Abstract
Background
Using contemporary data from NORIC (Norwegian Registry of Invasive Cardiology) we investigated the predictive value of patient age and time from ECG diagnosis to sheath insertion (ECG‐2‐sheath) in primary percutaneous coronary intervention for ST‐segment–elevation myocardial infarction (STEMI).
Methods and Results
Data from 11 226 patients collected from all centers offering 24/7/365 primary percutaneous coronary intervention service were explored. For patients aged <80 years the mortality rates were 5.6% and 7.6% at 30 days and 1 year, respectively. For octogenarians the corresponding rates were 15.0% and 24.2%. The Cox hazard ratio was 2.02 (1.93–2.11, P value <0.0001) per 10 years of patient age. Time from ECG‐2‐sheath was significantly associated with mortality with a 3.6% increase per 30 minutes of time. Using achievement of time goal <90 minutes in patients aged >80 years and mortality at 30 days, mortality was 10.5% and 17.7% for <90 or ≥90 minutes, respectively. The number needed to prevent 1 death was 39 in the whole population and 14 in the elderly.
Restricted mean survival gains during median 938 days of follow‐up in patients with ECG‐2‐sheath time <90 minutes were 24 and 76 days for patients aged <80 and ≥80 years, respectively.
Conclusions
Time from ECG‐diagnosis to sheath insertion is strongly correlated with mortality. This applies especially to octogenarians who derive the most in terms of absolute mortality reduction.
Registration
Keywords: octogenarians, primary PCI, revascularization, STEMI, timing
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Coronary Artery Disease, Acute Coronary Syndromes
Nonstandard Abbreviations and Acronyms
- ECG‐2‐sheath
ECG diagnosis to sheath insertion
- NORIC
Norwegian Registry of Invasive Cardiology
- OHCA
out‐of‐hospital cardiac arrest
- pPCI
primary percutaneous coronary intervention
Clinical Perspective
What Is New?
This study indicates that timely reperfusion with percutaneous coronary intervention in patients with ST‐segment–elevation myocardial infarction is even more important in octogenarians.
What Are the Clinical Implications?
These findings further support the new ST‐segment–elevation myocardial infarction guidelines' time limits also for octogenarians.
It is recommended that the prehospital management of patients with ST‐segment–elevation myocardial infarction (STEMI) is based on regional networks designed to deliver reperfusion therapy expeditiously and effectively, with efforts made to make primary percutaneous coronary intervention (pPCI) available to as many patients as possible. 1 Mortality rates have decreased considerably following the implementation of pPCI as the standard of care for urgent revascularization. 2 , 3 , 4 , 5 , 6 , 7 Several factors, including prehospital logistics, 8 , 9 center volumes 10 and improved cardiac care 11 contribute to this positive development. Key predictors as door‐to‐balloon time 12 and infarct size 13 serve as indicators of quality.
Limitations of the current reports on prognosis in STEMI are that they are based on selected samples from hospitals voluntary registries, 14 trials 15 and surveys, 16 and thereby lack full population coverage. Furthermore, as noted in the current European Society of Cardiology (ESC) STEMI guidelines, the selection of a 90 through 120 minutes from STEMI diagnosis to percutaneous coronary intervention (PCI)‐mediated reperfusion as the cut‐off to choose PCI or fibrinolysis is based on relatively old registries and trials with different treatment strategies from those presented in the current guidelines. 1 , 17 , 18 In addition to symptom to reperfusion time, patient age also predicts outcome. 19 , 20 However, contemporary data on timely access to reperfusion therapy and in‐hospital outcomes according to the age of older adults presenting with STEMI are limited.
Therefore, we investigated the predictive value of time from ECG diagnosis to sheath insertion (ECG‐2‐sheath), a mandatory data point in NORIC (Norwegian Registry of Invasive Cardiology), on mortality in patients, including patients aged ≥80 years, undergoing pPCI for STEMI in Norway.
Methods
Data and methods used in the analysis, and materials used to conduct the research are available for purposes of reproducing the results or replicating the procedure. However, our data permit does not allow for distribution to third‐party (explicitly states no data transfer abroad). Access is granted after proper application to the data authorities, in this case the Norwegian Institute of Public Health. After which we would be happy to accommodate the request.
Requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Norwegian Institute of Public Health at hkr.oppdrag@fhi.no.
The NCDR (Norwegian Cardiovascular Disease Registry) is a nationwide mandatory, nonconsensual person‐identifiable health registry 21 that collects data from all patient contacts with hospitals in Norway for cardiovascular diseases and procedures. NORIC is a national medical quality‐registry connected to NCDR which comprises clinical and procedural information from invasive cardiac procedures in the PCI hospitals in Norway. There are 9 centers offering pPCI in Norway, all of them are open 24/7/365.
The registry has collected data from 2013 and had a full national coverage from January 1, 2015. Norwegian PCI centers have provided pPCI 24/7/365 for many years, but the adaption to registration in NORIC was gradual, center‐by‐center. The coverage for the 5 included PCI centers in 2014 was 99%. Approximately the same applies for 2013. We have no reason to suspect that the achieved results in nonincluded centers from 2013 to 2014 would materially alter the results of the analyses in any direction, as audits from 2015 onwards have shown consistent results between centers.
Information about date of deaths for patients in NORIC is obtained through linkage to the National Population Register using the Norwegian national identity number.
All invasive coronary procedures are registered in a web‐based application directly in NORIC by the PCI‐operator forthwith including patient, logistical and procedural data.
In NORIC the patients with STEMI are categorized in 5 subgroups: 1, STEMI admitted for pPCI within 24 hours of symptoms; 2, STEMI with symptoms >24 hours; 3, admitted for rescue PCI following failed thrombolysis; 4, Invasive coronary assessment after successful thrombolysis; and 5, out‐of‐hospital cardiac arrest (OHCA) and STEMI.
Patient Selection
The current analysis includes all patients registered in NORIC from January 1, 2013 until May 27th, 2020, undergoing either successful or attempted pPCI for the indication STEMI with or without OHCA and a valid Norwegian identity number. Patients undergoing only coronary angiography (ie, false cath. laboratory activation, Takotsubo cardiomyopathy, moribund patients, etc), who had received thrombolytic therapy or presented later than 24 hours were excluded.
Ethical Considerations
NORIC 22 is a mandatory, nonconsensual person‐identifiable quality health registry organized under the NCDR. The Norwegian Institute of Public Health is the data controller for the registry. Therefore, there are no signed informed consent forms. The regional national ethical committee approves the use of the data for publication # 33912.
Statistical Analysis
We present baseline characteristics of patients with either numbers (%) for categorical or median (interquartile range) for continuous variables. Between group differences were assessed using Fisher exact test for categorical or Kruskal–Wallis test for continuous variables.
