Abstract
The Shigella outer membrane protein IcsA belongs to the family of type V secreted (autotransported) virulence factors. Members of this family mediate their own translocation across the bacterial outer membrane: the carboxy-terminal β domain forms a β barrel channel in the outer membrane through which the amino-terminal α domain passes. IcsA, which is localized at one pole of the bacterium, mediates actin assembly by Shigella, which is essential for bacterial intracellular movement and intercellular dissemination. Here, we characterize the transit of IcsA across the periplasm during its secretion. We show that an insertion in the dsbB gene, whose gene product mediates disulfide bond formation of many periplasmic intermediates, does not affect the surface expression or unipolar targeting of IcsA. However, IcsA forms one disulfide bond in the periplasm in a DsbA/DsbB-dependent fashion. Furthermore, cellular fractionation studies reveal that IcsA has a transient soluble periplasmic intermediate. Our data also suggest that IcsA is folded in a proteinase K-resistant state in the periplasm. From these data, we propose a novel model for the secretion of IcsA that may be applicable to other autotransported proteins.
Proteins of pathogenic gram-negative bacteria secreted by the type V secretion pathway (also known as autotransported proteins) constitute a growing family of virulence factors (12). Characteristic of this family is the capacity of each member to mediate its own translocation across the bacterial outer membrane. After transport across the inner membrane, which is thought to be Sec mediated, a carboxy-terminal β domain of the proprotein forms a β barrel channel of amphipathic antiparallel β sheets in the outer membrane through which the amino-terminal α domain is “threaded” until it reaches the surface of the bacterium (22). For most family members, in conjunction with translocation, the proprotein is proteolytically processed at the junction of the α and β domains; the α domain then either is released into the extracellular milieu or remains noncovalently associated with the β domain.
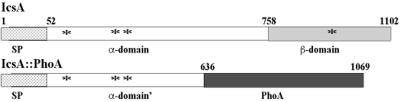
The Shigella protein IcsA (VirG) has been classified as a type V secreted protein (13, 15, 27, 12). The 1,102-amino-acid proprotein has a 52-amino-acid signal peptide that contains motifs characteristic of proteins that use the Sec machinery for secretion across the inner membrane, a 706-amino-acid α domain and a 344-amino-acid β domain (9, 27) (Fig. 1). Unlike many of the type V family members, cleavage of IcsA at the junction of the α and β domains is not autocatalytic, but rather is mediated by the protease IcsP (SopA) (6, 25). The cleaved α domain is released into the extracellular milieu (9). IcsP-mediated cleavage occurs slowly, so that during exponential growth, approximately 80% of IcsA is found uncleaved and associated with the bacterial outer membrane (25).
FIG. 1.
Schematic of native IcsA (top) and the IcsA-PhoA fusion protein (bottom) used in this study. Stippled bar, signal peptide (sp); open bar, α domain; gray bar, β domain; solid bar, alkaline phosphatase protein. Asterisks indicate the locations of cysteine residues in IcsA.
Shigella is a genus of facultative intracellular pathogens that use the host actin cytoskeleton for intracellular motility and intercellular dissemination. At the bacterial old pole, IcsA mediates the polymerization of host actin into polarized “actin tails”; assembly of these tails generates sufficient force to propel the bacterium through the host cytoplasm and into adjacent cells (28). The region of IcsA that is active in actin assembly is contained within the α domain (7; J. Magdalena and M. B. Goldberg, unpublished); however, the mature conformation of this domain is uncharacterized.
Insertion of the β domain of type V family members into the outer membrane is believed to occur spontaneously. The AMPHI algorithm predicts that the β barrel of most consists of an even number of amphipathic antiparallel β sheets, suggesting that the first and last β sheet spontaneously form hydrogen bonds in an antiparallel fashion (13). Analysis of protein fusions between PhoA and the β domain of IcsA and between the cholera toxin B subunit and the β domain of the immunoglobulin A1 (IgA1) protease of Neisseria gonorrhoeae had suggested that disulfide bond formation in the passenger domain inhibited its translocation across the outer membrane (15, 27). However, recent analysis of fusions of a single-chain antibody to the β domain of IgA1 protease indicates that certain folded passenger proteins can be translocated, albeit at reduced levels (29). Such findings suggest that proteins need not lack disulfide bonds to be competent for secretion through the β domain and that passenger domains of hybrid proteins are exposed to the periplasmic compartment during transport.
In this paper we examine the nature of native IcsA transit across the periplasm. We demonstrate that while IcsA does not require DsbB for surface expression or targeting to the old pole, it forms one intramolecular disulfide bond. In addition, in pulse-chase studies, we demonstrate that IcsA can be isolated from the periplasm in a soluble form. Furthermore, under steady-state conditions, proteinase K-resistant, truncated, soluble forms of IcsA can be isolated from the periplasm of either wild-type or dsbB Shigella. Taken together, these studies indicate that the α domain of IcsA is folded during secretion, that it forms an intramolecular disulfide bond, that its folding does not require disulfide bond formation, and that a soluble periplasmic state of IcsA is present during secretion. We propose a novel model for the secretion of IcsA that may be relevant to other autotransported proteins.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All Shigella flexneri strains were derived from serotype 2a wild-type strain 2457T (17). S. flexneri MBG283 is 2457T ΔicsA::Ω (26), MBG341 is 2457T icsP1 (25), MBG347 is MBG283(pWR100) icsP1 (26), and SS100 is 2457T galU2 (31). Escherichia coli HPT66, which is phoR araD1714 λ102ΔD1 dsbB::aph, was obtained from J. Beckwith. LDB660, which is dsbB::aph, was constructed by transduction of the dsbB::aph locus from HPT66 into 2457T.
