Abstract
Background
Waveform parameters provide approximate data about aortic wave reflection. However, their association with cardiovascular events remains controversial and their role in cardiovascular prediction is unknown.
Methods and Results
We analyzed participants aged between 40 and 69 from the population‐based CARTaGENE cohort. Baseline pulse wave analysis (central pulse pressure, augmentation index) and wave separation analysis (forward pressure, backward pressure, reflection magnitude) parameters were derived from radial artery tonometry. Associations between each parameter and major adverse atherosclerotic events (MACE; cardiovascular death, stroke, myocardial infarction) were obtained using adjusted Cox models. The incremental predictive value of each parameter compared with the 10‐year atherosclerotic cardiovascular disease score alone was assessed using hazard ratios, c‐index differences, continuous net reclassification indexes, and integrated discrimination indexes. From 17 561 eligible patients, 2315 patients had a MACE during a median follow‐up of 10.1 years. Central pulse pressure, forward pressure, and backward pressure, but not augmentation index and reflection magnitude, were significantly associated with MACE after full adjustment. All parameters except forward pressure statistically improved MACE prediction compared with the atherosclerotic cardiovascular disease score alone. The greatest prediction improvement was seen with augmentation index and reflection magnitude but remained small in magnitude. These 2 parameters enhanced predictive performance more strongly in patients with low baseline atherosclerotic cardiovascular disease scores. Up to 5.7% of individuals were reclassified into a different risk stratum by adding waveform parameters to atherosclerotic cardiovascular disease scores.
Conclusions
Some waveform parameters are independently associated with MACEs in a population‐based cohort. Augmentation index and reflection magnitude slightly improve risk prediction, especially in patients at low cardiovascular risk.
Keywords: cardiovascular prediction, central pressure, pulse wave analysis, wave separation analysis
Subject Categories: Cardiovascular Disease, Hemodynamics
Nonstandard Abbreviations and Acronyms
- AIx@75
augmentation index at 75 beats per minute
- cNRI
continuous net reclassification index
- cPP
central pulse pressure
- IDI
integrated discrimination index
- MACE
major adverse cardiovascular events
- Pb
backward pressure
- Pf
forward pressure
- RM
reflection magnitude
Clinical Perspective.
What Is New?
We conducted the largest study to date that evaluated the association of noninvasive pulse waveform parameters with cardiovascular events in a population‐based cohort.
Three waveform parameters (central pulse pressure, forward pressure, backward pressure) were independently associated with the incidence of major adverse cardiovascular events during a median follow‐up of 10 years.
The addition of 2 waveform parameters (augmentation index and areflection magnitude) to the atherosclerotic cardiovascular disease score improved cardiovascular prediction (especially in patients at low baseline risk) and reclassified up to 5.7% of patients in another atherosclerotic cardiovascular disease risk category.
What Are the Clinical Implications?
Cardiovascular prediction tools may be improved by the addition of noninvasive waveform parameters.
These parameters might be used to identify patients at increased cardiovascular risk among low‐risk populations.
Hypertension is the world's leading risk factor for global disease burden and is associated with several unfavorable outcomes such as cardiovascular disease, kidney failure, and cognitive impairment. 1 , 2 , 3 Although brachial cuff measurement of blood pressure (BP) has been used for more than a century, it is recognized as an imperfect marker of cardiovascular biomechanics. Indeed, brachial BP is an inaccurate and imprecise surrogate of intra‐aortic BP, 4 notably because of variability in reflected wave timing and pressure amplification. Furthermore, by focusing on the maximum (systolic) and minimum (diastolic) of the pulse waveform, it ignores the full extent of waveform characteristics and how it is differently affected by antihypertensive agents than brachial BP. 5 Despite all this, brachial‐cuff derived BP remains the sole BP parameter used in several cardiovascular prediction scores. 6 , 7 , 8
Noninvasive devices now allow the measurement of central waveform parameters derived from tonometry or cuff‐based methods. In this regard, conventional time‐domain pulse wave analysis yields parameters such as central pulse pressure (cPP) and augmentation index adjusted for 75 bpm (AIx@75; Figure 1). More recently, decomposition of the central waveform in a forward and backward component by wave separation analysis has allowed the derivation of additional markers of adverse aortic biomechanics (Figure 1). 9 , 10 Although these parameters have been associated with surrogates of cardiac load and end‐organ damage, 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 their epidemiological association with long‐term cardiovascular outcomes remains controversial. 11 , 12 , 21 , 22 , 23 , 24 , 25 , 26 , 27 Furthermore, several studies concerning these parameters were led in high‐risk populations (such as patients with chronic kidney disease or coronary artery disease) and are thus not generalizable to the overall population. Finally, in contrast to the aortic pulse wave velocity, 28 the incremental predictive value of these parameters over clinical scores that use the brachial cuff systolic BP has not been evaluated thoroughly. Further data are thus needed to support the use of these parameters in clinical practice.
Figure 1. Pulse wave and wave separation analysis.
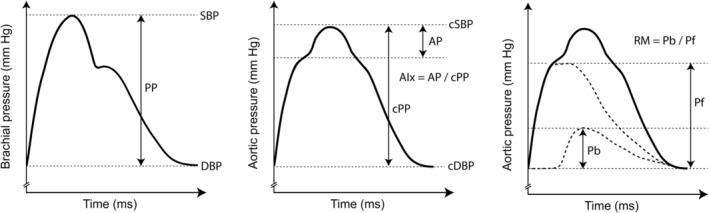
AIx indicates augmentation index; AP, augmented pressure; cPP, central pulse pressure; cDBP, central diastolic blood pressure; cSBP, central systolic blood pressure; DBP, diastolic blood pressure; Pb, backward pressure; Pf, forward pressure; PP, pulse pressure; RM, reflection magnitude; and SBP, systolic blood pressure.
The objectives of our study were to use the population‐based cohort CARTaGENE to (1) evaluate the independent association of each waveform parameter with major adverse cardiovascular events (MACE); (2) assess the incremental predictive value of waveform parameters for MACEs over the atherosclerotic cardiovascular disease (ASCVD) prediction score; and (3) examine whether baseline cardiovascular risk influences the predictive performance of waveform parameters.
METHODS
Data Availability
CARTaGENE data (https://cartagene.qc.ca) were used under license. Restrictions apply to its availability to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Design, Data Sources, and Population
This study was conducted using data from the population‐based survey CARTaGENE (https://cartagene.qc.ca), created to evaluate determinants of chronic diseases. Requests to access data used in this study from qualified researchers trained in human subject confidentiality protocols may be sent to CARTaGENE. Complete details concerning its recruitment and data collection processes have been previously published. 29 , 30 It formed a representative sample of the Quebec province by recruiting 19 996 individuals aged between 40 and 69 years in 2009 and 2010. Individuals in nursing homes, jails, or native reserves were excluded. For this substudy, all participants of CARTaGENE were included unless they had missing outcome, brachial BP, or radial tonometry data. Health questionnaires, physical measurements, medication usage, and blood samples were collected during a recruitment visit by a nurse. Consent for the CARTaGENE study and for follow‐up using medico‐administrative databases was obtained at recruitment. The study was authorized by the local institutional review committee and all authors adhered to the Helsinki Declaration.
