Abstract
Background
Function after acute stroke using the modified Rankin Scale (mRS) is usually assessed at a point in time. The analytical implications of serial mRS measurements to evaluate functional recovery over time is not completely understood. We compare repeated‐measures and single‐measure analyses of the mRS from a randomized clinical trial.
Methods and Results
Serial mRS data from AFFINITY (Assessment of Fluoxetine in Stroke Recovery), a double‐blind placebo randomized clinical trial of fluoxetine following stroke (n=1280) were analyzed to identify demographic and clinical associations with functional recovery (reduction in mRS) over 12 months. Associations were identified using single‐measure (day 365) and repeated‐measures (days 28, 90, 180, and 365) partial proportional odds logistic regression. Ninety‐five percent of participants experienced a reduction in mRS after 12 months. Functional recovery was associated with age at stroke <70 years; no prestroke history of diabetes, coronary heart disease, or ischemic stroke; prestroke history of depression, a relationship partner, living with others, independence, or paid employment; no fluoxetine intervention; ischemic stroke (compared with hemorrhagic); stroke treatment in Vietnam (compared with Australia or New Zealand); longer time since current stroke; and lower baseline National Institutes of Health Stroke Scale & Patient Health Questionnaire‐9 scores. Direction of associations was largely concordant between single‐measure and repeated‐measures models. Association strength and variance was generally smaller in the repeated‐measures model compared with the single‐measure model.
Conclusions
Repeated‐measures may improve trial precision in identifying trial associations and effects. Further repeated‐measures stroke analyses are required to prove methodological value.
Registration
URL: http://www.anzctr.org.au; Unique identifier: ACTRN12611000774921.
Keywords: cerebrovascular disease, functional outcomes, modified Rankin Scale, partial proportional odds, repeated measures, stroke
Subject Categories: Cerebrovascular Disease/Stroke, Quality and Outcomes, Clinical Studies
Nonstandard Abbreviations and Acronyms
- AFFINITY
Assessment of Fluoxetine in Stroke Recovery
- mRS
modified Rankin Scale
- NHMRC
National Health and Medical Research Council of Australia
- NIHSS
National Institutes of Health Stroke Scale
- PHQ‐9
Patient Health Questionnaire 9
Clinical Perspective.
What Is New?
The modified Rankin Scale (mRS) literature has shifted from dichotomous to ordinal analyses of the mRS, improving statistical precision; however, such studies typically still analyze a single measurement of mRS following stroke (eg, day 90).
Ordinal analyses of repeated measures of mRS over time may offer incremental improvements in statistical precision over ordinal single‐measure analyses; however, this has not been investigated previously.
Using a randomized control trial poststroke data set (n=1276) with 5 mRS measurements (days 1, 28, 90, 180, and 365) per individual, we compare associations from single‐measure and repeated‐measures models.
What Are the Clinical Implications?
Single‐measure and repeated‐measures models identified demographic and clinical associations with functional recovery that can be validated in future predictive studies.
The repeated‐measures model generally had improved precision (reduced variance) compared with the single‐measure model, which may be driven by increased use of available data; however, direction of associations was largely concordant between models.
This article invites trial analysts to consider repeated‐measures analysis of ordinal mRS to improve model precision and power, especially ex‐post if repeated‐measures data are already available.
The modified Rankin Scale (mRS) is the most widely used primary outcome measure in stroke trials and is considered a valid tool for evaluating stroke interventions. 1 , 2 The mRS has 7 categories to measure functional status, ranging from independent and free of disability (0) to death (6). Historically, in analyses of variables associated with the mRS, mRS scores were dichotomously transformed rather than ordinally preserved, and statistical methods were single measure (usually 3 or 6 months after enrollment) rather than longitudinal. 3 Numerous trials and systematic reviews have found variance in the statistical significance of stroke interventions (as measured by treatment effect on mRS), depending on trial design, choice of outcome (including simplified and utility‐weighted mRS) and statistical methods. 4 , 5 , 6 While ordinal mRS analyses have increased in number because of greater statistical power and association with long‐term clinical outcomes and costs, 5 , 7 these are usually single mRS measurement analyses. A 2018 literature review found 2 studies using repeated measures of the mRS to analyze recovery over time; however, these did not analyze ordinal mRS. 8 , 9 , 10 Further to this, our literature searches have not revealed studies comparing single‐measure and repeated‐measures ordinal logistic mRS analyses to identify associations with functional recovery. If collected, usage of all serial follow‐up data (rather than study end points) should strengthen validity of any identified associations with mRS by increasing statistical power 11 and is concordant with the Stroke Recovery and Rehabilitation Roundtable goals in applying repeated measures to understand the natural history of recovery and optimal timing of interventions. 12
Previous repeated‐measures analyses of the mRS have focused on either describing mRS over time or identifying treatment effect, rather than associations, and have not used ordinal logistic regression. A comparison of statistical methods 13 highlighted that multistate Markov models and partial proportional odds models were the most efficient in analyzing ordinal mRS, but noting that Markov models are designed for prediction of transitions between individual mRS categories 13 , 14 rather than identifying associations. A posttrial analysis of the National Institute of Neurological Disorders and Stroke rt‐PA Stroke Trial further supports use of the partial proportional odds model for analyzing mRS, finding lower prediction error and better model fit when compared with the proportional odds model (with violation of the proportional odds assumption). 15 Another posttrial analysis of the National Institute of Neurological Disorders and Stroke rt‐PA Stroke Trial used both repeated measures (baseline; day 7; months 3, 6, and 12) and single‐measure methods to compare treatment effects of thrombolysis on dichotomized mRS (mRS <1). 16 At 90 days, repeated‐measures analysis augmented treatment effect compared with single‐measure analysis, with larger odds ratios and greater precision. 16 Another study used repeated‐measures of mRS (baseline; months 1, 3, 6, 9, and 12) in generalized linear models to analyze thrombolysis and endovascular treatment effects on dichotomous and ordinal mRS but did not identify associations with functional recovery nor compare with single‐measure analysis. 17
