Abstract
Background
The sodium‐glucose cotransporter 2 inhibitor canagliflozin reduced the risk of first cardiovascular composite events in the CREDENCE (Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation) trial. In this post hoc analysis, we evaluated the effect of canagliflozin on total (first and recurrent) cardiovascular events.
Methods and Results
The CREDENCE trial compared canagliflozin or matching placebo in 4401 patients with type 2 diabetes, albuminuria, and estimated glomerular filtration rate of 30 to <90 mL/min per 1.73 m2, over a median of 2.6 years. The primary outcome was analyzed as a composite of any cardiovascular event including myocardial infarction, stroke, hospitalization for heart failure, hospitalization for unstable angina, and cardiovascular death. Negative binomial regression models were used to assess the effect of canagliflozin on the net burden of cardiovascular events. During the trial, 634 patients had 883 cardiovascular events, of whom 472 (74%) had just 1 cardiovascular event and 162 (26%) had multiple cardiovascular events. Canagliflozin reduced first cardiovascular events by 26% (hazard ratio, 0.74 [95% CI, 0.63–0.86]; P<0.001) and total cardiovascular events by 29% (incidence rate ratio, 0.71 [95% CI, 0.59–0.86]; P<0.001). The absolute risk difference per 1000 patients treated over 2.5 years was −44 (95% CI, −67 to −21) first cardiovascular events and −73 (95% CI, −114 to −33) total events.
Conclusions
Canagliflozin reduced cardiovascular events, with a larger absolute benefit for total cardiovascular than first cardiovascular events. These findings provide further support for the benefit of continuing canagliflozin therapy after an initial event to prevent recurrent cardiovascular events.
Registration Information
URL: https://www.clinicaltrials.gov; Unique Identifier: NCT02065791.
Keywords: canagliflozin, chronic kidney disease, diabetes, recurrent cardiovascular event
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- CREDENCE
Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation
- SGLT2
sodium‐glucose co‐transporter 2
Clinical Perspective.
What Is New?
Canagliflozin not only reduces the risk of a first cardiovascular (cardiovascular) event in patients with type 2 diabetes and chronic kidney disease but also reduces recurrent cardiovascular events, with a larger absolute benefit observed for total events.
What Are the Clinical Implications?
Canagliflozin should be continued after an incident cardiovascular event, as patients will continue to derive a clinical benefit from this treatment.
Cardiovascular (cardiovascular) disease is both highly prevalent and a major burden in patients with type 2 diabetes and chronic kidney disease (CKD). 1 The presence of CKD increases morbidity and mortality associated with cardiovascular disease. 2
Sodium‐glucose cotransporter 2 (SGLT2) inhibitors were first developed to lower blood glucose levels in patients with type 2 diabetes, but many also protect against a broad range of cardiovascular outcomes including hospitalization for heart failure and major adverse cardiac events during follow‐up. 3 , 4 The net burden of cardiovascular disease and the potential overall benefit that SGLT2 inhibition can afford can better to assessed by considering all cardiovascular events—first and recurrent—reflective of the total cardiovascular event burden. This analysis would help us understand whether those who experience a cardiovascular event while taking an SGLT2 inhibitor derive ongoing benefit from drug continuation. From a clinical, health economic, and health services perspective, it is not only first events that are important, but also subsequent events that contribute to reduced quality of life, poor health outcomes, and increased health expenditure.
It has been reported that canagliflozin reduced the first occurrence of the exploratory composite end point of cardiovascular death, nonfatal myocardial infarction (MI), nonfatal stroke, and hospitalization for heart failure or hospitalization for unstable angina in the CREDENCE (Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation) trial, with a 26% relative risk reduction. 5 In this secondary analysis, we sought to analyze both first and subsequent cardiovascular events for this composite cardiovascular outcome to better characterize the effect of canagliflozin on the total burden of cardiovascular events.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. The CREDENCE study was a double‐blind, randomized, multicenter, international clinical trial comparing the effects of canagliflozin versus placebo on kidney and cardiovascular outcomes in patients with type 2 diabetes and CKD. The trial was registered at ClinicalTrials.gov (NCT02065791), institutional review board approval was obtained, and all patients provided written informed consent. 6
Patients
The patients were at least 30 years old, with type 2 diabetes with a glycated hemoglobin level of 6.5% to 12.0%, and CKD, defined as an estimated glomerular filtration rate of 30 to <90 mL/min per 1.73 m−2 and high albuminuria (urine albumin: creatinine ratio of >300 to 5000 mg/g). Before randomization, all patients were required to be receiving a stable maximum labeled or tolerated dose of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker for at least 4 weeks. Treatment with dual renin‐angiotensin pathway inhibitors (eg, angiotensin‐converting enzyme inhibitor and angiotensin receptor blocker, or agents in either of these classes with a direct renin inhibitor) was not allowed, and patients receiving a mineralocorticoid receptor antagonist at baseline were excluded. Moreover, patients who had suspected nondiabetic kidney disease or type 1 diabetes, had been treated with immunosuppression for kidney disease, or had a history of dialysis or kidney transplantation were excluded. 6 Patients who had a prior cardiovascular event were eligible, unless it had occurred within the preceding 3 months.
