Abstract
Background
Anemia and blood loss occur often in patients with ST‐segment–elevation myocardial infarction (STEMI). In‐hospital hemoglobin drop is associated with 1‐year mortality in patients with acute coronary syndrome. However, data on the effect of hemoglobin reduction on myocardial salvage and long‐term outcomes are scarce. We investigated the impact of in‐hospital hemoglobin drop on myocardial salvage and 5‐year mortality in patients with STEMI treated with primary percutaneous coronary intervention.
Methods and Results
In‐hospital hemoglobin drop was defined as a decrease in hemoglobin levels from admission and nadir hemoglobin values. Patients were categorized as having the following: no drop, minimal drop (<3 g/dL), minor drop (≥3 to <5 g/dL), and major drop (≥5 g/dL). Myocardial area at risk and infarct size were measured using serial single‐photon emission computerized tomography imaging. The co‐primary outcomes were myocardial salvage and 5‐year all‐cause mortality. Of 1204 patients, 1169 (97.1%) showed a hemoglobin drop during hospitalization: minimal, minor, and major drop occurred in 894 (74.3%), 214 (17.8%), and 61 (5.1%) patients, respectively. Myocardial salvage was reduced in patients with minimal (median, 0.53 [interquartile range, 0.27–0.83]), minor (median, 0.40 [interquartile range, 0.18–0.62]), and major (median, 0.40 [interquartile range, 0.14–0.77]) drop compared with patients without drop (median, 0.70 [interquartile range, 0.44–1.0], P<0.001). After adjusting for covariates, hemoglobin drop remained an independent correlate of poor myocardial salvage. A drop of ≥3 g/dL was associated with reduced left ventricular function at 6 months and with increased mortality at 5‐year follow‐up after STEMI.
Conclusions
In patients with STEMI undergoing primary percutaneous coronary intervention, in‐hospital hemoglobin drop was associated with reduced myocardial salvage, left ventricular function, and increased long‐term mortality.
Keywords: blood loss, hemoglobin, infarct size, myocardial infarction, single‐photon emission computed tomography imaging
Subject Categories: Percutaneous Coronary Intervention, Revascularization, Stent, Myocardial Infarction, Nuclear Cardiology and PET
Nonstandard Abbreviations and Acronyms
- MACE
major adverse cardiovascular events
- PPCI
primary percutaneous coronary intervention
- REALITY
Restrictive and Liberal Transfusion Strategies in Patients With AMI
- WHO
World Health Organization
Clinical Perspective.
What Is New?
Our data show that an in‐hospital hemoglobin drop of ≥3 g/dL, which occurred in one fourth to one fifth of the study population, was associated with reduced myocardial salvage, reduced left ventricular function, and increased long‐term mortality in patients with acute myocardial infarction.
What Are the Clinical Implications?
These results indicate that precautionary measures should be implemented to strictly avoid blood loss in patients with myocardial infarction undergoing primary percutaneous coronary intervention.
Further, our results suggest using a dynamic (in‐hospital decline in hemoglobin levels ≥3 g/dL in comparison to admission levels) rather than a static hemoglobin cutoff in future randomized controlled trials comparing different transfusion strategies in patients with anemia and acute myocardial infarction.
Atherosclerosis and its complications such as myocardial infarction (MI) and stroke are major contributors to worldwide morbidity and mortality. 1 MI is characterized by abrupt interruption of coronary blood flow in the infarct‐related arteries leading to myocardial ischemia, injury/necrosis, and cardiac dysfunction. 2 , 3 Low hemoglobin levels occur often in patients with MI and may already be present on admission or may develop during the hospital stay because patients undergo invasive procedures and receive antiplatelet and anticoagulant therapies, which may lead to blood loss. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 However, a drop in hemoglobin levels may worsen the ischemic damage by further decreasing oxygen supply to the infarct area and by increasing myocardial oxygen demand as a result of an elevated cardiac output needed to maintain systemic perfusion. 14
To this point, studies that specifically examined the impact of in‐hospital hemoglobin reduction on myocardial recovery in patients with acute ST‐segment elevation myocardial infarction (STEMI) are lacking. We sought to investigate the association of in‐hospital hemoglobin drop with myocardial salvage assessed with serial single‐photon emission computed tomography (SPECT) imaging in patients with STEMI undergoing primary percutaneous coronary intervention (PPCI).
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Design
In this study, we analyzed prospectively collected data from patients with STEMI. Details and characteristics of the study were previously described. 15 , 16 , 17 In brief, patients with STEMI underwent PPCI and serial scintigraphic imaging at 2 tertiary cardiac care centers (Deutsches Herzzentrum München and Klinikum rechts der Isar, both at the Technical University of Munich) between January 2002 and December 2007. The diagnosis of STEMI was based on chest pain lasting ≥20 minutes and persistent ST‐segment elevation ≥1 mm in at least 2 extremities or ≥2 mm in at least 2 chest leads or new onset of left bundle branch block. 17 In a recent analysis by Sager and colleaguees, 17 200 of 1406 patients with STEMI were excluded because the time of day at symptom onset was not clearly documented. Hence, the remaining cohort of 1206 patients was included in this analysis with additional information (eg, about symptom onset) for further analysis. Two patients were excluded because admission hemoglobin measurements were not available. Hence, 1204 patients with STEMI who had scintigraphic data on the myocardial area at risk and infarct size and both 1‐ and 5‐year outcomes (ie, all‐cause mortality, MI, or major adverse cardiovascular events [MACE]) were included in this study. All patients gave written informed consent for PPCI and imaging procedures. The study protocol was approved by the institutional ethics committee (454/21 S‐KH) and conforms to the Declaration of Helsinki.
Angiography and PPCI
The culprit lesion was identified during coronary angiography by the presence of acute occlusion, intraluminal filling defects (or thrombus), ulcerated plaques with contrast‐filled pocket protruding into the plaque with or without delayed contrast washout, extraluminal contrast, dissection or intraluminal flaps. 17 Coronary artery disease in nonculprit lesions was defined as coronary stenosis of ≥50% lumen obstruction. 17 Left ventricular ejection fraction (LVEF) at baseline and after 6 months was measured on left ventricular angiograms using the area‐length method. 17 Unfractionated heparin was used for periprocedural anticoagulation. 17 The antithrombotic regimen included clopidogrel, a loading dose of 600 mg, and aspirin 325 to 500 mg. 17 Chronic antithrombotic therapy consisted of clopidogrel, 150 mg until discharge (no more than 3 days) mostly followed by 75 mg/d for ≥1 month, and aspirin 200 mg/d indefinitely. 17 Few patients were treated with ticlopidine (250 mg twice/d) instead of clopidogrel and aspirin 200 mg/d indefinitely. If indicated—mostly because of new onset of atrial fibrillation—patients were treated with phenprocoumon in combination with either aspirin and clopidogrel or aspirin and ticlopidin, respectively.