Data Collection
Demographics, medical history, and medication use were collected at recruitment with standardized questionnaires. Physical parameters (height, weight, body mass index [BMI]) were measured at baseline. Blood samples were drawn during this visit and were used to compute the lipid panel and estimated glomerular filtration rate (eGFR, using the Chronic Kidney Disease Epidemiology Collaboration formula from isotope dilution mass spectroscopy‐calibrated creatinine). 31 Diabetes was diagnosed when any of the following were present: a glycosylated hemoglobin A1c ≥6.5%, a fasting glucose ≥7.0 mmol/L, a nonfasting glucose ≥11.1 mmol/L, or use of hypoglycemic medication. 32 Prior cardiovascular disease was defined as prior stroke, acute coronary syndrome, heart failure in self‐reported questionnaire data or in administrative databases from 1998 to recruitment. The 10‐year ASCVD score was computed using the previously described variables (age, sex, race, total cholesterol, high‐density‐lipoprotein levels, brachial systolic BP [SBP], antihypertensive treatment, smoking status, diabetes). 8 Notably, this score includes brachial SBP as a continuous term and an interaction term between SBP and antihypertensive use. ASCVD was further categorized as low (<5% over 10 years), intermediate (5% to 20%) and high (>20%) in accordance to the American Heart Association guidelines. 8
BP measurements were taken during the recruitment visit. Brachial BP was measured with the Omron 907L device (Omron, Lake Forest, IL) in a seated position after 10 minutes of rest. The average of 3 measurements automatically taken at 2‐minute intervals was used. Central BP measurements were taken immediately afterwards using the radial applanation tonometry device SphygmoCor (AtCor Medical, Lisle, IL). This device records all radial waveforms over a 10‐second time frame and uses a generalized transfer function (calibrated with brachial SBP and diastolic BP; type I calibration) to generate a central waveform from each radial waveform. 33 , 34 All central waveforms are then overlaid to produce an averaged central waveform from which the SphygmoCor derives central BP and pulse wave analysis parameters (Figure 1): central pulse pressure (cPP: central SBP – central diastolic BP); and AIx@75 (AIx@75=[cPP–incident pressure wave height]÷cPP×100). Because wave separation analysis parameters are not automatically computed by the SphygmoCor, we used a custom MATLAB (The MathWorks Inc., Natick, MA) algorithm to extract and isolate central waveforms as shown in the SphygmoCor software output images and hence obtain machine readable (comma‐separated values format) pressure waveforms. These extracted central waveforms were then used to derive the forward (Pf) and backward (Pb) components of the pulse wave (Figure 1) and to compute reflection magnitude (RM=Pb÷Pf×100), based on a Windkessel model and as previously described. 9 , 10 , 12 , 35 , 36 , 37 , 38 This methodology has been validated against traditional measurement of Pf and Pb by echo‐Doppler derived flow curves from the left ventricular outflow tract and using data from the Asklepios cohort, 37 , 39 and from computational models. 35 We have previously used it in a population of patients with end‐stage renal disease. 36
Outcomes
MACE were defined as the composite of myocardial infarction (fatal or nonfatal), stroke (ischemic or hemorrhagic), or cardiovascular death, in accordance with the ASCVD score definition. 8 The incidence of MACE was assessed from recruitment until July 31, 2020, using data from provincial medico‐administrative databases (Régie de l'assurance maladie du Québec, Institut de la Statistique du Québec). Previously validated International Classification of Diseases, Tenth Revision (ICD‐10) diagnostic codes were employed. 40 , 41 These codes have been previously used by our team in this cohort. 42 , 43 MACE data were judged complete because the Quebec government is the sole provider of health care in the province and because emigration out of the province is low (<0.5%). 44
Statistical Analysis
Analyses were conducted with R Software 4.1.0 (The R Project for Statistical Computing). P values under 0.05 were considered significant. Adjustments for multiple comparisons were made using the Benjamini‐Hochberg procedure. Missing confounder data (BMI [0.9% missing], smoking status [0.6%], eGFR [2.4%], and lipid parameters [2.4%]) was assumed to be missing at random and was addressed using multiple imputation with the aregImpute function. Briefly, this function builds flexible additive models from bootstrapped samples of the population to predict each missing value. It then uses predictive mean matching to replace each missing value with an observed value drawn from a multinomial distribution in which each individual with a nonmissing value is weighed by the distance of its predicted value with the target predicted value. The imputation models included the outcome (MACEs), each pulse wave parameter, all adjustment covariates, and auxiliary variables. Three run‐in iterations were first conducted and then 10 imputed data sets were generated. Rubin's rules were used to combine the results of analyses conducted in each imputed data set. 45 , 46 ASCVD scores were computed separately in each imputed data set.
Independent associations between each waveform parameter and MACEs were assessed with Cox proportional hazard models censored for mortality or the end of follow‐up. Proportional hazards were verified using Schoenfeld residuals. Unadjusted, demographics‐adjusted (age, sex, race) and fully adjusted models (age, sex, race, height, weight, smoking, eGFR, diabetes, total cholesterol, high‐density lipoprotein levels, heart rate, statin use, brachial cuff SBP, prior cardiac disease, antihypertensive drug use) were used. Predictors of interest were assessed both linearly and nonlinearly (restricted cubic splines with 3 knots at standard locations). 45 Separate models were built for each parameter. All continuous confounders were treated nonlinearly with restricted cubic splines. Sensitivity analyses were conducted by (1) using diastolic BP instead of SBP in adjusted models; (2) excluding patients with a heart rate below 60 bpm (waveform images were extracted from a 1‐second pressure curve, therefore any with heart rate below 60 bpm had missing waveform data); (3) restricting analyses to patients without prior cardiovascular disease; and (4) only treating with splines the confounders that had a significant or near‐significant (P<0.10) nonlinear term in the fully adjusted model of any multiply imputed data set. Finally, an unadjusted model including all pulse waveform parameters was attempted but not interpretable due to multicollinearity.
