Abstract
Deoxycytosine methylase (Dcm) enzyme activity causes mutagenesis in vitro either directly by enzyme-induced deamination of cytosine to uracil in the absence of the methyl donor, S-adenosylmethionine (SAM), or indirectly through spontaneous deamination of [5-methyl]cytosine to thymine. Using a Lac reversion assay, we investigated the contribution of the first mechanism to Dcm mutagenesis in vivo by lowering the levels of SAM. Escherichia coli SAM levels were lowered by reducing SAM synthetase activity via the introduction of a metK84 allele or by hydrolyzing SAM using the bacteriophage T3 SAM hydrolase. The metK84 strains exhibited increased C-to-T mutagenesis. Expression of the T3 SAM hydrolase gene, under the control of the arabinose-inducible PBAD promoter, effectively reduced Dcm-mediated genomic DNA methylation. However, increased mutagenesis was not observed until extremely high arabinose concentrations were used, and genome methylation at Dcm sites was negligible.
Methylated cytosines were first shown to be associated with an increase in the frequency of C-to-T transition mutations in the Escherichia coli lacI gene (6). In humans, methylation occurs predominantly at CpG dinucleotides and plays an essential role in many processes, including development, recombination, and X-chromosome inactivation (20, 39, 41). These methylated sites exhibit high rates of C-to-T transition mutations in both germ line and cancerous tissues (21, 23, 49). In prokaryotes, cytosine methyltransferases (CMTases) are generally associated with restriction-modification systems, and as a consequence of their action they play a role in bacterial genome evolution (3, 15, 31). Various CMTase recognition sites are mutational hot spots in E. coli (6, 24, 46).
CMTase function is well conserved in prokaryotes and eukaryotes. Upon binding to the target sequence, the protein flips the target cytosine into a catalytic enzyme pocket, where it becomes covalently linked to cytosine C6. This interaction activates cytosine C5, enabling transfer of a methyl group from the CMTase cofactor, S-adenosylmethionine (SAM), to produce DNA-[5-methyl]cytosine (5MeC) and S-adenosylhomocysteine (SAH)(22, 45).
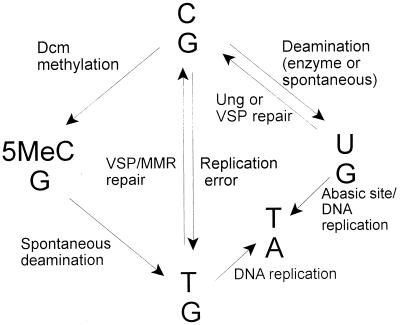
Various mechanisms have been hypothesized to explain the increase in mutagenesis at CMTase target cytosines. While DNA replication errors can account for some of the observed mutations, spontaneous hydrolytic deamination of 5MeC to thymine was initially proposed as the most likely mechanism responsible (6). Indeed, 5MeC-to-T deamination events occur more frequently than C-to-U events (43), and the deamination frequency is enhanced on single-stranded DNA during transcription (2). However, CMTases may also directly cause 5MeC-to-T mutation (48). A number of other factors can affect the outcome of CMTase activity. The efficiency of DNA repair at sites of deamination or replication error, T/G or U/G, dictates the fixation rate of mutation. In bacteria, at least three separate DNA repair pathways are capable of dealing with the resultant mismatched bases, very short patch repair (VSP), uracil-DNA-glycosyl transferase (Ung), and mismatch repair (MMR) (25, 27, 33). The efficiency of each of these pathways varies from site to site and with growth phase (Fig. 1). It has also been shown that some CMTases bind with higher affinity to mismatched DNA, a characteristic which may impede repair of the lesion (47).
FIG. 1.
Pathways to mutation at C · G.
SAM is a universal methyl donor, produced from methionine and ATP by SAM synthetase; it is required for a host of cellular methylation reactions (34, 49), including CMTase methylation activity. It is now evident that reduced levels of this cofactor have a profound effect on the ability of various CMTases to induce deamination at target Cs in vitro. HpaII methyltransferase (M.HpaII) activity in a plasmid-based neomycin reversion assay increases the C-to-U reversion frequency about 104-fold. This reaction occurs only in the absence of SAM (42).
The deamination mechanism was first described for M.HhaI (45). At low SAM and SAH concentrations, the CMTase interaction with cytosine results in the production of a transient covalent CMTase-dihydrocytosine intermediate, rapidly followed by an enzyme-catalyzed exchange of the 5-H of cytosine for protons of water to produce uracil. This reaction is inhibited by SAM or SAH.
In E. coli, deoxycytosine methylase (Dcm)-mediated cytosine methylation occurs at the internal cytosine residues of CCWGG sequences. In the event of 5MeC/G conversion to T/G, the Vsr protein nicks the DNA 5′ to the mismatched T, and DNA polymerase I then replaces a short stretch of nucleotides in a process known as VSP repair. If repair does not take place, a C-to-5MeC-to-T transition occurs. If spontaneous or enzyme-mediated deamination causing C-to-U transition takes place, it has been assumed that the Ung enzyme will excise the U, followed by removal of the abasic site and resynthesis of the correct DNA. However, Vsr can also recognize and cleave at U/G mismatches; whether it is as efficient as Ung in this regard remains unclear (12–14). It is interesting that the stability of 5MeC is similar to that of C in nondividing cells, implying that 5MeC-derived mutations occur during growth phase and are due to inefficient VSP repair (26), which may be reduced due to limited Vsr levels in exponentially growing cells (30).
