Abstract
N6-methyladenosine (m6A), the most common form of RNA modification, controls CD4+ T cell homeostasis by targeting the IL-7/STAT5/SOCS signaling pathways. The role of m6A modification in unconventional T cell development remains unknown. Using mice with T cell-specific deletion of RNA methyltransferase METTL14 (T-Mettl14−/−), we demonstrate that m6A modification is indispensable for iNKT cell homeostasis. Loss of METTL14-dependent m6A modification leads to the upregulation of apoptosis in double-positive thymocytes, which in turn decreases Vα14-Jα18 gene rearrangements, resulting in drastic reduction of iNKT numbers in the thymus and periphery. Residual T-Mettl14−/− iNKT cells exhibit increased apoptosis, impaired maturation, and decreased responsiveness to IL-2/IL-15 and TCR stimulation. Furthermore, METTL14 knockdown in mature iNKT cells diminishes their cytokine production, correlating with increased Cish expression and decreased TCR signaling. Collectively, our study highlights a critical role for METTL14-dependent-m6A modification in iNKT cell development and function.
Graphical abstract
In brief
Cao et al. show that T cell-specific deletion of METTL14, a component of RNA m6A writer complex, leads to severe defects in iNKT cell development, survival, and function. Mechanistically, METTL14-dependent m6A modification controls iNKT cell development in a cell-intrinsic manner by regulating the apoptosis pathway and TCR signaling pathway.
INTRODUCTION
N6-methyladenosine (m6A) is the most abundant modification in eukaryotic mRNA (Bolger et al., 2014; Roundtree et al., 2017). m6A modification influences mRNA splicing, stability, and translation efficiency (Meyer and Jaffrey, 2014; Roignant and Soller, 2017). It is also involved in long non-coding RNA (lncRNA)-mediated transcriptional repression (Shafik et al., 2016) and micro RNA genesis (Erson-Bensan and Begik, 2017). The machinery responsible for the dynamic deposition and recognition of m6A on mRNA consists of ‘‘writers’’ that install m6A modification on target RNAs, ‘‘reader’’ proteins that preferentially recognize m6A and enact downstream functions, and ‘‘eraser’’ RNA demethylases that remove the m6A modification (Fujii and Shimizu, 2019). The m6A modification is mainly catalyzed by the m6A writer complex composed of a methyltransferase-like (METTL) 3/METTL14 heterodimer and additional adaptor proteins (Ping et al., 2014). METTL3 contains the catalytic component and METTL14 binds and positions RNA for methylation and facilitates complex integrity (Liu et al., 2014).
The m6A modification is involved in diverse cellular processes including cancer progression and metastasis (Lan et al., 2019), viral replication (Manners et al., 2019), stem cell differentiation (Batista et al., 2014), and embryonic development (Alarcon et al., 2015; Batista et al., 2014; Geula et al., 2015). Within the immune system, m6A modification plays important roles in various immune cell types through the regulation of multiple signaling pathways. Previous studies with Mettl3−/− mice demonstrate that m6A mRNA methylation controls CD4+ T cell homeostasis by targeting the interleukin (IL)-7/STAT5/SOCS pathways (Li et al., 2017). METTL3-dependent m6A modification is critical for T regulatory cell (Treg)-suppressive functions via IL-2/STAT5 signaling (Tong et al., 2018). Similarly, METTL14-deficient Treg cells fail to inhibit T cell-mediated colitis in mice (Lu et al., 2020). m6A modification also controls early B cell development by regulating IL-7 signaling (Zheng et al., 2020). Mechanistically, the loss of m6A modification results in slower mRNA decay and increased expression of the STAT signaling inhibitory proteins SOCS1, SOCS2, and CISH, which in turn inhibit cytokine-mediated STAT5 activation and therefore T cell proliferation and T cell differentiation. A recent study showed that METTL3-dependent m6A modification also controls T follicular helper cell (TFH) differentiation by regulating the expression of TCF-1, which in turn controls the expression of TFH cell regulators, including BCL6, CXCR5, and ICOS (Yao et al., 2021). In addition, METTL3-mediated m6A RNA modification promotes anti-tumor immunity of natural killer cells by maintaining SHP-2/IL-15/AKT pathway (Song et al., 2021). Despite all these advances, the role of m6A modification in the development and function of unconventional T cells, including invariant NKT (iNKT) cells, remains unknown.
iNKT cells recognize self or foreign lipid antigens presented by CD1d, a non-classical major histocompatibility complex (MHC) class-I-like molecule (Kronenberg, 2005; Rossjohn et al., 2012). iNKT cells express semi-invariant T cell receptors (TCRs) formed by Vα14-Jα18 (Vα24-Jα18 in humans) TCRα-chain paired with Vβ8, Vβ7, and Vβ2 (Vβ11 in humans) (Bendelac et al., 2007). iNKT cells exhibit an effector-memory phenotype. Upon activation through TCR or cytokine signaling, iNKT cells rapidly produce a variety of cytokines, including interferon (IFN)-γ, IL-4, tumor necrosis factor, IL-17, and IL-10 (Crosby and Kronenberg, 2018). As innate-like lymphocytes, iNKT cells are mobilized as the first line of defense against cancers and infections (Juno et al., 2012; Slauenwhite and Johnston, 2015; Terabe and Berzofsky, 2018).
The developmental program of iNKT cells in the thymus diverges from conventional T cells at the double-positive (DP) stage. Unlike conventional T cells that are selected by MHC/peptide complexes on thymic epithelial cells, the positive selection of iNKT cells is mediated by CD1d-expressing DP thymocytes through homotypic interactions (Godfrey et al., 2010). iNKT cell development requires strong TCR signaling (Moran et al., 2011) to upregulate the expression of the transcription factor Egr2 (Lazarevic et al., 2009; Seiler et al., 2012) and PLZF (Kovalovsky et al., 2008; Savage et al., 2008), as well as signaling from the SLAM family receptors (Griewank et al., 2007; Sintes et al., 2013). Traditionally, iNKT cell development is divided into four stages: stage 0 (CD24hiCD44−NK1.1−), stage 1 (CD24loCD44− NK1.1−), stage 2 (CD24loCD44+NK1.1−), and stage 3 (CD24lo CD44+NK1.1+) (Benlagha et al., 2005; Pellicci et al., 2002). iNKT cells can also be functionally classified into NKT1, NKT2, and NKT17 subsets based on the expression of transcriptional factors, T-bet, PLZF, GATA3, and RORγt (Constantinides and Bendelac, 2013; Lee et al., 2013). Recently, single-cell RNA sequencing (scRNA-seq) revealed comprehensive transcriptional profiles linked to the development, proliferation, maturation, and function of various iNKT cell subsets (Baranek et al., 2020; Engel et al., 2016). In addition, iNKT cells share overlapping developmental pathways, particularly with respect to cytokines, cell surface markers, and transcription factors needed for differentiation, with other innate T cell populations, including MAIT cells and γδ T cells (Harsha Krovi et al., 2020; Lee et al., 2020). This suggests that iNKT cells can serve as a useful model for understanding the development of unconventional T cells.
In this study, we used mice with a T cell-specific deletion of Mettl14 (Mettl14fl/fl; CD4-Cre+, hereafter referred to as T-Mettl14−/−) to investigate the role of m6A modification on iNKT cell development and function. We found that loss of METTL14-dependent m6A modification results in a dramatic decrease in the number of iNKT cells with a blockade of maturation occurring in a cell-autonomous manner. RNA-seq analysis of Mettl14−/− DP thymocytes revealed the abnormal expression of m6A modified genes, such as Hmga1b and Xaf1, which could promote p53-mediated apoptosis. In addition, Mettl14−/− iNKT cells and Mettl14-knockdown (Mettl14KD) mature iNKT cells had increased Cish expression, which attenuated TCR and cytokine signaling, leading to impaired cell proliferation and function. Of note, knocking down Cish expression in Mettl14KD iNKT cell hybridomas restored their cytokine response. Collectively, our data demonstrate a critical role for METTL14-dependent m6A modification in iNKT cell development, survival, and function.
RESULTS
m6A is important for homeostasis of iNKT cells
As iNKT cells are innate-like T cells and have a different developmental program, they may harbor a different abundance in m6A modification compared with conventional T cells. Interestingly, we found no significant differences in m6A modification level among CD4+, CD8+ T cells, and iNKT cells. However, m6A abundance was increased in iNKT cells after TCR stimulation (Figure 1A), indicating that m6A modification may play a role in thymic selection and peripheral activation of iNKT cells. To understand the potential role of m6A modification in iNKT development and function, we decided to use a loss-of-function mouse model. Previous work has shown that deletion of METTL14, a key component of the m6A methyltransferase complex, abolished m6A modification (Liu et al., 2014). Therefore, mice with a T cell-specific deletion of Mettl14 (T-Mettl14−/−) were used for this study to determine the role of METTL14 in iNKT development and function.
Figure 1. iNKT cell development is severely impaired in T-Mettl14−/− mice.
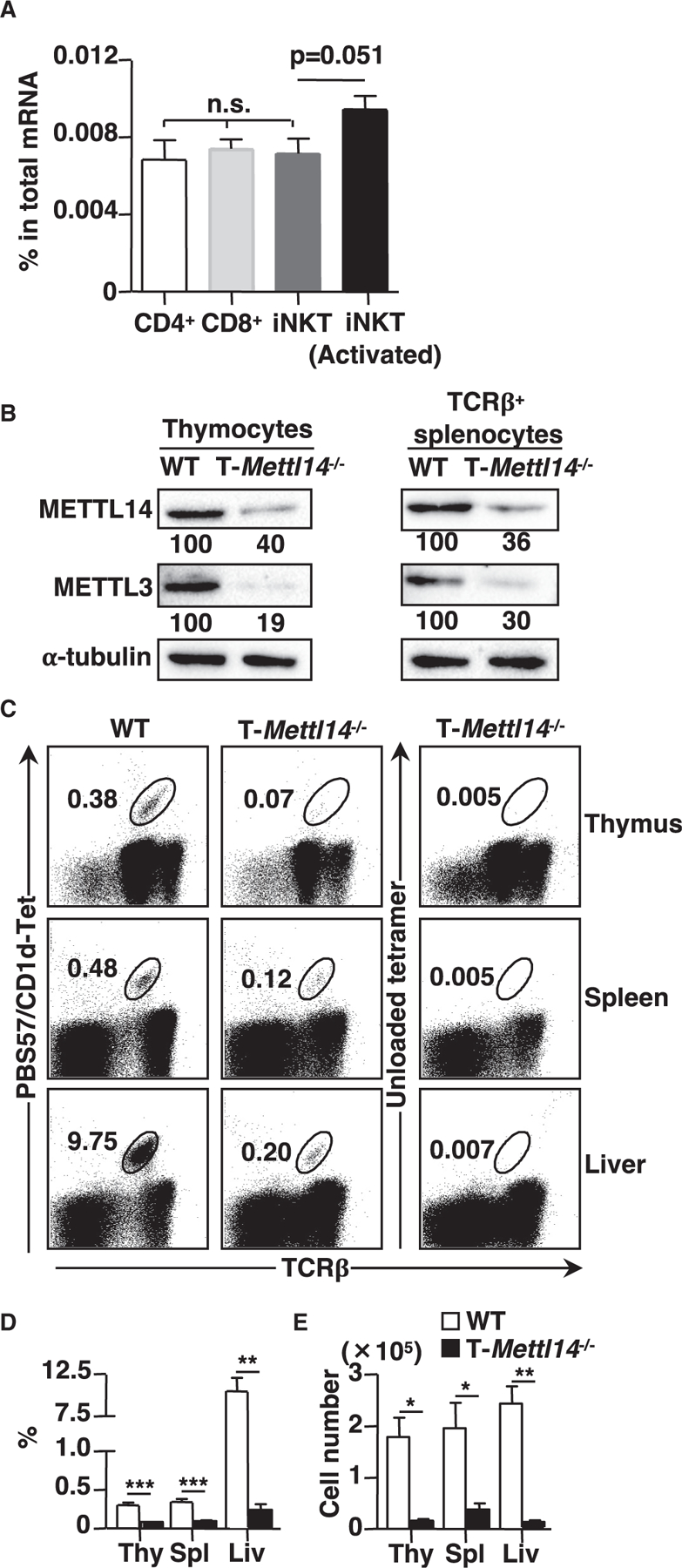
(A) m6A level in total mRNA of CD4+, CD8+ T cells, and iNKT cells (naive and activated) (n = 5–6).
(B) Immunoblot of METTL14 and METTL3 in total thymocytes and TCRβ+ splenocytes in WT and T-Mettl14−/− mice. Data representative of three independent experiments.
(C) Representative staining of lymphocytes in indicated organs from WT and T-Mettl14−/− mice with CD1d/PBS57 tetramer or unloaded CD1d tetramer.
(D and E) Summary of frequencies and cell numbers of iNKT cells in the indicated organs from WT and T-Mettl14−/− mice (n = 5–8). SEM is shown. *p < 0.05, **p < 0.01, ***p < 0.001.
We first confirmed that the protein level of METTL14 was significantly reduced in both thymocytes and TCRβ+ splenocytes from T-Mettl14−/− mice (Figure 1B). As METTL14 and METTL3 subunits are mutually required for protein stability (Kobayashi et al., 2018), the level of METTL3 protein was also decreased in both thymocytes and TCRβ+ splenocytes from T-Mettl14−/− mice (Figure 1B). T cell-specific ablation of METTL14 resulted in a 2- to 3-fold reduction of total thymocytes; consequently, the absolute cell number of DP, CD4SP, CD8SP, and DN thymocytes in T-Mettl14−/− mice were substantially reduced compared with wild-type (WT) littermates (T-Mettl14+/−) (Figure S1A). However, METTL14-deficiency had the most profound impact on iNKT cells; T-Mettl14−/− mice had an ~90% reduction in the percentage and cell number of iNKT cells in the thymus (Figures 1C–1E). Analysis of iNKT cells in the peripheral revealed that the percentage and absolute number of iNKT cells were also dramatically reduced (~5- to 10-fold) in the spleen and liver of T-Mettl14−/− mice as compared with WT littermates (Figures 1C–1E). In comparison, the total numbers of conventional CD4+ and CD8+ T cells were reduced by 2- to 3-fold in the spleen (Figure S1B). We also assessed the effect of METTL14-deletion on the development of other innate-like T cells, including CD1d-restricted type II NKT cells (defined as CD8−NK1.1+CD1d/PBS57 tetramer− T cells) (Zhao et al., 2014) and MAIT cells, and found that the frequency and absolute number of both T cell types were significantly reduced in the thymus and liver of T-Mettl14−/− mice (Figures S1C–S1H). Thus, m6A modification plays an important role in the development and homeostasis of innate-like T cells.
