or invasive candidal infection within 3 months posttransplant. Infections were defined using CDC/NHSN surveillance criteria19 and were determined via manual chart review by an infectious diseases-trained physician (JAA). Infections at any anatomical site, due to any organism (MDRO or non-MDRO) and due to any source (donor-derived or non-donor-derived), were included. Infections were evaluated through 90 days posttransplant, since the impact of the donor on the risk of recipient infection likely occurs in this early posttransplant period.20
The second primary outcome was graft failure or death within 12 months posttransplant. Graft failure was defined by re-listing for transplant for any organ recipient or return to dialysis for kidney transplant recipients.
2.4 ∣. Data collection
Data on exposed and unexposed SOT recipients were abstracted from the electronic medical record system at each hospital. Data on each recipient’s donor were abstracted from the donor medical record maintained by the GLDP (see Supporting Information Section B for a complete list of donor and recipient data elements that were collected). The standard perioperative antimicrobial prophylaxis employed for each organ type at each center, as well as the typical approach to treating positive donor cultures, is provided in the Supporting Information Section C.
2.5 ∣. Statistical analysis
SOT recipients were characterized by baseline clinical factors, such as demographics and comorbidities. Continuous variables were compared using a one-way ANOVA or Wilcoxon rank-sum test, and categorical variables were compared using the χ2 or Fisher exact test. For the primary analyses, we performed survival analyses. Time zero was defined as the day of transplantation, and the time at risk was measured in days. For the evaluation of posttransplant infection, the day on which the SOT recipient first met criteria for a bacterial or invasive candidal infection within 3 months posttransplant was the failure date, and subjects were censored at the time of death or at the end of 3 months of follow-up (whichever occurred first). For the evaluation of posttransplant graft and patient survival, the failure date was the day that the SOT recipient met criteria for graft failure or died (whichever occurred first), and subjects were censored at 12 months of follow-up if neither component of the outcome occurred in that timeframe.
For the unadjusted analyses, a Kaplan–Meier curve was plotted, stratified by exposure status, and a log rank test was performed. For the adjusted analyses, mixed effects multivariable frailty models using the Weibull distribution were developed for each outcome, with a random effect for donor. This random effect was included in order to account for possible clustering by donor, since several SOT recipients in the cohort received organs from the same donor.
For each of the multivariable analyses, bivariable regression was used to examine the relationship between the primary exposure (donor MDRO status), as well as other baseline donor and recipient factors, and the outcome. Variables were retained in the final multivariable model if they were confounders of the primary association or had a p value of <.05 in the multivariable model. The strength of each association was measured using a hazard ratio (HR), and a 95% confidence interval (CI) was calculated for each effect estimate.
Of note, we did not adjust our analyses for antimicrobials administered to the donors or recipients peri- or posttransplant, since these antimicrobial administrations would have occurred after the exposure of interest and would thus be on the causal pathway.
All analyses were performed using STATAv.15.0 (StataCorp, College Station, Texas).
2.6 ∣. Subgroup and stratified analyses
Several prespecified subgroup analyses were performed. First, there was a concern that those donors known to be infected or colonized with MDROs prior to organ procurement would only be accepted under specific scenarios for recipients (e.g., those SOT candidates unlikely to receive another offer), and there would thus be a confounding by indication bias. To address this, we performed a subgroup analysis in which we excluded those SOT recipients whose donors had positive hospital cultures (those taken prior to organ procurement). In the resulting subgroup, the exposed SOT recipients were those whose donors had positive OPO cultures (taken during organ procurement). The results of such OPO cultures could not have been known about at the time of organ offer and procurement, thus resulting in a pseudo-randomization of recipients to donors with and without MDROs on OPO cultures.
Second, we evaluated whether the anatomical site of donor MDRO growth impacted recipient outcomes by restricting the exposure to those recipients whose donors had an MDRO or a non-MDRO organism grow on a blood or allograft culture, since prior studies have suggested that these may be the highest risk scenarios for DDIs.3,5 An allograft culture was defined as a respiratory tract culture (e.g., sputum, tracheal aspirate, or bronchoalveolar lavage culture) for lung recipients and a genitourinary tract culture (e.g., urine or ureter culture) for kidney recipients.
Third, we evaluated the association between donor MDRO status and recipient outcomes after stratifying by (1) organ type, to determine whether any of the observed associations were being driven by specific organ groups and (2) MDRO type, to determine whether any of the observed associations were being driven by specific MDROs. Due to small numbers, only bivariable analyses were performed for these stratified analyses.
2.7 ∣. Exploratory analyses
We performed a secondary exploratory analysis to determine whether the observed association between donor MDROs and recipient infection was being driven by specific types of recipient infections, with a particular focus on DDIs (as defined in Supporting Information Section D20), certain anatomical sites of infection (e.g., lower respiratory tract infections), and/or certain etiologic organisms (e.g., Klebsiella species). Due to small numbers and the exploratory nature of these analyses, only unadjusted analyses were performed.