All‐cause mortality was investigated using the Cox proportional‐hazards (PH) model for estimation of hazard ratios (HR) with associated 95% CIs. The independent variables were age as both continuous and categorical variable with and without adjustment for patient sex.
The effect of age on mortality was assessed with Kaplan–Meier plots for different age groups and continuous spline estimates of Cox PH estimated HR.
To assess delays in reperfusion several time intervals were defined from the available time variables; symptom onset, diagnostic ECG (most often prehospital), hospital arrival, sheath‐insertion, and target vessel flow reestablished. The interval with good data completeness and deemed most robust was ECG‐2‐sheath insertion time, which we decided to use as a surrogate for the ESC guidelines time interval ECG‐to‐wire crossing. Differences in survival between those patients who achieved the recommended <90 minutes as well as 120 minutes time to sheath insertion and those who did not was modeled using Cox PH models as well as restricted mean survival time (difference in average time‐to‐event/area under the survival curve at a fixed time point) at median follow‐up.
To adjust for possible confounders in multivariate Cox PH models variable selection and regularization using least absolute shrinkage and selection operator with penalized maximum likelihood was performed. Input variables were clinically meaningful covariates available before patient admittance to the catheterization laboratory and following treatment: patient age, sex, smoking, diabetes, hypertension, peripheral artery disease, history of prior revascularization, cardiogenic shock, cardiac arrest, contrast use, fluoroscopy time, admittance outside of office hours (1600‐0800 hours), use of intracoronary imaging, history of heart failure, number of diseased coronary vessels, use of mechanical circulatory support, arterial access site, ECG‐2‐sheath time, in‐laboratory complications, and complete revascularization at index procedure (n=20). The method needs complete data and because of row‐wise deletion the number of patients was reduced to 4919.
For all relevant analyses described above a 2‐sided P value of 0.05 was selected.
All data handling and analyses were done using R version 4.0.2 (R Core Team [2020]. R Foundation for Statistical Computing, Vienna, Austria) and Rstudio (RStudio Team [2020]. RStudio, Inc., Boston, USA).
Results
Data from January 1, 2013 to May 27, 2020 were explored. During this time period 11 226 patients with valid Norwegian national identity numbers and with symptoms <24 hours undergoing pPCI including incomplete procedures (ie, wiring attempts but not successful revascularization) were included in the analyses. The incidence of pPCI for the years of 2015 through 2019 when the registry had full national coverage was 34, 32, 34, 36, and 35 per 100 000 people per year.
Demographics
Briefly 76% were men and median age was 64 years with a significant age difference between the sexes of 6 years (men 63 years and women 70 years of age, P value <0.0001). Cardiogenic shock was present in 5.1%, and 9.1% had OHCA (Table 1). For patients presenting with OHCA, the 30‐day and 1‐year mortality was 30.0% and 32.2%, respectively, while for cardiogenic shock it was 50.4% and 55.5%, respectively.
Table 1.
Baseline Characteristics
| Complete data (n) | Whole population (n=11 226) | Aged <80 y (n=9826) | Aged ≥80 y (n=1400) | P value | |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age (y), median (IQR) | 11 226 | 64.00 (56.00, 73.00) | 62.00 (54.00, 70.00) | 84.00 (81.00, 87.00) | <0.0001 |
| Sex, male n (%) | 11 226 | 8536 (76.04%) | 7777 (79.15%) | 759 (54.21%) | <0.0001 |
| BMI (kg/m2), median (IQR) | 9224 | 26.56 (24.22, 29.39) | 26.87 (24.49, 29.70) | 24.57 (22.41, 26.88) | <0.0001 |
| SBT (mm Hg), median (IQR) | 5936 | 130.00 (110.00, 149.00) | 130.00 (110.00, 149.00) | 127.50 (110.00, 148.75) | 0.25 |
| DBT (mm Hg), median (IQR) | 5800 | 80.00 (69.00, 90.00) | 80.00 (70.00, 90.00) | 73.00 (60.00, 84.00) | <0.0001 |
| Risk factors | |||||
| Diabetes, n (%) | 10 720 | 1467 (13.68%) | 1276 (13.57%) | 191 (14.50%) | 0.37 |
| Hypertension, n (%) | 10 409 | 4075 (39.15%) | 3370 (36.85%) | 705 (55.82%) | <0.0001 |
| PAD, n (%) | 10 063 | 434 (4.31%) | 336 (3.78%) | 98 (8.30%) | <0.0001 |
| Current smoker, n (%) | 9201 | 3734 (40.58%) | 3575 (43.62%) | 159 (15.82%) | <0.0001 |
| Prior MI, n (%) | 10 600 | 1464 (13.81%) | 1200 (12.89%) | 264 (20.51%) | <0.0001 |
| Prior PCI, n (%) | 11 079 | 1497 (13.51%) | 1270 (13.08%) | 227 (16.59%) | 0.0005 |
| Prior CABG, n (%) | 11 161 | 329 (2.95%) | 250 (2.56%) | 79 (5.69%) | <0.0001 |
| Prior stroke, n (%) | 10 533 | 454 (4.31%) | 343 (3.70%) | 111 (8.79%) | <0.0001 |
| Known left ventricular dysfunction (LVEF <50%) | 9718 | 590 (6.01%) | 472 (5.49%) | 118 (10.57%) | <0.0001 |
| Prehospital care and logistics | |||||
| Cardiac arrest, n (%) | 11 226 | 1018 (9.07%) | 940 (9.57%) | 78 (5.57%) | <0.0001 |
| Admittance between 1600 and 0800, n (%) | 11 226 | 7143 (63.63%) | 6307 (64.19%) | 836 (59.71%) | 0.001 |
| Time from symptoms to ECG (min), median (IQR) | 7566 | 90.00 (163) | 90.00 (155) | 115 (159) | <0.0001 |
