Abstract
Glutathione (GSH), a major antioxidant in mammalian cells, regulates several vital cellular processes, such as nutrient metabolism, protein synthesis, and immune responses. In addition to its role in antioxidant defense, GSH controls biological processes through its conjugation to reactive protein cysteines in a post-translational modification known as protein S-glutathionylation (PSSG). PSSG has recently been implicated in the pathogenesis of multiple diseases including idiopathic pulmonary fibrosis (IPF). Hallmarks of IPF include repeated injury to the alveolar epithelium with aberrant tissue repair, epithelial cell apoptosis and fibroblast resistance to apoptosis, and the accumulation of extracellular matrix and distortion of normal lung architecture. Several studies have linked oxidative stress and PSSG to the development and progression of IPF. Additionally, it has been suggested that the loss of epithelial cell homeostasis and increased apoptosis, accompanied by the release of various metabolites, creates a vicious cycle that aggravates disease progression. In this short review, we highlight some recent studies that link PSSG to epithelial cell apoptosis and highlight the potential implication of metabolites secreted by apoptotic cells.
Keywords: glutaredoxin, glutathione, apoptosis, idiopathic pulmonary fibrosis, epithelial cells
1. Glutathione and S-Glutathionylation
Tripeptide glutathione (GSH) is the most abundant antioxidant in mammalian cells [1]. Glutathione contains the amino acids glutamate, cysteine, and glycine, and exists in reduced (GSH) and oxidized disulfide (GSSG) forms. Glutathione molecules containing additional sulfur atoms and with unique chemical reactivity also exist, but will not be further discussed here [1,2,3,4,5]. Under homeostatic conditions, the ratio of GSH:GSSG heavily favors the former (99:1), reflecting a reducing environment; although, in other compartments such as the endoplasmic reticulum and extracellularly, this ratio is more oxidizing [6,7]. GSH exerts numerous vital functions in cells, including phase II xenobiotic metabolism and reactions with oxidants [1,8,9]. In addition, GSH regulates physiological processes that include cell proliferation [10,11,12], DNA and protein synthesis [13,14], cytokine production, and immune responses [15,16,17]. Through the γ-glutamyl cycle, GSH acts as a continuous source of cysteine that can be incorporated into GSH synthesis itself, or in the synthesis of other proteins [18]. One critical function of GSH that is now well established is its conjugation to protein thiols in a post-translational modification called protein S-glutathionylation (PSSG). Multiple protein cysteine oxidations occur, including of sulfenic acid residues (SOH) and S-nitrosylated residues (SNO). While SOH- and SNO-containing proteins impart unique biological functions, SOH and SNO moieties can also give rise to more stable PSSG. PSSG changes the structure and function of the protein of concern by affecting the charge and size. Additionally, PSSG is chemically important as it prevents the overoxidation of cysteines [4,19,20,21,22]. The forward S-glutathionylation reaction can be catalyzed by glutathione S-transferases, notably by GSTP, which is the highest-expressed GST in lung tissue (see [23] for recent review). Conversely, glutaredoxin under physiological conditions acts as a deglutathionylating enzyme re-establishing the reduced protein thiol, as will be further discussed below. Because PSSG is reversible, it facilitates the dynamic regulation of the protein structure and function, making this post-translational modification an ideal mechanism through which signals from oxidants are transduced into biological responses.
2. Alterations in Redox Homeostasis in Idiopathic Pulmonary Fibrosis
The lung is constantly exposed to high oxygen concentrations and has some of the highest GSH levels in the body [24,25,26]. As such, dysregulation of the GSH system has been implicated in diverse lung pathologies, including cystic fibrosis, chronic obstructive pulmonary disease, asthma, and lung fibrosis. Idiopathic pulmonary fibrosis (IPF) is a progressive and irreversible interstitial lung disease characterized by the deposition of the extracellular matrix and loss of lung function. IPF is an aging-associated disease and occurs primarily in older adults, preferentially males, and has increased in both prevalence and mortality in recent years [27]. Patients diagnosed with IPF have a poor prognosis with a median survival of 3-5 years following diagnosis. Relatively few therapeutic options exist, and care is focused on managing symptoms and slowing disease progression [28]. The key histopathological features of IPF include the loss of the alveolar epithelium, the appearance of honeycombed cysts myofibroblasts and fibroblast foci, and the excess deposition of the extracellular matrix in the distal regions of the lung. As its name suggests, the exact cause of IPF is not currently known. However, several processes are thought to be central to IPF pathogenesis, such as repeated cycles of epithelial cell injury and death, aberrant repair along with changes in epithelial cell plasticity, sustained transforming growth factor beta (TGFB) signaling, and fibroblast activation to a myofibroblast phenotype that is accompanied by resistance to apoptosis and senescence [29,30,31].
Changes in redox homeostasis have been observed in patients with IPF and mouse models of fibrosis. Oxidized proteins, hydrogen peroxide, nitric oxide, and 8-isoprostanes, a byproduct of lipid peroxidation, were significantly increased in the bronchoalveolar lavage fluid (BALF) and exhaled breath was found to condensate in patients with IPF compared to healthy controls [32,33,34]. In addition, blood samples from IPF patients showed evidence of increased oxidant formation [35]. Among the intracellular sources of oxidants in cells are the NADPH family of oxidases (NOX), which produce hydrogen peroxide and superoxide [36]. Notably, a prominent role for NOX4 in pulmonary fibrosis was identified [37,38]. NOX4 was increased in the alveolar epithelium of IPF lungs, and global deletion of NOX4 protected mice from bleomycin-induced fibrosis [39]. In a bleomycin mouse model of fibrosis, persistent fibrosis was detected in aged mice compared to young mice and was accompanied by an apoptosis-resistant population of myofibroblasts. Intriguingly, the apoptosis resistance of aged myofibroblasts was mediated by the elevated expression of NOX4, as well as an impaired capacity to induce NRF2, which is a transcription factor that augments the expression of numerous antioxidant and redox-controlling genes. A small-molecule NOX4 inhibitor diminished age-associated persistent bleomycin-induced fibrosis in mice, while NOX4 siRNA diminished senescence and apoptosis resistance in fibroblasts in association with a diminished expression of the antiapoptotic protein BCL2 [40]. Activation of NRF2 was also sufficient to reverse the myofibroblast phenotype [40,41]. These findings illuminate an age-dependent imbalance of the NOX4–NRF2 axis in the bleomycin model of fibrosis in mice. Importantly, concomitant increases in the expression of NOX4 and a diminished expression of NRF2 were also demonstrated in primary fibroblasts isolated from the lungs of IPF subjects, compared to fibroblasts from control subjects or lung tissues [40].