Measurement of Myocardial Area at Risk and Final Infarct Size Using SPECT
As previously described, 99mTc‐sestamibi SPECT imaging was performed. 15 , 16 , 17 In short, SPECT imaging was performed serially in each patient at two predefined time points by the following protocol: time point one: 99mTc‐sestamibi (27 mCi [1000 MBq]) was administered intravenously before PPCI and imaging was performed 6 to 8 hours after the injection to assess the perfusion defect (this estimates the myocardial area at risk); and time point two: 99mTc‐sestamibi was again administered intravenously 7 to 14 days after PPCI and imaging was performed 6 to 8 hours after the injection to assess the perfusion defect (this estimates the final infarct size). Perfusion defects were defined as <50% uptake of 99mTc‐sestamibi and were expressed as percentage of the left ventricle. 17 Myocardial salvage index (or relative salvage) was calculated as the initial myocardial area at risk minus the final infarct size divided by the initial myocardial area at risk. 17 The myocardial salvage index indicates the proportion of initial myocardial area at risk salvaged by therapy. All measurements were performed by investigators who were not aware of the clinical or angiographic data. 15 , 16 , 17
Laboratory Data, Medical History, and Definitions
Laboratory measurements were performed daily and extracted from each patient's chart up to 10 days of hospitalization. Baseline hemoglobin refers to the first available measurement of hemoglobin level recorded on admission. Nadir hemoglobin refers to the lowest concentration measured during hospitalization. Patients with hemoglobin drop were those where there was a decrease in hemoglobin levels from admission to nadir hemoglobin levels as previously described. 7 Based on this difference, patients were categorized into 4 groups: (1) no hemoglobin drop (patients with zero or negative difference between baseline and nadir hemoglobin values), (2) <3 g/dL hemoglobin drop, (3) ≥3 to <5 g/dL hemoglobin drop, and (4) ≥5 g/dL hemoglobin drop. Creatine kinase myocardial band (CK‐MB) was also measured daily and peak levels were defined as the highest value during hospitalization. 17 CK‐MB was used as an enzymatic estimate of infarct size. 17 Renal function was assessed by calculating the creatinine clearance according to the Cockroft‐Gault formula. According to the World Health Organization (WHO) definition, anemia was considered when serum hemoglobin level was <12 g/dL in women or <13 g/dL in men.
Study Outcomes and Follow‐Up
The co‐primary end points were myocardial salvage index (or relative salvage) and 5‐year all‐cause mortality. Secondary end points were: 1‐year all‐cause mortality, LVEF, nonfatal MI (1‐year and 5‐year follow‐up), and MACE (1‐year and 5‐year follow‐up). As a standard practice in our institutions at the time of patient discharge, coronary angiography 6 months after the index procedure was scheduled. Moreover, coronary angiography was performed whenever patients showed symptoms or signs of myocardial ischemia. 17 The 6‐month angiograms were used for the calculation of the LV‐EF at this time point. Nonfatal MI was diagnosed based on development of new abnormal Q waves in ≥ 2 contiguous chest or ≥2 adjacent extremity leads, or an elevation of CK‐MB >2 times (>3 times for 48 hours after a percutaneous coronary intervention procedure) the upper limit of normal in the presence of ischemia symptoms. 17 Follow‐up information was obtained by staff members who were not aware of the clinical data via phone calls 30 days after percutaneous coronary intervention, 1 year after percutaneous coronary intervention, and yearly thereafter. 17 Data on mortality were obtained from hospital records, death certificates, or phone contact with patients' relatives or referring physicians. 17
Statistical Analysis
Continuous data are presented as mean ± SD or median with 25th to 75th percentiles and 10th to 90th percentiles depending on normality of distribution and compared with 1‐way ANOVA, Kruskal–Wallis test (plus Dunn's multiple comparisons test), or Mann–Whitney test, as appropriate. 17 Discrete variables were presented as proportions (percentages) and compared with chi‐square test. 17 The association between the hemoglobin drop (as a continuous variable) and poor myocardial salvage (defined as relative salvage below median) was tested using the multiple logistic regression model (with myocardial salvage as a dichotomous variable). The following variables were included in the model: age, sex, diabetes, hypercholesterolemia, family history of cardiovascular disease, number of diseased vessels, previous MI, Killip class on admission, hemoglobin values on admission, creatinine clearance, door‐to‐balloon time, LVEF on admission, and no‐reflow after percutaneous coronary intervention. Long‐term clinical outcomes were assessed using the Kaplan–Meier method and log‐rank test. Multivariable Cox proportional hazards model was used to assess the association between hemoglobin drop (categorized as no drop, <3 g/dL hemoglobin drop, ≥3 to <5 g/dL hemoglobin drop, and ≥5 g/dL hemoglobin drop) and all‐cause mortality. Hazard ratios (HRs) were shown with 95% CIs. Age, heart rate (on admission), systolic blood pressure (on admission), creatinine clearance, sex, diabetes, Killip class on admission, previous MI, number of diseased vessels, hemoglobin values on admission, and the difference between admission hemoglobin and nadir hemoglobin values were entered into the model(s). Landmark analysis of all‐cause mortality was performed at 30 days after admission to the hospital, which on the one hand represents a common end point for early cardiovascular events, 18 and on the other hand shows the influence of mortality beyond the phase of acute MI and hospitalization. Assuming an HR of 2.0 and a type I error rate of 5%, we calculated a power of 85% to detect significant differences regarding outcomes in a sample size of 1157 individuals. A two‐sided P value of <0.05 was considered to indicate statistical significance. IBM SPSS Statistics version 27 and GraphPad Prism 8 (version 8.4.3) were used for statistical analysis and visualization of data.
Results
Baseline Data
Of 1204 patients included in this analysis, 1169 (97.1%) showed a hemoglobin drop during hospitalization. Patients were categorized into 4 groups: no hemoglobin drop (35 patients), <3 g/dL hemoglobin drop (894 patients), ≥3 to <5 g/dL hemoglobin drop (214 patients), and ≥5 g/dL hemoglobin drop (61 patients). Hemoglobin drop occurred early (within the first 48 hours) in the majority of patients (Figure S1). Baseline characteristics of patients according to the degree of hemoglobin drop are shown in Table 1. The baseline variables differed between the groups with respect to age, sex, frequency of diabetes, hypercholesterolemia, positive family history for cardiovascular diseases, creatinine clearance on admission, number of affected coronary vessels, previous MI, door‐to‐balloon time, Killip class on admission, occurrence of no‐reflow after PPCI, and LV‐EF on admission.
Table 1.
