Abstract
Simple Summary
Metastasis is the main cause of death in patients with malignant tumors worldwide. Mounting evidence suggests lipid droplet metabolism is involved in the process of metastasis. As a mechanism to selectively degrade lipid droplets, the current research on lipophagy and tumor metastasis is quite limited. This review summarizes the crosstalk among lipophagy, tumor lipid metabolism and cancer metastasis, which will provide a new reference for the development of effective targeted drugs.
Abstract
Obesity is a prominent risk factor for certain types of tumor progression. Adipocytes within tumor stroma contribute to reshaping tumor microenvironment (TME) and the metabolism and metastasis of tumors through the production of cytokines and adipokines. However, the crosstalk between adipocytes and tumor cells remains a major gap in this field. Known as a subtype of selective autophagy, lipophagy is thought to contribute to lipid metabolism by breaking down intracellular lipid droplets (LDs) and generating free fatty acids (FAs). The metastatic potential of cancer cells closely correlates with the lipid degradation mechanisms, which are required for energy generation, signal transduction, and biosynthesis of membranes. Here, we discuss the recent advance in the understanding of lipophagy with tumor lipid metabolism and review current studies on the roles of lipoghagy in the metastasis of certain human malignancies. Additionally, the novel candidate drugs targeting lipophagy are integrated for effective treatment strategies.
Keywords: lipophagy, lipid metabolism, cancer metastasis
1. Introduction
Cancer metastasis is the progression of cancer cells escaping from a primary site to distant organs that they gradually colonize [1]. Cancer metastasis is a dynamic process with multi-factor participation and multi-stage development involving tumor cells themselves, the interaction between the tumor and the microenvironment, etc. Given its systemic nature and the resistance of disseminated tumor cells to existing therapies, metastatic disease accounts for 90% malignant cancer-related deaths [2]. Thus, our capacity to interdict the process of metastasis largely determines the therapeutic efficacy. Based on the clinical realities, attention to implicating specific molecules in biological aspects of the metastasis has increased [3].
Lipophagy is a source of alternative energy in times of nutrient scarcity [4]. In a broad sense, lipophagy include two types: chaperone-mediated lipophagy and macrolipophagy; in a narrow sense, macrolipophagy is identified as lipophagy because it occurs more frequently [5]. During the selective autophagic degradation of lipid droplets (LDs), the phosphorylation of perilipins is firstly triggered by protein kinase A [6]. LD surface proteins PLIN2 and PLIN3 are next recognized by Hsc70 and bind to lysosomal membrane receptor LAMP2, contributing to a degradation progress called chaperone-mediated lipophagy [7]. Then, the LDs are exposed for degradation in two ways: (1) lipases represented by lipase degrade LDs directly into fatty acids (FAs) by lipolysis [8]; (2) Rab7 and Rab10 recruit lysosome and LC3-positive autophagic membranes to LDs, respectively [9,10], leading to a progress termed macrolipophagy. Lipophagy was firstly described in liver cells [4], since then, the function of lipophagy in adipocytes [11], neurons [12], renal tubular cells [13], prostate carcinoma cells [14], breast cancer cells [15] and foam macrophages [16] have been discovered gradually. Remarkably, an effective way to monitor the lipophagy process by a pH-sensitive spirocyclization strategy makes it easier to visualize the closely related organelles [17].
Notably, recent studies indicate that lipophagy not only supports fuels for cancer metastasis [15], but also participates in each step of metastasis [18,19]. So, understanding the latest potential roles of lipophagy in common malignancies to find new therapeutic targets is of great importance.
2. Overview of Lipophagy in Tumor Lipid Metabolism
2.1. Steps of Lipophagy
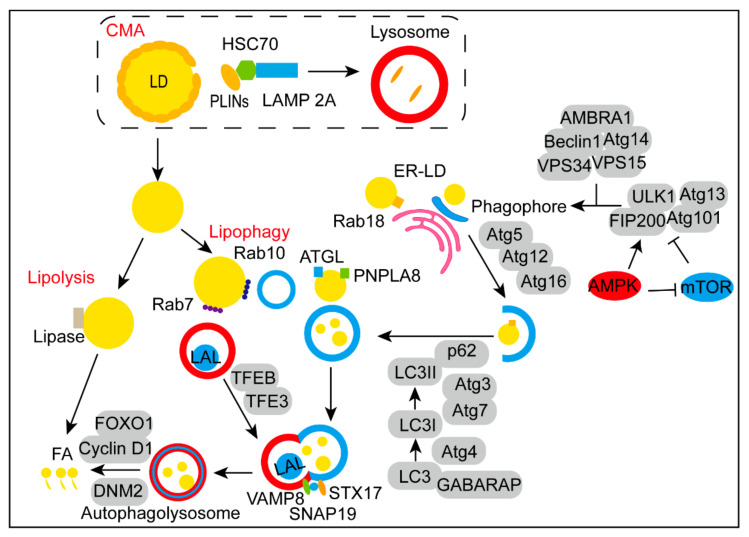
As a selective autophagy, the steps of lipophagy include initiation of autophagy, phagophore nucleation, expansion of phagophore, fusion of autolysosome and degradation of LDs, enjoying overlapping and special parts with autophagy (Figure 1).
Figure 1.
Steps of LDs degradation. After the degradation of PLIN2 and PLIN3 in lysosome via CMA mediated by HSC70, LDs are exposed for lipolysis and lipophagy. The activation of AMPK and mTOR recruits ULK1-Atg13-FIP200-Atg101 complex to trigger the initiation of autophagy. Next, the initiation complex composed of Beclin1, Atg14, VPS34, VPS15, and AMBRA1 is activated to form the origin of phagophore. Rab18 is essential in the spatiotemporal dynamic of ER-LD. During the elongation progress, the Atg5-Atg12-Atg16 complex promotes the autophagic vesicle formation. Simultaneously, Atg3, Atg4 and Atg7 are involved in the lipid-soluble form of LC3. Receptors such as p62 facilitate the localization of LC3-II on the autophagic membrane. On the surface of LDs, ATGL and PNPLA8 were identified as mammalian lipid receptors. Rab10 recruits LC3-positive phagophores to the circumference of LD, while Rab7 promotes the tethering and fusion of autophagosomes to lysosomes. TFEB and TFE3 are key regulators in autophagosome maturation and lysosomal biogenesis. Then, the VAMP8-SNAP29-STX17 complex mediates the form of autolysosome. Finally, LDs in the autolysosome degraded to FAs by LAL and regulated by FOXO1, Cyclin D1 and DNM2.
2.1.1. The Initiation of Autophagy via the Complex of ULK1-Atg13-FIP200-Atg101
Under tumor hypoxia and nutrient shortage circumstances, elevated levels of AMP/ATP and ADP/ATP ratios inhibit the mammalian target of rapamycin complex 1 (mTORC1) signal pathway by activating the energy sensor AMP-activated kinase (AMPK) pathway [20]. And the initiation of autophagy is triggered by the ULK1-Atg13-FIP200-Atg101 complex at the same time [21]. As the core component of the complex, the kinase activity of ULK1 is regulated by the phosphorylation level of Ser 317, Ser 467, Ser 574 and Ser757 [22]. Atg13, a lipid-binding protein, plays a mediating role and contains a HORMA domain [23]. FIP200 is the ortholog of Atg17 in mammals [24]. Atg101 binds to ATG13 through the HORMA domain to jointly maintain the stability of the complex [25]. Then the initiation complex composed of Beclin1, Atg14, VPS34, VPS15, and AMBRA1 is activated, leading to the accumulation of phosphatidylinositol 3,4,5-triphosphate (PI3P) at phagophore assembly site (PAS), which is the origin of phagophore [26]. Beclin1 is the core subunit of the complex [27], Atg14 determines the localization of the PI3K complex [28], VPS34 is mainly involved in the formation of endoplasmic reticulum (ER) membrane curvature [29], VPS15 plays a role in multiple membrane trafficking pathways [30], the major function of AMBRA1 is to positively regulate the lipid kinase activity [31].
2.1.2. The Rab18-NRZ-SNARE Complex Is Essential in Establishing ER-LD Contact
Acting as gatekeepers of LDs, the degradation of LD surface proteins (PLINs) is a prerequisite for lipophagy to occur [7]. Exposure of the LD surface allows the phagophore nucleation step. Vesicular expansion and cisternal expansion are two possible mechanisms of phagophore nucleation, they rely on the delivery of lipid bilayers and the fusion of small compartments, respectively [32]. The dissociation of LDs from the ER is prior to phagophore nucleation. When it comes to the spatiotemporal dynamic of ER-LD, several proteins are involved. The Rab18-NRZ-SNARE complex has been proved essential in aggregating and establishing ER-LD contact to maintain LD formation. The deficiency of Rab18 led to a markedly decreased number of mature LDs and increased ER stress [33]. It has been found that DFCP1 was a Rab18 effector for LD localization and played a positive role in enhancing ER-LD contacts [34]. LDAF1-seipin complex is the core protein machinery in facilitating LD biogenesis and determining the location in the ER [35]. Meanwhile, the deficiency of ORP5 promoted the accumulation of triglycerides (TGs) in LDs by increasing the expression of PI(4)P, which is important in the transportation of phosphatidylserine [36].
