Abstract
Simple Summary
Neuroblastoma is one of the most diffuse and the deadliest cancer in children. While many advances have been made in the last few decades to improve patients’ outcome, high-risk neuroblastoma (HR-NB) still shows a very aggressive pattern of development and poor prognosis, with only a 50% chance of 5-year survival. Moreover, while many factors contribute to defining the high-risk condition, MYCN status is well established as the major element in pathology disclosure. The aim of this review is to describe the current knowledge in the diagnosis, prognosis and therapeutic approaches of HR-NB, particularly in relation to MYCN. The review highlights how MYCN influences the HR-NB scenario and the new therapeutic approaches that are currently proposed to target it, in consideration of MYCN as a highly relevant target for HR-NB patient management.
Abstract
Among childhood cancers, neuroblastoma is the most diffuse solid tumor and the deadliest in children. While to date, the pathology has become progressively manageable with a significant increase in 5-year survival for its less aggressive form, high-risk neuroblastoma (HR-NB) remains a major issue with poor outcome and little survivability of patients. The staging system has also been improved to better fit patient needs and to administer therapies in a more focused manner in consideration of pathology features. New and improved therapies have been developed; nevertheless, low efficacy and high toxicity remain a staple feature of current high-risk neuroblastoma treatment. For this reason, more specific procedures are required, and new therapeutic targets are also needed for a precise medicine approach. In this scenario, MYCN is certainly one of the most interesting targets. Indeed, MYCN is one of the most relevant hallmarks of HR-NB, and many studies has been carried out in recent years to discover potent and specific inhibitors to block its activities and any related oncogenic function. N-Myc protein has been considered an undruggable target for a long time. Thus, many new indirect and direct approaches have been discovered and preclinically evaluated for the interaction with MYCN and its pathways; a few of the most promising approaches are nearing clinical application for the investigation in HR-NB.
Keywords: MYCN, high-risk neuroblastoma, pediatric tumor, neuroblastoma therapeutics, undruggable targets
1. Introduction
Neuroblastoma is one of the most diffuse neoplasia in children (<14 years), preceded only by leukemia, lymphomas and central nervous system neoplasms. In 2021, a total of 10,500 children in the USA were diagnosed with cancer, and 6% of these cases were neuroblastoma [1]. Notably, in the last fifteen years the 5-year survival rate has increased from 72% to 81%, highlighting an important advancement in the treatment and therapy of the pathology [1,2], but the problem still remains. In particular, high-risk neuroblastoma (HR-NB) patients, accounting for about half of the overall cases, can be considered more fragile and meaningful due to the poor outcome associated with this condition. In fact, patients affected by HR-NB normally show a strong reduction in 5-year survival with a mean of 50%, in comparison with low and intermediate-risk patients that reach about 90–85% [3]. HR-NB patients normally show mutations in major risk biomarkers such as MYCN amplification [4] and common segmental chromosomal aberration (SCA) [5]. The age of the patient at the pathology diagnosis is considered another important factor, and for this reason a proper evaluation of the tumor is fundamental not only for the treatment of the pathology, but also for better outcomes assessment.
The neuroblastoma risk classification system underwent modification in past decades, from the Evans staging system in 1971 to the most recent International Neuroblastoma Staging System (INSS) or International Neuroblastoma Risk Group Staging System (INRGSS), which was used for the first time in 2005 [6]. Recently, the Children’s Oncology Group revised this system, (Table 1) aiming to improve the correspondence between patient therapy and stage assignation and more highly considering the impact of the risk biomarkers [3]. The re-assignation of patients from INRGSS classes of risk [7] to newly identified classes highlighted that HR patients were well assigned using the previous version of INRGSS (only 3.4% of non-HR shifted to HR), and that MYCN is one of the most relevant factors occurring in a majority of case scenarios in the HR-NB patients [3].
Table 1.
International Neuroblastoma Risk Group Staging System revised and updated by the Children’s Oncology Group in 2021.
| INRGSS | Age | MYCN Amp | SCA at 1 p or 11 q | Ploidy | INPC | Differentiation | Risk Group |
|---|---|---|---|---|---|---|---|
| L1 | No | Any | Any | Any | LR | ||
| AMP | LR or HR | ||||||
| L2 | <18 months | No | Absent | DI > 1 | FH | IR | |
| Any | Any | Any | IR | ||||
| AMP | Any | Any | Any | HR | |||
| 18 months–5 years | No | Any | Any | FH | IR | ||
| UH | HR | ||||||
| ≥5 years | No | Any | Any | UH | Differentiating | IR | |
| Undifferentiated or poorly differentiated | HR | ||||||
| M | <12 months | No | Any | Any | Any | IR | |
| AMP | HR | ||||||
| 12 to <18 months | No | Absent | DI > 1 | FH | IR | ||
| Present | Any | Any | HR | ||||
| Any | DI = 1 | Any | HR | ||||
| Any | UH | HR | |||||
| Any | NA | ||||||
| At least 1 feature | Unfavorable | HR | |||||
| AMP | Any | Any | Any | HR | |||
| ≥18 months | Any | Any | Any | Any | HR | ||
| MS | <12 months | No bx | No bx | No bx | No bx | LR or IR | |
| No | Absent | DI > 1 | FH | LR or IR | |||
| Present | Any | Any | IR | ||||
| Any | DI = 1 | Any | IR | ||||
| Any | UH | IR | |||||
| AMP | Any | Any | Any | HR |
Abbreviation: AMP, amplification; bx, biopsy; SCA, segmental chromosome aberration; DI, DNA index; INPC, International Neuroblastoma Pathology Classification; FH, favorable INPC histology; UH unfavorable INPC histology; LR, low-risk; IR, intermediate-risk; HR, high-risk; NA, not applicable. Note: Any included unknown.
MYCN amplification occurs in almost 25% of all neuroblastoma cases and correlates with HR-NB and poor prognosis [8,9,10]. Interestingly, despite the expectations, MYCN amplification does not always result in a higher expression of mRNA or protein [11,12,13], highlighting that more complex interaction should be considered in HR-NB disclosure [14,15]. While the relevance of the correlation between MYCN amplification and over-expression is still under discussion, the role of N-Myc oncoprotein as a potential target for therapy is well established [16,17,18]. In the same way, the prediction potential of MYCN amplification is considered a standard tool to distinguish tumors subtype and patient prognosis in medical practice [19,20]. For this reason, MYCN emerges as a key component of HR-NB in diagnostic, prognostic and medical procedures, such as in pharmaceutical research.
2. Diagnosis of High-Risk Neuroblastoma
A proper diagnostic evaluation can be relevant to perform the best patient assignation to a risk group and to select the best therapy available. The most common and used methods for NB assessment include a combination of histological observation with imaging features and multiple laboratory tests such as fluorescence in situ hybridization (FISH), polymerase chain reaction polymerase chain reaction (PCR), multiplex ligation-dependent probe amplification (MLPA) or array comparative genomic hybridization (aCGH) [4,21,22]. In a similar manner to laboratory tests, imaging also offers a broad spectrum of techniques that have been developed or improved over the years, in order to provide reliable tools to assess neuroblastoma staging and follow-up [23,24].
For example, anatomical imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) represent the diagnostic standard for the precise localization of primary tumor mass and provide anatomical details for consequent loco-regional staging [25,26]. Interestingly, the modern CT-based approach has shown the ability to predict MYCN amplification (MNA) status in neuroblastoma through the generation of radiomics profiles of tumors [27], highlighting the great potential of this technique. In contrast, functional imaging analysis, using positron emission tomography (PET) or the combined approach PET/CT, is less useful when approaching the primary tumor, but it is more reliable for distant metastasis disclosure and proper tumor evaluation after anatomical distortions induced by surgery or radiation [24,28]. Moreover, the use of different tracers in PET, such as 123I-MIBG, 18F-FDG, and 99mTc-MDP, may allow us to obtain the best imaging performance on neuroblastoma tumors, differing in biological and molecular characteristics and further improving the value of this tool [28].
With the advancement of new technologies, the cellular, molecular, genetic and anatomical features of NB become constantly more fast and accessible for analysis, allowing proper patient assignation [3,29]. In particular, MNA status is considered the strongest indicator for both HR-NB assignation and poor prognosis [29,30,31], and a fast diagnosis can be particularly relevant in consideration of the increase in risk with the age of the patient [3,32,33].
Circulating Free DNA and Circulating Free Cells
Most of the tests for NB assessment require bioptic material. The biopsy procedure needed for the analysis is an invasive procedure and the tumor mass is not always accessible for recovery and analysis. Moreover, the analysis of a tumor with an abundance of non-malignant cells [34] can be confounding and show MNA heterogeneous pattern results [35,36]. For this reason, a new approach was developed involving the use of circulating free DNA (cfDNA) that was isolated from plasma or serum [37]. Using this so called “liquid biopsy” approach, it is possible to overcome the problem related to the invasiveness of the surgical procedure and the genetic heterogeneity found in solid tissue [38]. The technique is fast and fully reliable for the assessment of MYCN copy number using a PCR based analysis [39,40,41]. Moreover, it was demonstrated that cfDNA can be used in combination with specifically quantitative PCR (q-PCR) to perform an MNA analysis with high sensitivity and specificity in patients with advanced disease [42,43].
Interestingly, circulating messenger RNA (mRNA), circulating tumor cells (CTCs) and circulating NB exosomes can also be found in biological fluids and used as biomarkers for diagnosis and prognosis assessment [44]. In particular, CTCs can provide comprehensive tumor profiling involving RNA, protein and/or metabolic information, while cfDNA only contains a genomic statement [45,46]. Of course, this method is more expensive and slower than cfDNA use [47], but it can be extensively considered as complementary in HR-NB evaluation, as suggested in other cancer studies [48,49].
3. Current Therapies of High-Risk Neuroblastoma
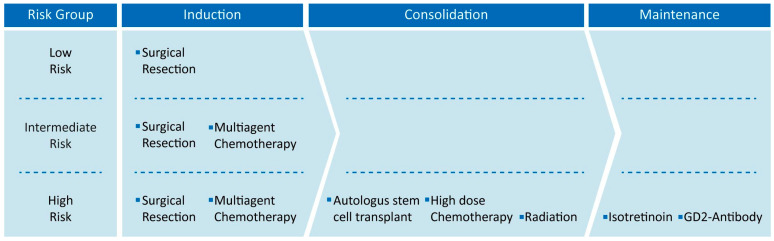
Current NB therapy in the majority of countries is constituted by three phases: induction, consolidation and maintenance therapies (Figure 1), and lasts approximately 18 months, varying by the patient risk [50,51]. Treatment strategies for each phase may include chemotherapy, surgical resection, high-dose chemotherapy with autologous stem cell rescue, radiation therapy, immunotherapy and isotretinoin, but the modality of the administration of the single procedures mostly depends on patient risk status [52]. Low- and intermediate-risk patients, for example, show high overall survival with minimal therapy approach, involving only surgical resection alone or combined with small chemotherapy administration in the induction phase [53,54,55]. On the other hand, the high-risk group need a more aggressive approach, and each phase is involved in the management of the pathology with the administration of any possible therapy available [52,56,57,58], being more challenging not only for the efficacy assessment, but also for the safety of the patient.
Figure 1.
Neuroblastoma therapies are reported in relationship to risk group. Each treatment is collected in its own neuroblastoma therapy phase from induction to maintenance. Low- and intermediate-risk patients only receive the induction phase, while high-risk patients receive all treatments.
3.1. Surgical Resection
Surgical resection is considered the first lane of treatment for neuroblastoma and is mostly relevant in HR patients [59]. In particular, the surgical approach may vary from complete tumor resection to gross tumor resection or biopsy only, and this can have a heavy impact on therapy outcome. Complete or gross tumor resection is not always possible but showed better outcome in comparison to less aggressive strategies such as partial resection or biopsy only in HR patients [60,61,62]. More in detail, tumor resection >90% was specifically associated with better EFS than more partial resection [63]. Unfortunately, surgery cannot be considered an independent procedure and to establish the proper timing for resection in function of chemotherapy is not clear [64,65]. In HR-NB, for example, preoperative chemotherapy is highly suggested for the management of an unresectable tumor [66,67]. More in general, despite many studies reporting that patient treatments with neo-adjuvant chemotherapy prior to surgery may facilitate tumor removal and improve post-surgery outcomes [59,68,69], the impact of surgical timing in primary tumor resection remains controversial and challenging [70]. In particular, defining proper chemotherapy strategies in terms of compound and the number of cycles to administer prior to and post-surgery is still a major issue [65]. While surgical procedure remains fundamental, the location of the primary tumor and the experience of the surgeon, such as the post-operative care of the patients, may impact the results of the therapy outcome. In the same way, amplification of the resection extent may increase both intra-operative and post-operative complication and reduce the therapy compliance or lead to the abandonment of such a life-saving procedure [62].
3.2. Multi-Agent Chemotherapy
Multimodal chemotherapies treatment strategies for HR-NB patients may vary from place to place, but they always involve a combination of chemotherapy and both high dose and frequent administration strategies. The most used North American model, for example, involves the use of a five-cycle strategy consisting in the administration of topotecan/cyclophosphamide for cycles 1 and 2, cisplatin/etoposide for cycles 3 and 5, and vincristine/doxorubicin/cyclophosphamide for cycle 4 [51], while the well-established COJEC system (cisplatin [C], vincristine [O], carboplatin [J], etoposide [E] and cyclophosphamide [C]) uses another mix of chemotherapy rapidly administered every 21 or 10 days [71,72]. Of course, the use of chemotherapy at a high concentration and frequency inevitably leads to a broad spectrum of side effects, and although the community effort is focused on increasing patient compliance, long-term toxicity still remains a major issue in HR patients [73]. The most common side effects include growth failure, thyroid dysfunction [74], hearing loss [75,76], ovarian/testicular failure [77], diabetes mellitus, pulmonary dysfunction [73,78], cardiac dysfunction [79,80], renal dysfunction, subsequent malignant neoplasm [81,82] and physiologic impairment [83]. Interestingly, endocrinopathies are one of the most prevalent complications after the treatment of HR-NB using modern therapies [78,84]. This condition is particularly relevant in consideration of the young age of the patient and can strongly impair growth and fertility in the adulthood [85].
3.3. Autologous Stem Cell Transplantation
An increase in chemotherapy drug dosage was postulated as a strategy for tumor treatment, highly in consideration of the advancement of support therapy. Preclinical studies on the dose-response of cytotoxic agents demonstrated how it is possible to maintain a linear range, highlighting the possibility of effectively increasing the dose and, thus, the effect [86,87]. Unfortunately, chemotherapy has many side effects and, particularly, myelotoxicity has been evaluated as the most dose-limiting toxicity for chemotherapeutic agents [88]. For this reason, the possibility of rescuing the stem cells from the patient and performing autologous stem cell transplantation (ASCT) after high-dose treatment could highly impact therapy outcome [89,90]. Many studies demonstrated how single [56,91] or more recent tandem transplantation [92] post-chemotherapy can increase event-free survival compared to chemotherapy alone, having a particular impact on HR-NB patients’ outcome. While ASCT remains as one of the most important advancements for HR-NB treatment, the procedure itself is not totally without risk. Infection management [93,94] such as stem cell availability, sorting procedure and processing [95] may be challenging, and advancements are needed to safely improve patient healthcare [89].
3.4. Radiation Therapy
Similar to surgery and chemotherapy, radiotherapy (RT) is considered one of the most relevant and common parts of the treatment of HR-NB in the multimodal approach [96,97]. While its function in supporting primary tumor removal is well established [98,99], its role in metastasis management after induction chemotherapy is still uncertain [100,101]. Many advancements were achieved to upgrade this technology, increasing performance both in dose shaping and specificity [102], for example, with the administration of proton therapy [100,103], or 131I-MIBG [104,105,106] instead of standard photon RT. Considering the improvement in HR-NB treatment and the increase in patient survival, more attention is paid to radiotherapy’s toxic effect. This effect may include growth and developmental failure, hypothyroidism, gastrointestinal dysfunction, neurocognitive defects, pulmonary and cardiac abnormalities, infertility and secondary cancers. The most frequent side effects are musculoskeletal abnormalities followed by the growth impairment of bones, including scoliosis, kyphosis or short stature [107,108,109], as well as general growth impairment effects [84,110]. Less frequently, sensorineural hearing loss, cardiac dysfunction and secondary malignancies can be observed [84,107], underlining a broad spectrum of toxic effects that must be properly taken into account for patient safety [111].
3.5. Anti-GD2 Immunotherapy
Immunotherapy and, in particular, the use of monoclonal antibody in cancer has increased in past years [112,113,114]. Unfortunately, pediatric tumors normally show few tumor-specific antigens, and for this reason immunotherapy is less frequent compared to adult counterparts [115]. Interestingly, neuroblastoma represents an exception; in fact, almost all tumorigenic cells show the expression of aberrant gangliosides [116,117]—such as disialoganglioside GD2—which are nearly absent in the majority of normal tissue [118]. Gangliosides are sialic acid-containing glycolipids [119] that are able to stimulate the immune response, and their use in immunotherapy was demonstrated to improve the outcome in a patient with HR-NB [114,120,121,122]. Dinutuximab, for example, is a commercial chimeric anti-GD2, successfully used for many years for the treatment of HR-NB [123,124,125]. Recently, a new humanized anti-GD2 antibody known as naxitamab has been approved by the Food and Drug Administration for the treatment of neuroblastoma and other GD2-related cancers [126,127]. However, anti-GD2 treatment was also found to be associated with several side effects. Significant neuropathic pains can be found in almost all patients treated with anti-GD2 [128,129], while mydriasis, light accommodation impairment [130] and severe demyelinating polyneuropathy are reported more rarely [131]. Moreover, anaphylactic reaction can occur in association with the development of circulating antibodies [132]. New strategies are in development to overcome this issue [133], but in general, less aggressive approaches appear to be less effective in immune stimulation [134].
3.6. Isotretinoin
Isotretinoin (13-cis-retinoic acid; 13-cisRA) is a retinoid that was first approved in 1982 by the Food and Drug Administration for the treatment of severe acne [135]. Despite its native application, isotretinoin has found many other applications, such as maintenance therapy in HR-NB [96,136]. In fact, it was demonstrated that 13-cis-retinoic acid is able to induce both cell differentiation and the arrest of proliferation in neuroblastoma [137,138,139]. Unfortunately, a major issue is represented by the development of tumor resistance by HR-NB relapsed patients [140,141], resulting in this therapy’s great limitation. Cheilitis or dry lips are the major adverse effects found in almost all the patients treated with isotretinoin, but sun sensitivity, xerosis and xerostomia are also very common [142]. While minimum side effects occur, the efficacy of isotretinoin alone is under discussion, and more frequently, combination treatment overcomes its use as a single agent [121,143,144,145].
