Abstract
Simple Summary
Drug resistance is the “Achilles’ heel” in current oncology. In this sense, the clinical management of HER2 breast carcinomas (tumors with overexpression/amplification of the ErbB2/HER2 oncogene) is still a challenge. Although a variety of anti-HER2 therapies (anti-HER2 antibodies, antibody–drug conjugates, and tyrosine kinase inhibitors) are available for these patients, the frequent appearance of innate and acquired tumor resistance to these treatments creates an urgent need to develop new and more effective therapeutic approaches. In this review, we discuss the most relevant clinical and pre-clinical advances in therapeutic strategies aimed at overcoming drug resistance in HER2 breast cancer patients in different stages of the disease.
Abstract
The prognosis and quality of life of HER2 breast cancer patients have significantly improved due to the crucial clinical benefit of various anti-HER2 targeted therapies. However, HER2 tumors can possess or develop several resistance mechanisms to these treatments, thus leaving patients with a limited set of additional therapeutic options. Fortunately, to overcome this problem, in recent years, multiple different and complementary approaches have been developed (such as antibody–drug conjugates (ADCs)) that are in clinical or preclinical stages. In this review, we focus on emerging strategies other than on ADCs that are either aimed at directly target the HER2 receptor (i.e., novel tyrosine kinase inhibitors) or subsequent intracellular signaling (e.g., PI3K/AKT/mTOR, CDK4/6 inhibitors, etc.), as well as on innovative approaches designed to attack other potential tumor weaknesses (such as immunotherapy, autophagy blockade, or targeting of other genes within the HER2 amplicon). Moreover, relevant technical advances such as anti-HER2 nanotherapies and immunotoxins are also discussed. In brief, this review summarizes the impact of novel therapeutic approaches on current and future clinical management of aggressive HER2 breast tumors.
Keywords: HER2 breast cancer, resistance, antiHER2 therapies
1. Introduction
HER2-positive breast cancers (HER2 BC), which account for about 15–20% of all breast tumors, are highly aggressive neoplasms with poor prognosis [1,2]. These tumors overexpress, due to gene amplification, the oncogenic Epidermal Growth Factor receptor-2 (ErbB2, also known as HER2), which mediates the activation of several intracellular pathways, including MAPK and/or PIK/AKT/mTOR, involved in angiogenesis, invasiveness, metabolic programming, and other processes [3]. HER2 plays a pivotal role in tumor biology, not only in breast cancers but also in other tumors, and, therefore, assessing its overexpression/amplification status is one of the gold standards in the pathological classification of breast carcinomas. In this sense, following the current ASCO/CAP guidelines [4], standardized immunohistochemical HER2 assessment classifies tumors into 0–3+ scores. Tumors with 0–1+ score (≤10% HER2 expression in tumor cells) are classified as HER2-negative, while 3+ (>10% of intense circumferential membrane staining) are considered positive. Additional study is necessary for 2+ HER2-expression tumors (weak-to-moderate complete membrane staining in 10% of tumor cells). In these cases, an equivocal result of HER2 gene amplification should be confirmed by ISH (in situ hybridization), where it is considered positive when the HER2/CEP17 (centromere chromosome 17) ratio is ≥2.0 and the HER2 copy number signals/cell is ≥4 [4].
From the clinical point of view, HER2 typification is an example of breast cancer precision oncology and a successful therapeutic target, since many anti-HER2 targeted treatments have been specifically developed. So far, current targeted HER2 therapies include (i) monoclonal antibodies (MAbs) such as trastuzumab (Herceptin), which was the first approved treatment by FDA (1998), and pertuzumab (Perjeta); (ii) tyrosine kinase inhibitors (TKIs) such as lapatinib, neratinib, and tucatinib; and (iii) antibody–drug conjugates such as ado–trastuzumab emtansine (T–DM1) or trastuzumab–deruxtecan (T–DXd) [5,6,7]. These therapies target different HER2 regions: MAbs bind to the extracellular HER2 region, mainly inhibiting receptor dimerization; TKIs attach to the intracellular tyrosine kinase domain, impeding the HER2-mediated intracellular pathways [5,6,7].
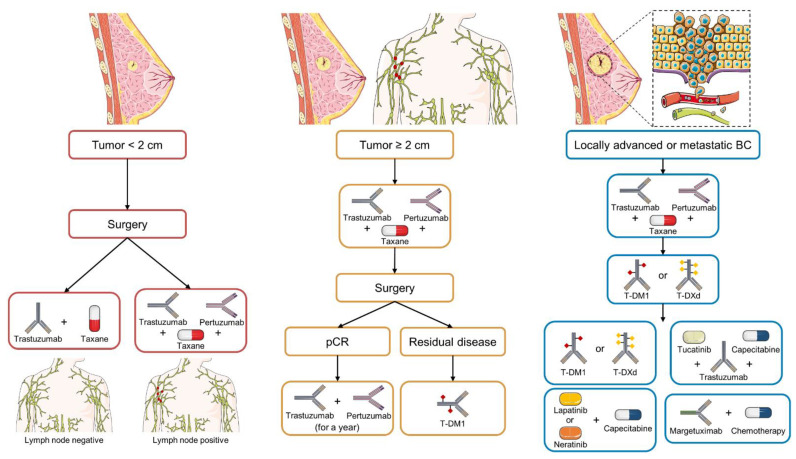
Accordingly, the first-line treatment in the case of neoadjuvant regimens in stage II/III breast cancer patients is currently based on the combination of MAbs (pertuzumab and trastuzumab) and chemotherapy (usually a taxane). The adjuvant treatment setting depends on whether the patient received neoadjuvant therapy. After neoadjuvant treatment, for those patients with residual disease, the recommendation is T-DM1 treatment in the second line, while patients who show a pathological complete response (pCR) receive trastuzumab plus pertuzumab for a year, and then, if necessary, the tyrosine kinase inhibitor neratinib can be applied [8,9]. In addition, patients with breast tumors smaller than 2 cm and who are lymph node negative receive trastuzumab and a taxane, and those with tumors larger than 2 cm or who are lymph node positive (not candidates for neoadjuvant) are treated with chemotherapy, trastuzumab, and pertuzumab [10]. Finally, CLEOPATRA phase III trial results showed the benefit of treatment with pertuzumab plus trastuzumab and docetaxel as the standard-of-care for metastatic patients who have not received any previous anti-HER2 treatment [11] (Figure 1).
Figure 1.
Current established treatment protocol for early-stage, advanced, or metastatic HER2 BC according to ASCO and NCCN guidelines. The different treatment options for each stage are presented sequentially based on their strength of recommendation. Icons designed with smart.servier.com and Adobe Illustrator.
Despite the benefits of anti-HER2 therapies in the survival rate of HER2 BC patients [3], unfortunately, some patients do not respond from the beginning, or they develop acquired resistance during the treatment [12]. Indeed, an ever-growing list of resistance mechanisms to standard anti-HER2 therapies has been reported (Table 1). Therefore, the development of novel treatment strategies to overcome these therapeutic failures continues to be a priority in current oncology. To achieve the highest survival rate of HER2 BC patients, in particular those in advanced/metastatic stages, it is necessary to understand and correctly classify the resistance mechanisms to conventional anti-HER2 drugs as well as to develop more effective and less toxic therapeutic approaches. All these aspects are reviewed in the next sections discussing the applicability of diverse novel strategies that are currently under validation in either clinical or preclinical settings. To this end, we performed a comprehensive review of the relevant literature published from 2010 until September 2022 by searching in the PubMed database using the following keywords: “HER2 Breast Cancer” AND “Treatment/drug resistance” OR “Novel/new therapy” OR “Clinical trial” OR “mechanism of resistance”.
Table 1.
Previously described mechanisms of resistance to anti-HER2 therapies.
| HER2-Targeted Therapy | Mechanism of Resistance | References |
|---|---|---|
| Trastuzumab/ Pertuzumab/TKIs/T-DM1 |
HER2 somatic mutations | [13,14,15,16] |
| Trastuzumab/T-DM1 | Expression of truncated receptor variants, such as p95HER2 and Δ16-HER2 | [14,17,18] |
| Trastuzumab/TKIs | Upregulation of ligands and/or other receptors of the HER family (e.g., EGFR, HER3) | [19,20,21,22] |
| Trastuzumab/TKIs/T-DM1 | Overactivation of PI3K/Akt/PTEN/mTOR signaling pathway (via PI3K mutations or loss of PTEN function) | [20,23,24,25,26] |
| Trastuzumab/Lapatinib | Stimulation of additional RTKs/growth factors (MET tyrosine kinase, insulin-like growth factor 1, VEGF, AXL) and/or downstream kinases (Src) or signaling pathways (MAPK) | [27,28,29,30] |
| Trastuzumab/TKIs | Inhibition of cell death mechanisms (e.g., overexpression of XIAP or MCL-1) | [31,32,33] |
| Trastuzumab/TKIs | Alterations in cell cycle regulators (loss of p27Kip1, upregulation of Cyclin E, or activation of CDK12) | [34,35,36] |
| Trastuzumab | Masking the trastuzumab binding site on HER2 receptor via overexpression of large glycoproteins (MUC4) | [37] |
| Trastuzumab | Alterations in the Fc receptor (including FCγRIIa polymorphisms) preventing trastuzumab antibody-derived cellular cytotoxicity (ADCC) | [38] |
| Lapatinib | Crosstalk with endocrine receptor signaling (ER, AR) | [39,40] |
| Trastuzumab/Lapatinib | Induction of protective autophagy | [41,42] |
| Trastuzumab/TKIs | Functional involvement of other genes within HER2 amplicon (e.g., GSDMB, STARD3, GRB7, CDK12) | [36,43,44,45,46,47,48] |
| Lapatinib | Phenotypic cell plasticity (epithelial mesenchymal transition and metabolic rewiring) | [49,50] |
| Trastuzumab/ Pertuzumab/T-DM1/TKIs |
Interaction with stromal and immune cells and microenvironmental response to stimuli (chemokines, hypoxia) | [36,51,52,53,54,55] |
| Trastuzumab | Modulation of specific miRNAs | [56] |
| Trastuzumab | Up/downregulation of different genes via alterations to transcriptome and chromatin landscape (e.g., PPP1R1B) | [57] |
2. Strategies Already in the Clinic or under Clinical Trials
2.1. Development of New HER2-Targeted Drugs
HER2-targeted agents differ in their composition and mechanism of action and can be broadly classified into the following categories: anti-HER2 and pan-HER antibodies (targeting various HER family receptors, such as Sym013), ADCs, antibodies fused to cytotoxic peptides, bispecific antibodies, immunotherapeutic approaches (including peptide vaccines and T-CARs), and TKIs, among others (reviewed in [58]). The importance of ADCs and HER2-directed immunotherapy agents in current and future treatments have been specifically reviewed [14,59]. In this review, we focus mostly on other approaches.
2.1.1. Tyrosine Kinase Inhibitors (TKIs)
Lapatinib (GW572016, Tyverb, GlaxoSmithKline), a dual HER1–HER2 reversible inhibitor, was the first TKI approved for metastatic HER2 BC and is still the only one recommended in combination with endocrine therapy (letrozole) and/or chemotherapy (capecitabine) for hormone receptor-positive (HR+)/HER2+ advanced or metastatic tumors [60]. However, due to its toxicity and the large number of resistance mechanisms documented (Table 1), this TKI is progressively being replaced by novel TKIs. These TKIs differ in their selectivity among HER receptors, binding strength, efficacy, and toxicity profiles (reviewed in [7,61]).
Neratinib (HKI-272; Nerlynx, Puma Biotechnology) is an oral pan-HER TKI that binds irreversibly to HER1, HER2, and HER4. The potent and extensive effect on diverse HER receptors might explain its frequent intestinal toxicity (mostly diarrhea). This agent is capable of overcoming trastuzumab resistance (either innate or acquired) in HER2 BC models [62]. In 2017, based on the results of ExteNET [63], the FDA approved neratinib treatment as extended adjuvant therapy for early-stage HER2 BC patients who have received at least one year of trastuzumab. In this trial, HR+ tumors responded better to neratinib than HR-negative ones. For metastatic patients previously treated with HER2-targeted drugs, the FDA approved neratinib plus capecitabine in 2020 [64]. Interestingly, neratinib shows good CNS penetration and promising therapeutic activity in patients with brain metastases, despite increased diarrhea as a side effect, compared to lapatinib [64,65]. In this setting, neratinib as monotherapy could also provide some therapeutic benefit [66]. However, it shows more efficacy in combination with capecitabine [65], and indeed, current clinical trials are also evaluating its utility in combination with other therapies: fulvestrant (NCT03289039 and NCT01670877, only in HER2-mutant patients) or T-DM1 (NCT02236000 or NCT01494662 in patients with brain metastasis) [7,67].
Tucatinib (ARRY-380, ONT-380, Tukysa, Seattle genetics) is an oral, reversible inhibitor that is >1000-fold more specific for HER2 than for EGFR, and thus shows reduced EGFR-associated toxicity. Preclinical studies showed strong antitumor activity in HER2-overexpressing cancers as a single agent and combined with trastuzumab [68,69,70], including those with truncating p95/p110 mutations, known to cause trastuzumab resistance [71]. Interestingly, tucatinib shows increased activity and central nervous system (CNS) penetration compared to lapatinib or neratinib in mice with intracranial HER2 cancers [72]. Moreover, it produces tumor regression when co-administered with either CDK4/6 inhibitors or hormonal therapy in xenografts models of HER2 cancer [69]. In the pivotal HER2CLIMB trial, patients with metastatic disease and previous anti-HER2 treatment who were given trastuzumab + capecitabine and tucatinib showed a significant increase in PFS and OS compared to the placebo arm; this was also seen in patients with brain metastasis [73]. In April 2020, the FDA approved the triple combination of tucatinib + trastuzumab + capecitabine for patients with advanced disease (including CNS metastasis) that had progressed from previous anti-HER2 treatments [74]. Ongoing clinical trials in different phases are investigating the utility of tucatinib in various drug combinations, such as chemotherapy, T-DM1, T-DXd, the aromatase inhibitor (letrozole), CDK4/6 inhibitors (palbociclib), or immunotherapy (Pembrolizumab) (revised in [67]).
Pyrotinib (SHR1528, Irene) is an irreversible pan-HER (HER1, 2, 4) inhibitor. Preclinical data showed HER2 cancer growth inhibition [75] and a synergistic effect with palbociclib in xenografts [76]. In 2020, it was approved by the Chinese State drug administration in combination with capecitabine as the second line of treatment for patients with advanced or metastatic cancer. In this setting, pyrotinib increased PFS compared to lapatinib, though pyrotinib showed higher rates of toxicity [77]. Multiple clinical trials are ongoing in China in metastatic patients with diverse drug combinations, including chemotherapies and other anti-HER2 agents. Some of the studies are designed to assess pyrotinib utility in patients who have progressed from previous HER2 therapy, where its efficacy in overcoming resistance to standard HER2 treatments is being evaluated [67].
Other irreversible pan-HER inhibitors under clinical evaluation are Canertinib (CI-1033), Poziotinib [78], epertinib (S-22611) [79], varlitinib (ASLAN001; NCT02396108), and TAS0728, which is effective in preclinical models of acquired resistance to HER2-targeted antibodies [80]. However, toxicity concerns are halting further studies on poziotinib [78].
While TKIs can have advantages over antibodies (oral administration, capability to reach CNS metastasis, and multiple HER family targets), they still have important limitations. They generally are not used as single agents, and their gastrointestinal and cardiac toxicity raises safety concerns when combined with other anti-HER2 approaches or chemotherapy. For instance, the combination of either pyrotinib or neratinib with capecitabine (compared to lapatinib) produces worrying rates of grade 3 diarrhea according to PHOEBE [77] and NALA trials [64]; thus, caution should be taken with these TKI + capecitabine combinations. Moreover, cross-resistance to diverse TKIs has been reported [81], and HER2 L755S mutation, although infrequent in breast cancer, can make tumors insensitive to lapatinib, neratinib, and tucatinib [82]. Fortunately, breast cancer cell lines with this mutation seem to be sensitive to either T-DM1, T-DXd [83], or poziotinib [84]. Similarly, in metastatic non-small-cell lung cancer patients with HER2 mutations, T-DM1 [85] and TDXd [86] produced promising anticancer activity, thus providing a new potential way to treat tumors with TKI-resistance mutations.