3 ∣. RESULTS
3.1 ∣. Study population
The cohort included 658 SOT recipients, including 418 (64%) from HUP, 167 (25%) from TUH, and 73 (11%) from HUH. The median age was 59 years old (IQR 48–65), and 231 (35%) were women. The cohort included 275 (42%) kidney transplant recipients, 182 (28%) liver transplant recipients, 105 (16%) heart transplant recipients, 131 (20%) lung transplant recipients, and five (1%) pancreas transplant recipients (see Table 1 for further baseline characteristics.)
TABLE 1.
Baseline characteristics of the solid organ transplant recipients and deceased organ donors stratified by donor MDRO status
| Baseline characteristica,b | Recipients with negative donor cultures (N 88) |
Recipients with non-MDRO positive donor cultures (N 477) |
Recipients with an MDRO on donor culture (N 93) |
p valuec |
|---|---|---|---|---|
| Recipient baseline characteristics | ||||
| Demographics | ||||
| Age (median, IQR), years | 62 (53–66) | 59 (47–65) | 56 (50–64) | .09 |
| Female gender | 30 (34%) | 168 (35%) | 33 (35%) | .98 |
| Black race | 27 (31%) | 158 (33%) | 27 (29%) | .70 |
| White race | 51 (58%) | 277 (58%) | 60 (65%) | .50 |
| Asian race | 5 (6%) | 13 (3%) | 3 (3%) | .35 |
| Hispanic ethnicity | 4 (5%) | 35 (7%) | 9 (10%) | .41 |
| Organ transplant type | ||||
| Kidney | 37 (42%) | 192 (40%) | 46 (49%) | .26 |
| Liver | 26 (30%) | 134 (28%) | 22 (24%) | .62 |
| Lung | 18 (20%) | 96 (20%) | 17 (18%) | .91 |
| Heart | 9 (10%) | 80 (17%) | 16 (17%) | .29 |
| Pancreas | 1 (1%) | 3 (1%) | 1 (1%) | .42 |
| Comorbidities | ||||
| Diabetes mellitus | 31 (35%) | 143 (30%) | 40 (43%) | .04 |
| Hypertension | 55 (63%) | 288 (60%) | 60 (65%) | .73 |
| Chronic kidney diseased | 49 (56%) | 240 (50%) | 49 (53%) | .63 |
| Cirrhosise | 24 (27%) | 122 (26%) | 20 (22%) | .64 |
| Structural lung diseasef | 23 (26%) | 111 (23%) | 19 (20%) | .66 |
| Heart failure or cardiomyopathyg | 13 (15%) | 109 (23%) | 21 (23%) | .24 |
| Charlson comorbidity index (median, IQR) | 4 (3–6) | 4 (2–6) | 4 (2-6) | .38 |
| Pretransplant characteristics | ||||
| Days on waitlist (median, IQR) | 271 (65–890) | 206 (46–778) | 236 (57–638) | .91 |
| Intensive care unit pretransplantationh | 10 (11%) | 42 (9%) | 8 (9%) | .73 |
| Mechanical ventilation pretransplantationh | 3 (3%) | 14 (3%) | 1 (1%) | .59 |
| Renal replacement therapy pretransplantationh | 39 (45%) | 183 (39%) | 40 (44%) | .39 |
| Pretransplantation infectionsi | ||||
| Lower respiratory tract infection | 9 (10%) | 42 (9%) | 8 (9%) | .90 |
| Skin or soft tissue infection | 2 (2%) | 6 (1%) | 4 (4%) | .08 |
| Urinary tract infection | 6 (7%) | 20 (4%) | 1 (1%) | .13 |
| Bloodstream infection | 1 (1%) | 12 (3%) | 0 (0%) | .31 |
| Intra-abdominal infection | 3 (3%) | 14 (3%) | 4 (4%) | .72 |
| Laboratory valuesj | ||||
| Albumin (median, IQR), g/dl | 3.6 (3.0–4.1) | 3.6 (2.8–4.1) | 3.6 (3.0–4.2) | .06 |
| CMV seropositive | 49 (56%) | 268 (56%) | 58 (62%) | .53 |
| EBV seropositive | 86 (98%) | 448 (94%) | 87 (94%) | .34 |
| HCV seropositive | 6 (10%) | 53 (16%) | 12 (16%) | .53 |
| Donor baseline characteristics | ||||
| Age (median, IQR), years | 43 (26-51) | 36 (27–50) | 32 (25–47) | .05 |
| Female gender | 46 (52%) | 174 (36%) | 39 (42%) | .02 |
| Comorbidities | ||||
| Diabetes mellitus | 4 (5%) | 51 (11%) | 9 (10%) | .20 |
| Hypertension | 29 (33%) | 105 (22%) | 26 (28%) | .06 |
| Donor substance use | ||||
| Tetrahydrocannabinol/marijuana usek | 10 (11%) | 63 (13%) | 20 (22%) | .08 |
| Injection drug use | 10 (11%) | 108 (23%) | 25 (27%) | .03 |
| Death mechanism | ||||
