Abstract
Bioactive components such as polyphenolics, flavonoids, bioactive peptides, pigments, and essential fatty acids were known to ward off some deadliest diseases. Nutraceuticals are those beneficial compounds that may be food or part of food that has come up with medical or health benefits. Nanoencapsulation and nanofabricated delivery systems are an imminent approach in the field of food sciences. The sustainable fabrication of nutraceuticals and biocompatible active components indisputably enhances the food grade and promotes good health. Nanofabricated delivery systems include carbohydrates-based, lipids (solid and liquid), and proteins-based delivery systems. Solid nano-delivery systems include lipid nanoparticles. Liquid nano-delivery systems include nanoliposomes and nanoemulsions. Physicochemical properties of nanoparticles such as size, charge, hydrophobicity, and targeting molecules affect the absorption, distribution, metabolism, and excretion of nano delivery systems. Advance research in toxicity studies is necessary to ensure the safety of the nanofabricated delivery systems, as the safety of nano delivery systems for use in food applications is unknown. Therefore, improved nanotechnology could play a pivotal role in developing functional foods, a contemporary concept assuring the consumers to provide programmed, high-priced, and high-quality research toward nanofabricated delivery systems.
Keywords: nano-formulation, nutraceuticals, prebiotics, nanofabricated delivery system, liposomes, nano-emulsions
1. Introduction
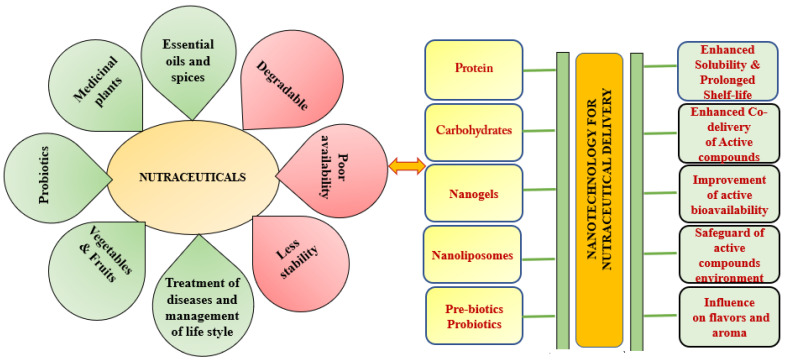
According to the description placed by National Nanotechnology Initiatives (NNI) in 1999, nanotechnology is defined as the appliance of systematic scientific knowledge to operate and manage matter to the nanoscale range to draw on size and structure-dependent properties and phenomena [1]. Food nanotechnology is an upcoming, promising, and fast-growing field bound to an extensive range of disciplines and has established an assortment of applications in the field of food sectors. Food nanotechnology has come into play for the last 10 years and globally opened up new opportunities and promises for novel discoveries in the Food industry. Numerous bioactive components from the Foods are well known from the ancient time for their beneficial activity for maintaining a healthy lifestyle, management of diseases, and also for their in-vitro biological functionalities; in contrast to this, many of the bioactive ingredients are not inevitably active after being ingested or consumed as part of food ingredients. The modern medicine system includes expensive disease treatment methods. People are unsatisfied with moving toward alternative substitutes (beneficial products or prebiotics and probiotics) in maintaining a healthy diet to protect against diseases [2]. Nutraceuticals are beneficial compounds generally attained from nutrients, herbal products, dietary supplements, and diets for genetically engineered “designed foods”. Food products include soups, cereals, and beverages [3,4].
Physico-chemical stability of these bioactive compounds and nutraceuticals are significantly affected by the harsh environmental condition during production, processing, and storage. In the meantime, due to the physiological conditions generated within the body system during digestion and absorption, the organic functions of bioactive compounds are altered. With an ever-rising demand for healthy foodstuff, substantial interest has been drawn to renovating food processing technologies to build up novel, purposeful, or functional food products with enhanced health benefits. In this perception, nanotechnology or nanofabricated bioactive compounds/nutraceuticals with superior delivery strategies have been launched in the field of the food sector to improve the biological efficacy and physicochemical stability of naturally available bioactive compounds/Nutraceuticals in food and also to shield the food quality [1,3]. Nanofabrication is a promising and exceptional tool in food biotechnology to augment the efficiency of biomolecules in the past, present, and future.
2. Nano Formulation of Bioactive Compounds
Bioactive compounds such as vitamins, flavonoids, phenolics, proteins, etc., are essential for proper cell growth and differentiation in living organisms; these also play a crucial role in combating diseases by scheming biological mechanisms (Figure 1 and Table 1). Recent studies on coupling the bioactive compounds with existing drugs such as cyclophosphamide and paclitaxel have enhanced breast cancer’s healing effect [13]. Consequently, combining medications with bioactive compounds opens a new window in the medical field to treat various diseases. In essence, the formulation of bioactive molecules and drugs also complements the negative aspect of the overdose of drug molecules [14]. On the other hand, the desired amount of bioactive compounds is not consumed in some cases. As a result, unregulated uptake also leads to deterioration in health. Henceforth functional food or food supplements are formulated to tackle these issues. Out of this biological importance, other fields such as biomedical, optoelectronics, and health care with light modification and functionalization were also the beneficiaries. They offered an excellent demand for bioactive compounds. Hence the development of nano-formulation bioactive compounds with an improved adaptation of nanofabrication tools of delivery strategies is in great need or challenge in scientific research [14,15]. Advancement in nano-formulation procedures certainly reduces the limitation of bioactive molecules such as bioavailability, specificity, and solubility. That paves new insights into discoveries of novel materials principally apt for diverse biomedical applications (Table 2).
Figure 1.
Bioactive compounds for different applications.
Table 1.
Biochemical profiling of principle bioactive compounds and their functions.
| Bioactive Compounds |
Health-Promoting Property | Occurrence | Molecular Weight (g/mol) |
Structure | Reference |
|---|---|---|---|---|---|
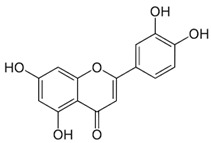
| Quercetin | Promoting cardiovascular health properties and helping in blood flow. | Fruits and vegetables, especially in onions, grapes, lemon tea, citrus, etc., | 302.236 |
|
[5] |
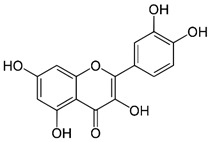
| Luteolin | Anticarcinogenic activity |
Green pepper carrots, Broccoli, oregano | 286.24 |
|
[6] |
| Kaempferol | Antioxidant activity and Anticarcinogenic activity | Tomatoes, apples, grapes, green tea, broccoli, lettuce, peaches | 286.23 |
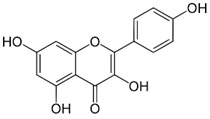
|
[7] |
| Curcumin | Antibacterial activity, antioxidant activity, and anti-inflammatory activity | Turmeric | 368.38 |
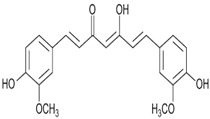
|
[8] |
| Berberine | Treatment of breast cancer, colon cancer, pancreatic cancer, gastric cancer, liver cancer, oral cancer, etc., | Widely present in barks, leaves, twigs, rhizomes, roots, and stems of several medicinal plant species. | 336.3612 g/mol |
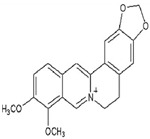
|
[9] |
| Rutin& Quercetin | Protection against cancer and some other diseases. Lowers cholesterol, mainly used in skin aging. | invasive plant species, Carpobrotus edulis | 610.517 g/mol |
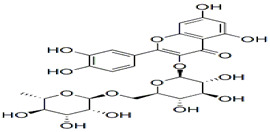
|
[10] |
| Astaxanthin | Protection from UV skin damage, Reduction in inflammation, Supports Immune system. | algae, yeast, salmon, trout, krill, shrimp, and crayfish | 596.841 g/mol |
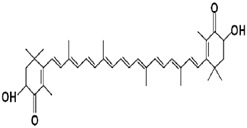
|
[11] |
| Vitamin E | helps maintain healthy skin and eyes, and strengthen the body’s natural defense against illness and infection (the immune system). | Plant-based oils, nuts, seeds, fruits, and vegetables. | 430.71 g/mol |
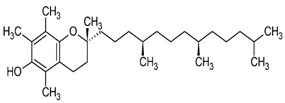
|
[12] |
Table 2.
Various nano-formulations systems of different bioactive compounds and their functional and physical properties.
| Nano formulated Bioactive Compounds | Solubility | Stability | Nanofabricated Method/Source | Bioavailability/Release Kinetics | Main Findings | References |
|---|---|---|---|---|---|---|
| Rutin and Quercetin | Water-insoluble | Sensitive to light, oxidation, pH | PLGA | Release of quercetin ~64% after 3 days of injection, in vivo | Study of anticancer activity | [16] |
| Curcumin | Poor water solubility | Sensitive to oxygen, light | Curcumin Hydrogel beads (CHBs) | Cucumin release was 67% after 2 h, and 67% after 4 h | Mask bitterness, enhance solubility, and increase the bioavailability | [17] |
| Vitamin E | Water- insoluble | Sensitive to oxygen, light, pH | Oil –in water emulsion | Bioaccessibility of developed emulsions was in the range of 65–85% | Increased storage stability (Vitamin E fortified emulsions) | [18] |
| Vitamin E | Water -insoluble | Sensitive to oxygen, light, pH | Spiral dextrin inclusion complexes | 95% of Vit E and 98% of soy isoflavone released after 80 min | Study of release kinetics of bioactive compound and Antioxidant capacity during the simulated gastrointestinal tract | [19] |
| Curcumin | Water-insoluble | Sensitive to light, heat, iron ion | Spi-Fuc polymer-core-shell nanoparticles | Release rates of curcumin were 96.25% and 82.69% after 4 h | Stability studies, delivering lipid soluble active ingredients | [20] |
| Curcumin and piperine | Water-insoluble | Sensitive to light, heat, iron ion | Nanoemulsion | Release of curcumin 40%, release of piperine 7.5% after 72 h | The activity of curcumin on HCT 116 Colorectal Cancer Model | [21] |
| Kaempferol glucoside | Water insoluble | Sensitive to oxidation, light, pH | Gold nanoparticles | - | Catalytic, antioxidant, and anticancer activities of gold nanoparticles | [22] |
| Berberine | Low water solubility | Sensitive to heat and pH | Liquid crystalline nanoparticle | 80% of berberine released after 24 h | Anticancer activity in MCF7 human breast cancer cells | [23] |
| Carvacrol and linalool | Water insoluble | Sensitive to oxidation, light, pH | β-cyclodextrin-grafted chitosan | Carvacrol released—49% after 600 min, linalool released—71% after 460 min | sustainable biopesticide aiming pest control | [24] |
| Astaxanthin | Low water solubility | Sensitive to oxygen, light, heat, pH | Lupin protein-based Pickering emulsion | Astaxanthin powder exhibited 80% bioaccessibility | Usage as a food ingredientLupin protein-based particles | [25] |
2.1. Nutraceuticals and Nanobiotechnology
Nutraceuticals are those beneficial compounds that may be a kind of food or part of food that has come up with medical or health benefits. Prevention and treatment of diseases are also involved in these nutraceuticals [2,26]. Moreover, consumers are more conscious of their health care and its administration and management. In approach to modern medicine, people are discontented with expensive disease treatment, so people are often moved towards substitutes of beneficial products. This makes nutraceuticals predominantly appealing. Nutraceuticals are beneficial compounds generally attained from dietary supplements, nutrients, herbal products, and diets for genetically engineered “designs foods”. Also, food products such as soups, cereals, and beverages [4,27]. In the current years, researchers have bought with a new plan that put forth their effort in bringing up an improved diet by substituting modern medicine with natural products. Furthermore, the R & D related and nutraceuticals market is vastly evolving [28,29].
