Abstract
Interstitial lung disease (ILD) is the leading cause of mortality in systemic sclerosis (SSc). Progressive pulmonary fibrosis (PPF) is defined as progression in 2 domains including clinical, radiological or lung-function parameters. Our aim was to assess predictors of functional decline in SSc-ILD patients and compare disease behavior to that in idiopathic pulmonary fibrosis (IPF) patients. Patients with normal forced vital capacity (FVC > 80% predicted; SSc-ILD: n = 31; IPF: n = 53) were followed for at least 1 year. Predictors of functional decline including clinical symptoms, comorbidities, lung-function values, high-resolution CT pattern, and treatment data were analyzed. SSc-ILD patents were significantly younger (59.8 ± 13.1) and more often women (93 %) than IPF patients. The median yearly FVC decline was similar in both groups (SSc-ILD = −67.5 and IPF = −65.3 mL/year). A total of 11 SSc-ILD patients met the PPF criteria for functional deterioration, presenting an FVC decline of −153.9 mL/year. Cough and pulmonary hypertension were significant prognostic factors for SSc-ILD functional progression. SSc-ILD patients with normal initial spirometry presenting with cough and PH are at higher risk for showing progressive functional decline.
Keywords: systemic sclerosis, interstitial lung disease, cough, pulmonary hypertension, predictors of treatment response
1. Introduction
Systemic sclerosis (SSc) is a chronic connective tissue disease with multiple organ involvement [1]. Skin and internal organ manifestations are common; however, SSc-associated interstitial lung disease (SSc-ILD) is the leading cause of mortality in SSc [2]. Another significant cardiopulmonary complication accounting for additional high mortality is pulmonary hypertension (PH) [3,4]. The clinical course of this disease shows a high variability in progression [5].
Goh criteria are widely used to assess the severity of pulmonary involvement in SSc based on the extent of lung fibrosis on high-resolution computed tomography (HRCT) scan and forced vital capacity (FVC) % predicted value [6]. According to these criteria, SSc-ILD patients are classified into limited or extensive disease subgroups; however, functional decline in patients with normal spirometry, progression of symptoms, or HRCT is not included in the disease stratification [4]. The SSc-ILD Safety and Efficacy of Nintedanib in Systemic Sclerosis (SENSCIS) clinical trial confirmed that the annual rate of FVC decline in placebo-treated patients was on average −93.3 mL/year, and 46% of participants without specific treatment lost over 5% of FVC during a 1-year follow-up [7]. Furthermore, recent studies additionally suggested that SSc-ILD patients might lose lung function even in the physiological range and should be followed more closely to prevent functional deterioration [8].
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrosing ILD of unknown origin and is associated with a poor outcome. IPF is characterized by a progressive functional decline that is mainly assessed using FVC and diffusing capacity of the lung for carbon monoxide (DLCO) [9]. FVC is currently the most extensively studied marker of disease progression in IPF, as this functional value is the only parameter accepted by regulatory bodies in predicting mortality [10].
Progressive pulmonary fibrosis (PPF) is defined by two parameters of the following three criteria: (1) worsening of respiratory symptoms; (2) physiological criteria of PFT decline (FVC ≥ 5%/year and/or DLCO ≥ 10%/year); and (3) radiological signs of disease progression within a 1-year follow-up [11]. PPF might show similar clinical, lung functional, and radiological features over long term with IPF, the prototype of rapidly progressive ILD.
Our goal was to assess the PPF criteria on lung functional decline and possible clinical predictors of functional progression in SSc-ILD patients with normal spirometry and compare data with that of IPF patients to identify possible common factors defining functional progression.
2. Materials and Methods
2.1. Study Design and Parameters
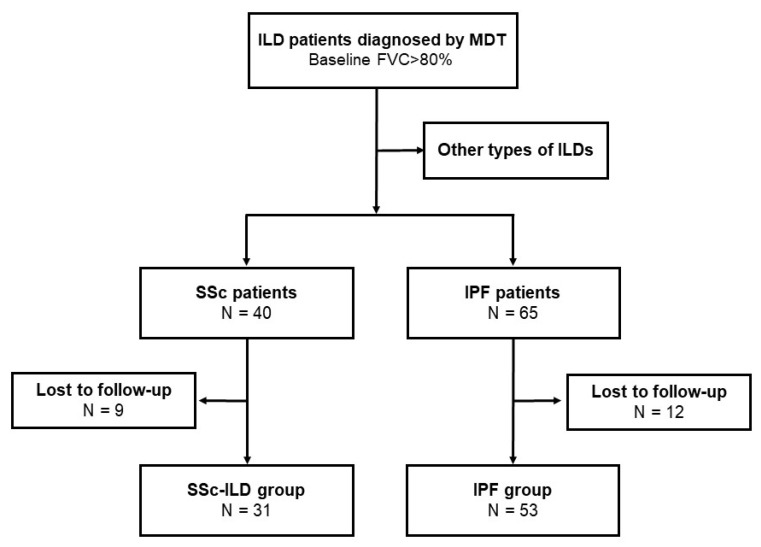
Our study was a retrospective longitudinal observational study. All patients referred between February 2015 and January 2021 to the ILD multidisciplinary team (MDT) of the Department of Pulmonology, Semmelweis University, Budapest, Hungary, were screened. We enrolled subjects in our study who had been diagnosed with either SSc-ILD or IPF, had physiologic spirometric lung-function parameters (forced vital capacity (FVC) > 80% of predicted value), and had at least 12 months of follow-up data. SSc-ILD patients (classified with both J84 and M34 codes according to the 10th edition of the International Classification of Diseases (ICD10)) were diagnosed between January 2017 and July 2019. The diagnosis of SSc was established previously based on the American College of Rheumatology/European League Against Rheumatism Collaborative Initiative (EULAR-ACR) criteria [12] by immunology and rheumatology specialists; the disease-specific therapy was coordinated at immunological–rheumatological centers in central Hungary. Patients diagnosed with IPF between February 2015 and January 2021 according to the 2011 American Thoracic Society/European Respiratory Society (ATS/ERS) IPF guideline included in the EMPIRE registry were reviewed and all entered into the analysis if they met the inclusion criteria of having lung-function follow-up of at least one year with a baseline FVC > 80% predicted [10,13]. The study’s functional and follow-up criteria were met by 53 IPF and 31 SSc-ILD patients (Figure 1).