Randomization and Study Treatment
After a 2‐week placebo run‐in period, patients were randomly assigned to canagliflozin or matching placebo in a double‐blind 1:1 ratio stratified by screening glomerular filtration rate categories (30–45, 45–60, or 60–90 mL/min per 1.73 m2). Use of other background therapies for glycemic, cardiovascular, and kidney risk management was encouraged according to local and international guidelines.
Follow‐Up
Patients were reviewed after randomization at weeks 3, 13, and 26 and then through alternating in‐clinic visits and telephone calls at 13‐week intervals. The occurrence of cardiovascular events was evaluated at every scheduled follow‐up.
Outcomes
The prespecified outcome of this analysis was total cardiovascular composite events, which included cardiovascular death, MI, stroke, hospitalization for heart failure, or hospitalization for unstable angina occurring at any time point during study follow‐up. These events were adjudicated by the Endpoint Adjudication Committee using rigorous definitions that were prespecified according to standardized criteria. 5
Statistical Analysis
Baseline clinical characteristics were assessed as frequencies for categorical variables, mean (SD) and medians (interquartile ranges) for continuous variables. Comparisons between baseline characteristics for patients with no events, a single event, or multiple events were made using the chi‐square test for categorical variables and Kruskal‐Wallis test for continuous variables. To improve the performance and validity of our models, a bundling approach was used for the composite cardiovascular outcome, whereby nonfatal events together with a cardiovascular death occurring on the same day were excluded, and only cardiovascular death was considered. For example, for coronary revascularization occurring after an MI that eventually resulted in the patient's death all on the same day, only the cardiovascular death would be included. 7
Overdispersion usually arises in such analysis because the repeated events on a subject may not be independent; thus, negative binomial regression was used instead of Poisson regression. 8 The model included an offset for duration of follow‐up. Incidence rate ratio and corresponding 95% CI for total events was calculated. In sensitivity analyses, the Wei, Lin, and Weissfeld method, 9 a marginal model that extends survival models on the basis of the Cox proportional hazard, was used to separately analyze the impact of canagliflozin on the risk of experiencing a first, second, or third event. An additional sensitivity analysis was performed that calculated total events using an Andersen‐Gill model. An on‐treatment analysis was performed to explore the effect of discontinued blinded study drug after an event. Mean cumulative frequencies were calculated using the Nelson‐Aalen estimator. 10 The Sankey diagram is a kind of flow diagram in which the width of the flow is proportional to the flow rate, which was used to show event sequence change according to the first event; it was plotted using R package networkD3. 11 All efficacy comparisons were performed according to the intention‐to‐treat principle. All tests were 2‐sided, with a P value <0.05 considered to be significant. Analyses were performed using SAS Enterprise Guide 7.15 (SAS Institute, Cary, NC) and R studio version 1.1.463 (R Foundation for Statistical Computing, Vienna, Austria).
Results
During the trial, a total of 883 cardiovascular events occurred in 634 patients, over a median follow‐up of 2.62 years. Of these, 634 (72%) were first events, and 249 (28%) were subsequent cardiovascular events. The majority of the first, second, and third cardiovascular events were either cardiovascular death or hospitalization for heart failure. When considering the entire trial population of 4401 subjects, 85.6% (n=3767) experienced no cardiovascular events, 10.7% (n=472) had a single event, and 3.7% (n=162) had at least 2 events. The maximum number of events experienced by a patient was 7 events.
Patients who experienced multiple cardiovascular events were older, and more likely to have a history of heart failure or cerebrovascular or peripheral diseases. These patients also had a higher body mass index, glycated hemoglobin, and systolic blood pressure; lower estimated glomerular filtration rate; longer diabetic duration; and higher urine albumin‐to‐creatinine ratio. With respect to baseline medications, they were more often on a loop diuretic, statin, β‐blocker, antithrombotic, insulin, or calcium channel blocker therapy and less likely to be on metformin and non–loop diuretics than those who did not have a cardiovascular event during study follow‐up (Table).