4. MYCN as Prognostic Indicator in High-Risk Neuroblastoma
Despite the advancement in medical standard therapy for the treatment of HR-NB, there is no specific therapy for MNA patients [92,146,147], but this aspect of the pathology becomes increasingly relevant in consideration of its prognostic effect. For example, the prognostic impact of MNA is particularly relevant in infants with stage M disease where both event-free survival (EFS) and overall survival (OS) are higher in an MYCN non-amplified tumor, that shows better outcomes in comparison with MNA patients (EFS and OS: 82.5% and 90.8% versus 36.9% and 44.8%) [3]. In a similar manner, the behavior of an L2 and MS stage tumor is mostly impacted by MYCN status. EFS and OS are lower at any age when MYCN is amplified, while the absence of amplification correlates with better survivability results for the patients [3]. Interestingly, MNA association with worse prognosis appears more pronounced in the context of other favorable prognostic features and can be considered as an indicator for aggressive intervention [148], highlighting how MYCN gene status impacts therapy selection and medical decision making.
While MNA is considered a staple in neuroblastoma diagnosis, as mentioned before, MYCN over-expression has a more controversial role; in a similar manner, MYCN expression has the same prognostic behavior. Some studies reported that MYCN expression is valuable for prognostic purpose, but only in a specific pathology context. In particular, a patient cohort showed that the lowest value in MNA cases has a more negative outcome when MYCN and mRNA levels are higher, in comparison to patients with higher MNA levels where mRNA expression cannot be considered as prognostic [11]. Otherwise N-Myc protein expression shows different prognostic value, being reported as an indicator of poor outcome regardless of MNA status [31]. These findings together suggest that deep investigation on how and when MNA or MYCN expression are predictors of patients’ outcomes needs to be improved, but their value as key elements of prognosis assessment is undeniable, remaining so in the establishment of HR-NB therapy administration.
5. MYCN Determines High-Risk Neuroblastoma
Despite MYCN amplification being the first discovered genetic mechanism in neuroblastoma, its role in driving the pathology is not fully understood [149,150,151]. In fact, MYCN amplification leads to deep remodeling of the cancer cell, influencing its apoptosis resistance, its undifferentiated status, its metabolic landscape and immune evasion.
Different studies showed that MYCN over-expression is an obstacle to neuronal differentiation. High-risk and MYCN amplified neuroblastoma present a different transcriptional profile, where different pathways and genes related to differentiation are particularly altered [152]. Indeed, MYCN amplified neuroblastoma cell lines fail to differentiate in response to 13-cis-retinoic acid [8]. The concomitant inhibition of MYCN and the administration of RA is able to reverse this block [152]. Moreover, the ectopic expression of MYCN in precursor cells blocks the differentiation in chromaffin cells.
In addition, MYCN controls both proliferation and apoptosis: many studies showed that over-expression disrupts the cell cycle, leading to maintained apoptosis inhibition and induced proliferation [153]. In fact, blocking MYCN leads to G1 phase cell accumulation and slows down the transition to S phase and PI3K repression (which is known to promote cell growth and proliferation) [154,155,156,157,158]. MYCN also positively affects the expression of other key cellular regulator such as E2 factor (E2F) and inhibitor of differentiation 2 (ID2), which are also involved in cell cycle progression [155,159,160]. As an additional mechanism, MYCN amplification is also associated to TERT expression and telomere anomalies [161,162].
Interestingly, MYCN can promote apoptosis and/or sensitizes cancer cells to cytotoxic drugs [163,164]. MYCN is able to promote the expression of phorbol-12-myristate-13-acetate-induced protein 1 (NOXA), which is a pro-apoptotic regulator. Moreover, E-box elements are present in the promoter of p53, which is the most known onco-suppressor able to stop cell proliferation and induce apoptosis (even if in a significant part of neuroblastoma p53 is found mutated) [164]. As it is known, the murine double minute 2 (MDM2) is a negative regulator of p53 and is over-expressed in different human malignant tumors [165]. In particular, MDM2 is able to reduce p53 levels using the mechanism of binding to p53 with consequent ubiquitination and proteosomal degradation [166,167]. However, MDM2 also promotes the stability of MYCN, while the latter induces MDM2 transcription [168,169,170]. Thus, MYCN can induce the transcription of p53 and MDM2, regulating the balance between proliferation and apoptosis. Over-expression is thought to alter this precarious equilibrium, inducing MDM2 expression and p53 blocking [168,169,170].
Early studies showed how metabolism is deeply altered in cancer cells. In fact, cancer cells are skewed towards rapid ATP production, which is generally obtained through the “Warburg Effect”, where cells rely on glycolysis and mitochondrial respiration is impaired [171,172,173,174]. Cancer cells then use the fatty acids and glutamine as a source for biosynthesis and ultimately sustain the cell growth and proliferation. Moreover, this alteration in the mitochondria leads to reactive oxygen species (ROS) production, while the fatty acid oxidation is used to replenish the NADPH pool in order to prevent excessive oxidative stress [175,176]. Neuroblastoma and, in particular, MYCN amplified tumors are heavily dependent in glutamine, and blocking MYCN leads to the arrest of glutamine transport [177,178]. In addition, MYCN promotes the glycolysis and fatty acid uptake and leads to mitochondria alteration [178,179]. MNA tumors also present different metabolic alterations, leading to an increase in iron uptake. For instance, it has recently been shown that MYCN induces massive lipid peroxidation and cysteine depletion. This leads MNA to be sensible to oxidative stress and especially to ferroptosis [180]. In this context, blocking MYCN in MNA neuroblastoma leads to ROS production (through TRAP1 decrease), which the cancer cell fails to handle, consequently undergoing apoptosis [181]. In addition, MYCN also blocks autophagy and mitophagy (an autophagy sub-pathway used by the cell to recycle damaged mitochondria), and it has been shown in inducible MYCN cell line (TET21N) that the MYCN blocking restores this pathway (OPTN transcription) [181]. All these studies show a strong rewiring of the metabolism by MYCN expression and the fine grain regulation of the redox equilibrium.
Different studies showed the role of the phosphatidylinositol 3-kinase (PI3K)/mTOR pathway in neuroblastoma [156,182,183]. In fact, the mTOR pathway is known to stabilize N-Myc, and its blocking affects cell growth. Moreover, retinoic acid has been described as being capable of mTOR inhibition. While the N-Myc protein is stabilized by the mTOR complex, it also regulates the expression of different MTOR genes in a positive loop [152,184]. Indeed, MNA cell lines have a higher expression of mTOR genes, and they are more resistant to mTOR inhibitors. Furthermore, it has been shown that mTOR is also negatively associated to the prognosis [152].
MYCN amplification impact is not limited to the cancer cell itself but also to the tumor microenvironment. In fact, MNA cancers remodel the external environment to sustain their growth and the immune evasion [185]. A significant portion of MNA tumors present PD-L1 expression and MHC I complex down-regulation, leading to a suppressive micro-environment [186,187]. In addition, MNAs are also enriched in M2 macrophages and CD4+ T helper 2 cells [185]. Macrophages are also responsible for maintaining a hypoxic environment and lead to the transcription of hypoxia inducible factor (HIF 2α), which ultimately leads to vascularization and metastasis spreading. Indeed, HR-NB that are fast growing are high in the immunostaining for HIF2 [188]. However, neuroblastoma also exploits other strategies such as expressing other immune-suppressive molecules (such as CD276), miRNAs and exosomes release [189]. Overall, both innate and adaptive immune systems seem to be down-regulated [185]. This complex landscape is probably at the origin of the fact that immune-therapy has shown modest results [185,190,191].
MYCN amplification also leads to extracellular matrix (ECM) modification. ECM is often altered in HR-NB with anomalous collagen I deposit and often correlates with bad prognosis [192,193,194,195]. These alterations are also promoted by the hypoxic and inflamed state of the tumor microenvironment [196,197,198]. Collagen I inhibition has shown a promising effect, allowing better chemotherapy delivery [199]. Moreover, different matrix metalloproteinases (MMPs) are altered in neuroblastoma which are linked to bad prognosis, angiogenesis and metastasis promotion [200,201,202].
HIF2 expression, ECM alteration and VEGF expression in HR-NB also lead to new vascularization. These tumors show more aggressive features such as more immature states and more easily spreading metastasis [203,204,205,206,207,208]. Moreover, there is evidence that PI3K kinases promote VEGF expression via MYCN [209,210]. Interestingly, blocking PI3K by SF1126 in neuroblastoma led to reduced MYCN expression, cell death and angiogenesis block, while temporarily increasing the macrophages’ M1 to M2 ratio, showing how all these mechanisms are interconnected [211,212,213,214].
Furthermore, the neuroblastoma tumor microenvironment presents an enrichment of cancer-associated fibroblasts (CAFs) and mesenchymal stromal cells (MSCs). This enrichment correlates with progression and it is important to sustain the tumor growth, micro-vessels formation and progression, showing correlation with poor outcome [215,216,217]. In fact, CAF produces TGFβ (a cytokine with immunosuppressive property) and CCL2, which recruit TAM to neuroblastoma [218,219,220]. In addition, studies have highlighted that the CAF area extension correlates with MYCN amplification [221,222,223]. MSCs are also involved in inducing an immune suppressive environment, leading to the recruitment of ulterior suppressive cells (T regulatory cells and macrophages).
Other than the direct action of MYCN on cellular process leading to neuroblastoma, in the past few years, many epigenetic mechanisms have been discovered regulating MYCN with a specific role in HR-NB development [224]. Micro RNAs (miRNAs) are small single-stranded RNA molecules that function as post-transcriptional RNA regulators [225]. MiRNAs targeting MYCN were found to be particularly important in regulating its expression in neuroblastoma in different ways [8,226]. Some miRNAs targeting MYCN, such as the let-7 family, work as inhibitors [227] and are down-regulated in neuroblastoma, inducing N-Myc protein expression [228,229,230]. These miRNA types are considered the most common, but other miRNAs were found to work with the opposite mechanism. For instance, miRNAs such as the family of miR-17-92 are substantially employed as MYCN up-regulators. Interestingly N-Myc is able to stimulate the expression of miR-17-92 cluster, suggesting the presence of a positive feedback mechanism of regulation between MYCN and the miR-17-92 cluster itself [230,231,232].
Moreover, the natural antisense transcript was found to be able to regulate MYCN expression. In particular, MYCN locus is able to generate an antisense transcript known as MYCNOS (or N-cym) [233]. This transcript originates from the opposite strand of the locus and regulates MYCN as either regulatory long non-coding RNA (lncRNA) or protein. In fact the lncRNA of MYCNOS regulates MYCN promoter through the recruiting of protein in this site [234], while the MYCNOS-encoded protein works as an inhibitor of glycogen synthase kinase 3 β (GSK3beta), stabilizing in this way the NMYC protein [235]. For this reason, high levels of MYCNOS can be found relative to MYCN over-expression and correlate with poor outcome in neuroblastoma [13,236]. Furthermore, MYCNOS itself was found to be regulated from some non-coding RNAs such as lncUSMycN [237] that are able to suppress its expression and indirectly regulate MYCN [234].
Methylation is another regulatory system used by cells to define gene expression and cellular function. While the correlation between MYCN and its methylation status in neuroblastoma is still unclear, many other genes involved in neuroblastoma transformation were well characterized in both MYCN amplified and non-amplified tumors [224,238]. Extensive methylation was found in many onco-suppressor genes with no particular correlation to MYCN status [239]. In particular, miRNAs were reported to be methylated in neuroblastoma cell lines, highlighting their role in tumor progression and poor prognosis [240].
6. MYCN as Therapeutic Target
Despite current therapeutic advances and ongoing clinical trials, NB remains a complex medical challenge, especially in the high-risk cases, and the discovery of new therapeutic approaches is needed to improve patient welfare and outcome (Table 2) [241].
Table 2.
Summary of current therapies and the new therapeutic approach in the treatment of neuroblastoma and high-risk neuroblastoma.
| Therapy | Therapeutic Strategy | Availability |
|---|---|---|
| Surgical Resection | Tumor mass removal by surgical resection | Standard Medical Practice |
| Multimodal Chemotherapy | Tumor cell elimination using non-specific chemical agents | Standard Medical Practice |
| Autologous stem cell transplantation | Stem cell reinfusion after high dose chemotherapy | Standard Medical Practice |
| Radiation Therapy | Tumor mass removal by radiations | Standard Medical Practice |
| Anti-GD2 Immunotherapy | Induction of immune system stimulation | Standard Medical Practice |
| Isotretinoin | Induction of tumor cell differentiation and proliferation arrest | Standard Medical Practice |
| BET Inhibitors | Inhibition of specific molecular pathway related to MYCN | Clinical Studies |
| HDACs Inhibitors | Inhibition of specific molecular pathway related to MYCN | Clinical Studies |
| PI3K/mTOR Inhibitors | Inhibition of specific molecular pathway related to MYCN | Clinical Studies |
| Aurora Kinase-A Inhibitors | Inhibition of specific molecular pathway related to MYCN | Clinical Studies |
| MDM2 inhibitors | Inhibition of specific molecular pathway related to MYCN | Clinical Studies |
| MYCN direct inhibitor | Specific MYCN expression inhibition or N-Myc protein degradation | Preclinical Studies |
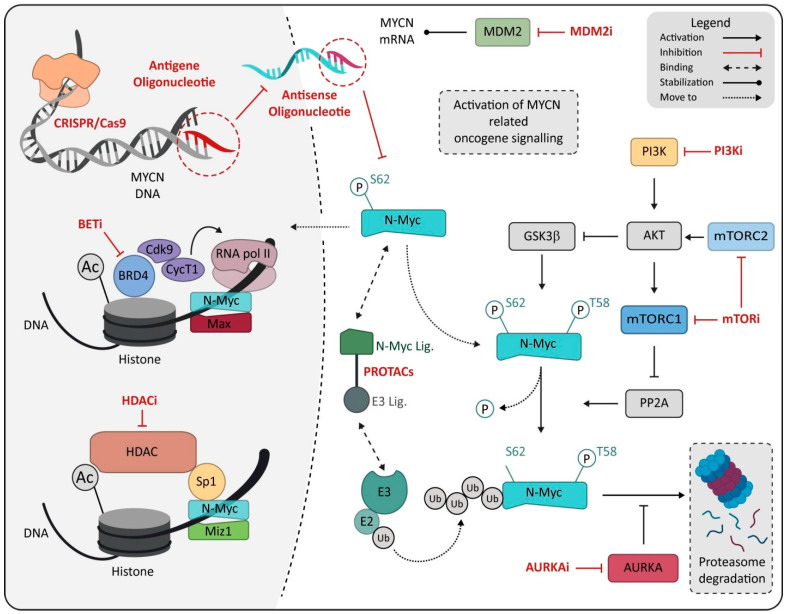
In this scenario, MYCN certainly represents an ideal therapeutic target given its correlation with rapid tumor progression, poor prognosis and the limited expression in normal cells and tissue, suggesting high tolerability for an MYCN-specific approach [8,242]. Many attempts and investigations have been made to develop specific inhibitors for N-Myc protein, but both direct or indirect N-Myc modulators (Figure 2) failed to result in an efficient or reliable N-Myc-specific therapy [243]. Unfortunately, N-Myc targeting shows a different issue. Some of these issues can resemble any transcription factor, while some challenges are more peculiar and reside in the lack of specific interaction site on the protein [244,245] or in the homology with the MYC family oncogene, increasing the difficulty of preserving the physiological function of c-Myc protein in normal tissue [153,246]. For this reason, new strategies have been proposed in the hope of overcoming the failure of the precedent attempt to make MYCN a fully available target for HR-NB [8,18,153,247].
Figure 2.
Schematic representation of drugs directly or indirectly targeting MYCN. Drugs are marked in red and reported as class, named on mechanism of action. Abbreviation: MDM2, murine double minute 2; PI3K, Phosphoinositide 3-kinases; PI3Ki, Phosphoinositide 3-kinases inhibitor; BETi, bromodomain and extra-terminal domain family inhibitor; BRD4, bromodomain-containing protein 4; mTORC1, mammalian target of rapamycin complex 1; mTORC2, mammalian target of rapamycin complex 2; mTORi, mammalian target of rapamycin inhibitor; PROTACs, proteolysis-targeting chimeras; MIZ1, MYC-interacting zinc-finger protein 1; HDAC, histone deacetylase; HDACi, histone deacetylase inhibitor; E3, E3 ubiquitin ligase; E2, E2 ubiquitin-conjugating enzyme; AURKA, Aurora kinase A; AURKAi, Aurora kinase A inhibitor; Ub, ubiquitin.
6.1. BET Inhibitors
Bromodomain and extra-terminal domain family (BET) is a group of epigenetic regulators that consist of four elements, BRD2, BRD3, BRD4 and BRDT [248]. These proteins can bind DNA and recruit P-TEFb complex to start the elongation of the transcription process, activating the RNA pol II and so regulating gene expression [249,250,251]. Interestingly, these factors are required for both MYCN transcription and MYCN-driven transcription, so that their inhibition can have a double effect [153,252,253].
Many BET inhibitors capable of inhibiting MYC or MYCN in vitro were discovered in the past few years [254,255] with a specific function in NB [252,256], but their application with clinical purpose was unsuccessful. However, new BET inhibitors, BMS-986158, BMS-986378 (NCT03936465) and GSK525762 (NCT01587703) are currently in clinical trial phase I with specific application in neuroblastoma (Table 3), maintaining the interest in this class of compound.
Table 3.
Inhibitor drugs under clinical studies for neuroblastoma treatment are reported (ClinicalTrials.Gov, updated 1 August 2022). Drug inhibitor target, clinical phases, trial status and ID are reported.
| Drug Name | Target | Clinical Phase | Status | NTC Number |
|---|---|---|---|---|
| Vorinostat | HDAC | Phase1|Phase2 | Recruiting|Completed|No longer available | NCT01019850|NCT03561259|NCT01838187|NCT01132911|NCT02559778|NCT03332667|NCT02035137|NCT01208454|NCT04308330 |
| Panobinostat | HDAC | Phase 2 | Terminated | NCT04897880 |
| BMS-986158 | BET | Phase 1 | Recruiting | NCT03936465 |
| BMS-986378 | BET | Phase1 | Recruiting | NCT03936465 |
| GSK525762 | BET | Phase1 | Completed | NCT01587703 |
| SF1126 | PI3K/mTOR | Phase 1 | Terminated | NCT02337309 |
| Samotolisib | PI3K/mTOR | Phase 2 | Recruiting | NCT03213678 |
| ALRN-6924 | Dual MDM2/MDMX | Phase 1 | Recruiting | NCT03654716 |
| LY3295668 | Aurora-A Kinase | Phase 1 | Active, no recruiting | NCT04106219 |
| Alisertib (MLN8237) | Aurora-A Kinase | Phase 1|Phase 2 | Complete | NCT02444884|NCT01601535|NCT01154816 |
Abbreviation: HDAC, histone deacetylase; BET, bromodomain and extra-terminal motif; PI3K Phosphoinositide 3-kinases; mTOR, mechanistic target of rapamycin.