2.1.3. Rab10 Is Important in “Lipophagic Junction”
Many ATGs are critical in the elongation of phagophores. During the progress, the Atg5-Atg12-Atg16 complex promotes the autophagic vesicle formation [37]. Atg12 conjugates with Atg5 through a ubiquitin-like conjugating system and then bound to the N-terminal region of Atg16, which is paramount in recruiting the complex to autophagosomal membranes [38]. During the elongation of phagophore, another critical step is the binding of LC3/GABARAP family proteins, also called ATG8 family proteins to phosphatidylethanolamine (PE). Furthermore, Atg3, Atg4 and Atg7 are involved in the transition of LC3 from a soluble form (LC3-I) to a lipid-soluble form (LC3-II). Membrane curvature-reading domains in the N-terminus of Atg3 are required for the lipidation preferentially occurred in lipid-deficient membranes [39]. The Atg4 cysteine proteases are required for proteolytic activation and delipidation [40]. Atg7 an essential autophagy effector enzyme, activates the conjugation between either ATG5 and ATG12 or ATG8 family proteins and PE [41]. In line with it, overexpression of ATG7 reversed the accumulation of hepatic LDs [42]. Then, by interacting with receptors including p62, NBR1, NDP52 and OPTN, LC3-II is located in the lipid bilayer of the phagophore and delivers the modified target protein to phagophore [43]. Worth mentioning, Rab10 recruits LC3-positive phagophores to the circumference of LD surface via the form of Rab10-EHBP1-EHD2 complex, resulting a “lipophagic junction” [44].
2.1.4. Rab7 Promotes “Lipophagic Synapses” in the Fusion of Autolysosome
Transcription factors TFEB and TFE3 are key regulators in autophagosome maturation and lysosomal biogenesis [45]. The local release of Ca2+ from lysosomes through mucolipin 1 (MCOLN1) activates calcium-dependent calcineurin, which binds and dephosphorylates TFEB, permitting its transcriptional activation [46]. The activated TFEB in nucleus next binds to CLEAR motifs, which comprise several promoters of lysosomal genes [47]. In addition, dephosphorylated TFE3 shuttles from cytoplasm rapidly to nucleus for combination of CLEAR elements, as a result, expanding the lysosomal compartment and facilitating autophagic flux [48]. Then the autophagosome docks with the lysosome with the help of dynein to form the autolysosome [49]. Importantly, Rab7, a fundamental component of LDs, promotes the progress termed “lipophagic synapses”, which induce the tethering and fusion of autophagosomes to lysosomes [50]. During the procedure, apart from VAMP8-SNAP29-STX17 complex [51], another complex YKT6-SNAP29-STX7 also mediates the fusion [52]. Molecules recruit to the progress include TECPR1 [53], EPG5 [54], PLEKHM1 [55] and GRASP55 [56], etc.
2.1.5. Lysosomal Acid Lipase Degrades the Internal Lipids Stored in LDs
After the formation of autolysosome, lysosomal acid lipase (LAL), localized in the lysosome originally, attacks and degrades the internal cholesterol esters (CEs) and TGs stored in LDs [50]. The FAs produced by degradation are then released to the cytoplasm. Forkhead homeobox type protein O1 (FOXO1), a critical mediator of the cellular stress response, triggers lipophagy by up-regulation of LAL and then facilitates the lipid degradation cascade [11]. In addition, Cyclin D1 interferes with lipophagy by enhancing the accumulation of LDs or disrupting the degradation of LDs in the autolysosome [57]. Following the breakdown of LDs is the formation of new lysosomes by recycling autolysosomal membranes. During autophagic flux, the large GTPase DNM2 acted as a regulator of lipophagic turnover by promoting the scission of nascent tubules from previous autolysosome [58]. Under nutrient-limiting conditions, acting as precursors of protolysosomes, short tubular structures bud into cytoplasm to maintain efficient lipophagy operation [58].
2.1.6. The Regulation of Lipophagy
On the one hand, many hormones are involved in the regulation of lipophagy. It is proved that β-adrenergic signaling enhances the lipophagy procedure in a Rab-7-dependent manner [59]. Stimulation of adipocytes with insulin or β-adrenergic receptor agonist isoproterenol benefits the supplementation of Rab18 to the surface of LDs [60]. Thyroid hormone 3 promotes lipophagy in a thyroid hormone receptor-dependent manner by activation of ANGPTL8 [61]. On the other hand, several transcriptional regulators and key enzymes are critical in the lipophagic progress. Under nutrient deprivation circumstances, the dephosphorylation of TFEB plays an essential role in enhancing lipophagy by the fasting transcriptional activator CREB and PPARα, while the activation of FXR could revise the procedure [62]. It is proved that SREBP-2 directly activates autophagy genes and increases LD turnover in the presence of cell-sterol depletion [63]. Lipophagic progress could be inhibited by abnormal accumulation of p62 and LDs as a result of superoxide dismutase 1 (SOD1) deficiency [64]. ATGL and PNPLA8 were identified as mammalian lipid receptors. Mechanistically, SIRT1 activity is required for ATGL-mediated induction of lipophagy [65] while PNPLA8 can interact with LC3 to induce lipophagy [66]. In addition, Muhammad et al. summarized varieties of natural compounds such as epigallocatechin-3-gallate (EGCG) [67], caffeine [68], bergamot [69] and resveratrol [70] could modulate lipophagy. It should be mentioned that researchs on the roles of dietary lipids level and stored lipid content in lipophagy are limited.
2.2. Associations between Lipophagy and Tumor Lipid Metabolism
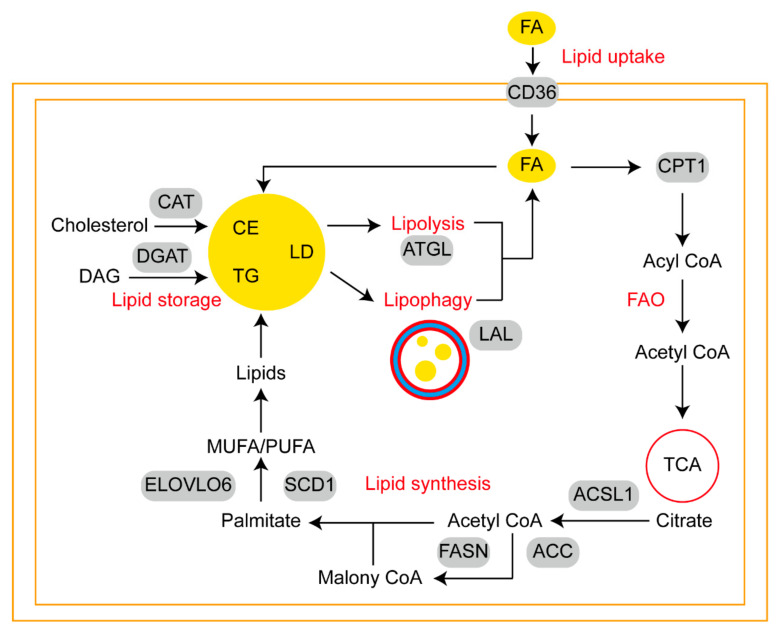
Cross-talk between adipocytes and cancer cells in the tumor microenvironment (TME) can create a metabolic symbiosis driving cancer metastasis. Lipophagy can occur in both adipocytes and tumor cells. Lipophagy may modulate differentiation process of adipocytes from adipocyte precursor cells. Research carried out by Hahm et al. showed that the expression of LC3-II, AMPK activity and formation of acidic vesicles appeared during the normal differentiation of mouse 3T3-L1 preadipocytes [71]. The inhibition of lipophagy resulted in the inhibition of the adipogenic differentiation of embryonic fibroblasts by preventing LDs aggregation [72]. Adipose tissue can be fertile ground for metastatic tumors, for example, melanoma actively absorbs lipids and is more likely to metastasize to adipocyte-rich tissue [73]. In similar, ovarian cancer has a clear predilection for metastasis to omentum, a fatty tissue characterized by immune structures called milky spots. Milky spots and adipocytes are responsible for the localization of disseminated cancer cells and subsequent growth respectively [74]. Lipids absorption by tumor cells facilitate remodeling of lipid metabolism (Figure 2), which includes five parts: (1) lipid uptake; (2) fatty acid oxidation (FAO); (3) lipid synthesis; (4) lipid storage; (5) lipolysis and lipophagy.
Figure 2.
Schematic diagram of lipid metabolism reprogramming in metastatic tumor cells. Extracellular FAs could be transported across the membrane by CD36 and FABPs. The entry of FAs into mitochondria for FAO is controlled by CPT1. ACSL1 catalyzes the conversion of citrate to substrates for lipid synthesis. ACC and FASN are key enzymes in the biosynthesis of palmitate. Then, ELOVL6 and SCD1 facilitate the conversion of monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA). Excess lipids can be stored in the forms of LD, which cores are CE and TG. Under stress conditions, LDs could be degraded by lipolysis and lipophagy to generate energy.
