Abstract
Background
Carotid artery stenosis (CAS) is a common cause of ischemic stroke, and the early detection of CAS may improve patient outcomes. Carotid Doppler ultrasound is commonly used to diagnose CAS. However, it is costly and may not be practical for regular screening practice. This article presents a novel noninvasive and noncontact detection technique using video‐based motion analysis (VMA) to extract useful information from subtle pulses on the skin surface to screen for CAS.
Methods and Results
We prospectively enrolled 202 patients with prior carotid Doppler ultrasound data. A short 30‐second video clip of the neck was taken using a commercial mobile device and analyzed by VMA with mathematical quantification of the amplitude of skin motion changes in a blinded manner. The first 40 subjects were used to set up the VMA protocol and define cutoff values, and the following 162 subjects were used for validation. Overall, 54% of the 202 subjects had ultrasound‐confirmed CAS. Using receiver operating characteristic curve analysis, the area under the curve of VMA‐derived discrepancy values to differentiate patients with and without CAS was excellent (area under the curve, 0.914 [95% CI, 0.874–0.954]; P<0.01). The best cutoff value of VMA‐derived discrepancy values to screen for CAS was 5.1, with a sensitivity of 87% and a specificity of 87%. The diagnostic accuracy was consistently high in different subject subgroups.
Conclusions
A simple and accurate screening technique to quickly screen for CAS using a VMA system is feasible, with acceptable sensitivity and specificity.
Keywords: carotid artery disease, mobile device, screening, video‐based motion analysis
Subject Categories: Diagnostic Testing
Nonstandard Abbreviations and Acronyms
- CAS
carotid artery stenosis
- NASCET
North American Symptomatic Carotid Endarterectomy Trial
- ROI
region of interest
- VMA
video‐based motion analysis
Clinical Perspective.
What Is New?
Carotid artery stenosis is a common cause of ischemic stroke, and the early detection of carotid artery stenosis may improve patient outcomes.
We developed a novel noninvasive and noncontact detection technique using video‐based motion analysis to extract useful information from subtle pulses on the skin surface to screen for carotid artery stenosis, with acceptable sensitivity and specificity.
What Are the Clinical Implications?
The video‐based motion analysis is a simple and accurate screening technique to quickly screen for carotid artery stenosis, and it is applicable in daily clinical practice.
This technique may be used in telehealth or a remote application in the future.
Carotid artery stenosis (CAS) is an important cause of ischemic stroke, 1 , 2 and the early diagnosis and treatment of CAS can improve long‐term outcomes. 3 , 4 , 5 , 6 CAS can be diagnosed by invasive angiography using the NASCET (North American Symptomatic Carotid Endarterectomy Trial) method 7 ; however, angiography cannot be used for the early detection of CAS. Carotid auscultation is limited by its low sensitivity and specificity in CAS screening, despite its availability. 8 Currently, carotid Doppler ultrasound is the preferred noninvasive tool to assess the severity of CAS 9 , 10 ; however, it is limited by patient obesity, neck immobility, and interobserver variability. 11 Computed tomography angiography and magnetic resonance angiography are accurate tools, but they are expensive and impractical for screening purposes. 9
CAS results in altered pulsation characteristics on the skin surface. Minute but distinct differences in the patterns between healthy and diseased vessels may in theory be detectable. Various methods of data processing, feature extraction, and pattern recognition for image classification have been developed. 12 , 13 Digital video processing techniques with motion magnification can be used to detect subtle movements, and video‐based motion analysis (VMA) has been applied in material engineering, structure stability, and infant monitoring. 14 , 15
We developed a novel noninvasive and noncontact detection technique to screen for CAS. This technique uses a short video clip of the neck, which is processed mathematically using an optical flow method 16 , 17 , 18 and principal component analysis. 19 The aim of this study was to investigate and validate the diagnostic power of this VMA‐based technique to identify the patients with CAS.
METHODS
Anonymized patient‐level data will be made available by the corresponding author on reasonable request.
Patients
We first prospectively enrolled 40 Taiwanese subjects (20 with CAS and 20 without CAS) at National Taiwan University Hospital to set up the video recording environment and protocol and calculate the appropriate discrepancy cutoff for the model algorithm. After the initial 40 cases, we further enrolled 162 subjects (89 with CAS and 73 without CAS) for validation. The inclusion criteria were as follows: (1) subjects who received carotid Doppler ultrasound within 6 months before enrollment; and (2) aged >20 years. The exclusion criteria were as follows: (1) a history of carotid stenting; and (2) refusal to participate. The baseline demographics, medical history, and laboratory studies were collected. All subjects provided written informed consent, and this study was approved by the Institutional Review Board of National Taiwan University Hospital and conducted under relevant guidelines and regulations.
Video Recording Setup
The system consisted of the video equipment with accessories and VMA software. Each patient was asked to lie in the supine position with his/her head placed inside a custom‐made box positioned to avoid movements during video recording (Figure 1A). An Apple iPhone 6 64GB (Apple Inc, Cupertino, CA) was mounted through a rectangular cutout on the top side of the box and used to record the video. Two fiber‐optic lights were installed inside the box on either side of the iPhone, oriented at an ≈45° angle in opposite directions to create a uniform light source. Each patient was asked to tilt his/her head backwards and lift his/her chin to stretch his/her neck. A 30‐second video clip was then recorded at 30 frames per second and pixel resolution of 1920×1080 (Figure 1B). The video file was uploaded to the cloud for processing using Intel Core i7‐3930K 6‐Core 3.2‐GHz desktop processors. A rectangular region of interest (ROI), bounded below the patient's chin and above the patient’s clothing, was manually boxed for VMA. The pixel numbers inside the ROI were sorted from top to bottom and then from left to right in ascending order.