Decreases in GSH have also been observed in fibrotic lungs. Sputum samples from patients with IPF showed significantly decreased GSH when compared to healthy controls, with a slight inverse correlation between sputum GSH and disease progression [42]. The same trend was observed in the lower respiratory tract, where epithelial lining fluid from IPF patients also demonstrated a fourfold decrease in GSH [43]. In addition, a decreased GSH [42] and a decreased GSH:GSSG ratio were observed in blood samples from patients with IPF.
In recent years, it has become evident that fibrosis may not simply result from an imbalance between the production of oxidants and antioxidant defenses, but that an altered redox environment affects sensitive proteins and pathways in a cell-specific and location-specific manner. This specificity of oxidant-induced targets is perhaps evidenced by the failure of broadly applied non-specific antioxidant therapies for fibrosis, such as N-acetyl-l-cysteine, which demonstrated no significant benefit in clinical trials [44,45]. These observations indicate that a greater understanding of the oxidative processes and affected cellular pathways in IPF is necessary to provide more promising therapeutic options. With numerous clinical and animal studies confirming a redox imbalance in IPF, some have tried to characterize an oxidative biomarker to predict disease progression and acute exacerbations [46]. However, no consensus about the utility of such biomarker has yet been reached and these endeavors have not yielded a widely utilized assay for clinical management.
3. Glutaredoxin and Lung Fibrosis
A key regulator of PSSG is the family of oxidoreductases called glutaredoxins (GLRX). Since the first description of GLRX [47], several isoforms of GLRX have been described and studied. Mammalian GLRX1, hereafter referred to as GLRX, is the main isoform in the cytosol, which can catalyze both the removal of glutathione from a protein thiol (deglutathionylation) and the reduction of disulfide bonds. The first step of the deglutathionylation reaction involves the breaking of a mixed protein-GSH disulfide by GLRX, which occurs rapidly due to the low pKa (approximately 3.5) of the GLRX active site cysteine [48]. As a result of this reaction, GLRX itself is glutathionylated, and must be reduced by a second molecule of GSH. The glutathione system, specifically GSH, Glutathione Reductase (GR), and NADPH, provides reduction equivalents to fuel these reactions [5,49].
In mammalian cells, GLRX is the primary enzyme responsible for deglutathionylation in the cytosol. Although they are members of the thioredoxin protein superfamily, GLRX reduces mixed protein-GSH disulfides much faster, and with greater specificity, than thioredoxins [50]. As such, the balance of glutathionylated proteins depends heavily on the expression and activity of GLRX. Mice with a global deletion of Glrx appear grossly normal, and do not display signs of increased oxidative stress under homeostatic conditions [51]. However, the consequences of an altered Glrx expression become evident in pathophysiological settings in which the Glrx status is linked to the aberrant activation of various pathways that control disease progression in diverse tissues. For example, GLRX-deficient mice fed a high-fat diet develop steatohepatitis [52], a condition that can lead to liver fibrosis. In contrast, the upregulation of GLRX enhances neuroinflammation and neuronal cell death, and contributes to the progression of Parkinson’s disease [53]. GLRX has also been implicated in angiogenesis, with increases in GLRX inhibiting vascularization and endothelial cell migration [54]. Interestingly, sexual dimorphism was recently reported for the role of GLRX in metabolic reprogramming of monocytes, where GLRX deficiency in female monocytes promotes dysregulated macrophage phenotypes and weight gain [55]. For more detailed information on GLRX in brain, liver, and heart pathophysiology, the reader is directed to the recent and comprehensive review by Matsui et al. [56].
Alterations in GLRX activity and expression have been associated with pulmonary fibrosis. GLRX expression and activity were decreased in patients with IPF compared to healthy controls in association with increases in PSSG. In addition, GLRX activity was directly correlated with lung function, while PSSG was inversely correlated with lung function in subjects with IPF. In models of bleomycin- or TGFB1-induced fibrosis, mice lacking Glrx displayed significantly increased collagen deposition, while transgenic mice overexpressing Glrx in airway epithelial cells showed attenuated collagen deposition. Strikingly, the oropharyngeal administration of recombinant GLRX was sufficient to reverse existing fibrotic changes [57]. The pathways in which disrupted PSSG and GLRX promote fibrogenesis remain incompletely understood and await elucidation of the S-glutathionylated proteome. However, one prominent PSSG target that contributes to the development of lung fibrosis is the death receptor FAS, as is described below.
4. Importance of Apoptosis in Lung Fibrosis
Epithelial cells and fibroblasts are cell types with critical roles in the pathogenesis of pulmonary fibrosis. In fibrotic lung disease, including IPF, disruptions of the distal conducting airway or alveolar epithelial cells represent common histopathological features. Inhaled insults lead to the death of epithelial cells; in an aging lung, the lack of normal epithelial restitution along with the concomitant activation and proliferation of myofibroblasts collectively promote fibrosis [58,59,60,61,62]. Apoptosis is a form of programmed cell death that is crucial for normal homeostasis and is dysregulated in diverse pathologies. It involves the tightly regulated recruitment and binding of specific receptor-ligand pairs to induce activation of proteolytic effector caspases for controlled degradation of cellular components. Two main mechanisms of apoptosis have been described in detail. The first involves the translocation of Bid to the mitochondria, resulting in the release of caspases to the cytosol, commonly referred to as the intrinsic pathway. In contrast, the extrinsic pathway involves the ligand binding of cell surface receptors, including FAS, TNF, D4, and D5, to form a death-induced signaling complex (DISC), which further recruits and activates caspases [63].
Numerous studies strongly support the importance of increased epithelial apoptosis, and the apoptosis resistance of myofibroblasts, in subsequent fibrogenesis and the importance of FAS herein. Upon administration of diphtheria toxin to transgenic mice expressing the diphtheria toxin receptor in type II epithelial cells or club cells, marked death of type II epithelial or club cells occurs with resultant prolonged fibrosis [61,62]. The functional role of FAS in the development of pulmonary fibrosis is evident from studies showing that agonistic FAS antibody (which mimics crosslinking between FASL and FAS) resulted in the apoptosis of epithelial cells, leading to fibrosis [58,64,65]. Conversely, bleomycin-induced fibrosis could be prevented using soluble anti-FAS, or anti-FAS Ligand antibodies, and did not occur in mice that lacked functional FAS (lpr) or FAS ligand (gld) [57,66]. Apoptosis of epithelial cells has been shown following co-culture with myofibroblasts isolated from patients with IPF that express FASL, while these myofibroblasts themselves are resistant to FASL-induced apoptosis [65,67]. The resistance of IPF myofibroblasts to FAS-induced apoptosis has been associated with an increased expression of multiple antiapoptotic genes and the downregulation of FAS [68,69]. The selective ablation of FAS in lung fibroblasts led to fibroblast and fibrosis persistence in mice [69], which confirmed the importance of diminished FAS expression to the apoptosis resistance of myofibroblasts. Epithelial cell death by IPF-derived fibroblasts has been linked to H2O2 produced by myofibroblasts [70]. Taken together, these findings demonstrate the importance of FAS in epithelial cell apoptosis and resistance to FAS-induced apoptosis in myofibroblasts in the pathogenesis of lung fibrosis. These findings suggest that avenues that dampen the extent of epithelial cell death and promote the apoptosis sensitivity of fibroblasts by targeting the FAS pathway in epithelial cells and fibroblasts, have the potential to attenuate fibrotic remodeling.
