Abstract
Neisserial lipooligosaccharides (LOSs) are a family of complex cell surface glycolipids. We used mass spectrometry techniques (electrospray ionization, collision-induced dissociation, and multiple step), combined with fluorophore-assisted carbohydrate electrophoresis monosaccharide composition analysis, to determine the structure of the two low-molecular-mass LOS molecules (LOSI and LOSII) expressed by Neisseria subflava 44. We determined that LOSI contains one glucose on both the α and β chains. LOSII is structurally related to LOSI and differs from it by the addition of a hexose (either glucose or galactose) on the α chain. LOSI and LOSII were able to bind monoclonal antibody (MAb) 25-1-LC1 when analyzed by Western blotting experiments. We used a set of genetically defined Neisseria gonorrhoeae mutants that expressed single defined LOS epitopes and a group of Neisseria meningitidis strains that expresses chemically defined LOS components to determine the structures recognized by MAb 25-1-LC1. We found that extensions onto the β-chain glucose of LOSI block the recognition by this MAb, as does further elongation from the LOSII α chain. The LOSI structure was determined to be the minimum structure that is recognized by MAb 25-1-LC1.
Neisseria gonorrhoeae and Neisseria meningitidis are important human pathogens. Although there are many more cases of gonorrhea than meningococcal meningitis, meningitis is a much more serious disease due to its associated mortality. The importance of lipooligosaccharide (LOS) in the pathogenesis and immunobiology of these microbes is well established (1, 2, 9, 16, 19, 23, 41). LOSs are a family of complex glycolipid molecules found on the outer surfaces of the outer membranes of gram-negative bacteria (1, 2, 9, 12, 16, 19, 22, 41, 53). They possess many antigenic determinants that are important in natural and acquired immunity (24, 37, 51, 52). In recent years, biologists have focused their efforts on the study of LOS as a potential vaccine candidate (5, 19, 20).
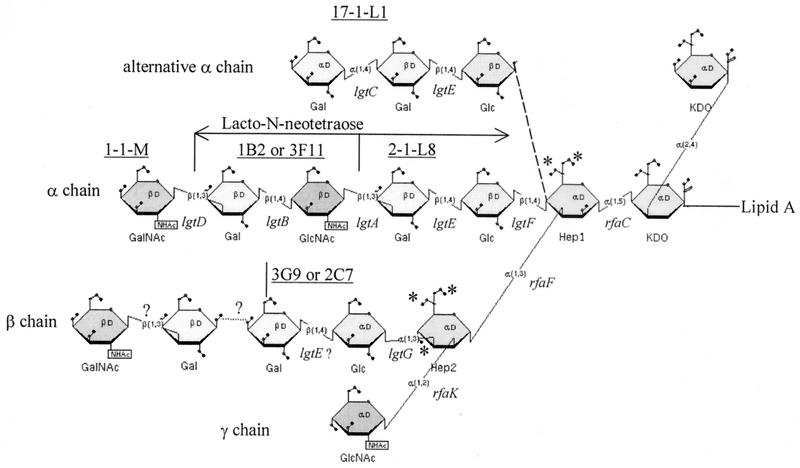
Gonococcal and meningococcal LOSs have been examined by chemical (11, 18, 22), biological (17), and immunological techniques (28, 42), as well as through visualization by silver staining sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (26, 43). LOS is an amphipathic molecule that consists of a hydrophilic carbohydrate moiety attached to a hydrophobic lipid A moiety through two molecules of the acidic sugar 3-deoxy-d-manno-2-octulosonic acid (KDO). The oligosaccharide (OS) is multiantenniary and branches at two basal heptose (Hep) residues, forming three elongation centers defined as the α, β, and γ chains (11, 22, 33, 50) (Fig. 1). The α chain elongates from Hep I and may contain several structures that are mimics of human carbohydrate epitopes (1, 30). β-Chain (extending from Hep II) expression is modulated by the expression of lgtG (4), and the β chain may be composed of a single glucose (Glc) or lactose, or Glc with additional sugars (12, 51). The γ chain has been found in all strains examined and consists of a GlcNAc or GlcNAc (acetate) linked to Hep II. Occasionally, this chain is elongated by the addition of galactose (15). Some positions of Hep I and Hep II are also available for phosphoethanolamine (PEA) or phosphate addition (10, 29). Most of the genes that mediate gonococcal and meningococcal LOS biosynthesis have been cloned and characterized (4, 7, 13, 14, 25, 27, 38, 40, 45, 54).
FIG. 1.
Composite structure of N. gonorrhoeae LOS. Dotted lines, alternative LOS structures. MAb reactivities are underlined. Genes involved in LOS biosynthesis are indicated. ∗, possible site to which PEA or phosphate can be added. The broken line indicates the addition of an alternate α chain, joined to HepI as a β1-4 linkage.
Monoclonal antibody (MAb) 1B2 binds to gonococcal LOSs that possess terminal lactosamine residues (16, 23); these LOSs are generally considered to be high-molecular-mass LOS molecules. Neisseria subflava 44 cells are able to react in colony blotting experiments with MAb 1B2. However, N. subflava 44 was found to only express low-molecular-mass LOS components when its LOS was analyzed by SDS-PAGE (D. C. Stein, unpublished observations). This suggested that other structural motifs could bind MAb 1B2. We obtained MAb 25-1-LC1, which reacts with several neisserial LOSs with different immunotypes. This suggested that this MAb binds to a common core epitope. Our studies indicated that N. subflava 44 LOS can bind this MAb very strongly, indicating that this strain's LOS would be a good candidate for determining the specificity of the MAb. This study was undertaken to define the LOS structure expressed by N. subflava 44, identify structures recognized by MAb 25-1-LC1, and determine the nature of this organism's ability to bind MAb 1B2.
MATERIALS AND METHODS
Bacterial strains, plasmids, oligonucleotides, and culture conditions.