2.2.1. Sources of Fatty Acids Available to Tumor Cells
Known as energy sources and membrane constituents, FAs are required to meet the metabolic demand of cancer cells or to adapt to reduced serum-derived lipid availability in TME [75]. A variety of FAs derived from dietary routes and endogenous. Nutrients consumed by tumor cells may affect cancer progression, dietary polyunsaturated fatty acid (PUFA) as a selective adjuvant antitumor modality may efficiently induce ferroptosis in acidic cancer cells [76]. During feeding, gut microbes produce short-chain fatty acids (SCFAs), which promote prostate cancer growth via IGF1 signaling [77]. Metastasis-associated tumor stromal cells, such as adipocytes, fibroblasts, and macrophages, are frequently sources of FAs. During adipocyte lipolysis, FAs provide breast cancer cells with metabolic substrates [78]. FAs are transferred from cancer-associated fibroblasts to cancer cells through vesicles, which promote tumor growth [79]. Macrophages are a rich source of FAs, in addition, increased levels of FAO in macrophages can effectively promote M2 polarization [80]. To some extent, cancer cell growth relies on FAs supplied through the bloodstream [81].
2.2.2. CD36 and Fatty Acid-Networks Binding Proteins Participate in Lipid Uptake
Tumor cells can support growth by ingesting FAs obtained from extracellular lipolysis. In normal physiological state, as essential membrane glycoprotein, CD36 mediates lipid uptake, immunological recognition, inflammation, molecular adhesion, and apoptosis in multiple cell types [82]. CD36, a scavenger receptor, is highly expressed in tumor cells at metastatic sites but low in the carcinoma in situ [83]. The large extracellular region of CD36 is used for the binding of FAs and oxidized LDL by forming hydrophobic cavities [84]. CD36 is transported across organelles and cell membranes by vesicles, regulating FA uptake and metabolic energy balance. It has been shown that uptake of PA by CD36 leads to AKT phosphorylation, which is positively correlates with gastric cancer metastasis [85]. The report by Ladanyi et al. suggested that ovarian cancer (OvCa) cells co-cultured with primary human omental adipocytes express high levels of CD36 in the plasma membrane, thereby facilitating exogenous FA uptake and OvCa metastasis [86]. In similar to the function of CD36, fatty acid-networks binding proteins (FABP) are lipid chaperones assisting in the trafficking of fatty acids [87]. Decreased expression of FABP2 and FABP3 was observed when adding lipids into MDA-MB-231 cells pretreated with fibroblast media. Concomitant with this was an increase in FAs uptake and a decline of fatty acid synthase (FASN) activity [88]. Targeting FABP4 can inhibit the ability of ovarian cancer cells to adapt and colonize in lipid-rich TME efficiently [89,90]. It is worth noting that activation of the FABP12/PPARγ pathway in prostate cancer cells facilitates metastatic transformation via energy derived from lipids [91].
2.2.3. Carnitine Palmityl Acyltransferase 1 Is the Key Enzyme in FAO
The ingested FAs are then utilized by FAO, also called β-oxidation, which mainly occurs in mitochondria. The progress requires long-chain acyl-coenzyme A synthase (ACSL) to activating acyl CoA, which is then catalyzed by carnitine palmityl acyltransferase 1 (CPT1) from cytoplasm to mitochondria. Each ACSL isoform is responsible for channeling long-chain FAs to a different metabolic fate. Its deregulation has also been observed frequently in variable cancers [92]. A miR-377-3p-CPT1 axis has been implicated in the metastasis of hepatocellular carcinoma through mediating FAO [93]. By repeating multiple rounds of enzymatic dehydrogenation, hydration, dehydrogenation and thiolysis, acetyl-CoA, NADH and FADH2 can enter the TCA cycle or maintain cellular homeostasis. YAP, a transcriptional coactivator, is selectively activated in lymph node-metastatic tumors, resulting in increased expression of genes involved in FAO signaling [94].
2.2.4. FASN Plays Important Roles in Lipid Synthesis
As ATP citrate lyase (ACLY) converts citrate to oxaloacetate, cytosolic acetyl-CoA is generated, which serves as a major substrate for lipid synthesis. The IKKβ-USP30-ACLY axis controls lipogenesis and tumorigenesis [95]. First committed step, the synthesis of malonyl-CoA is catalyzed by acetyl-CoA carboxylase (ACC). Lipogenesis and hepatocellular carcinoma are suppressed when ACC is inhibited by phosphorylation or ND-654 [96]. Multiple polymerizations of acetyl-CoA and malonyl-CoA under the catalytic action of FASN create saturated fatty acids. In the progression of breast cancer, CircWHSC1regulates the FASN/AMPK/mTOR axis via the sponge of miR-195-5p [97]. Palmitate, the product of FASN-mediated de novo lipogenesis, can either be elongated by very long chain fatty acids protein 6 (ELOVL6) or desaturated by stearoyl-CoA desaturases (SCDs). ELOVL6 regulates bortezomib resistance in multiple myeloma [98]. SCD1 has been identified as a mechanoresponsive enzyme in response to matrix stiffness and lipid metabolism and its overexpression is a prognostic marker associated with poor outcomes in HCC patients [99].
2.2.5. LD Is a Major Form of Lipid Storage
An excess of FAs can be harmful to cells due to lipotoxicity, so cells convert FAs into TGs and CEs for storage in LDs. The final step of TG synthesis is catalyzed by diacylglycerol acyltransferase (DGAT) while cholesterol acyltransferase (CAT) is the key enzyme in the form of CEs. By the way, some extra sphingolipids and cholesteryl esters can form lipid rafts in the plasma membrane that play a role in signal transduction [100]. As a result of the anabolic reaction, neutral lipids are continuously deposited in a lens-like microdomain between the ER bilayer membranes, the outer lobe of which buds to form a distinct spherical organelle, the LDs. Then, the initial LDs could be converted to expanding LDs and giant LDs. In response to an increase in cellular FAs levels, LD biogenesis is stimulated. Of note, acidic microenvironment caused by tumors favors the synthesis and release of LDs [101,102]. LDs vary in size and number in different cell types, revealing the capacity of managing lipid storage [103]. Moreover, LDs are thought to mediate lipid transport by directly interacting with most cellular organelles [104].
2.2.6. ATGL Predicts a Linear Relationship between Lipolysis and Lipophagy
Malnutrition and stress conditions mediate LDs degradation via lipolysis and lipophagy. In colon cancer cells, overexpression of key lipase ATGL is observed in obesity-promoted colonic tumorigenesis via lipid metabolic reprograming [105]. Meanwhile, Elevated DECR1 directly activates HSL to enhance lipolysis in Hela cells [106]. It has been demonstrated that, under glucose deprivation, choline kinase (CHK) promotes lipolysis, FAO and brain tumor growth by phosphorylating PLIN2/PLIN3 on the surface of LDs [107]. In a recent study, Satyanarayan and colleagues found that there was a linear relationship between lipase ATGL and lipophagy. By increasing the interaction between LC3 and lysosomes with LD, ATGL increases lipophagy and positively regulates autophagy in the liver [65]. Of note, studies indicated that lipolysis preferentially targeted larger-sized LDs in hepatocytes, while the resulting small LDs (<1 μm2) are more suitable for lipophagy [108]. Whether a similar phenomenon exists in tumor lipid metabolism remains to be further demonstrated.
3. Evidence of Lipophagy in Cancer Metastasis
According to integrative analysis of multiple omics data, it is revealed that lipid metabolism disturbances caused by lipophagy may confer pro-metastatic traits and directly impact the metastatic dissemination process [109]. The metastases are formed after the completion of a complex series of cellular biological events named as invasion-metastasis cascade. As the process unfolds, cells break free from the primary tumor [110]. The tumor cells then penetrate the blood vessels via angiogenesis and proteolysis [111]. Cancer cells are subsequently found in the circulation either as single cells or as emboli containing platelets or leukocytes to protect them from shear forces, immune attack, and anoikic [112]. Then, tumor cells arrest at distant organs and extravasate into the parenchyma. Following extravasation, the vast majority of cells die while the rest remain dormant or grow into a micrometastasis [113]. As a result of adaptation and remodeling of the microenvironment, cancer cells form a macrometastatic lesion [3]. This whole cascade is orchestrated by a cohort of molecular pathways such as epithelial–mesenchymal transition (EMT), together with genes related to oxidative stress, viral replication and signal transduction. Given the limited researchs are available on the role of lipophagy in cancer metastasis, we will clarify the role of lipophagy in several of the above-mentioned biological process related to cancer metastasis in this section.
3.1. The Interaction between Lipophagy and EMT
EMT is a cellular pathway that represents a salient property of primary tumor development and metastasis, in which cells lose their epithelial properties (characterized by the loss of membranous E-Cadherin) and acquire features of mesenchymal cells (featured by increased N-cadherin, vimentin expression along with migratory capability) [114]. Higher levels of MUFA together with enhanced de novo fatty acid synthesis is the unique signature of epithelial cells. By contrast, reduced lipogenesis, higher PUFA level and increased TGs synthesis and LDs formation are observed in mesenchymal cells [115]. There is evidence that the loss of C/EBPδ, a critical lipid metabolic regulator can induce significant oscillations in EMT gene networks, leading to cancer metastasis via enhanced oxLDL uptake [116]. In hepatocellular carcinoma, lipophagy has been proved to participate in fluid shear stress (a pathological factor in cancer metastasis)-induced EMT and cell migration [117]. It is revealed that inhibition of 14,15-epoxyeicosatrienoic acid, an important lipid signaling molecule could serve as a novel strategy to reverse EMT in breast cancer cells [118]. Exploring the role of the lipophagy portion of lipid signaling in EMT is integral to elucidating the mechanisms of cancer metastasis.