To assess the incremental predictive value of each waveform parameter over the ASCVD score, a transformation of ASCVD probabilities was initially required to obtain comparable linear predictors to include into Cox models (since original equations for ASCVD probabilities were sex and race stratified). A log transformation (using as a base the weighted S0 at 10 years across sex and race strata) was first applied to the expected survival (1–ASCVD probabilities) of each individual and a natural logarithmic transformation was applied afterwards. These transformed ASCVD probabilities were then used to assess prediction improvement using 4 distinct statistical modalities. 47 , 48 First, ASCVD score‐adjusted hazard ratios (HRs; and their associated likelihood ratio tests) between each parameter and MACEs were computed from Cox models to establish whether each parameter led or not to improved statistical prediction. 45 , 49 Second, the C‐index difference associated with the addition of each parameter to the ASCVD score in Cox models was computed using the CompareC package. 50 Third and fourth, the continuous net reclassification index (cNRI) and integrated discrimination index (IDI) associated with the addition of each parameter to the ASCVD score were computed using the improveProb function from the Hmisc package. 51 These 3 modalities were used to quantify the extent of prediction improvement brought by each pulse wave parameter. For this purpose, CIs and statistical significance tests for these 3 modalities were not computed in accordance with expert recommendations. 49 , 52 , 53 Similar analyses were conducted for traditional clinical risk factors (BMI, eGFR) not included in the ASCVD score, for comparison purposes. The performance of an optimal combination of parameters (selected using least absolute shrinkage and selection operator) was assessed similarly. 54 A sensitivity analysis was conducted by using crude ASCVD probabilities (treated with restricted cubic splines to account for nonlinearity) rather than twice log transformed ASCVD probabilities.
We also evaluated the predictive performance of waveform parameters according to the baseline ASCVD risk. First, a linear interaction term between each parameter and the ASCVD score was added to Cox models to generate an interaction P value and HRs at various levels of baseline risk. These effects were also displayed graphically. Then, HRs, C‐indexes, cNRIs, and IDIs were computed separately for each stratum of baseline cardiovascular risk (low: 0% to 5%; intermediate: 5% to 20%; high: ≥20%). 55 Finally, the impact of adding waveform parameters was illustrated using reclassification tables according to baseline ASCVD strata.
RESULTS
Population Characteristics
From the 19 996 patients recruited by CARTaGENE, 167 patients had missing follow‐up data and 2268 patients had missing brachial BP or waveform data. Thus, 17 561 patients (49% men) were included in this study with a median age of 53 years (interquartile range 48 to 61). During a median follow‐up of 10.1 years, 2315 patients had at least 1 MACE (first event: 1728 [74.6%] myocardial infarction, 510 [22.0%] stroke, 77 [3.3%] cardiovascular death). Complete population characteristics by MACE status are shown in Table 1. During follow‐up, 394 (2.2%) patients died from any cause. Table 1 also displays mean values for waveform parameters by MACE status. At baseline, patients with MACE during the follow‐up had significantly higher cPP (difference of 3.7 mm Hg [95% CI, 3.2, 4.2]), AIx@75 (0.9% [0.4, 1.3]), Pf (2.1 mm Hg [1.9, 2.4]), and Pb (1.6 mm Hg [1.3, 1.8]).
Table 1.
Population Characteristics
| Overall (n=17 561) | MACE (n=2315) | No MACE (n=15 246) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 53 [48–61] | 59 [52–65] | 53 [47–60] | <0.001 |
| Sex (male) | 8599 (49.0) | 1420 (61.3) | 7179 (47.1) | <0.001 |
| Black race | 358 (2.0) | 37 (1.6) | 321 (2.1) | 0.126 |
| Clinical | ||||
| Body mass index, kg/m2 | 27.5±5.3 | 28.5±5.4 | 27.4±5.2 | <0.001 |
| Height, m | 1.67±0.09 | 1.68±0.09 | 1.67±0.09 | 0.016 |
| Weight, kg | 78±17 | 81±17 | 77±17 | <0.001 |
| Smoking | 3276 (18.7) | 559 (24.1) | 2717 (17.8) | <0.001 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 88.0±14.7 | 84.5±15.8 | 88.5±14.4 | <0.001 |
| Diabetes | 1560 (8.9) | 421 (18.2) | 1139 (7.5) | <0.001 |
| Total cholesterol, mmol/L | 5.1±1.0 | 4.9±1.1 | 5.1±1.0 | <0.001 |
| High‐density lipoprotein cholesterol, mmol/L | 1.2±0.4 | 1.1±0.4 | 1.3±0.4 | <0.001 |
| Statin use | 3239 (18.4) | 807 (34.9) | 2432 (16.0) | <0.001 |
| Antihypertensive drug use | 3934 (22.4) | 987 (42.6) | 2947 (19.3) | <0.001 |
| Heart rate, bpm | 70±11 | 70±12 | 70±11 | 0.699 |
| Prior cardiovascular disease | 2448 (13.9) | 847 (36.6) | 1601 (10.5) | <0.001 |
| Brachial BP | ||||
| Brachial systolic BP, mm Hg | 124±16 | 128±16 | 124±15.3 | <0.001 |
| Brachial diastolic BP, mm Hg | 74±10 | 74±11 | 74±10 | 0.670 |
| Pulse wave analysis parameters | ||||
| Central pulse pressure, mm Hg | 39±10 | 43±11 | 39±10 | <0.001 |
| Augmentation index at 75 bpm (%) | 24±10 | 25±10 | 24±11 | <0.001 |
| Wave separation analysis parameters | ||||
| Forward pressure, mm Hg | 24±6 | 26±7 | 24±5 | <0.001 |
| Backward pressure, mm Hg | 17±5 | 18±6 | 16±5 | <0.001 |
| Reflection magnitude (%) | 68.5 [60.3–75.4] | 68.7 [60.7–75.8] | 68.4 [60.2–75.4] | 0.137 |
Characteristics are presented as counts (percentage) for categorical parameters and either as means±SD (for normally distributed data) or as medians (interquartile range) (for nonnormally distributed data). P values for MACE vs no‐MACE groups were computed using either X2, Student t test or Mann–Whitney tests. BP indicates blood pressure; bpm, beats per minute; and MACE, major adverse cardiovascular event.
Association of Waveform Parameters With MACE Incidence
We first assessed the independent associations of waveform parameters using Cox models with various levels of adjustment. When parameters were treated linearly (Table 2), higher cPP, Pf, and Pb were significantly associated with increases in MACE incidence, even after full adjustment for potential confounders (age, sex, race, height, weight, smoking, eGFR, diabetes, total cholesterol, high‐density lipoprotein levels, heart rate, statin use, SBP, prior cardiac disease, antihypertensive drug use). AIx@75 was associated with MACE incidence in unadjusted and demographics‐adjusted models but not with full adjustment. RM was not associated with MACEs in any model. Similar results were obtained in sensitivity analyses (Table S1) in which (1) SBP was substituted for diastolic BP, (2) patients with heart rates <60 were excluded, (3) patients with prior cardiovascular disease were excluded, or (4) spline treatment of confounders was reduced. Potential nonlinear relationships of waveform parameters with MACEs were evaluated using restricted cubic splines. As shown in Figure 2, cPP, Pf, and Pb displayed linear associations with MACE incidence whereas AIx@75 and RM were not associated with MACEs.
Table 2.