| Drug overdose | 8 (9%) | 121 (25%) | 24 (26%) | <.01 |
| Asphyxiation | 8 (9%) | 20 (4%) | 10 (11%) | .02 |
| Cardiovascular | 14 (16%) | 116 (24%) | 20 (22%) | .21 |
| Gunshot wound | 10 (11%) | 37 (8%) | 6 (6%) | .43 |
| Blunt injury | 18 (20%) | 64 (13%) | 12 (13%) | .21 |
| Intracranial hemorrhage | 27 (31%) | 112 (23%) | 19 (20%) | .24 |
| Donor type | ||||
| Donation after circulatory death | 12 (14%) | 41 (9%) | 16 (17%) | .03 |
| Expanded criteria donor | 20 (23%) | 68 (14%) | 15 (16%) | .13 |
| PHS-increased risk | 19 (22%) | 184 (39%) | 31 (33%) | .01 |
| Donor laboratory values | ||||
| CMV seropositive | 49 (56%) | 249 (52%) | 47 (51%) | .77 |
| EBV seropositive | 78 (89%) | 439 (92%) | 87 (94%) | .43 |
| HCV seropositive | 3 (3%) | 20 (4%) | 5 (5%) | .80 |
| Donor management | ||||
| Length of stay during terminal hospitalization (median, IQR), days | 3 (2–4) | 4 (2–5) | 4 (2-7) | <.01 |
| Corticosteroid administrationl | 51 (58%) | 296 (62%) | 55 (59%) | .71 |
| Antimicrobial administration during terminal hospitalization | 84 (95%) | 471 (99%) | 88 (97%) | .08 |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein–Barr virus; HCV, hepatitis C virus; IQR, interquartile range; MDRO, multidrug-resistant organism; PHS, Public Health Service.
Data are presented as numbers (percentages) except where noted.
Only those variables with a p value < .20, those of notable biologic importance, and those included in the final multivariable models are shown in this table.
The provided p value reflects a comparison of the three exposure groups.
Two-hundred thirty-nine (71%) of those with chronic kidney diseases were kidney transplant recipients.
One-hundred fifty-nine (96%) of those with cirrhosis were liver transplant recipients.
One-hundred nineteen (78%) of those with structural lung disease were lung transplant recipients.
One hundred (70%) of those with heart failure/cardiomyopathy were heart transplant recipients.
Assessed in the 24 h prior to transplantation.
Assessed in the 7 days prior to transplantation. In order to be categorized as having the infection pretransplantation, the transplant recipient had to be on ongoing antimicrobial therapy for the designated infection within 7 days of transplantation.
The most recent value prior to transplantation is included.
Defined by tetrahydrocannabinol detection on toxicology screen taken on admission to the donor’s terminal hospitalization.
Administered as part of “T4 protocol” by the organ procurement organization, in which high-dose corticosteroids, thyroxine (T4), insulin, and vasopressin are administered.
Among the SOT recipients, 93 (14%) had a donor with at least one MDRO on culture, 477 (73%) had a donor with at least one non-MDRO bacterial or candidal organism on culture, and 88 (13%) had a donor with no growth (or only contaminants) on culture. The most common MDROs on donor culture were MRSA (54, 8% of recipients) and ESC-R Enterobacterales (38, 6% of recipients). The most common anatomical site of MDRO growth was the respiratory tract (79, 12% of recipients).
3.2 ∣. Association between donor MDROs and post-transplant infection
Among the entire cohort, 300 (46%) SOT recipients developed a bacterial or invasive candidal infection within 3 months post-transplant (Table 2). The most common infection types were lower respiratory tract infections (140, 21%) and genitourinary tract infections (104, 16%). The most common etiologic organisms were Enterobacterales species (148, 22%), and the most common MDROs were ESC-R Enterobacterales species (43, 7%).
TABLE 2.