| ECG‐2‐sheath time (min), median (IQR) | 6832 | 75.00 (52.00, 105.00) | 75.00 (52.00, 104.00) | 81.00 (56.50, 110.00) | 0.0001 |
| Cardiogenic shock on arrival, n (%) | 11 226 | 576 (5.13%) | 490 (4.99%) | 86 (6.14%) | 0.07 |
| Medication at admittance | |||||
| ASA, n (%) | 5807 | 1411 (24.30%) | 1147 (22.71%) | 264 (34.92%) | <0.0001 |
| Statins, n (%) | 10 490 | 2234 (21.30%) | 1914 (20.87%) | 320 (24.22%) | <0.0001 |
| Angiographic data | |||||
| Extent of CAD | <0.0001 | ||||
| Single‐vessel CAD, n (%) | 11 217 | 6176 (55.06%) | 5571 (56.74%) | 605 (43.28%) | |
| Two‐vessel CAD, n (%) | 11 217 | 3049 (27.18%) | 2631 (26.79%) | 418 (29.90%) | |
| Three‐vessel CAD, n (%) | 11 217 | 1992 (17.76%) | 1617 (16.47%) | 375 (26.82%) | |
| Procedural data | |||||
| Radial access, n (%) | 11 226 | 9957 (88.70%) | 8795 (89.51%) | 1162 (83.00%) | <0.0001 |
| Fluoro time (min), median (IQR) | 11 150 | 9.43 (6.00, 15.11) | 9.30 (5.98, 14.80) | 10.72 (6.72, 17.31) | <0.0001 |
| Contrast volume (mL), median (IQR) | 11 220 | 130.00 (100.00, 180.00) | 132.00 (100.00, 180.00) | 130.00 (100.00, 180.00) | 0.02 |
| Complete revascularization, n (%) | 11 086 | 6785 (61.20%) | 6124 (63.06%) | 661 (48.07%) | <0.0001 |
| Culprit only, n (%) | 11 226 | 10 058 (89.60%) | 8823 (89.79%) | 1235 (88.21%) | 0.08 |
| No. of stents, mean (SD) | 11 197 | 1.65 (0.93) | 1.65 (0.93) | 1.70 (0.98) | 0.05 |
| MCS, n (%) | 11 226 | 291 (2.59%) | 263 (2.68%) | 18 (1.29%) | 0.001 |
N=11 226 patients undergoing primary percutaneous coronary intervention for ST‐segment–elevation myocardial infarction in Norway from 2013 to May 2020. Continuous variables are presented as median (interquartile range) with the exception of number of stents which is presented as mean (±SD). Categorical variables are presented as number (%) and number/total number in subgroups. P value is for Kruskal–Wallis test for continuous variables with the exception for t test for number of stents. P value for categorical variables is from Fisher exact test. A 2‐sided significance level 0.05 was selected. ASA indicates acetylsalicylic acid; BMI, body‐mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; DBT, diastolic blood pressure; ECG‐2‐sheath, ECG diagnosis to sheath insertion; LVEF, left ventricular ejection fraction; MCS, mechanical circulatory support; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; and SBT, systolic blood pressure.
Clinical and Procedural Characteristics of the Elderly ≥80 years of Age
The elderly had a more even sex‐distribution (54% men). In turns of coronary risk factors more elderly patients had hypertension, peripheral artery disease and previous revascularization procedures, but the presence of diabetes was similar with the whole population and the rate of current smoking was lower (16%). Radial access was used in 83% (in contrast to 90% in younger patients, P value <0.0001). While fluoroscopic time was increased (median 10 minutes 43 seconds versus 9 minutes 20 seconds, P value <0.0001), contrast use was somewhat lower in the elderly compared with the younger patients (median 130 versus 132 mL, P value 0.02). Culprit only PCI at index procedure was done in similarly rates (88% versus 90%, P value 0.08), but complete revascularization during hospital stay was achieved in only 48% versus 63%, reflecting a greater extent of coronary artery disease with 27% of the elderly presenting with 3‐vessel disease in contrast to 16% of patients <80 years of age (Table 1).
Whole Population and Sex‐Specific Mortality
Median follow‐up was 938 days (interquartile range 379 to 1592 and maximum 2703 days). The total mortality was 14.1% during the entire follow‐up.
Time‐restricted mortality at 30 days and 1 year was 6.8% and 9.7% for the whole population. For men, 30‐days and 1‐year mortality was 6.2% and 8.7%—while there was a significant higher 30‐days and 1‐year mortality for women at 8.7% and 12.8% respectively. Female sex was associated with an increased risk for death following pPCI with a Cox Hazards Ratio (HR) (95% CI) of 1.54 (1.39–1.71, P value <0.0001) which was completely attenuated by adjusting for age (HR 0.98 [0.88–1.10], P value 0.77). Data not shown in table, only reported in text.
Age at Admission
Mortality was strongly dependent on age (Figures 1, 2, 3). The Cox HR was 2.02 (1.93–2.11, P value <0.0001) per 10 year of patient age. For patients younger than 80 years the mortality rates were 5.6% and 7.6% at 30 days and 1 year, respectively. For patients 80 years of age or older the corresponding rates were 15.0% and 24.2%. As presented in Table 2, the HR for the elderly was 4.53, (4.09–5.03, P value <0.0001).
Figure 1. Survival (y‐axis) in days (x‐axis) following primary percutaneous coronary intervention for STEMI according to different age groups.
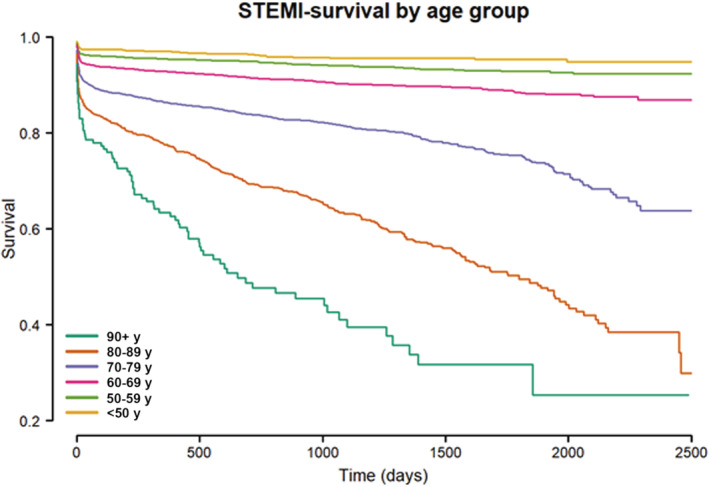
Y‐axis truncated at 20%. STEMI indicates ST‐segment–elevation myocardial infarction.
Figure 2. Survival (y‐axis) in days (x‐axis) following primary percutaneous coronary intervention for STEMI according to patients aged ≥80 or <80 years.
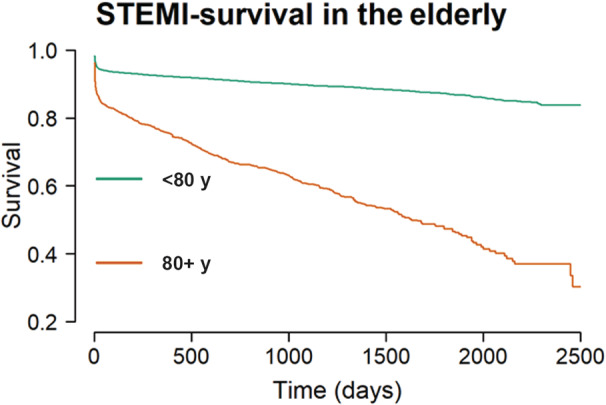
Y‐axis truncated at 20%. STEMI indicates ST‐segment–elevation myocardial infarction.