Impairment of iNKT cell homeostasis in T-Mettl14−/− mice is cell intrinsic
As iNKT cells were substantially reduced in T-Mettl14−/− mice, this led to the question of whether this deficiency was mediated by a cell-intrinsic or cell-extrinsic mechanism. We first assessed CD1d expression in DP thymocytes, the cell type responsible for positive selection of iNKT cells (Bendelac, 1995). CD1d expression was unaltered in T-Mettl14−/− DP thymocytes (Figure 2A). Similarly, the production of IL-2 was unchanged in the iNKT cell hybridoma DN32.D3 cocultured with WT or T-Mettl14−/− thymocytes pulsed with various concentrations of iNKT cell-specific ligand α-GalCer (Figure 2B), suggesting METTL14 deficiency does not affect the expression and antigen-presenting capacity of CD1d.
Figure 2. Defective development of iNKT cells in T-Mettl14−/− mice is cell intrinsic.
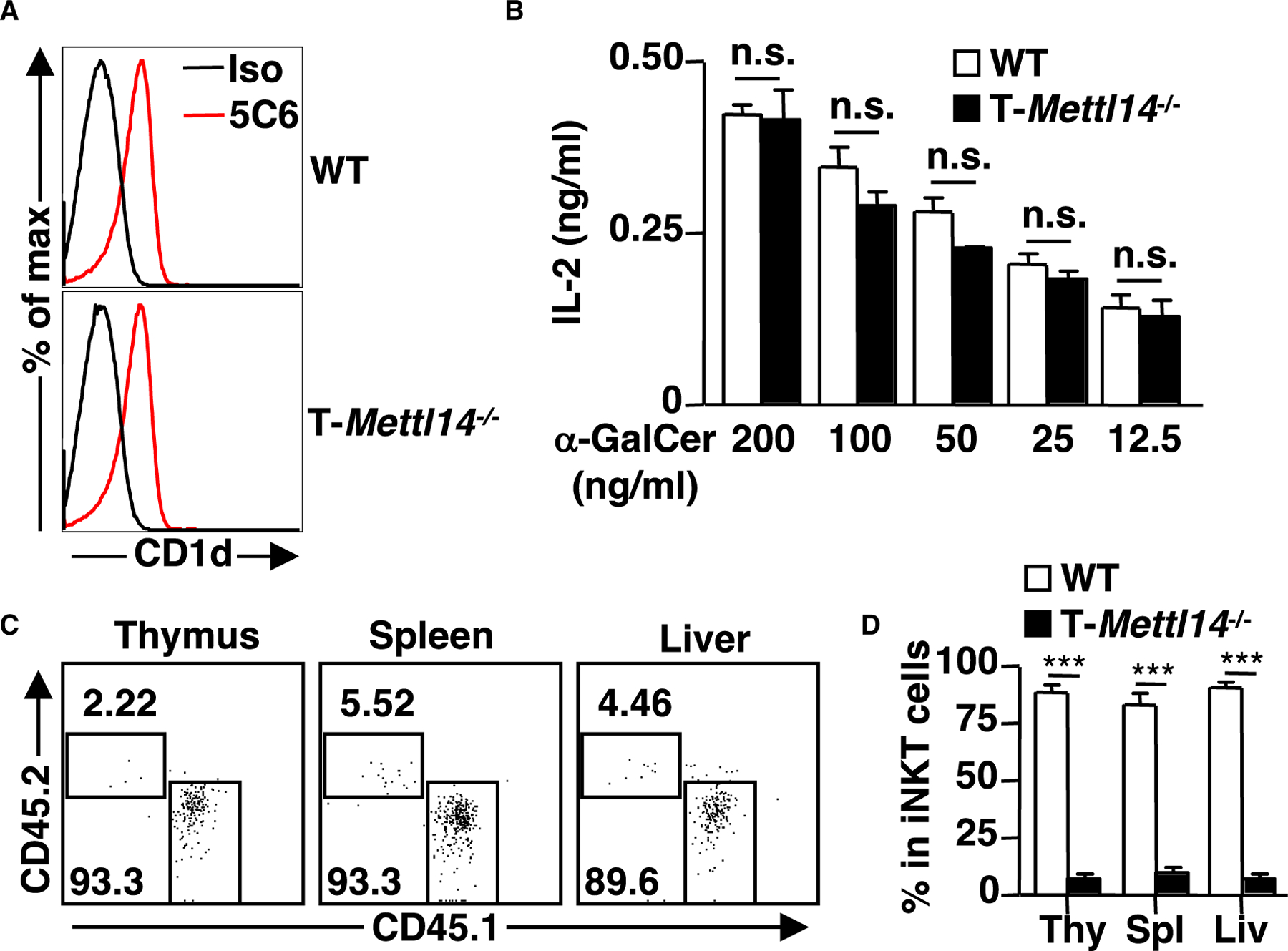
(A) Representative histogram of CD1d expression on DP thymocytes. CD1d was stained with α-CD1d or isotype control antibody (n = 8).
(B) IL-2 detected by ELISA following 48-h co-culture of iNKT cell hybridoma DN32.D3 with irradiated thymocytes pulsed with α-GalCer ranging from 200 ng/mL to 12.5 ng/mL. Data representative of three independent experiments.
(C) Flow cytometric analysis of iNKT cells in the Jα18−/− recipient mice after 6 weeks of reconstitution with 1:1 mixture of bone marrow cells from WT (CD45.1) and T-Mettl14−/− (CD45.2).
(D) Quantification of iNKT cell reconstitution in thymus, spleen, and liver in bone marrow chimera recipients (n = 7). SEM is shown. ***p < 0.001.
To determine whether METTL14 regulates iNKT cell development in a cell-intrinsic manner, we injected a 1:1 mixture of bone marrow cells (BM) from WT (CD45.1) and T-Mettl14−/− (CD45.2) mice into irradiated Jα18−/− mice lacking iNKT cells. We found that iNKT cells derived from T-Mettl14−/− BM only accounted for 2% to 5% of total reconstituted iNKT cells in the thymus, spleen, and liver of recipient mice (Figures 2C and 2D). Taken together, METTL14 deficiency affects iNKT cell development through a cell-intrinsic mechanism.
METTL14 deficiency impairs the maturation of iNKT cells
To further investigate the impact of m6A modification on iNKT cell development, the maturation status of iNKT cells was analyzed in the thymus of T-Mettl14−/− mice. The percentages of iNKT cells from T-Mettl14−/− mice in stage 0–2 were increased compared with WT littermate controls, while the percentage of stage 3 iNKT cells was significantly decreased (Figures 3A and 3B). In terms of absolute cell numbers, all stages of iNKT cells in T-Mettl14−/− thymus decreased substantially except for those in stage 1 (Figure 3C). Interestingly, this developmental defect was not due to the lack of Egr2 (Figures S2A and S2B), a transcriptional factor that determines both early and late stages of iNKT lineage differentiation (Lazarevic et al., 2009; Seiler et al., 2012).
Figure 3. Mettl14 deficiency impairs the maturation of iNKT cells.
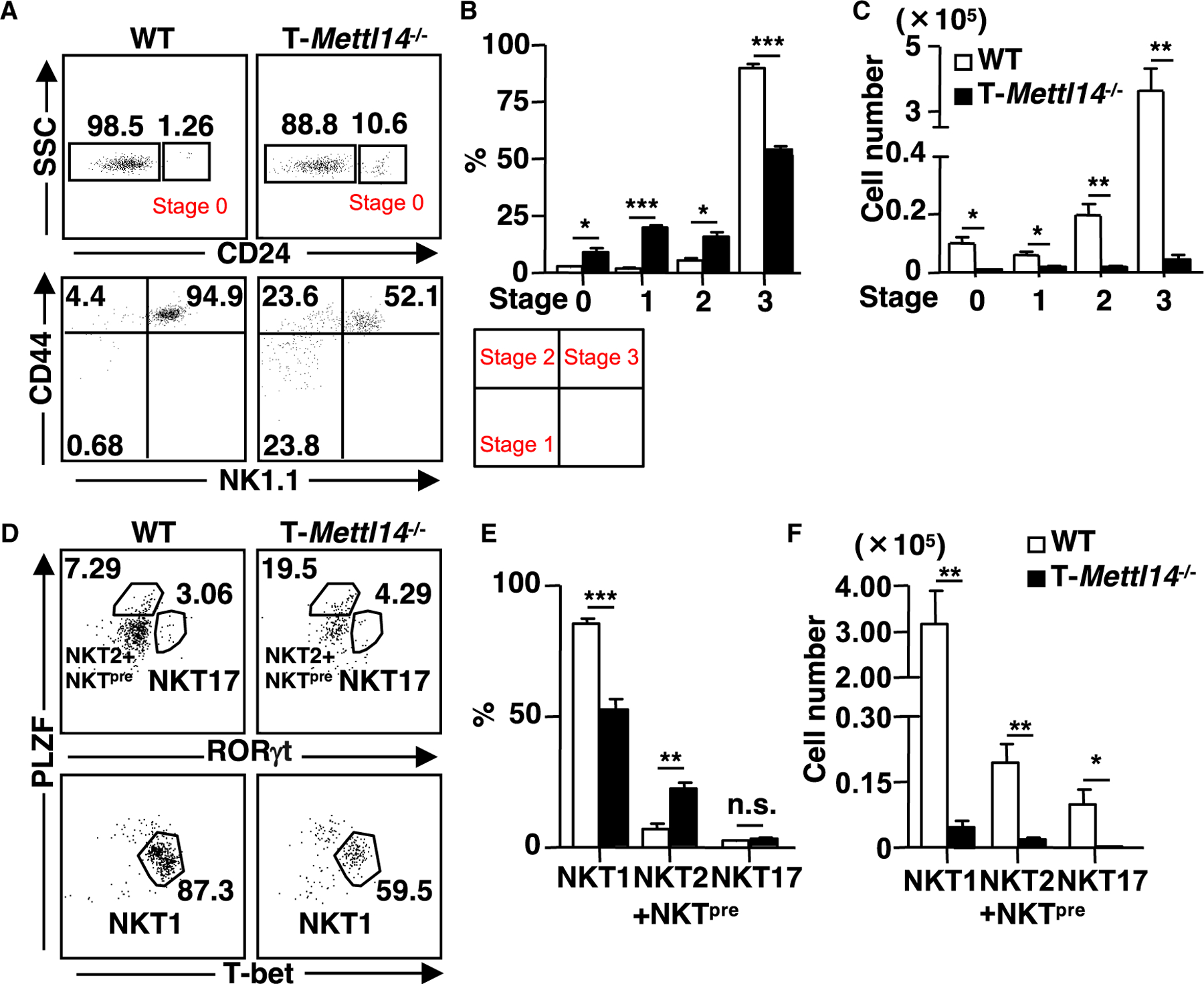
(A) Representative staining of iNKT cells (CD69+CD1d/PBS57 tetramer+) at various developmental stages in the thymus of WT and T-Mettl14−/− mice.
(B and C) Quantification of percentages and cell numbers of stages 0, 1, 2, and 3 iNKT cells in WT and T-Mettl14−/− mice (n = 3–4).
(D) Intracellular staining of PLZF, T-bet, and RORγt in thymic iNKT cells of WT and T-Mettl14−/− mice.
(E and F) Quantification of percentages and cell numbers of NKT1, NKT2+NKTpre, and NKT17 subsets (n = 6). SEM is shown. *p < 0.05, **p < 0.01, ***p < 0.001.
To further differentiate functional iNKT cell subsets, changes in the expression of three key transcription factors, PLZF, RORγt, and T-bet, were analyzed in thymic iNKT cells of T-Mettl14−/− mice (Lee et al., 2013). The proportion of the RORγt+ NKT17 subset was not altered, but PLZFhiRORγt− iNKT cells, enriched for NKT2 and NKT cell precursors (NKTpre) (Wang and Hogquist, 2018), were significantly increased and the T-bet+ NKT1 subset was significantly decreased in T-Mettl14−/− mice (Figures 3D and 3E). The absolute cell numbers of all three subsets were significantly decreased due to drastically lower iNKT cell numbers in T-Mettl14−/− mice (Figure 3F). These data showed that ablation of Mettl14 results in impaired iNKT cell maturation and differentiation.
m6A maintains DP thymocyte survival in part through regulation of the p53-mediated apoptosis pathway
In order to identify mechanisms by which METTL14 deficiency impaired iNKT cell development, DP thymocytes containing the precursor of iNKT cells from T-Mettl14−/− and littermate control mice were sorted for RNA-seq analysis. A total number of 117 differentially expressed genes (DEGs) were identified, of which 71 were protein-coding genes and 23 were long non-coding RNAs (lncRNAs) (Figures 4A and 4B). Interestingly, all differentially expressed lncRNAs were downregulated in the T-Mettl14−/− group, consistent with previous findings that m6A modification stabilizes lncRNAs (He et al., 2020). Using gene set enrichment analysis (GSEA) (Subramanian et al., 2005), both p53 hallmark and apoptosis hallmark gene sets were positively enriched within the T-Mettl14−/− group with a normalized enrichment score of 0.99 and 0.93, respectively (Figures 4C, 4D, S3A, and S3B). Several potential key regulators of these pathways were identified within the DEG list. Hmga1b, one of the most significantly downregulated genes in Mettl14−/− DP thymocytes, encodes a chromatin-associated protein that is known to interact with p53 and inhibit its apoptotic function (Frasca et al., 2006). Xaf1, encoding a pro-apoptotic protein that can induce p53-mediated apoptosis (Lee et al., 2014), was upregulated in Mettl14−/− DP thymocytes. Another upregulated gene, Trim25, encodes an E3 ubiquitin ligase that controls the Keap1/Nrf2 pathway, known to promote survival of tumor cells (Liu et al., 2020) and increase the apoptosis of iNKT cells (Pyaram et al., 2019).
Figure 4. m6A maintains DP thymocyte survival in part through regulation of the p53-mediated apoptosis pathway.