6.2. HDACs Inhibitors
It is known that MYCN can modify the genome in many ways [257]. One of these is represented by the ability to induce the transcriptional repression of tumor suppressor genes by N-Myc protein binding to the MIZ1 and SP1 transcriptional activators and resulting in the recruitment of histone deacetylases (HDACs) [258,259,260]. For this reason, HDACs inhibitors are considered a viable route to target MYCN-amplified neuroblastomas [8,261]. These inhibitors can modulate both histone and non-histone proteins inhibiting cancer-related processes, while stimulating the immune response and chemotherapy sensitivity [262,263]. The Food and Drug Administration has approved different HDAC inhibitors such as vorinostat, romidepsin, belinostat and panobinostat, mostly for hematological cancer types such as T-cell lymphoma or multiple myeloma [264,265,266]. In particular, in neuroblastoma, the HDAC-8 appears to correlate with poor prognosis, and many attempts were made to inhibit its function. The specific inhibitors 1-naphthohydroxamic acid (Cpd2) and PCI-34051, for example, showed potential HDAC-8 inhibitory activity and thereby decreased the neuroblastoma cell viability [267]. However, more common HDACs inhibitors such as vorinostat or parabinostat (NCT04897880) are currently in clinical trials for neuroblastoma and have successfully reached clinical phase 2 (Table 3).
6.3. PI3K/mTOR Inhibitors
Protein stabilization is fundamental for the correct function of proteins. Affecting this process represents a possibility to deregulate protein function and, therefore, the cellular process. In cerebellar neuron precursor for example, N-Myc protein stability is regulated by Phosphoinositide 3-kinases (PI3K) through AKT and glycogen synthase kinase 3β (GSK3β) [268,269], suggesting its possible role in inhibiting upstream N-Myc signaling. In a similar manner, N-Myc is also indirectly regulated upstream by mammalian target of rapamycin complexes (mTORC), which modulates cell growth and protein synthesis [270,271].
For this reason, several inhibitors interacting with this key element for protein function were developed in the past decade to increase protein degradation and limit their biological effects [272,273]. First generation inhibitors of mammalian target of rapamycin (mTOR), a core protein of mTORC, showed high efficacy in reducing cell viability and N-Myc level in MYCN-amplified NB cells [274]. More interestingly, compounds such as NVP-BEZ235 (dactolisib) and INK128 (sapanisertib) were found to be able to inhibit the activation of entire PI3K/mTOR pathway in specific MYCN tumors, further promoting their role in N-Myc down-regulation [182,275]. Several mTOR inhibitors have already been approved for the therapeutic treatments of different types of cancer [276,277], while in neuroblastoma new clinical trials are ongoing (NCT02337309, NCT03213678) making mTOR inhibition a very promising therapeutic avenue for MYCN-deregulated childhood cancers (Table 3).
6.4. Aurora Kinase-A Inhibitors
Aurora kinase A (AURKA) belongs to a family of serine/threonine kinases, named Aurora, mostly involved in cell division process through the regulation of centrosome formation, chromatin condensation and chromosome microtubule interaction [278]. AURKA expression was found to be altered in many cancers, making it a good candidate for therapy [279,280]. In neuroblastoma, N-Myc is able to interact with AURKA, leading to N-Myc stabilization and the limitation of cell cycle arrest in G2/M [281]. On the contrary, AURKA inhibition induces N-Myc degradation and stimulates cell death [282,283]. In combination, the use of AURKA and BRD4 inhibitors can reduce cell viability in a synergistic way in HR-NB cells [284,285], as well as in glioblastoma cells [286]. Despite the well described efficacy of AURKA inhibitors in combination, clinical trials are also available for a single agent. The selective inhibitor LY3295668 [287] for example is actively under examination alone in a clinical phase 1 study (Table 3) in relapse/refractory neuroblastoma (NCT04106219).
6.5. MDM2 Inhibitors
Tumor suppressor p53 is a critical protein for the regulation of apoptosis, cell cycle arrest or DNA damage repair process in response to DNA damage and cellular stress [288,289,290]. This protein has been reported to be mutated in almost 50% of human cancer [291], with related impairment of transcriptional activity [292,293]. In neuroblastoma, TP53 is rarely mutated [294], but it is normally associated with HR-NB and poor outcome [295,296]. The MDM2 oncogene is amplified and/or over-expressed in numerous human malignancies, including neuroblastoma [297,298], showing poor prognosis in this conditions [299]. MDM2 was discovered to be a negative regulator of p53 through a mechanism involving both transcription repression [300] or protein ubiquitination and degradation [301]. Interestingly, MDM2 may interact with N-Myc in a similar manner to p53 in neuroblastoma, resulting in MYCN mRNA stabilization and translation increase [302,303]. For this reason, the p53-MDM2-N-Myc pathway is very interesting as a target for new therapies [17,304]. For example, the MDM2 inhibitor DS-3032b was able to reactivate both in vitro and in vivo TP53 signaling in MYCN-amplified neuroblastoma [296]. A new dual MDM2/MDMX inhibitor ALRN-6924 [305] is under testing (NCT03654716) in a clinical phase 1 trial for neuroblastoma (Table 3), raising new hope for HR-NB treatment using TP53 reactivation.
6.6. MYCN Direct Inhibitor
Despite the difficulties in directly targeting the N-Myc protein, advances in chemistry and chemical genomics have created new instruments to overcome this issue in different ways [247,271,306]. Proteolysis targeting chimeras (PROTACs) induce protein degradation by exploiting the ubiquitination mechanism, resulting in viability for undruggable targets [307]. From a structural point of view, PROTACs are heterobifunctional molecules composed of an E3 ubiquitin ligase, covalently linked to another ligand with the ability to recognize the target and to drive the ubiquitination process [307,308]. As small molecules, 10058-F4 and 10074-G5 successfully showed in vitro the ability to bind N-Myc [309] and may be used to develop PROTACs. Further, the use of such molecules provides a new opportunity for screening strategy in MYCN therapeutics [310].
Directly targeting MYCN mRNA or MYCN gene at the level of DNA using MYCN-specific oligonucleotides is another highly promising and valuable approach for the specific, effective and safe treatment of MYCN-related HR-NB and other MYCN-expressing tumors. In recent years, different synthetic oligonucleotides have been developed for the specific silencing of target genes, making this technology always more affordable for both pre-clinical and clinical studies in cancer therapies [311,312]. An in vitro study on neuroblastoma cells with and without MYCN amplification showed that treatment with specific anti-MYCN small interfering RNAs (siRNAs) targeting the MYCN mRNA may cause cell growth arrest, the activation of apoptosis, and differentiation [313]. Moreover, synthetic miRNAs have recently been developed and showed in vitro the ability to stably interact with MYCN mRNA, which is promising for further biological study [314].
An innovative strategy consists in the specific gene expression inhibition at the level of the DNA of the MYCN gene through an antigene peptide nucleic acid (agPNA) oligonucleotide that is specific for MYCN [315]. Blocking the level of transcription by the antigene oligonucleotide strategy has shown pharmacological advantages over the translation block by antisense oligonucleotides. The chemical modification of peptide nucleic acids (PNAs) confer resistance to the degradation of the oligonucleotide by proteasome and nuclease and the ability to potently and specifically target DNA and resulted in relevant pharmacological optimal properties for the antigene strategy [316,317,318]. Antigene oligonucleotide therapy by targeting MYCN transcription has been demonstrated by the novel MYCN-specific agPNA BGA002 [152] in the preclinical treatment of neuroblastoma, and has also great potential in treating other aggressive MYCN-expressing tumors. BGA002 showed higher efficacy compared with MYCN antisense oligonucleotides [152]. BGA002 is able to specifically target a unique sequence on the human MYCN gene [152], resulting in a dose-dependent decrease in MYCN mRNA and protein. This effect causes a potent decrease in viability in a panel of 20 NB cell lines, in a block of different MYCN tumorigenic alterations and in the anti-tumor efficacy of BGA002 in vivo in a MNA NB mouse model [152]. Moreover, the block of MYCN by the anti-MYCN BGA002 is able to reactivate and restore the effectiveness of natural killer immune cells against NB, reverting the role of MYCN as a driver of a tumor immunosuppressive environment which impacts survival in several MYCN-positive tumors [156]. While MNA-NBs are generally resistant to retinoic acid (RA) treatment, the specific inhibition of MYCN expression by BGA002 has been shown to restore the RA response in MNA-NB, leading to a significant increase in survival in an MNA-NB mouse model [123]. The restoration of RA treatment could be beneficial not only for MNA-NB, but also for the treatment of different MNA tumors. BGA002 has been granted orphan drug designation from the European Medicines Agency (orphan registry: EU/3/12/1016) and from the Food and Drug Administration (orphan registry: DRU-2017-6085). Preclinical regulatory safety profile package studies also showed that BGA002 is well-tolerated, and it is now moving to phase I clinical trials in neuroblastoma patients.
Finally, regulating MYCN expression at the DNA amplification level was found to be possible. CRISPR/Cas9 technology is an editing tool that allows the cutting and/or addition of genomic fragments as needed [319]. Studies on cellular and animal models have shown that the CRISPR/Cas9 technique can be effective in treating cancers [320]. For this reason, several clinical trials are underway to evaluate the efficiency of this technology in treating cancers [321,322]. While no treatment is available using this system, a recent study showed that decreasing MYCN copy number by using MYCN-A3 alkylating agent can down-regulate MYCN expression and suppress NB growth in vitro and in a xenograft mouse model [323]. While this approach could be a new method of intervention, safety and toxicity aspects related to the unspecific genomic activities of CRISP/Cas9 technology should be further investigated.
7. Challenge
There are many challenges in managing neuroblastoma and especially HR-NB, beginning from diagnosis to prognosis.
7.1. Rarity of This Cancer
To address the low number of patients, a number of recent collaborations have sought. These organizations have run clinical trials on an international basis, including the International Society of Pediatric Oncology-Europe Neuroblastoma Association (SIOPEN) [324]; the Children’s Oncology Group (COG) in North America [325]; the European Neuroblastoma Study Group (ENSG) [72]; the German Pediatric Hematology and Oncology Group (GPOH) [7,326]; and the Neuroblastoma Committee of the Japanese Society of Pediatric Oncology (JNBSG) [327].
7.2. Diverse Prognosis
The clinical behavior of neuroblastoma is very heterogeneous with cases of spontaneous regression and fatal progression. Treatment is adjusted according to the combination of many prognostic variables, with the intensity of therapy guided by a risk assessment of the projected behavior of the disease. Any prognostic variable that can reliably guide risk group stratification and avoid the potential late effects following unnecessarily aggressive treatment in patients with a more favorable prognosis is highly desirable.
7.3. Initial Response Rates Are Not Optimal
The aim of induction therapy is to reduce the primary tumor size to facilitate successful surgery and to diminish the metastatic tumor load burden. A good response to initial induction therapy has been correlated with a better outcome [328], but it is difficult to compare initial response rates for the different response criteria used.
7.4. Risk of Relapse
Those high-risk patients that demonstrate a good response to induction and consolidation therapies are still at a significant risk of relapse, and this is due to the presence of minimal residual disease (MRD). The maintenance phase of treatment at the end of therapy aims to eradicate MRD.
7.5. Measurement of Disease Extent
The existence of residual disease is predictive of a poor outcome. The standardized operating procedures for detecting MRD by immunocytology using disialoganglioside GD2, and quantitative reverse transcriptase-PCR using tyrosine hydroxylase mRNA published by INRG, should facilitate the comparison of results [329]. Survival after relapse is poor, with no universally effective regime at present. According to the data of the Italian neuroblastoma registry, for stage 4 patients who had progressed or relapsed, the 10-year OS was only 2% [330].
7.6. CNS Relapse
Though CNS site in neuroblastoma at diagnosis is rare, it is a site of disease relapse. This could be due to the inability of many chemotherapy agents to cross the blood–brain barrier. Generally, CNS relapse is fatal; however, the outcome could be improved with the early recognition of disease at this site. Moreover, mIBG does not cross the intact blood–brain barrier and so diagnostic and surveillance mIBG imaging can miss CNS disease.
7.7. Minimizing Treatment-Related Morbidity
In the group of low- and intermediate-risk neuroblastoma, the aim has been to reduce treatment intensity for reducing toxicity and long-term effects. Neuroblastoma patients without MYCN gene amplification and even those with unresectable disease and no MYCN amplification have had OS rates of 95–100%. However, the treatment of these patients could induce long-term toxicity. The Childhood Cancer Survivor Study (CCSS) calculated the incidence of secondary neoplasms, which was 3.5% at 20 years and 7% at 30 years after diagnosis [331].
7.8. Distribution of Age of Patients
Approximately 3–4% of neuroblastoma cases occur in older children, adolescents and young adults [332,333]. Neuroblastoma in this age group has a different biology and clinical course and different responses to therapy [334]. As the number of patients in this group is small, the results of the studies are difficult to compare to the other groups.
7.9. Access to New Drugs
The prognosis of high-risk neuroblastoma is poor, so many research groups are searching for innovative therapeutic approaches. However, novel agents are mainly tested within clinical trials, as safety and efficacy data are required by continental and national drug regulatory authorities before the agents can be licensed and made commercially available. Pharmaceutical companies have little incentive for the development of new drugs for low-incidence diseases such as neuroblastoma where, even if a new drug was found to be effective, there would be little regain of the development costs.
8. Conclusions
Many advancements have been made in the last few decades from diagnostic to patient management and drug discovery in high-risk neuroblastoma. Improvements in outcome and general patient welfare are undeniable. New diagnostic procedures and our better understanding of biological markers and their role in determining the pathology enhance the incidence of entire treatment improving lifespan and quality of life. Despite all the advancements we have described, effectively targeting high-risk neuroblastoma remains challenging. Many new specific inhibitors are designed to specifically inhibit MYCN expression and its oncological effect, in consideration of its predominant role in defining high-risk designation and poor outcome.
Currently, while many of these inhibitors are in use for different type of tumors, only clinical trials for high-risk neuroblastoma that remains “orphan” from its own therapeutic agent are reported. Medical science needs to make many other efforts to overcome the issue linked to aggressive tumor and target therapy, especially in the field of genes and transcription factors such as MYCN. The availability of a more personalized medicine approach is always more concrete and provides us with new possibilities and insights on treating aggressive tumors such as HR-NB.
Author Contributions
D.B., L.M., S.R. and S.L. conducted data acquisition, statistical analysis, and manuscript writing and design; D.B. assembled the figures; A.P. and P.H. participated in manuscript revision and study supervision; R.T. performed study design and conceptualization, funding acquisition, manuscript revision and study supervision. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Publicly available datasets at https://www.clinicaltrials.gov/ (accessed on 1 August 2022) were used in this study.