Bacterial and candidal infections among solid organ transplant recipients within 3 months posttransplantation
| Infection typea | Recipients with negative donor cultures (N 88) |
Recipients with positive, non-MDRO, donor cultures (N 477) |
Recipients with MDRO on donor culture (N 93) |
p value |
|---|---|---|---|---|
| Any bacterial or candidal infection | 34 (39%) | 210 (44%) | 45 (48%) | .42 |
| Donor-derived infection | N/Ab | 24 (5%) | 7 (8%) | .33 |
| Infection type | ||||
| Lower respiratory tract infection | 14 (16%) | 104 (22%) | 23 (25%) | .33 |
| Genitourinary infection (UTI, pyelonephritis) | 15 (17%) | 69 (15%) | 20 (22%) | .22 |
| Surgical site infection | 10 (11%) | 43 (9%) | 6 (6%) | .51 |
| Bloodstream infection | 8 (9%) | 33 (7%) | 8 (9%) | .70 |
| Intra-abdominal infection | 4 (5%) | 30 (6%) | 1 (1%) | .10 |
| Skin or soft tissue infection | 7 (8%) | 27 (6%) | 3 (3%) | .42 |
| Clostridioides difficile infection | 4 (5%) | 22 (5%) | 3 (3%) | .91 |
| Etiologic organismc | ||||
| Candida spp | 11 (13%) | 52 (11%) | 11 (12%) | .89 |
| Coagulase-negative Staphylococci | 9 (10%) | 53 (11%) | 5 (5%) | .25 |
| Enterobacterales spp | 19 (22%) | 103 (22%) | 26 (28%) | .40 |
| Enterococcus spp | 10 (11%) | 48 (10%) | 8 (9%) | .83 |
| Pseudomonas spp | 5 (6%) | 48 (10%) | 8 (9%) | .42 |
| Staphylococcus aureus | 6 (7%) | 48 (10%) | 10 (11%) | .63 |
| Stenotrophomonas spp | 1 (1%) | 7 (1%) | 2 (2%) | .87 |
| Streptococcus spp | 1 (1%) | 14 (3%) | 2 (2%) | .79 |
| MDROs | ||||
| Any MDROd | 10 (11%) | 65 (14%) | 18 (19%) | .25 |
| MRSA | 1 (1%) | 16 (3%) | 6 (6%) | .16 |
| VRE | 6 (7%) | 19 (4%) | 7 (8%) | .23 |
| ESC-R Enterobacterales | 5 (6%) | 31 (7%) | 7 (8%) | .88 |
| CRE | 0 (0%) | 7 (1%) | 2 (2%) | .48 |
| MDR-Pseudomonas | 0 (0%) | 10 (2%) | 2 (2%) | .57 |
Abbreviations: CRE, carbapenem-resistant Enterobacterales; ESC-R, extended-spectrum cephalosporin-resistant; MDR, multidrug-resistant; MDRO, multidrug-resistant organism; MRSA, methicillin-resistant S. aureus; spp, species; UTI, urinary tract infection; VRE, vancomycin-resistant Enterococcus.
Data are presented as numbers (percentages) except where noted.
Solid organ transplant recipients whose donors did not have any positive cultures could not have a donor-derived infection, since it is defined by identifying the same organism on donor and recipient cultures.
Etiologic organism identified on recipient culture within 3 months posttransplant.
MDROs included MRSA, VRE, ESC-R Enterobacterales, CRE, MDR-Pseudomonas, and MDR-Acinetobacter.
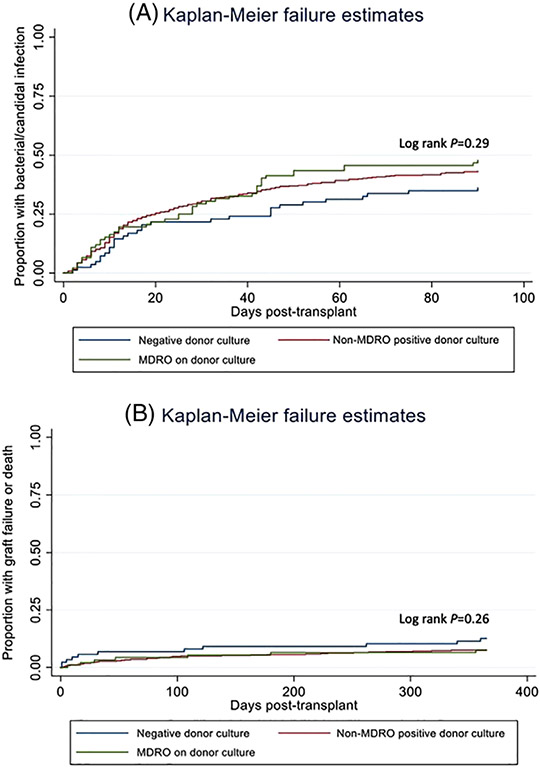
In the unadjusted analysis (Figure 1A), there was not a significant association between donor MDRO status and the hazard of post-transplant infection (log rank p = .29). However, on multivariable analysis (Table 3), there was a significantly increased hazard of infection among recipients whose donors had either an MDRO on culture (aHR 1.63, 95% CI 1.01–2.62, p = .04) or a non-MDRO positive culture (aHR 1.51, 95% CI 1.02–2.23, p = .04). (See Table S1 for full model.)
FIGURE 1.
