Abstract
Background
Understanding the magnitude of cardiovascular disease (CVD) inequalities is the first step toward addressing them. The linkage of socioeconomic and clinical data in universal health care settings provides critical information to characterize CVD inequalities.
Methods and Results
We employed a prospective cohort design using electronic health records data from all residents of Catalonia aged 18+ between January and December of 2019 (N=6 332 228). We calculated age‐adjusted sex‐specific prevalence of 5 CVD risk factors (diabetes, hypertension, hyperlipidemia, obesity, and smoking), and 4 CVDs (coronary heart disease, cerebrovascular disease, atrial fibrillation, and heart failure). We categorized income into high, moderate, low, and very low according to individual income (tied to prescription copayments) and receipt of welfare support. We found large inequalities in CVD and CVD risk factors among men and women. CVD risk factors with the largest inequalities were diabetes, smoking, and obesity, with prevalence rates 2‐ or 3‐fold higher for those with very low (versus high) income. CVDs with the largest inequalities were cerebrovascular disease and heart failure, with prevalence rates 2 to 4 times higher for men and women with very low (versus high) income. Inequalities varied by age, peaking at midlife (30–50 years) for most diseases, while decreasing gradually with age for smoking.
Conclusions
We found wide and heterogeneous inequalities by income in 5 CVD risk factors and 4 CVD. Our findings in a region with a high‐quality public health care system and universal coverage stress that strong equity‐promoting policies are necessary to reduce disparities in CVD.
Keywords: cardiovascular disease, health disparities, health equity, income, socioeconomic status
Subject Categories: Hypertension, Cardiovascular Disease, Epidemiology
Nonstandard Abbreviations and Acronyms
- CCHS
Catalan Health Surveillance System of the Government of Catalonia
- RII
relative index of inequality
- SII
slope index of inequality
Clinical Perspective.
What Is New?
There are large socioeconomic status inequalities in cardiovascular disease and associated risk factors in a population with universal access to health care.
Low‐income men were 2 to 4 times more likely to have cerebrovascular disease and heart failure than their high‐income counterparts; low‐income men and women were 3 to 4 times more likely to have diabetes compared with their high‐income counterparts.
For most conditions, inequalities were widest among midlife adults (30–50 years).
What Are the Clinical Implications?
Clinical interventions alone are not likely to reduce these disparities.
Although universal access to health care is important, it falls short of eliminating cardiovascular disease inequalities.
A combination of population‐based and targeted equity‐promoting policies is necessary to address the root causes of these inequalities.
Inequalities in cardiovascular disease (CVD) mortality have been documented in many countries. 1 , 2 , 3 Differences in socioeconomic status (SES), measured as level of education, income, and occupation, play a large role in the distribution of CVD risk and mortality, with a number of studies showing a higher prevalence of CVD in low‐SES (versus high‐SES) populations. 4 , 5 , 6 Lower SES groups are more likely to be exposed to risk factors and less likely to access timely and high‐quality health care. However, inequality in CVD varies across countries and is present even in those with universal health care. 7 , 8 , 9
In countries with universal health care, the pathway between SES and access to health care may be a less important contributor to health inequities, given universal access to health care services, regardless of their ability to pay. For example, Catalonia has a single payer universal public health care system, with almost complete coverage for all residents: health care services are free at the point of delivery except for drug prescriptions, which have a copay calculated according to individual income. 10 However, ever under these conditions, we have previously shown wide socioeconomic disparities in life expectancy in the overall population 11 of Catalonia and in its population with heart failure. 12 Inequalities in CVD and CVD risk may persist if other pathways remain important to disease causation. In fact, the theory on the fundamental causes of diseases posits that social inequalities may be replicated via new pathways and that only a comprehensive set of policies addressing the social determinants of health can generate meaningful change in health inequalities. 13
To better understand the patterns leading to inequalities in mortality in a context with universal health care, we studied inequalities in CVD, the most common cause of death in the region. 14 , 15 Specifically, the objective of this study is to examine inequalities by income in the prevalence of 5 CVD risk factors (diabetes, hypertension, hyperlipidemia, obesity, and smoking), and 4 CVDs (coronary heart disease, cerebrovascular disease, atrial fibrillation, and heart failure), in the entire adult population of Catalonia by leveraging exhaustive local databases that link demographic, socioeconomic, and clinical data.
METHODS
Study Design and Data Source
This is a prospective cohort study using data from the Catalan Health Surveillance System of the Government of Catalonia (CCHS). The CCHS is an electronic health records (EHR) system, where each resident of Catalonia is assigned a unique personal identification number, which can be used to track use of health care services by each individual. The CCHS data set contains individual‐level demographic and clinical data from more than 6 million adults (aged 18+) who are residents of Catalonia. The CCHS also collects data on categorized annual individual income and receipt of welfare support from the Catalan government, which is used to calculate copayments for drug prescriptions. Importantly, although all residents are included in the CCHS data set, visits and diagnoses received in private clinics are not captured in these data, but such diagnoses may be recorded later in the EHR, especially to be able to obtain prescriptions in the publicly funded system. More details on the CCHS have been published in previous studies. 11 , 12 , 16 Because of the sensitive nature of the data used in this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Catalan Health System (CatSalut).
Exposure
We used data on the categories of income and receipt of welfare support used to determine copayments, which include the following 4 groups: (1) high income defined as individuals with annual income higher than 100 000 euros, (2) moderate income defined as individuals with income between 18 000 and 100 000 euros, (3) low income defined as individuals with income <18 000 euros, and (4) very low income defined as those receiving welfare support from the government. Although these groups are broad, further disaggregation is not possible as these are the thresholds defined by law to assign copayments for prescriptions, and linked income data are available only using these categories. Nonetheless, we have previously found this categorization to be highly predictive of life expectancy in people with and without CVD. 11 , 12 We also had data on sex (men/women) and age (18–19, 20–24, 25–29… 80+).