Baseline and Procedural Characteristics
| No drop | Minimal drop (<3 g/dL) | Minor drop (≥3 and<5 g/dL) | Major drop (≥5 g/dL) | P value | |
|---|---|---|---|---|---|
| n=35 (2.9%) | n=894 (74.3%) | n=214 (17.8%) | n=61 (5.1%) | ||
| Hemoglobin on admission, mean (SD), g/dL | 12.2 (2.4) | 14.3 (1.5) | 14.7 (1.5) | 14.9 (1.5) | <0.001 |
| Nadir hemoglobin, mean (SD), g/dL | 12.2 (2.4) | 12.7 (1.6) | 11.0 (1.6) | 8.9 (1.6) | <0.001 |
| Age, mean (SD), y | 62.4 (10.6) | 61.3 (13.0) | 65.5 (12.7) | 65.4 (13.4) | <0.001 |
| Men, n (%) | 28 (80.0) | 701 (78.4) | 153 (71.5) | 36 (59.0) | 0.002 |
| Diabetes, n (%) | 4 (11.4) | 158 (17.7) | 47 (22.0) | 20 (32.8) | 0.011 |
| BMI, mean (SD), kg/m2 | 25.9 (3.3) | 26.8 (4.0) | 26.6 (3.9) | 26.2 (3.1) | 0.359 |
| Hypercholesterolemia, n (%) | 28 (80.0) | 485 (54.3) | 101 (47.2) | 35 (57.4) | 0.003 |
| Hypertension, n (%) | 24 (68.6) | 617 (69.0) | 153 (71.5) | 52 (85.2) | 0.060 |
| Current smoking, n (%) | 10 (28.6) | 391 (43.7) | 88 (41.1) | 27 (44.3) | 0.319 |
| Family history for cardiovascular disease, n (%) | 9 (25.7) | 370 (41.4) | 86 (40.2) | 15 (24.6) | 0.009 |
|
CrCL mean (SD), mL/min |
83.2 (30.5) | 90.0 (33.4) | 81.8 (36.9) | 75.3 (29.1) | <0.001 |
| No. of affected vessels (%) | 0.007 | ||||
| 1 | 11 (31.4) | 342 (38.3) | 62 (29.0) | 12 (19.7) | |
| 2 | 10 (28.6) | 279 (31.2) | 65 (30.4) | 22 (36.1) | |
| 3 | 14 (40.0) | 273 (30.5) | 87 (40.7) | 27 (44.3) | |
| Previous MI, n (%) | 13 (37.1) | 115 (12.9) | 15 (7.0) | 8 (13.1) | <0.001 |
| Previous CABG, n (%) | 2 (5.7) | 22 (2.5) | 11 (5.1) | 3 (4.9) | 0.136 |
| Time to admission, mean (SD), h | 5.0 (5.28) | 6.8 (6.1) | 6.3 (5.7) | 6.6 (6.1) | 0.242 |
| Door‐to‐balloon time, mean (SD), h | 1.4 (0.7) | 1.5 (0.9) | 1.3 (0.7) | 1.3 (0.8) | 0.010 |
| Killip class, n (%) | <0.001 | ||||
| I | 22 (62.9) | 698 (78.1) | 137 (64.0) | 36 (59.0) | |
| II | 9 (25.7) | 162 (18.1) | 49 (22.9) | 13 (21.3) | |
| III | 3 (8.6) | 18 (2.0) | 17 (7.9) | 1 (1.6) | |
| IV | 1 (2.9) | 16 (1.8) | 11 (5.1) | 11 (18.0) | |
| Baseline TIMI flow, n (%) | 0.630 | ||||
| 0 | 12 (34.4) | 411 (46.0) | 112 (52.6) | 31 (50.8) | |
| 1 | 3 (8.6) | 99 (11.1) | 27 (12.7) | 7 (11.5) | |
| 2 | 11 (31.4) | 209 (23.4) | 45 (21.1) | 13 (21.3) | |
| 3 | 9 (25.7) | 174 (19.5) | 29 (13.6) | 10 (16.4) | |
| Type of PCI, n (%) | 0.277 | ||||
| PTCA, n (%) | 5 (14.3) | 128 (14.3) | 22 (10.3) | 7 (11.5) | |
| Stenting, n (%) | 30 (85.7) | 766 (85.7) | 192 (89.7) | 54 (88.5) | |
| No reflow, n (%) | 5 (14.3) | 98 (11.0) | 46 (21.6) | 16 (26.2) | <0.001 |
| LVEF, mean (SD), % | 47.7 (13.0) | 49.8 (11.0) | 47.0 (12.3) | 45.5 (13.1) | <0.001 |
| Infarct vessel (culprit lesion), n (%) | 0.875 | ||||
| Left main | 0 (0) | 4 (0.5) | 0 (0) | 0 (0) | |
| LAD | 20 (57.1) | 400 (44.7) | 97 (45.3) | 22 (36.1) | |
| LCX | 3 (8.6) | 153 (17.1) | 34 (15.9) | 12 (19.7) | |
| RCA | 11 (31.4) | 322 (36.0) | 79 (36.9) | 26 (42.6) | |
| CABG surgery | 1 (2.9) | 15 (1.7) | 4 (1.9) | 1 (1.6) |
BMI indicates body mass index; CABG, coronary artery bypass graft; CrCl, creatinine clearance; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; RCA, right coronary artery; and TIMI, thrombolysis in myocardial infarction.
Prevalence of anemia with respect to sex both on admission and at the time point for nadir hemoglobin levels are shown in Table 2. According to the WHO definition, anemia on admission occurred in 127 patients (10.6%): 18 patients with no drop (51.4%), 91 patients with minimal drop (10.2%), 17 patients with minor drop (7.9%), and 1 patient with major drop (1.6%). Of those, 84 patients were men (66.1%). When we considered nadir hemoglobin levels, 697 patients met the criteria for anemia (57.9%): 18 patients with no drop (51.4%), 434 patients with minimal drop (48.6%), 185 patients with minor drop (86.4%), and 60 patients with major drop (98.4%). Of those, 488 individuals were men (70.0%).
Table 2.
