Abstract
BACKGROUND
Data on trends, predictors, and outcomes of heart failure (HF) readmissions after transcatheter aortic valve replacement (TAVR) remain limited. Moreover, the relationship between hospital TAVR discharge volume and HF readmission outcomes has not been established.
METHODS AND RESULTS
The Nationwide Readmission Database was used to identify 30‐day readmissions for HF after TAVR from October 1, 2015, to November 30, 2018, using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes. A total of 167 345 weighted discharges following TAVR were identified. The all‐cause readmission rate within 30 days of discharge was 11.4% (19 016). Of all the causes of 30‐day rehospitalizations, HF comprised 31.4% (5962) of all causes. The 30‐day readmission rate for HF did not show a significant decline during the study period (P trend=0.06); however, all‐cause readmission rates decreased significantly (P trend=0.03). HF readmissions were comparable between high‐ and low‐volume TAVR centers. Charlson Comorbidity Index >8, length of stay >4 days during the index hospitalization, chronic obstructive pulmonary disease, atrial fibrillation, chronic HF, preexisting pacemaker, complete heart block during index hospitalization, paravalvular regurgitation, chronic kidney disease, and end‐stage renal disease were independent predictors of 30‐day HF readmission after TAVR. HF readmissions were associated with higher mortality rates when compared with non‐HF readmissions (4.9% versus 3.3%; P<0.01). Each HF readmission within 30 days was associated with an average increased cost of $13 000 more than for each non‐HF readmission.
CONCLUSIONS
During the study period from 2015 to 2018, 30‐day HF readmissions after TAVR remained steady despite all‐cause readmissions decreasing significantly. All‐cause readmission mortality and HF readmission mortality also showed a nonsignificant downtrend. HF readmissions were comparable across low‐, medium‐, and high‐volume TAVR centers. HF readmission was associated with increased mortality and resource use attributed to the increased costs of care compared with non‐HF readmission. Further studies are needed to identify strategies to decrease the burden of HF readmissions and related mortality after TAVR.
Keywords: heart failure, TAVI, TAVR, transcatheter aortic valve implantation, transcatheter aortic valve replacement
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Heart Failure
Nonstandard Abbreviations and Acronyms
- NRD
Nationwide Readmission Database
- PPM
preexisting permanent pacemaker
- TAVR
transcatheter aortic valve replacement
Clinical Perspective.
What Is New?
At 30 days after transcatheter aortic valve replacement, 1 in 3 readmissions is attributed to heart failure and is associated with higher readmission mortality rates compared with non–heart failure readmission.
What Are the Clinical Implications?
High comorbidity burden, length of stay >4 days during the index hospitalization, anemia, atrial fibrillation, paravalvular regurgitation, history of heart failure, preexisting pacemaker, complete heart block during the index hospitalization, chronic kidney disease, and end‐stage renal disease are predictors of 30‐day heart failure readmission.
Since the first‐in‐human use of transcatheter aortic valve replacement (TAVR) in 2002, TAVR has emerged as the treatment of choice for severe aortic stenosis across the spectrum of surgical risk. 1 , 2 , 3 With the rapid advancements in device technology, expansion to lower risk patient groups, increased operator volume, and site experience, TAVR outcomes, including readmission rates, have improved significantly in recent years. 4 , 5 Although noncardiac readmissions after TAVR are more common, among cardiac causes of readmissions, heart failure (HF) remains one of the most common culprits. 6 , 7
HF readmissions after TAVR have been associated with significant mortality, morbidity, and health system resource use. 7 , 8 Although all‐cause readmission rates after TAVR have decreased, the trend of HF readmission has not been established. 5 , 6 Similarly, previous studies have reported that increased site TAVR volume may be associated with decreased operative mortality and reduced all‐cause unplanned readmission rates. 9 , 10 However, the impact of TAVR volume on HF rehospitalizations remains to be explored.
Given the scarcity of data on trends, outcomes, and predictors of HF readmissions, we aimed to study 30‐day hospital readmissions for HF after TAVR from a large contemporary data set, the Nationwide Readmission Database (NRD).
Methods
NRD data are publicly available. The specific data supporting this study's findings are available from the corresponding author upon request.
Study Data
The NRD is sponsored by the Agency for Healthcare Research and Quality and developed through the Federal–State Industry partnership. The database was developed for the HCUP (Healthcare Cost and Utilization Project), and house data on 35 million annual weighted discharges. The discharge data available from 28 states represent 59.7% of the US population and 58.7% of inpatient hospitalizations. The NRD is an all‐payer database that captures all admissions and readmissions with nationally representative weighting, allowing the analysis of causes for readmissions and resource use in terms of cost of care. Each patient is assigned a unique identifier code for tracing readmissions within a calendar year. The NRD days‐to‐event variable captures readmissions within a calendar year but not across different years. 11 Given the deidentified nature of the database, institutional review board approval and informed consent were not required for this study.
Study Design and Data Selection
For the study, International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes were used to identify patients undergoing TAVR (ICD‐10‐CM code 02RF3x) from October 1, 2015, to November 30, 2018 (Table S1). The discharge weights provided by the NRD were used to provide nationally representative data. The NRD contains data on total hospital charges, which is the amount billed by the hospital. However, charges differ from the actual cost, including the total expense of hospital services, counting utilities, wages, and supplies. To calculate the cost, HCUP provides cost‐to‐charge ratio files that provide hospital‐specific ratios or weighted average ratios to supplement the original NRD file. The cost information was obtained from accounting reports of the participating hospitals collected by the Centers for Medicare and Medicaid Services, with the imputation of missing values when necessary. 12 The cost data on readmission were missing for 61 cases and were not included in the cost calculation. We determined the adjusted cost of care by multiplying the element of the total charge provided by the NRD by the cost‐to‐charge ratios. We also adjusted hospitalization costs for inflation to January 2020 US dollars using the Bureau of Labor Statistics Consumer Price Index. 13 A detailed flowchart of the study methods is shown in Figure 1.
Figure 1. Study flow diagram.