Figure 1. Video recording and processing.
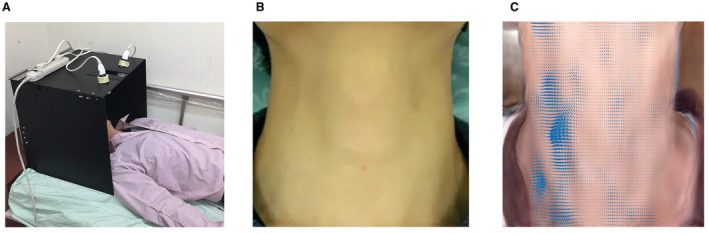
A, Video recording setup. B, Original video recording. C, Video processing and highlighting the movements of every pixel in each frame.
Video‐Based Motion Analysis
VMA involves a series of video processing techniques. Motion magnification was done first on video inputs by applying decomposition of different spatial frequency bands followed by temporal filtering. 12 Variations in pixel intensity in each spatial frequency band were extracted and handled individually using fast Fourier transform. They were magnified within a specific frequency range, such as heart rate of the patient, by choosing user‐specified parameters for motion magnification. Principal component analysis with Fourier transformation is used to recover the blood volume pulses that can be used for the heart rate estimation. 19
The magnified video was further processed using an optical flow method and principal component analysis. The optical flow method used temporal variations in pixel intensity of image sequences to determine the motion of objects between consecutive frames. This allowed for the construction of a flow vector field in which each vector was a velocity or displacement with up to one vector per pixel, highlighting the movement of every pixel in each frame (Figure 1C). Because large data sets of a video sequence are often difficult to interpret, the flow vector field generated by the optical flow method was further processed using principal component analysis to reduce dimensionality and maximize correlations among the input features. The output consisted of unique pulse information for each patient, and the video sequence was expressed as a temporal oscillating waveform after a series of video processing techniques. The resulting waveform was quantified to estimate its model coefficients using a nonlinear least‐squares fitting method. We were then able to approximate the oscillating waveform with quantified coefficients, including amplitude and frequency. The VMA analysis was performed with MATLAB R2015b software (The MathWorks, Inc, Natick, MA).
Criteria for CAS Detection
The selected ROIs were boxed within the original ROI to include the right and left carotid area. Its size was usually one quarter or one half that of the original ROI. The quantified coefficients calculated in VMA were compared between the original ROI and the selected ROIs. VMA‐derived discrepancy was defined as the difference in amplitude between the original ROI and the selected ROI. The highest VMA‐derived discrepancy of the subject was obtained for further analysis. Patients with CAS had larger discrepancies, as their pulse characteristics were not uniform in nature because of local vessel narrowing and hemodynamic change. Receiver operating characteristic (ROC) curve analysis was used to define the optimal cutoff VMA‐derived discrepancy value to detect CAS.
Carotid Doppler Ultrasonography
All subjects received standard carotid Doppler ultrasonography using an EPIQ Ultrasound System (Philips, Amsterdam, the Netherlands) before the video was recorded, and the results were blinded for the VMA. The extent and degree of CAS were described in accordance with the European Society of Cardiology guidelines. 20 , 21
Statistical Analysis
Data are expressed as mean±SD for normally distributed data. Comparisons of data between patients with and without CAS were made using the independent t test. Differences between proportions were assessed using the χ2 test or the Fisher exact test. One‐way ANOVA was used to analyze the data with the Scheffé method for post hoc analysis. The optimal cutoff VMA‐derived discrepancy value was determined using ROC curve analysis. Sensitivity, specificity, accuracy, positive predictive value, and negative predictive value were calculated using logistic regression with generalized estimating equations that accounted for clustering of the same patient. Univariable linear regression was used to assess associations between variables and VMA‐derived discrepancy values, and variables with P<0.1 were then selected for multivariable linear regression analysis. A 2‐sided P<0.05 was considered to be statistically significant. The statistical analyses were performed using SPSS v25 for Windows (IBM Corp, Armonk, NY).
RESULTS
Patients
A total of 202 patients were enrolled in this study, including 109 (54%) patients with and 93 (46%) patients without significant CAS (≥50%) detected by ultrasound. The baseline characteristics of the patients in the setup and validation cohorts are summarized in Table 1 and Table S1. The baseline characteristics were well balanced between both cohorts. Overall, the patients with CAS were significantly older, were predominantly women, had a lower body mass index, and more had a history of head and neck radiation (Table 1). Of the 109 patients with CAS, 34 (31%) had isolated right‐sided stenosis, 29 (27%) had isolated left‐sided stenosis, and 46 (42%) had bilateral stenosis (Table 1).
Table 1.