2.1.2. Novel Antibody-Based Therapies
In contrast to TKIs, therapeutic antibodies can effectively kill cancer cells in vivo through activation of antibody-dependent cellular cytotoxicity (ADCC) by effector lymphocytes. The development of novel antibody-based therapies is leading to a significant revolution in current and future HER2 BC patients, including those refractory to standard therapies. Margetuximab–cmkb (MGAH22, Margenza) is a novel chimeric antibody that binds to HER2 on the same epitope as trastuzumab but contains a constant FC region with a higher affinity for CD16a; thus, this antibody enhances ADCC via NK cells expressing CD16a receptor [87]. Mostly based on data from the SOPHIA trial (NCT02492711), in 2020, the FDA approved its use in combination with chemotherapy for the treatment of metastatic HER2 BC patients who have received at least two previous anti-HER2 therapies [88]. Other novel HER2-specific (e.g., 19H6-Hu) or pan-HER antibody mixtures (such as Sym013, which contains six humanized monoclonal antibodies targeting different epitopes of HER1, 2, and 3 receptors) are currently in preclinical or clinical studies [89,90]. Moreover, bispecific antibodies that bind either to various HER2 epitopes (such as ZW25 and azymetric) or HER2 plus immune receptors (e.g., PRS-343 targeting CD137) have shown promising preliminary results in trials [91,92].
Among antibody-derived approaches, ADCs are already a reality and have resulted in encouraging improvement in patient survival; these include T-DM1 (Kadcyla),T-deruxtecan (Enhertu) and Disitamab vedotin (RC48; Aidixi, approved in China for gastric and urothelial carcinoma), which are already used in the clinic, while a large list of other ADCs, including Trastuzumab Duocarmazine (SYD985), ALT-P7, ARX788, A166, BAT8001, and XMT-1522, among others, are under clinical evaluation (revised in [14,93]). Rather than attaching chemotherapeutic or cytotoxic agents to anti-HER2 antibodies (as ADCs do), immunotoxins contain an anti-HER2 antibody or its fragments fused with cell-death-inducing peptides. These peptides can originate from natural toxins (such as Pseudomonas exotoxin A “ETA”) or mammalian/human anti-tumor proteins (including granzyme B, caspases, endostatin, apoptosis-inducing factor, and RNases, among others) (reviewed in [94]). Many of these fusion proteins can selectively kill HER2 BC cells in vitro and/or in vivo using preclinical models [94]. However, only a few HER2-immunotoxins have so far been evaluated in clinical trials, where they just reached phase I with HER2 cancer patients. Specifically, ScFv (FRP5)–ETA [95] and MT–5111 (containing Shiga-like Toxin A subunit [96]) were generally well-tolerated, but limited efficacy was observed, and erb–38 rIT (using ETA) caused general hepatoxicity with low therapeutic benefit [97]. Therefore, immunotoxins require further refinement to reduce side effects, improve penetration into tumor tissues, and avoid neutralization by the immune system [94].
Despite encouraging clinical benefits observed by recent FDA-approved antibody-based approaches and those still under clinical trials, some resistance mechanisms to these new agents have already been reported. Fortunately, some of them could be potentially reversed by targeting other signaling pathways or by combination with other anti-HER2 drugs or treatments (revised in [14,93]).
2.1.3. Combination Therapy
As commented above, one effective way to overcome resistance to anti-HER2 single agents is the combination of diverse anti-HER2 drugs (antibodies, ADCs, and TKIs) with/without chemotherapy. This is a possibility in current practice (for example, combining tucatinib plus trastuzumab + capecitabine is approved for metastatic disease), and numerous clinical trials are evaluating the safety and effectiveness of diverse combinatorial strategies: for instance, tucatinib + TDM1 or tucatinib + T-DXd (HERCLIMB02-04 trials) (revised in [67]).
Importantly, the focus of many preclinical studies and clinical trials is to demonstrate the benefits of combining anti-HER2 agents with drugs/treatments targeting downstream signaling pathways or common molecular resistance mechanisms (described in the section below). While these combinations could be very effective, they should be designed carefully to avoid additive toxicities. For instance, the NTC03846583 clinical trial proposing the combination of tucatinib + trastuzumab and abemaciclib was withdrawn since metabolic interference could increase the plasma concentration and toxicity of both abemaciclib and tucatinib [98].
2.1.4. Immunotherapies
The therapeutic activation of the immune system to elicit tumor recognition and cancer cell killing is one of the greatest advances in current oncology. For HER2 BC, there are multiple and complementary approaches under pre-clinical or clinical evaluation. Extensive and updated reviews on the importance of different immunotherapies for HER2 BC treatment have been recently published elsewhere (see, for instance [99,100,101]); thus, only the most relevant results are briefly mentioned here. Techniques that use the HER2 molecule as the target for recognition by immune cells, such as HER2 vaccines, bispecific antibodies, and HER2-targeted chimeric antigen receptor T-cell therapy (CAR-T), are very promising, but none of them have been clinically approved so far [99,100]. Regarding HER2 CAR-Ts, this approach can effectively kill trastuzumab-resistant BC tumors in vivo [102]. Currently ongoing phase I clinical trials with HER2-CAR-T in patients with advanced HER2 BC include NCT369630 (including patients with CNS metastasis), NTC04511871 (using autologous T cells), and NCT04650451 (using the dual switch CAR-T BPX-603) [100]. The results of these trials will determine the safety of these approaches, as potentially lethal effects have been described with high doses of HER2-CAR-T in preclinical mouse in vivo studies [103]. Moreover, some mechanisms of resistance have already been described, such as disruption of IFN-gamma signaling, which is a general mechanism for cancer cells to elude immune killing produced by CAR-T cells or bispecific antibodies [104].
With respect to therapeutic vaccines, different technical approaches are used to induce HER2-mediated immunization, including HER2 peptides and DNA-based, viral-vector-mediated, and cell-based vaccines [100]. Vaccines based on E75 or GP2 peptides are currently being tested in multiple clinical trials, with very variable results depending on the study [99,105]. In a recent meta-analysis evaluating 24 clinical trials, You et al. [99] concluded that E75-based vaccines are generally safe, elicit strong immune response, and may provide significant, albeit reduced, clinical benefits. However, clinical trials testing distinct peptide vaccine formulations in combination with either trastuzumab or checkpoint inhibitors are underway [99,105]. Moreover, in addition to metastatic patients, peptide vaccines have been tested in the adjuvant setting to prevent progression to metastatic disease [99,100,105].
Rather than attacking the HER2 molecule, current immunotherapies aim to hamper immune checkpoint inhibitors, and these approaches have been successful in the treatment of triple negative breast cancer (TNBC) [106]. Whereas HER2 carcinomas are usually more inflamed than luminal A tumors [107], the use of immunotherapies in these tumors is still a controversial issue [108]. Four immune checkpoint antibodies are undergoing clinical trials, alone or in combination with other agents: Atezolizumab (binds to PD-L1 and produces dual blockage of PD-1 and CD80 receptors [109]); Pembrolizumab (anti-PD1 antibody that blocks interaction with PD-L1 and PD-L2 ligands [110]); durvalamab (anti-PD-L1 that inhibits interaction with PD-1 and CD80 receptors [111]); and avelumab (anti-PD-L1 [112]). Initial studies in the advanced setting such as PANACEA (Pembrolizumab + Trastuzumab) or KATE-2 (T-DM1 plus Atezolizumab or placebo), showed some clinical response of the immunotherapies only in PD-L1-positive tumors [113,114]. Multiple clinical trials are underway in previously treated advanced/metastatic patients, but since these patients generally have weakened immune systems, other studies are testing the effect of immune checkpoint inhibitors in the adjuvant setting (such as the Astefania trial) or the neoadjuvant setting (revised in [100]). The comprehensive review by Kyriazoglou and colleagues [101] summarizes the results of the clinical trials using these drugs in HER2 BC through 2020, and the authors conclude that checkpoint inhibitors are tolerable and safe, and, overall, show some positive results, but the clinical benefit is still debatable. It is required to optimize adequate biomarkers that can help with proper patient selection, such as tumor PD-L1 status, mutational burden (TMB), or the percentage of tumor-infiltrating lymphocytes (TILs) [101]. Further studies will clarify if these approaches could be useful in patients resistant to previous HER2-targeted drugs.
2.2. Therapies Not Directly Targeting HER2 Receptor
2.2.1. CDK4/6 Inhibitors
In HER2 BC, the cyclin D1-CDK4/6-pRb pathway is controlled by cyclin D1 overexpression (CCND1) or increased stability, leading to resistance to anti-HER2 therapies [115]. In this sense, preclinical studies revealed that resistant tumor cells exhibited augmented levels of cyclin D1 and CDK4, and the subsequent addition of a CDK4/6 inhibitor restored cell sensitivity to HER2-targeted therapies [115]. These data reinforced previous preclinical studies that pointed out the synergy between CDK4/6 inhibitors and anti-HER2 drugs [116,117].
Currently, three CDK4/6 inhibitors (abemaciclib, palbociclib, and ribociclib) have been approved by the FDA, but are in ongoing development for advanced HER2 BC [118,119]. In this regard, early clinical studies also propose the combination of CDK4/6 inhibitors plus anti-HER2 therapies, particularly in the subset of HER2 BC patients with estrogen receptor (ER)-positive expression [120,121,122]. At present, many clinical trials are evaluating the use of CDK4/6 inhibitors in advanced HER2 BC, highlighting three randomized trials: the PATINA study (NCT02947685) focuses on the benefit of the addition of palbociclib to trastuzumab/pertuzumab + endocrine therapy in HER2+ /HR+ invasive BC pretreated with chemotherapy plus an anti-HER2 therapy; the SOLTI-1303 PATRICIA trial (NCT02448420) studies the combination of palbociclib plus trastuzumab +/− letrozole in pretreated (2–4 prior lines of anti-HER2 drugs) advanced HER2 BC; and the MonarcHER study (NCT02675231) assesses the combination of abemaciclib plus trastuzumab +/− fulvestrant in HER2+ /HR+ locally advanced or metastatic BC pretreated with taxane plus two anti-HER2-based regimens. Initial results from the SOLTI-1303 PATRICIA and the MonarcHER2 trials have already been published, noting in the first case the need to previously classify BC by PAM50 because the PFS after treatment with palbociclib + trastuzumab was significantly higher in luminal vs. nonluminal BC [123]. In the case of the MonarcHER2 trial, the combination of abemaciclib with trastuzumab and fulvestrant revealed significant improvement to the PFS in comparison with standard-of-care chemotherapy [124]. However, the absence of a fulvestrant + trastuzumab arm in this trial limits the interpretation of the benefit of adding abemaciclib. Further, another trial was carried out (NCT02308020), in which treatment with abemaciclib alone or with trastuzumab was evaluated in HR+ /HER2+ BC patients with brain metastases, but no objective responses were observed [125]. Therefore, these data point out the clinical benefit of CDK4/6 inhibitors plus anti-HER2 therapies to overcome resistance in some cohorts, suggesting the need for further clinical trials to introduce CDK4/6 inhibitors in the therapeutic strategy of advanced HER2/HR BC. Indeed, many other nonrandomized trials are in current development: NCT03054363, NCT03530696, and NCT03709082.
2.2.2. PI3K/AKT/mTOR Inhibitors
The PI3K/AKT/mTOR pathway is frequently dysregulated in many cancer subtypes, including BC. Indeed, targeting this axis is an attractive approach to overcome therapy resistance in advanced breast cancer [126,127]. PIK3CA-activating or PTEN-inactivating mutations, which are two of the most frequent genetic alterations in breast cancer [128,129], lead to abnormal PI3K pathway activation and subsequently increase phosphatidylinositol 3,4,5-triphosphate (PIP3) levels [126]. PIP3 can interact with phosphoinositide-dependent kinase-1 (PDK1) to phosphorylate AKT. Furthermore, AKT may also be phosphorylated by mTORC2. Activated AKT acts as a key regulator, with many downstream effectors and substrates, including mTORC1, different transcription factors such as FoxO, GSK3 and NF-κB, MDM2, and BAD to promote the survival and tumorigenesis of the cell [126,127]. Particularly in HER2 BC, activation of the PI3K/AKT/mTOR pathway by genetic alterations is associated with resistance to anti-HER2 targeted therapies [122]. Indeed, preclinical studies have shown that PIK3CA or PTEN mutations have a negative result on trastuzumab’s therapeutic effect [23,24], and these data were subsequently confirmed by early clinical trials [130]. Moreover, PI3K/AKT/mTOR pathway hyperactivation has been identified as one of the main reasons for the oncogenicity associated with different mechanisms of resistance to anti-HER2 therapies, such as HER2 truncated mutant (p95-HER2) [131] or HER3 [132] overexpression. Therefore, many inhibitors that target different steps of this pathway (PI3K, AKT, or mTOR inhibitors) have been developed in recent years, with outstanding results in overcoming resistance to anti-HER2 drugs in preclinical studies [133,134,135,136]. Among them, the FDA approved alpelisib, a PI3K inhibitor, and everolimus, an mTOR inhibitor for the treatment of advanced HR+ /HER2− BC, but they have not yet been approved for advanced HER2+ BC.
In this sense, an important randomized phase III trial, BOLERO-3, has demonstrated the PFS benefit of the addition of everolimus to trastuzumab plus vinorelbine in HER2 locally advanced or metastatic BC resistant to trastuzumab (NCT01007942) [137]. Furthermore, data from BOLERO-3 and the randomized phase III BOLERO-1, which evaluated the use of everolimus as the first line in HER2 advanced BC (NCT00876395), showed that everolimus addition is especially effective in patients harboring PIK3CA mutations, loss of PTEN, or hyperactivation of the PI3K-AKT-mTOR pathway, supporting the relevance of including the status of these markers in BC diagnosis [138]. Other clinical trials to assess the use of everolimus or other mTOR inhibitors (ridaforolimus, temsirolimus, or TAK-228) have been carried out but center on HER2 metastatic BC resistant to trastuzumab (e.g., NCT01783756 [139], NCT00736970 [140], and NCT01111825). Moreover, currently, several clinical trials focusing on treatment with PI3K or AKT inhibitors in HER2 advanced BC resistant to therapy are ongoing. Among these, two trials stand out (NCT04208178 and ALPHABET, and NCT05063786) for studying the use of alpelisib (PI3K inhibitor) in HER2 advanced BC with PIK3CA mutations and resistance to trastuzumab and pertuzumab or to T-DM1, respectively.
Interestingly, inhibition of ataxia telangiectasia and Rad3-related kinase (ATR), a member of the phosphatidylinositol 3-kinase-related kinase (PIKKs) family and involved in DNA repair in response to DNA damage and replication stress [141,142], is currently being tested in combination with trastuzumab deruxtecan in HER2 advanced or metastatic BC pretreated with chemotherapy and anti-HER2 therapy in a phase I/Ib trial (DASH, NCT04704661).
2.2.3. Endocrine Therapy
There is complex bidirectional crosstalk between HER2 and the signaling pathways activated by hormonal receptors for estrogen (ER), progesterone (PgR), and androgens (AR) that can lead to cancer resistance to either antihormonal drugs [143] or anti-HER2 agents, and thus, co-targeting HER2 and HRs could lead to tumor regression [144]. Around 50% of HER2 tumors express ER, and most of these patients are treated with anti-HER2 antibodies (usually up to one year), whereas endocrine treatment requires at least 5 years [143]. It is interesting to note that around 20% of tumors negative for HER2 can become positive during progression, mostly after endocrine treatment [145,146]. For early-stage HR/HER2 tumors, neratinib could be used for extended adjuvant therapy, while in metastatic ER/HER2 tumors, the current guidelines recommend chemotherapy and anti-HER2 agents, either TKIs (lapatinib, neratinib, tucatinib, or pyrotinib), monoclonal antibodies (trastuzumab, pertuzumab, or margetuximab), or ADC (T-DM1 or T-DXd), regardless of ER status [143]. Among TKIs, lapatinib is the only one approved in combination with letrozole. While different clinical studies have proven the safety of combining endocrine therapy with anti-HER2 agents in advanced or metastatic settings, the relative benefit of endocrine treatment is still uncertain [143]. However, in these patients, the addition of CDK4/6 inhibitors seems to have clinical benefit in diverse trials [123,124].
Regarding androgens, AR is a potential marker for predicting pathological response in HER2 BC patients treated with trastuzumab plus pertuzumab neoadjuvant therapy [147], and pre-clinical studies with the AR antagonist enzalutamide proved that this agent can avoid trastuzumab resistance [148]. Consequently, phase II clinical trials showed that the combination of enzalutamide with trastuzumab in patients with advanced BC previously treated with trastuzumab was tolerable, and some patients had durable disease control [149].
2.2.4. Hsp90 Inhibitors
Heat shock protein 90 (HSP90) is an essential chaperone that enhances conformational maturation, stability, and activation of oncoproteins, including HER2 [150,151]. In addition, AKT and other members of the HER2 signaling pathway are also clients of HSP90 [152,153,154]. Therefore, HSP90 has been proposed as a potential therapeutic target to induce the degradation of HER2, and preclinical studies have confirmed the beneficial effect of HSP90 inhibitors, even in trastuzumab-resistant cancer cells [155,156,157,158]. Therefore, combinations of different HSP90 inhibitors (e.g., tanespimycin, AUY922, ganetespib, and alvespimycin) with anti-HER2-based drugs have been tested in clinical trials with HER2 BC patients (in different disease stages, including tumors refractory to anti-HER2 drugs) [159,160,161,162]. Unfortunately, despite promising efficacy in preclinical studies, so far none of the HSP90 inhibitors have obtained regulatory approval due to their high off-target toxicity and inadequate pharmacokinetic profiles [163]. Nonetheless, research on novel anti-HSP90 drugs continues and is now focusing on isoform-selective or C-terminal Hsp90 inhibitors that present limited cytotoxic side effects, such as NCT-547, which produces effective degradation of HER2 and the p95HER2 variant, leading to in vivo growth reduction of trastuzumab-resistant tumors [164].
