Abstract
Acute lung injury (ALI) is a common and devastating clinical disorder with a high mortality rate and no specific therapy. The pathophysiology of ALI is characterized by increased alveolar/capillary permeability, lung inflammation, oxidative stress and structural damage to lung tissues, which can progress to acute respiratory distress syndrome (ARDS). Adelmidrol (ADM), an analogue of palmitoylethanolamide (PEA), is known for its anti-inflammatory and antioxidant functions, which are mainly due to down-modulating mast cells (MCs) and promoting endogenous antioxidant defense. The aim of this study is to evaluate the protective effects of ADM in a mice model of ALI, induced by intratracheal administration of lipopolysaccharide (LPS) at the dose of 5 mg/kg. ADM 2% was administered by aerosol 1 and 6 h after LPS instillation. In this study, we clearly demonstrated that ADM reduced lung damage and airway infiltration induced by LPS instillation. At the same time, ADM counteracted the increase in MC number and the expression of specific markers of MC activation, i.e., chymase and tryptase. Moreover, ADM reduced oxidative stress by upregulating antioxidant enzymes as well as modulating the Nf-kB pathway and the resulting pro-inflammatory cytokine release. These results suggest that ADM could be a potential candidate in the management of ALI.
Keywords: acute lung injury, Adelmidrol, mast cells, inflammation, oxidative stress
1. Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), two acute inflammatory conditions, are a major cause of respiratory failure and one of the most challenging clinical conditions with significant morbidity and mortality [1]. ALI is characterized by alteration of the endothelium and alveolar epithelial barrier, resulting in increased microvascular permeability, pulmonary edema, and polymorphonuclear neutrophil infiltration, all of which contribute to decreased respiratory function [2]. Evidence has proposed that several pathophysiological pathways are activated during ALI, especially during the early phase of the disease [3,4], in which inflammatory response plays a key role [1]. ALI can be modeled in rodents by the administration of LPS through tracheal instillation [5,6,7,8]. Local administration of LPS causes an acute and vigorous migration of inflammatory cells into the lung tissue, leading to the overproduction of pro-inflammatory cytokines, including interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α [9,10]. Among the inflammatory cells, mast cells (MCs) stand out for their involvement in the pathophysiology of ALI [11]. In particular, MCs activation induce the release of the contents of their granules, including specific proteases such as chymase and tryptase that contribute to the progression of inflammatory diseases on the respiratory system [12,13]. Additionally, several studies support the role of oxidants and oxidative stress in the pathogenesis of ALI [14,15,16]. In the context of ALI/ARDS, there are many potential sources of reactive oxygen species (ROS), including leukocytes (neutrophils, monocytes, and macrophages), parenchymal cells (endothelial and epithelial cells, fibroblasts, and myocytes) and circulating oxidant-generating enzymes [16]. Excessive ROS production generated by the injured endothelium/epithelium, as well as recruited leukocytes, amplifies the tissue damage and pulmonary edema [17,18]. Thus, a cross-link between inflammatory response and oxidative stress is involved in the development of ALI [19,20]. Therefore, new approaches are needed to improve the clinical outcomes of the patients affected with the disease. In this regard, we investigated the properties of Adelmidrol (ADM), a palmitoylethanolamide (PEA) analogue that belongs to the ALIAmide family (Autacoid Local Injury Antagonist Amides) [21]. It is well known that ADM has important anti-inflammatory properties due to the regulation of MC activation [22,23,24]. Recently, it has also been shown that ADM is able to boost endogenous antioxidant defense [25], indirectly enhancing its protective function. Therefore, the aim of this study is to evaluate the beneficial effects of ADM in an LPS-induced ALI model, through the modulation of inflammatory and oxidative pathways.
2. Materials and Methods
2.1. Animals
Male CD1 mice (25–30 g, Envigo, Milan, Italy) were housed in a controlled environment, with food and water ad libitum. The University of Messina Review Board for animal care (OPBA) approved the study (ethical protocol code: 266/2021-PR). All in vivo experiments followed the new directives of the USA, Europe, Italy, and the ARRIVE guidelines.
2.2. Induction of Acute Lung Injury
For intratracheal (i.t.) instillation, animals were anesthetized with isoflurane (2%), and LPS was instilled as previously described [26,27]. Briefly, a 1 cm long ventral midline cervical incision was used to expose the trachea, and LPS was injected using a bent 27-gauge tuberculin needle. Escherichia coli LPS (026: B6L3755, Sigma Aldrich, St. Louis, MO, USA) was administered by a single i.t. instillation at the dose of 5 mg/kg suspended in saline solution (total volume = 0.05 mL per animal) [1]. Sham animals were subjected to the same procedure but received saline instead of LPS. ADM 2% in isotonic solution was administered by aerosol, with a Lovelace nebulizer (In-Tox Products, Albuquerque, NM, USA) being used to create an atmosphere in an exposure chamber (Research and Consulting Co., AG, Basel, Switzerland), as previously described by D’Amico et al. [28].
2.3. Experimental Groups
Mice were randomized into the following experimental groups (n = 12/group):
-
-
LPS group: Mice received LPS i.t. and were treated with the vehicle (saline);
-
-
LPS + ADM group: Mice received LPS i.t. and were treated with ADM 2% aerosol 1 h and 6 h after LPS instillation;
-
-
Sham group: Similar to LPS group, but mice received saline i.t. instead of LPS;
-
-
Sham + ADM: Mice received saline i.t. and were treated with ADM 2% aerosol 1 h and 6 h after saline instillation (data not shown, as no significant difference was ever observed between Sham and Sham + ADM).
At 24 h after induction, all animals were sacrificed, and bronchoalveolar lavage fluid (BALF) as well as lung tissues were collected for further analysis.
2.4. Proteins Concentration and Cell Counts in BALF
The cell count in BALF was carried out as previously described [1]. Briefly, BALF was collected by cannulating the trachea and lavaging the lung twice with 0.7 mL of phosphate-buffered saline (PBS) [1]. The washing solution were removed by aspiration and BALF was centrifugated at 800 rpm [29]. The supernatant was stored at −20 °C, while the pelleted cells were resuspended in PBS. Then, the total cells in BALF were enumerated by counting with a hemocytometer in the presence of the trypan blue stain. For differential cell counting, Wright’s Giemsa stain was performed, and the leukocyte and macrophage populations present in BALF were counted. After staining, the differential count was carried out by the standard morphological protocol under a light microscope [30]. To determine the protein concentration and to measure the pro-inflammatory cytokines, the supernatants in BALF were analyzed by a BCA Protein Assay Kit (ThermoFisher, 00161, Rome, Italy. while the levels of IL-6 (#DKW12-2060; Dakewe Biotech Co., Ltd., Bensheim, Germany), IL-1β (#MBS8800273; Biosource International, Camarillo, CA, USA) and TNF-α (#30907; BioLegend, San Diego, CA, USA)) were detected using ELISA [1,31].
2.5. Measurement of Lung Edema
At the end of experiment, wet lung weights were recorded. The lungs were subsequently dried for 48 h at 80 °C and weighed again. The water content in the lung tissues was calculated as the ratio of wet/dry weight of the lung [32].