Patient Characteristics and Results of the VMA
| Variable | Setup cohort (N=40) | Validation cohort (N=162) | Total cohort (N=202) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| With CAS (N=20) | Without CAS (N=20) | P value | With CAS (N=89) | Without CAS (N=73) | P value | With CAS (N=109) | Without CAS (N=93) | P value | |
| Patient characteristics | |||||||||
| Age, y | 69.5±10.5 | 67.5±10.9 | 0.568 | 70.2±10.3 | 65.7±11.0 | 0.008 | 70.1±10.3 | 66.1±11.0 | 0.008 |
| Male sex | 13 (65) | 16 (80) | 0.288 | 63 (71) | 66 (90) | 0.002 | 76 (70) | 82 (88) | 0.002 |
| BMI, kg/m2 | 25.5±3.1 | 25.8±3.6 | 0.813 | 24.4±4.9 | 26.2±3.2 | 0.007 | 24.6±4.6 | 26.1±3.2 | 0.009 |
| Diabetes | 6 (30) | 7 (35) | 0.736 | 33 (37) | 34 (47) | 0.222 | 39 (36) | 41 (44) | 0.229 |
| Hypertension | 16 (80) | 10 (50) | 0.047 | 67 (75) | 58 (80) | 0.529 | 83 (76) | 68 (73) | 0.621 |
| Dyslipidemia | 12 (60) | 19 (95) | 0.008 | 69 (78) | 49 (67) | 0.138 | 81 (74) | 68 (73) | 0.848 |
| Atrial fibrillation | 1 (5) | 1 (5) | 1.000 | 5 (6) | 2 (3) | 0.459 | 6 (6) | 3 (3) | 0.511 |
| Cervical irradiationhistory | 4 (20) | 0 (0) | 0.106 | 18 (20) | 0 (0) | <0.001 | 22 (20.2) | 0 (0) | <0.001 |
| Hemoglobin, g/dL | 12.9±2.1 | 13.5±1.8 | 0.336 | 13.2±2.0 | 13.7±1.8 | 0.145 | 13.2±2.1 | 13.6±1.8 | 0.087 |
| Creatinine, mg/dL | 1.4±0.7 | 1.1±0.6 | 0.282 | 1.5±1.8 | 1.6±2.2 | 0.686 | 1.4±1.6 | 1.5±2.0 | 0.859 |
| Fasting glucose, mg/dL | 106.7±26.5 | 108.1±26.2 | 0.868 | 111.2±34.4 | 121.8±41.8 | 0.088 | 110.3±33.0 | 118.7±39.2 | 0.102 |
| HbA1c, % | 6.2±1.0 | 6.1±0.09 | 0.621 | 6.3±1.1 | 6.1±0.9 | 0.322 | 6.3±1.1 | 6.1±0.9 | 0.257 |
| Total cholesterol, mg/dL | 171.2±35.8 | 148.6±37.4 | 0.058 | 151.1±30.2 | 149.9±42.1 | 0.833 | 154.8±32.1 | 149.6±41.0 | 0.316 |
| Triglyceride, mg/dL | 135.1±68.0 | 129.2±101.4 | 0.831 | 125.9±55.8 | 143.0±160.3 | 0.349 | 127.6±58.0 | 140.0±149.1 | 0.424 |
| LDL‐C, mg/dL | 99.8±25.0 | 78.7±29.7 | 0.020 | 83.2±24.1 | 82.2±29.6 | 0.804 | 82.3±25.0 | 81.4±29.5 | 0.208 |
| HDL‐C, mg/dL | 46.6±13.3 | 49.0±9.1 | 0.519 | 45.6±11.1 | 44.7±11.3 | 0.599 | 45.8±11.5 | 45.6±11.5 | 0.903 |
| Carotid Doppler ultrasound | |||||||||
| Isolated right CAS | 4 (20) | 0 (0) | N/A | 30 (34) | 0 (0) | N/A | 34 (31) | 0 (0) | N/A |
| Isolated left CAS | 6 (30) | 0 (0) | N/A | 23 (26) | 0 (0) | N/A | 29 (27) | 0 (0) | N/A |
| Bilateral CAS | 10 (50) | 0 (0) | N/A | 36 (40) | 0 (0) | N/A | 46 (42) | 0 (0) | N/A |
| Severity of CAS stenosis* | |||||||||
| Normal (0%–29%) | 0 (0) | 13 (65) | <0.001 | 0 (0) | 57 (78) | <0.001 | 0 (0) | 70 (75) | <0.001 |
| Mild (30%–49%) | 0 (0) | 7 (35) | 0 (0) | 16 (22) | 0 (0) | 23 (25) | |||
| Moderate (50%–69%) | 1 (5) | 0 (0) | 21 (24) | 0 (0) | 22 (20) | 0 (0) | |||
| Severe (70%–99%) | 14 (70) | 0 (0) | 52 (58) | 0 (0) | 66 | 0 (0) | |||
| Total occlusion (100%) | 5 (25) | 0 (0) | 16 (18) | 0 (0) | 21 (19) | 0 (0) | |||
| VMA | |||||||||
| Discrepancy value | 7.9±2.9 | 3.2±2.0 | <0.001 | 9.4±4.5 | 3.0±2.1 | <0.001 | 9.1±4.2 | 3.1±2.1 | <0.001 |
Data are given as mean±SD or number (percentage). BMI indicates body mass index; CAS, carotid artery stenosis; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; N/A, not applicable; and VMA, video‐based motion analysis.