Figure 3. Smoothed spline Cox proportional hazard model for patient age at admission (x‐axis) and log‐transformed hazard ratio for mortality (y‐axis).
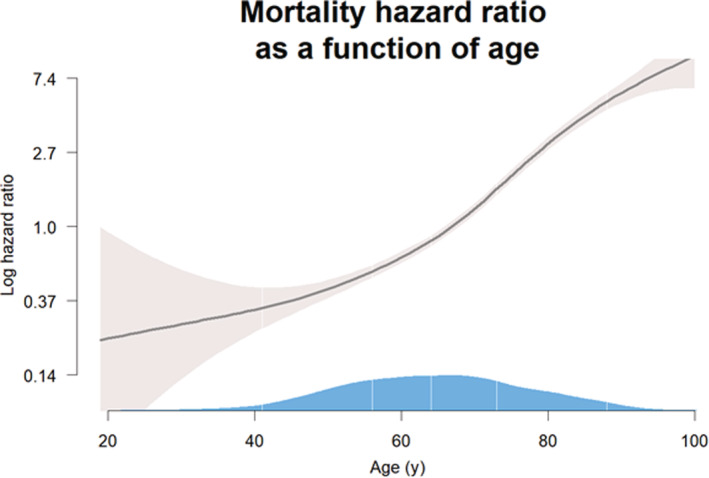
Gray shaded area is the 95% CI and the blue shaded area over the x‐axis the population density according to age, white lines denote the following percentiles from left to right: 2.5, 25, 50, 75, and 95.
Table 2.
Cox Proportional Hazards Models
| Univariate* | Hazard ratio (95% CI) | P value |
|---|---|---|
| Age continuous (per 10 y) | 2.03 (1.94–2.12) | <0.0001 |
| Age categorical (≥80 y) | 4.53 (4.09–5.03) | <0.0001 |
| Sex‐adjusted† | Hazard ratio (95% CI) | P value |
|---|---|---|
| Age continuous (per 10 y) | 2.02 (1.93–2.11) | <0.0001 |
| Age categorical (≥80 y) | 4.39 (3.94–4.89) | <0.0001 |
| Multivariate model‡ | Hazard ratio (95% CI) | P value |
|---|---|---|
| Age continuous (per 10 y) | 2.23 (2.08–2.39) | <0.0001 |
| Sex (men) | 0.87 (0.75–1.00) | 0.06 |
| Extent of CAD (per vessel) | 1.18 (1.08–1.28) | 0.0002 |
| Hypertension | 1.05 (0.91–1.22) | 0.47 |
| Diabetes | 1.60 (1.34–1.90) | <0.0001 |
| Current smoker | 1.39 (1.19–1.62) | <0.0001 |
| Prior revascularization | 1.08 (0.90–1.30) | 0.39 |
| Known reduced LVEF | 2.15 (1.76–2.63) | <0.0001 |
Analyses using the Cox proportional hazards model estimating hazard ratio and associated 95% CI. CAD indicates coronary artery disease; and LVEF, left ventricular ejection fraction.
Univariate model with age as explanatory variable.
Age as explanatory variable adjusted for patient sex.
Multivariate Cox proportional hazards model.
A smoothed spline Cox PH model (Figure 3) showed a highly significant (P value <0.0001), mostly linear relationship between patient age and increasing risk for log‐transformed mortality.
Multivariate Modeling
Multivariate models were designed using clinically relevant variables with few missing data and presented in Table 2. In summary, age, the presence of diabetes and known impaired left ventricular function was strongly associated with mortality. Both the presence of cardiogenic shock and OHCA profoundly affected the multivariate model and were not included as this arguably represents a different clinical entity (data not shown).
Time from Symptom Onset until ECG Diagnosis
Median (IQR) from symptom onset until ECG diagnosis was 90 (163) minutes and differed significantly between those younger than 80 years of age (median 90 minutes) and older (median 115 minutes), P value <0.0001. Cox PH modeling showed a linear relationship between symptoms‐to‐ECG delay in minutes and log‐transformed mortality with a HR (95% CI) of 1.10 (1.04–1.17) per quartile, P value 0.002. Data were reported in text only.
Time from ECG Diagnosis to Sheath Insertion
ECG‐2‐sheath and the relationship with mortality are presented in Table 3 and Figure 4. ECG‐2‐sheath time was significantly associated with mortality with HR: 1.012 (1.008–1.016, P=0.0001) per 10 minutes which translates into a 3.6% increase in mortality risk per 30 minutes of time from ECG to sheath insertion. This relationship was present irrespectively of patient age including the elderly with similar risk estimates. Per quartile of ECG‐2‐sheath time the HR (95% CI) for mortality increased by 1.21 (1.13–1.29, P‐value <0.0001). For patients younger than 80 years of age HR was 1.24 (1.13–1.34, P‐value <0.0001) and for the elderly 1.11 (1.00–1.24, P value 0.0495), respectively.
Table 3.
Time to Reperfusion
| Cox proportional hazard ratio | Whole population (n=6818) | Aged <80 y (n=5976) | Aged ≥80 y (n=842) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| HR per 10 min increase in ECG‐2‐sheath time (min)* | 1.012 (1.008–1.016) | <0.0001 | 1.013 (1.008–1.017) | <0.0001 | 1.010 (1.003–1.017) | 0.004 |
| HR for ECG‐2‐sheath time≥90 min* | 1.49 (1.28–1.72) | <0.0001 | 1.52 (1.26–1.83) | <0.0001 | 1.31 (1.04–1.66) | 0.02 |
| HR for ECG‐2‐sheath time≥120 min* | 1.73 (1.47–2.04) | <0.0001 | 1.93 (1.5–2.37) | <0.0001 | 1.32 (1.01–1.74) | 0.04 |
| Restricted mean survival time† | Days lost (95% CI) | P value | Days lost (95% CI) | P value | Days lost (95% CI) | P value |
|---|---|---|---|---|---|---|
| ECG‐2‐sheath time >90 min | 35 (23–48) | <0.0001 | 24 (12–35) | <0.0001 | 76 (23–128) | 0.005 |
| ECG‐2‐sheath time≥120 min | 54 (36–72) | <0.0001 | 46 (29–63) | <0.0001 | 71 (5–137) | 0.04 |
ECG‐2‐sheath indicates ECG diagnosis to sheath insertion; and HR, hazard ratio.
Sex‐adjusted hazard ratio estimates.
Truncation time was set to median follow‐up (938 days) to ensure stability of Kaplan–Meier estimates.
Figure 4. Smoothed spline Cox proportional hazard model of log‐transformed hazard ratio for mortality (y‐axis) according to time from ECG‐2‐sheath insertion (x‐axis).