Conflicts of Interest
A. Pession and R. Tonelli are Biogenera shareholders. The authors declare no potential conflict of interest. The companies had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
A. Pession, P. Hrelia and R. Tonelli are funded by the University of Bologna (ECOITONELL). D. Bartolucci and S. Lampis are funded by Biogenera SpA. L. Montemurro is funded by AGEOP. S. Raieli is funded by Oncodesign SA.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R., Ward E., Brawley O., Jemal A. Cancer Statistics, 2011: The Impact of Eliminating Socioeconomic and Racial Disparities on Premature Cancer Deaths. CA Cancer J. Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Irwin M.S., Naranjo A., Zhang F.F., Cohn S.L., London W.B., Gastier-Foster J.M., Ramirez N.C., Pfau R., Reshmi S., Wagner E., et al. Revised Neuroblastoma Risk Classification System: A Report from the Children’s Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021;39:3229–3241. doi: 10.1200/JCO.21.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambros P.F., Ambros I.M., Brodeur G.M., Haber M., Khan J., Nakagawara A., Schleiermacher G., Speleman F., Spitz R., London W.B., et al. International Consensus for Neuroblastoma Molecular Diagnostics: Report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br. J. Cancer. 2009;100:1471–1482. doi: 10.1038/sj.bjc.6605014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto N., Mayfield J.R., Raca G., Applebaum M.A., Chlenski A., Sukhanova M., Bagatell R., Irwin M.S., Little A., Rawwas J., et al. Segmental Chromosomal Aberrations in Localized Neuroblastoma Can Be Detected in Formalin-Fixed Paraffin-Embedded Tissue Samples and are Associated with Recurrence: Segmental Chromosomal Aberrations in Localized Neuroblastoma. Pediatr. Blood Cancer. 2016;63:1019–1023. doi: 10.1002/pbc.25934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokol E., Desai A. The Evolution of Risk Classification for Neuroblastoma. Children. 2019;6:27. doi: 10.3390/children6020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohn S.L., Pearson A.D.J., London W.B., Monclair T., Ambros P.F., Brodeur G.M., Faldum A., Hero B., Iehara T., Machin D., et al. The International Neuroblastoma Risk Group (INRG) Classification System: An INRG Task Force Report. J. Clin. Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang M., Weiss W.A. Neuroblastoma and MYCN. Cold Spring Harb. Perspect. Med. 2013;3:a014415. doi: 10.1101/cshperspect.a014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maris J.M., Hogarty M.D., Bagatell R., Cohn S.L. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 10.Rubie H., Hartmann O., Michon J., Frappaz D., Coze C., Chastagner P., Baranzelli M.C., Plantaz D., Avet-Loiseau H., Bénard J., et al. N-Myc Gene Amplification is a Major Prognostic Factor in Localized Neuroblastoma: Results of the French NBL 90 Study. Neuroblastoma Study Group of the Société Francaise d’Oncologie Pédiatrique. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997;15:1171–1182. doi: 10.1200/JCO.1997.15.3.1171. [DOI] [PubMed] [Google Scholar]
- 11.Tang X.X., Zhao H., Kung B., Kim D.Y., Hicks S.L., Cohn S.L., Cheung N.-K., Seeger R.C., Evans A.E., Ikegaki N. The MYCN Enigma: Significance of MYCN Expression in Neuroblastoma. Cancer Res. 2006;66:2826–2833. doi: 10.1158/0008-5472.CAN-05-0854. [DOI] [PubMed] [Google Scholar]
- 12.Nisen P.D., Waber P.G., Rich M.A., Pierce S., Garvin J.R., Gilbert F., Lanzkowsky P. N-Myc Oncogene RNA Expression in Neuroblastoma. J. Natl. Cancer Inst. 1988;80:1633–1637. doi: 10.1093/jnci/80.20.1633. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs J.F.M., van Bokhoven H., van Leeuwen F.N., Hulsbergen-van de Kaa C.A., de Vries I.J.M., Adema G.J., Hoogerbrugge P.M., de Brouwer A.P.M. Regulation of MYCN Expression in Human Neuroblastoma Cells. BMC Cancer. 2009;9:239. doi: 10.1186/1471-2407-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott D., Elsden J., Pearson A., Lunec J. Genes Co-Amplified with MYCN in Neuroblastoma: Silent Passengers or Co-Determinants of Phenotype? Cancer Lett. 2003;197:81–86. doi: 10.1016/S0304-3835(03)00086-7. [DOI] [PubMed] [Google Scholar]
- 15.Schwab M. MYCN in Neuronal Tumours. Cancer Lett. 2004;204:179–187. doi: 10.1016/S0304-3835(03)00454-3. [DOI] [PubMed] [Google Scholar]
- 16.Gherardi S., Valli E., Erriquez D., Perini G. MYCN-Mediated Transcriptional Repression in Neuroblastoma: The Other Side of the Coin. Front. Oncol. 2013;3:42. doi: 10.3389/fonc.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zafar A., Wang W., Liu G., Xian W., McKeon F., Zhou J., Zhang R. Targeting the P53-MDM2 Pathway for Neuroblastoma Therapy: Rays of Hope. Cancer Lett. 2021;496:16–29. doi: 10.1016/j.canlet.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolpaw A.J., Bayliss R., Büchel G., Dang C.V., Eilers M., Gustafson W.C., Hansen G.H., Jura N., Knapp S., Lemmon M.A., et al. Drugging the “Undruggable” MYCN Oncogenic Transcription Factor: Overcoming Previous Obstacles to Impact Childhood Cancers. Cancer Res. 2021;81:1627–1632. doi: 10.1158/0008-5472.CAN-20-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue Z.-X., Huang C., Gao C., Xing T.-Y., Liu S.-G., Li X.-J., Zhao Q., Wang X.-S., Zhao W., Jin M., et al. MYCN Amplification Predicts Poor Prognosis Based on Interphase Fluorescence in Situ Hybridization Analysis of Bone Marrow Cells in Bone Marrow Metastases of Neuroblastoma. Cancer Cell Int. 2017;17:43. doi: 10.1186/s12935-017-0412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell K., Gastier-Foster J.M., Mann M., Naranjo A.H., van Ryn C., Bagatell R., Matthay K.K., London W.B., Irwin M.S., Shimada H., et al. Association of MYCN Copy Number with Clinical Features, Tumor Biology, and Outcomes in Neuroblastoma: A Report from the Children’s Oncology Group. Cancer. 2017;123:4224–4235. doi: 10.1002/cncr.30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swift C.C., Eklund M.J., Kraveka J.M., Alazraki A.L. Updates in Diagnosis, Management, and Treatment of Neuroblastoma. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2018;38:566–580. doi: 10.1148/rg.2018170132. [DOI] [PubMed] [Google Scholar]
- 22.Zhan Y., Shi S., Ehlerding E.B., Graves S.A., Goel S., Engle J.W., Liang J., Tian J., Cai W. Radiolabeled, Antibody-Conjugated Manganese Oxide Nanoparticles for Tumor Vasculature Targeted Positron Emission Tomography and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces. 2017;9:38304–38312. doi: 10.1021/acsami.7b12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar-Sever Z., Biassoni L., Shulkin B., Kong G., Hofman M.S., Lopci E., Manea I., Koziorowski J., Castellani R., Boubaker A., et al. Guidelines on Nuclear Medicine Imaging in Neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:2009–2024. doi: 10.1007/s00259-018-4070-8. [DOI] [PubMed] [Google Scholar]
- 24.Kroiss A.S. Current Status of Functional Imaging in Neuroblastoma, Pheochromocytoma, and Paraganglioma Disease. Wien. Med. Wochenschr. 2019;169:25–32. doi: 10.1007/s10354-018-0658-7. [DOI] [PubMed] [Google Scholar]
- 25.Sarioglu F.C., Salman M., Guleryuz H., Ozer E., Cecen E., Ince D., Olgun N. Radiological Staging in Neuroblastoma: Computed Tomography or Magnetic Resonance Imaging? Pol. J. Radiol. 2019;84:e46–e53. doi: 10.5114/pjr.2019.82736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sofka C.M., Semelka R.C., Kelekis N.L., Worawattanakul S., Chung C.J., Gold S., Fordham L.A. Magnetic Resonance Imaging of Neuroblastoma Using Current Techniques. Magn. Reson. Imaging. 1999;17:193–198. doi: 10.1016/S0730-725X(98)00102-7. [DOI] [PubMed] [Google Scholar]
- 27.Wu H., Wu C., Zheng H., Wang L., Guan W., Duan S., Wang D. Radiogenomics of Neuroblastoma in Pediatric Patients: CT-Based Radiomics Signature in Predicting MYCN Amplification. Eur. Radiol. 2021;31:3080–3089. doi: 10.1007/s00330-020-07246-1. [DOI] [PubMed] [Google Scholar]
- 28.Sharp S.E., Parisi M.T., Gelfand M.J., Yanik G.A., Shulkin B.L. Functional-Metabolic Imaging of Neuroblastoma. Q. J. Nucl. Med. Mol. Imaging. 2013;57:6–20. [PubMed] [Google Scholar]
- 29.Campbell K., Shyr D., Bagatell R., Fischer M., Nakagawara A., Nieto A.C., Brodeur G.M., Matthay K.K., London W.B., DuBois S.G. Comprehensive Evaluation of Context Dependence of the Prognostic Impact of MYCN Amplification in Neuroblastoma: A Report from the International Neuroblastoma Risk Group (INRG) Project. Pediatr. Blood Cancer. 2019;66:e27819. doi: 10.1002/pbc.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanishevski D., McCarville M.B., Doubrovin M., Spiegl H.R., Zhao X., Lu Z., Federico S.M., Furman W.L., Murphy A.J., Davidoff A.M. Impact of MYCN Status on Response of High-Risk Neuroblastoma to Neoadjuvant Chemotherapy. J. Pediatr. Surg. 2020;55:130–134. doi: 10.1016/j.jpedsurg.2019.09.067. [DOI] [PubMed] [Google Scholar]
- 31.Chan H.S., Gallie B.L., DeBoer G., Haddad G., Ikegaki N., Dimitroulakos J., Yeger H., Ling V. MYCN Protein Expression as a Predictor of Neuroblastoma Prognosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1997;3:1699–1706. [PubMed] [Google Scholar]
- 32.Van Heerden J., Esterhuizen T.M., Hendricks M., Poole J., Büchner A., Naidu G., du Plessis J., van Emmenes B., Uys R., Hadley G.P., et al. Age at Diagnosis as a Prognostic Factor in South African Children with Neuroblastoma. Pediatr. Blood Cancer. 2021;68:e28878. doi: 10.1002/pbc.28878. [DOI] [PubMed] [Google Scholar]
- 33.Sokol E., Desai A.V., Applebaum M.A., Valteau-Couanet D., Park J.R., Pearson A.D.J., Schleiermacher G., Irwin M.S., Hogarty M., Naranjo A., et al. Age, Diagnostic Category, Tumor Grade, and Mitosis-Karyorrhexis Index are Independently Prognostic in Neuroblastoma: An INRG Project. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:1906–1918. doi: 10.1200/JCO.19.03285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathew P., Valentine M.B., Bowman L.C., Rowe S.T., Nash M.B., Valentine V.A., Cohn S.L., Castleberry R.P., Brodeur G.M., Look A.T. Detection of MYCN Gene Amplification in Neuroblastoma by Fluorescence in Situ Hybridization: A Pediatric Oncology Group Study. Neoplasia. 2001;3:105–109. doi: 10.1038/sj.neo.7900146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Squire J.A., Thorner P.A., Marrano P.A., Parkinson D.I., Ng Y.K., Gerrie B.L., Chilton-Macneill S., Zielenska M. Identification of MYCN Copy Number Heterogeneity by Direct FISH Analysis of Neuroblastoma Preparations. Mol. Diagn. 1996;1:281–289. doi: 10.1016/S1084-8592(96)70010-3. [DOI] [PubMed] [Google Scholar]
- 36.Marrano P., Irwin M.S., Thorner P.S. Heterogeneity of MYCN Amplification in Neuroblastoma at Diagnosis, Treatment, Relapse, and Metastasis. Genes Chromosom. Cancer. 2017;56:28–41. doi: 10.1002/gcc.22398. [DOI] [PubMed] [Google Scholar]
- 37.Marrugo-Ramírez J., Mir M., Samitier J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int. J. Mol. Sci. 2018;19:2877. doi: 10.3390/ijms19102877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Namløs H.M., Boye K., Mishkin S.J., Barøy T., Lorenz S., Bjerkehagen B., Stratford E.W., Munthe E., Kudlow B.A., Myklebost O., et al. Noninvasive Detection of CtDNA Reveals Intratumor Heterogeneity and is Associated with Tumor Burden in Gastrointestinal Stromal Tumor. Mol. Cancer Ther. 2018;17:2473–2480. doi: 10.1158/1535-7163.MCT-18-0174. [DOI] [PubMed] [Google Scholar]
- 39.Combaret V., Audoynaud C., Iacono I., Favrot M.-C., Schell M., Bergeron C., Puisieux A. Circulating MYCN DNA as a Tumor-Specific Marker in Neuroblastoma Patients. Cancer Res. 2002;62:3646–3648. [PubMed] [Google Scholar]
- 40.Combaret V., Bergeron C., Noguera R., Iacono I., Puisieux A. Circulating MYCN DNA Predicts MYCN-Amplification in Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005;23:8919–8920; author reply 8920. doi: 10.1200/JCO.2005.04.0170. [DOI] [PubMed] [Google Scholar]
- 41.Trigg R.M., Turner S.D., Shaw J.A., Jahangiri L. Diagnostic Accuracy of Circulating-Free DNA for the Determination of MYCN Amplification Status in Advanced-Stage Neuroblastoma: A Systematic Review and Meta-Analysis. Br. J. Cancer. 2020;122:1077–1084. doi: 10.1038/s41416-020-0740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gotoh T., Hosoi H., Iehara T., Kuwahara Y., Osone S., Tsuchiya K., Ohira M., Nakagawara A., Kuroda H., Sugimoto T. Prediction of MYCN Amplification in Neuroblastoma Using Serum DNA and Real-Time Quantitative Polymerase Chain Reaction. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005;23:5205–5210. doi: 10.1200/JCO.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Iehara T., Yagyu S., Gotoh T., Ouchi K., Yoshida H., Miyachi M., Kikuchi K., Sugimoto T., Hosoi H. A Prospective Evaluation of Liquid Biopsy for Detecting MYCN Amplification in Neuroblastoma Patients. Jpn. J. Clin. Oncol. 2019;49:743–748. doi: 10.1093/jjco/hyz063. [DOI] [PubMed] [Google Scholar]
- 44.Pinzani P., D’Argenio V., del Re M., Pellegrini C., Cucchiara F., Salvianti F., Galbiati S. Updates on Liquid Biopsy: Current Trends and Future Perspectives for Clinical Application in Solid Tumors. Clin. Chem. Lab. Med. 2021;59:1181–1200. doi: 10.1515/cclm-2020-1685. [DOI] [PubMed] [Google Scholar]
- 45.Rifatbegovic F., Frech C., Abbasi M.R., Taschner-Mandl S., Weiss T., Schmidt W.M., Schmidt I., Ladenstein R., Ambros I.M., Ambros P.F. Neuroblastoma Cells Undergo Transcriptomic Alterations upon Dissemination into the Bone Marrow and Subsequent Tumor Progression. Int. J. Cancer. 2018;142:297–307. doi: 10.1002/ijc.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reza K.K., Dey S., Wuethrich A., Wang J., Behren A., Antaw F., Wang Y., Sina A.A.I., Trau M. In Situ Single Cell Proteomics Reveals Circulating Tumor Cell Heterogeneity during Treatment. ACS Nano. 2021;15:11231–11243. doi: 10.1021/acsnano.0c10008. [DOI] [PubMed] [Google Scholar]
- 47.Lodrini M., Wünschel J., Thole-Kliesch T.M., Grimaldi M., Sprüssel A., Linke R.B., Hollander J.F., Tiburtius D., Künkele A., Schulte J.H., et al. Circulating Cell-Free DNA Assessment in Biofluids from Children with Neuroblastoma Demonstrates Feasibility and Potential for Minimally Invasive Molecular Diagnostics. Cancers. 2022;14:2080. doi: 10.3390/cancers14092080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beltran H., Jendrisak A., Landers M., Mosquera J.M., Kossai M., Louw J., Krupa R., Graf R.P., Schreiber N.A., Nanus D.M., et al. The Initial Detection and Partial Characterization of Circulating Tumor Cells in Neuroendocrine Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016;22:1510–1519. doi: 10.1158/1078-0432.CCR-15-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw J.A., Guttery D.S., Hills A., Fernandez-Garcia D., Page K., Rosales B.M., Goddard K.S., Hastings R.K., Luo J., Ogle O., et al. Mutation Analysis of Cell-Free DNA and Single Circulating Tumor Cells in Metastatic Breast Cancer Patients with High Circulating Tumor Cell Counts. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23:88–96. doi: 10.1158/1078-0432.CCR-16-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith V., Foster J. High-Risk Neuroblastoma Treatment Review. Children. 2018;5:114. doi: 10.3390/children5090114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DuBois S.G., Macy M.E., Henderson T.O. High-Risk and Relapsed Neuroblastoma: Toward More Cures and Better Outcomes. Am. Soc. Clin. Oncol. Educ. Book. 2022;42:768–780. doi: 10.1200/EDBK_349783. [DOI] [PubMed] [Google Scholar]
- 52.Tolbert V.P., Matthay K.K. Neuroblastoma: Clinical and Biological Approach to Risk Stratification and Treatment. Cell Tissue Res. 2018;372:195–209. doi: 10.1007/s00441-018-2821-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baker D.L., Schmidt M.L., Cohn S.L., Maris J.M., London W.B., Buxton A., Stram D., Castleberry R.P., Shimada H., Sandler A., et al. Outcome after Reduced Chemotherapy for Intermediate-Risk Neuroblastoma. N. Engl. J. Med. 2010;363:1313–1323. doi: 10.1056/NEJMoa1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubie H., de Bernardi B., Gerrard M., Canete A., Ladenstein R., Couturier J., Ambros P., Munzer C., Pearson A.D.J., Garaventa A., et al. Excellent Outcome with Reduced Treatment in Infants with Nonmetastatic and Unresectable Neuroblastoma without MYCN Amplification: Results of the Prospective INES 99.1. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29:449–455. doi: 10.1200/JCO.2010.29.5196. [DOI] [PubMed] [Google Scholar]
- 55.Strother D.R., London W.B., Schmidt M.L., Brodeur G.M., Shimada H., Thorner P., Collins M.H., Tagge E., Adkins S., Reynolds C.P., et al. Outcome after Surgery Alone or with Restricted Use of Chemotherapy for Patients with Low-Risk Neuroblastoma: Results of Children’s Oncology Group Study P9641. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:1842–1848. doi: 10.1200/JCO.2011.37.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matthay K.K., Villablanca J.G., Seeger R.C., Stram D.O., Harris R.E., Ramsay N.K., Swift P., Shimada H., Black C.T., Brodeur G.M., et al. Treatment of High-Risk Neuroblastoma with Intensive Chemotherapy, Radiotherapy, Autologous Bone Marrow Transplantation, and 13-Cis-Retinoic Acid. Children’s Cancer Group. N. Engl. J. Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 57.Yanik G., Naranjo A., Parisi M.T., Shulkin B.L., Nadel H., Gelfand M.J., Ladenstein R., Boubaker A., Poetschger U., Valteau-Couanet D., et al. Impact of Post-Induction Curie Scores in High-Risk Neuroblastoma. Biol. Blood Marrow Transplant. 2015;21:S107. doi: 10.1016/j.bbmt.2014.11.131. [DOI] [Google Scholar]