S. flexneri strains were grown in tryptic soy broth (TCS) or M9 minimal medium (19) supplemented with 0.2% casamino acids; 0.2% glucose; 0.5 mg of thiamine, 0.5 mg of tryptophan, and 0.5 mg of nicotinic acid per ml; 0.1 mM CaCl2; and 0.5 mM MgSO4. E. coli strains were grown in Luria-Bertani broth. pHEX3, a pSU18 (3) derivative, is a cloning vector. pBAD-PhoA is pACYC-derived vector carrying phoA under the control of the arabinose promoter (D. Ritz and J. Beckwith, unpublished). Where appropriate, antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 40 μg/ml; spectinomycin, 100 μg/ml; chloramphenicol, 25 μg/ml; and tetracyline, 15 or 2.5 μg/ml (chromosomal and plasmid copy, respectively).
Construction and identification of icsA::phoA fusion strains.
Molecular and genetic techniques were performed according to standard procedures (24). A TnphoA insertional library was generated in S. flexneri 2a SS100 with a replication-deficient lambda phage carrying TnphoA (31). P1L4 grown on this library was transduced into 2457T, selecting for kanamycin resistance, encoded by TnphoA. PhoA-positive colonies were identified by overlay of alkaline soft agar [0.75% agar in 100 mM 3-(cyclohexylamino)-1-propanesulfonic acid, pH 11.0] containing 40 μg of 5-bromo-4-chloro-3-indolylphosphate-p-toluidine salt (XP) per ml, which allowed specific detection of alkaline phosphatase activity. JS11.0, which is icsA::phoA, was generated in this manner.
Isolation of dsbB mutant strains.
A screen was performed to identify factors involved in secretion of IcsA into the periplasm. Random second-site mutations in JS11.0 were generated by P1L4 transduction of a mini-Tn10 transposon library into JS11.0 (31). In JS11.0, the IcsA-PhoA fusion protein is transported to the periplasm, but since it lacks the β domain of IcsA (Fig. 1), it is not translocated to the extracellular milieu; JS11.0 has 320 U of alkaline phosphatase activity. Congo red-positive transductants of JS11.0 were qualitatively analyzed for alkaline phosphatase activity, and those that were alkaline phosphatase negative were isolated. The Tn10 insertion in each of these was then moved into the 2457T background by P1L4 transduction. The locus that contains the insertion was subcloned into pHEX3 and sequenced (31). A large percentage of the Tn10 insertions were located in the dsbB gene. LDB143, which is 2457T dsbB1::Tn10 and representative of this group of mutants, was thus derived from a transductant isolated in this screen. In LDB143, the Tn10 insertion is located immediately after base 778 of dsbB (GenBank accession no. D38254) and is oriented in the same direction as that of dsbB transcription. LDB143 has 6.2 U of alkaline phosphatase activity.
Analysis of IcsA on the bacterial surface.
Analysis of the localization of IcsA on the surface of intact bacteria was performed by indirect immunofluorescence (9). The amount of IcsA on the surface of bacterial strains was determined by modified enzyme-linked immunosorbent assay (ELISA), using intact bacteria (30), which was performed in quadruplicate.
Protein preparation and analysis.
Whole cell (4), supernatant (1), and periplasmic (21) proteins were prepared from bacteria grown in TCS or M9 medium. 4-Acetamido-4′-maleimidylstilbene-2,2′-disulfonate (AMS)-mediated mobility shift assays were conducted as described (23), except that strains were grown to an optical density at 600 nm (OD600) of 0.5 in M9 medium that contained all amino acids and nutrients except methionine and cysteine.
For pulse-chase analysis, an overnight culture of MBG341 grown in M9 medium containing glucose, all vitamins, and all amino acids except methionine and cysteine was diluted 1:20 in the same medium and grown at 37°C to an OD600 of 0.800. Trans label (>1,000 mCi/mmol) (NEN-Dupont) was added to a final concentration of 100 μCi/ml of culture. After incubation for 10 s, 1 ml of the culture was removed. The culture was then chased with 5 mM nonradiolabeled methionine and cysteine, and at 5, 10, 20, 30, 60, 120, and 300 after addition of the nonradiolabeled amino acids, 1 ml of the culture was removed.
For total cellular protein analysis, the reactions were stopped by adding them to tubes containing 1/10 volume of trichloracetic acid. For fractionation work, the reactions were stopped by adding them to cooled tubes in an NaCl-H2O bath at −15°C containing chloramphenicol (100 μg/ml) and 10 mM sodium azide (14).
Periplasmic proteins were isolated essentially as described (16). The cells were spun in a cold microcentrifuge at the highest speed and washed once with ice-cold 10 mM Tris-Cl–30 mM NaCl (pH 7.5) containing 5 μg of leupeptin per ml, 20 μg of aprotinin per ml, 500 μM pepstatin, 10 mM sodium azide, and 1 mM phenylmethyl sulfonyl fluoride. The cell pellet was resuspended in 75 μl of ice-cold 0.1 M Tris-Cl (pH 8.0)–0.5 M sucrose–0.5 mM EDTA. Then 7.5 μl of 2-mg/ml lysozyme was added, and 75 μl of ice-cold water was added. The suspension was incubated on ice for 25 min, after which 2 μl of cold 1 M MgCl2 was added. Following centrifugation in a microcentrifuge for 5 min at maximum speed, the supernatant contained the periplasmic fraction, and the pellet contained the cytoplasmic and membrane fractions. The supernatant (periplasmic fraction) was then subjected to ultracentrifugation (TLA110 rotor; Beckman) at 100,000 rpm for 1 h. Immunoprecipitation was performed on the supernatant from ultracentrifugation using polyclonal antibodies to KdpE and β-lactamase (5′-3′ Inc., Boulder, Colo.) and monoclonal antibodies to IcsA; the antibody-protein complexes were precipitated with Gamma-Bind Plus (Pharmacia).