Outcomes
We examined 5 CVD risk factors: diabetes, hypertension, hyperlipidemia, obesity, and smoking; and 4 cardiovascular diseases: coronary heart disease, cerebrovascular disease, atrial fibrillation, and heart failure. These outcomes were defined according to International Classification of Diseases, Ninth Revision (ICD‐9), recorded in the CCHS data set. To calculate prevalences, we used diagnosis codes from health care encounters starting in 2011. Until 2017, health care services used a mix of classifications, including ICD‐9 and International Classification of Diseases, Tenth Revision (ICD‐10). For this study, all data were recoded to ICD‐9. Table S1 contains the specific codes used for each disease. Individuals were classified as a prevalent case if they had a diagnosis code (for a given risk factor or disease) by December 31, 2019. For secondary analysis, we also classified individuals as a newly diagnosed case if they were free of a given risk factor or disease by January 1, 2019, and then had a diagnosis code for that same risk factor or disease by December 31, 2019. Because newly diagnosed cases may not fully represent incident cases owing to length‐time bias, we focus most results on the analysis of prevalence but present incidence results in Figures S1 and S2.
Statistical Analysis
The main objective of this analysis was to examine socioeconomic inequalities in 5 cardiovascular risk factors and 4 cardiovascular diseases. We conducted our analyses in 3 steps. First, we calculated sex‐ and age‐specific prevalence of 5 CVD risk factors and 4 CVDs stratified by income (high, medium, low, and very low). Prevalence was calculated for each age (in the categories outlined previously), sex, and income group using the number of existing cases as of December 31, 2019, and the total population in the group as the denominator. We then calculated age‐adjusted rates using the direct method of standardization and the 2000 to 2025 World Health Organization World Standard Population as the referent population. 17 We plotted prevalences by sex and income group and compared rates across all outcomes.
Second, we computed 2 indices of inequality: the relative index of inequality (RII) and the slope index of inequality (SII). Both are measures that provide a description of the linear association between an ordinal or continuous SES indicator (in our case, income) and an outcome. The SII provides an absolute measure of inequality while the RII provides a relative measure. 18 To estimate the RII while accounting for age, we followed the approach by Moreno‐Betancur et al. 19 and fitted an overdispersed Poisson model, where each row is an age‐income group and where we model the counts of prevalent cases in each age‐income group with an offset for the population of that age‐income group. The model includes fixed effects for age categories and income as an ordinal variable. The exponentiated coefficient for income represents the ratio between the bottom and top of the hierarchy of income. To estimate the age‐adjusted SII, we followed the same approach by Moreno‐Betancur et al., 19 using an additive overdispersed Poisson model, where we obtained the SII for age category and then obtained a weighted sum of these SIIs, weighted using the 2000 to 2025 World Health Organization World Standard Population. For both the RII and SII calculations and to maximize the likelihood of model convergence, we pooled age categories into <40, 40 to 49, 50 to 59, 60 to 69, 70 to 79, and 80+.
Third and last, we repeated the RII models adding an interaction of income with age in order to estimate how these relative inequalities varied by age. In this model, age was operationalized as continuous (representing the midpoint of each age group), and introduced using linear, quadratic, and cubic polynomials, along with an interaction with income, to allow for flexibility in the modeling of the RII by age. We then used a linear combination of coefficients to calculate the predicted RII across ages. To avoid instability in coefficients, we show ages only where the sex/outcome combination had at least 5 cases of the outcome in each income group.
We performed 2 sets of sensitivity analyses. First, we repeated the calculation of age‐adjusted prevalences and the RII using newly diagnosed cases (as a proxy for incidence). For this, 1‐year cumulative incidence was calculated for each age, sex, and income group using new cases between January 1, 2019 and December 31, 2019, and the population free of the risk factor or disease by January 1, 2019 as the denominator in each income group. Age‐adjusted rates of newly diagnosed cases were then calculated using the 2000 to 2025 World Health Organization World Standard Population. The second sensitivity analysis aims to account for a potential underestimation of diagnosis among individuals who use private health care and whose diagnoses may not be captured in these data. For this, we conducted a sensitivity analysis including (in numerators and denominators) exclusively users of the public health care system, defined as those who have used primary health care, emergency room, specialty care or have been hospitalized, all in the publicly funded system at any point in 2019.
All analyses were conducted in R, version 4.0.1. Overdispersed Poisson models were fitted using the package glm2. This study was approved by the ethics committee of the Institut d'Investigació Biomédica de Bellvitge and conformed to the Declaration of Helsinki. Data were deidentified, thus informed consent was not obtained.
RESULTS
Study Population
A total of 6 332 228 individuals were included in this analysis (Table 1). The majority were in the low‐income group (61.0%), followed by moderate income (34.6%), with 3.3% and 1.1% in the very‐low‐ and high‐income groups, respectively. Women and younger adults were disproportionally represented in the low‐ and very‐low‐income groups. Among all residents, 15% were foreign born, with nearly half of those being from low‐income countries, 51% were actively employed, and 7% were receiving unemployment subsidies. Foreign‐born residents as well as those receiving unemployment benefits were disproportionally represented in the low‐ and very‐low‐income groups. High‐income individuals were less likely to be users of the public health care system (Table 1). Overall, in December 2019, 9.3% of the adult Catalan population had diabetes, 24.6% had hypertension, 20.6% had hyperlipidemia, 18.5% had obesity, and 20.9% of the population smoked. We also found prevalence rates of 3.8%, 3.8%, 3.3%, and 2.5% for ischemic heart disease, cerebrovascular disease, atrial fibrillation, and heart failure, respectively (Table 1).