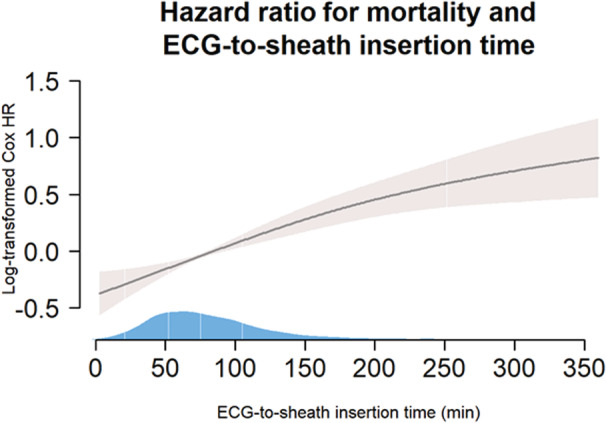
Gray shaded area is the 95% CI and the blue shaded area over the x‐axis the population density according to age, white lines denote the following percentiles from left to right: 2.5, 25, 50, and 75 (95th percentile not visible). HR indicates hazard ratio.
Survival in different age groups are presented in Table 3. When time from ECG‐diagnosis until sheath insertion was more than 90 and 120 minutes, the HR (95% CI) was 1.49 (1.28–1.72, P value <0.0001) and 1.73 (1.47–2.04, P value <0.0001), respectively.
The difference in restricted mean survival at the median follow‐up of 938 days showed that patients in the <90 minutes group had mean 35 days increased survival (P value <0.0001). For patients younger than 80 years, this difference was 24 days (P value <0.0001) while it was 76 days in the elderly (P value 0.005) (Figure 5). Estimates for sheath insertion more than 120 minutes after ECG diagnosis where 54 days lost (P value <0.0001) for the whole population and 46 days (P value <0.0001) for patients <80 years of age and 71 days (P value 0.04) for patients ≥80 years of age.
Figure 5. Restricted mean survival gain at the median follow‐up time of 938 days according to age and time from ECG to treatment in octogenarians compared with the younger population.
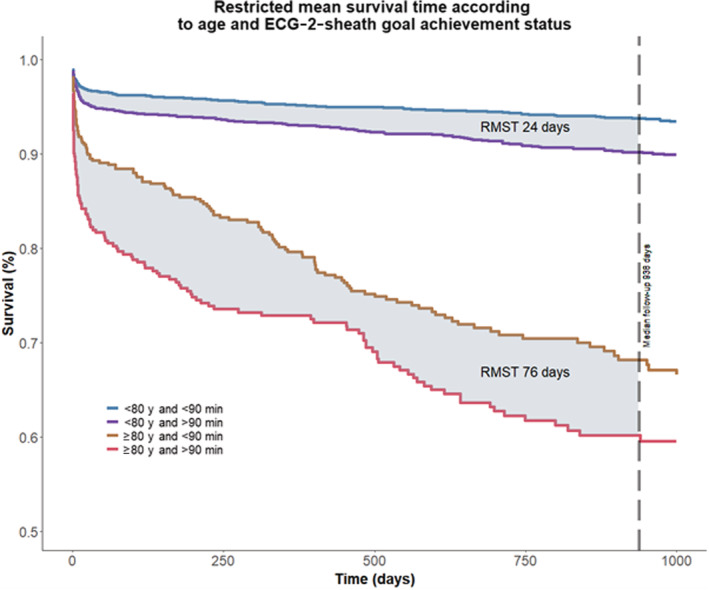
ECG‐2‐sheath indicates ECG diagnosis to sheath insertion; and RMST, restricted mean survival time.
Using achievement of time goal (<90 minutes) in the elderly ≥80 years of age and survival at 30 days in a 2×2 table shown in Tables S1 through S4, survival in patients with valid ECG‐2‐sheath time was 10.7% and 17.9% for <90 or ≥90 minutes, respectively (Chi‐square test, P value 0.0001). The number of patients, achieving the ECG‐2‐sheath time goal of less than 90 min, needed to prevent onedeath at 30 days, was 39 in the whole population and 14 in the elderly.
Data adjusted for comorbidities etc. using Cox PH model estimating hazard ratio and associated 95% CI are shown in Table S2.
Additional time points and intervals have been added in Table S3. Secondary analyses using Cox PH modeling of time to mortality according to door‐to‐flow time shown in Table S4.
Discussion
The main findings in this report are that both symptom to ECG and ECG‐2‐sheath time was significant longer in the elderly (≥80 years) than in the younger patients. Furthermore, time from ECG‐diagnosis to procedural start is strongly related to outcomes both in the general population, as well as in the elderly >80 years of age. Additionally, ECG‐2‐sheath time >90 minutes was associated with an even worse prognosis in the elderly aged ≥80 years compared with those <80 years confirming that achievement of guideline recommended time goals <90 minutes is associated with a major survival benefit, especially in the octogenarians.
The clinical characteristics with increased number of risk factors in the elderly ≥80 years of age are natural consequences of the aging process. Additionally, procedural characteristics with fewer patients being fully revascularized is also dependent on a higher relative number of patients with 3‐vessel disease.
Although ischemic heart disease develops on average 7 through 10 years later in women compared with men, acute myocardial infarction remains a leading cause of death also in women. Despite improved outcome for both women and men with an invasive strategy in acute coronary syndrome, 23 women with STEMI are less likely to receive invasive management, revascularization, or preventive medication at discharge. 24 , 25 In line with this, younger age has been associated with higher 30‐day mortality rates in women with STEMI even after adjustment for medications, primary PCI, and other coexisting comorbidities. This difference declines after age 60 and is no longer observed in the oldest women. 26 Thus, the results of the current study that show that although women have higher in‐hospital mortality than men, female sex itself is not an independent risk factor for in‐hospital mortality. 27 This is in accordance with a recent report from the Zwolle Myocardial Infarction Study Group showing that differences in mortality between men and women with STEMI treated with pPCI are age‐dependent. 28
Despite adjustment for known risk factors, age thus remains as the variable with the strongest risk prediction value in multivariate Cox modeling. In addition to previous ischemic heart disease, diabetes, Killip class, creatinine, hemoglobin, troponin on admission, symptom‐to‐balloon‐time and left ventricular ejection fraction, − age ‐ per se has been shown to be a predictor of both 1‐year mortality and hospitalization for heart failure. 29 In elderly patients with STEMI and multivessel disease, multivessel PCI is associated with better outcomes especially after staged procedures. 30 However, despite more favorable baseline characteristics, elderly patients with STEMI have worse survival and are at higher risk of stroke compared with patients with non–ST‐segment–elevation acute coronary syndromeafter PCI. 31 , 32 Heart failure on admission and previous coronary artery disease have in addition to age, been shown to be prognostic variables. This is in line with the increased mortality associated with diabetes and reduced left ventricular ejection fraction in the current study.
