Abstract
Background
Initial protocols for return to play cardiac testing in young competitive athletes following SARS‐CoV‐2 infection recommended cardiac troponin (cTn) to screen for cardiac involvement. This study aimed to define the diagnostic yield of cTn in athletes undergoing cardiovascular testing following SARS‐CoV‐2 infection.
Methods and Results
This prospective, observational cohort study from ORCCA (Outcomes Registry for Cardiac Conditions in Athletes) included collegiate athletes who underwent cTn testing as a component of return to play protocols following SARS‐CoV‐2 infection. The cTn values were stratified as undetectable, detectable but within normal limits, and abnormal (>99% percentile). The presence of probable or definite SARS‐CoV‐2 myocardial involvement was compared between those with normal versus abnormal cTn levels. A total of 3184/3685 (86%) athletes in the ORCCA database met the inclusion criteria for this study (age 20±1 years, 32% female athletes, 28% Black race). The median time from SARS‐CoV‐2 diagnosis to cTn testing was 13 days (interquartile range, 11, 18 days). The cTn levels were undetectable in 2942 athletes (92%), detectable but within normal limits in 210 athletes (7%), and abnormal in 32 athletes (1%). Of the 32 athletes with abnormal cTn testing, 19/32 (59%) underwent cardiac magnetic resonance imaging, 30/32 (94%) underwent transthoracic echocardiography, and 1/32 (3%) did not have cardiac imaging. One athlete with abnormal troponin met the criteria for definite or probable SARS‐CoV‐2 myocardial involvement. In the total cohort, 21/3184 (0.7%) had SARS‐CoV‐2 myocardial involvement, among whom 20/21 (95%) had normal troponin testing.
Conclusions
Abnormal cTn during routine return to play cardiac screening among competitive athletes following SARS‐CoV‐2 infection appears to have limited diagnostic utility.
Keywords: athletes, return‐to‐play, SARS‐CoV‐2, troponin
Subject Categories: Diagnostic Testing, Exercise
Elevation of cardiac troponin (cTn) during the acute phase of SARS‐CoV‐2 infection is both common and prognostic of myocardial injury in hospitalized patients. 1 This finding emerged early during the COVID‐19 pandemic and stimulated concerns for adverse cardiac sequalae in young competitive athletes with SARS‐CoV‐2 infection. 2 , 3 Accordingly, return to play (RTP) cardiac screening protocols were developed to screen for SARS‐CoV‐2 cardiac involvement. 2 , 4 Initial RTP screening recommendations suggested cardiac “triad testing” with a 12‐lead ECG, transthoracic echocardiography (TTE), and cTn testing in all athletes with SARS‐CoV‐2 infection. In addition, it was recommended that athletes with high‐sensitivity cTn values >99th percentile be managed as presumptive myocarditis, thereby necessitating extended restriction from exercise. 3 , 4
Subsequent data acquired from large cohort studies of young and otherwise healthy athletes undergoing postinfectious RTP evaluation have established prevalence estimates of SARS‐CoV‐2 cardiac involvement ranging from 0.5% to 3.0%. 5 , 6 , 7 Although the clinical significance of SARS‐CoV‐2 cardiac involvement remains incompletely delineated, available clinical surveillance data document no associated adverse cardiac events. 7 Lower than anticipated disease prevalence estimates coupled with a paucity of corollary adverse outcomes have led to revised RTP screening recommendations. RTP cardiac testing is now recommended only for athletes with moderate or severe acute infectious symptoms or cardiopulmonary symptoms on return to exercise. 8 , 9 This important shift, designed to reduce low‐yield testing, focused on who should be screened with comparatively less emphasis on how screening should be done. Data‐driven refinements in post–SARS‐CoV‐2 RTP screening protocols that maximize diagnostic accuracy and reduce resource use are of paramount importance. Unlike ECG and TTE, cTn has not been rigorously examined as a screening tool nor have normative data been established among young competitive athletes. We therefore sought to examine the prevalence and clinical relevance of abnormal cTn among young competitive athletes undergoing cardiac screening following SARS‐CoV‐2 infection.