Table 1.
Baseline Characteristics of Patients With No Events, a Single Event, or Multiple Events
| Characteristic | No events (n=3767) | Single event (n=472) | Multiple events (n=162) | P value* |
|---|---|---|---|---|
| Age, y | 62.7 (9.3) | 64.6 (8.6) | 65.7 (7.6) | <0.0001 |
| Female sex, n (%) | 1296 (34.4) | 146 (30.9) | 52 (32.1) | 0.285 |
| Race, n (%) | 0.007 | |||
| Asian | 759 (20.1) | 85 (18.0) | 33 (20.4) | |
| Black or African American | 175 (4.6) | 39 (8.3) | 10 (6.2) | |
| Other† | 330 (8.8) | 32 (6.8) | 7 (4.3) | |
| White | 2503 (66.4) | 316 (66.9) | 112 (69.1) | |
| Smoking, n (%) | 550 (14.6) | 62 (13.1) | 27 (16.7) | 0.509 |
| History of hypertension, n (%) | 3641 (96.7) | 459 (97.2) | 160 (98.8) | 0.276 |
| Cardiovascular disease history, n (%) | 1798 (47.7) | 316 (66.9) | 106 (65.4) | <0.001 |
| History of coronary, n (%) | 1034 (27.4) | 210 (44.5) | 69 (42.6) | <0.001 |
| History of cerebrovascular, n (%) | 563 (14.9) | 101 (21.4) | 36 (22.2) | <0.001 |
| History of peripheral disease, n (%) | 825 (21.9) | 161 (34.1) | 60 (37.0) | <0.001 |
| History of heart failure, n (%) | 508 (13.5) | 95 (20.1) | 49 (30.2) | <0.001 |
| NYHA I, n (%) | 162 (4.3) | 31 (6.6) | 11 (6.8) | <0.001 |
| NYHA II, n (%) | 287 (7.6) | 45 (9.5) | 27 (16.7) | <0.001 |
| NYHA III, n (%) | 48 (1.3) | 15 (3.2) | 7 (4.3) | <0.001 |
| History of microvascular disease, n (%) | ||||
| Retinopathy | 1587 (42.1) | 217 (46.0) | 78 (48.1) | 0.104 |
| Neuropathy | 1805 (47.9) | 254 (53.8) | 88 (54.3) | 0.019 |
| Duration of diabetes, y | 15.6 (8.6) | 16.6 (8.6) | 17.5 (9.4) | 0.0027 |
| Body mass index, kg/m2 | 31.2 (6.1) | 32.1 (6.8) | 32.1 (6.1) | 0.0042 |
| Systolic BP, mm Hg | 139.6 (15.4) | 142.5 (16.4) | 142.2 (17.6) | <0.0001 |
| Glycated hemoglobin, % | 8.2 (1.3) | 8.4 (1.4) | 8.6 (1.3) | 0.0002 |
| eGFR, mL/min per 1.73 m2 | 56.8 (18.3) | 52.9 (17.8) | 51.5 (16.9) | <0.0001 |
| UACR, mg/g, median (IQR) | 882.0 (448.0–1753.0) | 1049.5 (528.5–2141.0) | 1438.0 (720.0–2900.0) | <0.0001 |
| Medications, n (%) | ||||
| Diuretic | 1714 (45.5) | 247 (52.3) | 96 (59.3) | <0.001 |
| Loop diuretic | 756 (20.1) | 137 (29.0) | 62 (38.3) | <0.001 |
| Non–loop diuretics | 958 (25.4) | 110 (23.3) | 34 (21.0) | 0.289 |
| β‐Blocker | 1434 (38.1) | 240 (50.8) | 96 (59.3) | <0.001 |
| Statin | 2569 (68.2) | 339 (71.8) | 128 (79.0) | 0.005 |
| Antithrombotic | 2179 (57.8) | 326 (69.1) | 119 (73.5) | <0.001 |
| ACEI | 1642 (43.6) | 202 (42.8) | 78 (48.1) | 0.478 |
| ARB | 2128 (56.5) | 267 (56.6) | 85 (52.5) | 0.597 |
| Insulin | 2403 (63.8) | 354 (75.0) | 127 (78.4) | <0.001 |
| Metformin | 2219 (58.9) | 253 (53.6) | 73 (45.1) | <0.001 |
ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blocker; BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; and UACR, urine albumin‐to‐creatinine ratio.
P<0.05 is consistent with heterogeneity across the 3 groups.
Other includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiple, other, unknown, and not reported.