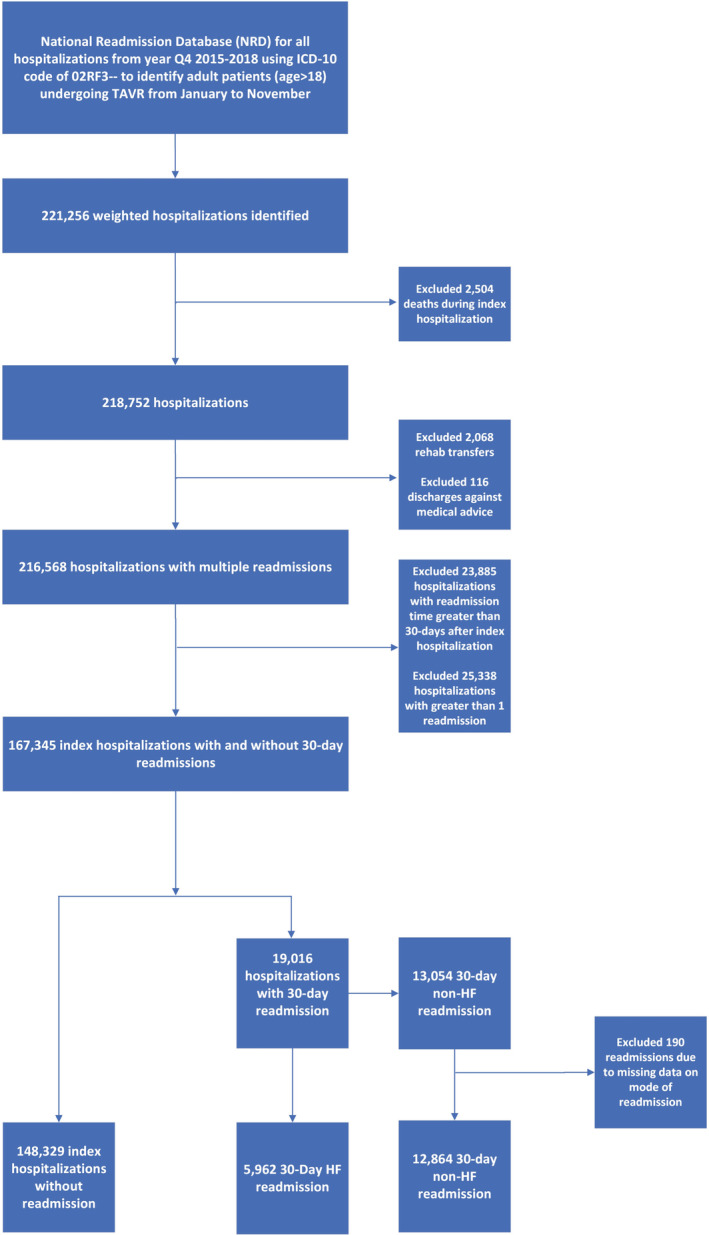
Reported numbers are based on weighted hospitalizations. HF indicates heart failure; NRD, Nationwide Readmission Database; and TAVR, transcatheter aortic valve replacement.
Study Definitions
Index admissions were defined for patients undergoing TAVR and discharged alive with no missing variables critical for identifying readmissions (ie, length of stay [LOS], mortality, or days‐to‐event variables). Index admissions were identified per calendar year from January to November, where December admissions were excluded to allow for analysis of 30‐day readmission data. We further excluded patients who left against medical advice or who were transferred to a rehabilitation facility. Readmission was defined as emergent nonelective or elective readmissions within 30 days of discharge. In patients who had multiple 30‐day hospitalizations, only the first hospitalization was included in the analysis. Readmission mortality was defined as any death occurring in the hospital within 30 days of discharge (excluding deaths occurring outside the hospital and in the emergency department). HF readmission was defined as unplanned emergent readmission for acute HF. Readmissions for elective causes with a secondary diagnosis of HF were categorized as non‐HF readmissions. Based on a prior publication, cutoffs were prespecified, and hospitals performing <50 procedures were categorized as low‐volume TAVR centers, 51 to 100 procedures were considered medium‐volume centers, and hospitals performing >100 procedures were categorized as high‐volume centers. 10
Study End Point
The primary outcome was 30‐day readmissions for HF after TAVR discharge. Secondary outcomes included predictors of HF readmissions, temporal trends, in‐hospital complications related to HF readmissions compared with non‐HF readmissions, the association of readmissions with hospital TAVR discharge volume, and resource use in terms of adjusted hospitalization cost for HF readmissions and non‐HF readmissions.
Statistical Analysis
Categorical variables were presented as frequencies and percentages, and continuous variables were reported as medians with an interquartile range (IQR). The Shapiro–Wilk test was used to assess the normality of continuous data. Baseline characteristics were compared using the Pearson χ2 and Fisher exact tests for categorical variables and the Mann–Whitney U and Kruskal–Wallis tests for continuous variables. The P value for the slope was used to assess temporal trends. A multivariable logistic regression model was developed to compute independent predictors of 30‐day HF readmission by using the enter regression method. The index hospitalization characteristics of patients readmitted with HF were compared with those who were not readmitted. From the index hospitalization group, we excluded index cases that were readmitted for non‐HF causes. Baseline variables that were nonsignificant on univariate analysis (P>0.05) and variables with <10 observations were excluded. As the overall missing values were minimal, we used listwise deletion and did not include missing values in the logistic regression analysis. The logistic regression model included other important variables, including age, baseline comorbidities, and index hospitalization characteristics shown in Table 1. R's MatchIt package was used for propensity matching. 14 To account for potential confounding and selection bias, a propensity score–matching model was developed using logistic regression to derive 2 matched groups for comparative outcomes analysis of patients readmitted with HF compared with patients who were not readmitted with HF. A nearest‐neighbor 1:1 variable ratio, parallel, balanced propensity‐matching model without replacement was made using caliper of width equal to 0.2 of the SDs of the logit of the propensity score. Age, sex, and baseline comorbidities related to the readmission hospitalizations were included in propensity matching. Outcomes during rehospitalization for HF and non‐HF 30‐day readmissions were reported. The variables with missing data were categorized as “others/missing” before matching. Index hospitalization characteristics or variables directly related to the outcome for the readmissions were not included. A second multivariable logistic regression model adjusted for age, sex, and baseline comorbidities was also developed that used nonweighted data from the year 2018 to assess readmission outcomes for high and medium discharge volume hospitals compared with low‐volume hospitals as a reference group. For missing values in the nonweighted data, listwise deletion was used, and missing values were not included in the logistic regression analysis.
Table 1.