Kaplan–Meier curves depicting (A) time to posttransplant bacterial or invasive candidal infection stratified by donor multidrug-resistant organism status (unadjusted) and (B) time to posttransplant graft failure or mortality stratified by donor multidrug-resistant organism status (unadjusted)
TABLE 3.
Mixed effect multivariable frailty model evaluating the association between donor culture results and hazard of (a) bacterial or invasive candidal infection and (b) graft failure or death following transplantation among solid organ transplant recipients
| (a) Bacterial or invasive candidal infection | ||||||
|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
|||||
| Variable | HR | 95% CI | p value | aHR | 95% CI | p value |
| Negative donor cultures | Ref. | Ref. | ||||
| Non-MDRO bacteria or Candida on donor cultures | 1.30 | 0.87–1.93 | .20 | 1.51 | 1.02–2.23 | .04 |
| MDRO on donor cultures | 1.47 | 0.91–2.38 | .11 | 1.63 | 1.01–2.62 | .04 |
| (b) Graft failure or death | ||||||
| Unadjusted |
Adjusted |
|||||
| HR | 95% CI | p value | aHR | 95% CI | p value | |
| Negative donor cultures | Ref. | Ref. | ||||
| Non-MDRO bacteria or Candida on donor cultures | 0.57 | 0.28–1.16 | .12 | 0.60 | 0.30–1.23 | .16 |
| MDRO on donor cultures | 0.59 | 0.22–1.58 | .30 | 0.45 | 0.15–1.36 | .16 |
Abbreviations: aHR, adjust hazard ratio; CI, confidence interval; HR, hazard ratio; MDRO, multidrug-resistant organism; Ref., reference.
3.3 ∣. Association between donor MDROs and posttransplant graft failure and death
Among the entire cohort, 57 (9%) recipients developed graft failure or death within 12 months, of which 27 (4%) represented deaths. In the unadjusted analysis (Figure 1B), there was no significant association between donor MDRO status and the hazard of graft failure or death within 12 months posttransplant (log rank p = .26). Similarly, on the multivariable analysis (Table 3), there remained no significant association between donor MDRO status and the hazard of graft failure or death (aHR 0.60, 95% CI 0.30–1.23, p = .16 for non-MDRO positive donor cultures; aHR 0.45, 95% CI 0.15–1.36, p = .16 for MDROs on donor culture). (see Table S2 for full model.)
3.4 ∣. Subgroup and stratified analyses
3.4.1 ∣. MDROs on donor OPO cultures
After restricting the cohort to only those SOT recipients whose donors had entirely negative hospital cultures, there were 252 recipients remaining in the cohort, of which 14 (6%) had a donor with an MDRO on OPO culture, 150 (60%) had a donor with non-MDRO positive OPO cultures, and 88 (35%) had donors with no growth (or only contaminants) on OPO cultures. On both unadjusted (Figure S1) and multivariable analyses (Table S3), there was a significantly increased hazard of bacterial or invasive candidal infection associated with donors who had MDROs on OPO culture (aHR 5.59, 95% CI 1.44–21.67, p = .01). Conversely, there was no significant association between donor OPO culture status and the hazard of graft failure or death on either unadjusted or multivariable analyses (aHR 0.42, 0.05–3.76, p = .44, Table S4).
3.4.2 ∣. MDROs on donor blood or allograft cultures
In this subgroup analysis, there were 40 (6%) SOT recipients whose donors had an MDRO on blood or allograft culture, 171 (26%) recipients whose donors had a non-MDRO positive blood or allograft culture, and 447 (68%) recipients whose donors had no growth (or only contaminants) on blood and allograft cultures. On multivariable analysis, there was no significant association between donor blood or allograft MDRO status and the hazard of infection (aHR 1.11, 0.79–1.57, p = .53 for non-MDRO positive blood or allograft cultures; aHR 1.21, 95% CI 0.74–1.98, p = .46 for MDROs on blood or allograft culture, Table S5) or the hazard of graft failure or death (aHR 1.16, 95% CI 0.52-2.55, p = .72 for non-MDRO positive blood or allograft cultures; aHR 1.05, 95% CI 0.29–3.81, p = .95 for MDROs on donor blood or allograft culture, Table S6).
3.4.3 ∣. Stratified analysis by organ type
After stratifying by organ transplant type, we found on bivariable analyses that (1) there was a significant increase in the hazard of posttransplant infection associated with MDROs on donor culture among liver transplant recipients (HR 3.77, 95% CI 0.98–14.43, p = .05); (2) there were no significant associations between donor MDRO status and the hazard of graft failure or death among any of the organ types (data not shown).