METHODS
The data that support the findings of this study are available from the corresponding author on reasonable request. This prospective observational cohort study included collegiate athlete data submitted to the ORCCA (Outcomes Registry for Cardiac Conditions in Athletes) from September 1, 2020, to November 1, 2021. A detailed description of the ORCCA study, including definitions of SARS‐CoV‐2 myocardial and myopericardial involvement, has previously been published. 7 Athletes were included in this study if they had confirmed SARS‐CoV‐2 infection by laboratory testing and underwent cTn testing during RTP evaluation. Troponin values were categorized as undetectable, detectable/normal (<99% upper limit of normal [ULN]), and abnormal (>99% ULN) per local institutional assays. Categorical variables are presented as number (percentage), and continuous variables are presented as mean (SD) or median (interquartile range [IQR]). Mann–Whitney U tests and Fisher exact tests were used for comparisons of continuous and categorical variables, respectively. Statistical analyses were performed using R: A Language and Environment for Statistical Computing (R Core Team, Vienna, Austria; https://www.R‐project.org/). This study was approved by the Massachusetts General Brigham Institutional Review Board (Protocol 2020P002667), and the need for informed consent was waived.
RESULTS
A total of 3184/3685 (86%) athletes in the ORCCA database (age 20±1 years, 32% female athletes, 28% Black race) representing 44 colleges/universities had cTn testing performed during RTP cardiac screening. Among athletes with known symptom status (n=2848), 32% were asymptomatic, 31% had mild symptoms, 26% had moderate symptoms, and 11% had cardiopulmonary symptoms during the initial infection or on return to exercise. Median time from SARS‐CoV‐2 diagnosis to cTn testing was 13 days (IQR 11, 18 days) with high‐sensitivity cTn assays comprising 12% of overall testing. The cTn levels were undetectable in 2942 athletes (92%), detectable but within normal limits in 210 athletes (7%), and abnormal in 32 athletes (1%; Figure 1), with 7/32 (22%) abnormal cTn values measured with a high‐sensitivity assay. The magnitude of elevation for abnormal troponins is presented in Figure 2. A total of 3017/3184 athletes (95%) underwent cardiac imaging: 2890 (91%) with TTE, 506 (16%) with cardiac magnetic resonance imaging (CMR), and 379 (12%) with both.
Figure 1. Results of troponin‐inclusive return to play testing in athletes.
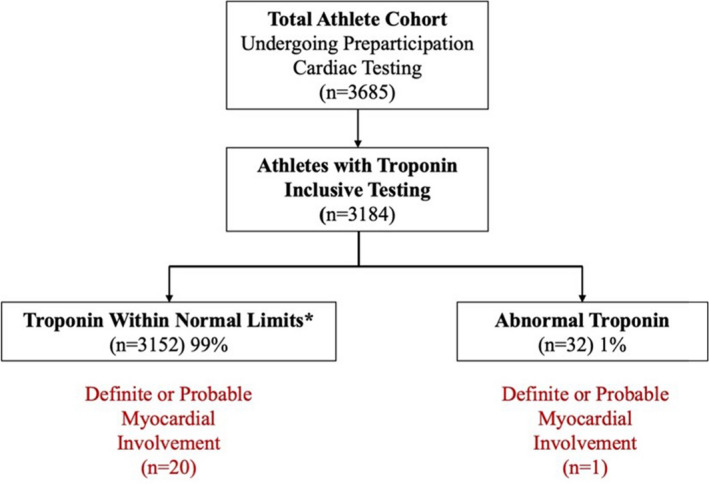
*Includes undetectable (n=2942) and detectable (n=210).
Figure 2. Magnitude of troponin elevation in athletes with abnormal troponin levels.
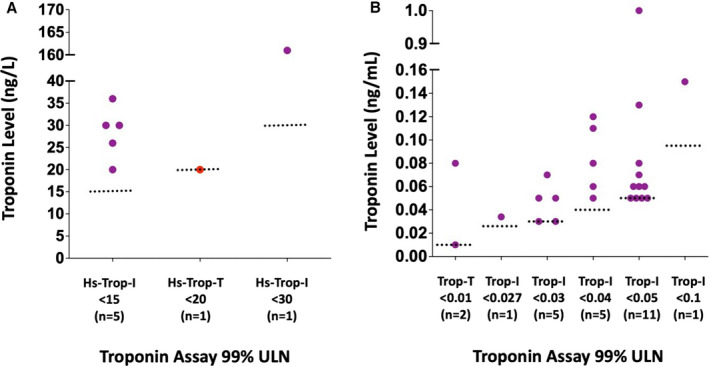
A, High‐sensitivity troponin assays. B, Traditional troponin assays. Red dots indicate probable or definite SARS‐CoV‐2 myocardial involvement. Purple dots = athletes with abnormal troponin but no evidence of probable or definite SARS‐CoV‐2 myocardial involvement; Red dots = athletes with abnormal troponin and evidence of probable or definite SARS‐CoV‐2 myocardial involvementHs indicates high sensitivity; Trop, troponin; Trop‐I, troponin‐I; Trop‐T, troponin‐T; and ULN, upper limit of normal.