The cumulative event curves for first and recurrent cardiovascular events by randomized group are shown in Figure 1. As previously reported, 5 there were 88 fewer first cardiovascular events in the canagliflozin group compared with the placebo group, resulting in a 26% hazard reduction (events/1000 patient‐years, 49.4 versus 66.9, respectively; hazard ratio, 0.74 [95% CI, 0.63–0.86]; P<0.001). In addition to the reduction in first cardiovascular events, there were 51 fewer subsequent events in the canagliflozin group (99 events in the canagliflozin group versus 150 events in the placebo group), resulting in a 139 fewer total cardiovascular events during the trial follow‐up (total events 372 versus 511, respectively; incidence rate ratio, 0.71 [95% CI, 0.59–0.86]; P<0.001; Figure 2). Total events for each of the composite end points were also reduced with canagliflozin (Figure 3 and Figures S1 through S2).
Figure 1. Cumulative rate of total (first and subsequent) cardiovascular events and time to cardiovascular composite outcomes.
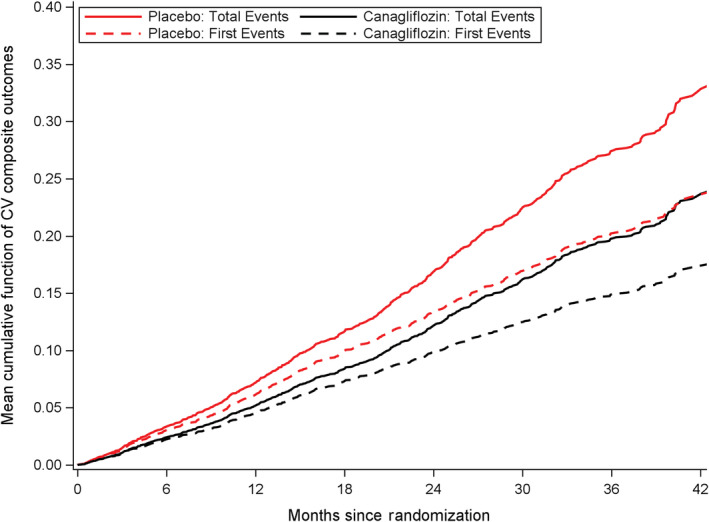
CV indicates cardiovascular.
Figure 2. Total primary end point events by randomized therapy.
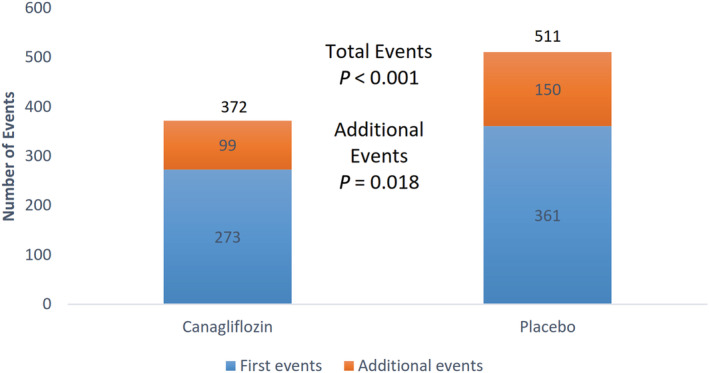
P value was produced by negative binomial regression.
Figure 3. Forest plot of total cardiovascular composite end points and individual component end points.
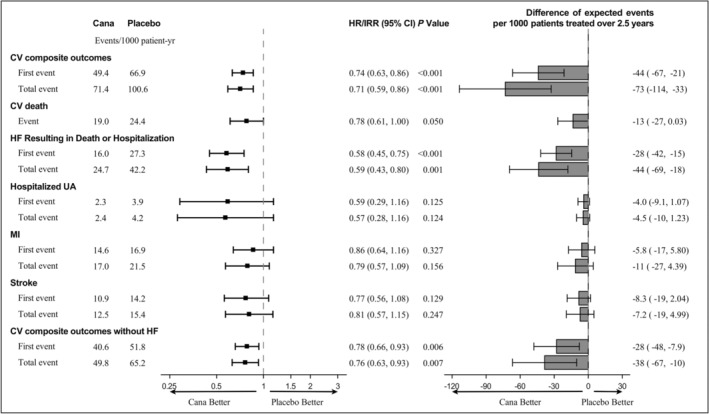
CV indicates cardiovascular; HF, heart failure; HR, hazard ratio; IRR, incidence rate ratio; MI, myocardial infarction; and UA, unstable angina.