Common associations in stroke functional recovery models include age, preadmission comorbidities, prestroke dependency, and neurologic deficit. 18 , 19 , 20 , 21 , 22 mRS score is commonly recorded before the trial and at 90 days (and recommended at 90 days), 23 , 24 and models typically predict a single measure in time (eg, day 90 mRS). 18 , 19 , 20 , 21 , 22 , 25 However, individualized understanding of functional recovery over time may be clinically important to assist personalized rehabilitation and discharge. Understanding an individual’s ordinal mRS score may assist with this, 26 while acknowledging that mRS as a global measure of function will not fully characterize physical or cognitive abilities. 27 Recovery is often not linear nor the same for each person, 28 and thus mRS should be assessed several times to reflect potential fluctuations. Previous longitudinal stroke studies have found that older age, male sex, previous ischemic stroke, peripheral artery disease, and diabetes are associated with mRS scores that either stay constant or increase over time, although broadly mRS scores decrease with time. 8 , 9 , 29 , 30
The AFFINITY (Assessment of Fluoxetine in Stroke Recovery) trial found no benefit from 6 months of daily treatment with 20 mg of oral fluoxetine in reducing disability (measured by mRS at 6 months). This article is a secondary post hoc cohort level analysis of the AFFINITY trial data with repeated measures of mRS at baseline (day 1) and days 28, 90, 180, and 365. Our aims are 2‐fold: (1) to identify baseline demographic and clinical associations with functional recovery (reduction in mRS score) over 12 months after the index stroke and use the statistical power of multiple measurements to compare findings of repeated‐measures and single‐measure analysis; and (2) to descriptively analyze the functional recovery of participants over 12 months. While recovery mostly occurs in the first 3 months, the literature is limited in reporting 12‐month follow‐up, which should capture most long‐term stroke‐related disability. 31 Our results may provide stakeholders with prognostic information, guide future stroke trial analyses, and identify associations for validation in future studies to develop a clinical model for predicting stroke recovery.
Methods
Data Availability
The data that support the study findings are available from the corresponding author upon reasonable request. One of the authors (A.C.) had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Reporting Checklist
The Strengthening the Reporting of Observational Studies in Epidemiology 32 guidelines were used.
Trial Registration
The AFFINITY trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12611000774921) and Institutional Review Board ethics approval was obtained from the Royal Perth Hospital Ethics Committee (approval number EC2011/131), with written informed consent obtained from participants.
Participants
AFFINITY was a randomized, double‐blind control trial that investigated the efficacy of 6 months of daily treatment with 20 mg of oral fluoxetine in addition to usual care compared with placebo, with 6 months of follow‐up without fluoxetine. 33 The trial was conducted in Australia, Vietnam, and New Zealand, recruiting 1280 participants from 2013 to 2019. Participants were aged ≥18 years with a clinical diagnosis of ischemic or hemorrhagic stroke within the past 2 to 15 days and persisting neurological deficit with mRS ≥1. For this analysis, participants with an incorrect diagnosis of stroke (n=4) were excluded, leaving 1276 participants. A modified Consolidated Standards of Reporting Trials flow diagram can be viewed in Figure S1. Further details on trial methods, recruitment, and design have been published previously. 33
Outcomes
For single measure analysis, the outcome variable was mRS at day 365. For repeated‐measures analysis, the outcome variable was mRS at day 28, 90, 180 or 365. mRS was ordinally preserved without transformation. Trained clinical staff used the validated simplified mRS questionnaire to measure mRS. 34 Ninety‐eight percent of the trial population was followed up to day 365.
Statistical Analysis
Baseline cohort characteristics were summarized using mean and SD for age at stroke, median with interquartile range for stroke scores and counts with percentage for binary variables (Table 1). The distribution of mRS scores (by category) over 12 months is visualized in the Figure, with a Sankey diagram visualizing transitions in mRS over 12 months in Figure S2. Patterns of mRS change (Worsening, Improving, Constant) between follow‐up intervals are summarized with counts (percentages), mean mRS change, and SD (Table 2). Associations between key baseline independent variables (demographic, clinical) and mRS scores were analyzed using partial proportional odds ordinal logistic regression, as recommended by the Optimizing Analysis of Stroke Trials Collaboration 35 and validated in previous studies, 13 , 15 identifying associations rather than treatment effect and transitions. 13 With <5% (n=30) of participants missing baseline (day 1) characteristics or day 365 mRS data, complete case analysis without imputation was used.
Table 1.
Baseline Characteristics for Regression Variables of Interest (n=1276)
| Factor | Values, n (%) |
|---|---|
| Male sex | 801 (62.8) |
| Age at stroke, y, mean (SD) | 64.0 (12.5) |
| Race | |
| Asian | 727 (57.0) |
| White | 518 (40.6) |
| Other race | 31 (2.4) |
| Country treated in | |
| Australia | 528 (40.4) |
| New Zealand | 42 (3.3) |
| Vietnam | 706 (55.3) |
| Paid employment | |
| Full‐time | 385 (30.2) |
| Part‐time | 144 (11.3) |
| Unemployed or disabled | 37 (2.9) |
| Retired | 677 (53.1) |
| Other employment | 33 (2.6) |
| Functional status | |
| Independent prior* | 1260 (98.7) |
| Previous medical history | |
| History of diabetes | 289 (22.6) |
| History of coronary heart disease | 114 (8.9) |
| History of ischemic stroke | 161 (12.6) |
| History of intracranial hemorrhage | 19 (1.5) |
| History of depression | 49 (3.8) |
| Stroke diagnosis | |
| Intracerebral hemorrhage | 185 (14.5) |
| Ischemic stroke | 1091 (85.5) |
| NIHSS total score†, median (IQR) | 6.0 (3.0–9.0) |
| PHQ‐9 total score‡, median (IQR) | 4.0 (1.0–7.0) |
IQR indicates interquartile range; NIHSS, National Institutes of Health Stroke Scale; and PHQ‐9, 9‐item Patient Health.