Histone deacetylase (HDAC) inhibitors have also been assessed to overcome therapy resistance in BC [165]. Indeed, HDAC blocking results in HSP90 acetylation, causing dissociation of HSP90 from HER2, with subsequent HER2 degradation [165,166]; also, its combination with anti-HER2 drugs promotes apoptosis by FOXO3-mediated Bim1 expression [167]. Two clinical trials with HDAC inhibitors (entinostat or panobinostat) in combination with anti-HER2 drugs were performed, but results showed modest or no clinical benefit (NCT01434303 [168] and NCT00788931).
2.2.5. Other Receptor Tyrosine Kinase (RTKs) Inhibitors
Several receptor tyrosine kinases (RTKs) have been involved in resistance to anti-HER2 therapies by bypassing HER2 inhibition [169,170]. Hence, insulin-like growth factor 1 receptor (IGF1R) inhibition by cixutumumab has been tested in combination with lapatinib plus capecitabine in locally advanced or metastatic HER2 BC resistant to trastuzumab, but with poor results (NCCTG N0733 and NCT00684983 [171]). Moreover, the multitarget tyrosine kinase inhibitor pazopanib, which blocks vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), and c-KIT, in combination with lapatinib, has also been proven in HER2 invasive BC pretreated with chemotherapy and trastuzumab, with a worse or similar PFS (Progression-free survival) compared to lapatinib treatment alone (lapatinib plus placebo) (NCT00558103).
2.2.6. Non-Receptor Tyrosine Kinase Inhibitors
Src is a membrane non-receptor tyrosine kinase that is activated by several receptor tyrosine kinases (RTKs), including HER2, leading to the regulation of the Ras, focal adhesion kinase (FAK), and PI3K pathways and, therefore, enhancing the malignant behavior of the cell [172,173]. In this sense, Src hyperactivation has also been related to limited response to trastuzumab [173,174]. Consequently, several preclinical studies have shown the efficacy of Src targeting by specific inhibitors in combination with anti-HER2 agents [174]. In terms of clinical trials, the Src inhibitor dasatinib, approved by the FDA for leukemia, has been assessed as first line in combination with trastuzumab plus paclitaxel in HER2 metastatic BC (NCT01306942) with awaited results [175], and as second line alone in HER2 recurrent, locally advanced, or metastatic BC pretreated with chemotherapy (taxane and/or anthracycline, NCT00371345) with poor results.
Bruton’s tyrosine kinase (BTK) is a non-receptor tyrosine kinase that plays a vital role in the development and maturation of B cells [176]. Recently, an alternative isoform of BTK (BTK-C) was found to be overexpressed in breast cancer cells and was associated with cell survival [177]. Subsequently, preclinical studies have revealed that BTK inhibition by ibrutinib overcomes resistance to lapatinib in HER2 BC by avoiding activation of the AKT signaling pathway by NRG or EGF [178]. A phase I/II trial testing the combination of ibrutinib plus trastuzumab in T-DM1 pretreated HER2 metastatic BC is currently ongoing (NCT03379428).
2.2.7. FASN Inhibitors
Fatty acid synthase (FASN) regulates the expression, activity, and cellular location of HER2 [179]. In fact, FASN inhibition suppresses HER2 expression by upregulating the transcription factor PEA3, a repressor of HER2 [179,180]. Further, preclinical studies have demonstrated the efficacy of targeting FASN to revert the resistance to anti-HER2 based regimens in HER2 BC [181,182]. This research has paved the way for the use of FASN inhibitors such as TVB-2640 in clinical trials in combination with trastuzumab in HER2 metastatic BC pretreated with anti-HER2 drugs (NCT03179904, currently ongoing).
2.2.8. PARP Inhibitors
Poly (ADP-Ribose) polymerase (PARP) is pivotal for the base excision repair pathway [183]. PARP inhibition has been shown to be a promising therapeutic strategy in homologous recombination repair-deficient tumors, such as BRCA1 and BRCA2-mutated breast cancer [183]. Indeed, the FDA approved several PARP inhibitors in recent years to treat HER2-negative, BRCA1 and BRCA2-mutated BC [183]. However, recent preclinical research has pointed out the effectiveness of using PARP inhibitors in trastuzumab-resistant HER2 BC via inhibition of NF-κB signaling [184]. In this regard, a phase Ib/II trial is being performed, assessing the combination of the PARP inhibitor niraparib plus trastuzumab in HER2 metastatic BC pretreated with anti-HER2 drugs (NCT03368729).
3. Strategies in Pre-Clinical Development
Although a wide spectrum of therapeutic strategies has entered clinical trials, preclinical research continues to unravel the molecular mechanisms that underlie anti-HER2 therapy resistance, and this knowledge has led to the development of novel potential therapeutic approaches against other molecular targets. Indeed, protective autophagy and/or the role of other genes located on the HER2 amplicon that are co-amplified/overexpressed with the receptor have emerged as responsible for anti-HER2 therapeutic failure. For instance, insights about novel therapeutic approaches such as nanotherapy or even microbiota have been strategically proposed to overcome this resistance.
3.1. Protective Autophagy Blockade
Autophagy is a multistep process whereby cells degrade dysfunctional or unnecessary intracellular components under stress conditions. Further, it may act as a pro-survival (by obtaining energy) or a pro-death (by degrading cellular components) mechanism depending on the cellular context [185]. In HER2 BC, autophagy has been considered a tumor-inhibiting process because of the activation of several oncoproteins involved in HER2 signaling, such as both mTOR and AKT, which provokes autophagy inhibition [185,186]. However, protective autophagy has come out as a novel resistance mechanism to anti-HER2 therapies [41,42]. In this sense, preclinical studies showed that trastuzumab- or lapatinib-refractory HER2 BC cells exhibit an increase in autophagosome formation, which was essential for their survival [41,42]. Therefore, blocking this protective autophagic process might be a suitable approach to restore sensitivity. Indeed, preclinical studies demonstrated that the combination of autophagy inhibitors, such as chloroquine, plus anti-HER2 therapies boosts tumor cell death [41,42,187,188].
Few clinical trials have been performed analyzing the use of chloroquine or hydroxychloroquine in breast cancer, and none of them so far have distinguished between breast cancer subtypes [185]. Due to the importance of the cellular context in autophagy-mediated drug resistance, future clinical trials need to consider the cancer subtype and the previous treatment history. In this regard, new trials are ongoing to evaluate the usage of hydroxychloroquine in HR+ /HER2− BC (NCT03032406, NCT03774472, and NCT04316169) due to autophagy to promote resistance to other drugs [185,186]. However, further research is crucial to decode the mechanisms whereby autophagy promotes resistance to anti-HER2 therapies to test their combination with autophagy inhibitors in clinical trials and to define a patient profile better suited to this therapeutic approach.
3.2. Targeting HER2 Amplicon and Neighbor Genes
The consensus “HER2 amplicon” in breast cancers located at the 17q12–21 chromosomal region contains (besides ERBB2) at least five other genes (STARD3, TCAP, PNMT, PGAP3, and MIEN1). Interestingly, in more than 60% of HER2-amplified breast tumors, the amplicon is larger, encompassing up to 27 genes [48]. While HER2 is considered the main oncogene within this amplicon, studies have shown that some ERBB2-amplified breast cancer cell lines are not truly addicted to the HER2 oncogene but require other genes for survival against therapeutic challenge [48,189]. In fact, multiple studies indicate that other genes within the HER2 amplicon or adjacent chromosomal regions play important roles in the pathobiology and the treatment behavior of HER2 tumors, supporting that these genes are not mere “passenger” genes [48]. Specifically, Sahlberg and colleagues proved that silencing either STARD3, GRB7, PSMD3, PERLD1, PPP1R1B, THRA, or GSDMA partially sensitized specific breast cancer cell lines to anti-HER2 agents [48]. Thus, identification of the co-amplified genes that affect cancer development and drug response is a suitable approach for the development of new therapeutic strategies in these tumors. In this sense, among 22 genes closely associated with the HER2 amplicon, the overexpression of GSDMB (Gasdermin-B; located at 17q12) had the strongest statistical association with poor prognosis in diverse microarray datasets [43]. Further validation using a GSDMB-specific monoclonal antibody and FISH probe confirmed that GSDMB amplification and protein overexpression occurs in more than 60% of HER2 BC [43,45]. Importantly, in these tumors, GSDMB overexpression correlates to distant metastasis, poor prognosis (relapse-free survival and pathologic complete response), and reduced response to standard treatment (trastuzumab plus chemotherapy) independent of HR status in both neoadjuvant and adjuvant settings [43]. In fact, GSDMB upregulation is functionally involved in mediating HER2 BC’s aggressive behavior in multiple ways, since it enhances cancer cell migration and invasion, in vivo tumorigenesis (in GEMMs and orthotopic xenografts [44,190]), metastasis [44,45], and resistance (innate and acquired) to anti-HER2 therapies [43,188]. GSDMB induces survival to anti-HER2 agents through the promotion of pro-survival autophagy in breast and gastric HER2 cancers [188]. These pro-tumor functions could be partially reversed by GSDMB silencing via si/shRNAs [44,45,188]. To further prove that GSDMB is a novel and feasible therapeutic target in HER2 BCs, an innovative nanotherapy (nanoparticles loaded with a specific anti-GSDMB antibody) was generated and showed effectiveness in reducing GSDMB pro-tumoral activities, including resistance to therapy (discussed below in Section 3.3) [45].
Paradoxically, GSDMB shares a potentially activatable pro-cell-death function with other members of the Gasdermin family. Indeed, Gasdermins are the key effectors of a lytic and pro-inflammatory cell death mechanism termed pyroptosis, and thus, triggering pyroptosis in tumors has been shown to induce a potent antitumor immune response and cancer regression (revised in [191]). Since the HER2 amplicon frequently contains two GSDM genes (GSDMA and GSDMB), rather than inhibiting GSDMA–B expression, activating their pyroptotic activity via therapeutic intervention (either with GSDM-targeted nanotherapies, chemotherapy, or immunotherapy) could be a promising approach for future treatment of HER2 BC resistant to standard therapies (revised in [191]).
Aside GSDMs, there are other frequently amplified genes near the HER2 amplicon that could affect HER2 BC clinical behavior, such as TOP2A (in 17q21.2 [192]) or genes within the 17q23 amplicon such as RPS6KB1, PPM1D, or MIR21 [193,194]. PPM1D (located at the 17q23 region) encodes the wildtype p53-induced phosphatase1 (WIP1), a protein that negatively regulates p53 function, enhances cell cycle progression, and accelerates tumor incidence in HER2 BC mouse models [195]. In HER2/WIP1-amplified tumors, pharmacological WIP1 inhibition reduced cell proliferation and, importantly, also partially restored sensitivity to trastuzumab [193,194], thus suggesting that the WIP1 oncogene could be another potential therapeutic target to reverse anti-HER2 therapy resistance.
3.3. Targeted Nanotherapy
Nanotechnology has come out as an interesting way to reduce toxicity and improve different pharmacokinetic and pharmacodynamic aspects of current treatments. These new strategies are based on nanoparticle delivery systems (nanocarriers) composed of biomaterials/biodegradable materials that enhance the action range by increasing the drug–site contact time [196,197]. In recent years, several non-targeted nanotherapeutics, which consisted of improved chemotherapy-delivery systems (such as DOXIL, MYOCET, NANOXEL, etc.), have been approved by regulatory agencies [196]. However, new problems and challenges such as the lack of target specificity and novel toxicities appeared related to these novel treatments [196]. For this reason, current research is focused on targeted nanocarriers directed against HER2 BC cells.
Multiple designs of targeting nanocarriers for HER2 BC have been developed not only as therapy but also as tumor imaging techniques, with the HER2 BC targeting ligand the main difference between designs. In this regard, antibodies are the most common ligand to target HER2 BC cells, with the employment of trastuzumab in the lead, usually combined with other therapeutical agents [196]. For instance, Lin and colleagues developed a nanocarrier based on a liposome–PEG–PEI complex that was conjugated with trastuzumab plus curcumin or doxorubicin, proving that the nanomedicine increased the therapeutic effect compared to trastuzumab alone both in vitro and in vivo, specifically in HER2 BC cell lines [198]. Interestingly, trastuzumab-conjugated nanocarriers have also been studied in other HER2-positive cancers, such as HER2 gastric cancers, showing promising results in overcoming resistance to trastuzumab [199]. Additionally, photothermal and photodynamic nanotherapy has emerged in the last years, and, particularly in this context, several studies have succeeded in the use of anti-HER2 nanoparticles that accumulated in breast tumors, and after irradiation or illumination, they selectively induced tumor inhibition even in trastuzumab-resistant tumors [200,201,202].
In addition to entire HER2 antibodies, other molecules that have been successfully used as ligands to target HER2 receptor include antibody fragments, nucleic acids, peptides, DARPINS (Designed Ankyrin Repeat Proteins, which are small single-chain scaffold non-immunoglobulin proteins that are less immunogenic than antibodies), or affibodies and ADAPTs (albumin binding domain (ABD)-derived affinity proteins) [196]. Their application as an alternative anti-HER2 therapy or even as a delivery strategy for other therapeutical agents opens the door to a wide variety of treatments to restore sensitivity. Examples of these approaches are DARPin_9-29 (which binds to a HER2 extracellular region different to the pertuzumab epitope) coupled with gold nanorods, which can reduce in vivo HER2 tumor growth [203]; or the ZHER2:2891 affibody conjugated with 5-Fluorouracil, which is effective in killing HER2 cancer cells [204]. Moreover, Truffi et al. conjugated multiple half chains of trastuzumab onto magnetic iron oxide nanoparticles (MNP-HC), achieving an increased effect compared to trastuzumab alone, even in trastuzumab-resistant cells [205]. Further, no resistance to therapy was found after treatment with nanoparticles carrying a siHER2, proving more durable HER2 inhibition than conventional anti-HER2 drugs [206]. Similarly, the use of branched polyethylenimine-functionalized carbon dot (BP-CD) nanoparticles that carry a siHER3 together with trastuzumab showed an improved effect compared to trastuzumab alone [207]. Lastly, the use of anti-HER2 peptides as ligand has allowed, for instance, for improving the efficacy and drug delivery of liposomal doxorubicin [208]. In addition, Xiang et al. developed magnetosome nanoparticles functionalized with an anti-EGFR/HER2 peptide that had promising results for magnetic resonance imaging of EGFR and HER2 tumors [209].
In parallel to anti-HER2 targeted nanomedicines, nanotherapies that specifically attack other molecules important for HER2 tumor behavior have obtained promising preclinical results, even as a novel way to limit resistance to anti-HER2 drugs [45]. In this sense, Molina-Crespo et al. generated biocompatible nanocapsules functionalized with hyaluronic acid that carry an antibody directed against the GSDMB protein (which induces resistance to trastuzumab and lapatinib [45,188]). This targeted nanomedicine (termed AbGB-NCs) increased the sensitivity to trastuzumab in vitro, but also reduced cancer cell migration as well as in vivo breast tumor growth and lung metastasis in orthotopic xenografts, specifically in GSDMB-overexpressing cells, without systemic toxicity [45]. Other novel key molecules that can be targeted by nanotherapy include WIP1 and miRNAs (which otherwise are almost unreachable). Indeed, the specific delivery of miR-21 and WIP1 inhibitors by pH-sensitive nanoparticles has been successfully used to overcome resistance to trastuzumab [194].
Despite the ease of the design and the development of nanocarriers, this technology faces some important challenges in becoming a reality in clinical practice. Among them, the lack of standardization in terms of particle size, toxicity, and surface charge, together with problems with short half-life, stability, and the rate of drug release, suggests that this technology needs further refinement before entering clinical trials [196]. However, the recent approvals by regulatory agencies of lipid nanoparticles conjugated with siRNA (Patisiran) for the treatment of hereditary transthyretin amyloidosis [210] and the two COVID-19 nanoparticle-based vaccines [211] have paved the way for the expansion of this technology in the following years.
