Abstract
Objective:
Clostridioides difficile infection (CDI) is the leading cause of infectious nosocomial diarrhea. Although initial fidaxomicin or vancomycin treatment is recommended by most major guidelines to treat severe CDI, there exists varied recommendations for first-episode non-severe CDI. Given the discrepancy in current treatment guidelines, we sought to evaluate the use of initial vancomycin versus metronidazole for first-episode non-severe CDI.
Methods:
We conducted a retrospective cohort study of all adult inpatients with first-episode CDI at our institution from January 2013 to May 2018. The initial vancomycin versus initial metronidazole cohorts were examined using a multivariate logistic regression model.
Results:
The study cohort of 737 patients had a median age of 72.3 years, and 357 of these patients (48.4%) had hospital-acquired infection. Among 326 patients with non-severe CDI, recurrence, new incident infection, and 30-day mortality rates were 16.2%, 10.9%, and 5.3%, respectively, when treated with initial metronidazole, compared to 20.0%, 1.4%, and 10.0%, respectively, when treated with initial vancomycin. In an adjusted multivariable analysis, the use of initial vancomycin for the treatment of non-severe CDI was associated with a reduction in new incident infection (adjusted odds ratio [ORadj], 0.11; 95% confidence interval [CI], 0.02–0.86; P = .035), compared to initial metronidazole.
Conclusions:
Initial vancomycin was associated with a reduced rate of new incident infection in the treatment of adult inpatients with first-episode non-severe CDI. These findings support the use of initial vancomycin for all inpatients with CDI, when fidaxomicin is unavailable.
Clostridioides difficile infection (CDI) manifests itself in patients with an altered gut microbiota, and it is commonly caused by exposure to antibiotics. 1 As the leading cause of infectious nosocomial diarrhea, CDI continues to be a major burden within healthcare institutions and in the community. 1 Despite undergoing lengthy courses of antimicrobial therapy, however, recurrence is common, with approximately 20% of patients experiencing debilitating episodes of repeated disease. 2 Recurrent CDI is associated with a greater risk for future recurrence and severe complications such as hypotension, perforation, and toxic megacolon. 2 Therefore, early interventions aimed at preventing recurrent CDI and reinfection are needed to improve outcomes, decrease the number of patient days spent in isolation, and improve quality of life.
St. Joseph’s Healthcare Hamilton is a multisite teaching hospital located in Hamilton, Ontario, Canada, with a dedicated Clostridioides difficile consultation service for inpatients and referred outpatients. Although the consultation team may offer fecal microbiota transplantation to patients with multiple CDI episodes or refractory disease, 3,4 clinical interventions for first-episode CDI primarily involve the use of antibiotics. In the past, metronidazole was indicated for non-severe CDI at our institution, while vancomycin was reserved for febrile patients with elevated white counts or for recurrent episodes. In recent years, however, there has been a shift to initial vancomycin to treat first-episode non-severe CDI at our institution, which is consistent with guidelines published by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA), when fidaxomicin is unavailable. 5 Importantly, guidelines published by the European Society of Clinical Microbiology and Infectious Diseases, the World Society of Emergency Surgery, and the Australasian Society of Infectious Diseases still recommend the use of metronidazole for the first episode of non-severe disease. 6–8
Although initial fidaxomicin or vancomycin treatment is recommended by most major guidelines to treat severe CDI, there exists a discrepancy in treatment recommendations for first-episode non-severe CDI. 5–8 Few studies have been conducted with long-term follow-up evaluating the use of initial vancomycin compared to initial metronidazole to treat non-severe inpatient CDI. In 2 large randomized controlled trials with follow-up of 3 weeks and 4 weeks, respectively, subgroup analyses stratified by severity did not demonstrate significant differences in relapse rates between the vancomycin and metronidazole treatment groups for non-severe CDI. 9,10 Similarly, 2 cohort studies reported that relapse rates and mortality did not differ among patients with mild-to-moderate CDI treated with metronidazole or vancomycin. 11,12 Therefore, we sought to identify predictors of adverse clinical outcomes and evaluate the use of initial vancomycin versus metronidazole for the treatment of first-episode non-severe CDI among adult inpatients.