Independence was defined as estimated mRS ≤2 before stroke.
Higher scores indicate greater neurological impairment.
Higher scores indicate more depressive symptoms.
Figure 1. Distribution of mRS scores at days 1, 28, 90, 180, and 365 (n=1276).
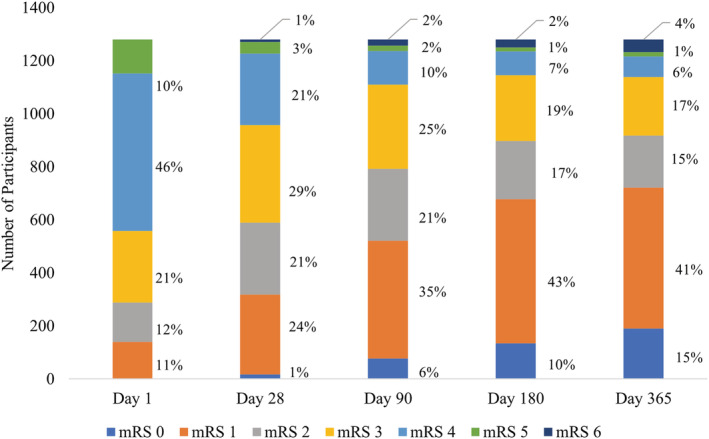
Visualization of the distribution of mRS scores by category at different follow‐up points during the AFFINITY trial. AFFINITY indicates Assessment of Fluoxetine in Stroke Recovery; and mRS, modified Rankin Scale.
Table 2.
Patterns of Follow‐Up Change in mRS Between Days 1, 28, 90, 180, and 365
| Day 1 (n=1276) | Day 28 (n=1263) | Day 90 (n=1253) | Day 180 (n=1249) | Day 365 (n=1246) | |
|---|---|---|---|---|---|
| Worsening, n (%) | … | 24 (2) | 34 (3) | 44 (4) | 66 (5) |
| No change, n (%) | … | 536 (42) | 676 (54) | 853 (68) | 1002 (80) |
| Improving, n (%) | … | 703 (56) | 543 (43) | 352 (28) | 178 (15) |
| Incremental deaths, n (%) | 9 (1) | 12 (1) | 7 (1) | 18 (1) | |
| mRS, mean (SD) | 3.3 (0.0) | 2.6 (0.0) | 2.1 (0.0) | 1.8 (0.0) | 1.7 (0.0) |
| mRS, median (IQR) | 4 (1) | 3 (1) | 2 (2) | 1 (2) | 1 (2) |
| mRS change, mean (SD) | … | −0.8 (0.9) | −0.5 (0.8) | −0.3 (0.6) | −0.1 (0.6) |
Worsening, no change, and improving refer to the difference between the participant’s mRS score at one assessment compared with the previous. For example, at day 90, 34 (3%) individuals had a worse (higher) mRS score than at day 28. Incremental deaths refer to new deaths between follow‐ups. IQR indicates interquartile range; and mRS, modified Rankin Scale.
Baseline variables were selected according to clinical input from the AFFINITY Steering Committee, and associations with functional outcomes after stroke in the literature. 36 Regressor variables included demographics (age >70 years, sex, country of treatment), prestroke comorbidities (diabetes, coronary heart disease, ischemic stroke, depression), prestroke function (relationship status [single compared with relationship partner], living status [home alone compared with living with other people], employment, independence [mRS ≤2]), and current stroke (fluoxetine intervention, ischemic or haemorrhagic classification, stroke severity [National Institutes of Health Stroke Scale (NIHSS)] and mental health [Patient Health Questionnaire 9 (PHQ‐9)]).
Regression analysis sought to identify associations or the unadjusted relationship between 2 variables of interest, explored within strata of a few confounding variables. 37 Partial proportional odds models identified associations between baseline variables and a single measure of mRS at day 365 and between baseline variables and repeated measures of the mRS at days 28, 90, 180, and 365. The Brant test was used to determine whether the explanatory variables satisfied the proportional odds assumption, finding violations for age at stroke >70 years, the NIHSS, and time since stroke. For these variables, associations for cumulative logit models corresponding to different categories of the mRS (eg, mRS 0–1 versus 2–6) are presented. Unadjusted associations reflect conditional total effects and are provided for all variables. Adjusted estimates (with covariates age at stroke >70 and sex) were only provided for nonexogenous variables (excluding age at stroke >70 years, sex, country of treatment, fluoxetine intervention, and time since stroke). A directed acyclic graph informing our adjusted regression analysis can be found in Figure S3. Clustered standard errors by country were used for both single‐measure and repeated‐measures models. Results are reported with odds ratios (ORs) and CIs. An OR <1 indicates lower odds and vice versa. For interpreting the cumulative logit ORs, an OR >1 indicates higher odds of having an mRS greater than the lower bound (eg, for the cumulative logit mRS 0 versus 1–6, an OR >1 indicates higher odds of an mRS >0).
Analyses were performed using Stata 16.1 (StataCorp, College Station, TX), using the gologit2 commands for partial proportional odds models. 38
Results
Table 1 summarizes baseline cohort characteristics for regression variables. Sixty‐three percent of participants were men, with an average age of 64 years. Fifty‐five percent of participants were recruited in Vietnam, 42% in Australia, and 3% in New Zealand. Before the stroke, 99% of participants were independent, 30% in full‐time paid employment, and 53% retired. Most strokes were ischemic (85%). The median NIHSS score was 6 (moderate stroke severity); and the median PHQ‐9 score was 4 (depressive disorder is unlikely).
The Figure presents the distribution of mRS scores over 12 months. Except for death (mRS 6), which increased over time, there was a general reduction in severity of mRS scores.
Table 2 presents patterns of recovery as measured by the mRS over 12 months. Most improvement in mRS scores occurred within 90 days, with a small number of participants (n=60) with worse mRS scores at day 365 compared with day 1 (77% attributable to death). Differentiation between ischemic and hemorrhagic stroke can be seen in Tables S1 and S2. Participants with hemorrhagic strokes had more severe strokes (higher mean mRS at baseline) but had a slightly faster rate of recovery (as measured by change in mean mRS) than ischemic strokes.