3.4. Other Approaches
Accumulating evidence shows that resistance to anti-HER2 treatment is a complex process that involves signaling crosstalk with diverse cells within the tumor microenvironment. In fact, macrophages, alveolar epithelial cells, and fibroblastic reticular cells have been shown to act as drivers of both preexisting and acquired resistance to anti-HER2 agents, and thus, microenvironment and tumor heterogeneity should be considered when designing new treatments [212]. The phenotypic plasticity of tumor cells, which is controlled by both intrinsic and microenvironmental cues, can result in the appearance of mesenchymal-like or cancer stem cell-like cells with reduced sensitivity to anti-HER2 drugs [213,214]. Interestingly, the phenotypic evolution of HER2 tumors could lead to the emergence of novel druggable targets, such as PDGFR-B, that can counteract HER2-targeted treatment desensitization [215]. Intrinsic mechanisms such as metabolic rewiring of tumor cells are another source of drug resistance, in particular, lipid metabolism plays an important role in this process. Thus, dysregulation of cholesterol homeostasis using drugs such as lovastatin, in combination with lapatinib, shows an enhanced therapeutic effect over lapatinib alone in HER2 BC patients (revised in [216]). Extrinsic factors such as the gut microbiota can also affect therapy response. In this sense, there is an association between trastuzumab efficacy and gut microbiota due to its modulation of the host immune system. In fact, antibiotic-treated microbiota showed reduced dendritic cell activation as well as IL12p70 release, which is a mechanism needed for trastuzumab efficacy [217]. Therefore, the alteration of the gut microbiota might be a promising strategy to enhance trastuzumab effectiveness or even overcome resistance in the future.
4. Conclusions
Treatment options for HER2 BC patients have increased steadily in recent years, and with a myriad of novel approaches under preclinical investigation or clinical validation (Figure 2), it is expected that in a few years, the conventional anti-HER2 antibodies and TKIs will be replaced by more effective and innovative therapies (ADCs, bispecific antibodies, CAR-T cells, nanotherapy, and immunotherapy). However, it is also anticipated that new mechanisms of resistance might also arise to these novel agents; thus, a combination of diverse approaches (including targeted therapies, chemotherapy, hormone therapy, and novel techniques) is likely to be the usual practice in the future of diverse clinical settings. Whereas new agents (such ADCs) are already making a strong impact on overall survival and quality of life of HER2 BC patients in particular scenarios, successfully treating metastatic disease, in particular to the CNS, will be the toughest challenge in the near future.
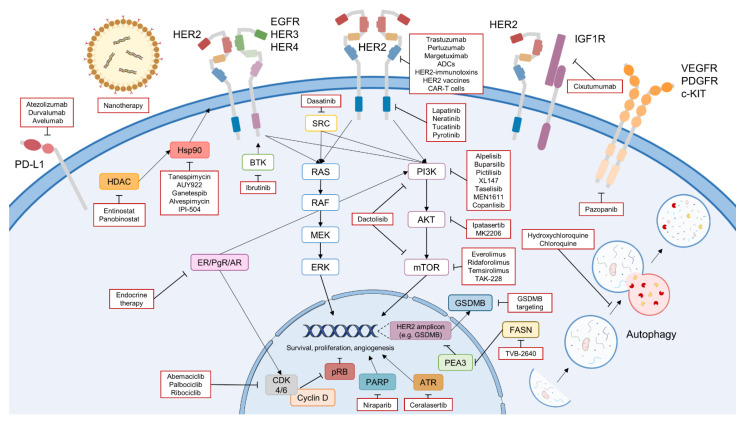
Figure 2.
Overview of the novel therapeutic strategies to overcome resistance to anti-HER2 therapies in HER2 BC (highlighted in red). The figure includes the therapies under clinical validation, including novel anti-HER2 drugs (novel TKIs, antibodies, ADCs, CAR-T cells, etc.), immunotherapies, CDK4/6 inhibitors, PI3K/AKT/mTOR inhibitors, endocrine therapy, Hsp90 and HDAC inhibitors, other RTKs inhibitors, non-receptor tyrosine kinase inhibitors, FASN inhibitors, and PARP inhibitors. Furthermore, novel approaches under preclinical validation are also pointed out, such as nanotherapy, inhibition of pro-survival autophagy, and targeting of HER2 amplicon genes (e.g., GSDMB). Icons designed with smart.servier.com and Adobe Illustrator.
Acknowledgments
We are grateful to members of Gema Moreno-Bueno’s laboratory for constructive suggestions. We would like to thank the guest editors Ana Rovira and Joan Albanell for giving us the opportunity to participate in the Cancers Special Issue “Anti-HER2 Therapy Resistance in Breast Cancer”.
Author Contributions
Conceptualization; original draft preparation; writing—review and editing, M.G.-C., D.S. and G.M.-B. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study has been supported by the Ministerio de Ciencia, Innovación y Universidades, Agencia Estatal de Investigación (MICINN-AEI, PID2019-104644RB-I00), the Instituto de Salud Carlos III (CIBERONC, CB16/12/00295), and by the AECC Scientific Foundation (FCAECC PROYE19036MOR and GCTRA18014MATI). DS’s contract is funded by CIBERONC, partly supported by FEDER funds.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wolff A.C., Hammond M.E.H., Hicks D.G., Dowsett M., McShane L.M., Allison K.H., Allred D.C., Bartlett J.M.S., Bilous M., Fitzgibbons P., et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast. J. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 2.Yersal O., Barutca S. Biological Subtypes of Breast Cancer: Prognostic and Therapeutic Implications. World J. Clin. Oncol. 2014;5:412–424. doi: 10.5306/wjco.v5.i3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vernieri C., Milano M., Brambilla M., Mennitto A., Maggi C., Cona M.S., Prisciandaro M., Fabbroni C., Celio L., Mariani G., et al. Resistance Mechanisms to Anti-HER2 Therapies in HER2-Positive Breast Cancer: Current Knowledge, New Research Directions and Therapeutic Perspectives. Crit. Rev. Oncol. Hematol. 2019;139:53–66. doi: 10.1016/j.critrevonc.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Wolff A.C., Elizabeth Hale Hammond M., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., Bilous M., Ellis I.O., Fitzgibbons P., Hanna W., et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/ College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 5.Pernas S., Tolaney S.M. Clinical Trial Data and Emerging Strategies: HER2-Positive Breast Cancer. Breast Cancer Res. Treat. 2022;193:281–291. doi: 10.1007/s10549-022-06575-7. [DOI] [PubMed] [Google Scholar]
- 6.Meisel J.L., Venur V.A., Gnant M., Carey L. Evolution of Targeted Therapy in Breast Cancer: Where Precision Medicine Began. ASCO Educ. B. 2018;38:78–86. doi: 10.1200/EDBK_201037. [DOI] [PubMed] [Google Scholar]
- 7.Schlam I., Swain S.M. HER2-Positive Breast Cancer and Tyrosine Kinase Inhibitors: The Time Is Now. NPJ Breast Cancer. 2021;7:56. doi: 10.1038/s41523-021-00265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grogan Fleege N.M., Cobain E.F. Breast Cancer Management in 2021: A Primer for the Obstetrics and Gynecology. Best Pract. Res. Clin. Obstet. Gynaecol. 2022;82:30–45. doi: 10.1016/j.bpobgyn.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Giordano S.H., Franzoi M.A.B., Temin S., Anders C.K., Chandarlapaty S., Crews J.R., Kirshner J.J., Krop I.E., Lin N.U., Morikawa A., et al. Systemic Therapy for Advanced Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022;40:2612–2635. doi: 10.1200/JCO.22.00519. [DOI] [PubMed] [Google Scholar]
- 10.Morganti S., Bianchini G., Giordano A., Giuliano M., Curigliano G., Criscitiello C. How I Treat HER2-Positive Early Breast Cancer: How Long Adjuvant Trastuzumab Is Needed? ESMO Open. 2022;7:100428. doi: 10.1016/j.esmoop.2022.100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swain S.M., Miles D., Kim S.B., Im Y.H., Im S.A., Semiglazov V., Ciruelos E., Schneeweiss A., Loi S., Monturus E., et al. Pertuzumab, Trastuzumab, and Docetaxel for HER2-Positive Metastatic Breast Cancer (CLEOPATRA): End-of-Study Results from a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 12.Yang J., Ju J., Guo L., Ji B., Shi S., Yang Z., Gao S., Yuan X., Tian G., Liang Y., et al. Prediction of HER2-Positive Breast Cancer Recurrence and Metastasis Risk from Histopathological Images and Clinical Information via Multimodal Deep Learning. Comput. Struct. Biotechnol. J. 2022;20:333–342. doi: 10.1016/j.csbj.2021.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaibar M., Beltrán L., Romero-Lorca A., Fernández-Santander A., Novillo A., Selli C. Somatic Mutations in HER2 and Implications for Current Treatment Paradigms in HER2-Positive Breast Cancer. J. Oncol. 2020;2020:6375956. doi: 10.1155/2020/6375956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díaz-Rodríguez E., Gandullo-Sánchez L., Ocaña A., Pandiella A. Novel ADCs and Strategies to Overcome Resistance to Anti-HER2 ADCs. Cancers. 2022;14:154. doi: 10.3390/cancers14010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocco E., Lopez S., Santin A.D., Scaltriti M. Prevalence and Role of HER2 Mutations in Cancer. Pharmacol. Ther. 2019;199:188–196. doi: 10.1016/j.pharmthera.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veeraraghavan J., Mistry R., Nanda S., Sethunath V., Shea M., Mitchell T., Anurag M., Mancini M.A., Stossi F., Osborne C.K., et al. Abstract PD3-09: HER2 L755S Mutation Is Acquired upon Resistance to Lapatinib and Neratinib and Confers Cross-Resistance to Tucatinib and Trastuzumab in HER2-Positive Breast Cancer Cell Models. Cancer Res. 2021;81:PD3-09. doi: 10.1158/1538-7445.SABCS20-PD3-09. [DOI] [Google Scholar]
- 17.Hart V., Gautrey H., Kirby J., Tyson-Capper A. Review HER2 Splice Variants in Breast Cancer: Investigating Their Impact on Diagnosis and Treatment Outcomes. Oncotarget. 2020;11:4338–4357. doi: 10.18632/oncotarget.27789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arribas J., Baselga J., Pedersen K., Parra-Palau J.L. P95HER2 and Breast Cancer. Cancer Res. 2011;71:1515–1519. doi: 10.1158/0008-5472.CAN-10-3795. [DOI] [PubMed] [Google Scholar]
- 19.Ritter C.A., Perez-Torres M., Rinehart C., Guix M., Dugger T., Engelman J.A., Arteaga C.L. Human Breast Cancer Cells Selected for Resistance to Trastuzumab in Vivo Overexpress Epidermal Growth Factor Receptor and ErbB Ligands and Remain Dependent on the ErbB Receptor Network. Clin. Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 20.Rexer B.N., Arteaga C.L. Intrinsic and Acquired Resistance to HER2-Targeted Therapies in HER2 Gene-Amplified Breast Cancer: Mechanisms and Clinical Implications. Crit. Rev. Oncog. 2012;17:1–16. doi: 10.1615/CritRevOncog.v17.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veeraraghavan J., Liao F.-T., Gordon T., Selenica P., Nanda S., Qin L., Zhu Y., Patel J.A., Gazzo A., Stossi F., et al. Abstract LB517A: The Role of EGFR in Resistance to Tucatinib and Its Therapeutic Implications. Cancer Res. 2022;82:LB517A. doi: 10.1158/1538-7445.AM2022-LB517A. [DOI] [Google Scholar]
- 22.D’Amato V., Raimondo L., Formisano L., Giuliano M., De Placido S., Rosa R., Bianco R. Mechanisms of Lapatinib Resistance in HER2-Driven Breast Cancer. Cancer Treat. Rev. 2015;41:877–883. doi: 10.1016/j.ctrv.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Nagata Y., Lan K.-H., Zhou X., Tan M., Esteva F.J., Sahin A.A., Klos K.S., Li P., Monia B.P., Nguyen N.T., et al. PTEN Activation Contributes to Tumor Inhibition by Trastuzumab, and Loss of PTEN Predicts Trastuzumab Resistance in Patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Berns K., Horlings H.M., Hennessy B.T., Madiredjo M., Hijmans E.M., Beelen K., Linn S.C., Gonzalez-Angulo A.M., Stemke-Hale K., Hauptmann M., et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Li G., Guo J., Shen B.-Q., Yadav D.B., Sliwkowski M.X., Crocker L.M., Lacap J.A., Phillips G.D.L. Mechanisms of Acquired Resistance to Trastuzumab Emtansine in Breast Cancer Cells. Mol. Cancer Ther. 2018;17:1441–1453. doi: 10.1158/1535-7163.MCT-17-0296. [DOI] [PubMed] [Google Scholar]
- 26.Eli L.D., Kavuri S.M. Mechanisms of Neratinib Resistance in HER2-Mutant Metastatic Breast Cancer. Cancer Drug Resist. 2022;5:873–881. doi: 10.20517/cdr.2022.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahta R., Yuan L.X.H., Zhang B., Kobayashi R., Esteva F.J. Insulin-like Growth Factor-I Receptor/Human Epidermal Growth Factor Receptor 2 Heterodimerization Contributes to Trastuzumab Resistance of Breast Cancer Cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 28.Rexer B.N., Ham A.J.L., Rinehart C., Hill S., De Matos Granja-Ingram N., González-Angulo A.M., Mills G.B., Dave B., Chang J.C., Liebler D.C., et al. Phosphoproteomic Mass Spectrometry Profiling Links Src Family Kinases to Escape from HER2 Tyrosine Kinase Inhibition. Oncogene. 2011;30:4163–4174. doi: 10.1038/onc.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L., Greger J., Shi H., Liu Y., Greshock J., Annan R., Halsey W., Sathe G.M., Martin A.M., Gilmer T.M. Novel Mechanism of Lapatinib Resistance in HER2-Positive Breast Tumor Cells: Activation of AXL. Cancer Res. 2009;69:6871–6878. doi: 10.1158/0008-5472.CAN-08-4490. [DOI] [PubMed] [Google Scholar]
- 30.Minuti G., Cappuzzo F., Duchnowska R., Jassem J., Fabi A., Obrien T., Mendoza A.D., Landi L., Biernat W., Czartoryska-Arukowicz B., et al. Increased MET and HGF Gene Copy Numbers Are Associated with Trastuzumab Failure in HER2-Positive Metastatic Breast Cancer. Br. J. Cancer. 2012;107:793–799. doi: 10.1038/bjc.2012.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aird K.M., Ding X., Baras A., Wei J., Morse M.A., Clay T., Lyerly H.K., Devi G.R. Trastuzumab Signaling in ErbB2-Overexpressing Inflammatory Breast Cancer Correlates with X-Linked Inhibitor of Apoptosis Protein Expression. Mol. Cancer Ther. 2008;7:38–47. doi: 10.1158/1535-7163.MCT-07-0370. [DOI] [PubMed] [Google Scholar]
- 32.Bashari M.H., Fan F., Vallet S., Sattler M., Arn M., Luckner-Minden C., Schulze-Bergkamen H., Zörnig I., Marme F., Schneeweiss A., et al. Mcl-1 Confers Protection of Her2-Positive Breast Cancer Cells to Hypoxia: Therapeutic Implications. Breast Cancer Res. 2016;18:26. doi: 10.1186/s13058-016-0686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Floros K.V., Jacob S., Kurupi R., Fairchild C.K., Hu B., Puchalapalli M., Koblinski J.E., Dozmorov M.G., Boikos S.A., Scaltriti M., et al. Targeting Transcription of MCL-1 Sensitizes HER2-Amplified Breast Cancers to HER2 Inhibitors. Cell Death Dis. 2021;12:179. doi: 10.1038/s41419-021-03457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahta R., Takahashi T., Ueno N.T., Hung M.C., Esteva F.J. P27kip1 Down-Regulation Is Associated with Trastuzumab Resistance in Breast Cancer Cells. Cancer Res. 2004;64:3981–3986. doi: 10.1158/0008-5472.CAN-03-3900. [DOI] [PubMed] [Google Scholar]