Of 32 athletes, 19 (59%) with abnormal cTn testing underwent CMR (median time from cTn to CMR 6 days [IQR 3, 9 days]), 30/32 (94%) underwent TTE, and 1/32 (3%) did not have cardiac imaging. Of the athletes who underwent CMR, 18/19 (95%) had unremarkable imaging, whereas 1 met criteria for definite or probable SARS‐CoV‐2 myocardial involvement. 7 No significant differences were identified in the presence of cardiopulmonary symptoms (9.4% versus 9.8%; P=0.999) or the time from initial infection to cTn testing (median 14 days [IQR 11, 18 days] versus 13 days [IQR 11, 18 days]; P=0.924) between athletes with abnormal versus undetectable or detectable/normal cTn values.
In the total cohort, definite or probable SARS‐CoV‐2 myocardial involvement was found in 21/3184 (0.7%) athletes, of whom 20/21 (95%) had normal cTn values. The exception was an asymptomatic athlete with a borderline elevated high‐sensitivity cTn value of 20 ng/L (laboratory ULN <20; Figure 2) and a CMR that demonstrated increased T2 signal intensity consistent with edema but with no late gadolinium enhancement or left ventricular dysfunction.
DISCUSSION
This study examined the yield of cTn testing among young competitive athletes during RTP cardiac screening following SARS‐CoV‐2 infection. Key findings and their clinical and scientific implications can be summarized as follows. First, ≈1% of athletes had abnormal cTn values following SARS‐CoV‐2 infection, with only 1 athlete having evidence of cardiac involvement. Several complementary plausible explanations for this observation are noteworthy. As physiologic cTn elevation is common among healthy people without underlying heart disease after even modest bouts of exercise, we suspect that some proportion of the observed cTn elevations are attributable to this phenomenon. 10 In addition, there exist no published cTn reference standards that account for age, sex, and habitual physical activity pattern among young competitive athletes. It is therefore possible that laboratory reference standards derived from alternative source populations are not appropriate for use in this context. Second, these results suggest that the majority of abnormal cTn values in this cohort are likely unrelated to underlying SARS‐CoV‐2 myocardial involvement. This finding draws into question the role cTn testing as a screening tool during post–SARS‐CoV‐2 RTP evaluation in this population. Although cTn testing likely provides important clinical information among the small minority of athletes who present with a clinical syndrome suggestive of myocarditis, 11 its use as a screening modality in patients with a low pretest probability of clinical myocarditis appears limited and should be discouraged. Our results should inform future RTP screening recommendations for athletes after SARS‐CoV‐2 infection.
Limitations
This study has several important limitations. First, evolving RTP recommendations throughout the study period may have contributed to variability of cardiac screening practices among participating institutions. However, this unavoidable reality would not be expected to impact the relationships between cTn and accompanying clinical data. Second, although we acknowledge that cTn assays varied across participating institutions, we attempted to account for this potential source of variability by using individual laboratory’s 99th percentile values. Third, cTn levels were drawn a median of 13 days (IQR 11, 18 days) from infection onset, and therefore the possibility exists that early elevations in cTn related to acute infection may have resolved before testing. Fourth, physical activity levels and the timing of this in relation to cTn testing was not available. Finally, an important minority of athletes with elevated cTn (13/32) values did not undergo CMR to assess for SARS‐CoV‐2 myocardial involvement, and thus cases of myocardial involvement could have been missed. However, 12 of these 13 athletes underwent TTE without findings to suggest clinically relevant SARS‐CoV‐2 myocardial involvement.
CONCLUSIONS
Findings from this study suggest that cTn testing during routine RTP cardiac screening following SARS‐CoV‐2 infection is of limited diagnostic utility, particularly in athletes with a low pretest probability of clinical myocarditis. Future iterations of postinfectious RTP cardiac testing recommendations should consider endorsing a more limited role of cTn testing confined to athletes with high clinical pretest probability of disease. Defining normal cTn reference ranges in young athletic populations represents an important area of future work.