The evidence supporting the C-to-U mutagenic potential of prokaryotic CMTases is mainly derived from in vitro studies using purified proteins and plasmid DNAs as substrates (1, 42, 45, 46, 50). In this study we have investigated the in vivo effects of Dcm in strains devoid of VSP and/or Ung repair activity. The mutagenic effect of Dcm activity is monitored using an episome which contains a Dcm recognition site within the lacZ gene sequence. C-to-T transition mutations at this site convert the strain to a Lac+ phenotype (36). In an effort to detect enzyme-mediated deamination events, we also examined the effects of reduced SAM levels on Dcm-induced mutagenesis in vivo. Reduction of E. coli SAM levels was accomplished by reducing SAM synthesis using a metK84 allele (17, 34), or by hydrolyzing cellular SAM using the bacteriophage T3 SAM hydrolase (T3SH) gene expressed from a plasmid. SAM synthetase is the product of the metK gene, and while mutants completely deficient in enzyme activity have not been isolated, E. coli metK84 strains produce markedly reduced levels of SAM (17) and exhibit a complex phenotype if grown in minimal medium, including a cell division defect which produces an excessively filamentous phenotype (34). T3SH hydrolyzes SAM to methylthioadenosine and homoserine and has been used previously to deplete SAM pools in E. coli (37, 44).
MATERIALS AND METHODS
Bacterial strains, phage lysates, and plasmids.
E. coli strains and plasmids are described in Table 1. BD2314 was obtained from the E. coli Genetic Stock Center, Yale University, New Haven, Conn., and GM3888 dcm-6 zed-501::Tn10 is available from M. G. Marinus (http://users.umassmed.edu/martin.marinus/dstrains.html). All mutant alleles were transferred to the test strains using standard P1 transduction protocols (32). Since the metK84 allele is not marked or directly selectable in rich medium, a serA::kan derivative was produced to allow a subsequent cotransduction of the metK84 mutation with serA+. The metK84 strains are notoriously unstable and accumulate suppressor mutations very rapidly (34). To ensure the integrity of our test strains, we prepared fresh sets of transductants for each experiment. Purified metK84 transductants were tested for kanamycin sensitivity, filamentous growth in minimal glucose medium containing low concentrations of leucine (15 μg/ml), and growth on minimal glucose plates containing gamma glutamyl methyl ester (500 μg/ml) (34). To verify that the ung allele conferred the appropriate mutation spectrum, an increase in C-to-T transversions, it was introduced into E. coli strains containing different episomal lacZ mutations, including CC102 (GC to AT), CC106 (AT to GC), and CC110 (+A frameshift) (7, 8). As expected, an increased reversion frequency was produced only in CC102 ung.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| Strains | ||
| CSH142 | Δ(gpt-lac)5 | 32 |
| CC102 | CSH142, F′ lacZ−YA, proAB; G · C to A · T | 8 |
| CC106 | CSH142, F′ lacZ−YA, proAB; A · T to G · C | 8 |
| CC110 | CSH142, F′ lacZ−YA, proAB; +A frameshift | 7 |
| CC112 | Δ(gpt-lac)5 argE(Am), F′lacZ−YA, proAB; CCAGG to CTAGG | 40 |
| CC112Δ | CC112Δ(supD-dcmvsr-fla) zee-3129::Tn10 | 40 |
| CC112dcm-6 | CC112dcm-6 zed-501::Tn10 | This work |
| BD2314 | ung-152::Tn10 | 11 |
| MEW30 | metK84 | 34 |
| MEW305 | serA::λplacMu9kan | 34 |
| CC500 | CSH142, F′ lacZ−YA, proAB; CCAGG to CTAGG | 36 |
| CC501 | CSH142serA::λplacMu9kan | This work |
| CC502 | metK84 | This work |
| CC503 | vsr::kan | This work |
| CC504 | ung::Tn10 | This work |
| CC505 | vsr::kan metK84 | This work |
| CC506 | ung::Tn10 vsr::kan | This work |
| CC507 | ung::Tn10 metK84 | This work |
| CC508 | ung::Tn10 vsr::kan metK84 | This work |
| LMG194 | Δara | 18 |
| CC509 | CC503 Δara | This work |
| CC510 | CC506 Δara | This work |
| Plasmids | ||
| pACYC184 | Cmr and Tetr | 5 |
| pDV101 | E. coli dcm gene in pACYC184 (Cmr) | 29 |
| pDV102 | E. coli dcm and vsr genes in pACYC184 (Cmr) | 29 |
| pBAD24 | Ampr | 18 |
| pBAD-T3SH | Bacteriophage T3 SAM hydrolase gene in pBAD24 | 37 |
The pBAD-T3SH plasmid contains the bacteriophage T3 SAM hydrolase gene under the control of the PBAD promoter (18). Expression of this gene in E. coli grown in the presence of arabinose (Luria-Bertani [LB] broth plus ampicillin at 100 μg/ml and 13.3 mM arabinose) leads to a limited filamentation phenotype (37). This change in morphology was used to confirm the presence of the T3SH gene in our test strains.
Growth of cultures.
The CC112 strain and its derivatives were transformed with various plasmids and grown overnight at 37°C in minimal medium containing 0.2% glucose, ampicillin (100 μg/ml), and chloramphenicol (20 μg/ml). Overnight cultures of metK84 strains and their isogenic counterparts were diluted 103-fold into LB medium and grown for 15 h at 37°C. Analyses of mutation rates in metK84 strains were carried out in LB medium to avoid any artifactual increases in mutation rate which may arise solely as a result of the very extensive cell filamentation associated with this strain. Previous studies have shown that decreasing leucine levels in minimal-glucose medium correlate with decreasing SAM synthetase levels in metK84 strains and increasing filamentation (34). SAM synthetase levels are lowered by approximately 50% in metK84 strains grown in LB medium (17). A comparison of mutation rates in the metK84 strains grown under nonfilamenting conditions, either in LB medium or minimal-glucose medium containing leucine (100 μg/ml), indicated that the levels of mutagenesis were similar (data not shown).