Methods
Study population
We conducted a retrospective cohort study of all adult inpatients with first-episode CDI at our institution from January 1, 2013 to May 31, 2018. Patients were identified from hospital infection control registries maintained by Infection Prevention and Control, and all entries were validated against data reported to the Ontario Ministry of Health and Long-Term Care. A CDI episode was characterized clinically as 3 or more type 5–7 diarrheal stools on the Bristol stool scale, together with a positive Clostridioides difficile toxin gene loop-mediated isothermal amplification assay. 13 Patients with a prior history of CDI were excluded from the study. Similarly, colonized patients who had a positive toxin gene assay but did not meet the clinical criteria of 3 or more type 5–7 loose stools were excluded.
Data abstracted included: demographics, specimen collection date, date of symptom onset, source of acquisition, unit in which CDI was first diagnosed (medical, surgical, or nephrology), clinical indicators at the time of diagnosis, CDI treatment, number of isolation days, and outcomes including recurrence, new incident infection, and death. Time to treatment and time to recurrence were measured from the start of the initial CDI diagnosis. Hospital-acquired infection (HAI) was defined as CDI present >72 hours after admission, whereas non-HAI was defined as CDI present ≤72 hours after admission. 14 CDI severity was defined according to IDSA and SHEA guidelines. 13 A patient with white blood cell count ≥15×109/L or a serum creatinine level >1.5 mg/dL was considered to have severe CDI. 13
Exposures and outcomes
The primary exposures in this study were the treatment of first-episode non-severe CDI with initial vancomycin or metronidazole. The outcomes of interest were recurrence, new incident infection, and all-cause 30-day mortality. All patients were followed for 26 weeks from the date of their initial CDI diagnosis. Recurrence was defined as a CDI case occurring after an episode with positive assay result in the previous 2–8 weeks, while new incident infection was defined as a CDI case occurring after more than 8 weeks. 13 All-cause 30-day mortality was defined as death for any reason within 30 days of CDI diagnosis.
Statistical analysis
Logistic regression was used to determine prognostic factors for the composite outcome of recurrence or all-cause 30-day mortality for the overall cohort. Recurrence was selected as a patient-important outcome because it is associated with a greater risk for complications and adverse outcomes. 2 All-cause 30-day mortality was included in the composite outcome because it is a potential competing event for recurrence. All factors were determined a priori based on published guidelines. 6–8,13 Univariable analysis with P < .20 was used, followed by stepwise selection in a multivariable logistic regression model. The number of variables entered into the model enabled 80% study power with 15 events per factor.
To evaluate the impact of initial vancomycin treatment on adverse clinical outcomes in patients with first-episode non-severe CDI, the initial vancomycin versus initial metronidazole cohorts were first examined in an unadjusted logistic regression analysis for any combination of recurrence, new incident infection, and all-cause 30-day mortality. Prognostic factors for the overall cohort were then added to the adjusted multivariable analysis.
The study protocol was approved by the Hamilton Integrated Research Ethics Board (project no. 2018-3543). The Hamilton Integrated Research Ethics Board has categorized this retrospective cohort study as minimal risk, defined as no potential for negative impact on the health and safety of the participant, and waiver of consent for participation was obtained.