All bacterial strains used in this study are listed in Table 1. All Neisseria strains were grown in phosphate-buffered gonococcal broth (39) plus growth supplements (49) and 0.042% sodium bicarbonate or on gonococcal agar base (Difco) plates at 37°C in a CO2 incubator.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant features | Reference or source |
|---|---|---|
| N. meningitidis | ||
| 35E | L2 LOS | 35 |
| M981 | L5 LOS | 29 |
| 8032 | Nontypeable LOS | W. Zollinger |
| N. subflava 44 | H. Feldmana | |
| N. gonorrhoeae | ||
| F62 | Full α chain, no β chain; LOS reacts with MAbs 1B2 and 1-1-M | 47, 50 |
| F62 ΔlgtA | 239-bp ApoI deletion in lgtA; truncated lactosyl α chain; LOS reacts with MAb 2-1-L8 | 4 |
| F62 ΔlgtA ΔlgtE | 622-bp BspEI and AgeI deletion in lgtE of F62 ΔlgtA; only Glc in α chain; LOS reacts with MAb 25-1-LC1 | 3 |
| F62 ΔlgtA ΔlgtF | 240-bp BsiWI and BsrGI deletion in lgtF of F62 ΔlgtA; lacks α and β chains; LOS reacts with MAb B5 | 3 |
| F62 ΔlgtA ΔlgtF ΔrfaK | 459-bp DraI deletion of F62 ΔlgtA ΔlgtF; lacks α, β, and γ chains | 3 |
| F62 ΔlgtA lgtG+ | lgtG fixed in expression state; lactose in both α and β chains; LOS reacts with MAb 3G9 | 3 |
| F62 ΔlgtA ΔlgtE lgtG+ | 622-bp BspEI and AgeI deletion in lgtE of F62ΔlgtAlgtG+; α and β chains contain only one Glc, LOS reacts with MAbs 25-1-LC1 | 3 |
| F62 ΔlgtA ΔlgtF lgtG+ | 240-bp BsiWI and BsrGI deletion in lgtF of F62 ΔlgtA lgtG+; lacks α and β chains; LOS reacts with MAb B5 | 3 |
| F62 ΔlgtD | lgtD gene fixed off; only expresses lacto-N-neotetraose LOS | 44 |
| 15253 lgtE | 15253 lgtE mutant; α and β chains contain only one Glc; LOS reacts with MAb 25-1-LC1 | 4 |
Albany Medical Center, Albany, N.Y.
Chemicals and reagents.
All chemicals used for this study were reagent grade or better and were purchased from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise specified. Tris-Tricine gels (16.5%) and running buffer were obtained from Bio-Rad Laboratories (Richmond, Calif.). The fluorophore-assisted carbohydrate electrophoresis (FACE) monosaccharide composition analysis kit was from Glyco Inc. (Novato, Calif.). Acetic acid was from Fisher Scientific Co. (Silver Spring, Md.). MAbs 25-1-LC1 and 2-1-L8 were made in the laboratory of Wendell Zollinger, Walter Reed Army Institute of Research, Washington, D.C. MAb B5 was a gift from Margaret A. Gidney, Institute for Biological Sciences, National Research Council, Ottawa, Canada.
LOS purification and SDS-PAGE analysis.
LOSs were purified from acetone-powdered organisms by the hot phenol-water method (48). Proteinase K-treated whole-cell lysates were prepared from 18- to 20-h cultures by the procedure of Hitchcock and Brown (21). Approximately 0.1 μg of LOS was subjected to SDS-PAGE on a 16.5% Tris-Tricine gel in Tris-Tricine running buffer in accordance with the protocol suggested by manufacturer. The gel was fixed overnight in 40% ethanol–5% acetic acid, and the LOS was visualized by silver staining (46).
MS analysis of LOS structure.
LOS was subject to mild acid hydrolysis, extraction, and methylation (6) to produce samples for characterization by mass spectrometry (MS). The methods used in this study were described previously (31).
Preparation of MAb 25-1-LC1.
BALB/c mice were immunized intraperitoneally at weeks 0 and 3 with a saline suspension (0.1 ml/mouse) of N. meningitidis containing an equal mixture of strain M981 (L5) and strain 8032 (L nontypeable). The suspension contained approximately 105 live bacteria per ml. At weeks 5, 7, and 10, the mice were immunized intraperitoneally with a saline solution of LOS (200 μg/ml) prepared from strains M981 and 8032 (0.1 ml/mouse). Spleens were harvested 3 days after the final immunization, and lymphocytes were fused with P3X63-Ag 8.653 mouse myeloma cells at a ratio of 4:1, as previously described (32). Positive clones were selected by an enzyme-linked immunosorbent assay using plates coated with M981 LOS or 8032 LOS. One clone, producing MAb 25-1-LC1, was selected for further analysis. Western blot analysis was used to confirm the binding of the MAb to one of the LOS bands expressed by M981 LOS. Ascitic fluid was produced by injecting 5 × 106 hybridoma cells into pristine-primed BALB/c mice. Ascitic fluid was collected, and aliquots were stored at −20°C.
FACE monosaccharide composition analysis.
Purified LOS (∼5 μg) was hydrolyzed in 1% acetic acid for 2 h at 80°C. The hydrolysate was centrifuged (12,000 × g, 20 min), and the supernatant containing the OSs was collected. For sugar composition analysis, the OS was treated in accordance with the procedure provided by Glyko Inc., with the only difference being that the OS was hydrolyzed with 4 N HCl for 2 h instead of with 2 N trifluoroacetic acid for 5 h.
Western blot and colony blot analyses.
After SDS-PAGE, LOSs were electrotransferred onto an Immobilon-P membrane (Millipore Corp., Bedford, Mass.) in a Tris-glycine-methanol buffer (0.025 M Tris, 0.192 M glycine, 20% methanol) at a constant voltage of 100 V for 1 h in accordance with the protocol provided by Bio-Rad Corp. After being air dried for 1 h, the membrane was processed by the same procedure as that used for colony bloting, which was described previously (39).
RESULTS
SDS-PAGE profile of N. subflava 44.
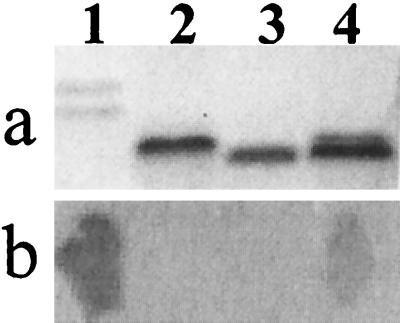
N. gonorrhoeae strain F62 expresses two predominant LOS components that can be visualized based on their different electrophoretic mobilities on SDS-PAGE gels. The data presented in Fig. 2A show the typical electrophoretic pattern obtained with LOS isolated from this strain. The chemical structures of these two components have been determined (50), and it is the faster-migrating component that binds MAb 1B2. Derivatives of F62 that lack the ability to make this LOS component have been constructed (3), and they produce truncated LOS molecules (Fig. 2A, lanes 2 and 3). N. subflava produced two small low-molecular-mass LOS components (Fig. 2A, lane 4).
FIG. 2.
SDS-PAGE profiles and colony blot analysis of various Neisseria strains. LOS samples from different Neisseria strains were analyzed on a Tris-Tricine gel, and their reactivities with MAb 1B2 were detected with a colony blot of a duplicate gel. SDS-PAGE gel; (b) colony blot with MAb 1B2. Lanes: 1, N. gonorrhoeae F62; 2, N. gonorrhoeae F62 ΔlgtA; 3, N. gonorrhoeae F62 ΔlgtA ΔlgtE; 4, N. subflava 44.
