Abstract
7-Ketocholesterol (7KC) is one of the oxysterols produced by the auto-oxidation of cholesterol during the dysregulation of cholesterol metabolism which has been implicated in the pathological development of osteoporosis (OP). Oxiapoptophagy involving oxidative stress, autophagy, and apoptosis can be induced by 7KC. However, whether 7KC produces negative effects on MC3T3-E1 cells by stimulating oxiapoptophagy is still unclear. In the current study, 7KC was found to significantly decrease the cell viability of MC3T3-E1 cells in a concentration-dependent manner. In addition, 7KC decreased ALP staining and mineralization and down-regulated the protein expression of OPN and RUNX2, inhibiting osteogenic differentiation. 7KC significantly stimulated oxidation and induced autophagy and apoptosis in the cultured MC3T3-E1 cells. Pretreatment with the anti-oxidant acetylcysteine (NAC) could effectively decrease NOX4 and MDA production, enhance SOD activity, ameliorate the expression of autophagy-related factors, decrease apoptotic protein expression, and increase ALP, OPN, and RUNX2 expression, compromising 7KC-induced oxiapoptophagy and osteogenic differentiation inhibition in MC3T3-E1 cells. In summary, 7KC may induce oxiapoptophagy and inhibit osteogenic differentiation in the pathological development of OP.
Keywords: oxiapoptophagy, 7-Ketocholesterol, oxidative stress, osteogenic differentiation, osteoporosis
1. Introduction
Osteoporosis (OP), commonly marked by a constant decrease in bone mass and changes in bone/skeletal tissue structure, is a common bone/skeletal disease [1]. More often than not, patients do not realize OP at the early stage until accidental fractural damages occur [2]. The pathogenesis and progression of OP might be affected by aging, genetic factors, bad living habits, and nutritional deficiency, among which, the imbalance of bone homeostasis is one of the key factors [3,4]. There is increasing evidence that lipid metabolism disorders, such as atherosclerosis (AS) and OP, share common underlying pathogenesis involving bone and vascular mineralization, as well as age-related degenerative processes [5,6,7]. However, the specific mechanism of action between lipid and bone metabolism disorders remains unclear.
Oxysterols are the lipid oxidation products of 27C cholesterol obtained from certain diets and cholesterol metabolism by enzymatic or non-enzymatic mechanisms [8]. Endogenous oxysterols are generated by the latter mechanism, with oxidation occurring in the sterol ring. Many factors, such as singlet oxygen and hydrogen peroxide, can induce the oxidation of cholesterol in lipoproteins, cell membranes, and food [9]. Oxysterols can be formed in foods containing cholesterol during prolonged storage or heating. In addition, cholesterol in the stomach can also be transformed into oxidized cholesterol due to an acidic pH and the presence of oxygen and iron ions [10]. Oxysterols can trigger oxidation, inducing pathological damage in some organelles (mitochondria, lysosomes, and peroxisomes) and cell death. Oxysterols have been implicated in important physiological and pathological activities, including cholesterol metabolism homeostasis, cell differentiation and proliferation, inflammation, and OP [11]. Evidence shows that oxidized sterols contribute to atherosclerotic vascular modeling and remodeling, specifically mediating many key steps: dysfunction of endothelial cells, adhesion of circulating blood cells, formation of foam cells and fibrous cap, re-constructure of blood vessels and extracellular matrix (ECM), and cellular apoptosis and unstable plaques [12,13,14,15,16].
7-Ketocholesterol (7KC), a widely studied oxysterol mainly formed by auto-oxidation [17], is often found in industrial foods [18]. 7KC is found to accumulate in the vascular wall, retina, and brain [17]. For example, 7KC is abundant in the circulation of hypercholesterolemia and atherosclerotic lesions [19,20]. 7KC induces oxidative stress, triggers inflammation, and can decrease cell mobility, causing pathological cell injury via aberrant signaling pathways and leading cells to a special form of death. These pathological changes can be referred to as oxiapoptophagy (OXIdative stress + APOPTOsis + autoPHAGY). This form of death is related to the challenges of oxidative stress, apoptosis, and autophagy [21,22,23]. According to previous research, 7KC, 7β-hydroxycholesterol (7β-OHC), and 24(S)-hydroxycholesterol (24S-OHC) have a biological effect on activating oxiapoptophagy [24]. 7KC may exhibit a regulatory activity in macrophage reprogramming and oxiapoptophagy in several cell types/lines [25,26,27,28]. In atherosclerotic plaques, 7KC induces calcium-containing apoptosis and promotes the deposition of cell bodies, which in turn leads to vascular calcification.
Interestingly, 7KC is reported to be an inhibitor of the adipogenic differentiation of adipose-derived stem cells by mediating the Wnt/β-catenin and MAPK signaling pathways [29]. Both adipocytes and osteoblasts can be differentiated from bone marrow-derived mesenchymal stem cells (BMSCs). OP can cause an adipo-osteogenic imbalance and the effects of 7KC on osteogenic differentiation are still unknown. However, it was recently reported that 7KC can promote osteoclast differentiation by increasing the expression of the miR-107-5p/MKP1 axis [30]. Whether 7KC can induce oxiapoptophagy in osteoblasts is still needed for further investigation. In this study, the focus is on the biological actions of 7KC on the induction of oxiapoptophagy and osteogenic differentiation in the cultured pre-osteoblast MC3T3-E1 cells.
2. Materials and Methods
2.1. Cell Culture
MC3T3-E1 cells were purchased and cultured as in a previous study [7]. Briefly, cells were cultured in α-MEM under standard conditions. An osteogenic induction medium (OIM) was supplemented for 15 days. Cells were pre-incubated with the anti-oxidant N-acetylcysteine (NAC, 2.5 mM, Sigma-Aldrich, St. Louis, MO, USA) overnight and then 7KC (Sigma-Aldrich, St. Louis, MO, USA) with different concentrations (0, 10, 20, and 40 μM) was added.
2.2. Determination of Cell Viability
Cell viability was measured by a CCK-8 kit (Solarbio, Beijing, China). A total of 1 × 104 cells/wells were inoculated on 96-well plates, and then 7KC was added in different concentrations (0, 2.5, 5, 10, 20, and 40 μM). After 24 h or 48 h, the medium was sucked out and cleaned two times with phosphate buffered solution (PBS) (Beyotime), and 10% CCK-8 medium was added to each well. The cells were incubated at 37 °C for 1 h, and then the absorbance was measured at a 450 nm wavelength under the guidelines of a microplate reader (Varioskanlux, Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Analysis of ROS Levels
The cells were inoculated with 1 × 105 cells/well in 24-well plates. Then, 500 μL of medium containing different drug concentrations was added to the plates, incubated for 24 h, and washed twice with PBS. The solution contained 10 μM of 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA, Solarbio, Beijing, China) in a serum-free medium and was incubated for 30 min in the dark. The cells were washed in PBS three times. Next, 4% paraformaldehyde (Beyotime, Shanghai, China) was added to the system and the cells were fixed at room temperature for 10 min. After the cells had been washed in PBS three times, 4’,6-diamidino-2-phenylindole (DAPI, Solarbio, Beijing, China) was used for staining for 10 min, the cells were washed three times with PBS again, and the fluorescence intensity was observed using a fluorescence microscope and photographed. ImageJ/Fiji, an open-source image processing package, was used to analyze the mean fluorescence intensity.