Results
In total, 737 inpatients with first-episode CDI were included in our study. Their demographics, clinical data at the time of CDI diagnosis, initial CDI treatment, and outcomes are summarized in Table 1. Patients had a median age of 72.3 years (IQR, 61.2–83.3) and 326 (44.2%) were classified to have non-severe CDI. Moreover, 357 (48.4%) patients were classified as having hospital-acquired infection, and 309 (41.9%) and 71 (9.6%) were classified as having acquired CDI in the community and other institutions, respectively. Most patients, 496 (67.3%), were initially diagnosed with CDI in a medical setting. At the time of CDI diagnosis, patients had an average temperature of 37.1°C (SD, 0.8) and a median white blood cell count of 12.3×109/L (interquartile range [IQR], 8.7–18.1). The median time to treatment following CDI diagnosis was 2 days (IQR, 1–5). Overall, 539 patients (73.1%) received initial metronidazole treatment, and 130 (17.6%) received initial vancomycin. Patients experienced a recurrence rate of 14.5%, a new incident infection rate of 7.3%, and a 30-day mortality rate of 12.8%. The median time to recurrence was 27 days (IQR, 22–36) and the median length of time spent in isolation was 17 days (IQR, 7–34).
Table 1.
Description of Patients With Clostridioides difficile Infection (n = 737)
| Variable | Total a |
|---|---|
| Demographics | |
| Age, median y (IQR) | 72.3 (61.2–83.3) |
| Sex, male, no. (%) | 336 (45.6) |
| Hospital-acquired infection, no. (%) | 357 (48.4) |
| Medical, no. (%) | 496 (67.3) |
| Surgical, no. (%) | 156 (21.2) |
| Nephrology, no. (%) | 83 (11.3) |
| Non-severe infection, no. (%) | 326 (44.2) |
| Clinical data | |
| Temperature, mean °C (SD) | 37.1 (0.8) |
| Albumin, mean g/L (SD) | 23.1 (6.2) |
| Creatinine, median mg/dL (IQR) | 1.1 (0.7–2.1) |
| White blood cell count, median ×109/L (IQR) | 12.3 (8.7–18.1) |
| Initial treatment | |
| Metronidazole, no. (%) | 539 (73.1) |
| Vancomycin, no. (%) | 130 (17.6) |
| Metronidazole and vancomycin, no. (%) | 62 (8.4) |
| Time to treatment, median d (IQR) | 2 (1–5) |
| Outcomes | |
| Recurrence, no. (%) | 107 (14.5) |
| New incident infection, no. (%) | 54 (7.3) |
| 30-day mortality, no. (%) | 94 (12.8) |
| Time to recurrence, median d (IQR) | 27 (22–36) |
| Isolation days, median d (IQR) | 17 (7–34) |
Note. IQR, interquartile range.
Sums may not add up to total due to missing data. Percentages exclude missing data.
Overall cohort
The results of the multivariable logistic regression model to determine prognostic factors for the composite outcome of recurrence or all-cause 30-day mortality for the overall cohort are summarized in Table 2. Predictors of the composite outcome were: age ≥65 years (adjusted odds ratio [ORadj], 2.02; 95% confidence interval [CI], 1.37–2.96; P < .001), hospital-acquired infection (ORadj, 1.98; 95% CI, 1.41–2.77; P < .001), and white blood cell count ≥15×109/L (ORadj, 1.64; 95% CI, 1.16–2.30; P = .005).
Table 2.
Multivariable Predictors of the Composite Outcome of Recurrence or 30-Day Mortality for Patients in the Overall Cohort With Clostridioides difficile Infection
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Age ≥65 y | 2.02 (1.37 – 2.96) | <.001 |
| Hospital-acquired infection | 1.98 (1.41 – 2.77) | <.001 |
| White blood cell count ≥15×109/L | 1.64 (1.16 – 2.30) | .005 |
Note. CI, confidence interval.