Nutraceuticals have been widely used in nanotechnology due to their ability to improve bioavailability, ingredient solubility, and stability. There are various unique properties associated with nanoparticles that involve small size and a high surface-to-volume ratio. Nanomaterials possess physio-chemical properties that show unfavorable effects on human health [30]. There are specific safety measures to bring up varieties of new marketable nutraceuticals, and formulation estimation of their efficacy is required. In this article, in agreement with European food safety Authority recommendations and high throughput screening fashion, they have worked upon an in-vitro-based approach that evaluates the safety and efficacy of new formulations.
This type of study plays a significant role in its time and cost-effectiveness. It is an essential tool that helps support companies as it comes up with the design of a safe concept presently under development in Europe [31]. In the field of nanoscience, food industries and nutraceuticals open a wide variety of technology to this. There has been explained with the new probability by nanotechnology in consultation to modified properties of nutraceuticals and its application in the development of nutritional supplements, food industry, and nano-formulations. In nanotechnology, nanoparticles have got various properties that complete the application in the food sector and nutraceuticals [32].
This review article is required to present advanced nano-based nutraceuticals with enhanced solubility, bioavailability, efficiency, improved encapsulation, sustained and targeted drug, increased stability, microbial contamination with improved pharmacological activity, and protection against degradation [33]. This article is also helpful in the knowledge of how nanomaterials for encapsulating enzymes, antibodies, peptides, etc., and nanoparticles play an essential role in the application of the food packaging industry. There are enormous benefits that have come across with nanotechnology and nanomaterials in health sectors by improving quality of life and treatment strategies [34].
2.2. Definition and General Classification of Nutraceuticals
In 1989, Stephen De Felice M.D derived the term nutraceuticals from ‘nutrition’ and ‘pharmaceutics’ [35]. A nutraceutical is defined as any substance that is a food or a part of food and provides health or medical benefits, including the treatment and prevention of disease. The term Nutraceutical can be used as a marketing term. Dietary supplements such as MSM, Glucosamine, and chondroitin are most popular and are used for joint health. Functional food for one consumer can act as a nutraceutical for one another. Examples of nutraceuticals include fortified dairy products (e.g., milk) and the city’s fruits (e.g., orange juice). Several nutraceuticals are studied under cancer therapies and are beneficial in curing those diseases [36,37]. Nutraceuticals’ definition mainly depends on their source. They are broadly classified primarily based on their pharmacological condition, the chemical constitution of the products, and natural source. Nutraceuticals are commonly classified into dietary supplements, medicinal foods, pharmaceuticals, and functional foods [38,39].
2.3. Dietary Supplements
Dietary supplements are the type of nutraceuticals that contain nutrients derived from food products. These dietary supplements are intended to complement or supplement the diet and are distinct from traditional foods [40,41]. Dietary supplements include capsules, pills, tablets, powders, liquids, or extracts. It contains many valuable supplements that include minerals, vitamins, herbs, amino acids, fiber, enzymes, and other plants. These dietary supplements are regulated or controlled as foods by the U.S. Food and Drug Administration [FDA]. Because of their critical role in cellular growth and biotransformation or metabolism, membrane shape, and function, LCPUFAs are extremely important for prenatal and infant growth and development [42]. Given that they are high in arachidonic acid and docosahexaenoic acid, the LCPUFAs (“walnuts, flaxseed oil, soybean oil and canola oil”) help in promoting brain growth and irreversible damage [43]. The five essential fatty acids for human consumption are saturated palmitic acid, stearic acid, monounsaturated oleic acid, polyunsaturated linoleic acid, and linolenic acid [44]. Arachidonic acid, eicosatetraenoic acid, and docosahexaenoic acid are newly synthesized secure, less exorbitant, and feasible plant-based products of these fatty acids [45]. Higher concentrations of eicosatetraenoic acid and docosahexaenoic acid were generated in Brassica juncea through the use of an extreme acquiesce [43]. These dietary supplements also include medicines and other products [46].
2.4. Functional Foods
Functional foods offer health benefits beyond their nutritional value, including whole foods and fortified [47,48]. Proponents of functional foods also enriched with dietary components say that they promote optimal health and may help reduce the risk of chronic diseases. It gives health benefits compared to its traditional nutrients [49]. According to the Canadian Health Organization (CHO), functional foods are “regular recipes that seem to have elements introduced in order to give it a proper scientific or physiological advantage, aside from a merely dietary effect”. According to Japan, all pragmatic ingredients have to be available and arising in their herbal version [43] instead of a tablet, capsule, or powder [50] consumed in the nutrition plan on even a regular or daily basis [51] and should revise a biological system in order to avoid disease or disorder-like instances [43].
2.5. Medicinal Nutraceuticals and Pharmaceuticals
Medicinal foods are specially formulated and intended for the dietary management of a disease that has acquired distinctive nutritional needs and cannot be met by the regular diet itself [52]. These medicinal foods are absorbed internally under the consultation of a qualified physician. It acts as precise dietary management of a particular disease under which the nutritional requirements of individuals are confirmed by medical evaluation and based on recognized scientific principles [53]. Pharmaceuticals are used in diagnosing, preventing, or treating the disease and for modifying, restoring, and correcting organic functions [54]. These are enormously helpful medical products obtained from animals or crops. The term is derived from the combination of the words ‘farm’ and ‘pharmaceuticals’ [55]. Consumers’ well-being is increasing, with a focus on scientific research and asserts reassurance of bioactive molecules, along with novel therapeutic dose and distribution forms [56,57]. The ability of innovative medical and drug technologies to diagnose disease and eventually expand the average lifespan has also provided us with a unique outlook. All food products could have nutritious, sensory characteristics, and preventative measures features. Countless biologically active components have already been commercialized as pharmaceuticals (pills, capsules, solutions, gels, liquors, powders, granules, etc.). These are known as “nutraceuticals”, and they help to improve public health. These provide a physiological reward or grant immunity against serious illness, support healthy, and slow the aging process [56,57].
2.6. Role of Nutraceuticals in Human Health
Nutraceuticals are concentrated compounds from food sources that can provide health benefits, including disease prevention and health promotion [58]. Examples of nutraceuticals include substances such as antioxidants, probiotics, and omega 3. It combines nutrition and pharmaceuticals [59]. Nutraceuticals play a preeminent, significant, and valuable role in maintaining normal physiological function, which helps to keep healthy human beings; nutraceuticals are part of food [60]. There has been a fast growth in the nutraceuticals market worldwide that are present in the population and the health trends. In nutraceuticals, the food products used can be classified as fiber, polyunsaturated fatty acids, and other diverse types of herbal/natural foods [61]. Nutraceuticals are the source that is crucially utilized to regulate some of the century’s major problems that involve cancer, diabetes, cardiovascular diseases, osteoporosis, cholesterol, arthritis, etc. [62,63]. Furthermore, nutraceuticals have led to a new era of health and medicine in the food industry that has become a research-oriented sector. In this decade, people are more conscious of the correlation between life-threatening diseases and eating habits differing and increasing daily [29]. For this reason, people have become more attentive to the quality consumed and show interest in the foods that have beneficial effects on one’s health and avoid the onset of life-threatening diseases [64,65]. The above features help the researchers study the potential effects of beneficial nutraceuticals on their mechanism of action and human health. On an identical note, industries, mainly the food industry, were much more prominent and were interested in developing food products that attract consumers or people to purchase them for their health [66,67].
It is a comprehensive technology that has allowed researchers to improve enormous limitations related to the use of nutraceuticals to retinue their encapsulation into these structures, which involves their stability, poor bioavailability, and low solubility [68,69]. It is mandatory to know about the fact of bioavailability on a notice that it’s a fraction of taken compound, which is absorbed and available for physiological functions and is the critical characteristic for nutraceuticals compounds, because of its effectiveness which is strictly associated to their bioavailability [70,71]. Few other important factors deal with the bioavailability of nutraceuticals, and the factors are various endogenous and exogenous, which are involved in the biochemical transformation that undergoes into food storage, Epithelial cells, physio-chemical features, and so on [72,73,74]. Due to this, more advanced technologies have been established to exert their beneficial effects when introduced into the organism. Different nano-formulations have been developed between them to increase the needful effects of nutraceuticals [75,76]. In the presence of the highest quantity of bioavailability, there promotes a controlled release and targeted delivery of encapsulated bioactive compounds, which increases the biological efficacy of food. Nanotechnology develops the framework of the agriculture and food industry by offering various advantages [77,78]. To design the nutraceutical delivery system, multiple criteria should be enrolled into an account which involves the formulation it must have good physical and chemical properties, sustainable production costs, and food-grade materials used [79,80].
2.7. Favorable Nutraceuticals Properties on Human Health
Nutraceuticals are those substances that have a physiological benefit or protect against chronic diseases. Most therapeutics are involved in it and have had various beneficial effects on human health in recent years. These includes diabetes, arthritis, inflammation, cholesterol, and many other disease conditions [81,82]. One of the best nutraceutical products is natural honey which can be used to feed infants, as it is known to be a food with great benefits and beneficial nutritional properties for human health [83]. Natural honey is made up of antioxidants and enzymes that are essential to the digestive process. Using raw honey allows for cloudiness. Since the extract inhibits the angiotensin-I converting enzyme (ACE1), it is also used to treat high blood pressure. Finally, it is important to emphasize that the skin of the allis shad, which is a nutraceutical, has a reduced benefit compared to some traditional medicines that have been used in the treatment of type 2 diabetes as conditions such as cardiovascular disease and eye disease Side effects on the patient provides problems and ulcers [84]. The honey obtained has antimicrobial activity and can be used to disinfect the infected wound [85]. Thanks to the increase in experimental evidence of the efficacy of nutraceuticals in the prevention or treatment of various pathological conditions, numerous efforts have been made to apply nanotechnology to encapsulate these natural products [86]. A combination of nutraceuticals (bergamot extract, chlorogenic acid, phytosterols, and vitamin C) was administered to overweight subjects with dyslipidemia, increased glucose, and lipid metabolism compared to subjects treated with overnight placebo [87]. Numerous scientific studies have conclusively shown that controlling blood cholesterol levels can reduce the likelihood of developing cardiovascular diseases [88,89,90]. It is unacceptable that the food industry is responsible for producing a significant part of its waste. It has a major impact on the environment and can be used as an essential source of nutraceuticals. The example concerns a citrus peel, fruit waste rich in phenolic compounds with strong antioxidant activity [87,91]. The antioxidant activity of natural compounds has multiple beneficial effects on human health [92].