Prevalence of Anemia on Admission and on Nadir With Respect to Sex
| No drop | Minimal drop (<3 g/dL) | Minor drop (≥3 and <5 g/dL) | Major drop (≥5 g/dL) | P value | |
|---|---|---|---|---|---|
| n=35 | n=894 | n=214 | n=61 | ||
| Anemia on admission | |||||
| Sex, n (%) | |||||
| Men | 11 (31.4) | 60 (6.7) | 12 (5.6) | 1 (1.6) | <0.001 |
| Women | 7 (20.0) | 31 (3.5) | 5 (2.3) | 0 | <0.001 |
| Anemia on nadir | |||||
| Sex, n (%) | |||||
| Men | 11 (31.4) | 311 (34.8) | 131 (61.2) | 35 (57.4) | <0.001 |
| Women | 7 (20.0) | 123 (13.8) | 54 (25.2) | 25 (41.0) | <0.001 |
In‐Hospital Hemoglobin Drop and Myocardial Salvage
Scintigraphic data and CK‐MB values are shown in Figure 1 and Figure S2. In patients with no hemoglobin drop, minimal drop, minor drop, and major hemoglobin drop, the myocardial area at risk or initial perfusion defect before PPCI (median) was 25.0% (25th–75th percentile, 8.0%–39.0%), 22.0% (25th–75th percentile, 11.0%–39.0%), 29.0% (25th–75th percentile, 17.0%–51.0%), and 26.3% (25th–75th percentile, 16.0%–43.0%) of the left ventricle, respectively. There was a significant difference in the area at risk in patients with minimal drop versus those with minor hemoglobin drop (Figure 1A). Moreover, we found that the myocardial area at risk was significantly smaller in patients with a hemoglobin drop of <3 g/dL compared with patients with a drop of ≥3 g/dL (median, 22.0% [25th–75th percentile, 11.0%–39.0%] versus 29.0% [25th–75th percentile, 16.7%–48.0%], respectively) (Figure S2A).
Figure 1. In‐hospital hemoglobin drop and indices of infarct size and myocardial salvage.
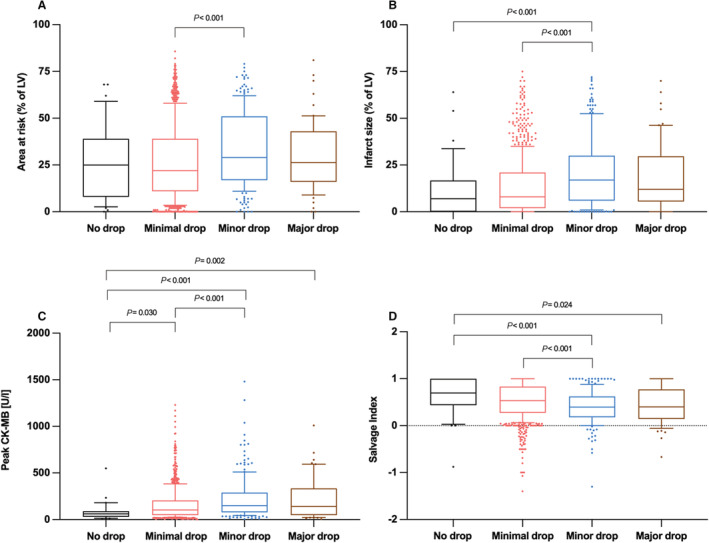
A, Initial myocardial area at risk (percentage of the left ventricle [LV]) assessed by first scintigraphic imaging before primary percutaneous coronary intervention (PPCI). B, Final infarct size assessed by second scintigraphic imaging 7 to 14 days after PPCI (percentage of the left ventricle). C, Peak blood creatine kinase MB (CK‐MB) values (U/L) (enzymatic infarct size). D, Myocardial salvage index (initial area at risk minus the final infarct size divided by initial myocardial area at risk) as a measure of the proportion of myocardial area at risk salvaged by treatment. Data are expressed as median with 25th to 75th percentiles (boxes), 10th to 90th percentiles (bars), and values outside the given percentiles (dots).
In patients with no hemoglobin drop and minimal, minor, and major hemoglobin drop, the final infarct size in the 7 to 14 days scintigraphy (median) was 7.0% (25th–75th percentile, 0.0%–16.7%), 8.0% (25th–75th percentile, 2.0%–21.0%), 17.0% (25th–75th percentile, 6.0%–30.0%), and 12.0% (25th–75th percentile, 6.0%–29.0%) of the left ventricle, respectively. Final infarct sizes were greater among patients with minor hemoglobin drop compared with patients with no or minimal hemoglobin drop (Figure 1B). Further, the final infarct size was smaller in the group with a hemoglobin drop of <3 g/dL versus the group with a drop of ≥3 g/dL as assessed by scintigraphy (median, 8.0% [25th–75th percentile, 2.0%–20.8%] and 16.0% [25th–75th percentile, 6.0–30.0%], respectively) (Figure S2B).
Peak CK‐MB values, an enzymatic estimate of infarct size, are shown in Figure 1C. Specifically, in patients with no hemoglobin drop and minimal, minor, and major hemoglobin drop, peak CK‐MB values (median) were 61.0 U/L (25th–75th percentile, 30.0–89.0 U/L), 101.0 U/L (25th–75th percentile, 46.0–202.0 U/L), 147.5 U/L (25th–75th percentile, 75.5–283.5 U/L), and 132.0 U/L (25th–75th percentile, 41.0–329.0 U/L), respectively. When we compared patients with a hemoglobin drop <3 g/dL with patients with a drop of ≥3 g/dL, we found lower peak CK‐MB values in the group with a <3 g/dL drop (median, 98.0 U/L [25th–75th percentile, 46.0–197.0 U/L] and 147.0 U/L [25th–75th percentile, 73.0–300.0 U/L], respectively) (Figure S2C).
The myocardial salvage index (median)—the co‐primary outcome—was 0.70 (25th–75th percentile, 0.44–1.00), 0.53 (25th–75th percentile, 0.27–0.83), 0.40 (25th–75th percentile, 0.18–0.62), and 0.40 (25th–75th percentile, 0.14–0.77) in patients with no hemoglobin drop and minimal, minor, and major hemoglobin drop, respectively (Figure 1D). Myocardial salvage index was significantly lower in patients with minor or major hemoglobin drop compared with patients with no hemoglobin drop and in patients with minor hemoglobin drop compared with minimal hemoglobin drop. The salvage index was significantly lower in patients with a hemoglobin drop of ≥3g/dL compared with patients with a drop of <3 g/dL (median): 0.40 [25th–75th percentile, 0.17–0.65] versus 0.54 [25th–75th percentile, 0.27–0.84], P<0.001) (Figure S2D).
The association between hemoglobin drop and myocardial salvage was assessed using the multivariable logistic regression model (see Methods for variables entered into the model). In the univariable logistic regression model, hemoglobin drop was associated with poor myocardial salvage (odds ratio [OR], 1.22 [95% CI, 1.12–1.33], P<0.001; for 1 g/dL hemoglobin drop). After adjustment, hemoglobin drop remained an independent correlate of poor myocardial salvage (OR, 1.23 [95% CI, 1.11–1.37], P<0.001; for 1 g/dL hemoglobin drop) (Table 3).
Table 3.