The risk differences for every 1000 patients treated for 2.5 years with canagliflozin for the 5 components of the composite primary end point are shown in Figure 3. Approximately 73 total cardiovascular composite events could be prevented per 1000 patients within that time frame: 44 heart failures resulting in death or hospitalization, 13 cardiovascular deaths, 11 MIs, 7 strokes, and 5 hospitalizations for unstable angina, separately. The cardiovascular disease spectrum interface is shown by a Sankey diagram (Figure 4).
Figure 4. Sankey diagram for illustration of cardiovascular disease spectrum interface.
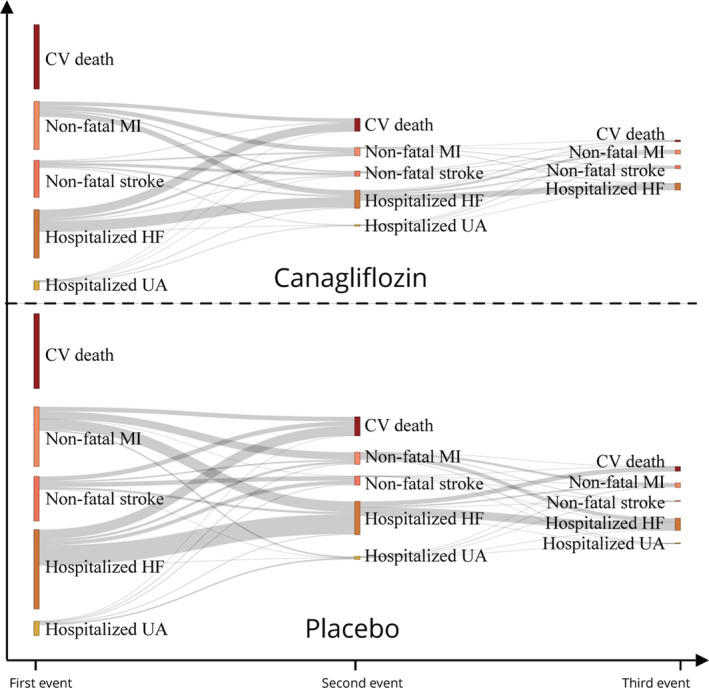
The Sankey plot depicts event transitions from the first to third events. Longer indicates more events. CV indicates cardiovascular; HF, heart failure; MI, myocardial infarction; and UA, unstable angina.
The results of sensitivity analyses including the Wei‐Lin‐Weissfeld model, the Anderson‐Gill model, and an on‐treatment analysis using a cardiovascular composite, were consistent with the overall results The hazard ratio for the second event and third event in the Wei‐Lin‐Weissfeld model is 0.59 (95% CI, 0.43–0.80; P=0.001) and 0.65 (95% CI, 0.37–1.15; P=0.141) (Figure S3 and Table S1). Subgroup analysis also showed consistent results among patient groups defined by different baseline characteristics and according to baseline cardiovascular history (Figures S4 and S5).
Discussion
In this secondary analysis of the CREDENCE trial, we showed that among patients with type 2 diabetes and CKD, canagliflozin reduced not only the relative risk of the first cardiovascular event but also the risk of total cardiovascular events. The absolute benefit was increased by more than one‐third for the prevention of total events compared with that of the first event only. Taken together, these findings suggest that canagliflozin treatment should be continued even after individuals experience a first event. The results of this analysis would suggest that a significant number of recurrent cardiovascular events would be avoided by continuing treatment in this high‐risk group.