Table 1.
Descriptive Table of Population Demographics, Cardiovascular Disease Risk Factors and Diseases by Income Group
| All | High | Moderate | Low | Very low | |
|---|---|---|---|---|---|
| N=6 262 290 | 70 487 (1.13%) | 2 164 781 (34.6%) | 3 820 804 (61.0%) | 206 218 (3.29%) | |
| Sex | |||||
| Men | 48.5% | 64.1% | 55.1% | 45.0% | 39.8% |
| Women | 51.5% | 35.9% | 44.9% | 55.0% | 60.2% |
| Age, y, mean (SD) and % | 50.0 (18.4) | 53.3 (15.4) | 51.5 (15.9) | 49.0 (19.8) | 51.2 (16.1) |
| <45 | 42.0 | 23.3 | 35.6 | 46.4 | 33.6 |
| 45–64 | 34.7 | 55.3 | 42.2 | 29.5 | 46.3 |
| 65–74 | 11.8 | 13.9 | 13.6 | 10.7 | 12.1 |
| 75–84 | 7.56 | 5.41 | 6.35 | 8.38 | 5.82 |
| >84 | 3.97 | 2.17 | 2.29 | 5.05 | 2.24 |
| Foreign born | 15.2% | 6.47% | 5.00% | 20.8% | 21.7% |
| Foreign ‐born from a low‐income country | 6.90% | 0.18% | 1.12% | 9.91% | 13.9% |
| Actively employed | 51.0% | 69.7% | 64.4% | 45.7% | 2.37% |
| Receiving unemployment subsidies | 7.10% | 2.54% | 2.43% | 7.48% | 50.6% |
| User of the public health care system* | 69.8% | 41.4% | 64.8% | 72.6% | 79.5% |
| Cardiovascular risk factors—unadjusted prevalence | |||||
| Diabetes | 9.33% | 5.46% | 7.73% | 10.1% | 13.4% |
| Hypertension | 24.6% | 18.9% | 22.9% | 25.5% | 28.3% |
| Hyperlipidemia | 20.6% | 15.9% | 19.9% | 20.9% | 24.0% |
| Obesity | 18.5% | 7.73% | 15.2% | 20.1% | 28.0% |
| Smoking | 20.9% | 10.7% | 19.9% | 21.0% | 32.8% |
| Cardiovascular diseases—unadjusted prevalence | |||||
| Ischemic heart disease | 3.84% | 3.41% | 3.48% | 4.03% | 4.28% |
| Cerebrovascular disease | 3.79% | 2.37% | 2.97% | 4.21% | 5.08% |
| Atrial fibrillation | 3.30% | 2.62% | 2.76% | 3.64% | 2.92% |
| Heart failure | 2.46% | 0.90% | 1.47% | 3.01% | 3.03% |
Defined as those who have used primary health care, emergency room, or specialty care or have been hospitalized, all in the publicly funded system at any point in 2019.
Inequalities by Income in Cardiovascular Risk Factors and Diseases
Age‐adjusted prevalence rates for the 5 CVD risk factors showed large income‐based inequalities for both men and women (Figure 1), with higher rates for the lower income groups, and a gradual decrease in prevalence with higher income categories. Men and women of high income had prevalence rates for diabetes of 3.8% and 2.2%, respectively whereas rates were almost 3 to 4 times higher in men and women of very low income. For hypertension and hyperlipidemia, we observed higher prevalence rates but relatively smaller gaps between the income groups, particularly for men. For obesity, we observed very wide inequalities, particularly for women, with women of very low income having almost 6 times higher prevalence of obesity than women of high income. Finally, smoking prevalence for very‐low‐income men was more than 4 times higher than for men of high income (Figure 1). We also observed similarly wide inequalities in the age‐adjusted prevalences of the 4 cardiovascular diseases (Figure 2). Specifically, the prevalence of ischemic heart disease, cerebrovascular disease, and heart failure among men of very low income was 1.6, 2.4, and 4.2 times higher than among men of high SES. These inequalities were also wide among women, with rates 2.5, 2.5, and 3.4, higher for very‐low‐income women compared with high‐income women. We did not observe inequalities in the prevalence of atrial fibrillation among men, as the prevalence was similar across income groups, but did observe a social gradient in women (Figure 2).
Figure 1. Age‐adjusted prevalence of 5 cardiovascular disease risk factors by sex and income.