3.2. Oxidative Stress and Lipophagy
Oxidative stress refers to a state in which the oxidative and anti-oxidative effects are unbalanced. The accumulation of reactive oxygen species (ROS) is the main cause of it [119]. To optimize ROS-driven proliferation, cancer cells enhance the capacity of anti-oxidation while avoiding ROS thresholds that are necessary for senescence, apoptosis, or ferroptosis [120]. Ferroptosis is a type of nonapoptotic cell death caused by lipid peroxidation. Recent studies revealed that lipophagy promoted RSL3-induced lipid peroxidation and subsequent ferroptosis in hepatocytes, which can be prevented either by enhancing TPD52-dependent lipid storage or blocking ATG5- and RAB7A-dependent lipid degradation [121]. Lipophagy mediated by PGRMC1 is required for ferroptosis in paclitaxel-tolerant persister cancer cells [18]. Interestingly, a recent study revealed a unique relationship between ATG14 and lipophagy in HeLa cells. In response to ATG14 overexpression, lipophagy and intracellular FAs accumulation occur, which lead to oxidative stress and apoptosis, while inhibition of ATG14 maintains cell survival [122]. In addition, it was previously found that oxidative and ER stress pathways played crucial regulatory roles in high glucose-induced the activation of lipophagy and lipid metabolism via enhancing the DNA binding capacity of carbohydrate response element-binding protein at PPARγ promoter region, which in turn induced transcriptional activation of the key genes related to lipogenesis and lipophagy [123]. Similar pathogenic mechanisms have not been investigated in tumor patients with obesity, which is a risk factor in certain cancers.
3.3. Lipophagy in Viral Replication
Lipophagy is a selective autophagic process that not only facilitates the clearance of lipid aggregates, but also provides energy for the replication of multiple pathogenic microorganisms. In the latest study, inflammatory mediators released by LDs and their surface antiviral proteins form a line of defense during microbial infection, thereby acting as a molecular switch in innate immunity [124]. Hepatitis virus infection often exacerbates the vascular metastasis of liver cancer. In liver cancer cells, lipophagy leads to a significant decrease in LD volume and TG, which contributes to the replication of hepatitis C virus (HCV) [125]. In a recent study, LDs-associated ABHD5 was essential for the assembly and release of HCV, the underlying mechanisms by which it mobilized LDs-associated lipids metabolism is possibly via the regulation of ATGL activity [126]. Additionally, inhibition of autophagy increases cellular oxidation, which prevents the lipophagy of virus [127]. Furthermore, the small GTPase Rab11a has been identified to function as a proviral host factor in stimulating viral replication [128], and there is evidence that the silence of Rab11b leads to the accumulation of LC3-II [129], whether Rab11a is essential in viral replication through lipophagy remains to be further explored.
3.4. Lipophagy in Signal Transduction
As a result of lipophagy, excessive FAs are produced in lysosomes. Researchers recently discovered that lipid-filled lysosomes fuse with plasma membranes for extracellular export, altering systemic lipid homeostasis and intercellular communication [130]. P53 is a tumor-suppressor that plays a direct role in the regulation of autophagy and FAO-related genes, as well as promoting lipid degradation afterwards [131]. LC3-II and perilipins, both of which are necessary for lipophagy, can be downregulated by inhibition of p53, which is required for the induction of lipophagy in hepatoma cells. Thereby, p53 exerts a unique tumor-suppressor role through lipophagy [132]. It has been documented that LAL deficiency can increase the risk of tumorigenesis and metastasis via mTOR signaling [133]. Moreover, by activating PI3K/AKT/mTOR, Apolipoprotein C-II promotes gastric cancer peritoneal metastatic spread [134]. By altering lipid metabolism, cancer stem cells (CSCs) can both satisfy their energy demands and produce biomass, as well as stimulate a number of key oncogenic signaling pathways, such as Wnt/β-catenin and Hippo/YAP signaling [135]. However, whether these signaling pathways affect malignant metastasis via lipophagy needs to be further studied.
4. Potential Treatment Strategies through Targeting Lipophagy
With the increasing understanding of the mechanism of lipophagy, scholars try to find ways to effectively control metastasis by inhibiting the lipophagy pathway. There have been recent proposals that LDs can serve as functional markers of CSCs, whose biogenesis is a multistep process involving the enzyme diacylglycerol acyltransferase 2 (DGAT2). Pretreatment with PF-06424439, an inhibitor of DGAT2, in conjunction with X-ray exposure can enhance the radiosensitivity of breast cancer cells and improve the effectiveness of radiotherapy [136]. When used alone and in combination with carboplatin, AACOC3 effectively inhibited tumor growth and LD biogenesis in ovarian cancer [137]. Taking metformin works in reducing adipocyte secretion of MCP-1 and prevents ovarian cancer metastasis by inhibiting MCP-1/CCR-2 axis [138]. By activating a variety of pathways involved in apoptosis and lipophagy, simvastatin can kill radioresistant breast cancer cells, as well as inhibit their migration abilities and vimentin expression [139]. An inhibitor of PFKFB3 called PFK-15 promotes lipophagy in gynecologic cancers and is also chemosensitive [140]. Resveratrol, a tumor-suppressor, reverses lysophosphatidic acid induced cell migration and platinum resistance via rescuing hedgehog-mediated autophagy in ovarian cancer therapy [141]. Hydroxychloroquine (HCQ) and chloroquine are inhibitors of autophagy used in the cancer clinic. Goldberg and colleagues have successfully demonstrated the safety of HCQ with and without erlotinib in the treatment of non-small-cell lung cancer patients [142]. Over expression of CCAAT enhancer-binding protein a in hepatocellular carcinoma weakens tumorigenesis by increasing lipophagy, which can be abrogated by chloroquine treatment [143]. Celastrol, a triterpene derived from the traditional Chinese medicine, has been identified as a lipophagy-based anticancer therapeutic approach in ccRCC [144]. Additional studies indicated that melatonin promoted “tumor slimming” and suppressed ccRCC development via PGC1A/UCP1-mediated autophagy and lipid browning [145]. Aside from developing new targeted drugs, researchs on new applications of old drugs are also urgently needed.
5. Conclusions
The above summarized the crosstalk among lipophagy, tumor lipid metabolism and cancer metastasis. A better understanding of the crosstalk mechanism will lead to new and exciting therapeutic possibilities for cancer elimination. Despite the fact that the related mechanisms of lipophagy have been illustrated, as well as some proteins or genes that are key participants in lipophagy have been validated, the overall landscape of lipophagy requires further research. The researchs on the roles of lipophagy in tumor cells and tumor stromal cells including tumor-associated adipocytes, tumor-associated fibroblasts and tumor-associated macrophages are also warranted. Unfortunately, the mechanism of lipophagy related to tumor angiogenesis and immune escape is still lacking, which are closely related to the metastatic potential of tumors. With the development of new sequencing technologies and probe labels, more pivotal factors involving lipophagy and cancer metastasis will be formulated, and new personalized clinical treatment options will be developed, based on the sufficient fundamental research.
Abbreviations
LD: Lipid droplet; FA, fatty acid; PLIN, lipid droplets surface protein; ER, endoplasmic reticulum; TG, triglycerides; LAL, lysosomal acid lipase; CE, cholesterol esters; TME, tumor microenvironment; FAO, fatty acid oxidation; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; FABP, fatty acid-networks binding proteins; FASN, fatty acid synthase; ACSL, long-chain acyl-coenzyme A synthase; CPT1, carnitine palmityl acyltransferase 1; ACLY, ATP citrate lyase; ACC, acetyl-CoA carboxylase; ELOVL6, very long chain fatty acids protein 6; SCD, stearoyl-CoA desaturase; DGAT, diacylglycerol acyltransferase; CAT, cholesterol acyltransferase; EMT, epithelial–mesenchymal transition; ROS, reactive oxygen species.