2.4. Protein Expression Detected by Western Blot
MC3T3-E1 cells (5 × 105 cells/wells) were cultured for 24 h before the experiment. Then, the cells were harvested and lysed on ice in RIPA Lysis Buffer (Beyotime) for 30 min. The lysates were collected after centrifugation and the protein concentration was determined. An electrophoretic separation on 25 µg of each sample was performed by a 10–12% SDS-PAGE gel they were then transferred onto PVDF membranes. After the membranes were incubated for 1 h in TBS plus 5% skim milk, the primary antibodies (all were diluted by 1:1000) anti-NOX4 (Affinity, Jiangsu, China), anti-runx2 (Affinity), anti-LC3-I/II (Affinity), anti-P62 (Affinity), anti-Bax (Affinity), anti-Bcl-2 (Affinity), anti-OPN (Beyotime), anti-Beclin 1 (Beyotime), anti-β-actin (Solarbio), and anti-Cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA) were incubated with the membranes at 4 °C overnight. HRP-labeled goat and rabbit secondary antibody (1:5000; Boster, Wuhan, China) was added and incubated at room temperature for 1 h. Finally, protein bands were measured and analyzed by enhanced chemiluminescence detection systems and Fiji ImageJ.
2.5. Apoptosis Analysis
The apoptosis rates were determined by employing the Annexin V apoptosis kit which was obtained from BD Biosciences (San Jose, CA, USA). The cells (5 × 105 cells/wells) were grown until confluence of about 80% was reached. After the proper treatment, the cells were collected. Under the guideline of the kit’s instructions, the cells were moved for apoptotic determination by flow cytometry (BD FACS Canto II, San Jose, CA, USA).
2.6. ALP Staining
MC3T3-E1 cells were inoculated with 1 × 105 cells/wells and cultured for 24 h before experiments. After 14 days of incubation, cells were collected and washed with the Beyotime. The ALP activity was then measured using an ALP activity assay kit (Beyotime). Finally, the absorption at the 405 nm wavelength was measured under the guidelines of a microplate reader (Varioskanlux, Thermo Fisher Scientific, Waltham, MA, USA).
2.7. Determination of MDA and SOD Activities
MC3T3-E1 cells were inoculated with 2 × 106 cells/wells for 24 h before experiments. Then, the cells were harvested and lysed on ice in RIPA Lysis Buffer (Beyotime) for 30 min. The lysates were collected after centrifugation, and the protein concentration was determined. Then, the SOD and MDA activities were measured using a SOD kit (Beijing Solarbio, China) and an MDA kit (Beijing Solarbio, China). Finally, the absorption of SOD and MDA at 560 nm and 532 nm, respectively, was measured under the guidelines of a microplate meter (Varioskanlux).
2.8. Mineralization Detection
MC3T3-E1 cells (1 × 105 cells/well) were cultured for 24 h. After 14 days of incubation, the cells were washed and fixed with 4% paraformaldehyde (Beyotime, Shanghai, China). Then, the cells were stained with alizarin red S (Solarbio, Beijing, China) at 37 °C for 30 min. After washing with PBS, orange calcified nodules were observed under an inverted microscope. Data were analyzed under the guideline of the kit’s instructions.
2.9. Statistical Analysis
Data were indicated as the mean ± standard deviation (SD). Statistical analysis was conducted by the GraphPad Prism v7 software (GraphPad Software Inc., La Jolla, CA, USA). The one-way analysis of variance (ANOVA) and subsequent Bonferroni’s multiple comparisons test were analyzed. p < 0.05 indicated a statistical difference.
3. Results
3.1. 7KC Inhibited the Viability of MC3T3-E1 Cells
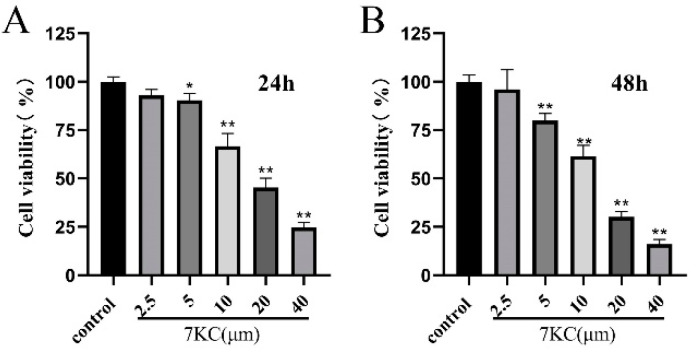
MC3T3-E1 cell viability at 24 h and 48 h, respectively, after the intervention of 7KC (0, 2.5, 5, 10, 20, or 40 μM), is shown in Figure 1A,B. At concentrations above 5 μM, 7KC significantly decreased MC3T3-E1 cell viability within 24 h or 48 h, compared with that in the control group.
Figure 1.
7KC inhibited MC3T3-E1 cell viability. (A,B) MC3T3-E1 cells were treated with 7KC (0, 2.5, 5, 10, 20, or 40 μM) for 24 h and 48 h. * p < 0.05; ** p < 0.01.
3.2. 7KC Inhibited the Osteogenic Differentiation of MC3T3-E1 Cells
In order to explore the biological actions of 7KC on MC3T3-E1 cell differentiation, ALP activity detection and mineral assays were conducted. Results showed that 7KC significantly attenuated ALP activity and alizarin red S staining (Figure 2A–C), suggesting that cell differentiation was inhibited. The expression of OPN (Figure 2D,E) and RUNX2 (Figure 2D,F) in MC3T3-E1 cells also decreased after 7KC administration. This suggested that 7KC could attenuate the osteogenic differentiation of MC3T3-E1 cells.
Figure 2.
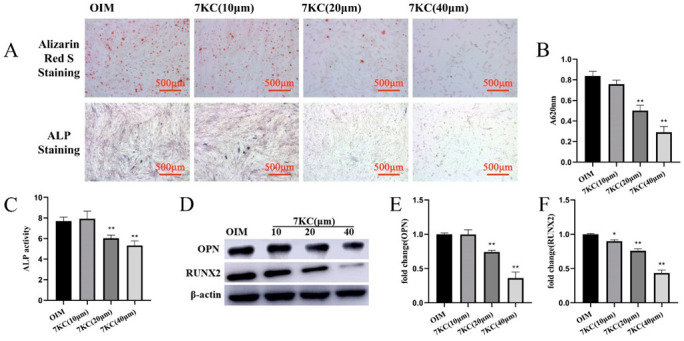
7KC inhibited the osteogenic differentiation of MC3T3-E1 cells. MC3T3-E1 cells were treated with 7KC (0, 10, 20, or 40 μM). (A–C) ALP activity detection and the mineral assays were performed (×50 magnification); ALP activity detection and quantitative analysis of the mineralized area. The protein expressions of OPN (D,E) and RUNX2 (D,F) were analyzed by Western blot. Data were analyzed and compared with the OIM group. * p < 0.05; ** p < 0.01. OIM, osteogenic induction medium.