Univariate and Multivariate Association of In‐Hospital Hemoglobin Drop and poor Myocardial Salvage in the Logistic Regression Model
| OR (95% CI) | P value | |
|---|---|---|
| Univariable model | ||
| In‐hospital hemoglobin drop (per g/dL) | 1.22 (1.12–1.33) | <0.001 |
| Multivariable model | ||
| In‐hospital hemoglobin drop (per g/dL) | 1.23 (1.11–1.37) | <0.001 |
| Hemoglobin on admission (per g/dL) | 0.93 (0.84–1.03) | 0.144 |
| Age (per year) | 0.99 (0.98–1.01) | 0.473 |
| Sex (if woman) | 0.48 (0.37–0.68) | <0.001 |
| Diabetes (if yes) | 0.71 (0.51–0.99) | 0.049 |
| Hypercholesterolemia (if yes) | 0.90 (0.70–1.17) | 0.436 |
| Family history for cardiovascular disease (if yes) | 0.96 (0.73–1.25) | 0.752 |
| Creatinine clearance (per mL/min) | 0.99 (0.99–1.00) | 0.214 |
| Three‐vessel disease (reference 1‐vessel disease) | 1.20 (0.86–1.70) | 0.287 |
| Previous MI (if yes) | 1.76 (1.15–2.69) | 0.009 |
| Door to balloon (per h) | 1.17 (1.00–1.37) | 0.045 |
| Killip class IV (reference Killip class I) | 1.09 (0.49–2.42) | 0.826 |
| No reflow (if yes) | 2.00 (1.36–2.95) | <0.001 |
| LVEF (per %) | 0.96 (0.95–0.0.97) | <0.001 |
LVEF indicates left ventricular ejection fraction; MI, myocardial infarction; and OR, odds ratio.
In‐Hospital Hemoglobin Drop and 1‐ and 5‐Year Mortality
The median follow‐up for all‐cause mortality was 1341 days. One‐year follow‐up for all‐cause mortality was available in 1074 patients (89.2%). At 1 year, 44 patients had died: 1 patient in the group with no hemoglobin drop, 21 patients in the group with minimal hemoglobin drop, 17 patients in the group with minor hemoglobin drop, and 5 patients in the group with major hemoglobin drop (Kaplan–Meier estimates of 1‐year mortality: 3.2%, 2.4%, 8.1%, and 8.3%, respectively [log‐rank test, P<0.001]) (Figure 2A). At 1 year, 303 MACE occurred in 8 patients in the group with no hemoglobin drop, 206 patients in the group with minimal hemoglobin drop, 69 patients in the group with minor hemoglobin drop, and 20 patients in the group with major hemoglobin drop (Kaplan–Meier estimates of 1‐year MACE: 25.0%, 24.9%, 33.2%, and 33.8%, respectively; log‐rank test, P=0.005) (Figure S3A). At 1 year, 32 MIs occurred in 2 patients in the group with no hemoglobin drop, 20 patients in the group with minimal hemoglobin drop, 7 patients in the group with minor hemoglobin drop, and 3 patients in the group with major hemoglobin drop (Kaplan–Meier estimates of 1‐year MI: 6.3%, 2.3%, 3.3%, and 5.0%, respectively; log‐rank test, P=0.29) (Figure S4A).
Figure 2. In‐hospital hemoglobin drop and 1‐year (A) and 5‐year (B) all‐cause mortality.
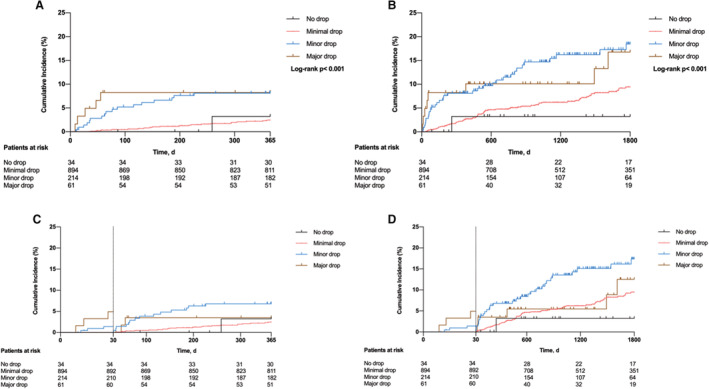
Landmark analysis at 30 days for 1‐year ( C ) and 5‐year ( D ) all‐cause mortality.
Five‐year follow‐up for all‐cause mortality was available in 451 (37.5%) patients. At 5 years, 104 patients had died: 1 patient in the group with no hemoglobin drop, 63 patients in the group with minimal hemoglobin drop, 32 patients in the group with minor hemoglobin drop, and 8 patients in the group with major hemoglobin drop (Kaplan–Meier estimates of 5‐year mortality: 3.2%, 9.4%, 18.5%, and 16.8%, respectively; log‐rank test, P<0.001) (Figure 2B). A landmark analysis comparing early (within 30 days) with late (beyond 30 days) events (6 versus 16 and 34 events after 1 and 5 years, respectively) revealed that patients presenting with a hemoglobin drop ≥3 g/dL displayed the highest mortality when only events that occurred after 30 days were included (Figure 2C and 2D). At 5 years, 375 MACE occurred: 10 patients in the group with no hemoglobin drop, 259 patients in the group with minimal hemoglobin drop, 83 patients in the group with minor hemoglobin drop, and 23 patients in the group with major hemoglobin drop (Kaplan–Meier estimates of 5‐year MACE: 32.9%, 33%, 42.5% and 43.5%, respectively; log‐rank test, P=0.005) (Figure S3B). At 5 years, 41 MIs occurred in 2 patients in the group with no hemoglobin drop, 27 patients in the group with minimal hemoglobin drop, 8 patients in the group with minor hemoglobin drop, and 4 patients in the group with major hemoglobin drop (Kaplan–Meier estimates of 5‐year MI: 6.3%, 3.6%, 4.1%, and 7.2%, respectively; log‐rank test, P=0.32) (Figure S4B). Kaplan–Meier curves for all‐cause mortality, MACE, and MI are shown in Figure 2 and Figures S3 and S4, respectively.
In the multivariable Cox proportional hazards model, we observed a trend toward higher risk of death from any cause within the first year, particularly in individuals with minor or major hemoglobin drop. The hemoglobin drop was also associated with long‐term all‐cause mortality (Table 4). After adjustment, the association between hemoglobin drop and 5‐year mortality was significant for minor (adjusted HR, 13.31 [95% CI, 1.64–107.98]; P=0.016) and major (HR, 10.98 [95% CI, 1.23–98.24]; P=0.035) hemoglobin drop. The association between hemoglobin drop with mortality was assessed in the patient group with hemoglobin drop <3 g/dL versus those with a hemoglobin drop ≥3 g/dL. Patients with a ≥3 g/dL hemoglobin drop had a 92% higher adjusted risk for 5‐year mortality compared with patients with a hemoglobin drop <3 g/dL (adjusted HR, 1.92 [95% CI, 1.22–3.00]; P=0.004).