Author Contributions
Conceptualization, B.Y.; writing—original draft preparation, H.Y. and Y.S.; writing—review and editing, T.X., Y.J. and L.Y.; supervision, B.Y.; project administration, Y.Y.; funding acquisition, Y.Y. and Y.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project was funded by National Natural Science Foundation of China (No. 82173288, 82103435, and 81972554), Natural Science Foundation of Jiangsu (No. BK20201208).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 2.Gupta G.P., Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Valastyan S., Weinberg R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A.M., Czaja M.J. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S., Peng X., Yang S., Li X., Huang M., Wei S., Liu J., He G., Zheng H., Yang L., et al. The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders. Cell Death Dis. 2022;13:132. doi: 10.1038/s41419-022-04593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souza S.C., Muliro K.V., Liscum L., Lien P., Yamamoto M.T., Schaffer J.E., Dallal G.E., Wang X., Kraemer F.B., Obin M., et al. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J. Biol. Chem. 2002;277:8267–8272. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- 7.Kaushik S., Cuervo A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 2015;17:759–770. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang A., Mottillo E.P. Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochem. J. 2020;477:985–1008. doi: 10.1042/BCJ20190468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tapia D., Jiménez T., Zamora C., Espinoza J., Rizzo R., González-Cárdenas A., Fuentes D., Hernández S., Cavieres V.A., Soza A., et al. KDEL receptor regulates secretion by lysosome relocation- and autophagy-dependent modulation of lipid-droplet turnover. Nat. Commun. 2019;10:735. doi: 10.1038/s41467-019-08501-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerda-Troncoso C., Varas-Godoy M., Burgos P.V. Pro-Tumoral Functions of Autophagy Receptors in the Modulation of Cancer Progression. Front. Oncol. 2020;10:619727. doi: 10.3389/fonc.2020.619727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lettieri Barbato D., Tatulli G., Aquilano K., Ciriolo M.R. FoxO1 controls lysosomal acid lipase in adipocytes: Implication of lipophagy during nutrient restriction and metformin treatment. Cell Death Dis. 2013;4:e861. doi: 10.1038/cddis.2013.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y., Propson N.E., Du S., Xiong W., Zheng H. Autophagy deficiency modulates microglial lipid homeostasis and aggravates tau pathology and spreading. Proc. Natl. Acad. Sci. USA. 2021;118:e2023418118. doi: 10.1073/pnas.2023418118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto T., Takabatake Y., Minami S., Sakai S., Fujimura R., Takahashi A., Namba-Hamano T., Matsuda J., Kimura T., Matsui I., et al. Eicosapentaenoic acid attenuates renal lipotoxicity by restoring autophagic flux. Autophagy. 2021;17:1700–1713. doi: 10.1080/15548627.2020.1782034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panda P.K., Patra S., Naik P.P., Praharaj P.P., Mukhopadhyay S., Meher B.R., Gupta P.K., Verma R.S., Maiti T.K., Bhutia S.K. Deacetylation of LAMP1 drives lipophagy-dependent generation of free fatty acids by Abrus agglutinin to promote senescence in prostate cancer. J. Cell. Physiol. 2020;235:2776–2791. doi: 10.1002/jcp.29182. [DOI] [PubMed] [Google Scholar]
- 15.Pizato N., Kiffer L., Luzete B.C., Assumpção J.A.F., Correa L.H., Melo H.A.B., Sant’Ana L.P., Ito M.K., Magalhães K.G. Omega 3-DHA and Delta-Tocotrienol Modulate Lipid Droplet Biogenesis and Lipophagy in Breast Cancer Cells: The Impact in Cancer Aggressiveness. Nutrients. 2019;11:1199. doi: 10.3390/nu11061199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong S.J., Lee M.N., Oh G.T. The Role of Macrophage Lipophagy in Reverse Cholesterol Transport. Endocrinol. Metab. (Seoul Korea) 2017;32:41–46. doi: 10.3803/EnM.2017.32.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng F., Niu J., Zhang H., Yang R., Lu Q., Niu G., Liu Z., Yu X. A pH-Sensitive Spirocyclization Strategy for Constructing a Single Fluorescent Probe Simultaneous Two-Color Visualizing of Lipid Droplets and Lysosomes and Monitoring of Lipophagy. Anal. Chem. 2021;93:11729–11735. doi: 10.1021/acs.analchem.1c01842. [DOI] [PubMed] [Google Scholar]
- 18.You J.H., Lee J., Roh J.L. PGRMC1-dependent lipophagy promotes ferroptosis in paclitaxel-tolerant persister cancer cells. J. Exp. Clin. Cancer Res. CR. 2021;40:350. doi: 10.1186/s13046-021-02168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontaine A., Bellanger D., Guibon R., Bruyère F., Brisson L., Fromont G. Lipophagy and prostate cancer: Association with disease aggressiveness and proximity to periprostatic adipose tissue. J. Pathol. 2021;255:166–176. doi: 10.1002/path.5754. [DOI] [PubMed] [Google Scholar]
- 20.Lin S.C., Hardie D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018;27:299–313. doi: 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y., Iemura S., Natsume T., Takehana K., Yamada N., et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell. 2009;20:1981–1991. doi: 10.1091/mbc.e08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roach P.J. AMPK -> ULK1 -> autophagy. Mol. Cell. Biol. 2011;31:3082–3084. doi: 10.1128/MCB.05565-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim B.W., Jin Y., Kim J., Kim J.H., Jung J., Kang S., Kim I.Y., Kim J., Cheong H., Song H.K. The C-terminal region of ATG101 bridges ULK1 and PtdIns3K complex in autophagy initiation. Autophagy. 2018;14:2104–2116. doi: 10.1080/15548627.2018.1504716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morselli E., Shen S., Ruckenstuhl C., Bauer M.A., Mariño G., Galluzzi L., Criollo A., Michaud M., Maiuri M.C., Chano T., et al. p53 inhibits autophagy by interacting with the human ortholog of yeast Atg17, RB1CC1/FIP200. Cell Cycle (Georget. Tex.) 2011;10:2763–2769. doi: 10.4161/cc.10.16.16868. [DOI] [PubMed] [Google Scholar]
- 25.Qi S., Kim D.J., Stjepanovic G., Hurley J.H. Structure of the Human Atg13-Atg101 HORMA Heterodimer: An Interaction Hub within the ULK1 Complex. Structure. 2015;23:1848–1857. doi: 10.1016/j.str.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das G., Shravage B.V., Baehrecke E.H. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb. Perspect. Biol. 2012;4:a008813. doi: 10.1101/cshperspect.a008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H.D., Qin Z.H. Beclin 1, Bcl-2 and Autophagy. Adv. Exp. Med. Biol. 2019;1206:109–126. doi: 10.1007/978-981-15-0602-4_5. [DOI] [PubMed] [Google Scholar]
- 28.Itakura E., Kishi C., Inoue K., Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell. 2008;19:5360–5372. doi: 10.1091/mbc.e08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B.C., Nandakumar R., Reinert L.S., Huang J., Laustsen A., Gao Z.L., Sun C.L., Jensen S.B., Troldborg A., Assil S., et al. STEEP mediates STING ER exit and activation of signaling. Nat. Immunol. 2020;21:868–879. doi: 10.1038/s41590-020-0730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thoresen S.B., Pedersen N.M., Liestøl K., Stenmark H. A phosphatidylinositol 3-kinase class III sub-complex containing VPS15, VPS34, Beclin 1, UVRAG and BIF-1 regulates cytokinesis and degradative endocytic traffic. Exp. Cell Res. 2010;316:3368–3378. doi: 10.1016/j.yexcr.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Fimia G.M., Stoykova A., Romagnoli A., Giunta L., Di Bartolomeo S., Nardacci R., Corazzari M., Fuoco C., Ucar A., Schwartz P., et al. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447:1121–1125. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- 32.Reggiori F., Klionsky D.J. Autophagosomes: Biogenesis from scratch? Curr. Opin. Cell Biol. 2005;17:415–422. doi: 10.1016/j.ceb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Xu D., Li Y., Wu L., Li Y., Zhao D., Yu J., Huang T., Ferguson C., Parton R.G., Yang H., et al. Rab18 promotes lipid droplet (LD) growth by tethering the ER to LDs through SNARE and NRZ interactions. J. Cell Biol. 2018;217:975–995. doi: 10.1083/jcb.201704184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li D., Zhao Y.G., Li D., Zhao H., Huang J., Miao G., Feng D., Liu P., Li D., Zhang H. The ER-Localized Protein DFCP1 Modulates ER-Lipid Droplet Contact Formation. Cell Rep. 2019;27:343–358.e345. doi: 10.1016/j.celrep.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Chung J., Wu X., Lambert T.J., Lai Z.W., Walther T.C., Farese R.V., Jr. LDAF1 and Seipin Form a Lipid Droplet Assembly Complex. Dev. Cell. 2019;51:551–563.e557. doi: 10.1016/j.devcel.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du X., Zhou L., Aw Y.C., Mak H.Y., Xu Y., Rae J., Wang W., Zadoorian A., Hancock S.E., Osborne B., et al. ORP5 localizes to ER-lipid droplet contacts and regulates the level of PI(4)P on lipid droplets. J. Cell Biol. 2020;219:e201905162. doi: 10.1083/jcb.201905162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romanov J., Walczak M., Ibiricu I., Schüchner S., Ogris E., Kraft C., Martens S. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J. 2012;31:4304–4317. doi: 10.1038/emboj.