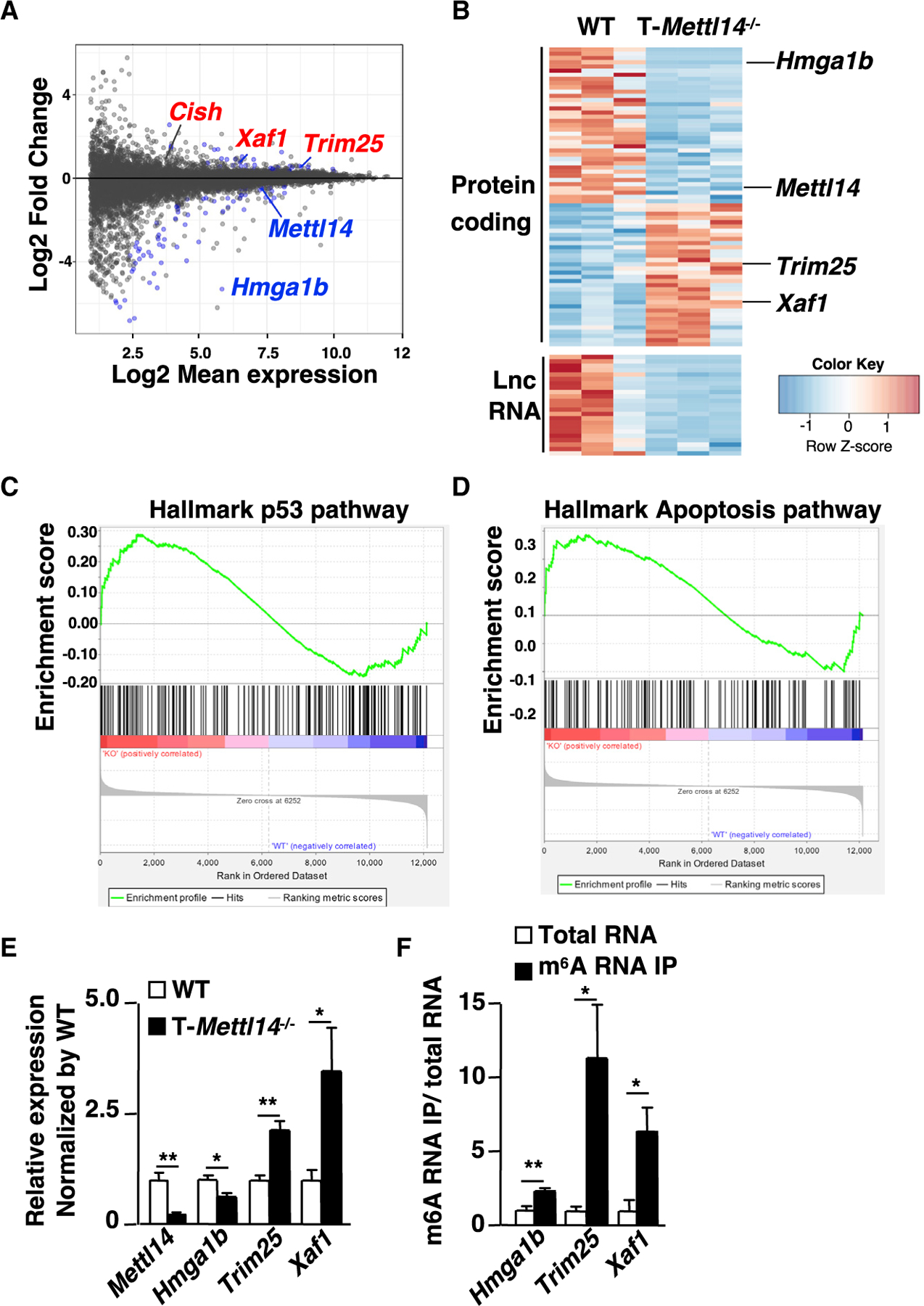
(A) RNA-seq results of DP thymocytes from WT and T-Mettl14−/− mice with indicated gene labeling (n = 3). Data shown are fold change of T-Mettl14−/−/WT.
(B) Heatmap of differentially expressed protein-coding genes and long non-coding RNA. Gene set enrichment analysis showing enrichment for hallmark p53 pathway (C) and apoptosis pathway (D) in T-Mettl14−/− DP thymocytes.
(E) Relative expression of Mettl14, Hmga1b, Trim25, and Xaf1 in DP thymocytes of T-Mettl14−/− mice detected by qPCR.
(F) Relative expression of Hmga1b, Trim25, and Xaf1 in the m6A RNA immunoprecipitation versus total thymocyte RNA in WT mice detected by qPCR (n = 3–4). SEM is shown. *p < 0.05, **p < 0.01.
Using qPCR, we confirmed downregulation of Mettl14 and Hmga1b and upregulation of Trim25 and Xaf1 in METTL14-deficient DP thymocytes (Figure 4E). To determine whether these genes may be direct targets of m6A modification, m6A-RNA-IP was performed using total RNA from B6 thymocytes in combination with cDNA generation/qPCR. Hmga1b, Trim25, and Xaf1 were enriched within the m6A-RNA-IP group, suggesting they are in fact direct targets of m6A modification (Figure 4F).
Decreased Hmga1b and increased Xaf1 expression suggest that activation of the p53-mediated apoptotic pathway may be enhanced in T-Mettl14−/− DP thymocytes. Indeed, higher percentages of Annexin V+ apoptotic cells were detected in ex vivo-isolated, in vitro-medium cultured, and anti-CD3/anti-CD4 stimulated T-Mettl14−/− DP thymocytes as compared with WT DP thymocytes (Figures 5A and 5B). As reactive oxygen species (ROS) are downstream mediators of p53-dependent apoptosis (Johnson et al., 1996), intracellular ROS between T-Mettl14−/− and WT DP thymocytes was compared using the cell-permeant florescent probe CM-H2DCFDA. ROS levels were elevated in T-Mettl14−/− DP thymocytes (Figure 5C), which correlated with increased apoptosis (Figure 5D). Interestingly, treatment with antioxidant N-acetyl-L-cysteine (NAC), a precursor of glutathione that is known to rescue thymocyte apoptosis by scavenging ROS (Pathak and Khandelwal, 2006) (Figure 5D), led to a decreased percentage of Annexin V+ cells in T-Mettl14−/− DP thymocytes to a level comparable with WT DP thymocytes.
Figure 5. Elevated apoptosis in T-Mettl14−/− DP thymocytes is rescued by the scavenger of ROS.
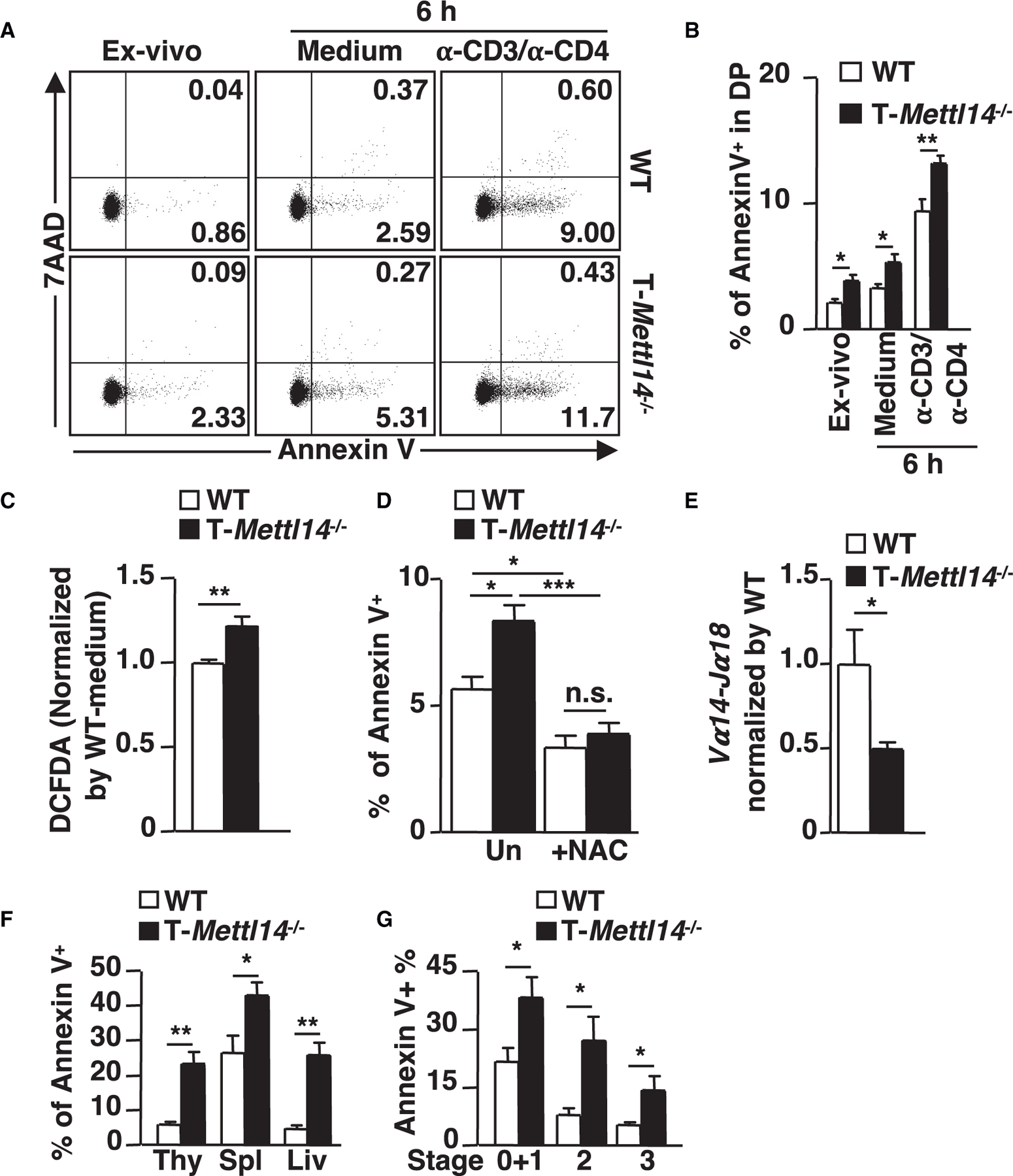
(A) Representative staining of apoptosis markers of naive and activated DP thymocytes.
(B) Percentage of Annexin V+ cells in ex vivo, 6-h medium culture, or anti-CD3/anti-CD4-stimulated DP thymocytes from WT and T-Mettl14−/− mice (n = 5–6).
(C) Intracellular ROS in DP thymocytes detected by DCFDA (n = 5).
(D) Percentage of Annexin V+ population in DP thymocytes after 6-h incubation with or without NAC (n = 5).
(E) Relative expression of Vα14-Jα18 in DP thymocytes of T-Mettl14−/− mice (n = 5).
(F) Percentage of Annexin V+ population of iNKT cells in WT and T-Mettl14−/− mice from thymus, spleen, and liver (n = 7).
(G) Percentage of Annexin V+ population of iNKT thymocytes at different developmental stages (n = 7). SEM is shown. *p < 0.05, **p < 0.01, ***p < 0.001.
The enhanced level of apoptosis likely shortens the lifespan of T-Mettl14−/− DP thymocytes, which in turns reduces the efficiency of distal TCR recombination that iNKT cells harbor and therefore leads to an overall decreased number of thymic iNKT cells (Baldwin et al., 2004). Indeed, the mRNA level of Vα14-Jα18 recombination, critical for the development of iNKT cells, was significantly decreased in CD69− preselection DP thymocytes isolated from T-Mettl14−/− mice (Figure 5E). We also evaluated whether the residual Mettl14−/− iNKT cells are more susceptible to apoptosis. iNKT cells in the thymus, spleen, and liver of T-Mettl14−/− mice had a higher percentage of Annexin V+ cells. In addition, all stages of thymic iNKT cells in T-Mettl14−/− mice exhibited enhanced apoptosis (Figures 5F and 5G). These data suggest that disruption of METTL14-dependent m6A modification results in altered expression of the key molecules involved in the p53-mediated apoptotic pathway, which may contribute to enhanced apoptosis of DP thymocytes and iNKT cells.
Upregulation of the m6A target gene Cish in METTL14-deficient thymocytes correlates with decreased TCR signaling and impaired cytokine response
Absence of m6A modification in T-Mettl3−/− mice resulted in elevated expression of several SOCS family genes, including Socs1, Socs3, and Cish, in CD4+ T cells (Li et al., 2017). We explored whether SOCS family genes were also upregulated in T-Mettl14−/− DP thymocytes, which contain iNKT cell precursors. The expression of Cish, but not other SOCS family genes, was drastically increased in both naive and TCR-stimulated T-Mettl14−/− DP thymocytes, compared with their WT counterparts (Figures 6A and S4A). In addition, Cish was a direct target of m6A modification in DP thymocytes (Figure 6B), similar to the finding in CD4+ T cells (Li et al., 2017).
Figure 6. Upregulation of m6A target gene Cish in Mettl14-deficient thymocytes correlates with decreased TCR signaling and impaired cytokine response.