- 58.Yanik G.A., Parisi M.T., Naranjo A., Nadel H., Gelfand M.J., Park J.R., Ladenstein R.L., Poetschger U., Boubaker A., Valteau-Couanet D., et al. Validation of Postinduction Curie Scores in High-Risk Neuroblastoma: A Children’s Oncology Group and SIOPEN Group Report on SIOPEN/HR-NBL1. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018;59:502–508. doi: 10.2967/jnumed.117.195883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rojas Y., Jaramillo S., Lyons K., Mahmood N., Wu M.-F., Liu H., Vasudevan S.A., Guillerman R.P., Louis C.U., Russell H.V., et al. The Optimal Timing of Surgical Resection in High-Risk Neuroblastoma. J. Pediatr. Surg. 2016;51:1665–1669. doi: 10.1016/j.jpedsurg.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Vollmer K., Gfroerer S., Theilen T.-M., Bochennek K., Klingebiel T., Rolle U., Fiegel H. Radical Surgery Improves Survival in Patients with Stage 4 Neuroblastoma. World J. Surg. 2018;42:1877–1884. doi: 10.1007/s00268-017-4340-9. [DOI] [PubMed] [Google Scholar]
- 61.Englum B.R., Rialon K.L., Speicher P.J., Gulack B., Driscoll T.A., Kreissman S.G., Rice H.E. Value of Surgical Resection in Children with High-Risk Neuroblastoma. Pediatr. Blood Cancer. 2015;62:1529–1535. doi: 10.1002/pbc.25504. [DOI] [PubMed] [Google Scholar]
- 62.Qi Y., Zhan J. Roles of Surgery in the Treatment of Patients with High-Risk Neuroblastoma in the Children Oncology Group Study: A Systematic Review and Meta-Analysis. Front. Pediatr. 2021;9:1059. doi: 10.3389/fped.2021.706800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Von Allmen D., Davidoff A.M., London W.B., van Ryn C., Haas-Kogan D.A., Kreissman S.G., Khanna G., Rosen N., Park J.R., la Quaglia M.P. Impact of Extent of Resection on Local Control and Survival in Patients from the COG A3973 Study with High-Risk Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017;35:208–216. doi: 10.1200/JCO.2016.67.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brodeur G.M., Seeger R.C., Barrett A., Berthold F., Castleberry R.P., D’Angio G., de Bernardi B., Evans A.E., Favrot M., Freeman A.I. International Criteria for Diagnosis, Staging, and Response to Treatment in Patients with Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1988;6:1874–1881. doi: 10.1200/JCO.1988.6.12.1874. [DOI] [PubMed] [Google Scholar]
- 65.Ryan A.L., Akinkuotu A., Pierro A., Morgenstern D.A., Irwin M.S. The Role of Surgery in High-Risk Neuroblastoma. J. Pediatr. Hematol. Oncol. 2020;42:1–7. doi: 10.1097/MPH.0000000000001607. [DOI] [PubMed] [Google Scholar]
- 66.Brodeur G.M., Pritchard J., Berthold F., Carlsen N.L., Castel V., Castelberry R.P., de Bernardi B., Evans A.E., Favrot M., Hedborg F. Revisions of the International Criteria for Neuroblastoma Diagnosis, Staging, and Response to Treatment. J. Clin. Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 67.Chui C. Effects of Preoperative Chemotherapy on Neuroblastoma with MYCN Amplification: A Surgeon’s Perspective. World J. Pediatr. Surg. 2020;3:e000129. doi: 10.1136/wjps-2020-000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adkins E.S., Sawin R., Gerbing R.B., London W.B., Matthay K.K., Haase G.M. Efficacy of Complete Resection for High-Risk Neuroblastoma: A Children’s Cancer Group Study. J. Pediatr. Surg. 2004;39:931–936. doi: 10.1016/j.jpedsurg.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 69.Varan A., Ali V., Kesik V., Vural K., Şenocak M.E., Emin Ş.M., Kale G., Gulsev K., Akyüz C., Canan A., et al. The Efficacy of Delayed Surgery in Children with High-Risk Neuroblastoma. J. Cancer Res. Ther. 2015;11:268–271. doi: 10.4103/0973-1482.151852. [DOI] [PubMed] [Google Scholar]
- 70.Fischer J., Pohl A., Volland R., Hero B., Dübbers M., Cernaianu G., Berthold F., von Schweinitz D., Simon T. Complete Surgical Resection Improves Outcome in INRG High-Risk Patients with Localized Neuroblastoma Older than 18 Months. BMC Cancer. 2017;17:520. doi: 10.1186/s12885-017-3493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peinemann F., Tushabe D.A., van Dalen E.C., Berthold F. Rapid COJEC versus Standard Induction Therapies for High-Risk Neuroblastoma. Cochrane Database Syst. Rev. 2015;5:CD010774. doi: 10.1002/14651858.CD010774.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pearson A.D.J., Pinkerton C.R., Lewis I.J., Imeson J., Ellershaw C., Machin D., European Neuroblastoma Study Group. Children’s Cancer and Leukaemia Group (CCLG formerly United Kingdom Children’s Cancer Study Group) High-Dose Rapid and Standard Induction Chemotherapy for Patients Aged over 1 Year with Stage 4 Neuroblastoma: A Randomised Trial. Lancet Oncol. 2008;9:247–256. doi: 10.1016/S1470-2045(08)70069-X. [DOI] [PubMed] [Google Scholar]
- 73.Hobbie W.L., Li Y., Carlson C., Goldfarb S., Laskin B., Denburg M., Goldmuntz E., Mostoufi-Moab S., Wilkes J., Smith K., et al. Late Effects in Survivors of High-Risk Neuroblastoma Following Stem Cell Transplant with and without Total Body Irradiation. Pediatr. Blood Cancer. 2022;69:e29537. doi: 10.1002/pbc.29537. [DOI] [PubMed] [Google Scholar]
- 74.Armstrong A.E., Danner-Koptik K., Golden S., Schneiderman J., Kletzel M., Reichek J., Gosiengfiao Y. Late Effects in Pediatric High-Risk Neuroblastoma Survivors After Intensive Induction Chemotherapy Followed by Myeloablative Consolidation Chemotherapy and Triple Autologous Stem Cell Transplants. J. Pediatr. Hematol. Oncol. 2018;40:31–35. doi: 10.1097/MPH.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 75.Bertolini P., Lassalle M., Mercier G., Raquin M.A., Izzi G., Corradini N., Hartmann O. Platinum Compound-Related Ototoxicity in Children: Long-Term Follow-up Reveals Continuous Worsening of Hearing Loss. J. Pediatr. Hematol. Oncol. 2004;26:649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 76.Gurney J.G., Tersak J.M., Ness K.K., Landier W., Matthay K.K., Schmidt M.L., Children’s Oncology Group Hearing Loss, Quality of Life, and Academic Problems in Long-Term Neuroblastoma Survivors: A Report from the Children’s Oncology Group. Pediatrics. 2007;120:e1229–e1236. doi: 10.1542/peds.2007-0178. [DOI] [PubMed] [Google Scholar]
- 77.Sklar C.A., Mertens A.C., Mitby P., Whitton J., Stovall M., Kasper C., Mulder J., Green D., Nicholson H.S., Yasui Y., et al. Premature Menopause in Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. JNCI J. Natl. Cancer Inst. 2006;98:890–896. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 78.Laverdière C., Cheung N.-K.V., Kushner B.H., Kramer K., Modak S., LaQuaglia M.P., Wolden S., Ness K.K., Gurney J.G., Sklar C.A. Long-Term Complications in Survivors of Advanced Stage Neuroblastoma. Pediatr. Blood Cancer. 2005;45:324–332. doi: 10.1002/pbc.20331. [DOI] [PubMed] [Google Scholar]
- 79.Mulrooney D.A., Armstrong G.T., Huang S., Ness K.K., Ehrhardt M.J., Joshi V.M., Plana J.C., Soliman E.Z., Green D.M., Srivastava D., et al. Cardiac Outcomes in Adult Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy: A Cross-Sectional Study. Ann. Intern. Med. 2016;164:93–101. doi: 10.7326/M15-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Armstrong G.T., Oeffinger K.C., Chen Y., Kawashima T., Yasui Y., Leisenring W., Stovall M., Chow E.J., Sklar C.A., Mulrooney D.A., et al. Modifiable Risk Factors and Major Cardiac Events among Adult Survivors of Childhood Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Applebaum M.A., Vaksman Z., Lee S.M., Hungate E.A., Henderson T.O., London W.B., Pinto N., Volchenboum S.L., Park J.R., Naranjo A., et al. Neuroblastoma Survivors are at Increased Risk for Second Malignancies: A Report from the International Neuroblastoma Risk Group Project. Eur. J. Cancer. 2017;72:177–185. doi: 10.1016/j.ejca.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Federico S.M., Allewelt H.B., Spunt S.L., Hudson M.M., Wu J., Billups C.A., Jenkins J., Santana V.M., Furman W.L., McGregor L.M. Subsequent Malignant Neoplasms in Pediatric Patients Initially Diagnosed with Neuroblastoma. J. Pediatr. Hematol. Oncol. 2015;37:e6–e12. doi: 10.1097/MPH.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng D.J., Krull K.R., Chen Y., Diller L., Yasui Y., Leisenring W., Brouwers P., Howell R., Lai J.-S., Balsamo L., et al. Long-Term Psychological and Educational Outcomes for Survivors of Neuroblastoma: A Report from the Childhood Cancer Survivor Study. Cancer. 2018;124:3220–3230. doi: 10.1002/cncr.31379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cohen L.E., Gordon J.H., Popovsky E.Y., Gunawardene S., Duffey-Lind E., Lehmann L.E., Diller L.R. Late Effects in Children Treated with Intensive Multimodal Therapy for High-Risk Neuroblastoma: High Incidence of Endocrine and Growth Problems. Bone Marrow Transplant. 2014;49:502–508. doi: 10.1038/bmt.2013.218. [DOI] [PubMed] [Google Scholar]
- 85.Institute of Medicine (US) National Research Council (US) National Cancer Policy Board . In: Childhood Cancer Survivorship: Improving Care and Quality of Life. Hewitt M., Weiner S.L., Simone J.V., editors. National Academies Press (US); Washington, DC, USA: 2003. [PubMed] [Google Scholar]
- 86.Frei E., Teicher B.A., Holden S.A., Cathcart K.N., Wang Y.Y. Preclinical Studies and Clinical Correlation of the Effect of Alkylating Dose. Cancer Res. 1988;48:6417–6423. [PubMed] [Google Scholar]
- 87.Cheung N.V., Heller G. Chemotherapy Dose Intensity Correlates Strongly with Response, Median Survival, and Median Progression-Free Survival in Metastatic Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1991;9:1050–1058. doi: 10.1200/JCO.1991.9.6.1050. [DOI] [PubMed] [Google Scholar]
- 88.Daniel D., Crawford J. Myelotoxicity from Chemotherapy. Semin. Oncol. 2006;33:74–85. doi: 10.1053/j.seminoncol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Fish J., Grupp S. Stem Cell Transplantation for Neuroblastoma. Bone Marrow Transplant. 2008;41:159–165. doi: 10.1038/sj.bmt.1705929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mora J. Autologous Stem-Cell Transplantation for High-Risk Neuroblastoma: Historical and Critical Review. Cancers. 2022;14:2572. doi: 10.3390/cancers14112572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yalçin B., Kremer L.C.M., van Dalen E.C. High-Dose Chemotherapy and Autologous Haematopoietic Stem Cell Rescue for Children with High-Risk Neuroblastoma. Cochrane Database Syst. Rev. 2015;8:CD006301. doi: 10.1002/14651858.CD006301.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park J.R., Kreissman S.G., London W.B., Naranjo A., Cohn S.L., Hogarty M.D., Tenney S.C., Haas-Kogan D., Shaw P.J., Kraveka J.M., et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients with High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA. 2019;322:746–755. doi: 10.1001/jama.2019.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Powell J.L., Bunin N.J., Callahan C., Aplenc R., Griffin G., Grupp S.A. An Unexpectedly High Incidence of Epstein-Barr Virus Lymphoproliferative Disease after CD34+ Selected Autologous Peripheral Blood Stem Cell Transplant in Neuroblastoma. Bone Marrow Transplant. 2004;33:651–657. doi: 10.1038/sj.bmt.1704402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khan S., AlSayyad K., Siddiqui K., AlAnazi A., AlSeraihy A., AlAhmari A., ElSolh H., Ghemlas I., AlSaedi H., AlJefri A., et al. Pediatric High Risk Neuroblastoma with Autologous Stem Cell Transplant—20 Years of Experience. Int. J. Pediatr. Adolesc. Med. 2021;8:253–257. doi: 10.1016/j.ijpam.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rill D.R., Santana V.M., Roberts W.M., Nilson T., Bowman L.C., Krance R.A., Heslop H.E., Moen R.C., Ihle J.N., Brenner M.K. Direct Demonstration That Autologous Bone Marrow Transplantation for Solid Tumors Can Return a Multiplicity of Tumorigenic Cells. Blood. 1994;84:380–383. doi: 10.1182/blood.V84.2.380.380. [DOI] [PubMed] [Google Scholar]
- 96.Simon T., Hero B., Schulte J.H., Deubzer H., Hundsdoerfer P., von Schweinitz D., Fuchs J., Schmidt M., Prasad V., Krug B., et al. 2017 GPOH Guidelines for Diagnosis and Treatment of Patients with Neuroblastic Tumors. Klin. Padiatr. 2017;229:147–167. doi: 10.1055/s-0043-103086. [DOI] [PubMed] [Google Scholar]
- 97.Braunstein S.E., London W.B., Kreissman S.G., Villablanca J.G., Davidoff A.M., DeSantes K., Castleberry R.P., Murray K., Diller L., Matthay K., et al. Role of the Extent of Prophylactic Regional Lymph Node Radiotherapy on Survival in High-Risk Neuroblastoma: A Report from the COG A3973 Study. Pediatr. Blood Cancer. 2019;66:e27736. doi: 10.1002/pbc.27736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jo J.H., Ahn S.D., Koh M., Kim J.H., Lee S.-W., Song S.Y., Yoon S.M., Kim Y.S., Kim S.S., Park J.-H., et al. Patterns of Recurrence after Radiation Therapy for High-Risk Neuroblastoma. Radiat. Oncol. J. 2019;37:224–231. doi: 10.3857/roj.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ferris M.J., Danish H., Switchenko J.M., Deng C., George B.A., Goldsmith K.C., Wasilewski K.J., Cash W.T., Khan M.K., Eaton B.R., et al. Favorable Local Control from Consolidative Radiation Therapy in High-Risk Neuroblastoma Despite Gross Residual Disease, Positive Margins, or Nodal Involvement. Int. J. Radiat. Oncol. Biol. Phys. 2017;97:806–812. doi: 10.1016/j.ijrobp.2016.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jazmati D., Butzer S., Hero B., Doyen J., Ahmad Khalil D., Steinmeier T., Schulze Schleithoff S., Eggert A., Simon T., Timmermann B. Long-Term Follow-up of Children with Neuroblastoma Receiving Radiotherapy to Metastatic Lesions within the German Neuroblastoma Trials NB97 and NB 2004. Strahlenther. Onkol. 2021;197:683–689. doi: 10.1007/s00066-020-01718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu K.X., Naranjo A., Zhang F.F., DuBois S.G., Braunstein S.E., Voss S.D., Khanna G., London W.B., Doski J.J., Geiger J.D., et al. Prospective Evaluation of Radiation Dose Escalation in Patients with High-Risk Neuroblastoma and Gross Residual Disease After Surgery: A Report from the Children’s Oncology Group ANBL0532 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:2741–2752. doi: 10.1200/JCO.19.03316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao Q., Liu Y., Zhang Y., Meng L., Wei J., Wang B., Wang H., Xin Y., Dong L., Jiang X. Role and Toxicity of Radiation Therapy in Neuroblastoma Patients: A Literature Review. Crit. Rev. Oncol. Hematol. 2020;149:102924. doi: 10.1016/j.critrevonc.2020.102924. [DOI] [PubMed] [Google Scholar]
- 103.Hattangadi J.A., Rombi B., Yock T.I., Broussard G., Friedmann A.M., Huang M., Chen Y.-L.E., Lu H.-M., Kooy H., MacDonald S.M. Proton Radiotherapy for High-Risk Pediatric Neuroblastoma: Early Outcomes and Dose Comparison. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:1015–1022. doi: 10.1016/j.ijrobp.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 104.Weiss B.D., Yanik G., Naranjo A., Zhang F.F., Fitzgerald W., Shulkin B.L., Parisi M.T., Russell H., Grupp S., Pater L., et al. A Safety and Feasibility Trial of 131I-MIBG in Newly Diagnosed High-Risk Neuroblastoma: A Children’s Oncology Group Study. Pediatr. Blood Cancer. 2021;68:e29117. doi: 10.1002/pbc.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weyl Ben-Arush M., Ben Barak A., Bar-Deroma R., Ash S., Goldstein G., Golan H., Houri H., Waldman D., Nevo N., Bar Shalom R., et al. Targeted Therapy with Low Doses of 131I-MIBG is Effective for Disease Palliation in Highly Refractory Neuroblastoma. Isr. Med. Assoc. J. 2013;15:31–34. [PubMed] [Google Scholar]
- 106.Genolla J., Rodriguez T., Minguez P., Lopez-Almaraz R., Llorens V., Echebarria A. Dosimetry-Based High-Activity Therapy with 131I-Metaiodobenzylguanidine (131I-MIBG) and Topotecan for the Treatment of High-Risk Refractory Neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:1567–1575. doi: 10.1007/s00259-019-04291-x. [DOI] [PubMed] [Google Scholar]
- 107.Ducassou A., Gambart M., Munzer C., Padovani L., Carrie C., Haas-Kogan D., Bernier-Chastagner V., Demoor C., Claude L., Helfre S., et al. Long-Term Side Effects of Radiotherapy for Pediatric Localized Neuroblastoma: Results from Clinical Trials NB90 and NB94. Strahlenther. Onkol. Organ Dtsch. Rontgenges. Al. 2015;191:604–612. doi: 10.1007/s00066-015-0837-z. [DOI] [PubMed] [Google Scholar]
- 108.Yu J.I., Lim D.H., Jung S.H., Sung K.W., Yoo S.-Y., Nam H. The Effects of Radiation Therapy on Height and Spine MRI Characteristics in Children with Neuroblastoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2015;114:384–388. doi: 10.1016/j.radonc.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 109.Paulino A.C., Mayr N.A., Simon J.H., Buatti J.M. Locoregional Control in Infants with Neuroblastoma: Role of Radiation Therapy and Late Toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2002;52:1025–1031. doi: 10.1016/S0360-3016(01)02713-4. [DOI] [PubMed] [Google Scholar]