Figure 1.
Patient selection for analysis. FVC, forced vital capacity; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; MDT, multidisciplinary team; SSc-ILD, systemic-sclerosis-associated interstitial lung disease.
Baseline characteristics including smoking history, symptoms, detailed pulmonary function test (PFT) values (FVC, forced expiratory volume in 1st second (FEV1), total lung capacity (TLC), DLCO, carbon monoxide transfer coefficient (KLCO)). HRCT pattern and treatment data were analyzed and compared between the study populations. Arterial blood gas (ABG) analysis, body mass index (BMI), and 6-minute walk test (6MWT) results were examined. The gender, age, and physiology (GAP) index was calculated as a prognostic staging system [14]. At each follow-up visit, routine PFTs, ABGs, and the 6MWT were documented. Methods of the measurements (PFTs, ABGs, 6MWT, and HRCT) were described in detail in our previous study [8]. The median follow-up was 34 months and the functional decline of PPF was established throughout the follow-up according to the criteria of ≥5% FVC decline and/or ≥10% DLCO decline within 1 year. A PPF diagnosis was not made because no additional second criterion of worsening symptoms and/or progression on HRCT was assessed [11].
2.2. Statistical Analysis
Data were analyzed using Graph Pad software (GraphPad Prism 5.0 Software, Inc., La Jolla, CA, USA) and SPSS v25 (IBM Corporation, Armonk, NY, USA). Continuous variables were expressed as the mean ± standard deviation (SD) or median (interquartile range (IQR)) and were compared using a t-test or a Mann–Whitney U-test according to the distribution of the variable. The test for normality was performed using the Kolmogorov–Smirnov test. Categorical variables are presented as percentages (%) expressed for the entire study population (all patients) or respective subgroups as indicated and were compared using a chi-squared test or two-tailed Fisher’s exact test. Multiple logistic regression analysis was used to assess predictors of possible functional progression including age (as a continuous variable), sex (male/female), smoking history (present/absent), cough (present/absent), PH (present/absent), baseline FVC, TLC, DLCO, KLCO (all functional parameters in % predicted as continuous variables), and therapy (applied/none). A p-value < 0.05 was defined as statistically significant.
3. Results
The characteristics of the patients are summarized in Table 1. The mean age of the SSc-ILD patients was significantly lower than in IPF patients, with female predominance. IPF patients had been more frequently smokers and a higher proportion of patients qualified as overweight. A majority of the patients in both groups were in GAP stage I at baseline.
Table 1.
Patient characteristics.
| Characteristics | IPF (n = 53) | SSc-ILD (n = 31) | p-Value |
|---|---|---|---|
| Age (years) | 68.9 ± 8.5 | 59.8 ± 13.1 | 0.001 |
| Sex (male:female) | 28:25 | 2:29 | <0.001 |
| Smoking history | |||
| Ever smoker | 33 (62.3) | 7 (22.6) | 0.001 |
| Non-smoker | 20 (37.7) | 24 (77.4) | |
| BMI (kg/m2) | 27.7 ± 4.4 | 25.2 ± 4.4 | 0.006 |
| Overweight (25.0–29.9 kg/m2) | 21 (39.6%) | 5 (16.2%) | 0.025 |
| Signs and symptoms | |||
| Dyspnea | 52 (98.1) | 11 (35.5) | <0.001 |
| Cough | 26 (49.1) | 9 (29.0) | 0.108 |
| Finger clubbing | 12 (22.6) | 0 | NA |
| Crackles | 47 (88.7) | 12 (38.7) | <0.001 |
| Raynaud phenomenon | 0 | 23 (74.2) | NA |
| GAP score | |||
| Stage I | 50 (94.3) | 31 (100.0) | NA |
| Stage II | 3 (5.7) | 0 | NA |
| Stage III | 0 | 0 | NA |
| Specific comorbidities | |||
| PH | 7 (13.2) | 5 (16.1) | 0.712 |
| GERD | 6 (11.3) | 6 (19.4) | 0.345 |
| HRCT pattern | |||
| UIP/pUIP | 26/25 (96.2) | 0/3 (9.7) | <0.001 |
| NSIP | 0 | 26 (83.9) | NA |
| Other | 2 (3.8) | 2 (6.5) | 0.578 |
| Therapy | |||
| Nintedanib | 39 (73.6) | 0 | NA |
| Pirfenidone | 8 (15.0) | 0 | NA |
| ISU | 0 | 26 (83.9) | NA |
| Biological treatment | 0 | 9 (29.0) | NA |
| None | 14 (26.4) | 3 (9.7) | NA |
Data are presented as n (%) or mean ± SD. BMI, body mass index; GAP, Gender–Age–Physiology index; GERD, gastro-esophageal reflux disease; HRCT, high-resolution computed tomography; IPF, idiopathic pulmonary fibrosis; NA, not assessed; NSIP, non-specific interstitial pneumonia; PH, pulmonary hypertension; pUIP, probable usual interstitial pneumonia; SSc-ILD, systemic-sclerosis-associated interstitial lung disease; UIP, usual interstitial pneumonia; ISU, immunosuppressive therapy. GAP index: stage I = 0–3 points; stage II = 4–5 points; stage III = 6–8 points. Statistically significant values are highlighted in bold.
ILD-associated symptoms showed a significant difference between the two groups. More patients presented with respiratory symptoms such as dyspnea, cough, and crackles at the time of the diagnosis in the IPF group. On the other hand, the Raynaud phenomenon was only observed in the SSc-ILD group. No difference between groups was observed regarding the presence of PH and gastroesophageal reflux disease The Scl-70 antibody was present in 48% of SSc-ILD patients. The HRCT pattern was in line with actual guidelines and the literature, as usual interstitial pneumonia (UIP) or probable (p)UIP was the predominant pattern in IPF, while non-specific interstitial pneumonia (NSIP) predominated in SSc-ILD.