Cells containing pBAD24 or pBADT3SH were grown overnight in LB medium containing ampicillin at 100 μg/ml (LBAmp), diluted to 10−6 in LBAmp with glucose (0.2%) or arabinose at various concentrations, and grown for 18 h at 37°C in a rotary shaker. Regardless of the method used to reduce SAM levels, the resultant cultures were used to produce samples for Lac reversion assays and for genomic DNA analysis to monitor the extent of DNA methylation.
Lac reversion assays.
A papillation assay was performed as follows. Strain CC112, described previously (40), carries an episome which allows Lac reversion via C-to-T mutation at a Dcm site in the lacZ gene (aaC CAG Ggg). The strain carries a plasmid that contains a glutamic acid-inserting amber suppressor tRNA gene which allows the mutation of the CAG to TAG to be detected as a Lac+ revertant. Five-microliter aliquots of each overnight culture were spotted onto papillation medium and incubated at 37°C until papillae were visible (35).
CC500 carries an episome, pro462, which contains a Dcm recognition site (CCA GGg) within the lacZ gene to monitor C-to-T transition mutations. In the event of a C-to-T mutation at the second cytosine, the proline residue (CCA) will be replaced by a leucine (CTA) which will change the phenotype from Lac− to Lac+ (36). Overnight cultures of all strains grown in LB medium were assayed for Lac reversion as previously described (32). All strains containing the metK84 allele grew more slowly on the minimal lactose plates and were incubated for 40 h instead of 36 h.
DNA methylation assay.
The extent of DNA methylation was assessed using the Bio-Rad CHEF-DRIII pulsed-field electrophoresis system and protocols. Approximately 5 × 108 cells were transferred to a microcentrifuge tube and centrifuged for 1 min at 13,000 × g. The cells were resuspended in 0.5 ml of cell suspension buffer (10 mM Tris [pH 7.2], 20 mM NaCl, and 50 mM EDTA) and equilibrated to 50°C in a water bath. The cell suspension was then combined with an equal volume of 2% agarose (pulsed-field gel electrophoresis grade), mixed, and transferred to a plug mold (100 μl of suspension per plug). The agarose was allowed to solidify at 4°C for 10 min. Each set of 10 plugs was then placed in a 50-ml conical centrifuge, followed by a series of incubations and washes at room temperature. The plugs were incubated in lysozyme buffer (10 mM Tris [pH 7.2], 50 mM NaCl, 0.2% sodium deoxycholate, 0.5% sodium lauryl sarcosine, and 1 mg of lysozyme per ml) for 1 h at 37°C. The plugs were rinsed with 25 ml of 1× wash buffer (20 mM Tris [pH 8.0] and 50 mM EDTA) followed by an incubation in 5 ml of proteinase K reaction buffer (100 mM EDTA [pH 8.0], 0.2% sodium deoxycholate, 1% sodium lauryl sarcosine, and 1 mg of proteinase K per ml) at 50°C overnight. Each sample was then washed four times in 50 ml of 1× wash buffer for 1 h with gentle agitation; phenylmethylsulfonyl fluoride (1 mM final concentration) was added to the third wash. Before digestion with restriction enzymes, the plugs were cut into quarters and washed in 0.1× wash buffer for 30 min with agitation. Each quarter plug was incubated in 250 μl of the appropriate 1× restriction enzyme buffer for 1 h with agitation. The quarter plug was then incubated in 75 μl of fresh 1× restriction enzyme buffer plus 10 U of restriction enzyme overnight at 37°C and then rinsed with 250 μl of 1× wash buffer. Each quarter plug was then inserted into a well in a 1.2% agarose–Tris-borate-EDTA gel and sealed into position using molten 1.2% agarose. Molecular weight markers were also loaded as plugs. Electrophoresis was carried out in 0.5× Tris-borate-EDTA at 14°C, 6 V/cm for 22 h with a switch time of 50 to 90 s.
RESULTS
The frequency of C-to-T mutations increased in a VSP-deficient strain.
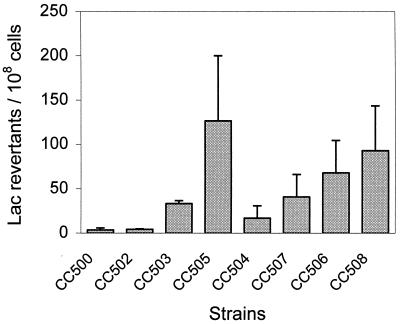
The wild-type strain CC500 exhibited a very low mutation frequency at the CCAGG site under study (Fig. 2). However, when the cells were VSP repair deficient, as in CC503, the mutation frequency was ninefold higher than that observed in a wild-type strain (Fig. 2), indicating the level of mutagenesis that results from Dcm activity. Therefore, using the pro462 episome, we could easily measure the mutagenic outcome of wild-type levels of Dcm activity in the absence of VSP repair.
FIG. 2.
Number of Lac revertants per 108 cells (plus standard error of the mean) resulting from C-to-T transition mutations at a Dcm recognition site, CCAGG, in an episomally located lac gene, in various genetic backgrounds. CC500 (wild type), CC502 (metK84), CC503 (vsr), CC505 (vsr metK84), CC504 (ung), CC507 (ung metK84), CC506 (ung vsr), CC508 (ung vsr metK84). Six replicate cultures for each strain were analyzed in triplicate.
C-to-T transition mutations were enhanced in metK strains in vivo.
The consequences of the metK84 allele on Dcm function were assessed by comparing CC503 (vsr) with CC505, a vsr metK84 strain (Fig. 2). In this instance, C-to-T transition mutations in the vsr metK84 strain increased approximately fourfold over the isogenic vsr strain and were 35-fold higher than those in the CC500 strain. The fourfold difference in mutation frequency was consistently larger than that observed when the CC500 strain was compared to the metK84 strain, CC502, which exhibited a 1.2-fold increase in C-to-T mutations over the CC500 strain (Fig. 2).