2.6. Histological Examination
Lung sections were stained with Hematoxylin and Eosin (H&E) for histological analysis [33,34,35,36] and with toluidine blue to determine MC degranulation [37]. Every section was examined using a Leica DM6 microscope; (Leica Microsystems SpA, Milan, Italy) associated with Leica LAS X Navigator software (Leica Microsystems SpA, Milan, Italy). Every slide was viewed at a magnification of 10× and morphological changes were evaluated by two blinded investigators [38,39,40,41]. Lung injury score was measured according to the methods reported previously [42,43]. The criteria are as follows: 0 = no damage, l = mild damage, 2 = moderate damage, 3 = severe damage, 4 = very severe histologic changes.
2.7. Myeloperoxidase (MPO) Assay
The MPO activity was measured as previously described [44,45,46] and represented in units per gram of wet tissue weight, defined as the amount of enzyme capable of decomposing 1 μmol of peroxide per minute at 37 °C.
2.8. Immunohistochemical Localization of Chymase and Tryptase
Immunohistochemical analysis was performed as previously described [47,48,49]. Primary antibodies anti-MC chymase (1:100, Santa Cruz Biotechnology (SCB) Heidelberg, Germany, #sc59586) and anti-MC tryptase (1:100, SCB, #sc59587) were incubated overnight on the lung tissue sections. Images were collected using a Leica DM6 microscope; a 10× magnification is shown (Leica Microsystems SpA, Milan, Italy) following a typical procedure [50,51,52,53]. The positive pixel intensity value obtained was connected to the histogram profile [54,55].
2.9. Measurement of Oxidative Stress
The malondialdehyde (MDA; #A003-1-2, Nanjing, China), glutathione (GSH; #A006-2-1, Nanjing, China) and catalase (CAT; #A007-1-1, Nanjing, China) levels in the lung tissues were measured using activity assay kits (Nanjing Jiancheng Bioengineering Institute) [52,56,57,58].
2.10. Analysis of Western Blots
Western blots were performed on lung samples as described in our previous studies [59,60,61]. The following antibodies were used: anti-IkBα (1:1000, SCB, #sc1643), anti-NF-kB p65 (1:1000; SCB, #sc8414), anti-Nrf2 (1:5000; SCB, #sc365949), anti-HO-1 (1:5000; SCB, #sc136960), MnSOD (1:5000 SCB #sc137254), anti-β-actin (1:5000; SCB, #sc8432) and anti-lamin A/C antibody (1:5000; Sigma-Aldrich, St. Louis, MO, USA). The membranes were then incubated with IgG peroxidase-conjugated secondary antibody-conjugated bovine mouse IgG or IgG peroxidase-conjugated goat anti-rabbit (1:2000, Jackson ImmunoResearch, Baltimore, MD, USA) [58,62,63,64]. Protein expression was quantified by densitometry with BIORAD ChemiDocTM XRS + software and normalized to housekeeping genes β-actin and lamin A/C as previously reported [64,65]. Images of blot signals were imported to analysis software (Image Quant TL, v2003, Rome, Italy.) [41,60].
2.11. Materials
Unless, otherwise stated, all compounds used in this study were purchased from Sigma-Aldrich Company Ltd. (Milan, Italy). ADM was obtained from Epitech Group SpA.
2.12. Statistical Evaluation
All values are expressed as mean ± standard error of the mean (SEM) of N observations. The images shown are representative of the last three experiments performed on diverse experimental days on tissue sections collected from all animals in each group. For in vivo studies, N represents the number of animals used. The results were analyzed by one-way ANOVA followed by a Bonferroni post hoc test for multiple comparisons. A p value less than 0.05 was considered significant.
3. Results
3.1. ADM 2% Aerosol on Histopathological Analysis and Neutrophil Activity
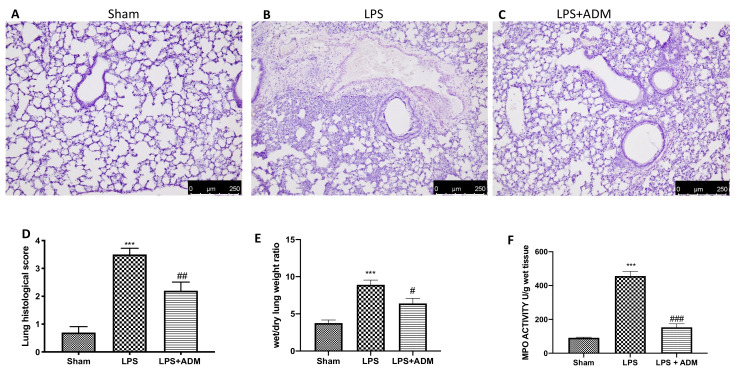
First, we analyzed the ADM effects on histopathological damage, including alveolar congestion, bleeding, neutrophil infiltration and thickness of alveolar wall/hyaline membrane formation. H&E exhibited extensive tissue damage and extracellular matrix deposition in the lungs of LPS-treated animals (Figure 1B,D) compared to the sham groups (Figure 1A,D). Aerosol treatment with ADM 2% significantly minimized lung damage (Figure 1C,D). We also evaluated the presence of lung edema by the ratio of wet/dry weight of the lung and neutrophil infiltration by the MPO assay. The ratio of wet/dry weight of the lung and MPO activity were increased by i.t. injection of LPS, while ADM 2% significantly reduced both parameters. (Figure 1E,F).
Figure 1.
Histological analysis: sham (A), LPS (B), LPS + ADM 2% (C). Histological score (D). Wet/dry lung weight ratio (E). MPO activity (F). A 10× magnification is shown. Data are expressed as the mean ± SEM of N = 6 mice/group. *** p < 0.001 vs. sham; # p < 0.05 vs. LPS; ## p < 0.01 vs. LPS; ### p < 0.001 vs. LPS.
3.2. ADM 2% Aerosol on Inflammatory Cells and Pro-Inflammatory Cytokines in BALF
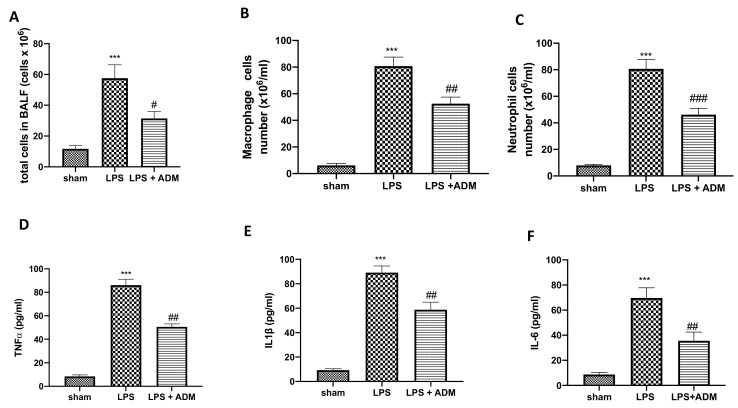
To determine whether ADM was able to reduce cell infiltration, we measured inflammatory cell counts in the BALF 24 h after LPS i.t. instillation. We found a substantial increase in total cell counts (Figure 2A), macrophages (Figure 2B) and neutrophils (Figure 2C) in BALF taken from LPS-treated animals compared to Sham mice. The number of inflammatory cells in BALF was significantly reduced after ADM 2% aerosol (Figure 2A–C). Additionally, we examined BALF levels of the pro-inflammatory cytokines. TNF-α (Figure 2D), IL-1β (Figure 2E) and IL-6 (Figure 2F) levels were significantly increased in the LPS group compared to Sham mice. On the contrary, cytokines release in BALF was markedly reduced in mice treated with ADM (Figure 2D–F).