5. Glutathionylation and Epithelial Cell Apoptosis
Several studies have noted a link between the glutathione system and cell death by apoptosis. Deletion of the GSH synthesis enzyme GCLC in mice is embryonically lethal due to the high rates of apoptosis [71]. The reduction in cytosolic GSH is a hallmark of cells undergoing apoptosis [72], and GSH depletion through cysteine starvation has been shown to induce apoptosis [73]. Conversely, increased GSH levels are associated with resistance to apoptosis [74,75]. Further, there is evidence that apoptotic proteins can themselves be glutathionylated. Our laboratory has previously shown that the activation of apoptosis in airway epithelial cells induced by FASL is accompanied by increases in total PSSG [76]. In this model, FAS was found to be glutathionylated at cysteine 294 following the binding of FASL, thereby promoting caspase activation and apoptosis. Increases in FAS-SSG were not linked to increased oxidant production, but rather, were linked to the degradation of GLRX by caspases. Epithelial cells lacking Glrx were more susceptible to FASL-induced apoptosis and displayed elevated FAS-SSG compared to WT counterparts, while the overexpression of Glrx attenuated epithelial cell apoptosis in association with diminished FAS-SSG. A follow-up study conducted by our group discovered the existence of distinct pools of FAS in epithelial cells and that upon ligation of FAS, the latent FAS localized in the endoplasmic reticulum undergoes oxidative processing in a reaction that requires protein disulfide isomerase A3 (PDIA3) and GSTP, leading to the formation of FAS-SSG. Knock down, ablation, or pharmacological inhibition of PDIA3 or GSTP attenuated FAS-SSG and epithelial apoptosis [77]. Of relevance are further studies demonstrating that the pharmacological inhibition of PDIA3 or GSTP using clinically relevant compounds attenuated fibrosis in multiple mouse models [77,78]. Besides FAS, caspase-3 is also regulated via PSSG. In endothelial cells during tumor necrosis factor alpha (TNF-A)-induced apoptosis, caspase-3-SSG prevented its cleavage by caspase-8 and attenuated apoptosis [79]. Control of caspase-3-SSG was GLRX dependent, with the expression of GLRX in turn regulated by TNF-A. Collectively, these observations highlight the importance of the PSSG of key proteins in the apoptosis pathway in regulating the extent of apoptosis.
6. Release of Metabolites by Apoptotic Cells: Potential for Regulation by Glutaredoxin
As described above, pulmonary fibrosis is the culmination of enhanced apoptosis of epithelial cells, an acquired state of apoptosis resistance of myofibroblasts, and aberrant cross talk between epithelial cells, fibroblasts, and likely additional cell types not discussed here. These observations raise questions about whether signals originating from apoptotic epithelial cells impinge on the biology of fibroblasts and other cell types within the lung. A recent study shed light on this question by subjecting a variety of cells to multiple inducers of apoptosis and conducted a metabolite analysis of the medium. They reported that a number of metabolites were consistently released from multiple cell types subjected to various apoptotic stimuli, including the stimulation of FAS. These metabolites included spermidine, creatine, dihydroxyacetone phosphate (DHAP), fructose-1,6-bisphosphate (FBP), UDP-glucose, glycerol-3-phosphate, (G3P), guanosine 5′ monophosphate (GMP), and inosine monophosphate (IMP). The authors also reported that six of these metabolites together profoundly affected gene expression profiles in macrophages and that these effects could be recapitulated by three out of six metabolites, namely spermidine, GMP, and IMP. Importantly, these metabolites were also anti-inflammatory in vivo and elicited protective effects in arthritis and lung transplant rejections in mouse models. The pannexin 1 channel, which opens in a caspase-dependent manner, was required for metabolite release [80].
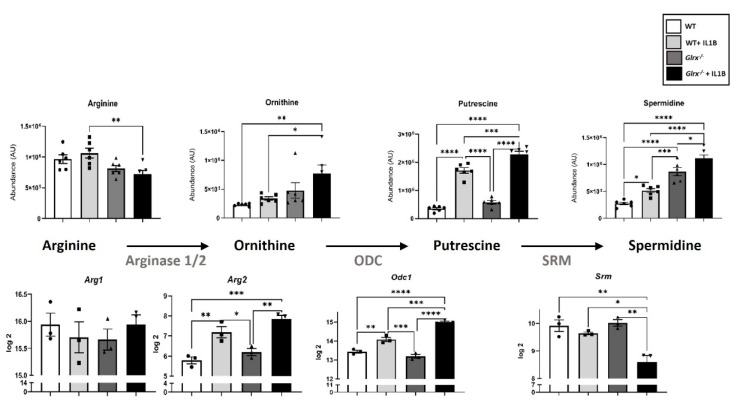
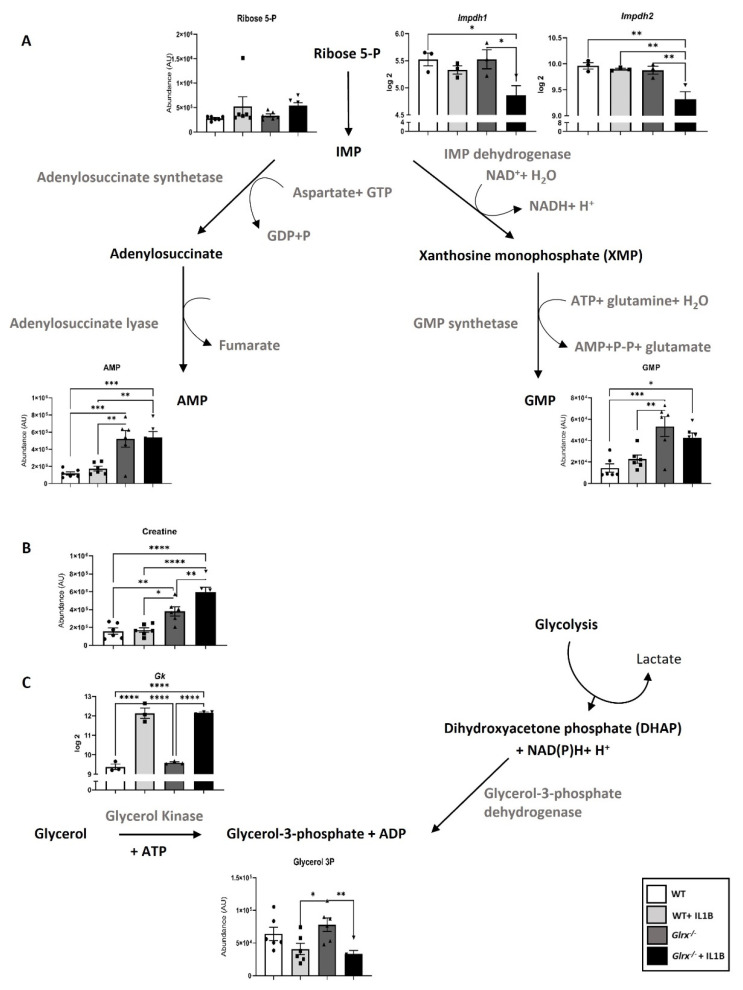