3.3. 7KC Induced Oxiapoptophagy in MC3T3-E1 Cells
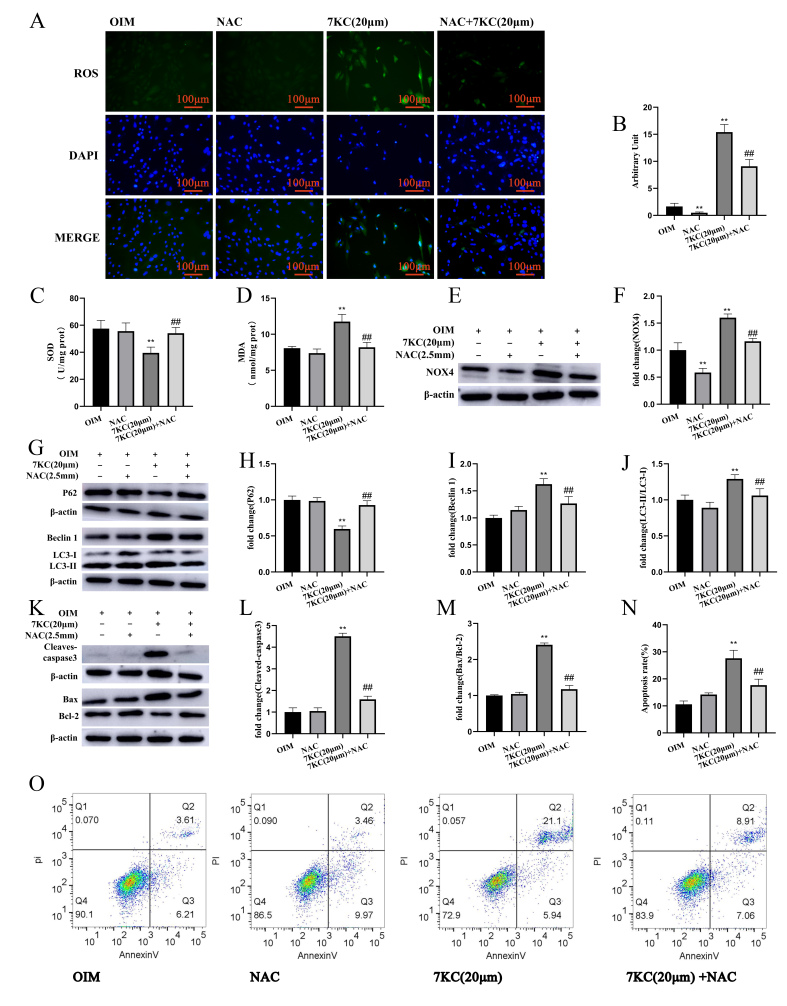
7KC is proven to produce oxiapoptophagy [19,21,31]. To explore the biological actions of 7KC on MC3T3-E1 cells, DCFH-DA staining was employed, and the results show that 7KC increased ROS levels in MC3T3-E1 cells in a concentration-dependent manner (Figure 3A,B). The SOD activity and MDA level of MC3T3-E1 cells treated by 7KC significantly decreased (Figure 3C,D). NADPH oxidase 4 (NOX4) plays an important role in the production of ROS, and it has been reported that 7KC can increase NOX4 expression in vascular smooth muscle cells [32,33]. Here, 7KC stimulated NOX4 expression in MC3T3-E1 cells (Figure 3E,F). In addition, 7KC increased the ratio of LC3-II/LC3-I protein expression, enhanced Beclin 1 expression, and significantly decreased P62 protein expression (Figure 3G–J). 7KC treatment also resulted in an increased apoptosis-related protein Bax/Bcl-2 ratio and cleaved caspase-3 protein expression (Figure 3K–M). Flow cytometry showed that 7KC could significantly increase the apoptosis of MC3T3-E1 cells (Figure 3N,O). Collectively, treatment with 7KC in MC3T3-E1 cells might induce oxidative stress, autophagy, and apoptosis, known as oxiapoptophagy.
Figure 3.
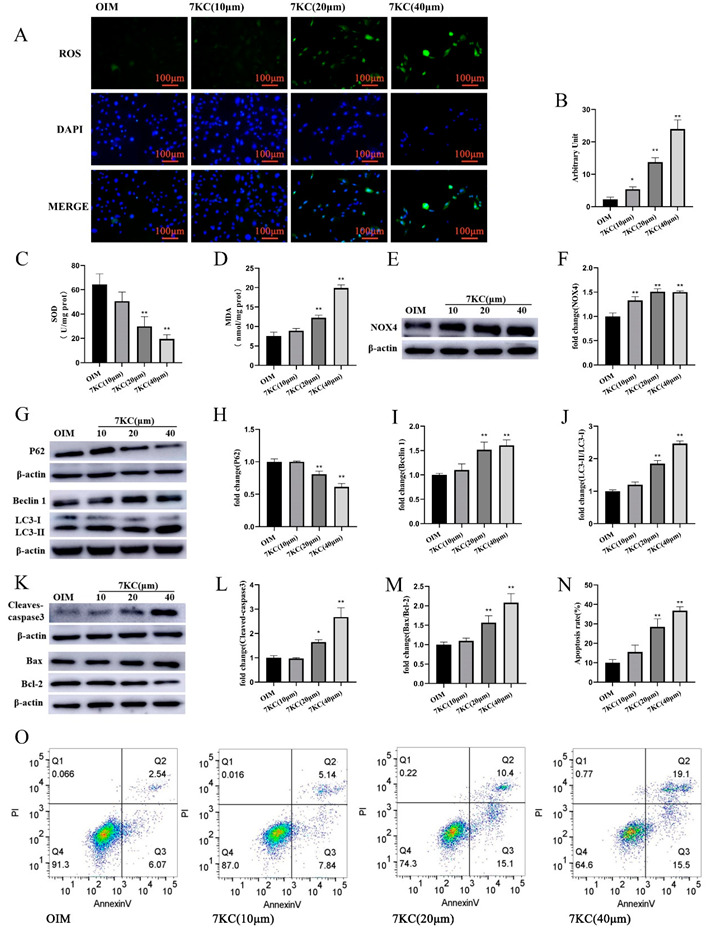
7KC regulated oxiapoptophagy in MC3T3-E1 cells. (A) Cells were prepared for staining with DCFH-DA and DAPI, respectively, and analyzed by a fluorescence microscope (×200 magnification). (B) Quantitative analysis of ROS production. (C,D) SOD activity detection, and MDA level measurement were conducted using the kits. (E,F) The protein expression of NOX4 was analyzed by Western blot. (G–J) The protein expression of LC3I/II, Beclin1, and P62 was detected. (K–M) The protein expression of Bax/Bcl-2 and cleaved caspase-3 was measured. (N,O) Apoptosis analysis was conducted by flow cytometry. Data were analyzed and compared with the OIM group. * p < 0.05; ** p < 0.01. OIM, osteogenic induction medium; NC, negative control.