Baseline Characteristics and Predictors of 30‐Day Readmission for HF After Transcatheter Aortic Valve Replacement
| Univariate analysis, 30‐day HF readmission | P value | Adjusted multivariable analysis | P value | |||
|---|---|---|---|---|---|---|
| Without readmission, n=148 329; median (IQR) or n (%) | With 30‐day HF readmission, n=5962; median (IQR) or n (%) | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | |||
| Age, y | 81 (75–86) | 83 (76–87) | ||||
| Age categories, y | ||||||
| ≤64 | 7512 (5.1) | 239 (4.0) | Reference | Reference | 0.16 | |
| 65–74 | 26 974 (18.2) | 1020 (17.1) | 1.19 (1.03–1.37) | 0.02 | 1.12 (0.96–1.31) | 0.52 |
| 75–84 | 62 842 (42.4) | 2321 (38.9) | 1.16 (1.02–1.33) | 0.03 | 0.95 (0.81–1.11) | 0.99 |
| ≥85 | 51 001 (34.4) | 2382 (40.0) | 1.47 (1.28–1.68) | <0.01 | 1.00 (0.85–1.18) | 0.16 |
| Charlson Comorbidity Index | 7 (6–8) | 8 (7–9) | ||||
| Charlson Comorbidity Index score >8 | 57 915 (39.0) | 3415 (57.3) | 2.09 (1.99–2.21) | <0.01 | 1.29 (1.20–1.39) | <0.01 |
| Elective index admission | 120 366 (81.4) | 4037 (68.0) | 0.49 (0.46–0.51) | <0.01 | 0.92 (0.86–0.98) | 0.01 |
| Female sex | 67 678 (45.6) | 2766 (46.4) | 1.03 (0.98–1.09) | 0.25 | … | … |
| Primary payer | ||||||
| Medicare | 134 856 (90.9) | 5527 (92.7) | Reference | Reference | ||
| Medicaid | 1588 (1.1) | 52 (0.9) | 0.79 (0.61–1.05) | 0.10 | 0.82 (0.61–1.10) | 0.19 |
| Private insurance | 8613 (5.8) | 274 (4.6) | 0.78 (0.69–0.88) | <0.01 | 0.99 (0.86–1.13) | 0.82 |
| Self‐pay | 534 (0.4) | 22 (0.4) | 0.99 (0.64–1.52) | 0.95 | 0.89 (0.57–1.39) | 0.60 |
| Other† | 2561 (1.7) | 79 (1.3) | 0.76 (0.61–0.96) | 0.02 | 0.86 (0.68–1.08) | 0.18 |
| Others/missing‡ | 177 (0.1) | 9 (0.1) | … | … | … | … |
| Median quartile of income | ||||||
| 0–25th percentile | 30 144 (20.3) | 1247 (20.9) | Reference | |||
| 25–50th percentile | 40 254 (27.1) | 1574 (26.4) | 0.95 (0.88–1.02) | 0.14 | … | … |
| 50–75th percentile | 40 177 (27.1) | 1645 (27.6) | 0.99 (0.92–1.07) | 0.79 | … | … |
| 75–100th percentile | 35 869 (24.2) | 1413 (23.7) | 0.95 (0.88–1.03) | 0.22 | … | … |
| Others/missing† | 1885 (1.3) | 84 (1.4) | … | … | … | … |
| Hospital size | ||||||
| Small | 6699 (4.5) | 243 (4.1) | Reference | |||
| Medium | 30 564 (20.6) | 1257 (21.1) | 1.13 (0.98–1.30) | 0.08 | … | … |
| Large | 111 066 (74.9) | 4462 (74.8) | 1.11 (0.97–1.26) | 0.13 | … | … |
| Hospital teaching | ||||||
| Metropolitan nonteaching | 15 961 (10.8) | 607 (10.2) | Reference | |||
| Metropolitan teaching | 130 993 (88.3) | 5290 (88.7) | 1.06 (0.98–1.16) | 0.17 | … | … |
| Nonmetropolitan hospital | 1375 (0.9) | 65 (1.1) | 1.24 (0.96–1.61) | 0.11 | … | … |
| Anemias | 6034 (4.1) | 548 (9.2) | 2.39 (2.18–2.62) | <0.01 | 1.70 (1.55–1.88) | <0.01 |
| Alcohol use | 94 (0.1) | <11 (<0.1)* | 0.27 (0.04‐1.91) | 0.25 | … | … |
| Hypertension | 132 235 (89.1) | 5391 (90.4) | 1.15 (1.05–1.26) | <0.01 | 0.99 (0.90–1.09) | 0.83 |
| Diabetes | 25 956 (17.5) | 929 (15.6) | 0.87 (0.81–0.94) | <0.01 | 0.98 (0.91–1.06) | 0.64 |
| Coronary artery disease | 104 023 (70.1) | 4141 (69.5) | 0.97 (0.92–1.03) | 0.27 | … | … |
| Cerebrovascular disease | 16 403 (11.1) | 543 (9.1) | 0.81 (0.74–0.88) | <0.01 | 0.69 (0.63–0.76) | <0.01 |
| Chronic obstructive pulmonary disease | 42 845 (28.9) | 2162 (36.3) | 1.40 (1.33–1.48) | <0.01 | 1.23 (1.16–1.31) | <0.01 |
| Pulmonary circulation disorder | 28 272 (19.1) | 1404 (23.6) | 1.31 (1.23–1.39) | <0.01 | 0.93 (0.88–1.00) | 0.05 |
| Obesity | 28 406 (19.2) | 945 (15.9) | 0.81 (0.74–0.85) | <0.01 | 0.76 (0.70–0.82) | <0.01 |
| Prior MI | 18 723 (12.6) | 803 (13.5) | 1.08 (0.99–1.16) | 0.06 | … | … |
| Prior PCI | 29 895 (20.2) | 1156 (19.4) | 0.95 (0.89–1.02) | 0.15 | … | … |
| Prior CABG | 27 210 (18.3) | 1123 (18.8) | 1.03 (0.97–1.10) | 0.35 | … | … |
| Preexisting pacemaker | 14 949 (10.1) | 1265 (21.2) | 2.40 (2.25–2.56) | <0.01 | 2.18 (2.04–2.33) | <0.01 |
| Pacemaker implanted during index hospitalization | 14 747 (9.9) | 831 (13.9) | 1.47 (1.36–1.58) | <0.01 | 1.08 (0.97–1.20) | 0.15 |
| Complete heart block during index hospitalization | 13 648 (9.2) | 785 (13.2) | 1.51 (1.39–1.62) | <0.01 | 1.20 (1.08–1.33) | <0.01 |
| Prior ICD | 3854 (2.6) | 13 (0.2) | 1.96 (1.74–2.21) | <0.01 | 1.77 (1.55–2.01) | <0.01 |
| Weight loss | 4576 (3.1) | 518 (8.7) | 2.99 (2.72–3.29) | <0.01 | 1.71 (1.54–1.89) | <0.01 |
| Peripheral vascular disease | 31 157 (21.0) | 935 (15.7) | 0.71 (0.65–0.75) | <0.01 | 0.60 (0.55–0.64) | <0.01 |
| Atrial fibrillation | 59 545 (40.1) | 3562 (59.7) | 2.21 (2.10–2.33) | <0.01 | 1.63 (1.54–1.73) | <0.01 |
| Liver disease | 4480 (3.0) | 259 (4.3) | 1.46 (1.28–1.66) | <0.01 | 1.25 (1.09–1.43) | <0.01 |
| Chronic kidney disease | 32 721 (22.1) | 2140 (35.9) | 1.98 (1.87–2.09) | <0.01 | 1.47 (1.37–1.57) | <0.01 |
| End‐stage renal disease | 5826 (3.9) | 402 (6.7) | 1.77 (1.59–1.96) | <0.01 | 1.56 (1.39–1.76) | <0.01 |
| Paravalvular regurgitation | 613 (0.4) | 57 (1.0) | 2.33 (1.77–3.06) | <0.01 | 2.07 (1.56–2.75) | <0.01 |
| Mitral stenosis | 1327 (0.9) | 84 (1.4) | 1.58 (1.27–1.98) | <0.01 | 1.32 (1.04–1.67) | 0.02 |
| Mitral regurgitation | 10 476 (7.1) | 522 (8.8) | 1.26 (1.15–1.38) | <0.01 | 1.04 (0.95–1.15) | 0.38 |
| HF with reduced EF | 18 265 (12.3) | 1550 (26.0) | 2.50 (2.36–2.66) | <0.01 | 3.24 (3.00–3.51) | <0.01 |
| HF with preserved EF | 64 057 (43.2) | 3383 (56.7) | 1.73 (1.64–1.82) | <0.01 | 2.69 (2.52–2.87) | <0.01 |
| Length of stay >4 d during index hospitalization | 51 076 (34.4) | 3531 (59.2) | 2.77 (2.62–2.92) | <0.01 | 1.76 (1.65–1.88) | <0.01 |
| Nonhome/facility discharge during index hospitalization | 54 641 (36.8) | 112 (3363) | 2.22 (2.11–2.34) | <0.01 | 1.40 (1.31–1.48) | <0.01 |
Descriptive statistics and regression model are based on weighted data. CABG indicates coronary artery bypass graft surgery; EF, ejection fraction; HF, heart failure; ICD, implantable cardioverter defibrillator; IQR, interquartile range; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
Observations <11 are not reported per Healthcare Cost and Utilization Project guidelines.