The identity and importance of metabolites released from apoptotic epithelial cells in fibrotic lung tissues remain unknown, and the importance of oxidative signals in promoting metabolite formation and release also require additional investigation. We conducted a multi-omics study in airway epithelial cells derived from WT or Glrx−/− mice stimulated with the pro-fibrotic cytokine interleukin 1 beta (IL1B), and discovered that multiple metabolic pathways were perturbed in the absence of Glrx or in response to IL1B [17]. Notably, glycolysis was enhanced in epithelial cells lacking Glrx, with further increases occurring following IL1B stimulation. When further assessing the metabolite profiles in this study, we observed that some of the metabolites released by apoptotic cells were also detected intracellularly and depended on the GLRX status. As stated above, Glrx−/− epithelial cells are more prone to FASL-induced apoptosis [76]. The results in Figure 1 show that Glrx−/− epithelial cells had higher levels of spermidine. Stimulating WT or Glrx−/− cells with IL1B increased spermidine levels in WT and Glrx−/− cells, with further increases apparent in Glrx−/− cells. In mammalian cells, arginine is a major source of spermidine, via the arginase (ARG)-dependent conversion of arginine to ornithine, the subsequent ornithine decarboxylase (ODC)-dependent conversion of ornithine to putrescine, and finally via spermidine synthase (SRM) conversion of putrescine to spermidine (Figure 1). While arginine, ornithine, and putrescine levels were not different between unstimulated WT or Glrx−/− epithelial cells, in response to IL1B arginine diminished while ornithine and putrescine levels increased in cells lacking Glrx compared to their WT counterparts. Supporting these observations, mRNA levels of arginase 2 (Arg2) were increased in control or IL1B-stimulated Glrx−/− cells compared to the respective WT groups. Ornithine decarboxylase (Odc) was increased in IL1B-stimulated Glrx−/− cells compared to the respective WT group. In contrast, spermidine synthase (Srm) decreased in Glrx−/− cells stimulated with IL1B compared to the WT group (Figure 1). Collectively, these findings suggest that a metabolic pathway that yields spermidine is regulated by GLRX status. Among the other metabolites released by apoptotic cells were GMP, AMP, creatine, and G3P [80]. Of interest, Glrx−/− cells exhibited higher levels of GMP, AMP (Figure 2A), and creatine (Figure 2B), while G3P levels decreased in Glrx−/− cells following IL1B stimulation (Figure 2C). Inosine monophosphate dehydrogenases 1 and 2 (Impdh1/2) catalyze the conversion of ribose-5-phosphate to inosine monophosphate (IMP), which can subsequently be metabolized to xanthosine monophosphate and GMP via Inosine-5′-monophosphate dehydrogenase (IMPDH) and GMP synthase. In response to IL1B, the expression of Impdh1 and Impdh2 significantly decreased compared to unstimulated Glrx−/− cells or the WT groups (Figure 2A). While the expression of arginine:glycine amidinotransferase and guanidinoacetate N-methyltransferase, which generate creatine, remained unchanged in our study (not shown), the expression of glycerol kinase (Gk), which catalyzes the conversion of glycerol to G3P, significantly increased in response to IL1B in both WT and Glrx−/− cells (Figure 2C). Overall, these findings illustrate the modulation of multiple pathways that regulate the synthesis and utilization of diverse metabolites released by apoptosis cells and their regulation by GLRX status.
Figure 1.
GLRX-dependent modulation of metabolic pathways that use arginine and give rise to spermidine in airway basal cells stimulated with IL1B. WT or Glrx−/− airway basal cells were stimulated with 10 ng/mL of IL1B for 24 h prior to the assessment of gene expression or metabolite analysis in cell lysates. We refer the reader to Aboushousha et al. [17] for technical details about this study. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ANOVA.
Figure 2.
GLRX-dependent modulation of metabolic pathways that regulate AMP, GMP (A), creatine (B), and glycerol-3-phosphate (C) in airway basal cells stimulated with IL1B. WT or Glrx−/− airway basal cells were stimulated with 10 ng/mL of Il1B for 24 h prior to the assessment of gene expression or metabolite analysis in cell lysates. We refer the reader to Aboushousha et al. [17] for technical details about this study. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ANOVA.