Antioxidants act as a barrier to protect the organism and as free radical scavengers, preventing oxidative damage [93,94]. In addition, their effectiveness helps prevent cancer and cardiovascular disease, and their anti-inflammatory effects and ability to establish and prevent Alzheimer’s disease are better known [95]. It has been scientifically proven that phenolic compounds isolated from the lily of the valley (Citrus maxima) benefit citrus peel. Honey, for example, has a significant antibacterial effect and can be used to disinfect infected wounds. It also shows inhibitory activity against amylase and glucosidase, indicating its usefulness in treating type 2 diabetes [96]. The extract is also used to treat high blood pressure as it inhibits angiotensin I converting enzyme (ACE1) [97]. Finally, because the shells of the Maddocks are marked. Since the extract inhibits the angiotensin-I converting enzyme (ACE1), it is also used to treat high blood pressure. Finally, it is important to emphasize that as a nutraceutical, the shell of the allis shad offers the benefit of reducing side effects for the patient; compared to some traditional drugs that have been used as nutraceuticals in the treatment of type 2 diabetes, it has the advantage of having fewer side effects on patients than other typical drugs used to treat type 2 diabetes [98]. Another study by Barreca et al. [99] showed how antioxidants can be extracted from pistachio waste; Only by using organic solvents was it possible to extract about 20 compounds with cytoprotective and antioxidant properties from the ripe pistachio shell [100]. They are catechin, gallic acid, isoehamnetin-3-O-glucoside, quercetin-3-O-rutinoside and naringin [101]. In addition, nectarines, olive leaves, and tomato skins were considered, since the new claims of nutraceuticals and additives [102] have currently been detected in the polyphenolic extracts from these food wastes, which confirmed a specific positive activity in insulinemia and postprandial glycemia [103].
3. Nanotechnology as a Nutraceutical Properties Enhancement Strategy
Credit to the increase of experimental data on the efficacy of nutraceuticals in preventing or treating several pathological states [97], a great deal of effort has gone into applying nanotechnology to encapsulate such natural products [104]. The encapsulation era is a very promising method in nutraceutical delivery because it offers several advantages [105]. A unique interest has been drawn internationally to Eucalyptus plants in various fields of industry. Amongst them are prescription drugs, perfumery, nutraceuticals, and fixtures. Consequently, they represent a quick-developing supply of wood and a source of oil used for several [106].
Nanoencapsulation techniques produce active component nanosuspensions with a coating or encapsulated with either wall materials in dried or liquid [107] The chemical structure of nutraceuticals can be safeguarded with the possibility of associated environmental agents, which involves light, oxygen, pH, radicals, and temperature. It enables site-specific delivery, increases bioavailability, and permits the controlled release of encapsulated compounds (Figure 2) [108]. Regarding the ability of nanosystems to withstand the release of supplied active compounds, it is bound to the beneficial site of action and encapsulation of nutraceuticals with a specific time or concentration release profile, and it is the main challenge that has to be performed [109].
Figure 2.
Applications of nutraceuticals and nanotechnology for nutraceuticals delivery.
Subsequently, an ideal delivery system must exist and be able to release its contents, leading to certain stimuli that involve enzymes, moisture, pH, temperature, and preserving nutraceuticals from the same stimuli [55]. In addition, the encapsulation of nutraceutical compounds leads to an enrichment of their solubility; as a result, when nutraceuticals are loaded into the carrier, the characteristics obtained depend in particular on the physicochemical properties of the vesicle relative to an entrapped compound [96]. Nanosystems also offer the possibility of adding lipid and water-soluble molecules, thus supporting their synergistic effect [110]. They also show promise in physiochemical stability and help avoid undesirable taste and odor changes that can result from adding nutraceuticals to food products. In a drug delivery system, the materials used to implement it can be of a variety of natures (lipid, polymeric, protein), so long as they are generally recognized as safe status (GRAS) [111]. In addition, the mode of drug delivery and its composition are preferred based on the chemical and physical properties of the encapsulated compound, the target to be reached, and its application type [112]. Accordingly, nanoencapsulation of nutraceuticals has been shown to have beneficial adverse effects on human health, thus helping to reduce side effects [96]. Main nanocarriers, mainly used for encapsulating nutraceuticals, have to be analyzed.
3.1. Nano Probiotics
Micro and nano probiotics are live micro-organisms intended to have health benefits when consumed or applied to the body. Examples of probiotics involve yogurt, kefir, sauerkraut, tempeh, kimchi, etc., probiotics have become a field of interest to research in recent times [113]. It is considered a desirable alternative to antibiotics because of their beneficial effects on the economy and the safety of farm animals [114]. The International Scientific Association for Probiotics and Prebiotics (ISAPP) has introduced a wide range of products containing probiotics that have to contain health-promoting effects. It includes drugs, baby formulas (e.g., first milk), therapeutic supplements, and foods (e.g., fermented dairy with reportedly health-beneficial effects) [115]. Probiotics come from different sources that involve several foods, natural environments, and human guts. The main property of probiotics is the ability to survive through the gastrointestinal tract; it promotes health benefits to the host etc., [116] (Table 3). The probiotics can also include fungal and yeast species such as Saccharomyces cerevisiae and Kluyveromyces.
Table 3.
List of probiotics and their applications.
| Type | Micro-Organisms | Activity | Study | Reference |
|---|---|---|---|---|
| Probiotic | Lactobacilli plantarum C70 | Anticancer effect | Lactobacilli plantarum C70 by releasing the exopolysaccharide, caused 73.1% and 88.1% cytotoxic properties against breast and colon cancers, respectively. | [117] |
| Probiotic | Lactobacilli cocktail | Anticancer effect | HT-29, a human colorectal carcinoma cell line, was controlled by Lactobacilli cocktail via the modulation of the Notch and Wnt/β-catenin signaling pathways | [118] |
| Probiotic | L. rhamnosus | Anticancer effect | The bioconversion of cranberry proanthocyanidins to Lactobacillus rhamnosus could result in the IC50 values of 20.1 and 47.8 µg/mL | [119] |
| Probiotic | Eurotium cristatum | Anti-obesity effect | The administration of Eurotium cristatum showed anti-obesity activity in mice fed a high-fat diet (HFD) through the modulation of gut microbiota | [120] |
3.2. Nano Prebiotics
Nano-prebiotics are foods that promote the growth of beneficial bacteria in the gut. These are a type of fire that the human body cannot digest; they serve as food for probiotics and are tiny living organisms that involve bacteria and yeast (Table 4).
Table 4.
List of prebiotics and their applications.
| Type | Active Compounds | Activity | Study | Reference |
|---|---|---|---|---|
| Prebiotic | Chondroitin Sulfate Disaccharide | Anticancer effect | The growth of HT-29, human colon cancer cell line, was controlled by Chondroitin sulfate (CS)-Keel disaccharide (CSD) generated by chondroitin AC lyase, estimated at 80% antiproliferative activity. | [121] |
| Prebiotic | Blueberry anthocyanins | Antioxidant effect | The density and composition of intestinal microbiota in human models were increased by consumption of high purity blueberry anthocyanins through the increase in the modulatory and prebiotic activities. | [122] |
| Prebiotic | Short-chain fatty acids | Antiproliferative effects | The administration of short-chain fatty acids (SCFAs) prevented the expression of genes involved in the human colorectal cancer cell. | [123] |
| Prebiotic | Oligosaccharides | Antioxidant effect | The water-soluble oligosaccharide of EMOS-1a showed a 1420% proliferation level | [124] |
Prebiotics are found in various sources, including non-digestible carbohydrates, non-digestible oligosaccharides, unrefined wheat, unrefined barley, and soybeans [125]. Some compounds found in prebiotics are xylooligosaccharides, lactulose, and non-starch polysaccharides (NSP) [126].
3.3. Food Grade-Nanofabricated Delivery Systems
Incorporating functional food components for the better functionality of food products to meet consumer needs for a healthy lifestyle is in great demand. It has become a delightful trend in the market. These programmed food or functional food components is not limited only to their inhibitory action against unsolicited microbial growth. Still, it enhances the product’s taste, flavor, and color and attributes beneficial health effects. Active food constituents possess some properties such as low water solubility, unappetizing sensory character, constrained oral bioavailability, and intolerance to chemical degradation, so most of these functional food constituents are antagonistic to the food matrix. Nanoencapsulation technology deals with encapsulating bioactive compounds using different nano-fabricated delivery systems [127]. Even though these delivery systems can be used as biocompatible and biodegradable to make them reachable for food applications, these delivery systems are generally classified into carbohydrates, lipids, or protein-based [128].
3.4. Carbohydrate/Polysaccharides Delivery Systems
Carbohydrate delivery systems can interact with a wide range of bioactive components because these carbohydrate-based delivery systems possess exceptional properties like biodegradability, abundance, and a high potential for adaptation to enviable functionality so that these delivery systems are more appropriate for high-temperature stability over lipid and protein-based carriers which undergo degradation and denaturation. Cyclodextrin (α, β, γ) is a carbohydrate-based delivery system. It is a prominent condensed cone-shaped oligosaccharide with a hydrophobic central cavity and hydrophilic end on the other. So, in carbohydrate-based delivery systems, the present trend is to study these cyclodextrins. These are useful for the confinement of less soluble, temperature susceptible, or chemically liable food bioactive. On the other hand, a mixture or combination of two or more carbohydrate-based delivery systems gives inventive frontiers for increased efficiency. Nanofabrication techniques like spray drying, electro-spraying, coacervation, and electro-spinning are extensively used to fabricate carbohydrate-based delivery systems [129,130] (Table 5) (Figure 3).
Table 5.
Nutraceutical encapsulation by the carbohydrate-based delivery system.
| Nutraceuticals | Encapsulating Materials | Encapsulation Techniques | Target | Reference |
|---|---|---|---|---|
| Catechin | Azivash (Corchorus olitorius L.) gum-polyvinyl alcohol | Electrospinning process | Simulated gastric fluid and simulated intestinal fluid, EE%. | [131] |
| Hesperetin (HSP) | Nanofibers: Basil seed mucilage/polyvinylalcohol | Electrospinning | Characterization of nanofibers. Release models, EE% | [132] |
| Rutin | Quinoa and maize starch NPs | Ultrasonication | Characterization, EE%, simulated in vitro digestion. | [133] |
| Saffron Bioactive components | Nanoparticles: chitosan (CS) and gum arabic (GA) | Ionic gelation (IG) | Characterization NPs, EE%, Release of Saffron in acidic and natural media | [134] |
Figure 3.
Polysaccharides-based nanofabrication systems.
3.5. Protein-Based Delivery Systems
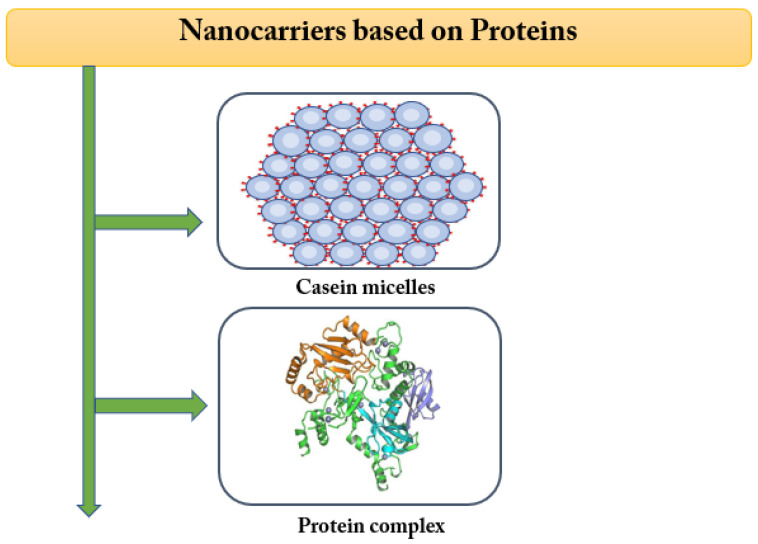
Protein-based delivery systems also have captivated great recognition. The exterior surface of protein-based delivery systems possesses many functional groups that facilitate their ability to encapsulate both hydrophilic and lipophilic bioactive constituents. They are mainly obtained from plant, animal, and microbial sources. Coacervation, gelation, spray drying, and electro-hydrodynamic process are commonly used methods for fabricating protein-based delivery systems. [135]. In recent years plant-protein-based delivery systems have also emerged in the field of nanotechnology to shield and manage lipophilic bioactive compounds (polyphenols, polyunsaturated fatty acids, vitamins, carotenoids), has increased attention in nutraceuticals, food, and pharmaceutical fields.