- 35.Scaltriti M., Eichhorn P.J., Cortés J., Prudkin L., Aurac C., Jiménez J., Chandarlapaty S., Serra V., Prat A., Ibrahim Y.H., et al. Cyclin E Amplification/Overexpression Is a Mechanism of Trastuzumab Resistance in HER2+ Breast Cancer Patients. Proc. Natl. Acad. Sci. USA. 2011;108:3761–3766. doi: 10.1073/pnas.1014835108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conlon N.T., Kooijman J.J., van Gerwen S.J.C., Mulder W.R., Zaman G.J.R., Diala I., Eli L.D., Lalani A.S., Crown J., Collins D.M. Comparative Analysis of Drug Response and Gene Profiling of HER2-Targeted Tyrosine Kinase Inhibitors. Br. J. Cancer. 2021;124:1249–1259. doi: 10.1038/s41416-020-01257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercogliano M.F., De Martino M., Venturutti L., Rivas M.A., Proietti C.J., Inurrigarro G., Frahm I., Allemand D.H., Deza E.G., Ares S., et al. TNFα-Induced Mucin 4 Expression Elicits Trastuzumab Resistance in HER2-Positive Breast Cancer. Clin. Cancer Res. 2017;23:636–648. doi: 10.1158/1078-0432.CCR-16-0970. [DOI] [PubMed] [Google Scholar]
- 38.Musolino A., Naldi N., Bortesi B., Pezzuolo D., Capelletti M., Missale G., Laccabue D., Zerbini A., Camisa R., Bisagni G., et al. Immunoglobulin g Fragment c Receptor Polymorphisms and Clinical Efficacy of Trastuzumab-Based Therapy in Patients with HER-2/Neu-Positive Metastatic Breast Cancer. J. Clin. Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 39.Xia W., Bacus S., Hegde P., Husain I., Strum J., Liu L., Paulazzo G., Lyass L., Trusk P., Hill J., et al. A Model of Acquired Autoresistance to a Potent ErbB2 Tyrosine Kinase Inhibitor and a Therapeutic Strategy to Prevent Its Onset in Breast Cancer. Proc. Natl. Acad. Sci. USA. 2006;103:7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y.C., Morrison G., Gillihan R., Guo J., Ward R.M., Fu X., Botero M.F., Healy N.A., Hilsenbeck S.G., Phillips G.L., et al. Different Mechanisms for Resistance to Trastuzumab versus Lapatinib in HER2- Positive Breast Cancers—Role of Estrogen Receptor and HER2 Reactivation. Breast Cancer Res. 2011;13:R121. doi: 10.1186/bcr3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen S., Zhu X., Qiao H., Ye M., Lai X., Yu S., Ding L., Wen A., Zhang J. Protective Autophagy Promotes the Resistance of HER2-Positive Breast Cancer Cells to Lapatinib. Tumor Biol. 2016;37:2321–2331. doi: 10.1007/s13277-015-3800-9. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez-Martin A., Oliveras-Ferraros C., Menendez J.A. Autophagy Facilitates the Development of Breast Cancer Resistance to the Anti-HER2 Monoclonal Antibody Trastuzumab. PLoS ONE. 2009;4:e6251. doi: 10.1371/journal.pone.0006251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hergueta-Redondo M., Sarrio D., Molina-Crespo Á., Vicario R., Bernadó-Morales C., Martínez L., Rojo-Sebastián A., Serra-Musach J., Mota A., Martínez-Ramírez Á., et al. Gasdermin B Expression Predicts Poor Clinical Outcome in HER2-Positive Breast Cancer. Oncotarget. 2016;7:56295–56308. doi: 10.18632/oncotarget.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hergueta-Redondo M., Sarrió D., Molina-Crespo Á., Megias D., Mota A., Rojo-Sebastian A., García-Sanz P., Morales S., Abril S., Cano A., et al. Gasdermin-B Promotes Invasion and Metastasis in Breast Cancer Cells. PLoS ONE. 2014;9:e90099. doi: 10.1371/journal.pone.0090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molina-Crespo A., Cadete A., Sarrio D., Gamez-Chiachio M., Martinez L., Chao K., Olivera A., Gonella A., Díaz E., Palacios J., et al. Intracellular Delivery of an Antibody Targeting Gasdermin-B Reduces HER2 Breast Cancer Aggressiveness. Clin. Cancer Res. 2019;25:4846–4858. doi: 10.1158/1078-0432.CCR-18-2381. [DOI] [PubMed] [Google Scholar]
- 46.Asif K., Memeo L., Palazzolo S., Frión-Herrera Y., Parisi S., Caligiuri I., Canzonieri V., Granchi C., Tuccinardi T., Rizzolio F. STARD3: A Prospective Target for Cancer Therapy. Cancers. 2021;13:4693. doi: 10.3390/cancers13184693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nencioni A., Cea M., Garuti A., Passalacqua M., Raffaghello L., Soncini D., Moran E., Zoppoli G., Pistoia V., Patrone F., et al. Grb7 Upregulation Is a Molecular Adaptation to HER2 Signaling Inhibition Due to Removal of Akt-Mediated Gene Repression. PLoS ONE. 2010;5:e9024. doi: 10.1371/journal.pone.0009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahlberg K.K., Hongisto V., Edgren H., Mäkelä R., Hellström K., Due E.U., Moen Vollan H.K., Sahlberg N., Wolf M., Børresen-Dale A.-L., et al. The HER2 Amplicon Includes Several Genes Required for the Growth and Survival of HER2 Positive Breast Cancer Cells. Mol. Oncol. 2013;7:392–401. doi: 10.1016/j.molonc.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Creedon H., Gómez-Cuadrado L., Tarnauskaite Ž., Balla J., Canel M., MacLeod K.G., Serrels B., Fraser C., Unciti-Broceta A., Tracey N., et al. Identification of Novel Pathways Linking Epithelial-to-Mesenchymal Transition with Resistance to HER2-Targeted Therapy. Oncotarget. 2016;7:11539–11552. doi: 10.18632/oncotarget.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng W.W., Wilkins O., Bang S., Ung M., Li J., An J., del Genio C., Canfield K., DiRenzo J., Wells W., et al. CD36-Mediated Metabolic Rewiring of Breast Cancer Cells Promotes Resistance to HER2-Targeted Therapies. Cell Rep. 2019;29:3405–3420.e5. doi: 10.1016/j.celrep.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luen S.J., Salgado R., Fox S., Savas P., Eng-Wong J., Clark E., Kiermaier A., Swain S.M., Baselga J., Michiels S., et al. Tumour-Infiltrating Lymphocytes in Advanced HER2-Positive Breast Cancer Treated with Pertuzumab or Placebo in Addition to Trastuzumab and Docetaxel: A Retrospective Analysis of the CLEOPATRA Study. Lancet Oncol. 2017;18:52–62. doi: 10.1016/S1470-2045(16)30631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zazo S., González-Alonso P., Martín-Aparicio E., Chamizo C., Luque M., Sanz-Álvarez M., Mínguez P., Gómez-López G., Cristóbal I., Caramés C., et al. Autocrine CCL5 Effect Mediates Trastuzumab Resistance by ERK Pathway Activation in HER2-Positive Breast Cancer. Mol. Cancer Ther. 2020;19:1696–1707. doi: 10.1158/1535-7163.MCT-19-1172. [DOI] [PubMed] [Google Scholar]
- 53.Chandran V.I., Mansson A.S., Barbachowska M., Cerezo-Magana M., Nodin B., Joshi B., Koppa D.A.N., Saad O.M., Gluz O., Isaksson K., et al. Hypoxia Attenuates Trastuzumab Uptake and Trastuzumab-Emtansine (T-DM1) Cytotoxicity through Redistribution of Phosphorylated Caveolin-1. Mol. Cancer Res. 2020;18:644–656. doi: 10.1158/1541-7786.MCR-19-0856. [DOI] [PubMed] [Google Scholar]
- 54.Dreyer T.F., Kuhn S., Stange C., Heithorst N., Schilling D., Jelsma J., Sievert W., Seitz S., Stangl S., Hapfelmeier A., et al. The Chemokine CX3CL1 Improves Trastuzumab Efficacy in HER2 Low–Expressing Cancer In Vitro and In Vivo. Cancer Immunol. Res. 2021;9:779–789. doi: 10.1158/2326-6066.CIR-20-0327. [DOI] [PubMed] [Google Scholar]
- 55.Mukhopadhyay P., Verma U., Story M., Ding L., Snider A.-M., Avila K., Tripathy D. Upregulation and Targeting of Chemokine Receptor CXCR4 in Acquired Trastuzumab Resistance. Cancer Res. 2007;67:2338. [Google Scholar]
- 56.Isca C., Piacentini F., Mastrolia I., Masciale V., Caggia F., Toss A., Piombino C., Moscetti L., Barbolini M., Maur M., et al. Circulating and Intracellular miRNAs as Prognostic and Predictive Factors in HER2-Positive Early Breast Cancer Treated with Neoadjuvant Chemotherapy: A Review of the Literature. Cancers. 2021;13:4894. doi: 10.3390/cancers13194894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murad R., Avanes A., Ma X., Geng S., Mortazavi A., Momand J. Transcriptome and Chromatin Landscape Changes Associated with Trastuzumab Resistance in HER2+ Breast Cancer Cells. Gene. 2021;799:145808. doi: 10.1016/j.gene.2021.145808. [DOI] [PubMed] [Google Scholar]
- 58.Kunte S., Abraham J., Montero A.J. Novel HER2–Targeted Therapies for HER2–Positive Metastatic Breast Cancer. Cancer. 2020;126:4278–4288. doi: 10.1002/cncr.33102. [DOI] [PubMed] [Google Scholar]
- 59.Costa R.L.B., Czerniecki B.J. Clinical Development of Immunotherapies for HER2+ Breast Cancer: A Review of HER2-Directed Monoclonal Antibodies and Beyond. NPJ Breast Cancer. 2020;6:10. doi: 10.1038/s41523-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnston S., Pippen J., Pivot X., Lichinitser M., Sadeghi S., Dieras V., Gomez H.L., Romieu G., Manikhas A., Kennedy M.J., et al. Lapatinib Combined with Letrozole versus Letrozole and Placebo as First-Line Therapy for Postmenopausal Hormone Receptor-Positive Metastatic Breast Cancer. J. Clin. Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 61.Le Du F., Diéras V., Curigliano G. The Role of Tyrosine Kinase Inhibitors in the Treatment of HER2+ Metastatic Breast Cancer. Eur. J. Cancer. 2021;154:175–189. doi: 10.1016/j.ejca.2021.06.026. [DOI] [PubMed] [Google Scholar]
- 62.Canonici A., Gijsen M., Mullooly M., Bennett R., Bouguern N., Pedersen K., O’Brien N.A., Roxanis I., Li J.L., Bridge E., et al. Neratinib Overcomes Trastuzumab Resistance in HER2 Amplified Breast Cancer. Oncotarget. 2013;4:1592–1605. doi: 10.18632/oncotarget.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan A., Delaloge S., Holmes F.A., Moy B., Iwata H., Harvey V.J., Robert N.J., Silovski T., Gokmen E., von Minckwitz G., et al. Neratinib after Trastuzumab-Based Adjuvant Therapy in Patients with HER2-Positive Breast Cancer (ExteNET): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2016;17:367–377. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- 64.Saura C., Oliveira M., Feng Y.H., Dai M.S., Chen S.W., Hurvitz S.A., Kim S.B., Moy B.M., Delaloge S., Gradishar W., et al. Neratinib plus Capecitabine versus Lapatinib plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated with ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020;38:3138–3149. doi: 10.1200/JCO.20.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freedman R.A., Gelman R.S., Anders C.K., Melisko M.E., Parsons H.A., Cropp A.M., Silvestri K., Cotter C.M., Componeschi K.P., Marte J.M., et al. TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients with Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J. Clin. Oncol. 2019;37:1081–1089. doi: 10.1200/JCO.18.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freedman R.A., Gelman R.S., Wefel J.S., Melisko M.E., Hess K.R., Connolly R.M., Van Poznak C.H., Niravath P.A., Puhalla S.L., Ibrahim N., et al. Translational Breast Cancer Research Consortium (TBCRC) 022: A Phase II Trial of Neratinib for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J. Clin. Oncol. 2016;34:945–952. doi: 10.1200/JCO.2015.63.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ulrich L., Okines A.F.C. Treating Advanced Unresectable or Metastatic HER2-Positive Breast Cancer: A Spotlight on Tucatinib. Breast Cancer Targets Ther. 2021;13:361–381. doi: 10.2147/BCTT.S268451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kulukian A., Lee P., Taylor J., Rosler R., De Vries P., Watson D., Forero-Torres A., Peterson S. Preclinical Activity of HER2-Selective Tyrosine Kinase Inhibitor Tucatinib as a Single Agent or in Combination with Trastuzumab or Docetaxel in Solid Tumor Models. Mol. Cancer Ther. 2020;19:976–987. doi: 10.1158/1535-7163.MCT-19-0873. [DOI] [PubMed] [Google Scholar]
- 69.O’Brien N.A., Huang H.K.T., McDermott M.S.J., Madrid A.M., Luo T., Ayala R., Issakhanian S., Gong K.W., Lu M., Zhang J., et al. Tucatinib Has Selective Activity in HER2-Positive Cancers and Significant Combined Activity with Approved and Novel Breast Cancer–Targeted Therapies. Mol. Cancer Ther. 2022;21:751–761. doi: 10.1158/1535-7163.MCT-21-0847. [DOI] [PubMed] [Google Scholar]
- 70.O’Brien N.A., Conklin D., McDermott M., Luo T., Ayala R., Issakhanian S., Salgar S., Hurvitz S., Slamon D.J. Abstract P6-17-11: The Small Molecule Inhibitor of HER2, Tucatinib, Has Potent and Highly Selective Activity in Preclinical Modes of HER2-Driven Cancer. Cancer Res. 2019;79:P6-17-11. doi: 10.1158/1538-7445.SABCS18-P6-17-11. [DOI] [Google Scholar]
- 71.Pheneger T., Bouhana K., Anderson D., Garrus J., Ahrendt K., Allen S., von Carlowitz I., Greschuk J., Gross S., Hoffman K., et al. Abstract #1795: In Vitro and in Vivo Activity of ARRY-380: A Potent, Small Molecule Inhibitor of ErbB2. Cancer Res. 2009;69:1795. [Google Scholar]
- 72.Dinkel V., Anderson D., Winski S., Winkler J., Koch K., Lee P.A. Abstract 852: ARRY-380, a Potent, Small Molecule Inhibitor of ErbB2, Increases Survival in Intracranial ErbB2+ Xenograft Models in Mice. Cancer Res. 2012;72:852. doi: 10.1158/1538-7445.AM2012-852. [DOI] [Google Scholar]
- 73.Murthy R.K., Loi S., Okines A., Paplomata E., Hamilton E., Hurvitz S.A., Lin N.U., Borges V., Abramson V., Anders C., et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 74.Lee A. Tucatinib: First Approval. Drugs. 2020;80:1033–1038. doi: 10.1007/s40265-020-01340-w. [DOI] [PubMed] [Google Scholar]
- 75.Li X., Yang C., Wan H., Zhang G., Feng J., Zhang L., Chen X., Zhong D., Lou L., Tao W., et al. Discovery and Development of Pyrotinib: A Novel Irreversible EGFR/HER2 Dual Tyrosine Kinase Inhibitor with Favorable Safety Profiles for the Treatment of Breast Cancer. Eur. J. Pharm. Sci. 2017;110:51–61. doi: 10.1016/j.ejps.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 76.Zhang K., Hong R., Kaping L., Xu F., Xia W., Qin G., Zheng Q., Lu Q., Zhai Q., Shi Y., et al. CDK4/6 Inhibitor Palbociclib Enhances the Effect of Pyrotinib in HER2-Positive Breast Cancer. Cancer Lett. 2019;447:130–140. doi: 10.1016/j.canlet.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Xu B., Yan M., Ma F., Hu X., Feng J., Ouyang Q., Tong Z., Li H., Zhang Q., Sun T., et al. Pyrotinib plus Capecitabine versus Lapatinib plus Capecitabine for the Treatment of HER2-Positive Metastatic Breast Cancer (PHOEBE): A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2021;22:351–360. doi: 10.1016/S1470-2045(20)30702-6. [DOI] [PubMed] [Google Scholar]
- 78.Park Y.H., Lee K.H., Sohn J.H., Lee K.S., Jung K.H., Kim J.H., Lee K.H., Ahn J.S., Kim T.Y., Kim G.M., et al. A Phase II Trial of the Pan-HER Inhibitor Poziotinib, in Patients with HER2-Positive Metastatic Breast Cancer Who Had Received at Least Two Prior HER2-Directed Regimens: Results of the NOV120101-203 Trial. Int. J. Cancer. 2018;143:3240–3247. doi: 10.1002/ijc.31651. [DOI] [PubMed] [Google Scholar]