Figure 2.
Cell infiltration expression in BALF. Total cell number (A), Macrophages (B), Neutrophils (C). Expression of proinflammatory cytokine: TNF-α (D), IL-1β (E), IL-6 (F). Data are expressed as the mean ± SEM of N = 6 mice/group. *** p < 0.001 vs. sham; # p < 0.05 vs. LPS; ## p < 0.01 vs. LPS; ### p < 0.001 vs. LPS.
3.3. ADM 2% Aerosol on MC Number
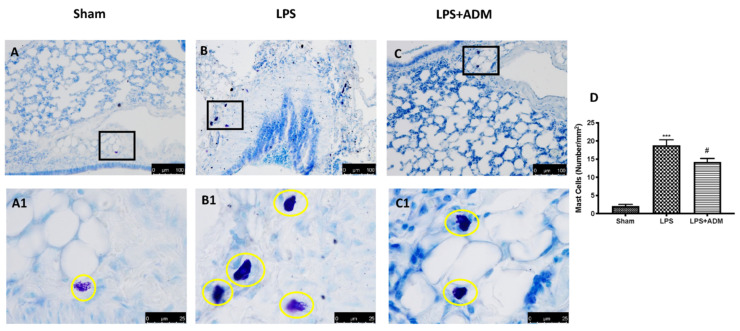
Toluidine blue staining of lung sections was used to assess the MC number. We detected a higher number of MCs in the LPS group (Figure 3B,B1,D), compared to Sham animals (Figure 3A,A1,D). ADM 2% aerosol reduced in a significant manner MC hyperplasia in lung tissues (Figure 3C,C1,D).
Figure 3.
Mast cells indicated by toluidine blue staining: Sham (A), LPS (B), LPS + ADM 2% (C), mast cell count (D). A 20× and 100× magnification is shown. Data are expressed as the mean ± SEM of N = 6 mice/group. *** p < 0.001 vs. sham; # p < 0.05 vs. LPS.
3.4. ADM 2% Aerosol on Chymase and Tryptase Expression
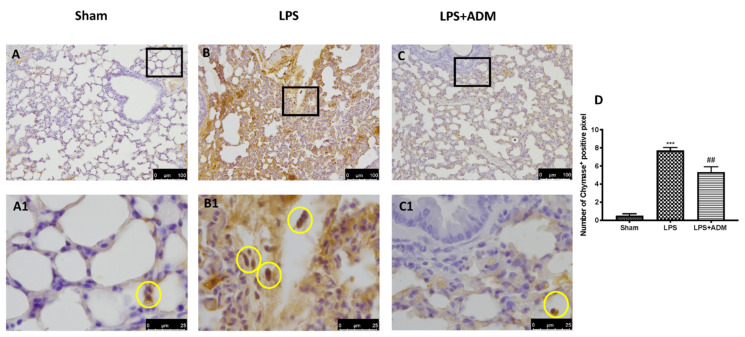
To confirm the activity of MCs and their activation, we evaluated the chymase and tryptase expressions by immunohistochemical analysis. LPS instillation enhanced chymase activity in the lungs (Figure 4B,B1,D), compared to Sham mice (Figure 4A,A1,D). At the same way, LPS increased tryptase expression in the lungs (Figure 5B,B1,D) compared to Sham mice (Figure 5A,A1,D). ADM 2% aerosol was able to reduce both preformed mediators expression (Figure 4C,C1,D for chymase; Figure 5C,C1,D for tryptase).
Figure 4.
Immunohistochemical analysis for chymase: Sham (A,A1), LPS (B,B1), LPS + ADM 2% (C,C1), graphical quantification (D). A 20× and 100× magnification is shown. Data are expressed as the mean ± SEM of N = 6 mice/group. *** p < 0.001 vs. sham; ## p < 0.01 vs. LPS.
Figure 5.
Immunohistochemical analysis for tryptase: Sham (A,A1), LPS (B,B1), LPS + ADM 2% (C,C1), graphical quantification (D). A 20× and 100× magnification is shown. Data are expressed as the mean ± SEM of N = 6 mice/group. *** p < 0.001 vs. sham; ## p < 0.01 vs. LPS.
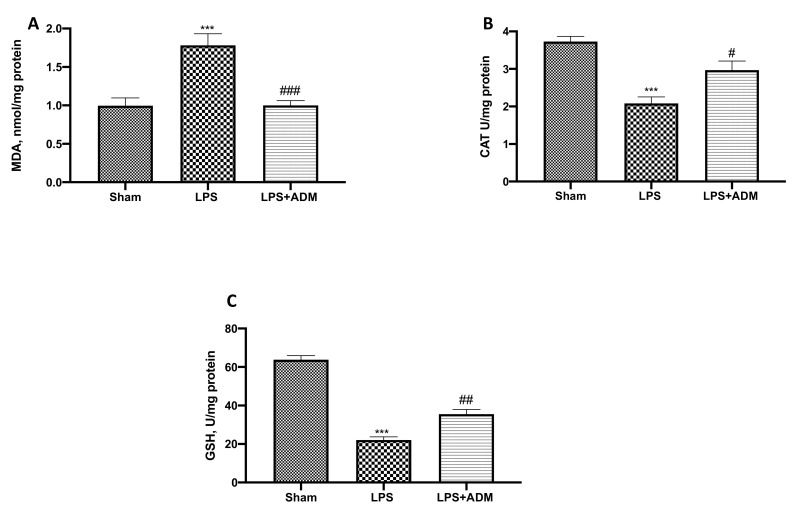
3.5. ADM 2% Aerosol on Oxidative Stress
To evaluate the effect of ADM on oxidative stress, we performed MDA activity as an indicator of lipid peroxidation. LPS-treated mice showed increased MDA levels, while the LPS + ADM group showed lower levels of MDA (Figure 6A). Additionally, we investigated CAT and GSH levels for the oxidative response. LPS induced an important decrease in CAT (Figure 6B) and GSH (Figure 6C) levels, compared to the Sham groups. Both levels of antioxidant indicators were markedly increased by ADM 2% aerosol treatment.
Figure 6.
Markers of oxidative stress: MDA (A), CAT (B), and GSH (C). Data are expressed as the mean ± SEM of N = 6 mice/group. *** p < 0.001 vs. sham; # p < 0.05 vs. LPS; ## p < 0.01 vs. LPS; ### p < 0.001 vs. LPS.