Independent Association of Waveform Parameters With MACEs
| Parameter | Unadjusted HR | Demographics adjusted HR | Fully adjusted HR | Crude P value | Corrected P value |
|---|---|---|---|---|---|
| Pulse wave analysis parameters | |||||
| Central pulse pressure | 1.35 (1.31, 1.40) | 1.13 (1.08, 1.17) | 1.17 (1.08, 1.26) | <0.001 | <0.001 |
| Augmentation index at 75 beats per minute | 1.08 (1.04, 1.13) | 1.14 (1.08, 1.19) | 1.05 (1.00, 1.11) | 0.061 | 0.076 |
| Wave separation analysis parameters | |||||
| Forward pressure | 1.36 (1.31, 1.41) | 1.12 (1.08, 1.17) | 1.12 (1.04, 1.20) | 0.002 | 0.004 |
| Backward pressure | 1.30 (1.26, 1.35) | 1.11 (1.06, 1.15) | 1.11 (1.03, 1.19) | 0.003 | 0.005 |
| Reflection magnitude | 1.03 (0.99, 1.07) | 1.03 (0.98, 1.07) | 1.03 (0.97, 1.08) | 0.339 | 0.339 |
Associations are presented as hazard ratios (95% CI) for 1 SD increase. Crude P values were computed from the fully adjusted model. Corrected P values were obtained after a Benjamini‐Hochberg procedure using the P values from the fully adjusted model. The demographics adjusted model includes age, sex, and race. The fully adjusted model includes age, sex, race, height, weight, smoking, diabetes, total cholesterol, high‐density‐lipoprotein levels, estimated glomerular filtration rate, heart rate, statin use, prior cardiovascular disease, antihypertensive drug use, and systolic blood pressure. The fully adjusted model for body mass index omitted height and weight. HR indicates hazard ratio; and MACE, major adverse cardiovascular event.
Figure 2. Nonlinear association of waveform parameters with MACE incidence.
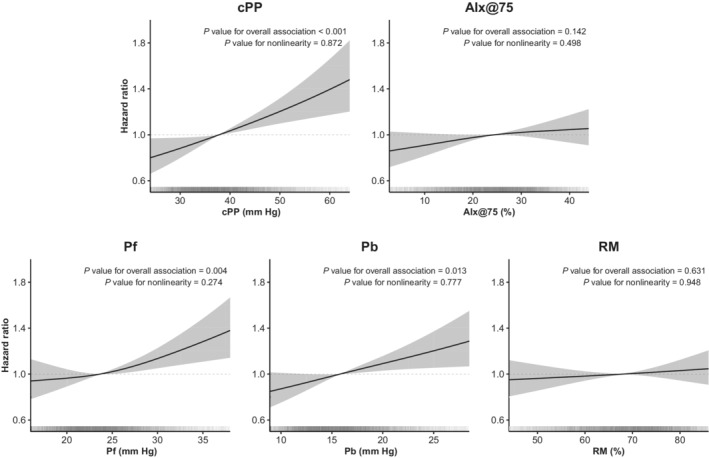
Hazard ratios are displayed after full adjustment for age, sex, race, weight, height, smoking, diabetes, total cholesterol, high‐density‐lipoprotein levels, estimated glomerular filtration rate, heart rate, statin use, prior cardiovascular disease, antihypertensive drug use, and systolic blood pressure. Shaded areas indicate the 95% CI. Vertical lines at the bottom of each plot represent the distribution of each parameter. AIx@75 indicates augmentation index at 75 beats per minute; cPP, central pulse pressure; MACE, major adverse cardiovascular event; Pb, backward pressure; Pf, forward pressure; and RM, reflection magnitude.
Incremental Value of Waveform Parameters for MACE Prediction
We then used a predictive approach to evaluate the incremental value of waveform parameters over a commonly used clinical prediction score (the ASCVD score). Four statistical modalities were used for this purpose. As displayed in Table 3, all parameters except Pf led to significant ASCVD‐score adjusted HRs and likelihood ratio tests. In the 3 other modalities, AIx@75 and RM led to the largest improvement in prediction improvement, but the improvement nevertheless remained small in magnitude (maximal ΔC‐index: 0.19%, cNRI: 0.066 and IDI: 0.11%). A least absolute shrinkage and selection operator‐derived combination of parameters (including cPP, AIx@75, Pb, and RM) led to a performance similar to AIx@75. Traditional cardiovascular risk factors not included in the ASCVD also displayed a performance similar to waveform parameters. As sensitivity analysis (Table S2), replacing transformed ASCVD probabilities with a direct inclusion into models using restricted cubic splines yielded results similar to the principal ones.
Table 3.
Predictive Value of Waveform Parameters for MACEs
| Parameters | ASCVD‐adjusted | Δ C‐index (%) | Continuous NRI | IDI (%) | |
|---|---|---|---|---|---|
| HR (95% CI) | LR Test | ||||
| Pulse wave analysis parameters | |||||
| cPP | 1.06 (1.02, 1.08) | 0.003 | 0.03 | 0.006 | 0.10 |
| AIx@75 | 1.10 (1.06, 1.15) | <0.001 | 0.19 | 0.066 | 0.11 |
| Wave separation analysis parameters | |||||
| Forward pressure | 1.04 (1.00, 1.08) | 0.072 | −0.08 | −0.029 | 0.05 |
| Pb | 1.07 (1.03, 1.11) | <0.001 | 0.05 | 0.016 | 0.10 |
| RM | 1.08 (1.03, 1.12) | <0.001 | 0.12 | 0.053 | 0.07 |
| Combined* | 1.11 (1.06, 1.15) | <0.001 | 0.19 | 0.066 | 0.14 |
| Clinical | |||||
| Body mass index | 1.07 (1.02, 1.11) | 0.003 | 0.09 | 0.085 | 0.08 |
| Estimated glomerular filtration rate | 0.91 (0.87, 0.95) | <0.001 | 0.15 | 0.025 | 0.18 |
Hazard ratios are presented for 1 SD increase. Likelihood ratio tests are presented as the maximal P value observed across the 10 multiply imputed data sets. AIx@75 indicates augmentation index at 75 beats per minute; ASCVD, atherosclerotic cardiovascular disease; cPP, central pulse pressure; HR, hazard ratio; IDI, integrated discrimination index; LR, likelihood ratio; MACE, major adverse cardiovascular event; NRI, net reclassification index; Pb, bpressure; and RM, reflection magnitude.
Combined performance of waveform parameters was derived using a least absolute shrinkage and selection operator procedure that selected cPP, AIx@75, Pb, and RM.