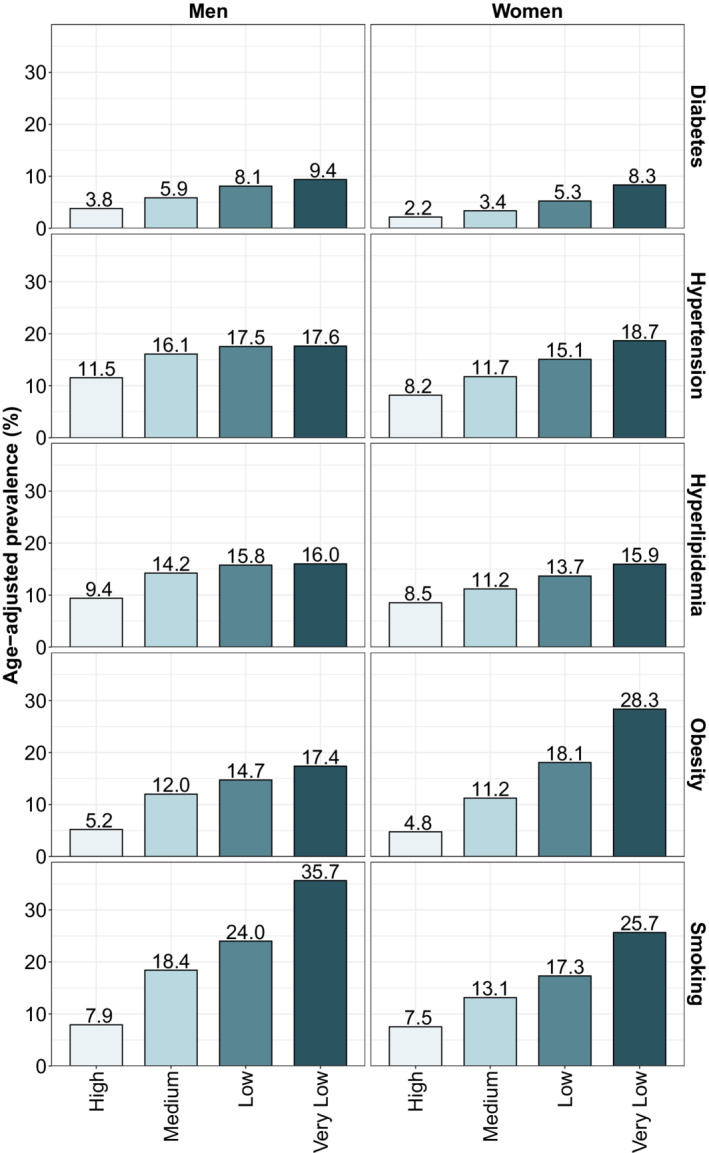
Prevalence was standardized using the direct method of standardization and the 2000 to 2025 World Health Organization's World Standard Population.
Figure 2. Age‐adjusted prevalence of 4 cardiovascular diseases by sex and income.
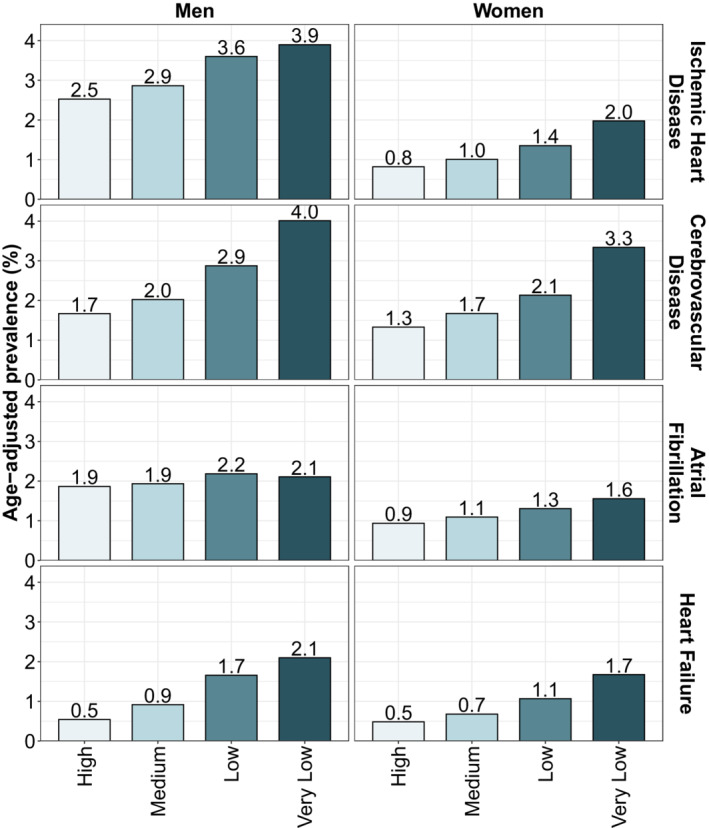
Prevalence was standardized using the direct method of standardization and the 2000 to 2025 World Health Organization's World Standard Population.
Relative and Slope Indices of Inequality by Outcome and Sex
Table 2 shows the age‐adjusted RII and SII for all outcomes, stratified by sex. For risk factors, we found RIIs ranging from 1.28 (hypertension in men) to 3.67 (obesity in women), meaning that the prevalence at the bottom versus top of the income distribution was between 1.28 (for hypertension in men) and 3.67 (for obesity in women) times higher. In general, we found higher RIIs for women (up to 2 times higher in the case of obesity), except for smoking, where men had a slightly higher RII. For CVD, we found that heart failure had the highest RIIs, at 4.65 and 3.51 for men and women, respectively, whereas atrial fibrillation had the lowest, at 1.36 and 1.66, respectively. We also found wide absolute inequalities measured through the SII. Specifically, inequalities in prevalences at the bottom versus top of the income distribution ranged from 4.3% higher (hyperlipidemia in men) to 21.4% higher (obesity in women). Women had widest absolute inequalities in obesity followed by smoking, and men had widest absolute inequalities in smoking followed by obesity. Results for CVD mirrored those for the RII, although models for heart failure failed to converge.
Table 2.
