Abstract
Plants of the species Fridericia chica (Bonpl.) L. G. Lohmann (Bignoniaceae), which are widely distributed in Brazil and named crajiru in the state of Amazonas, are known in folk medicine as a traditional medicine in the form of a tea for the treatment of intestinal colic, diarrhea, and anemia, among other diseases. The chemical analysis of extracts of the leaves has identified phenolic compounds, a class of secondary metabolites that provide defense for plants and benefits to the health of humans. Several studies have shown the therapeutic efficacy of F. chica extracts, with antitumor, antiviral, wound healing, anti-inflammatory, and antioxidant activities being among the therapeutic applications already proven. The healing action of F. chica leaf extract has been demonstrated in several experimental models, and shows the ability to favor the proliferation of fibroblasts, which is essential for tissue repair. The anti-inflammatory activity of F. chica has been clearly demonstrated by several authors, who suggest that it is related to the presence of 3-deoxyanthocyanidins, which is capable of inhibiting pro-inflammatory pathways such as the kappa B (NF-kB) nuclear transcription factor pathway. Another important effect attributed to this species is the antioxidant effect, attributed to phenolic compounds interrupting chain reactions caused by free radicals and donating hydrogen atoms or electrons. In conclusion, the species Fridericia chica has great therapeutic potential, which is detailed in this paper with the objective of encouraging new research and promoting the sum of efforts for the inclusion of herbal medicines in health systems around the world.
Keywords: Fridericia chica, phytochemistry, anti-inflammatory, antioxidant, wound healing
1. Introduction
Fridericia chica (Bonpl.) L. G. Lohmann (Figure 1) is a liana or creeper that belongs to the family Bignoniaceae, which comprises about 120 genera and 800 species [1]. The largest number of species of this family is found mainly in tropical and subtropical regions, with Brazil and the African continent as the two major centers of geographical distribution. In Brazil, the species of the family Bignoniaceae do not have a unique habitat and are widely distributed in the region of the legal Amazon, as well as throughout the southern region of the country as far down as Rio Grande do Sul. In addition, plants of this family can be found in the Cerrado and Atlantic Forest biomes [2,3,4]. F. chica was first described by Cronquist [5] as Arrabidaea chica, belonging to the division Magnoliophyta, class Magnoliopsida, subclass Asteridae, order Scrophulariales, family bignoniaceae, and genus Arrabidaea. However, its classification has been altered due to recent taxonomic changes, such as the inclusion of several species of the genus Arrabidaea in the genus Fridericia. This change has affected the classification of its varieties A. chica var. acutifolia (DC.) Bureau, A. chica var. angustifolia Bureau & K. Schum, A. chica var. cuprea Bureau & K. Schum, A. chica var. thyrsoidea (DC.) Bureau and A. chica var. viscida Donn.Sm [6].
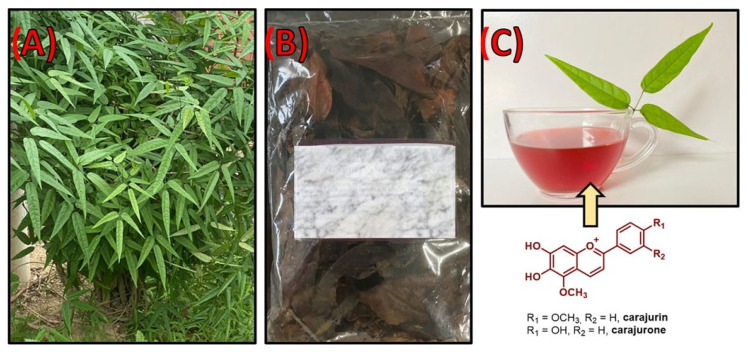
Figure 1.
Fridericia chica (Bonpl.) L. G. Lohmann: specimen and presentations forms. (A) Arboreal specimen of adult F. chica in situ in the city of Manaus, Amazonas Brazil. (B) Dried leaves of F. chica as marketed in the city of Manaus, Brazil. (C) Tea obtained from the dried leaves of F. chica and its main compounds responsible for the characteristic color. Photos: Author.
Botanically, Fridericia chica are described as woody, shrub-like, or arboreal plants, as well as climbing plants, with that leaves measure between 18–20 cm in length when mature. They have opposite crossed leaves, compound bi- or trifoliate, however, with the terminal foliole modified in tendrils in the elevated part of the branches. The folioles have an oblong-lanceolate shape, cartaceous, with an obtuse base, acute apex and herbaceous consistency. The surface of the folioles is smooth, and the rib is of the peninerveal type, i.e., the secondary ribs branch from the main rib [3,7,8,9]. The flowers are campanulate, resemble the shape of a bell, and have pink or violet coloration [10,11].
F. chica occurs in Central American countries, the Caribbean, and mainly in South American countries, such as Guyana and French Guiana, and especially in Brazil. In the latter it has wide geographical distribution, with confirmed occurrence in all regions and phytogeographic domains, including the Amazon, the Caatinga, the Cerrado, the Atlantic Forest, and the Pantanal biomes. With regard to the type of vegetation, F. chica can be found in floodplains or forests of various types, such as riparian, flooded forests, and terra firma, among others [6].
2. Revision Strategy
An extensive literature review was carried out using different scientific electronic sources, including databases such as Scifinder, Pubmed, Scopus, Web of Science, and Google Scholar. The study databases included original papers published in peer reviewed journals, books, dissertations, and theses, and all data of scientific interest written or translated into English published prior to July 2022 was considered. The keywords “Arrabidae chica” and “Fridericia chica” alone and in combination with änflammation”, inflammatory”, änti-inflammatory”, “oxidative stress”, “antioxidant”, “wound healing”, and “healing”. In addition, the names of all phytochemical compounds were used in the search.
3. Ethnobotany
In Brazil, F. chica is popularly known as cajuru, carajiru, carajunu, carajuru, chica, china, cipó-cruz, cipó-pau, coá-piranga, crajiru, crajuru, cuica, guajuru, guajuru-piranga, guarajuru, oajuru, oajuru-piranga, paripari, pariri, and piranga, among others [2,6,10]. Its mature leaves, collected from plants ranging from 50 cm to 2 m, are used in the form of tea (dried form), which has an astringent function and can be used against intestinal colic, diarrhea, anemia, uterine inflammation, hemorrhages, leukemia, jaundice, albuminuria, for vaginal hygiene via bathing, or in the form of tincture for topical use directly on skin lesions or even in ointments, creams [10,12], and in the form of soap with an antiacne effect [13]. It is used as an anti-inflammatory, antioxidant, antidiabetic medicine, and a disinfectant [3,10,14,15].
4. Chemical Composition
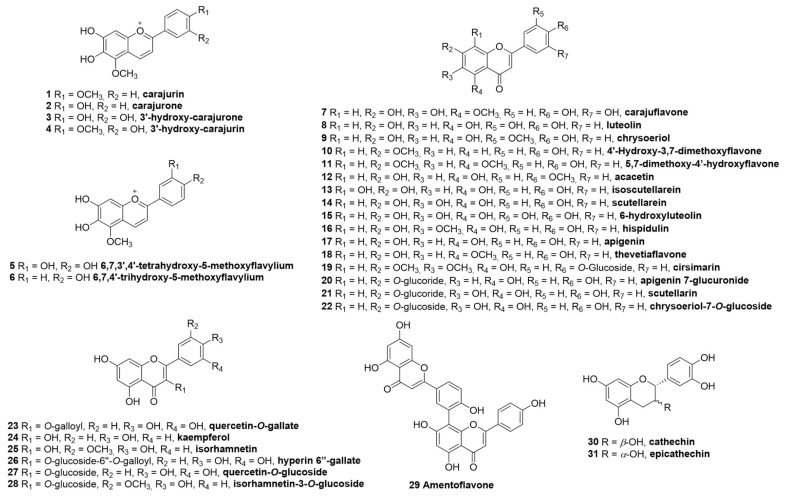
Chemically, F. chica is rich in polyphenols, especially flavonoids. Among the flavonoids, derivatives of the class of anthocyanins stand out, which in this plant are abundant in the form of 3-deoxyanthocyanins. These are involved in plant growth and development, including UV protection, stimulation of pollination and seed dispersal, and as a defense mechanism [16]. The 3-deoxyanthocyanins are the substances that give the tea its red color, especially the main compounds carajurin (1) and carajurone (2) [17], and to a lesser extent four other substances, 3’- hydroxy-carajurone (3), 3’-hydroxy-carajurin (4), 6,7,3’,4’-tetrahydroxy-5-methoxyflavylium (5), and 6,7,4’-trihydroxy-5-methoxyflavylium (6) [18]. Substances 1 and 2 were the first to be described in this species.
In addition to anthocyanidins, other flavonoids (aglycones and glycosides) have been isolated and/or characterized using high-performance liquid chromatography coupled to mass spectrometry (HPLC-MS) analysis, among them the flavones carajuflavone (7), luteolin (8) [13], chrysoeriol (9) [19], 4′-hydroxy-3,7-dimethoxyflavone (10) [20], 5,7-dimethoxy-4′-hydroxyflavone (11) [19], acacetin (12) [17], isoscutellarein (13), scutellarein (14), 6-hydroxyluteolin (15), hispidulin (16), apigenin (17) [21], thevetiaflavone (18) [13], cirsimarin (19) [19], apigenin 7-glucuronide (20), scutellarin (21) [22], chrysoeriol-O-glucoside (22) [19]. Flavonols are a more restricted group, being so far characterized as quercetin-O-gallate (23), kaempferol (24), isorhamnetin (25) hyperin 6”-gallate (26), quercetin-O-glucoside (27), and isorhamnetin-3-O-glucoside (28) [19]. The biflavonoid amentoflavone (29) and the flavans-3-ols catechin (30) and epicatechin (31) [19] are other flavonoid derivatives identified in F. chica (Figure 2).
Figure 2.
Polyphenols (1–31) of F. chica.
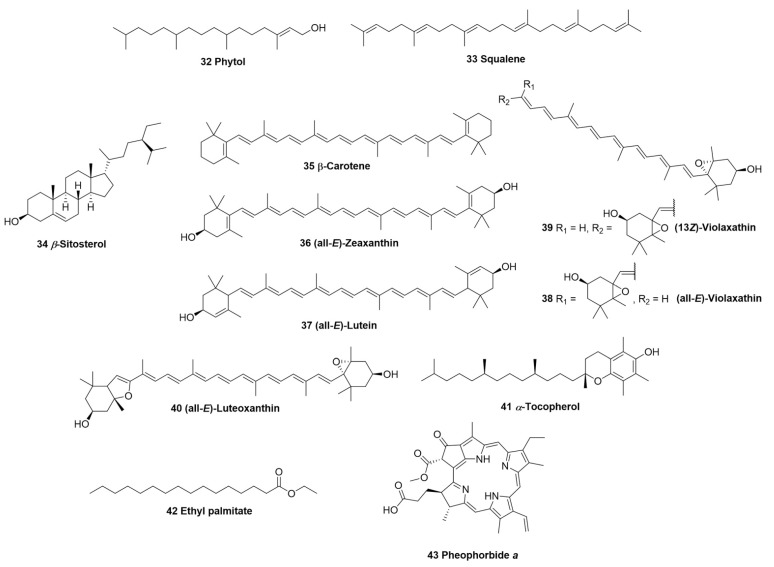
With respect to other classes of natural products, the characterization of terpenes, including carotenoids, and other classes such as fatty acids and their derivatives, tocochromanols and alkaloids, stands out. The diterpene phytol (32), the triterpene squalene (33), and the steroidal β-sitosterol (34) [23] are described as structures of a terpenic nature of up to 30 carbons described in F. chica. On the other hand, a number of tetraterpenes have been identified using HPLC-MS, among them the main compounds β-carotene (35), (all-E)-zeaxanthin (36), (all-E)-lutein (37), (all-E)-violaxathin (38), (13Z)-violaxathin (39), and (all-E)-luteoxanthin (40) [22]. In addition to antioxidant carotenoids, tocochromanol α-tocopherol (41) has been described as well [23]. Additionally, the identification of the fatty acid ethyl ester ethyl palmitate (42) has been reported [23], as has the alkaloid pheophorbide α (43) [24]. In Table 1, the 43 compounds already identified in F. chica and their biological activities already studied from these molecules alone (isolated from F. chica extract or other sources) are shown. It is worth noting that despite the tea being the most popular use of F. chica, phytochemical studies were focused on hydroalcoholic and/or organic extracts, possibly aiming for the enhancement of extraction yields. Moreover, tea preparations may contain the most polar compounds, specifically the positively charged 3-deaoxyanthocyanins, information that is corroborated by the bright red color of the dried leaf infusions. Thus, the composition of the extract is of great importance, as these compounds can exert pharmacological effects with summative, potentiating or deleterious characteristics (Figure 3).