Incremental Value of Waveform Parameters by Baseline ASCVD Risk
We also explored whether baseline ASCVD risk influenced the predictive performance of waveform parameters. As displayed in Table S3, there were significant interactions between baseline ASCVD and 3 waveform parameters (AIx@75, Pf, RM). AIx@75 and RM were more predictive of MACEs at lower baseline ASCVD values whereas Pf was more predictive at higher ASCVD values. Although interactions between cPP, Pb, and ASCVD values did not reach significance, HRs for these 2 parameters were greater at higher ASCVD values. These relations are graphically displayed in Figure 3, which represents the observed MACE probability for a given baseline ASCVD score according to 3 levels of each waveform parameters. Three graphical patterns hence emerged. First, cPP and Pb discriminated risk only at higher baseline ASCVD values. Second, AIx@75 and RM discriminated risk at all ASCVD values but more strongly at lower ones. Finally, Pf displayed inverse risk discrimination according to baseline risk. When predictive performance was stratified by categorical level of baseline risk (Table S4; low risk=9374 patients; intermediate risk=7087 patients; high risk=1100 patients), AIx@75 and RM statistically improved prediction in the lower risk strata but not in other risk categories. The magnitude of this prediction improvement was more pronounced in the lower risk strata (for AIx@75, ΔC‐index: 1.12, cNRI: 0.155, and IDI: 0.17).
Figure 3. MACE risk according to baseline ASCVD score and waveform parameters.
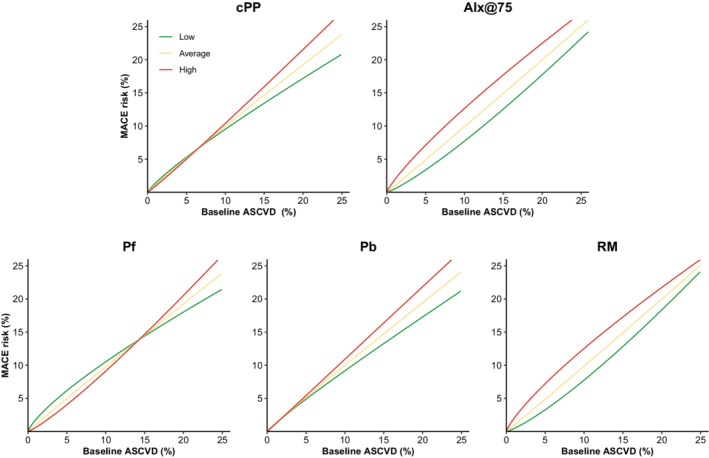
The figure displays the relationship between baseline ASCVD score (in percentage) and observed cardiovascular risk (in percentage) according to 3 levels of each waveform parameter. For each parameter, the low value was defined as 2 SDs below mean; the average value as the mean; and the high value as 2 SDs above mean. AIx@75 indicates augmentation index at 75 beats per minute; ASCVD, atherosclerotic cardiovascular disease; cPP, central pulse pressure; MACE, major adverse cardiovascular event; Pb, backward pressure; Pf, forward pressure; PP, pulse pressure; and RM, reflection magnitude.
Finally, we evaluated the absolute effect of waveform parameters on risk reclassification (Tables S5 through S9). For example, adding AIx@75 to the ASCVD score reclassified 1001 individuals (5.7% of the cohort) to a different risk category. Notably, 300 low‐risk individuals (3.2% of the low‐risk group) were classified to intermediate risk by adding AIx@75. Similar results were obtained for other parameters.
DISCUSSION
In this study of a large population‐based cohort, 3 waveform parameters (cPP, Pf, Pb) were independently associated with MACEs during a follow‐up of 10 years. In contrast, AIx@75 and RM improved risk prediction across the full spectrum of baseline risk, especially in participants with low baseline cardiovascular risk.
Over the past decades, the concept of pulse wave reflection has gained traction as a determinant of adverse arterial biomechanics. With each cardiac beat, the left ventricle generates a forward wave that travels in major arteries and encounters sites of reflection such as diameter reductions, changes in stiffness, and bifurcations. These sites generate multiple reflected waves that summate into 1 backward wave that travels into the proximal aorta and adds itself to the forward wave to form the central SBP. 13 Several studies have shown that an increased degree of reflection adversely affects the left ventricle structure and coronary perfusion. 14 , 16 , 56 , 57 Traditional analysis of the central pulse wave provides a first way to clinically assess wave reflection. Central pulse pressures and the augmentation index indeed does illustrate wave reflection at some degree but are influenced by clinical factors such as height and heart rate. For example, although arterial stiffness increases with age, the AIx@75 reaches a ceiling at ≈60 years old, especially in women. 58 , 59 , 60 , 61 To overcome these limitations, wave separation analysis was recently adapted to directly estimate the forward and backward waves and their ratios from radial waveforms transformed using a generalized transfer function. 12
A first finding of our study is the linear association between 3 pulse wave parameters (cPP, Pf, and Pb) and MACEs after full adjustment and correction for multiple comparisons. Among these, cPP was evaluated in 2 past meta‐analyses that reported similar HRs for cardiovascular events than ours (1.14 and 1.12 per 10 mm Hg versus 1.16 per 10 mm Hg in our study). 22 , 23 These 2 meta‐analyses also reported significant associations between AIx@75 and cardiovascular events (HRs of 1.32 and 1.18 per 10% increase), which we did not observe. Nevertheless, the inclusion of studies with less generalizable populations (notably patients with kidney disease or undergoing coronary angiography) and various outcome definitions in the meta‐analyses may explain these discrepancies. Similarly, some studies in these meta‐analyses used a more restricted set of adjustment parameters that better correspond to our demographics‐adjusted model in which we did observe a significant associated between AIx@75 and MACEs. In contrast, Weber et al. evaluated the 5 parameters we studied, but in a smaller study (725 patients). Interestingly, they observed that Pb and cPP were usually more strongly associated with MACEs than AIx@75 and RM, especially in the subset of patients with normal or slightly impaired systolic function. 12 Nevertheless, their study differs from ours regarding the population (patients undergoing coronary angiography) and outcome (including coronary, cerebrovascular, and peripheral revascularization). Similar discrepancies in sample sizes, populations, adjustment factors, and outcomes may also explain the slightly larger but often nonsignificant associations observed by other previous studies concerning AIx@75, Pb, Pf, and RM. 11 , 25 , 26 Taken together with previous studies, our results reinforce the hypothesis that some waveform parameters are associated with MACEs. Furthermore, our findings highlight the need for large and generalizable population‐based cohorts in the evaluation of potential cardiovascular risk factors.
A novel result of our study is the improvement in MACE prediction seen with some waveform parameters. Indeed, all parameters except Pf statistically improved prediction as ascertained by the significance of their HRs and likelihood ratio tests. Further graphical evaluation also revealed that AIx@75 and RM discriminated risk across the spectrum of baseline ASCVD risk (albeit more strongly at lower values), in contrast to other parameters. Nevertheless, whether this statistical increase in prediction performance has a magnitude of clinical relevance is not certain. For this purpose, we used 3 other statistical modalities (ΔC‐indexes, cNRIs, and IDIs) to quantify the extent of prediction improvement brought by each pulse wave parameter. Although the largest values of these 3 modalities were generally observed for AIx@75 and RM across all baseline risk strata, they remained smaller than the ones observed in a previous meta‐analysis concerning the predictive value of aortic pulse wave velocity 28 and were equivalent or slightly smaller than the ones observed for central SBP in the same CARTaGENE cohort. 43 As such, based on previous calculations, our values of cNRIs, IDIs, and ΔC‐indexes translate into a Cohen effect size <0.2, corresponding to a weak effect. 47 Although the predictive value of waveform parameters may be small in magnitude, their addition to the ASCVD score did reclassify a clinically significant proportion of individuals. For example, adding AIx@75 reclassified more than 5% of individuals into another risk category, which could have a nonnegligible impact on clinical practice. Thus, although the individual role of waveform parameters on prediction might be small, its populational impact may be larger.