SSc therapy in 26 patients’ was conventional immunosuppressive therapy (ISU), while biological treatment was given in 9 cases, including 7 subjects with combined ISU and biological therapy. Antifibrotic agents (nintedanib or pirfenidone) were applied in 39 cases in the IPF group, while 14 patients did not receive IPF-specific treatment.
The functional parameters for the patients are summarized in Table 2. The values for FVC and FEV1 were similar in both groups, while TLC was significantly impaired and below the normal range in IPF patients. Baseline CO diffusion parameters showed a significant difference; DLCO was decreased in the IPF group and SSc-ILD patients had lower KLCO values. In the ABG, IPF patients had significantly lower pO2 values compared to SSc-ILD patients. The 6MWT distance was similar for both groups, representing a diminished functional capacity in SSc-ILD patients since normal percentile values are age-dependent [15,16]. Post-exercise desaturation in the 6MWT was present in both groups to a similar degree.
Table 2.
Baseline lung function, ABG, and 6MWT functional parameters.
| Values | IPF (n = 53) | SSc-ILD (n = 31) | p-Value |
|---|---|---|---|
| Lung-function parameters | |||
| FVC (mL) | 3035.7 ± 836.2 | 2725.5 ± 655.6 | 0.080 |
| FVC (% pred) | 96.4 ± 13.9 | 98.7 ± 12.2 | 0.263 |
| FEV1 (mL) | 2488.5 ± 696.4 | 2301.6 ± 569.2 | 0.209 |
| FEV1 (% pred) | 98.6 ± 16.2 | 99.7 ± 13.3 | 0.748 |
| FEV1/FVC (%) | 82.6 ± 8.2 | 84.5 ± 5.2 | 0.329 |
| TLC (mL) | 4648.9 ± 1358.4 | 4263.9 ± 823.3 | 0.157 |
| TLC (% pred) | 79.5 ± 14.4 | 88.4 ± 15.4 | 0.022 |
| Diffusion parameters | |||
| DLCO (mmol/min/kPa) | 5.9 ± 1.8 | 6.4 ± 1.6 | 0.201 |
| DLCO (% pred) | 74.1 ± 17.6 | 83.7 ± 18.3 | 0.020 |
| KLCO (mmol/min/kPa/L) | 1.3 ± 0.3 | 1.4 ± 0.3 | 0.042 |
| KLCO (% pred) | 88.8 ± 24.2 | 71.2 ± 16.4 | <0.001 |
| ABGs | |||
| pH | 7.4 ±0.0 | 7.4 ±0.0 | 0.655 |
| pCO2 (mmHg) | 37.7 ± 5.5 | 37.1 ± 2.3 | 0.119 |
| pO2 (mmHg) | 67.8 ± 11.0 | 78.6 ± 8.6 | <0.001 |
| 6MWT | |||
| Distance (m) | 454.4 ± 103.1 | 449.3 ± 70.8 | 0.502 |
| Initial SpO2 (%) | 95.3 ± 2.9 | 94.9 ± 3.0 | 0.463 |
| Final SpO2 (%) | 88.8 ± 9.0 | 89.2 ± 10.8 | 0.407 |
| Initial HR (1/min) | 81.0 ± 13.9 | 84.4 ± 14.3 | 0.425 |
| Final HR (1/min) | 111.2 ± 19.7 | 106.5 ± 20.2 | 0.443 |
| Initial Borg score (0–10) | 0 (0–0) | 0 (0–0) | 0.885 |
| Final Borg score (0–10) | 2 (0–4) | 1.5 (1–3) | 0.924 |
Data are presented as mean ± SD, median (IQR). 6MWT, 6-minute walk test; ABGs, arterialized capillary blood gases; DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HR, heart rate; IPF, idiopathic pulmonary fibrosis; KLCO, transfer coefficient of the lung for carbon monoxide; pCO2; partial pressure of carbon dioxide; pO2, partial pressure of oxygen; SpO2, oxygen saturation; SSc-ILD, systemic-sclerosis-associated interstitial lung disease; TLC, total lung capacity. Statistically significant values are highlighted in bold.
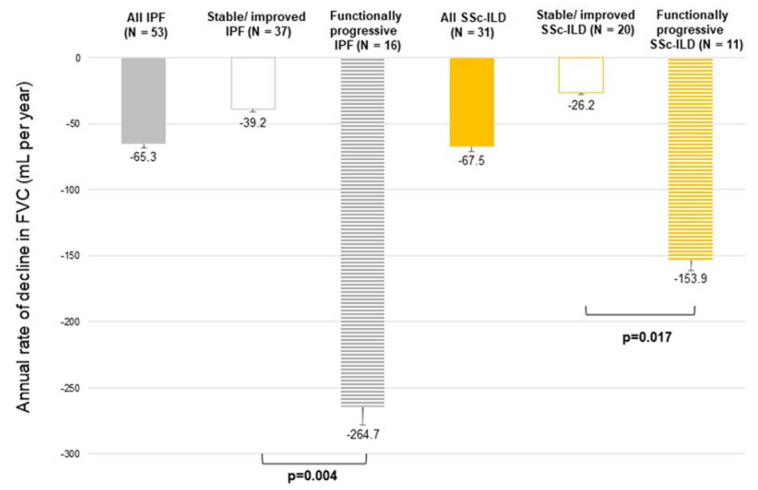
The annual median decline in FVC for SSc-ILD was −67.5 (−146.0 to −4.0) mL/year and −65.3 (−173.8 to −65.3) mL/year in the IPF group. During the follow-up period, 11 (35%) SSc-ILD patients met the criteria of PPF for functional decline: 7 patients presented with ≥5% FVC and 6 patients presented with ≥10% DLCO predicted value decline, including 3 patients that met both criteria. In IPF patients, FVC and/or DLCO decline, as defined for PFF, was present in 16 (30.2%) cases: 14 patients presented with ≥5% FVC and 7 patients presented with ≥10% DLCO predicted value decline, including 5 patients meeting both criteria.