- 79.MacPherson I.R., Spiliopoulou P., Rafii S., Saggese M., Baird R.D., Garcia-Corbacho J., Italiano A., Bonneterre J., Campone M., Cresti N., et al. A Phase I/II Study of Epertinib plus Trastuzumab with or without Chemotherapy in Patients with HER2-Positive Metastatic Breast Cancer. Breast Cancer Res. 2019;22:1–9. doi: 10.1186/s13058-019-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Irie H., Kawabata R., Fujioka Y., Nakagawa F., Itadani H., Nagase H., Ito K., Uchida J., Ohkubo S., Matsuo K. Acquired Resistance to Trastuzumab/Pertuzumab or to T-DM1 in Vivo Can Be Overcome by HER2 Kinase Inhibition with TAS0728. Cancer Sci. 2020;111:2123–2131. doi: 10.1111/cas.14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Breslin S., Lowry M.C., O’Driscoll L. Neratinib Resistance and Cross-Resistance to Other HER2-Targeted Drugs Due to Increased Activity of Metabolism Enzyme Cytochrome P4503A4. Br. J. Cancer. 2017;116:620–625. doi: 10.1038/bjc.2016.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veeraraghavan J., Mistry R., Nanda S., Sethunath V., Shea M., Mitchell T., Anurag M., Mancini M.A., Stossi F., Osborne C.K., et al. Abstract 1911: HER2 L755S Mutation Is Associated with Acquired Resistance to Lapatinib and Neratinib, and Confers Cross-Resistance to Tucatinib in HER2-Positive Breast Cancer Models. Cancer Res. 2020;80:1911. doi: 10.1158/1538-7445.AM2020-1911. [DOI] [Google Scholar]
- 83.Mukohara T., Hosono A., Mimaki S., Nakayama A., Kusuhara S., Funasaka C., Nakao T., Fukasawa Y., Kondoh C., Harano K., et al. Effects of Ado-Trastuzumab Emtansine and Fam-Trastuzumab Deruxtecan on Metastatic Breast Cancer Harboring HER2 Amplification and the L755S Mutation. Oncologist. 2021;26:635–639. doi: 10.1002/onco.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalra R., Chen C.H., Wang J., Salam A.B., Dobrolecki L.E., Lewis A., Sallas C., Yates C.C., Gutierrez C., Karanam B., et al. Poziotinib Inhibits HER2-Mutant–Driven Therapeutic Resistance and Multiorgan Metastasis in Breast Cancer. Cancer Res. 2022;82:2928–2939. doi: 10.1158/0008-5472.CAN-21-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li B.T., Shen R., Buonocore D., Olah Z.T., Ni A., Ginsberg M.S., Ulaner G.A., Offin M., Feldman D., Hembrough T., et al. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J. Clin. Oncol. 2018;36:2532–2537. doi: 10.1200/JCO.2018.77.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li B.T., Smit E.F., Goto Y., Nakagawa K., Udagawa H., Mazières J., Nagasaka M., Bazhenova L., Saltos A.N., Felip E., et al. Trastuzumab Deruxtecan in HER2-Mutant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2021;386:241–251. doi: 10.1056/NEJMoa2112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nordstrom J.L., Gorlatov S., Zhang W., Yang Y., Huang L., Burke S., Li H., Ciccarone V., Zhang T., Stavenhagen J., et al. Anti-Tumor Activity and Toxicokinetics Analysis of MGAH22, an Anti-HER2 Monoclonal Antibody with Enhanced Fcγ Receptor Binding Properties. Breast Cancer Res. 2011;13:1–14. doi: 10.1186/bcr3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Royce M., Osgood C.L., Amatya A.K., Fiero M.H., Chang C.J.G., Ricks T.K., Shetty K.A., Kraft J., Qiu J., Song P., et al. FDA Approval Summary: Margetuximab plus Chemotherapy for Advanced or Metastatic HER2-Positive Breast Cancer. Clin. Cancer Res. 2022;28:1487–1492. doi: 10.1158/1078-0432.CCR-21-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang X., Chen J., Weng Z., Li Q., Zhao L., Yu N., Deng L., Xu W., Yang Y., Zhu Z., et al. A New Anti-HER2 Antibody That Enhances the Anti-Tumor Efficacy of Trastuzumab and Pertuzumab with a Distinct Mechanism of Action. Mol. Immunol. 2020;119:48–58. doi: 10.1016/j.molimm.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 90.Berlin J., Tolcher A.W., Ding C., Whisenant J.G., Horak I.D., Wood D.L., Nadler P.I., Hansen U.H., Lantto J., Skartved N.J.Ø., et al. First-in-Human Trial Exploring Safety, Antitumor Activity, and Pharmacokinetics of Sym013, a Recombinant Pan-HER Antibody Mixture, in Advanced Epithelial Malignancies. Investig. New Drugs. 2022;40:586–595. doi: 10.1007/s10637-022-01217-7. [DOI] [PubMed] [Google Scholar]
- 91.Meric-Bernstam F., Beeram M., Mayordomo J.I., Hanna D.L., Ajani J.A., Blum Murphy M.A., Murthy R.K., Piha-Paul S.A., Bauer T.M., Bendell J.C., et al. Single Agent Activity of ZW25, a HER2-Targeted Bispecific Antibody, in Heavily Pretreated HER2-Expressing Cancers. J. Clin. Oncol. 2018;36:2500. doi: 10.1200/JCO.2018.36.15_suppl.2500. [DOI] [Google Scholar]
- 92.Hinner M.J., Aiba R.S.B., Jaquin T.J., Berger S., Durr M.C.C., Schlosser C., Allersdorfer A., Wiedenmann A., Matschiner G., Julia Schuler C., et al. Tumor-Localized Costimulatory T-Cell Engagement by the 4-1BB/HER2 Bispecific Antibody-Anticalin Fusion PRS-343. Clin. Cancer Res. 2019;25:5878–5889. doi: 10.1158/1078-0432.CCR-18-3654. [DOI] [PubMed] [Google Scholar]
- 93.Yu J., Fang T., Yun C., Liu X., Cai X. Antibody-Drug Conjugates Targeting the Human Epidermal Growth Factor Receptor Family in Cancers. Front. Mol. Biosci. 2022;9:847835. doi: 10.3389/fmolb.2022.847835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mahmoudi R., Dianat-Moghadam H., Poorebrahim M., Siapoush S., Poortahmasebi V., Salahlou R., Rahmati M. Recombinant Immunotoxins Development for HER2-Based Targeted Cancer Therapies. Cancer Cell Int. 2021;21:1–17. doi: 10.1186/s12935-021-02182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.von Minckwitz G., Harder S., Hövelmann S., Jäger E., Al-Batran S.E., Loibl S., Atmaca A., Cimpoiasu C., Neumann A., Abera A., et al. Phase I Clinical Study of the Recombinant Antibody Toxin ScFv(FRP5)-ETA Specific for the ErbB2/HER2 Receptor in Patients with Advanced Solid Malignomas. Breast Cancer Res. 2005;7:R617–R626. doi: 10.1186/bcr1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wainberg Z.A., Mita M.M., Barve M.A., Hamilton E.P., Brenner A.J., Valdes F., Ahn D., Hubbard J., Starr J., Burnett C., et al. Abstract CT130: Phase 1 Study of the Novel Immunotoxin MT-5111 in Patients with HER-2+tumors. Cancer Res. 2021;81:CT130. doi: 10.1158/1538-7445.AM2021-CT130. [DOI] [Google Scholar]
- 97.Pai-Scherf L.H., Villa J., Pearson D., Watson T., Liu E., Willingham M.C., Pastan I. Hepatotoxicity in Cancer Patients Receiving Erb-38, a Recombinant Immunotoxin That Targets the ErbB2 Receptor. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999;5:2311–2315. [PubMed] [Google Scholar]
- 98.Posada M.M., Morse B.L., Turner P.K., Kulanthaivel P., Hall S.D., Dickinson G.L. Predicting Clinical Effects of CYP3A4 Modulators on Abemaciclib and Active Metabolites Exposure Using Physiologically Based Pharmacokinetic Modeling. J. Clin. Pharmacol. 2020;60:915–930. doi: 10.1002/jcph.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.You Z., Zhou W., Weng J., Feng H., Liang P., Li Y., Shi F. Application of HER2 Peptide Vaccines in Patients with Breast Cancer: A Systematic Review and Meta-Analysis. Cancer Cell Int. 2021;21:489. doi: 10.1186/s12935-021-02187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agostinetto E., Montemurro F., Puglisi F., Criscitiello C., Bianchini G., Del Mastro L., Introna M., Tondini C., Santoro A., Zambelli A. Immunotherapy for HER2-Positive Breast Cancer: Clinical Evidence and Future Perspectives. Cancers. 2022;14:2136. doi: 10.3390/cancers14092136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kyriazoglou A., Kaparelou M., Goumas G., Liontos M., Zakopoulou R., Zografos E., Zygogianni A., Dimopoulos M.A., Zagouri F. Immunotherapy in HER2-Positive Breast Cancer: A Systematic Review. Breast Care. 2022;17:63–70. doi: 10.1159/000514860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Szöőr Á., Tóth G., Zsebik B., Szabó V., Eshhar Z., Abken H., Vereb G. Trastuzumab Derived HER2-Specific CARs for the Treatment of Trastuzumab-Resistant Breast Cancer: CAR T Cells Penetrate and Eradicate Tumors That Are Not Accessible to Antibodies. Cancer Lett. 2020;484:1–8. doi: 10.1016/j.canlet.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 103.Globerson-Levin A., Waks T., Eshhar Z. Elimination of Progressive Mammary Cancer by Repeated Administrations of Chimeric Antigen Receptor-Modified T Cells. Mol. Ther. 2014;22:1029–1038. doi: 10.1038/mt.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arenas E.J., Martínez-Sabadell A., Rius Ruiz I., Román Alonso M., Escorihuela M., Luque A., Fajardo C.A., Gros A., Klein C., Arribas J. Acquired Cancer Cell Resistance to T Cell Bispecific Antibodies and CAR T Targeting HER2 through JAK2 Down-Modulation. Nat. Commun. 2021;12:1–13. doi: 10.1038/s41467-021-21445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tobias J., Garner-Spitzer E., Drinić M., Wiedermann U. Vaccination against Her-2/Neu, with Focus on Peptide-Based Vaccines. ESMO Open. 2022;7:100361. doi: 10.1016/j.esmoop.2021.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Hegg R., Im S.-A., Shaw Wright G., et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 107.Salgado R., Denkert C., Campbell C., Savas P., Nuciforo P., Aura C., de Azambuja E., Eidtmann H., Ellis C.E., Baselga J., et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015;1:448–455. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holgado E., Perez-Garcia J., Gion M., Cortes J. Is There a Role for Immunotherapy in HER2-Positive Breast Cancer? NPJ Breast Cancer. 2018;4:2–4. doi: 10.1038/s41523-018-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S., Sosman J.A., McDermott D.F., Powderly J.D., Gettinger S.N., et al. Predictive Correlates of Response to the Anti-PD-L1 Antibody MPDL3280A in Cancer Patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Robert C., Ribas A., Wolchok J.D., Hodi F.S., Hamid O., Kefford R., Weber J.S., Joshua A.M., Hwu W.J., Gangadhar T.C., et al. Anti-Programmed-Death-Receptor-1 Treatment with Pembrolizumab in Ipilimumab-Refractory Advanced Melanoma: A Randomised Dose-Comparison Cohort of a Phase 1 Trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 111.Stewart R., Morrow M., Hammond S.A., Mulgrew K., Marcus D., Poon E., Watkins A., Mullins S., Chodorge M., Andrews J., et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol. Res. 2015;3:1052–1062. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 112.Boyerinas B., Jochems C., Fantini M., Heery C.R., Gulley J.L., Tsang K.Y., Schlom J. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol. Res. 2015;3:1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Loi S., Giobbie-Hurder A., Gombos A., Bachelot T., Hui R., Curigliano G., Campone M., Biganzoli L., Bonnefoi H., Jerusalem G., et al. Pembrolizumab plus Trastuzumab in Trastuzumab-Resistant, Advanced, HER2-Positive Breast Cancer (PANACEA): A Single-Arm, Multicentre, Phase 1b-2 Trial. Lancet Oncol. 2019;20:371–382. doi: 10.1016/S1470-2045(18)30812-X. [DOI] [PubMed] [Google Scholar]
- 114.Emens L.A., Esteva F.J., Beresford M., Saura C., De Laurentiis M., Kim S.-B., Im S.-A., Wang Y., Salgado R., Mani A., et al. Trastuzumab Emtansine plus Atezolizumab versus Trastuzumab Emtansine plus Placebo in Previously Treated, HER2-Positive Advanced Breast Cancer (KATE2): A Phase 2, Multicentre, Randomised, Double-Blind Trial. Lancet Oncol. 2020;21:1283–1295. doi: 10.1016/S1470-2045(20)30465-4. [DOI] [PubMed] [Google Scholar]
- 115.Goel S., Wang Q., Watt A.C., Tolaney S.M., Dillon D.A., Li W., Ramm S., Palmer A.C., Yuzugullu H., Varadan V., et al. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors. Cancer Cell. 2016;29:255–269. doi: 10.1016/j.ccell.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Finn R.S., Dering J., Conklin D., Kalous O., Cohen D.J., Desai A.J., Ginther C., Atefi M., Chen I., Fowst C., et al. PD 0332991, a Selective Cyclin D Kinase 4/6 Inhibitor, Preferentially Inhibits Proliferation of Luminal Estrogen Receptor-Positive Human Breast Cancer Cell Lines in Vitro. Breast Cancer Res. 2009;11:1–13. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Witkiewicz A.K., Cox D., Knudsen E.S. CDK4/6 Inhibition Provides a Potent Adjunct to Her2-Targeted Therapies in Preclinical Breast Cancer Models. Genes Cancer. 2014;5:261–272. doi: 10.18632/genesandcancer.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Corona S.P., Ravelli A., Cretella D., Cappelletti M.R., Zanotti L., Dester M., Gobbi A., Petronini P.G., Generali D. CDK4/6 Inhibitors in HER2-Positive Breast Cancer. Crit. Rev. Oncol. Hematol. 2017;112:208–214. doi: 10.1016/j.critrevonc.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 119.O’Sullivan C.C., Suman V.J., Goetz M.P. The Emerging Role of CDK4/6i in HER2-Positive Breast Cancer. Ther. Adv. Med. Oncol. 2019;11:1758835919887665. doi: 10.1177/1758835919887665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patnaik A., Rosen L.S., Tolaney S.M., Tolcher A.W., Goldman J.W., Gandhi L., Papadopoulos K.P., Beeram M., Rasco D.W., Hilton J.F., et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non–Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov. 2016;6:740–753. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 121.DeMichele A., Clark A.S., Tan K.S., Heitjan D.F., Gramlich K., Gallagher M., Lal P., Feldman M., Zhang P., Colameco C., et al. CDK 4/6 Inhibitor Palbociclib (PD0332991) in Rb+ Advanced Breast Cancer: Phase II Activity, Safety, and Predictive Biomarker Assessment. Clin. Cancer Res. 2015;21:995–1001. doi: 10.1158/1078-0432.CCR-14-2258. [DOI] [PubMed] [Google Scholar]
- 122.Pernas S., Tolaney S.M. HER2-Positive Breast Cancer: New Therapeutic Frontiers and Overcoming Resistance. Ther. Adv. Med. Oncol. 2019;11:175883591983351. doi: 10.1177/1758835919833519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ciruelos E., Villagrasa P., Pascual T., Oliveira M., Pernas S., Paré L., Escrivá-De-Romaní S., Manso L., Adamo B., Martínez E., et al. Palbociclib and Trastuzumab in HER2-Positive Advanced Breast Cancer: Results from the Phase II SOLTI-1303 PATRICIA Trial. Clin. Cancer Res. 2020;26:5820–5829. doi: 10.1158/1078-0432.CCR-20-0844. [DOI] [PubMed] [Google Scholar]
- 124.Tolaney S.M., Wardley A.M., Zambelli S., Hilton J.F., Troso-Sandoval T.A., Ricci F., Im S.A., Kim S.B., Johnston S.R., Chan A., et al. Abemaciclib plus Trastuzumab with or without Fulvestrant versus Trastuzumab plus Standard-of-Care Chemotherapy in Women with Hormone Receptor-Positive, HER2-Positive Advanced Breast Cancer (MonarcHER): A Randomised, Open-Label, Phase 2 Trial. Lancet Oncol. 2020;21:763–775. doi: 10.1016/S1470-2045(20)30112-1. [DOI] [PubMed] [Google Scholar]
- 125.Tolaney S.M., Sahebjam S., Le Rhun E., Bachelot T., Kabos P., Awada A., Yardley D., Chan A., Conte P., Diéras V., et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor-Positive Breast Cancer. Clin. Cancer Res. 2020;26:5310–5319. doi: 10.1158/1078-0432.CCR-20-1764. [DOI] [PubMed] [Google Scholar]