7. Conclusions and Future Directions
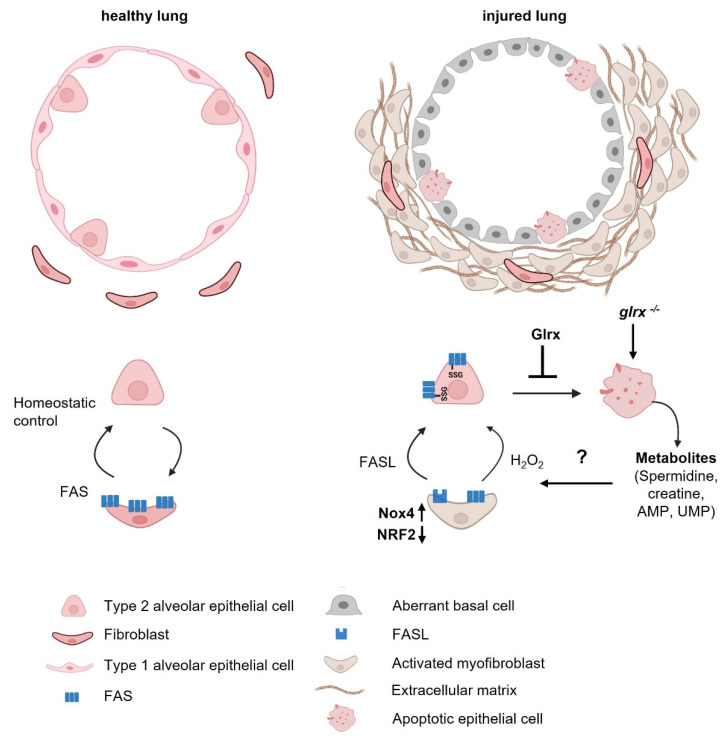
Pulmonary fibrosis is an insidious disease that develops in response to diverse insults against the backdrop of aging. Aberrant death of epithelia, disrupted differentiation patterns of progenitor epithelial cells, emerging populations of apoptosis-resistant myofibroblasts, and the production of an excessive extracellular matrix are some of the contributory processes that promote the loss of normal alveolar architecture. These abnormalities occur in an increased oxidative environment, represented by increases in the oxidation of GSH, enhanced S-glutathionylation, and disruptions in GLRX (Figure 3). Remarkably, the exact PSSG targets in different cell populations or the extracellular environment of the fibrotic lung remain unknown. The implications of enhanced PSSG for dying epithelial cells also require further study. In this manuscript, we addressed the importance of GLRX for epithelial cell apoptosis and the production of metabolites in apoptosis-prone epithelial cells. The exact PSSG target(s) that regulates the formation and utilization of these metabolites remain(s) to be formally tested. The implications of elevated levels of these metabolites in Glrx−/− cells, their secretion, and implications for (myo)fibroblast biology also warrant further investigation. Additional studies will also be required to assess whether other pro-fibrotic stimuli besides IL1B can regulate the aforementioned pathways and metabolites. Of note, a recent study reported that the supplementation of primary human corneal fibroblasts with arginine increased a number of metabolites, including spermidine, and was associated with increases in collagen deposition [81]. Conversely, treatment of mice with spermidine following the development of bleomycin-induced fibrosis caused decreases in alveolar epithelial cell apoptosis, and ultimately decreased inflammation and collagen deposition [82]. An intricate link exists between redox-dependent processes including S-glutathionylation, energy metabolism, and cell death, all of which are processes that are disrupted in lung fibrosis. Multi-omics-based approaches that aim at identifying oxidation-sensitive nodes that intersect these pathways in different cell populations disrupted in the fibrotic lung have great potential to shed further light on disease pathogenesis, and may yield therapeutic strategies not realized to date. With the availability of high resolution redox-mapping [83], single cell-based mass spectrometry [84], and organoid technology to expand diseased cell populations [85,86], these studies are now possible and will yield new insights into redox, metabolic, and cellular perturbations with unprecedented detail.
Figure 3.
Schematic overview of disrupted epithelial-fibroblast homeostasis in pulmonary fibrosis. Left: top—depiction of the healthy alveolus lined with alveolar type 1 (AT1) cells interspersed with alveolar type 2 (AT2) progenitor cells and sporadic fibroblasts. Bottom—homeostatic control between AT2 cells and fibroblasts. Right: top—depiction of a disrupted alveolus in pulmonary fibrosis showing loss of AT1 cells, dying AT2 cells, emerging aberrant basal cells, activated fibroblasts, and excessive deposition of extracellular matrix. Bottom—disrupted crosstalk between AT2 cells and fibroblasts in fibrosis. Enhanced FASL and NOX4 expression in fibroblasts and secretion of H2O2 promote killing of alveolar epithelial cells, while the diminished FAS expression on activated myofibroblasts conversely promotes their apoptosis-resistance. Enhanced FAS-SSG on epithelial cells promotes their death, which can be ameliorated by the deglutathionylase, GLRX. Additionally depicted is the GLRX-sensitive production of apoptosis-associated metabolites in dying alveolar epithelial cells. Future studies should address the impact of apoptosis-associated metabolites in the emergence of aberrant basal cells and myofibroblasts and the disrupted cross talk between epithelial cells and fibroblasts. This image was generated using Biorender.
Abbreviations
| AMP | adenosine monophosphate |
| AT1 | alveolar type 1 cells |
| AT2 | alveolar type 2 cells |
| BALF | bronchoalveolar lavage |
| DHAP | dihydroxyacetone phosphate |
| DISC | death-induced signaling complex |
| FBP | fructose-1,6-bisphosphate |
| G3P | glycerol-3-phosphate |
| GK | glycerol kinase |
| GLRX | glutaredoxin |
| GMP | guanosine 5′ monophosphate |
| GSH | glutathione |
| GSSG | oxidized glutathione |
| GSTP | glutathione S-transferases P |
| GR | glutathione reductase |
| IMP | inosine monophosphate |
| IMPDH | inosine monophosphate dehydrogenases |
| IPF | idiopathic pulmonary fibrosis |
| NOX | NADPH family of oxidases |
| ODC | ornithine decarboxylase |
| PDIA3 | protein disulfide isomerase A3 |
| PSSG | protein S-glutathionylation |
| SRM | spermidine synthase |
| TNF-A | tumor necrosis factor alpha |
| TGFB | transforming growth factor beta |
Conflicts of Interest
Yvonne Janssen-Heininger holds the following patents: United States Patent No. 8,679,811, “Treatments Involving Glutaredoxins and Similar Agents”; United States Patent No. 8,877,447, “Detection of Glutathionylated Proteins”; and United States Patents 9,907,828 and 10,688,150, “Treatments of oxidative stress conditions”. Yvonne Janssen-Heininger received consulting fees from Celdara Medical LLC for their contributions to the proposed commercialization of glutaredoxin for the treatment of pulmonary fibrosis.