Another finding of our study is the contrast between traditional (independent associations) and predictive analyses; a subject that has been discussed extensively in the past years. 62 , 63 , 64 Indeed, the 3 parameters independently associated with MACEs underperformed in predictive analyses. This phenomenon has been previously observed with BMI, a universally recognized causal determinant of cardiovascular disease 65 , 66 , 67 that did not improve risk prediction in a previous large meta‐analysis. 68 It has been hypothesized that the effect of BMI on cardiovascular health is reflected by other variables already included in the ASCVD score, hence decreasing its predictive value. 69 Inversely, AIx@75 and RM led to the greatest predictive improvement in our study but lacked a strong independent relationship in the adjusted models. To explain this finding, we hypothesize that these 2 biomarkers encompass the cardiovascular effects of several parameters included in our adjusted model but not fully accounted for by the routinely used ASCVD score. These findings thus highlight the need for a formal predictive assessment of risk biomarkers in addition to routinely used prediction scores rather than fully adjusted models that are not well representative of the clinical practice.
Another major finding of our study is the interaction between waveform parameters and baseline ASCVD scores. For AIx@75 and RM, this interaction led to higher predictive performance at lower baseline cardiovascular risk, especially for ΔC‐indexes and cNRIs. As such, the use of these 2 parameters could reveal patients at increased cardiovascular risk among the low ASCVD score population. They hence exemplify the concept of early vascular aging, which was recently demonstrated using aortic pulse wave velocity. 70 , 71 We could theorize that in individuals at low cardiovascular risk, the first signs of cardiovascular adaptations may be visible only in the waveform and not in absolute BP values, which remain rather constant. As the cardiovascular risk increases, cardiovascular adaptations compensation mechanisms start to fail and as such, BP starts to increase. At this later stage, wave parameters may not be as useful as they approach their ceilings, as it is described with AIx@75 after 60 years of age. 59 , 60 , 61 Alternatively, we observed that some parameters had a greater performance in higher‐risk patients or even had inverse relationships in each extreme of cardiovascular risk. Nevertheless, owing to the population‐based nature of our cohort, few patients had high baseline cardiovascular risk. Thus, extrapolation of our findings in this population should await further confirmation. Globally, these results reveal that each pulse wave parameter performs differently at a given level of cardiovascular risk and highlight the need for a right predictive biomarker in each risk strata.
Our study has several strengths. To our knowledge, it is the largest study to evaluate the relationship of 5 pulse wave parameters with incident cardiovascular events. Furthermore, it was conducted in a population‐based cohort representative of the Quebec population and is therefore easily generalizable. It is also the first to directly assess the predictive performance of these parameters and evaluate its interplay with baseline ASCVD risk. Other strengths of our study include (1) a robust statistical methodology including adjustment for several potential confounders, correction for multiple comparisons, and numerous sensitivity analyses; (2) the use of restricted cubic splines to detect nonlinear relationships in predictors and confounders; (3) the evaluation of predictive performance with several modalities; (4) a largely recognized MACE definition (alike to the one used to derive the ASCVD score 8 ) and its identification using validated medico‐administrative codes with data from the single provider of health care in Quebec; and (5) the evaluation of pulse waveform parameters with algorithms easily incorporable into central BP devices.
Some limitations of this study are nevertheless worth considering. By its observational nature, causality cannot be proved for identified associations. Also, residual confounding cannot be excluded concerning association analyses. Self‐reporting may have slightly biased the measurement of some clinical variables. Furthermore, the population‐based nature of our cohort and its lower age range (40 – 69 years) yielded few patients at high cardiovascular risk; extrapolation of our results to this population should therefore be done cautiously. Participants with reduced left ventricular function (known to be associated with reduced AIx@75) could not be discerned among patients with heart failure at baseline. The lack of gold standard measurement of arterial stiffness (carotid‐femoral pulse wave velocity) has prevented us from comparing its association and predictive value to our parameters of interest. The correlation between each pulse waveform parameter also prevented us from evaluating several parameters simultaneously into a statistical model. Finally, all analyzed waveform parameters were estimated from radial tonometry‐derived central waveforms, instead of being directly derived from an invasive aortic waveform. Hence, they were subject to a calibration procedure, which may have influenced their accuracy.
CONCLUSIONS
In conclusion, using a large prospective population‐based study, we observed that 3 waveform parameters are independently associated with MACEs during a 10‐year follow‐up. Although the addition of AIx@75 and RM to the ASCVD score had a slight statistical effect on MACE prediction, it allowed the reclassification of a nonnegligible proportion of individuals into a different risk category. Furthermore, the performance of these 2 parameters was more pronounced in individuals at low cardiovasular risk. Hence, our findings suggest a role for parameters of wave reflection in the identification of higher‐risk individuals among low‐risk populations.
Sources of Funding
Funding for this project was provided by the Canadian Institutes of Health Research (project # PJT‐173313) and the Heart & Stroke Foundation of Canada (project # G‐20‐0028656).
Disclosures
None.
Supporting information
Tables S1–S9
Acknowledgments
R Goupil holds a research scholarship from the Fonds de recherche du Québec–Santé and is a recipient of the Société québécoise d'hypertension artérielle–Bourse Jacques‐de‐Champlain scholarship. AC Nadeau‐Fredette holds a research scholarship from the Fonds de recherche du Québec–Santé and is a recipient of the Société québécoise de néphrologie scholarship.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026603
For Sources of Funding and Disclosures, see page 10.