APPENDIX
Members of the ORCCA (Outcomes Registry for Cardiac Conditions in Athletes) Study Group
Steering Committee
Irfan M. Asif, MD; Aaron L. Baggish, MD; James Borchers, MD; Jonathan A. Drezner, MD; Katherine M. Edenfield, MD; Michael S. Emery, MD, MS; Kyle Goerl, MD; Brian Hainline, MD; Kimberly G. Harmon, MD; Pei‐Ni Jone, MD; Jonathan H. Kim, MD, MSc; Stephanie Kliethermes, PhD; William E. Kraus, MD; Rachel Lampert, MD; Matthew Leiszler, MD; Benjamin D. Levine, MD; Matthew W. Martinez, MD; Nathaniel Moulson, MD; Francis G. O'Connor, MD, MPH; Manesh R. Patel, MD; Bradley J. Petek, MD; Dermot Phelan, MD; Lawrence D. Rink, MD; Herman A. Taylor, MD, MPH.
Site Investigators
Carl Ade, PhD; Aryan Aiyer, MD; Jarrah Alfadhli, MD; Chloe Amaradio; Scott Anderson, ATC; Stephanie Arlis‐Mayor, MD; Jonathan S. Aubry, MD; Andrea Austin, MSN, RN; Brenden J. Balcik, MD; Timothy Beaver, MD; Nicolas Benitez; Brant Berkstresser; Thomas M. Best, MD, PhD; Tiffany Bohon, MD; Jonathan P. Bonnet, MD, MPH; Elizabeth Boyington; James Bray, MD; Jenna Bryant, MD; Jeffrey Bytomski, DO; Sean Carnahan, DO; Rachel Chamberlain, MD; Samantha Charters, ATC; Nicholas Chill, MD; Daniel E. Clark, MD; Douglas Comeau, DO; Laura E. Cook, MD; Deanna Corey, MD; Amy Costa, MD; Marshall Crowther, MD; Tarun Dalia, MD; Craig Davidson, MD; Kaitlin Davitt, MS, ATC; Annabelle De St. Maurice, MD, MPH; Peter N. Dean, MD; Jeffrey M. Dendy, MD; Katelyn DeZenzo; Courtney Dimitris; Jeanne Doperak, DO; Calvin Duffaut, MD; Craig Fafara; Katherine Fahy, MD; Jason Ferderber, MD; Megan Finn; Frank A. Fish, MD; R. Warne Fitch, MD; Angelo Galante, MD; Todd Gerlt, ATC; Amy Gest, MPA; Carla Gilson, ATC; Jeffrey Goldberger, MD; Joshua Goldman, MD, MBA; Erich Groezinger, MS; Jonathan R. Guin, MD; Heather Halseth; Joshua Hare, MD; Beth Harness, ATC; Nicolas Hatamiya, DO; Julie Haylett, RN, MSN; Neal Hazen, MBA, PT, ATC, LAT; Sean G. Hughes, MD; Yeun Hiroi, BS; Amy Hockenbrock, MD; Amanda Honsvall, MD; Jennifer Hopp, MD; Julia Howard, ATC; Samantha Huba; Mustafa Husaini, MD; Lindsay Huston, MD; Calvin Hwang, MD; Laura Irvin, DO; Val Gene Iven, MD; Robert Jones, MD; Donald Joyce, MD; Kristine Karlson, MD; Jeremy Kent, MD; Christian F. Klein, MD; Chris Klenck, MD; Michele Kirk, MD; Jordan Knight; Laura Knippa, RN; Madeleine Knutson, ATC; Louis E. Kovacs, MD; Yumi Kuscher; Andrea Kussman, MD; Chrissy Landreth; Amy Leu, DO; Dylan Lothian; Maureen Lowery, MD; Andrew Lukjanczuk, MS, ATC, LAT; John M. MacKnight, MD; Lawrence M. Magee, MD; Marja‐Liisa Magnuson, DO, MS; Aaron V. Mares, MD; Anne Marquez; Grant McKinley, MD; Scott Meester, MD; Megan Meier, MD; Pranav Mellacheruvu, BS; Christopher Miles, MD; Emily Miller, MD; Hannah Miller, MSEd, ATC, LAT; Raul Mitrani, MD; Aaron J. Monseau, MD; Benjamin Moorehead, MD; Robert J. Myerburg, MD; Greg Mytyk, ATC; Andrew Narver, DO; Aurelia Nattiv, MD; Laika Nur, MD; Brooke E. Organ, MD; Meredith Pendergast, ATC; Frank A. Pettrone, MD; Jordan Pierce, ATC; Sourav K. Poddar, MD; Diana Priestman; Ian Quinn, DO; Fred Reifsteck, MD; Morgan Restivo; James B. Robinson, MD; Ryan Roe, PA‐C; Thomas Rosamond, MD; Carrie Rubertino Shearer; Diego Riveros, MD; Miguel Rueda; Takamasa Sakamoto, MEd, ATC; Brock Schnebel, MD; Ankit B. Shah, MD, MPH; Alan Shahtaji, DO; Kevin Shannon, MD; Polly Sheridan‐Young, PA‐C; Jonathon H. Soslow, MD; Siobhan M. Statuta, MD; Mark Stovak, MD; Andrei Tarsici; Kenneth S. Taylor, MD; Kim Terrell; Matt Thomason, ATC; Jason Tso, MD; Daniel Vigil, MD; Francis Wang, MD; Jennifer Winningham, MS, ATC, LAT; Susanna T. Zorn, MD.