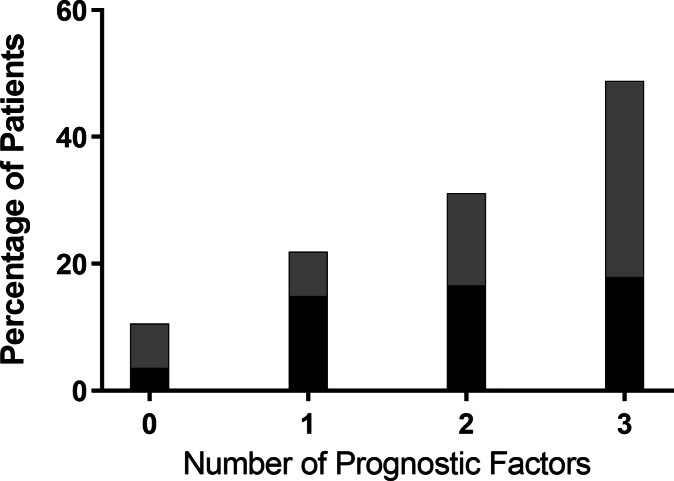
Figure 1 illustrates the proportion of patients in the overall cohort who experienced recurrence or the composite outcome of recurrence or 30-day mortality, given the number of prognostic factors in the multivariable logistic regression model. At a score of 0 prognostic factors, the recurrence rate was 3.5% and the rate of recurrence or 30-day mortality was 10.5%. At a score of 1, 2, or 3 prognostic factors, the recurrence rate increased to 14.8%, 16.5%, and 17.9%, respectively. Similarly, at 1, 2, or 3 prognostic factors, the rate of the composite outcome of recurrence or 30-day mortality increased to 21.8%, 31.0%, and 48.7%, respectively.
Fig. 1.
Proportion of patients in the overall cohort who experienced recurrence or the composite outcome of recurrence or 30-day mortality given the number of prognostic factors.
Non-severe CDI cohort
In total, 326 (44.2%) patients were classified as having non-severe CDI. Among them, 247 (75.8%) were treated with initial metronidazole and 70 (21.5%) were treated with initial vancomycin. Among patients with first-episode non-severe CDI treated with initial metronidazole, 16.2% had recurrence, 10.9% had new incident infection, and the 30-day mortality rate was 5.3%. For patients with first-episode non-severe CDI treated with initial vancomycin, 20.0% had recurrence, 1.4% had new incident infection, and the 30-day mortality rate was 10.0%. The use of initial vancomycin for treatment of first-episode non-severe CDI was associated with a reduction in new incident infection (ORadj, 0.11; 95% CI, 0.02–0.86; P = .035) (Table 3).
Table 3.
Predictors of New Incident Infection for Patients With Non-severe Clostridioides difficile Infection
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value |
| Initial vancomycin | 0.12 (0.02–0.89) | .038 | 0.11 (0.02–0.86) | .035 |
| Age ≥65 y | 0.56 (0.25–1.27) | .165 | ||
| Hospital-acquired infection | 1.36 (0.61–3.05) | .452 | ||
Note. CI, confidence interval.
Discussion
Although clinical trials have shown vancomycin to be superior to metronidazole for the treatment of severe CDI, 10 some studies have suggested that metronidazole and vancomycin are equally effective for the treatment of non-severe CDI. 9–12 Coupled with the higher cost of vancomycin and concerns associated with vancomycin-resistant enterococci, metronidazole has been the drug of choice for the treatment of non-severe CDI. 15 Given that some guidelines recommend the use of metronidazole for non-severe CDI, 6–8 Figure 1 illustrates a scoring rule which could help identify low-risk patient populations who are likely to do well on initial metronidazole.