Table 1.
Phytochemical components of F. chica extracts and their biological activities.
| Compound No. | Name | Source | Isolation/Detection | Biological Activity * |
|---|---|---|---|---|
| Anthocyanins | ||||
| 1 | Carajurin | leaves, MeOH | Isolation [17] | Anti-inflammatory [17] |
| 2 | Carajurone | leaves, MeOH | Isolation [17] | N.A. |
| 3 | 3’-Hydroxy-carajurone | leaves, MeOH | Isolation [17] | N.A. |
| 4 | 3’-Hydroxy-carajurin | leaves, MeOH | Isolation [17] | N.A. |
| 5 | 6,7,3’,4’-Tetrahydroxy-5-methoxyflavylium | leaves, DCM fraction | Isolation [18] | N.A. |
| 6 | 6,7,4’-Trihydroxy-5-methoxyflavylium | leaves, DCM fraction | Isolation [18] | N.A. |
| Flavones | ||||
| 7 | Carajuflavone | leaves, AcOEt fraction | Isolation [1] | N.A. |
| 8 | Luteolin | leaves, AcOEt fraction | Isolation [1] | Anti-inflammatory [25] Antioxidant [26] |
| 9 | Chrysoeriol | leaves, 70% EtOH | HPLC-MS detection [19] |
Anti-inflammatory [27] Antioxidant [28] |
| 10 | 4′-Hydroxy-3,7-dimethoxyflavone | leaves, EtOH | Isolation [20] | N.A. |
| 11 | 5,7-dimethoxy-4′-hydroxyflavone | leaves, 70% EtOH | HPLC-MS detection [19] |
N.A. |
| 12 | Acacetin | leaves, MeOH | Isolation [17] | Anti-inflammatory [29] Antioxidant [30] |
| 13 | Isoscutellarein | leaves, 90% EtOH | HPLC-MS detection [21] |
Antioxidant [31] |
| 14 | Scutellarein | leaves, 90% EtOH | HPLC-MS detection, isolation [21] | Anti-inflammatory [32,33] Antioxidant [34] |
| 15 | 6-Hydroxyluteolin | leaves, 90% EtOH | HPLC-MS detection [21] |
N.A. |
| 16 | Hispidulin | leaves, 90% EtOH | HPLC-MS detection [21] |
Anti-inflammatory [35,36] Antioxidant [37] |
| 17 | Apigenin | leaves, 90% EtOH | HPLC-MS detection, isolation [21] | Anti-inflammatory [38,39] Antioxidant [40] Healing [41] |
| 18 | Thevetiaflavone | leaves, AcOEt fraction | Isolation [1] | Antioxidant [42] |
| 19 | Cirsimarin | leaves, 70% EtOH | HPLC-MS detection [19] |
Anti-inflammatory [43] Antioxidant [44] Healing [45] |
| 20 | Apigenin 7-glucuronide | leaves, 80% MeOH | HPLC-MS detection [22] |
Anti-inflammatory [46] Antioxidant [47] |
| 21 | Scutellarin | leaves, 80% MeOH | HPLC-MS detection [22] |
Anti-inflammatory [48] Antioxidant [34] Healing [49] |
| 22 | Chrysoeriol-O-glucoside | leaves, 70% EtOH | HPLC-MS detection [19] |
N.A. |
| Flavonols | ||||
| 23 | Quercetin-O-gallate | leaves, 70% EtOH | HPLC-MS detection [19] |
N.A. |
| 24 | Kaempferol | leaves, 70% EtOH | HPLC-MS detection [19] |
Anti-inflammatory [50,51] Antioxidant [31] Healing [52] |
| 25 | Isorhamnetin | leaves, 70% EtOH | HPLC-MS detection [19] |
Anti-inflammatory [53] Antioxidant [54] |
| 26 | Hyperin 6”-gallate | leaves, 70% EtOH | HPLC-MS detection [19] |
Antioxidant [55] |
| 27 | Quercetin-O-glucoside | leaves, 70% EtOH | HPLC-MS detection [19] |
Antioxidant [56] |
| 28 | Isorhamnetin-3-O-glucoside | leaves, 70% EtOH | HPLC-MS detection [19] |
Antioxidant [57] |
| Flavone dimer | ||||
| 29 | Amentoflavone | leaves, 70% EtOH | HPLC-MS detection [19] |
Anti-inflammatory [58] Antioxidant [59] |
| Flavan-3-ols | ||||
| 30 | Catechin | leaves, 70% EtOH | HPLC-MS detection [19] |
Anti-inflammatory [60] Anti-oxidant [61] |
| 31 | Epicatechin | leaves, 70% EtOH | HPLC-MS detection [19] |
Anti-inflammatory [62] Antioxidant [63] |
| Terpenes | ||||
| 32 | Phytol | leaves, 70% EtOH, hexane fraction | GC-MS detection [23] | Anti-inflammatory [64] Antioxidant [65] |
| 33 | Squalene | leaves, 70% EtOH, hexane fraction | GC-MS detection [23] | Antioxidant [66] Anti-inflammatory [67] |
| 34 | β-Sitosterol | leaves, 70% EtOH, hexane fraction | GC-MS detection [23] | Anti-inflammatory [68] |
| 35 | β-Carotene | leaves, acetone | HPLC-MS detection [22] |
Anti-inflammatory [69] Antioxidant [69] Healing [70] |
| 36 | (all-E)-Zeaxanthin | leaves, acetone | HPLC-MS detection [22] |
Anti-inflammatory [71] Antioxidant [72] |
| 37 | (all-E)-Lutein | leaves, acetone | HPLC-MS detection [22] |
Anti-inflammatory [73] Antioxidant [74] |
| 38 | (all-E)-Violaxanthin | leaves, acetone | HPLC-MS detection [22] |
Anti-inflammatory [75] Antioxidant [75] |
| 39 | (13Z)-Violaxanthin | leaves, acetone | HPLC-MS detection [22] |
Antioxidant [76] |
| 40 | (all-E)-Luteoxanthin | leaves, acetone | HPLC-MS detection [22] |
N.A. |
| Tocochromanol | ||||
| 41 | α-Tocopherol | leaves, 70% EtOH, hexane fraction | GC-MS detection [23] | Antioxidant [77] Healing [78] |
| Fatty acid | ||||
| 42 | Ethyl palmitate | leaves, 70% EtOH, hexane fraction | GC-MS detection [23] | Anti-inflammatory [79] |
| Alkaloid | ||||
| 43 | Pheophorbide a | leaves, 90% EtOH | Isolation [24] | Anti-inflammatory [80] Antioxidant [81] Healing [82] |
* Biological activities concern anti-inflammatory, antioxidant, and healing activity described in previous articles using the isolated components obtained from F. chica or other plants. N.A., not available.
Figure 3.
Terpenes (32–40), tocochromanol (41), fatty acid (42), and alkaloid (43) of F. chica.
5. Pharmacological Properties
Historically, plants have been an important source of active ingredients for drug development, and scientific research has been directed towards the prospect of new drugs based on natural products [83]. Thus, the connection between plants and health is responsible for the beginning of a new generation of therapy that integrates drugs derived from plants or the use of plants themselves or their parts [84]. The public health system in Brazil offers twelve herbal medicines as treatment options, among them several with anti-inflammatory potential, such as Aloe vera, Schinus terebenthifolius, and Uncaria tomentosa [85,86].
The use of plants as a medicine occurs in many traditional societies [87]; therefore, it is important to stimulate scientific studies that prove existing popular knowledge about plants and their effectiveness in the treatment of diseases [88]. In this sense, several studies have been carried out with different extracts of F. chica leaves in order to prove the effectiveness of the plant for the therapeutic purposes for which it is popularly used (Table 2).
Table 2.
Pharmacological properties of extracts of Fridericia chica.
| Authors | Type of Study | Action | Etiological Agent | Plant Material/Part | Treatment |
|---|---|---|---|---|---|
| [20] | In vitro study | Antifungal and antiprotozoal | Trichophyton mentagrophytes (fungus) and Trypanosoma cruzi | Ethanolic extract of leaves | 4 mg/mL (trypanocide); 3.125 mg/mL (fungicide) |
| [89] | In vitro study | Antiprotozoal | Leishmania amazonensis and Leishmania infantum | Hexanic leaf extract | 37.2 µg/mL (L. amazonensis); 18.6 µg/mL (L. infantum) |
| [90] | In vitro and in vivo study | Antiprotozoal and healing | Leishmania amazonensi; Swiss Webster mouse | Ethanolic extract of leaves and fractions | Leishmanicidal effect: 60–155.9 μg/mL; healing effect: 10 mg/g |
| [24] | In vitro study | Antiprotozoal | Trypanosoma cruzi | Hydroethanolic extract of leaves and fractions | 24.8 μg/mL–213 μg/mL (hydroethanolic extract); 2.3 μg/mL–10 μg/mL (feoforbide-a) |
| [91] | In vivo Study | Antimicrobial | Helicobacter pylori 43,504 and Enterococcus faecalis 29,212 | Hydroethanolic extract of leaves | 12.5 μg/mL (H. pylori); 100 μg/mL (E. faecalis) |
| [92] | In vitro study | Antimicrobial | Staphylococcus sp. | Hydroalcoholic extract | 250 μg gallic acid equivalent (GAE)/mL–MIC; 1000 μg GAE/mL–MBC |
| [93] | In vitro study | Antimicrobial | Candida sp. | Dichloromethane extract of leaves | 0.007–0.03 mg/mL |
| [94] | In vitro study | Antiviral | aMPV cepa SHS/669/03 | Ethanolic extract of leaves | 2.5 μg/mL |
| [95] | In vitro study | Antiviral | Human Herpes Virus type 1 (HHV-1); murine Encephalomyocarditis virus (EMCV); Vaccinia Virus strain Western Reserve (VACV-WR) | Ethanolic extract of leaves | EC50: 245.7 μg/mL (HHV-1); 86.3 μg/mL (VACV-WR) |
| [96] | In vivo study | Antitumor | Solid Ehrlich tumor | Ethanolic extract and aqueous extract of leaves | 30 mg/kg body weight (10 days of oral treatment) |
| [97] | In vivo study | Antitumor | 7,12-dimethyl injection-induced breast cancer-1,2-benzanthracene (DMBA) | Hydroalcoholic extract of leaves | Oral administration for 16 weeks: extract at a dose of 300 mg/kg; 7,12-dimethyl-1,2-benzanthracene (DMBA) associated with vincristine 250 µg /mL |
| [98] | In vitro study | Antitumor and fibroblast proliferation | Human tumor cell lines: MCF-7 (breast), NCI-ADR/RES (ovary with multiple drug resistance phenotype), UACC-62 (melanoma), NCI–h460 (lung), PC-3 (prostate), HT29 (colon), OVCAR-03 (ovary), 786-0 (kidney) and K562 (leukemia). Fibroblasts: obtained from 3T3 mice |
Crude leaf extracts (without and with enzyme treatment) | 0.25; 2.5 and 25 μg/mL without enzymatic treatment (fibroblast proliferation); 7.4 and 8.7 μg/mL with enzymatic treatment (cytostatic effect for UACC-62–melanoma lineage) |
| [99] | Survey of use by health professionals | Anti-inflammatory | Oral diseases | Leaves | Tea (no dose determined) |
| [19] | In vivo study | Analgesic and anti-inflammatory | Osteoarthritis induced by sodium monoiodoacetate | Hydroalcoholic extract of leaves | Oral administration s. i.d. for 25 days: 50 mg/kg, 150 mg/kg, 450 mg/kg |
| [100] | Survey of studies with herbal medicines | Anti-acne | Does not apply | Not mentioned | Not informed |
| [101] | Survey of traditional use | Treatment of skin irritation and healing | Measles and smallpox | Leaves | Infusion and bath (measles and smallpox); leaves macerated and applied to the affected area (lesions) |
| [102] | Review | Treatment of tuberculosis-related symptoms | Mycobacterium tuberculosis | Not specified | Not specified |
| [103] | Survey of traditional use | Anemia and weakness | Does not apply | Leaves | Not specified |
| [104] | In vivo study | Antihypertensive | Does not apply | Hydroalcoholic extract of leaves | Oral administration of the extract at doses of 100 mg/kg; 250 mg/ kg and 500 mg/kg |
| [105] | Survey of traditional use | (1) Anemia, weakness, restoration facial color in malaria patients; (2) ovarian cysts, cystitis, hepatitis, liver, diarrhea; (3) flu, cough, anemia; (4) aids getting pregnant, ulcers, (5) vaginal itching | Does not apply | Leaves | (1) maceration or tea; (2) tea or infusion; (3) syrup; (4) bottled; (5) bath. No dose determined |
| [21] | Ex vivo study | Photoprotection | Does not apply | Various parts | Topical application of nonionic cream with 2.5% ethylacetate fraction and 2.5% hexane fraction |
| [106] | In vitro study | Photosensitization | MCF-7 cells of human breast adenocarcinoma | Extract nanoemulsion produced from aerial parts | CC50: 1.3 μg ACE/mL |
| [107] | In vitro study | Anti-hepatoxic | Does not apply | Leaves | 0.25–1.25 mg/mL |
| [108] | In vivo study | Anti-hepatoxic | Carbon tetrachloride | Ethanolic extract of leaves | 300, 500 and 600 mg/kg |
| [109] | In vitro study | Antioxidant | Free Radical DPPH (1,1-diphenyl-2-picrylidazyl | Ethanolic extract of leaves and fractions | 5, 10, 25, 50, 125 and 250 µg/mLin ethanol |
| [14] | In vitro study | Antioxidant | Free Radical DPPH (1,1-diphenyl-2-picrylidazyl | Methanolic extract of leaves | 0.25; 2.5; 25 and 250 μg/mL |
Among biological effects from F. chica leaf extracts, anti-inflammatory, antioxidant, and healing properties are well evidenced from previous studies (Table 2), and result from the leaves’ phytochemical composition. Studies have shown that the compounds identified in F. chica leaf extracts, as well as in several other plants, are responsible for the above-mentioned pharmacological activities (Table 1) when evaluated in isolation (Table 1).