Time from Symptom Onset until ECG‐Diagnosis
Median time from symptom onset until ECG diagnosis differed significantly between those <80 years of age with additional 25 minutes in the elderly (shown in text in Results section). This in accordance with a recent published study in elderly patients treated with reperfusion therapy for STEMI. 33 Since patient‐delay is also related to mortality—albeit weaker than for door‐to‐balloon delay, some of the difference in mortality could be attributable to delayed time from symptoms to ECG. This delay might be explained by atypical presentation, 34 cognitive status, 35 or earlier time to death among older adults. 36 However, using other forms of first medical contact than the organized emergency medical service is also associated increased time delay to diagnosis is verified. 37 Intense measures must therefore be taken to educate the public about the prognostic importance of an early and correct first action with an exclusive use of the emergency medical service when suspecting a myocardial infarction. Any effort must thus be made to keep the respective time intervals between the onset of symptoms and the beginning of reperfusion therapy as short as possible. On the other hand, there have been some unintended consequences of trying to reduce door‐to‐balloon time, as many as one third of activations of STEMI teams are now false alarms. 38
Time to Reperfusion
The goal of reperfusion therapy is to restore blood flow to ischemic, but still viable, myocardium and reduce infarct size in a timely fashion, reducing the time to treatment and maximizing myocardial salvage—in keeping with the mantra that “time is muscle”. Contemporary guidelines explicitly list maximal tolerated transfer delays to be shorter than 120 minutes, or even 60 minutes in cases where the patient first presents directly to a PCI‐capable hospital or when presenting within 2 hours after symptom onset. 1 Maximum time from first medical contact to ECG diagnosis should be <10 minutes. If reperfusion with PCI can be performed within 120 minutes pPCI is the choice of strategy. In patients admitted directly to PCI hospitals this should be <60 minutes, and in transferred patients <90 minutes.
The ESC guideline recommended time limit of 120 minutes from ECG diagnoses until wire‐crossing, was not logged in NORIC until recently. Time of ECG is defined as the time of recording. However, ECG‐2‐sheath insertion time is a good surrogate for time to wire crossing in well‐established centers with experienced operators where sheath‐to‐wire crossing time is low and comparable with the time from ECG recording to established STEMI diagnosis.
In the current report 80% of the patients received pPCI within 115 minutes, with the longest delays in north of rural Norway. This is a quality indicator of a well‐functioning pPCI network. 34
In a recent study with 2823 patients admitted for pPCI for STEMI, a longer symptom‐to‐balloon time ≥180 minutes was the only component associated with higher in‐hospital major adverse cardiovascular and cerebrovascular events in the present study. 39 Previously, the issue of interest was the door‐to‐balloon time. However, although door‐to‐balloon times have improved significantly for patients undergoing primary PCI for STEMI, in‐hospital mortality has remained virtually unchanged. 40 This underlines that the whole chain of treatment from symptoms to reperfusion should be in focus, justifying a time from prehospital ECG to wire crossing of <90 minutes as the most important prognostic factor, as demonstrated in the current study, also in the elderly (aged ≥ 80 years) where it is associated with an average survival benefit of 76 days at 2.5 years of follow‐up.
Strength and Limitations
Our study has several limitations including a selected population of patients with STEMI, who survived until hospital admission. We excluded both false scrambling of catheterization laboratory as well as patients who were dead at admission from the analyses. Only patients with a valid Norwegian national identity number were subject to this analysis, ie, excluding tourists and patients not entered correctly in the system. While NORIC has >99% coverage nationally, not all variables are complete.
On the other hand, a >99% national coverage of all pPCI procedures and use of the National Population Register, which includes all deaths should reassure robust data with generalizability. Data are entered into NORIC by the actual PCI‐operator (usually) the same day. The technical aspects and variables of the procedure and time delays are thus more likely to be correct than if data were entered by nonphysician personnel using the electronic patient record system at a later time point.
Data are not externally monitored. However, data are continuously validated using automatic internal logical algorithms. In studies using general registries and medical records coverage might be low, but this is not the case in NORIC.
The use of “ECG‐2‐sheath insertion” is not a standard time metric for studies evaluating primary PCI. However, we used this time metric and not the other time intervals because this is the most robust representation of the true pre‐ and intrahospital logistical chain. The time of first balloon inflation is not logged in NORIC. Total ischemic time depends on both accurate time dating of symptom onset, which is subject to several challenges including, but not limited to recollection bias, patient sedation and agitation, crescendo or stuttering chest pain onset etc. as well as established flow. In cases with thrombolysis in myocardial infarction 3 flow at angiography, this time variable is set to “missing” by default. A minor part of the patients admitted with STEMI was not treated with PCI.
Most patients have thrombolysis in myocardial infarction (TIMI) flow 0, but some patients had TIMI 1‐2 as usual in STEMI registries and studies. In addition, many patients continue to experience chest pain and ST‐segment elevation after reestablished thrombolysis in myocardial infarction 3 flow, eg, due to microvascular obstruction, intramyocardial hemorrhage etc. When considering these points we concluded that time to flow was not robust. In addition, the degree of flow is subject to inter‐observer variation in scoring.
The time point for insertion of the vascular sheath is more objective, happens in all patients, noted by the nurses on in‐laboratory case forms and presumably has more at‐random distribution of missing data. Also, considering that all ECGs are automatically timestamped on recording we would argue that ECG‐2‐sheath insertion is more robust and more accurately describes the logistical chain. The degree of missing data is not materially different between the different time intervals (Table S3). In addition, median time from sheath insertion to flow is 11 minutes, arguably close to the speculated time from ECG‐recording to STEMI‐diagnosis by hospital physician after electronic transfer which in essence equates this interval with the ESC guideline recommended ECG‐diagnosis‐to‐wire crossing.
Conclusions
Patient age at admission, but not sex adjusted for age, was a strong predictor for mortality in patients undergoing pPCI for STEMI. Time from ECG‐diagnosis to sheath insertion was strongly correlated with mortality. Achievement of guideline recommended time goals <90 minutes for time from ECG to pPCI was associated with a major survival benefit, especially in the octogenarians.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S4
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.024849
For Sources of Funding and Disclosures, see page 10.