3.4. NAC Antagonized 7KC-Induced Autophagy, Apoptosis, and Differentiation Inhibition of MC3T3-E1 Cells
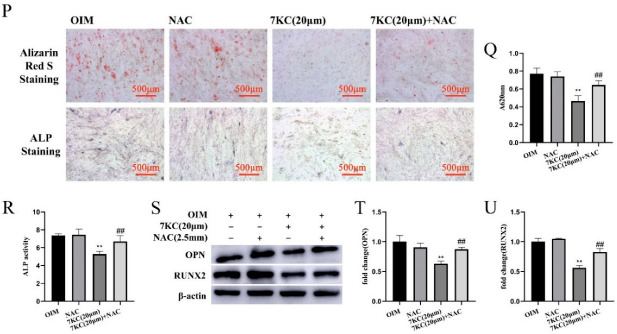
To explore the role of oxidative stress in 7KC-induced oxiapoptophagy and differentiation inhibition, a ROS scavenger (NAC) was added. The results show that NAC preincubation can decrease the ROS levels in 7KC-induced MC3T3-E1 cells, decrease the NOX4 protein expression, increase the SOD activity, and decrease the MDA production (Figure 4A–F). NAC can ameliorate the increased ratio of LC3-II/LC3-I, increase the expression of Beclin 1, and decrease the expression of P62 induced by 7KC (Figure 4G–J). NAC preincubation can also decrease the protein expression of BAX and Cleaved caspase-3 and increase the protein expression of anti-apoptotic protein Bcl-2 (Figure 4K–M). Flow cytometry detection results indicate that NAC preincubation can significantly reduce the apoptosis of 7KC-treated MC3T3-E1 cells (Figure 4N,O). Furthermore, NAC improves the osteogenic differentiation inhibition of MC3T3-E1 cells by 7KC, as shown by the increased activity of alkaline phosphatase and formation of mineralization (Figure 4P-R) as well as the increased protein expression of OPN (Figure 4S,T) and RUNX2 (Figure 4S,U).
Figure 4.
NAC inhibited oxiapoptophagy and differentiation in 7KC-induced MC3T3-E1 cells. (A) Cells were prepared for staining with DCFH-DA and DAPI, respectively, and analyzed by a fluorescence microscope (×200 magnification). (B) The production of ROS was analyzed by fluorescence intensity. (C,D) SOD activity detection and MDA level measurement were conducted using the kits. (E,F) The protein expression of NOX4 was analyzed by Western blot. (G–J) The protein expression of LC3I/II, Beclin1, and P62 was detected. (K–M) The protein expression of Bax/Bcl-2 and cleaved caspase-3 was measured. (N,O) Apoptosis analysis was conducted by flow cytometry. (P–R) ALP activity detection and the mineral assays were performed (×50 magnification) and analyzed. The protein expression of OPN (S,T) and RUNX2 (S,U) was determined by Western blotting. Data were analyzed for comparison with the OIM group. ** p < 0.01. Compared with the 7KC (20 μM) group, ## p < 0.01. OIM, osteogenic induction medium; NAC, 2.5 mM NAC.
4. Discussion
Previous studies indicate a correlation of AS with an enhanced fracture risk for bone [5]. However, the biological action of oxysterol, a critical regulator implicated in the pathophysiological process of AS, on bone metabolism has not been fully elucidated. Current results indicate that 7KC lowered the viability, increased the generation of ROS, induced oxiapoptophagy, and attenuated the osteogenic differentiation in pre-osteoblast MC3T3-E1 cells. In addition, pre-incubation with NAC strongly attenuates 7KC-induced pathological changes.
Oxysterols are considered to be a class of factors associated with human physiological and pathological processes. For example, oxysterols were recently demonstrated to function as the ligands that interact with the liver X receptors (LXRα/β), which are involved in the regulation of cholesterol metabolism by mediating the transcription of specific genes, such as ABCA1, ABCG1, and SREBP-1c [34]. Oxysterols may exhibit pro-apoptotic and pro-autophagic activities, depending on the difference in oxysterol types, cell lines, and treatment concentrations [35]. The main types of cell death induced by oxysterols, apoptosis, autophagy, and necrosis, have been studied [10,36]. However, cell necrosis may only be induced by high concentrations of oxysterols in some cell lines [37]. It has been demonstrated that 27-hydroxycholesterol may decrease osteoblast differentiation, increase osteoclastogenesis, and increase bone resorption by interacting with estrogen receptors and LXRs [38]. 25-Hydroxycholesterol is reported to increase apoptosis, promote osteogenic differentiation, and induce vascular calcification in vascular smooth muscle cells (VSMCs) by triggering endoplasmic reticulum stress [39]. 7KC is reported to promote cell apoptosis in mesenchymal stem cells derived from adipose tissue [40]. Similarly, 7KC can induce oxiapoptophagy in BMSCs from patients with acute myeloid leukemia by mediating the Sonic Hedgehog pathway [19]. However, whether 7KC affects osteogenic differentiation by inducing oxiapoptophagy is still unclear.
To investigate the toxicity of 7KC on MC3T3-E1 cells, we measured the effect of 7KC on cell viability. We found that 7KC exhibited cytotoxicity at a concentration as low as 5 μM. At concentrations of 20 μM and 40 μM, 7KC induced apoptosis by 26.3% and 35.6%, respectively. It is reported that 7KC can induce cytotoxicity at a concentration range of 5 to 80 μg/mL in different cell lines in 48 h [41]. In U937 cells, 7KC induces cell apoptosis with maximal proportions (40 ± 5%) at the concentration of 40 μg/mL in 30 h [42]. In VSMCs, 7KC promotes cell apoptosis at a high concentration of 30 μM in 6 days [43]. In human umbilical venous endothelial cells, 7KC at concentrations of 10 μg/mL and 20 μg/mL does not produce effects on cell apoptosis. At a concentration of 40 μg/mL, however, 7KC could significantly induce DNA fragmentation in 20 h [44].