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otomo C., Metlagel Z., Takaesu G., Otomo T. Structure of the human ATG12~ATG5 conjugate required for LC3 lipidation in autophagy. Nat. Struct. Mol. Biol. 2013;20:59–66. doi: 10.1038/nsmb.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nath S., Dancourt J., Shteyn V., Puente G., Fong W.M., Nag S., Bewersdorf J., Yamamoto A., Antonny B., Melia T.J. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat. Cell Biol. 2014;16:415–424. doi: 10.1038/ncb2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M., Hou Y., Wang J., Chen X., Shao Z.M., Yin X.M. Kinetics comparisons of mammalian Atg4 homologues indicate selective preferences toward diverse Atg8 substrates. J. Biol. Chem. 2011;286:7327–7338. doi: 10.1074/jbc.M110.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collier J.J., Suomi F., Oláhová M., McWilliams T.G., Taylor R.W. Emerging roles of ATG7 in human health and disease. EMBO Mol. Med. 2021;13:e14824. doi: 10.15252/emmm.202114824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L., Li P., Fu S., Calay E.S., Hotamisligil G.S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ro S.H., Jang Y., Bae J., Kim I.M., Schaecher C., Shomo Z.D. Autophagy in Adipocyte Browning: Emerging Drug Target for Intervention in Obesity. Front. Physiol. 2019;10:22. doi: 10.3389/fphys.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z., Schulze R.J., Weller S.G., Krueger E.W., Schott M.B., Zhang X., Casey C.A., Liu J., Stöckli J., James D.E., et al. A novel Rab10-EHBP1-EHD2 complex essential for the autophagic engulfment of lipid droplets. Sci. Adv. 2016;2:e1601470. doi: 10.1126/sciadv.1601470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malek M., Wawrzyniak A.M., Ebner M., Puchkov D., Haucke V. Inositol triphosphate-triggered calcium release from the endoplasmic reticulum induces lysosome biogenesis via TFEB/TFE3. J. Biol. Chem. 2022;298:101740. doi: 10.1016/j.jbc.2022.101740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medina D.L., Di Paola S., Peluso I., Armani A., De Stefani D., Venditti R., Montefusco S., Scotto-Rosato A., Prezioso C., Forrester A., et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Settembre C., Fraldi A., Medina D.L., Ballabio A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013;14:283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martina J.A., Diab H.I., Lishu L., Jeong A.L., Patange S., Raben N., Puertollano R. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci. Signal. 2014;7:ra9. doi: 10.1126/scisignal.2004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reggiori F. 1. Membrane origin for autophagy. Curr. Top. Dev. Biol. 2006;74:1–30. doi: 10.1016/s0070-2153(06)74001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouimet M., Franklin V., Mak E., Liao X., Tabas I., Marcel Y.L. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Itakura E., Kishi-Itakura C., Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256–1269. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Matsui T., Jiang P., Nakano S., Sakamaki Y., Yamamoto H., Mizushima N. Autophagosomal YKT6 is required for fusion with lysosomes independently of syntaxin 17. J. Cell Biol. 2018;217:2633–2645. doi: 10.1083/jcb.201712058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wetzel L., Blanchard S., Rama S., Beier V., Kaufmann A., Wollert T. TECPR1 promotes aggrephagy by direct recruitment of LC3C autophagosomes to lysosomes. Nat. Commun. 2020;11:2993. doi: 10.1038/s41467-020-16689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z., Miao G., Xue X., Guo X., Yuan C., Wang Z., Zhang G., Chen Y., Feng D., Hu J., et al. The Vici Syndrome Protein EPG5 Is a Rab7 Effector that Determines the Fusion Specificity of Autophagosomes with Late Endosomes/Lysosomes. Mol. Cell. 2016;63:781–795. doi: 10.1016/j.molcel.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 55.McEwan D.G., Popovic D., Gubas A., Terawaki S., Suzuki H., Stadel D., Coxon F.P., Miranda de Stegmann D., Bhogaraju S., Maddi K., et al. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol. Cell. 2015;57:39–54. doi: 10.1016/j.molcel.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X., Wang L., Lak B., Li J., Jokitalo E., Wang Y. GRASP55 Senses Glucose Deprivation through O-GlcNAcylation to Promote Autophagosome-Lysosome Fusion. Dev. Cell. 2018;45:245–261.e246. doi: 10.1016/j.devcel.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu H., Ploeger J.M., Kamarajugadda S., Mashek D.G., Mashek M.T., Manivel J.C., Shekels L.L., Lapiro J.L., Albrecht J.H. Evidence for a Novel Regulatory Interaction Involving Cyclin D1, Lipid Droplets, Lipolysis, and Cell Cycle Progression in Hepatocytes. Hepatol. Commun. 2019;3:406–422. doi: 10.1002/hep4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulze R.J., McNiven M.A. A well-oiled machine: DNM2/dynamin 2 helps keep hepatocyte lipophagy running smoothly. Autophagy. 2014;10:388–389. doi: 10.4161/auto.27486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carmona-Gutierrez D., Zimmermann A., Madeo F. A molecular mechanism for lipophagy regulation in the liver. Hepatology. 2015;61:1781–1783. doi: 10.1002/hep.27738. [DOI] [PubMed] [Google Scholar]
- 60.Pulido M.R., Diaz-Ruiz A., Jiménez-Gómez Y., Garcia-Navarro S., Gracia-Navarro F., Tinahones F., López-Miranda J., Frühbeck G., Vázquez-Martínez R., Malagón M.M. Rab18 dynamics in adipocytes in relation to lipogenesis, lipolysis and obesity. PLoS ONE. 2011;6:e22931. doi: 10.1371/journal.pone.0022931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinha R.A., Singh B.K., Yen P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018;14:259–269. doi: 10.1038/nrendo.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seok S., Fu T., Choi S.E., Li Y., Zhu R., Kumar S., Sun X., Yoon G., Kang Y., Zhong W., et al. Transcriptional regulation of autophagy by an FXR-CREB axis. Nature. 2014;516:108–111. doi: 10.1038/nature13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seo Y.K., Jeon T.I., Chong H.K., Biesinger J., Xie X., Osborne T.F. Genome-wide localization of SREBP-2 in hepatic chromatin predicts a role in autophagy. Cell Metab. 2011;13:367–375. doi: 10.1016/j.cmet.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurahashi T., Hamashima S., Shirato T., Lee J., Homma T., Kang E.S., Fujii J. An SOD1 deficiency enhances lipid droplet accumulation in the fasted mouse liver by aborting lipophagy. Biochem. Biophys. Res. Commun. 2015;467:866–871. doi: 10.1016/j.bbrc.2015.10.052. [DOI] [PubMed] [Google Scholar]
- 65.Sathyanarayan A., Mashek M.T., Mashek D.G. ATGL Promotes Autophagy/Lipophagy via SIRT1 to Control Hepatic Lipid Droplet Catabolism. Cell Rep. 2017;19:1–9. doi: 10.1016/j.celrep.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim K.Y., Jang H.J., Yang Y.R., Park K.I., Seo J., Shin I.W., Jeon T.I., Ahn S.C., Suh P.G., Osborne T.F., et al. SREBP-2/PNPLA8 axis improves non-alcoholic fatty liver disease through activation of autophagy. Sci. Rep. 2016;6:35732. doi: 10.1038/srep35732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim H.S., Montana V., Jang H.J., Parpura V., Kim J.A. Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: A potential role for reducing lipid accumulation. J. Biol. Chem. 2013;288:22693–22705. doi: 10.1074/jbc.M113.477505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sinha R.A., Farah B.L., Singh B.K., Siddique M.M., Li Y., Wu Y., Ilkayeva O.R., Gooding J., Ching J., Zhou J., et al. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology. 2014;59:1366–1380. doi: 10.1002/hep.26667. [DOI] [PubMed] [Google Scholar]
- 69.Parafati M., Lascala A., Morittu V.M., Trimboli F., Rizzuto A., Brunelli E., Coscarelli F., Costa N., Britti D., Ehrlich J., et al. Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. J. Nutr. Biochem. 2015;26:938–948. doi: 10.1016/j.jnutbio.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y., Chen M.L., Zhou Y., Yi L., Gao Y.X., Ran L., Chen S.H., Zhang T., Zhou X., Zou D., et al. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol. Nutr. Food Res. 2015;59:1443–1457. doi: 10.1002/mnfr.201500016. [DOI] [PubMed] [Google Scholar]
- 71.Hahm J.R., Noh H.S., Ha J.H., Roh G.S., Kim D.R. Alpha-lipoic acid attenuates adipocyte differentiation and lipid accumulation in 3T3-L1 cells via AMPK-dependent autophagy. Life Sci. 2014;100:125–132. doi: 10.1016/j.lfs.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Baerga R., Zhang Y., Chen P.H., Goldman S., Jin S. Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy. 2009;5:1118–1130. doi: 10.4161/auto.5.8.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang M., Di Martino J.S., Bowman R.L., Campbell N.R., Baksh S.C., Simon-Vermot T., Kim I.S., Haldeman P., Mondal C., Yong-Gonzales V., et al. Adipocyte-Derived Lipids Mediate Melanoma Progression via FATP Proteins. Cancer Discov. 2018;8:1006–1025. doi: 10.1158/2159-8290.CD-17-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clark R., Krishnan V., Schoof M., Rodriguez I., Theriault B., Chekmareva M., Rinker-Schaeffer C. Milky spots promote ovarian cancer metastatic colonization of peritoneal adipose in experimental models. Am. J. Pathol. 2013;183:576–591. doi: 10.1016/j.ajpath.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Röhrig F., Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 76.Dierge E., Debock E., Guilbaud C., Corbet C., Mignolet E., Mignard L., Bastien E., Dessy C., Larondelle Y., Feron O. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 2021;33:1701–1715.e5. doi: 10.1016/j.cmet.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 77.Matsushita M., Fujita K., Hayashi T., Kayama H., Motooka D., Hase H., Jingushi K., Yamamichi G., Yumiba S., Tomiyama E., et al. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Prostate Cancer Growth via IGF1 Signaling. Cancer Res. 2021;81:4014–4026. doi: 10.1158/0008-5472.CAN-20-4090. [DOI] [PubMed] [Google Scholar]