3.4.4 ∣. Stratified analysis by MDRO type
After stratifying by MDRO type, we found on bivariable analyses that (1) there was a significant increase in the hazard of posttransplant infection associated with VRE on donor culture (HR 10.58, 95% CI 3.48–32.17, p < .01); (b) there was no significant association between the hazard of posttransplant infection and either ESC-R Enterobacterales on donor culture (HR 1.19, 95% CI 0.64–2.23, p = .58) or MDR-Gram negatives on donor culture (including ESC-R Enterobacterales, MDR-Pseudomonas, and MDR-Acinetobacter) (HR 1.22, 95% CI 0.66–2.56, p = .52); (c) there was no significant association between donor culture status and the hazard of graft failure or death with any MDRO type (data not shown).
3.5 ∣. Exploratory analysis of DDIs
There were 31 (5%) SOT recipients in the cohort who developed a probable DDI, of which nine (28% of DDIs) were due to MDROs. The MDROs causing DDIs were MRSA (4, 13% of DDIs), VRE (2, 6% of DDIs), ESC-R Enterobacterales (2, 6% of DDIs), and Candida glabrata (1, 3% of DDIs). These MDROs causing probable DDIs were originally cultured from the donor’s respiratory tract (6, 19% of DDIs), blood (1, 3% of DDIs), and perfusate fluid (2, 6% of DDIs), and occurred among four lung, three kidney, one liver, and one heart transplant recipient.
On unadjusted analysis, there was a non-significant increase in probable DDIs among SOT recipients whose donors had MDROs on culture (7, 8%) compared to those whose donors had non-MDRO positive cultures (24, 5%) (unadjusted p = .33) (Table 2). When DDIs were excluded from the outcome of “recipient infection,” there was no longer a significant association between donor MDROs and the hazard of post-transplant bacterial or invasive candidal infection (aHR 1.36, 95% CI 0.82–2.25, p = .23).
4 ∣. DISCUSSION
In this study, we found that SOT recipients whose donors were infected or colonized with MDROs had a significantly increased hazard of bacterial or invasive candidal infection in the first 3 months posttransplant, but no significant change in their hazard of graft failure or death in the first year posttransplant, compared to those recipients whose donors had negative cultures.
Notably, in the primary analysis, the hazard of posttransplant infection was not remarkably different between recipients whose donor had an MDRO and recipients whose donor had a more antibiotic-susceptible organism on culture. However, when evaluating those SOT recipients whose donors had negative hospital cultures but positive OPO cultures, there was a significant risk associated with donor MDROs that was not observed with donors who had non-MDRO positive cultures. In this latter scenario, the recipient transplant centers would not have been aware of the donor MDRO at the time of organ acceptance. This suggests that (1) the increased risk of posttransplant infection is present even when donors are assigned to recipients independent of the donor MDRO status, (2) donor MDRO status is not merely a marker for sicker transplant candidates, and (3) the risk of posttransplant infection may be increased when donor culture results are not known at the time of transplant. It further suggests that earlier identification of donor MDROs may potentially mitigate the risk of posttransplant infection and DDIs, perhaps through earlier intervention in the recipient.
In exploratory analyses, there was no single infection type that was identified as independently driving the association between donor MDROs and recipient infections, though DDIs were numerically more common among SOT recipients whose donors had MDROs on culture. Additionally, the association between donor MDROs and recipient infections was no longer present after excluding DDIs from the infection outcome, suggesting DDIs play an important role in this pathway. We would hypothesize—though it is not directly evaluated in this study—that donor MDROs may be associated with more DDIs than antibiotic-susceptible donor organisms because the standard peri-transplant antimicrobial prophylaxis given to recipients does not have activity against the majority of MDROs (save for MRSA when vancomycin is administered). It is also conceivable that donor infection or colonization with an MDRO causes a nonspecific increase in the risk of recipient infections (that are not donor-derived) by altering alloresponses21,22 or compromising anastomotic integrity.23,24
We did not observe a significant increase in posttransplant infections when the donor had an MDRO specifically on blood or allograft culture, despite prior literature suggesting these may be the highest risk scenarios for DDIs.2,3,5 However, the number of SOT recipients in this study with positive donor blood or allograft cultures was small (6%), so the study may not have been adequately powered to observe this effect. Further, at the included study sites, it is standard to treat SOT recipients with antimicrobials targeted to the donor organism when the donor has a positive blood or allograft culture; this practice may have reduced the risk of DDI and posttransplant infection sufficiently such that it was no longer a notable risk factor. It was also noteworthy that we found no association between donor MDR-Gram negatives and the risk of infection posttransplant, despite prior case reports that describe particularly poor outcomes with donor MDR-Gram negatives.2,3,5 The lack of association in our study may have again been due to limited power (6% of SOT recipients had a donor with an MDR-Gram negative on culture), but it is not routine practice at the included study sites to treat all MDR-Gram negatives on donor cultures in the recipient, unless identified from the blood or allograft, so our results do raise the question of whether MDR-Gram negatives on donor culture may be less threatening than perceived.8 Conversely, we did find an association between donor VRE and recipient infection in our unadjusted secondary analyses, suggesting further study of the impact of donor VRE on recipient outcomes is needed.25 Additionally, future studies evaluating how antimicrobials administered to the recipient affect the association between donor MDROs and recipient infections are needed to better understand these outcomes.