Table 3 summarizes associations between baseline characteristics and mRS scores using single‐measure and repeated‐measures methods. For single‐measure analysis, lower mRS scores at day 365 were associated with male sex, age <70 at stroke, no history of diabetes, coronary heart disease, ischemic stroke, or depression; no fluoxetine intervention, ischemic stroke (compared with hemorrhagic), treatment in Vietnam (compared with Australia or New Zealand); lower baseline NIHSS or PHQ‐9 scores; and prestroke independence, living alone, having a partner, and paid employment. Participants >70 years at stroke were associated with higher mRS categories. For the single‐measure model, association of mRS with age at stroke >70 years increased in the cumulative logit models mRS 0 versus 1 to 6 to mRS 0 to 3 versus 4 to 6, before decreasing, whereas in the repeated‐measures model, age at stroke >70 years had varying strengths of positive associations at different cumulative logit models. Participants with higher NIHSS scores were associated with higher mRS categories, with the association strength decreasing at higher mRS categories. This was consistent across univariable and multivariable single‐measure and repeated‐measures regressions.
Table 3.
Multivariable Partial Proportional Odds Regression of Day 365 mRS on Baseline Characteristics, Unadjusted and Adjusted Results of Single Measure (n=1246) and Repeated Measures Analysis (n=1276)
| Factor | Univariable single measure model [OR, σ2, (95% CI)] | Adjusted single measure model [OR, σ2, (95% CI)] | Univariable repeated measures model [OR, σ2, (95% CI)] | Adjusted repeated measures model [OR, σ2, (95% CI)] |
|---|---|---|---|---|
| Demographics | ||||
| Male sex* | 0.76†, 0.00, (0.70–0.81) | … | 0.76†, 0.00, (0.75–0.77) | … |
| Age greater than 70 y* |
2.52, 1.75, (0.90–7.04) †† |
… |
2.07, 1.02, (0.8–5.38) †† |
… |
| Before stroke | ||||
| Diabetes* | 1.73†, 0.00, (1.61–1.87) | 1.73†, 0.02, (1.45–2.06) | 1.44†, 0.01, (1.28–1.61) | 1.43†, 0.02, (1.18–1.74) |
| Coronary heart disease* | 2.47†, 0.16, (1.79–3.4) | 1.88†, 0.09, (1.37–2.57) | 2.05†, 0.08, (1.56–2.69) | 1.66†, 0.04, (1.30–2.12) |
| Ischemic stroke* | 1.65†, 0.03, (1.32–2.06) | 1.43§, 0.04, (1.11–1.85) | 1.43§, 0.03, (1.13–1.8) | 1.29, 0.03, (1.00–1.66) |
| Depression* | 1.05, 0.03, (0.78–1.42) | 0.92, 0.02, (0.70–1.22) | 0.97, 0.01, (0.82–1.15) | 0.88, 0.00, (0.76–1.00) |
| Independence* | 0.09†, 0.00, (0.02–0.37) | 0.15§, 0.01, (0.04–0.51) | 0.12†, 0.00, (0.06–0.24) | 0.18†, 0.00, (0.10–0.32) |
| Paid employment* | 0.52‡‡, 0.02, (0.29–0.92) | 0.75, 0.02, (0.52–1.09) | 0.62, 0.03, (0.37–1.04) | 0.86, 0.02, (0.60–1.22) |
| Partner* | 0.71†, 0.00, (0.63–0.8) | 0.87§, 0.00, (0.79–0.96) | 0.77§, 0.00, (0.7–0.84) | 0.92‡‡, 0.00, (0.85–1.00) |
| Living alone* | 1.34§, 0.02, (1.11–1.61) | 1.00, 0.00, (0.87–1.14) | 1.23†, 0.00, (1.13–1.34) | 0.97‡‡, 0.00, (0.95–0.99) |
| Current stroke (baseline) | ||||
| Fluoxetine intervention* | 1.06, 0.00, (0.97–1.15) | … | 1.02, 0.00, (0.93–1.12) | … |
| Haemorrhagic stroke* | 1.3, 0.08, (0.86–1.97) | 1.35‡‡, 0.03, (1.03–1.75) | 1.48§, 0.04, (1.14–1.93) | 1.52†, 0.01, (1.33–1.74) |
| Treated in Australia* | 1.47†, 0.01, (1.25–1.73) | … | 1.28†, 0.01, (1.12–1.46) | … |
| Treated in New Zealand‡‡ | 1.83†, 0.13, (1.25–2.68) | … | 1.77§, 0.05, (1.38–2.26) | … |
| PHQ‐9† Score | 1.04§, 0.00, (1.02–1.06) | 1.04†, 0.00, (1.01–1.07) | 1.05§, 0.00, (1.03–1.06) | 1.05§, 0.00, (1.03–1.07) |
| NIHSS Score | ||||
| Time since stroke (reference: day 28) | ||||
| Day 90 | … | … |
2.37, 2.30, (0.67–8.31) †† |
… |
| Day 180 | … | … |
3.19, 6.21, (0.69–14.75) †† |
… |
| Day 365 | … | … | … | |
Results are presented to 2 decimal places. Adjusted estimates were not provided for exogenous variables (age at stroke greater than 70 years, sex, fluoxetine intervention, location of treatment and time since stroke). NIHSS indicates National Institutes of Health Research Stroke Scale (higher scores indicates greater neurological impairment). NIHSS indicates National Institutes of Health Research Stroke Scale (higher scores indicates greater neurological impairment); OR, odds ratio; and PHQ‐9, 9‐item Patient Health Questionnaire (higher scores indicates more depressive symptoms).
Dummy Variable = 1 if true. Partner refers to participants who declared they were married or had a partner, relative to single participants. Living alone was relative to participants living in institutions or sharing a residence. Independence was defined as an estimated mRS score <2 before stroke. Haemorrhagic stroke was relative to ischemic strokes. Treatment in Australia and New Zealand was relative to Vietnam.