- 78.Balaban S., Shearer R.F., Lee L.S., van Geldermalsen M., Schreuder M., Shtein H.C., Cairns R., Thomas K.C., Fazakerley D.J., Grewal T., et al. Adipocyte lipolysis links obesity to breast cancer growth: Adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017;5:1. doi: 10.1186/s40170-016-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li C., Teixeira A.F., Zhu H.J., Ten Dijke P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol. Cancer. 2021;20:154. doi: 10.1186/s12943-021-01463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu L., Zhang X., Zheng L., Zhao H., Yan G., Zhang Q., Zhou Y., Lei J., Zhang J., Wang J., et al. RIPK3 Orchestrates Fatty Acid Metabolism in Tumor-Associated Macrophages and Hepatocarcinogenesis. Cancer Immunol. Res. 2020;8:710–721. doi: 10.1158/2326-6066.CIR-19-0261. [DOI] [PubMed] [Google Scholar]
- 81.Lee H., Woo S.M., Jang H., Kang M., Kim S.Y. Cancer depends on fatty acids for ATP production: A possible link between cancer and obesity. Semin. Cancer Biol. 2022 doi: 10.1016/j.semcancer.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 82.Silverstein R.L., Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pascual G., Avgustinova A., Mejetta S., Martín M., Castellanos A., Attolini C.S., Berenguer A., Prats N., Toll A., Hueto J.A., et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 84.Armesilla A.L., Vega M.A. Structural organization of the gene for human CD36 glycoprotein. J. Biol. Chem. 1994;269:18985–18991. doi: 10.1016/S0021-9258(17)32263-9. [DOI] [PubMed] [Google Scholar]
- 85.Pan J., Fan Z., Wang Z., Dai Q., Xiang Z., Yuan F., Yan M., Zhu Z., Liu B., Li C. CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3β/β-catenin pathway. J. Exp. Clin. Cancer Res. CR. 2019;38:52. doi: 10.1186/s13046-019-1049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ladanyi A., Mukherjee A., Kenny H.A., Johnson A., Mitra A.K., Sundaresan S., Nieman K.M., Pascual G., Benitah S.A., Montag A., et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018;37:2285–2301. doi: 10.1038/s41388-017-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Furuhashi M., Hotamisligil G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lopes-Coelho F., André S., Félix A., Serpa J. Breast cancer metabolic cross-talk: Fibroblasts are hubs and breast cancer cells are gatherers of lipids. Mol. Cell. Endocrinol. 2018;462:93–106. doi: 10.1016/j.mce.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 89.Mukherjee A., Chiang C.Y., Daifotis H.A., Nieman K.M., Fahrmann J.F., Lastra R.R., Romero I.L., Fiehn O., Lengyel E. Adipocyte-Induced FABP4 Expression in Ovarian Cancer Cells Promotes Metastasis and Mediates Carboplatin Resistance. Cancer Res. 2020;80:1748–1761. doi: 10.1158/0008-5472.CAN-19-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R., Romero I.L., Carey M.S., Mills G.B., Hotamisligil G.S., et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu R.Z., Choi W.S., Jain S., Dinakaran D., Xu X., Han W.H., Yang X.H., Glubrecht D.D., Moore R.B., Lemieux H., et al. The FABP12/PPARγ pathway promotes metastatic transformation by inducing epithelial-to-mesenchymal transition and lipid-derived energy production in prostate cancer cells. Mol. Oncol. 2020;14:3100–3120. doi: 10.1002/1878-0261.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quan J., Bode A.M., Luo X. ACSL family: The regulatory mechanisms and therapeutic implications in cancer. Eur. J. Pharmacol. 2021;909:174397. doi: 10.1016/j.ejphar.2021.174397. [DOI] [PubMed] [Google Scholar]
- 93.Zhang T., Zhang Y., Liu J., Ma Y., Ye Q., Yan X., Ding L. MicroRNA-377-3p inhibits hepatocellular carcinoma growth and metastasis through negative regulation of CPT1C-mediated fatty acid oxidation. Cancer Metab. 2022;10:2. doi: 10.1186/s40170-021-00276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee C.K., Jeong S.H., Jang C., Bae H., Kim Y.H., Park I., Kim S.K., Koh G.Y. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363:644–649. doi: 10.1126/science.aav0173. [DOI] [PubMed] [Google Scholar]
- 95.Gu L., Zhu Y., Lin X., Lu B., Zhou X., Zhou F., Zhao Q., Prochownik E.V., Li Y. The IKKβ-USP30-ACLY Axis Controls Lipogenesis and Tumorigenesis. Hepatology. 2021;73:160–174. doi: 10.1002/hep.31249. [DOI] [PubMed] [Google Scholar]
- 96.Lally J.S.V., Ghoshal S., DePeralta D.K., Moaven O., Wei L., Masia R., Erstad D.J., Fujiwara N., Leong V., Houde V.P., et al. Inhibition of Acetyl-CoA Carboxylase by Phosphorylation or the Inhibitor ND-654 Suppresses Lipogenesis and Hepatocellular Carcinoma. Cell Metab. 2019;29:174–182.e175. doi: 10.1016/j.cmet.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Humbert M., Seiler K., Mosimann S., Rentsch V., Sharma K., Pandey A.V., McKenna S.L., Tschan M.P. Reducing FASN expression sensitizes acute myeloid leukemia cells to differentiation therapy. Cell Death Differ. 2021;28:2465–2481. doi: 10.1038/s41418-021-00768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lipchick B.C., Utley A., Han Z., Moparthy S., Yun D.H., Bianchi-Smiraglia A., Wolff D.W., Fink E., Liu L., Furdui C.M., et al. The fatty acid elongase ELOVL6 regulates bortezomib resistance in multiple myeloma. Blood Adv. 2021;5:1933–1946. doi: 10.1182/bloodadvances.2020002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu H.H., Xu Y., Li C.J., Hsu S.J., Lin X.H., Zhang R., Chen J., Chen J., Gao D.M., Cui J.F., et al. An SCD1-dependent mechanoresponsive pathway promotes HCC invasion and metastasis through lipid metabolic reprogramming. Mol. Ther. J. Am. Soc. Gene Ther. 2022;30:2554–2567. doi: 10.1016/j.ymthe.2022.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li B., Qin Y., Yu X., Xu X., Yu W. Lipid raft involvement in signal transduction in cancer cell survival, cell death and metastasis. Cell Prolif. 2022;55:e13167. doi: 10.1111/cpr.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pillai S., Mahmud I., Mahar R., Griffith C., Langsen M., Nguyen J., Wojtkowiak J.W., Swietach P., Gatenby R.A., Bui M.M., et al. Lipogenesis mediated by OGR1 regulates metabolic adaptation to acid stress in cancer cells via autophagy. Cell Rep. 2022;39:110796. doi: 10.1016/j.celrep.2022.110796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ippolito L., Comito G., Parri M., Iozzo M., Duatti A., Virgilio F., Lorito N., Bacci M., Pardella E., Sandrini G., et al. Lactate Rewires Lipid Metabolism and Sustains a Metabolic-Epigenetic Axis in Prostate Cancer. Cancer Res. 2022;82:1267–1282. doi: 10.1158/0008-5472.CAN-21-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walther T.C., Chung J., Farese R.V., Jr. Lipid Droplet Biogenesis. Annu. Rev. Cell Dev. Biol. 2017;33:491–510. doi: 10.1146/annurev-cellbio-100616-060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olzmann J.A., Carvalho P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019;20:137–155. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iftikhar R., Penrose H.M., King A.N., Samudre J.S., Collins M.E., Hartono A.B., Lee S.B., Lau F., Baddoo M., Flemington E.F., et al. Elevated ATGL in colon cancer cells and cancer stem cells promotes metabolic and tumorigenic reprogramming reinforced by obesity. Oncogenesis. 2021;10:82. doi: 10.1038/s41389-021-00373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou H., Zhang J., Yan Z., Qu M., Zhang G., Han J., Wang F., Sun K., Wang L., Yang X. DECR1 directly activates HSL to promote lipolysis in cervical cancer cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2022;1867:159090. doi: 10.1016/j.bbalip.2021.159090. [DOI] [PubMed] [Google Scholar]
- 107.Liu R., Lee J.H., Li J., Yu R., Tan L., Xia Y., Zheng Y., Bian X.L., Lorenzi P.L., Chen Q., et al. Choline kinase alpha 2 acts as a protein kinase to promote lipolysis of lipid droplets. Mol. Cell. 2021;81:2722–2735.e2729. doi: 10.1016/j.molcel.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 108.Schott M.B., Weller S.G., Schulze R.J., Krueger E.W., Drizyte-Miller K., Casey C.A., McNiven M.A. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. J. Cell Biol. 2019;218:3320–3335. doi: 10.1083/jcb.201803153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haerinck J., Berx G. Partial EMT takes the lead in cancer metastasis. Dev. Cell. 2021;56:3174–3176. doi: 10.1016/j.devcel.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 111.Reymond N., d’Água B.B., Ridley A.J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer. 2013;13:858–870. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- 112.Keller L., Pantel K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer. 2019;19:553–567. doi: 10.1038/s41568-019-0180-2. [DOI] [PubMed] [Google Scholar]