Funding Statement
This work was supported by NIH grant R35HL135828 (Y-JH).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Forman H.J., Zhang H., Rinna A. Glutathione, Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meister A., Tate S.S. Glutathione and related gamma-glutamyl compounds, Biosynthesis and utilization. Annu. Rev. Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- 3.Wang W., Ballatori N. Endogenous glutathione conjugates, Occurrence and biological functions. Pharmacol. Rev. 1998;50:335–356. [PubMed] [Google Scholar]
- 4.Chia S.B., Elko E.A., Aboushousha R., Manuel A.M., van de Wetering C., Druso J.E., van der Velden J., Seward J.D., Anathy V., Irvin C.J., et al. Dysregulation of the glutaredoxin/S-glutathionylation redox axis in lung diseases. Am. J. Physiol. Cell Physiol. 2020;318:C304–C327. doi: 10.1152/ajpcell.00410.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/S0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 6.Giustarini D., Galvagni F., Tesei A., Farolfi A., Zanoni M., Pignatta S., Milzani A., Marone M.I., Dalle-Donne I., Nassini R., et al. Glutathione, glutathione disulfide, and S-glutathionylated proteins in cell cultures. Free Radic. Biol. Med. 2015;89:972–981. doi: 10.1016/j.freeradbiomed.2015.10.410. [DOI] [PubMed] [Google Scholar]
- 7.Montero D., Tachibana C., Winther J.R., Appenzeller-Herzog C. Intracellular glutathione pools are heterogeneously concentrated. Redox Biol. 2013;1:508–513. doi: 10.1016/j.redox.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastore A., Federici G., Bertini E., Piemonte F. Analysis of glutathione, Implication in redox and detoxification. Clin. Chim. Acta. 2003;333:19–39. doi: 10.1016/S0009-8981(03)00200-6. [DOI] [PubMed] [Google Scholar]
- 9.Lushchak V.I. Glutathione homeostasis and functions, Potential targets for medical interventions. J. Amino Acids. 2012;2012:736837. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallardó F.V., Markovic J., García J.L., Viña J. Role of nuclear glutathione as a key regulator of cell proliferation. Mol. Aspects Med. 2009;30:77–85. doi: 10.1016/j.mam.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Vivancos P., de Simone A., Kiddle G., Foyer C.H. Glutathione--linking cell proliferation to oxidative stress. Free Radic. Biol. Med. 2015;89:1154–1164. doi: 10.1016/j.freeradbiomed.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Markovic J., Mora N.J., Broseta A.M., Gimeno A., de-la-Concepción N., Viña J., Pallardó F.V. The depletion of nuclear glutathione impairs cell proliferation in 3t3 fibroblasts. PLoS ONE. 2009;4:e6413. doi: 10.1371/journal.pone.0006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen C.K. Cellular thiols and redox-regulated signal transduction. Curr. Top Cell Regul. 2000;36:1–30. doi: 10.1016/s0070-2137(01)80001-7. [DOI] [PubMed] [Google Scholar]
- 14.Wu G., Fang Y.Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 15.Pena L.R., Hill D.B., McClain C.J. Treatment with glutathione precursor decreases cytokine activity. JPEN J. Parenter Enteral Nutr. 1999;23:1–6. doi: 10.1177/014860719902300101. [DOI] [PubMed] [Google Scholar]
- 16.Alam K., Ghousunnissa S., Nair S., Valluri V.L., Mukhopadhyay S. Glutathione-redox balance regulates c-rel-driven IL-12 production in macrophages, Possible implications in antituberculosis immunotherapy. J. Immunol. 2010;184:2918–2929. doi: 10.4049/jimmunol.0900439. [DOI] [PubMed] [Google Scholar]
- 17.Aboushousha R., Elko E., Chia S.B., Manuel A.M., van de Wetering C., van der Velden J., MacPherson M., Erickson C., Reisz A.J., D’Alessandro A., et al. Glutathionylation chemistry promotes interleukin-1 beta-mediated glycolytic reprogramming and pro-inflammatory signaling in lung epithelial cells. FASEB J. 2021;35:e21525. doi: 10.1096/fj.202002687RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalinina E., Novichkova M. Glutathione in Protein Redox Modulation through S-Glutathionylation and S-Nitrosylation. Molecules. 2021;26 doi: 10.3390/molecules26020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong Y., Uys J.D., Tew K.D., Townsend D.M. S-glutathionylation: From molecular mechanisms to health outcomes. Antioxid Redox Signal. 2011;15:233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grek C.L., Zhang J., Manevich Y., Townsend D.M., Tew K.D. Causes and consequences of cysteine S-glutathionylation. J. Biol. Chem. 2013;288:26497–26504. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurd T.R., Costa N.J., Dahm C.C., Beer S.M., Brown S.E., Filipovska A., Murphy M.P. Glutathionylation of mitochondrial proteins. Antioxid Redox Signal. 2005;7:999–1010. doi: 10.1089/ars.2005.7.999. [DOI] [PubMed] [Google Scholar]
- 23.van de Wetering C., Elko E., Berg M., Schiffers C.H.J., Stylianidis V., van den Berge M., Nawijn M., Wouters E.F.M., Janssen-Heininger Y.M.W., Reynaert N.L. Glutathione S-transferases and their implications in the lung diseases asthma and chronic obstructive pulmonary disease: Early life susceptibility? Redox Biol. 2021;43:101995. doi: 10.1016/j.redox.2021.101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantin A.M., North S.L., Hubbard R.C., Crystal R.G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 25.Bargagli E., Olivieri C., Bennett D., Prasse A., Muller-Quernheim J., Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: A review. Respir Med. 2009;103:1245–1256. doi: 10.1016/j.rmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Comhair S.A., Lewis M.J., Bhathena P.R., Hammel J.P., Erzurum S.C. Increased glutathione and glutathione peroxidase in lungs of individuals with chronic beryllium disease. Am. J. Respir. Crit. Care Med. 1999;159:1824–1829. doi: 10.1164/ajrccm.159.6.9810044. [DOI] [PubMed] [Google Scholar]
- 27.Lederer D.J., Martinez F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018;379:797–798. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 28.Mora A.L., Rojas M., Pardo A., Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 2017;16:810. doi: 10.1038/nrd.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borok Z., Whitsett J.A., Bitterman P.B., Thannickal V.J., Kotton D.N., Reynolds S.D., Krasnow M.A., Bianchi D.W., Morrisey E.E., Hogan B.L., et al. Cell plasticity in lung injury and repair: Report from an NHLBI workshop, April 19–20, 2010. Proc. Am. Thorac. Soc. 2011;8:215–222. doi: 10.1513/pats.201012-067CB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willis B.C., Borok Z. TGF-beta-induced EMT: Mechanisms and implications for fibrotic lung disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 31.Doolin M.T., Smith I.M., Stroka K.M. Fibroblast to myofibroblast transition is enhanced by increased cell density. Mol. Biol. Cell. 2021;32:ar41. doi: 10.1091/mbc.E20-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenz A.G., Costabel U., Maier K.L. Oxidized BAL fluid proteins in patients with interstitial lung diseases. Eur. Respir. J. 1996;9:307–312. doi: 10.1183/09031936.96.09020307. [DOI] [PubMed] [Google Scholar]