The severity of CAS was defined as the greatest carotid stenosis in bilateral extracranial carotid arteries.
VMA‐Derived Discrepancy Value to Identify CAS
The VMA‐derived discrepancy value was significantly higher in the patients with CAS in the setup, validation, and total cohorts (Table 1 and Figure 2A and 2B). The discrepancy value showed good diagnostic power to identify the patients with CAS. In ROC curve analysis, the area under the curve of VMA‐derived discrepancy value to differentiate patients with and without CAS was 0.904 (95% CI, 0.815–0.993; P<0.001) in the 40 setup cohort patients, 0.912 (95% CI, 0.867–0.958; P<0.001) in the 162 validation cohort patients, and 0.914 (95% CI, 0.874–0.954; P<0.001) in the 202 total cohort patients (Figure 3).
Figure 2. Discrepancy values detected by video motion analysis in patients with and without carotid artery stenosis (CAS).
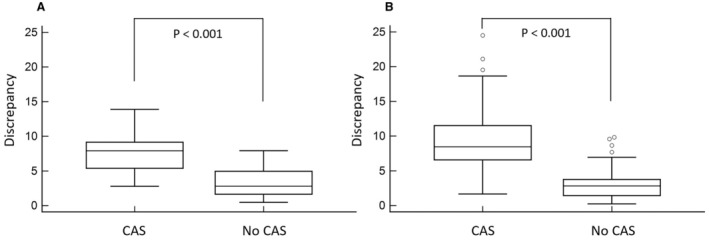
The video‐based motion analysis–derived discrepancy values of patients with and without CAS in the setup cohort (A) and the validation cohort (B).
Figure 3. Receiver operating characteristic curves of video motion analysis for detecting carotid artery stenosis (CAS).
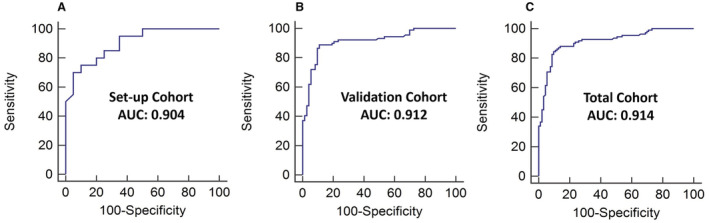
The area under the curve (AUC) of video‐based motion analysis–derived discrepancy values in differentiating patients with and without CAS was 0.904 in the setup cohort (A), 0.912 in the validation cohort (B), and 0.914 in the total cohort (C).
VMA‐Derived Discrepancy in Different Severity of CAS
The relationship between the severity of carotid stenosis and VMA‐derived discrepancy is illustrated in Figure 4. The VMA‐derived discrepancy values were 2.96±2.00 in CAS 0% to 29% (N=70), 3.48±2.21 in CAS 30% to 49% (N=23), 8.38±5.69 in CAS 50% to 69% (N=22), 9.52±4.14 in CAS 70% to 99% (N=66), and 8.46±2.63 in CAS 100% (N=21). The VMA‐derived discrepancy was significantly higher in patients with >50% stenosis compared with those with ≤49% stenosis. However, there was no significant difference among patients with different CAS severity.
Figure 4. The video‐based motion analysis (VMA)–derived discrepancy in different severity of carotid artery stenosis.
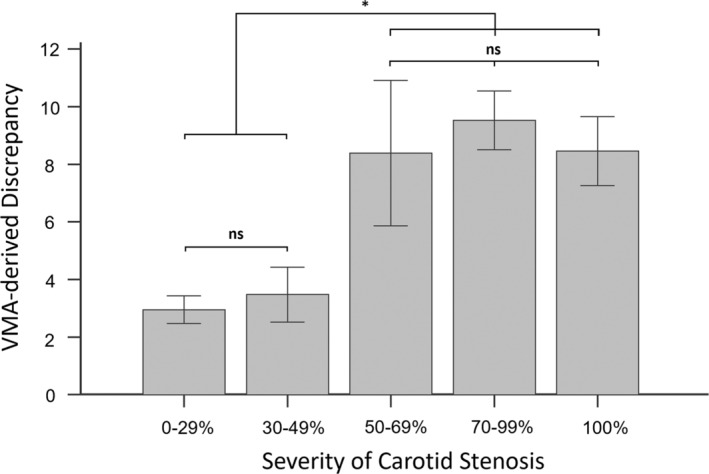
The VMA‐derived discrepancy was significantly higher in patients with >50% stenosis. Ns indicates nonsignificance. *P<0.05.