P<0.001.
mRS 0 vs 1 to 6: Odds ratio greater than 1 indicates higher odds of having an mRS greater than 0 (and vice‐versa).
P<0.01.
mRS 0 to 1 vs 2 to 6: Odds ratio greater than 1 indicates higher odds of having an mRS greater than 1 (and vice‐versa).
mRS 0 to 2 vs 3 to 6: Odds ratio greater than 1 indicates higher odds of having an mRS greater than 2 (and vice‐versa).
mRS 0 to 3 vs 4 to 6: Odds ratio greater than 1 indicates higher odds of having an mRS greater than 3 (and vice‐versa).
mRS 0 to 4 vs 5 to 6: Odds ratio greater than 1 indicates higher odds of having an mRS greater than 4 (and vice‐versa).
mRS 0 to 5 vs 6: Odds ratio greater than 1 indicates higher odds of having an mRS greater than 5 (and vice‐versa).
P<0.05.
Repeated‐measures analysis indicated that greater recovery time since stroke was generally associated with lower mRS scores. Greater time since stroke (at days 90, 180, and 365) was associated with lower mRS categories in all cumulative logit models, except for mRS 0 to 5 versus 6 (at days 90, 180, and 365) and mRS 0 to 4 versus 5 to 6 (day 365). The association of greater time since stroke with a lower mRS category increased in strength over time for all cumulative logit models from mRS 0 versus 1 to 6 to mRS 0 to 3 versus 4 to 6. Initially at days 90 and 180, greater time since stroke was associated with a lower mRS category for the cumulative logit model mRS 0 to 4 versus 5 to 6; however, this association changed direction at day 365. The association of greater time since stroke with a higher mRS category increased in strength over time for the cumulative logit model mRS 0 to 5 versus 6.
The direction of associations was largely concordant between single‐measure and repeated‐measures models except for a history of depression (positive OR in the univariable single‐measure model, but negative OR in the univariable repeated‐measures model and the multivariable single and repeated‐measures model). However, the size of associations was generally reduced (ORs closer to unity) in the repeated‐measures model, with lower variance (increased precision) and smaller CIs.
Discussion
This article has presented poststroke recovery over 12 months for an international cohort of 1276 participants with acute stroke of mild to moderate severity. Most (81% of average 12‐month mRS score improvement) recovery occurred in the first 3 months after stroke, in line with literature and recommended timing for functional assessment. 23 , 24 , 31 Nonetheless, small functional gains were made in the “late subacute” (4–6 months) and “chronic” phase (>6 months) as defined by the Stroke Recovery and Rehabilitation Roundtable. 12 This was reflected in the associations of time since stroke (at days 90, 180, and 365) with lower mRS categories in all cumulative logit models, except for mRS 0 to 5 versus 6 (at days 90, 180, and 365) and mRS 0 to 4 versus 5 to 6 (day 365). This can be explained by an increase in deaths over time compared with the day 28 reference category (sample total of 1% at day 28, 2% at day 90, 2% at day 180, and 4% at day 365) and an increase in mRS 5 and 6 (sample total of 5% at day 365) compared with day 28 (sample total of 4%), respectively. We note that the follow‐up points are not evenly spaced in time, meaning that the strength of the time associations are not directly comparable and are more heavily weighted toward early recovery (eg, from day 1 to day 28 compared with day 28 to day 90). In comparison with the National Institute of Neurological Disorders and Stroke control group at 12 months (mRS 0 [11%], mRS 1 [17%], mRS 2 [12%], mRS 3 [13%], mRS 4 [11%], mRS 5 [5%], and mRS 6 [29%]), 33 our 12‐month results were more heavily weighted toward lower mRS scores, indicating greater recovery (mRS 0 [15%], mRS 1 [41%], mRS 2 [15%], mRS 3 [17%], mRS 4 [6%], mRS 5 [1%], and mRS 6 [4%]). This may reflect that AFFINITY enrolled less severe strokes compared with the National Institute of Neurological Disorders and Stroke. 33
Identified associations with mRS align with a similar study, which found that younger age, lower baseline NIHSS, no prestroke health problems, previous stroke, diabetes, and coronary heart disease were associated with lower 6‐month mRS scores. 39 Another study found younger age and lower baseline NIHSS was associated with improved recovery (measured by the Barthel Index). 29 The small association with male sex and improved recovery may be supported by literature suggesting female sex is associated with worse functional outcomes following stroke; however, this has not been fully explained. 40 Our finding that fluoxetine was associated with higher mRS scores may be related to the increased likelihood of adverse events (including seizures and falls) in AFFINITY. 33 With mixed literature comparing functional outcomes of ischemic and hemorrhagic stroke, 41 , 42 , 43 our results suggest hemorrhagic strokes are associated with higher mRS scores over 12 months.
While mRS scores have been used to predict poststroke depression at 6 months, 44 PHQ‐9 at baseline had not been identified in the literature as an association with mRS or functional disability. A systematic review and meta‐analysis of the impact of stroke on physical disability 45 concluded depression following stroke was associated with worse long‐term disability. However, our repeated‐measures results show that a history of depression was imprecisely associated with slightly lower mRS scores. This may be attributable to treatment of underlying (or poststroke) depression with fluoxetine (possibly reflected by the improved mood and emotional control in AFFINITY), although only 3% of participants had a prestroke diagnosis of depression, limiting analysis. 33 Our work also supports previous research suggesting independence (measured by prestroke mRS) is strongly associated with poststroke function, 46 which may explain why living alone was associated with improved function. However, 99% of participants were independent before the stroke. Having a marital partner has been linked to improved poststroke survival and recovery from disability, 47 , 48 aligning with our findings.