For proteinase K analyses, bacterial proteins were fractionated as described by Neu and Heppel (21), with the following modifications. Prior to hypertonic treatment, all bacterial samples were washed in the presence of 2.5 μg of proteinase K (Sigma) per ml to remove surface-localized IcsA. During hypertonic treatment, bacteria were incubated in the presence of either proteinase K at 20 μg/ml (proteinase K-treated samples) or 50 mM N-α-p-tosyl-l-arginine methyl ester (TAME) (control samples) for 10 min at 25°C. At the end of hypertonic treatment, 50 mM TAME was immediately added to all bacterial samples to stop the proteinase K reaction. Finally, the periplasmic proteins were released by hypotonic osmotic shock.
For proteinase K analysis of the isolated α domain of IcsA, strain 2457T was grown in supplemented M9 medium to an OD600 of 0.8. The cells were removed by centrifugation, and the culture supernatant was passed through a 0.22-μm cellulose-acetate filter (Corning) to remove any remaining intact cells. The supernatant fraction was then concentrated 50-fold using a Centriprep 20 concentrator (Amicon). The resulting fraction was left untreated or treated with 0.1 M dithiothreitol (DTT) or 0.1 M DTT plus 2% sodium dodecyl sulfate (SDS) prior to boiling for 5 min. These three fractions were each then digested with proteinase K (20 μg/ml) and the digestions were stopped with 50 mM TAME.
Western blot analysis was performed using monoclonal antibodies VIF6 and VIF8 that were column purified from SCID mouse ascites, rabbit IcsA antiserum (9), rabbit IcsA β domain antiserum (26), rabbit KdpE antiserum (L.D. Brandon, unpublished data), rabbit bovine alkaline phosphatase antiserum (5′-3′ Inc.), or rabbit β-galactosidase antiserum (5′-3′ Inc.). Enhanced chemiluminescence was performed using ECL (Amersham) for polyclonal antisera or Super Signal (Pierce) for monoclonal antibodies. Enzymatic assays for alkaline phosphatase and β-galactosidase activity of various strains were performed as described (18, 19).
Construction of leader peptidase (LepB)-resistant IcsA.
To generate a LepB-resistant form of IcsA, the upstream region of IcsA (bases 51 to 877, GenBank accession no. M22802) was subcloned as an SphI-XbaI fragment into pALTER-1 (Promega). Ala49 and Ala51 were changed to arginines by site-directed mutagenesis according to the manufacturer's instructions (Altered Sites II in vitro mutagenesis system; Promega) using primer 5′-CCCGAAAGAGGAGTACGAAAACGTATTGGCCCCCCGAG-3′. An NheI site was introduced upstream of the icsA ATG start site for cloning purposes, using primer 5′-GAATTTGATTCATGCACTATGCTAGCAGTAAGTGGTTGAT-3′. The construct was verified by sequencing, and the NheI-XbaI fragment was then exchanged into pMBG472 (26), in which icsA is expressed under the control of the arabinose promoter. This vector and pBAD-phoA (gift of J. Beckwith) were introduced into MBG347 to generate LDB631, and pMBG472 and pBAD-phoA were introduced into MBG347 to generate LDB632.
RESULTS
Presence of IcsA in the soluble periplasmic fraction.
Studies in which passenger proteins have been fused to the β domain of autotransported proteins suggest that these proteins have a periplasmic intermediate. To date, native forms of IcsA and other type V secreted proteins have not been isolated from the soluble periplasmic fraction under steady-state conditions (13, 27). To explore the nature of the IcsA periplasmic intermediate, we examined the distribution of IcsA in subcellular fractions of S. flexneri proteins by pulse-chase analysis (Fig. 2).
FIG. 2.
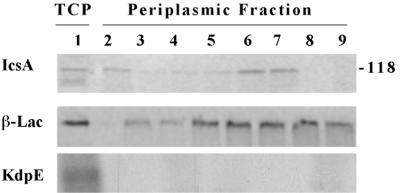
IcsA in the soluble periplasmic fraction; Pulse-chase and immunoprecipitation analyses of MBG341. Lane 1, total cellular protein (TCP) after pulse for 10 s. Lanes 2 to 9, periplasmic proteins: lane 2, after pulse for 10 s; lanes 3 to 9, after pulse for 10 s and chase for 5, 10, 20, 30, 60, 120, and 300 s, respectively. (A) Immunoprecipitated with monoclonal antibodies to IcsA; (B) immunoprecipitated with polyclonal antibodies to β-lactamase (β-Lac); and (C) immunoprecipitated with polyclonal antibodies to KdpE. Apparent molecular masses of standard proteins run in parallel are indicated (in kilodaltons). Results from one representative experiment of three are shown.
To provide a marker for periplasmic proteins, we employed MBG341 (25), in which a cassette that encodes β-lactamase is inserted in the icsP gene. Since IcsA is inefficiently cleaved from the bacterial surface of strain MBG341 (26), its use also enabled us to minimize contamination of the soluble periplasmic fraction with the cleaved form of IcsA from the external milieu. This was important because in preliminary experiments conducted with 2457T and MBG341, it was apparent that small amounts of IcsA that had been cleaved from the bacterial surface by IcsP contaminated the periplasmic fraction of 2457T but not of MBG341 (data not shown). As previously reported for icsP mutant strains (25), MBG341 allows surface presentation and unipolar targeting of IcsA.