Optimal VMA‐Derived Discrepancy Cutoff Value
The optimal cutoff VMA‐derived discrepancy value was calculated using ROC curve analysis in the first 40 cases. On the basis of the Youden J statistic, VMA‐derived discrepancy value of 5.5 was first determined to be the optimal cutoff, with 75% sensitivity and 90% specificity. However, we think the 75% sensitivity is too low for screening purpose. We therefore calculated several different cutoffs (4.1, 5.1, and 7.9) using the ROC curve (Table 2), and decided to use 5.1 as the best cutoff with balanced sensitivity (80% [95% CI, 56%–94%]) and specificity (80% [95% CI, 56%–94%]), with a positive predictive value of 80% (95% CI, 62%–91%) and negative predictive value of 80% (95% CI, 62%–91%). The cutoff value of discrepancy in the following validation cohort was then set at 5.1. In the overall cohort, including the initial 40 setup and 162 validation cases, the 5.1 discrepancy value showed high sensitivity (87% [95% CI, 79%–93%]) and specificity (87% [95% CI, 79%–93%]), with a positive predictive value of 89% (95% CI, 82%–93%) and negative predictive value of 85% (95% CI, 78%–90%) in detecting CAS (Table 2).
Table 2.
Diagnostic Performance of VMA in CAS Screening at Different Discrepancy Cutoff Values
| Variable | Discrepancy cutoff value | |||
|---|---|---|---|---|
| ≥4.1 | ≥5.1 | ≥5.5 | ≥7.9 | |
| Setup cohort (N=40, including 20 with CAS and 20 without CAS) | ||||
| Sensitivity, % | 90 (95% CI, 68–99) | 80 (95% CI, 56–94) | 75 (95% CI, 51–91) | 55 (95% CI, 32–77) |
| Specificity, % | 65 (95% CI, 41–85) | 80 (95% CI, 56–94) | 90 (95% CI, 68–99) | 95 (95% CI, 75–100) |
| Positive predictive value, % | 72 (95% CI, 58–83) | 80 (95% CI, 62–91) | 88 (95% CI, 66–97) | 92 (95% CI, 61–99) |
| Negative predictive value, % | 87 (95% CI, 63–96) | 80 (95% CI, 62–91) | 78 (95% CI, 62–89) | 68 (95% CI, 56–78) |
| Validation cohort (N=162, including 89 with CAS and 73 without CAS) | ||||
| Sensitivity, % | 91 (95% CI, 83–96) | 89 (95% CI, 80–95) | 87 (95% CI, 78–93) | 55 (95% CI, 44–66) |
| Specificity, % | 78 (95% CI, 65–86) | 89 (95% CI, 80–95) | 80 (95% CI, 81–96) | 96 (95% CI, 88–99) |
| Positive predictive value, % | 83 (95% CI, 76–88) | 91 (95% CI, 84–95) | 92 (95% CI, 84–96) | 94 (95% CI, 84–98) |
| Negative predictive value, % | 88 (95% CI, 78–93) | 87 (95% CI, 78–92) | 85 (95% CI, 76–90) | 64 (95% CI, 58–69) |
| Total cohort (N=202, including 109 with CAS and 93 without CAS) | ||||
| Sensitivity, % | 91 (95% CI, 84–96) | 87 (95% CI, 79–93) | 84 (95% CI, 76–91) | 55 (95% CI, 45–65) |
| Specificity, % | 74 (95% CI, 64–83) | 87 (95% CI, 79–93) | 90 (95% CI, 82–95) | 96 (95% CI, 89–99) |
| Positive predictive value, % | 80 (95% CI, 74–85) | 89 (95% CI, 82–93) | 91 (95% CI, 85–95) | 94 (95% CI, 85–98) |
| Negative predictive value, % | 87 (95% CI, 79–93) | 85 (95% CI, 78–90) | 83 (95% CI, 76–88) | 64 (95% CI, 60–69) |
CAS indicates carotid artery stenosis; and VMA, video‐based motion analysis.
Potential Factors Complicating the VMA‐Derived Discrepancy Value
In univariable linear regression analysis, female sex, lower body mass index, history of cervical irradiation, and presence of CAS were significantly associated with higher VMA‐derived discrepancy values. However, in multivariable linear regression analysis, only the presence of CAS (β, 6.09 [95% CI, 5.05–7.14]; P<0.001) was significantly associated with higher discrepancy values (Table 3).
Table 3.
Univariable and Multivariable Linear Regression of Predictors Associated With Higher VMA‐Derived Discrepancy Values
| Univariable linear regression | Multivariable linear regression | |||
|---|---|---|---|---|
| VMA‐derived discrepancy values | ||||
| β (95% CI) | P value | β (95% CI) | P value | |
| Sex (men) | −1.42 (−2.94 to 0.10) | 0.067 | 0.24 (−0.96 to 1.43) | 0.696 |
| Age, y | 0.05 (−0.01 to 0.10) | 0.127 | ||
| BMI, kg/m2 | −0.17 (−0.32 to −0.02) | 0.030 | −0.04 (−0.16 to 0.08) | 0.507 |
| Atrial fibrillation, n (%) | 0.50 (−2.57 to 3.56) | 0.750 | ||
| Cervical irradiation history, n (%) | 2.64 (0.65 to 4.64) | 0.010 | −0.53 (−2.15 to 0.08) | 0.468 |
| CAS, n (%) | 6.00 (5.05 to 6.96) | <0.001 | 6.09 (5.05 to 7.14) | <0.001 |
BMI indicates body mass index; CAS, carotid artery stenosis; and VMA, video‐based motion analysis.
This analysis was performed in the whole cohort (N=202, including 109 patients with CAS and 93 patients without CAS). Sex, BMI, cervical irradiation history, and CAS were entered into the multivariable regression model.