In recent years, however, guidelines, such as those published by the IDSA and SHEA, have recommended initial fidaxomicin (preferred) or vancomycin (alternative) over metronidazole for the treatment of non-severe CDI. 5 Our findings support the recommendation to use vancomycin when fidaxomicin is unavailable, which is applicable to institutions that rely on metronidazole and vancomycin for the treatment of CDI. A strength of our investigation was the long duration of follow-up, 26 weeks in a pragmatic real-world setting, which allowed for conclusions about the impact of initial vancomycin treatment on new incident infection, defined as a CDI episode occurring >8 weeks after the initial infection. In an adjusted analysis, the use of initial vancomycin for the treatment of first-episode non-severe CDI was associated with a reduction in new incident infection. At our institution, there has been an overall decline in new incident infection rates in recent years, though it is unclear whether this should be attributed to the increased use of initial vancomycin treatment or other factors. A possible explanation for the lower new incident infection rate experienced by patients who received initial vancomycin could be the broader spectrum antimicrobial activity of metronidazole, which may be more disruptive to gut flora and potentially predispose patients for new incident infection. 2,16,17
Our observational study had several limitations. Patients were not randomized to treatment groups, for instance, and they might have differed on key prognostic factors that may have influenced patient outcomes. To mitigate differences between groups, an adjusted analysis was done using prognostic factors found for the overall cohort. However, a limitation of our analysis was the exclusion of comorbidities in the database. Another limitation was the possible misclassification of exposures because the treatment of CDI can evolve over a patient’s stay. Patients were analyzed in a manner analogous to the intention-to-treat approach, based on the initial treatment assignment, and any treatment switches after 72 hours were disregarded. Furthermore, although there was no observed increase in instances of vancomycin-resistant enterococci throughout our investigation, testing for this organism was not systematic. Finally, our investigation could have included some patients colonized with Clostridioides difficile instead of true infection. This is unlikely, however, because diagnosis of CDI was confirmed by 1 of 3 experienced infectious disease physicians, all cases were followed by a dedicated CDI consultation team, and CDI diagnosis required the presence of a positive toxin gene assay together with a clinical presentation of ≥3 loose stools (ie, type 5–7 on the Bristol stool scale).
In conclusion, initial vancomycin was associated with a reduction in new incident infection in the treatment of adult inpatients with first-episode non-severe CDI. These findings support the use of initial vancomycin for all inpatients with CDI, when fidaxomicin is unavailable.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
References
- 1. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med 2014;370:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hopkins RJ, Wilson RB. Treatment of recurrent Clostridium difficile colitis: a narrative review. Gastroenterol Rep 2018;6:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang K, Beckett P, Abouanaser S, Stankus V, Lee C, Smieja M. Prolonged oral vancomycin for secondary prophylaxis of relapsing Clostridium difficile infection. BMC Infect Dis 2019;19:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2016;315:142. [DOI] [PubMed] [Google Scholar]
- 5. Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 2021. doi: 10.1093/cid/ciab549. [DOI] [PubMed]
- 6. Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014;20:1–26. [DOI] [PubMed] [Google Scholar]
- 7. Sartelli M, Di Bella S, McFarland LV, et al. 2019 update of the WSES guidelines for management of Clostridioides (Clostridium) difficile infection in surgical patients. World J Emerg Surg 2019;14(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trubiano JA, Cheng AC, Korman TM, et al. Australasian Society of Infectious Diseases updated guidelines for the management of Clostridium difficile infection in adults and children in Australia and New Zealand: CDI management guidelines. Intern Med J 2016;46:479–493. [DOI] [PubMed] [Google Scholar]
- 9. Zar FA, Bakkanagari SR, Moorthi KMLST, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis 2007;45:302–307. [DOI] [PubMed] [Google Scholar]
- 10. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014;59:345–354. [DOI] [PubMed] [Google Scholar]
- 11. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med 2017;177:546. [DOI] [PubMed] [Google Scholar]
- 12. Le F, Arora V, Shah DN, Salazar M, Palmer HR, Garey KW. A real-world evaluation of oral vancomycin for severe Clostridium difficile infection: implications for antibiotic stewardship programs. Pharmacotherapy 2012;32:129–134. [DOI] [PubMed] [Google Scholar]
- 13. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;66(7):e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xia Y, Tunis M, Frenette C, et al. Epidemiology of Clostridioides difficile infection in Canada: a six-year review to support vaccine decision-making. CCDR 2019;45:191–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rea MC, Dobson A, O’Sullivan O, et al. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc Nat Acad Sci 2011;108 suppl 1:4639–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One 2010;5:e9836. [DOI] [PMC free article] [PubMed]