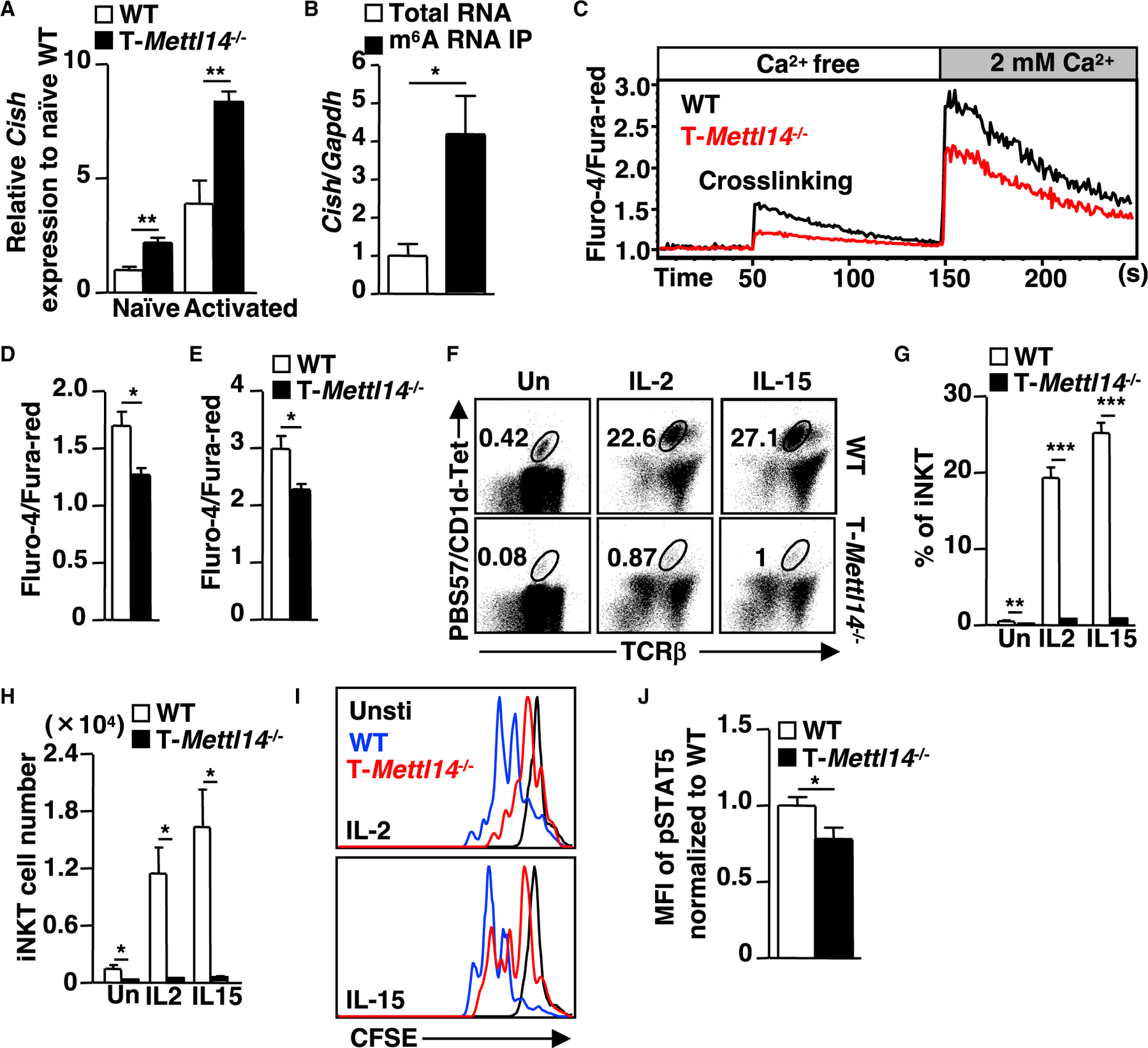
(A) Relative expression of Cish in naive and activated (anti-CD3/anti-CD4) DP thymocytes (n = 5–9).
(B) Relative expression of Cish in the m6A RNA immunoprecipitation of total thymocytes RNA in WT mice by qPCR quantification (n = 4).
(C) Intracellular calcium flux in DP thymocytes in response to crosslinking of anti-CD3/anti-CD4 in T-Mettl14−/− mice.
(D and E) Quantification of maximum calcium flux on crosslinking and addition of Ca2+ in T-Mettl14−/− DP cells (n = 5–6).
(F) Representative staining of thymic iNKT cell expansion at day 3 after stimulation with IL-2 or IL-15.
(G and H) Percentage and cell number of thymic iNKT cells on D0 and D3 after stimulation with IL-2 or IL-15 (n = 5).
(I) Representative figure of cell trace distribution in thymic iNKT cells on day 3 post-stimulation with IL-2 or IL-15 (n = 5–6). Unstimulated thymocytes were used as controls.
(J) Bar graph of pSTAT5 in iNKT thymocytes after 20 min of IL-15 stimulation (n = 5). SEM is shown. *p < 0.05, **p < 0.01, ***p < 0.001.
Cish is induced by TCR stimulation in T cells (Li et al., 2000) and plays an important role in regulating TCR and cytokine signaling (Matsumoto et al., 1997; Yoshimura et al., 1995) by targeting PLC-γ1 for proteasomal degradation (Palmer et al., 2015) and disrupting IL-stimulated STAT5 phosphorylation (Aman et al., 1999). To explore the functional consequence of Cish upregulation, we first evaluated the TCR-mediated calcium flux in DP thymocytes. T-Mettl14−/− DP thymocytes exhibited attenuated calcium influx compared with WT DP thymocytes both at TCR crosslinking and at Ca2+ addition stages (Figures 6C–6E). Next, we assessed the proliferative response of thymic iNKT cells to IL-2 or IL-15 stimulation (Gordy et al., 2011; Matsuda et al., 2002). While there was a 5-fold reduction in the percentage of iNKT cells in T-Mettl14−/− mice before stimulation, the differences widened after stimulation with IL-2 or IL-15, resulting in a 25-fold decrease in the percentage and 30-fold decrease in cell number of iNKT cells in T-Mettl14−/− mice (Figures 6F–6H). The diminished cytokine response in Mettl14−/− iNKT cells was further demonstrated by using cells labeled with CellTrace fluorescent dye (CFSE) to show that T-Mettl14−/− iNKT cells were less able to proliferate in response to IL-2 and IL-15 stimulation, compared with WT iNKT cells (Figure 6I). Moreover, phosphorylation of STAT5, a downstream target in cytokine stimulation, was also decreased in IL-15-stimulated Mettl14−/− thymic iNKT cells (Figure 6J). These data suggest that elevated Cish expression due to the loss of m6A modification may play a role in suppressing TCR and cytokine-mediated signaling in iNKT cells prior to egress into the periphery.
METTL14 deficiency impairs the function of mature iNKT cells
Having found METTL14-dependent m6A modification is indispensable for thymic homeostasis and development of iNKT cells, next we addressed whether the function of peripheral iNKT cells in T-Mettl14−/− mice was affected. α-GalCer was injected into WT and T-Mettl14−/− mice to activate iNKT cells in vivo and IFN-g and IL-4 production by iNKT cells was evaluated using intracellular cytokine staining. We found that hepatic iNKT cells from α-GalCer-injected T-Mettl14−/− mice had a significantly lower percentage of IFN-γ and IL-4 single and double producing cells as compared with iNKT cells from α-GalCer-injected WT mice (Figures 7A and 7B). To determine whether METTL14-deficiency affects the expansion of iNKT cells in vivo, we evaluated iNKT cell proliferation and apoptosis on day 3 after α-GalCer injection. While the percentage of splenic iNKT cells increased approximately 20-fold in α-GalCer-injected WT mice, the percentage of iNKT cells in T-Mettl14−/− was only increased by 4-fold, compared with naive counterparts (Figures 1D, 1E, and S5A–S5C). In addition, splenic iNKT cells in α-GalCer-injected T-Mettl14−/− mice had a lower percentage of Ki67+ and higher percentage of Annexin V+ cells compared with WT control (Figures S5D and S5E). These results indicate that the function of residual iNKT cells is impaired in T-Mettl14−/− mice.
Figure 7. Mettl14-deficiency impairs mature iNKT cell function.
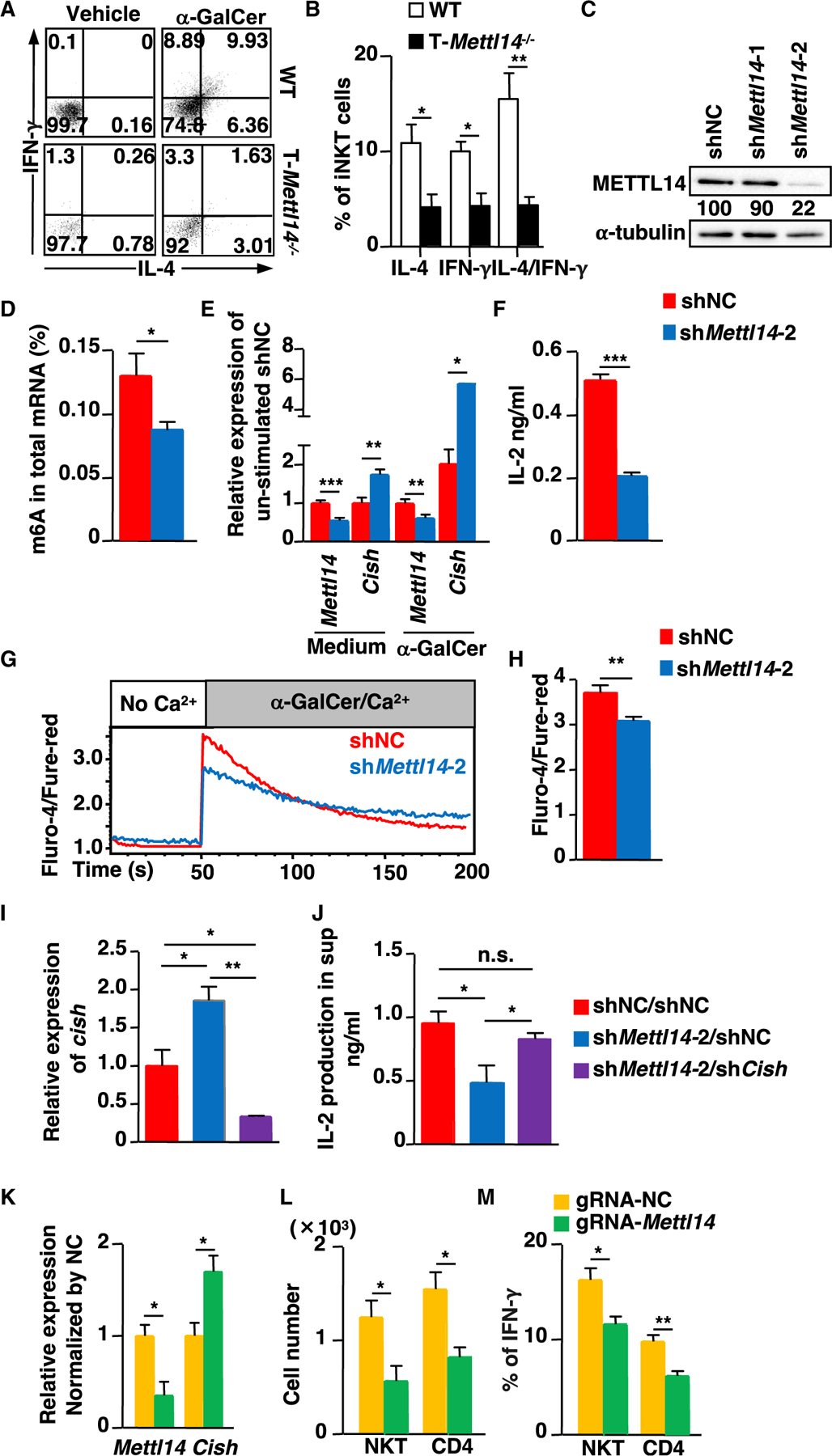
(A) Dot plots of IFN-γ and IL-4 in residual iNKT cells from T-Mettl14−/− mice after in vivo α-GalCer stimulation.
(B) Quantification of the percentage of IFN-γ and IL-4 producing iNKT cells in α-GalCer-immunized WT and T-Mettl14−/− mice (n = 4).
(C) Expression of METTL14 in DN32.D3 cells transduced with lentivirus coding Mettl14-specific shRNA (shMettl14-1 or shMettl14-2) or control shRNA (shNC).
(D) m6A level in mRNA of DN32.D3 cells transduced with shMettl14-2 and shNC.
(E) Relative expression of Mettl14 and Cish in DN32.D3-shMettl14-2 in medium or stimulation with α-GalCer for 24 h.
(F) Production of IL-2 in DN32.D3-shMettl14-2 after stimulation with α-GalCer for 24 h quantified by ELISA.
(G) Intracellular calcium flux in DN32.D3-shMettl14-2 cells in response to α-GalCer/Ca2+.
(H) Quantification of maximum calcium flux upon α-GalCer stimulation. Data representative of three to six independent experiments. shNC or shMettl14-2-treated DN32.D3 were spin-transduced with retrovirus carrying shCish or shNC. Zsgreen+ cells were sorted and cultured.
(I) Relative expression of Cish in the indicated groups.
(J) IL-2 production in shNC/shNC-treated (‘‘WT’’ control) and shNC and shCish-treated Mettl14KD DN32.D3 cells after stimulation with α-GalCer for 24 h. Vα14Tg splenocytes were nucleofected with rCas9/gRNA complex and maintained in complete RPMI supplemented with IL-2 for 3 days.
(K) Mettl14 and Cish expression by qPCR on day 3 after nucleofection.
(L) Absolute cell numbers of iNKT and CD4+ T cells after nucleofection.
(M) IFN-γ production in iNKT and CD4+ T cells after nucleofection (n = 3–4). SEM is shown. *p < 0.05, **p < 0.01, ***p < 0.001.
Since differentiation and maturation of iNKT cells is impaired in T-Mettl14−/− mice, the effect of METTL14-dependent m6A modification on the function of mature iNKT cells was further assessed using the iNKT cell hybridoma DN32.D3 that stably expressed a short hairpin RNA (shRNA) targeting Mettl14. Two Mettl14-shRNAs were tested, but only sh2 was able to substantially knock down the expression of METTL14 in DN32.D3 cells (Figure 7C). In addition, m6A modification level was significantly decreased in Mettl14 knockdown (Mettl14KD) DN32.D3 cells (Figure 7D). Knocking down Mettl14 in DN32.D3 cells significantly increased the expression of Cish in both medium and α-GalCer stimulated conditions (Figure 7E), while other Socs family genes were slightly upregulated in the stimulated group (Figure S4B). In addition, production of IL-2 was significantly decreased in Mettl14KD DN32.D3 cells after stimulation with α-GalCer (Figure 7F), which was consistent with their reduced calcium influx (Figures 7G–7H). To determine whether Cish upregulation contributed to the decreased TCR signaling and cytokine response in Mettl14KD DN32.D3 cells, we knocked down Cish in Mettl14KD DN32.D3 cells using retrovirus expressing shRNA targeting Cish. We found knocking down Cish in Mettl14KD DN32.D3 cells (shMettl14-2/shCish) could restore IL-2 production to the level comparable to the control group (shNC/shNC) (Figures 7I and 7J).