The annual median decline in FVC for the functionally progressive SSc-ILD subgroup was −153.9 (−278.3 to −121.4) mL/year, which was significantly higher compared to −26.2 (−75.4 to −1.6) mL/year in the stable/improved SSc-ILD subgroup (p = 0.017). In the functionally progressive IPF and stable/improved IPF subgroups, the respective data were −264.7 (−404.9 to −204.6) vs. −39.2 (−85.7 to +7.5) mL/year (p = 0.004). FVC decline for all patients, including functionally stable/improved and functionally progressive subgroups regarding IPF and SSc-ILD, are presented in Figure 2.
Figure 2.
Annual rate of decline in FVC (mL per year) in IPF and SSc-ILD patients. FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis; SSc-ILD, systemic-sclerosis-associated interstitial lung disease.
The functionally progressive IPF and SSc-ILD subgroups’ baseline characteristics and functional data are presented in Table 3. While in IPF, the functional progression was not associated with any difference in baseline characteristics or treatment, in SSc-ILD, cough and the presence of PH was significantly more common in the subgroup of patients showing functional decline over the one-year follow-up.
Table 3.
Patient characteristics; HRCT pattern; treatment; and baseline lung function, ABG, and 6MWT functional parameters of the IPF and SSc-ILD subgroups.
| Values | Functionally Stable/Improved IPF (n = 37) |
Functionally Progressive IPF (n = 16) |
Functionally Stable/Improved SSc-ILD (n = 20) | Functionally Progressive SSc-ILD (n = 11) |
|---|---|---|---|---|
| Age (years) | 67.6 ± 9.1 | 71.8 ± 6.5 | 59.8 ± 13.1 | 59.7 ± 12.6 |
| Sex (male:female) | 20:17 | 8:8 | 1:19 | 1:10 |
| Smoking history | ||||
| Ever smoker | 23 (62.2) | 10 (62.5) | 3 (15.0) | 4 (36.4) |
| Non-smoker | 14 (37.8) | 6 (37.5) | 17 (85.0) | 7 (63.6) |
| BMI (kg/m2) | 28.3 ± 4.4 | 26.8 ± 4.3 | 25.2 ± 4.4 | 23.7 ± 4.1 |
| Overweight (25.0–29.9 kg/m2) | 15 (40.5%) | 6 (37.5%) | 5 (45.5%) | 0 |
| Signs and symptoms | ||||
| Dyspnea | 37 (100) | 15 (93.8) | 7 (35.0) | 4 (36.4) |
| Cough | 19 (51.4) | 7 (43.8) | 2 (10.0) * | 7 (63.6) * |
| Finger clubbing | 6 (16.2) | 6 (37.5) | 0 | 0 |
| Crackles | 33 (89.2) | 14 (87.5) | 7 (35.0) | 5 (45.5) |
| Raynaud phenomenon | 0 | 0 | 16 (80.0) | 7 (63.6) |
| GAP score | ||||
| Stage I | 35 (94.6) | 15 (93.8) | 20 (100.0) | 11 (100.0) |
| Stage II | 2 (5.4) | 1 (6.2) | 0 | 0 |
| Stage III | 0 | 0 | 0 | 0 |
| Specific comorbidities | ||||
| PH | 5 (13.5) | 2 (13.3) | 1 (5.0) # | 4 (36.4) # |
| GERD | 5 (13.5) | 1 (6.2) | 3 (15.0) | 3 (27.3) |
| HRCT pattern | ||||
| UIP/pUIP | 16/21 (100) | 10/4 (87.5) | 0/3 (15.0) | 0 |
| NSIP | 0 | 0 | 16 (80.0) | 10 (90.9) |
| Other | 0 | 2 (12.5) | 1 (5.0) | 1 (9.1) |
| Therapy | ||||
| Nintedanib | 27 (73.0) | 12 (75.0) | 0 | 0 |
| Pirfenidone & | 3 (8.1) | 5 (31.2) | 0 | 0 |
| ISU | 0 | 0 | 18 (90.0) | 8 (72.7) |
| Biological treatment | 0 | 0 | 7 (35.0) | 2 (18.2) |
| None | 10 (27.0) | 4 (25.0) | 1 (5.0) | 2 (18.2) |
| Lung-function parameters | ||||
| FVC (mL) | 3057.0 ± 840.8 | 2986.3 ± 850.7 | 2770.5 ± 681.2 | 2642.7 ± 631.4 |
| FVC (% pred) | 95.5 ± 12.8 | 98.4 ± 16.5 | 98.9 ± 13.7 | 98.4 ± 9.7 |