Uracil DNA glycosylase activity played a minor role in the repair of C-to-U mutations at the Dcm site under study.
The C-to-T mutations detected in the lac reversion assay could potentially arise via 5mC-to-T or C-to-U-to-T events, and we have assumed that any pre mutagenic C-to-U lesions will be repaired by either the VSP or Ung repair pathways. To address the contribution of Ung to DNA repair, ung versions of the test strains were analyzed. In the ung strain CC504 and the ung metK84 strain CC507, the mutation frequencies were approximately 5- and 10-fold higher, respectively, than those measured in the corresponding ung+ isogenic strains CC500 and CC502 (Fig. 2). In CC506, a vsr ung strain, the mutation frequency showed a less dramatic twofold increase when compared to the isogenic ung+ strain CC503 (Fig. 2). CC508, the ung vsr met strain, also exhibited little change in mutation rate when compared to CC505, its isogenic ung+ counterpart (Fig. 2), as evidenced by a slight reduction in the Lac reversion frequency. While the additional ung::tet mutation revealed the underlying level of spontaneous deamination events normally corrected by Ung, it also suggested that Ung-mediated DNA repair is less important than VSP repair at the Dcm site used in this study, particularly in the VSP− strains. In contrast, a previous study found that the Ung repair system was responsible for the majority of DNA repair at a Dcm site (28). This study used a strain containing a dcm-6 allele (9) to reduce VSP repair, which may have concealed the importance of this repair pathway.
CC112 dcm-6 possesses VSP repair function.
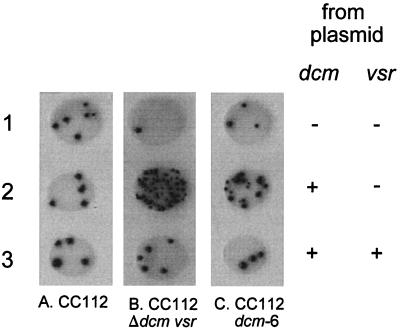
We measured the level of VSP activity in a strain containing the dcm-6 allele, which contains two mutations in the dcm gene, a Gln to Arg change at codon 26 and a Trp to an opal stop at codon 45 (9). Strains that possess this allele do not produce Dcm as measured by a methylase assay (9) and by Western blot analysis (data not shown). The opal stop codon is thought to have a polar effect upon the expression of vsr (9). We compared the levels of VSP repair function in strains containing either wild-type dcm or the dcm-6 allele with a strain deleted for dcm and vsr. The strains were transformed with various plasmids to restore Dcm and/or Vsr function as appropriate. CC112 dcm+ vsr+ exhibits a low level of Lac reversion in the presence of all plasmids tested, indicating that the chromosomal copy of vsr produces enough Vsr to repair the majority of lesions occurring at the Dcm test site (Fig. 3 column A, rows 1 through 3). When CC112Δ (dcm vsr) is examined in the presence of the same plasmids, the expression of plasmid-encoded dcm in the absence of vsr leads to a high level of mutagenesis (Fig. 3, column B, row 2), whereas the expression of dcm and vsr from a plasmid does not alter the mutation frequency, again due to efficient VSP repair (Fig. 3, column B, row 3). Analysis of CC112 dcm-6 indicates that in the absence or presence of both the dcm and vsr genes only background levels of mutagenesis are detectable (Fig. 3, column C, rows 1 and 3), as seen in the other strains. However, the expression of dcm in CC112 dcm-6 (Fig. 3, column C, row 2) results in a much less dramatic increase in mutagenesis than that observed in CC112Δ overexpressing dcm (Fig. 3, column B, row 2). This suggests that moderate levels of VSP repair are present in the strain carrying the dcm-6 allele.
FIG. 3.
Effect of the dcm-6 allele on VSP repair. Five-microliter aliquots of saturated bacterial cultures were spotted onto papillation medium. Columns: A, CC112; B, CC112 Δdcm vsr; C, CC112 dcm-6. Strains containing plasmids: row 1, pACYC184; row 2, pDV101; row 3, pDV102.
Genomic DNA methylation in metK strains.
To assess the effectiveness of our experimental approach at reducing methyl group availability to Dcm, we examined the levels of Dcm methylation of genomic DNA in metK84 strains grown in LB using a clamped homogeneous electric field (CHEF) agarose gel analysis of genomic DNA. MvaI cuts DNA at Dcm sites irrespective of Dcm methylation of the DNA while EcoRII cleaves only unmethylated Dcm sites. Genomic DNA from all metK84 strains tested was cleaved by MvaI but was insensitive to EcoRII hydrolysis under the growth conditions used for the experiment (data not shown). Therefore, the reduced SAM synthetase levels achieved in metK84 strains grown in LB are not sufficient to alter Dcm methylation of DNA as detected by Dcm methylation-sensitive restriction enzyme analysis.
Induction of SAM hydrolase reduces Dcm methylation of genomic DNA.
To reduce Dcm methylation levels more effectively without the risk of extensive filamentation, we used T3SH. The gene for T3SH is expressed from the plasmid pBAD24 (18), under the control of the PBAD promoter, which allows extensive modulation of the levels of the protein using arabinose. Recent studies have shown that T3SH expression from this plasmid lowers SAM levels in E. coli and reduces methylation of adenines in chromosomal DNA (36). The CC509 vsr Δara strain transformed with pBAD24 or pBAD-T3SH was grown in LBAmp with increasing concentrations of arabinose (0 to 13.3 mM) and analyzed for Dcm methylation of chromosomal DNA. All DNA plugs were checked for cleavage at Dcm sites using the methylation-insensitive restriction enzyme MvaI. All genomic DNA samples were cleaved to completion with MvaI, indicating the purity of the DNA plug preparations (Fig. 4A).
FIG. 4.