- 113.Obenauf A.C., Massagué J. Surviving at a Distance: Organ-Specific Metastasis. Trends Cancer. 2015;1:76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bakir B., Chiarella A.M., Pitarresi J.R., Rustgi A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020;30:764–776. doi: 10.1016/j.tcb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Giudetti A.M., De Domenico S., Ragusa A., Lunetti P., Gaballo A., Franck J., Simeone P., Nicolardi G., De Nuccio F., Santino A., et al. A specific lipid metabolic profile is associated with the epithelial mesenchymal transition program. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864:344–357. doi: 10.1016/j.bbalip.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 116.Wang D., Cheng X., Li Y., Guo M., Zhao W., Qiu J., Zheng Y., Meng M., Ping X., Chen X., et al. C/EBPδ-Slug-Lox1 axis promotes metastasis of lung adenocarcinoma via oxLDL uptake. Oncogene. 2020;39:833–848. doi: 10.1038/s41388-019-1015-z. [DOI] [PubMed] [Google Scholar]
- 117.Su G., Feng T., Pei T., Yang F., Sun D., Yu H., Wang X., Gao W., He J., Shen Y., et al. Autophagy modulates FSS-induced epithelial-mesenchymal transition in hepatocellular carcinoma cells. Mol. Carcinog. 2021;60:607–619. doi: 10.1002/mc.23327. [DOI] [PubMed] [Google Scholar]
- 118.Luo J., Yao J.F., Deng X.F., Zheng X.D., Jia M., Wang Y.Q., Huang Y., Zhu J.H. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin αvβ3 and activating FAK/PI3K/AKT signaling. J. Exp. Clin. Cancer Res. CR. 2018;37:23. doi: 10.1186/s13046-018-0694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dodson M., Castro-Portuguez R., Zhang D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bai Y., Meng L., Han L., Jia Y., Zhao Y., Gao H., Kang R., Wang X., Tang D., Dai E. Lipid storage and lipophagy regulates ferroptosis. Biochem. Biophys. Res. Commun. 2019;508:997–1003. doi: 10.1016/j.bbrc.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 122.Mukhopadhyay S., Schlaepfer I.R., Bergman B.C., Panda P.K., Praharaj P.P., Naik P.P., Agarwal R., Bhutia S.K. ATG14 facilitated lipophagy in cancer cells induce ER stress mediated mitoptosis through a ROS dependent pathway. Free Radic. Biol. Med. 2017;104:199–213. doi: 10.1016/j.freeradbiomed.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 123.Zhao T., Wu K., Hogstrand C., Xu Y.H., Chen G.H., Wei C.C., Luo Z. Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARγ pathways. Cell. Mol. Life Sci. 2020;77:1987–2003. doi: 10.1007/s00018-019-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bosch M., Sánchez-Álvarez M., Fajardo A., Kapetanovic R., Steiner B., Dutra F., Moreira L., López J.A., Campo R., Marí M., et al. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science. 2020;370 doi: 10.1126/science.aay8085. [DOI] [PubMed] [Google Scholar]
- 125.McLauchlan J. Lipid droplets and hepatitis C virus infection. Biochim. Biophys. Acta. 2009;1791:552–559. doi: 10.1016/j.bbalip.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 126.Bley H., Schöbel A., Herker E. Whole Lotta Lipids-from HCV RNA Replication to the Mature Viral Particle. Int. J. Mol. Sci. 2020;21:2888. doi: 10.3390/ijms21082888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu K., Czaja M.J. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013;20:3–11. doi: 10.1038/cdd.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang K., Li S., Worku T., Hao X., Yang L., Zhang S. Rab11a is required for porcine reproductive and respiratory syndrome virus induced autophagy to promote viral replication. Biochem. Biophys. Res. Commun. 2017;492:236–242. doi: 10.1016/j.bbrc.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 129.Fu M., Zhang J., Zhao M., Zhang S., Dai L., Ouyang X., Yu Y., Wen B., Zhou D., Sun Y., et al. Coxiella burnetii Plasmid Effector B Promotes LC3-II Accumulation and Contributes to Bacterial Virulence in a SCID Mouse Model. Infect. Immun. 2022;90:e0001622. doi: 10.1128/iai.00016-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cui W., Sathyanarayan A., Lopresti M., Aghajan M., Chen C., Mashek D.G. Lipophagy-derived fatty acids undergo extracellular efflux via lysosomal exocytosis. Autophagy. 2021;17:690–705. doi: 10.1080/15548627.2020.1728097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goldstein I., Rotter V. Regulation of lipid metabolism by p53—Fighting two villains with one sword. Trends Endocrinol. Metab. TEM. 2012;23:567–575. doi: 10.1016/j.tem.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 132.Xu Z., Wu W., Yan H., Hu Y., He Q., Luo P. Regulation of p53 stability as a therapeutic strategy for cancer. Biochem. Pharmacol. 2021;185:114407. doi: 10.1016/j.bcp.2021.114407. [DOI] [PubMed] [Google Scholar]
- 133.Zhao T., Du H., Ding X., Walls K., Yan C. Activation of mTOR pathway in myeloid-derived suppressor cells stimulates cancer cell proliferation and metastasis in lal(-/-) mice. Oncogene. 2015;34:1938–1948. doi: 10.1038/onc.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang C., Yang Z., Xu E., Shen X., Wang X., Li Z., Yu H., Chen K., Hu Q., Xia X., et al. Apolipoprotein C-II induces EMT to promote gastric cancer peritoneal metastasis via PI3K/AKT/mTOR pathway. Clin. Transl. Med. 2021;11:e522. doi: 10.1002/ctm2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yi M., Li J., Chen S., Cai J., Ban Y., Peng Q., Zhou Y., Zeng Z., Peng S., Li X., et al. Emerging role of lipid metabolism alterations in Cancer stem cells. J. Exp. Clin. Cancer Res. CR. 2018;37:118. doi: 10.1186/s13046-018-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nisticò C., Pagliari F., Chiarella E., Fernandes Guerreiro J., Marafioti M.G., Aversa I., Genard G., Hanley R., Garcia-Calderón D., Bond H.M., et al. Lipid Droplet Biosynthesis Impairment through DGAT2 Inhibition Sensitizes MCF7 Breast Cancer Cells to Radiation. Int. J. Mol. Sci. 2021;22:10102. doi: 10.3390/ijms221810102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roy D., Mondal S., Khurana A., Jung D.B., Hoffmann R., He X., Kalogera E., Dierks T., Hammond E., Dredge K., et al. Loss of HSulf-1: The Missing Link between Autophagy and Lipid Droplets in Ovarian Cancer. Sci. Rep. 2017;7:41977. doi: 10.1038/srep41977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun C., Li X., Guo E., Li N., Zhou B., Lu H., Huang J., Xia M., Shan W., Wang B., et al. MCP-1/CCR-2 axis in adipocytes and cancer cell respectively facilitates ovarian cancer peritoneal metastasis. Oncogene. 2020;39:1681–1695. doi: 10.1038/s41388-019-1090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aschenbrenner B., Negro G., Savic D., Sorokin M., Buzdin A., Ganswindt U., Cemazar M., Sersa G., Skvortsov S., Skvortsova I. Simvastatin is effective in killing the radioresistant breast carcinoma cells. Radiol. Oncol. 2021;55:305–316. doi: 10.2478/raon-2021-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mondal S., Roy D., Sarkar Bhattacharya S., Jin L., Jung D., Zhang S., Kalogera E., Staub J., Wang Y., Xuyang W., et al. Therapeutic targeting of PFKFB3 with a novel glycolytic inhibitor PFK158 promotes lipophagy and chemosensitivity in gynecologic cancers. Int. J. Cancer. 2019;144:178–189. doi: 10.1002/ijc.31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ferraresi A., Esposito A., Girone C., Vallino L., Salwa A., Ghezzi I., Thongchot S., Vidoni C., Dhanasekaran D.N., Isidoro C. Resveratrol Contrasts LPA-Induced Ovarian Cancer Cell Migration and Platinum Resistance by Rescuing Hedgehog-Mediated Autophagy. Cells. 2021;10:3213. doi: 10.3390/cells10113213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goldberg S.B., Supko J.G., Neal J.W., Muzikansky A., Digumarthy S., Fidias P., Temel J.S., Heist R.S., Shaw A.T., McCarthy P.O., et al. A phase I study of erlotinib and hydroxychloroquine in advanced non-small-cell lung cancer. J. Thorac. Oncol. 2012;7:1602–1608. doi: 10.1097/JTO.0b013e318262de4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu G.D., Ang Y.H., Zhou J., Tamilarasi J., Yan B., Lim Y.C., Srivastava S., Salto-Tellez M., Hui K.M., Shen H.M., et al. CCAAT/enhancer binding protein α predicts poorer prognosis and prevents energy starvation-induced cell death in hepatocellular carcinoma. Hepatology. 2015;61:965–978. doi: 10.1002/hep.27593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang C.J., Zhu N., Long J., Wu H.T., Wang Y.X., Liu B.Y., Liao D.F., Qin L. Celastrol induces lipophagy via the LXRα/ABCA1 pathway in clear cell renal cell carcinoma. Acta Pharmacol. Sin. 2021;42:1472–1485. doi: 10.1038/s41401-020-00572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiao W., Xiong Z., Xiong W., Yuan C., Xiao H., Ruan H., Song Z., Wang C., Bao L., Cao Q., et al. Melatonin/PGC1A/UCP1 promotes tumor slimming and represses tumor progression by initiating autophagy and lipid browning. J. Pineal Res. 2019;67:e12607. doi: 10.1111/jpi.12607. [DOI] [PubMed] [Google Scholar]