References
- 1. Kalla K, Christ G, Karnik R, Malzer R, Norman G, Prachar H, Schreiber W, Unger G, Glogar HD, Kaff A, et al. Implementation of guidelines improves the standard of care: the Viennese registry on reperfusion strategies in ST‐elevation myocardial infarction (Vienna STEMI registry). Circulation. 2006;113:2398–2405. doi: 10.1161/CIRCULATIONAHA.105.586198 [DOI] [PubMed] [Google Scholar]
- 2. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. ESC scientific document group. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 3. Krumholz HM, Wang Y, Chen J, Drye EE, Spertus JA, Ross JS, Curtis JP, Nallamothu BK, Lichtman JH, Havranek EP, et al. Reduction in acute myocardial infarction mortality in the United States: risk‐standardized mortality rates from 1995–2006. JAMA. 2009;302:767–773. doi: 10.1001/jama.2009.1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7 [DOI] [PubMed] [Google Scholar]
- 5. Jernberg T, Johanson P, Held C, Svennblad B, Lindbäck J, Wallentin L, SWEDEHEART/RIKS‐HIA . Association between adoption of evidence‐based treatment and survival for patients with ST‐elevation myocardial infarction. JAMA. 2011;305:1677–1684. doi: 10.1001/jama.2011.522 [DOI] [PubMed] [Google Scholar]
- 6. Bradley EH, Herrin J, Elbel B, McNamara RL, Magid DJ, Nallamothu BK, Wang Y, Normand SL, Spertus JA, Krumholz HM. Hospital quality for acute myocardial infarction: Correlation among process measures and relationship with short‐term mortality. JAMA. 2006;296:72–78. doi: 10.1001/jama.296.1.72 [DOI] [PubMed] [Google Scholar]
- 7. Sinnaeve P, Van de Werf F. Primary PCI and the indistinct 120 min time limit. Eur Heart J. 2020;41:867–869. doi: 10.1093/eurheartj/ehz755 [DOI] [PubMed] [Google Scholar]
- 8. Larsen AI, Melberg TH, Bonarjee V, Barvik S, Nilsen DW. Change to a primary PCI program increases number of patients offered reperfusion therapy and significantly reduces mortality: a real‐life experience evaluating the initiation of a primary PCI service at a single center without on‐site heart surgery in Western Norway. Int J Cardiol. 2008;127:208–213. doi: 10.1016/j.ijcard.2007.05.118 [DOI] [PubMed] [Google Scholar]
- 9. Terkelsen CJ, Sørensen JT, Maeng M, Jensen LO, Tilsted HH, Trautner S, Vach W, Johnsen SP, Thuesen L, Lassen JF. System delay and mortality among patients with STEMI treated with primary percutaneous coronary intervention. JAMA. 2010;304:763–771. doi: 10.1001/jama.2010.1139 [DOI] [PubMed] [Google Scholar]
- 10. Kumbhani DJ, Cannon CP, Fonarow GC, Liang L, Askari AT, Peacock WF, Peterson ED, Bhatt DL. Get with the guidelines steering committee and investigators. Association of hospital primary angioplasty volume in ST‐segment elevation myocardial infarction with quality and outcomes. JAMA. 2009;302:2207–2213. doi: 10.1001/jama.2009.1715 [DOI] [PubMed] [Google Scholar]
- 11. Murphy NF, MacIntyre K, Stewart S, Capewell S, McMurray JJ. Reduced between‐hospital variation in short term survival after acute myocardial infarction: the result of improved cardiac care? Heart. 2005;91:726–730. doi: 10.1136/hrt.2004.042929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lambert L1, Brown K, Segal E, Brophy J, Rodes‐Cabau J, Bogaty P. Association between timeliness of reperfusion therapy and clinical outcomes in ST‐elevation myocardial infarction. JAMA. 2010;303:2148–2155. doi: 10.1001/jama.2010.712 [DOI] [PubMed] [Google Scholar]
- 13. Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, et al. Relationship between infarct size and outcomes following primary PCI: patient‐level analysis from 10 randomized trials. J Am Coll Cardiol. 2016;67:1674–1683. doi: 10.1016/j.jacc.2016.01.069 [DOI] [PubMed] [Google Scholar]
- 14. Eagle KA, Goodman SG, Avezum A, Budaj A, Sullivan CM, López‐Sendón J, for the GRACE Investigators . Practice variation and missed opportunities for reperfusion in ST‐segment‐elevation myocardial infarction: Findings from the global registry of acute coronary events (GRACE). Lancet. 2002;359:373–377. doi: 10.1016/S0140-6736(02)07595-5 [DOI] [PubMed] [Google Scholar]
- 15. Kociol RD, Lopes RD, Clare R, Thomas L, Mehta RH, Kaul P, Pieper KS, Hochman JS, Weaver WD, Armstrong PW, et al. International variation in and factors associated with hospital readmission after myocardial infarction. JAMA. 2012;307:66–74. doi: 10.1001/jama.2011.1926 [DOI] [PubMed] [Google Scholar]
- 16. Schiele F, Hochadel M, Tubaro M, Meneveau N, Wojakowski W, Gierlotka M, Polonski L, Bassand JP, Fox KA, Gitt AK. Reperfusion strategy in Europe: temporal trends in performance measures for reperfusion therapy in ST‐elevation myocardial infarction. Eur Heart J. 2010;31:2614–2624. doi: 10.1093/eurheartj/ehq305 [DOI] [PubMed] [Google Scholar]
- 17. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6 [DOI] [PubMed] [Google Scholar]
- 18. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST‐elevation myocardial infarction: An update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016;133:1135–1147. doi: 10.1161/CIR.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 19. Kvakkestad KM, Abdelnoor M, Claussen PA, Eritsland J, Fossum E, Halvorsen S. Long‐term survival in octogenarians and older patients with ST‐elevation myocardial infarction in the era of primary angioplasty: a prospective cohort study. Eur Heart J Acute Cardiovasc Care. 2016;5:243–252. doi: 10.1177/2048872615574706 [DOI] [PubMed] [Google Scholar]
- 20. Pascual I, Hernandez‐Vaquero D, Almendarez M, Lorca R, Escalera A, Díaz R, Alperi A, Carnero M, Silva J, Morís C, et al. Observed and expected survival in men and women after suffering a STEMI. J Clin Med. 2020;9:1174. doi: 10.3390/jcm9041174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Website for the Norwegian Cardiovascular Disease Registry . Available at: https://www.fhi.no/en/hn/health‐registries/cardiovascular‐disease‐registry/about‐the‐norwegian‐cardiovascular‐disease‐registry. Accessed August 11, 2022.
- 22. Website for the Norwegian Registry for Invasive Cardiology . Available at: https://helse‐bergen.no/avdelinger/hjarteavdelinga/noric. Accessed August 11, 2022.