Furthermore, we found that 7KC induced oxiapoptophagy in MC3T3-E1 by up-regulating NOX4 expression and increasing intracellular ROS, thus reducing the survival rate of MC3T3-E1 cells. The presence of the ROS scavenger NAC could compromise these alterations. ROS are produced in cells under various stresses [45]. However, the excessive production of ROS can destroy the balance between oxidant and antioxidant systems and altered ROS generation can lead to the oxidative-stress-induced OP by promoting lipid peroxidation, reducing antioxidant enzyme expression, inducing osteoblast apoptosis, and inhibiting bone formation [46,47,48,49]. It was initially found that 7KC can increase the expression of NOX4, contributing to the overproduction of ROS in human aortic smooth muscle cells [50]. Consistently, 7KC enhances ROS production, promotes the translocation of NOX components from the cytosol to the cellular membrane, inhibits HO-1 expression, and stimulates lysozyme release in human neutrophils [51]. In human red blood cells, 7KC is reported to activate NOX and induce eryptosis by mediating Rac GTPase and PKCζ [52]. NOX4 is an important source of ROS production and is highly expressed in MC3T3-E1 cells [53]. NOX4 has been involved in the glucocorticoid-induced apoptosis of MC3T3-E1 cells [54]. It was found that simvastatin can protect osteoblasts against H2O2-induced oxidative damage by inhibiting NOX4 expression [55]. In addition, iron overload can stimulate ROS generation, enhance NOX4 expression, and induce apoptosis in MC3T3-E1 cells. NAC can scavenge ROS, inhibit NOX4 expression, and ameliorate iron-overload-induced apoptosis [56].
Oxidative stress can inhibit osteoblast differentiation. Although bone and vascular cells share common processes of osteogenesis and mineralization, reduced bone mineralization (osteoporosis) is associated with increased calcification in humans and mice [57,58]. These paradoxical results in skeletal and vascular niches suggest that different internal signaling pathways orchestrated by tissue-specific microenvironments control the production of mineralization in different ways [59]. Mechanically, this discrepancy might be related to the totally different actions of oxidative stress on RUNX2 expression, which is an important regulator of osteogenic differentiation and mineralization in osteocytes and VSMC [57,60]. Growing evidence demonstrates that oxysterols play a critical role in vascular calcification [6]. Studies have shown that 7KC is also highly involved in PI-induced calcification of vascular smooth muscle cells by inducing lysosomal dysfunction and apoptosis [43,61,62]. A study found that 7KC promotes VSMC cell calcification through lysosomal dysfunction-dependent oxidative stress [43]. Additionally, 7KC may play a key pathogenic role in atherosclerotic plaque calcification development [63]. Our study showed that 7KC inhibited the ALP activity and OPN and RUNX2 expression, thereby inhibiting the osteogenic differentiation of MC3T3-E1 cells. However, these effects could be attenuated by NAC.
Bone homeostasis is maintained by the balance between bone formation and bone resorption. Osteoblasts are differentiated from BMSCs and exhibit critical roles in bone formation. Osteogenic differentiation is complex and orchestrated by various signaling pathways, such as Wnt/β-catenin, TGFβ/BMP, MAPK, and Notch signaling pathways [64]. Early osteogenic biomarkers, such as ALP, RUNX2, and SP7, and late biomarkers, such as OPN and OCN, have been used to evaluate the biological effects of potential candidates [65]. RUNX2 is an important transcription factor mastering osteogenic differentiation that regulates the transcriptional expression of these biomarkers [66,67]. ALP, RUNX2, and OCN build a transcriptional network, governing osteogenesis. In the current study, these biomarkers were purposely employed to evaluate the effects of 7KC on osteogenic differentiation. However, their potential mechanisms still need further investigation. Additionally, although our study shows that 7KC can induce MC3T3-E1 oxiapoptophagy and osteogenic differentiation inhibition, these processes are related to the excessive production of ROS. However, the relationship between oxidative stress, apoptosis, autophagy, and osteogenic differentiation was not addressed in detail. More careful studies are required in the future.
5. Conclusions
7KC, an oxysterol formed by autoxidation, is involved in the pathophysiological processes of many diseases, such as cardiovascular, neuronal, and retinal diseases. In this study, we observed that 7KC significantly enhanced oxidative stress, induced autophagy, and promoted apoptosis in MC3T3-E1 cells. Meanwhile, 7KC attenuated the expression of osteogenic biomarkers, such as ALP, RUNX2, and OPN, indicating inhibitory activity against osteogenic differentiation. It is important to explore the association of oxysterols, particularly 7KC, with the metabolism of osteoblasts/osteoclasts in bone tissues. Oxysterols can be potent intermediates for the synthesis of steroid hormones or bile acids. Additionally, exogenous or endogenous oxidized cholesterol products are easily accessible. The accumulation of these oxysterols, such as 7KC, may exhibit adverse effects. It is essential to understand the underlying regulatory mechanism of oxysterols as they might become potential targets for the therapeutic management of diseases, including OP.
Acknowledgments
We thank Wei Li, who is working in the Department of Rehabilitation at Gannan Medical University, for the help in data analysis.
Author Contributions