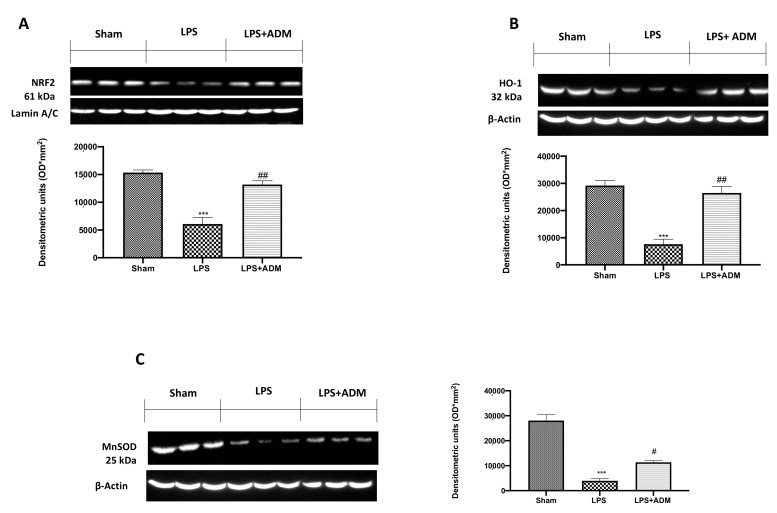
3.6. ADM 2% Aerosol on Nrf2 Pathway
To confirm the antioxidant effect of ADM, we evaluated the Nrf2 pathway by Western blot analysis. Our results showed an important reduction in Nrf2 expression in the vehicle group compared to the sham group, while ADM was able to upregulate Nrf2 expression (Figure 7A). Consequently, we evaluated HO-1 (Figure 7B) and MnSOD (Figure 7C) expression, which are regulated by the Nrf-2 pathway. Our results showed an important decrease in HO-1 and MnSOD expression in the LPS group, compared to sham mice; on the contrary, ADM partially restored the expression of both endogenous enzymes (Figure 7B,C).
Figure 7.
Western blot analysis for: Nrf2 (A); HO-1 (B); MnSOD (C). A demonstrative blot of lysates with a densitometric analysis for all animals is shown. Data are expressed as the mean ± SEM of N = 6 mice/group. *** p < 0.001 vs. sham; # p < 0.05 vs. LPS; ## p < 0.01 vs. LPS.
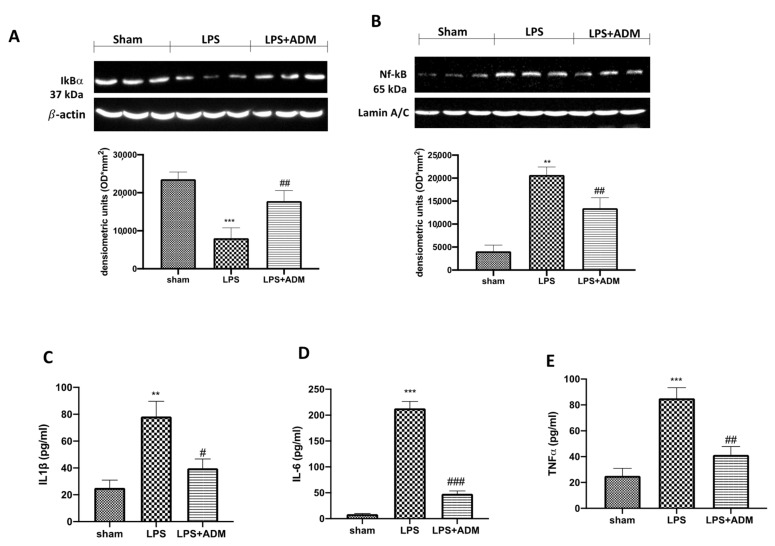
3.7. ADM 2% Aerosol on Inflammatory Pathway
Additionally, we investigated one of the key inflammatory pathways involved in LPS-induced ALI, the NF-κB pathway. Our Western blot analysis showed a basal expression of IκB-α in Sham mice, while LPS i.t. instillation significantly decreased IκB-α expression in lung samples (Figure 8A). At the same time, nuclear NF-κB expression was significantly higher in LPS-treated animals compared to the sham group (Figure 8B). ADM treatment reduced IKB-α degradation and, consequently, nuclear translocation of NF-κB induced by LPS (Figure 8A,B). Additionally, to confirm the anti-inflammatory effect of ADM, we measured the levels of pro-inflammatory cytokines in lung tissues. We found that IL-1β (Figure 8C), IL-6 (Figure 8D) and TNF-α (Figure 8E) levels were markedly increased in the LPS group, compared to the sham mice. On the contrary, ADM was able to decrease the lung levels of these pro-inflammatory cytokines (Figure 8C–E).
Figure 8.
Western blot analysis for NF-κB (B) and IκB-α (A). Levels of inflammatory cytokine in lung tissues: IL-1β (C) IL-6 (D), TNF-α (E). A demonstrative blot of lysates for NF-kB and IκB-α with a densitometric analysis for all animals is shown. Data are expressed as the mean ± SEM of N = 6 mice/group. ** p < 0.01 vs. sham; *** p < 0.001 vs. sham; # p< 0.05 vs. LPS; ## p < 0.01 vs. LPS; ### p < 0.001 vs. LPS.
4. Discussion
ALI is an acute inflammatory illness that can advance to a severe stage known as ARDS, which is marked by a high death rate. These clinical syndromes are characterized by a loss of barrier functionality by alveolar epithelial and pulmonary capillary endothelial cells, resulting in respiratory failure in critically ill individuals. To study the molecular mechanisms underlying ALI, the experimental endotoxin (bacterial LPS) model by intratracheal instillation in mice was used [7,66]. In experimental ALI, the lung parenchyma is damaged by the generation of a complex network of inflammatory cytokines and chemokine, including IL-1β, IL-6, and TNF-α [9]. Moreover, activation of oxidative stress with excessive release of ROS produced by activated pulmonary macrophages and transmigrated neutrophils in the interstitial and alveolar compartments has been demonstrated [66,67]. Then, an imbalance is created between the oxidant/antioxidant system, which, combined with the activated inflammatory response, causes diffuse alveolar damage with intrapulmonary hemorrhage, edema and fibrin deposition. Therefore, this study was designed to evaluate the effects of ADM in controlling the inflammatory and oxidative response in LPS-induced ALI. The anti-inflammatory and antioxidant properties of ADM, a member of the ALIAmide family, have been extensively demonstrated in previous studies [23,25,34,50,68,69]. First, histopathological investigation showed that ADM 2% aerosol administration significantly repaired the morphological and histological alterations in lung tissue, induced by LPS instillation. Extensive neutrophil infiltration, the large release of inflammatory mediators, an increase in capillary permeability, and severe interstitial edema are all thought to play important roles in the pathogenesis of ALI [70,71,72,73]. ADM 2% treatment was able to reduce all these parameters, as demonstrated by a reduction in MPO activity and levels of cell infiltration in BALF, as well as significant decrease in pro-inflammatory cytokines and lung edema. ALI and ARDS are often characterized by inappropriately/chronically activated MCs [74]. Many proteases, such as chymase and tryptase, are substances secreted by MCs activation, and contribute to inflammatory cells infiltration, cytokine production, and increased vascular permeability, exacerbating inflammation [75]. At this regard, the anti-inflammatory properties of ADM are mainly due to the control of MC activation and, as expected, our results confirmed a reduced number of MCs after ADM treatment. Consequently, we observed an important reduction in chymase and tryptase expression after ADM 2% administration, confirming the control of ADM on MCs activity. Additionally, it has also been demonstrated that ADM increased endogenous levels of antioxidant enzymes [25], indirectly modulating the NF-κB pathway. Indeed, ALI is characterized by excessive ROS production, causing imbalance to antioxidant system, and resulting in the release of substances modulating the endothelial dysfunction and disruption responsible for the principal clinical manifestations of the syndrome. ADM 2% aerosol administration also had positive results on endogenous levels of enzymes involved in oxidative stress; in fact, the treatment significantly counteracted the LPS-induced down-regulation of antioxidant indicators, as shown by the effect of CAT and GSH levels, as well as HO-1 and MnSOD expressions. These antioxidant enzymes are regulated by the Nrf2 pathway. Moreover, the functional crosstalk between Nrf2 and NF-κB is well known. The absence of Nrf2 is associated with increased oxidative stress, leading to an increase in cytokine production, as NF-κB is more readily activated in oxidative conditions [76,77]. Our Western blot analysis showed that ADM 2% was able to upregulate Nrf2 expression, responsible for antioxidant response, as well as to modulate the NF-κB pathway. To confirm the protective function of ADM, we also investigated the levels of proinflammatory cytokines in lung tissue. Again, ADM treatment significantly counteracted the LPS-induced increase in inflammatory mediator levels.