- 110.Sutton E.J., Tong R.T., Gillis A.M., Henning T.D., Weinberg V.A., Boddington S., Haas-Kogan D.A., Matthay K., Sha V., Gooding C., et al. Decreased Aortic Growth and Middle Aortic Syndrome in Patients with Neuroblastoma after Radiation Therapy. Pediatr. Radiol. 2009;39:1194–1202. doi: 10.1007/s00247-009-1351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stauder M.C., Laack N.N.I., Moir C.R., Schomberg P.J. Excellent Local Control and Survival after Intraoperative and External Beam Radiotherapy for Pediatric Solid Tumors: Long-Term Follow-up of the Mayo Clinic Experience. J. Pediatr. Hematol. Oncol. 2011;33:350–355. doi: 10.1097/MPH.0b013e3182148dad. [DOI] [PubMed] [Google Scholar]
- 112.Massimino M., Bode U., Biassoni V., Fleischhack G. Nimotuzumab for Pediatric Diffuse Intrinsic Pontine Gliomas. Expert Opin. Biol. Ther. 2011;11:247–256. doi: 10.1517/14712598.2011.546341. [DOI] [PubMed] [Google Scholar]
- 113.Baroni L.V., Alderete D., Solano-Paez P., Rugilo C., Freytes C., Laughlin S., Fonseca A., Bartels U., Tabori U., Bouffet E., et al. Bevacizumab for Pediatric Radiation Necrosis. Neuro-Oncol. Pract. 2020;7:409–414. doi: 10.1093/nop/npz072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheung N.-K.V., Cheung I.Y., Kushner B.H., Ostrovnaya I., Chamberlain E., Kramer K., Modak S. Murine Anti-GD2 Monoclonal Antibody 3F8 Combined with Granulocyte-Macrophage Colony-Stimulating Factor and 13-Cis-Retinoic Acid in High-Risk Patients with Stage 4 Neuroblastoma in First Remission. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Majzner R.G., Heitzeneder S., Mackall C.L. Harnessing the Immunotherapy Revolution for the Treatment of Childhood Cancers. Cancer Cell. 2017;31:476–485. doi: 10.1016/j.ccell.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 116.Ladisch S., Wu Z.L., Feig S., Ulsh L., Schwartz E., Floutsis G., Wiley F., Lenarsky C., Seeger R. Shedding of GD2 Ganglioside by Human Neuroblastoma. Int. J. Cancer. 1987;39:73–76. doi: 10.1002/ijc.2910390113. [DOI] [PubMed] [Google Scholar]
- 117.Sabbih G.O., Danquah M.K. Neuroblastoma GD2 Expression and Computational Analysis of Aptamer-Based Bioaffinity Targeting. Int. J. Mol. Sci. 2021;22:9101. doi: 10.3390/ijms22169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mujoo K., Cheresh D.A., Yang H.M., Reisfeld R.A. Disialoganglioside GD2 on Human Neuroblastoma Cells: Target Antigen for Monoclonal Antibody-Mediated Cytolysis and Suppression of Tumor Growth. Cancer Res. 1987;47:1098–1104. [PubMed] [Google Scholar]
- 119.Ahmed M., Cheung N.-K.V. Engineering Anti-GD2 Monoclonal Antibodies for Cancer Immunotherapy. FEBS Lett. 2014;588:288–297. doi: 10.1016/j.febslet.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 120.Cheung N.K., Saarinen U.M., Neely J.E., Landmeier B., Donovan D., Coccia P.F. Monoclonal Antibodies to a Glycolipid Antigen on Human Neuroblastoma Cells. Cancer Res. 1985;45:2642–2649. [PubMed] [Google Scholar]
- 121.Yu A.L., Gilman A.L., Ozkaynak M.F., London W.B., Kreissman S.G., Chen H.X., Smith M., Anderson B., Villablanca J.G., Matthay K.K., et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ladenstein R., Pötschger U., Valteau-Couanet D., Luksch R., Castel V., Ash S., Laureys G., Brock P., Michon J.M., Owens C., et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1) Cancers. 2020;12:309. doi: 10.3390/cancers12020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barker E., Mueller B.M., Handgretinger R., Herter M., Yu A.L., Reisfeld R.A. Effect of a Chimeric Anti-Ganglioside GD2 Antibody on Cell-Mediated Lysis of Human Neuroblastoma Cells. Cancer Res. 1991;51:144–149. [PubMed] [Google Scholar]
- 124.Uttenreuther-Fischer M.M., Huang C.S., Yu A.L. Pharmacokinetics of Human-Mouse Chimeric Anti-GD2 MAb Ch14.18 in a Phase I Trial in Neuroblastoma Patients. Cancer Immunol. Immunother. 1995;41:331–338. doi: 10.1007/BF01526552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Navid F., Sondel P.M., Barfield R., Shulkin B.L., Kaufman R.A., Allay J.A., Gan J., Hutson P., Seo S., Kim K., et al. Phase I Trial of a Novel Anti-GD2 Monoclonal Antibody, Hu14.18K322A, Designed to Decrease Toxicity in Children with Refractory or Recurrent Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014;32:1445–1452. doi: 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheung I.Y., Kushner B.H., Modak S., Basu E.M., Roberts S.S., Cheung N.-K.V. Phase I Trial of Anti-GD2 Monoclonal Antibody Hu3F8 plus GM-CSF: Impact of Body Weight, Immunogenicity and Anti-GD2 Response on Pharmacokinetics and Survival. Oncoimmunology. 2017;6:e1358331. doi: 10.1080/2162402X.2017.1358331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Markham A. Naxitamab: First Approval. Drugs. 2021;81:291–296. doi: 10.1007/s40265-021-01467-4. [DOI] [PubMed] [Google Scholar]
- 128.Blom T., Lurvink R., Aleven L., Mensink M., Wolfs T., Dierselhuis M., van Eijkelenburg N., Kraal K., van Noesel M., van Grotel M., et al. Treatment-Related Toxicities During Anti-GD2 Immunotherapy in High-Risk Neuroblastoma Patients. Front. Oncol. 2020;10:601076. doi: 10.3389/fonc.2020.601076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sorkin L.S., Otto M., Baldwin W.M., Vail E., Gillies S.D., Handgretinger R., Barfield R.C., Yu H.M., Yu A.L. Anti-GD(2) with an FC Point Mutation Reduces Complement Fixation and Decreases Antibody-Induced Allodynia. Pain. 2010;149:135–142. doi: 10.1016/j.pain.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tse B.C., Navid F., Billups C.A., O’Donnell T., Hoehn M.E. Ocular Abnormalities in Patients Treated with a Novel Anti-GD2 Monoclonal Antibody, Hu14.18K322A. J. Am. Assoc. Pediatric Ophthalmol. Strabismus. 2015;19:112–115. doi: 10.1016/j.jaapos.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yuki N., Yamada M., Tagawa Y., Takahashi H., Handa S. Pathogenesis of the Neurotoxicity Caused by Anti-GD2 Antibody Therapy. J. Neurol. Sci. 1997;149:127–130. doi: 10.1016/S0022-510X(97)05390-2. [DOI] [PubMed] [Google Scholar]
- 132.Ceylan K., Jahns L.J., Lode B.N., Ehlert K., Kietz S., Troschke-Meurer S., Siebert N., Lode H.N. Inflammatory Response and Treatment Tolerance of Long-Term Infusion of the Anti-GD2 Antibody Ch14.18/CHO in Combination with Interleukin-2 in Patients with High-Risk Neuroblastoma. Pediatr. Blood Cancer. 2018;65:e26967. doi: 10.1002/pbc.26967. [DOI] [PubMed] [Google Scholar]
- 133.Terme M., Dorvillius M., Cochonneau D., Chaumette T., Xiao W., Diccianni M.B., Barbet J., Yu A.L., Paris F., Sorkin L.S., et al. Chimeric Antibody c.8B6 to O-Acetyl-GD2 Mediates the Same Efficient Anti-Neuroblastoma Effects as Therapeutic Ch14.18 Antibody to GD2 without Antibody Induced Allodynia. PLoS ONE. 2014;9:e87210. doi: 10.1371/journal.pone.0087210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fleurence J., Fougeray S., Bahri M., Cochonneau D., Clémenceau B., Paris F., Heczey A., Birklé S. Targeting O-Acetyl-GD2 Ganglioside for Cancer Immunotherapy. J. Immunol. Res. 2017;2017:5604891. doi: 10.1155/2017/5604891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zaenglein A.L., Pathy A.L., Schlosser B.J., Alikhan A., Baldwin H.E., Berson D.S., Bowe W.P., Graber E.M., Harper J.C., Kang S., et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2016;74:945–973.e33. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 136.Veal G.J., Errington J., Rowbotham S.E., Illingworth N.A., Malik G., Cole M., Daly A.K., Pearson A.D.J., Boddy A.V. Adaptive Dosing Approaches to the Individualization of 13-Cis-Retinoic Acid (Isotretinoin) Treatment for Children with High-Risk Neuroblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19:469–479. doi: 10.1158/1078-0432.CCR-12-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reynolds C.P., Kane D.J., Einhorn P.A., Matthay K.K., Crouse V.L., Wilbur J.R., Shurin S.B., Seeger R.C. Response of Neuroblastoma to Retinoic Acid In Vitro and In Vivo. Prog. Clin. Biol. Res. 1991;366:203–211. [PubMed] [Google Scholar]
- 138.Armstrong J.L., Ruiz M., Boddy A.V., Redfern C.P.F., Pearson A.D.J., Veal G.J. Increasing the Intracellular Availability of All-Trans Retinoic Acid in Neuroblastoma Cells. Br. J. Cancer. 2005;92:696–704. doi: 10.1038/sj.bjc.6602398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reynolds C.P. Differentiating Agents in Pediatric Malignancies: Retinoids in Neuroblastoma. Curr. Oncol. Rep. 2000;2:511–518. doi: 10.1007/s11912-000-0104-y. [DOI] [PubMed] [Google Scholar]
- 140.Matthay K.K. Targeted Isotretinoin in Neuroblastoma: Kinetics, Genetics or Absorption. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19:311–313. doi: 10.1158/1078-0432.CCR-12-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reynolds C.P., Lemons R.S. Retinoid Therapy of Childhood Cancer. Hematol. Oncol. Clin. N. Am. 2001;15:867–910. doi: 10.1016/S0889-8588(05)70256-2. [DOI] [PubMed] [Google Scholar]
- 142.Pile H.D., Sadiq N.M. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Isotretinoin. [Google Scholar]
- 143.Pinto N., DuBois S.G., Marachelian A., Diede S.J., Taraseviciute A., Glade Bender J.L., Tsao-Wei D., Groshen S.G., Reid J.M., Haas-Kogan D.A., et al. Phase I Study of Vorinostat in Combination with Isotretinoin in Patients with Refractory/Recurrent Neuroblastoma: A New Approaches to Neuroblastoma Therapy (NANT) Trial. Pediatr. Blood Cancer. 2018;65:e27023. doi: 10.1002/pbc.27023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pennington B., Ren S., Barton S., Bacelar M., Edwards S.J. Dinutuximab Beta for Treating Neuroblastoma: An Evidence Review Group and Decision Support Unit Perspective of a NICE Single Technology Appraisal. PharmacoEconomics. 2019;37:985–993. doi: 10.1007/s40273-018-0744-0. [DOI] [PubMed] [Google Scholar]
- 145.Shusterman S., Naranjo A., van Ryn C., Hank J.A., Parisi M.T., Shulkin B.L., Servaes S., London W.B., Shimada H., Gan J., et al. Antitumor Activity and Tolerability of Hu14.18-IL2 with GMCSF and Isotretinoin in Recurrent or Refractory Neuroblastoma: A Children’s Oncology Group Phase II Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019;25:6044–6051. doi: 10.1158/1078-0432.CCR-19-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Whittle S.B., Smith V., Doherty E., Zhao S., McCarty S., Zage P.E. Overview and Recent Advances in the Treatment of Neuroblastoma. Expert Rev. Anticancer Ther. 2017;17:369–386. doi: 10.1080/14737140.2017.1285230. [DOI] [PubMed] [Google Scholar]
- 147.Ozkaynak M.F., Gilman A.L., London W.B., Naranjo A., Diccianni M.B., Tenney S.C., Smith M., Messer K.S., Seeger R., Reynolds C.P., et al. A Comprehensive Safety Trial of Chimeric Antibody 14.18 With GM-CSF, IL-2, and Isotretinoin in High-Risk Neuroblastoma Patients Following Myeloablative Therapy: Children’s Oncology Group Study ANBL0931. Front. Immunol. 2018;9:1355. doi: 10.3389/fimmu.2018.01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tonini G.P., Boni L., Pession A., Rogers D., Iolascon A., Basso G., Cordero di Montezemolo L., Casale F., Pession A., Perri P., et al. MYCN Oncogene Amplification in Neuroblastoma is Associated with Worse Prognosis, except in Stage 4s: The Italian Experience with 295 Children. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997;15:85–93. doi: 10.1200/JCO.1997.15.1.85. [DOI] [PubMed] [Google Scholar]
- 149.Brodeur G.M. Neuroblastoma: Biological Insights into a Clinical Enigma. Nat. Rev. Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 150.Brodeur G.M., Seeger R.C., Schwab M., Varmus H.E., Bishop J.M. Amplification of N-Myc in Untreated Human Neuroblastomas Correlates with Advanced Disease Stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 151.Seeger R.C., Brodeur G.M., Sather H., Dalton A., Siegel S.E., Wong K.Y., Hammond D. Association of Multiple Copies of the N-Myc Oncogene with Rapid Progression of Neuroblastomas. N. Engl. J. Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 152.Lampis S., Raieli S., Montemurro L., Bartolucci D., Amadesi C., Bortolotti S., Angelucci S., Scardovi A.L., Nieddu G., Cerisoli L., et al. The MYCN Inhibitor BGA002 Restores the Retinoic Acid Response Leading to Differentiation or Apoptosis by the MTOR Block in MYCN-Amplified Neuroblastoma. J. Exp. Clin. Cancer Res. 2022;41:160. doi: 10.1186/s13046-022-02367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rickman D.S., Schulte J.H., Eilers M. The Expanding World of N-MYC–Driven Tumors. Cancer Discov. 2018;8:150–163. doi: 10.1158/2159-8290.CD-17-0273. [DOI] [PubMed] [Google Scholar]
- 154.Harashima H., Dissmeyer N., Schnittger A. Cell Cycle Control across the Eukaryotic Kingdom. Trends Cell Biol. 2013;23:345–356. doi: 10.1016/j.tcb.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 155.Woo C.-W., Tan F., Cassano H., Lee J., Lee K.C., Thiele C.J. Use of RNA Interference to Elucidate the Effect of MYCN on Cell Cycle in Neuroblastoma. Pediatr. Blood Cancer. 2008;50:208–212. doi: 10.1002/pbc.21195. [DOI] [PubMed] [Google Scholar]
- 156.Cage T.A., Chanthery Y., Chesler L., Grimmer M., Knight Z., Shokat K., Weiss W.A., Gustafson W.C. Downregulation of MYCN through PI3K Inhibition in Mouse Models of Pediatric Neural Cancer. Front. Oncol. 2015;5:111. doi: 10.3389/fonc.2015.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bosch A., Li Z., Bergamaschi A., Ellis H., Toska E., Prat A., Tao J.J., Spratt D.E., Viola-Villegas N.T., Castel P., et al. PI3K Inhibition Results in Enhanced Estrogen Receptor Function and Dependence in Hormone Receptor-Positive Breast Cancer. Sci. Transl. Med. 2015;7:283ra51. doi: 10.1126/scitranslmed.aaa4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bouchard C., Dittrich O., Kiermaier A., Dohmann K., Menkel A., Eilers M., Lüscher B. Regulation of Cyclin D2 Gene Expression by the Myc/Max/Mad Network: Myc-Dependent TRRAP Recruitment and Histone Acetylation at the Cyclin D2 Promoter. Genes Dev. 2001;15:2042–2047. doi: 10.1101/gad.907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ren B., Cam H., Takahashi Y., Volkert T., Terragni J., Young R.A., Dynlacht B.D. E2F Integrates Cell Cycle Progression with DNA Repair, Replication, and G(2)/M Checkpoints. Genes Dev. 2002;16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lasorella A., Stegmüller J., Guardavaccaro D., Liu G., Carro M.S., Rothschild G., de la Torre-Ubieta L., Pagano M., Bonni A., Iavarone A. Degradation of Id2 by the Anaphase-Promoting Complex Couples Cell Cycle Exit and Axonal Growth. Nature. 2006;442:471–474. doi: 10.1038/nature04895. [DOI] [PubMed] [Google Scholar]
- 161.Kuzyk A., Gartner J., Mai S. Identification of Neuroblastoma Subgroups Based on Three-Dimensional Telomere Organization. Transl. Oncol. 2016;9:348–356. doi: 10.1016/j.tranon.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Valentijn L.J., Koster J., Zwijnenburg D.A., Hasselt N.E., van Sluis P., Volckmann R., van Noesel M.M., George R.E., Tytgat G.A.M., Molenaar J.J., et al. TERT Rearrangements are Frequent in Neuroblastoma and Identify Aggressive Tumors. Nat. Genet. 2015;47:1411–1414. doi: 10.1038/ng.3438. [DOI] [PubMed] [Google Scholar]
- 163.Ham J., Costa C., Sano R., Lochmann T.L., Sennott E.M., Patel N.U., Dastur A., Gomez-Caraballo M., Krytska K., Hata A.N., et al. Exploitation of the Apoptosis-Primed State of MYCN-Amplified Neuroblastoma to Develop a Potent and Specific Targeted Therapy Combination. Cancer Cell. 2016;29:159–172. doi: 10.1016/j.ccell.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen L., Iraci N., Gherardi S., Gamble L.D., Wood K.M., Perini G., Lunec J., Tweddle D.A. P53 is a Direct Transcriptional Target of MYCN in Neuroblastoma. Cancer Res. 2010;70:1377–1388. doi: 10.1158/0008-5472.CAN-09-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hou H., Sun D., Zhang X. The Role of MDM2 Amplification and Overexpression in Therapeutic Resistance of Malignant Tumors. Cancer Cell Int. 2019;19:216. doi: 10.1186/s12935-019-0937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kracikova M., Akiri G., George A., Sachidanandam R., Aaronson S.A. A Threshold Mechanism Mediates P53 Cell Fate Decision between Growth Arrest and Apoptosis. Cell Death Differ. 2013;20:576–588. doi: 10.1038/cdd.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Muller P.A.J., Vousden K.H. P53 Mutations in Cancer. Nat. Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 168.Barbieri E., Mehta P., Chen Z., Zhang L., Slack A., Berg S., Shohet J.M. MDM2 Inhibition Sensitizes Neuroblastoma to Chemotherapy-Induced Apoptotic Cell Death. Mol. Cancer Ther. 2006;5:2358–2365. doi: 10.1158/1535-7163.MCT-06-0305. [DOI] [PubMed] [Google Scholar]
- 169.Yogev O., Barker K., Sikka A., Almeida G.S., Hallsworth A., Smith L.M., Jamin Y., Ruddle R., Koers A., Webber H.T., et al. P53 Loss in MYC-Driven Neuroblastoma Leads to Metabolic Adaptations Supporting Radioresistance. Cancer Res. 2016;76:3025–3035. doi: 10.1158/0008-5472.CAN-15-1939. [DOI] [PubMed] [Google Scholar]