| FEV1 (mL) | 2497.6 ± 707.6 | 2467.5 ± 692.0 | 2354.5 ± 609.4 | 2200.9 ± 510.8 |
| FEV1 (% pred) | 96.8 ± 15.0 | 102.7 ± 18.5 | 100.0 ± 14.7 | 98.6 ± 11.3 |
| FEV1/FVC (%) | 82.0 ± 8.7 | 82.9 ± 5.2 | 84.8 ± 4.5 | 83.6 ± 6.4 |
| TLC (mL) | 4683.5 ± 1459.1 | 4568.8 ± 1130.2 | 4343.0 ± 884.2 | 4199.1 ± 635.5 |
| TLC (% pred) | 79.7 ± 16.1 | 78.8 ± 9.5 | 89.7 ± 17.5 | 88.1 ± 10.7 |
| Diffusion parameters | ||||
| DLCO (mmol/min/kPa) | 6.1 ± 1.8 | 5.3 ± 1.6 | 6.3 ± 1.5 | 6.6 ± 1.6 |
| DLCO (% pred) | 76.6 ± 18.0 | 68.3 ± 15.5 | 82.5 ± 18.4 | 88.6 ± 14.5 |
| KLCO (mmol/min/kPa/L) | 1.3 ± 0.4 | 1.2 ± 0.2 | 1.4 ± 0.3 | 1.5 ± 0.3 |
| KLCO (% pred) | 89.2 ± 23.6 | 87.8 ± 26.3 | 70.3 ± 16.3 | 75.6 ± 12.9 |
| ABGs | ||||
| pH | 7.4 ±0.0 | 7.4 ±0.0 | 7.4 ±0.0 | 7.4 ±0.0 |
| pCO2 (mmHg) | 38.5 ± 2.6 | 35.3 ± 10.0 | 37.7 ± 1.9 | 36.0 ± 2.8 |
| pO2 (mmHg) | 69.7 ± 10.2 | 62.2 ± 11.8 | 77.0 ± 6.4 | 81.4 ± 11.7 |
| 6MWT | ||||
| Distance (m) | 459.7 ± 102.5 | 442.5 ± 107.1 | 454.7 ± 68.8 | 438.4 ± 81.8 |
| Initial SpO2 (%) | 95.6 ± 2.3 | 94.6 ± 3.8 | 95.6 ± 1.9 | 93.6 ± 4.4 |
| Final SpO2 (%) | 89.4 ± 8.9 | 87.4 ± 9.3 | 93.6 ± 5.3 | 82.0 ± 14.0 |
| Initial HR (1/min) | 80.4 ± 12.9 | 82.3 ± 16.4 | 85.6 ± 15.6 | 82.4 ± 13.2 |
| Final HR (1/min) | 109.7 ± 19.7 | 114.7 ± 19.7 | 106.0 ± 16.7 | 107.2 ± 27.2 |
| Initial Borg score (0–10) | 0 (0–0) | 0.5 (0–0) | 0 (0–0) | 0 (0–1) |
| Final Borg score (0–10) | 1.5 (0–4) | 2.8 (0–4) | 2 (1–3) | 1 (0–3) |
Data are presented as n (%) or mean ± SD, median (IQR). 6MWT, 6-minute walk test; ABGs, arterialized capillary blood gases; BMI, body mass index; DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GAP, Gender–Age–Physiology index; GERD, gastro-esophageal reflux disease; HR, heart rate; HRCT, high-resolution computed tomography; IPF, idiopathic pulmonary fibrosis; ISU, immunosuppressive therapy; KLCO, transfer coefficient of the lung for carbon monoxide; NSIP, non-specific interstitial pneumonia; pCO2; partial pressure of carbon dioxide; PH, pulmonary hypertension; pO2, partial pressure of oxygen; pUIP, probable usual interstitial pneumonia; SpO2, oxygen saturation; SSc-ILD, systemic-sclerosis-associated interstitial lung disease; TLC, total lung capacity; UIP, usual interstitial pneumonia;. GAP index: stage I = 0–3 points; stage II = 4–5 points; stage III = 6–8 points. Statistically significant values are highlighted in bold: * p = 0.002; # p = 0.023. & All treatment was included: a nintedanib-to-pirfenidone or pirfenidone-to-nintedanib change in therapy resulted in the higher number of treated vs. untreated IPF patients.
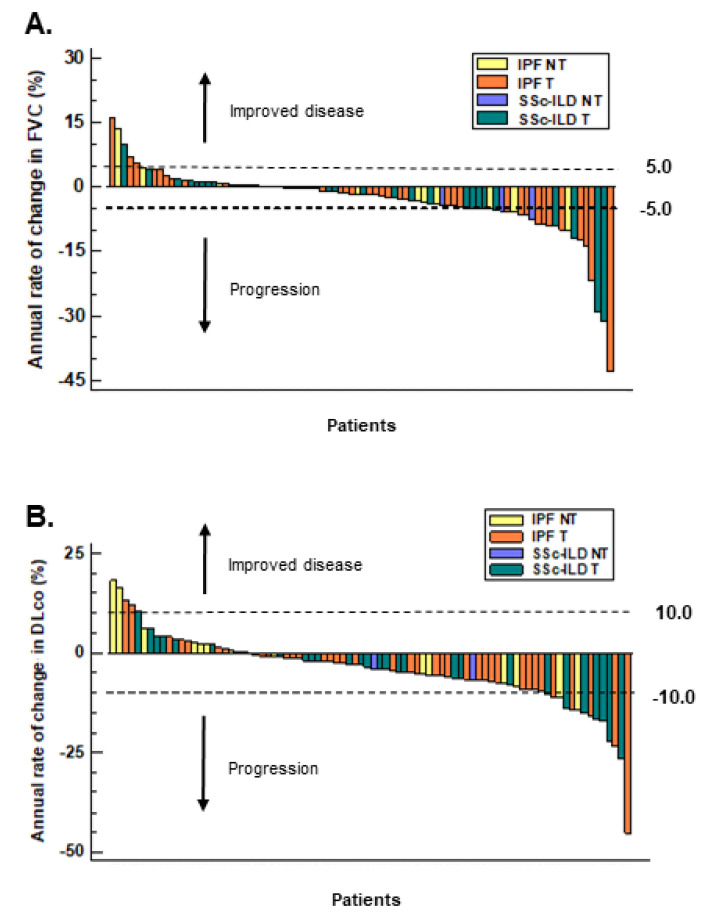
A multiple logistic regression did not show a significant baseline predictor of progression in the IPF group; however, in the SSc-ILD group, cough and PH were prognostic factors for functional progression (odds ratio: 36.2 (95% confidence interval: 1.8–711.9) and 36.4 (95% confidence interval: 1.1–1184.9), respectively). Most patients had a dry cough (SSc-ILD: n = 7; IPF: n = 17), and in the functionally progressive subgroups, a dry cough was predominant (SSc-ILD: 85.7% and IPF: 71.4%). The functional decline in individual patients according to treatment is presented in Figure 3.
Figure 3.