5.1. Healing Activity
The tissue repair process is divided into three successive and overlapping phases that present specific characteristics: (1) the inflammatory phase, (2) the proliferative phase, and (3) the remodeling phase. In the inflammatory phase, there is an increase in vascular permeability, which promotes chemotaxis and the consequent intense migration of leukocytes, mainly neutrophils and macrophages, to the site of tissue damage in response to the release of pro-inflammatory chemical mediators. The proliferative phase is characterized by the reconstitution of epithelial tissue, angiogenesis and migration of endothelial cells, granulation tissue formation, and extracellular matrix deposition, mainly collagen produced by activated fibroblasts. In the remodeling phase, the initial deposited collagen (type III) is degraded by collagenases and reabsorbed, and the gradual replacement of type III collagen by type I collagen occurs until the proportion found in healthy tissue is reached, providing reorganization of the extracellular matrix and marking the success of tissue repair [110,111].
Several herbal medicines have been explored in order to evaluate their action on tissue healing, mainly due to their lower cost and fewer observed adverse effects [112]. Tissue regeneration studies with F. chica leaves have addressed the action of different extracts and formulations in both in vitro and in vivo experiments using experimental animals in order to investigate different phases of the healing process. Among the main experimental procedures in the bioprospecting of new components with healing potential, cell proliferation assays using fibroblasts and the production of extracellular matrix components such as collagen are considered highly reliable models. Fibroblasts are connective tissue cells that are essential for the formation of the dermis and are responsible for structural firmness. After injury, fibroblasts proliferate and migrate to the injured site, where they produce a large amount of extracellular matrix material, such as type I and III collagen, which helps to isolate and repair the injured tissue. The use of a methanolic extract of F. chica leaves stimulates the growth of fibroblasts in vitro in a concentration-dependent manner and increases collagen production [14].
From this tissue regeneration property, several in vivo studies with experimental animals have been developed to evaluate the healing potential of F. chica in different tissue types. In treatment of gastric ulcers induced by ethanol and indomethacin in Wistar/Uni rats, administration of the hydroalcoholic extract of F. chica leaves reduced about 60% of lesions [113]. The methanolic extract administered orally was able to reduce up to 96% of the ulcerative effect of ethanol in a murine model [14]. The ethanol-induced model is associated with gastric mucosal injury induced by oxidative stress (via alcohol); thus, the gastroprotective effect of F. chica may occur via antioxidant action. In skin lesions of Swiss mice, treatment with ethanolic extract of F. chica leaves has not yet shown promising results. During 21 days of observation, it was found that the extract did not accelerate total tissue regeneration and presented a profile that was similar to the control group [90]. Other studies have investigated the effect of F. chica leaves on the healing process of tendon injuries in rats. Observed results included an increase in the total collagen content on the seventh and twenty-first day of the healing process, an increase in the synthesis of collagenase type IV (matrix metalloproteinase-2, an enzyme that participates in the extracellular matrix remodeling process), and better organization of collagen fibers. The treated groups presented a lower inflammatory reaction, which promoted the recovery of the injured animals’ ability to walk [114,115].
In addition to the studies developed in different tissues, investigations of the healing properties of Fridericia chica have used different types of formulations for administration of the plant extract and for carrying out these studies. The choice of formulation is extremely important, as it influences the stability, distribution, solubility, bioavailability, and protection of the active compound. Topical formulations such as cream and gel containing F. chica leaf extract are being developed for the treatment of skin lesions [90,116]. For the treatment of gastric ulcers, a nanoformulation of F. chica extract has been elaborated in chitosan triphosphate-based nanoparticles for oral administration. Nanoformulation was responsible for a greater fibroblast proliferation effect, and the anti-ulcerogenic effect of F. chica leaf extract in a nanoformulation was more efficient compared to hydroalcoholic extract [113].
From the 43 compounds identified from F. chica leaves, seven molecules (comprising the classes of flavones, flavonols, terpenes, tochromanols, and alkaloids) have been described to present healing properties when evaluated alone. Among them, kaempferol (compound 24—Table 1) is a flavonol which has been described as improving repair of diabetic excisional and nondiabetic incisional wounds in rats, working as an effective topical wound healing agent [52]. Another component present in F. chica leaf extract in considering amount is α-tocopherol (compound 41, Table 1), belonging to the tocochromanol class. Shuid and colleagues [78] evaluated the effects of α-tocopherol supplementation on osteoporotic fracture healing in a rat model, showing an improvement in fracture healing associated with the antioxidatant property. Other compounds have been shown to induce healing activity, such as apigenin (compound 17, Table 1), with anti-hyaluronidase and anti-collagenase activities [41], scutellarin (compound 21, Table 1) with angiogenic properties [49], and pheophorbide a (compound 43, Table 1), which decreased the length of the inflammation stage [82].
5.2. Anti-Inflammatory Activity
Inflammation is defined as a protective response of the host to various interactions with the external environment. The assembly of this response requires the participation of cells and molecules, including macrophages and neutrophils, along with cytokines, such as interleukin (IL)-1β and IL-6 [117,118,119,120,121]. These cells recognize and discriminate stimuli as damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) through pattern recognition receptors (PRR) [122]. The inflammatory response, despite being an effective defense mechanism, can occur in an exacerbated or perpetuated manner for a longer period of time than is necessary, causing important tissue damage and contributing to the development of diseases such as rheumatoid arthritis [123,124].
The participation of inflammation in the pathophysiology of multiple diseases stimulates the constant development of anti-inflammatory drugs, and nature represents an important source of plants with therapeutic potential that needs to be explored [125,126]. Plants such as Aloe vera have demonstrated efficacy in controlling the inflammatory response and are considered by the Brazilian public health system as a treatment option [127]. Flavonoids, a class of secondary metabolites belonging to the group of phenolic compounds, are anti-inflammatory agents and can act to block arachidonic acid cascade through inhibition of the COX and lipoxygenase pathways [128,129]. The species Fridericia chica is used in traditional medicine as an anti-inflammatory agent, which has been confirmed in several studies using different extracts prepared from the leaves of this plant [15,17,130].
The chemical characterization of extracts of F. chica leaves has allowed the identification of 3-deoxyanthocyanidins, to which are attributed the greater part of the anti-inflammatory effect of this plant [4,17,18]. In 2001, Zorn et al. [17] demonstrated that both the lipophilic extract of Fridericia chica leaves (200 mg/mL) and one of its isolated components (carajurine) at a concentration of 500 µM had the ability to completely inhibit nuclear factor kappa B (NF-κB), which is responsible for the transcription of genes that encode several pro-inflammatory mediators and which participates in the pathogenesis of diseases such as multiple sclerosis [131,132,133]. Several authors have suggested that other unidentified substances present in the extract of F. chica leaves may contribute to the anti-inflammatory activity of this species [17].
Several models of inflammation have been used for the study of anti-inflammatory drugs, one of which is the experimental model of inflammation induced by snake venom. Its pathogenesis may involve the participation of the enzyme phospholipase A2, which stimulates the release of arachidonic acid [134,135]. In 2009, Oliveira et al. [15] evaluated the anti-inflammatory effect of an aqueous extract of the leaves of Fridericia chica in mice inoculated with the venom of the snakes Bothrops atrox and Crotalus durissus ruruima, administered via the subcutaneous and intraperitoneal routes at different times. After treatment with the extract, it was possible to observe that it showed inhibitory activity, especially in the processes of myocytolysis and granulocyte migration, thus resulting in a decrease in inflammation. This effect is attributed to substances present in the extract, flavonoids among them.
The effectiveness of Fridericia chica leaves as an anti-inflammatory agent has been demonstrated in other models, such as inflammatory angiogenesis, which was induced in a murine model by a sponge implant (polyether-polyurethane) and in the proliferative process of human tumor cell lines in vitro, in which Michel et al. (2015) [130] observed a reduction in the amount of neutrophils at the site of the sponge implants and a great decrease in angiogenesis after treatment with aqueous and ethanolic extracts. Flavone escutelarin derivatives, found in the hydroethanolic extract of Fridericia chica leaves, have been associated with a reduction of parameters linked to inflammatory response in the model of intestinal mucositis induced by 5-fluorouracil in a study by [136].
Among phytochemical compounds present in F. chica leaf extracts, 25 have been described as presenting anti-inflammatory behavior (Table 1). The anthocyanins comprise the class of most abundant compounds in F. chica leaves, with carajurin composing one of the major molecules found in extract of the leaves [17]. Carajurin (compound 1, Table 1) is capable of inhibiting transcription factor NF-κB, an important signaling pathway associated with pro-inflammatory response [17]. F. chica leaf extracts consist of a high range of flavonoid compounds, such as flavones and flavonols (Table 1). Regarding this class, the literature on their biological potentials is extensive, with a total of 14 flavonoids from F. chica leaf extracts having been shown to suppress inflammation through different cell signaling pathways. An example is luteolin (compound 8, Table 1), a flavone which has been shown to interact with several inflammatory targets such as matrix metalloprotease 9 (MMP9) and mitogen-activated protein kinase 1 (MAPK1) [25]. Chrysoeriol (compound 9, Table 1), another flavone, ameliorates TPA-induced ear edema in mice, and its inhibition of JAK2/STAT3 and IκB/p65 NF-κB pathways is involved in the anti-inflammatory effects [27]. Hispidulin, a flavone found in a number of plants, among them in F. chica, was evaluated by Yu et al. (2020) [36] in relation to protection against neuroinflammation using the immortalized cell line of murine microglial BV2 (BV2 cells). Hispidulin has been shown to inhibit NO and ROS production, which suppressed the expression of the inflammation-related enzymes iNOS and COX-2 [137] in a dose-dependent manner. Corroborating these data, Torres et al. (2018) [138] showed a decrease in the enzymatic activity of lipoxygenase in the presence of ethanolic extract of F. chica leaves. They observed that treatment with hispidulin in the presence of LPS inhibited the production of TNF- α, IL-6, IL-1 β, and PGE2 in a dose-dependent manner. In addition, hispidulin showed activity in reducing NF-κB, and decreased the levels of phosphorylation of IκB kinase in BV2 cells. Based on the above examples, there is other evidence of the effect of the species F. chica leaves in the modulation of the cytokine profile, among them that demonstrated by Lima (2020) [139], which suggests an increase in the production of IL-10 and a decrease in the production of IL-1β in RAW 264.7 cells activated with LPS in the presence of a hydroethanolic extract of F. chica. The authors attributed this effect to 5 O-methylescutelarin, a derivative of the flavone escutelarin. The aforementioned studies corroborate the therapeutic potential described in traditional medicine for F. chica.