Repeated measures capture within‐person and interperson variation, enabling research to be sensitive to clinically important fluctuations in mRS scores during stroke recovery. Repeated measures of the same state improve precision. While this is limited by different measurements in time, this partially corrects single measures impacted by temporal confounders. Repeated measurements also improve precision by requiring fewer participants, as subjects act as their own “controls.” 11 The direction of associations was concordant between single‐measure and repeated‐measures analysis except for a history of depression, which was associated with higher mRS scores in the univariable single‐measure regression and a negative association in the repeated‐measures regressions. However, precision of associations varied, with generally smaller associations, reduced variance (increased precision) and CIs in the repeated‐measures model compared with the single‐measure model.
Limitations
Our results may be less generalizable to more severe strokes, with a median baseline NIHSS of 6, and more participants (72%) recovering functional independence (mRS 0–2) at 6 months than estimated in original power calculations (42%). 33 While all mRS data were measured using the simplified mRS, variation in interrater and intrarater reliability may account for some change over time; however, data collector identity was unavailable. The simplified mRS is a patient‐reported, not clinician‐reported outcome, more closely reflecting patients’ subjective experiences and perceptions but less objectively reflecting functional ability (eg, in disorders of neglect). Interobserver variability in mRS assessment across countries 49 may bias estimates for different locations and cultural groups. Additionally, as a secondary analysis of a randomized clinical trial, our results may not be generalizable to wider populations. Our model did not incorporate repeated measures of independent variables (eg, PHQ‐9 at day 180), as these were measured later in the recovery period and were less complete than mRS data. Including repeated measures of independent variables may be an avenue of investigation for future stroke recovery modeling. We also note that the mRS curve is unlikely to be linear (with 1 study suggesting an S‐shaped curve 50 ); however, research in this area is still new, with optimal weightings yet to be decided. 4 , 50 Finally, we note that the adjusted OR is difficult to interpret because of its noncollapsible nature 51 ; hence, we have provided both unadjusted and adjusted estimates to mitigate this.
Conclusions
Most participants improved or remained stable through follow‐up over 12 months after stroke, with improved functional outcomes associated with various clinical and demographic variables. Repeated‐measures analyses generally demonstrated improvement in association precisio. Additional repeated‐measures analyses of stroke trials will improve understanding of its methodological value. Stroke trial analyses with repeated‐measures data ex‐post may wish to consider the potential of integrating follow‐up assessments to improve precision, validity, and reliability of conclusions.
Sources of Funding
The original AFFINITY trial was funded by the National Health and Medical Research Council of Australia (NHMRC) Project Grant 1059094. This article was completed by Alexander Chye with funding from the George Institute. The remaining authors contributed meaningful edits and feedback without compensation.
Disclosures
Drs Hankey and Hackett report grants from the NHMRC, Vetenskapsrådet (the Swedish Research Council), and the United Kingdom National Institute for Health Research Technology, during the original AFFINITY study. Dr Hankey reports personal fees from the American Heart Association, outside the submitted work. Dr Hackett reports Career Development Fellowship APP1141328 from the NHMRC; Dr Jan reports Principal Research Fellowship from the NHMRC (1119443). Drs Etherton‐Beer, Billot, Anderson, Jan, and Lung report grants from the NHMRC, during the original AFFINITY study. Dr Anderson reports grants from the NHMRC, grants from Takeda, and personal fees from Takeda, outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figures S1–S3
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.025425
For Sources of Funding and Disclosures, see page 9.
References
- 1. Broderick JP, Adeoye O, Elm J. Evolution of the modified rankin scale and its use in future stroke trials. Stroke. 2017;48:2007–2012. doi: 10.1161/STROKEAHA.117.017866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: implications for stroke clinical trials ‐ a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6 [DOI] [PubMed] [Google Scholar]
- 3. Nunn A, Bath PM, Gray LJ. Analysis of the modified Rankin scale in randomised controlled trials of acute ischaemic stroke: a systematic review. Stroke Res Treat. 2016. doi: 10.1155/2016/9482876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rebchuk AD, O’Neill ZR, Szefer EK, Hill MD, Field TS. Health utility weighting of the modified rankin scale: a systematic review and meta‐analysis. JAMA Netw Open. 2020;3:203767. doi: 10.1001/jamanetworkopen.2020.3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ganesh A, Luengo‐Fernandez R, Wharton RM, Rothwell PM. Ordinal vs dichotomous analyses of modified rankin scale, 5‐year outcome, and cost of stroke. Neurology. 2018;91:1951–1960. doi: 10.1212/WNL.0000000000006554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bath PMW, Lees KR, Schellinger PD, Altman H, Bland M, Hogg C, Howard G, Saver JL. Statistical analysis of the primary outcome in acute stroke trials. Stroke. 2012;43:1171–1178. doi: 10.1161/STROKEAHA.111.641456 [DOI] [PubMed] [Google Scholar]