- 126.Dong C., Wu J., Chen Y., Nie J., Chen C. Activation of PI3K/AKT/MTOR Pathway Causes Drug Resistance in Breast Cancer. Front. Pharmacol. 2021;12:1–16. doi: 10.3389/fphar.2021.628690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guerrero-Zotano A., Mayer I.A., Arteaga C.L. PI3K/AKT/MTOR: Role in Breast Cancer Progression, Drug Resistance, and Treatment. Cancer Metastasis Rev. 2016;35:515–524. doi: 10.1007/s10555-016-9637-x. [DOI] [PubMed] [Google Scholar]
- 128.Mukohara T. Pi3k Mutations in Breast Cancer: Prognostic and Therapeutic Implications. Breast Cancer Targets Ther. 2015;7:111–123. doi: 10.2147/BCTT.S60696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kechagioglou P., Papi R.M., Provatopoulou X., Kalogera E., Papadimitriou E., Grigoropoulos P., Nonni A., Zografos G., Kyriakidis D., Gounaris A. Tumor Suppressor PTEN in Breast Cancer: Heterozygosity, Mutations and Protein Expression. Anticancer Res. 2014;34:1387–1400. [PubMed] [Google Scholar]
- 130.Razis E., Bobos M., Kotoula V., Eleftheraki A.G., Kalofonos H.P., Pavlakis K., Papakostas P., Aravantinos G., Rigakos G., Efstratiou I., et al. Evaluation of the Association of PIK3CA Mutations and PTEN Loss with Efficacy of Trastuzumab Therapy in Metastatic Breast Cancer. Breast Cancer Res. Treat. 2011;128:447–456. doi: 10.1007/s10549-011-1572-5. [DOI] [PubMed] [Google Scholar]
- 131.Pedersen K., Angelini P.-D., Laos S., Bach-Faig A., Cunningham M.P., Ferrer-Ramón C., Luque-García A., García-Castillo J., Parra-Palau J.L., Scaltriti M., et al. A Naturally Occurring HER2 Carboxy-Terminal Fragment Promotes Mammary Tumor Growth and Metastasis. Mol. Cell. Biol. 2009;29:3319–3331. doi: 10.1128/MCB.01803-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dey N., Williams C., Leyland-Jones B., De P. A Critical Role for HER3 in HER2-Amplified and Non-Amplified Breast Cancers: Function of a Kinase-Dead RTK. Am. J. Transl. Res. 2015;7:733–750. [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang C., Xu B., Liu P. Addition of the P110α Inhibitor BYL719 Overcomes Targeted Therapy Resistance in Cells from Her2-Positive-PTEN-Loss Breast Cancer. Tumor Biol. 2016;37:14831–14839. doi: 10.1007/s13277-016-5381-7. [DOI] [PubMed] [Google Scholar]
- 134.Maira S.M., Pecchi S., Huang A., Burger M., Knapp M., Sterker D., Schnell C., Guthy D., Nagel T., Wiesmann M., et al. Identification and Characterization of NVP-BKM120, an Orally Available Pan-Class I PI3-Kinase Inhibitor. Mol. Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 135.O’Brien N.A., McDonald K., Tong L., Von Euw E., Kalous O., Conklin D., Hurvitz S.A., Di Tomaso E., Schnell C., Linnartz R., et al. Targeting PI3K/MTOR Overcomes Resistance to HER2-Targeted Therapy Independent of Feedback Activation of AKT. Clin. Cancer Res. 2014;20:3507–3520. doi: 10.1158/1078-0432.CCR-13-2769. [DOI] [PubMed] [Google Scholar]
- 136.Brünner-Kubath C., Shabbir W., Saferding V., Wagner R., Singer C.F., Valent P., Berger W., Marian B., Zielinski C.C., Grusch M., et al. The PI3 Kinase/mTOR Blocker NVP-BEZ235 Overrides Resistance against Irreversible ErbB Inhibitors in Breast Cancer Cells. Breast Cancer Res. Treat. 2011;129:387–400. doi: 10.1007/s10549-010-1232-1. [DOI] [PubMed] [Google Scholar]
- 137.André F., O’Regan R., Ozguroglu M., Toi M., Xu B., Jerusalem G., Masuda N., Wilks S., Arena F., Isaacs C., et al. Everolimus for Women with Trastuzumab-Resistant, HER2-Positive, Advanced Breast Cancer (BOLERO-3): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet Oncol. 2014;15:580–591. doi: 10.1016/S1470-2045(14)70138-X. [DOI] [PubMed] [Google Scholar]
- 138.André F., Hurvitz S., Fasolo A., Tseng L.M., Jerusalem G., Wilks S., O’Regan R., Isaacs C., Toi M., Burris H., et al. Molecular Alterations and Everolimus Efficacy in Human Epidermal Growth Factor Receptor 2-Overexpressing Metastatic Breast Cancers: Combined Exploratory Biomarker Analysis from BOLERO-1 and BOLERO-3. J. Clin. Oncol. 2016;34:2115–2124. doi: 10.1200/JCO.2015.63.9161. [DOI] [PubMed] [Google Scholar]
- 139.Hurvitz S., Singh R., Adams B., Taguchi J.A., Chan D., Dichmann R.A., Castrellon A., Hu E., Berkowitz J., Mani A., et al. Phase Ib/II Single-Arm Trial Evaluating the Combination of Everolimus, Lapatinib and Capecitabine for the Treatment of HER2-Positive Breast Cancer with Brain Metastases (TRIO-US B-09) Ther. Adv. Med. Oncol. 2018;10:1758835918807339. doi: 10.1177/1758835918807339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Seiler M., Ray-Coquard I., Melichar B., Yardley D.A., Wang R.X., Dodion P.F., Lee M.A. Oral Ridaforolimus plus Trastuzumab for Patients with HER2+ Trastuzumab-Refractory Metastatic Breast Cancer. Clin. Breast Cancer. 2015;15:60–65. doi: 10.1016/j.clbc.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 141.Yap T.A., Krebs M.G., Postel-Vinay S., El-Khouiery A., Soria J.C., Lopez J., Berges A., Amy Cheung S.Y., Irurzun-Arana I., Goldwin A., et al. Ceralasertib (AZD6738), an Oral ATR Kinase Inhibitor, in Combination with Carboplatin in Patients with Advanced Solid Tumors: A Phase I Study. Clin. Cancer Res. 2021;27:5213–5224. doi: 10.1158/1078-0432.CCR-21-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Menolfi D., Zha S. ATM, ATR and DNA-PKcs Kinases-the Lessons from the Mouse Models: Inhibition = Deletion. Cell Biosci. 2020;10:1–15. doi: 10.1186/s13578-020-0376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alataki A., Dowsett M. Human Epidermal Growth Factor Receptor-2 and Endocrine Resistance in Hormone-Dependent Breast Cancer. Endocr. Relat. Cancer. 2022;29:R105–R122. doi: 10.1530/ERC-21-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mehta A., Tripathy D. Co-Targeting Estrogen Receptor and HER2 Pathways in Breast Cancer. Breast. 2014;23:2–9. doi: 10.1016/j.breast.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 145.Priedigkeit N., Hartmaier R.J., Chen Y., Vareslija D., Basudan A., Watters R.J., Thomas R., Leone J.P., Lucas P.C., Bhargava R., et al. Intrinsic Subtype Switching and Acquired ERBB2/HER2 Amplifications and Mutations in Breast Cancer Brain Metastases. JAMA Oncol. 2017;3:666–671. doi: 10.1001/jamaoncol.2016.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Priedigkeit N., Ding K., Horne W., Kolls J.K., Du T., Lucas P.C., Blohmer J.U., Denkert C., Machleidt A., Ingold-Heppner B., et al. Acquired Mutations and Transcriptional Remodeling in Long-Term Estrogen-Deprived Locoregional Breast Cancer Recurrences. Breast Cancer Res. 2021;23:1. doi: 10.1186/s13058-020-01379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li J., Zhang S., Ye C., Liu Q., Cheng Y., Ye J., Liu Y., Duan X., Xin L., Zhang H., et al. Androgen Receptor: A New Marker to Predict Pathological Complete Response in HER2-Positive Breast Cancer Patients Treated with Trastuzumab Plus Pertuzumab Neoadjuvant Therapy. J. Pers. Med. 2022;12:261. doi: 10.3390/jpm12020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gordon M.A., D’Amato N.C., Gu H., Babbs B., Wulfkuhle J., Petricoin E.F., Gallagher I., Dong T., Torkko K., Liu B., et al. Synergy between Androgen Receptor Antagonism and Inhibition of MTOR and HER2 in Breast Cancer. Mol. Cancer Ther. 2017;16:1389–1400. doi: 10.1158/1535-7163.MCT-17-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wardley A., Cortes J., Provencher L., Miller K., Chien A.J., Rugo H.S., Steinberg J., Sugg J., Tudor I.C., Huizing M., et al. The Efficacy and Safety of Enzalutamide with Trastuzumab in Patients with HER2+ and Androgen Receptor-Positive Metastatic or Locally Advanced Breast Cancer. Breast Cancer Res. Treat. 2021;187:155–165. doi: 10.1007/s10549-021-06109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang Y., Fu P., Fan W. Novel Targeted Therapies to Overcome Trastuzumab Resistance in HER2- Overexpressing Metastatic Breast Cancer. Curr. Drug Targets. 2013;14:889–898. doi: 10.2174/13894501113149990161. [DOI] [PubMed] [Google Scholar]
- 151.Maloney A., Workman P. HSP90 as a New Therapeutic Target for Cancer Therapy: The Story Unfolds. Expert Opin. Biol. Ther. 2002;2:3–24. doi: 10.1517/14712598.2.1.3. [DOI] [PubMed] [Google Scholar]
- 152.Basso A.D., Solit D.B., Munster P.N., Rosen N. Ansamycin Antibiotics Inhibit Akt Activation and Cyclin D Expression in Breast Cancer Cells That Overexpress HER2. Oncogene. 2002;21:1159–1166. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Münster P.N., Marchion D.C., Basso A.D., Rosen N. Degradation of HER2 by Ansamycins Induces Growth Arrest and Apoptosis in Cells with HER2 Overexpression via a HER3, Phosphatidylinositol 3′-Kinase-AKT-Dependent Pathway. Cancer Res. 2002;62:3132–3137. [PubMed] [Google Scholar]
- 154.De Mattos-Arruda L., Cortes J. Breast Cancer and HSP90 Inhibitors: Is There a Role beyond the HER2-Positive Subtype? Breast. 2012;21:604–607. doi: 10.1016/j.breast.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 155.Zsebik B., Citri A., Isola J., Yarden Y., Szöllosi J., Vereb G. Hsp90 Inhibitor 17-AAG Reduces ErbB2 Levels and Inhibits Proliferation of the Trastuzumab Resistant Breast Tumor Cell Line JIMT-1. Immunol. Lett. 2006;104:146–155. doi: 10.1016/j.imlet.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 156.Scaltriti M., Serra V., Normant E., Guzman M., Rodriguez O., Lim A.R., Slocum K.L., West K.A., Rodriguez V., Prudkin L., et al. Antitumor Activity of the Hsp90 Inhibitor IPI-504 in HER2-Positive Trastuzumab-Resistant Breast Cancer. Mol. Cancer Ther. 2011;10:817–824. doi: 10.1158/1535-7163.MCT-10-0966. [DOI] [PubMed] [Google Scholar]
- 157.Chandarlapaty S., Scaltriti M., Angelini P., Ye Q., Guzman M., Hudis C.A., Norton L., Solit D.B., Arribas J., Baselga J., et al. Inhibitors of HSP90 Block P95-HER2 Signaling in Trastuzumab-Resistant Tumors and Suppress Their Growth. Oncogene. 2010;29:325–334. doi: 10.1038/onc.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Canonici A., Qadir Z., Conlon N.T., Collins D.M., O’Brien N.A., Walsh N., Eustace A.J., O’Donovan N., Crown J. The HSP90 Inhibitor NVP-AUY922 Inhibits Growth of HER2 Positive and Trastuzumab-Resistant Breast Cancer Cells. Investig. New Drugs. 2018;36:581–589. doi: 10.1007/s10637-017-0556-7. [DOI] [PubMed] [Google Scholar]
- 159.Jhaveri K., Wang R., Teplinsky E., Chandarlapaty S., Solit D., Cadoo K., Speyer J., D’Andrea G., Adams S., Patil S., et al. A Phase I Trial of Ganetespib in Combination with Paclitaxel and Trastuzumab in Patients with Human Epidermal Growth Factor Receptor-2 (HER2)-Positive Metastatic Breast Cancer. Breast Cancer Res. 2017;19:1–8. doi: 10.1186/s13058-017-0879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Modi S., Stopeck A., Linden H., Solit D., Chandarlapaty S., Rosen N., D’Andrea G., Dickler M., Moynahan M.E., Sugarman S., et al. HSP90 Inhibition Is Effective in Breast Cancer: A Phase II Trial of Tanespimycin (17-AAG) plus Trastuzumab in Patients with HER2-Positive Metastatic Breast Cancer Progressing on Trastuzumab. Clin. Cancer Res. 2011;17:5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 161.Modi S., Stopeck A.T., Gordon M.S., Mendelson D., Solit D.B., Bagatell R., Ma W., Wheler J., Rosen N., Norton L., et al. Combination of Trastuzumab and Tanespimycin (17-AAG, KOS-953) Is Safe and Active in Trastuzumab-Refractory HER-2-Overexpressing Breast Cancer: A Phase I Dose-Escalation Study. J. Clin. Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 162.Kong A., Rea D., Ahmed S., Beck J.T., López R.L., Biganzoli L., Armstrong A.C., Aglietta M., Alba E., Campone M., et al. Phase 1B/2 Study of the HSP90 Inhibitor AUY922 plus Trastuzumab in Metastatic HER2-Positive Breast Cancer Patients Who Have Progressed on Trastuzumab-Based Regimen. Oncotarget. 2016;7:37680–37692. doi: 10.18632/oncotarget.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sanchez J., Carter T.R., Cohen M.S., Blagg B.S.J. Old and New Approaches to Target the Hsp90 Chaperone. Curr. Cancer Drug Targets. 2020;20:253–270. doi: 10.2174/1568009619666191202101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Park J.M., Kim Y.J., Park S., Park M., Farrand L., Nguyen C.T., Ann J., Nam G., Park H.J., Lee J., et al. A Novel HSP90 Inhibitor Targeting the C-Terminal Domain Attenuates Trastuzumab Resistance in HER2-Positive Breast Cancer. Mol. Cancer. 2020;19:4–11. doi: 10.1186/s12943-020-01283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Damaskos C., Valsami S., Kontos M., Spartalis E., Kalampokas T., Kalampokas E., Athanasiou A., Moris D., Daskalopoulou A., Davakis S., et al. Histone Deacetylase Inhibitors: An Attractive Therapeutic Strategy against Breast Cancer. Anticancer Res. 2017;37:35–46. doi: 10.21873/anticanres.11286. [DOI] [PubMed] [Google Scholar]
- 166.Kotwal A., Amere Subbarao S. Hsp90 Regulates HDAC3-Dependent Gene Transcription While HDAC3 Regulates the Functions of Hsp90. Cell. Signal. 2020;76:109801. doi: 10.1016/j.cellsig.2020.109801. [DOI] [PubMed] [Google Scholar]
- 167.Lee J., Bartholomeusz C., Mansour O., Humphries J., Hortobagyi G.N., Ordentlich P., Ueno N.T. A Class I Histone Deacetylase Inhibitor, Entinostat, Enhances Lapatinib Efficacy in HER2-Overexpressing Breast Cancer Cells through FOXO3-Mediated Bim1 Expression. Breast Cancer Res. Treat. 2014;146:259–272. doi: 10.1007/s10549-014-3014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lim B., Murthy R.K., Jackson S., Willey J.S., Lee J., Alvarez R.H., Barcenas C.H., Karuturi M.S., Ibrahim N.K., Booser D.J., et al. Open-Label Phase Ib Study of Entinostat (E), and Lapatinib (L) Alone, and in Combination with Trastuzumab (T) in Patients (Pts) with HER2+ Metastatic (MHER2+) Breast Cancer after Progression on Trastuzumab. J. Clin. Oncol. 2016;34:609. doi: 10.1200/JCO.2016.34.15_suppl.609. [DOI] [Google Scholar]
- 169.Vu T., Claret F.X. Trastuzumab: Updated Mechanisms of Action and Resistance in Breast Cancer. Front. Oncol. 2012;2:62. doi: 10.3389/fonc.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Alexander P.B., Chen R., Gong C., Yuan L., Jasper J.S., Ding Y., Markowitz G.J., Yang P., Xu X., McDonnell D.P., et al. Distinct Receptor Tyrosine Kinase Subsets Mediate Anti-HER2 Drug Resistance in Breast Cancer. J. Biol. Chem. 2017;292:748–759. doi: 10.1074/jbc.M116.754960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haddad T.C., He J., O’Sullivan C.C., Chen B., Northfelt D., Dueck A.C., Ballman K.V., Tenner K.S., Linden H., Sparano J.A., et al. Randomized Phase II Trial of Capecitabine and Lapatinib with or without IMC-A12 (Cituxumumab) in Patients with HER2-Positive Advanced Breast Cancer Previously Treated with Trastuzumab and Chemotherapy: NCCTG N0733 (Alliance) Breast Cancer Res. Treat. 2021;188:477–487. doi: 10.1007/s10549-021-06221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mayer E.L., Krop I.E. Advances in Targeting Src in the Treatment of Breast Cancer and Other Solid Malignancies. Clin. Cancer Res. 2010;16:3526–3532. doi: 10.1158/1078-0432.CCR-09-1834. [DOI] [PubMed] [Google Scholar]