Despite being encountered in minor proportions in F. chica, terpenes may play roles in its biological activities, including in anti-inflammatory response. β-carotene (compound 35, Table 1) was capable of inhibiting LPS-stimulated Cox2, Nos2, and Tnfalpha gene expression from macrophages in vitro [69]. Another terpene, phytol (compound 32, Table 1), has been described as inducing anti-inflammatory activity in vitro and in vivo, with higher potency in the presence of others non-steroidal anti-inflammatories [64]. Among fatty acids, ethyl palmitate (compound 42, Table 1) reduced plasma levels of tumor necrosis factor-α (TNF-α) and IL-6, decreased NF-κB expression in liver and lung tissues, and ameliorated histopathological changes in an LPS-induced endotoxemia rat model [79]. The alkaloid pheophorbide a (compound 43, Table 1), was found to inhibit nitric oxide production and suppress the expression of iNOS proteins in LPS-stimulated macrophages [80].
5.3. Antioxidant Activity
The term oxidative stress, first proposed in 1985, refers to the imbalance between the production of oxidizing and antioxidant molecules; it favors oxidation reactions and compromises cell signaling and redox control [140,141,142]. The aforementioned changes result in excessive production of free radicals, which can contribute to the generation of reactive oxygen species, thus resulting in structural damage to biological systems [143,144,145]. Reactive species are defined as atoms, molecules, or ions resulting from oxygen, usually with a high reactivity capacity, which can cause damage to cellular constituents, such as lipid peroxidation and oxidation of DNA bases [146,147,148,149]. Free radicals and other reactive species are essential for cellular homeostasis, but when generated excessively they contribute to cellular aging and the development of diseases, as is observed in the development of hemolytic anemia due to deficiency of the enzyme glucose-6-phosphate dehydrogenase. In addition, oxidative stress is part of the pathophysiology of several chronic degenerative diseases, including cancer, diabetes, and hypertension [150,151,152,153,154,155,156].
Antioxidants are compounds that are capable of slowing, preventing, or removing damage caused by redox imbalance, and thereby regulating the development of oxidative stress [157]. The large number of adverse effects observed with the use of synthetic antioxidants have driven the search for other alternatives, among them substances isolated from natural products [158,159,160]. The main antioxidants present in plants are phenolic compounds, which represent a family of secondary metabolites with the property of interrupting chain reactions caused by free radicals by donating hydrogen atoms or electrons [161,162,163]. To this large group of compounds belong several of the flavonoids, which are present in medicinal plants such as the species F. chica [20,21,91,164,165,166].
The antioxidant potential of F. chica has already been evaluated in several studies. In 2012, Do Amaral et al. [109] identified the presence of phenolic compounds in an ethanolic extract of the leaves of this plant. In addition, Gemelli et al. (2015) [166] and Campos de Siqueira et al. (2019) [22] examined the antioxidant potential in the aqueous and hydroethanolic leaf extract, respectively. The anti-inflammatory property of 3-deoxyanthocyanidins, which are phenolic pigments with the ability to stabilize free radicals by donating hydrogen radicals, was proposed by Zorn et al. in 2001 [17]. However, the antioxidant capacity of these flavonoids was identified much later, by Do Amaral et al. in 2012 [109], and later confirmed by Dos Santos et al. in 2013 [166]. Another important finding was that reported by Olivero-Verbel et al. in 2021 [167], which suggests the ability of aqueous extract of F. chica leaves to induce nuclear translocation of DAF-16 in a prominent manner, thus suggesting the potential of this extract in the regulation of oxidative stress [17,22,109,166,167,168].
As already mentioned, flavonoids are phenolic compounds which have a great antioxidant potential. Among them are flavones, the antioxidant capacity of which is related to the presence of free hydroxyl groups in their A and B rings [161,162]. Luteolin (compound 8—Table 1), one of the most abundant flavones, was identified by Do Amaral et al. (2012) [116], who evaluated the antioxidant activity of the ethanolic extract of F. chica leaves in the presence of the free radical DPPH (2,2-diphenyl-1-picrylhydrazil) using Gingkgo bibola as a positive control and demonstrated that the antioxidant capacity of F. chica extract is superior. This antioxidant effect was attributed mainly to the flavones luteolin and apigenin. In 2015, Gemelli et al. [168] confirmed the presence of luteolin in the aqueous extract of F. chica leaves and demonstrated its antioxidant potential by DNA damage assay in CHO cells (hamster ovary cells) [109,168].
The leaves of Fridericia chica have a high flavones content along with the presence of apigenin (compound 17, Table 1), with high antioxidant activity [40], as evidenced by Siraichi et al. in 2013 [21], confirming what had already been proposed by Do Amaral et al. in 2012 [109]. Other important flavone identified in the extract of F. chica leaves are scutellarin (compound 21, Table 1) and scutellarein (compound 14, Table 1), which are present in other medicinal plants such as Scutellaria barbata and S. lateriflora. Scutellarin is a glucoronid form of scutellarein, which means that although both molecules present antioxidant activity, the aglycone form (scutellarein) has a stronger activity [34]. The research of Campos de Siqueira et al. (2019) [22], who identified carotenoids such as β-carotene and α-carotene in a hydrometanolic extract of F. chica, was not the first time that escutelarin was identified in the species F. chica, as in 2013 Siraichi et al. [21] attributed the antioxidant effect of the species to the presence of this flavone [21,22,109].
Terpenes are well known antioxidant agents, and have been shown to provide relevant protection under oxidative stress conditions. The terpene zeaxanthin (compound 36, Table 1) protects against chronic eye and cardiovascular diseases due its antioxidant property by directly quenching reactive oxygen species (ROS) and by facilitating glutathione synthesis [72]. Violaxanthin (compound 38, Table 1) possesses potent lipid peroxidation inhibitory activity [76]. Other compounds from different classes, such as α-Tocopherol and Pheophorbide a (compounds 41 and 43, respectively, in Table 1) have shown antioxidant activity as well, and are present in others plant species [77,81].
Martins et al., in 2016 [169] and Ribeiro et al., working in 2018 [170], demonstrated the antioxidant capacity of a hydroethanolic extract of Fridericia chica in the presence of both the free radical DPPH and the oxidative damage induced by ultraviolet radiation. The use of several methodologies to evaluate the effects of F. chica extracts for the control of oxidative stress allowed for a more complete view of this mechanism. Other authors, such as Torres et al. in 2018 [138] and Teixeira et al. in 2017 [171], have included other free radical neutralization assays, such as ABTS (2,2’-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) and FRAP (ferric reducing antioxidant power), thus reaffirming the biological effects of F. chica [138,169,170,171].
In 2009, the Brazilian Ministry of Health published a list of 71 species that are part of the Report on Medicinal Plants of Interest to the SUS (RENISUS); F. chica is among them due to the large amount of scientific evidence pointing to its different biological effects [172,173]. One of the most important biological effects of this species is the antioxidant effect, which can contribute to the control and prevention of degenerative diseases, as already mentioned. Encouraging the development of research related to F. chica is be essential for its inclusion in the list of herbal medicines prescribed within the Brazilian health services, through which twelve herbal medicines are already available that are part of the National List of Essential Medicines (RENAME) [127].
6. Conclusions
The World Health Organization (WHO) has encouraged the inclusion of medicinal plants in primary health care networks, and in Brazil their use is regulated by the National Health Surveillance Agency (ANVISA). The public health system of Brazil has offered herbal medicines since 2007, and 12 herbal medicines have been made available in the public network by the Ministry of Health (MS). The species Fridericia chica (Bonpl.) L. G. Lohmann is traditionally used in folk medicine, especially in the Amazon region, and is part of the 71 species of interest to the SUS (Brazilian public health system). Among these species, Aloe vera, Schinus terebenthifolius, and Uncaria tomentosa are used as commercial herbal pharmaceutical formulations in the Brazilian Health System. Schinus terebenthifolius has healing and anti-inflammatory action and is used as an antiseptic topical for gynecological applications, available in the forms of cream and ovules. Uncaria tomentosa is indicated as an assistant in cases of arthritis and osteoarthritis thanks its anti-inflammatory and immunomodulatory activity, and is available in capsule, pill, and gel forms. Aloe vera is indicated for the topical treatment of first and second degree burns and as an adjunct in cases of psoriasis vulgaris, and is available in cream form [85]. Therefore, the present review indicates the potential inclusion of F. chica on the list of herbal medicines prescribed within the scope of Brazilian Health System services, as the extract can be used in different pharmacological formulations such as topical or oral administrations in order to treat diseases associated with inflammatory response and wounded tissue.
Scientific evidence indicates the different biological effects of F. chica, which proves the therapeutic effect of this species in folk medicine, which is explained especially by the presence of a wide range of biologically active compounds that are known from other plant species. The present review has shown that from all compounds identified in F. chica leaf extract, over 70% are known to exert anti-inflammatory, antioxidant, and/or healing properties based on previous studies. Therefore, the pharmacological properties of F. chica leaf extract are directly associated with the phytochemical composition, with different molecules working in association in order to observe the conclusive therapeutical potential. Although carajurin is found as the major component of the extract with anti-inflammatory activity, its synergism with other less abundant components is important to the overall pharmacological properties of the extract. Therefore, the proportion of every molecule within the extract and its biological potency should be more closely investigated. It is of great importance to encourage more studies related to the chemical profile, standardization of extracts, and proof of pharmacological activities of F. chica. Concerning the traditional use of F. chica leaf extract as tea, more studies should be performed in order to evaluate the phytochemical composition of aqueous infusions, considering that almost all studies on leaves have used extract compositions with other types of solvents. New research needs to be developed in order to add scientific evidence that favors the use of this and other medicinal species, both in Brazil and in other regions of the world.
The scientific investigations cited in this study contribute to the construction of arguments, providing a solid basis to attribute anti-inflammatory, antioxidant, and healing properties to Fridericia chica, thus demonstrating the scientific bases that justify its use in traditional medicine.
Acknowledgments
The authors would also like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the payment of scholarships to MAS (No. 163843/2020-1), WM (No. 309207/2020-7) and HK (No. 305942/2020-4).