Relative and Slope Index of Inequality for the Prevalence of 5 Cardiovascular Risk Factors and 4 Cardiovascular Diseases in Men and Women in Catalonia, 2019
| Outcome | Relative index of inequality (95% CI) | Slope index of inequality (95% CI) | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Cardiovascular risk factors | ||||
| Diabetes | 2.38 (1.89; 3.00) | 3.66 (2.93; 4.56) | 6.64 (4.78; 8.50) | 6.10 (4.43; 7.77) |
| Hypertension | 1.28 (1.14; 1.44) | 1.94 (1.66; 2.27) | 4.54 (0.72; 8.37) | 10.47 (8.19; 12.75) |
| Hyperlipidemia | 1.33 (1.15; 1.55) | 1.71 (1.48; 1.97) | 4.30 (0.55; 8.05) | 7.13 (5.63; 8.62) |
| Obesity | 1.87 (1.55; 2.26) | 3.67 (3.02; 4.45) | 9.69 (6.43; 12.95) | 21.4 (18.94; 23.85) |
| Smoking | 2.33 (1.91; 2.85) | 2.14 (1.78; 2.58) | 16.81 (12.3; 21.31) | 11.75 (9.57; 13.94) |
| Cardiovascular diseases | ||||
| Ischemic heart disease | 1.77 (1.48; 2.11) | 2.46 (2.03; 2.99) | 2.08 (1.23; 2.93) | 1.22 (0.75; 1.68) |
| Cerebrovascular disease | 2.75 (2.13; 3.55) | 2.23 (1.79; 2.79) | 2.64 (1.60; 3.68) | 1.72 (1.00; 2.45) |
| Atrial fibrillation | 1.36 (1.19; 1.56) | 1.66 (1.44; 1.91) | 0.73 (0.08; 1.37) | 0.75 (0.36; 1.14) |
| Heart failure | 4.65 (3.20; 6.75) | 3.51 (2.55; 4.82) | N/A | N/A |
All models adjusted by age and stratified by sex. The slope index of inequality is age adjusted using the World Health Organization's 2000 to 2025 World Standard Population. For the RII and the SII the null (references) are 1 and 0, respectively. N/A indicates a model that did not converge (SII for heart failure); RII, relative index of inequality; and SII, slope index of inequality.
Age‐Varying Relative Inequalities
Figure 3 shows how the RIIs for prevalence changed by age for each outcome and sex. For diabetes, hypertension, hyperlipidemia, and obesity, we found that the widest inequalities were observed among midlife adults, ranging from 30 to 50 years, especially in women. For smoking, we found that inequality decreased gradually with age in women, with the widest inequalities in young adults and the narrowest in the elderly, whereas they were stable across ages for men. Ischemic heart disease, cerebrovascular disease and heart failure followed a similar pattern to risk factors, with inequalities peaking at 40 to 50 years of age and declining after that, although we lack data to properly estimate inequalities at younger age groups.
Figure 3. Relative index of inequality for income for the prevalence of 5 cardiovascular disease risk factors and 4 cardiovascular diseases, by age.
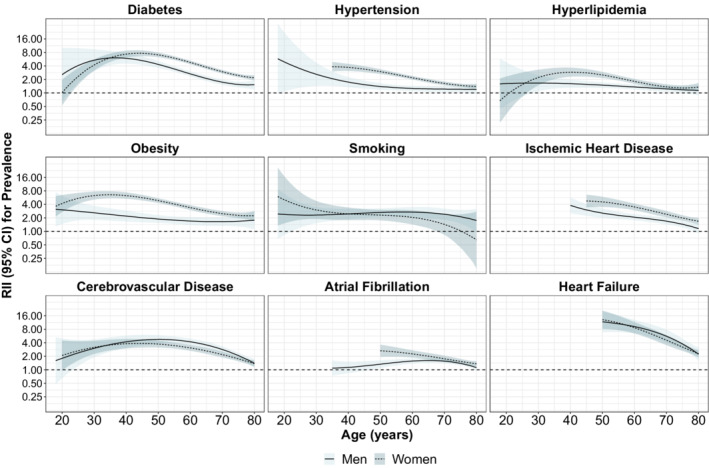
The relative index of inequality is calculated from a model with income (as an ordinal variable), with linear, quadratic and cubic polynomials for age, stratified by sex. We showed ages for which the sex/outcome combination has at least 5 cases in each income group. RII indicates relative index of inequality.
Sensitivity Analyses
Figures S1, S2 and Table S2 show results for the first sensitivity analysis, which uses newly diagnosed cases. Figure S3 compares RIIs for prevalence and newly diagnosed cases. In general, we found very similar patterns of inequality by risk factor, disease, or sex. Figure S4 shows the results of the second sensitivity analysis comparing RII for the full sample versus RII restricted to people who had at least had 1 event of health care use in 2019 in the publicly‐funded system. We found that the RIIs using the full and restricted samples were strongly correlated (Spearman's rho=0.97 and 0.92 for men and women, respectively). However, we also found that the RIIs were narrower in the restricted (versus full) sample, ranging now from 1.03 (hypertension in men) to 1.41 (diabetes in women) for risk factors, and from 1.07 (atrial fibrillation in men) to 1.59 (heart failure in men) for CVD. However, all of them remained statistically significant.
DISCUSSION
In this study of income‐based inequalities in cardiovascular risk factors and disease among more than 6 million adults in Catalonia, we found 4 key results. First, we found a clear social gradient in the prevalence of 5 CVD risk factors and 4 CVDs. In all cases, and after adjusting for age, individuals in lower income groups had a higher prevalences than individuals in the high‐income group. Second, CVD risk factors with the largest inequalities were diabetes, smoking, and obesity with prevalence rates twice or 3 times higher for individuals with very low income (versus those with high income). CVDs with the largest inequalities were cerebrovascular disease and heart failure with prevalence rates 2 to 4 times higher for men and women with very low income (versus those with high income). Third, we found that, in general, women had wider inequalities for diabetes, hypertension, hyperlipidemia, and obesity, and men had wider inequalities for smoking. Moreover, inequalities in atrial fibrillation were much narrower among men than women, whereas inequalities in the other 3 CVDs were similar in magnitude across sexes. Fourth, we found that in most cases, inequalities were widest among midlife adults (30–50 years), except for smoking, for which inequalities were wider among the youngest population.