- 170.Qi D.-L., Cobrinik D. MDM2 but Not MDM4 Promotes Retinoblastoma Cell Proliferation through P53-Independent Regulation of MYCN Translation. Oncogene. 2017;36:1760–1769. doi: 10.1038/onc.2016.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cairns R.A., Harris I.S., Mak T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 172.Diers A.R., Broniowska K.A., Chang C.-F., Hogg N. Pyruvate Fuels Mitochondrial Respiration and Proliferation of Breast Cancer Cells: Effect of Monocarboxylate Transporter Inhibition. Biochem. J. 2012;444:561–571. doi: 10.1042/BJ20120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carracedo A., Cantley L.C., Pandolfi P.P. Cancer Metabolism: Fatty Acid Oxidation in the Limelight. Nat. Rev. Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zirath H., Frenzel A., Oliynyk G., Segerström L., Westermark U.K., Larsson K., Munksgaard Persson M., Hultenby K., Lehtiö J., Einvik C., et al. MYC Inhibition Induces Metabolic Changes Leading to Accumulation of Lipid Droplets in Tumor Cells. Proc. Natl. Acad. Sci. USA. 2013;110:10258–10263. doi: 10.1073/pnas.1222404110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond Aerobic Glycolysis: Transformed Cells Can Engage in Glutamine Metabolism That Exceeds the Requirement for Protein and Nucleotide Synthesis. Proc. Natl. Acad. Sci. USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Qing G., Li B., Vu A., Skuli N., Walton Z.E., Liu X., Mayes P.A., Wise D.R., Thompson C.B., Maris J.M., et al. ATF4 Regulates MYC-Mediated Neuroblastoma Cell Death upon Glutamine Deprivation. Cancer Cell. 2012;22:631–644. doi: 10.1016/j.ccr.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wahlström T., Henriksson M.A. Impact of MYC in Regulation of Tumor Cell Metabolism. Biochim. Biophys. Acta. 2015;1849:563–569. doi: 10.1016/j.bbagrm.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 179.Tao L., Mohammad M.A., Milazzo G., Moreno-Smith M., Patel T.D., Zorman B., Badachhape A., Hernandez B.E., Wolf A.B., Zeng Z., et al. MYCN-Driven Fatty Acid Uptake is a Metabolic Vulnerability in Neuroblastoma. Nat. Commun. 2022;13:3728. doi: 10.1038/s41467-022-31331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Alborzinia H., Flórez A.F., Kreth S., Brückner L.M., Yildiz U., Gartlgruber M., Odoni D.I., Poschet G., Garbowicz K., Shao C., et al. MYCN Mediates Cysteine Addiction and Sensitizes Neuroblastoma to Ferroptosis. Nat. Cancer. 2022;3:471–485. doi: 10.1038/s43018-022-00355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Montemurro L., Raieli S., Angelucci S., Bartolucci D., Amadesi C., Lampis S., Scardovi A.L., Venturelli L., Nieddu G., Cerisoli L., et al. A Novel MYCN-Specific Antigene Oligonucleotide Deregulates Mitochondria and Inhibits Tumor Growth in MYCN-Amplified Neuroblastoma. Cancer Res. 2019;79:6166–6177. doi: 10.1158/0008-5472.CAN-19-0008. [DOI] [PubMed] [Google Scholar]
- 182.Vaughan L., Clarke P.A., Barker K., Chanthery Y., Gustafson C.W., Tucker E., Renshaw J., Raynaud F., Li X., Burke R., et al. Inhibition of MTOR-Kinase Destabilizes MYCN and is a Potential Therapy for MYCN-Dependent Tumors. Oncotarget. 2016;7:57525–57544. doi: 10.18632/oncotarget.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schramm A., Köster J., Marschall T., Martin M., Schwermer M., Fielitz K., Büchel G., Barann M., Esser D., Rosenstiel P., et al. Next-Generation RNA Sequencing Reveals Differential Expression of MYCN Target Genes and Suggests the MTOR Pathway as a Promising Therapy Target in MYCN-Amplified Neuroblastoma. Int. J. Cancer. 2013;132:E106–E115. doi: 10.1002/ijc.27787. [DOI] [PubMed] [Google Scholar]
- 184.Yue M., Jiang J., Gao P., Liu H., Qing G. Oncogenic MYC Activates a Feedforward Regulatory Loop Promoting Essential Amino Acid Metabolism and Tumorigenesis. Cell Rep. 2017;21:3819–3832. doi: 10.1016/j.celrep.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 185.Raieli S., di Renzo D., Lampis S., Amadesi C., Montemurro L., Pession A., Hrelia P., Fischer M., Tonelli R. MYCN Drives a Tumor Immunosuppressive Environment Which Impacts Survival in Neuroblastoma. Front. Oncol. 2021;11:625207. doi: 10.3389/fonc.2021.625207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nallasamy P., Chava S., Verma S.S., Mishra S., Gorantla S., Coulter D.W., Byrareddy S.N., Batra S.K., Gupta S.C., Challagundla K.B. PD-L1, Inflammation, Non-Coding RNAs, and Neuroblastoma: Immuno-Oncology Perspective. Semin. Cancer Biol. 2018;52:53–65. doi: 10.1016/j.semcancer.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Melaiu O., Mina M., Chierici M., Boldrini R., Jurman G., Romania P., D’Alicandro V., Benedetti M.C., Castellano A., Liu T., et al. PD-L1 is a Therapeutic Target of the Bromodomain Inhibitor JQ1 and, Combined with HLA Class I, a Promising Prognostic Biomarker in Neuroblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23:4462–4472. doi: 10.1158/1078-0432.CCR-16-2601. [DOI] [PubMed] [Google Scholar]
- 188.Noguera R., Fredlund E., Piqueras M., Pietras A., Beckman S., Navarro S., Påhlman S. HIF-1α and HIF-2α are Differentially Regulated In Vivo in Neuroblastoma: High HIF-1α Correlates Negatively to Advanced Clinical Stage and Tumor Vascularization. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:7130–7136. doi: 10.1158/1078-0432.CCR-09-0223. [DOI] [PubMed] [Google Scholar]
- 189.Burr M.L., Sparbier C.E., Chan K.L., Chan Y.-C., Kersbergen A., Lam E.Y.N., Azidis-Yates E., Vassiliadis D., Bell C.C., Gilan O., et al. An Evolutionarily Conserved Function of Polycomb Silences the MHC Class I Antigen Presentation Pathway and Enables Immune Evasion in Cancer. Cancer Cell. 2019;36:385–401.e8. doi: 10.1016/j.ccell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Merchant M.S., Wright M., Baird K., Wexler L.H., Rodriguez-Galindo C., Bernstein D., Delbrook C., Lodish M., Bishop R., Wolchok J.D., et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016;22:1364–1370. doi: 10.1158/1078-0432.CCR-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Majzner R.G., Simon J.S., Grosso J.F., Martinez D., Pawel B.R., Santi M., Merchant M.S., Geoerger B., Hezam I., Marty V., et al. Assessment of Programmed Death-Ligand 1 Expression and Tumor-Associated Immune Cells in Pediatric Cancer Tissues. Cancer. 2017;123:3807–3815. doi: 10.1002/cncr.30724. [DOI] [PubMed] [Google Scholar]
- 192.Hahn M., Glass T., Koke J. Extracellular Matrix Effects on a Neuroblastoma Cell Line. Cytobios. 2000;102:7–19. [PubMed] [Google Scholar]
- 193.Meyer A., van Golen C.M., Kim B., van Golen K.L., Feldman E.L. Integrin Expression Regulates Neuroblastoma Attachment and Migration. Neoplasia. 2004;6:332–342. doi: 10.1593/neo.03445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Young S.A., McCabe K.E., Bartakova A., Delaney J., Pizzo D.P., Newbury R.O., Varner J.A., Schlaepfer D.D., Stupack D.G. Integrin A4 Enhances Metastasis and May Be Associated with Poor Prognosis in MYCN-Low Neuroblastoma. PLoS ONE. 2015;10:e0120815. doi: 10.1371/journal.pone.0120815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Erdreich-Epstein A., Shimada H., Groshen S., Liu M., Metelitsa L.S., Kim K.S., Stins M.F., Seeger R.C., Durden D.L. Integrins Alpha(v)Beta3 and Alpha(v)Beta5 are Expressed by Endothelium of High-Risk Neuroblastoma and Their Inhibition is Associated with Increased Endogenous Ceramide. Cancer Res. 2000;60:712–721. [PubMed] [Google Scholar]
- 196.Pickup M.W., Mouw J.K., Weaver V.M. The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Willumsen N., Thomsen L.B., Bager C.L., Jensen C., Karsdal M.A. Quantification of Altered Tissue Turnover in a Liquid Biopsy: A Proposed Precision Medicine Tool to Assess Chronic Inflammation and Desmoplasia Associated with a pro-Cancerous Niche and Response to Immuno-Therapeutic Anti-Tumor Modalities. Cancer Immunol. Immunother. 2018;67:1–12. doi: 10.1007/s00262-017-2074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ng M.R., Brugge J.S. A Stiff Blow from the Stroma: Collagen Crosslinking Drives Tumor Progression. Cancer Cell. 2009;16:455–457. doi: 10.1016/j.ccr.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 199.Diop-Frimpong B., Chauhan V.P., Krane S., Boucher Y., Jain R.K. Losartan Inhibits Collagen I Synthesis and Improves the Distribution and Efficacy of Nanotherapeutics in Tumors. Proc. Natl. Acad. Sci. USA. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sugiura Y., Shimada H., Seeger R.C., Laug W.E., DeClerck Y.A. Matrix Metalloproteinases-2 and -9 are Expressed in Human Neuroblastoma: Contribution of Stromal Cells to Their Production and Correlation with Metastasis. Cancer Res. 1998;58:2209–2216. [PubMed] [Google Scholar]
- 201.Sans-Fons M.G., Sole S., Sanfeliu C., Planas A.M. Matrix Metalloproteinase-9 and Cell Division in Neuroblastoma Cells and Bone Marrow Macrophages. Am. J. Pathol. 2010;177:2870–2885. doi: 10.2353/ajpath.2010.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ara T., Fukuzawa M., Kusafuka T., Komoto Y., Oue T., Inoue M., Okada A. Immunohistochemical Expression of MMP-2, MMP-9, and TIMP-2 in Neuroblastoma: Association with Tumor Progression and Clinical Outcome. J. Pediatr. Surg. 1998;33:1272–1278. doi: 10.1016/S0022-3468(98)90167-1. [DOI] [PubMed] [Google Scholar]
- 203.Bergers G., Benjamin L.E. Tumorigenesis and the Angiogenic Switch. Nat. Rev. Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 204.Ramani P., Nash R., Radevsky L., Patel A., Luckett M., Rogers C. VEGF-C, VEGF-D and VEGFR-3 Expression in Peripheral Neuroblastic Tumours. Histopathology. 2012;61:1006–1016. doi: 10.1111/j.1365-2559.2012.04307.x. [DOI] [PubMed] [Google Scholar]
- 205.Chlenski A., Liu S., Cohn S.L. The Regulation of Angiogenesis in Neuroblastoma. Cancer Lett. 2003;197:47–52. doi: 10.1016/S0304-3835(03)00082-X. [DOI] [PubMed] [Google Scholar]
- 206.Meitar D., Crawford S.E., Rademaker A.W., Cohn S.L. Tumor Angiogenesis Correlates with Metastatic Disease, N-Myc Amplification, and Poor Outcome in Human Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1996;14:405–414. doi: 10.1200/JCO.1996.14.2.405. [DOI] [PubMed] [Google Scholar]
- 207.Ribatti D., Vacca A., Nico B., de Falco G., Giuseppe Montaldo P., Ponzoni M. Angiogenesis and Anti-Angiogenesis in Neuroblastoma. Eur. J. Cancer. 2002;38:750–757. doi: 10.1016/S0959-8049(01)00337-9. [DOI] [PubMed] [Google Scholar]
- 208.Rössler J., Taylor M., Geoerger B., Farace F., Lagodny J., Peschka-Süss R., Niemeyer C.M., Vassal G. Angiogenesis as a Target in Neuroblastoma. Eur. J. Cancer. 2008;44:1645–1656. doi: 10.1016/j.ejca.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 209.Chanthery Y.H., Gustafson W.C., Itsara M., Persson A., Hackett C.S., Grimmer M., Charron E., Yakovenko S., Kim G., Matthay K.K., et al. Paracrine Signaling through MYCN Enhances Tumor-Vascular Interactions in Neuroblastoma. Sci. Transl. Med. 2012;4:115ra3. doi: 10.1126/scitranslmed.3002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kang J., Rychahou P.G., Ishola T.A., Mourot J.M., Evers B.M., Chung D.H. N-Myc is a Novel Regulator of PI3K-Mediated VEGF Expression in Neuroblastoma. Oncogene. 2008;27:3999–4007. doi: 10.1038/onc.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Singh A.R., Joshi S., Burgoyne A.M., Sicklick J.K., Ikeda S., Kono Y., Garlich J.R., Morales G.A., Durden D.L. Single Agent and Synergistic Activity of the “First-in-Class” Dual PI3K/BRD4 Inhibitor SF1126 with Sorafenib in Hepatocellular Carcinoma. Mol. Cancer Ther. 2016;15:2553–2562. doi: 10.1158/1535-7163.MCT-15-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Joshi S., Singh A.R., Durden D.L. Pan-PI-3 Kinase Inhibitor SF1126 Shows Antitumor and Antiangiogenic Activity in Renal Cell Carcinoma. Cancer Chemother. Pharmacol. 2015;75:595–608. doi: 10.1007/s00280-014-2639-x. [DOI] [PubMed] [Google Scholar]
- 213.Joshi S., Singh A.R., Zulcic M., Durden D.L. A Macrophage-Dominant PI3K Isoform Controls Hypoxia-Induced HIF1α and HIF2α Stability and Tumor Growth, Angiogenesis, and Metastasis. Mol. Cancer Res. 2014;12:1520–1531. doi: 10.1158/1541-7786.MCR-13-0682. [DOI] [PubMed] [Google Scholar]
- 214.Singh A.R., Joshi S., George E., Durden D.L. Anti-Tumor Effect of a Novel PI3-Kinase Inhibitor, SF1126, in (12) V-Ha-Ras Transgenic Mouse Glioma Model. Cancer Cell Int. 2014;14:105. doi: 10.1186/s12935-014-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xing F., Saidou J., Watabe K. Cancer Associated Fibroblasts (CAFs) in Tumor Microenvironment. Front. Biosci. Landmark Ed. 2010;15:166–179. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yazhou C., Wenlv S., Weidong Z., Licun W. Clinicopathological Significance of Stromal Myofibroblasts in Invasive Ductal Carcinoma of the Breast. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2004;25:290–295. doi: 10.1159/000081394. [DOI] [PubMed] [Google Scholar]
- 217.Tuxhorn J.A., Ayala G.E., Smith M.J., Smith V.C., Dang T.D., Rowley D.R. Reactive Stroma in Human Prostate Cancer: Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- 218.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R., Carey V.J., Richardson A.L., Weinberg R.A. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 219.Silzle T., Kreutz M., Dobler M.A., Brockhoff G., Knuechel R., Kunz-Schughart L.A. Tumor-Associated Fibroblasts Recruit Blood Monocytes into Tumor Tissue. Eur. J. Immunol. 2003;33:1311–1320. doi: 10.1002/eji.200323057. [DOI] [PubMed] [Google Scholar]
- 220.Yingling J.M., Blanchard K.L., Sawyer J.S. Development of TGF-Beta Signalling Inhibitors for Cancer Therapy. Nat. Rev. Drug Discov. 2004;3:1011–1022. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 221.Hashimoto O., Yoshida M., Koma Y.-I., Yanai T., Hasegawa D., Kosaka Y., Nishimura N., Yokozaki H. Collaboration of Cancer-Associated Fibroblasts and Tumour-Associated Macrophages for Neuroblastoma Development. J. Pathol. 2016;240:211–223. doi: 10.1002/path.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kakarla S., Song X.-T., Gottschalk S. Cancer-Associated Fibroblasts as Targets for Immunotherapy. Immunotherapy. 2012;4:1129–1138. doi: 10.2217/imt.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fakhrai H., Dorigo O., Shawler D.L., Lin H., Mercola D., Black K.L., Royston I., Sobol R.E. Eradication of Established Intracranial Rat Gliomas by Transforming Growth Factor Beta Antisense Gene Therapy. Proc. Natl. Acad. Sci. USA. 1996;93:2909–2914. doi: 10.1073/pnas.93.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Braoudaki M., Hatziagapiou K., Zaravinos A., Lambrou G.I. MYCN in Neuroblastoma: “Old Wine into New Wineskins”. Diseases. 2021;9:78. doi: 10.3390/diseases9040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bartel D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Buechner J., Einvik C. N-Myc and Noncoding RNAs in Neuroblastoma. Mol. Cancer Res. 2012;10:1243–1253. doi: 10.1158/1541-7786.MCR-12-0244. [DOI] [PubMed] [Google Scholar]
- 227.Beckers A., van Peer G., Carter D.R., Mets E., Althoff K., Cheung B.B., Schulte J.H., Mestdagh P., Vandesompele J., Marshall G.M., et al. MYCN-Targeting MiRNAs are Predominantly Downregulated during MYCN-driven Neuroblastoma Tumor Formation. Oncotarget. 2015;6:5204–5216. doi: 10.18632/oncotarget.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Molenaar J.J., Domingo-Fernández R., Ebus M.E., Lindner S., Koster J., Drabek K., Mestdagh P., van Sluis P., Valentijn L.J., van Nes J., et al. LIN28B Induces Neuroblastoma and Enhances MYCN Levels via Let-7 Suppression. Nat. Genet. 2012;44:1199–1206. doi: 10.1038/ng.2436. [DOI] [PubMed] [Google Scholar]
- 229.Powers J.T., Tsanov K.M., Pearson D.S., Roels F., Spina C.S., Ebright R., Seligson M., de Soysa Y., Cahan P., Theißen J., et al. Multiple Mechanisms Disrupt the Let-7 MicroRNA Family in Neuroblastoma. Nature. 2016;535:246–251. doi: 10.1038/nature18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Misiak D., Hagemann S., Bell J.L., Busch B., Lederer M., Bley N., Schulte J.H., Hüttelmaier S. The MicroRNA Landscape of MYCN-Amplified Neuroblastoma. Front. Oncol. 2021;11:647737. doi: 10.3389/fonc.2021.647737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mogilyansky E., Rigoutsos I. The MiR-17/92 Cluster: A Comprehensive Update on Its Genomics, Genetics, Functions and Increasingly Important and Numerous Roles in Health and Disease. Cell Death Differ. 2013;20:1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mestdagh P., Boström A.-K., Impens F., Fredlund E., van Peer G., de Antonellis P., von Stedingk K., Ghesquière B., Schulte S., Dews M., et al. The MiR-17-92 MicroRNA Cluster Regulates Multiple Components of the TGF-β Pathway in Neuroblastoma. Mol. Cell. 2010;40:762–773. doi: 10.1016/j.molcel.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Armstrong B.C., Krystal G.W. Isolation and Characterization of Complementary DNA for N-Cym, a Gene Encoded by the DNA Strand Opposite to N-Myc. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 1992;3:385–390. [PubMed] [Google Scholar]
- 234.Vadie N., Saayman S., Lenox A., Ackley A., Clemson M., Burdach J., Hart J., Vogt P.K., Morris K.V. MYCNOS Functions as an Antisense RNA Regulating MYCN. RNA Biol. 2015;12:893–899. doi: 10.1080/15476286.2015.1063773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Suenaga Y., Islam S.M.R., Alagu J., Kaneko Y., Kato M., Tanaka Y., Kawana H., Hossain S., Matsumoto D., Yamamoto M., et al. NCYM, a Cis-Antisense Gene of MYCN, Encodes a de Novo Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas. PLoS Genet. 2014;10:e1003996. doi: 10.1371/journal.pgen.1003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.O’Brien E.M., Selfe J.L., Martins A.S., Walters Z.S., Shipley J.M. The Long Non-Coding RNA MYCNOS-01 Regulates MYCN Protein Levels and Affects Growth of MYCN-Amplified Rhabdomyosarcoma and Neuroblastoma Cells. BMC Cancer. 2018;18:217. doi: 10.1186/s12885-018-4129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liu P.Y., Atmadibrata B., Mondal S., Tee A.E., Liu T. NCYM is Upregulated by LncUSMycN and Modulates N-Myc Expression. Int. J. Oncol. 2016;49:2464–2470. doi: 10.3892/ijo.2016.3730. [DOI] [PubMed] [Google Scholar]