The CRISPR-Cas9-mediated gene targeting method (Seki and Rutz, 2018) was also used to delete Mettl14 in splenic iNKT cells from Vα14Tg mice. An ~70% reduction in Mettl14 transcript level was observed in iNKT cells transfected with rCas9/gRNA-Mettl14 complex as compared with iNKT cells transfected with negative control (NC) (Figure 7K). Consistent with the Mettl14KD results in the iNKT hybridoma cell line (DN32.D3), the expression of Cish was also significantly increased in rCas9/gRNA-Mettl14-treated primary splenic iNKT cells (Figure 7K). In addition, the total number of iNKT cells was significantly decreased in the rCas9/gRNA-Mettl4 treated group (Figure 7L). The percentage of IFN-γ-producing iNKT cells was also reduced in the rCas9/gRNA-Mettl14-treated group upon anti-CD3 stimulation (Figure 7M). These data suggested that METTL14-dependent m6A modification affects the function of mature iNKT cells by regulating Cish expression.
DISCUSSION
In this study, we described the critical requirement for METTL14-dependent m6A modifications in iNKT cell development and function. Our data suggest that the drastic reduction of iNKT cell population in T-Mettl14−/− mice is likely due to the enhanced apoptosis of DP thymocytes mediated by p53 apoptotic pathway activation and impaired responses to IL-2/IL-15 and TCR stimulation as a consequence of elevated Cish gene expression. Knocking down Mettl14 in mature iNKT cells also leads to decreased TCR signaling and cytokine production, which is correlated with increased expression of Cish, as knocking down Cish expression restored the function of iNKT cells.
Previous study with T-Mettl3−/− mice showed that thymocyte populations did not change significantly in number or percentage (Li et al., 2017) but in T-Mettl14−/− mice, all thymocyte subsets were decreased in cell number, with particularly striking effects on iNKT cells (Figures 1C–1E, and S1A). CD4+ T cells in T-Mettl3−/− mice did not show increased apoptosis after ex vivo culture. In contrast, T-Mettl14−/− mice had elevated apoptosis in both DP thymocytes (Figures 5A and 5B) and iNKT cells (Figures 5F and 5G) due to the upregulation of pro-apoptotic genes and downregulation of anti-apoptotic genes (Figure 4E). Furthermore, CD4+ T cells in T-Mettl3−/− mice failed to expand upon stimulation with IL-7 because of elevated expression of several SOCS genes, including Socs1, Socs3, and Cish. Cish was the only upregulated gene in the SOCS family in T-Mettl14−/− DP thymocytes after stimulation (Figures 6A and S4A), leading to an attenuated response to cytokine and TCR stimulation (Figures 6C–6J). The differences observed in T-Mettl3−/− and T-Mettl14−/− mice could in part be due to incomplete deletion of loxP flanked genes during T cell development in these mouse models. In addition, recent studies have indicated that METTL3 can directly control the translation process of certain mRNA by recruiting eIF3 without the coordination with METTL14 in some human cancer cells (Choe et al., 2018; Lin et al., 2016). Further study is required to determine specific genes targeted by these two METTLs in the development and function of various T cell subsets.
GSEA analysis of RNA-seq results showed that the p53 pathway and apoptosis pathways were enhanced in T-Mettl14−/− DP thymocytes. Therefore, the apoptotic-related genes, such as Hmga1b, Xaf1 and Trim25, were analyzed to confirm their mRNAs were m6A targets (Figures 4E and 4F). Increased apoptosis was consistently observed in T-Mettl14−/− DP thymocytes in both naive and activated states (Figures 5A and 5B). While our data indicated that METTL14 regulates iNKT cell development mostly in a cell-intrinsic fashion (Figure 2), the relative overall reduction in DP thymocytes, which are critical for iNKT cell positive selection, may also contribute to the drastic reduction of iNKT cells in T-Mettl14−/− mice. It has been shown that mature iNKT cells have a greatly increased ROS level compared with CD4+ and CD8+ T cells (Kim et al., 2017). In addition, iNKT cells are more susceptible to oxidative stress compared with conventional T cells (Kim et al., 2017). The elevated apoptosis in T-Mettl14−/− DP thymocytes was completely rescued by NAC treatment, indicating m6A may control homeostasis of DP thymocytes by restraining p53-mediated apoptosis. Interestingly, deficiency of Mettl3 also leads to enhanced apoptosis by modulating p53 signaling pathway in HepG2 cells (Dominissini et al., 2012) and the arsenite-transformed human keratinocytes (Zhao et al., 2020).
Other genes regulated by m6A modification may be involved in the control of iNKT cell development and function, such as c-myc, c-myb, Pten, and Bcl-2. c-Myc, c-Myb, and PTEN are required for iNKT cell development and homeostasis (Dose et al., 2009; Hu et al., 2010; Kishimoto et al., 2007; Mycko et al., 2009) and BCL-2 is an anti-apoptotic molecule that maintains iNKT cell survival (Zhu et al., 2018). In addition, iNKT cell development and function require nuclear factor-κB signaling (Stanic et al., 2004), which is modulated by m6A modification (Wang et al., 2019). However, none of these genes were differentially expressed in T-Mettl14−/− DP thymocytes compared with their WT counterparts based on RNA-seq data. Notably, the GSEA analysis revealed that c-Myc-target genes, which have been reported to maintain iNKT cell differentiation and function (Dose et al., 2009; Mycko et al., 2009), were significantly decreased (Figure S6A). In addition, Notch signaling, which has been shown to inhibit iNKT cell differentiation and function (Oh et al., 2015), was upregulated in T-Mettl14−/− DP thymocytes (Figure S6B). Therefore, m6A modification may regulate iNKT cell development through multiple signaling pathways.
lncRNAs are a class of non-coding RNA transcripts that are longer than 200 base pairs and have metabolic and regulatory functions (Fernandes et al., 2019). m6A modification increases the stability of lncRNA (He et al., 2020). The lncRNAs in T-Mettl14−/− DP were all downregulated due to the absence of the m6A modification. One lncRNA, Gm15441, was shown to attenuate inflammasome activation in response to PPARα agonism and fasting (Brocker et al., 2020), which might explain the activation of pro-inflammatory pathways in our GSEA analysis (Figure S6C). However, little is known about the role of lncRNAs in NKT cell development and function, which warrants further investigation.
Alterations in m6A patterns are associated with human diseases such as cancer initiation and progression. There is growing interest in developing m6A writer-based strategies to combat cancer (He and He, 2021). Specifically, inhibitors targeting METTL3 catalytic function have been developed (Dolbois et al., 2021; Moroz-Omori et al., 2021) and have shown promise in the treatment of acute myeloid leukemia (Yankova et al., 2021). These inhibitors could potentially be useful to delineate the temporal requirement for m6A modification in T cell development, homeostasis, and function. In this study, we demonstrated m6A modification was critical for the development and function of iNKT cells through orchestrating the regulation of multiple genes involved in cell survival and TCR signaling. As iNKT cells are innate-like T cells playing important roles in autoimmune disease, infection, and tumor immunity (Bedard et al., 2017; Fujii and Shimizu, 2019; Galvez et al., 2021; Van Kaer and Wu, 2018), clinical application of targeting m6A methyltransferases should consider the impact on the homeostasis and function of iNKT cells and the benefits to the treatment of chronic disease.
Limitations of the study
In this study, we showed that genes involved in several signaling pathways are differentially regulated in Mettl14-deficient DP thymocytes, including pathways associated with c-Myc, Notch, and inflammation. However, we have not directly investigated the impact of these pathways on iNKT cell development and function in T-Mettl14−/− mice. Furthermore, due to the very limited number of residual thymic iNKT cells in T-Mettl14−/− mice, we used total thymocytes instead of purified iNKT cells in most in vitro assays, and therefore cannot exclude the possibility that other cell types may affect iNKT cell responses in these experiments. Finally, since Mettl14 was deleted in all the CD4 expressing populations, some of the observed phenotypic changes in iNKT cells may be due to changes in their interactions with other T cells.
STAR☆METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Chyung-Ru Wang (chyung-ru-wang@northwestern.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The accession number of RNAseq data for DP is GSE189339 in NCBI (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE189339).
Scripts used for alignment and counting can be found here (https://github.com/ebartom/NGSbartom). Downstream analysis was performed as described in method details.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
C57BL/6 (B6), Vα14-Jα18 transgenic (Vα14Tg) mice and CD45.1 congenic B6 mice were purchased from The Jackson Laboratory. Mettl14fl/fl; CD4-Cre+ mice have been described previously (Lu et al., 2020) and were backcrossed for 10 generations onto B6 background. Littermates were used as controls. Jα18−/− mice on the B6 background were kindly provided by Dr. Luc Van Kaer (Vanderbilt University). All mice for each experiment were sacrificed between 2–4 months of age. Both males and females were used. All mouse experiments were approved by the institutional animal care and use committee (IACUC) at Northwestern University. All animals were housed in a specific-pathogen-free facility according to institutional guidelines.
Cell culture and E. coli culture
Splenocytes from Vα14Tg mice were stimulated with plate-bound anti-CD3 (5 μg/mL) and soluble anti-CD28 (2 μg/mL) for 24 h and subjected to nucleofection. Then the splenocytes were maintained in complete RPMI supplemented with 10% FBS, 2 mM Glutamine, 10 mM HEPES, 55 μM 2-ME, 100 units/mL penicillin and 100 μg/mL streptomycin in the presence of IL-2 (200 U/mL) in 5% CO2 incubator at 37°C. Thymocytes were labeled with CellTrace™ CFSE (Invitrogen) according to the manufacture’s protocol and cultured in the presence of IL-2 (200 U/mL) or IL-15 (100 ng/mL). iNKT hybridoma (DN32.D3) cells, 293T cells and Phoenix-Eco cells were maintained in DMEM supplemented with 10% FBS, 10 mM HEPES, 100 units/mL penicillin and 100 μg/mL streptomycin or additional 5 ug/mL puromycin for selecting stably transduced DN32.D3 hybridoma cell line. Stbl3™ E. coli was cultured in LB medium at 37°C with vigorous agitation.
METHOD DETAILS
T cell enrichment, stimulation, RNA extraction and m6A quantification
CD4+ and CD8+ T cells from B6 mice were enriched with anti-CD4 (L3T4) and anti-CD8α (Ly-2) MicroBeads (Miltenyi Biotec), respectively. iNKT cells were enriched from Vα14Tg mice by negative selection with biotinylated antibody specific to B220 (RA3–6B2), CD19 (6D5), CD8α (53–6.7), CD8β (53–5.8), CD11b (M1/70), CD11c (N418), Ter119 (TER-119), and I-A/I-E (M5/114.15.2) in combination with Dynabeads™ Biotin Binder (Invitrogen) followed by staining of iNKT cells for cell sorting (resting and in vivo activated). Total RNA was isolated from purified CD4+, CD8+ and iNKT cells with Qiagen RNAeasy® mini kit and subjected to mRNA enrichment (Mathur et al., 2021). mRNA concentration was quantified by Qubit™ RNA High Sensitivity (Thermo Fisher). Quantification of m6A in total mRNA was performed based on the protocol of manufacture by enzyme-linked immunosorbent assay (ELISA) (EPIGENTEK) (Song et al., 2021).
Immunoblotting
Total thymocytes, sorted TCRβ+ splenocytes and DN32.D3 cells were lysed in RIPA buffer and immunoblotting was performed using polyclonal antibody against METTL14 (Sigma) and METTL3 (Proteintech). Anti-α-tubulin (DM1A, Calbiochem) was used as a loading control according to the protocol described previously (Sena et al., 2013).
Antibodies and flow cytometry
Fluorescein isothiocynate (FITC)-conjugated anti-CD1d (5C6) was generated in our lab (Mandal et al., 1998). PE and Allophycocyanin (APC)-conjugated PBS57/mCD1d-tetramer, mCD1d unloaded tetramer, 5-OP-RU and 6-FP-loaded MR1 tetramers were obtained from the NIH tetramer core facility. Single cell suspensions from various organs were prepared and stained as described previously (Goossens et al., 1990; Zimmer et al., 2006). Cells were stained with the following surface markers: anti-TCRβ (H57–597), anti-CD8α (53–6.7), anti-CD4 (RM4–5), anti-NK1.1 (PK136), anti-CD24 (M1/69), anti-CD44 (IM7) anti-F4/80 (BM8), anti-CD69 (H1.2F3), anti-B220 (RA3–6B2), anti-CD45.1 (A20), anti-CD45.2 (104), anti-EGR2 (erongr2) and Annexin V/7AAD. Intracellular staining of anti-PLZF (9E12), anti-T-bet (4B10) and anti-RORγt (Q31–378) was performed following the protocol of Foxp3 Staining buffer set (eBiosciences). Anti-IL-4 (11B11) and anti-IFN-γ (XMG1.2) or corresponding isotype controls were stained after treatment with 2% PFA and 0.15% saponin in PBS-BSA.
Staining of pSTAT5 after IL-15 stimulation was performed following the BD Phosflow protocol. Briefly, thymocytes were incubated in complete RPMI with 100 ng/mL of IL-15 for 20 min (in water bath) together with staining of APC-CD1d/PBS57 tetramer and anti-TCRβ. Cells were fixed with Phosflow™ Fix Buffer I (BD) for 10 min in a 37°C water bath followed by cell permeabilization with prechilled Perm/Wash buffer™ on ice for 30 min pSTAT5 (pY694) was stained after washing with BD Pharmingen™ Stain Buffer. All antibodies were purchased from BD, BioLegend or eBiosciences. Flow cytometry was performed using a FACSCanto II and data were analyzed using FlowJo software.
Antigen presentation assay
Thymocytes isolated from WT and T-Mettl14−/− mice were pulsed with α-GalCer ranging from 200 ng/mL to 12.5 ng/mL overnight and irradiated before co-culture with iNKT cell hybridoma (DN32.D3, 5×104/well). DN32.D3 cells with knockdown of Mettl14 (Mettl14KD) were stimulated with α-GalCer (200 ng/mL) and supernatants were collected at 24 h for quantification of IL-2 by ELISA as described previously (Weng et al., 2021) or subjected to RNA extraction/qPCR analysis.
Generation of bone marrow chimeras
Bone marrow (BM) cells isolated from WT (CD45.1) and T-Mettl14−/− (CD45.2) mice were depleted of mature T cells using anti-Thy-1.2 (AT83.A-6) plus rabbit complement (Cedarlane Laboratories). 1 × 107 BM cells mixed in 1:1 ratio were injected via tail vein into irradiated Jα18−/− recipient mice. Six weeks after the BM reconstitution, lymphocytes from recipient mice were isolated from various organs and stained for FACS analysis.