“Other” variable includes Workers’ Compensation and other government programs.
The missing values were recoded as “others/missing.”
For weighted analysis from quarter 4 of 2015 to 2018, complete data were available for all variables except for primary expected payer (0.1%), disposition (<0.1%), elective index admission (0.4%), elective readmissions (<0.1%), and median household income (1.3%). In the unweighted sample of 2018 data, all variables had complete data except for primary expected payer (0.1%), elective index admission (<0.1%), elective readmission (0.1%), and median household income (1.2%). All missing values are reported in Table 1 and Tables S1, S2, and S3 and have been recoded as “others/missing.”
For all analyses, a 2‐tailed P value of 0.05 was considered statistically significant. Analyses were performed using SPSS version 27 and R software for statistical computing version 4.3. Discharge weights provided by the NRD were used for all weighted analyses except for annual hospital discharge volume analysis for which only unweighted data from the year 2018 were used.
Results
Baseline Characteristics of the Study Population
A total of 167 345 weighted hospitalizations for TAVR were identified. Of the included patients, 148 329 did not get readmitted. The all‐cause readmission rate within 30 days of discharge was 11.4% (19 016). The incidence of HF readmissions was 3.6% (5962), accounting for 31.4% of all‐cause 30‐day readmissions. Non‐HF readmissions were 7.8% (13 054). HF readmission occurred at a median of 9 days (IQR, 4–17 days) after discharge, whereas non‐HF readmission occurred at a median of 10 days (IQR, 4–19 days; P<0.01). Patients readmitted with HF had a higher comorbidity burden with a Charlson comorbidity median score of 8 (IQR, 7–9) versus 7 (6, 7, 8) among patients with other causes of readmission (P<0.01). The baseline characteristics are summarized in Table 1 and Table S2.
Predictors of 30‐Day HF Readmissions
A total of 148 329 index hospitalizations (after excluding index cases that were readmitted for non‐HF causes, n=13 054) were compared with 5962 30‐day HF readmissions. Independent predictors of 30‐day HF readmission after TAVR included the following: Charlson comorbidity score >8 (odds ratio [OR], 1.29; 95% CI, 1.20–1.39), LOS >4 days on index hospitalization (OR, 1.76; 95% CI, 1.65–1.88), nonhome/facility discharge on index admission (OR, 1.40; 95% CI, 1.31–1.48), diagnosis of anemia (OR, 1.70; 95% CI, 1.55–1.88), chronic obstructive pulmonary disease (OR, 1.23; 95% CI, 1.16–1.31), diagnosis of atrial fibrillation (OR, 1.63; 95% CI, 1.54–1.73), paravalvular regurgitation (OR, 2.07; 95% CI, 1.56–2.75), preexisting permanent pacemaker (PPM) on index hospitalization (OR, 1.08; 95% CI, 0.97–1.20), complete heart block during index hospitalization (OR, 1.20; 95% CI, 1.08–1.33), chronic kidney disease (CKD; OR, 1.47; 95% CI, 1.37–1.57), and end‐stage renal disease (ESRD; OR, 1.56; 95% CI, 1.39–1.76). In contrast, elective index admission (OR, 0.92; 95% CI, 0.86–0.98) was associated with a lower likelihood of readmission within a month after discharge (Table 1, Figure 2).
Figure 2. Independent predictors of 30‐day readmissions for HF.
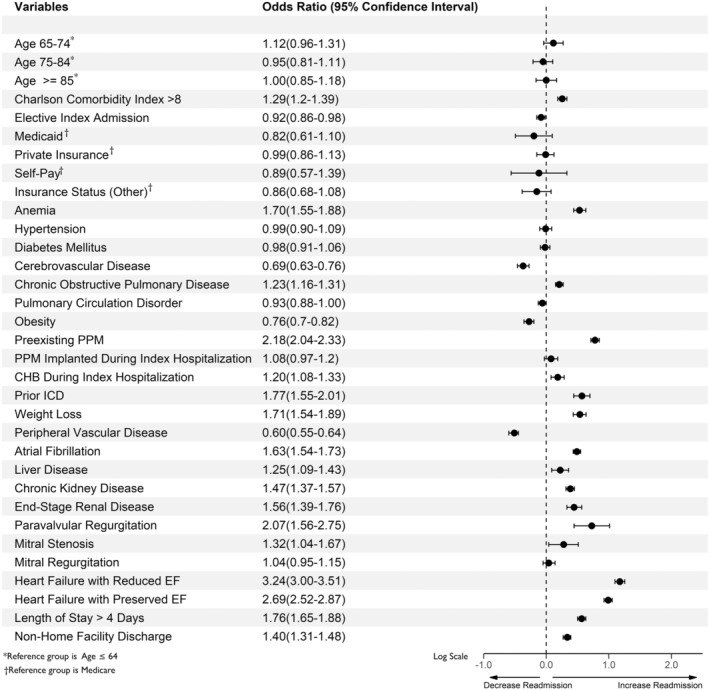
Estimates are based on weighted data. CHB indicates complete heart block; EF, ejection fraction; ICD, implantable cardioverter defibrillator; and PPM, permanent pacemaker.
Outcomes of HF Readmissions Compared With Readmissions Without HF
A total of 5962 HF readmissions were compared with non‐HF readmissions (12 864) after excluding patients with missing data on mode of readmission (n=190). The detailed baseline characteristics of HF versus non‐HF readmissions before and after propensity matching are given in Table S3. The covariate balance before and after propensity matching are shown in Figure S1. Non–propensity‐matched and propensity‐matched outcomes mirrored each other with minimal differences. HF readmissions were associated with higher mortality when compared with non‐HF readmissions (4.9% versus 3.3%; P<0.01). Similarly, more patients with HF readmissions were discharged to facilities than non‐HF readmission patients (68.3% versus 56.8%; P<0.01). Moreover, cardiogenic shock (3.2% versus 1.0%; P<0.01) and acute kidney injury (35.1% versus 20.8%; P<0.01) were higher on readmission with HF (Table 2).
Table 2.