References
- 1. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm hg, 1990–2015. JAMA. 2017;317:165–182. doi: 10.1001/jama.2016.19043 [DOI] [PubMed] [Google Scholar]
- 2. Hughes D, Judge C, Murphy R, Loughlin E, Costello M, Whiteley W, Bosch J, O'Donnell MJ, Canavan M. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta‐analysis. JAMA. 2020;323:1934–1944. doi: 10.1001/jama.2020.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi‐Kangevari M, Abd‐Allah F, Abdelalim A, Abdollahi M, Abdollahpour I, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/s0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Picone DS, Schultz MG, Otahal P, Aakhus S, Al‐Jumaily AM, Black JA, Bos WJ, Chambers JB, Chen CH, Cheng HM, et al. Accuracy of cuff‐measured blood pressure: systematic reviews and meta‐analyses. J Am Coll Cardiol. 2017;70:572–586. doi: 10.1016/j.jacc.2017.05.064 [DOI] [PubMed] [Google Scholar]
- 5. Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M, Investigators C, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the conduit artery function evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496 [DOI] [PubMed] [Google Scholar]
- 6. Hippisley‐Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. doi: 10.1136/bmj.j2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ESC Cardiovascular risk collaboration . SCORE2 risk prediction algorithms: new models to estimate 10‐year risk of cardiovascular disease in Europe. Eur Heart J. 2021;42:2439–2454. doi: 10.1093/eurheartj/ehab309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 9. Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A. Quantification of wave reflection in the human aorta from pressure alone: a proof of principle. Hypertension. 2006;48:595–601. doi: 10.1161/01.HYP.0000238330.08894.17 [DOI] [PubMed] [Google Scholar]
- 10. Westerhof N, Sipkema P, van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res. 1972;6:648–656. doi: 10.1093/cvr/6.6.648 [DOI] [PubMed] [Google Scholar]
- 11. Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, et al. Wave reflection and arterial stiffness in the prediction of 15‐year all‐cause and cardiovascular mortalities: a community‐based study. Hypertension. 2010;55:799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end‐organ damage and clinical outcomes. Hypertension. 2012;60:534–541. doi: 10.1161/HYPERTENSIONAHA.112.194571 [DOI] [PubMed] [Google Scholar]
- 13. Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, Roman MJ, Safar ME, Segers P, Smulyan H. Role of pulse pressure amplification in arterial hypertension: experts' opinion and review of the data. Hypertension. 2009;54:375–383. doi: 10.1161/hypertensionaha.109.134379 [DOI] [PubMed] [Google Scholar]
- 14. Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. doi: 10.1016/j.jacc.2007.07.032 [DOI] [PubMed] [Google Scholar]
- 15. Fukuta H, Ohte N, Wakami K, Asada K, Goto T, Mukai S, Tani T, Kimura G. Impact of arterial load on left ventricular diastolic function in patients undergoing cardiac catheterization for coronary artery disease. Circ J. 2010;74:1900–1905. doi: 10.1253/circj.cj-10-0283 [DOI] [PubMed] [Google Scholar]
- 16. Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O'Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–1024. doi: 10.1097/HJH.0b013e3282f62a9b [DOI] [PubMed] [Google Scholar]
- 17. Iketani T, Takazawa K, Ibukiyama C. The influence of changes in loading patterns on left ventricular relaxation in humans. Jpn Circ J. 1998;62:581–585. doi: 10.1253/jcj.62.581 [DOI] [PubMed] [Google Scholar]
- 18. Saba PS, Roman MJ, Pini R, Spitzer M, Ganau A, Devereux RB. Relation of arterial pressure waveform to left ventricular and carotid anatomy in normotensive subjects. J Am Coll Cardiol. 1993;22:1873–1880. doi: 10.1016/0735-1097(93)90772-s [DOI] [PubMed] [Google Scholar]
- 19. Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the strong heart study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078 [DOI] [PubMed] [Google Scholar]
- 20. Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461–467. doi: 10.1097/hjh.0b013e3283220ea4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benetos A, Gautier S, Labat C, Salvi P, Valbusa F, Marino F, Toulza O, Agnoletti D, Zamboni M, Dubail D, et al. Mortality and cardiovascular events are best predicted by low central/peripheral pulse pressure amplification but not by high blood pressure levels in elderly nursing home subjects: the PARTAGE (predictive values of blood pressure and arterial stiffness in institutionalized very aged population) study. J Am Coll Cardiol. 2012;60:1503–1511. doi: 10.1016/j.jacc.2012.04.055 [DOI] [PubMed] [Google Scholar]
- 22. Li WF, Huang YQ, Feng YQ. Association between central haemodynamics and risk of all‐cause mortality and cardiovascular disease: a systematic review and meta‐analysis. J Hum Hypertens. 2019;33:531–541. doi: 10.1038/s41371-019-0187-x [DOI] [PubMed] [Google Scholar]
- 23. Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with central haemodynamics: a systematic review and meta‐analysis. Eur Heart J. 2010;31:1865–1871. doi: 10.1093/eurheartj/ehq024 [DOI] [PubMed] [Google Scholar]
- 24. Mitchell GF, Hwang SJ, Larson MG, Hamburg NM, Benjamin EJ, Vasan RS, Levy D, Vita JA. Transfer function‐derived central pressure and cardiovascular disease events: the Framingham heart study. J Hypertens. 2016;34:1528–1534. doi: 10.1097/HJH.0000000000000968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, Hamburg NM, Vasan RS, Mitchell GF. Components of hemodynamic load and cardiovascular events: the Framingham heart study. Circulation. 2015;131:354–361; discussion 361. doi: 10.1161/CIRCULATIONAHA.114.011357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zamani P, Jacobs DR Jr, Segers P, Duprez DA, Brumback L, Kronmal RA, Lilly SM, Townsend RR, Budoff M, Lima JA, et al. Reflection magnitude as a predictor of mortality: the multi‐ethnic study of atherosclerosis. Hypertension. 2014;64:958–964. doi: 10.1161/HYPERTENSIONAHA.114.03855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manisty C, Mayet J, Tapp RJ, Parker KH, Sever P, Poulter NR, Thom SA, Hughes AD, Investigators A. Wave reflection predicts cardiovascular events in hypertensive individuals independent of blood pressure and other cardiovascular risk factors: an ASCOT (Anglo‐Scandinavian cardiac outcome trial) substudy. J Am Coll Cardiol. 2010;56:24–30. doi: 10.1016/j.jacc.2010.03.030 [DOI] [PubMed] [Google Scholar]
- 28. Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17 635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Awadalla P, Boileau C, Payette Y, Idaghdour Y, Goulet JP, Knoppers B, Hamet P, Laberge C. Cohort profile of the CARTaGENE study: Quebec's population‐based biobank for public health and personalized genomics. Int J Epidemiol. 2013;42:1285–1299. doi: 10.1093/ije/dys160 [DOI] [PubMed] [Google Scholar]
- 30. Desbiens LC, Goupil R, Madore F, Mac‐Way F. Incidence of fractures in middle‐aged individuals with early chronic kidney disease: a population‐based analysis of CARTaGENE. Nephrol, Dial, Transplant. 2020;35:1712–1721. doi: 10.1093/ndt/gfz259 [DOI] [PubMed] [Google Scholar]
- 31. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2018;43:S1–S325. [DOI] [PubMed] [Google Scholar]
- 33. Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827 [DOI] [PubMed] [Google Scholar]
- 34. Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106 [DOI] [PubMed] [Google Scholar]
- 35. Hametner B, Schneider M, Parragh S, Wassertheurer S. Computational assessment of model‐based wave separation using a database of virtual subjects. J Biomech. 2017;64:26–31. doi: 10.1016/j.jbiomech.2017.08.027 [DOI] [PubMed] [Google Scholar]