Cells were metabolically labeled with [35S]-methionine-cysteine for 10 s, and the label was then chased with nonradiolabeled methionine and cysteine for up to 5 min. Protein fractionation of MBG341 was performed, with isolation of the periplasmic, pellet, and supernatant fractions. The pellet fraction contains inner membranes, outer membranes, and cytoplasmic proteins. The periplasmic and supernatant fractions were analyzed for the presence of IcsA using immunoprecipitation. The periplasmic fraction was similarly analyzed for the presence of the periplasmic marker β-lactamase and the cytoplasmic marker KdpE, a transcriptional activator (20). IcsA was found in the soluble periplasmic fraction, migrating at the size of its mature form (Fig. 2). The amount of IcsA in the periplasm peaked at chase times of 30 to 60 s (Fig. 2, lanes 6 and 7). IcsA was observed as a weak signal in the supernatant at 120 to 300 s (data not shown), which suggests that periplasmic IcsA chases into the outer membrane, where a proportion of it is cleaved, and therefore that the periplasmic state is not the result of an off-pathway. That the band immunoprecipitated by IcsA antibody comigrated with IcsA was verified by Western blot analysis (data not shown). As expected, β-lactamase was found in the periplasmic fraction, while KdpE was not (Fig. 2). The absence of KdpE in this fraction indicates that the IcsA found in the periplasmic fraction could only be attributed to proteins isolated from this fraction and not to contaminating soluble proteins isolated from the cytoplasmic fraction. These data indicate that in strains that are wild type with respect to icsA, IcsA is transiently detectable in its mature form in the soluble periplasmic fraction.
DsbB-dependent disulfide bond formation in IcsA during secretion.
There are four cysteine residues in mature IcsA: three within the α domain (residues 130, 376, and 380) and one within the β domain (residue 1016) (asterisks, Fig. 1). The presence of IcsA in the periplasm during secretion suggested that native IcsA might form a disulfide bond(s). To examine this, IcsA was evaluated for free cysteine residues under reducing and nonreducing conditions in the wild-type background and in the dsbB1 mutant LDB143 (Fig. 3). LDB143 had been derived from a screen for genetic loci involved in secretion of IcsA (see Materials and Methods). DsbB is involved in periplasmic disulfide bond formation in that it reoxidizes the periplasmic disulfide oxidoreductase DsbA, which oxidizes target proteins; dsb mutants accumulate reduced forms of periplasmic and outer membrane proteins.
FIG. 3.
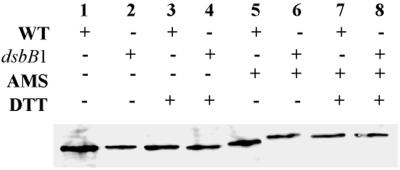
Disulfide bond formation in IcsA; gel mobility of reduced and nonreduced IcsA treated with an alkylating agent (AMS). Western blot analysis of IcsA in total cellular protein extracts from 2457T (wild type [WT]) (lanes 1, 3, 5, and 7) and LDB143 (dsbB1) (lanes 2, 4, 6, and 8) is shown. Results from one representative experiment of three are shown.
To determine the number of free cysteines, the gel mobility of IcsA was analyzed in the presence and absence of AMS under reducing and nonreducing conditions in the wild-type background and in strain LDB143 (dsbB1) (Fig. 3). AMS specifically alkylates free cysteine residues, resulting in an increase in molecular mass of 0.5 kDa for each alkylated cysteine (23). In the wild-type background, in the absence of DTT, IcsA showed a noticeable reduction in gel mobility after treatment with AMS (Fig. 3, lane 5), which indicates that native IcsA contains at least one free cysteine. Under strongly reducing conditions, IcsA shows a further reduction in gel mobility after treatment with AMS (Fig. 3, lane 7), which indicates that cysteines are made accessible by reduction, i.e., that IcsA forms a disulfide bond. In the dsbB1 strain, in the presence of AMS but absence of DTT, IcsA showed a mobility shift equivalent to that of IcsA in the wild-type background following AMS treatment under strongly reducing conditions (Fig. 3, lanes 6 to 8), which demonstrates that IcsA disulfide bond formation is dependent on DsbB.
Of note, these and subsequent studies were conducted on strains grown in M9 medium that contained all nutrients and amino acids except methionine and cysteine. This was to ensure that the observed phenotypes could be attributed to the dsbB mutation and not to oxidoreductase properties found in rich medium or in minimal medium that contains methionine and/or cysteine (5). dsb strains grown in the presence of methionine and/or cysteine will form disulfide bonds, albeit slowly.
Surface presentation and unipolar targeting of IcsA are dsbB independent.
In wild-type Shigella, IcsA is localized to one pole on the bacterial surface. To test whether dsbB was required for efficient translocation of IcsA to the bacterial surface, we tested whether presentation of IcsA on the bacterial surface was reduced in the dsbB background. To rule out any polar effects that might be mediated by the transposon insertion in LDB143, we constructed a strain with a nonpolar dsbB insertion (LDB660) and examined its phenotype in parallel.
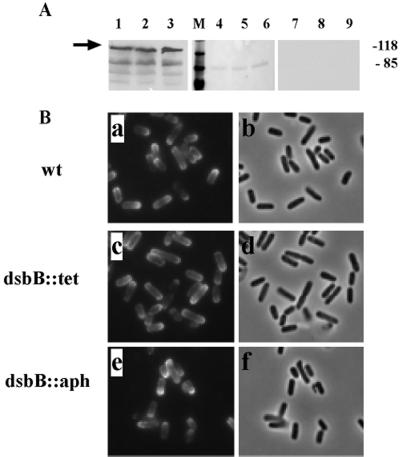
Presentation of IcsA on the bacterial surface was determined by modified ELISA of intact bacteria. From approximately 5 × 105 bacteria, the signal was 0.35 ± 0.07 arbitrary units (mean ± standard deviation) from the wild type, 0.31 ± 0.08 arbitrary units from LDB660 (dsbB::aph), and 0.28 ± 0.09 arbitrary units from LDB143 (dsbB1::Tn10). With serial dilutions of bacteria or antibody, the signal decreased approximately equivalently for the three strains. Thus, while the amount of IcsA on the dsbB mutants tended to be lower than that on the wild type, the differences were small and significant only for LDB143. Overall, these data indicate that translocation of IcsA to the bacterial surface is largely dsbB independent. Of note, in contrast to the observed IcsP-mediated cleavage of IcsA from the bacterial surface that is known to occur on bacteria grown in rich medium (6, 25), IcsA cleavage from the surface of each of these strains (including the wild type) was markedly reduced in minimal medium (Fig. 4A), which suggests that IcsP may be poorly expressed or relatively inactive under these growth conditions.