Conceptualization, L.W.; methodology, L.W.; software, J.O.; validation, J.H., Q.Z., S.Z., L.L., W.S. and Z.C.; formal analysis, J.H., Q.Z., S.Z., L.L., W.S. and Z.C.; investigation, J.O., Y.X. and Q.R.; resources, L.W.; data curation, L.W.; writing—original draft preparation, J.O., Y.X. and Q.R.; writing—review and editing, J.H., Q.Z., S.Z., L.L., W.S. and Z.C.; visualization, J.H., Q.Z., S.Z., L.L., W.S. and Z.C.; supervision, L.W.; project administration, L.W.; funding acquisition, L.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Animal Care and Use Committee of Gannan Medical University (protocol code GMU202011, 16 March 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was financially supported by NSFC, grant number 82060407, and Jiangxi Provincial Natural Science, grant number 20212ACB206002.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eun S.Y., Cheon Y.H., Park G.D., Chung C.H., Lee C.H., Kim J.Y., Lee M.S. Anti-Osteoporosis Effects of the Eleutherococcus senticosus, Achyranthes japonica, and Atractylodes japonica Mixed Extract Fermented with Nuruk. Nutr. 2021;13:3904. doi: 10.3390/nu13113904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Che Y., Yang J., Tang F., Wei Z., Chao Y., Li N., Li H., Wu S., Dong X. New Function of Cholesterol Oxidation Products Involved in Osteoporosis Pathogenesis. Int. J. Mol. Sci. 2022;23:2020. doi: 10.3390/ijms23042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z., Xie Z., Sun J., Huang S., Chen Y., Li C., Sun X., Xia B., Tian L., Guo C., et al. Gut Microbiome Reveals Specific Dysbiosis in Primary Osteoporosis. Front. Cell. Infect. Microbiol. 2020;10:160. doi: 10.3389/fcimb.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang T.L., Shen H., Liu A., Dong S.S., Zhang L., Deng F.Y., Zhao Q., Deng H.W. A road map for understanding molecular and genetic determinants of osteoporosis. Nat. Rev. Endocrinol. 2020;16:91–103. doi: 10.1038/s41574-019-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian L., Yu X. Lipid metabolism disorders and bone dysfunction--interrelated and mutually regulated (review) Mol. Med. Rep. 2015;12:783–794. doi: 10.3892/mmr.2015.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gargiulo S., Gamba P., Testa G., Leonarduzzi G., Poli G. The role of oxysterols in vascular ageing. J. Physiol. 2016;594:2095–2113. doi: 10.1113/JP271168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng T., Ji G., Chen J., Lai J., Liu T., Mo J., Jin Q. MicroRNA-142 protects MC3T3-E1 cells against high glucose-induced apoptosis by targeting β-catenin. Exp. Ther. Med. 2020;20:125. doi: 10.3892/etm.2020.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas C., Leleu D., Masson D. Cholesterol and HIF-1alpha: Dangerous Liaisons in Atherosclerosis. Front. Immunol. 2022;13:868958. doi: 10.3389/fimmu.2022.868958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luu W., Sharpe L.J., Capell-Hattam I., Gelissen I.C., Brown A.J. Oxysterols: Old Tale, New Twists. Annu. Rev. Pharmacol. Toxicol. 2016;56:447–467. doi: 10.1146/annurev-pharmtox-010715-103233. [DOI] [PubMed] [Google Scholar]
- 10.de Freitas F.A., Levy D., Zarrouk A., Lizard G., Bydlowski S.P. Impact of Oxysterols on Cell Death, Proliferation, and Differentiation Induction: Current Status. Cells. 2021;10:2301. doi: 10.3390/cells10092301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vejux A., Abed-Vieillard D., Hajji K., Zarrouk A., Mackrill J.J., Ghosh S., Nury T., Yammine A., Zaibi M., Mihoubi W., et al. 7-Ketocholesterol and 7β-hydroxycholesterol: In vitro and animal models used to characterize their activities and to identify molecules preventing their toxicity. Biochem. Pharmacol. 2020;173:113648. doi: 10.1016/j.bcp.2019.113648. [DOI] [PubMed] [Google Scholar]
- 12.Luchetti F., Crinelli R., Cesarini E., Canonico B., Guidi L., Zerbinati C., Di Sario G., Zamai L., Magnani M., Papa S., et al. Endothelial cells, endoplasmic reticulum stress and oxysterols. Redox. Biol. 2017;13:581–587. doi: 10.1016/j.redox.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilar-Ballester M., Herrero-Cervera A., Vinue A., Martinez-Hervas S., Gonzalez-Navarro H. Impact of Cholesterol Metabolism in Immune Cell Function and Atherosclerosis. Nutrients. 2020;12:2021. doi: 10.3390/nu12072021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poli G., Sottero B., Gargiulo S., Leonarduzzi G. Cholesterol oxidation products in the vascular remodeling due to atherosclerosis. Mol Asp. Med. 2009;30:180–189. doi: 10.1016/j.mam.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Negre-Salvayre A., Auge N., Camare C., Bacchetti T., Ferretti G., Salvayre R. Dual signaling evoked by oxidized LDLs in vascular cells. Free. Radic. Biol. Med. 2017;106:118–133. doi: 10.1016/j.freeradbiomed.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Gargiulo S., Testa G., Gamba P., Staurenghi E., Poli G., Leonarduzzi G. Oxysterols and 4-hydroxy-2-nonenal contribute to atherosclerotic plaque destabilization. Free. Radic. Biol. Med. 2017;111:140–150. doi: 10.1016/j.freeradbiomed.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Ghzaiel I., Sassi K., Zarrouk A., Ghosh S., Dias I.H.K., Nury T., Ksila M., Essadek S., Tahri Joutey M., Brahmi F., et al. Sources of 7-ketocholesterol, metabolism and inactivation strategies: Food and biomedical applications. Redox. Exp. Med. 2022;2022:R40–R56. doi: 10.1530/REM-22-0005. [DOI] [Google Scholar]
- 18.Risso D., Leoni V., Canzoneri F., Arveda M., Zivoli R., Peraino A., Poli G., Menta R. Presence of cholesterol oxides in milk chocolates and their correlation with milk powder freshness. PLoS ONE. 2022;17:e0264288. doi: 10.1371/journal.pone.0264288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paz J.L., Levy D., Oliveira B.A., de Melo T.C., de Freitas F.A., Reichert C.O., Rodrigues A., Pereira J., Bydlowski S.P. 7-Ketocholesterol Promotes Oxiapoptophagy in Bone Marrow Mesenchymal Stem Cell from Patients with Acute Myeloid Leukemia. Cells. 2019;8:482. doi: 10.3390/cells8050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosa-Fernandes L., Maselli L.M.F., Maeda N.Y., Palmisano G., Bydlowski S.P. Outside-in, inside-out: Proteomic analysis of endothelial stress mediated by 7-ketocholesterol. Chem. Phys. Lipids. 2017;207:231–238. doi: 10.1016/j.chemphyslip.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Nury T., Zarrouk A., Yammine A., Mackrill J.J., Vejux A., Lizard G. Oxiapoptophagy: A type of cell death induced by some oxysterols. Br. J. Pharm. 2021;178:3115–3123. doi: 10.1111/bph.15173. [DOI] [PubMed] [Google Scholar]
- 22.Nury T., Yammine A., Ghzaiel I., Sassi K., Zarrouk A., Brahmi F., Samadi M., Rup-Jacques S., Vervandier-Fasseur D., Pais de Barros J.P., et al. Attenuation of 7-ketocholesterol- and 7beta-hydroxycholesterol-induced oxiapoptophagy by nutrients, synthetic molecules and oils: Potential for the prevention of age-related diseases. Ageing. Res. Rev. 2021;68:101324. doi: 10.1016/j.arr.2021.101324. [DOI] [PubMed] [Google Scholar]