When the gonococcal cells were analyzed by colony-blotting experiments with MAb 1B2, only F62 cells gave a detectable signal (Fig. 2B, lanes 1 to 3). While LOS isolated from N. subflava 44 possessed SDS-PAGE mobilities similar to those of the genetically defined structural derivatives of F62 (F62 ΔlgtA ΔlgtE and F62 ΔlgtA), N. subflava 44 cells were able to bind MAb 1B2 in a colony-blotting experiment (Fig. 2B). When LOS was purified and analyzed by Western blotting of SDS-PAGE gels, only LOS isolated from F62 was able to bind MAb 1B2 (data not shown). These data suggested that LOS isolated from N. subflava 44, while possessing some structural similarities to that from F62, as evidenced by its ability to bind MAb 1B2, was structurally different.
Characterization of N. subflava 44 LOS structures by MS.
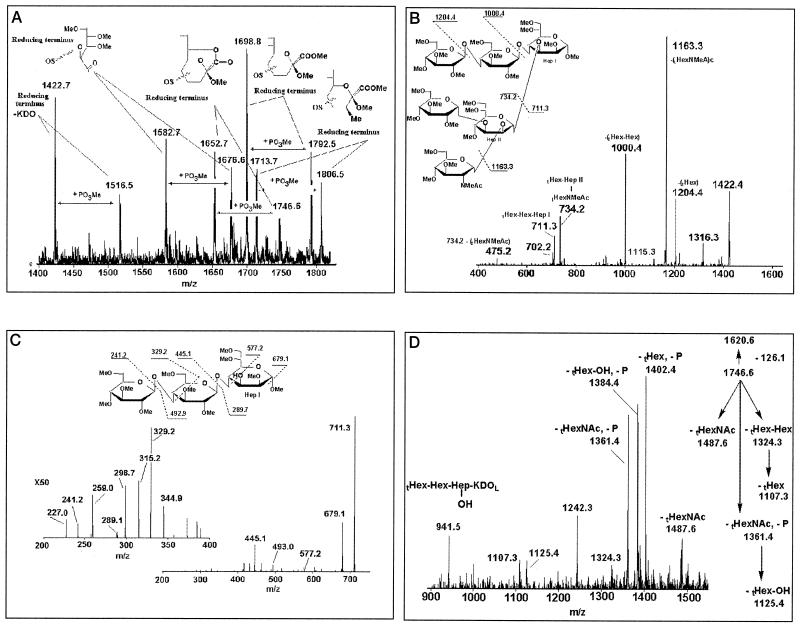
In order to determine why N. subflava 44 could bind MAb 1B2 when analyzed on a colony blot yet failed to bind this MAb when analyzed on a Western blot, we used MS to determine the structure of the LOS expressed by N. subflava 44. LOS was subjected to mild-acid hydrolysis, extraction, and methylation to produce samples for characterization by MS. Since the release and derivatization chemistry modifies the nascent reducing terminal KDO by adding chemical artifact peaks to the mass spectrum, additional m/z peaks arise from the multiple charge states commonly observed in electrospray ionization. In the study of N. subflava 44 OS, the variations were of two types: phosphorylation of the Hep II moiety and addition of monomers to the nonreducing termini of the α and β chains (glycoform distributions). MS profiles allowed us to identify the natural abundance of parent structures by the summed intensity of the associated peaks, and this measured abundance was consistent across multiple isolations and sample preparations. In the single-charge range of the spectrum (Fig. 3A, m/z 1,400 to 1,800), five principal ions were observed and each was paired with a satellite ion due to phosphorylation (94 Da; plus PO3Me). The five principal OS fragments are structurally related and differ only by the modification or elimination of the KDO. The KDO analogs and their phosphorylated satellite peaks were identified as follows: KDO methyl ketoside methylester, m/z 1,698.9/1,792.6; KDO lactone, m/z 1,652.7/1,746.6; KDO with acetone eliminated, m/z 1,582.7/1,676.5; KDO methyl addition product, m/z 1,713.7/1,806.6. A final pair of ions suggests that the OS has completely lost its terminal KDO moiety (m/z 1,422.7/1,516.6) (Fig. 3A). While this spread of signal across multiple peaks did diminish sensitivity, we were able to demonstrate a structural relationship when collision-induced spectra were compared (data not shown).
FIG. 3.
MS analysis of N. subflava 44 LOS structure. (A) MS profile with a structural representation of the reducing-end KDO. The structural assignment was based on the mass difference between fragments and MSn analysis of each product. (B) MS2 characterization of OS lacking a KDO (m/z 1,422.4). Methylation differentiates terminal and branched residues, identifying overall topology and glycosidic sequence. (C) MS3 analysis of the α-chain product (m/z 711.3). Linkage assignment from resonance activation of smaller-mass products provides enhanced structural detail. (D) MS2 analysis showing more-informative fragments of minor abundance that provide localization of the phosphorylated residue, Hep II.
The collision spectra of the five principal peaks (Fig. 3A; m/z 1,422.7, 1,582.7, 1,652.7, 1,698.8, and 1,713.7) and those of the peaks for the associated phosphorylated satellite ions indicated a common structural motif. That motif was clearly identified by MS2 analysis of the ion lacking a KDO moiety, m/z 1,422.7 (Fig. 3B). The loss of two nonreducing terminal residues (terminal HexNAc [tHexNAc] and terminal hexose [tHex]), the loss of a tHex disaccharide (tHex-Hex), and the elimination of a terminal trisaccharide (tHexNAc-Hex-Hep) from the parent ion allowed us to define the core topology for the α and β chains of this hexasaccharide, (Fig. 3B). The structure was further clarified by studying the fragments arising from the rupture between Hep I and Hep II, separating the α-chain fragment from the β-chain fragment (represented in peaks of m/z 711.3 and 734.2, respectively). Selection and resonance activation of the α-chain fragment, m/z 711.3, provided a terminal relationship (tHex-Hex m/z 241.2/445.1), as well as linkage information for this trisaccharide (Fig. 3C). The increments of +88 and +132 Da are due to the respective sequence fragments defined by cross-ring cleavages (m/z 329.2 and 577.2), indicating that the linkage between each monomer is (1–4) (36). Selection and activation of the alternate Hep-Hep rupture fragment, m/z 734.2, provided detailed characterization of this trisaccharide, (data not shown), indicating that the β chain contained a single Hex and that the γ chain contained a single HexNAc.
The replacement of protons on a phosphoryl group (R-OPO3H2) during methylation provided increments in the mass of the residue of 94 Da. Collisional activation of these analogs causes elimination, (−126 Da), leaving an unsaturated OS product. Even though this appears to be the most favored path to disassembly, other less-abundant fragments were also observed, allowing us to localize the original phosphate site. Thus, phosphorylation on the Hep II moiety of the OS lacking KDO (m/z 1,516.6), as well as on those of the other KDO analogs, was indicated. With KDO as a lactone (m/z 1,652.7), phosphorylation was indicated by the 94-Da increment (m/z 1,746.6), and MS2 (two-step) analysis of this product indicated a single major elimination fragment (m/z 1,620.5; data not shown). Amplification of the mass range between m/z 900 and 1,500 in this spectrum showed a series of nonreducing terminal losses (tHex, tHexNAc, and tHex-Hex), which were not phosphorylated and which, by their differences, indicate residue location (Fig. 3D). The fragment at m/z 941.5 indicates that phosphorylation occurs in the Hep II portion of the OS, and the sequential loss of the other terminal moieties places the site on Hep II. While the exact carbon on Hep II could not be ascertained from these studies, its identification as C-4 cannot be supported by these spectra.