Author Contributions
M.A.S., A.D.d.S.J.B. and D.C.d.M.S. conceived the main idea and designed the topics. A.D.d.S.J.B., D.C.d.M.S. and R.D.U. conducted the bibliography search. A.D.d.S.J.B., D.C.d.M.S., R.D.U., F.C.M.C., W.M.M., F.M.A.d.S., H.H.F.K., A.L.B. and M.A.S. wrote the review. A.D.d.S.J.B., D.C.d.M.S., H.H.F.K. and M.A.S. designed the figures and tables of this review. F.C.M.C., W.M.M., F.M.A.d.S., H.H.F.K., A.L.B. and M.A.S. revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The following agencies founded the present work: Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) for the funding, CT&I Priority Areas Call FAPEAM 010/2021 for MAS and HHFK, and HHFK’s research under the Universal Call FAPEAM-006/2019. HHFK and WMM acknowledge FAPEAM for funding via the calls PAPAC 005/2019, PRO-ESTADO-002/2008; 007/2018; 005/2019 and POSGRAD 2020-2021.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Takemura O.S., Iinuma M., Tosa H., Miguel O.G., Moreira E.A., Nozawa Y. A flavone from leaves of Arrabidaea chica f. cuprea. Phytochemistry. 1995;38:1299–1300. doi: 10.1016/0031-9422(94)00786-S. [DOI] [Google Scholar]
- 2.Pauletti P.M., Bolzani V.S., Young M.C.M. Chemical constituents of Arrabidaea samydoides (Bignonia-ceae) Química Nova. 2003;26:641–643. doi: 10.1590/S0100-40422003000500003. [DOI] [Google Scholar]
- 3.Lorenzi H., Matos F.J. Plantas Medicinais No Brasil: Nativas e Exóticas. Instituto Plantarum de Estudos da Flora; São Paulo, Brazil: 2002. p. 512. [Google Scholar]
- 4.Chapman E., Perkin A.G., Robinson R. The colouring matters of carajura. J. Chem. Soc. 1927;49:3015. doi: 10.1039/JR9270003015. [DOI] [Google Scholar]
- 5.Cronquist A. An Integrated System of Classification of Flowering Plants. Columbia University Press; New York, NY, USA: 1981. pp. 248–250. [Google Scholar]
- 6.Lohmann L.G. Bignoniaceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro; Rio de Janeiro, Brazil: 2015. [Google Scholar]
- 7.Behrens M.D., Tellis C.J.M. Arrabidaea chica (Humb. & Bonpl.) B. Verlot (Bignoniaceae) Rev. Fitos. 2012;7:236–244. [Google Scholar]
- 8.Joly A.B. Botânica: Introdução à Taxonomia Vegetal. Editora Nacional; São Paulo, Brazil: 1993. p. 776. [Google Scholar]
- 9.Puhl M.C.M.N., Milaneze-Gutierre M.A., Nakamura C.V., Cortez D.A.G. Morpho-anatomy of leaves and young stems of Arrabidaea chica (Humb. & Bonpl.) B. Verl. (Bignoniaceae) Lat. Am. J. Pharm. 2007;26:224–229. [Google Scholar]
- 10.Corrêa M.P., Pena L.A. Dicionário das Plantas Úteis do Brasil e das Exóticas Cultivadas. Ministério da Agricultura, Instituto Brasileiro de Desenvolvimento Florestal; Rio de Janeiro, Brazil: 1984. pp. 1934–1974. [Google Scholar]
- 11.Ferreira F.A.G., Carvalho C.M., Costa J.C., Ferreira J.M.R., Silva F. Comprovação do potencial medici-nal de Arrabidaea chica (Bignoniaceae) Sci. Prima. 2013;1:1–6. [Google Scholar]
- 12.Borrás M.R.L. Plantas da Amazônia: Medicinais ou Mágicas—Plantas Comercializadas no Mercado Municipal Adolpho Lisboa. Editora Valer, Governo do Estado do Amazonas; Manaus, Brazil: 2003. p. 322. [Google Scholar]
- 13.Moreira L.S., Silva S.A. Ação Inibitória do Crajiru Arrabdaea chica (Humb&Bonpl.) B.Verlt Sobre Staphylococcus sp. como Microoganismo Oportunista no Tratamento da Acne Vulgar. Revista Olhar Científico. 2016;2:210. [Google Scholar]
- 14.Jorge M.P., Madjarof C., Gois Ruiz A.L., Fernandes A.T., Ferreira Rodrigues R.A., de Oliveira Sousa I.M., Foglio M.A., de Carvalho J.E. Evaluation of wound healing properties of Arrabidaea chica Verlot extract. J. Ethnopharmacol. 2008;118:361–366. doi: 10.1016/j.jep.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira D.P.C., Borrás M.R.L., Ferreira L.C.L., López-Lozano J.L. Anti-inflammatory activity of the aqueous extract of Arrabidaea chica (Humb. & Bonpl.) B. Verl. on the self-induced inflammatory process from venoms amazonians snakes. Rev. Bras. de Farmacogn. 2009;19:643–649. [Google Scholar]
- 16.Buer C.S., Imin N., Djordjevic M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010;52:98–111. doi: 10.1111/j.1744-7909.2010.00905.x. [DOI] [PubMed] [Google Scholar]
- 17.Zorn B., García-Piñeres A.J., Castro V., Murillo R., Mora G., Merfort I. 3-Desoxyanthocyanidins from Arrabidaea chica. Phytochemistry. 2001;56:831–835. doi: 10.1016/S0031-9422(01)00038-3. [DOI] [PubMed] [Google Scholar]
- 18.Devia B., Llabres G., Wouters J., Dupont L., Escribano-Bailon M.T., de Pascual-Teresa S., Angenot L., Tits M. New 3-deoxyanthocyanidins from leaves of Arrabidaea chica. Phytochem. Anal. PCA. 2002;13:114–120. doi: 10.1002/pca.632. [DOI] [PubMed] [Google Scholar]
- 19.Vasconcelos C.C., Lopes A., Sousa E., Camelo D.S., Lima F., Rocha C., Silva G., Garcia J., Cartágenes M. Effects of Extract of Arrabidaea chica Verlot on an Experimental Model of Osteoarthritis. Int. J. Mol. Sci. 2019;20:4717. doi: 10.3390/ijms20194717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbosa W.L.R., Pinto L.N., Quignard E., Vieira J.M.S., Silva-Júnior O.C., Albuquerque S. Arrabidaea chica (HBK) Verlot: Phytochemical approach, antifungal and trypanocidal activities. Braz. J. Pharmacogn. 2008;18:544–548. doi: 10.1590/S0102-695X2008000400008. [DOI] [Google Scholar]
- 21.Siraichi J.T., Felipe D.F., Brambilla L.Z., Gatto M.J., Terra V.A., Cecchini A.L., Cortez L.E., Ro-drigues-Filho E., Cortez D.A. Antioxidant capacity of the leaf extract obtained from Arrabidaea chica cultivat-ed in Southern Brazil. PLoS ONE. 2013;8:e72733. doi: 10.1371/journal.pone.0072733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Siqueira F.C., Leitão D.D.S.T.C., Mercadante A.Z., Chiste R.C., Lopes A.S. Profile of phenolic compounds and carotenoids of Arrabidaea chica leaves and the in vitro singlet oxygen quenching capacity of their hydrophilic extract. Food Res. Int. 2019;126:108597. doi: 10.1016/j.foodres.2019.108597. [DOI] [PubMed] [Google Scholar]
- 23.Vasconcelos C.C., Lopes A., de Jesus Garcia Ataide E., Carvalho K., de Brito M., Rodrigues M.S., de Mo-rais S.V., Silva G., da Rocha C.Q., Garcia J., et al. Arrabidaea chica Verlot fractions re-duce MIA-induced osteoarthritis progression in rat knees. Inflammopharmacology. 2021;29:735–752. doi: 10.1007/s10787-021-00803-0. [DOI] [PubMed] [Google Scholar]
- 24.Miranda N., Gerola A.P., Novello C.R., Ueda-Nakamura T., de Oliveira Silva S., Dias-Filho B.P., Hioka N., de Mello J., Nakamura C.V. Pheophorbide a, a compound isolated from the leaves of Arrabidaea chica, induces photodynamic inactivation of Trypanosoma cruzi. Photodiagnosis Photodyn. Ther. 2017;19:256–265. doi: 10.1016/j.pdpdt.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Huang X.F., Zhang J.L., Huang D.P., Huang A.S., Huang H.T., Liu Q., Liu X.H., Liao H.L. A net-work pharmacology strategy to investigate the anti-inflammatory mechanism of luteolin combined with in vitro transcriptomics and proteomics. Int. Immunopharmacol. 2020;86:106727. doi: 10.1016/j.intimp.2020.106727. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y.C., Gan F.F., Shelar S.B., Ng K.Y., Chew E.H. Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;59:272–280. doi: 10.1016/j.fct.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 27.Wu J.Y., Chen Y.J., Bai L., Liu Y.X., Fu X.Q., Zhu P.L., Li J.K., Chou J.Y., Yin C.L., Wang Y.P., et al. Chrysoeriol ameliorates TPA-induced acute skin inflammation in mice and inhibits NF-κB and STAT3 pathways. Phytomedicine Int. J. Phytother. Phytopharm. 2020;68:153173. doi: 10.1016/j.phymed.2020.153173. [DOI] [PubMed] [Google Scholar]
- 28.Nickavar B., Rezaee J., Nickavar A. Effect-Directed Analysis for the Antioxidant Compound in Salvia verticillata. Iran. J. Pharm. Res. IJPR. 2016;15:241–246. [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W.C., Liou C.J. Dietary acacetin reduces airway hyperresponsiveness and eosinophil infiltration by modulating eotaxin-1 and th2 cytokines in a mouse model of asthma. Evid. Based Compl. Alter. Med. eCAM. 2012;2012:910520. doi: 10.1155/2012/910520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.More G., Lall N., Hussein A., Tshikalange T.E. Antimicrobial Constituents of Artemisia afra Jacq. ex Willd. against Periodontal Pathogens. Evid. Based Compl. Alter. Med. eCAM. 2012;2012:252758. doi: 10.1155/2012/252758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarian M.N., Ahmed Q.U., Mat So’ad S.Z., Alhassan A.M., Murugesu S., Perumal V., Syed Mohamad S., Khatib A., Latip J. Antioxidant and Antidiabetic Effects of Flavonoids: A Structure-Activity Relationship Based Study. BioMed Res. Int. 2017;2017:8386065. doi: 10.1155/2017/8386065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandith H., Zhang X., Thongpraditchote S., Wongkrajang Y., Gritsanapan W., Baek S.J. Effect of Siam weed extract and its bioactive component scutellarein tetramethyl ether on anti-inflammatory activity through NF-κB pathway. J. Ethnopharmacol. 2013;147:434–441. doi: 10.1016/j.jep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y., Ren N., Li S., Chen M., Pu P. Novel anti-obesity effect of scutellarein and potential underlying mechanism of actions. Biomed. Pharmacother. 2019;117:109042. doi: 10.1016/j.biopha.2019.109042. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q., Li X., Ouyang X., Chen D. Dual Effect of Glucuronidation of a Pyrogallol-Type Phytophenol Antiox-idant: A Comparison between Scutellarein and Scutellarin. Molecules. 2018;23:3225. doi: 10.3390/molecules23123225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ana Silvia G.R., Gabriela T.T., Maribel H.R., Nayeli M.B., José Luis T.E., Alejandro Z., Manasés G.C. Effect of Terpenoids and Flavonoids Isolated from Baccharis conferta Kunth on TPA-Induced Ear Edema in Mice. Molecules. 2020;25:1379. doi: 10.3390/molecules25061379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu C.I., Cheng C.I., Kang Y.F., Chang P.C., Lin I.P., Kuo Y.H., Jhou A.J., Lin M.Y., Chen C.Y., Lee C.H. Hispidulin Inhibits Neuroinflammation in Lipopolysaccharide-Activated BV2 Microglia and Attenu-ates the Activation of Akt, NF-κB, and STAT3 Pathway. Neurotox. Res. 2020;38:163–174. doi: 10.1007/s12640-020-00197-x. [DOI] [PubMed] [Google Scholar]
- 37.Dabaghi-Barbosa P., Mariante Rocha A., Franco da Cruz Lima A., Heleno de Oliveira B., Benigna Martinelli de Oliveira M., Gunilla Skare Carnieri E., Cadena S.M., Eliane Merlin Rocha M. Hispidulin: Antioxidant properties and effect on mitochondrial energy metabolism. Free. Radic. Res. 2005;39:1305–1315. doi: 10.1080/13561820500177659. [DOI] [PubMed] [Google Scholar]
- 38.Lee J.H., Zhou H.Y., Cho S.Y., Kim Y.S., Lee Y.S., Jeong C.S. Anti-inflammatory mechanisms of apig-enin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharmacal Res. 2007;30:1318–1327. doi: 10.1007/BF02980273. [DOI] [PubMed] [Google Scholar]
- 39.Funakoshi-Tago M., Nakamura K., Tago K., Mashino T., Kasahara T. Anti-inflammatory activity of structur-ally related flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharmacol. 2011;11:1150–1159. doi: 10.1016/j.intimp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Fidelis Q.C., Faraone I., Russo D., Aragão Catunda-Jr F.E., Vignola L., de Carvalho M.G., de Tommasi N., Milella L. Chemical and Biological insights of Ouratea hexasperma (A. St.-Hil.) Baill.: A source of bioactive compounds with multifunctional properties. Nat. Prod. Res. 2019;33:1500–1503. doi: 10.1080/14786419.2017.1419227. [DOI] [PubMed] [Google Scholar]