FIG. 4.
Effects of the dsbB mutation on the surface presentation and unipolar targeting of IcsA. (A) Expression and surface cleavage of IcsA. Lanes 1 to 3, Western blot analysis of total cellular protein extracts (arrow indicates IcsA); lanes 4 to 9, supernatant proteins on nitrocellulose, stained with Ponceau S, showing presence of protein (lanes 4 to 6), or probed by Western blot for IcsA, showing lack of secreted and cleaved IcsA (lanes 7 to 9). Lanes 1, 4, and 7, 2457T (wild type [wt]); lanes 2, 5, and 8, LDB143 (dsbB1); lanes 3, 6, and 9, LDB660 (dsb::aph). Apparent molecular masses of standard proteins run in parallel are indicated (in kilodaltons). (B) Localization of IcsA on the bacterial surface. Indirect immunoflourescence (a, c, and e) and corresponding fields by phase microscopy (b, d, and f). (a and b) 2457T; (c and d) LDB143; (e and f) LDB660.
To determine whether dsbB was required for unipolar targeting of IcsA, we examined the surface localization of IcsA in the dsbB mutant background. As shown in Fig. 4, unipolar targeting of IcsA in LDB660 (dsbB::aph) and LDB143 (dsbB1::Tn10) was identical to that in 2457T (wild type) (Fig. 4B). Moreover, the distribution of IcsA on the surface of all these strains grown in M9 media was consistent with the IcsP− phenotype (Fig. 4B) (25).
Presence of proteinase K-resistant IcsA fragments in the soluble periplasmic fraction.
Since IcsA forms disulfide bonds and can be found in a soluble form in the periplasm, we were interested in the folded state of IcsA during its translocation through the periplasm. To this end, subcellular fractionation was performed in the presence of proteinase K. Since we wished to examine species of IcsA found only in the periplasm, surface IcsA was removed from all bacteria by washing with proteinase K prior to hypertonic treatment of the bacteria. During hypertonic treatment, bacteria were incubated in either the presence or the absence of proteinase K. Finally, to limit proteolytic cleavage to proteins located in the periplasm, proteinase K was inactivated prior to the hypotonic extraction of periplasmic contents. That the approach leads to proteinase K-mediated cleavage of periplasmic contents that are specific to those organisms treated with proteinase K during hypertonic treatment is demonstrated by the presence of proteolytic fragments of alkaline phosphatase in the presence but not in the absence of proteinase K treatment (Fig. 5A, middle panel).
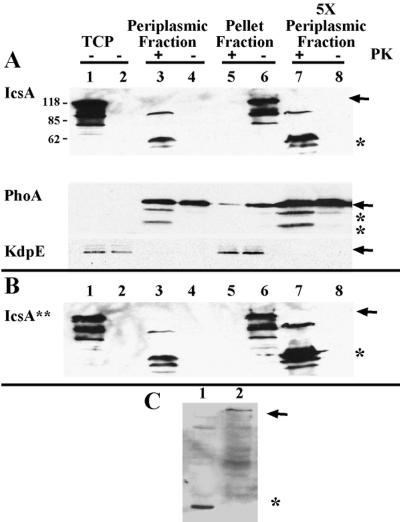
FIG. 5.
Folded state of IcsA in the periplasm. (A) Proteinase K treatment of native IcsA from LDB632 (IcsA) and (B) the LepB-resistant form of IcsA from LDB631 (IcsA∗∗). Shown are periplasmic proteins (lanes 3, 4, 7, and 8), and pellet fractions, which contain cytoplasmic and inner and outer membrane fractions (lanes 5 and 6), from LDB631 and LDB632 in the presence (lanes 3, 5, and 7) or absence (lanes 4, 6, and 8) of proteinase K. Western blot analysis was done with polyclonal antibodies to IcsA, PhoA, or KdpE. Protein (10 μg) was loaded into each of lanes 1 to 6; 50 μg of protein was loaded into each of lanes 7 and 8. Lane 1, total cellular protein (TCP) of 2457T (wild type); lane 2, total cellular protein of MBG283 (icsA); lanes 3 to 8, LDB631 (IcsA∗∗) or LDB632 (IcsA). Arrows indicate full-length proteins. Asterisks indicate proteins truncated by proteinase K treatment. Results from one representative experiment of three are shown. (C) Western blot analysis of pellet fraction of proteins isolated from LDB631 in the presence (lane 1) or absence (lane 2) of proteinase K, using polyclonal antibodies to the β domain of IcsA. Arrow indicates full-length IcsA. Asterisk indicates protein truncated by proteinase K treatment.
Native IcsA was expressed under the control of a tightly regulated arabinose promoter in an icsA icsP mutant background that also contained phoA under the control of the arabinose promoter (strain LDB632). In the soluble periplasmic fraction, while IcsA was not isolated in the absence of proteinase K (Fig. 5A, top panel, lanes 4 and 8), it was detected in the presence of proteinase K as a 62-kDa band (Fig. 5A, top panel, lanes 3 and 7) using antisera to the α domain of IcsA. When antiserum that recognizes the β domain of IcsA (which migrates as a 35-kDa band [26]) was used, a 35-kDa fragment was seen in the pellet fraction of proteins prepared in the presence of proteinase K but not in its absence (Fig. 5C, lanes 1 and 2). The intact protein was seen at the predicted size of 120 kDa in the pellet fraction of proteins prepared in the absence of proteinase K (Fig. 5A, lane 6, and Fig. 5C, lane 2). Alkaline phosphatase (a periplasmic marker) was also susceptible to proteinase K treatment (Fig. 5A, lanes 3 and 7), while the cytoplasmic marker KdpE was not (Fig. 5A, lane 5).