- 7. Wilson A, Bath PM, Berge E, Cadilhac DA, Cuche M, Ford GA, Macisaac R, Quinn TJ, Taylor M, Walters M, et al. Understanding the relationship between costs and the modified Rankin Scale: a systematic review, multidisciplinary consensus and recommendations for future studies. Eur Stroke J. 2017;2:3–12. doi: 10.1177/2396987316684705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li N, Elashoff RM, Li G, Saver J. Joint modeling of longitudinal ordinal data and competing risks survival times and analysis of the NINDS rt‐PA stroke trial. Stat Med. 2010;29:546–557. doi: 10.1002/sim.3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newman GC, Bang H, Hussain SI, Toole JF. Association of diabetes, homocysteine, and HDL with cognition and disability after stroke. Neurology. 2007;69:2054–2062. doi: 10.1212/01.wnl.0000280457.29680.9c [DOI] [PubMed] [Google Scholar]
- 10. Potts JE. A Comparison of Techniques Used in the Longitudinal Analysis of the Modified Rankin Scale in Stroke Randomised Control Trials. Keele University. Available at: https://eprints.keele.ac.uk/5599/1/PottsPhD2018.pdf. Accessed April 20, 2021. [Google Scholar]
- 11. Bramwell AT, Bittner AC, Morrissey SJ. Repeated‐measures analysis: issues and options. Int J Ind Ergon. 1992;10:185–197. doi: 10.1016/0169-8141(92)90032-U [DOI] [Google Scholar]
- 12. Bernhardt J, Hayward S, Kwakkel G, Ward N, Wolf SL, Borschmann K, Krakauer JW, Boyd LA, Carmichael ST, Corbett D, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Neurorehabil Neural Repair. 2017;31:793–799. doi: 10.1177/1747493017711816 [DOI] [PubMed] [Google Scholar]
- 13. Cassarly C, Martin RH, Chimowitz M, Peña EA, Ramakrishnan V, Palesch YY. Comparison of multistate Markov modeling with contemporary outcomes in a reanalysis of the NINDS tissue plasminogen activator for acute ischemic stroke treatment trial. PLoS One. 2017;12. doi: 10.1371/journal.pone.0187050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cassarly C, Martin RH, Chimowitz M, Peña EA, Ramakrishnan V, Palesch YY. Treatment effect on ordinal functional outcome using piecewise multistate Markov model with unobservable baseline: an application to the modified Rankin scale. J Biopharm Stat. 2019;29:82–97. doi: 10.1080/10543406.2018.1489404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desantis SM, Lazaridis C, Palesch Y, Ramakrishnan V. Regression analysis of ordinal stroke clinical trial outcomes: an application to the NINDS t‐PA Trial. Int J Stroke. 2014;9:226–231. doi: 10.1111/ijs.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng W, Vasquez G, Suri MFK, Lakshminarayan K, Qureshi AI. Repeated‐measures analysis of the national institute of neurological disorders and stroke rt‐PA stroke trial. J Stroke Cerebrovasc Dis. 2011;20:241–242. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 17. Palesch YY, Yeatts SD, Tomsick TA, Foster LD, Demchuk AM, Khatri P, Hill MD, Jauch EC, Jovin TG, Yan B, et al. Twelve‐month clinical and quality‐of‐life outcomes in the interventional management of stroke III trial. Stroke. 2015;46:1321–1327. doi: 10.1161/STROKEAHA.115.009180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Donnell MJ, Fang J, D’Uva C, Saposnik G, Gould L, McGrath E, Kapral MK. The PLAN score: a bedside prediction rule for death and severe disability following acute ischemic stroke. Arch Intern Med. 2012;172:1548–1556. doi: 10.1001/2013.jamainternmed.30 [DOI] [PubMed] [Google Scholar]
- 19. Saposnik G, Kapral MK, Liu Y, Hall R, O’Donnell M, Raptis S, Tu JV, Mamdani M, Austin PC. IScore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation. 2011;123:739–749. doi: 10.1161/CIRCULATIONAHA.110.983353 [DOI] [PubMed] [Google Scholar]
- 20. de Ridder IR, Dijkland SA, Scheele M, den Hertog HM, Dirks M, Westendorp WF, Nederkoorn PJ, van de Beek D, Ribbers GM, Steyerberg EW, et al. Development and validation of the Dutch Stroke Score for predicting disability and functional outcome after ischemic stroke: a tool to support efficient discharge planning. Eur Stroke J. 2018;3:165–173. doi: 10.1177/2396987318754591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ntaios G, Faouzi M, Ferrari J, Lang W, Vemmos K, Michel P. An integer‐based score to predict functional outcome in acute ischemic stroke: the ASTRAL score. Neurology. 2012;78:1916–1922. doi: 10.1212/WNL.0b013e318259e221 [DOI] [PubMed] [Google Scholar]
- 22. Counsell C, Dennis M, McDowall M, Warlow C. Predicting outcome after acute and subacute stroke: development and validation of new prognostic models. Stroke. 2002;33:1041–1047. doi: 10.1161/hs0402.105909 [DOI] [PubMed] [Google Scholar]
- 23. Savitsz SI, Benatar M, Saver JL, Fisher M. Outcome analysis in clinical trial design for acute stroke: physicians’ attitudes and choices. Cerebrovas Dis. 2008;26:156–162. doi: 10.1159/000139663 [DOI] [PubMed] [Google Scholar]
- 24. Duncan PW, Jorgensen HS, Wade DT. Outcome measures in acute stroke trials: a systematic review and some recommendations to improve practice. Stroke. 2000;31:1429–1438. doi: 10.1161/01.str.31.6.1429 [DOI] [PubMed] [Google Scholar]
- 25. Fahey M, Crayton E, Wolfe C, Douiri A. Clinical prediction models for mortality and functional outcome following ischemic stroke: a systematic review and meta‐analysis. PLoS One. 2018;13:e0185402. doi: 10.1371/journal.pone.0185402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stinear CM, Smith MC, Byblow WD. Prediction tools for stroke rehabilitation. Stroke. 2019;50:3314–3322. doi: 10.1161/STROKEAHA.119.025696 [DOI] [PubMed] [Google Scholar]
- 27. Visvanathan A, Whiteley W, Mead G, Lawton J, Doubal FN, Dennis M. Reporting “specific abilities” after major stroke to better describe prognosis. J Stroke Cerebrovasc Dis. 2020;29:104993. doi: 10.1016/j.jstrokecerebrovasdis.2020 [DOI] [PubMed] [Google Scholar]