- 23.Li B., Yang J., Lu Z., Liu B., Liu F. A study on the mechanism of rapamycin mediating the sensitivity of pancreatic cancer cells to cisplatin through PI3K/AKT/mTOR signaling pathway. JBUON. 2019;24:739–745. [PubMed] [Google Scholar]
- 24.Nury T., Zarrouk A., Mackrill J.J., Samadi M., Durand P., Riedinger J.M., Doria M., Vejux A., Limagne E., Delmas D., et al. Induction of oxiapoptophagy on 158N murine oligodendrocytes treated by 7-ketocholesterol-, 7β-hydroxycholesterol-, or 24(S)-hydroxycholesterol: Protective effects of α-tocopherol and docosahexaenoic acid (DHA; C22:6 n-3) Steroids. 2015;99:194–203. doi: 10.1016/j.steroids.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Ravi S., Duraisamy P., Krishnan M., Martin L.C., Manikandan B., Raman T., Sundaram J., Arumugam M., Ramar M. An insight on 7- ketocholesterol mediated inflammation in atherosclerosis and potential therapeutics. Steroids. 2021;172:108854. doi: 10.1016/j.steroids.2021.108854. [DOI] [PubMed] [Google Scholar]
- 26.Khatib S., Vaya J. Oxysterols and symptomatic versus asymptomatic human atherosclerotic plaque. Biochem. Biophys. Res. Commun. 2014;446:709–713. doi: 10.1016/j.bbrc.2013.12.116. [DOI] [PubMed] [Google Scholar]
- 27.Tani M., Kamata Y., Deushi M., Osaka M., Yoshida M. 7-Ketocholesterol enhances leukocyte adhesion to endothelial cells via p38MAPK pathway. PLoS ONE. 2018;13:e0200499. doi: 10.1371/journal.pone.0200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao X., Zhong J., Maiseyeu A., Gopalakrishnan B., Villamena F.A., Chen L.C., Harkema J.R., Sun Q., Rajagopalan S. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ. Res. 2014;115:770–780. doi: 10.1161/CIRCRESAHA.115.304666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murdolo G., Piroddi M., Tortoioli C., Bartolini D., Schmelz M., Luchetti F., Canonico B., Papa S., Zerbinati C., Iuliano L., et al. Free Radical-derived Oxysterols: Novel Adipokines Modulating Adipogenic Differentiation of Adipose Precursor Cells. J. Clin. Endocrinol. Metab. 2016;101:4974–4983. doi: 10.1210/jc.2016-2918. [DOI] [PubMed] [Google Scholar]
- 30.Li G., Sul O.J., Yu R., Choi H.S. 7-Ketocholesterol-Induced Micro-RNA-107-5p Increases Number and Activity of Osteoclasts by Targeting MKP1. Int. J. Mol. Sci. 2022;23:3697. doi: 10.3390/ijms23073697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yammine A., Zarrouk A., Nury T., Vejux A., Latruffe N., Vervandier-Fasseur D., Samadi M., Mackrill J.J., Greige-Gerges H., Auezova L., et al. Prevention by Dietary Polyphenols (Resveratrol, Quercetin, Apigenin) Against 7-Ketocholesterol-Induced Oxiapoptophagy in Neuronal N2a Cells: Potential Interest for the Treatment of Neurodegenerative and Age-Related Diseases. Cells. 2020;9:2346. doi: 10.3390/cells9112346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piera-Velazquez S., Jimenez S.A. Oxidative Stress Induced by Reactive Oxygen Species (ROS) and NADPH Oxidase 4 (NOX4) in the Pathogenesis of the Fibrotic Process in Systemic Sclerosis: A Promising Therapeutic Target. J. Clin. Med. 2021;10:4791. doi: 10.3390/jcm10204791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He C., Zhu H., Zhang W., Okon I., Wang Q., Li H., Le Y.Z., Xie Z. 7-Ketocholesterol induces autophagy in vascular smooth muscle cells through Nox4 and Atg4B. Am. J. Pathol. 2013;183:626–637. doi: 10.1016/j.ajpath.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma L.Q., Nelson E.R. Oxysterols and nuclear receptors. Mol. Cell. Endocrinol. 2019;484:42–51. doi: 10.1016/j.mce.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Levy D., Correa de Melo T., Ohira B.Y., Fidelis M.L., Ruiz J.L.M., Rodrigues A., Bydlowski S.P. Oxysterols selectively promote short-term apoptosis in tumor cell lines. Biochem. Biophys. Res. Commun. 2018;505:1043–1049. doi: 10.1016/j.bbrc.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Green D.R., Llambi F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015;7:80. doi: 10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vejux A., Malvitte L., Lizard G. Side effects of oxysterols: Cytotoxicity, oxidation, inflammation, and phospholipidosis. Braz. J. Med. Biol. Res. 2008;41:545–556. doi: 10.1590/S0100-879X2008000700001. [DOI] [PubMed] [Google Scholar]
- 38.Nelson E.R., DuSell C.D., Wang X., Howe M.K., Evans G., Michalek R.D., Umetani M., Rathmell J.C., Khosla S., Gesty-Palmer D., et al. The oxysterol, 27-hydroxycholesterol, links cholesterol metabolism to bone homeostasis through its actions on the estrogen and liver X receptors. Endocrinol. 2011;152:4691–4705. doi: 10.1210/en.2011-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong Q., Chen Y., Liu W., Liu X., Chen A., Yang X., Li Y., Wang S., Fu M., Ou J.S., et al. 25-Hydroxycholesterol promotes vascular calcification via activation of endoplasmic reticulum stress. Eur. J. Pharmacol. 2020;880:173165. doi: 10.1016/j.ejphar.2020.173165. [DOI] [PubMed] [Google Scholar]
- 40.Levy D., Ruiz J.L., Celestino A.T., Silva S.F., Ferreira A.K., Isaac C., Bydlowski S.P. Short-term effects of 7-ketocholesterol on human adipose tissue mesenchymal stem cells in vitro. Biochem. Biophys. Res. Commun. 2014;446:720–725. doi: 10.1016/j.bbrc.2014.01.132. [DOI] [PubMed] [Google Scholar]
- 41.Lizard G., Monier S., Cordelet C., Gesquière L., Deckert V., Gueldry S., Lagrost L., Gambert P. Characterization and comparison of the mode of cell death, apoptosis versus necrosis, induced by 7beta-hydroxycholesterol and 7-ketocholesterol in the cells of the vascular wall. Arterioscler. Thromb. Vasc. Biol. 1999;19:1190–1200. doi: 10.1161/01.ATV.19.5.1190. [DOI] [PubMed] [Google Scholar]
- 42.Miguet C., Monier S., Bettaieb A., Athias A., Besséde G., Laubriet A., Lemaire S., Néel D., Gambert P., Lizard G. Ceramide generation occurring during 7beta-hydroxycholesterol- and 7-ketocholesterol-induced apoptosis is caspase independent and is not required to trigger cell death. Cell. Death. Differ. 2001;8:83–99. doi: 10.1038/sj.cdd.4400792. [DOI] [PubMed] [Google Scholar]
- 43.Sudo R., Sato F., Azechi T., Wachi H. 7-Ketocholesterol-induced lysosomal dysfunction exacerbates vascular smooth muscle cell calcification via oxidative stress. Genes Cells. 2015;20:982–991. doi: 10.1111/gtc.12301. [DOI] [PubMed] [Google Scholar]