Dcm methylation of genomic DNA is reduced upon induction of T3 SAM hydrolase. (A) MvaI, a methylation-insensitive enzyme, cleaves at Dcm sites in genomic DNA from CC509 containing pBAD24 (lanes 1 to 6) or pBADT3SH (lanes 7 to 12). (B) EcoRII, a methylation-sensitive enzyme, does not cut DNA from CC509 containing pBAD24 (lanes 1 to 6). EcoRII cleaves at unmethylated Dcm sites in CC509 overexpressing T3SH (lanes 7 to 12). Lane U, typical sample of uncut DNA. (A and B) Arabinose concentrations: Lanes 1 and 7, 0 mM (plus 0.2% glucose); lanes 2 and 8, 1.3 μM; lanes 3 and 9, 13 μM; lanes 4 and 10, 130 μM; lanes 5 and 11, 1.3 mM; lanes 6 and 12, 13.3 mM.
The presence of pBAD24 had no effect on Dcm DNA methylation (Fig. 4B, lanes 1 to 6). The DNA appears to be cut to the same extent at all arabinose concentrations used, probably at naturally occurring unmethylated Dcm sites (38). CC509 transformed with pBAD-T3SH shows an increasing sensitivity to EcoRII as the levels of T3SH are increased using higher arabinose concentrations (Fig. 4B, lanes 7 to 12). Using this approach we were able to detect reduced Dcm methylation at arabinose concentrations of 13 and 130 μM which exhibited no enhanced cell filamentation (Fig. 4B, lanes 9 and 10, respectively). We also observed some EcoRII hydrolysis of the DNA prepared from the strain grown in LB containing 0.2% glucose, indicating that there is some limited expression of the T3 SAM hydrolase gene under repressing conditions (Fig. 4B, lane 7). At higher concentrations of the sugar (1.3 and 13.3 mM), EcoRII sensitivity of the genomic DNA samples increased and cell filamentation became more obvious (Fig. 4B, lanes 11 and 12).
Overexpression of the SAM hydrolase gene alters the C-to-T mutation rate.
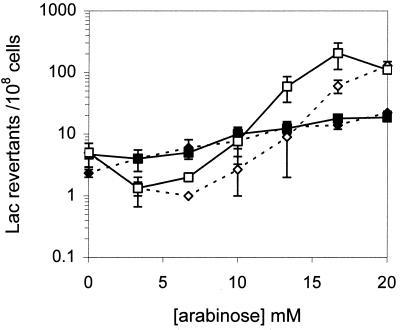
C-to-T mutations were monitored in CC509 (vsr Δara) containing pBAD24 or pBAD-T3SH and grown in LBAmp containing glucose or a range of arabinose concentrations. The levels of mutagenesis were similar for the CC509 strain carrying either plasmid grown in LBAmp glucose (0.2%) to repress expression from the Pbad promoter maximally (Fig. 5, 0 mM arabinose). At 1.3 μM arabinose, CC509/pBADT3SH exhibited a reduction in mutation frequency from five to one Lac revertants per 108 cells (not shown on graph); genomic DNA methylation appears normal at this concentration (Fig. 4B, lane 8). Arabinose (3.3 and 6.7 mM) also produced a modest reduction in mutation frequency in strains carrying pBAD-T3SH, indicative of reduced Dcm methyltransferase activity (Fig. 5). Figure 4B, lane 10, demonstrates that even at 0.13 mM arabinose, T3SH production was sufficient to reduce methylation at Dcm sites severely. At higher concentrations of arabinose (16.7 and 20 mM), the mutation frequency increased approximately 10-fold in the cultures producing T3SH. Mutagenesis was also enhanced in strains carrying the control plasmid, pBAD24, although the shape of the dose response curve was different; in this instance there was a slight but consistent increase in mutagenesis which correlated with increasing sugar concentrations (Fig. 5). Overexpressing the T3SH gene in CC510, an ung vsr Δara strain, produced a dose response curve that was similar in shape to the isogenic vsr Δara strain but exhibited slightly higher levels of mutagenesis (Fig. 5).
FIG. 5.
The effect of reduced SAM levels on C-to-T transition mutations. A T3SH-containing plasmid was compared to a pBAD24 control plasmid under various induction conditions. Lac revertants were measured per 108 cells (± standard error of the mean) due to C-to-T transition mutations at a Dcm recognition site, CCAGG, in an episomally located lac gene in two strains, CC509 vsr Δara and CC510 vsr ung Δara. Four replicate cultures were assayed in triplicate. CC509/pBAD24 ⧫; CC509/pBAD-T3SH ◊; CC510/pBAD24 ■; CC510/pBAD-T3SH □.
To ensure that the increasing SAM hydrolase levels do not have an adverse effect on MMR activity, due to decreased adenine methylation, the level of rifampin resistance (RifR) was also measured. Specific mutations in the rpoB gene confer Rifr; these mutations do not occur at Dcm recognition sites (19). Since Dcm-mediated mutagenesis does not result in Rifr, an increase in the frequency of Rifr mutants is indicative of a defect in MMR. We did not detect an increase in Rifr in any of the test strains (data not shown).
DISCUSSION
The purpose of this study was to ascertain whether the E. coli cytosine methyltransferase Dcm has the ability to mediate the deamination of C-to-U directly, leading to an increase in C-to-T transition mutations. Previous studies have suggested that this particular mutagenic event might be possible if SAM pools were to become limiting in vivo. This hypothesis is based mainly upon in vitro analyses including genetic and biochemical analyses of various bacterial methyltransferases, M.MspI (4, 50), M.HpaII (1, 42), and M.HhaI (45), that provide evidence that the enzymes are capable of mediating deamination of C leading to mutagenesis. A subsequent in vivo study did not support these findings (46).