The promising role of plant proteins from legumes, nuts, tuber, oil/edible seeds, and cereals is aggravated by their sustainable, eco-friendly, and vigorous nature collating with other sources. Before using raw materials carriers, particular challenges need to be addressed to overcome the adverse consequences by modifying approaches to improve their techno-functionality and limitations, aiming to fabricate innovative production of plant-based carriers (emulsions, hydrogels, structures, and self-assembled films). This plant protein delivery system is a limited application that only acts as a carrier for lipophilic compounds. Nevertheless, bottomless insights into new purification or extraction technologies and protein sources are necessary to validate their functional properties for the evolution of an effective delivery platform [136] (Figure 4).
Figure 4.
Protein-based nanofabrication system. Adapted with permission from Ref. Singh et al. [136]. Copyright © 2021, Controlled Release Society).
The spatial shape, size, colloidal stability, water dispersibility, etc., of protein-based nanocarriers, were determined by the physicochemical molecular principles of protein. Some of the available preparative methods could be used to fabricate several protein-based nanocarriers, including antisolvent precipitation, pH–driven, gelation, and electrospray methods. Recent studies have evidenced that protein-based nanocarriers provide numerous advantages in aid of the application of bioactive compounds to the food, medical, and cosmetic sectors [137].
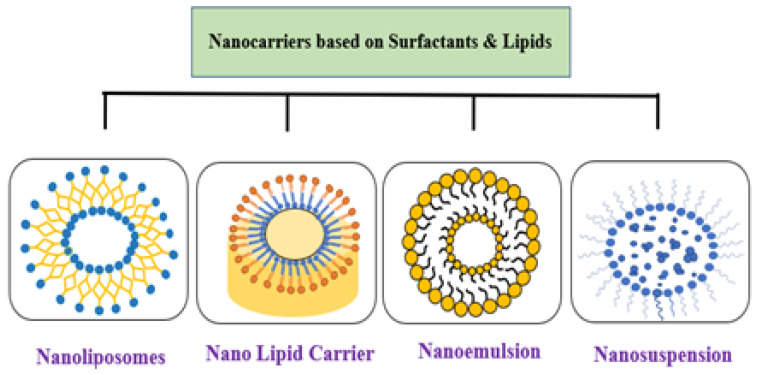
3.6. Lipid-Based Nano Delivery Systems
Compared to protein and carbohydrate-based delivery systems, the lipid-based nano delivery system is the most auspicious encapsulation system that offers peerless advantages over the other two systems due to its ability to entrap materials with different solubilities enhanced targeted delivery and ability to guard a constituent against free radical’s pH and enzymes [138]. Lipid-based nanocarriers have been fabricated for food applications such as nanoemulsions, nanoliposomes, nanostructured lipid carriers (NLC), and solid lipid nanoparticles [139] (Figure 5).
Figure 5.
Lipid-based nanofabrication system.
3.7. Nano-Emulsions
Nano-emulsions are liquid droplets with colloidal dispersion sizes less than 100 nm. They can be either oil-in-water or water-in-oil (w/o) [140]. They are made by entrapping bioactive constituents within the distribute phase, which is self-stabilized by surfactants/biopolymers in the continuous phase [141]. They are made by entrapping bioactive constituents within the distribute phase, which is self-stabilized by surfactants/biopolymers in the continuous phase [141]. These can be manufactured using high-energy emulsification techniques such as homogenization, ultrasonication, and microfluidization.
3.8. Nanoliposomes
Liposomes are nano-sized spherical vesicles composed of a phospholipid, a lipid bilayer with an aqueous core. Nanoliposomes have a superior surface area and tolerable stability profile to safeguard their size within nanometric scales such as small liposomes (20–100 nm) and large liposomes (>100 nm) [105]. These carries mainly contain lipids and phospholipids. Conversely, some include molecules such as antioxidants, carbohydrates, protein, or sterols in their structure. Their amphiphilic nature makes them potent nanocarriers since these have the potential to encapsulate and massive release hydrophobic and hydrophilic compounds concurrently, providing mutual benefit. Distinguishing phospholipids/bilayer structure is highly attuned to the skin surface, allowing them to act as penetration enhancers of nutraceuticals and bioactive compounds towards targeted sites [142]. Some techniques such as microfluidization, extrusion, and ultrasonication are usually used for nanoliposomes production [143]. The main restrictions lie with poor solubility towards low pH and enzymes after intake, low loading ability, and higher cost of food-grade fabrication wall materials. The present trend is to tackle these barriers for the expansion of nanoliposomes optimistic nanocarriers on a large scale [144].
Examples of drugs in liposomal carriers are doxorubicin, amphotericin B, nystatin, and vincristine. In addition, ZawnVillines 2018 [145] has reported that carotenoids encapsulated in lipid bilayers can impair carotenoid stability. This, in turn, affects the properties of liposomes. This is because types of carotenoids and different concentrations could modulate the structure, hydrophobicity, and dynamics of the liposomal membrane [146]. In turn, the structural integrity of the liposome bilayers can protect the encapsulated compounds from degradation, thereby enhancing their physicochemical stability. In recent years, some liposomes have been manufactured for the simultaneous delivery of bioactive compounds. For example, pectin-coated phosphatidylcholine (PC) liposomes have been engineered to encapsulate two important lysozyme and nisin compounds [147].
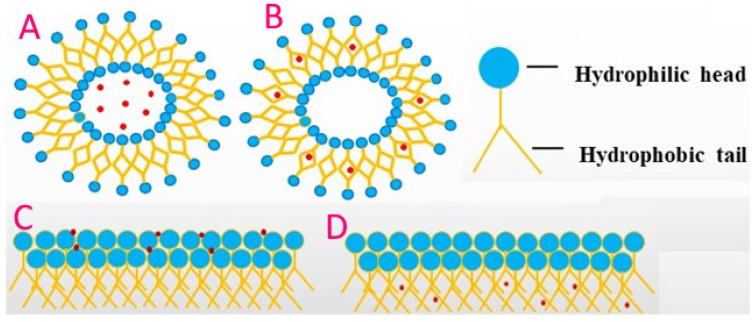
Furthermore, liposomes containing both the compounds lysozyme and nisin showed higher stability and antimicrobial activity compared to liposomes containing lysozyme, representing a promising co-delivery system for bioactive substances [148]. Water-soluble vitamin E derivative of PEG, d--tocopheryl polyethylene glycol 1000 succinate (TPGS). Such fabricated liposomes are more stable in biological environments and have proven to be a valuable, safe tool for incorporation into liposome membranes [149]. Some of the beneficial activities of liposomes include increased bioavailability and absorption rate compared to oral supplements; The micronized encapsulation protects against the harsh environment of the gastrointestinal tract and improves absorption [150]. It also has the potential to hold both hydrophilic and hydrophobic molecules. A list of Nutraceuticals encapsulated by liposomes is shown in Figure 6.
Figure 6.
Schematic representation of bioactive compounds encapsulated in various nano-fabricated liposome systems. (A), bioactive compound entrapped in the interior of liposome region; (B), bioactive compound entrapped in the fatty acid region of liposome region; (C), bioactive compound entrapped in the hydrophilic head region of phospholipid bilayer; (D), bioactive compound entrapped in the hydrophobic head region of the phospholipid bilayer.
3.9. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carrier (NLC)
(SLNs) and (NLC) are the other two important lipid-based nano delivery systems. SLNs are submicron emulsions established by homogenizing two immiscible phases in the presence of amphiphilic surfactant. These SLNs facilitate the maintenance of the liquid phase during the process. The lipid phase is melted at an elevated temperature to offer higher encapsulation efficiency, a slower degradation rate, and flexibility in the release profile [151]. The commonly used method for large-scale production of SLNs for food processing homogenization, the major problem associated with SLNs is the low encapsulation load. So that the manufacturing of nanostructured based lipid carrier as partially crystallized lipid nanocarriers provide an effective solution with enhanced physical stability, improved dispersibility, and higher encapsulation load as well as to move up a remarkable trap of hydrophilic and hydrophobic nutrients [152,153,154].
3.10. Nanogels
Nanogels are nanoparticles synthesized by combining hydrogel and a cross-linked chemically or physically with hydrophilic polymer chains [155]. It also signifies a biocompatible and biodegradable drug delivery system obtained from a nano-sized polymeric network; the swelling attains it under the action of a suitable solvent [156]. There are different ways to be administered these drug delivery systems. Nanogels can be expressed as they must be responsive to various stimuli involving magnetic field, temperature, and pH [157]. For example, the nanogels, which are made of dextran and ovalbumin, were produced by Maillard reaction and were effectively employed to improve curcumin bio-accessibility in the gastrointestinal tract, as was established in the form of an in vitro reproduced model [137].
4. Future Perspectives and Regulatory Outlook
The toxicological valuation of nano-fabricated material and their different delivery systems for bioactive compounds and nutraceuticals holds immense importance and vigilant assessment. The utilization of nano-fabricated materials may be allied with numerous safety reservations and necessitate implementing practices that perilously take safety and human health into consideration. The use of nano-fabricated materials in the food industry in food packaging is efficiently browbeaten with rapid growth but still coupled with end-user regulatory and safety issues that should be assessed and addressed critically [158] (Figure 6).
Health concerns, authoritarian policies, and safety must be well-thought-out during the production, processing, and wrapping of nano-based programmed food products. In terms of regulatory aspects of nanomaterials and nanostructure in the medical and food sector, there is no explicit legislation applied worldwide. Many countries still do not have specific authorization for nano-encapsulated products’ safety and risk assessments. The lack of reliability in the functional switching information between the countries is an outlined risk to mankind and the environment. It may perhaps minimize the promotion of novel and valuable products globally [159]. Due to the lack of specified regulations for food nanotechnology, European Union regulations and U.S. Food and Drug Administration has established guidelines regarding the use of nanotechnology in food and to get safety assessment approval before its use. In one of the articles on the nature of nanotechnology, Steffen Foss Hansen put forth a framework named ‘React Now,’ which stands for (Registration, Evaluation, Authorization, Categorization, and Tools to Evaluate Nanomaterials Opportunities and Weaknesses). All users of nano-fabricated materials should be evaluated according to the Nano-Risk-Cat-methodology [159]. Therefore, organizations and companies functioning with nano-fabricated materials have to carefully think about all the above-mentioned aspects to contest food safety concerns and ensure successful promotions of nano-fabricated programmed food products or nutraceuticals in the marketplace.