The patients in the CREDENCE study represent a population at high risk for cardiovascular events, as indicated by the absolute risk for 3‐point major adverse cardiac events being nearly 1.5 times higher than that observed in the CANVAS (Canagliflozin Cardiovascular Assessment Study) program. 12 These rates are consistent with the accepted understanding that patients with CKD, particularly those with type 2 diabetes, are at a higher risk of cardiovascular events than those with preserved kidney function. Our previous analyses of the CREDENCE trial have focused on the effect of canagliflozin on first events, demonstrating a significant benefit on cardiorenal outcomes, but did not evaluate the ongoing benefit of canagliflozin following an incident event. It has previously been reported that ≈6% of patients with diabetes and a prior cardiovascular event will experience a recurrent major cardiovascular event every year. 13 Specifically, survivors of MI are at increased risk of recurrent MI and have an annual mortality rate of 5%. This rate is 6 times that seen in patients of the same age who do not have coronary artery disease. Similarly, patients who have suffered a stroke remain at an increased risk of further stroke (about 7% per annum). 14 Moreover, beyond the first cardiovascular event, the number of recurrent cardiovascular events predicts mortality even after accounting for the risk associated with the first event, with an 8‐fold increase risk of death in those with ≥4 recurrent events compared with those with no recurrent cardiovascular events in patients with heart failure. 15 Beyond mortality risk, heart failure events are associated with an impaired quality of life 16 and higher health care costs, 17 making prevention of these events important for patients, clinicians, and the health care system. In this trial, heart failure events were the predominant type of recurrent cardiovascular event, regardless of the nature of the first event, and accounted for nearly one‐third of the total cardiovascular burden. Patients experiencing recurrent heart failure events are at high risk for subsequent events. This new information is therefore important in demonstrating that patients experiencing an event while on canagliflozin will continue to receive benefit from the medication and do not represent a resistant population. Inclusion of total cardiovascular events, rather than just first cardiovascular event, in this analysis has enabled us to better estimate the absolute benefit of canagliflozin therapy in reducing all cardiovascular events in patients with type 2 diabetes and CKD. 18 Similar analyses from recent trials where first and recurrent cardiovascular events have been reported within this class in non‐CKD cohorts also showed significant cardiovascular benefit in patients with diabetes and heart failure. 18 , 19 , 20
SGLT2 inhibitors may prevent cardiovascular and kidney events through multiple pathways. Our previous work using mediation analysis suggests that the beneficial effect of canagliflozin in reducing heart failure events is mainly mediated by the effects on improved volume status, kidney function, and possibly uric acid levels. 21 The beneficial effect on adverse kidney events is mainly mediated through improved volume status, uric acid, and systolic blood pressure. 22 These mechanisms could partially explain the ongoing beneficial effect of canagliflozin in patients and thus the reduction in total cardiovascular events observed in those with diabetes and CKD. 6
The absolute benefits of canagliflozin in preventing total cardiovascular events in patients with type 2 diabetes and reduced kidney function have broader health service and implementation implications. Diabetes and CKD frequently coexist, and each disease independently increases the risk of cardiovascular events. 23 As such, patients with comorbid diabetes, CKD, and cardiovascular disease represent an increasingly important public health priority. In the United States national health insurance system, Medicare, non–dialysis‐requiring CKD, diabetes, and heart failure together account for close to two‐thirds of all health costs. Although patients with all 3 diseases represent only 2.1% of the population, they account for 9.0% of the total Medicare cost in the United States alone. 17 Moreover, there is growing evidence that the health care of these complex patients is often suboptimal, with treatment target gaps and failure to meet recommended health indicators such as glycated hemoglobin and underrecognition of CKD with subsequent late referral to nephrologists. 24 It is evident that patients with concomitant diabetes and CKD are a population with disproportionate rates of events, in whom optimal treatment should be prioritized. Given the broad cardiovascular and kidney benefits of canagliflozin and other SGLT2 inhibitors 3 , 25 in those with type 2 diabetes, CKD, and cardiovascular disease, commencement and continuation of these therapies should be strongly encouraged unless absolutely contraindicated.
There are limitations to this study. First, it is a post hoc analysis of a randomized controlled trial, and although we used multiple statistical models to validate our results, it should be regarded as exploratory. Second, after a first nonfatal event, a small proportion of subjects (<10%) discontinued the double‐blind study drug, which would be anticipated to lead to underestimation of the real effects of treatment on recurrent events. However, discontinuation rates were low, and an on‐treatment analysis showed findings consistent with the intention to treat analysis. Third, we used a broad composite outcome (cardiovascular death, MI, stroke, hospitalization for heart failure, or hospitalization for unstable angina) as the main analysis. These results, however, are consistent with the findings for the traditional 3‐point major adverse cardiac event outcome, shown in Figure S5. Finally, the duration of follow‐up was short, and the analyses benefit of lifelong therapy with canagliflozin on first and all recurrent cardiovascular events are therefore likely underestimated.
In conclusion, canagliflozin significantly reduced total cardiovascular events in those with type 2 diabetes and CKD, with absolute benefits significantly greater than was evident when considering only first events. These results provide further support for continuation of canagliflozin after a cardiovascular event, as patients will continue to derive benefit from ongoing treatment.
Sources of Funding
CREDENCE was sponsored by Janssen Research & Development, LLC.