- 41.Süntar I., Küpeli Akkol E., Keles H., Yesilada E., Sarker S.D. Exploration of the wound healing potential of Helichrysum graveolens (Bieb.) Sweet: Isolation of apigenin as an active component. J. Ethnopharmacol. 2013;149:103–110. doi: 10.1016/j.jep.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Yao H., Yuan Z., Wei G., Chen C., Duan J., Li Y., Wang Y., Zhang C., Liu Y. Thevetiaflavone from Wikstroemia indica ameliorates PC12 cells injury induced by OGD/R via improving ROS mediated mitochondrial dysfunction. Mol. Med. Rep. 2017;16:9197–9202. doi: 10.3892/mmr.2017.7712. [DOI] [PubMed] [Google Scholar]
- 43.Han H.S., Shin J.S., Lee S.B., Park J.C., Lee K.T. Cirsimarin, a flavone glucoside from the aerial part of Cirsium japonicum var. ussuriense (Regel) Kitam. ex Ohwi, suppresses the JAK/STAT and IRF-3 signaling pathway in LPS-stimulated RAW 264.7 macrophages. Chem. Interact. 2018;293:38–47. doi: 10.1016/j.cbi.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Zhang E., Wang Y., Xie F., Zhuang X., Wang X., Yu X. Development and Validation of a UPLC-MS/MS Method for the Quantitative Determination and Pharmacokinetic Analysis of Cirsimarin in Rat Plasma. BioMed Res. Int. 2021;2021:9953664. doi: 10.1155/2021/9953664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serpeloni J.M., Oliveira L., Fujiike A., Tuttis K., Ribeiro D.L., Camara M., Rocha C., Cólus I. Flavone cirsimarin impairs cell proliferation, migration, and invasion in MCF-7 cells grown in 2D and 3D models. Toxicology. 2022;83:105416. doi: 10.1016/j.tiv.2022.105416. [DOI] [PubMed] [Google Scholar]
- 46.Hu W., Wang X., Wu L., Shen T., Ji L., Zhao X., Si C.L., Jiang Y., Wang G. Apigenin-7-O-β-D-glucuronide inhibits LPS-induced inflammation through the inactivation of AP-1 and MAPK signaling path-ways in RAW 264.7 macrophages and protects mice against endotoxin shock. Food Funct. 2016;7:1002–1013. doi: 10.1039/C5FO01212K. [DOI] [PubMed] [Google Scholar]
- 47.Li S., Xu T., Liu S., Liu Z., Pi Z., Song F., Jin Y. Exploring the potential pharmacodynamic material basis and pharmacologic mechanism of the Fufang-Xialian-Capsule in chronic atrophic gastritis by network pharma-cology approach based on the components absorbed into the blood. R. Soc. Open Sci. 2018;5:171806. doi: 10.1098/rsos.171806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo Z., Hu Z., Bian Y., Su W., Li X., Li S., Wu J., Shi L., Song Y., Zheng G., et al. Scutellarin Attenuates the IL-1β-Induced Inflammation in Mouse Chondrocytes and Prevents Osteoarthritic Progression. Front. Pharmacol. 2020;11:107. doi: 10.3389/fphar.2020.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Z.X., Huang D.Y., Li H.X., Zhang L.N., Lv Y.H., Cui H.D., Zheng J.H. Scutellarin promotes in vitro angiogenesis in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2010;400:151–156. doi: 10.1016/j.bbrc.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 50.Kadioglu O., Nass J., Saeed M.E., Schuler B., Efferth T. Kaempferol Is an Anti-Inflammatory Compound with Activity towards NF-κB Pathway Proteins. Anticancer. Res. 2015;35:2645–2650. [PubMed] [Google Scholar]
- 51.Park M.Y., Ji G.E., Sung M.K. Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Dig. Dis. Sci. 2012;57:355–363. doi: 10.1007/s10620-011-1883-8. [DOI] [PubMed] [Google Scholar]
- 52.Özay Y., Güzel S., Yumrutaş Ö., Pehlivanoğlu B., Erdoğdu İ.H., Yildirim Z., Türk B.A., Darcan S. Wound Healing Effect of Kaempferol in Diabetic and Nondiabetic Rats. J. Surg. Res. 2019;233:284–296. doi: 10.1016/j.jss.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Chirumbolo S. Anti-inflammatory action of isorhamnetin. Inflammation. 2014;37:1200–1201. doi: 10.1007/s10753-014-9846-9. [DOI] [PubMed] [Google Scholar]
- 54.Bakır T., Sönmezoğlu I., Imer F., Apak R. Antioxidant/prooxidant effects of α-tocopherol, quercetin and iso-rhamnetin on linoleic acid peroxidation induced by Cu(II) and H2O2. Int. J. Food Sci. Nutr. 2014;65:226–234. doi: 10.3109/09637486.2013.845654. [DOI] [PubMed] [Google Scholar]
- 55.Marzouk M.S., Moharram F.A., Haggag E.G., Ibrahim M.T., Badary O.A. Antioxidant flavonol glycosides from Schinus molle. Phytother. Res. 2006;20:200–205. doi: 10.1002/ptr.1834. [DOI] [PubMed] [Google Scholar]
- 56.Lesjak M., Beara I., Simin N., Pintać D., Majkić T., Bekvalac K., Orčić D., Mimica-Dukić N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods. 2018;40:68–75. doi: 10.1016/j.jff.2017.10.047. [DOI] [Google Scholar]
- 57.Kim J.S., Kwon Y.S., Sa Y.J., Kim M.J. Isolation and identification of sea buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and α-glucosidase inhibitory effect. J. Agric. Food Chem. 2011;59:138–144. doi: 10.1021/jf103130a. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z., Sun T., Niu J.G., He Z.Q., Liu Y., Wang F. Amentoflavone protects hippocampal neurons: Anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen. Res. 2015;10:1125–1133. doi: 10.4103/1673-5374.160109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bajpai V.K., Park I., Lee J., Shukla S., Nile S.H., Chun H.S., Khan I., Oh S.Y., Lee H., Huh Y.S., et al. Anti-oxidant and antimicrobial efficacy of a biflavonoid, amentoflavone from Nandina domestica in vitro and in minced chicken meat and apple juice food models. Food Chem. 2019;271:239–247. doi: 10.1016/j.foodchem.2018.07.159. [DOI] [PubMed] [Google Scholar]
- 60.Sánchez-Fidalgo S., da Silva M.S., Cárdeno A., Aparicio-Soto M., Salvador M.J., Frankland Sawaya A.C., Souza-Brito A.R., de la Lastra C.A. Abarema cochliacarpos reduces LPS-induced inflammatory response in murine peritoneal macrophages regulating ROS-MAPK signal pathway. J. Ethnopharmacol. 2013;149:140–147. doi: 10.1016/j.jep.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Pereira R.B., Sousa C., Costa A., Andrade P.B., Valentão P. Glutathione and the antioxidant potential of binary mixtures with flavonoids: Synergisms and antagonisms. Molecules. 2013;18:8858–8872. doi: 10.3390/molecules18088858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vasconcelos P.C.D.P., Seito L.N., Di Stasi L.C., Akiko Hiruma-Lima C., Pellizzon C.H. Epicatechin used in the treatment of intestinal inflammatory disease: An analysis by experimental models. Evid. Based Compl. Altern. Med. 2012;2012:508902. doi: 10.1155/2012/508902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yadav R., Kumar D., Kumari A., Yadav S.K. Encapsulation of catechin and epicatechin on BSA NPS im-proved their stability and antioxidant potential. EXCLI J. 2014;13:331–346. [PMC free article] [PubMed] [Google Scholar]
- 64.Islam M.T., Ayatollahi S.A., Zihad S., Sifat N., Khan M.R., Paul A., Salehi B., Islam T., Mubarak M.S., Martins N., et al. Phytol anti-inflammatory activity: Pre-clinical assessment and possible mechanism of action elucidation. Cell. Mol. Biol. 2020;66:264–269. doi: 10.14715/cmb/2020.66.4.31. [DOI] [PubMed] [Google Scholar]
- 65.Santos C.C., Salvadori M.S., Mota V.G., Costa L.M., de Almeida A.A., de Oliveira G.A., Costa J.P., de Sousa D.P., de Freitas R.M., de Almeida R.N. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013;2013:949452. doi: 10.1155/2013/949452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amarowicz R. Squalene: A natural antioxidant? Eur. J. Lipid Sci. Technol. 2009;111:411–412. doi: 10.1002/ejlt.200900102. [DOI] [Google Scholar]
- 67.Cárdeno A., Aparicio-Soto M., Montserrat-de la Paz S., Bermudez B., Muriana F.J.G., Alarcón-de-la-Lastra C. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct. Foods. 2015;14:779–790. doi: 10.1016/j.jff.2015.03.009. [DOI] [Google Scholar]
- 68.Loizou S., Lekakis I., Chrousos G.P., Moutsatsou P. Beta-sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol. Nutr. Food Res. 2010;54:551–558. doi: 10.1002/mnfr.200900012. [DOI] [PubMed] [Google Scholar]
- 69.Kawata A., Murakami Y., Suzuki S., Fujisawa S. Anti-inflammatory Activity of β-Carotene, Lycopene and Tri-n-butylborane, a Scavenger of Reactive Oxygen Species. In Vivo. 2018;32:255–264. doi: 10.21873/invivo.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiedor J., Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuzcu M., Orhan C., Muz O.E., Sahin N., Juturu V., Sahin K. Lutein and zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model. BMC Ophthalmol. 2017;17:129. doi: 10.1186/s12886-017-0524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murillo A.G., Hu S., Fernandez M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants. 2019;8:390. doi: 10.3390/antiox8090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung R., Leanderson P., Lundberg A.K., Jonasson L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis. 2017;262:87–93. doi: 10.1016/j.atherosclerosis.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 74.Wisniewska-Becker A., Nawrocki G., Duda M., Subczynski W.K. Structural aspects of the antioxidant activi-ty of lutein in a model of photoreceptor membranes. Acta Biochim. Pol. 2012;59:119–124. doi: 10.18388/abp.2012_2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Freitas de Lima F., Lescano C.H., Arrigo J., Cardoso C., Coutinho J.P., Moslaves I., Ximenes T., Kadri M., Weber S.S., Perdomo R.T., et al. Anti-inflammatory, anti-proliferative and cytoprotective potential of the Attalea phalerata Mart. ex Spreng. pulp oil. PLoS ONE. 2018;13:e0195678. doi: 10.1371/journal.pone.0195678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Araki M., Kaku N., Harada M., Ando Y., Yamaguchi R., Shindo K. Production of Auroxanthins from Violaxanthin and 9-cis-Violaxanthin by Acidic Treatment and the Antioxidant Activities of Violaxanthin, 9-cis-Violaxanthin, and Auroxanthins. J. Agric. Food Chem. 2016;64:9352–9355. doi: 10.1021/acs.jafc.6b04506. [DOI] [PubMed] [Google Scholar]
- 77.Serbinova E.A., Packer L. Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Methods Enzymol. 1994;234:354–366. doi: 10.1016/0076-6879(94)34105-2. [DOI] [PubMed] [Google Scholar]
- 78.Shuid A.N., Mohamad S., Muhammad N., Fadzilah F.M., Mokhtar S.A., Mohamed N., Soelaiman I.N. Effects of α-tocopherol on the early phase of osteoporotic fracture healing. J. Orthop.Res. Off. Publ. Orthop. Res. Soc. 2011;29:1732–1738. doi: 10.1002/jor.21452. [DOI] [PubMed] [Google Scholar]
- 79.Saeed N.M., El-Demerdash E., Abdel-Rahman H.M., Algandaby M.M., Al-Abbasi F.A., Abdel-Naim A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012;264:84–93. doi: 10.1016/j.taap.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 80.Islam M.N., Ishita I.J., Jin S.E., Choi R.J., Lee C.M., Kim Y.S., Jung H.A., Choi J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;55:541–548. doi: 10.1016/j.fct.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 81.Lanfer-Marquez U.M., Barros R.M.C., Sinnecker P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005;38:885–891. doi: 10.1016/j.foodres.2005.02.012. [DOI] [Google Scholar]
- 82.Liapina E.A., Machneva T.V., Larkina E.A., Tkachevskaia E.P., Osipov A.N., Mironov A.F. Effect of the photosensitizers pheophorbid a and protoporphyrin IX on skin wound healing by the action of low-intensity laser irradiation. Biofizika. 2010;55:350–355. [PubMed] [Google Scholar]
- 83.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Corrêa M.F.P., Melo G.O., Costa S.S. Natural products from plant origin potentially usefull in the asthma therapy. Braz. J. Pharmacogn. 2008;18:785–797. doi: 10.1590/S0102-695X2008000500025. [DOI] [Google Scholar]
- 85.Brasil. Relação Nacional de Medicamentos Essenciais: Rename 2013. Brasília, DF: Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Assistência Farmacêutica e Insumos Estra-tégicos, 8th ed. [(accessed on 15 May 2022)];2013 Available online: https://www.prefeitura.sp.gov.br/cidade/secretarias/upload/rename/livro-rename-2013-atualizado.pdf.