In the present study we have assessed the effect of E. coli Dcm on C-to-T transition mutations at a Dcm site in an episomally located lacZ gene. To do this we used CC503, a vsr::kan strain, to eliminate VSP repair. In CC503 the frequency of C-to-T transitions is ninefold higher than in the wild-type strain, CC500 (Fig. 2), while the mutation frequency is increased only fivefold in CC504, an ung strain. In agreement with the previous plasmid-based studies, we show that chromosomally encoded Dcm significantly enhances the C-to-T mutation rate at target Cs. Our results suggest that Vsr plays a more significant role in DNA repair at this site than Ung. Previously, with a dcm-6 strain in a Kanr reversion assay, it was suggested that VSP repair was less efficient than Ung at Dcm sites (28). This discrepancy may be due to differences in sequence context around the Dcm site or, more likely, to VSP repair in strains carrying the dcm-6 allele. The original characterization of the dcm-6 allele, using a phage recombinogenic assay, indicated a reduced level of Vsp repair in strains carrying this gene (9). Subsequent studies using a Kanr reversion assay have made the assumption that levels of repair are sufficiently low as to be insignificant (28). Our Lac reversion analysis indicates that strains carrying the dcm-6 allele still have significant levels of VSP repair (Fig. 3), making it unsuitable for our study. Therefore, we have used a vsr::kan allele. This allows the study of wild-type levels of Dcm without the need to introduce a plasmid containing dcm, and more importantly, due to the insertion of the kanamycin cassette into the vsr gene, there is no VSP repair (10).
Using a SAM synthetase mutant allele, metK84, that possesses low SAM synthase activity (34) we have investigated the consequences of reduced SAM pools on CMTase-mediated deamination events in vivo. The introduction of the metK84, allele into the test strain to produce CC502 increases the reversion rate less than twofold (Fig. 2). However, C-to-T mutagenesis increases 35-fold over the wild type in CC505, a vsr metK84 strain, and is fourfold higher than in the isogenic vsr strain CC503 (Fig. 2). The increase in mutation conferred by the additional metK84 allele in a VSP-deficient strain suggests that Dcm-mediated mutagenesis is enhanced. However, genomic DNA methylation did not appear to be significantly reduced in the presence of the metK84 allele. The substantial variability in mutation frequency observed using the double mutant, vsr metK84, is consistent with the occurrence of suppressor mutations that overcome the effects of the metK84 mutation in the population.
We also determined the contribution of the Ung repair system to repair of C-to-U premutagenic lesions arising at the target C. Due to extensive variability in the number of Lac revertants produced by the ung strains, no statistically significant differences were found between isogenic pairs. However, the patterns of mutation seen in Fig. 2 are reproducible. Our results indicate that Ung contributes to repair at this site and that C-to-U mutations may be more prevalent in strains carrying the metK84 allele. However, VSP repair makes a more significant contribution to the repair of premutagenic lesions than Ung, suggesting that VSP repair is primarily responsible for repair of C-to-U as well as 5MeC-to-T lesions at the Dcm site under investigation. The Ung enzyme may be unable to recognize U/G mismatches in the context of a Dcm site.
T3 SAM hydrolase has been used previously to investigate the role of SAM as an endogenous alkylating agent. Although adenine methylation by the Dam methyltransferase was inhibited, no significant changes in mutation frequency indicative of defective mismatch repair or alkylation damage were observed (37). In the present study we were able to correlate increasing induction of the T3SH gene with severe reductions in Dcm-mediated DNA methylation (Fig. 4B) and with changes in mutation frequency. A modest decline in the C-to-T mutation frequency was observed at a low arabinose concentration (1.3 μM) that did not induce a detectable reduction in genomic DNA methylation and at sugar concentrations (3.33 and 6.7 mM) that correlated with reduced Dcm-mediated DNA methylation (Fig. 5). This suggests that even subtle reductions in Dcm DNA methylation resulting in less 5MeC in the DNA reduce the potential for mutation via spontaneous deamination of 5MeC-to-T. However, at higher induction levels mutagenesis increased 10-fold (Fig. 5). It is unlikely that this increase was due to cell filamentation since the previous study using the T3 SAM hydrolase in a lac reversion assay did not detect increased mutagenesis under the same limited filamenting conditions (37). We also overexpressed the plasmids in the vsr ung Δara strain, CC510; although the outcome was very similar to that observed in the vsr Δara isogenic strain, the levels of mutagenesis were generally higher. Therefore, the increased mutagenicity may indicate a switch from 5MeC-to-T to C-to-U-to-T Dcm-mediated deamination. The lesions are not repaired in the absence of Vsr, indicating that the VSP repair constitutes the major DNA repair pathway at this site and that Ung has a more limited role, even when the genome is hypomethylated.
It had previously been suggested that the high mutation rates at CpG dinucleotides in human tumor cells may be the result of enhanced CMTase-mediated C-to-U deamination events induced by an altered SAM/SAH environment within the tumor cells. Subsequently, it has been shown that the SAM/SAH ratio is not significantly altered in these cells, reducing the likelihood that C-to-U mutations via this mechanism contribute to mutagenesis (16). Similarly, although we have observed an increase in C-to-T mutagenesis in VSP repair-deficient strains when SAM pools are reduced, it is doubtful that these changes in mutation frequency are physiologically relevant. Revertant or suppressor mutations which overcome the effect of the metK84 allele give the cells a selective advantage, and they rapidly take over the population. Using the T3 SAM hydrolase, we have confirmed the predictions from previous in vitro studies and have demonstrated that Dcm can directly mediate deamination in vivo when SAM levels are rapidly and drastically reduced. However, the massive reductions in SAM pools required to increase Dcm-mediated deamination of DNA significantly are unlikely to occur naturally in bacteria.
ACKNOWLEDGMENTS.