A leader peptidase (LepB)-resistant form of IcsA (LDB631) was analyzed in a similar fashion. LepB cleaves IcsA after Ala51 in the sequence Ala49-Phe-Ala-Thr-Pro53. The LepB-resistant form of IcsA was constructed by changing Ala49 and Ala51 (at positions −3 and −1, respectively) to arginine residues; as a result, the IcsA preprotein would be predicted to be resistant to LepB cleavage and to remain stably associated with the inner membrane and the periplasm during secretion (8). By indirect immunofluorescence analysis, native IcsA (strain LDB632) was seen translocated to the bacterial surface, while the LepB-resistant form of IcsA (strain LDB631) was not, whereas by Western blot analysis, IcsA was expressed in each of these strains (data not shown). This suggested that the LepB-resistant form was indeed trapped in the membranes and the periplasm during secretion.
When the proteinase K experiments were conducted with the LepB-resistant form of the IcsA construct (strain LDB631), greater amounts of the truncated forms of IcsA were found in the periplasmic fraction in the presence of proteinase K (Fig. 5B); however, the periplasmic bands migrated at the same positions as they had in the wild-type IcsA strain. Thus, fragments of IcsA can be released from the periplasm with proteinase K treatment when the signal peptide is not cleaved, which enriches for the preprotein found in the periplasm (LDB631), and when the signal peptide is processed normally (LDB632).
To determine whether the folded periplasmic state of IcsA required disulfide bond formation, native IcsA was also expressed in a strain that was dsbB::aph but otherwise isogenic to LDB632. IcsA isolated from the periplasmic compartment of this strain (LDB662) showed the same proteinase K protection pattern as IcsA isolated from LDB632, which is wild type with respect to dsbB (Fig. 6A).
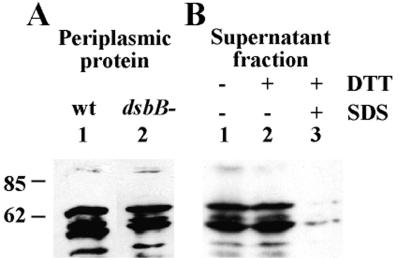
FIG. 6.
Folded state of IcsA in the periplasm and the external milieu. (A) Proteinase K treatment of periplasmic IcsA in a dsbB+ (lane 1) and dsbB mutant (lane 2) background. (B) Proteinase K treatment of IcsA isolated from the culture supernatant of wild-type (wt) Shigella. Lanes: untreated (lane 1), treated with DTT (lane 2) or treated with DTT and then denatured with SDS (lane 3). Sizes are shown in kilodaltons.
The α domain of IcsA is normally cleaved from the bacterial surface by IcsP. The pattern of folding of the isolated α domain was analyzed from protein that had been harvested from the culture supernatant. The α domain, which had been untreated (Fig. 6B, lane 1), reduced with DTT (lane 2), or reduced with DTT and then denatured with SDS (lane 3), was subjected to proteinase K treatment. Surprisingly, isolated α domain showed a proteinase K-resistant pattern identical to that of protein isolated from the periplasmic compartment (Fig. 6B, lane 1, and 6A, lane 1, respectively). Moreover, after subjection to strongly reducing conditions, isolated α domain showed the same proteinase K-resistant pattern as untreated protein (Fig. 6B, lanes 2 and 1, respectively). However, the 62-kDa fragment of the α domain was vulnerable to proteinase K cleavage when it had been both reduced and denatured before proteinase treatment (Fig. 6B, lane 3).
These data demonstrate that IcsA assumes a folded state so that it is resistant to proteolysis by proteinase K and suggest that IcsA may assume a conformation that protects it from degradation during the course of translocation from the cytoplasm to the outer membrane. This conformation is maintained after its localization to the bacterial surface. Finally, while disulfide bonds may stabilize the folded structure of IcsA, surface-presented and periplasmic forms of IcsA do not require the formation of disulfide bonds to assume a proteinase K-resistant conformation.
DISCUSSION
The mechanism of secretion of members of the family of gram-negative autotransported (type V secreted) proteins is unique, involving assisted translocation across the cytoplasmic membrane and self-translocation across the outer membrane. To date, the intermediate process of transit across the periplasm has been poorly understood. Full-length, mature, soluble, periplasmic forms of native IcsA and other autotransported proteins have not previously been reported. While passenger proteins fused to the β domain of these proteins are able to form disulfide bonds in the periplasm, these studies only indirectly suggest that a periplasmic intermediate state would occur during secretion of the native proteins.
Here, we examine the periplasmic state of native IcsA. The data presented indicate that the secreted protein IcsA can be found in the soluble periplasmic fraction in a folded, protease-resistant state. The 62-kDa band detected in proteinase K-treated periplasmic fractions consists of α domain, since it is recognized by α domain antiserum. The β domain that is left behind in the membrane migrates at the size of intact β domain (Fig. 5C) (26). The isolation from the periplasm of a 62-kDa α domain fragment that is relatively resistant to proteinase K digestion suggests that the bulk of the 72-kDa α domain is in a folded conformation in the periplasm (Fig. 7, panel 3). The species of IcsA detected in the LepB-resistant mutant migrated at the same size as those detected in the native IcsA construct, which indicates that the 62-kDa band does not contain any of the signal peptide, but rather contains only the α domain, since the signal peptide portion would have been cleaved in the native construct. This further indicates that, while the LepB site mutation led to trapping of IcsA in the process of secretion, it did not alter per se the region of IcsA that was accessible to proteinase K in the periplasm. Further, the presence of the 35-kDa β domain band in the pellet fraction indicates that the C-terminal proteinase K cleavage occurred near the junction of the α and β domains, indicating that the β domain was inaccessible because of its prior insertion into the outer membrane.
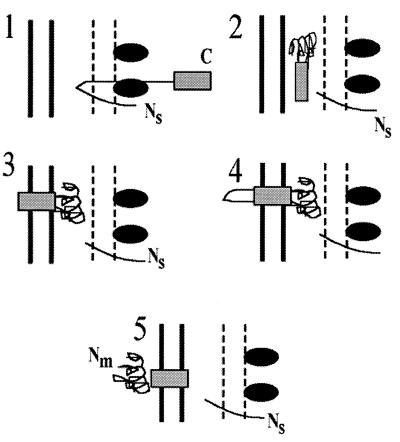
FIG. 7.