RNA-seq analysis
DP thymocytes from T-Mettl14−/− and littermate controls were sorted by BD FACS Aria with purity >98%. RNA was extracted with RNAeasy® mini kit (Qiagen). Libraries were generated by using the Illumina Truseq preparation kit and sequenced on HiSeq4000. Reads were analyzed with Ceto pipeline (https://github.com/ebartom/NGSbartom) using STAR and HTseq for alignment on mm10 mouse genome and reading counting, as described previously (Weng et al., 2021). Paired differential expression analysis was performed using DESeq2 to account for batch differences from two different sorting times (Love et al., 2014). Gene enrichment analysis was performed using GSEA (Subramanian et al., 2005). Data can be accessed at GSE189339.
Real-time PCR
Total RNA from sorted DP thymocytes (CD69-), DN32.D3 and Vα14Tg splenocytes was extracted using RNeasy kit (Qiagen) and subjected to cDNA generation using superscript III reverse transcriptase (Invitrogen). Real-time PCR was performed using SYBR green kit (Quantabio) with indicated primers in Key resources table and β-actin was used as control. Primers for Trim25 and Xaf1 were purchased from Bio-Rad.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Biotin anti-mouse/human CD45R/B220 antibody | BioLegend | RRID: AB_312988 |
| Biotin anti-mouse CD19 antibody | BioLegend | RRID: AB_313638 |
| Biotin anti-mouse CD8α antibody | BioLegend | RRID: AB_312742 |
| Biotin anti-mouse CD8β.2 antibody | BioLegend | RRID: AB_10641695 |
| Biotin anti-mouse CD11b antibody | BioLegend | RRID: AB_312786 |
| Biotin anti-mouse CD11c antibody | BioLegend | RRID: AB_313772 |
| Biotin anti-mouse Ter-119/Erythroid cells antibody | BioLegend | RRID: AB_313704 |
| Biotin anti-mouse I-A/I-E antibody | BioLegend | RRID: AB_313318 |
| Biotin anti-mouse CD4 antibody | BioLegend | RRID: AB_2561504 |
| Biotin rat anti-mouse IL-2 | BD Biosciences | RRID: AB_395384 |
| anti-mouse CD3ε antibody | BioXcell | RRID: AB_1107634 |
| Purified rat anti-mouse IL-2 | BD Biosciences | RRID: AB_395383 |
| anti-Thy-1.2 (AT83.A-6) | Self-made | RRID: CVCL_9186 |
| Rabbit anti-mouse METTL14 antibody | MilliporeSigma | RRID: AB_10672401 |
| Rabbit anti-mouse METTL3/MT-A70 antibody | Proteintech | RRID: AB_2142033 |
| Mouse anti-mouse α-tubulin antibody | Calbiochem | RRID: AB_2617116 |
| m6A-specific antibody | Synaptic Systems | RRID: AB_2279214 |
| FITC anti-mouse CD1d | Self-made | (Mandal et al., 1998) |
| BV421 anti-mouse TCRβ antibody | BD Biosciences | RRID: AB_2737830 |
| BV510 anti-mouse CD8α antibody | BD Biosciences | RRID: AB_2687548 |
| PerCP anti-mouse CD4 antibody | BioLegend | RRID: AB_893331 |
| PE anti-NK1.1 antibody | BioLegend | RRID: AB_313394 |
| FITC anti-mouse CD24 antibody | BioLegend | RRID: AB_312838 |
| PerCP anti-mouse CD69 antibody | BioLegend | RRID: AB_940497 |
| PE-Cy7 anti-mouse/human CD44 antibody | BioLegend | RRID: AB_830786 |
| PerCP anti-mouse F4/80 antibody | BioLegend | RRID: AB_893495 |
| PerCP anti-mouse/human CD45R/B220 antibody | BioLegend | RRID: AB_893355 |
| BV510 anti-mouse CD45.1 antibody | BioLegend | RRID: AB_2563378 |
| PerCP anti-mouse CD45.2 antibody | BioLegend | RRID: AB_893351 |
| PE anti-mouse EGR2 antibody | Thermo Fisher Scientific | RRID: AB_10717803 |
| PE anti-mouse PLZF antibody | BioLegend | RRID: AB_2561966 |
| PE-Cy7 anti-T-bet | BioLegend | RRID: AB_2561760 |
| PerCP anti-mouse RORγt | BD Biosciences | RRID: AB_2737720 |
| PE anti-mouse IL-4 antibody | BioLegend | RRID: AB_315317 |
| FITC anti-mouse IFN-γ antibody | BioLegend | RRID: AB_315399 |
| PE Rat IgG1, κ Isotype Ctrl antibody | BioLegend | RRID: AB_326513 |
| FITC Rat IgG1, κ Isotype Ctrl Antibody | BioLegend | RRID: AB_326511 |
| PE Mouse Anti-Stat5 (pY694) | BD Biosciences | RRID: AB_10894188 |
| PE anti-mouse CD4 | BioLegend | RRID: AB_312692 |
|
Bacterial and virus strains | ||
| Lentivirus harboring shRNA targeting murine Mettl14 (shNC, Mettl14-sh1 and sh2) | MilliporeSigma | Cat#: TRCN0000084996 |
| Retrovirus harboring shRNA targeting murine Cish and shNC | TAKARA | Cat#: 632455 |
| Stbl3™ E. coli | Thermo Fisher Scientific | C737303 |
|
Chemicals, peptides, and recombinant proteins | ||
| PE-conjugated PBS57/mCD1d-tetramer | NIH Tetramer core facility | N/A |
| APC-conjugated PBS57/mCD1d-tetramer | NIH Tetramer core facility | N/A |
| PE-conjugated mCD1d unloaded tetramer | NIH Tetramer core facility | N/A |
| APC-conjugated mCD1d unloaded tetramer | NIH Tetramer core facility | N/A |
| PE 5-OP-RU MR1 tetramers | NIH Tetramer core facility | N/A |
| PE 6-FP-loaded MR1 tetramers | NIH Tetramer core facility | N/A |
| N6-methyladenosine 5′-monophosphate sodium | MilliporeSigma | Cat#: M2780–10MG |
| FITC Annexin V | BioLegend | Cat#: 640905 |
| 7AAD | BD Biosciences | Cat#: 559925 |
| Recombinant Murine IL-15 | Peprotech | Cat#: 210–15 |
| Recombinant murine IL-2 | Peprotech | Cat#: 212–12 |
| α-GalCer | MilliporeSigma | Cat#: 867000P |
| rabbit complement | Cedarlane Laboratories | Cat#: CL3051 |
| CM-H2DCFDA | Thermo Fisher Scientific | Cat#: C6827 |
| N-acetyl cysteine (NAC) | MilliporeSigma | Cat#: 38520–57-9 |
| Fluro-4 | Thermo Fisher Scientific | Cat#: F14201 |
| Fura-red | Thermo Fisher Scientific | Cat#: F3020 |
| BamH I | NEB | Cat#: R0136S |
| EcoRI | NEB | Cat#: R0101S |
| Lipofectamine LTX | Thermo Fisher Scientific | Cat#: 15338100 |
| TrueCut™ Cas9 Protein v2 | Thermo Fisher Scientific | Cat#: A36498 |
|
Critical commercial assays | ||
| TRIzol | Thermo Fisher Scientific | Cat#: 15596026 |
| Qiagen RNAeasy® mini kit | Qiagen | Cat#: 74004 |
| Dynabeads™ mRNA purification kit | Thermo Fisher Scientific | Cat#: 61006 |
| Qubit™ RNA High Sensitivity (HS) Kits | Thermo Fisher Scientific | Q32852 |
| Enzyme-linked immunosorbent assay | EPIGENTEK | Cat#: P-9005–96 |
| Foxp3 Staining buffer set | Thermo Fisher Scientific | Cat#: 00–5523-00 |
| Illumina Truseq preparation kit | Illumina | Cat#: RS-122–2001 |
| Superscript III reverse transcriptase | Thermo Fisher Scientific | Cat#: 18080–044 |
| PerfeCTa SYBR Green FastMix | Quantabio | Cat#: 95071–012 |
| Protein G Dynabeads | Thermo Fisher Scientific | Cat#: 88847 |
| Trizol LS | Thermo Fisher Scientific | Cat#: 10296010 |
| CellTrace™ Violet cell proliferation Kit | Thermo Fisher Scientific | Cat#: C34557 |
| P4 primary cell 4D-NucleofectorTM X Kit S | Lonza | Cat#: V4XP-4032 |
|
Deposited data | ||
| Raw and processed file: RNA-Seq for DP thymocytes in T-Mettl14−/− mice | This paper | GSE189339 |
|
Experimental models: Cell lines | ||
| iNKT cell hybridoma (DN32.D3) | (Bendelac, 1995; Lantz and Bendelac, 1994) | N/A |
| 293T cells | ATCC | ATCC: CRL-3216 |
| Phoenix-Eco | ATCC | ATCC: CRL-3214 |
| J1j.10 | ATCC | ATCC: TIB-184 |
|
Experimental models: Organisms/strains | ||
| T-Mettl14−/− mice | (Lu et al., 2020) | N/A |
| C57BL/6J (B6) mice | Jackson Laboratory | JAX: 000664 |
| C57BL/6-Tg(Cd4-TcraDN32D3)1Aben/J (Vα14Tg) mice | Jackson Laboratory | JAX: 014639 |
| B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) mice | Jackson Laboratory | JAX: 002014 |
| Jα18−/− mice | (Cui et al., 1997) | N/A |
|
Recombinant DNA | ||
| pLKO.1 | MilliporeSigma | Cat#: TRCN0000084996 |
| pMD2.G | Addgene | Addgene: 12259 |
| psPAX2 | Addgene | Addgene: 12260 |
| pSIREN-RetroQ-Zsgreen | Takara | Takara: 632455 |
| pCL-Eco | Addgene | Addgene: 12371 |
|
Software and algorithms | ||
| FlowJo 10 | BD Biosciences | https://www.flowjo.com/solutions/flowjo/downloads/ |
| Ceto pipeline (Trimmomatic, STAR, and HTseq) | (Anders et al., 2015; Bolger et al., 2014; Dobin et al., 2013) | https://github.com/ebartom/NGSbartom |
| DESeq2 | (Love et al., 2014) | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| GSEA | (Subramanian et al., 2005) | https://www.gsea-msigdb.org/gsea/index.jsp |
| GSEA | (Subramanian et al., 2005) | https://www.gsea-msigdb.org/gsea/index.jsp |
| Microsoft Excel | Microsoft | https://www.microsoft.com/en-us/microsoft-365/microsoft-office |
| GraphPad 6.0 | GraphPad Software Inc | https://www.graphpad.com/ |
| Oligonucleotides | ||
| Vα14-Jα18 forward primer: TAAGCACAGCACGCTGCACAT | (Koseki et al., 1991) | N/A |
| Vα14-Jα18 reverse primer: CAATCAGCTGAGTCCCAGCT | (Koseki et al., 1991) | N/A |
| Cish forward primer: CTCTGGGACATGGTCCTTTG | (Li et al., 2017) | N/A |
| Cish reverse primer: GGCATCTTCTGTAGGTGCTG | (Li et al., 2017) | N/A |
| Socs1 forward primer: CGTCCTGCCGCCAGATGAG | (Li et al., 2017) | N/A |
| Socs1 reverse primer: GCCAACAGACCCCAAGGAG | (Li et al., 2017) | N/A |
| Socs2 forward primer: CTGCGCGAGCTCAGTCAAAC | (Li et al., 2017) | N/A |
| Socs2 reverse primer: GTCTGAATGCGAACTATCTC | (Li et al., 2017) | N/A |
| Socs3 forward primer: AGATTTCGCTTCGGGACTAG | (Li et al., 2017) | N/A |
| Socs3 reverse primer: GGAGCCAGCGTGGATCTGC | (Li et al., 2017) | N/A |
| Hmga1b forward primer: CAGCTCCAGGGAGGAAACC | (You et al., 2014) | N/A |
| Hmga1b reverse primer: AGGACTCCTGGGAGATGC | (You et al., 2014) | N/A |
| Mettl14 forward primer: CTGAGAGTGCGGATAGCATTG | (Cheng et al., 2021) | N/A |
| Mettl14 reverse primer: GAGCAGATGTATCATAGGAAGCC | (Cheng et al., 2021) | N/A |
| Gapdh forward primer: GTTGTCTCCTGCGACTTCA | (Prima et al., 2017) | N/A |
| Gapdh reverse primer: GGTGGTCCAGGGTTTCTTA | (Prima et al., 2017) | N/A |
| β-actin forward primer: CTTCTTTGCAGCTCCTTCGTT | (Heller et al., 2014) | N/A |
| β-actin reverse primer: AGGAGTCCTTCTGACCCATTC | (Heller et al., 2014) | N/A |
| Trim25 forward primer: | Bio-Rad | N/A |
| Trim25 reverse primer: | Bio-Rad | N/A |
| Xaf1 forward primer: | Bio-Rad | N/A |
| Xaf1 reverse primer: | Bio-Rad | N/A |
| shRNA for Mettl14-1: CCGGCTATGATACATCTGCTCCAAACTCGAGTTT GGAGCAGATGTATCATAGTTTTTG | MilliporeSigma | N/A |
| shRNA for Mettl14-2: CCGGGCATTGGTGCTGTGTTAAATACTCGAGTA TTTAACACAGCACCAATGCTTTTTG | MilliporeSigma | N/A |
| shCish: GATCCGGTACATTCCTAGTTCGAGATTCAAGAG ATCTCGAACTAGGAATGTACCTTTTTTG | (Funakoshi-Tago et al., 2017) | N/A |
|
Other | ||
| LB medium | Thermo Fisher Scientific | Cat#: 12780–052 |
| RPMI 1640 | CORNING | Cat#: 15–040-CV |
| FBS | PEAK serum | Cat#: PS-FB1 |
| L-Glutamine 100* | Hyclone | Cat#: SH30034.01 |
| HEPES | Hyclone | Cat#: SH3023701 |
| 2-Mercaptoethanol | Gibco | Cat#: 21985–023 |
| Penicillin-Streptomycin (10,000 U/mL) | Hyclone | Cat#: SV30010 |
| DPBS, no calcium, no magnesium | Thermo Fisher Scientific | Cat#: 14190250 |
| DMEM media | CORNING | Cat#: 10–013-CV |
| anti-CD4 (L3T4) Microbeads | Miltenyi Biotec | Cat#: 130–117-043 |
| anti-CD8α (Ly-2) Microbeads | Miltenyi Biotec | Cat#: 130–117-044 |
| Dynabeads™ Biotin Binder | Thermo Fisher Scientific | Cat#: 11047 |
| Bovine serum albumin (BSA) | MilliporeSigma | Cat#: A9418–500G |
| Paraformaldehyde (PFA) | MilliporeSigma | Cat#: P6148–500G |
| Saponin | poreSigma | Cat#: SAE0073–10G |
| Phosflow™ Fix Buffer I | BD | Cat#: 557870 |
| Perm/Wash buffer™ | BD | Cat#: 554723 |
| Pharmingen™ Stain Buffer | BD | Cat#: 554657 |
| Alkaline Phosphatase Streptavidin | Jackson Immunoresearch | Cat#: 016050084 |
| Phosphatase substrate | MilliporeSigma | Cat#: S0942–200TAB |
| RNasin® Ribonuclease Inhibitors | Promega | Cat#: N261B |
| Monensin | AMRESCO | Cat#: K645–500mg |
| Puromycin dihydrochloride | MilliporeSigma | Cat#: P8833–10MG |
| Ficoll-Paque | MilliporeSigma | Cat#: GE17–1440-02 |
m6A RNA-immunoprecipitation (RNA-IP) and m6A target qPCR analysis
Total RNA was extracted from B6 thymocytes with Trizol (Thermo Fisher), treated with DNase and then incubated with m6A-specific antibody (Sigma) premixed with protein G Dynabeads (Thermo Fisher) and RNasin® Ribonuclease Inhibitors (Promega) overnight on a 360° rotator in 4°C. m6A modified RNA was eluted with 6.7 mM N6-methyladenosine 5′-monophosphate sodium salt in IP buffer and re-extracted with Trizol LS (Thermo Fisher). cDNA was generated from total RNA and m6A modified RNA and subjected to qPCR analysis with indicated primers in Key resources table.