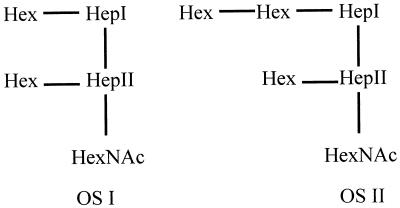
While MS studies indicated that there were two major components found in N. subflava 44 LOS, several larger components were present in LOS purified from this organism, but they were present in quantities too small to allow for their structural determination (data not shown). The structure described in Fig. 4 provides the carbon backbone for the largest molecule and has been named LOSII. Hep I is linked through KDO to lipid A. The α chain consisted of a Hex disaccharide; a Hex monosaccharide accounted for the β chain. Approximately one-third of this OS was phosphorylated. Further study showed the presence of a component that possessed a single Hex on the α chain (named LOSI).
FIG. 4.
Structural schematic of OSs produced by N. subflava 44. The carbohydrate backbones of the two predominant OSs expressed are depicted. Both OSs possess α, β, and γ chains analogous to those seen in N. gonorrhoeae and N. meningitidis.
FACE monosaccharide composition analysis of N. subflava 44 LOS.
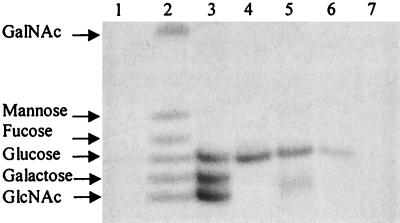
MS analysis allowed us to define the chemical backbone of the LOS components expressed by N. subflava 44. However, this type of analysis did not allow us to define the nature of the Hex and N-acetylhexosamine contained within this LOS. We identified the composition of the sugars contained in these structures by FACE monosaccharide composition analysis. N. subflava 44 LOS was hydrolyzed to individual sugars by treatment with HCl. Under these conditions, KDO is destroyed and N-acetyl groups are extracted from sugars containing this modification. OS samples were re-N-acetylated by acetic anhydride in sodium bicarbonate buffer prior to analysis on FACE gels. Figure 5 shows the results of monosaccharide composition analysis of LOS isolated from N. subflava 44. Since this methodology is able to detect ∼5-ng amounts of sugars, it is necessary to include in these analyses reagent controls, as some commercial sources of reagents have detectable levels of glucose in them. The absence of any detectable signal in lanes 1 (reagent control; 1% acetic acid) and 7 (high-pressure liquid chromatography water) demonstrated that all of the signal obtained was derived from the LOS sample.
FIG. 5.
FACE monosaccharide composition analysis of LOSs. Lanes: 1, 1% acetic acid; 2, MONO ladder standard 2 (100 pmol of each monosaccharide); 3, N. gonorrhoeae F62 ΔlgtD LOS; 4, N. subflava 44 LOS; 5, N. gonorrhoeae F62 ΔlgtA ΔlgtE LOS; 6, N. gonorrhoeae F62 ΔlgtA ΔlgtF lgtG+ LOS; 7, blank control (high-pressure liquid chromatography-grade H2O).
N. gonorrhoeae F62 ΔlgtD contains glucose, galactose, and N-acetylglucosamine in a molar ratio of 1:2:2. The data presented in Fig. 5, lane 3, indicated that FACE analysis was able to detect the presence of all three of these sugars in LOS isolated from this strain. FACE analysis was also able to correctly detect the presence of the constituents found in LOSs isolated from F62 ΔlgtA ΔlgtE and F62 ΔlgtA ΔlgtF lgtG+ (Fig. 5, lanes 5 and 6). The amount of N-acetylglucosamine detected in each of these LOSs was less than expected. We have determined that the γ-chain N-acetylglucosamine is underrepresented under the hydrolysis conditions employed (data not shown).
FACE analysis indicated that the predominant sugar contained in N. subflava 44 is glucose (Fig. 5, lane 4). We were able to detect a very small amount galactose when larger quantities of LOS were examined. Likewise we were able to detect N-acetylglucosamine (data not shown). Since LOSI possessed one Hex on both the α and β chains and one N-acetylhexosamine in the γ chain and since LOSII possesses one more Hex on the α chain than does LOSI, we have concluded that both Hex's of LOSI are glucose and that LOSII is a disaccharide containing either Glc or Gal as the terminal Hex on the α chain.
Characterization of structures recognized by MAb 25-1-LC1.
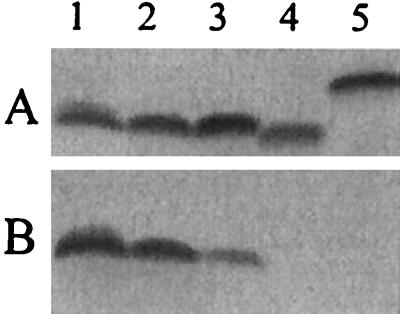
We have previously determined that MAb 25-1-LC1 reacted quite strongly with LOS expressed by N. subflava 44. In order to define the structures found in this LOS that are responsible for its binding, the SDS-PAGE and MAb binding profiles of LOS isolated from N. subflava were compared to the SDS-PAGE and MAb binding profiles obtained from various strains of N. gonorrhoeae whose LOSs has been structurally modified by genetic means (3). The data indicated that LOS with glucose on both the α and β chains bound MAb 25-1-LC1 (i.e., LOS isolated from N. gonorrhoeae 15253 lgtE and N. gonorrhoeae F62 ΔlgtA ΔlgtE lgtG+ (Fig. 6, lanes 2 and 3). However, LOS isolated from N. gonorrhoeae F62 ΔlgtA ΔlgtF lgtG+ failed to bind this MAb (Fig. 6, lane 4). These data indicate that when the α- and β-chain glucoses are present, LOS can bind MAb 25-1-LC1.
FIG. 6.
SDS-PAGE and Western blot analysis of LOS isolated from various neisserial species. (A) Silver stain of an SDS-PAGE gel. (B) Western blot of a duplicate gel obtained with MAb 25-1-LC1. Lanes: 1, N. subflava 44; 2, N. gonorrhoeae 15253 lgtE; 3, N. gonorrhoeae F62 ΔlgtA ΔlgtE lgtG+; 4, N. gonorrhoeae F62 ΔlgtA ΔlgtF lgtG+; 5, N. gonorrhoeae F62 ΔlgtA lgtG+.