Model for IcsA secretion. 1, IcsA is secreted across the inner membrane to the periplasm by the Sec machinery, where the signal peptide is cleaved. 2, IcsA can be found in a soluble proteinase K-resistant, folded state within the periplasm. 3, the β domain (solid box) becomes rapidly associated with the outer membrane. 4 and 5, the α domain of the mature protein (Nm) is relatively slowly translocated through the β domain, placing the α domain onto the bacterial surface, where it maintains or resumes the folded state it had in the periplasm. Ns, amino terminus of signal peptide; Nm, amino terminus of mature IcsA; C, carboxy terminus of IcsA; solid box, β domain of IcsA; ovals, Sec machinery.
These observations, in conjunction with the ability to detect IcsA in proteinase K-treated periplasmic fractions from the native IcsA construct, suggest that the insertion of the β domain into the outer membrane is a relatively rapid process, while the passage of the α domain through the β domain is the rate-limiting step in secretion. This is further substantiated by the observation that soluble periplasmic forms of native IcsA are not observed under steady-state conditions and can only be isolated by more sensitive assays (i.e., pulse-chase experiments). Thus, the relatively small population of IcsA found in the soluble fraction suggests that, upon secretion across the inner membrane, IcsA rapidly becomes associated with the outer membrane.
These data support a model in which, following IcsA secretion across the inner membrane, IcsA is transiently associated with the periplasmic compartment, where it folds into a structure that is resistant to periplasmic proteases. The β domain becomes associated with the outer membrane, and the amino terminus of the mature α domain passes through the “channel” formed by the β domain (Fig. 7). In conjunction with these translocation events across the inner and outer membranes, IcsA forms one disulfide bond in the periplasm, which likely stabilizes the folded structure and mediates its resistance to proteolysis by periplasmic proteases. Finally, IcsA that is on the bacterial surface maintains the folded conformation that it had formed in the periplasm. Whether the α domain is transiently unfolded to pass through the channel created by the β domain is not known.
Oxidation of sulfhydryl groups on cysteine residues to form intramolecular disulfide bonds is mediated by the periplasmic disulfide oxidoreductase DsbA (10); reoxidation of DsbA is mediated by the inner membrane protein DsbB (11). Disulfide bonds are formed in many secreted proteins, including many virulence factors of enteric pathogens, as they pass through the periplasmic compartment. Data presented here demonstrate that IcsA does not require DsbB for its presentation or unipolar localization on the bacterial surface and suggest that IcsA assumes a folded conformation even in the absence of disulfide bond formation, as has been reported for other outer membrane proteins that normally contain disulfide bonds (2). The lack of requirement for DsbB in these processes infers DsbA independence as well. Of note, methionine and cysteine in the growth medium can mediate disulfide bond formation independently of Dsb (5); in this work, all assays for disulfide bond formation were performed in the absence of methionine and cysteine. However, our data reveal that IcsA forms at least one disulfide bond. IcsA has relatively few cysteine residues compared to many secreted proteins, only three in the α domain and one in the β domain. Presumably, the cysteine in the β domain (Fig. 1) would be inaccessible for disulfide bond formation, which suggests that the disulfide bond is formed between two of the three cysteines in the α domain.
The question of whether IcsA requires an intramolecular disulfide bond for its activity, as indicated by its ability to assemble actin tails in the cytoplasm of infected cells, is more difficult to analyze. While dsbB strains are able to form actin tails in the cytoplasm of infected cells (data not shown), it is not possible to determine whether the cytoplasm of these cells contains oxidoreductase properties that may bypass the requirement for Dsb-mediated disulfide bond formation. The studies of Yu et al. (32), in which dsbA Shigella was able to move within the cytoplasm of epithelial cells, must be interpreted with the same caution. This is exemplified by our observation that in rich medium or M9 medium containing complete amino acids, IcsA forms a disulfide bond in a DsbB-independent manner (data not shown).
All type V secretion family members have a carboxy-terminal β domain that is thought to form a β barrel channel of amphipathic antiparallel β sheets in the outer membrane, through which the α domain passes. Many family members have a signal peptide similar in organization to that of IcsA, with a carboxy-terminal region that contains motifs characteristic of proteins that use the Sec machinery and an extensive amino-terminal tail of unknown function. From these domain similarities, it seems likely that secretion of other type V secretion family members will involve many of the secretion properties shown here for IcsA.
ACKNOWLEDGMENTS
We thank Dana Boyd, Daniel Ritz, and Jon Beckwith for E. coli HPT66 and pBAD-phoA and Renate Lux and Shahid Khan for pHEX3. We thank Thao Pham for technical assistance and Eric Rubin and Cathy Lee for their critical commentary on the text.
This work was supported by NIH grant AI35817 (M.B.G.), American Heart Association Established Investigator (M.B.G.) and Grant-In-Aid (M.B.G.) awards, and the National Foundation for Infectious Diseases Colin L. Powell Minority Postdoctoral Fellowship in Tropical Disease Research (L.D.B.).