Reassuringly, we found in all primary and secondary analyses that donor MDROs did not significantly impact the hazard of graft failure or death following transplantation. This suggests that the increased risk of posttransplant infection does not result in worse long-term outcomes for these recipients. Of note, this study did not evaluate whether outcomes are altered for those SOT recipients who develop an MDRO DDI, but rather demonstrates that outcomes are unchanged for the overall cohort of SOT recipients whose donors were infected or colonized with an MDRO.
There are several limitations of this study. First, as this was a retrospective observational study, the outcomes observed are only relevant in the context of the organ selection, donor management, and recipient management practices utilized at the study sites, and the results may not be generalizable to other institutions with dissimilar practices. Second, the hospital cultures collected from deceased donors were not standardized, and the number of hospital cultures collected from each donor may have varied depending on the level of clinical concern for infection, resulting in a degree of ascertainment bias. However, all donors underwent standardized OPO culture collection, so there was an opportunity for every donor to have the exposure of interest captured. Third, probable DDIs were determined based only on the identification and antimicrobial susceptibility profiles of each organism since whole genome sequencing was beyond the scope of this study; future studies using molecular methods to establish donor-origin will be needed to verify our DDI findings.4,6,25 Fourth, due to missing data, we were not able to assess the impact of specific donor or recipient antibiotic regimens on recipient outcomes. Finally, because this was an observational study—and SOT recipients cannot be randomized to donors with and without MDROs—it is possible that there were unmeasured confounders even in the multivariable adjusted analyses, and that donor MDRO status is a marker for a separate prognostic factor.
In conclusion, we found that donor MDROs were associated with an increased risk of early posttransplant bacterial or invasive candidal infection among recipients, but there was no significant change in the risk of graft failure or death through 12 months posttransplant. This study suggests that interventions directed at reducing the risk of posttransplant infection and DDIs, such as earlier identification of donor MDROs and earlier appropriate treatment of the recipient, may make it possible to safely use organs from donors infected or colonized with MDROs and thereby maintain and expand the donor pool over time.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Transplant Foundation’s Innovative Research Grant Program, an affiliate of the Gift of Life Donor Program (Donation and Transplantation Grant to Judith A. Anesi); Antibacterial Resistance Leadership Group (grant number: 5 UM 1AI104681-05 with a subaward fellowship grant to Judith A. Anesi); the National Institutes of Health (grant numbers: K24-AI080942 to Ebbing Lautenbach and K01-AI137317 to Judith A. Anesi); and by a CDC Cooperative Agreement FOA#CK16-004-Epicenters for the Prevention of Healthcare Associated Infections (to Ebbing Lautenbach). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ABBREVIATIONS:
- aHR
adjusted hazard ratio
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CRE
carbapenem-resistant Enterobacterales
- DDI
donor-derived infection
- ESC-R
extended-spectrum cephalosporin-resistant
- GLDP
Gift of Life Donor Program
- HUH
Hahnemann University Hospital
- HUP
Hospital of the University of Pennsylvania
- MDR
multidrug-resistant
- MDRO
multidrug-resistant organism
- MRSA
methicillin-resistant Staphylococcus aureus
- NHSN
National Healthcare Safety Network
- OPO
organ procurement organization
- SOT
solid organ transplant
- Spp
species
- TUH
Temple University Hospital
- VRE
vancomycin-resistant Enterococcus species
Footnotes
CONFLICT OF INTEREST
Emily Blumberg receives research support from Merck, Takeda, and Hologic, is a member of a Data and Safety Monitoring Board (DSMB) for Amplyx, and is a member of Scientific Advisory Committees for Merck and Takeda. Jennifer Han was affiliated with the University of Pennsylvania during the conduct of this project, is now employed by, and holds shares in, the GlaxoSmithKline group of companies. Ebbing Lautenbach is a member of a DSMB for Merck and consultant for Shionogi. None of the associations mentioned above are relevant to this article. All other authors report no conflicts of interest relevant to this article.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Wolfe CR, Ison MG, Practice ASTIDCo. Donor-derived infections: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13547. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JD, Sifri CD. Multidrug-resistant bacterial donor-derived infections in solid organ transplantation. Curr Infect Dis Rep. 2016;18(6):18. [DOI] [PubMed] [Google Scholar]
- 3.Mularoni A, Bertani A, Vizzini G, et al. Outcome of transplantation using organs from donors infected or colonized with carbapenem-resistant Gram-negative bacteria. Am J Transplant. 2015;15(10):2674–2682. [DOI] [PubMed] [Google Scholar]