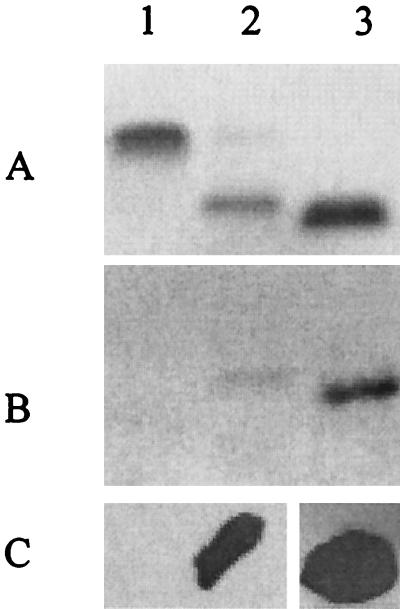
To further characterize the epitope recognized by MAb 25-1-LC1, we wished to determine if other additions to and/or modifications of the α chain might affect the ability of this MAb to bind to LOS. While N. meningitidis 35E (L2) LOS and N. meningitidis M981 (L5) LOS both possess a single glucose in the β chain, they differ in the structures of their α chains. L2 LOS possesses Galβ1–4GlcNAcβ1–3Galβ1–4Glc in the α chain, while L5 LOS possesses Galβ1–4GlcNAcβ1–3Galβ1–4Glcβ1–4Glc in the α chain (10, 29). LOS isolated from these strains was examined by SDS-PAGE and Western blot analysis using MAb 25-1-LC1. The data presented in Fig. 7 show that the high-molecular-mass LOS isolated from L2 and L5 strains failed to bind MAb 25-1-LC1. This indicates that further elongation onto the α-chain lactose blocks the epitope recognized by MAb 25-1-LC1. The data in Fig. 7 also show that the smaller LOS expressed by L5, which contains two glucoses in the α chain (29), reacts with MAb 25-1-LC1. This indicated that the presence of the second glucose in the α chain, like the presence of galactose, does not interfere with the ability of MAb 25-1-LC1 to bind this LOS.
FIG. 7.
SDS-PAGE and Western blot analysis of LOS. (A) Silver stain; (B) Western blot with MAb 25-1-LC1; (C) colony blot with MAb 25-1-LC1. Lanes: 1, N. meningitidis 35E (L2); 2, N. meningitidis M981 (L5); 3, N. subflava 44 LOS.
Requirement of the first glucose of the α chain for β-chain extensions.
The experiments described above demonstrate that MAb 25-1-LC1 is able to bind neisserial LOS when the α chain contains a glucose or a sucrose. In order to determine if any α-chain sugars were required for MAb 25-1-LC1 binding, we tested LOS isolated from F62 ΔlgtA ΔlgtF lgtG+ for its ability to bind MAb 25-1-LC1. The data in Fig. 6, lane 4, show that F62 ΔlgtA ΔlgtF lgtG+ LOS failed to bind MAb 25-1-LC1.
However, when the α chain is deleted, it is possible that the β chain may not be added because of a biosynthetic requirement for the presence of at least the glucose of the α chain before the first β-chain glycosyltransferase can act. Furthermore, in the absence of the addition of the β-chain glucose, a PEA can be variably added. Plested et al. (34) described MAb B5, which requires PEA on the 3 position of Hep II for antibody recognition. If F62 ΔlgtA ΔlgtF lgtG+ had a glucose instead of a PEA on the β chain, LOS isolated from this strain should now bind this MAb. The data in Fig. 8 show that F62 ΔlgtA ΔlgtF lgtG+ could bind MAb B5. This indicates that in N. gonorrhoeae, when lgtF was defective, no β-chain extension by LgtG occurred. A similar phenomenon was also found in N. meningitidis (25). This demonstrates that the α-chain glucose, added by LgtF, is needed before LgtG (which is constitutively expressed in this strain) can begin β-chain elongation.
FIG. 8.
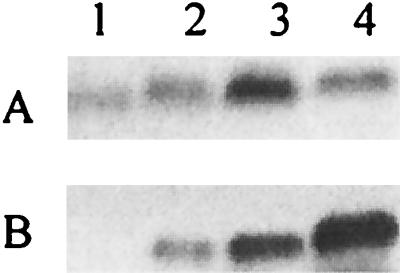
SDS-PAGE and Western blot analysis of LOS. (A) Silver stain; (B) Western blot with MAb B5. Lanes: 1, N. gonorrhoeae F62 ΔlgtA ΔlgtF ΔrfaK; 2, N. gonorrhoeae F62 ΔlgtA ΔlgtF; 3, N. gonorrhoeae F62 ΔlgtA ΔlgtF lgtG+; 4, N. gonorrhoeae F62 ΔlgtA ΔlgtE.
DISCUSSION
Previous studies in our laboratory have identified several nonpathogenic Neisseria spp. that possess the genetic potential for expressing gonococcus- and meningococcus-like LOS structures (3). Our genetic analysis of one of these strains, N. subflava 44, indicated that it possesses functional lgtA, lgtB, lgtE, and lgtG genes (3). Therefore, this strain should express the same lacto-N-neotetraose structure found in the α chain of N. gonorrhoeae F62 and N. meningitidis MC58. However, SDS-PAGE analysis of its LOS indicated that it produced two major small isoforms, which we have called LOSI and LOSII. In this study, MS techniques showed that LOSI possesses one Hex on both the α and β chains and that LOSII contains an additional Hex on the α chain. There is no indication that phosphorylation of LOSI occurs, while about one-third of the OSs in LOSII are phosphorylated at Hep II.
Banerjee and coworkers (4) demonstrated that, in the absence of lgtG activity, the β chain consists solely of a PEA added to C-3 of Hep II. The fact that N. subflava LOS contains a glucose added to C-3 in the β chain precludes the presence of PEA on this residue. Since only one-third of the LOSs expressed by N. subflava 44 contain PEA yet all of the molecules possess glucose on C-3 of the β chain, this modification is at a different location and its addition may not be required for the export of LOS to the surface of the gonococcus. These observations also demonstrate that PEA addition is another potential source of structural variability in neisserial LOS.
Using FACE monosaccharide composition analysis, we show that the predominant Hex in N. subflava 44 LOS was glucose; galactose made up less than 10% of the total sugar. The FACE analysis methods we employed allow for rapid compositional determinations of an unknown LOS structure. By combining genetic, structural, and compositional analysis, we conclude that LOSI has one glucose on both the α and β chains and that LOSII is structurally related to LOSI but differs from it by the addition of either a glucose or galactose on the α chain. The location of galactose in the LOS cannot be determined by these analysis. However, since a small amount of high-molecular-mass LOS that can bind MAb 1B2 is made and since N. subflava possesses an intact lgt gene cluster needed to make this molecule, it strongly suggests that the galactose is located at the reducing terminus of the molecule. This structural heterogeneity would not be detected by MS or SDS-PAGE because these LOS components would have the same masses and gel mobilities. This points to the need for better reagents that can recognize these types of differences in the LOS structures.