- 28. Persson HC, Opheim A, Lundgren‐Nilsson Å, Murpy MA, Danielsson A, Sunnerhagen KS. Upper extremity recovery after ischaemic and haemorrhagic stroke: part of the SALGOT study. Eur Stroke J. 2016;1:310–319. doi: 10.1177/2396987316672809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Douiri A, Grace J, Sarker SJ, Tilling K, McKevitt C, Wolfe CDA, Rudd AG. Patient‐specific prediction of functional recovery after stroke. Int J Stroke. 2017;12:539–548. doi: 10.1177/1747493017706241 [DOI] [PubMed] [Google Scholar]
- 30. Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007;68:1583–1587. doi: 10.1212/01.wnl.0000260967.77422.97 [DOI] [PubMed] [Google Scholar]
- 31. Ganesh A, Luengo‐Fernandez R, Wharton RM, Gutnikov SA, Silver LE, Mehta Z, Rothwell PM, Study the OV . Time course of evolution of disability and cause‐specific mortality after ischemic stroke: implications for trial design. J Am Heart Assoc. 2017;6:e005788. doi: 10.1161/JAHA.117.005788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elm von E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 33. Hankey GJ, Hackett ML, Almeida OP, Flicker L, Mead GE, Dennis MS, Etherton‐Beer C, Ford AH, Billot L, Jan S, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double‐blind, placebo‐controlled trial. Lancet Neurol. 2020;19:651–660. doi: 10.1016/S1474-4422(20)30207-6 [DOI] [PubMed] [Google Scholar]
- 34. Bruno A, Shah N, Lin C, Close B, Hess DC, Davis K, Baute V, Switzer JA, Waller JL, Nichols FT. Improving modified rankin scale assessment with a simplified questionnaire. Stroke. 2010;41:1048–1050. doi: 10.1161/STROKEAHA.109.571562 [DOI] [PubMed] [Google Scholar]
- 35. Optimising Analysis of Stroke Trials (OAST) Collaboration , PMW B, Gray LJ, Collier T, Pocock S, Carpenter J. Can we improve the statistical analysis of stroke trials. Stroke. 2007;38:1911–1915. doi: 10.1161/STROKEAHA.106.474080 [DOI] [PubMed] [Google Scholar]
- 36. Veerbeek JM, Kwakkel G, van Wegen EEH, Ket JCF, Heymans MW. Early prediction of outcome of activities of daily living after stroke. Stroke. 2011;42:1482–1488. doi: 10.1161/STROKEAHA.110.604090 [DOI] [PubMed] [Google Scholar]
- 37. Laubach ZM, Murray EJ, Hoke KL, Safran RJ, Perng W. A biologist’s guide to model selection and causal inference. Proc Biol Sci. 2021;288. doi: 10.1098/rspb.2020.2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams R. Understanding and interpreting generalized ordered logit models. J Math Sociol. 2016;40:7–20. doi: 10.1080/0022250X.2015.1112384 [DOI] [Google Scholar]
- 39. Hofstad H, Naess H, Gjelsvik BEB, Eide GE, Skouen JS. Subjective health complaints predict functional outcome six months after stroke. Acta Neurol. Scand. 2017;135:161–169. doi: 10.1111/ane.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gall SL, Tran PL, Martin K, Blizzard L, Srikanth V. Sex differences in long‐term outcomes after stroke: functional outcomes, handicap, and quality of life. Stroke. 2012;43:1982–1987. doi: 10.1161/STROKEAHA.111.632547 [DOI] [PubMed] [Google Scholar]
- 41. Perna R, Temple J. Rehabilitation outcomes: ischemic versus hemorrhagic strokes. Behav Neurol. 2015. doi: 10.1155/2015/891651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salvadori E, Papi G, Insalata G, Rinnoci V, Donnini I, Martini M, Falsini C, Hakiki B, Romoli A, Barbato C, et al. Comparison between ischemic and hemorrhagic strokes in functional outcome at discharge from an intensive rehabilitation hospital. Diagnostics. 2020;11. doi: 10.3390/diagnostics11010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paolucci S, Antonucci G, Grasso MG, Bragoni M, Coiro P, de Angelis D, Fusco FR, Morelli D, Venturiero V, Troisi E, et al. Functional outcome of ischemic and hemorrhagic stroke patients after inpatient rehabilitation. Stroke. 2003;34:2861–2865. doi: 10.1161/01.STR.0000102902.39759 [DOI] [PubMed] [Google Scholar]
- 44. López‐Espuela F, Roncero‐Martín R, Canal‐Macías ML, Moran JM, Vera V, Gomez‐Luque A, Lendinez‐Mesa A, Pedrera‐Zamorano JD, Casado‐Naranjo I, Lavado‐García J. Depressed mood after stroke: Predictive factors at six months follow‐up. Int J Environ Res Public Health. 2020;17:1–11. doi: 10.3390/ijerph17249542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blöchl M, Meissner S, Nestler S. Does depression after stroke negatively influence physical disability? A systematic review and meta‐analysis of longitudinal studies. J Affect Disord. 2019;247:45–56. doi: 10.1016/j.jad.2018.12.082 [DOI] [PubMed] [Google Scholar]
- 46. Quinn TJ, Taylor‐Rowan M, Coyte A, Clark AB, Musgrave SD, Metcalf AK, Day DJ, Bachmann MO, Warburton EA, Potter JF, et al. Pre‐stroke modified Rankin Scale: evaluation of validity, prognostic accuracy, and association with treatment. Front Neurol. 2017;8:275. doi: 10.3389/fneur.2017.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu Q, Wang X, Wang Y, Wang C, Zhao X, Liu L, Li Z, Meng X, Guo L, Wang Y. Association between marriage and outcomes in patients with acute ischemic stroke. J Neurol. 2018;265:942–948. doi: 10.1007/s00415-018-8793-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dupre ME, Lopes RD. Marital history and survival after stroke. J Am Heart Assoc. 2016;5:e004647. doi: 10.1161/JAHA.116.004647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quinn TJ, Dawson J, Walters MR, Lees KR. Variability in modified Rankin scoring across a large cohort of international observers. Stroke. 2008;39:2975–2979. doi: 10.1161/STROKEAHA.108.515262 [DOI] [PubMed] [Google Scholar]
- 50. Ganesh A, Luengo‐Fernandez R, Pendlebury ST, Rothwell PM. Weights for ordinal analyses of the modified Rankin Scale in stroke trials: a population‐based cohort study. EClinicalMedicine. 2020;23:100415. doi: 10.1016/j.eclinm.2020.100415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol. 2019;34:211–219. doi: 10.1007/s10654-019-00494-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3
Data Availability Statement
The data that support the study findings are available from the corresponding author upon reasonable request. One of the authors (A.C.) had full access to all the data in the study and takes responsibility for its integrity and the data analysis.