Hospital Outcomes and Resource Use Associated With 30‐Day Readmission After Transcatheter Aortic Valve Replacement
| Crude analysis | 1:1 Propensity matching | |||||
|---|---|---|---|---|---|---|
| Without HF readmission, n=12 864 | With HF readmission, n=5962 | P value | Without HF readmission, n=5962 | With HF readmission, n=5962 | P value | |
| Died during hospitalization | 420 (3.3) | 292 (4.9) | <0.01 | 230 (3.9) | 292 (4.9) | 0.01 |
| Discharge disposition | <0.01 | <0.01 | ||||
| Routine home discharge | 5556 (43.2) | 1887 (31.6) | 2365 (39.7) | 1887 (31.6) | ||
| SNF/facility discharge | 7304 (56.8) | 4076 (68.3) | 3595 (60.3) | 4076 (68.3) | ||
| Vascular complications | 680 (5.3) | 231 (3.9) | <0.01 | 296 (5.0) | 231 (3.9) | <0.01 |
| Cardiogenic shock | 125 (1.0) | 189 (3.2) | <0.01 | 75 (1.3) | 189 (3.2) | <0.01 |
| Acute kidney injury | 2680 (20.8) | 2092 (35.1) | <0.01 | 1599 (26.8) | 2092 (35.1) | <0.01 |
| Permanent pacemaker | 1481 (11.5) | 374 (6.3) | <0.01 | 607 (10.2) | 374 (6.3) | <0.01 |
| Urinary tract infection | 1609 (12.5) | 685 (11.5) | 0.31 | 836 (14.0) | 685 (11.5) | <0.01 |
| Pneumonia | 887 (6.9) | 732 (12.3) | <0.01 | 463 (7.8) | 732 (12.3) | <0.01 |
| Gastrointestinal bleed | 839 (6.5) | 236 (4.0) | <0.01 | 463 (7.8) | 236 (4.0) | <0.01 |
| Ischemic stroke | 701 (5.4) | 119 (2.0) | <0.01 | 224 (3.7) | 119 (2.0) | <0.01 |
| Hemorrhagic stroke | 114 (0.9) | 48 (0.8) | 0.04 | 25 (0.4) | 48 (0.8) | 0.01 |
| Resource use | ||||||
| LOS (days) | 3 (5–6) | 5 (3–8) | <0.01 | 4 (2–6) | 5 (3–8) | <0.01 |
| Hospitalization cost (USD) | $11 351 ($6403–$20 440) | $12 928 ($7087–$24 780) | <0.01 | $11 935 ($6540–$21 303) | $12 673 ($6768–$25 172) | <0.01 |
Data are provided as number (percentage) or median (interquartile range). HF indicates heart failure; LOS, length of stay; and SNF, skilled nursing facility.
Temporal Trends for All‐Cause and HF Readmissions
Temporal trends showed that all‐cause readmissions decreased significantly from 12.3% to 11% (P trend=0.03). However, HF readmissions showed a nonsignificant downward trend from 4.1% in 2015 to 3.4% in 2018 (P trend=0.06; Figure 3). Similarly, all‐cause readmission mortality and HF readmission mortality showed a nonsignificant downward trend from 2015 to 2018 ([5.9% to 3.6% [P trend=0.2] and 8.9% to 5% [P trend=0.3], respectively]). Across all years, HF readmission mortality was significantly higher than all‐cause readmission mortality (Figure 3A and 3B).
Figure 3. Temporal trends in HF and all‐cause readmissions after transcatheter aortic valve replacement.
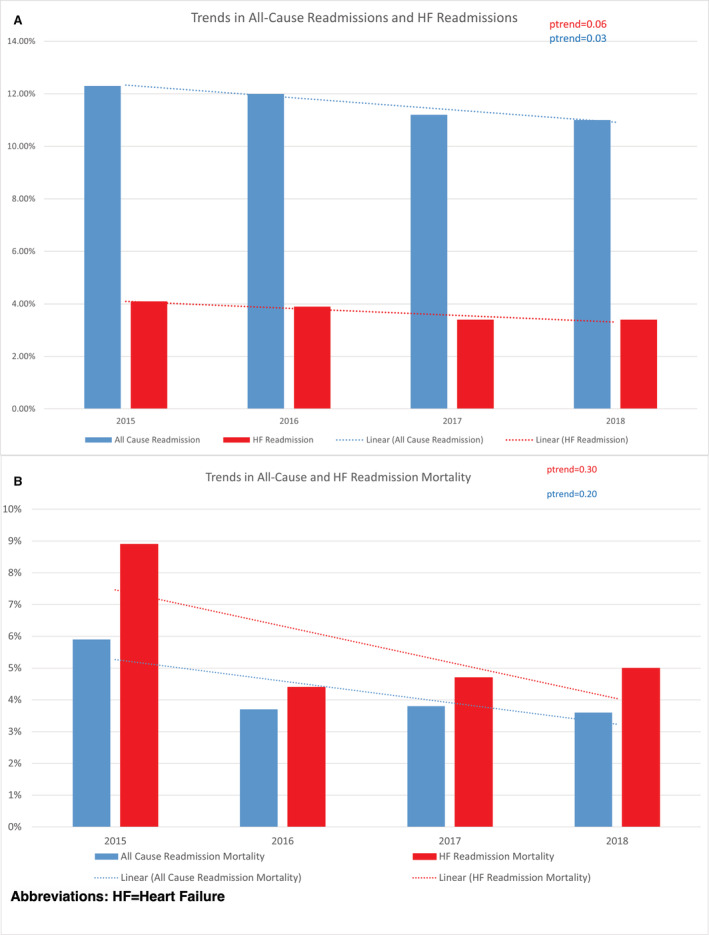
Estimates are based on weighted data. HF indicates heart failure.
Association of Hospital TAVR Discharge Volume With All‐Cause and HF Readmissions
In terms of hospital TAVR volumes, 3371 cases of TAVR were performed in low‐volume, 9653 cases in medium‐volume, and 21 716 cases in high‐volume hospitals during 2018, respectively. The detailed baseline characteristics are given in Table S4. Adjusted all‐cause readmissions were similar in high‐volume centers (OR, 0.97; 95% CI, 0.86–1.10) compared with low‐volume centers. Similarly, HF readmission rates were comparable across low‐, medium‐, and high‐volume centers (Table S5).
Resource Use for HF Readmissions Compared With Readmissions Without HF
Each 30‐day HF readmission was associated with a $12 928 greater increase in the cost of care than each non‐HF readmission (P≤0.01). Similarly, LOS on readmission was significantly higher for HF readmission compared with non‐HF readmissions (5 days versus 3 days; P<0.01; Table 2, Figure S2).
Discussion
We report 6 principal findings from our analysis of TAVR hospitalizations using a large, contemporary, nationwide readmission data set. First, 3.6% of 30‐day readmissions are attributed to HF and account for 31.4% of all‐cause readmissions. Second, all‐cause readmission rates after TAVR showed a significant downtrend, whereas HF rehospitalizations remained steady. Third, Charlson Comorbidity Index >8, LOS >4 days during the index hospitalization, anemia, chronic obstructive pulmonary disease, atrial fibrillation, paravalvular regurgitation, HF with reduced ejection fraction, HF with preserved ejection fraction, preexisting PPM, complete heart block during the index hospitalization, CKD, and ESRD were independent predictors of 30‐day HF readmission after TAVR. Fourth, patients with HF readmissions have significantly higher readmission mortality compared with non‐HF readmissions. Fifth, there was no difference between HF readmission rates, all‐cause mortality, or HF readmission mortality between high‐, medium‐ and low‐volume TAVR centers. Sixth, post‐TAVR HF readmissions are associated with significantly higher cost and duration of hospital stay during readmissions than non‐HF readmissions.