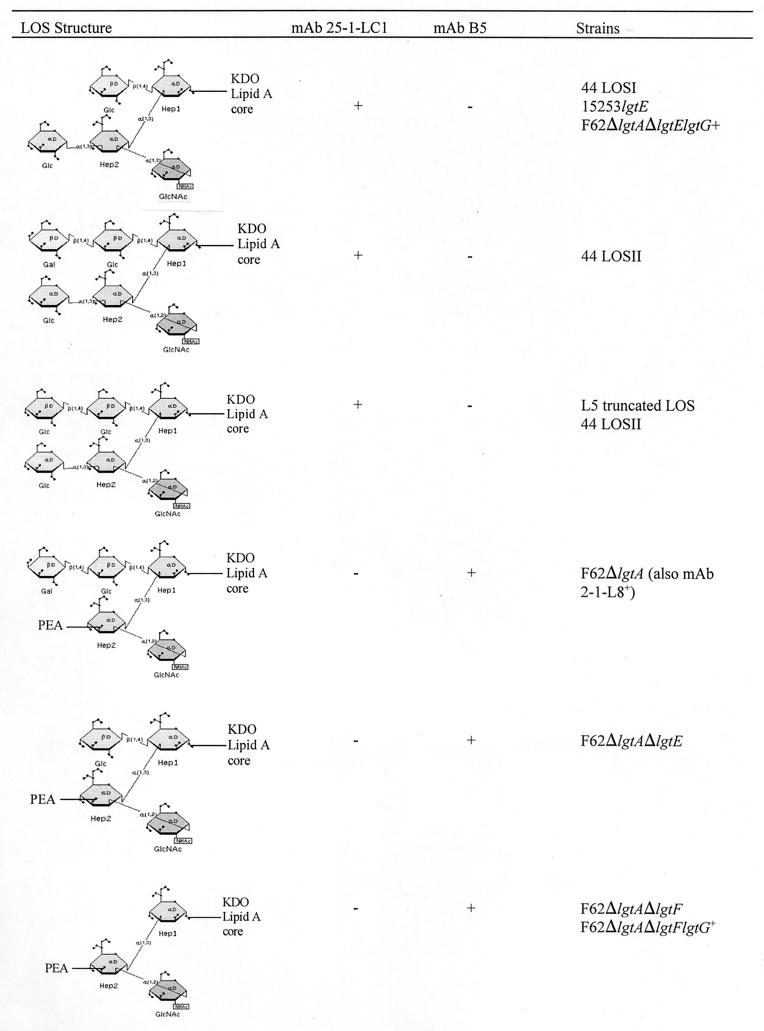
Both LOSI and LOSII of N. subflava 44 bound MAb 25-1-LC1, suggesting that this antibody has a specificity for core elements of neisserial LOS. To determine the specificity of this MAb, several isogenic strains of N. gonorrhoeae F62 and 15253 that expressed single genetically defined structures were constructed. These reactivities are summarized in Fig. 9. LOS from strains 15253 lgtE and F62 ΔlgtA ΔlgtE lgtG+ possess the same structure as isoform LOSI and they bind MAb 25-1-LC1 with the same intensity as N. subflava 44. N. meningitidis M981 (L5) LOS possesses a single glucose in the β chain and elongates the α chain from the first glucose. While the predominant bands expressed by these strains failed to bind MAb 25-1-LC1, a truncated LOS expressed by N. meningitidis M981 (L5), which contains two glucoses in the α chain, reacted with MAb 25-1-LC1. These experiments indicated that (i) the β-chain glucose is necessary for binding by MAb 25-1-LC1, (ii) LOSI is the minimum structure recognized by MAb 25-1-LC1, and (iii) further extension of the β chain and further extension of the α chain block the epitope bound by MAb 25-1-LC1.
FIG. 9.
LOS structure and MAb binding. Shown is a diagrammatic representation of some of the LOS components expressed by the strains and their reactivities with specific MAbs. +, the antibody can bind the structure; −, absence of binding by the structure.
LOS isolated from N. gonorrhoeae F62 ΔlgtA ΔlgtF lgtG+ failed to bind MAbs 2C7, 3G9, and 25-1-LC1 but bound MAb B5, which requires the presence of PEA on the 3 position of Hep II for binding. The fact that LOS isolated from F62 ΔlgtA ΔlgtF lgtG+ can bind MAb B5 indicates that, when the α chain is truncated and lacks the first glucose, β-chain extension does not occur, even when the strain possesses a functional lgtG gene. This has allowed us to further define the order by which sugars are added onto a growing OS. This sequence can be defined as follows: after the addition of Hep I, Hep II is added, the γ-chain GlcNAc is added, the α-chain glucose is added, and then further extension of the α chain or β chain is possible.
Yamasaki et al. (52) characterized MAb 2C7, which recognizes a similar gonococcal structure for antibody recognition (lactose extensions off of both Hep I and Hep II). This antibody binds LOS when the β chain is extended by the addition of galactose. In our studies, MAb 2C7 did not bind to N. subflava LOS, indicating that one can use the differential binding of MAb 2C7 and MAb 25-1-LC1 to differentiate β chains that contain glucose or lactose.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health to D.C.S. (AI 24452) and a grant to V.R. (GM45054).
We thank Paul Rick, Uniformed Services University, for allowing us to use his FACE system.