Sources of Funding
This work was funded in part by a grant from the American Medical Society for Sports Medicine (AMSSM) Foundation and AMSSM Collaborative Research Network. Dr Moulson is supported by the University of British Columbia Clinician Investigator Program.
Disclosures
Dr Patel is on the advisory boards of Amgen, Bayer, Janssen, Heartflow, and Medscape. They report grant funding from the National Heart, Lung, and Blood Institute, Bayer, Janssen, Heartflow, and Idorsia. Dr Patel’s research is also supported by the Joel Cournette Foundation for research on athletes’ hearts. Dr Baggish has received funding from the National Institute of Health/National Heart, Lung, and Blood Institute, the National Football Players Association, and the American Heart Association and receives compensation for his role as team cardiologist from the US Olympic Committee/US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. Dr Harmon discloses stock options for 98point6 for which she is also on the medical advisory board. The remaining authors have no disclosures to report.
Acknowledgments
Special thanks to the collaborators for ORCCA (Outcomes Registry for Cardiac Conditions in Athletes).
For Sources of Funding and Disclosures, see page 5.
Contributor Information
Aaron L. Baggish, Email: abaggish@partners.org.
for the ORCCA Investigators:
Irfan M. Asif, Aaron L. Baggish, James Borchers, Jonathan A. Drezner, Katherine M. Edenfield, Michael S. Emery, Kyle Goerl, Brian Hainline, Kimberly G. Harmon, Pei‐Ni Jone, Jonathan H. Kim, Stephanie Kliethermes, William E. Kraus, Rachel Lampert, Matthew Leiszler, Benjamin D. Levine, Matthew W. Martinez, Nathaniel Moulson, Francis G. O'Connor, Manesh R. Patel, Bradley J. Petek, Dermot Phelan, Lawrence D. Rink, Herman A. Taylor, Carl Ade, Aryan Aiyer, Jarrah Alfadhli, Chloe Amaradio, Scott Anderson, Stephanie Arlis‐Mayor, Jonathan S. Aubry, Andrea Austin, Brenden J. Balcik, Timothy Beaver, Nicolas Benitez, Brant Berkstresser, Thomas M. Best, Tiffany Bohon, Jonathan P. Bonnet, Elizabeth Boyington, James Bray, Jenna Bryant, Jeffrey Bytomski, Sean Carnahan, Rachel Chamberlain, Samantha Charters, Nicholas Chill, Daniel E. Clark, Douglas Comeau, Laura E. Cook, Deanna Corey, Amy Costa, Marshall Crowther, Tarun Dalia, Craig Davidson, Kaitlin Davitt, Annabelle De St. Maurice, Peter N. Dean, Jeffrey M. Dendy, Katelyn DeZenzo, Courtney Dimitris, Jeanne Doperak, Calvin Duffaut, Craig Fafara, Katherine Fahy, Jason Ferderber, Megan Finn, Frank A. Fish, R. Warne Fitch, Angelo Galante, Todd Gerlt, Amy Gest, Carla Gilson, Jeffrey Goldberger, Joshua Goldman, Erich Groezinger, Jonathan R. Guin, Heather Halseth, Joshua Hare, Beth Harness, Nicolas Hatamiya, Julie Haylett, Neal Hazen, Sean G. Hughes, Yeun Hiroi, Amy Hockenbrock, Amanda Honsvall, Jennifer Hopp, Julia Howard, Samantha Huba, Mustafa Husaini, Lindsay Huston, Calvin Hwang, Laura Irvin, Val Gene Iven, Robert Jones, Donald Joyce, Kristine Karlson, Jeremy Kent, Christian F. Klein, Chris Klenck, Michele Kirk, Jordan Knight, Laura Knippa, Madeleine Knutson, Louis E. Kovacs, Yumi Kuscher, Andrea Kussman, Chrissy Landreth, Amy Leu, Dylan Lothian, Maureen Lowery, Andrew Lukjanczuk, John M. MacKnight, Lawrence M. Magee, Marja‐Liisa Magnuson, Aaron