Table 4.
Association of Hemoglobin Drop With 1‐ and 5‐y All‐Cause Mortality After Adjustment in the Multivariable Cox Proportional Hazard Model
|
1‐y mortality HR (95% CI) |
P value |
5‐y mortality HR (95% CI) |
P value | |
|---|---|---|---|---|
| No hemoglobin drop | Reference | Reference | ||
| Minimal hemoglobin drop (hemoglobin drop <3 g/dL) | 2.83 (0.31–26.10) | 0.360 | 6.38 (0.82–49.61) | 0.075 |
| Minor hemoglobin drop (hemoglobin drop ≥3 to <5 g/dL) | 7.75 (0.77–78.50) | 0.083 | 13.31 (1.64–107.98) | 0.016 |
| Major hemoglobin drop (hemoglobin drop ≥5 g/dL) | 8.24 (0.71–95.69) | 0.090 | 10.98 (1.23–98.24) | 0.035 |
Age, heart rate (on admission), systolic blood pressure (on admission), creatinine clearance, sex, diabetes, Killip class on admission, previous myocardial infarction, number of diseased vessels, hemoglobin values on admission, and the difference between admission hemoglobin and nadir hemoglobin values were entered into the analysis. HR indicates hazard ratio.
In‐Hospital Hemoglobin Drop and Left Ventricular Function
Angiographic data of LVEF on admission were available in 1146 of 1204 patients (95.2%): 31 (2.7%) patients with no hemoglobin drop, 853 (74.4%) patients with minimal hemoglobin drop, 205 (17.9%) patients with minor hemoglobin drop, and 57 (5.0%) patients with major hemoglobin drop. LVEF values (median) were 49.0% (25th–75th percentile, 42.0%–56.0%), 50.0% (25th–75th percentile, 44.0%–57.0%), 46.0% (25th–75th percentile, 38.0%–55.0%), and 46.0% (25th–75th percentile, 36.0%–55.0%), respectively, with a significant difference between groups with minimal and minor hemoglobin drop (Figure 3A). Data on LVEF at 6‐month follow‐up were available in 470 of the 1204 patients (39%): 16 (3.4%) patients with no hemoglobin drop, 360 (76.6%) patients with minimal hemoglobin drop, 73 (15.5%) patients with minor hemoglobin drop, and 21 (4.5%) with major hemoglobin drop. In these groups, LVEF values at 6 months (median) were 62.0% (25th–75th percentile, 54.7%–68.1%), 61.0% (25th–75th percentile, 50.3%–68.0%), 53.2% (25th–75th percentile, 45.1%–64.0%), and 57.0% (25th–75th percentile, 46.2%–64.6%), respectively (Figure 3B). At 6 months, LVEF was lowest in the group with minor hemoglobin drop, with a significant reduction compared with the group with minimal hemoglobin drop.
Figure 3. In‐hospital hemoglobin drop and left ventricular ejection fraction (LVEF) at (A) baseline, (B) 6 months, and (C) comparison between baseline and 6 months.
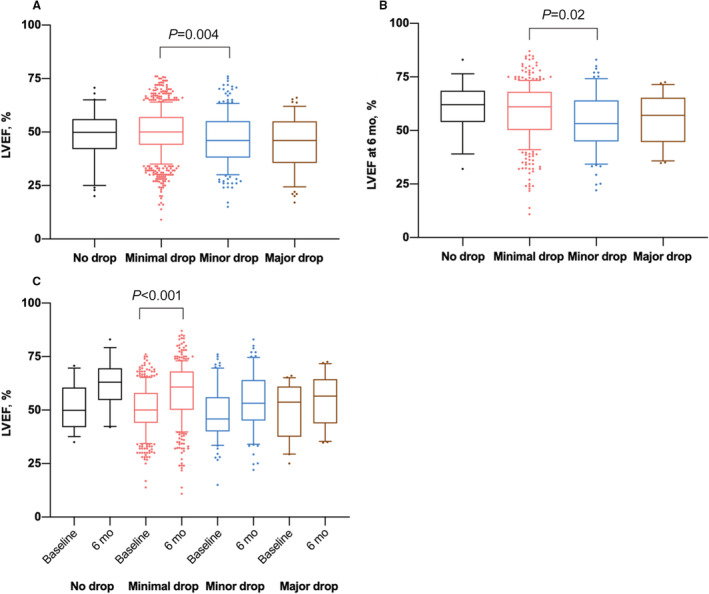
Data are median with 25th to 75th percentiles (boxes), 10th to 90th percentiles (bars), and values outside the given percentiles (dots).
Data on LVEF both at baseline and 6‐month follow‐up were available in 447 patients (37.1%): 13 (2.9%) patients with no hemoglobin drop, 343 (76.7%) patients with minimal hemoglobin drop, 71 (15.9%) patients with minor hemoglobin drop, and 20 (4.5%) patients with major hemoglobin drop (Figure 3C). A significant recovery (improvement compared with baseline values) of the LVEF at 6‐month follow‐up occurred only in patients with minimal hemoglobin drop, whereas there was no significant recovery in the other groups.
Discussion
The impact of hemoglobin drop on myocardial salvage in the setting of STEMI has not been investigated. The current study assessed the association between hemoglobin drop and myocardial salvage and long‐term clinical outcome in patients with STEMI undergoing PPCI. We report the following main findings: First, patients with STEMI with a hemoglobin drop ≥3 g/dL during hospitalization showed a greater infarct size and reduced myocardial salvage compared with patients with STEMI without hemoglobin drop or a drop <3 g/dL during hospitalization. Thus, each g/dL hemoglobin drop during hospitalization was associated with an OR of 1.23 for poor myocardial salvage. Second, LVEF at 6 months after MI recovered in patients with hemoglobin reduction <3 g/dL but not in those with a hemoglobin drop ≥3 g/dL.
Third, a hemoglobin drop of ≥3 g/dL was associated with increased 5‐year all‐cause mortality.