REFERENCES
- 1.Apicella M A, Mandrell R E. Molecular mimicry as a factor in the pathogenesis of human neisserial infections: in vitro and in vivo modification of the lipooligosaccharide of Neisseria gonorrhoeae by N-acetylneuraminic acid. Pediatr Infect Dis J. 1989;8:901–902. doi: 10.1097/00006454-198912000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Apicella M A, Westerink M A J, Morse S A, Schneider H, Rice P A, Griffiss J M. Bactericidal antibody response of normal human serum to the lipooligosaccharides of Neisseria gonorrhoeae. J Infect Dis. 1986;153:520–526. doi: 10.1093/infdis/153.3.520. [DOI] [PubMed] [Google Scholar]
- 3.Arking D, Tong Y, Stein D C. Analysis of lipooligosaccharide biosynthesis in the Neisseriaceae. J Bacteriol. 2001;183:934–941. doi: 10.1128/JB.183.3.934-941.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee A, Wang R, Uljohn S, Rice P A, Gotschlich E C, Stein D C. Identification of the gene (lgtG) encoding the lipooligosaccharide β chain synthesizing glucosyl transferase from Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1998;95:10872–10877. doi: 10.1073/pnas.95.18.10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brossay L, Paradis G, Pepin A, Mourad W, Cote L, Hebert J. Idiotype and anti-anti-idiotype antibodies to Neisseria gonorrhoeae lipooligosaccharides with bactericidal activity but no cross-reactivity with red blood cell antigens. J Immunol. 1993;151:234–243. [PubMed] [Google Scholar]
- 6.Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res. 1984;131:209–217. [Google Scholar]
- 7.Drazek E S, Stein D C, Deal C D. A mutation in the Neisseria gonorrhoeae rfaD homolog results in altered lipooligosaccharide expression. J Bacteriol. 1995;177:2321–2327. doi: 10.1128/jb.177.9.2321-2327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erwin A L, Haynes P A, Rice P A, Gotschlich E C. Conservation of the lipooligosaccharide synthesis locus lgt among strains of Neisseria gonorrhoeae: requirement for lgtE in synthesis of the 2C7 epitope and of the beta chain of strain 15253. J Exp Med. 1996;184:1233–1241. doi: 10.1084/jem.184.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estabrook M M, Griffiss J M, Jarvis G A. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect Immun. 1997;65:4436–4444. doi: 10.1128/iai.65.11.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamian A, Beurret M, Michon F, Brisson J-R, Jennings H J. Structure of the L2 lipopolysaccharide core oligosaccharide of Neisseria meningitidis. J Biol Chem. 1992;267:922–925. [PubMed] [Google Scholar]
- 11.Gibson B W, Melaugh W, Phillips N J, Apicella M A, Campagnari A A, Griffiss J M. Investigation of the structural heterogeneity of lipooligosaccharides from pathogenic Haemophilus and Neisseria species and of R-type lipopolysaccharides from Salmonella typhimurium by electrospray mass spectrometry. J Bacteriol. 1993;175:2702–2712. doi: 10.1128/jb.175.9.2702-2712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson B W, Webb J W, Yamasaki R, Fisher S J, Burlingame A L, Mandrell R E, Schneider H, Griffiss J M. Structure and heterogeneity of the oligosaccharides from the lipopolysaccharides of a pyocin-resistant Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1989;86:17–21. doi: 10.1073/pnas.86.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert M, Watson D C, Cunningham A M, Jennings M P, Young N M, Wakarchuk W W. Cloning of the lipooligosaccharide alpha-2, 3-sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J Biol Chem. 1996;271:28271–28276. doi: 10.1074/jbc.271.45.28271. [DOI] [PubMed] [Google Scholar]
- 14.Gotschlich E C. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J Exp Med. 1994;180:2181–2190. doi: 10.1084/jem.180.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiss J, Brandt B, Saunders N, Zollinger W. Structural relationships and sialylation among meningococcal L1, L8, and L3, 7 lipooligosaccharide serotypes. J Biol Chem. 2000;275:9716–9724. doi: 10.1074/jbc.275.13.9716. [DOI] [PubMed] [Google Scholar]
- 16.Griffiss, J. M. The role of bacterial lipooligosaccharides in the pathogenesis of human disease. Trends Glycosci. Glycotechnol. 7:461–478.
- 17.Griffiss J M, Brandt B, Engstrom J, Schneider H, Zollinger W, Gibson B. Structural relationships and sialylation among meningococcal lipooligosaccharide (LOS) serotypes. In: Evans J S, Yost S E, Maiden M C, Feavers I M, editors. Proceedings of the Ninth International Pathogenic Neisseria Conference. United Kingdom: Merieux; 1994. p. 12. [Google Scholar]
- 18.Griffiss J M, O'Brien J P, Yamaski R, Williams G D, Rice P A, Schneider H. Physical heterogeneity of neisserial lipooligosaccharide reflects oligosaccharides that differ in apparent molecular weight, chemical composition, and antigenic expression. Infect Immun. 1987;55:1792–1800. doi: 10.1128/iai.55.8.1792-1800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulati S, McQuillen D P, Mandrell R E, Jani D B, Rice P A. Immunogenicity of Neisseria gonorrhoeae lipooligosaccharide epitope 2C7, widely expressed in vivo with no immunochemical similarity to human glycosphingolipids. J Infect Dis. 1996;174:1223–1237. doi: 10.1093/infdis/174.6.1223. . (Erratum, 175:1027, 1997.) [DOI] [PubMed] [Google Scholar]
- 20.Gulati S, McQuillen D P, Sharon J, Rice P A. Experimental immunization with a monoclonal anti-idiotope antibody that mimics the Neisseria gonorrhoeae lipooligosaccharide epitope 2C7. J Infect Dis. 1996;174:1238–1248. doi: 10.1093/infdis/174.6.1238. [DOI] [PubMed] [Google Scholar]
- 21.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John C M, Griffiss J M, Apicella M A, Mandrell R E, Gibson B W. The structural basis for pyocin resistance in Neisseria gonorrhoeae lipooligosaccharides. J Biol Chem. 1991;266:19303–19311. [PubMed] [Google Scholar]
- 23.John C M, Schneider H, Griffiss J M. Neisseria gonorrhoeae that infect men have lipooligosaccharides with terminal N-acetyl-lactosamine repeats. J Biol Chem. 1999;274:1017–1025. doi: 10.1074/jbc.274.2.1017. [DOI] [PubMed] [Google Scholar]
- 24.Joiner K A, Scales R, Warren K A, Frank M M, Rice P A. Mechanism of action of blocking IgG for Neisseria gonorrhoeae. J Clin Investig. 1985;76:1765–1772. doi: 10.1172/JCI112167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahler C M, Carlson R W, Rahman M M, Martin L E, Stephens D S. Two glycosyltransferase genes, lgtF and rfaK, constitute the lipooligosaccharide ice (inner core extension) biosynthesis operon of Neisseria meningitidis. J Bacteriol. 1996;178:6677–6684. doi: 10.1128/jb.178.23.6677-6684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J J, Mandrell R E, Hu Z, Westerink M A, Poolman J T, Griffiss J M. Electromorphic characterization and description of conserved epitopes of the lipooligosaccharides of group A Neisseria meningitidis. Infect Immun. 1988;56:2631–2638. doi: 10.1128/iai.56.10.2631-2638.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levin J C, Stein D C. Cloning, complementation, and characterization of an rfaE homolog from Neisseria gonorrhoeae. J Bacteriol. 1996;178:4571–4575. doi: 10.1128/jb.178.15.4571-4575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandrell R E, Griffiss J M, Macher B A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes J. Exp Med. 1988;168:107–126. doi: 10.1084/jem.168.1.107. . (Erratum, 168:1517.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michon F, Beurret M, Gamian A, Brisson J R, Jennings H J. Structure of the L5 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J Biol Chem. 1990;265:7243–7247. [PubMed] [Google Scholar]