Our results are broadly consistent with other studies in the region that examined inequalities life expectancy, risk factors for CVD, and CVD conditions. 11 , 12 , 20 In 2016, life expectancy among Catalan low‐income men and women was 12 and 9 years lower than their high‐income counterparts, respectively. 11 Our study indicates that inequalities in CVD risk factors and diseases may be important drivers of these inequalities in life expectancy. A previous study using the Spanish National Health Survey found similar patterns of inequality for CVD risk factors among men and women. 20 In that study, RII values were smaller compared with ours, possibly because of differences in methodology, specifically in the exposures (social class instead of income) and outcomes (self‐reported instead of EHR diagnoses). Overall, our results highlight the importance of monitoring health inequalities even in regions with relatively low income inequality and universal health care system. 21 In this context, low‐income individuals are still exposed to the social determinants of health or structural drivers of cardiovascular disease morbidity and mortality. 22 These social determinants of health include neighborhood and physical environment factors such as walkability and access to recreation, 23 which interact with individual‐level socioeconomic conditions in important ways. 24 , 25 , 26
We found that women had wider inequalities compared with men in most of the outcomes, particularly in diabetes, hypertension, hyperlipidemia, obesity, ischemic heart disease, and atrial fibrillation. Inequality in smoking was wider among men, primarily driven by very high prevalence among men with low and very low SES. This pattern was also consistent with previous studies that showed wider inequalities in CVD risk factors for women except for smoking in Spain. 20 Smoking prevalence and inequality in prevalence continues to be higher among men despite the rising in women‐to‐men smoking ratio in the past few decades. 27 In a previous study examining several decades of smoking prevalence by sex and socioeconomic status, inequalities in smoking were inverted (higher prevalence among higher SES) for older women. 27 Smoking prevalence among women was historically low in Spain until the late 1960s, the last decade under the Franco dictatorship. This was an era of adoption of new social norms that, concurrent with an increase in tobacco advertisement directed toward women, led to an uptake of smoking especially among highly educated women. 27 , 28 However, new dynamics in smoking inequalities in the past few decades have led to an adoption of a pattern similar to that of men, with lower SES women having higher prevalence rates in recent years.
Patterns of inequality by age and sex differed by risk factors/diseases. For example, for diabetes, inequality was wider for women among almost all age groups, whereas for cerebrovascular disease, inequality was wider for men (versus women) between the ages of 30 and 60, and for ischemic heart disease the age curves were shifted with inequality among younger men being wider compared with younger women but wider among older women compared with men. Differences in inequalities among men and women by age indicate a combination of differences in the age of onset of disease and differences in survival across sex and income groups. For example, ischemic heart disease develops earlier in men, in part owing to biological differences 29 but also to higher rates of smoking among men, a gendered behavior. 27 Shifting inequality in ischemic heart disease among men and women across age groups could be the result of low‐income men dying at younger ages, compared with low‐income women and high‐income men, which leads to reductions in the RII for men in older age groups compared with women. Low‐income men may also be more likely to delay seeking care, as indicated by data that show they receive relatively fewer ambulatory‐based care visits but more urgent care and emergency department visits, 12 which may increase their risk of death. Last, the pattern of inequality by age for smoking reflects the aforementioned historical dynamics of adoption of smoking by social class, starting with the highest income and then transitioning to lower income. 27 , 30
Strengths and Limitations
The main limitation of this study is our reliance on EHR and diagnosis as identified on these records. However, this approach is being increasingly used to define the health status of populations where these databases are available. 31 , 32 Estimates from these large data sets may be more valid than self‐reported health status collected via surveys. Previous analysis using CCHS data reached similar findings 16 as those from studies performing detailed phenotyping of participants. 33 We also cannot rule out reverse causality, that is, that individuals with CVD risk factors and especially those with prevalent CVD may be more likely to have a downward SES trend via job loss, 34 although this may be less of an issue in a country with a strong social protection system. The consistency between inequality patterns for prevalence and incidence is reassuring, as the incidence measure is less vulnerable to reverse causality. However, our incident cases may just be newly recorded prevalent cases (eg, someone with undiagnosed diabetes getting diagnosed would count as an incidence case), so we cannot rule out this phenomenon. In addition, we cannot rule out unmeasured confounders in the association between income and CVD outcomes. It is possible that structural factors such as generational poverty and disadvantage, which may lead to present‐day income and poor CVD outcomes, are the true drivers behind the association. 35 However, this study does not aim to make causal arguments but rather provide a description of inequalities in CVD in the context of a universal health care system.
Another limitation of EHR in our case may be differential health care use by income group, which is plausible given higher rates of private health insurance among the wealthier individuals in this setting. 36 Our analysis restricting the sample to individuals who had used the publicly funded system at least once in the past year found narrower inequalities. There may be 2 reasons behind this. First, restricting the sample to only those using the public system most likely excluded people at the extremes of income, those at the top of the income groups who use private clinics, and those at the bottom of the income groups who face other barriers to care. Previous research in Spain has shown that people of higher SES are more likely to use the private system, but that visits to specialists do not differ by SES. 37 Second, restricting the analysis to users of the public system—who may also be more likely to have CVD—may have reduced heterogeneity in the population studied thus resulting in narrower inequalities. Despite that, (1) the inequalities remain significant even after controlling for this differential use, and (2) these differences do not vary by sex and risk factor or disease. Last, our measure of SES is crude, as it includes very wide income bands, with unbalanced groups. However, as this type of measurement error is nondifferential (as decisions about thresholds are exogenous to the prevalence of CVD and its risk factors), our results may be biased toward the null and may be a conservative estimate of actual inequalities. One of the disadvantages of our chosen measures of inequality, the RII and the SII, is the assumption of linearity of the SES‐outcome associations. However, from our results (Figures 1 and 2), it seems that this assumption is plausible. The main strength of our study is the inclusion of more than 6 million adults, representing almost the entire adult population of Catalonia, ruling out concerns about selection bias and generalizability. Our results quantify inequalities in the unique context of Catalonia, a region with universal coverage of health care and strong social protection.