5. Conclusions
In this review, we focused on the biological functionality of bioactive compounds and nutraceuticals and their beneficial activity for maintaining a healthy lifestyle. Nutraceutical compounds derived from natural origin can treat several pathologies, inflammation, cancer, and cardiovascular diseases. In contrast, we reviewed the physicochemical instability of these significant compounds and declined bioavailability due to environmental conditions. Furthermore, we addressed the promising contribution of food technology throughout the sustained release/targeted delivery of bioactive compounds/nutraceuticals and about their defense from harsh environments through the use of various nanofabricated delivery systems. Various delivery systems such as carbohydrates, proteins, lipids (nano-emulsion, liposome-mediated delivery systems), and many other techniques have drawn scientific interest and allowed researchers to eradicate many of the limitations in using nutraceuticals. Food nanotechnology could be an alternative method to generate future trends in nutraceuticals and uphold bioactive compounds’ stability. However, certain regulatory aspects and safety issues need to be addressed by the European Union regulations and U.S. Food and Drug Administration to make the best use of nanofabricated materials as nanofabricated delivery systems to enhance the activity and retain the shelf-life of bioactive compounds and nutraceuticals to treat various disease and to protect or improve food constituents, flavor and taste for the betterment of mankind in maintaining a healthy standard of living.
Acknowledgments
We are very thankful to Bhagya Lakshmi Neel Warne, for her timely support of the corrections of the manuscript.
Author Contributions
Conceptualization, V.B.R., M.S. and K.S.; methodology, R.P., R.L. and N.L.S.; software, V.K.G., B.S.I. and Z.U.; validation, V.K.G., V.B.R., B.S.I. and Z.U.; formal analysis, R.P., R.L., M.S. and N.L.S.; investigation, R.P., R.L. and N.L.S.; resources, V.B.R., R.P., K.S.; data curation, R.P., R.L. and N.L.S.; writing—original draft preparation, R.P., R.L. and N.L.S.; writing—review and editing, V.K.G., B.S.I., V.B.R., M.S., K.S., R.P. and Z.U; visualization, V.B.R., M.S., K.S. and Z.U; supervision, V.B.R., M.S. and K.S.; project administration, V.B.R., M.S., R.P. and K.S.; funding acquisition, V.B.R., M.S. and K.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data that support the findings are available within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Luo Y., Wang Q., Zhang Y. Biopolymer-Based Nanotechnology Approaches to Deliver Bioactive Compounds for Food Applications: A Perspective on the Past, Present, and Future. J. Agric. Food Chem. 2020;68:12993–13000. doi: 10.1021/acs.jafc.0c00277. [DOI] [PubMed] [Google Scholar]
- 2.Daliu P., Santini A., Novellino E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019;12:1–7. doi: 10.1080/17512433.2019.1552135. [DOI] [PubMed] [Google Scholar]
- 3.Sarwal A., Rawat N., Singh G., Sinha V.R., Sharma S., Kumar D. NanoNutraceuticals. CRC Press; Boca Raton, FL, USA: 2018. Nanonutraceuticals in Central Nervous System Disorders; pp. 91–104. [DOI] [Google Scholar]
- 4.Sasi S. Nutraceuticals—A review. Int. J. Ind. Biotechnol. Biomater. 2017;3:25–29. [Google Scholar]
- 5.Deepika, Maurya P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules. 2022;27:2498. doi: 10.3390/molecules27082498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagi S.S. Quercetin: A Potential Flavanol with Multiple Health Benefits. Arch. Food Sci. Nutr. Res. 2021;2:1002. [Google Scholar]
- 7.Yang L., Gao Y., Bajpai V.K., El-Kammar H.A., Simal-Gandara J., Cao H., Cheng K.W., Wang M., Arroo R.R., Zou L., et al. Advance toward isolation, extraction, metabolism and health benefits of kaempferol, a major dietary flavonoid with future perspectives. Crit. Rev. Food Sci. Nutr. 2021:1–17. doi: 10.1080/10408398.2021.1980762. [DOI] [PubMed] [Google Scholar]
- 8.Guest P.C., Sahebkar A. Studies on Biomarkers and New Targets in Aging Research in Iran. Springer; Cham, Switzerland: 2021. Research in the Middle East into the Health Benefits of Curcumin; pp. 1–13. [DOI] [PubMed] [Google Scholar]
- 9.Xu X., Yi H., Wu J., Kuang T., Zhang J., Li Q., Du H., Xu T., Jiang G., Fan G. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed. Pharmacother. 2021;133:110984. doi: 10.1016/j.biopha.2020.110984. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L., Cai L., Ruan H., Zhang L., Wang J., Jiang H., Wu Y., Feng S., Chen J. Electrospun chitosan oligosaccharide/polycaprolactone nanofibers loaded with wound-healing compounds of Rutin and Quercetin as antibacterial dressings. Int. J. Biol. Macromol. 2021;183:1145–1154. doi: 10.1016/j.ijbiomac.2021.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Raza S.H.A., Naqvi S.R.Z., Abdelnour S.A., Schreurs N., Mohammedsaleh Z.M., Khan I., Shater A.F., El-Hack M.E.A., Khafaga A.F., Quan G., et al. Beneficial effects and health benefits of Astaxanthin molecules on animal production: A review. Res. Vet. Sci. 2021;138:69–78. doi: 10.1016/j.rvsc.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Zaaboul F., Liu Y. Vitamin E in foodstuff: Nutritional, analytical, and food technology aspects. Compr. Rev. Food Sci. Food Saf. 2022;21:964–998. doi: 10.1111/1541-4337.12924. [DOI] [PubMed] [Google Scholar]
- 13.Mangla B., Kohli K. Combination of natural agent with synthetic drug for the breast cancer therapy. Int. J. Drug Dev. Res. 2018;10:22–26. [Google Scholar]
- 14.Majumdar M., Shivalkar S., Pal A., Verma M.L., Sahoo A.K., Roy D.N. Biotechnological Production of Bioactive Compounds. Elsevier; Amsterdam, The Netherlands: 2019. Nanotechnology for enhanced bioactivity of bioactive compounds; pp. 433–466. [DOI] [Google Scholar]
- 15.McClements D.J., Decker E., Weiss J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007;72:R109–R124. doi: 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghanbari-Movahed M., Mondal A., Farzaei M.H., Bishayee A. Quercetin- and rutin-based nano-formulations for cancer treatment: A systematic review of improved efficacy and molecular mechanisms. Phytomedicine. 2022;97:153909. doi: 10.1016/j.phymed.2021.153909. [DOI] [PubMed] [Google Scholar]
- 17.Sharma M., Inbaraj B.S., Dikkala P.K., Sridhar K., Mude A.N., Narsaiah K. Preparation of Curcumin Hydrogel Beads for the Development of Functional Kulfi: A Tailoring Delivery System. Foods. 2022;11:182. doi: 10.3390/foods11020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv S., Zhang Y., Tan H., Zhang R., McClements D.J. Vitamin E Encapsulation within Oil-in-Water Emulsions: Impact of Emulsifier Type on Physicochemical Stability and Bioaccessibility. J. Agric. Food Chem. 2019;67:1521–1529. doi: 10.1021/acs.jafc.8b06347. [DOI] [PubMed] [Google Scholar]
- 19.Wang P.-P., Luo Z.-G., Peng X.-C. Encapsulation of Vitamin E and Soy Isoflavone Using Spiral Dextrin: Comparative Structural Characterization, Release Kinetics, and Antioxidant Capacity during Simulated Gastrointestinal Tract. J. Agric. Food Chem. 2018;66:10598–10607. doi: 10.1021/acs.jafc.8b00644. [DOI] [PubMed] [Google Scholar]
- 20.Fan L., Lu Y., Ouyang X.-K., Ling J. Development and characterization of soybean protein isolate and fucoidan nanoparticles for curcumin encapsulation. Int. J. Biol. Macromol. 2021;169:194–205. doi: 10.1016/j.ijbiomac.2020.12.086. [DOI] [PubMed] [Google Scholar]
- 21.Bolat Z.B., Islek Z., Demir B.N., Yilmaz E.N., Sahin F., Ucisik M.H. Curcumin- and Piperine-Loaded Emulsomes as Combinational Treatment Approach Enhance the Anticancer Activity of Curcumin on HCT116 Colorectal Cancer Model. Front. Bioeng. Biotechnol. 2020;8:50. doi: 10.3389/fbioe.2020.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oueslati M.H., Ben Tahar L., Harrath A.H. Catalytic, antioxidant and anticancer activities of gold nanoparticles synthesized by kaempferol glucoside from Lotus leguminosae. Arab. J. Chem. 2020;13:3112–3122. doi: 10.1016/j.arabjc.2018.09.003. [DOI] [Google Scholar]
- 23.Loo Y.S., Madheswaran T., Rajendran R., Bose R.J. Encapsulation of berberine into liquid crystalline nanoparticles to enhance its solubility and anticancer activity in MCF7 human breast cancer cells. J. Drug Deliv. Sci. Technol. 2020;57:101756. doi: 10.1016/j.jddst.2020.101756. [DOI] [Google Scholar]
- 24.Campos E., Proença P.L.F., Oliveira J.L., Pereira A., Ribeiro L.N.D.M., Fernandes F.O., Gonçalves K.C., Polanczyk R.A., Pasquoto-Stigliani T., Lima R., et al. Carvacrol and linalool co-loaded in β-cyclodextrin-grafted chitosan nanoparticles as sustainable biopesticide aiming pest control. Sci. Rep. 2018;8:7623. doi: 10.1038/s41598-018-26043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgos-Díaz C., Opazo-Navarrete M., Soto-Añual M., Leal-Calderon F., Bustamante M. Food-grade Pickering emulsion as a novel astaxanthin encapsulation system for making powder-based products: Evaluation of astaxanthin stability during processing, storage, and its bioaccessibility. Food Res. Int. 2020;134:109244. doi: 10.1016/j.foodres.2020.109244. [DOI] [PubMed] [Google Scholar]
- 26.Aronson J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017;83:8–19. doi: 10.1111/bcp.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prabu S.L., Suriyaprakash T., Dinesh K., Suresh K., Ragavendran T. Nutraceuticals: A review. Elixir Pharm. 2012;46:8372–8377. [Google Scholar]
- 28.Crawford C., Boyd C., Paat C.F., Meissner K., Lentino C., Teo L., Berry K., Deuster P. Dietary Ingredients as an Alternative Approach for Mitigating Chronic Musculoskeletal Pain: Evidence-Based Recommendations for Practice and Research in the Military. Pain Med. 2019;20:1236–1247. doi: 10.1093/pm/pnz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanella M., Ciappellano S.G., Venturini M., Tedesco E., Manodori L., Benetti F. Nutraceuticals and nanotechnology. Diet. Ingred. Suppl. 2015;26:26–31. [Google Scholar]
- 30.Subramani T., Ganapathyswamy H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J. Food Sci. Technol. 2020;57:3545–3555. doi: 10.1007/s13197-020-04360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A.R., Desu P.K., Nakkala R.K., Kondi V., Devi S., Alam M.S., Hamid H., Athawale R.B., Kesharwani P. Nanotechnology-based approaches applied to nutraceuticals. Drug Deliv. Transl. Res. 2022;12:485–499. doi: 10.1007/s13346-021-00960-3. [DOI] [PubMed] [Google Scholar]