VMA Diagnostic Performance in Different Subgroups
Subgroup analysis was performed to further examine the VMA performance in different patient subgroups, and the results are summarized in Table 4. The diagnostic performance remained good in all subgroups. In patients with bilateral CAS, the sensitivity remained high as 89% (95% CI, 75%–96%).
Table 4.
VMA Performance in CAS Screening Among Different Subgroups
| Subgroups | Sensitivity, % | Specificity, % | Positive predictive value, % | Negative predictive value, % |
|---|---|---|---|---|
| Aged ≥65 y (N=122) | 89 (95% CI, 80–95) | 94 (95% CI, 83–99) | 96 (95% CI, 88–98) | 85 (95% CI, 75–92) |
| Aged <65 y (N=80) | 83 (95% CI, 67–94) | 80 (95% CI, 65–90) | 77 (95% CI, 65–86) | 85 (95% CI, 73–92) |
| Male sex (N=158) | 89 (95% CI, 80–95) | 88 (95% CI, 78–94) | 87 (95% CI, 79–92) | 90 (95% CI, 82–95) |
| Female sex (N=44) | 82 (95% CI, 65–93) | 82 (95% CI, 48–98) | 93 (95% CI, 79–98) | 60 (95% CI, 41–77) |
| Obesity (BMI >25 kg/m2) (N=97) | 87 (95% CI, 73–95) | 90 (95% CI, 79–97) | 89 (95% CI, 77–95) | 89 (95% CI, 79–94) |
BMI indicates body mass index; CAS, carotid artery stenosis; and VMA, video‐based motion analysis.
This analysis was the subgroup analysis of the whole cohort (N=202, including 109 patients with CAS and 93 patients without CAS).
DISCUSSION
The results of the present study clearly demonstrated the accuracy of our noninvasive VMA technique to detect CAS. CAS accounts for 10% to 20% of all ischemic strokes, and signals the risk of early recurrent stroke. 22 , 23 Timely carotid revascularization by either carotid endarterectomy or carotid artery stenting and optimized medical therapy can improve the outcomes of patients with CAS. 9 , 24 , 25 , 26 , 27 However, asymptomatic CAS may be underdiagnosed, with the reported prevalence ranging from 0% to 0.2% in patients aged <50 years, and from 5.0% to 7.5% in those aged ≥80 years. 28 Furthermore, in high‐risk patients, such as those with prior head and neck radiotherapy, the incidence of CAS is even higher (17%–25%). 29 As the annual risk of stroke is ≈2% to 5% in patients with asymptomatic CAS, 30 , 31 early screening and detection of CAS is an important but unmet clinical need.
Increased blood velocity attributable to CAS can produce local bruit on simple auscultation; however, its sensitivity and specificity are low. 8 Carotid Doppler ultrasonography is the current tool of choice for the noninvasive screening of CAS in clinical practice. 10 However, interobserver variability as well as issues such as patient obesity and/or poor neck mobility are major limitations. 11 In addition, the performance and interpretation of neck ultrasound require specialized equipment and personnel, and such may not be universally available. Other diagnostic modalities, such as computed tomography angiography and magnetic resonance angiography, are even more expensive and invasive, and hence impractical for CAS screening. An easily performed, low‐cost, noninvasive tool without the need of professional execution or interpretation will be useful for this purpose. We proposed an easy and reproducible video magnification technique in the present study, which may even be analyzed remotely.
Video magnification techniques can enhance tiny variations to a visible level and reveal subtle motion differences. Such techniques have been applied to investigate conditions such as peripheral artery disease, 32 heart failure, 33 and neuromuscular disorders. 34 , 35 The cervical carotid artery is a superficial vessel. Changes in the velocity and pattern of blood flow through CAS lesions may result in differences in the motion of the overlying skin or soft tissue; however, these differences are too subtle to be detected by the naked eye. Through motion magnification, these minute changes in pulse characteristics can be detected and used for early noninvasive screening. The video clips of the patients' necks in this study were recorded using a commercial smart phone camera and processed digitally. The quantification data were then fed to the screening algorithm, and the established threshold value was used to recognize abnormalities.
In digital video processing, slight perturbations in the background can trigger a significant response to slight changes in the source video. In our pilot 40 cases, we found that the magnified video could be of poor quality when the complete frame included the foreground (the patient's neck) and background (clothing and bedsheet). Therefore, instead of using the complete frame for VMA, we manually boxed a rectangular ROI bounded by the patient's chin and collar. Future refinements of the recording setup, such as creating uniform background conditions, may eliminate the need to manually select the ROI before VMA, and this, in turn, may help to realize the goal of a fully automated screening device.
In the present study, our VMA technique demonstrated excellent power for detecting >50% diameter carotid stenosis with high sensitivity and specificity. Its performance was consistent across all patient subsets, including patients with bilateral disease (accounting for 42% of our cohort). We believe that our VMA technique can be incorporated into routine clinical practice in the future. This video‐based system is resource and personnel independent. Clinicians can simply take videos of the patients' neck using commercial mobile devices, upload the videos for cloud computation, and then receive evaluation reports within 5 minutes. The video recording may even be done by the patients at home, and processed in a remote telehealth manner. A suspected >50% diameter CAS will then prompt the physician for a diagnostic confirmation, with either carotid Doppler ultrasonography or other imaging modalities.