V. Mares, Anne Marquez, Grant McKinley, Scott Meester, Megan Meier, Pranav Mellacheruvu, Christopher Miles, Emily Miller, Hannah Miller, Raul Mitrani, Aaron J. Monseau, Benjamin Moorehead, Robert J. Myerburg, Greg Mytyk, Andrew Narver, Aurelia Nattiv, Laika Nur, Brooke E. Organ, Meredith Pendergast, Frank A. Pettrone, Jordan Pierce, Sourav K. Poddar, Diana Priestman, Ian Quinn, Fred Reifsteck, Morgan Restivo, James B. Robinson, Ryan Roe, Thomas Rosamond, Carrie Rubertino Shearer, Diego Riveros, Miguel Rueda, Takamasa Sakamoto, Brock Schnebel, Ankit B. Shah, Alan Shahtaji, Kevin Shannon, Polly Sheridan‐Young, Jonathon H. Soslow, Siobhan M. Statuta, Mark Stovak, Andrei Tarsici, Kenneth S. Taylor, Kim Terrell, Matt Thomason, Jason Tso, Daniel Vigil, Francis Wang, Jennifer Winningham, and Susanna T. Zorn
REFERENCES
- 1. Shi S, Qin MU, Shen BO, Cai Y, Liu T, Yang F, Gong W, Liu XU, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baggish A, Drezner JA, Kim J, Martinez M, Prutkin JM. Resurgence of sport in the wake of COVID‐19: cardiac considerations in competitive athletes. Br J Sports Med. 2020;54:1130–1131. doi: 10.1136/bjsports-2020-102516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phelan D, Kim JH, Chung EH. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID‐19) infection. JAMA Cardiol. 2020;5:1085–1086. doi: 10.1001/jamacardio.2020.2136 [DOI] [PubMed] [Google Scholar]
- 4. American Medical Society for Sports Medicine and the American College of Cardiology . Cardiac Considerations for College Student‐Athletes during the COVID‐19 Pandemic. 2020.
- 5. Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M, Jeudy J, Mattson SE, Law IH, Borchers J, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS‐CoV‐2 infection: results from the big ten COVID‐19 cardiac registry. JAMA Cardiol. 2021;6:1078–1087. doi: 10.1001/jamacardio.2021.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, Phelan D, Kim JH, Meeuwisse W, Sills AK, et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID‐19 infection who received systematic return‐to‐play cardiac screening. JAMA Cardiol. 2021;6:745–752. doi: 10.1001/jamacardio.2021.0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moulson N, Petek BJ, Drezner JA, Harmon KG, Kliethermes SA, Patel MR, Baggish AL, Asif IM, Borchers J, Edenfield KM, et al. SARS‐CoV‐2 cardiac involvement in young competitive athletes. Circulation. 2021;144:256–266. doi: 10.1161/CIRCULATIONAHA.121.054824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim JH, Levine BD, Phelan D, Emery MS, Martinez MW, Chung EH, Thompson PD, Baggish AL. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021;6:219–227. doi: 10.1001/jamacardio.2020.5890 [DOI] [PubMed] [Google Scholar]
- 9. American Medical Society for Sports Medicine and the American College of Cardiology . Cardiac Considerations for College Student‐Athletes during the COVID‐19 Pandemic. 2022.
- 10. Shave R, Baggish A, George K, Wood M, Scharhag J, Whyte G, Gaze D, Thompson PD. Exercise‐induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol. 2010;56:169–176. doi: 10.1016/j.jacc.2010.03.037 [DOI] [PubMed] [Google Scholar]
- 11. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210 [DOI] [PubMed] [Google Scholar]