- 32.Sahani S., Sharma Y.C. Advancements in applications of nanotechnology in global food industry. Food Chem. 2021;342:128318. doi: 10.1016/j.foodchem.2020.128318. [DOI] [PubMed] [Google Scholar]
- 33.Manocha S., Dhiman S., Grewal A.S., Guarve K. Nanotechnology: An approach to overcome bioavailability challenges of nutraceuticals. J. Drug Deliv. Sci. Technol. 2022;72:103418. doi: 10.1016/j.jddst.2022.103418. [DOI] [Google Scholar]
- 34.Bronzwaer S., Kass G., Robinson T., Tarazona J., Verhagen H., Verloo D., Vrbos D., Hugas M. Food Safety Regulatory Research Needs 2030. EFSA J. 2019;17:e170622. doi: 10.2903/j.efsa.2019.e170622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitman M. Understanding the perceived need for complementary and alternative nutraceuticals: Lifestyle issues. Clin. J. Oncol Nurs. 2001;5:190–194. [PubMed] [Google Scholar]
- 36.Ghani U., Naeem M., Rafeeq H., Imtiaz U., Amjad A., Ullah S., Rehman A., Qasim F. A Novel Approach towards Nutraceuticals and Biomedical Applications. Sch. Int. J. Biochem. 2019;2:245–252. doi: 10.36348/SIJB.2019.v02i10.001. [DOI] [Google Scholar]
- 37.Palmer M.E., Haller C., McKinney P.E., Klein-Schwartz W., Tschirgi A., Smolinske S.C., Woolf A., Sprague B.M., Ko R., Everson G., et al. Adverse events associated with dietary supplements: An observational study. Lancet. 2003;361:101–106. doi: 10.1016/S0140-6736(03)12227-1. [DOI] [PubMed] [Google Scholar]
- 38.Dable-Tupas G., Otero M.C.B., Bernolo L. Functional Foods and Nutraceuticals. Springer; Cham, Switzerland: 2020. Functional foods and health benefits; pp. 1–11. [Google Scholar]
- 39.Crowe K.M., Francis C. Position of the Academy of Nutrition and Dietetics: Functional Foods. J. Acad. Nutr. Diet. 2013;113:1096–1103. doi: 10.1016/j.jand.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Dwyer J.T., Coates P.M., Smith M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients. 2018;10:41. doi: 10.3390/nu10010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rawson E.S., Miles M.P., Larson-Meyer D.E. Dietary supplements for health, adaptation, and recovery in athletes. Int. J. Sport Nutr. Exerc. Metab. 2018;28:188–199. doi: 10.1123/ijsnem.2017-0340. [DOI] [PubMed] [Google Scholar]
- 42.Psota T.L., Gebauer S.K., Kris E.P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am. J. Cardiol. 2006;98:3–18. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Chandra S., Saklani S., Kumar P., Kim B., Coutinho H.D.M. Nutraceuticals: Pharmacologically Active Potent Dietary Supplements. BioMed Res. Int. 2022;2022:2051017. doi: 10.1155/2022/2051017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbadi A., Domergue F. Biosynthesis of very long- chain polyunsaturated fatty acids in transgenic oilseeds: Constraints on their accumulation. Plant Cell. 2004;16:2734–2748. doi: 10.1105/tpc.104.026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu G., Truksa M., Datla N. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat. Biotechnol. 2005;23:1013–1017. doi: 10.1038/nbt1107. [DOI] [PubMed] [Google Scholar]
- 46.Das L., Bhaumik E., Raychaudhuri U., Chakraborty R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012;49:173–183. doi: 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siciliano R., Reale A., Mazzeo M., Morandi S., Silvetti T., Brasca M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients. 2021;13:1225. doi: 10.3390/nu13041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adefegha A. Functional Foods and Nutraceuticals as Dietary Intervention in Chronic Diseases; Novel Perspectives for Health Promotion and Disease Prevention. J. Diet. Suppl. 2018;15:977–1009. doi: 10.1080/19390211.2017.1401573. [DOI] [PubMed] [Google Scholar]
- 49.Gruss S.M., Nhim K., Gregg E., Bell M., Luman E., Albright A. Public Health Approaches to Type 2 Diabetes Prevention: The US National Diabetes Prevention Program and Beyond. Curr. Diabetes Rep. 2019;19:78. doi: 10.1007/s11892-019-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Felice S. FIM rationale and proposed guidelines for the nutraceutical research & education act–NREA; Proceedings of the FIM’s 10th Nutraceutical Conference; The Waldorf-Astoria, New York, NY, USA. 10–11 November 2002; [(accessed on 12 August 2022)]. Available online: https://fimdefelice.org/fim-rationale-and-proposed-guidelines-for-the-nutraceutical-research-education-act-nrea/ [Google Scholar]
- 51.Rajasekaran A., Sivagnanam G., Xavier R. Nutraceuticals as therapeutic agents, a review research. Res. J. Pharm. Technol. 2008;1:328–340. [Google Scholar]
- 52.Ozdal T., Tomas M., Toydemir G., Kamiloglu S., Capanoglu E. Aromatic Herbs in Food. Academic Press; Cambridge, MA, USA: 2021. Introduction to nutraceuticals, medicinal foods, and herbs; pp. 1–34. [DOI] [Google Scholar]
- 53.Jagannathan R., Patel S.A., Ali M.K., Narayan K.M.V. Global Updates on Cardiovascular Disease Mortality Trends and Attribution of Traditional Risk Factors. Curr. Diabetes Rep. 2019;19:44. doi: 10.1007/s11892-019-1161-2. [DOI] [PubMed] [Google Scholar]
- 54.Inoue M., Sumii Y., Shibata N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega. 2020;5:10633–10640. doi: 10.1021/acsomega.0c00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonçalves R.F.S., Martins J.T., Duarte C.M.M., Vicente A.A., Pinheiro A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018;78:270–291. doi: 10.1016/j.tifs.2018.06.011. [DOI] [Google Scholar]
- 56.Gopi S., Balakrishnan P. Handbook of Nutraceuticals and Natural Products. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2022. [Google Scholar]
- 57.Shahgholian N. Handbook of Nutraceuticals and Natural Products: Biological, Medicinal, and Nutritional Properties and Applications. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2002. Introduction to Nutraceuticals and Natural Products; pp. 1–14. [Google Scholar]
- 58.Durazzo A., Lucarini M., Santini A. Nutraceuticals in Human Health. Foods. 2020;9:370. doi: 10.3390/foods9030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Natarajan T.D., Ramasamy J.R., Palanisamy K. Nutraceutical potentials of synergic foods: A systematic review. J. Ethn. Foods. 2019;6:1–7. doi: 10.1186/s42779-019-0033-3. [DOI] [Google Scholar]
- 60.Ghaffari S., Roshanravan N. The role of nutraceuticals in prevention and treatment of hypertension: An updated review of the literature. Food Res. Int. 2020;128:108749. doi: 10.1016/j.foodres.2019.108749. [DOI] [PubMed] [Google Scholar]
- 61.Roshanravan N., Mohammadi H. Nutraceuticals for Prenatal, Maternal and Offspring’s Nutritional Health. CRC Press; Boca Raton, FL, USA: 2019. The Role of Nutraceuticals in Gestational Diabetes Mellitus; pp. 153–167. [DOI] [Google Scholar]
- 62.Allahveran A., Farokhzad A., Asghari M., Sarkhosh A. Foliar application of ascorbic and citric acids enhanced ‘Red Spur’apple fruit quality, bioactive compounds and antioxidant activity. Physiol. Mol. Biol. Plants. 2018;24:433–440. doi: 10.1007/s12298-018-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langer R., Peppas N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003;49:2990–3006. doi: 10.1002/aic.690491202. [DOI] [Google Scholar]
- 64.Estevinho L.M. Special issue “Nutraceuticals in human health and disease”. Int. J. Mol. Sci. 2018;19:1213. doi: 10.3390/ijms19041213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ting Y., Jiang Y., Ho C.-T., Huang Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods. 2014;7:112–128. doi: 10.1016/j.jff.2013.12.010. [DOI] [Google Scholar]
- 66.Meléndez-Martínez A.J., Böhm V., Borge G.I.A., Cano M.P., Fikselová M., Gruskiene R., Lavelli V., Loizzo M.R., Mandić A.I., Brahm P.M., et al. Carotenoids: Considerations for Their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals, and Novel Foods in the Context of Sustainability, Circular Economy, and Climate Change. Annu. Rev. Food Sci. Technol. 2021;12:433–460. doi: 10.1146/annurev-food-062220-013218. [DOI] [PubMed] [Google Scholar]
- 67.Leonard N.B. Handbook of Nutraceuticals and Functional Foods. CRC Press; Boca Raton, FL, USA: 2000. Stability testing of nutraceuticals and functional foods. [Google Scholar]
- 68.Zheng B., McClements D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules. 2020;25:2791. doi: 10.3390/molecules25122791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones D., Caballero S., Davidov-Pardo G. Advances in Food and Nutrition Research. Volume 88. Academic Press; Cambridge, MA, USA: 2019. Chapter Six—Bioavailability of nanotechnology-based bioactives and nutraceuticals; pp. 235–273. [DOI] [PubMed] [Google Scholar]
- 70.Punia S., Sandhu K.S., Kaur M., Siroha A.K. Nanobiotechnology in Bioformulations. Springer; Cham, Switzerland: 2019. Nanotechnology: A successful approach to improve nutraceutical bioavailability; pp. 119–133. [Google Scholar]
- 71.Helal N.A., Eassa H.A., Amer A.M., Eltokhy M.A., Edafiogho I., Nounou M.I. Nutraceuticals’ novel formulations: The good, the bad, the unknown and patents involved. Recent Pat. Drug Deliv. Formul. 2019;13:105–156. doi: 10.2174/1872211313666190503112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prasad R., Kumar V., Kumar M., Choudhary D.K., editors. Nanobiotechnology in Bioformulations. Springer International Publishing; Cham, Switzerland: 2019. [Google Scholar]
- 73.Huang Q., Yu H., Ru Q. Bioavailability and Delivery of Nutraceuticals Using Nanotechnology. J. Food Sci. 2010;75:R50–R57. doi: 10.1111/j.1750-3841.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- 74.Pandey M., Verma R.K., Saraf S.A. Nutraceuticals: New era of medicine and health. Asian J. Pharm. Clin. Res. 2010;3:11–15. [Google Scholar]
- 75.Rachmale M., Rajput N., Jadav T., Sahu A.K., Tekade R.K., Sengupta P. Implication of metabolomics and transporter modulation based strategies to minimize multidrug resistance and enhance site-specific bioavailability: A needful consideration toward modern anticancer drug discovery. Drug Metab. Rev. 2022;54:101–119. doi: 10.1080/03602532.2022.2048007. [DOI] [PubMed] [Google Scholar]