Limitations
There are several limitations to this study. First, this was a small study with a limited number of cases, which may have interfered with the interpretation of the results. Second, we did not analyze the effects of the neck length and the neck angle on the VMA‐derived discrepancy, which may in theory confound the results. Third, the subjects enrolled in this study were patients with high cardiovascular risk. Further studies recruiting patients with low cardiovascular risk are necessary to validate our VMA technique in general population. Fourth, patients with restenosis after prior carotid interventions were not included. Although we believe that such patients should be managed in a specialty clinic with dedicated facilities, the efficacy of VMA technique remains unknown in this specific group of patients.
CONCLUSIONS
We demonstrated that our novel noninvasive and noncontact VMA detection technique could accurately detect CAS and may be applied in future CAS screening.
Sources of Funding
This research was sponsored by the Ministry of Science and Technology in Taiwan through grants MOST‐108‐2823‐8‐002‐008 and MOST‐108‐2221‐E‐002‐153‐MY3.
Disclosures
Drs Hsiao and Kao cofounded a startup company, Pulxion, in 2020 to translate research work into clinical solutions.
Supporting information
Table S1
Acknowledgments
We thank the staff of the Eighth Core Lab, Department of Medical Research, National Taiwan University Hospital, for technical support during the study. We also acknowledge the Taiwan Health Foundation, Dr. T. Y. Lin's Medical Research Foundation, and the Yuanta Cultural and Educational Foundation for their support.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Jonas DE, Feltner C, Amick HR, Sheridan S, Zheng ZJ, Watford DJ, Carter JL, Rowe CJ, Harris R. Screening for asymptomatic carotid artery stenosis: a systematic review and meta‐analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:336–346. doi: 10.7326/M14-0530 [DOI] [PubMed] [Google Scholar]
- 2. Longstreth WT Jr, Shemanski L, Lefkowitz D, O'Leary DH, Polak JF, Wolfson SK Jr. Asymptomatic internal carotid artery stenosis defined by ultrasound and the risk of subsequent stroke in the elderly. The Cardiovascular Health Study. Stroke. 1998;29:2371–2376. doi: 10.1161/01.str.29.11.2371 [DOI] [PubMed] [Google Scholar]
- 3. Brott TG, Hobson RW II, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, et al. Stenting versus endarterectomy for treatment of carotid‐artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, Moore WS, Hill MD, Mantese VA, Clark WM, et al. Long‐term results of stenting versus endarterectomy for carotid‐artery stenosis. N Engl J Med. 2016;374:1021–1031. doi: 10.1056/NEJMoa1505215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. International Carotid Stenting Study investigators , Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, Lo TH, Gaines P, Dorman PJ, Macdonald S, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–997. doi: 10.1016/S0140-6736(10)60239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonati LH, Dobson J, Featherstone RL, Ederle J, van der Worp HB, de Borst GJ, Mali WP, Beard JD, Cleveland T, Engelter ST, et al. Long‐term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet. 2015;385:529–538. doi: 10.1016/S0140-6736(14)61184-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. North American Symptomatic Carotid Endarterectomy Trial C , Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox AJ, Rankin RN, Hachinski VC, Wiebers DO, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high‐grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701 [DOI] [PubMed] [Google Scholar]
- 8. Hankey GJ, Warlow CP. Symptomatic carotid ischaemic events: safest and most cost effective way of selecting patients for angiography, before carotid endarterectomy. BMJ. 1990;300:1485–1491. doi: 10.1136/bmj.300.6738.1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO) the Task Force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095 [DOI] [PubMed] [Google Scholar]
- 10. Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, Berry E, Young G, Rothwell P, Roditi G, et al. Accurate, practical and cost‐effective assessment of carotid stenosis in the UK. Health Technol Assess. 2006;10:iii–iv, ix–x, 1–182. doi: 10.3310/hta10300 [DOI] [PubMed] [Google Scholar]
- 11. Mead GE, Lewis SC, Wardlaw JM. Variability in Doppler ultrasound influences referral of patients for carotid surgery. Eur J Ultrasound. 2000;12:137–143. doi: 10.1016/s0929-8266(00)00111-7 [DOI] [PubMed] [Google Scholar]
- 12. Fedotov A. Selection of parameters of bandpass filtering of the ECG signal for heart rhythm monitoring systems. Biomed Eng. 2016;50:114–118. doi: 10.1007/s10527-016-9600-8 [DOI] [Google Scholar]
- 13. Szelag E, Kanabus M, Kolodziejczyk I, Kowalska J, Szuchnik J. Individual differences in temporal information processing in humans. Acta Neurobiol Exp (Wars). 2004;64:349–366. [DOI] [PubMed] [Google Scholar]
- 14. Wu H‐Y, Rubinstein M, Shih E, Guttag J, Durand F, Freeman W. Eulerian video magnification for revealing subtle changes in the world. ACM Trans Graph. 2012;31:1–8. doi: 10.1145/2185520.2185561 [DOI] [Google Scholar]
- 15. Elgharib M, Hefeeda M, Durand F, Freeman WT. Video magnification in presence of large motions. Paper/Poster Presented at: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. 2015.