5. Conclusions
In conclusion, our data demonstrated that ADM 2% aerosol was able to reduce lung damage and cell infiltration, as well as the overexpression of proinflammatory cytokines. The protective effects of ADM 2% aerosol, probably due to the control of MC degranulation and the upregulation of endogenous antioxidant enzymes, modulate the inflammatory and oxidative response. Therefore, we suggest that ADM 2% aerosol can be considered as a potential candidate in the management of ALI.
Acknowledgments
We would like to thank Valentina Malvagni for editorial support with the manuscript.
Author Contributions
Conceptualization, R.D.; methodology, T.G. and M.C.; software, R.F.; validation, A.F.P. and E.G.; formal analysis, R.C. and D.I.; investigation, A.F.P.; resources, R.S. and M.C.; data curation, R.D.; writing original draft preparation, L.I. and M.C.; writing—review and editing, R.D.P.; visualization, S.C. (Stefano Coaccioli); supervision, D.I.; project administration, S.C. (Salvatore Cuzzocrea); funding acquisition, S.C. (Salvatore Cuzzocrea). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the University of Messina Review Board for the care of animals. Animal care conformed to Italian regulations on the use of animals for experimental and scientific purposes (D.Lgs 2014/26 and EU Directive 2010/63).
Informed Consent Statement
Not applicable.
Data Availability Statement
For a rule of our laboratory the datasets used in the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
Salvatore Cuzzocrea is a coinventor on patent WO2013121449 A8 (Epitech Group Srl), which deals with methods and compositions for the modulation of amidases capable of hydrolyzing N-acylethanolamines employable in the treatment of inflammatory diseases. This invention is wholly unrelated to the present study. Moreover, Cuzzocrea is also, with Epitech Group, a coinventor on the patents EP 2 821 083, MI2014 A001495, and 102015000067344, which are unrelated to the study. The remaining authors report no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peritore A.F., D’Amico R., Siracusa R., Cordaro M., Fusco R., Gugliandolo E., Genovese T., Crupi R., Di Paola R., Cuzzocrea S., et al. Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach. Int. J. Mol. Sci. 2021;22:5533. doi: 10.3390/ijms22115533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung P.O., Lee H.H., Kung Y.C., Tsai M.F., Chou T.C. Therapeutic effect of C-phycocyanin extracted from blue green algae in a rat model of acute lung injury induced by lipopolysaccharide. Evid.-Based Complement. Altern. Med. 2013;2013:916590. doi: 10.1155/2013/916590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domscheit H., Hegeman M.A., Carvalho N., Spieth P.M. Molecular Dynamics of Lipopolysaccharide-Induced Lung Injury in Rodents. Front. Physiol. 2020;11:36. doi: 10.3389/fphys.2020.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menezes S.L., Bozza P.T., Neto H.C., Laranjeira A.P., Negri E.M., Capelozzi V.L., Zin W.A., Rocco P.R. Pulmonary and extrapulmonary acute lung injury: Inflammatory and ultrastructural analyses. J. Appl. Physiol. 2005;98:1777–1783. doi: 10.1152/japplphysiol.01182.2004. [DOI] [PubMed] [Google Scholar]
- 5.Wyns H., Plessers E., De Backer P., Meyer E., Croubels S. In Vivo porcine lipopolysaccharide inflammation models to study immunomodulation of drugs. Vet Immunol. Immunopathol. 2015;166:58–69. doi: 10.1016/j.vetimm.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Matute-Bello G., Frevert C.W., Martin T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Bai C., Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev. Respir. Med. 2010;4:773–783. doi: 10.1586/ers.10.71. [DOI] [PubMed] [Google Scholar]
- 8.Lee J.-W., Seo K.-H., Ryu H.W., Yuk H.J., Park H.A., Lim Y., Ahn K.-S., Oh S.-R. Anti-inflammatory effect of stem bark of Paulownia tomentosa Steud. in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages and LPS-induced murine model of acute lung injury. J. Ethnopharmacol. 2018;210:23–30. doi: 10.1016/j.jep.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Goodman R.B., Pugin J., Lee J.S., Matthay M.A. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14:523–535. doi: 10.1016/S1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 10.Cuzzocrea S., Nocentini G., Di Paola R., Agostini M., Mazzon E., Ronchetti S., Crisafulli C., Esposito E., Caputi A.P., Riccardi C. Proinflammatory role of glucocorticoid-induced TNF receptor-related gene in acute lung inflammation. J. Immunol. 2006;177:631–641. doi: 10.4049/jimmunol.177.1.631. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y., Jin Y., Han D., Zhang G., Cao S., Xie J., Xue J., Li Y., Meng D., Fan X. Mast cell-induced lung injury in mice infected with H5N1 influenza virus. J. Virol. 2012;86:3347–3356. doi: 10.1128/JVI.06053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caughey G.H. Mast cell proteases as protective and inflammatory mediators. Adv. Exp. Med. Biol. 2011;716:212–234. doi: 10.1007/978-1-4419-9533-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundequist A., Pejler G. Biological implications of preformed mast cell mediators. Cell. Mol. Life Sci. 2011;68:965–975. doi: 10.1007/s00018-010-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellner M., Noonepalle S., Lu Q., Srivastava A., Zemskov E., Black S.M. Pulmonary Vasculature Redox Signaling in Health and Disease. Volume 967. Springer; Cham, Switzerland: 2017. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS) pp. 105–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H., Lv H., Li H., Ci X., Peng L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-kappaB pathways. Cell Commun. Signal. 2019;17:98. doi: 10.1186/s12964-019-0366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow C.W., Herrera Abreu M.T., Suzuki T., Downey G.P. Oxidative stress and acute lung injury. Am. J. Respir. Cell Mol. Biol. 2003;29:427–431. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- 17.Han Y.K., Kim J.S., Lee G.B., Lim J.H., Park K.M. Oxidative stress following acute kidney injury causes disruption of lung cell cilia and their release into the bronchoaveolar lavage fluid and lung injury, which are exacerbated by Idh2 deletion. Redox Biol. 2021;46:102077. doi: 10.1016/j.redox.2021.102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan D., Wang D., Zhu L. Protective role of scutellarin on LPS induced—Acute lung injury and regulation of apoptosis, oxidative stress and reduction of mitochondrial dysfunction. Saudi. J. Biol. Sci. 2022;29:371–378. doi: 10.1016/j.sjbs.2021.08.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui Y.R., Qu F., Zhong W.J., Yang H.H., Zeng J., Huang J.H., Liu J., Zhang M.Y., Zhou Y., Guan C.X. Beneficial effects of aloperine on inflammation and oxidative stress by suppressing necroptosis in lipopolysaccharide-induced acute lung injury mouse model. Phytomedicine. 2022;100:154074. doi: 10.1016/j.phymed.2022.154074. [DOI] [PubMed] [Google Scholar]