Disclosures
Dr Arnott is supported by an National Health and Medical Research Council/Medical Research Future Fund Priority Investigator Grant and a New South Wales Health Early‐Mid Career Research Grant. She is an employee of the George Institute for Global Health. She has received honoraria from Amgen. Dr Cannon has received research grants from Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Merck, Janssen, and Takeda; and has received consulting fees from Aegerion, Alnylam, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Corvidia, GlaxoSmithKline, Innovent, Eisai, Eli Lilly, Kowa, Merck, Pfizer, Regeneron, and Sanofi. Dr Zeeuw reports serving on advisory boards and as a speaker for Bayer, Boehringer Ingelheim, Fresenius, Mundipharma, and Mitsubishi Tanabe; serving on Steering Committees and as a speaker for AbbVie and Janssen; and serving on Data Safety and Monitoring Committees for Bayer. Dr Heerspink has served as a consultant for Abbvie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Gilead, Janssen, Merck, and Mitsubishi‐Tanabe and has received grant support from Abbvie, AstraZeneca, Boehringer Ingelheim, and Janssen. Dr Charytan has served on clinical events committees or data safety and monitoring boards for PLC Medical, AstraZeneca, Allena Pharmaceuticals, and Merck; served on steering committees for Zoll Medical and Janssen Pharmaceuticals; and reported consulting fees or travel fees from Daichi Sankyo, Fresenius, and Medtronic/Coviden. He was on the steering committee for the kidney outcome trial of an SGLT2 inhibitor (canagliflozin; CREDENCE, 43 NCT02065791). Dr Barraclough is supported by a New South Wales Health Early‐Mid Career Research Grant and is an employee of the George Institute for Global Health. Dr Figtree reports receiving research support from the cofunded National Health and Medical Research Council and Heart Foundation (Australia) Practitioner Fellowship and the Heart Research Australia, and compensation from Janssen for serving on the Adjudication 1 Panel of the CANVAS program. Dr Bakris works for the University of Chicago Medicine. He is a consultant for Merck, Bayer, Vascular Dynamics, KBP Biosciences, Ionis, Alnylam, and Astra Zeneca. He has research support and is on the steering committee of trials for Bayer and Vascular Dynamics. He is the editor of the American Journal of Nephrology.
Dr Zinman is a consultant for Janssen, Eli Lilly, Boehringer Ingelheim, and Novo Nordisk. Dr Jardine is responsible for research projects that have received funding from Amgen, Baxter, CSL, Dimerix, Eli Lilly, Gambro, and MSD; has received advisory, steering committee, and scientific presentation fees from Akebia, Amgen, Astra Zeneca, Baxter, Bayer, Boehringer Ingelheim, Cesas Linx, Chinook, CSL, Janssen, Medscape, MSD, Roche, and Vifor; with any consultancy, honoraria or travel support is paid to her institution. Dr Perkovic has received fees for advisory boards, steering committee roles, or scientific presentations from Abbvie, Astellas, Astra Zeneca, Bayer, Baxter, BMS, Boehringer Ingelheim, Dimerix, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Retrophin, Sanofi, Servier, Vifor, and Tricida. Dr Neal is supported by an Australian National Health and Medical Research Council Principal Research Fellowship; holds a research grant for this study from Janssen; and has held research grants for other large‐scale cardiovascular outcome trials from Roche, Servier, and Merck Schering Plow; and his institution has received consultancy, honoraria, or travel support for contributions he has made to advisory boards and the continuing medical education programs of Abbott, Janssen, Novartis, Pfizer, Roche, and Servier. Dr Mahaffey's financial disclosures can be viewed at http://med.stanford.edu/profiles/kenneth‐mahaffey. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figures S1–S5
Acknowledgments
The authors thank all investigators, study teams, and patients for participating in these studies. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Maqbool M, Cooper ME, Jandeleit‐Dahm KAM. Cardiovascular disease and diabetic kidney disease. Semin Nephrol. 2018;38:217–232. doi: 10.1016/j.semnephrol.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 2. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80 [DOI] [PubMed] [Google Scholar]
- 3. Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSP, Rodgers A, Mahaffey KW, Cannon CP, Perkovic V, et al. Sodium‐glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta‐analysis. J Am Heart Assoc. 2020;9:e014908. doi: 10.1161/JAHA.119.014908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McMurray JJV, Wheeler DC, Stefánsson BV, Jongs N, Postmus D, Correa‐Rotter R, Chertow GM, Greene T, Held C, Hou F‐F, et al. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation. 2021;143:438–448. doi: 10.1161/CIRCULATIONAHA.120.051675 [DOI] [PubMed] [Google Scholar]
- 5. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 6. Jardine MJ, Mahaffey KW, Neal B, Agarwal R, Bakris GL, Brenner BM, Bull S, Cannon CP, Charytan DM, de Zeeuw D, et al. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol. 2017;46:462–472. doi: 10.1159/000484633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE‐IT. J Am Coll Cardiol. 2019;73:2791–2802. doi: 10.1016/j.jacc.2019.02.032 [DOI] [PubMed] [Google Scholar]
- 8. Pedan A. Analysis of count data using the SAS® system. 2001.