Readmission Rates and Temporal Trends
Outcomes after TAVR are increasingly in focus as the indication expanded from high‐risk and intermediate‐risk cohorts to low‐risk cohorts. Over time, the LOS has declined, and conscious sedation is increasingly used in current practice. 5 Previous studies have reported a wide range for all‐cause 30‐day readmission rates from 9% to 19%. 5 , 15 , 16 , 17 , 18 Kolte et al analyzed data from the 2013 US NRD and showed an all‐cause readmission rate of 17.9%, with 38.2% of readmissions attributed to cardiovascular causes. In their study, HF was the most common cause of all‐cause readmission in 22.5% of the cases. 6 Our contemporary analysis from the most recent NRD data shows a much lower all‐cause readmission rate of 11.4% and an HF rehospitalization rate of 3.6% at 30 days. Our study complements the Society of Thoracic Surgeons/American College of Cardiology (STS/ACC) Transcatheter Valve Therapies (TVT) Registry study, which showed that all‐cause readmission rates have decreased over time, with our study providing the most recent data. 5 However, it is important to note that a similar significant downtrend has not been noted with HF readmissions. HF readmissions are the most common causes of rehospitalizations in patients with TAVR, with reported rates between 20% and 40% of all‐cause readmissions (Kolte et al, 22.5% 6 ; Nombela‐Franco et al, 30.4% 15 ). Moreover, Tripathi and colleagues reported a readmission rate of 77% for all cardiovascular causes, including HF at 90 days after discharge from their analysis of NRD data from 2016 to 2017. 16 The variation in reported HF readmissions in the aforementioned studies is attributed to the heterogeneity in the time frame (30 days versus 90 days) and the type of study (single center versus administrative data sets). The HF rehospitalization rate of 3.6% at 30 days is consistent with prior studies using administrative claim codes. 17
Predictors of Readmission
We identified important independent predictors of 30‐day rehospitalizations with HF. We reinforce the finding of an earlier study that postprocedure paravalvular leak leads to a 2‐fold higher risk of readmission with HF. 18 Multiple prior studies have reported anemia as a significant predictor of HF rehospitalizations. Anemia can lead to a high output state and precipitate HF exacerbation. Moreover, blood transfusions to treat anemia can lead to volume overload and precipitation of HF, leading to early readmissions. 7 Similarly, pulmonary hypertension is also a well‐known predictor of HF readmission, which has been previously identified. 7 , 19 , 20 In our univariate analysis, a significantly higher percentage of patients with HF readmissions had pulmonary hypertension. Pulmonary hypertension attributed to postcapillary or combined pre‐ and postcapillary causes is associated with poor outcomes and is also a risk factor for mortality after TAVR. 21 Hence, it is suggested that patients undergo an evaluation to identify precapillary, postcapillary, or combined capillary pulmonary hypertension and risk stratify these patients. 7 Expectedly, chronic HF was associated with increased 30‐day readmissions, with HF with reduced ejection fraction being a stronger predictor than HF with preserved ejection fraction. Atrial fibrillation is a disease of the elderly and common comorbidity in patients with aortic stenosis and is an independent predictor of HF readmission. Furthermore, preexisting PPM on index hospitalization was an independent predictor of readmission along with complete heart block. However, although significant on univariate analysis, new PPM implantation during the first hospitalization was not predictive of HF readmission. Previous studies reported a nonsignificant association between preexisting PPM and all‐cause readmissions after TAVR at 30 days but a significant association at 90 days. 6 , 16 Finally, increased LOS >4 days during the index hospitalization increases the likelihood of 30‐day readmission, whereas Charlson Comorbidity Index >8 predicts a 1.4 times higher risk of 30‐day rehospitalization after TAVR. Our study supports the findings of the prior studies, which reported increased LOS during index hospitalization and higher comorbidity burden as predictors of readmissions. 6 , 16
Frailty is prevalent in the TAVR population 22 and is a risk factor for death and disability after TAVR. Weight loss is an indicator of frailty in the elderly population. 23 We report weight loss to be a significant predictor of readmission. Interventions to address sarcopenia should focus on diet and exercise with cardiac rehabilitation being 1 such intervention after TAVR. 24
The CKD‐ESRD subgroup is associated with poor outcomes both in hospital and at 1 year. We report an increased risk for HF‐related readmissions in this subgroup. CKD and ESRD are well‐known risk factors for readmission in the HF population. 25 This could be related to a cardiorenal syndrome where worsening kidney function can precipitate HF. TAVR has a beneficial effect on improving kidney function in a majority of patients, but a quarter of patients experience deteriorating kidney function. 26 It is likely that this subgroup could be at higher risk for readmissions from the cardiorenal syndrome and more so in those with worsening CKD or acute kidney injury after TAVR. 27
Readmission Mortality
The significantly increased mortality rate in those with 30‐day HF readmission after TAVR is concerning. This finding warrants further exploration. Prior studies suggest that cardiovascular mortality accounts for ≈72% of deaths for HF readmission compared with 19% for non‐HF readmissions. 15 Our study complements the findings of a prior study by Durand et al 7 that reported a worse prognosis with single and multiple HF readmissions at 30‐day follow‐up.
Paravalvular leak and worsening or residual valve lesions are known predictors of mortality after TAVR. The presence of a moderate or severe paravalvular leak is a predictor of both short‐term and long‐term mortality in addition to HF readmissions as discussed previously. 28 On univariate analysis, valve disease especially involving the mitral valve was significantly higher in the HF readmission group. However, the adjusted analysis did not reveal a significant association for mitral regurgitation. Persistent mitral regurgitation after TAVR is associated with poor functional class and mortality. 29 , 30 Mitral stenosis is also associated with an increased risk of mortality and HF rehospitalization at 1 year. 31 Similarly, worsening tricuspid regurgitation is shown to be a predictor of all‐cause and cardiovascular mortality after TAVR. 32 These residual valve lesions may predict 30‐day HF readmission mortality and readmissions when evaluated for a longer time frame. Cardiac amyloidosis is increasingly recognized as a coexistent pathology in the TAVR population and is seen in 1 in 8 patients referred for TAVR. Patients with amyloidosis may be at risk for HF readmission and higher mortality because of the continued remodeling despite decreasing the afterload. 33 Our study could not evaluate the role of amyloidosis because of the very low numbers, which could be attributed to underdiagnosis.
Hospital Volume and Readmission
There has been a great interest in studying hospital TAVR discharge volumes and outcomes. 9 , 10 Studies to date suggest an inverse relationship between hospital volume and mortality, with higher volume centers having less mortality. Our study did not find a significant mortality difference between high‐ and medium‐volume hospitals compared with low‐volume hospitals. A 2014 NRD analysis by Khera et al reported an inverse relationship between hospital discharge volume and all‐cause readmission rates after TAVR (25% lower admission in high‐volume centers compared with low‐volume centers). 10 Novel to our study is the lack of association between hospital TAVR discharge volume not only for all‐cause but also HF readmissions. We hypothesize that the patient‐level characteristics discussed previously play a significant role in HF readmissions rather than hospital‐level factors and should be the focus of future interventions. 7
Cost and LOS
HF hospitalizations are a significant burden on the health care system given that each readmission leads to ≈$13 000 excess cost per readmission. Our reported cost estimates adjusted for inflation agree with earlier reported estimates. 6 , 16 It is perhaps attributed to the increased incidence of cardiogenic shock requiring intensive care unit admissions, higher complications such as acute kidney injury, and the increased use of mechanical circulatory devices such as percutaneous left ventricular assist device and Impella that increase the duration of hospital stays attributed to HF rehospitalization, which led to an increased cost of hospitalizations. 34 , 35 Early follow‐up (<1 week) is a key intervention associated with reduced readmission after HF hospitalization. 36 A similar intervention can be considered part of the transition of care planning after hospital discharge after TAVR in those at high risk for HF readmission. Our study data may help identify a specific subset of patients—those with a prolonged index hospital stay, higher Charlson Comorbidity Index, anemia, chronic obstructive pulmonary disease, preexisting PPM, atrial fibrillation, valvular disease, kidney disease, and chronic HF—who will benefit from interventions to prevent readmission, including early discharge follow‐up. We suggest further research to develop postdischarge interventions for this cohort to help mitigate the risk of readmissions and consequently reduce the cost.