Annual changes in FVC (A) and DLCO (B) % of the predicted value in all IPF and SSc-ILD patients according to specific treatment groups. DLCO, diffusing capacity for carbon monoxide; FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis; NT, no treatment; SSc-ILD, systemic-sclerosis-associated interstitial lung disease; T, treatment.
4. Discussion
Our study confirmed that around one-third of SSc-ILD patients who had initial physiologic lung function parameters showed a decline in FVC and/or DLCO over one year. While the disease course of SSc-ILD is heterogeneous, development of PPF might be a leading cause of death in this rare patient group. Although pulmonary functional decline is only one out of the three criteria defining PPF, PFT is an important non-invasive measurement that, in combination with symptoms, might promote clinicians to engage in early treatment interventions if PPF is confirmed [11].
According to our data, SSc patients with HRCT-confirmed ILD who are experiencing cough as the leading respiratory symptom might be at higher risk of developing PPF even with normal lung function results when presented at the respiratory specialist. Similarly, cough was also studied in a subgroup analysis of the SENSCIS trial and was found to be an important prognostic factor for functional decline and progression in SSc-ILD patients [17]. However, patients enrolled in the SENSCIS trial had less preserved lung function and patients with cough had an average decline of −95.6 mL/year, which was slightly higher than in our study. Importantly, nintedanib was less effective in SSc-ILD patients with a cough compared to patients without a cough. These two independent observations could mean that cough is an independent negative prognostic factor for functional decline. Cough was also found to be a marker of unfavorable therapeutic response in the Scleroderma Lung Study (SLS) II and was strongly correlated with lung-function decline [18,19,20]. It is important to note that the mean FVC decline in prospective average population cohort studies ranged in men between −47.2 and −78.4 mL/year, while in women it ranged between −14.1 and −65.6 mL/year, underlining functional loss in non-progressive patients within the expected range [21].
Additionally, our study confirmed the important prognostic role of PH for functional decline in SSc-ILD, which, to the best of our knowledge, has not been previously established in the literature. On the other hand, PH is a well-known severe complication of SSc, and several large investigations have confirmed its contribution to mortality [22,23,24]. However, our study was not powered to investigate PH as a risk of mortality due to the short observation period.
Surprisingly, we found that the 6MWT results did not differ between the SSc-ILD and IPF patients, although the SSc-ILD patients were significantly younger and therefore were expected to have better functional performance compared to older IPF patients. The underlying mechanisms might have included vascular manifestations such as PH and musculoskeletal conditions affecting exercise limitation [25,26,27].
IPF can be regarded as the prototype of rapidly progressive fibrotic lung disease. The common cellular mechanism in IPF and SSc-ILD is fibroblast activation and recruitment, as well as myofibroblast differentiation leading to interstitial extracellular matrix accumulation and lung fibrosis [9,15]. Our data confirmed that functional decline in our total IPF patient population was lower than in the nintedanib-treated population of the Safety and Efficacy of nintedanib at High Dose in IPF Patients (INPULSIS) trial (FVC decline of −65.3 vs. adjusted annual rate of change of −114.7 mL/year) [28]. Importantly, no difference was found in FVC decline between IPF patients receiving antifibrotics and the treatment-free subgroups. This highlighted that despite therapy, some patients might deteriorate even while receiving frontline antifibrotic therapy; therefore, further additional risk factors need to be identified. Notably, the original trials did not focus on patients with physiological lung function. The total IPF group (including untreated individuals) revealed a limited FVC decline; the stable/improved subgroup only showed a marginal loss in this parameter, while the progressive subgroup presented with a similar decline to that observed in patients on a placebo in clinical trials [28,29]. This real-world observation of IPF patients supported the beneficial effect of antifibrotic treatment, similar to our previous study on functionally advanced IPF cases [13].
Treatment of SSc-ILD is challenging. Our data confirmed better functional outcome in ISU and/or biological-therapy-treated SSc-ILD, emphasizing early and specific SSc treatment [8,30]. An SENSCIS subanalysis evaluated the effectiveness of nintedanib as an antifibrotic agent among SSc-ILD patients with or without baseline mycophenolate mofetil (MMF) treatment [7]. The inclusion criteria were: an FVC of at least 40% of the predicted value, a DLCO of 30 to 89% of the predicted value, and a minimum of 10% fibrotic lung involvement on HRCT. At the end of the 52-week period, patients in the nintedanib + MMF group showed a significantly lower annual rate of decline in FVC than patients in the placebo + MMF group (−40.2 vs. −66.5 mL/year), and an even higher functional decline was present in the absence of ISU treatment (SSc-ILD on placebo: −119.3 mL/year). PPF SSc-ILD patients on the ISU therapy MMF with the addition of nintedanib had the highest advantage in lung-function preservation [7,31].
Treatment of PPF is still not well established; however, antifibrotic therapy might be an option in selected cases [11]. Nintedanib proved to be effective in reducing the annual rate of FVC decline in different fibrosing ILD patients in the Efficacy and Safety of Nintedanib in Patients with Progressive Fibrosing ILDs (INBUILD) trial and in the INPULSIS 1-2 IPF trials [28,32].
Optimal timing and dosing of ISU and/or biological therapy in SSc is of the utmost importance. Higher awareness of therapy initiation is needed in patients with normal lung function presenting with cough and in the presence of PH, and close pulmonary follow-up of these patients is suggested [33].
The limitations of our study were the retrospective single-center design and the low number of patients, which did not allow for a better stratification according to clinical features or treatment. Further prospective studies are needed to establish new markers of progression and to develop guidelines for the optimal timing of treatment introduction, with the adequate therapies being a combination of ISU and/or biological therapy and/or antifibrotics in the case of PPF in this special subgroup of SSc-ILD patients [34].