- 4.Wendt JM, Kaul D, Limbago BM, et al. Transmission of methicillin-resistant Staphylococcus aureus infection through solid organ transplantation: confirmation via whole genome sequencing. Am J Transplant. 2014;14(11):2633–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Procaccio F, Masiero L, Vespasiano F, et al. Organ donor screening for carbapenem-resistant Gram-negative bacteria in Italian intensive care units: the DRIn study. Am J Transplant. 2020;20(1):262–273. [DOI] [PubMed] [Google Scholar]
- 6.Altman DR, Sebra R, Hand J, et al. Transmission of methicillin-resistant Staphylococcus aureus via deceased donor liver transplantation confirmed by whole genome sequencing. Am J Transplant. 2014;14(11):2640–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). Transmission of multidrug-resistant Escherichia coli through kidney transplantation—California and Texas, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(50):1642–1646. [PubMed] [Google Scholar]
- 8.Anesi JA, Han JH, Lautenbach E, et al. Impact of deceased donor multidrug-resistant bacterial organisms on organ utilization. Am J Transplant. 2020;20(9):2559–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anesi JA, Blumberg EA, Han JH, et al. Risk factors for multidrug-resistant organisms among deceased organ donors. Am J Transplant. 2019;19(9):2468–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma X, Wu Y, Li L, et al. First multicenter study on multidrug resistant bacteria carriage in Chinese ICUs. BMC Infect Dis. 2015;15:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maechler F, Pena Diaz LA, Schroder C,Geffers C, Behnke M,Gastmeier P. Prevalence of carbapenem-resistant organisms and other Gram-negative MDRO in German ICUs: first results from the national noso-comial infection surveillance system (KISS). Infection. 2015;43(2):163–168. [DOI] [PubMed] [Google Scholar]
- 12.Jolivet S, Lolom I, Bailly S, et al. Impact of colonization pressure on acquisition of extended-spectrum beta-lactamase-producing Enterobacterales and meticillin-resistant Staphylococcus aureus in two intensive care units: a 19-year retrospective surveillance. J Hosp Infect. 2020;105(1):10–16. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez SA, Trotter JF. The rise of the opioid epidemic and hepatitis C-positive organs: a new era in liver transplantation. Hepatology. 2018;67(4):1600–1608. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg D, Lynch R. Improvements in organ donation: riding the coattails of a national tragedy. Clin Transplant. 2020;34(1):e13755. [DOI] [PubMed] [Google Scholar]
- 15.Phillips KG, Ranganath NK, Malas J, et al. Impact of the opioid epidemic on heart transplantation: donor characteristics and organ discard. Ann Thorac Surg. 2019;108(4):1133–1139. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Cohen SH, King JH, Monchaud C, Nguyen H, Flynn NM. Injecting drug use and community-associated methicillin-resistant Staphylococcus aureus infection. Diagn Microbiol Infect Dis. 2008;60(4):347–350. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz I,Gavalda J, Monforte V, et al. Donor-to-host transmission of bacterial and fungal infections in lung transplantation. Am J Transplant. 2006;6(1):178–182. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention National Healthcare Safety Network (NHSN) Patient Safety Component Manual. Multidrug-resistant organism & Clostridioides difficile infection (MDRO/CDI) module web site. January 2019. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Accessed September 15, 2021.
- 19.Centers for Disease Control and Prevention CDC/NHSN surveillance definitions for specific types of infections. 2020. http://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf. Accessed September 15, 2021.
- 20.Kaul DR, Vece G, Blumberg E, et al. Ten years of donor-derived disease: a report of the disease transmission advisory committee. Am J Transplant. 2021;21(2):689–702. [DOI] [PubMed] [Google Scholar]
- 21.Wu JF, Muthusamy A, Al-Ghalith GA,et al. Urinary microbiome associated with chronic allograft dysfunction in kidney transplant recipients. Clin Transplant. 2018;32(12):e13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alegre ML, Mannon RB, Mannon PJ. The microbiota, the immune system and the allograft. Am J Transplant. 2014;14(6):1236–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger MF, Badell IR. Single donor-derived pseudomonas aeruginosa pseudoaneurysms in two kidney transplant recipients: a case report of dichotomous allograft outcomes. Transplant Proc. 2017;49(10):2357–2361. [DOI] [PubMed] [Google Scholar]
- 24.Nelson PW, Delmonico FL,Tolkoff-Rubin NE, et al. Unsuspected donor pseudomonas infection causing arterial disruption after renal transplantation. Transplantation. 1984;37(3):313–314. [DOI] [PubMed] [Google Scholar]
- 25.Bashir A, Attie O, Sullivan M, et al. Genomic confirmation of vancomycin-resistant Enterococcus transmission from deceased donor to liver transplant recipient. PLoS One. 2017;12(3):e0170449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.