- 23. Alfredsson J, Lindbäck J, Wallentin L, Swahn E. Similar outcome with an invasive strategy in men and women with non‐ST‐elevation acute coronary syndromes: from the Swedish web‐system for enhancement and development of evidence‐based Care in Heart Disease Evaluated According to recommended therapies (SWEDEHEART). Eur Heart J. 2011;32:3128–3136. doi: 10.1093/eurheartj/ehr349 [DOI] [PubMed] [Google Scholar]
- 24. DeFilippis EM, Collins BL, Singh A, Biery DW, Fatima A, Qamar A, Berman AN, Gupta A, Cawley M, Wood MJ, et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: the mass general Brigham YOUNG‐MI registry. Eur Heart J. 2020;41:4127–4137. doi: 10.1093/eurheartj/ehaa662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khan E, Brieger D, Amerena J, Atherton JJ, Chew DP, Farshid A. Differences in management and outcomes for men and women with ST‐elevation myocardial infarction. Med J Aust. 2018;209:118–123. doi: 10.5694/mja17.01109 [DOI] [PubMed] [Google Scholar]
- 26. Cenko E, Yoon J, Kedev S, Stankovic G, Vasiljevic Z, Krljanac G, Kalpak O, Ricci B, Milicic D, Manfrini O, et al. Sex differences in outcomes after STEMI: effect modification by treatment strategy and age. JAMA Intern Med. 2018;178:632–639. doi: 10.1001/jamainternmed.2018.0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park JS, Kim YJ, Shin DG, Jeong MH, Ahn YK, Chung WS, Seung KB, Kim CJ, Cho MC, Jang YS, et al. Gender differences in clinical features and in‐hospital outcomes in ST‐segment elevation acute myocardial infarction: from the Korean acute myocardial infarction registry (KAMIR) study. Clin Cardiol. 2010;33:E1–E6. doi: 10.1002/clc.20557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Otten AM, Maas AH, Ottervanger JP, Kloosterman A, van 't Hof AW, Dambrink JH, Gosselink AT, Hoorntje JC, Suryapranata H, de Boer MJ, et al. Is the difference in outcome between men and women treated by primary percutaneous coronary intervention age dependent? Gender difference in STEMI stratified on age. Eur Heart J Acute Cardiovasc Care. 2013;2:334–341. doi: 10.1177/2048872612475270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bulluck H, Zheng H, Chan MY, Foin N, Foo DC, Lee CW, Lim ST, Sahlen A, Tan HC, Tan JW, et al. Independent predictors of cardiac mortality and hospitalization for heart failure in a multi‐ethnic Asian ST‐segment elevation myocardial infarction population treated by primary percutaneous coronary intervention. Sci Rep. 2019;9:10072. doi: 10.1038/s41598-019-46486-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de La Torre Hernandez JM, Gomez Hospital JA, Baz JA, Brugaletta S, Perez de Prado A, Linares JA, Lopez Palop R, Cid B, Garcia Camarero T, Diego A, et al. Multivessel disease in patients over 75years old with ST elevated myocardial infarction. Current management strategies and related clinical outcomes in the ESTROFA MI+75 nation‐wide registry. Cardiovasc Revasc Med. 2018;19:580–588. doi: 10.1016/j.carrev.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 31. Renilla A1, Barreiro M, Barriales V, Torres F, Alvarez P, Lambert JL. Management and risk factors for mortality in very elderly patients with acute myocardial infarction. Geriatr Gerontol Int. 2013;13:146–151. doi: 10.1111/j.1447-0594.2012.00876.x [DOI] [PubMed] [Google Scholar]
- 32. Morici N, Savonitto S, Ferri LA, Grosseto D, Bossi I, Sganzerla P, Tortorella G, Cacucci M, Ferrario M, Crimi G, et al. Outcomes of elderly patients with ST‐elevation or non‐ST‐elevation acute coronary syndrome undergoing percutaneous coronary intervention. Am J Med. 2019;132:209–216. doi: 10.1016/j.amjmed.2018.10.027 [DOI] [PubMed] [Google Scholar]
- 33. Turk J, Fourny M, Yayehd K, Picard N, Ageron FX, Boussat B, Belle L, Vanzetto G, Puymirat E, Labarère J, et al. Age‐related differences in reperfusion therapy and outcomes for ST‐segment elevation myocardial infarction. J Am Geriatr Soc. 2018;66:1325–1331. doi: 10.1111/jgs.15383 [DOI] [PubMed] [Google Scholar]
- 34. Sappa R, Grillo MT, Cinquetti M, Prati G, Spedicato L, Nucifora G, Perkan A, Zanuttini D, Sinagra G, Proclemer A. Short and long‐term outcome in very old patients with ST‐elevation myocardial infarction after primary percutaneous coronary intervention. Int J Cardiol. 2017;249:112–118. doi: 10.1016/j.ijcard.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 35. Guagliumi G, Stone GW, Cox DA, Stuckey T, Tcheng JE, Turco M, Musumeci G, Griffin JJ, Lansky AJ, Mehran R, et al. Outcome in elderly patients undergoing primary coronary intervention for acute myocardial infarction: results from the controlled abciximab and device investigation to lower late angioplasty complications (CADILLAC) trial. Circulation. 2004;110:1598–1604. doi: 10.1161/01.CIR.0000142862.98817.1F [DOI] [PubMed] [Google Scholar]
- 36. Newell MC, Henry JT, Henry TD, Duval S, Browning JA, Christiansen EC, Larson DM, Berger AK. Impact of age on treatment and outcomes in ST‐elevation myocardial infarction. Am Heart J. 2011;161:664–672. doi: 10.1016/j.ahj.2010.12.018 [DOI] [PubMed] [Google Scholar]
- 37. Thylén I, Ericsson M, Hellström Ängerud K, Isaksson RM, Sederholm Lawesson S, SymTime study group . First medical contact in patients with STEMI and its impact on time to diagnosis; an explorative cross‐sectional study. BMJ Open. 2015;5:e007059. doi: 10.1136/bmjopen-2014-007059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bates E, Jacobs AK. Time to treatment in patients with STEMI. N Engl J Med. 369(10):889–892. doi: 10.1056/NEJMp1308772 [DOI] [PubMed] [Google Scholar]
- 39. Nozari Y, Geraiely B, Alipasandi K, Mortazavi SH, Omidi N, Aghajani H, Amirzadegan A, Pourhoseini H, Salarifar M, Alidoosti M, et al. Time to treatment and in‐Hospital major adverse cardiac events among patients with ST‐segment elevation myocardial infarction who underwent primary percutaneous coronary intervention (PCI) according to the 24/7 primary PCI Service registry in Iran: cross‐sectional study. Interact J Med Res. 2020;9:e20352. doi: 10.2196/20352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Menees DS, Peterson ED, Wang Y, Curtis JP, Messenger JC, Rumsfeld JS, Gurm HS. Door‐to‐balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;5(369):901–909. doi: 10.1056/NEJMoa1208200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