- 20.Cui X.F., Lin P., Yu J., Liu L., Wang Z.Y., Tang X.J. Dimethyl fumarate attenuates lipopolysaccharide-induced acute lung injury by inhibiting inflammation and oxidative stress. J. Biol. Regul. Homeost Agents. 2021;35 doi: 10.23812/21-148-L. [DOI] [PubMed] [Google Scholar]
- 21.D’Amico R., Impellizzeri D., Cuzzocrea S., Di Paola R. ALIAmides Update: Palmitoylethanolamide and Its Formulations on Management of Peripheral Neuropathic Pain. Int. J. Mol. Sci. 2020;21:5330. doi: 10.3390/ijms21155330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Impellizzeri D., Di Paola R., Cordaro M., Gugliandolo E., Casili G., Morittu V.M., Britti D., Esposito E., Cuzzocrea S. Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of acute and chronic inflammation. Biochem. Pharmacol. 2016;119:27–41. doi: 10.1016/j.bcp.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Cordaro M., Impellizzeri D., Gugliandolo E., Siracusa R., Crupi R., Esposito E., Cuzzocrea S. Adelmidrol, a Palmitoylethanolamide Analogue, as a New Pharmacological Treatment for the Management of Inflammatory Bowel Disease. Mol. Pharmacol. 2016;90:549–561. doi: 10.1124/mol.116.105668. [DOI] [PubMed] [Google Scholar]
- 24.D’Amico R., Siracusa R., Fusco R., Cordaro M., Genovese T., Peritore A.F., Gugliandolo E., Crupi R., Impellizzeri D., Cuzzocrea S., et al. Protective effects of Colomast®, A New Formulation of Adelmidrol and Sodium Hyaluronate, in A Mouse Model of Acute Restraint Stress. Int. J. Mol. Sci. 2020;21:8136. doi: 10.3390/ijms21218136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fusco R., Cordaro M., Genovese T., Impellizzeri D., Siracusa R., Gugliandolo E., Peritore A.F., D’Amico R., Crupi R., Cuzzocrea S. Adelmidrol: A new promising antioxidant and anti-inflammatory therapeutic tool in pulmonary fibrosis. Antioxidants. 2020;9:601. doi: 10.3390/antiox9070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro A., Mayer C., Wilson C., Martin R., MacFarlane P. Intratracheal LPS administration attenuates the acute hypoxic ventilatory response: Role of brainstem IL-1β receptors. Respir. Physiol. Neurobiol. 2017;242:45–51. doi: 10.1016/j.resp.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Gugliandolo E., Fusco R., Ginestra G., D’amico R., Bisignano C., Mandalari G., Cuzzocrea S., Di Paola R. Involvement of TLR4 and PPAR-α receptors in host response and NLRP3 inflammasome activation, against pulmonary infection with pseudomonas aeruginosa. Shock. 2019;51:221–227. doi: 10.1097/SHK.0000000000001137. [DOI] [PubMed] [Google Scholar]
- 28.D’Amico R., Monaco F., Fusco R., Peritore A.F., Genovese T., Impellizzeri D., Crupi R., Interdonato L., Sforza A.M., Gugliandolo E., et al. Exposure to Atrazine Induces Lung Inflammation through Nrf2-HO1 and Beclin 1/LC3 Pathways. Cell Physiol. Biochem. 2021;55:413–427. doi: 10.33594/000000393. [DOI] [PubMed] [Google Scholar]
- 29.Conte E., Iemmolo M., Fagone E., Gili E., Fruciano M., Genovese T., Esposito E., Cuzzocrea S., Vancheri C. Thymosin beta4 reduces IL-17-producing cells and IL-17 expression, and protects lungs from damage in bleomycin-treated mice. Immunobiology. 2014;219:425–431. doi: 10.1016/j.imbio.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Saadat S., Beheshti F., Askari V.R., Hosseini M., Mohamadian Roshan N., Boskabady M.H. Aminoguanidine affects systemic and lung inflammation induced by lipopolysaccharide in rats. Respir. Res. 2019;20:96. doi: 10.1186/s12931-019-1054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Impellizzeri D., D’Amico R., Fusco R., Genovese T., Peritore A.F., Gugliandolo E., Crupi R., Interdonato L., Di Paola D., Di Paola R., et al. Acai Berry Mitigates Vascular Dementia-Induced Neuropathological Alterations Modulating Nrf-2/Beclin1 Pathways. Cells. 2022:11. doi: 10.3390/cells11162616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genovese T., Cuzzocrea S., Di Paola R., Failla M., Mazzon E., Sortino M.A., Frasca G., Gili E., Crimi N., Caputi A.P., et al. Inhibition or knock out of inducible nitric oxide synthase result in resistance to bleomycin-induced lung injury. Respir. Res. 2005;6:58. doi: 10.1186/1465-9921-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzon E., Esposito E., Impellizzeri D., Di Paola R., Melani A., Bramanti P., Pedata F., Cuzzocrea S. CGS 21680, an agonist of the adenosine (A2A) receptor, reduces progression of murine type II collagen-induced arthritis. J. Rheumatol. 2011;38:2119–2129. doi: 10.3899/jrheum.110111. [DOI] [PubMed] [Google Scholar]
- 34.Di Paola D., Natale S., Iaria C., Cordaro M., Crupi R., Siracusa R., D’Amico R., Fusco R., Impellizzeri D., Cuzzocrea S., et al. Intestinal Disorder in Zebrafish Larvae (Danio rerio): The Protective Action of N-Palmitoylethanolamide-oxazoline. Life. 2022;12:125. doi: 10.3390/life12010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Paola D., Natale S., Iaria C., Crupi R., Cuzzocrea S., Spano N., Gugliandolo E., Peritore A.F. Environmental Co-Exposure to Potassium Perchlorate and Cd Caused Toxicity and Thyroid Endocrine Disruption in Zebrafish Embryos and Larvae (Danio rerio) Toxics. 2022;10:198. doi: 10.3390/toxics10040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genovese T., Impellizzeri D., D’Amico R., Fusco R., Peritore A.F., Di Paola D., Interdonato L., Gugliandolo E., Crupi R., Di Paola R., et al. Role of Bevacizumab on Vascular Endothelial Growth Factor in Apolipoprotein E Deficient Mice after Traumatic Brain Injury. Int. J. Mol. Sci. 2022;23:4162. doi: 10.3390/ijms23084162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordaro M., Fusco R., D’Amico R., Siracusa R., Peritore A.F., Gugliandolo E., Genovese T., Crupi R., Mandalari G., Cuzzocrea S., et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants. 2020;9:992. doi: 10.3390/antiox9100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Paola D., Iaria C., Lanteri G., Cordaro M., Crupi R., Siracusa R., D’Amico R., Fusco R., Impellizzeri D., Cuzzocrea S. Sensitivity of Zebrafish Embryogenesis to Risk of Fotemustine Exposure. Fishes. 2022;7:67. doi: 10.3390/fishes7020067. [DOI] [Google Scholar]