- 44.Lemaire S., Lizard G., Monier S., Miguet C., Gueldry S., Volot F., Gambert P., Néel D. Different patterns of IL-1beta secretion, adhesion molecule expression and apoptosis induction in human endothelial cells treated with 7alpha-, 7beta-hydroxycholesterol, or 7-ketocholesterol. FEBS Lett. 1998;440:434–439. doi: 10.1016/S0014-5793(98)01496-3. [DOI] [PubMed] [Google Scholar]
- 45.Kiffin R., Bandyopadhyay U., Cuervo A.M. Oxidative stress and autophagy. Antioxid. Redox. Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 46.Schoppa A.M., Chen X., Ramge J.M., Vikman A., Fischer V., Haffner-Luntzer M., Riegger J., Tuckermann J., Scharffetter-Kochanek K., Ignatius A. Osteoblast lineage Sod2 deficiency leads to an osteoporosis-like phenotype in mice. Dis. Model. Mech. 2022 doi: 10.1242/dmm.049392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y.F., Chang Y.Y., Zhang X.M., Gao M.T., Zhang Q.L., Li X., Zhang L., Yao W.F. Salidroside protects against osteoporosis in ovariectomized rats by inhibiting oxidative stress and promoting osteogenesis via Nrf2 activation. Phytomedicine. 2022;99:154020. doi: 10.1016/j.phymed.2022.154020. [DOI] [PubMed] [Google Scholar]
- 48.Badila A.E., Radulescu D.M., Ilie A., Niculescu A.G., Grumezescu A.M., Radulescu A.R. Bone Regeneration and Oxidative Stress: An Updated Overview. Antioxidants. 2022;11:318. doi: 10.3390/antiox11020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anagnostis P., Florentin M., Livadas S., Lambrinoudaki I., Goulis D.G. Bone Health in Patients with Dyslipidemias: An Underestimated Aspect. Int. J. Mol. Sci. 2022;23:1639. doi: 10.3390/ijms23031639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedruzzi E., Guichard C., Ollivier V., Driss F., Fay M., Prunet C., Marie J.C., Pouzet C., Samadi M., Elbim C., et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alba G., Reyes-Quiróz M.E., Sáenz J., Geniz I., Jiménez J., Martín-Nieto J., Pintado E., Sobrino F., Santa-María C. 7-Keto-cholesterol and 25-hydroxy-1 cholesterol rapidly enhance ROS production in human neutrophils. Eur. J. Nutr. 2016;55:2485–2492. doi: 10.1007/s00394-015-1142-4. [DOI] [PubMed] [Google Scholar]
- 52.Attanzio A., Frazzitta A., Cilla A., Livrea M.A., Tesoriere L., Allegra M. 7-Keto-Cholesterol and Cholestan-3beta, 5alpha, 6beta-Triol Induce Eryptosis through Distinct Pathways Leading to NADPH Oxidase and Nitric Oxide Synthase Activation. Cell. Physiol. Biochem. 2019;53:933–947. doi: 10.33594/000000186. [DOI] [PubMed] [Google Scholar]
- 53.Fan S., Pan H., Huang J., Lei Z., Liu J. Hyperoside exerts osteoprotective effect on dexamethasone-induced osteoblasts by targeting NADPH Oxidase 4 (NOX4) to inhibit the reactive oxygen species (ROS) accumulation and activate c-Jun N-terminal kinase (JNK) pathway. Bioengineered. 2022;13:8657–8666. doi: 10.1080/21655979.2022.2054499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai S.C., Xu Q., Li H., Qin Y.F., Song L.C., Wang C.G., Cui W.H., Zheng Z., Yan D.W., Li Z.J., et al. NADPH Oxidase Isoforms Are Involved in Glucocorticoid-Induced Preosteoblast Apoptosis. Oxid. Med. Cell. Longev. 2019;2019:9192413. doi: 10.1155/2019/9192413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang W., Shang W.L., Li D.H., Wu W.W., Hou S.X. Simvastatin protects osteoblast against H2O2-induced oxidative damage via inhibiting the upregulation of Nox4. Mol. Cell. Biochem. 2012;360:71–77. doi: 10.1007/s11010-011-1045-5. [DOI] [PubMed] [Google Scholar]
- 56.Xu G., Li X., Zhu Z., Wang H., Bai X. Iron Overload Induces Apoptosis and Cytoprotective Autophagy Regulated by ROS Generation in Mc3t3-E1 Cells. Biol. Trace. Elem. Res. 2021;199:3781–3792. doi: 10.1007/s12011-020-02508-x. [DOI] [PubMed] [Google Scholar]
- 57.Byon C.H., Javed A., Dai Q., Kappes J.C., Clemens T.L., Darley-Usmar V.M., McDonald J.M., Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parhami F., Morrow A.D., Balucan J., Leitinger N., Watson A.D., Tintut Y., Berliner J.A., Demer L.L. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler. Thromb. Vasc. Biol. 1997;17:680–687. doi: 10.1161/01.ATV.17.4.680. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y., Zhao X., Wu H. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arterioscler. Thromb. Vasc. Biol. 2020;40:1078–1093. doi: 10.1161/ATVBAHA.120.313131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deng L., Huang L., Sun Y., Heath J.M., Wu H., Chen Y. Inhibition of FOXO1/3 promotes vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2015;35:175–183. doi: 10.1161/ATVBAHA.114.304786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saito E., Wachi H., Sato F., Seyama Y. 7-ketocholesterol, a major oxysterol, promotes pi-induced vascular calcification in cultured smooth muscle cells. J. Atheroscler. Thromb. 2008;15:130–137. doi: 10.5551/jat.E556. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe Y., Yamaguchi T., Ishihara N., Nakamura S., Tanaka S., Oka R., Imamura H., Sato Y., Ban N., Kawana H., et al. 7-Ketocholesterol induces ROS-mediated mRNA expression of 12-lipoxygenase, cyclooxygenase-2 and pro-inflammatory cytokines in human mesangial cells: Potential role in diabetic nephropathy. Prostaglandins. Other. Lipid. Mediat. 2018;134:16–23. doi: 10.1016/j.prostaglandins.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Anderson A., Campo A., Fulton E., Corwin A., Jerome W.G., 3rd, O’Connor M.S. 7-Ketocholesterol in disease and aging. Redox. Biol. 2020;29:101380. doi: 10.1016/j.redox.2019.101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morsczeck C. Mechanisms during Osteogenic Differentiation in Human Dental Follicle Cells. Int. J. Mol. Sci. 2022;23:5945. doi: 10.3390/ijms23115945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y., Xia C., Chen Y., Jiang T., Hu Y., Gao Y. Resveratrol Synergistically Promotes BMP9-Induced Osteogenic Differentiation of Mesenchymal Stem Cells. Stem. Cells. Int. 2022;2022:8124085. doi: 10.1155/2022/8124085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zha K., Tian Y., Panayi A.C., Mi B., Liu G. Recent Advances in Enhancement Strategies for Osteogenic Differentiation of Mesenchymal Stem Cells in Bone Tissue Engineering. Front. Cell Dev. Biol. 2022;10:824812. doi: 10.3389/fcell.2022.824812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim H.J., Kim W.J., Ryoo H.M. Post-Translational Regulations of Transcriptional Activity of RUNX2. Mol. Cells. 2020;43:160–167. doi: 10.14348/molcells.2019.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.