REFERENCES
- 1.Allaoui A, Mounier J, Prevost M-C, Sansonetti P J, Parsot C. icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol Microbiol. 1992;6:1605–1616. doi: 10.1111/j.1365-2958.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 2.Bardwell J C, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 3.Bartolome B, Jubete Y, Martinez E, de-la-Cruz F. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene. 1991;102:75–78. doi: 10.1016/0378-1119(91)90541-i. [DOI] [PubMed] [Google Scholar]
- 4.Bernardini M L, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus-of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debarbieux L, Beckwith J. On the functional interchangeability, oxidant versus reductant, of members of the thioredoxin superfamily. J Bacteriol. 2000;182:723–727. doi: 10.1128/jb.182.3.723-727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egile C, d'Hauteville H, Parsot C, Sansonetti P J. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol Microbiol. 1997;23:1063–1073. doi: 10.1046/j.1365-2958.1997.2871652.x. [DOI] [PubMed] [Google Scholar]
- 7.Egile C, Loisel T P, Laurent V, Li R, Pantaloni D, Sansonetti P J, Carlier M-F. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J Cell Biol. 1999;146:1319–1332. doi: 10.1083/jcb.146.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fikes J D, Barkocy-Gallagher G A, Klapper G A, Bassford J P J. Maturation of Escherichia coli maltose-binding protein by signal peptidase I in vivo: sequence requirements for efficient processing and demonstration of an alternate cleavage site. J Biol Chem. 1990;265:3417–3423. [PubMed] [Google Scholar]
- 9.Goldberg M B, Barzu O, Parsot C, Sansonetti P J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J Bacteriol. 1993;175:2189–2196. doi: 10.1128/jb.175.8.2189-2196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grauschopf U, Winter J R, Korber P, Zander T, Dallinger P, Bardwell J C. Why is DsbA such an oxidizing disulfide catalyst? Cell. 1995;83:947–955. doi: 10.1016/0092-8674(95)90210-4. [DOI] [PubMed] [Google Scholar]
- 11.Guilhot C, Jander G, Martin N L, Beckwith J. Evidence that the pathway of disulfide bond formation involves interactions between the cysteines of DsbB and DsbA. Proc Natl Acad Sci USA. 1995;92:9895–9899. doi: 10.1073/pnas.92.21.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson I R, Nataro J P, Kaper J B, Meyer T F, Farrand S K, Burns D L, Finlay B B, St. Geme J W., III Renaming protein secretion in the Gram-negative bacteria. Trends Microbiol. 2000;8:352. [PubMed] [Google Scholar]
- 13.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 14.Jacob-Dubuisson F, Striker R, Hultgren S. Chaperone-assisted self-assembly of pili independent of cellular energy. J Biol Chem. 1994;269:12447–12455. [PubMed] [Google Scholar]
- 15.Jose J, Kramer J, Klauser T, Pohlner J, Meyer T F. Absence of periplasmic DsbA oxidoreductase facilitates export of cysteine-containing passenger proteins to the Escherichia coli cell surface via the Iga beta autotransporter pathway. Gene. 1996;178:107–110. doi: 10.1016/0378-1119(96)00343-5. [DOI] [PubMed] [Google Scholar]
- 16.Koshland D, Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980;20:749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- 17.LaBrec E H, Schneider H, Magnani T J, Formal S B. Epithelial cell penetration as an essential step in the pathogenesis of bacillary dysentery. J Bacteriol. 1964;88:1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michaelis S, Inouye H, Oliver D, Beckwith J. Mutations that alter the signal sequence of alkaline phosphatase. J Bacteriol. 1983;154:366–374. doi: 10.1128/jb.154.1.366-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 20.Nakashima K, Suguira A, Momoi H, Mizuno T. Phosphotransfer signal transduction between two regulatory factors involved in the osmoregulated kdp operon in Escherichia coli. Mol Microbiol. 1992;6:1777–1784. doi: 10.1111/j.1365-2958.1992.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 21.Neu H C, Heppel L A. The release of enzymes from Escherichia coli by osmotic shock during the formation of spheroplasts. J Biol Chem. 1965;240:3685–3692. [PubMed] [Google Scholar]
- 22.Pohlner J, Halter R, Beyreuther K, Meyer T F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 23.Ritz D, Patel H, Doan B, Zheng M, Asland F, Storz G, Beckwith J. Thioredoxin 2 is involved in the oxidative stress response in Escherichia coli. J Biol Chem. 2000;275:2505–2512. doi: 10.1074/jbc.275.4.2505. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Shere K D, Sallustio S, Manessis A, D'Aversa T G, Goldberg M B. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol Microbiol. 1997;25:451–462. doi: 10.1046/j.1365-2958.1997.4681827.x. [DOI] [PubMed] [Google Scholar]
- 26.Steinhauer J, Agha R, Pham T, Varga A W, Goldberg M B. The unipolar Shigella surface protein IcsA is directly targeted to the old pole: IcsP cleavage of IcsA occurs over the entire bacterial surface. Mol Microbiol. 1999;32:367–378. doi: 10.1046/j.1365-2958.1999.01356.x. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Lett M-C, Sasakawa C. Extracellular transport of VirG protein in Shigella. J Biol Chem. 1995;270:30874–30880. doi: 10.1074/jbc.270.52.30874. [DOI] [PubMed] [Google Scholar]
- 28.Theriot J A, Mitchison T J, Tilney L G, Portnoy D A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992;357:257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- 29.Veiga E, de Lorenzo V, Fernandez L A. Probing secretion and translocation of a beta-autotransporter using a reporter single-chain Fv as a cognate passenger domain. Mol Microbiol. 1999;33:1232–1243. doi: 10.1046/j.1365-2958.1999.01571.x. [DOI] [PubMed] [Google Scholar]
- 30.Way S S, Borczuk A C, Goldberg M B. Thymic independence of adaptive immunity to the intracellular pathogen Shigella flexneri serotype 2a. Infect Immun. 1999;67:3970–3979. doi: 10.1128/iai.67.8.3970-3979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Way S S, Sallustio S, Magliozzo R S, Goldberg M B. The impact of either elevated or decreased levels of cytochrome bd expression on Shigella flexneri virulence. J Bacteriol. 1999;181:1229–1237. doi: 10.1128/jb.181.4.1229-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J. Inactivation of DsbA, but not DsbC and DsbD, affects the intracellular survival and virulence of Shigella flexneri. Infect Immun. 1998;66:3909–3917. doi: 10.1128/iai.66.8.3909-3917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]