Limitations
Previous studies have shown that aortic valve gradient, postprocedure left ventricular ejection fraction, and the presence of amyloidosis are significant predictors of HF readmission. 37 We could not study these factors because of the nonavailability or undercoding of ICD‐10‐CM codes for these conditions. 7 Our study looked at unweighted data for a national analysis of the association between TAVR hospital volume and readmission rates. However, the NRD is not designed to study hospital‐level outcome data. The NRD cannot capture deaths that occur outside of the hospital. 38 Studies are needed to assess the impact of hospital TAVR volume on readmission outcomes. Moreover, data on medication use and blood chemistry are lacking; hence, we could not factor in the role of medication noncompliance. 8 TAVR‐based outcomes such as patient prosthesis mismatch and valve dysfunction/thrombosis data could not be evaluated because of lack of echocardiographic data. Valve‐related readmissions contribute to <10% of all readmissions, according to a recent study. 5 The NRD is an administrative claim–based database that uses ICD‐10‐CM codes for diagnosis. Although procedural codes are less prone to error, coding errors and variability cannot be excluded entirely. The NRD collects data on in‐patient discharges, and each admission is registered as an independent event. Furthermore, emergency room visits after TAVR are not captured by the NRD and hence are not included in our analysis. Similar to any observational, retrospective study, association does not imply causation, and conclusions are hypothesis generating and should be drawn cautiously.
Conclusions
The incidence of 30‐day readmission for HF after TAVR was 3.6%, which accounts for 31.4% of all‐cause readmissions. Although 30‐day all‐cause readmissions after TAVR have decreased in recent years, HF readmissions have not shown a significant downward trend. Increased hospital TAVR volume is not associated with reduced HF readmissions. Patient characteristics associated with 30‐day HF readmissions include LOS >4 days during the index hospitalization, anemia, chronic obstructive pulmonary disease, paravalvular leak, atrial fibrillation, HF with reduced ejection fraction, HF with preserved ejection fraction, preexisting pacemaker, complete heart block on index hospitalization, CKD, and ESRD. Given the retrospective nature of the study, our study findings should be considered hypothesis generating. Further prospective studies are needed to identify strategies to decrease the burden of HF readmissions and readmission mortality after TAVR.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1‐S5
Figures S1‐S2
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024890
For Sources of Funding and Disclosures, see page 13.
REFERENCES
- 1. Cribier A, Eltchaninoff H, Tron C. First human transcatheter implantation of an aortic valve prosthesis in a case of severe calcific aortic stenosis. Ann Cardiol Angeiol (Paris). 2003;52:173–175. doi: 10.1016/s0003-3928(03)00062-3 [DOI] [PubMed] [Google Scholar]
- 2. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 4. Russo MJ, JM MC, Thourani VH, Guerrero M, Genereux P, Nguyen T, Hong KN, Kodali S, Leon MB. Case volume and outcomes after TAVR with balloon‐expandable prostheses. J Am Coll Cardiol. 2019;73:427–440. doi: 10.1016/j.jacc.2018.11.031 [DOI] [PubMed] [Google Scholar]
- 5. Sanchez CE, Hermiller JBJ, Pinto DS, Chetcuti SJ, Arshi A, Forrest JK, Huang J, Yakubov SJ. Predictors and risk calculator of early unplanned hospital readmission following contemporary self‐expanding transcatheter aortic valve replacement from the STS/ACC TVT registry. Cardiovasc Revasc Med. 2020;21:263–270. doi: 10.1016/j.carrev.2019.05.032 [DOI] [PubMed] [Google Scholar]
- 6. Kolte D, Khera S, Sardar MR, Gheewala N, Gupta T, Chatterjee S, Goldsweig A, Aronow WS, Fonarow GC, Bhatt DL, et al. Thirty‐day readmissions after transcatheter aortic valve replacement in the United States: Insights from the Nationwide readmissions database, Circulation. Cardiovasc Interv Ther. 2017;10. doi: 10.1161/CIRCINTERVENTIONS.116.004472 [DOI] [PubMed] [Google Scholar]
- 7. Durand E, Doutriaux M, Bettinger N, Tron C, Fauvel C, Bauer F, Dacher J‐N, Bouhzam N, Litzler P‐Y, Cribier A, et al. Incidence, prognostic impact, and predictive factors of readmission for heart failure after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2017;10:2426–2436. doi: 10.1016/j.jcin.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 8. Auffret V, Bakhti A, Leurent G, Bedossa M, Tomasi J, Belhaj Soulami R, Verhoye J‐P, Donal E, Galli E, Loirat A, Sharobeem S, Sost G, le Guellec M, Boulmier D, le Breton H Determinants and impact of heart failure readmission following transcatheter aortic valve replacement. Circ Cardiovasc Interv 2020;13:e008959. DOI: 10.1161/CIRCINTERVENTIONS.120.008959 [DOI] [PubMed] [Google Scholar]
- 9. Vemulapalli S, Carroll JD, Mack MJ, Li Z, Dai D, Kosinski AS, Kumbhani DJ, Ruiz CE, Thourani VH, Hanzel G, et al. Procedural volume and outcomes for transcatheter aortic‐valve replacement. N Engl J Med. 2019;380:2541–2550. doi: 10.1056/NEJMsa1901109 [DOI] [PubMed] [Google Scholar]
- 10. Khera S, Kolte D, Gupta T, Goldsweig A, Velagapudi P, Kalra A, Tang GHL, Aronow WS, Fonarow GC, Bhatt DL, et al. Association between hospital volume and 30‐day readmissions following transcatheter aortic valve replacement. JAMA Cardiol. 2017;2:732–741. doi: 10.1001/jamacardio.2017.1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Overview of the Nationwide Readmisison Database (NRD) . Agency for Healthcare Research and Quality, Rockville, MD. 2020.
- 12. Cost‐to‐Charge Ratio Files . Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. 2020. [PubMed]
- 13. Consumer Price Index: U.S. Bureau of Labor Statistics; 2020 [updated Jun. 10, 2020. Available from https://www.bls.gov/cpi/.]