- 86.Marmitt D.J., Rempel C., Goettert M.I., Silva A.C. Medicinal Plants RENISUS with Potential Anti-inflammatory: Systematic Review in Three Scientific Databases. Rev. Fitos. 2015;9:73–159. [Google Scholar]
- 87.Lopes G.F.G., Pantoja S.C.S. Levantamento das espécies de plantas medicinais utilizadas pela população de Santa Cruz–Rio de Janeiro–RJ. Rev. Eletrônica Novo Enfoque. 2013;16:62–80. [Google Scholar]
- 88.Feijó A.M., Heiden G. Medicinal plants used by elderly people with Diabetes mellitus in the treatment of the disease symptoms. Rev. Bras. Plantas Med. 2012;14:50–56. doi: 10.1590/S1516-05722012000100008. [DOI] [Google Scholar]
- 89.Rodrigues I.A., Azevedo M.M., Chaves F.C., Alviano C.S., Alviano D.S., Vermelho A.B. Arrabidaea chica hexanic extract induces mitochondrion damage and peptidase inhibition on Leishmania spp. BioMed. Res. Int. 2014:985171. doi: 10.1155/2014/985171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cortez de Sá J., Almeida-Souza F., Mondêgo-Oliveira R., Oliveira I., Lamarck L., Magalhães I., Ataídes-Lima A.F., Ferreira H., Abreu-Silva A.L. Leishmanicidal, cytotoxicity and wound healing potential of Arrabidaea chica Verlot. BMC Compl. Altern. Med. 2016;16:1–11. doi: 10.1186/s12906-015-0973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mafioleti L., da Silva Junior I.F., Colodel E.M., Flach A., Martins D.T. Evaluation of the toxicity and anti-microbial activity of hydroethanolic extract of Arrabidaea chica (Humb. & Bonpl.) B. Verl. J. Ethnopharmacol. 2013;150:576–582. doi: 10.1016/j.jep.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 92.Torres C.A., Zampini I.C., Nuñez M.B., Isla M.I., Castro M.P., Gonzalez A.M. In vitro antimicrobial activity of 20 selected climber species from the Bignoniaceae family. Nat. Prod. Res. 2013;27:2144–2148. doi: 10.1080/14786419.2013.782490. [DOI] [PubMed] [Google Scholar]
- 93.Hofling J.F., Anibal P.C., Obando-Pereda G.A., Peixoto I.A.T., Furletti V.F., Foglio M.A., Gonçalves R.B. Antimicrobial potential of some plant extracts against Candida species. Braz. J. Biol. 2010;70:1065–1068. doi: 10.1590/S1519-69842010000500022. [DOI] [PubMed] [Google Scholar]
- 94.Kohn L.K., Foglio M.A., Rodrigues R.A., Sousa I.M.O., Martini M.C., Padilla M.A., Lima-Neto D.F., Arns C.W. In-Vitro Antiviral Activities of Extracts of Plants of The Brazilian Cerrado against the Avian Metapneumovirus (aMPV) Rev. Bras. De Ciência Avícola. 2015;17:275–280. doi: 10.1590/1516-635X1703275-280. [DOI] [Google Scholar]
- 95.Brandão G.C., Kroon E.G., Santos J.R., Stehmann J.R., Lombardi J.A., Oliveira A.B. Antiviral activities of plants occurring in the state of Minas Gerais, Brazil: Part 2. Screening Bignoniaceae species. Rev. Bras. Farmacogn. 2010;20:742–750. doi: 10.1590/S0102-695X2010005000035. [DOI] [Google Scholar]
- 96.Ribeiro A.F.C., Telles T.C., Ferraz V.P., Souza-Fagundes E.M., Cassali G.D., Carvalho A.T., Melo M.M. Effect of Arrabidaea chica extracts on the Ehrlich solid tumor development. Rev. Bras. de Farmacogn. 2012;22:364–373. doi: 10.1590/S0102-695X2011005000225. [DOI] [Google Scholar]
- 97.Rocha K.B.F., Oliveira C.N., Azevedo I.M., Macedo R., Medeiros A.C. Effect of Arrabidaea chica extract against chemically induced breast cancer in animal model. Acta Cirúrgica Bras. 2019;34:e201901001. doi: 10.1590/s0102-865020190100000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taffarello D., Jorge M.P., Sousa I.M.O., Duarte M.C.T., Figueira G.M., Queiroz N.C.A., Rodrigues R.A.F., Carvalho J.E., Goes A.L.T.R., Foglio M.A. Activity of Arrabidaea chica (Humb. & Bonpl.) Verlot ex-tracts obtained by biotechnological processes on fibroblast and human tumor cells. Química Nova. 2013;36:431–436. [Google Scholar]
- 99.Evangelista S.S., Sampaio F.C., Parente R.C., Bandeira M.F.C.L. Phytotherapics in Odontology: Ethnobo-tanical study in Manaus. Rev. Bras. de Plantas Med. 2013;15:513–519. doi: 10.1590/S1516-05722013000400007. [DOI] [Google Scholar]
- 100.Elisabetsky E., Wannmacher L. The status of ethnopharmacology in Brazil. J. Ethnopharmacol. 1993;38:137–143. doi: 10.1016/0378-8741(93)90008-S. [DOI] [PubMed] [Google Scholar]
- 101.Odonne G., Valadeau C., Alban-Castillo J., Stien D., Sauvain M., Bourdy G. Medical ethnobotany of the Chayahuita of the Paranapura basin (Peruvian Amazon) J. Ethnopharmacol. 2013;146:127–153. doi: 10.1016/j.jep.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 102.Sharifi-Rad J., Salehi B., Stojanović-Radić Z., Fokou P.V.T., Sharifi-Rad M., Mahady G.B., Iriti M. Me-dicinal plants used in the treatment of tuberculosis-Ethnobotanical and ethnopharmacological approaches. Biotechnol. Adv. 2020;44:107629. doi: 10.1016/j.biotechadv.2020.107629. [DOI] [PubMed] [Google Scholar]
- 103.Moraes L.L.C., Fritas J.L., Matos-Filho J.R., Silva R.B.L., Borges C.H.A., Santos A.C. A Ethno-knowledge of medicinal plants in a community in the eastern Amazon. Rev. Ciên. Agrár. 2019;42:565–573. [Google Scholar]
- 104.Cartagenes M.d.S.S., Lima N.F.M.C., França L.G., Pessoa D.L.R., Amaral F.M.M., Abreu I.C., Silva S.N., Borges M.O.R., Medeiros I.A. Avaliação da atividade anti-hipertensiva do extrato de Arrabi-daea chica Verlot em ratos espontaneamente hipertensos. Rev. Ciên. Saúde. 2015;16:1–11. [Google Scholar]
- 105.Coelho-Ferreira M. Medicinal knowledge and plant utilization in an Amazonian coastal community of Marudá, Pará State (Brazil) J. Ethnopharmacol. 2009;126:159–175. doi: 10.1016/j.jep.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 106.Rodrigues M.C., Muehlmann L.A., Longo J.P.F., Silva R.C., Graebner I.B., Degterev I.A., Lucci C.M., Azevedo R.B., Garcia M.P. Photodynamic Therapy Based on Arrabidaea chica (Crajiru) Extract Nanoemulsion: In vitro Activity against Monolayers and Spheroids of Human Mammary Adenocarcinoma MCF-7 Cells. J. Nanomed. Nanotechnol. 2015;6:286. doi: 10.4172/2157-7439.1000286. [DOI] [Google Scholar]
- 107.Souza H.Q., Hidalgo A.F., Chaves F.C.M. Preparo do corante de crajirú (Arrabidaea chica (Bonpl.) B. Verl.) e sua aplicação em Histologia. J. Braz. Assoc. Hortic. Sci. 2007;25:S36. [Google Scholar]
- 108.Lima de Medeiros B.J., Dos Santos Costa K., Alves Ribeiro J.F., Carrera Silva J.O., Ramos Barbosa W.L., Tavares Carvalho J.C. Liver protective activity of a hydroethanolic extract of Arrabidaea chica (Humb. and Bonpl.) B. Verl. (pariri) Pharmacogn. Res. 2011;3:79–84. doi: 10.4103/0974-8490.81954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Do Amaral R.R., Santos A.A., Saravia A., Botas G., Cruz R.A., Fernandes C.P., Boylan F. Biological activities of Arrabidaea chica (Bonpl.) B. Verl. leaves. Lat. Am. J. Pharm. 2012;31:451–455. [Google Scholar]
- 110.Gonzalez A.C.O., Costa T.F., Andrade Z.A., Medrado A.R.A.P. Wound healing—A literature review. An. Bras. de Dermatol. 2016;91:614–620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pristo I. Cicatrização de feridas: Fases e fatores de influência. Acta Vet. Bras. 2012;6:267–271. [Google Scholar]
- 112.Martelli A., Andrade T.A.M., dos Santos G.M.T. Perspectivas na utilização de fitoterápicos na cicatriza-ção tecidual: Revisão sistemática. Arch. Health Investig. 2018;7:344–350. doi: 10.21270/archi.v7i8.3047. [DOI] [Google Scholar]
- 113.Servat-Medina L., González-Gómez A., Reyes-Ortega F., Sousa I.M., Queiroz N., Zago P.M., Jorge M.P., Monteiro K.M., de Carvalho J.E., San Román J., et al. Chitosan-tripolyphosphate nanoparticles as Arrabidaea chica standardized extract carrier: Synthesis, characterization, biocompatibility, and antiulcerogenic activity. Int. J. Nanomed. 2015;10:3897–3909. doi: 10.2147/IJN.S83705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aro A.A., Freitas K.M., Foglio M.A., Carvalho J.E., Dolder H., Gomes L., Vidal B.C., Pimentel E.R. Effect of the Arrabidaea chica extract on collagen fiber organization during healing of partially transected ten-don. Life Sci. 2013;92:799–807. doi: 10.1016/j.lfs.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 115.Aro A.A., Simões G.F., Esquisatto M.A., Foglio M.A., Carvalho J.E., Oliveira A.L., Gomes L., Pimen-tel E.R. Arrabidaea chica extract improves gait recovery and changes collagen content during healing of the Achilles tendon. Injury. 2013;44:884–892. doi: 10.1016/j.injury.2012.08.055. [DOI] [PubMed] [Google Scholar]
- 116.Pires A., Westin C.B., Hernandez-Montelongo J., Sousa I., Foglio M.A., Moraes A.M. Flexible, dense and porous chitosan and alginate membranes containing the standardized extract of Arrabidaea chica Verlot for the treatment of skin lesions. Mater. Sci. Engineering. C Mater. Biol. Appl. 2020;112:110869. doi: 10.1016/j.msec.2020.110869. [DOI] [PubMed] [Google Scholar]
- 117.Zhang H., Sun S.C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015;5:63. doi: 10.1186/s13578-015-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 119.Kuprash D.V., Nedospasov S.A. Molecular and Cellular Mechanisms of Inflammation. Biochemistry. 2016;81:1237–1239. doi: 10.1134/S0006297916110018. [DOI] [PubMed] [Google Scholar]
- 120.Groh L., Keating S.T., Joosten L., Netea M.G., Riksen N.P. Monocyte and macrophage immunometabo-lism in atherosclerosis. Semin. Immunopathol. 2018;40:203–214. doi: 10.1007/s00281-017-0656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kiripolsky J., McCabe L.G., Kramer J.M. Innate immunity in Sjögren’s syndrome. Clin. Immunol. 2017;182:4–13. doi: 10.1016/j.clim.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scrivo R., Vasile M., Bartosiewicz I., Valesini G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 2011;10:369–374. doi: 10.1016/j.autrev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 123.Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brubaker S.W., Bonham K.S., Zanoni I., Kagan J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Placha D., Jampilek J. Chronic Inflammatory Diseases, Anti-Inflammatory Agents and Their Delivery Nanosystems. Pharmaceutics. 2021;13:64. doi: 10.3390/pharmaceutics13010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tasneem S., Liu B., Li B., Choudhary M.I., Wang W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019;139:126–140. doi: 10.1016/j.phrs.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 127.Brasil. Relação Nacional de Medicamentos Essenciais: RENAME 2014. Brasília: Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Assistência Farmacêutica e Insumos Estratégi-cos. 9th edição. [(accessed on 1 July 2022)];2015 Available online: https://bvsms.saude.gov.br/bvs/publicacoes/relacao_nacional_medicamentos_essenciais_rename_2014.pdf.