We thank Elaine B. Newman for supplying the metK84 strain and for her advice on the use of this strain, Lan Jie for supplying various strains and lysates, and Leona Samson for supplying the pBAD-T3SH plasmid. Thanks also to Photini Pitsikas and Derek C. Bell for reading the manuscript. The CHEF-DRIII pulsed-field gel electrophoresis system was kindly made available to us by the Concordia University Center for Structural and Functional Genomics.
This research was funded by grants to C.G.C. from the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research.
REFERENCES
- 1.Bandaru B, Wyszynski M, Bhagwat A S. HpaII methyltransferase is mutagenic in Escherichia coli. J Bacteriol. 1995;177:2950–2952. doi: 10.1128/jb.177.10.2950-2952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beletskii A, Bhagwat A S. Transcription-induced mutations: increase in C to T mutations in the nontranscribed strand during transcription in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:13919–13924. doi: 10.1073/pnas.93.24.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhagwat A S, McClelland M. DNA mismatch correction by Very Short Patch repair may have altered the abundance of oligonucleotides in the E. coli genome. Nucleic Acids Res. 1992;20:1663–1668. doi: 10.1093/nar/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya S K, Dubey A K. Kinetic mechanism of cytosine DNA methyltransferase MspI. J Biol Chem. 1999;274:14743–14749. doi: 10.1074/jbc.274.21.14743. [DOI] [PubMed] [Google Scholar]
- 5.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulondre C, Miller J H, Farabaugh P J, Gilbert W. Molecular basis of base substitution hot spots in Escherichia coli. Nature. 1978;274:775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- 7.Cupples C G, Cabrera M, Cruz C, Miller J H. A set of lacZ mutations in Escherichia coli that allows rapid detection of specific frameshift mutations. Genetics. 1990;125:275–280. doi: 10.1093/genetics/125.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cupples C G, Miller J H. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc Natl Acad Sci USA. 1989;86:5345–5329. doi: 10.1073/pnas.86.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dar M E, Bhagwat A S. Mechanism of expression of DNA repair gene vsr, an Escherichia coli gene that overlaps the DNA cytosine methylase gene, dcm. Mol Microbiol. 1993;9:823–833. doi: 10.1111/j.1365-2958.1993.tb01741.x. [DOI] [PubMed] [Google Scholar]
- 10.Doiron K M J, Lavigne-Nicolas J, Cupples C G. Effect of interaction between 5-azacytidine and DNA (cytosine-5) methyltransferase on C-to-G and C-to-T mutations in Escherichia coli. Mutat Res. 1999;429:37–44. doi: 10.1016/s0027-5107(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 11.Duncan B K. Isolation of insertion, deletion, and nonsense mutations of the uracil-DNA glycosylase (ung) gene of Escherichia coli K-12. J Bacteriol. 1985;164:689–695. doi: 10.1128/jb.164.2.689-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox K R, Allinson S L H, Sahagun-Krause H, Brown T. Recognition of GT mismatches by Vsr mismatch endonuclease. Nucleic Acids Res. 2000;28:2535–2540. doi: 10.1093/nar/28.13.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabbara S, Wyszynski M, Bhagwat A S. A DNA repair process in Escherichia coli corrects U:G and T:G mismatches to C:G at sites of cytosine methylation. Mol Gen Genet. 1994;243:244–248. doi: 10.1007/BF00280322. [DOI] [PubMed] [Google Scholar]
- 14.Gläsner W, Hennecke F, Fritz H-J. Enzymatic properties and biological functions of Vsr DNA mismatch endonuclease. In: Lilley D M J, Heumann H, Suck D, editors. Structural tools for the analysis of protein-nucleic acid complexes. Basel, Switzerland: Birkhäuser Verlag; 1992. pp. 165–173. [Google Scholar]
- 15.Gläsner W F, Merkl R, Schellenberger V, Fritz H-J. Substrate preferences of Vsr DNA mismatch endonuclease and their consequences for the evolution of the Escherichia coli K-12 genome. J Mol Biol. 1995;245:1–7. doi: 10.1016/s0022-2836(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalgo M L, Jones P A. Mutagenic and epigenetic effects of DNA methylation. Mutat Res. 1997;386:107–118. doi: 10.1016/s1383-5742(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 17.Greene R C, Hunter J S V, Coch E-H. Properties of metK mutants of Escherichia coli K-12. J Bacteriol. 1973;115:57–67. doi: 10.1128/jb.115.1.57-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin D J, Gross C A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988;202:45–48. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- 20.Jones P A. The DNA methylation paradox. Trends Genet. 1999;15:34–37. doi: 10.1016/s0168-9525(98)01636-9. [DOI] [PubMed] [Google Scholar]
- 21.Jones P A, Rideout III W M, Shen J-C, Spruck C H, Tsai Y C. Methylation, mutation and cancer. Bioessays. 1992;14:33–36. doi: 10.1002/bies.950140107. [DOI] [PubMed] [Google Scholar]
- 22.Klimasauskas S, Kumar S, Roberts R J, Cheng X. Hhal methyl-transferase flips its target base out of the DNA helix. Cell. 1994;76:357–369. doi: 10.1016/0092-8674(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 23.Laird P W, Jaenisch R. DNA methylation and cancer. Hum Mol Genet. 1994;3:1487–1495. doi: 10.1093/hmg/3.suppl_1.1487. [DOI] [PubMed] [Google Scholar]
- 24.Lieb M. Spontaneous mutation at a 5-methylcytosine hotspot is prevented by very short patch (VSP) mismatch repair. Genetics. 1991;128:23–27. doi: 10.1093/genetics/128.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieb M, Bhagwat A S. Very short patch repair: reducing the cost of cytosine methylation. Mol Microbiol. 1996;20:467–473. doi: 10.1046/j.1365-2958.1996.5291066.x. [DOI] [PubMed] [Google Scholar]