- 14. Ho D, Imai K, King G, Stuart EA. MatchIt: Nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42(SE‐Articles):1–28. DOI: 10.1186/s40246-018-0156-4 [DOI] [Google Scholar]
- 15. Nombela‐Franco L, del Trigo M, Morrison‐Polo G, Veiga G, Jimenez‐Quevedo P, Abdul‐Jawad Altisent O, Campelo‐Parada F, Biagioni C, Puri R, DeLarochellière R, et al. Incidence, causes, and predictors of early (≤30 days) and late unplanned hospital readmissions after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8:1748–1757. doi: 10.1016/j.jcin.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 16. Tripathi B, Nerusu LA, Sawant AC, Atti L, Sharma P, Pershad A. Transcatheter aortic valve implantation readmissions in the current era (from the National Readmission Database). Am J Cardiol. 2020;130:115–122. doi: 10.1016/j.amjcard.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 17. Holmes DRJ, Brennan JM, Rumsfeld JS, Dai D, O'Brien SM, Vemulapalli S, Edwards FH, Carroll J, Shahian D, Grover F, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–1028. doi: 10.1001/jama.2015.1474 [DOI] [PubMed] [Google Scholar]
- 18. Pibarot P, Hahn RT, Weissman NJ, Arsenault M, Beaudoin J, Bernier M, Dahou A, Khalique OK, Asch FM, Toubal O, et al. Association of Paravalvular Regurgitation with 1‐year outcomes after transcatheter aortic valve replacement with the SAPIEN 3 valve. JAMA Cardiol. 2017;2:1208–1216. doi: 10.1001/jamacardio.2017.3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luçon A, Oger E, Bedossa M, Boulmier D, Verhoye JP, Eltchaninoff H, Iung B, Leguerrier A, Laskar M, Leprince P, et al. Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: study from the France 2 registry. Circ Cardiovasc Interv. 2014;7:240–247. doi: 10.1161/CIRCINTERVENTIONS.113.000482 [DOI] [PubMed] [Google Scholar]
- 20. O'Sullivan CJ, Wenaweser P, Ceylan O, Rat‐Wirtzler J, Stortecky S, Heg D, Spitzer E, Zanchin T, Praz F, Tüller D, et al. Effect of pulmonary hypertension hemodynamic presentation on clinical outcomes in patients with severe symptomatic aortic valve stenosis undergoing transcatheter aortic valve implantation: Insights from the new proposed pulmonary hypertension Classificat. Circ Cardiovasc Interv. 2015;8:e002358. doi: 10.1161/CIRCINTERVENTIONS.114.002358 [DOI] [PubMed] [Google Scholar]
- 21. Lachmann M, Rippen E, Schuster T, Xhepa E, von Scheidt M, Pellegrini C, Trenkwalder T, Rheude T, Stundl A, Thalmann R, et al. Subphenotyping of patients with aortic stenosis by unsupervised agglomerative clustering of echocardiographic and hemodynamic data. JACC Cardiovasc Interv. 2021;14:2127–2140. doi: 10.1016/j.jcin.2021.08.034 [DOI] [PubMed] [Google Scholar]
- 22. Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, et al. Frailty in older adults undergoing aortic valve replacement: The FRAILTY‐AVR study. J Am Coll Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 23. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: Evidence for a phenotype. The journals of gerontology . Series A, Biological sciences and medical sciences. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 24. Epstein E, Rosander A, Pazargadi A, Taub P. Cardiac rehab for functional improvement. Curr Heart Fail Rep. 2020;17:161–170. doi: 10.1007/s11897-020-00462-2 [DOI] [PubMed] [Google Scholar]
- 25. Fudim M, O'Connor CM, Dunning A, Ambrosy AP, Armstrong PW, Coles A, Ezekowitz JA, Greene SJ, Metra M, Starling RC, et al. Aetiology, timing and clinical predictors of early vs. late readmission following index hospitalization for acute heart failure: Insights from ASCEND‐HF. Eur J Heart Fail. 2018;20:304–314. doi: 10.1002/ejhf.1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Witberg G, Steinmetz T, Landes U, Pistiner Hanit R, Green H, Goldman S, Vaknin‐Assa H, Codner P, Perl L, Rozen‐Zvi B, et al. Change in kidney function and 2‐year mortality after transcatheter aortic valve replacement. JAMA Netw Open. 2021;4:e213296. doi: 10.1001/jamanetworkopen.2021.3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vavilis G, Evans M, Jernberg T, Rück A, Szummer K. Risk factors for worsening renal function and their association with long‐term mortality following transcatheter aortic valve implantation: Data from the SWEDEHEART registry. Open Heart. 2017;4:e000554. doi: 10.1136/openhrt-2016-000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lerakis S, Hayek SS, Douglas PS. Paravalvular aortic leak after transcatheter aortic valve replacement. Circulation. 2013;127:397–407. doi: 10.1016/j.jacc.2019.06.076 [DOI] [PubMed] [Google Scholar]
- 29. Cortés C, Amat‐Santos IJ, Nombela‐Franco L, Muñoz‐Garcia AJ, Gutiérrez‐Ibanes E, de La Torre Hernandez JM, Córdoba‐Soriano JG, Jimenez‐Quevedo P, Hernández‐García JM, Gonzalez‐Mansilla A, et al. Mitral regurgitation after transcatheter aortic valve replacement: Prognosis, imaging predictors, and potential management. JACC Cardiovasc Interv. 2016;9:1603–1614. doi: 10.1016/j.jcin.2016.05.025 [DOI] [PubMed] [Google Scholar]
- 30. Witberg G, Codner P, Landes U, Schwartzenberg S, Barbanti M, Valvo R, De Backer O, Ooms JF, Islas F, Marroquin L, et al. Effect of transcatheter aortic valve replacement on concomitant mitral regurgitation and its impact on mortality. J Am Coll Cardiol Intv 2021;14:1181–1192. DOI: 10.1016/j.jcin.2021.02.030 [DOI] [PubMed] [Google Scholar]
- 31. Joseph L, Bashir M, Xiang Q, Yerokun BA, Matsouaka RA, Vemulapalli S, Kapadia S, Cigarroa JE, Zahr F. Prevalence and outcomes of mitral stenosis in patients undergoing transcatheter aortic valve replacement: Findings from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry. JACC Cardiovasc Interv. 2018;11:693–702. doi: 10.1016/j.jcin.2018.01.245 [DOI] [PubMed] [Google Scholar]
- 32. Cremer PC, Wang TKM, Rodriguez LL, Lindman BR, Zhang Y, Zajarias A, Hahn RT, Lerakis S, Malaisrie SC, Douglas PS, et al. Incidence and clinical significance of worsening tricuspid regurgitation following surgical or transcatheter aortic valve replacement: Analysis from the PARTNER IIA trial. Circ Cardiovasc Interv 2021;14:e010437, e011430. DOI: 10.1161/CIRCINTERVENTIONS.120.010437 [DOI] [PubMed] [Google Scholar]
- 33. Nitsche C, Scully PR, Patel KP, Kammerlander AA, Koschutnik M, Dona C, Wollenweber T, Ahmed N, Thornton GD, Kelion AD, et al. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol. 2021;77:128–139. doi: 10.1016/j.jacc.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Afzal A, Nisar T, Jamil AK, Kluger AY, Felius J, Hall SA, Kale P. Use of intra‐aortic balloon pump in heart failure admissions requiring inotropic support in the United States between 2004–2014. J Heart Lung Transplant. 2019;38:S377–S378. doi: 10.1016/j.healun.2019.01.960 [DOI] [Google Scholar]
- 35. Khera R, Cram P, Lu X, Vyas A, Gerke A, Rosenthal GE, Horwitz PA, Girotra S. Trends in the use of percutaneous ventricular assist devices: Analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175:941–950. doi: 10.1001/jamainternmed.2014.7856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533 [DOI] [PubMed] [Google Scholar]
- 37. Castaño A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–2887. doi: 10.1093/eurheartj/ehx350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nazir S, Ahuja KR, Alamir MA. Is the national readmission database the right database to identify valve‐in‐valve transcatheter aortic valve replacement patients? Eur Heart J. 2020;41:4357. doi: 10.1093/eurheartj/ehaa578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S5
Figures S1‐S2