- 128.Chi Y.S., Jong H.G., Son K.H., Chang H.W., Kang S.S., Kim H.P. Effects of naturally occurring prenyl-ated flavonoids on enzymes metabolizing arachidonic acid: Cyclooxygenases and lipoxygenases. Biochem. Pharmacol. 2001;62:1185–1191. doi: 10.1016/S0006-2952(01)00773-0. [DOI] [PubMed] [Google Scholar]
- 129.Jang D.S., Cuendet M., Hawthorne M.E., Kardono L.B., Kawanishi K., Fong H.H., Mehta R.G., Pez-zuto J.M., Kinghorn A.D. Prenylated flavonoids of the leaves of Macaranga conifera with inhibitory activi-ty against cyclooxygenase-2. Phytochemistry. 2002;61:867–872. doi: 10.1016/S0031-9422(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 130.Michel A.F., Melo M.M., Campos P.P., Oliveira M.S., Oliveira F.A., Cassali G.D., Ferraz V.P., Cota B.B., Andrade S.P., Souza-Fagundes E.M. Evaluation of anti-inflammatory, antiangiogenic and antiprolifera-tive activities of Arrabidaea chica crude extracts. J. Ethnopharmacol. 2015;165:29–38. doi: 10.1016/j.jep.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 131.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tak P.P., Firestein G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 134.Furtado M.F.D., Silva W.D., Colleto G.M.D.D. Controle de qualidade dos venenos animais e dos corres-pondentes antivenenos. I Padronização dos métodos de ensaio das atividades bioquímicas e farmacológicas dos venenos de algumas espécies do gênero Bothrops e Crotalus usando amostras secas a temperaturas ambiente ou lioflizadas. Memórias Inst. Butantan. 1991;53:149–159. [Google Scholar]
- 135.Koutoulogenis G.S., Kokotou M.G., Hayashi D., Mouchlis V.D., Dennis E.A., Kokotos G. 2-Oxoester Phospholipase A2 Inhibitors with Enhanced Metabolic Stability. Biomolecules. 2020;10:491. doi: 10.3390/biom10030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takenaka I.K.T.M., Amorim J.M., de Barros P.A.V., Brandao G.C., Contarini S.M.L., de Sales Souza e Melo É., Fernandes S.O.A. Chemical characterization and anti-inflammatory assessment of the hydroethanol-ic extract of Fridericia chica. Rev. Bras. de Farmacogn. 2020;30:559–567. doi: 10.1007/s43450-020-00085-7. [DOI] [Google Scholar]
- 137.Murakami A., Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer. 2007;121:2357–2363. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- 138.Torres C.A., Pérez Zamora C.M., Nuñez M.B., Gonzalez A.M. In vitro antioxidant, antilipoxygenase and antimicrobial activities of extracts from seven climbing plants belonging to the Bignoniaceae. J. Integr. Med. 2018;16:255–262. doi: 10.1016/j.joim.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 139.Lima J., de Oliveira R.G., Silva V.C., de Sousa P.T., Jr., Violante I., Macho A., Martins D. Anti-inflammatory activity of 4’,6,7-trihydroxy-5-methoxyflavone from Fridericia chica (Bonpl.) L.G.Lohmann. Nat. Prod. Res. 2020;34:726–730. doi: 10.1080/14786419.2018.1495636. [DOI] [PubMed] [Google Scholar]
- 140.Sies H. Oxidative stress. Elsevier; Amsterdam, The Netherlands: 1985. Oxidative Stress: Introductory Remarks; pp. 1–8. [Google Scholar]
- 141.Sies H. Biological redox systems and oxidative stress. Cell. Mol. Life Sci. CMLS. 2007;64:2181–2188. doi: 10.1007/s00018-007-7230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sies H., Berndt C., Jones D.P. Oxidative Stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 143.Miguel M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011;1:7–15. [Google Scholar]
- 144.Carvalho A.V., Cavalcante M.A., Santana C.L., Alves R.M. Physical and chemical characteristics of ma-trices of yellow mobin fruits in the state of Pará. Aliment. Nutr. Araraquara. 2011;22:45–53. [Google Scholar]
- 145.Aprioku J.S. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J. Reprod. Infertil. 2013;14:158–172. [PMC free article] [PubMed] [Google Scholar]
- 146.Lü J.M., Lin P.H., Yao Q., Chen C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Halliwell B. Biochemistry of oxidative stress. Pt 5Biochem. Soc. Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 148.El-Beltagi H.S., Mohamed H.I. Reactive oxygen species, lipid peroxidation and antioxidative defense mechanism. Not. Bot. Horti Agrobot. Cluj-Napoca. 2013;41:44–57. doi: 10.15835/nbha4118929. [DOI] [Google Scholar]
- 149.Salisbury D., Bronas U. Reactive oxygen and nitrogen species: Impact on endothelial dysfunction. Nurs. Res. 2015;64:53–66. doi: 10.1097/NNR.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 150.Halliwell B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012;70:257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 151.Venkataraman K., Khurana S., Tai T.C. Oxidative stress in aging--matters of the heart and mind. Int. J. Mol. Sci. 2013;14:17897–17925. doi: 10.3390/ijms140917897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Katoch N., Kaur P., Kashyap P., Gupta S., Dahiya R.S. Role of oxidative stress in cardiovascular diseases. Res. J. Pharm. Biol. Chem. Sci. 2013;4:870–881. [Google Scholar]
- 153.Fibach E., Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Curr. Mol. Med. 2008;8:609–619. doi: 10.2174/156652408786241384. [DOI] [PubMed] [Google Scholar]
- 154.Federico A., Morgillo F., Tuccillo C., Ciardiello F., Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 155.Afanas’ev I. New nucleophilic mechanisms of ros-dependent epigenetic modifications: Comparison of aging and cancer. Aging Dis. 2013;5:52–62. doi: 10.14336/ad.2014.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Oguntibeju O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019;11:45–63. [PMC free article] [PubMed] [Google Scholar]
- 157.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. Oxford Scholarship Online; Oxford, UK: 2015. [Google Scholar]
- 158.Augustyniak A., Bartosz G., Cipak A., Duburs G., Horáková L., Luczaj W., Majekova M., Odysseos A.D., Rackova L., Skrzydlewska E., et al. Natural and synthetic antioxidants: An updated overview. Free. Radic. Res. 2010;44:1216–1262. doi: 10.3109/10715762.2010.508495. [DOI] [PubMed] [Google Scholar]
- 159.Arulselvan P., Fard M.T., Tan W.S., Gothai S., Fakurazi S., Norhaizan M.E., Kumar S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016;2016:5276130. doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Processing. 2011;89:217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- 161.Durazzo A., Lucarini M., Souto E.B., Cicala C., Caiazzo E., Izzo A.A., Novellino E., Santini A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. PTR. 2019;33:2221–2243. doi: 10.1002/ptr.6419. [DOI] [PubMed] [Google Scholar]
- 162.Mark R., Lyu X., Lee J.J., Parra-Saldívar R., Chen W.N. Sustainable production of natural phenolics for functional food applications. J. Funct. Foods. 2019;57:233–254. doi: 10.1016/j.jff.2019.04.008. [DOI] [Google Scholar]
- 163.Caleja C., Ribeiro A., Barreiro M.F., Ferreira I. Phenolic Compounds as Nutraceuticals or Functional Food Ingredients. Curr. Pharm. Des. 2017;23:2787–2806. doi: 10.2174/1381612822666161227153906. [DOI] [PubMed] [Google Scholar]
- 164.Silva E.M., Souza J.N.S., Rogez H., Rees J.F., Larondelle Y. Antioxidant activities and polyphenolic con-tents of fifteen selected plant species from the Amazonian region. Food Chem. 2007;101:1012–1018. doi: 10.1016/j.foodchem.2006.02.055. [DOI] [Google Scholar]
- 165.Paula J.T., Paviani L.C., Foglio M.A., Sousa I.M.O., Duarte G.H.B., Jorge M.P., Eberlin M.N., Ca-bral F.A. Extraction of anthocyanins and luteolin from Arrabidaea chica by sequential extraction in fixed bed using supercritical CO2, ethanol and water as solvents. J. Supercrit. Fluids. 2014;86:100–107. doi: 10.1016/j.supflu.2013.12.008. [DOI] [Google Scholar]
- 166.Dos Santos V.C., Longo T.B., Garcia A.L., Richter M.F., Guecheva T.N., Henriques J.A., Ferraz A., Picada J.N. Evaluation of the mutagenicity and genotoxicity of Arrabidaea chica Verlot (Bignoneaceae), an Amazon plant with medicinal properties. J. Toxicol. Environ. Health Part A. 2013;76:381–390. doi: 10.1080/15287394.2012.761947. [DOI] [PubMed] [Google Scholar]
- 167.Gemelli T.F., Prado L., Santos F.S., de Souza A.P., Guecheva T.N., Henriques J.A., Ferraz A., Corrêa D.S., Dihl R.R., Picada J.N. Evaluation of Safety of Arrabidaea chica Verlot (Bignoniaceae), a Plant with Healing Properties. J. Toxicol. Environ. Health Part A. 2015;78:1170–1180. doi: 10.1080/15287394.2015.1072070. [DOI] [PubMed] [Google Scholar]
- 168.Olivero-Verbel J., De la Parra-Guerra A., Caballero-Gallardo K., Sierra-Marquez L., Fuentes-Lopez K., Franco-Marmolejo J., Jannasch A.S., Sepulveda M.S., Stashenko E. The aqueous extract of Fridericia chica grown in northern Colombia ameliorates toxicity induced by Tergitol on Caenorhabditis elegans. Comparative biochemistry and physiology. Toxicol. Pharmacol. CBP. 2021;244:109026. doi: 10.1016/j.cbpc.2021.109026. [DOI] [PubMed] [Google Scholar]
- 169.Martins F.J., Caneschi C.A., Vieira J.L., Barbosa W., Raposo N.R. Antioxidant activity and potential pho-toprotective from amazon native flora extracts. J. Photochem. Photobiol. B Biol. 2016;161:34–39. doi: 10.1016/j.jphotobiol.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 170.Ribeiro F.M., Volpato H., Lazarin-Bidóia D., Desoti V.C., de Souza R.O., Fonseca M., Ueda-Nakamura T., Nakamura C.V., Silva S.O. The extended production of UV-induced reactive oxygen species in L929 fi-broblasts is attenuated by posttreatment with Arrabidaea chica through scavenging mechanisms. J. Photochem. Photobiol. B Biol. 2018;178:175–181. doi: 10.1016/j.jphotobiol.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 171.Teixeira T.S., Vale R.C., Almeida R.R., Ferreira T.P.S., Guimarães L.G.L. Antioxidant Potential and its Correlation with the Contents of Phenolic Compounds and Flavonoids of Methanolic Extracts from Different Medicinal Plants. Rev. Virtual Química. 2017;9:1546–1559. doi: 10.21577/1984-6835.20170090. [DOI] [Google Scholar]
- 172.Brasil. Programa Nacional de Plantas Medicinais e Fitoterápicos. Brasília: Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Assistência Farmacêutica e Insumos Estratégicos. [(accessed on 3 June 2022)];2009 Available online: https://bvsms.saude.gov.br/bvs/publicacoes/programa_nacional_plantas_medicinais_fitoterapicos.pdf.
- 173.Panizza S.T. Como Prescrever ou Recomendar Plantas Medicinais. Conbrafito; São Luís, Brazil: 2010. p. 15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.