- 76.Balkrishna A., Sakat S.S., Joshi K., Joshi K., Sharma V., Ranjan R., Bhattacharya K., Varshney A. Cytokines driven anti-Inflammatory and anti-psoriasis like efficacies of nutraceutical sea buckthorn (hippophaerhamnoides) oil. Front. Pharm. 2019;10:1186. doi: 10.3389/fphar.2019.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Younis S.A., Kim K.-H., Shaheen S.M., Antoniadis V., Tsang Y.F., Rinklebe J., Deep A., Brown R.J. Advancements of nanotechnologies in crop promotion and soil fertility: Benefits, life cycle assessment, and legislation policies. Renew. Sustain. Energy Rev. 2021;152:111686. doi: 10.1016/j.rser.2021.111686. [DOI] [Google Scholar]
- 78.Bortolotti M., Mercatelli D., Polito L. Momordica charantia, a Nutraceutical Approach for Inflammatory Related Diseases. Front. Pharmacol. 2019;10:486. doi: 10.3389/fphar.2019.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonçalves A., Estevinho B.N., Rocha F. Methodologies for simulation of gastrointestinal digestion of different controlled delivery systems and further uptake of encapsulated bioactive compounds. Trends Food Sci. Technol. 2021;114:510–520. doi: 10.1016/j.tifs.2021.06.007. [DOI] [Google Scholar]
- 80.DeRosa G., Limas C.P., Macías P.C., Estrella A., Maffioli P. Dietary and nutraceutical approach to type 2 diabetes. Arch. Med Sci. 2014;10:336–344. doi: 10.5114/aoms.2014.42587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sachdeva V., Roy A., Bharadvaja N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020;21:884–896. doi: 10.2174/1389201021666200130113441. [DOI] [PubMed] [Google Scholar]
- 82.Castrogiovanni P., Trovato F.M., Loreto C., Nsir H., Szychlinska M.A., Musumeci G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016;17:2042. doi: 10.3390/ijms17122042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pashte V.V., Pashte S.V., Said P.P. Nutraceutical properties of natural honey to fight health issues: A comprehensive review. J. Pharmacogn. Phytochem. 2020;9:234–242. [Google Scholar]
- 84.Abbas M., Shah I., Nawaz M.A., Pervez S., Hussain Y., Niaz K., Khan F. Nutraceuticals and Cancer Signaling. Springer; Cham, Switzerland: 2021. Honey Against Cancer; pp. 401–418. [Google Scholar]
- 85.Ajibola A., Chamunorwa J.P., Erlwanger K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. 2012;9:61. doi: 10.1186/1743-7075-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cianciosi D., Forbes-Hernández T.Y., Ansary J., Gil E., Amici A., Bompadre S., Simal-Gandara J., Giampieri F., Battino M. Phenolic compounds from Mediterranean foods as nutraceutical tools for the prevention of cancer: The effect of honey polyphenols on colorectal cancer stem-like cells from spheroids. Food Chem. 2020;325:126881. doi: 10.1016/j.foodchem.2020.126881. [DOI] [PubMed] [Google Scholar]
- 87.Cicero A.F.G., Fogacci F., Bove M., Giovannini M., Borghi C. Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phytother. Res. 2019;33:2094–2101. doi: 10.1002/ptr.6402. [DOI] [PubMed] [Google Scholar]
- 88.Martínez-Puc J.F., Cetzal-Ix W., Basu S.K., Enríquez-Nolasco J.R., Magaña-Magaña M.A. Nutraceutical and medicinal properties of native stingless bees honey and their contribution to human health. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases, Academic Press: Cambridge, MA, USA, 2022; pp. 481–489
- 89.Poli A., Barbagallo C.M., Cicero A.F., Corsini A., Manzato E., Trimarco B., Bernini F., Visioli F., Bianchi A., Canzone G., et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018;134:51–60. doi: 10.1016/j.phrs.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 90.Cicero A.F.G., Fogacci F., Stoian A.P., Vrablik M., Al Rasadi K., Banach M., Toth P.P., Rizzo M. Nutraceuticals in the Management of Dyslipidemia: Which, When, and for Whom? Could Nutraceuticals Help Low-Risk Individuals with Non-optimal Lipid Levels? Curr. Atheroscler. Rep. 2021;23:57. doi: 10.1007/s11883-021-00955-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y., Pan H., Hao S., Pan D., Wang G., Yu W. Evaluation of phenolic composition and antioxidant properties of different varieties of Chinese citrus. Food Chem. 2021;364:130413. doi: 10.1016/j.foodchem.2021.130413. [DOI] [PubMed] [Google Scholar]
- 92.Alcaide-Hidalgo J.M., Romero M., Duarte J., López-Huertas E. Antihypertensive Effects of Virgin Olive Oil (Unfiltered) Low Molecular Weight Peptides with ACE Inhibitory Activity in Spontaneously Hypertensive Rats. Nutrients. 2020;12:271. doi: 10.3390/nu12010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Akbari B., Baghaei-Yazdi N., Bahmaie M., Abhari F.M. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors. 2022;48:611–633. doi: 10.1002/biof.1831. [DOI] [PubMed] [Google Scholar]
- 94.Sahebkar A., Serban M.-C., Gluba-Brzózka A., Mikhailidis D.P., Cicero A.F., Rysz J., Banach M. Lipid-modifying effects of nutraceuticals: An evidence-based approach. Nutrition. 2016;32:1179–1192. doi: 10.1016/j.nut.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Bianconi V., Mannarino M.R., Sahebkar A., Cosentino T., Pirro M. Cholesterol-lowering nutraceuticals affecting vascular function and cardiovascular disease risk. Curr. Cardiol. Rep. 2018;20:1–20. doi: 10.1007/s11886-018-0994-7. [DOI] [PubMed] [Google Scholar]
- 96.Paolino D., Mancuso A., Cristiano M., Froiio F., Lammari N., Celia C., Fresta M. Nanonutraceuticals: The New Frontier of Supplementary Food. Nanomaterials. 2021;11:792. doi: 10.3390/nano11030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu X., Raghuvanshi R., Ceylan F.D., Bolling B.W. Quercetin and Its Metabolites Inhibit Recombinant Human Angiotensin-Converting Enzyme 2 (ACE2) Activity. J. Agric. Food Chem. 2020;68:13982–13989. doi: 10.1021/acs.jafc.0c05064. [DOI] [PubMed] [Google Scholar]
- 98.Cimaglia P., Sega F.V.D., Vitali F., Lodolini V., Bernucci D., Passarini G., Fortini F., Marracino L., Aquila G., Rizzo P., et al. Effectiveness of a Novel Nutraceutical Compound Containing Red Yeast Rice, Polymethoxyflavones and Antioxidants in the Modulation of Cholesterol Levels in Subjects with Hypercholesterolemia and Low-Moderate Cardiovascular Risk: The NIRVANA Study. Front. Physiol. 2019;10:217. doi: 10.3389/fphys.2019.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barreca D., Laganà G., Leuzzi U., Smeriglio A., Trombetta D., Bellocco E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016;196:493–502. doi: 10.1016/j.foodchem.2015.09.077. [DOI] [PubMed] [Google Scholar]
- 100.Mandalari G., Barreca D., Gervasi T., Roussell M.A., Klein B., Feeney M.J., Carughi A. Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects. Plants. 2021;11:18. doi: 10.3390/plants11010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumar K., Yadav A.N., Kumar V., Vyas P., Dhaliwal H.S. Food waste: A potential bioresource for extraction of NutraceuticalsAnd bioactive compounds. Bioresour. Bioprocess. 2017;4:18. doi: 10.1186/s40643-017-0148-6. [DOI] [Google Scholar]
- 102.Tenore G.C., Caruso D., D’Avino M., Buonomo G., Caruso G., Ciampaglia R., Schiano E., Maisto M., Annunziata G., Novellino E. A Pilot Screening of Agro-Food Waste Products as Sources of Nutraceutical Formulations to Improve Simulated Postprandial Glycaemia and Insulinaemia in Healthy Subjects. Nutrients. 2020;12:1292. doi: 10.3390/nu12051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Varzakas T., Zakynthinos G., Verpoort F. Plant Food Residues as a Source of Nutraceuticals and Functional Foods. Foods. 2016;5:88. doi: 10.3390/foods5040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Enrico C. Studies in Natural Products Chemistry. Volume 62. Elsevier; Amsterdam, The Netherlands: 2019. Nanotechnology-Based Drug Delivery of Natural Compounds and Phytochemicals for the Treatment of Cancer and Other Diseases; pp. 91–123. [DOI] [Google Scholar]
- 105.Khorasani S., Danaei M., Mozafari M. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018;79:106–115. doi: 10.1016/j.tifs.2018.07.009. [DOI] [Google Scholar]
- 106.Salehi B., Venditti A., Sharifi-Rad M., Kręgiel D., Sharifi-Rad J., Durazzo A., Martins N. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019;20:1305. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tahir A., Ahmad R.S., Imran M., Ahmad M.H., Khan M.K., Muhammad N., Nisa M.U., Nadeem M.T., Yasmin A., Tahir H.S., et al. Recent approaches for utilization of food components as nano-encapsulation: A review. Int. J. Food Prop. 2021;24:1074–1096. doi: 10.1080/10942912.2021.1953067. [DOI] [Google Scholar]
- 108.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., del Pilar Rodriguez-Torres M., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martínez-Ballesta M., Gil-Izquierdo Á., García-Viguera C., Domínguez-Perles R. Nanoparticles and controlled delivery for bioactive compounds: Outlining challenges for new “smart-foods” for health. Foods. 2018;7:72. doi: 10.3390/foods7050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Persano F., Gigli G., Leporatti S. Lipid-polymer hybrid nanoparticles in cancer therapy: Current overview and future directions. Nano Express. 2021;2:012006. doi: 10.1088/2632-959X/abeb4b. [DOI] [Google Scholar]
- 111.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sosa-Hernández J.E., Villalba-Rodríguez A.M., Romero-Castillo K.D., Zavala-Yoe R., Bilal M., Ramirez-Mendoza R.A., Parra-Saldivar R., Iqbal H.M. Poly-3-hydroxybutyrate-based constructs with novel characteristics for drug delivery and tissue engineering applications—A review. Polym. Eng. Sci. 2020;60:1760–1772. doi: 10.1002/pen.25470. [DOI] [Google Scholar]
- 113.Sonawane P.G., Doshi Y.S., Shah M.U., Bajaj M., Kevadia V. Probiotics: The Nano Soldiers for Periodontium. J. PEARLDENT. 2017;8:1. doi: 10.5958/2229-4457.2017.00001.0. [DOI] [Google Scholar]
- 114.Wang X., Cheng F., Wang X., Feng T., Xia S., Zhang X. Chitosan decoration improves the rapid and long-term antibacterial activities of cinnamaldehyde-loaded liposomes. Int. J. Biol. Macromol. 2021;168:59–66. doi: 10.1016/j.ijbiomac.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 115.Hills S., Walker M., Barry A.E. Sport as a vehicle for health promotion: A shared value example of corporate social responsibility. Sport Manag. Rev. 2019;22:126–141. doi: 10.1016/j.smr.2018.10.001. [DOI] [Google Scholar]
- 116.Khangwal I., Yadav M., Mandeep, Shukla P. Microbial Enzymes and Biotechniques. Springer; Singapore: 2020. Probiotics and Prebiotics: Techniques Used and Its Relevance; pp. 193–206. [DOI] [Google Scholar]