Conclusions
In this study including the whole population of more than 6 million adult residents of Catalonia, a region with universal health coverage, we found large SES inequalities in 9 CVD risk factors and conditions among men and women. The wide inequalities found in this study demonstrates that although universal access to health care is important, it falls short of eliminating CVD inequalities. This finding expands the body of literature that points to the need of strong equity‐promoting policies with the goal of reducing disparities primarily in CVD risk factors.
Sources of Funding
Bilal and Mullachery were supported by the Office of the Director of the National Institutes of Health under award number DP5OD26429. Mullachery is also supported by the Cotswold Postdoctoral Fellowship, award number 284134.
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S4
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026587
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Zhang Y‐B, Chen C, Pan X‐F, Guo J, Li Y, Franco OH, Liu G, Pan A. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ. 2021;373:n604. doi: 10.1136/bmj.n604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang S, Zhai H, Wei L, Shen B, Wang J. Socioeconomic status predicts the risk of stroke death: a systematic review and meta‐analysis. Prev Med Rep. 2020;19:101124. doi: 10.1016/j.pmedr.2020.101124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh GK, Siahpush M, Azuine RE, Williams SD. Increasing area deprivation and socioeconomic inequalities in heart disease, stroke, and cardiovascular disease mortality among working age populations, United States, 1969–2011. Int J MCH AIDS. 2015;3:119–133. [PMC free article] [PubMed] [Google Scholar]
- 4. Kunst AE, Del Rios M, Groenhof F, Mackenbach JP. Socioeconomic inequalities in stroke mortality among middle‐aged men: an international overview. Stroke. 1998;29:2285–2291. doi: 10.1161/01.STR.29.11.2285 [DOI] [PubMed] [Google Scholar]
- 5. Kunst AE, Groenhof F, Andersen O, Borgan JK, Costa G, Desplanques G, Filakti H, Giraldes MR, Faggiano F, Harding S. Occupational class and ischemic heart disease mortality in the United States and 11 European countries. Am J Public Health. 1999;89:47–53. doi: 10.2105/AJPH.89.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Girolamo C, Nusselder WJ, Bopp M, Brønnum‐Hansen H, Costa G, Kovács K, Leinsalu M, Martikainen P, Pacelli B, Valverde JR. Progress in reducing inequalities in cardiovascular disease mortality in Europe. Heart. 2020;106:40–49. doi: 10.1136/heartjnl-2019-315129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Lengerke T, Gohl D, Babitsch B. Re‐revisiting the behavioral model of health care utilization by Andersen: a review on theoretical advances and perspectives. In: Janssen C, Swart E, von Lengerke T, eds. Health Care Utilization in Germany. New York, NY: Springer; 2014:11–28. doi: 10.1007/978-1-4614-9191-0_2 [DOI] [Google Scholar]
- 8. Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low‐income Americans. J Gen Intern Med. 2006;21:71–77. doi: 10.1111/j.1525-1497.2005.00278.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grintsova O, Maier W, Mielck A. Inequalities in health care among patients with type 2 diabetes by individual socio‐economic status (SES) and regional deprivation: a systematic literature review. Int J Equity Health. 2014;13:1–14. doi: 10.1186/1475-9276-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. García‐Altés A, Ruiz‐Muñoz D, Colls C, Mias M, Bassols NM. Socioeconomic inequalities in health and the use of healthcare services in Catalonia: analysis of the individual data of 7.5 million residents. J Epidemiol Community Health. 2018;72:871–879. doi: 10.1136/jech-2018-210817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bilal U, Cainzos‐Achirica M, Cleries M, Santaeugènia S, Corbella X, Comin‐Colet J, Vela E. Socioeconomic status, life expectancy and mortality in a universal healthcare setting: an individual‐level analysis of >6 million Catalan residents. Prev Med. 2019;123:91–94. doi: 10.1016/j.ypmed.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 12. Cainzos‐Achirica M, Capdevila C, Vela E, Cleries M, Bilal U, Garcia‐Altes A, Enjuanes C, Garay A, Yun S, Farre N. Individual income, mortality and healthcare resource use in patients with chronic heart failure living in a universal healthcare system: a population‐based study in Catalonia, Spain. Int J Cardiol. 2019;277:250–257. doi: 10.1016/j.ijcard.2018.10.099 [DOI] [PubMed] [Google Scholar]
- 13. Phelan JC, Link BG. Controlling disease and creating disparities: a fundamental cause perspective. J Gerontol B Psychol Sci Soc Sci. 2005;60:S27–S33. doi: 10.1093/geronb/60.Special_Issue_2.S27 [DOI] [PubMed] [Google Scholar]
- 14. Cainzos‐Achirica M, Bilal U. Further improvements in coronary heart disease mortality in Spain: context, paradoxes, and pathways forward. Rev Esp Cardiol (Engl Ed). 2021;74:823–826. doi: 10.1016/j.rec.2021.05.011 [DOI] [PubMed] [Google Scholar]
- 15. Hervella MI, Carratalá‐Munuera C, Orozco‐Beltrán D, López‐Pineda A, Bertomeu‐González V, Gil‐Guillén VF, Pascual R, Quesada JA. Trends in premature mortality due to ischemic heart disease in Spain from 1998 to 2018. Rev Esp Cardiol (Engl Ed). 2021;74:838–845. doi: 10.1016/j.rec.2020.09.034 [DOI] [PubMed] [Google Scholar]
- 16. Satish P, Vela E, Bilal U, Cleries M, Kanaya AM, Kandula N, Virani SS, Islam N, Valero‐Elizondo J, Yahya T. Burden of cardiovascular risk factors and disease in five Asian groups in Catalonia: a disaggregated, population‐based analysis of 121 000 first‐generation Asian immigrants. Eur J Prev Cardiol. 2022;29:916–924. doi: 10.1093/eurjpc/zwab074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmad OB, Boschi‐Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. Age Standardization of Rates: A New WHO Standard. Geneva: World Health Organization; 2001:9. [Google Scholar]
- 18. Regidor E. Measures of health inequalities: part 2. J Epidemiol Community Health. 2004;58:900–903. doi: 10.1136/jech.2004.023036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moreno‐Betancur M, Latouche A, Menvielle G, Kunst AE, Rey G. Relative index of inequality and slope index of inequality: a structured regression framework for estimation. Epidemiology. 2015;26:518–527. doi: 10.1097/EDE.0000000000000311 [DOI] [PubMed] [Google Scholar]
- 20. Gullón P, Díez J, Cainzos‐Achirica M, Franco M, Bilal U. Social inequities in cardiovascular risk factors in women and men by autonomous regions in Spain. Gac Sanit. 2021;35:326–332. doi: 10.1016/j.gaceta.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palomino JC, Rodriguez JG, Sebastian R. The COVID‐19 shock on the labour market: poverty and inequality effects across Spanish regions. SSRN 3775091. 2021.