NAC treatment and ROS detection
Thymocytes were incubated with 3 μM CM-H2DCFDA (Thermol Fisher) for 20 min at 37°C and stained for FACS analysis. Thymocytes were incubated for 6 h in complete RPMI medium with or without supplement with 20 mM N-acetyl cysteine (NAC, Sigma) and stained for apoptosis analysis.
Stimulation of DP thymocytes
Sorted DP thymocytes from WT and T-Mettl14−/− mice were stimulated with plate-bound anti-CD3 (5 μg/mL) and soluble anti-CD4 (2 μg/mL) for 6 h. Cells were then subjected to Annexin V staining or qPCR analysis. Thymocytes were labeled with CellTrace™ CFSE (Invitrogen) according to the manufacture’s protocol and cultured in the presence of IL-2 (200 U/mL) or IL-15 (100 ng/mL). After 3 days, cells were harvested and stained for FACS analysis.
Calcium flux
Thymocytes were labeled with 4 μg/mL Fluro-4 (Invitrogen) and 8 μg/mL Fura-red (Molecular Probes) for 45 min and incubated with biotinylated anti-CD3 (145–2C11) and anti-CD4 (RM4–4) antibodies plus fluorescent-conjugated anti-CD4 (GK1.5) and anti-CD8α (53–6.7). Labeled cells were re-suspended in DPBS without Ca2+ and Mg2+, crosslinked by the addition of streptavidin, and then analyzed by flow cytometry. DN32.D3 cells were incubated with 4 μg/mL Fluro-4 and 8 μg/mL Fura-red for 45 min and re-suspended with Ca2+-free DPBS after wash. Ca2+/α-GalCer was introduced for stimulation for flow cytometry analysis.
In vivo treatment of α-GalCer
5 μg of α-GalCer in 200 μL PBS was injected into the tail vein of mice. Lymphocytes were isolated from the liver and spleen in one hour. Cells were cultured for 2 h followed by Monensin treatment for additional 4 h before subjecting to intracellular cytokine staining. Splenic iNKT cells were stained for surface markers, Annexin V and Ki67 on day 3 after in vivo α-GalCer injection.
Stable knockdown of Mettl14 and Cish in DN32.D3 NKT cell line
Lentivirus targeting Mettl14 or negative control (NC) were packaged with a combination of a lentiviral backbone plasmid (pLKO.1, Sigma-Aldrich) and two packaging plasmids, pMD2.G and psPAX2 in 293T cells. pSIREN-RetroQ-Zsgreen plasmid (shLuc as NC) was gifted by Dr. Philip Lazarus (Washington State University) and shCish was subcloned into BamH I and EcoR I sites. Retrovirus were packaged with cotransfection of pSIREN-RetroQ-Zsgreen and pCL-Eco in Phoenix-Eco cells using Lipofectamine LTX reagent according to the manufacture’s protocol. The sequences were listed in Key resources table. DN32.D3 cells were transduced by lentivirus encoding Mettl14-targeting or NC shRNA (Sigma). Transduced DN32.D3 were selected using complete RPMI 1640 medium supplemented with 5 μg/mL puromycin. Stably transduced cell lines were further spin-transduced with retrovirus expressing shRNA targeting Cish, and Zsgreen+ cells were sorted for culture and downstream analysis.
Nucleofection of rCas9/Mettl14-gRNA complex in primary iNKT cells
Splenocytes from Vα14Tg mice were isolated with Ficoll-Paque (Sigma) and stimulated as described previously (Weng et al., 2021). Nucleofection with rCas9 (Thermo Fisher)/gRNA targeting Mettl14 or scrambled control (Integrated DNA Technologies) was performed using P4 primary cell 4D-NucleofectorTM X Kit S with program CM-137 (Nucleofection Kit; Lonza) (Seki and Rutz, 2018). Splenocytes were further cultured in complete RPMI supplemented with rIL-2 (200 U/mL) for 3 days.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistics
Statistical analyses were carried out using GraphPad Prism 6.0 (GraphPad Software). Student’s t test was used to determine the difference between two groups. A p value < 0.05 was considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001). Bar graphs represented mean ± standard error of the mean (SEM). All the statistical details can be found in figure legends.
Supplementary Material
Highlights.
RNA m6A methylation plays essential roles in iNKT cell development and function
Reduced m6A RNA methylation enhances iNKT cell apoptosis in thymus and periphery
Loss of METTL14 diminishes TCR signaling and cytokine response in iNKT cells
ACKNOWLEDGMENTS
We thank Dr. Chuan He for the T-Mettl14−/− mice, Dr. Albert Bendelac for DN32.D3 NKT cell hybridoma, Dr. Luc Van Kaer for Jα18−/− mice, Dr. Elizabeth Bartom for the use of her RNA-seq analysis pipeline, the NIH tetramer core facility for CD1d and MR1 tetramers, Northwestern University flow cytometry core facility for cell sorting, NUSeq core facility for library construction and sequencing, and Northwestern IT Research Computing Services for maintaining the High Performance Computing Cluster Quest. This work was supported by NIH grants R01 AI141083 and R01 AI057460 to C.-R.W.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.111156.
DECLARATION OF INTERESTS
H.H. is an employee of Genentech.
REFERENCES
- Alarcón CR, Lee H, Goodarzi H, Halberg N, and Tavazoie SF (2015). N6-methyladenosine marks primary microRNAs for processing. Nature 519, 482–485. 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MJ, Migone TS, Sasaki A, Ascherman DP, Zhu MH, Soldaini E, Imada K, Miyajima A, Yoshimura A, and Leonard WJ (1999). CIS associates with the interleukin-2 receptor beta chain and inhibits interleukin-2-dependent signaling. J. Biol. Chem 274, 30266–30272. 10.1074/jbc.274.42.30266. [DOI] [PubMed] [Google Scholar]
- Anders S, Pyl PT, and Huber W (2015). HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin TA, Hogquist KA, and Jameson SC (2004). The fourth way? Harnessing aggressive tendencies in the thymus. J. Immunol 173, 6515–6520. 10.4049/jimmunol.173.11.6515. [DOI] [PubMed] [Google Scholar]
- Baranek T, Lebrigand K, de Amat Herbozo C, Gonzalez L, Bogard G, Dietrich C, Magnone V, Boisseau C, Jouan Y, Trottein F, et al. (2020). High dimensional single-cell analysis reveals iNKT cell developmental trajectories and effector fate decision. Cell Rep 32, 108116. 10.1016/j.celrep.2020.108116. [DOI] [PubMed] [Google Scholar]
- Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, Bouley DM, Lujan E, Haddad B, Daneshvar K, et al. (2014). m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15, 707–719. 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard M, Salio M, and Cerundolo V (2017). Harnessing the power of invariant natural killer T cells in cancer immunotherapy. Front. Immunol 8, 1829. 10.3389/fimmu.2017.01829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A (1995). Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med 182, 2091–2096. 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, and Teyton L (2007). The biology of NKT cells. Annu. Rev. Immunol 25, 297–336. 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Benlagha K, Wei DG, Veiga J, Teyton L, and Bendelac A (2005). Characterization of the early stages of thymic NKT cell development. J. Exp. Med 202, 485–492. 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, and Usadel B (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker CN, Kim D, Melia T, Karri K, Velenosi TJ, Takahashi S, Aibara D, Bonzo JA, Levi M, Waxman DJ, and Gonzalez FJ (2020). Long non-coding RNA Gm15441 attenuates hepatic inflammasome activation in response to PPARA agonism and fasting. Nat. Commun 11, 5847. 10.1038/s41467-020-19554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Wu Y, Xiao T, Xue J, Sun J, Xia H, Ma H, Lu L, Li J, Shi A, et al. (2021). METTL3-mediated m(6)A modification of ZBTB4 mRNA is involved in the smoking-induced EMT in cancer of the lung. Mol. Ther. Nucleic Acids 23, 487–500. 10.1016/j.omtn.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Choe J, Lin S, Zhang W, Liu Q, Wang L, Ramirez-Moya J, Du P, Kim W, Tang S, Sliz P, et al. (2018). mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature 561, 556–560. 10.1038/s41586-018-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides MG, and Bendelac A (2013). Transcriptional regulation of the NKT cell lineage. Curr. Opin. Immunol 25, 161–167. 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby CM, and Kronenberg M (2018). Tissue-specific functions of invariant natural killer T cells. Nat. Rev. Immunol 18, 559–574. 10.1038/s41577-018-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, and Taniguchi M (1997). Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science 278, 1623–1626. 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolbois A, Bedi RK, Bochenkova E, Müller A, Moroz-Omori EV, Huang D, and Caflisch A (2021). 1, 4, 9-Triazaspiro[5.5]undecan-2-one derivatives as potent and selective METTL3 inhibitors. J. Med. Chem 64, 12738–12760. 10.1021/acs.jmedchem.1c00773. [DOI] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. (2012). Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206. 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- Dose M, Sleckman BP, Han J, Bredemeyer AL, Bendelac A, and Gounari F (2009). Intrathymic proliferation wave essential for Valpha14+ natural killer T cell development depends on c-Myc. Proc. Natl. Acad. Sci. USA 106, 8641–8646. 10.1073/pnas.0812255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel I, Seumois G, Chavez L, Samaniego-Castruita D, White B, Chawla A, Mock D, Vijayanand P, and Kronenberg M (2016). Innate-like functions of natural killer T cell subsets result from highly divergent gene programs. Nat. Immunol 17, 728–739. 10.1038/ni.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erson-Bensan AE, and Begik O (2017). m6A Modification and Implications for microRNAs. MicroRNA 6, 97–101. 10.2174/2211536606666170511102219. [DOI] [PubMed] [Google Scholar]
- Fernandes JCR, Acuña SM, Aoki JI, Floeter-Winter LM, and Muxel SM (2019). Long non-coding RNAs in the regulation of gene expression: physiology and disease. Noncoding RNA 5, 17. 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca F, Rustighi A, Malaguarnera R, Altamura S, Vigneri P, Del Sal G, Giancotti V, Pezzino V, Vigneri R, and Manfioletti G (2006). HMGA1 inhibits the function of p53 family members in thyroid cancer cells. Cancer Res 66, 2980–2989. 10.1158/0008-5472.CAN-05-2637. [DOI] [PubMed] [Google Scholar]
- Fujii SI, and Shimizu K (2019). Immune networks and therapeutic targeting of iNKT cells in cancer. Trends Immunol 40, 984–997. 10.1016/j.it.2019.09.008. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Moriwaki T, Ueda F, Tamura H, Kasahara T, and Tago K (2017). Phosphorylated CIS suppresses the Epo or JAK2 V617F mutant-triggered cell proliferation through binding to EpoR. Cell. Signal 31, 41–57. 10.1016/j.cellsig.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Gálvez NMS, Bohmwald K, Pacheco GA, Andrade CA, Carreño LJ, and Kalergis AM (2021). Type I natural killer T cells as key regulators of the immune response to infectious diseases. Clin. Microbiol. Rev 34, e00232–20. 10.1128/CMR.00232-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V, Peer E, Mor N, Manor YS, et al. (2015). Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science 347, 1002–1006. 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Stankovic S, and Baxter AG (2010). Raising the NKT cell family. Nat. Immunol 11, 197–206. 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- Goossens PL, Jouin H, Marchal G, and Milon G (1990). Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J. Immunol. Methods 132, 137–144. 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- Gordy LE, Bezbradica JS, Flyak AI, Spencer CT, Dunkle A, Sun J, Stanic AK, Boothby MR, He YW, Zhao Z, et al. (2011). IL-15 regulates homeostasis and terminal maturation of NKT cells. J. Immunol 187, 6335–6345. 10.4049/jimmunol.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, and Bendelac A (2007). Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity 27, 751–762. 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsha Krovi S, Zhang J, Michaels-Foster MJ, Brunetti T, Loh L, Scott-Browne J, and Gapin L (2020). Thymic iNKT single cell analyses unmask the common developmental program of mouse innate T cells. Nat. Commun 11, 6238. 10.1038/s41467-020-20073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He PC, and He C (2021). m(6) A RNA methylation: from mechanisms to therapeutic potential. EMBO J 40, e105977. 10.15252/embj.2020105977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He RZ, Jiang J, and Luo DX (2020). The functions of N6-methyladenosine modification in lncRNAs. Genes Dis 7, 598–605. 10.1016/j.gendis.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller JJ, Schjerven H, Li S, Lee A, Qiu J, Chen ZME, Smale ST, and Zhou L (2014). Restriction of IL-22-producing T cell responses and differential regulation of regulatory T cell compartments by zinc finger transcription factor Ikaros. J. Immunol 193, 3934–3946. 10.4049/jimmunol.1401234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Simmons A, Yuan J, Bender TP, and Alberola-Ila J (2010). The transcription factor c-Myb primes CD4+CD8+ immature thymocytes for selection into the iNKT lineage. Nat. Immunol 11, 435–441. 10.1038/ni.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TM, Yu ZX, Ferrans VJ, Lowenstein RA, and Finkel T (1996). Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 93, 11848–11852. 10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juno JA, Keynan Y, and Fowke KR (2012). Invariant NKT cells: regulation and function during viral infection. PLoS Pathog 8, e1002838. 10.1371/journal.ppat.1002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Kumar A, Chang CH, and Pyaram K (2017). Reactive oxygen species regulate the inflammatory function of NKT cells through promyelocytic leukemia zinc finger. J. Immunol 199, 3478–3487. 10.4049/jimmunol.1700567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto H, Ohteki T, Yajima N, Kawahara K, Natsui M, Kawarasaki S, Hamada K, Horie Y, Kubo Y, Arase S, et al. (2007). The Pten/PI3K pathway governs the homeostasis of Valpha14iNKT cells. Blood 109, 3316–3324. 10.1182/blood-2006-07-038059. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ohsugi M, Sasako T, Awazawa M, Umehara T, Iwane A, Kobayashi N, Okazaki Y, Kubota N, Suzuki R, et al. (2018). The RNA meth-yltransferase complex of WTAP, METTL3, and METTL14 regulates mitotic clonal expansion in adipogenesis. Mol. Cell Biol 38, e00116–18. 10.1128/MCB.00116-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki H, Asano H, Inaba T, Miyashita N, Moriwaki K, Lindahl KF, Mizutani Y, Imai K, and Taniguchi M (1991). Dominant expression of a distinctive V14+ T-cell antigen receptor alpha chain in mice. Proc. Natl. Acad. Sci. USA 88, 7518–7522. 10.1073/pnas.88.17.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, et al. (2008). The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol 9, 1055–1064. 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M (2005). Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol 23, 877–900. 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Lan Q, Liu PY, Haase J, Bell JL, Hüttelmaier S, and Liu T (2019). The critical role of RNA m(6)A methylation in cancer. Cancer Res 79, 1285–1292. 10.1158/0008-5472.CAN-18-2965. [DOI] [PubMed] [Google Scholar]