- 33.Psathakis K., Mermigkis D., Papatheodorou G., Loukides S., Panagou P., Polychronopoulos V., Siafakas N.M., Bouros D. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur. J. Clin. Investig. 2006;36:362–367. doi: 10.1111/j.1365-2362.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- 34.Montaldo C., Cannas E., Ledda M., Rosetti L., Congiu L., Atzori L. Bronchoalveolar glutathione and nitrite/nitrate in idiopathic pulmonary fibrosis and sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:54–58. [PubMed] [Google Scholar]
- 35.Teramoto S., Fukuchi Y., Uejima Y., Shu C.Y., Orimo H. Superoxide anion formation and glutathione metabolism of blood in patients with idiopathic pulmonary fibrosis. Biochem. Mol. Med. 1995;55:66–70. doi: 10.1006/bmme.1995.1033. [DOI] [PubMed] [Google Scholar]
- 36.Veith C., Boots A.W., Idris M., van Schooten F.J., van der Vliet A. Redox Imbalance in Idiopathic Pulmonary Fibrosis, A Role for Oxidant Cross-Talk Between NADPH Oxidase Enzymes and Mitochondria. Antioxid Redox Signal. 2019;31:1092–1115. doi: 10.1089/ars.2019.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amara N., Goven D., Prost F., Muloway R., Crestani B., Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733–738. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T.R., Horowitz J.C., Pennathur S., Martinez J.F., Thannickal J.V. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carnesecchi S., Deffert C., Donati Y., Basset O., Hinz B., Preynat-Seauve O., Guichard C., Arbiser L.J., Banfi B., Pache C.J., et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal. 2011;15:607–619. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hecker L., Logsdon N.J., Kurundkar D., Kurundkar A., Bernard K., Hock T., Meldrum E., Sanders Y.Y., Thannickal J.V. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med. 2014;6:231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Artaud-Macari E., Goven D., Brayer S., Hamimi A., Besnard V., Marchal-Somme J., Ali A.E., Crestani B., Kerdine-Römer S., Boutten A., et al. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2013;18:66–79. doi: 10.1089/ars.2011.4240. [DOI] [PubMed] [Google Scholar]
- 42.Beeh K.M., Beier J., Haas I.C., Kornmann O., Micke P., Buhl R. Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2002;19:1119–1123. doi: 10.1183/09031936.02.00262402. [DOI] [PubMed] [Google Scholar]
- 43.Cantin A.M., Hubbard R.C., Crystal R.G. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- 44.Bando M., Hosono T., Mato N., Nakaya T., Yamasawa H., Ohno S., Sugiyama Y. Long-term efficacy of inhaled N-acetylcysteine in patients with idiopathic pulmonary fibrosis. Intern. Med. 2010;49:2289–2296. doi: 10.2169/internalmedicine.49.4011. [DOI] [PubMed] [Google Scholar]
- 45.Martinez F.J., de Andrade J.A., Anstrom K.J., King T.E., Jr., Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2093–2101. doi: 10.1056/NEJMoa1401739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paliogiannis P., Fois A.G., Collu C., Bandinu A., Zinellu E., Carru C., Pirina P., Mangoni A.A., Zinellu A. Oxidative stress-linked biomarkers in idiopathic pulmonary fibrosis, A systematic review and meta-analysis. Biomark Med. 2018;12:1175–1184. doi: 10.2217/bmm-2018-0108. [DOI] [PubMed] [Google Scholar]
- 47.Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc. Natl. Acad. Sci. USA. 1976;73:2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mieyal J.J., Starke D.W., Gravina S.A., Hocevar B.A. Thioltransferase in human red blood cells: Kinetics and equilibrium. Biochemistry. 1991;30:8883–8891. doi: 10.1021/bi00100a023. [DOI] [PubMed] [Google Scholar]
- 49.Xiao W., Wang R.S., Handy D.E., Loscalzo J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid Redox Signal. 2018;28:251–272. doi: 10.1089/ars.2017.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandes A.P., Holmgren A. Glutaredoxins, Glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 51.Ho Y.S., Xiong Y., Ho D.S., Gao J., Chua B.H., Pai H., Mieyal J.J. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic. Biol. Med. 2007;43:1299–1312. doi: 10.1016/j.freeradbiomed.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shao D., Han J., Hou X., Fry J., Behring J.B., Seta F., Long T.M., Roy K.H., Cohen A.R., Matsui R., et al. Glutaredoxin-1 Deficiency Causes Fatty Liver and Dyslipidemia by Inhibiting Sirtuin-1. Antioxid Redox Signal. 2017;27:313–327. doi: 10.1089/ars.2016.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorelenkova Miller O., Behring J.B., Siedlak S.L., Jiang S., Matsui R., Bachschmid M.M., Zhu X.W., Mieyal J.J. Upregulation of Glutaredoxin-1 Activates Microglia and Promotes Neurodegeneration: Implications for Parkinson’s Disease. Antioxid Redox Signal. 2016;25:967–982. doi: 10.1089/ars.2015.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murdoch C.E., Shuler M., Haeussler D.J., Kikuchi R., Bearelly P., Han J., Watanabe Y., Fuster J.J., Walsh K., Ho Y.S., et al. Glutaredoxin-1 up-regulation induces soluble vascular endothelial growth factor receptor 1, attenuating post-ischemia limb revascularization. J. Biol. Chem. 2014;289:8633–8644. doi: 10.1074/jbc.M113.517219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahn Y.J., Wang L., Tavakoli S., Nguyen H.N., Short J.D., Asmis R. Glutaredoxin 1 controls monocyte reprogramming during nutrient stress and protects mice against obesity and atherosclerosis in a sex-specific manner. Nat. Commun. 2022;13:790. doi: 10.1038/s41467-022-28433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsui R., Ferran B., Oh A., Croteau D., Shao D., Han J., Pimentel D.R., Bachschmid M.M. Redox Regulation via Glutaredoxin-1 and Protein S-Glutathionylation. Antioxid Redox Signal. 2020;32:677–700. doi: 10.1089/ars.2019.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anathy V., Lahue K.G., Chapman D.G., Chia S.B., Casey D.T., Aboushousha R., van der Velden J.L., Elko E., Hoffman S.M., McMillan D.H., et al. Reducing protein oxidation reverses lung fibrosis. Nature Med. 2018;24:1128–1135. doi: 10.1038/s41591-018-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matute-Bello G., Wurfel M.M., Lee J.S., Park D.R., Frevert C.W., Madtes D.K., Shapiro S.D., Martin T.R. Essential role of MMP-12 in Fas-induced lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2007;37:210–221. doi: 10.1165/rcmb.2006-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuwano K., Kunitake R., Maeyama T., Hagimoto N., Kawasaki M., Matsuba T., Yoshimi M., Inoshima T., Yoshida K., Hara N. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L316–L325. doi: 10.1152/ajplung.2001.280.2.L316. [DOI] [PubMed] [Google Scholar]