- 9. Wei L‐J, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–1073. doi: 10.1080/01621459.1989.10478873 [DOI] [Google Scholar]
- 10. Nelson WB. Recurrent Events Data Analysis for Product Repairs, Disease Recurrences, and Other Applications. SIAM; 2003. https://epubs.siam.org/doi/book/10.1137/1.9780898718454 [Google Scholar]
- 11. Allaire J, Ellis P, Gandrud C, Kuo K, Lewis B, Owen J, Russell K, Rogers J, Sese C, Yetman C. Package ‘networkD3’. D3 JavaScript Network Graphs from R. 2017.
- 12. Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 13. Giorda CB, Avogaro A, Maggini M, Lombardo F, Mannucci E, Turco S, Alegiani SS, Raschetti R, Velussi M, Ferrannini E, et al. Recurrence of cardiovascular events in patients with type 2 diabetes: epidemiology and risk factors. Diabetes Care. 2008;31:2154–2159. doi: 10.2337/dc08-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. WHO . Prevention of recurrences of myocardial infarction and stroke study. https://wwwwhoint/cardiovascular_diseases/priorities/secondary_prevention/country/en/index1html.
- 15. Lee DS, Austin PC, Stukel TA, Alter DA, Chong A, Parker JD, Tu JV. “Dose‐dependent” impact of recurrent cardiac events on mortality in patients with heart failure. Am J Med. 2009;122:162.e161–162.e169. doi: 10.1016/j.amjmed.2008.08.026 [DOI] [PubMed] [Google Scholar]
- 16. Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, Haass M. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87:235–241. doi: 10.1136/heart.87.3.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foley RN, Collins AJ. The growing economic burden of diabetic kidney disease. Curr Diabetes Rep. 2009;9:460–465. doi: 10.1007/s11892-009-0075-9 [DOI] [PubMed] [Google Scholar]
- 18. Jhund PS, Ponikowski P, Docherty KF, Gasparyan SB, Böhm M, Chiang C‐E, Desai AS, Howlett J, Kitakaze M, Petrie MC, et al. Dapagliflozin and recurrent heart failure hospitalizations in heart failure with reduced ejection fraction: an analysis of DAPA‐HF. Circulation. 2021;143:1962–1972. doi: 10.1161/CIRCULATIONAHA.121.053659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGuire DK, Zinman B, Inzucchi SE, Wanner C, Fitchett D, Anker SD, Pocock S, Kaspers S, George JT, von Eynatten M, et al. Effects of empagliflozin on first and recurrent clinical events in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a secondary analysis of the EMPA‐REG OUTCOME trial. Lancet Diabetes Endocrinol. 2020;8:949–959. doi: 10.1016/s2213-8587(20)30344-2 [DOI] [PubMed] [Google Scholar]
- 20. Kaku K, Wanner C, Anker SD, Pocock S, Yasui A, Mattheus M, Lund SS. The effect of empagliflozin on the total burden of cardiovascular and hospitalization events in the Asian and non‐Asian populations of the EMPA‐REG OUTCOME trial of patients with type 2 diabetes and cardiovascular disease. Diabetes Obes Metab. 2022;24:662–674. doi: 10.1111/dom.14626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Woodward M, Perkovic V, Figtree GA, Heerspink HJL, Mahaffey KW, de Zeeuw D, Vercruysse F, Shaw W, Matthews DR, et al. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail. 2020;8:57–66. doi: 10.1016/j.jchf.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 22. Li J, Neal B, Perkovic V, de Zeeuw D, Neuen BL, Arnott C, Simpson R, Oh R, Mahaffey KW, Heerspink HJ. Mediators of the effects of canagliflozin on kidney protection in patients with type 2 diabetes. Kidney Int. 2020;98:769–777. doi: 10.1016/j.kint.2020.04.051 [DOI] [PubMed] [Google Scholar]
- 23. Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non‐diabetic people: a population‐based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8 [DOI] [PubMed] [Google Scholar]
- 24. Lo C, Ilic D, Teede H, Cass A, Fulcher G, Gallagher M, Johnson G, Kerr PG, Mathew T, Murphy K. The perspectives of patients on health‐care for co‐morbid diabetes and chronic kidney disease: a qualitative study. PLoS One. 2016;11:e0146615. doi: 10.1371/journal.pone.0146615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neuen B, Young T, Heerspink H, Neal B, Perkovic V, Billot L, Mahaffey K, Charytan D, Wheeler D, Arnott C, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2019;7:845–854. doi: 10.1016/S2213-8587(19)30256-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S5