- 22. Powell‐Wiley TM, Baumer Y, Baah FO, Baez AS, Farmer N, Mahlobo CT, Pita MA, Potharaju KA, Tamura K, Wallen GR. Social determinants of cardiovascular disease. Circ Res. 2022;130:782–799. doi: 10.1161/CIRCRESAHA.121.319811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization . Closing the Gap in a Generation: Health Equity Through Action on the Social Determinants of Health: Commission on Social Determinants of Health Final Report. World Health Organization; 2008. [Google Scholar]
- 24. Diez‐Roux AV, Nieto FJ, Muntaner C, Tyroler HA, Comstock GW, Shahar E, Cooper LS, Watson RL, Szklo M. Neighborhood environments and coronary heart disease: a multilevel analysis. Am J Epidemiol. 1997;146:48–63. doi: 10.1093/oxfordjournals.aje.a009191 [DOI] [PubMed] [Google Scholar]
- 25. Singh GK, Siahpush M, Kogan MD. Neighborhood socioeconomic conditions, built environments, and childhood obesity. Health Aff. 2010;29:503–512. doi: 10.1377/hlthaff.2009.0730 [DOI] [PubMed] [Google Scholar]
- 26. Bilal U, Auchincloss AH, Diez‐Roux AV. Neighborhood environments and diabetes risk and control. Curr Diab Rep. 2018;18:1–10. doi: 10.1007/s11892-018-1032-2 [DOI] [PubMed] [Google Scholar]
- 27. Bilal U, Beltrán P, Fernández E, Navas‐Acien A, Bolumar F, Franco M. Gender equality and smoking: a theory‐driven approach to smoking gender differences in Spain. Tob Control. 2016;25:295–300. doi: 10.1136/tobaccocontrol-2014-051892 [DOI] [PubMed] [Google Scholar]
- 28. Amos A, Haglund M. From social taboo to “torch of freedom”: the marketing of cigarettes to women. Tob Control. 2000;9:3–8. doi: 10.1136/tc.9.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062 [DOI] [PubMed] [Google Scholar]
- 30. Dixon J, Banwell C. Theory driven research designs for explaining behavioural health risk transitions: the case of smoking. Soc Sci Med. 2009;68:2206–2214. doi: 10.1016/j.socscimed.2009.03.025 [DOI] [PubMed] [Google Scholar]
- 31. Ohlmeier C, Langner I, Hillebrand K, Schmedt N, Mikolajczyk R, Riedel O, Garbe E. Mortality in the German Pharmacoepidemiological Research Database (GePaRD) compared to national data in Germany: results from a validation study. BMC Public Health. 2015;15:1–7. doi: 10.1186/s12889-015-1943-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blak B, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. J Innov Health Inform. 2011;19:251–255. doi: 10.14236/jhi.v19i4.820 [DOI] [PubMed] [Google Scholar]
- 33. Patel AP, Wang M, Kartoun U, Ng K, Khera AV. Quantifying and understanding the higher risk of atherosclerotic cardiovascular disease among South Asian individuals: results from the UK Biobank prospective cohort study. Circulation. 2021;144:410–422. doi: 10.1161/CIRCULATIONAHA.120.052430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krug G, Eberl A. What explains the negative effect of unemployment on health? An analysis accounting for reverse causality. Res Soc Stratif Mobil. 2018;55:25–39. doi: 10.1016/j.rssm.2018.03.001 [DOI] [Google Scholar]
- 35. Glass TA, Bilal U. Are neighborhoods causal? Complications arising from the ‘stickiness’ of ZNA. Soc Sci Med. 2016;166:244–253. doi: 10.1016/j.socscimed.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 36. Borrell C, Fernandez E, Schiaffino A, Benach J, Rajmil L, Villalbí JR, Segura A. Social class inequalities in the use of and access to health services in Catalonia, Spain: what is the influence of supplemental private health insurance? Int J Qual Health Care. 2001;13:117–125. doi: 10.1093/intqhc/13.2.117 [DOI] [PubMed] [Google Scholar]
- 37. Regidor E, Martínez D, Calle ME, Astasio P, Ortega P, Domínguez V. Socioeconomic patterns in the use of public and private health services and equity in health care. BMC Health Serv Res. 2008;8:1–9. doi: 10.1186/1472-6963-8-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S4