- 117.Ayyash M., Abu-Jdayil B., Itsaranuwat P., Galiwango E., Tamiello-Rosa C., Abdullah H., Esposito G., Hunashal Y., Obaid R.S., Hamed F. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int. J. Biol. Macromol. 2020;144:938–946. doi: 10.1016/j.ijbiomac.2019.09.171. [DOI] [PubMed] [Google Scholar]
- 118.Ghanavati R., Asadollahi P., Shapourabadi M.B., Razavi S., Talebi M., Rohani M. Inhibitory effects of Lactobacilli cocktail on HT-29 colon carcinoma cells growth and modulation of the Notch and Wnt/β-catenin signaling pathways. Microb. Pathog. 2020;139:103829. doi: 10.1016/j.micpath.2019.103829. [DOI] [PubMed] [Google Scholar]
- 119.Rupasinghe H.P.V., Parmar I., Neir S.V. Biotransformation of Cranberry Proanthocyanidins to Probiotic Metabolites by Lactobacillus rhamnosus Enhances Their Anticancer Activity in HepG2 Cells In Vitro. Oxidative Med. Cell. Longev. 2019;2019:4750795. doi: 10.1155/2019/4750795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kang D., Su M., Duan Y., Huang Y. Eurotium cristatum, a potential probiotic fungus from Fuzhuan brick tea, alleviated obesity in mice by modulating gut microbiota. Food Funct. 2019;10:5032–5045. doi: 10.1039/C9FO00604D. [DOI] [PubMed] [Google Scholar]
- 121.Rani A., Baruah R., Goyal A. Prebiotic Chondroitin Sulfate Disaccharide Isolated from Chicken Keel Bone Exhibiting Anticancer Potential Against Human Colon Cancer Cells. Nutr. Cancer. 2019;71:825–839. doi: 10.1080/01635581.2018.1521446. [DOI] [PubMed] [Google Scholar]
- 122.Zhou L., Xie M., Yang F., Liu J. Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. LWT. 2020;117:108621. doi: 10.1016/j.lwt.2019.108621. [DOI] [Google Scholar]
- 123.Ohara T., Mori T. Antiproliferative Effects of Short-chain Fatty Acids on Human Colorectal Cancer Cells via Gene Expression Inhibition. Anticancer Res. 2019;39:4659–4666. doi: 10.21873/anticanres.13647. [DOI] [PubMed] [Google Scholar]
- 124.Li E., Yang S., Zou Y., Cheng W., Li B., Hu T., Li Q., Wang W., Liao S., Pang D. Purification, Characterization, Prebiotic Preparations and Antioxidant Activity of Oligosaccharides from Mulberries. Molecules. 2019;24:2329. doi: 10.3390/molecules24122329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Davani-Davari D., Negahdaripour M., Karimzadeh I., Seifan M., Mohkam M., Masoumi S.J., Ghasemi Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8:92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pandey K., Naik S., Vakil B. Probiotics, prebiotics and synbiotics—a review. J. Food Sci. Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nuruzzaman M., Rahman M.M., Liu Y., Naidu R. Nanoencapsulation, Nano-guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016;64:1447–1483. doi: 10.1021/acs.jafc.5b05214. [DOI] [PubMed] [Google Scholar]
- 128.Xiao J., Cao Y., Huang Q. Edible Nanoencapsulation Vehicles for Oral Delivery of Phytochemicals: A Perspective Paper. J. Agric. Food Chem. 2017;65:6727–6735. doi: 10.1021/acs.jafc.7b02128. [DOI] [PubMed] [Google Scholar]
- 129.Fathi M., Martín A., McClements D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014;39:18–39. doi: 10.1016/j.tifs.2014.06.007. [DOI] [Google Scholar]
- 130.Samaranayaka A.G., Li-Chan E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods. 2011;3:229–254. doi: 10.1016/j.jff.2011.05.006. [DOI] [Google Scholar]
- 131.Hoseyni S.Z., Jafari S.M., Tabarestani H.S., Ghorbani M., Assadpour E., Sabaghi M. Release of catechin from Azivash gum-polyvinyl alcohol electrospun nanofibers in simulated food and digestion media. Food Hydrocoll. 2021;112:106366. doi: 10.1016/j.foodhyd.2020.106366. [DOI] [Google Scholar]
- 132.Kurd F., Fathi M., Shekarchizadeh H. Nanoencapsulation of hesperetin using basil seed mucilage nanofibers: Characterization and release modeling. Food Biosci. 2019;32:100475. doi: 10.1016/j.fbio.2019.100475. [DOI] [Google Scholar]
- 133.Remanan M.K., Zhu F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2021;353:128534. doi: 10.1016/j.foodchem.2020.128534. [DOI] [PubMed] [Google Scholar]
- 134.Rajabi H., Jafari S.M., Rajabzadeh G., Sarfarazi M., Sedaghati S. Chitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive components. Colloids Surf. A Physicochem. Eng. Asp. 2020;578:123644. doi: 10.1016/j.colsurfa.2019.123644. [DOI] [Google Scholar]
- 135.Fathi M., Donsi F., McClements D.J. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018;17:920–936. doi: 10.1111/1541-4337.12360. [DOI] [PubMed] [Google Scholar]
- 136.Ahmad U., Ali A., Khan M.M., Siddiqui M.A., Akhtar J., Ahmad F.J. Nanotechnology-Based Strategies for Nutraceuticals: A Review of Current Research Development. Nanosci. Technol. Int. J. 2019;10:133–155. doi: 10.1615/NanoSciTechnolIntJ.2019030098. [DOI] [Google Scholar]
- 137.Zhang R., Han Y., Xie W., Liu F., Chen S. Advances in Protein-Based Nanocarriers of Bioactive Compounds: From Microscopic Molecular Principles to Macroscopical Structural and Functional Attributes. J. Agric. Food Chem. 2022;70:6354–6367. doi: 10.1021/acs.jafc.2c01936. [DOI] [PubMed] [Google Scholar]
- 138.Mozafari M.R., Flanagan J., Matia-Merino L., Awati A., Omri A., Suntres Z.E., Singh H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J. Sci. Food Agric. 2006;86:2038–2045. doi: 10.1002/jsfa.2576. [DOI] [Google Scholar]
- 139.Akhavan S., Assadpour E., Katouzian I., Jafari S.M. Lipid Nano Scale Cargos for The Protection and Delivery of Food Bioactive Ingredients and Nutraceuticals. Trends Food Sci. Technol. 2018;74:132–146. doi: 10.1016/j.tifs.2018.02.001. [DOI] [Google Scholar]
- 140.Livney Y.D. Nanostructured delivery systems in food: Latest developments and potential future directions. Curr. Opin. Food Sci. 2015;3:125–135. doi: 10.1016/j.cofs.2015.06.010. [DOI] [Google Scholar]
- 141.Assadpour E., Jafari S.M. An overview of specialized equipment for nanoencapsulation of food ingredients. In: Jafari S., editor. Nanoencapsulation of Food Ingredients by Specialized Equipment. Academic Press; Cambridge, MA, USA: 2019. pp. 1–30. [DOI] [Google Scholar]
- 142.Rashidi L. Different nano-delivery systems for delivery of nutraceuticals. Food Biosci. 2021;43:101258. doi: 10.1016/j.fbio.2021.101258. [DOI] [Google Scholar]
- 143.Ajeeshkumar K.K., Aneesh P.A., Raju N., Suseela M., Ravishankar C.N., Benjakul S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021;20:1280–1306. doi: 10.1111/1541-4337.12725. [DOI] [PubMed] [Google Scholar]
- 144.Rostamabadi H., Falsafi S.R., Jafari S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control. Release. 2019;298:38–67. doi: 10.1016/j.jconrel.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 145.Xia S., Tan C., Zhang Y., Abbas S., Feng B., Zhang X., Qin F. Modulating effect of lipid bilayer–carotenoid interactions on the property of liposome encapsulation. Colloids Surf. B Biointerfaces. 2015;128:172–180. doi: 10.1016/j.colsurfb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 146.Sercombe L., Veerati T., Moheimani F., Wu S.Y., Sood A.K., Hua S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lopes N.A., Pinilla C.M.B., Brandelli A. Antimicrobial activity of lysozyme-nisin co-encapsulated in liposomes coated with polysaccharides. Food Hydrocoll. 2019;93:1–9. doi: 10.1016/j.foodhyd.2019.02.009. [DOI] [Google Scholar]
- 148.Zhou W., Cheng C., Ma L., Zou L., Liu W., Li R., Cao Y., Liu Y., Ruan R., Li J. The Formation of Chitosan-Coated Rhamnolipid Liposomes Containing Curcumin: Stability and In Vitro Digestion. Molecules. 2021;26:560. doi: 10.3390/molecules26030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dalmoro A., Bochicchio S., Lamberti G., Bertoncin P., Janssens B., Barba A.A. Micronutrients encapsulation in enhanced nanoliposomal carriers by a novel preparative technology. RSC Adv. 2019;9:19800–19812. doi: 10.1039/C9RA03022K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Peng Y., Meng Q., Zhou J., Chen B., Xi J., Long P., Zhang L., Hou R. Nanoemulsion delivery system of tea polyphenols enhanced the bioavailability of catechins in rats. Food Chem. 2019;242:527–532. doi: 10.1016/j.foodchem.2017.09.094. [DOI] [PubMed] [Google Scholar]
- 151.Katouzian I., Esfanjani A.F., Jafari S.M., Akhavan S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017;68:14–25. doi: 10.1016/j.tifs.2017.07.017. [DOI] [Google Scholar]
- 152.Fathi M., Mozafari M.R., Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012;23:13–27. doi: 10.1016/j.tifs.2011.08.003. [DOI] [Google Scholar]
- 153.Dhiman N., Awasthi R., Sharma B., Kharkwal H., Kulkarni G.T. Lipid nanoparticles as carriers for bioactive delivery. Front. Chem. 2021;9:580118. doi: 10.3389/fchem.2021.580118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang Z., Li X., Sang S., McClements D.J., Chen L., Long J., Jiao A., Wang J., Jin Z., Qiu C. A review of nanostructured delivery systems for the encapsulation, protection, and delivery of silymarin: An emerging nutraceutical. Food Res. Int. 2022;156:111314. doi: 10.1016/j.foodres.2022.111314. [DOI] [PubMed] [Google Scholar]
- 155.Peres L.B., dos Anjos R.S., Tappertzhofen L.C., Feuser P.E., de Araújo P.H., Landfester K., Muñoz-Espí R. pH-responsive physically and chemically cross-linked glutamic-acid-based hydrogels and nanogels. Eur. Polym. J. 2018;101:341–349. doi: 10.1016/j.eurpolymj.2018.02.039. [DOI] [Google Scholar]
- 156.Sahu P., Kashaw S.K., Sau S., Kushwah V., Jain S., Agrawal R.K., Iyer A.K. pH Responsive 5-Fluorouracil Loaded Biocompatible Nanogels for Topical Chemotherapy of Aggressive Melanoma. Colloids Surf. B Biointerfaces. 2019;174:232–245. doi: 10.1016/j.colsurfb.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang J., Xu W., Zhang N., Yang C., Xu H., Wang Z., Li B., Ding J., Chen X. X-ray-responsive polypeptide nanogel for concurrent chemoradiotherapy. J. Control. Release. 2021;332:1–9. doi: 10.1016/j.jconrel.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 158.Mullen E., Morris M. Green Nanofabrication Opportunities in the Semiconductor Industry: A Life Cycle Perspective. Nanomaterials. 2021;11:1085. doi: 10.3390/nano11051085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bazana M.T., Codevilla C.F., de Menezes C.R. Nanoencapsulation of bioactive compounds: Challenges and perspectives. Curr. Opin. Food Sci. 2019;26:47–56. doi: 10.1016/j.cofs.2019.03.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings are available within the manuscript.