Anemia is common in patients with acute MI and may: (1) be already present on admission (baseline anemia), or (2) develop during the hospital stay with possible contributions of periprocedural and postprocedural bleeding from invasive procedures and intensified anticoagulant therapy. However, lack of oxygen carriers/supply may further deteriorate cardiac function by exacerbating myocardial ischemia even after coronary flow is re‐established. Our data show that the prevalence of anemia was 10.5% on admission and anemia developed in 57.9% of the patients with STEMI at some point during hospitalization. These data are in line with previous studies which reported that anemia was present in ≈20% of patients with acute MI and the proportion of patients with anemia increased to 48% during hospitalization. 6
Anemia often triggers red blood cell transfusions, which are administered in up to 10% of patients with acute coronary syndrome, 19 although a clear hemoglobin cutoff value mandating transfusion is not yet standardized. In most studies, a liberal blood transfusion strategy (transfusion triggered by hemoglobin levels <9 g/dL) was compared with a restrictive blood transfusion strategy (transfusion triggered by hemoglobin levels <7.0 g/dL). Since data from large randomized controlled trials including patients with acute coronary syndrome are missing, current guidelines recommend a restrictive policy of transfusion in patients with anemia with acute MI. 19 This recommendation is substantiated by the recently published REALITY (Restrictive and Liberal Transfusion Strategies in Patients With AMI) trial. 20 This trial enrolled patients with acute MI and anemia at any time during admission and randomly assigned them to either a restrictive (transfusion administered when hemoglobin was ≤8 g/dL) or a liberal (transfusion administered when hemoglobin was ≤10 g/dL) transfusion strategy. The study showed that a restrictive transfusion strategy was noninferior to the liberal strategy in preventing 30‐day major adverse cardiac events supporting the use of a restrictive strategy for blood transfusion in anemic patients with MI.
A recent study investigated the impact of blood loss/hemoglobin drop during the index hospital stay in patients with acute coronary syndrome. The authors found that in patients with acute coronary syndrome, an in‐hospital hemoglobin drop of ≥3 g/dL occurs frequently and was associated with increased risk for 1‐year mortality. 7 Another study that also explored the impact of changes in hemoglobin levels during hospital stay on long‐term outcome in patients with MI found that development of anemia was associated with increased long‐term mortality. 21 In line with these 2 previous trials, our study also shows that a hemoglobin drop of ≥3 g/dL was associated with decreased long‐term survival. However, how a drop in hemoglobin leads to increased mortality remains unclear. One may speculate that lack of oxygen carriers attributable to a loss in the acute setting may worsen myocardial ischemia and may even promote extension of the infarcted area. Our scintigraphic and biomarker findings support such a mechanism. SPECT imaging and blood peak CK‐MB levels showed a larger infarct size in patients with a hemoglobin drop of ≥3 g/dL. Importantly, we found that myocardial salvage was reduced in patients with a hemoglobin drop of ≥3 g/dL. In line, we also found that reduced myocardial salvage and larger infarct size were associated with lack of recovery of ventricular function after acute MI and lower LVEF at 6 months after the acute event in patients with a hemoglobin drop ≥3 g/dL. Our findings suggest that reduced myocardial salvage and deteriorated cardiac function may provide an explanation for increased long‐term mortality associated with hemoglobin drop in patients with STEMI.
The fact that acute hemoglobin drop seems to impact mortality may have implications for treatment strategies of these patients. It may be suggested that blood transfusion should not be triggered by the presence of anemia alone but rather by the degree of acute hemoglobin loss. Whether blood transfusions can reverse the deleterious effect of an acute hemoglobin drop of ≥3 g/dL remains to be tested in future randomized controlled trials.
This study has several limitations. First, this is a retrospective analysis of patients presenting with STEMI treated with PPCI in 2 tertiary cardiac care centers between 2002 and 2007 and not a trial specifically designed to investigate the association between hemoglobin drop and the infarct size or clinical outcome. Second, considering the time of patients' inclusion, the therapy of patients does not adhere to current guidelines. In particular, less potent P2Y12 inhibitors (mainly clopidogrel) and less advanced stent technology than currently recommended were used. Moreover, in almost all patients, a femoral artery route was used for PPCI. These factors may have had an impact on periprocedural and postprocedural ischemic and bleeding complications. Our mortality data are, however, in line with analyses from contemporary clinical trials. 7 , 12 Third, data on preexisting diseases were not available. Preexisting bleeding diathesis, for instance, may also have impacted the acute reduction of hemoglobin levels during hospitalization. Moreover, details on blood transfusion, follow‐up hemoglobin levels, or potential bleeding events following the index hospitalization with PPCI were not available. Further, in patients presenting with low hemoglobin levels, the cause of anemia is unknown (no information, for instance, on iron status). Finally, our study shows associations but no causality in the relationship between hemoglobin drop and myocardial salvage, LVEF, or long‐term mortality.
Conclusions
In patients with STEMI undergoing PPCI, in‐hospital hemoglobin drop was associated with reduced myocardial salvage, poor recovery, and reduced LVEF and increased long‐term mortality.
Sources of Funding
A.D. reports funding from the Clinician Scientist Excellence Program of the German Center for Cardiovascular Research (DZHK; FKZ 81X3600507) and the “Deutsche Herzstiftung” (522/20 S) outside of this current work. H.B.S. has received funding from the European Research Council under the European Union's Horizon 2020 Research and Innovation Programme (STRATO, grant agreement number 759272), the “Else‐Kröner‐Fresenius‐Stiftung” (2020_EKSE.07), and the “Deutsche Herzstiftung” (F/28/17). This study was further supported by the “Deutsche Forschungsgemeinschaft” (German Research Foundation [DFG]) (H.B.S.: SA 1668/5–1 and H.B.S. and T.K.: CRC 1123 [B02]). T.K. is supported by the Corona Foundation (Junior Research Group Translational Cardiovascular Genomics). Additionally, we gratefully acknowledge the support of the German Federal Ministry of Education and Research (BMBF), to H.S., within the framework of ERA‐NET on Cardiovascular Disease (Druggable‐MI‐genes: 01KL1802) and the scheme of target validation (BlockCAD: 16GW0198K), as well as the DZHK Munich Heart Alliance, within the framework of the e:Med research and funding concept (AbCD‐Net: 01ZX1706C). The British Heart Foundation/DZHK Collaboration, for which we are co‐applicants, has also provided support and funding. H.S. has received further support from the DFG as part of the “Sonderforschungsbereich” 1123 (B02) and the “Sonderforschungsbereich” TRR 267 (B05). Bavarian State Ministry of Health and Care also funded this work as part of “DigiMed Bayern” (grant number DMB‐1805‐0001). The German Federal Ministry of Economics and Energy has supported this work within ModulMax (grant number ZF4590201BA8).
Disclosures
H.B.S. reports grants from the European Research Council, the “Else‐Kröner‐Fresenius‐Stiftung,” the “Deutsche Herzstiftung,” and the DFG during the conduct of the study. H.B.S. received lecture fees from Novo Nordisk Pharma GmbH and AstraZeneca GmbH. T.K. received lecture fees from Bayer AG, Pharmaceuticals. H.S. reports grants and personal fees from Astra‐Zeneca and personal fees from Vifor Pharma, Boehringer Ingelheim, Brahms, Medtronic, Sanofi‐Aventis, MSD SHARPE & DOHME, Bristol‐Mayers Squibb, Servier, Bayer Vital GmbH, Daiichi‐Sankyo, AMGEN, Novartis, Synlab, and Pfizer, outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Figure S1–S4
Supplemental Material is available at https://doi.org/10.1161/JAHA.121.024857
For Sources of Funding and Disclosures, see page 11.