- 26.Lieb M, Rehmat S. 5-Methylcytosine is not a mutation hot spot in nondividing Escherichia coli. Proc Natl Acad Sci USA. 1997;94:940–945. doi: 10.1073/pnas.94.3.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- 28.Lutsenko E, Bhagwat A S. Principal causes of hot spots for cytosine to thymine mutations at sites of cytosine methylation in growing cells: model, its experimental support and implications. Mutat Res. 1999;437:11–20. doi: 10.1016/s1383-5742(99)00065-4. [DOI] [PubMed] [Google Scholar]
- 29.Macintyre G, Doiron K M J, Cupples C G. The Vsr endonuclease of Escherichia coli: an efficient DNA repair enzyme and a potent mutagen. J Bacteriol. 1997;179:6048–6052. doi: 10.1128/jb.179.19.6048-6052.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macintyre G, Pitsikas P, Cupples C G. Growth phase-dependent regulation of Vsr endonuclease may contribute to 5-methylcytosine mutational hot spots in Escherichia coli. J Bacteriol. 1999;181:4435–4436. doi: 10.1128/jb.181.14.4435-4436.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merkl R, Kroger R, M, Rice P, Fritz H-J. Statistical evaluation and biological interpretation of non-random abundance in the E. coli K-12 genome of tetra- and pentanucleotide sequences related to VSP DNA mismatch repair. Nucleic Acids Res. 1992;20:1657–1662. doi: 10.1093/nar/20.7.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 33.Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination and cancer biology. Annu Rev Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 34.Newman E B, Budman L I, Chan E C, Greene R C, Lin R T, Woldringh C L, D'Ari R. Lack of S-adenosylmethionine results in a cell division defect in Escherichia coli. J Bacteriol. 1998;180:3614–3619. doi: 10.1128/jb.180.14.3614-3619.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nghiem Y M, Cabrera M, Cupples C G, Miller J H. The mutY gene: a locus in Escherichia coli that generates G.C → T.A transversions. Proc Natl Acad Sci USA. 1988;85:2709–2713. doi: 10.1073/pnas.85.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petropolous L, Vidmar J J, Passi E, Cupples C G. A simple assay for monitoring the mutagenic effects of 5-methyl-cytosine deamination in Escherichia coli. Mutat Res. 1994;304:181–185. doi: 10.1016/0027-5107(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 37.Posnick L M, Samson L D. Influence of S-adenosylmethionine pool size on spontaneous mutation, Dam methylation, and cell growth of Escherichia coli. J Bacteriol. 1999;181:6756–6762. doi: 10.1128/jb.181.21.6756-6762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ringquist S, Smith C L. The Escherichia coli chromosome contains specific, unmethylated dam and dcm sites. Proc Natl Acad Sci USA. 1992;89:4539–4543. doi: 10.1073/pnas.89.10.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson K D, Jones P A. DNA methylation: past, present and future. Carcinogenesis. 2000;21:461–467. doi: 10.1093/carcin/21.3.461. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz S M, Létourneau S, Cupples C G. Isolation and characterization of an Escherichia coli strain with a high frequency of C-to-T mutations at 5-methylcytosines. J Bacteriol. 1993;175:4985–4989. doi: 10.1128/jb.175.16.4985-4989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selker E U. Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Genet. 1990;24:579–613. doi: 10.1146/annurev.ge.24.120190.003051. [DOI] [PubMed] [Google Scholar]
- 42.Shen J-C, Rideout III W M, Jones P A. High frequency mutagenesis by a DNA methyltransferase. Cell. 1992;71:1073–1080. doi: 10.1016/s0092-8674(05)80057-1. [DOI] [PubMed] [Google Scholar]
- 43.Shen J-C, Rideout III W M, Jones P A. The rate of hydrolytic deamination of 5-methylcytosine in double-stranded DNA. Nucleic Acids Res. 1994;22:972–976. doi: 10.1093/nar/22.6.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Val D L, Cronan J E., Jr In vivo evidence that S-adenosylmethionine and fatty acid synthesis intermediates are the substrates for the LuxI family of autoinducer synthases. J Bacteriol. 1998;180:2644–2651. doi: 10.1128/jb.180.10.2644-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J C, Santi D V. Kinetic and catalytic mechanism of HhaI methyltransferase. J Biol Chem. 1987;262:4778–4786. [PubMed] [Google Scholar]
- 46.Wyszynski M, Gabbara S, Bhagwat A S. Cytosine deaminations catalyzed by DNA cytosine methyltransferases are unlikely to be the major cause of mutational hot spots at sites of cytosine methylation in Escherichia coli. Proc Natl Acad Sci USA. 1994;91:1574–1578. doi: 10.1073/pnas.91.4.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang A S, Shen J-C, Zingg J-M, Mi S, Jones P A. HhaI and HpaII DNA methyl-transferases bind DNA mismatches, methylate uracil and block DNA repair. Nucleic Acids Res. 1995;23:1380–1387. doi: 10.1093/nar/23.8.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yebra M J, Bhagwat A S. A cytosine methyltransferase converts 5-methylcytosine in DNA to thymine. Biochemistry. 1995;34:14572–14557. doi: 10.1021/bi00045a016. [DOI] [PubMed] [Google Scholar]
- 49.Zingg J-M, Jones P A. Genetic and epigenetic aspects of DNA methylation on genome expression, evolution, mutation and carcinogenesis. Carcinogenesis. 1997;18:869–882. doi: 10.1093/carcin/18.5.869. [DOI] [PubMed] [Google Scholar]
- 50.Zingg J-M, Shen J-C, Jones P A. Enzyme-mediated cytosine deamination by the bacterial methyltransferase M.MspI. Biochem J. 1998;332:223–230. doi: 10.1042/bj3320223. [DOI] [PMC free article] [PubMed] [Google Scholar]