5. Conclusions
Progression in ILDs has an unfavorable effect on several clinical outcomes. Regular measurements of FVC are one of the most important parameters accepted for monitoring functional decline among IPF and SSc-ILD subjects. Patient-reported symptoms such as cough (especially dry cough) and the presence of PH as a lung-related comorbidity should be taken into consideration in connection with the disease progression in SSc-ILD patients. Defining high-risk patients for PPF is of utmost importance because timely and optimal introduction of ISU and/or biological and possibly antifibrotic agents might prevent disease deterioration. However, the timing and combination of treatments require further research in SSc-ILD and other PPF. Close monitoring and regular follow-ups are required in patients with normal lung function, especially in patients presenting with cough and PH.
Acknowledgments
Department of Pulmonology, Semmelweis University is a member of the European Reference Network for Respiratory Diseases (ERN-LUNG).
Author Contributions
Conceptualization, T.N. and V.M.; methodology, software, and validation, T.N., B.C., L.P. and V.M.; formal analysis, V.M.; resources, V.M. data curation, T.N., N.M.T., E.P., A.N., E.B., Á.D.T., D.L.T., E.K., P.M.-H. and V.M.; writing—original draft preparation, T.N., B.C. and V.M.; writing—review and editing, Á.D.T., D.L.T., G.N., E.K., P.M.-H. and V.M.; visualization, T.N., A.B., K.V., N.E. and A.N.; supervision, N.E., K.V., E.B., A.B. and V.M.; T.N., N.M.T., E.P., L.P., N.E., K.V., E.B., A.B., Á.D.T., D.L.T., G.N., E.K., P.M.-H. and V.M. were treating physicians or radiologists and members of the multidisciplinary ILD team at Semmelweis University. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committees of TUKEB and Semmelweis University (Study No. 69/2015—24 February 2015; 181/2021—23 November 2021).
Informed Consent Statement
Informed consent was obtained from all IPF subjects involved in the study. In other cases, informed consent was not needed due to the retrospective design.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
T.N. and L.P. were supported by the “Development of scientific workshops of medical, health sciences and pharmaceutical educations” (EFOP-3.6.3-VEKOP-1-00009). T.N. and A.N. were supported by the Hungarian Respiratory Society and Hungarian Respiratory Foundation. The EMPIRE registry was supported with funding from Boehringer Ingelheim (BI) and F. Hoffmann-La Roche (Roche). BI and Roche had no role in the study design, analysis, or interpretation of the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bergamasco A., Hartmann N., Wallace L., Verpillat P. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin. Epidemiol. 2019;11:257–273. doi: 10.2147/CLEP.S191418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denton C.P., Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 3.Panagopoulos P., Goules A., Hoffmann-Vold A.M., Matteson E.L., Tzioufas A. Natural history and screening of interstitial lung disease in systemic autoimmune rheumatic disorders. Ther. Adv. Musculoskelet. Dis. 2021;13:1759720x211037519. doi: 10.1177/1759720X211037519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cottin V., Brown K.K. Interstitial lung disease associated with systemic sclerosis (SSc-ILD) Respir. Res. 2019;20:13. doi: 10.1186/s12931-019-0980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vonk M.C., Walker U.A., Volkmann E.R., Kreuter M., Johnson S.R., Allanore Y. Natural variability in the disease course of SSc-ILD: Implications for treatment. Eur. Respir. Rev. 2021;30:200340. doi: 10.1183/16000617.0340-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goh N.S., Desai S.R., Veeraraghavan S., Hansell D.M., Copley S.J., Maher T.M., Corte T.J., Sander C.R., Ratoff J., Devaraj A., et al. Interstitial lung disease in systemic sclerosis: A simple staging system. Am. J. Respir. Crit. Care Med. 2008;177:1248–1254. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 7.Highland K.B., Distler O., Kuwana M., Allanore Y., Assassi S., Azuma A., Bourdin A., Denton C.P., Distler J.H.W., Hoffmann-Vold A.M., et al. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: A subgroup analysis of the SENSCIS trial. Lancet Respir. Med. 2021;9:96–106. doi: 10.1016/S2213-2600(20)30330-1. [DOI] [PubMed] [Google Scholar]
- 8.Nagy A., Nagy T., Kolonics-Farkas A.M., Eszes N., Vincze K., Barczi E., Tarnoki A.D., Tarnoki D.L., Nagy G., Kiss E., et al. Autoimmune Progressive Fibrosing Interstitial Lung Disease: Predictors of Fast Decline. Front. Pharmacol. 2021;12:778649. doi: 10.3389/fphar.2021.778649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richeldi L., Collard H.R., Jones M.G. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 10.Raghu G., Collard H.R., Egan J.J., Martinez F.J., Behr J., Brown K.K., Colby T.V., Cordier J.F., Flaherty K.R., Lasky J.A., et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghu G., Remy-Jardin M., Richeldi L., Thomson C.C., Inoue Y., Johkoh T., Kreuter M., Lynch D.A., Maher T.M., Martinez F.J., et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022;205:e18–e47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Hoogen F., Khanna D., Fransen J., Johnson S.R., Baron M., Tyndall A., Matucci-Cerinic M., Naden R.P., Medsger T.A., Jr., Carreira P.E., et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013;72:1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 13.Barczi E., Starobinski L., Kolonics-Farkas A., Eszes N., Bohacs A., Vasakova M., Hejduk K., Müller V. Long-Term Effects and Adverse Events of Nintedanib Therapy in Idiopathic Pulmonary Fibrosis Patients with Functionally Advanced Disease. Adv. Ther. 2019;36:1221–1232. doi: 10.1007/s12325-019-00906-9. [DOI] [PubMed] [Google Scholar]