References
- 1. Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8 [DOI] [PubMed] [Google Scholar]
- 2. Sager HB, Nahrendorf M. Inflammation: a trigger for acute coronary syndrome. Q J Nucl Med Mol Imaging. 2016;60:185–193. [PubMed] [Google Scholar]
- 3. Sager HB, Kessler T, Schunkert H. Monocytes and macrophages in cardiac injury and repair. J Thorac Dis. 2017;9:S30–S35. doi: 10.21037/jtd.2016.11.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farhan S, Baber U, Mehran R. Anemia and acute coronary syndrome: time for intervention studies. J Am Heart Assoc. 2016;5:e004908. doi: 10.1161/JAHA.116.004908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, Gibson CM, Braunwald E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–2049. doi: 10.1161/01.CIR.0000162477.70955.5F [DOI] [PubMed] [Google Scholar]
- 6. Bassand JP. Impact of anaemia, bleeding, and transfusions in acute coronary syndromes: a shift in the paradigm. Eur Heart J. 2007;28:1273–1274. doi: 10.1093/eurheartj/ehm132 [DOI] [PubMed] [Google Scholar]
- 7. Leonardi S, Gragnano F, Carrara G, Gargiulo G, Frigoli E, Vranckx P, Di Maio D, Spedicato V, Monda E, Fimiani L, et al. Prognostic implications of declining hemoglobin content in patients hospitalized with acute coronary syndromes. J Am Coll Cardiol. 2021;77:375–388. doi: 10.1016/j.jacc.2020.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salisbury AC, Alexander KP, Reid KJ, Masoudi FA, Rathore SS, Wang TY, Bach RG, Marso SP, Spertus JA, Kosiborod M. Incidence, correlates, and outcomes of acute, hospital‐acquired anemia in patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2010;3:337–346. doi: 10.1161/CIRCOUTCOMES.110.957050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kansagara D, Dyer E, Englander H, Fu R, Freeman M, Kagen D. Treatment of anemia in patients with heart disease: a systematic review. Ann Intern Med. 2013;159:746–757. doi: 10.7326/0003-4819-159-11-201312030-00007 [DOI] [PubMed] [Google Scholar]
- 10. Mahendiran T, Nanchen D, Gencer B, Meier D, Klingenberg R, Raber L, Carballo D, Matter CM, Luscher TF, Windecker S, et al. Prognosis of patients with chronic and hospital‐acquired Anaemia after acute coronary syndromes. J Cardiovasc Transl Res. 2020;13:618–628. doi: 10.1007/s12265-019-09934-w [DOI] [PubMed] [Google Scholar]
- 11. Nagao K, Watanabe H, Morimoto T, Inada T, Hayashi F, Nakagawa Y, Furukawa Y, Kadota K, Akasaka T, Natsuaki M, et al. Prognostic impact of baseline hemoglobin levels on long‐term thrombotic and bleeding events after percutaneous coronary interventions. J Am Heart Assoc. 2019;8:e013703. doi: 10.1161/JAHA.119.013703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ndrepepa G, Neumann FJ, Menichelli M, Holdenrieder S, Richardt G, Mayer K, Cassese S, Xhepa E, Kufner S, Wiebe J, et al. Prognostic value of haemoglobin drop in patients with acute coronary syndromes. Eur J Clin Invest. 2021;51:e13670. doi: 10.1111/eci.13670 [DOI] [PubMed] [Google Scholar]
- 13. Velasquez‐Rodriguez J, Diez‐Delhoyo F, Valero‐Masa MJ, Vicent L, Devesa C, Sousa‐Casasnovas I, Juarez M, Angulo‐Llanos R, Fernandez‐Aviles F, Martinez‐Selles M. Prognostic impact of age and hemoglobin in acute ST‐segment elevation myocardial infarction treated with reperfusion therapy. Am J Cardiol. 2017;119:1909–1916. doi: 10.1016/j.amjcard.2017.03.018 [DOI] [PubMed] [Google Scholar]
- 14. Metivier F, Marchais SJ, Guerin AP, Pannier B, London GM. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant. 2000;15(suppl 3):14–18. doi: 10.1093/oxfordjournals.ndt.a027970 [DOI] [PubMed] [Google Scholar]
- 15. Ndrepepa G, Alger P, Fusaro M, Kufner S, Seyfarth M, Keta D, Mehilli J, Schomig A, Kastrati A. Impact of perfusion restoration at epicardial and tissue levels on markers of myocardial necrosis and clinical outcome of patients with acute myocardial infarction. EuroIntervention. 2011;7:128–135. doi: 10.4244/EIJV7I1A21 [DOI] [PubMed] [Google Scholar]
- 16. Ndrepepa G, Tiroch K, Fusaro M, Keta D, Seyfarth M, Byrne RA, Pache J, Alger P, Mehilli J, Schomig A, et al. 5‐year prognostic value of no‐reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55:2383–2389. doi: 10.1016/j.jacc.2009.12.054 [DOI] [PubMed] [Google Scholar]
- 17. Sager HB, Husser O, Steffens S, Laugwitz KL, Schunkert H, Kastrati A, Ndrepepa G, Kessler T. Time‐of‐day at symptom onset was not associated with infarct size and long‐term prognosis in patients with ST‐segment elevation myocardial infarction. J Transl Med. 2019;17:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, et al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;137:961–972. doi: 10.1161/CIRCULATIONAHA.117.033502 [DOI] [PubMed] [Google Scholar]
- 19. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 20. Ducrocq G, Gonzalez‐Juanatey JR, Puymirat E, Lemesle G, Cachanado M, Durand‐Zaleski I, Arnaiz JA, Martinez‐Selles M, Silvain J, Ariza‐Sole A, et al. Effect of a restrictive vs Liberal blood transfusion strategy on major cardiovascular events among patients with acute myocardial infarction and anemia: the REALITY randomized clinical trial. JAMA. 2021;325:552–560. doi: 10.1001/jama.2021.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aronson D, Suleiman M, Agmon Y, Suleiman A, Blich M, Kapeliovich M, Beyar R, Markiewicz W, Hammerman H. Changes in haemoglobin levels during hospital course and long‐term outcome after acute myocardial infarction. Eur Heart J. 2007;28:1289–1296. doi: 10.1093/eurheartj/ehm013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1–S4