- 39.D’Iglio C., Albano M., Famulari S., Savoca S., Panarello G., Di Paola D., Perdichizzi A., Rinelli P., Lanteri G., Spano N., et al. Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021;11:16315. doi: 10.1038/s41598-021-95814-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Paola D., Abbate J.M., Iaria C., Cordaro M., Crupi R., Siracusa R., D’Amico R., Fusco R., Impellizzeri D., Cuzzocrea S., et al. Environmental Risk Assessment of Dexamethasone Sodium Phosphate and Tocilizumab Mixture in Zebrafish Early Life Stage (Danio rerio) Toxics. 2022;10:279. doi: 10.3390/toxics10060279. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Di Paola D., Capparucci F., Abbate J.M., Cordaro M., Crupi R., Siracusa R., D’Amico R., Fusco R., Genovese T., Impellizzeri D., et al. Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio) Toxics. 2022;10:81. doi: 10.3390/toxics10020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M., Zhong H., Zhang X., Huang X., Wang J., Li Z., Chen M., Xiao Z. EGCG promotes PRKCA expression to alleviate LPS-induced acute lung injury and inflammatory response. Sci. Rep. 2021;11:11014. doi: 10.1038/s41598-021-90398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su X., Wang L., Song Y., Bai C. Inhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharide. Intensive Care Med. 2004;30:133–140. doi: 10.1007/s00134-003-2001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cordaro M., Siracusa R., Fusco R., D’Amico R., Peritore A.F., Gugliandolo E., Genovese T., Scuto M., Crupi R., Mandalari G. Cashew (Anacardium occidentale L.) nuts counteract oxidative stress and inflammation in an acute experimental model of Carrageenan-induced Paw edema. Antioxidants. 2020;9:660. doi: 10.3390/antiox9080660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mariotto S., Esposito E., Di Paola R., Ciampa A., Mazzon E., de Prati A.C., Darra E., Vincenzi S., Cucinotta G., Caminiti R., et al. Protective effect of Arbutus unedo aqueous extract in carrageenan-induced lung inflammation in mice. Pharmacol. Res. 2008;57:110–124. doi: 10.1016/j.phrs.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Fusco R., Cordaro M., Siracusa R., Peritore A.F., Gugliandolo E., Genovese T., D’Amico R., Crupi R., Smeriglio A., Mandalari G., et al. Consumption of Anacardium Occidentale L. (Cashew Nuts) Inhibits Oxidative Stress through Modulation of the Nrf2/HO-1 and NF-kB Pathways. Molecules. 2020;25:4426. doi: 10.3390/molecules25194426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cordaro M., Siracusa R., Impellizzeri D., D’Amico R., Peritore A.F., Crupi R., Gugliandolo E., Fusco R., Di Paola R., Schievano C. Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Arthritis Res. Ther. 2019;21:1–17. doi: 10.1186/s13075-019-2048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galuppo M., Esposito E., Mazzon E., Di Paola R., Paterniti I., Impellizzeri D., Cuzzocrea S. MEK inhibition suppresses the development of lung fibrosis in the bleomycin model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011;384:21–37. doi: 10.1007/s00210-011-0637-7. [DOI] [PubMed] [Google Scholar]
- 49.D’Amico R., Gugliandolo E., Cordaro M., Fusco R., Genovese T., Peritore A.F., Crupi R., Interdonato L., Di Paola D., Cuzzocrea S., et al. Toxic Effects of Endocrine Disruptor Exposure on Collagen-Induced Arthritis. Biomolecules. 2022;12:564. doi: 10.3390/biom12040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siracusa R., Impellizzeri D., Cordaro M., Gugliandolo E., Peritore A.F., Di Paola R., Cuzzocrea S. Topical application of adelmidrol+ trans-traumatic acid enhances skin wound healing in a streptozotocin-induced diabetic mouse model. Front. Pharmacol. 2018;9:871. doi: 10.3389/fphar.2018.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Amico R., Fusco R., Cordaro M., Siracusa R., Peritore A.F., Gugliandolo E., Crupi R., Scuto M., Cuzzocrea S., Di Paola R., et al. Modulation of NLRP3 Inflammasome through Formyl Peptide Receptor 1 (Fpr-1) Pathway as a New Therapeutic Target in Bronchiolitis Obliterans Syndrome. Int. J. Mol. Sci. 2020;21:2144. doi: 10.3390/ijms21062144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Paola D., Capparucci F., Lanteri G., Cordaro M., Crupi R., Siracusa R., D’Amico R., Fusco R., Impellizzeri D., Cuzzocrea S., et al. Combined Toxicity of Xenobiotics Bisphenol A and Heavy Metals on Zebrafish Embryos (Danio rerio) Toxics. 2021;9:344. doi: 10.3390/toxics9120344. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Di Paola D., Capparucci F., Lanteri G., Crupi R., Marino Y., Franco G.A., Cuzzocrea S., Spano N., Gugliandolo E., Peritore A.F. Environmental Toxicity Assessment of Sodium Fluoride and Platinum-Derived Drugs Co-Exposure on Aquatic Organisms. Toxics. 2022;10:272. doi: 10.3390/toxics10050272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varghese F., Bukhari A.B., Malhotra R., De A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE. 2014;9:e96801. doi: 10.1371/journal.pone.0096801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Amico R., Gugliandolo E., Siracusa R., Cordaro M., Genovese T., Peritore A.F., Crupi R., Interdonato L., Di Paola D., Cuzzocrea S., et al. Toxic Exposure to Endocrine Disruptors Worsens Parkinson’s Disease Progression through NRF2/HO-1 Alteration. Biomedicines. 2022;10:1073. doi: 10.3390/biomedicines10051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J., Cui R., Feng Y., Gao W., Bi J., Li Z., Liu C. Serotonin exhibits accelerated bleomycin-induced pulmonary fibrosis through TPH1 knockout mouse experiments. Mediat. Inflamm. 2018;2018:7967868. doi: 10.1155/2018/7967868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saini R., Verma S., Singh A., Gupta M.L. Role of Active Principles of Podophyllum hexandrum in Amelioration of Radiation Mediated Lung Injuries by Reactive Oxygen/Nitrogen Species Reduction. CellBio. 2013;2:36989. doi: 10.4236/cellbio.2013.23012. [DOI] [Google Scholar]