- 16. Zainal Z, Ramli R, Mustafa MM. Improved heading direction interpretation via optical flow using selected region of interest. Procedia Eng. 2012;41:1298–1306. doi: 10.1016/j.proeng.2012.07.314 [DOI] [Google Scholar]
- 17. Sharmin N, Brad R. Optimal filter estimation for Lucas‐Kanade optical flow. Sensors. 2012;12:12694–12709. doi: 10.3390/s120912694 [DOI] [Google Scholar]
- 18. Kayaba H, Kokumai Y. Non‐contact full field vibration measurement based on phase‐shifting. Paper/Poster presented at: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. 2017.
- 19. Yu Y‐P, Raveendran P, Lim C‐L, Kwan B‐H. Dynamic heart rate estimation using principal component analysis. Biomed Opt Express. 2015;6:4610–4618. doi: 10.1364/BOE.6.004610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sprynger M, Rigo F, Moonen M, Aboyans V, Edvardsen T, de Alcantara ML, Brodmann M, Naka KK, Kownator S, Simova I, et al. Focus on echovascular imaging assessment of arterial disease: complement to the ESC guidelines (PARTIM 1) in collaboration with the Working Group on Aorta and Peripheral Vascular Diseases. Eur Heart J Cardiovasc Imaging. 2018;19:1195–1221. doi: 10.1093/ehjci/jey103 [DOI] [PubMed] [Google Scholar]
- 21. Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, Carroll BA, Eliasziw M, Gocke J, Hertzberg BS, et al. Carotid artery stenosis: gray‐scale and Doppler US diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229:340–346. doi: 10.1148/radiol.2292030516 [DOI] [PubMed] [Google Scholar]
- 22. Flaherty ML, Kissela B, Khoury JC, Alwell K, Moomaw CJ, Woo D, Khatri P, Ferioli S, Adeoye O, Broderick JP, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology. 2013;40:36–41. doi: 10.1159/000341410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population‐based incidence studies. Neurology. 2004;62:569–573. doi: 10.1212/01.wnl.0000110311.09970.83 [DOI] [PubMed] [Google Scholar]
- 24. Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, Taylor DW, Haynes RB, Finan JW, Hachinski VC, et al. The north American symptomatic carotid endarterectomy trial: surgical results in 1415 patients. Stroke. 1999;30:1751–1758. doi: 10.1161/01.str.30.9.1751 [DOI] [PubMed] [Google Scholar]
- 25. Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D; Group MRCACSTC . Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1 [DOI] [PubMed] [Google Scholar]
- 26. Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Ansel G, Strickman NE, Wang H, Cohen SA, et al. Long‐term results of carotid stenting versus endarterectomy in high‐risk patients. N Engl J Med. 2008;358:1572–1579. doi: 10.1056/NEJMoa0708028 [DOI] [PubMed] [Google Scholar]
- 27. Silver FL, Mackey A, Clark WM, Brooks W, Timaran CH, Chiu D, Goldstein LB, Meschia JF, Ferguson RD, Moore WS, et al. Safety of stenting and endarterectomy by symptomatic status in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST). Stroke. 2011;42:675–680. doi: 10.1161/STROKEAHA.110.610212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O'Leary DH, Rosvall M, Sitzer M, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta‐analysis. Stroke. 2010;41:1294–1297. doi: 10.1161/STROKEAHA.110.581058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu J, Cao Y. Radiation‐induced carotid artery stenosis: a comprehensive review of the literature. Interv Neurol. 2014;2:183–192. doi: 10.1159/000363068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Norris JW, Zhu CZ, Bornstein NM, Chambers BR. Vascular risks of asymptomatic carotid stenosis. Stroke. 1991;22:1485–1490. doi: 10.1161/01.str.22.12.1485 [DOI] [PubMed] [Google Scholar]
- 31. Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, Barnett HJ. The causes and risk of stroke in patients with asymptomatic internal‐carotid‐artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 2000;342:1693–1700. doi: 10.1056/NEJM200006083422302 [DOI] [PubMed] [Google Scholar]
- 32. Bennett SL, Goubran RA, Bennett B, Bennett RA, Knoefel F. The use of a thermal camera and Eulerian enhancement in the examination of pedal pulse and microvascular health. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:1385–1388. doi: 10.1109/EMBC.2016.7590966 [DOI] [PubMed] [Google Scholar]
- 33. Abnousi F, Kang G, Giacomini J, Yeung A, Zarafshar S, Vesom N, Ashley E, Harrington R, Yong C. A novel noninvasive method for remote heart failure monitoring: the EuleriAn video magnification apPLications in heart failure studY (AMPLIFY). NPJ Digit Med. 2019;2:80. doi: 10.1038/s41746-019-0159-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Hillegondsberg L, Carr J, Brey N, Henning F. Using Eulerian video magnification to enhance detection of fasciculations in people with amyotrophic lateral sclerosis. Muscle Nerve. 2017;56:1063–1067. doi: 10.1002/mus.25690 [DOI] [PubMed] [Google Scholar]
- 35. Adleberg J, O'Connell Ferster AP, Benito DA, Sataloff RT. Detection of muscle tension dysphonia using Eulerian video magnification: a pilot study. J Voice. 2020;34:622–628. doi: 10.1016/j.jvoice.2019.02.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