- 14.Ley B., Ryerson C.J., Vittinghoff E., Ryu J.H., Tomassetti S., Lee J.S., Poletti V., Buccioli M., Elicker B.M., Jones K.D., et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 15.Khanna D., Tashkin D.P., Denton C.P., Renzoni E.A., Desai S.R., Varga J. Etiology, Risk Factors, and Biomarkers in Systemic Sclerosis with Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2020;201:650–660. doi: 10.1164/rccm.201903-0563CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winstone T.A., Assayag D., Wilcox P.G., Dunne J.V., Hague C.J., Leipsic J., Collard H.R., Ryerson C.J. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: A systematic review. Chest. 2014;146:422–436. doi: 10.1378/chest.13-2626. [DOI] [PubMed] [Google Scholar]
- 17.Volkmann E.R., Kreuter M., Hoffmann-Vold A.M., Wijsenbeek M., Smith V., Khanna D., Denton C.P., Wuyts W.A., Miede C., Alves M., et al. Dyspnoea and cough in patients with systemic sclerosis-associated interstitial lung disease in the SENSCIS trial. Rheumatology. 2022:keac091. doi: 10.1093/rheumatology/keac091. online ahead of print . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng J.Z., Wilcox P.G., Glaspole I., Corte T.J., Murphy D., Hague C.J., Ryerson C.J. Cough is less common and less severe in systemic sclerosis-associated interstitial lung disease compared to other fibrotic interstitial lung diseases. Respirology. 2017;22:1592–1597. doi: 10.1111/resp.13084. [DOI] [PubMed] [Google Scholar]
- 19.Tashkin D.P., Roth M.D., Clements P.J., Furst D.E., Khanna D., Kleerup E.C., Goldin J., Arriola E., Volkmann E.R., Kafaja S., et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): A randomised controlled, double-blind, parallel group trial. Lancet Respir. Med. 2016;4:708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tashkin D.P., Volkmann E.R., Tseng C.H., Roth M.D., Khanna D., Furst D.E., Clements P.J., Theodore A., Kafaja S., Kim G.H., et al. Improved Cough and Cough-Specific Quality of Life in Patients Treated for Scleroderma-Related Interstitial Lung Disease: Results of Scleroderma Lung Study II. Chest. 2017;151:813–820. doi: 10.1016/j.chest.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas E.T., Guppy M., Straus S.E., Bell K.J.L., Glasziou P. Rate of normal lung function decline in ageing adults: A systematic review of prospective cohort studies. BMJ Open. 2019;9:e028150. doi: 10.1136/bmjopen-2018-028150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer A., Swigris J.J., Bolster M.B., Chung L., Csuka M.E., Domsic R., Frech T., Hinchcliff M., Hsu V., Hummers L.K., et al. Pulmonary hypertension and interstitial lung disease within PHAROS: Impact of extent of fibrosis and pulmonary physiology on cardiac haemodynamic parameters. Clin. Exp. Rheumatol. 2014;32:109–114. [PubMed] [Google Scholar]
- 23.Launay D., Sobanski V., Hachulla E., Humbert M. Pulmonary hypertension in systemic sclerosis: Different phenotypes. Eur. Respir. Rev. 2017;26:170056. doi: 10.1183/16000617.0056-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odler B., Foris V., Gungl A., Müller V., Hassoun P.M., Kwapiszewska G., Olschewski H., Kovacs G. Biomarkers for Pulmonary Vascular Remodeling in Systemic Sclerosis: A Pathophysiological Approach. Front. Physiol. 2018;9:587. doi: 10.3389/fphys.2018.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bournia V.K., Kallianos A., Panopoulos S., Gialafos E., Velentza L., Vlachoyiannopoulos P.G., Sfikakis P.P., Trakada G. Cardiopulmonary exercise testing and prognosis in patients with systemic sclerosis without baseline pulmonary hypertension: A prospective cohort study. Rheumatol. Int. 2022;42:303–309. doi: 10.1007/s00296-021-04937-w. [DOI] [PubMed] [Google Scholar]
- 26.Garin M.C., Highland K.B., Silver R.M., Strange C. Limitations to the 6-minute walk test in interstitial lung disease and pulmonary hypertension in scleroderma. J. Rheumatol. 2009;36:330–336. doi: 10.3899/jrheum.080447. [DOI] [PubMed] [Google Scholar]
- 27.Vandecasteele E., De Pauw M., De Keyser F., Decuman S., Deschepper E., Piette Y., Brusselle G., Smith V. Six-minute walk test in systemic sclerosis: A systematic review and meta-analysis. Int. J. Cardiol. 2016;212:265–273. doi: 10.1016/j.ijcard.2016.03.084. [DOI] [PubMed] [Google Scholar]
- 28.Richeldi L., du Bois R.M., Raghu G., Azuma A., Brown K.K., Costabel U., Cottin V., Flaherty K.R., Hansell D.M., Inoue Y., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 29.King T.E., Jr., Bradford W.Z., Castro-Bernardini S., Fagan E.A., Glaspole I., Glassberg M.K., Gorina E., Hopkins P.M., Kardatzke D., Lancaster L., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann-Vold A.M., Allanore Y., Alves M., Brunborg C., Airó P., Ananieva L.P., Czirják L., Guiducci S., Hachulla E., Li M., et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann. Rheum. Dis. 2021;80:219–227. doi: 10.1136/annrheumdis-2020-217455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Distler O., Highland K.B., Gahlemann M., Azuma A., Fischer A., Mayes M.D., Raghu G., Sauter W., Girard M., Alves M., et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N. Engl. J. Med. 2019;380:2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 32.Flaherty K.R., Wells A.U., Cottin V., Devaraj A., Walsh S.L.F., Inoue Y., Richeldi L., Kolb M., Tetzlaff K., Stowasser S., et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 33.Kowal-Bielecka O., Fransen J., Avouac J., Becker M., Kulak A., Allanore Y., Distler O., Clements P., Cutolo M., Czirjak L., et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 2017;76:1327–1339. doi: 10.1136/annrheumdis-2016-209909. [DOI] [PubMed] [Google Scholar]
- 34.Nagy A., Palmer E., Polivka L., Eszes N., Vincze K., Barczi E., Bohacs A., Tarnoki A.D., Tarnoki D.L., Nagy G., et al. Treatment and Systemic Sclerosis Interstitial Lung Disease Outcome: The Overweight Paradox. Biomedicines. 2022;10:434. doi: 10.3390/biomedicines10020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.