- 30.Moran A P, Prendergast M M, Appelmelk B J. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol Med Microbiol. 1996;16:105–116. doi: 10.1111/j.1574-695X.1996.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 31.Muhlecker W, Gulati S, McQuillen D P, Ram S, Rice P A, Reinhold V N. An essential saccharide binding domain for the mAb 2C7 established for Neisseria gonorrhoeae LOS by ES-MS and MSn. Glycobiology. 1999;9:157–171. doi: 10.1093/glycob/9.2.157. [DOI] [PubMed] [Google Scholar]
- 32.Narayanan R B, Drabick J J, Wiliams J C, Fortier A H, Meltzer M S, Sadoff J C, Bolt R C, Nacy N A. Immunotherapy of tularemia: characterization of monoclonal antibodies reactive with Francisella tularensis. J Leukoc Biol. 1993;53:112–116. doi: 10.1002/jlb.53.1.112. [DOI] [PubMed] [Google Scholar]
- 33.Phillips N J, John C M, Reinders L G, Gibson B W, Apicella M A, Griffiss J M. Structural models for the cell surface lipooligosaccharides of Neisseria gonorrhoeae and Haemophilus influenzae. Biomed Environ Mass Spectrom. 1990;19:731–745. doi: 10.1002/bms.1200191112. [DOI] [PubMed] [Google Scholar]
- 34.Plested J S, Makepeace K, Jennings M P, Gidney M A J, Lacelle S, Brisson J-R, Cox A D, Martin A, Bird A G, Tang C M, Mackinnon F M, Richards J C, Moxon E R. Conservation and accessibility of an inner core lipopolysaccharide epitope of Neisseria meningitidis. Infect Immun. 1999;67:5417–5426. doi: 10.1128/iai.67.10.5417-5426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poolman J T, Hopman C T P, Zanen H C. Problems in the definition of meningococcal serotypes. FEMS Microbiol Lett. 1982;13:339–348. [Google Scholar]
- 36.Reinhold V N, Reinhold B B, Chan S. Carbohydrate sequence analysis by electrospray ionization mass spectrometry. Methods Enzymol. 1996;271:377–402. doi: 10.1016/s0076-6879(96)71018-2. [DOI] [PubMed] [Google Scholar]
- 37.Rice P A, Kasper D L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection: the role of gonococcal endotoxin. J Clin Investig. 1977;60:1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandlin R C, Apicella M A, Stein D C. Cloning of a gonococcal DNA sequence that complements the lipooligosaccharide defects of Neisseria gonorrhoeae 1291d and 1291e. Infect Immun. 1993;61:3360–3368. doi: 10.1128/iai.61.8.3360-3368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandlin R C, Danaher R C, Stein D C. Genetic basis of pyocin resistance in Neisseria gonorrhoeae. J Bacteriol. 1994;176:6869–6876. doi: 10.1128/jb.176.22.6869-6876.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandlin R C, Stein D C. Role of phosphoglucomutase in lipooligosaccharide biosynthesis in Neisseria gonorrhoeae. J Bacteriol. 1994;176:2930–2937. doi: 10.1128/jb.176.10.2930-2937.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider H, Griffiss J M, Boslego J W, Hitchcock P J, McJunkin K O, Apicella M A. Expression of paragloboside-like lipooligosaccharide may be a necessary component of gonococcal pathogenesis in men. J Exp Med. 1991;174:1601–1605. doi: 10.1084/jem.174.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider H, Griffiss J M, Mandrell R E, Jarvis G A. Elaboration of a 3.6-kilodalton lipooligosaccharide, antibody against which is absent from human sera, is associated with serum resistance of Neisseria gonorrhoeae. Infect Immun. 1985;50:672–677. doi: 10.1128/iai.50.3.672-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider H, Hale T L, Zollinger W D, Seid R C, Hammack C A, Griffiss J M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984;45:544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song W, Ma L, Chen R, Stein D C. Role of lipooligosaccharide in Opa-independent invasion of Neisseria gonorrhoeae into human epithelial cells. J Exp Med. 2000;191:949–959. doi: 10.1084/jem.191.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stojiljkovic I, Hwa V, Larson J, Lin L, So M, Nassif X. Cloning and characterization of the Neisseria meningitidis rfaC gene encoding alpha-1,5 heptosyltransferase I. FEMS Microbiol Lett. 1997;151:41–49. doi: 10.1111/j.1574-6968.1997.tb10392.x. [DOI] [PubMed] [Google Scholar]
- 46.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipooligosaccharide in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 47.West S E, Clark V L. Genetic loci and linkage associations in Neisseria gonorrhoeae and Neisseria meningitidis. Clin Microbiol Rev. 1989;2(Suppl.):S92–S103. doi: 10.1128/cmr.2.suppl.s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1972;5:83–91. [Google Scholar]
- 49.White L A, Kellogg D S., Jr Neisseria gonorrhoeae identification in direct smears by a fluorescent antibody-counterstain method. Appl Microbiol. 1965;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamasaki R, Bacon B E, Nasholds W, Schneider H, Griffiss J M. Structural determination of oligosaccharides derived from lipooligosaccharide of Neisseria gonorrhoeae F62 by chemical, enzymatic, and two-dimensional NMR methods. Biochemistry. 1991;30:10566–10575. doi: 10.1021/bi00107a028. . (Erratum, 31:316, 1992.) [DOI] [PubMed] [Google Scholar]
- 51.Yamasaki R, Kerwood D E, Schneider H, Quinn K P, Griffiss J M, Mandrell R E. The structure of lipooligosaccharide produced by Neisseria gonorrhoeae, strain 15253, isolated from a patient with disseminated infection: evidence for a new glycosylation pathway of the gonococcal lipooligosaccharide. J Biol Chem. 1994;269:30345–30351. [PubMed] [Google Scholar]
- 52.Yamasaki R, Koshino H, Kurono S, Nishinaka Y, McQuillen D P, Kume A, Gulati S, Rice P A. Structural and immunochemical characterization of Neisseria gonorrhoeae epitope defined by a monoclonal antibody 2C7; the antibody recognizes a conserved epitope on specific lipo-oligosaccharides in spite of the presence of human carbohydrate epitopes. J Biol Chem. 1999;274:36550–36558. doi: 10.1074/jbc.274.51.36550. [DOI] [PubMed] [Google Scholar]
- 53.Yamasaki R, Nasholds W, Schneider H, Apicella M A. Epitope expression and partial structural characterization of F62 lipooligosaccharide (LOS) of Neisseria gonorrhoeae: IgM monoclonal antibodies (3F11 and 1-1-M) recognize non-reducing termini of the LOS components. Mol Immunol. 1991;28:1233–1242. doi: 10.1016/0161-5890(91)90010-h. [DOI] [PubMed] [Google Scholar]
- 54.Zhou D, Lee N G, Apicella M A. Lipooligosaccharide biosynthesis in Neisseria gonorrhoeae: cloning, identification and characterization of the alpha 1,5 heptosyltransferase I gene (rfaC) Mol Microbiol. 1994;14:609–618. doi: 10.1111/j.1365-2958.1994.tb01300.x. [DOI] [PubMed] [Google Scholar]