- 60.Lee C.G., Kang H.R., Homer R.J., Chupp G., Elias J.A. Transgenic modeling of transforming growth factor-beta (1): Role of apoptosis in fibrosis and alveolar remodeling. Proc. Am. Thorac. Soc. 2006;3:418–423. doi: 10.1513/pats.200602-017AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sisson T.H., Mendez M., Choi K., Subbotina N., Courey A., Cunningham A., Dave A., Engelhardt J.F., Liu X., White E.S., et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perl A.K., Riethmacher D., Whitsett J.A. Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis. Am. J. Respir. Crit. Care Med. 2011;183:511–521. doi: 10.1164/rccm.201005-0744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D’Arcy M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 64.Hagimoto N., Kuwano K., Miyazaki H., Kunitake R., Fujita M., Kawasaki M., Kaneko Y., Hara N. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am. J. Respir. Cell Mol. Biol. 1997;17:272–278. doi: 10.1165/ajrcmb.17.3.2893. [DOI] [PubMed] [Google Scholar]
- 65.Wallach-Dayan S.B., Golan-Gerstl R., Breuer R. Evasion of myofibroblasts from immune surveillance, A mechanism for tissue fibrosis. Proc. Natl. Acad. Sci. USA. 2007;104:20460–20465. doi: 10.1073/pnas.0705582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuwano K., Hagimoto N., Kawasaki M., Yatomi T., Nakamura N., Nagata S., Suda T., Kunitake R., Maeyama T., Miyazaki H., et al. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J. Clin. Investig. 1999;104:13–19. doi: 10.1172/JCI5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Golan-Gerst l.R., Wallach-Dayan S.B., Zisman P., Cardoso W.V., Goldstein R.H., Breuer R. Cellular FLICE-like inhibitory protein deviates myofibroblast fas-induced apoptosis toward proliferation during lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2012;47:271–279. doi: 10.1165/rcmb.2010-0284RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wynes M.W., Edelman B.L., Kostyk A.G., Edwards M.G., Coldren C., Groshong S.D., Cosgrove G.P., Redente E.F., Bamberg A., Brown K.K., et al. Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis, Implications for fibroblast accumulation in idiopathic pulmonary fibrosis. J. Immunol. 2011;187:527–537. doi: 10.4049/jimmunol.1100447. [DOI] [PubMed] [Google Scholar]
- 69.Redente E.F., Chakraborty S., Sajuthi S., Black B.P., Edelman B.L., Seibold M.A., Riches D.W. Loss of Fas signaling in fibroblasts impairs homeostatic fibrosis resolution and promotes persistent pulmonary fibrosis. JCI Insight. 2020;6 doi: 10.1172/jci.insight.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waghray M., Cui Z., Horowitz J.C., Subramanian I.M., Martinez F.J., Toews G.B., Thannickal V.J. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. Faseb j. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 71.Shi Z.Z., Osei-Frimpong J., Kala G., Kala S.V., Barrios R.J., Habib G.M., Lukin D.J., Danney C.M., Matzuk M.M., Lieberman M.W. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc. Natl. Acad. Sci. USA. 2000;97:5101–5106. doi: 10.1073/pnas.97.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Franco R., Cidlowski J.A. Apoptosis and glutathione, Beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 73.Armstrong J.S., Whiteman M., Yang H., Jones D.P., Sternberg P., Jr. Cysteine starvation activates the redox-dependent mitochondrial permeability transition in retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2004;45:4183–4189. doi: 10.1167/iovs.04-0570. [DOI] [PubMed] [Google Scholar]
- 74.Cazanave S., Berson A., Haouzi D., Vadrot N., Fau D., Grodet A., Lettéron P., Feldmann G., El-Benna J., Fromenty B., et al. High hepatic glutathione stores alleviate Fas-induced apoptosis in mice. J. Hepatol. 2007;46:858–868. doi: 10.1016/j.jhep.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 75.Friesen C., Kiess Y., Debatin K.M. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ. 2004;11 Suppl 1:S73–S85. doi: 10.1038/sj.cdd.4401431. [DOI] [PubMed] [Google Scholar]
- 76.Anathy V., Aesif S.W., Guala A.S., Havermans M., Reynaert N.L., Ho Y.S., Budd R.C., Janssen-Heininger Y.M. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J. Cell Biol. 2009;184:241–252. doi: 10.1083/jcb.200807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McMillan D.H., van der Velden J.L., Lahue K.G., Qian X., Schneider R.W., Iberg M.S., Nolin J.D., Abdalla S., Casey D.T., Tew K.D., et al. Attenuation of lung fibrosis in mice with a clinically relevant inhibitor of glutathione-S-transferase π. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar A., Elko E., Bruno S.R., Mark Z.F., Chamberlain N., Mihavics B.K., Chandrasekaran R., Walzer J., Ruban M., Gold C., et al. Inhibition of PDIA3 in club cells attenuates osteopontin production and lung fibrosis. Thorax. 2022;77:669–678. doi: 10.1136/thoraxjnl-2021-216882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan S., Berk B.C. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis, Key role for glutaredoxin in the death pathway. Circ. Res. 2007;100:213–219. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- 80.Medina C.B., Mehrotra P., Arandjelovic S., Perry J.S.A., Guo Y., Morioka S., Barron B., Walk S.F., Ghesquière B., Krupnick S.A., et al. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020;580:130–135. doi: 10.1038/s41586-020-2121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McKay T.B., Priyadarsini S., Rowsey T., Karamichos D. Arginine Supplementation Promotes Extracellular Matrix and Metabolic Changes in Keratoconus. Cells. 2021:10. doi: 10.3390/cells10082076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baek A.R., Hong J., Song K.S., Jang A.S., Kim D.J., Chin S.S., Park S.W. Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)-induced cell death in mice. Exp. Mol. Med. 2020;52:2034–2045. doi: 10.1038/s12276-020-00545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao H., Jedrychowski M.P., Schweppe D.K., Huttlin E.L., Yu Q., Heppner D.E., Li J., Long J., Mills E.L., Szpyt J., et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell. 2020;180:968–983. doi: 10.1016/j.cell.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tracey L.J., An Y., Justice M.J. CyTOF, An Emerging Technology for Single–Cell Proteomics in the Mouse. Curr. Protoc. 2021;1:e118. doi: 10.1002/cpz1.118. [DOI] [PubMed] [Google Scholar]
- 85.Li F., Zhang P., Wu S., Yuan L., Liu Z. Advance in Human Epithelial-Derived Organoids Research. Mol. Pharm. 2021;18:3931–3950. doi: 10.1021/acs.molpharmaceut.1c00452. [DOI] [PubMed] [Google Scholar]
- 86.Kong J., Wen S., Cao W., Yue P., Xu X., Zhang Y., Luo L., Chen T., Li L., Wang F., et al. Lung organoids, useful tools for investigating epithelial repair after lung injury. Stem. Cell Res. Ther. 2021;12:95. doi: 10.1186/s13287-021-02172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]