- Lantz O, and Bendelac A (1994). An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8- T cells in mice and humans. J. Exp. Med 180, 1097–1106. 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, and Glimcher LH (2009). The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat. Immunol 10, 306–313. 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Lee E, Han SK, Choi YH, Kwon DI, Choi H, Lee K, Park ES, Rha MS, Joo DJ, et al. (2020). Single-cell RNA sequencing identifies shared differentiation paths of mouse thymic innate T cells. Nat. Commun 11, 4367. 10.1038/s41467-020-18155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Han J, Jeong SI, Her NG, Lee JH, Ha TK, Kang MJ, Ryu BK, and Chi SG (2014). XAF1 directs apoptotic switch of p53 signaling through activation of HIPK2 and ZNF313. Proc. Natl. Acad. Sci. USA 111, 15532–15537. 10.1073/pnas.1411746111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Holzapfel KL, Zhu J, Jameson SC, and Hogquist KA (2013). Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol 14, 1146–1154. 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, Bailis W, Cao G, Kroehling L, Chen Y, et al. (2017). m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 548, 338–342. 10.1038/nature23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen S, Xu X, Sundstedt A, Paulsson KM, Anderson P, Karlsson S, Sjögren HO, and Wang P (2000). Cytokine-induced Src homology 2 protein (CIS) promotes T cell receptor-mediated proliferation and prolongs survival of activated T cells. J. Exp. Med 191, 985–994. 10.1084/jem.191.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Choe J, Du P, Triboulet R, and Gregory RI (2016). The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol. Cell 62, 335–345. 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. (2014). A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol 10, 93–95. 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tao S, Liao L, Li Y, Li H, Li Z, Lin L, Wan X, Yang X, and Chen L (2020). TRIM25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat. Commun 11, 348. 10.1038/s41467-019-14190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TX, Zheng Z, Zhang L, Sun HL, Bissonnette M, Huang H, and He C (2020). A new model of spontaneous colitis in mice induced by deletion of an RNA m(6)A methyltransferase component METTL14 in T cells. Cell Mol. Gastroenterol. Hepatol 10, 747–761. 10.1016/j.jcmgh.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Chen XR, Alegre ML, Chiu NM, Chen YH, Castaño AR, and Wang CR (1998). Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol. Immunol 35, 525–536. 10.1016/s0161-5890(98)00055-8. [DOI] [PubMed] [Google Scholar]
- Manners O, Baquero-Perez B, and Whitehouse A (2019). m(6)A: wide-spread regulatory control in virus replication. Biochim. Biophys. Acta. Gene Regul. Mech 1862, 370–381. 10.1016/j.bbagrm.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur L, Jung S, Jang C, and Lee G (2021). Quantitative analysis of m(6) A RNA modification by LC-MS. STAR Protoc 2, 100724. 10.1016/j.xpro.2021.100724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, Surh CD, and Kronenberg M (2002). Homeostasis of V alpha 14i NKT cells. Nat. Immunol 3, 966–974. 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Masuhara M, Mitsui K, Yokouchi M, Ohtsubo M, Misawa H, Miyajima A, and Yoshimura A (1997). CIS, a cytokine inducible SH2 protein, is a target of the JAK-STAT5 pathway and modulates STAT5 activation. Blood 89, 3148–3154. [PubMed] [Google Scholar]
- Meyer KD, and Jaffrey SR (2014). The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol 15, 313–326. 10.1038/nrm3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, and Hogquist KA (2011). T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med 208, 1279–1289. 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz-Omori EV, Huang D, Kumar Bedi R, Cheriyamkunnel SJ, Bochenkova E, Dolbois A, Rzeczkowski MD, Li Y, Wiedmer L, and Caflisch A (2021). METTL3 inhibitors for epitranscriptomic modulation of cellular processes. ChemMedChem 16, 3035–3043. 10.1002/cmdc.202100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycko MP, Ferrero I, Wilson A, Jiang W, Bianchi T, Trumpp A, and MacDonald HR (2009). Selective requirement for c-Myc at an early stage of V(alpha)14i NKT cell development. J. Immunol 182, 4641–4648. 10.4049/jimmunol.0803394. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Ahn S, Jin YH, Ishifune C, Kim JH, Yasutomo K, and Chung DH (2015). Notch 1 and Notch 2 synergistically regulate the differentiation and function of invariant NKT cells. J. Leukoc. Biol 98, 781–789. 10.1189/jlb.1A0914-459RR. [DOI] [PubMed] [Google Scholar]
- Palmer DC, Guittard GC, Franco Z, Crompton JG, Eil RL, Patel SJ, Ji Y, Van Panhuys N, Klebanoff CA, Sukumar M, et al. (2015). Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance. J. Exp. Med 212, 2095–2113. 10.1084/jem.20150304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak N, and Khandelwal S (2006). Influence of cadmium on murine thymocytes: potentiation of apoptosis and oxidative stress. Toxicol. Lett 165, 121–132. 10.1016/j.toxlet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Pellicci DG, Hammond KJL, Uldrich AP, Baxter AG, Smyth MJ, and Godfrey DI (2002). A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(-)CD4(+) CD1d-dependent precursor stage. J. Exp. Med 195, 835–844. 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al. (2014). Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 24, 177–189. 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prima V, Kaliberova LN, Kaliberov S, Curiel DT, and Kusmartsev S (2017). COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. USA 114, 1117–1122. 10.1073/pnas.1612920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyaram K, Kumar A, Kim YH, Noel S, Reddy SP, Rabb H, and Chang CH (2019). Keap1-Nrf2 system plays an important role in invariant natural killer T cell development and homeostasis. Cell Rep 27, 699–707.e4. 10.1016/j.celrep.2019.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roignant JY, and Soller M (2017). m(6)A in mRNA: an ancient mechanism for fine-tuning gene expression. Trends Genet 33, 380–390. 10.1016/j.tig.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Rossjohn J, Pellicci DG, Patel O, Gapin L, and Godfrey DI (2012). Recognition of CD1d-restricted antigens by natural killer T cells. Nat. Rev. Immunol 12, 845–857. 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree IA, Evans ME, Pan T, and He C (2017). Dynamic RNA modifications in gene expression regulation. Cell 169, 1187–1200. 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, and Bendelac A (2008). The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity 29, 391–403. 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MP, Mathew R, Liszewski MK, Spooner CJ, Spooner C, Barr K, Meng F, Singh H, and Bendelac A (2012). Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nat. Immunol 13, 264–271. 10.1038/ni.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki A, and Rutz S (2018). Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. J. Exp. Med 215, 985–997. 10.1084/jem.20171626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, et al. (2013). Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236. 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafik A, Schumann U, Evers M, Sibbritt T, and Preiss T (2016). The emerging epitranscriptomics of long noncoding RNAs. Biochim. Biophys. Acta 1859, 59–70. 10.1016/j.bbagrm.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Sintes J, Cuenca M, Romero X, Bastos R, Terhorst C, Angulo A, and Engel P (2013). Cutting edge: Ly9 (CD229), a SLAM family receptor, negatively regulates the development of thymic innate memory-like CD8+ T and invariant NKT cells. J. Immunol 190, 21–26. 10.4049/jimmunol.1202435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauenwhite D, and Johnston B (2015). Regulation of NKT cell localization in homeostasis and infection. Front. Immunol 6, 255. 10.3389/fimmu.2015.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Song J, Cheng M, Zheng M, Wang T, Tian S, Flavell RA, Zhu S, Li HB, Ding C, et al. (2021). METTL3-mediated m(6)A RNA methylation promotes the anti-tumour immunity of natural killer cells. Nat. Commun 12, 5522. 10.1038/s41467-021-25803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic AK, Bezbradica JS, Park JJ, Van Kaer L, Boothby MR, and Joyce S (2004). Cutting edge: the ontogeny and function of Va14Ja18 natural T lymphocytes require signal processing by protein kinase C theta and NF-kappa B. J. Immunol 172, 4667–4671. 10.4049/jimmunol.172.8.4667. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, and Mesirov JP (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550. 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabe M, and Berzofsky JA (2018). Tissue-specific roles of NKT cells in tumor immunity. Front. Immunol 9, 1838. 10.3389/fimmu.2018.01838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Cao G, Zhang T, Sefik E, Amezcua Vesely MC, Broughton JP, Zhu S, Li H, Li B, Chen L, et al. (2018). m(6)A mRNA methylation sustains Treg suppressive functions. Cell Res 28, 253–256. 10.1038/cr.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kaer L, and Wu L (2018). Therapeutic potential of invariant natural killer T cells in autoimmunity. Front. Immunol 9, 519. 10.3389/fimmu.2018.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, and Hogquist KA (2018). CCR7 defines a precursor for murine iNKT cells in thymus and periphery. Elife 7, e34793. 10.7554/eLife.34793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hu X, Huang M, Liu J, Gu Y, Ma L, Zhou Q, and Cao X (2019). Mettl3-mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat. Commun 10, 1898. 10.1038/s41467-019-09903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng X, Kumar A, Cao L, He Y, Morgun E, Visvabharathy L, Zhao J, Sena LA, Weinberg SE, Chandel NS, and Wang CR (2021). Mitochondrial metabolism is essential for invariant natural killer T cell development and function. Proc. Natl. Acad. Sci. USA 118, e2021385118. 10.1073/pnas.2021385118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, Pilka ES, Aspris D, Leggate D, Hendrick AG, et al. (2021). Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature 593, 597–601. 10.1038/s41586-021-03536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Yang Y, Guo W, Xu L, You M, Zhang YC, Sun Z, Cui X, Yu G, Qi Z, et al. (2021). METTL3-dependent m(6)A modification programs T follicular helper cell differentiation. Nat. Commun 12, 1333. 10.1038/s41467-021-21594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Ohkubo T, Kiguchi T, Jenkins NA, Gilbert DJ, Copeland NG, Hara T, and Miyajima A (1995). A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J 14, 2816–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You XY, Huang JH, Liu B, Liu SJ, Zhong Y, and Liu SM (2014). HMGA1 is a new target of miR-195 involving isoprenaline-induced cardiomyocyte hypertrophy. Biochemistry 79, 538–544. 10.1134/S0006297914060078. [DOI] [PubMed] [Google Scholar]
- Zhao J, Weng X, Bagchi S, and Wang CR (2014). Polyclonal type II natural killer T cells require PLZF and SAP for their development and contribute to CpG-mediated antitumor response. Proc. Natl. Acad. Sci. USA 111, 2674–2679. 10.1073/pnas.1323845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Sun D, Zhao M, Lai Y, Liu Y, and Zhang Z (2020). N(6)-methyladenosine mediates arsenite-induced human keratinocyte transformation by suppressing p53 activation. Environ. Pollut 259, 113908. 10.1016/j.envpol.2019.113908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Zhang L, Cui XL, Yu X, Hsu PJ, Lyu R, Tan H, Mandal M, Zhang M, Sun HL, et al. (2020). Control of early B cell development by the RNA N(6)-methyladenosine methylation. Cell Rep 31, 107819. 10.1016/j.celrep.2020.107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Xie X, Zhang L, Wang H, Jie Z, Zhou X, Shi J, Zhao S, Zhang B, Cheng X, and Sun SC (2018). TBK-binding protein 1 regulates IL-15-induced autophagy and NKT cell survival. Nat. Commun 9, 2812. 10.1038/s41467-018-05097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer MI, Colmone A, Felio K, Xu H, Ma A, and Wang CR (2006). A cell-type specific CD1d expression program modulates invariant NKT cell development and function. J. Immunol 176, 1421–1430. 10.4049/jimmunol.176.3.1421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number of RNAseq data for DP is GSE189339 in NCBI (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE189339).
Scripts used for alignment and counting can be found here (https://github.com/ebartom/NGSbartom). Downstream analysis was performed as described in method details.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