- 36. Fortier C, Cote G, Mac‐Way F, Goupil R, Desbiens LC, Desjardins MP, Marquis K, Hametner B, Wassertheurer S, Schultz MG, et al. Prognostic value of carotid and radial artery reservoir‐wave parameters in end‐stage renal disease. J Am Heart Assoc. 2019;8:e012314. doi: 10.1161/JAHA.119.012314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hametner B, Wassertheurer S, Kropf J, Mayer C, Holzinger A, Eber B, Weber T. Wave reflection quantification based on pressure waveforms alone‐‐methods, comparison, and clinical covariates. Comput Methods Programs Biomed. 2013;109:250–259. doi: 10.1016/j.cmpb.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 38. Hametner B, Weber T, Mayer C, Kropf J, Wassertheurer S. Calculation of arterial characteristic impedance: a comparison using different blood flow models. Math Comput Model Dyn Syst. 2013;19:329–330. [Google Scholar]
- 39. Hametner B, Parragh S, Mayer C, Weber T, Van Bortel L, De Buyzere M, Segers P, Rietzschel E, Wassertheurer S. Assessment of model based (input) impedance, pulse wave velocity, and wave reflection in the Asklepios cohort. PloS one. 2015;10:e0141656. doi: 10.1371/journal.pone.0141656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCormick N, Bhole V, Lacaille D, Avina‐Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One. 2015;10:e0135834. doi: 10.1371/journal.pone.0135834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McCormick N, Lacaille D, Bhole V, Avina‐Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One. 2014;9:e92286. doi: 10.1371/journal.pone.0092286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dupuis ME, Nadeau‐Fredette AC, Madore F, Agharazii M, Goupil R. Association of glomerular hyperfiltration and cardiovascular risk in middle‐aged healthy individuals. JAMA Netw Open. 2020;3:e202377. doi: 10.1001/jamanetworkopen.2020.2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lamarche F, Agharazii M, Madore F, Goupil R. Prediction of cardiovascular events by type I central systolic blood pressure: a prospective study. Hypertension. 2021;77:319–327. doi: 10.1161/HYPERTENSIONAHA.120.16163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Statistics Canada . Estimates of the components of demographic growth, annual. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000801&request_locale=en. 2017. Accessed December 12 2018.
- 45. Harrell F. Regression Modeling Strategies. 2nd ed. Springer International Publishing; 2015. [Google Scholar]
- 46. Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons; 2004. [Google Scholar]
- 47. Pencina MJ, D'Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–481. doi: 10.1093/aje/kws207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014;25:114–121. doi: 10.1097/ede.0000000000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kang L, Chen W. compareC: Compare two correlated C indices with right‐censored survival outcome.2015. https://cran.r‐project.org/package=compareC. [DOI] [PMC free article] [PubMed]
- 51. Harrell FE Jr. Hmisc: Harrell Miscellaneous. 2021. https://cran.r‐project.org/package=Hmisc.
- 52. Kerr KF, McClelland RL, Brown ER, Lumley T. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol. 2011;174:364–374. doi: 10.1093/aje/kwr086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pepe MS, Fan J, Feng Z, Gerds T, Hilden J. The net reclassification index (NRI): a misleading measure of prediction improvement even with independent test data sets. Stat Biosci. 2015;7:282–295. doi: 10.1007/s12561-014-9118-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Friedman J, Hastie T, Tibshirani R, Narasimhan B, Tay K, Simon N, Qian J, Yang J. Glmnet: Lasso and Elastic‐Net Regularized Generalized Linear Models. 2021. https://cran.r‐project.org/web/packages/glmnet/glmnet.pdf.
- 55. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gillebert TC, Lew WY. Influence of systolic pressure profile on rate of left ventricular pressure fall. Am J Physiol. 1991;261:H805–H813. doi: 10.1152/ajpheart.1991.261.3.H805 [DOI] [PubMed] [Google Scholar]
- 57. Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M. Influence of aortic impedance on the development of pressure‐overload left ventricular hypertrophy in rats. Circulation. 1996;94:3362–3368. doi: 10.1161/01.cir.94.12.3362 [DOI] [PubMed] [Google Scholar]
- 58. Torjesen AA, Wang N, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Forward and backward wave morphology and central pressure augmentation in men and women in the Framingham heart study. Hypertension. 2014;64:259–265. doi: 10.1161/HYPERTENSIONAHA.114.03371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Janner JH, Godtfredsen NS, Ladelund S, Vestbo J, Prescott E. Aortic augmentation index: reference values in a large unselected population by means of the SphygmoCor device. Am J Hypertens. 2010;23:180–185. doi: 10.1038/ajh.2009.234 [DOI] [PubMed] [Google Scholar]
- 60. Reference Values for Arterial Stiffness' Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore longitudinal study of aging. Hypertension. 2013;62:934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101 [DOI] [PubMed] [Google Scholar]
- 63. Feng Z. Classification versus association models: should the same methods apply? Scand J Clin Lab Invest Suppl. 2010;242:53–58. doi: 10.3109/00365513.2010.493387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Poldrack RA, Huckins G, Varoquaux G. Establishment of best practices for evidence for prediction: a review. JAMA Psychiatry. 2020;77:534–540. doi: 10.1001/jamapsychiatry.2019.3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mongraw‐Chaffin ML, Peters SAE, Huxley RR, Woodward M. The sex‐specific association between BMI and coronary heart disease: a systematic review and meta‐analysis of 95 cohorts with 1·2 million participants. Lancet Diabetes Endocrinol. 2015;3:437–449. doi: 10.1016/s2213-8587(15)00086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Riaz H, Khan MS, Siddiqi TJ, Usman MS, Shah N, Goyal A, Khan SS, Mookadam F, Krasuski RA, Ahmed H. Association between obesity and cardiovascular outcomes: a systematic review and meta‐analysis of Mendelian randomization studies. JAMA Netw Open. 2018;1:e183788. doi: 10.1001/jamanetworkopen.2018.3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/s0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. The Emerging Risk Factors Collaboration . Separate and combined associations of body‐mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/s0140-6736(11)60105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. The Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration . Metabolic mediators of the effects of body‐mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. doi: 10.1016/s0140-6736(13)61836-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bruno RM, Nilsson PM, Engstrom G, Wadstrom BN, Empana JP, Boutouyrie P, Laurent S. Early and supernormal vascular aging: clinical characteristics and association with incident cardiovascular events. Hypertension. 2020;76:1616–1624. doi: 10.1161/HYPERTENSIONAHA.120.14971 [DOI] [PubMed] [Google Scholar]
- 71. Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: a tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension. 2009;54:3–10. doi: 10.1161/HYPERTENSIONAHA.109.129114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9
Data Availability Statement
CARTaGENE data (https://cartagene.qc.ca) were used under license. Restrictions apply to its availability to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.