- 58.Di Paola D., Iaria C., Capparucci F., Cordaro M., Crupi R., Siracusa R., D’Amico R., Fusco R., Impellizzeri D., Cuzzocrea S., et al. Aflatoxin B1 Toxicity in Zebrafish Larva (Danio rerio): Protective Role of Hericium erinaceus. Toxins. 2021;13:710. doi: 10.3390/toxins13100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peritore A.F., Crupi R., Scuto M., Gugliandolo E., Siracusa R., Impellizzeri D., Cordaro M., D’Amico R., Fusco R., Di Paola R., et al. The Role of Annexin A1 and Formyl Peptide Receptor 2/3 Signaling in Chronic Corticosterone-Induced Depression-Like behaviors and Impairment in Hippocampal-Dependent Memory. CNS Neurol. Disord.-Drug Targets. 2020;19:27–43. doi: 10.2174/1871527319666200107094732. [DOI] [PubMed] [Google Scholar]
- 60.Fusco R., Gugliandolo E., Siracusa R., Scuto M., Cordaro M., D’Amico R., Evangelista M., Peli A., Peritore A.F., Impellizzeri D., et al. Formyl Peptide Receptor 1 Signaling in Acute Inflammation and Neural Differentiation Induced by Traumatic Brain Injury. Biology. 2020;9:238. doi: 10.3390/biology9090238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marfella R., Esposito K., Nappo F., Siniscalchi M., Sasso F.C., Portoghese M., Di Marino M.P., Baldi A., Cuzzocrea S., Di Filippo C., et al. Expression of angiogenic factors during acute coronary syndromes in human type 2 diabetes. Diabetes. 2004;53:2383–2391. doi: 10.2337/diabetes.53.9.2383. [DOI] [PubMed] [Google Scholar]
- 62.Cordaro M., Siracusa R., D’Amico R., Genovese T., Franco G., Marino Y., Di Paola D., Cuzzocrea S., Impellizzeri D., Di Paola R., et al. Role of Etanercept and Infliximab on Nociceptive Changes Induced by the Experimental Model of Fibromyalgia. Int. J. Mol. Sci. 2022;23:6139. doi: 10.3390/ijms23116139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Paola D., Iaria C., Capparucci F., Arangia A., Crupi R., Cuzzocrea S., Spano N., Gugliandolo E., Peritore A.F. Impact of Mycotoxin Contaminations on Aquatic Organisms: Toxic Effect of Aflatoxin B1 and Fumonisin B1 Mixture. Toxins. 2022;14:518. doi: 10.3390/toxins14080518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Paola D., Natale S., Gugliandolo E., Cordaro M., Crupi R., Siracusa R., D’Amico R., Fusco R., Impellizzeri D., Cuzzocrea S., et al. Assessment of 2-Pentadecyl-2-oxazoline Role on Lipopolysaccharide-Induced Inflammation on Early Stage Development of Zebrafish (Danio rerio) Life. 2022;12:128. doi: 10.3390/life12010128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Siracusa R., Fusco R., Peritore A.F., Cordaro M., D’Amico R., Genovese T., Gugliandolo E., Crupi R., Smeriglio A., Mandalari G. The antioxidant and anti-inflammatory properties of Anacardium occidentale L. cashew nuts in a mouse model of colitis. Nutrients. 2020;12:834. doi: 10.3390/nu12030834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rittirsch D., Flierl M.A., Day D.E., Nadeau B.A., McGuire S.R., Hoesel L.M., Ipaktchi K., Zetoune F.S., Sarma J.V., Leng L., et al. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J. Immunol. 2008;180:7664–7672. doi: 10.4049/jimmunol.180.11.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kabir K., Gelinas J.P., Chen M., Chen D., Zhang D., Luo X., Yang J.H., Carter D., Rabinovici R. Characterization of a murine model of endotoxin-induced acute lung injury. Shock. 2002;17:300–303. doi: 10.1097/00024382-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Di Paola R., Fusco R., Impellizzeri D., Cordaro M., Britti D., Morittu V.M., Evangelista M., Cuzzocrea S. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res. Ther. 2016;18:291. doi: 10.1186/s13075-016-1189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campolo M., Siracusa R., Cordaro M., Filippone A., Gugliandolo E., Peritore A.F., Impellizzeri D., Crupi R., Paterniti I., Cuzzocrea S. The association of adelmidrol with sodium hyaluronate displays beneficial properties against bladder changes following spinal cord injury in mice. PLoS ONE. 2019;14:e0208730. doi: 10.1371/journal.pone.0208730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parekh D., Dancer R.C., Thickett D.R. Acute lung injury. Clin. Med. 2011;11:615. doi: 10.7861/clinmedicine.11-6-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma C., Dong L., Li M., Cai W. Qidonghuoxue Decoction Ameliorates Pulmonary Edema in Acute Lung Injury Mice through the Upregulation of Epithelial Sodium Channel and Aquaporin-1. Evid.-Based Complement. Altern. Med. 2020;2020:2492304. doi: 10.1155/2020/2492304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He Y.Q., Zhou C.C., Deng J.L., Wang L., Chen W.S. Tanreqing Inhibits LPS-Induced Acute Lung Injury In Vivo and In Vitro Through Downregulating STING Signaling Pathway. Front. Pharmacol. 2021;12:746964. doi: 10.3389/fphar.2021.746964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J., Lu K., Sun F., Tan S., Zhang X., Sheng W., Hao W., Liu M., Lv W., Han W. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J. Transl. Med. 2021;19:96. doi: 10.1186/s12967-021-02745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Virk H., Arthur G., Bradding P. Mast cells and their activation in lung disease. Transl. Res. 2016;174:60–76. doi: 10.1016/j.trsl.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Huang P., Liu D., Gan X., Zhang R., Gao W., Xia Z., Hei Z. Mast cells activation contribute to small intestinal ischemia reperfusion induced acute lung injury in rats. Injury. 2012;43:1250–1256. doi: 10.1016/j.injury.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 76.Genovese T., D’Amico R., Fusco R., Impellizzeri D., Peritore A.F., Crupi R., Interdonato L., Gugliandolo E., Cuzzocrea S., Paola R.D., et al. Acai (Euterpe Oleraceae Mart.) Seeds Regulate NF-kappaB and Nrf2/ARE Pathways Protecting Lung against Acute and Chronic Inflammation. Cell Physiol. Biochem. 2022;56:1–20. doi: 10.33594/000000529. [DOI] [PubMed] [Google Scholar]
- 77.Cordaro M., D’Amico R., Morabito R., Fusco R., Siracusa R., Peritore A.F., Impellizzeri D., Genovese T., Crupi R., Gugliandolo E., et al. Physiological and Biochemical Changes in NRF2 Pathway in Aged Animals Subjected to Brain Injury. Cell Physiol. Biochem. 2021;55:160–179. doi: 10.33594/000000353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For a rule of our laboratory the datasets used in the current study are available from the corresponding author on reasonable request.