- 173.Peiró G., Ortiz-Martínez F., Gallardo A., Pérez-Balaguer A., Sánchez-Payá J., Ponce J.J., Tibau A., López-Vilaro L., Escuin D., Adrover E., et al. Src, a Potential Target for Overcoming Trastuzumab Resistance in HER2-Positive Breast Carcinoma. Br. J. Cancer. 2014;111:689–695. doi: 10.1038/bjc.2014.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang S., Huang W.C., Li P., Guo H., Poh S.B., Brady S.W., Xiong Y., Tseng L.M., Li S.H., Ding Z., et al. Combating Trastuzumab Resistance by Targeting SRC, a Common Node Downstream of Multiple Resistance Pathways. Nat. Med. 2011;17:461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ocana A., Gil-Martin M., Antolín S., Atienza M., Montaño Á., Ribelles N., Urruticoechea A., Falcón A., Pernas S., Orlando J., et al. Efficacy and Safety of Dasatinib with Trastuzumab and Paclitaxel in First Line HER2-Positive Metastatic Breast Cancer: Results from the Phase II GEICAM/2010-04 Study. Breast Cancer Res. Treat. 2019;174:693–701. doi: 10.1007/s10549-018-05100-z. [DOI] [PubMed] [Google Scholar]
- 176.Wang X., Kokabee L., Kokabee M., Conklin D.S. Bruton’s Tyrosine Kinase and Its Isoforms in Cancer. Front. Cell Dev. Biol. 2021;9:668996. doi: 10.3389/fcell.2021.668996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Eifert C., Wang X., Kokabee L., Kourtidis A., Jain R., Gerdes M.J., Conklin D.S. A Novel Isoform of the B Cell Tyrosine Kinase BTK Protects Breast Cancer Cells from Apoptosis. Genes. Chromosomes Cancer. 2013;52:961–975. doi: 10.1002/gcc.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang X., Wong J., Sevinsky C.J., Kokabee L., Khan F., Sun Y., Conklin D.S. Bruton’s Tyrosine Kinase Inhibitors Prevent Therapeutic Escape in Breast Cancer Cells. Mol. Cancer Ther. 2016;15:2198–2208. doi: 10.1158/1535-7163.MCT-15-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Menendez J.A., Vellon L., Mehmi I., Oza B.P., Ropero S., Colomer R., Lupu R. Inhibition of Fatty Acid Synthase (FAS) Suppresses HER2/Neu (ErbB-2) Oncogene Overexpression in Cancer Cells. Proc. Natl. Acad. Sci. USA. 2004;101:10715–10720. doi: 10.1073/pnas.0403390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee J.S., Sul J.Y., Park J.B., Lee M.S., Cha E.Y., Song I.S., Kim J.R., Chang E.S. Fatty Acid Synthase Inhibition by Amentoflavone Suppresses HER2/Neu (ErbB2) Oncogene in SKBR3 Human Breast Cancer Cells. Phyther. Res. 2013;27:713–720. doi: 10.1002/ptr.4778. [DOI] [PubMed] [Google Scholar]
- 181.Blancafort A., Giró-Perafita A., Oliveras G., Palomeras S., Turrado C., Campuzano Ò., Carrión-Salip D., Massaguer A., Brugada R., Palafox M., et al. Dual Fatty Acid Synthase and HER2 Signaling Blockade Shows Marked Antitumor Activity against Breast Cancer Models Resistant to Anti-HER2 Drugs. PLoS ONE. 2015;10:e0131241. doi: 10.1371/journal.pone.0131241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vazquez-Martin A., Colomer R., Brunet J., Menendez J.A. Pharmacological Blockade of Fatty Acid Synthase (FASN) Reverses Acquired Autoresistance to Trastuzumab (HerceptinTM) by Transcriptionally Inhibiting “HER2 Super-Expression” Occurring in High-Dose Trastuzumab-Conditioned SKBR3/Tzb100 Breast Cancer Cells. Int. J. Oncol. 2007;31:769–776. doi: 10.3892/ijo.31.4.769. [DOI] [PubMed] [Google Scholar]
- 183.Cortesi L., Rugo H.S., Jackisch C. An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target. Oncol. 2021;16:255–282. doi: 10.1007/s11523-021-00796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wielgos M.E., Zhang Z., Rajbhandari R., Cooper T.S., Zeng L., Forero A., Esteva F.J., Kent Osborne C., Schiff R., LoBuglio A.F., et al. Trastuzumab-Resistant HER2 þ Breast Cancer Cells Retain Sensitivity to Poly (Adp-Ribose) Polymerase (Parp) Inhibition. Mol. Cancer Ther. 2018;17:921–930. doi: 10.1158/1535-7163.MCT-17-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zambrano J., Yeh E.S. Autophagy and Apoptotic Crosstalk: Mechanism of Therapeutic Resistance in HER2-Positive Breast Cancer. Breast Cancer Basic Clin. Res. 2016;10:13–23. doi: 10.4137/BCBCR.S32791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Janser Félice A., Tschan Mario P., Rupert L. The Role of Autophagy in HER2-Targeted Therapy. Swiss Med. Wkly. 2019;149:w20138. doi: 10.4414/smw.2019.20138. [DOI] [PubMed] [Google Scholar]
- 187.Cufí S., Vazquez-Martin A., Oliveras-Ferraros C., Corominas-Faja B., Cuyàs E., López-Bonet E., Martin-Castillo B., Joven J., Menendez J.A. The Anti-Malarial Chloroquine Overcomes Primary Resistance and Restores Sensitivity to Trastuzumab in HER2-Positive Breast Cancer. Sci. Rep. 2013;3:1–13. doi: 10.1038/srep02469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gámez-Chiachio M., Molina-Crespo Á., Ramos-Nebot C., Martinez-Val J., Martinez L., Gassner K., Llobet F.J., Gonzalo-Consuegra C., Cordani M., Bernadó-Morales C., et al. Gasdermin B Over-Expression Arbitrates HER2-Targeted Therapy Resistance by Inducing Protective Autophagy. bioRxiv. 2021 doi: 10.1101/2021.07.01.450506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shiu K.K., Wetterskog D., Mackay A., Natrajan R., Lambros M., Sims D., Bajrami I., Brough R., Frankum J., Sharpe R., et al. Integrative Molecular and Functional Profiling of ERBB2-Amplified Breast Cancers Identifies New Genetic Dependencies. Oncogene. 2014;33:619–631. doi: 10.1038/onc.2012.625. [DOI] [PubMed] [Google Scholar]
- 190.Sarrió D., Rojo-Sebastián A., Teijo A., Pérez-López M., Díaz-Martín E., Martínez L., Morales S., García-Sanz P., Palacios J., Moreno-Bueno G. Gasdermin-B Pro-Tumor Function in Novel Knock-in Mouse Models Depends on the in Vivo Biological Context. Front. Cell Dev. Biol. 2022;10:813929. doi: 10.3389/fcell.2022.813929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sarrió D., Martínez-Val J., Molina-Crespo Á., Sánchez L., Moreno-Bueno G. The Multifaceted Roles of Gasdermins in Cancer Biology and Oncologic Therapies. Biochim. Biophys. Acta-Rev. Cancer. 2021;1876:188635. doi: 10.1016/j.bbcan.2021.188635. [DOI] [PubMed] [Google Scholar]
- 192.Fountzilas G., Christodoulou C., Bobos M., Kotoula V., Eleftheraki A.G., Xanthakis I., Batistatou A., Pentheroudakis G., Xiros N., Papaspirou I., et al. Topoisomerase II Alpha Gene Amplification Is a Favorable Prognostic Factor in Patients with HER2-Positive Metastatic Breast Cancer Treated with Trastuzumab. J. Transl. Med. 2012;10:212. doi: 10.1186/1479-5876-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Maoz M., Devir M., Inbar M., Inbar-Daniel Z., Sherill-Rofe D., Bloch I., Meir K., Edelman D., Azzam S., Nechushtan H., et al. Clinical Implications of Sub-Grouping HER2 Positive Tumors by Amplicon Structure and Co-Amplified Genes. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-55455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu Y., Xu J., Choi H.H., Han C., Fang Y., Li Y., Van der Jeught K., Xu H., Zhang L., Frieden M., et al. Targeting 17q23 Amplicon to Overcome the Resistance to Anti-HER2 Therapy in HER2+ Breast Cancer. Nat. Commun. 2018;9:4718. doi: 10.1038/s41467-018-07264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Demidov O.N., Kek C., Shreeram S., Timofeev O., Fornace A.J., Appella E., Bulavin D.V. The Role of the MKK6/P38 MAPK Pathway in Wip1-Dependent Regulation of ErbB2-Driven Mammary Gland Tumorigenesis. Oncogene. 2007;26:2502–2506. doi: 10.1038/sj.onc.1210032. [DOI] [PubMed] [Google Scholar]
- 196.Kumar G., Nandakumar K., Mutalik S., Rao C.M. Biologicals to Direct Nanotherapeutics towards HER2-Positive Breast Cancers. Nanomed. Nanotechnol. Biol. Med. 2020;27:102197. doi: 10.1016/j.nano.2020.102197. [DOI] [PubMed] [Google Scholar]
- 197.Luque-Bolivar A., Pérez-Mora E., Villegas V.E., Rondón-Lagos M. Resistance and Overcoming Resistance in Breast Cancer. Breast Cancer Targets Ther. 2020;12:211–229. doi: 10.2147/BCTT.S270799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lin Y.L., Tsai N.M., Chen C.H., Liu Y.K., Lee C.J., Chan Y.L., Wang Y.S., Chang Y.C., Lin C.H., Huang T.H., et al. Specific Drug Delivery Efficiently Induced Human Breast Tumor Regression Using a Lipoplex by Non-Covalent Association with Anti-Tumor Antibodies. J. Nanobiotechnol. 2019;17:25. doi: 10.1186/s12951-019-0457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kubota T., Kuroda S., Kanaya N., Morihiro T., Aoyama K., Kakiuchi Y., Kikuchi S., Nishizaki M., Kagawa S., Tazawa H., et al. HER2-Targeted Gold Nanoparticles Potentially Overcome Resistance to Trastuzumab in Gastric Cancer. Nanomed. Nanotechnol. Biol. Med. 2018;14:1919–1929. doi: 10.1016/j.nano.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 200.Deken M.M., Kijanka M.M., Beltrán Hernández I., Slooter M.D., de Bruijn H.S., van Diest P.J., van Bergen en Henegouwen P.M.P., Lowik C.W.G.M., Robinson D.J., Vahrmeijer A.L., et al. Nanobody-Targeted Photodynamic Therapy Induces Significant Tumor Regression of Trastuzumab-Resistant HER2-Positive Breast Cancer, after a Single Treatment Session. J. Control. Release. 2020;323:269–281. doi: 10.1016/j.jconrel.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nunes T., Pons T., Hou X., Van Do K., Caron B., Rigal M., Di Benedetto M., Palpant B., Leboeuf C., Janin A., et al. Pulsed-Laser Irradiation of Multifunctional Gold Nanoshells to Overcome Trastuzumab Resistance in HER2-Overexpressing Breast Cancer. J. Exp. Clin. Cancer Res. 2019;38:1–13. doi: 10.1186/s13046-019-1305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Carpin L.B., Bickford L.R., Agollah G., Yu T.K., Schiff R., Li Y., Drezek R.A. Immunoconjugated Gold Nanoshell-Mediated Photothermal Ablation of Trastuzumab-Resistant Breast Cancer Cells. Breast Cancer Res. Treat. 2011;125:27–34. doi: 10.1007/s10549-010-0811-5. [DOI] [PubMed] [Google Scholar]
- 203.Proshkina G.M., Shramova E.I., Shilova M.V., Zelepukin I.V., Shipunova V.O., Ryabova A.V., Deyev S.M., Kotlyar A.B. DARPin_9-29-Targeted Gold Nanorods Selectively Suppress HER2-Positive Tumor Growth in Mice. Cancers. 2021;13:5235. doi: 10.3390/cancers13205235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lan K.-H., Tsai C.-L., Chen Y.-Y., Lee T.-L., Pai C.-W., Chao Y., Lan K.-L. Affibody-Conjugated 5-Fluorouracil Prodrug System Preferentially Targets and Inhibits HER2-Expressing Cancer Cells. Biochem. Biophys. Res. Commun. 2021;582:137–143. doi: 10.1016/j.bbrc.2021.09.078. [DOI] [PubMed] [Google Scholar]
- 205.Truffi M., Colombo M., Sorrentino L., Pandolfi L., Mazzucchelli S., Pappalardo F., Pacini C., Allevi R., Bonizzi A., Corsi F., et al. Multivalent Exposure of Trastuzumab on Iron Oxide Nanoparticles Improves Antitumor Potential and Reduces Resistance in HER2-Positive Breast Cancer Cells. Sci. Rep. 2018;8:6563. doi: 10.1038/s41598-018-24968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gu S., Ngamcherdtrakul W., Reda M., Hu Z., Gray J.W., Yantasee W. Lack of Acquired Resistance in HER2-Positive Breast Cancer Cells after Long-Term HER2 siRNA Nanoparticle Treatment. PLoS ONE. 2018;13:e0198141. doi: 10.1371/journal.pone.0198141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shu M., Gao F., Yu C., Zeng M., He G., Wu Y., Su Y., Hu N., Zhou Z., Yang Z., et al. Dual-Targeted Therapy in HER2-Positive Breast Cancer Cells with the Combination of Carbon Dots/HER3 SiRNA and Trastuzumab. Nanotechnology. 2020;31:335102. doi: 10.1088/1361-6528/ab8a8a. [DOI] [PubMed] [Google Scholar]
- 208.Zahmatkeshan M., Gheybi F., Rezayat S.M., Jaafari M.R. Improved Drug Delivery and Therapeutic Efficacy of PEgylated Liposomal Doxorubicin by Targeting Anti-HER2 Peptide in Murine Breast Tumor Model. Eur. J. Pharm. Sci. 2016;86:125–135. doi: 10.1016/j.ejps.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 209.Xiang Z., Yang X., Xu J., Lai W., Wang Z., Hu Z., Tian J., Geng L., Fang Q. Tumor Detection Using Magnetosome Nanoparticles Functionalized with a Newly Screened EGFR/HER2 Targeting Peptide. Biomaterials. 2017;115:53–64. doi: 10.1016/j.biomaterials.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 210.Adams D., Gonzalez-Duarte A., O’Riordan W.D., Yang C.-C., Ueda M., Kristen A.V., Tournev I., Schmidt H.H., Coelho T., Berk J.L., et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 211.Nanomedicine and the COVID-19 Vaccines. Nat. Nanotechnol. 2020;15:963. doi: 10.1038/s41565-020-00820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Janiszewska M., Stein S., Metzger Filho O., Eng J., Kingston N.L., Harper N.W., Rye I.H., Alečković M., Trinh A., Murphy K.C., et al. The Impact of Tumor Epithelial and Microenvironmental Heterogeneity on Treatment Responses in HER2+ Breast Cancer. JCI Insight. 2021;6:e147617. doi: 10.1172/jci.insight.147617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nami B., Ghanaeian A., Black C., Wang Z. Epigenetic Silencing of HER2 Expression during Epithelial-Mesenchymal Transition Leads to Trastuzumab Resistance in Breast Cancer. Life. 2021;11:868. doi: 10.3390/life11090868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pupa S.M., Ligorio F., Cancila V., Franceschini A., Tripodo C., Vernieri C., Castagnoli L. HER2 Signaling and Breast Cancer Stem Cells: The Bridge behind HER2-Positive Breast Cancer Aggressiveness and Therapy Refractoriness. Cancers. 2021;13:4778. doi: 10.3390/cancers13194778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Giusti V., Ruzzi F., Landuzzi L., Ianzano M.L., Laranga R., Nironi E., Scalambra L., Nicoletti G., De Giovanni C., Olivero M., et al. Evolution of HER2-Positive Mammary Carcinoma: HER2 Loss Reveals Claudin-Low Traits in Cancer Progression. Oncogenesis. 2021;10:77. doi: 10.1038/s41389-021-00360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wahdan-Alaswad R., Liu B., Thor A.D. Targeted Lapatinib Anti-HER2/ErbB2 Therapy Resistance in Breast Cancer: Opportunities to Overcome a Difficult Problem. Cancer Drug Resist. 2020;3:179–198. doi: 10.20517/cdr.2019.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Di Modica M., Gargari G., Regondi V., Bonizzi A., Arioli S., Belmonte B., De Cecco L., Fasano E., Bianchi F., Bertolotti A., et al. Gut Microbiota Condition the Therapeutic Efficacy of Trastuzumab in HER2-Positive Breast Cancer. Cancer Res. 2021;81:2195–2206. doi: 10.1158/0008-5472.CAN-20-1659. [DOI] [PubMed] [Google Scholar]