- 238.Decock A., Ongenaert M., Vandesompele J., Speleman F. Neuroblastoma Epigenetics: From Candidate Gene Approaches to Genome-Wide Screenings. Epigenetics. 2011;6:962–970. doi: 10.4161/epi.6.8.16516. [DOI] [PubMed] [Google Scholar]
- 239.Westerlund I., Shi Y., Toskas K., Fell S.M., Li S., Surova O., Södersten E., Kogner P., Nyman U., Schlisio S., et al. Combined Epigenetic and Differentiation-Based Treatment Inhibits Neuroblastoma Tumor Growth and Links HIF2α to Tumor Suppression. Proc. Natl. Acad. Sci. USA. 2017;114:E6137–E6146. doi: 10.1073/pnas.1700655114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Parodi F., Carosio R., Ragusa M., di Pietro C., Maugeri M., Barbagallo D., Sallustio F., Allemanni G., Pistillo M.P., Casciano I., et al. Epigenetic Dysregulation in Neuroblastoma: A Tale of MiRNAs and DNA Methylation. Biochim. Biophys. Acta. 2016;1859:1502–1514. doi: 10.1016/j.bbagrm.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 241.Louis C.U., Shohet J.M. Neuroblastoma: Molecular Pathogenesis and Therapy. Annu. Rev. Med. 2015;66:49–63. doi: 10.1146/annurev-med-011514-023121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pession A., Tonelli R. The MYCN Oncogene as a Specific and Selective Drug Target for Peripheral and Central Nervous System Tumors. Curr. Cancer Drug Targets. 2005;5:273–283. doi: 10.2174/1568009054064606. [DOI] [PubMed] [Google Scholar]
- 243.Fletcher J.I., Ziegler D.S., Trahair T.N., Marshall G.M., Haber M., Norris M.D. Too Many Targets, Not Enough Patients: Rethinking Neuroblastoma Clinical Trials. Nat. Rev. Cancer. 2018;18:389–400. doi: 10.1038/s41568-018-0003-x. [DOI] [PubMed] [Google Scholar]
- 244.Andresen C., Helander S., Lemak A., Farès C., Csizmok V., Carlsson J., Penn L.Z., Forman-Kay J.D., Arrowsmith C.H., Lundström P., et al. Transient Structure and Dynamics in the Disordered C-Myc Transactivation Domain Affect Bin1 Binding. Nucleic Acids Res. 2012;40:6353–6366. doi: 10.1093/nar/gks263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bayliss R., Burgess S.G., Leen E., Richards M.W. A Moving Target: Structure and Disorder in Pursuit of Myc Inhibitors. Biochem. Soc. Trans. 2017;45:709–717. doi: 10.1042/BST20160328. [DOI] [PubMed] [Google Scholar]
- 246.Kohl N.E., Legouy E., DePinho R.A., Nisen P.D., Smith R.K., Gee C.E., Alt F.W. Human N-Myc is Closely Related in Organization and Nucleotide Sequence to c-Myc. Nature. 1986;319:73–77. doi: 10.1038/319073a0. [DOI] [PubMed] [Google Scholar]
- 247.Esposito M.R., Aveic S., Seydel A., Tonini G.P. Neuroblastoma Treatment in the Post-Genomic Era. J. Biomed. Sci. 2017;24:14. doi: 10.1186/s12929-017-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Filippakopoulos P., Picaud S., Mangos M., Keates T., Lambert J.-P., Barsyte-Lovejoy D., Felletar I., Volkmer R., Müller S., Pawson T., et al. Histone Recognition and Large-Scale Structural Analysis of the Human Bromodomain Family. Cell. 2012;149:214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Patel M.C., Debrosse M., Smith M., Dey A., Huynh W., Sarai N., Heightman T.D., Tamura T., Ozato K. BRD4 Coordinates Recruitment of Pause Release Factor P-TEFb and the Pausing Complex NELF/DSIF to Regulate Transcription Elongation of Interferon-Stimulated Genes. Mol. Cell. Biol. 2013;33:2497–2507. doi: 10.1128/MCB.01180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yang Z., Yik J.H.N., Chen R., He N., Jang M.K., Ozato K., Zhou Q. Recruitment of P-TEFb for Stimulation of Transcriptional Elongation by the Bromodomain Protein Brd4. Mol. Cell. 2005;19:535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 251.Shapiro G.I., LoRusso P., Dowlati A., Do K.T., Jacobson C.A., Vaishampayan U., Weise A., Caimi P.F., Eder J.P., French C.A., et al. A Phase 1 Study of RO6870810, a Novel Bromodomain and Extra-Terminal Protein Inhibitor, in Patients with NUT Carcinoma, Other Solid Tumours, or Diffuse Large B-Cell Lymphoma. Br. J. Cancer. 2021;124:744–753. doi: 10.1038/s41416-020-01180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Puissant A., Frumm S.M., Alexe G., Bassil C.F., Qi J., Chanthery Y.H., Nekritz E.A., Zeid R., Gustafson W.C., Greninger P., et al. Targeting MYCN in Neuroblastoma by BET Bromodomain Inhibition. Cancer Discov. 2013;3:308–323. doi: 10.1158/2159-8290.CD-12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jang M.K., Mochizuki K., Zhou M., Jeong H.-S., Brady J.N., Ozato K. The Bromodomain Protein Brd4 is a Positive Regulatory Component of P-TEFb and Stimulates RNA Polymerase II-Dependent Transcription. Mol. Cell. 2005;19:523–534. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 254.Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W.B., Fedorov O., Morse E.M., Keates T., Hickman T.T., Felletar I., et al. Selective Inhibition of BET Bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nicodeme E., Jeffrey K.L., Schaefer U., Beinke S., Dewell S., Chung C.-W., Chandwani R., Marazzi I., Wilson P., Coste H., et al. Suppression of Inflammation by a Synthetic Histone Mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Henssen A., Althoff K., Odersky A., Beckers A., Koche R., Speleman F., Schäfers S., Bell E., Nortmeyer M., Westermann F., et al. Targeting MYCN-Driven Transcription By BET-Bromodomain Inhibition. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016;22:2470–2481. doi: 10.1158/1078-0432.CCR-15-1449. [DOI] [PubMed] [Google Scholar]
- 257.He S., Liu Z., Oh D.-Y., Thiele C.J. MYCN and the Epigenome. Front. Oncol. 2013;3:1. doi: 10.3389/fonc.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Iraci N., Diolaiti D., Papa A., Porro A., Valli E., Gherardi S., Herold S., Eilers M., Bernardoni R., della Valle G., et al. A SP1/MIZ1/MYCN Repression Complex Recruits HDAC1 at the TRKA and P75NTR Promoters and Affects Neuroblastoma Malignancy by Inhibiting the Cell Response to NGF. Cancer Res. 2011;71:404–412. doi: 10.1158/0008-5472.CAN-10-2627. [DOI] [PubMed] [Google Scholar]
- 259.Lu Z., Tian Y., Salwen H.R., Chlenski A., Godley L.A., Raj J.U., Yang Q. Histone Lysine Methyltransferase EHMT2 is Involved in Proliferation, Apoptosis, Cell Invasion and DNA Methylation of Human Neuroblastoma Cells. Anti-Cancer Drugs. 2013;24:484–493. doi: 10.1097/CAD.0b013e32835ffdbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lodrini M., Oehme I., Schroeder C., Milde T., Schier M.C., Kopp-Schneider A., Schulte J.H., Fischer M., de Preter K., Pattyn F., et al. MYCN and HDAC2 Cooperate to Repress MiR-183 Signaling in Neuroblastoma. Nucleic Acids Res. 2013;41:6018–6033. doi: 10.1093/nar/gkt346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bishayee K., Nazim U.M., Kumar V., Kang J., Kim J., Huh S.-O., Sadra A. Reversing the HDAC-Inhibitor Mediated Metabolic Escape in MYCN-Amplified Neuroblastoma. Biomed. Pharmacother. Biomed. Pharmacother. 2022;150:113032. doi: 10.1016/j.biopha.2022.113032. [DOI] [PubMed] [Google Scholar]
- 262.West A.C., Johnstone R.W. New and Emerging HDAC Inhibitors for Cancer Treatment. J. Clin. Investig. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Phimmachanh M., Han J.Z.R., O’Donnell Y.E.I., Latham S.L., Croucher D.R. Histone Deacetylases and Histone Deacetylase Inhibitors in Neuroblastoma. Front. Cell Dev. Biol. 2020;8:578770. doi: 10.3389/fcell.2020.578770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gallinari P., di Marco S., Jones P., Pallaoro M., Steinkühler C. HDACs, Histone Deacetylation and Gene Transcription: From Molecular Biology to Cancer Therapeutics. Cell Res. 2007;17:195–211. doi: 10.1038/sj.cr.7310149. [DOI] [PubMed] [Google Scholar]
- 265.Eckschlager T., Plch J., Stiborova M., Hrabeta J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017;18:1414. doi: 10.3390/ijms18071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ramaiah M.J., Tangutur A.D., Manyam R.R. Epigenetic Modulation and Understanding of HDAC Inhibitors in Cancer Therapy. Life Sci. 2021;277:119504. doi: 10.1016/j.lfs.2021.119504. [DOI] [PubMed] [Google Scholar]
- 267.Rettig I., Koeneke E., Trippel F., Mueller W.C., Burhenne J., Kopp-Schneider A., Fabian J., Schober A., Fernekorn U., von Deimling A., et al. Selective Inhibition of HDAC8 Decreases Neuroblastoma Growth In Vitro and In Vivo and Enhances Retinoic Acid-Mediated Differentiation. Cell Death Dis. 2015;6:e1657. doi: 10.1038/cddis.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kenney A.M., Widlund H.R., Rowitch D.H. Hedgehog and PI-3 Kinase Signaling Converge on Nmyc1 to Promote Cell Cycle Progression in Cerebellar Neuronal Precursors. Dev. Camb. Engl. 2004;131:217–228. doi: 10.1242/dev.00891. [DOI] [PubMed] [Google Scholar]
- 269.Manning B.D., Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Gustafson W.C., Weiss W.A. Myc Proteins as Therapeutic Targets. Oncogene. 2010;29:1249–1259. doi: 10.1038/onc.2009.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Borgenvik A., Čančer M., Hutter S., Swartling F.J. Targeting MYCN in Molecularly Defined Malignant Brain Tumors. Front. Oncol. 2020;10:626751. doi: 10.3389/fonc.2020.626751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zheng Y., Jiang Y. MTOR Inhibitors at a Glance. Mol. Cell. Pharmacol. 2015;7:15–20. [PMC free article] [PubMed] [Google Scholar]
- 273.Wu C.-C., Hou S., Orr B.A., Kuo B.R., Youn Y.H., Ong T., Roth F., Eberhart C.G., Robinson G.W., Solecki D.J., et al. MTORC1-Mediated Inhibition of 4EBP1 is Essential for Hedgehog Signaling-Driven Translation and Medulloblastoma. Dev. Cell. 2017;43:673–688.e5. doi: 10.1016/j.devcel.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Johnsen J.I., Segerström L., Orrego A., Elfman L., Henriksson M., Kågedal B., Eksborg S., Sveinbjörnsson B., Kogner P. Inhibitors of Mammalian Target of Rapamycin Downregulate MYCN Protein Expression and Inhibit Neuroblastoma Growth In Vitro and In Vivo. Oncogene. 2008;27:2910–2922. doi: 10.1038/sj.onc.1210938. [DOI] [PubMed] [Google Scholar]
- 275.Zhang H., Dou J., Yu Y., Zhao Y., Fan Y., Cheng J., Xu X., Liu W., Guan S., Chen Z., et al. MTOR ATP-Competitive Inhibitor INK128 Inhibits Neuroblastoma Growth via Blocking MTORC Signaling. Apoptosis Int. J. Program. Cell Death. 2015;20:50–62. doi: 10.1007/s10495-014-1066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Huang Z., Wu Y., Zhou X., Qian J., Zhu W., Shu Y., Liu P. Clinical Efficacy of MTOR Inhibitors in Solid Tumors: A Systematic Review. Future Oncol. 2015;11:1687–1699. doi: 10.2217/fon.15.70. [DOI] [PubMed] [Google Scholar]
- 277.Hua H., Kong Q., Zhang H., Wang J., Luo T., Jiang Y. Targeting MTOR for Cancer Therapy. J. Hematol. Oncol. 2019;12:71. doi: 10.1186/s13045-019-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Carmena M., Earnshaw W.C. The Cellular Geography of Aurora Kinases. Nat. Rev. Mol. Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 279.Bischoff J.R., Anderson L., Zhu Y., Mossie K., Ng L., Souza B., Schryver B., Flanagan P., Clairvoyant F., Ginther C., et al. A Homologue of Drosophila Aurora Kinase is Oncogenic and Amplified in Human Colorectal Cancers. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Du R., Huang C., Liu K., Li X., Dong Z. Targeting AURKA in Cancer: Molecular Mechanisms and Opportunities for Cancer Therapy. Mol. Cancer. 2021;20:15. doi: 10.1186/s12943-020-01305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Otto T., Horn S., Brockmann M., Eilers U., Schüttrumpf L., Popov N., Kenney A.M., Schulte J.H., Beijersbergen R., Christiansen H., et al. Stabilization of N-Myc is a Critical Function of Aurora A in Human Neuroblastoma. Cancer Cell. 2009;15:67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 282.Brockmann M., Poon E., Berry T., Carstensen A., Deubzer H.E., Rycak L., Jamin Y., Thway K., Robinson S.P., Roels F., et al. Small Molecule Inhibitors of Aurora-a Induce Proteasomal Degradation of N-Myc in Childhood Neuroblastoma. Cancer Cell. 2013;24:75–89. doi: 10.1016/j.ccr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gustafson W.C., Meyerowitz J.G., Nekritz E.A., Chen J., Benes C., Charron E., Simonds E.F., Seeger R., Matthay K.K., Hertz N.T., et al. Drugging MYCN through an Allosteric Transition in Aurora Kinase A. Cancer Cell. 2014;26:414–427. doi: 10.1016/j.ccr.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Felgenhauer J., Tomino L., Selich-Anderson J., Bopp E., Shah N. Dual BRD4 and AURKA Inhibition is Synergistic against MYCN-Amplified and Nonamplified Neuroblastoma. Neoplasia. 2018;20:965–974. doi: 10.1016/j.neo.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pastor E.R., Mousa S.A. Current Management of Neuroblastoma and Future Direction. Crit. Rev. Oncol. Hematol. 2019;138:38–43. doi: 10.1016/j.critrevonc.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 286.Čančer M., Drews L.F., Bengtsson J., Bolin S., Rosén G., Westermark B., Nelander S., Forsberg-Nilsson K., Uhrbom L., Weishaupt H., et al. BET and Aurora Kinase A Inhibitors Synergize against MYCN-Positive Human Glioblastoma Cells. Cell Death Dis. 2019;10:881. doi: 10.1038/s41419-019-2120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Du J., Yan L., Torres R., Gong X., Bian H., Marugán C., Boehnke K., Baquero C., Hui Y.-H., Chapman S.C., et al. Aurora A-Selective Inhibitor LY3295668 Leads to Dominant Mitotic Arrest, Apoptosis in Cancer Cells, and Shows Potent Preclinical Antitumor Efficacy. Mol. Cancer Ther. 2019;18:2207–2219. doi: 10.1158/1535-7163.MCT-18-0529. [DOI] [PubMed] [Google Scholar]
- 288.Bálint E., Vousden K.H. Activation and Activities of the P53 Tumour Suppressor Protein. Br. J. Cancer. 2001;85:1813–1823. doi: 10.1054/bjoc.2001.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hernández Borrero L.J., El-Deiry W.S. Tumor Suppressor P53: Biology, Signaling Pathways, and Therapeutic Targeting. Biochim. Biophys. Acta Rev. Cancer. 2021;1876:188556. doi: 10.1016/j.bbcan.2021.188556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Harris S.L., Levine A.J. The P53 Pathway: Positive and Negative Feedback Loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 291.Nag S., Qin J., Srivenugopal K.S., Wang M., Zhang R. The MDM2-P53 Pathway Revisited. J. Biomed. Res. 2013;27:254–271. doi: 10.7555/JBR.27.20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Bullock A.N., Fersht A.R. Rescuing the Function of Mutant P53. Nat. Rev. Cancer. 2001;1:68–76. doi: 10.1038/35094077. [DOI] [PubMed] [Google Scholar]
- 293.Hainaut P., Hollstein M. P53 and Human Cancer: The First Ten Thousand Mutations. Adv. Cancer Res. 2000;77:81–137. doi: 10.1016/s0065-230x(08)60785-x. [DOI] [PubMed] [Google Scholar]
- 294.Vogan K., Bernstein M., Leclerc J.M., Brisson L., Brossard J., Brodeur G.M., Pelletier J., Gros P. Absence of P53 Gene Mutations in Primary Neuroblastomas. Cancer Res. 1993;53:5269–5273. [PubMed] [Google Scholar]
- 295.Wang H., Wang X., Xu L., Zhang J. TP53 and TP53-Associated Genes are Correlated with the Prognosis of Paediatric Neuroblastoma. BMC Genom. Data. 2022;23:41. doi: 10.1186/s12863-022-01059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Arnhold V., Schmelz K., Proba J., Winkler A., Wünschel J., Toedling J., Deubzer H.E., Künkele A., Eggert A., Schulte J.H., et al. Reactivating TP53 Signaling by the Novel MDM2 Inhibitor DS-3032b as a Therapeutic Option for High-Risk Neuroblastoma. Oncotarget. 2018;9:2304–2319. doi: 10.18632/oncotarget.23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Corvi R., Savelyeva L., Breit S., Wenzel A., Handgretinger R., Barak J., Oren M., Amler L., Schwab M. Non-Syntenic Amplification of MDM2 and MYCN in Human Neuroblastoma. Oncogene. 1995;10:1081–1086. [PubMed] [Google Scholar]
- 298.Cattelani S., Defferrari R., Marsilio S., Bussolari R., Candini O., Corradini F., Ferrari-Amorotti G., Guerzoni C., Pecorari L., Menin C., et al. Impact of a Single Nucleotide Polymorphism in the MDM2 Gene on Neuroblastoma Development and Aggressiveness: Results of a Pilot Study on 239 Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008;14:3248–3253. doi: 10.1158/1078-0432.CCR-07-4725. [DOI] [PubMed] [Google Scholar]
- 299.Rayburn E., Zhang R., He J., Wang H. MDM2 and Human Malignancies: Expression, Clinical Pathology, Prognostic Markers, and Implications for Chemotherapy. Curr. Cancer Drug Targets. 2005;5:27–41. doi: 10.2174/1568009053332636. [DOI] [PubMed] [Google Scholar]
- 300.Momand J., Zambetti G.P., Olson D.C., George D., Levine A.J. The Mdm-2 Oncogene Product Forms a Complex with the P53 Protein and Inhibits P53-Mediated Transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-R. [DOI] [PubMed] [Google Scholar]
- 301.Haupt Y., Maya R., Kazaz A., Oren M. Mdm2 Promotes the Rapid Degradation of P53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 302.Gu L., Zhang H., He J., Li J., Huang M., Zhou M. MDM2 Regulates MYCN MRNA Stabilization and Translation in Human Neuroblastoma Cells. Oncogene. 2012;31:1342–1353. doi: 10.1038/onc.2011.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Slack A., Lozano G., Shohet J.M. MDM2 as MYCN Transcriptional Target: Implications for Neuroblastoma Pathogenesis. Cancer Lett. 2005;228:21–27. doi: 10.1016/j.canlet.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 304.Konopleva M., Martinelli G., Daver N., Papayannidis C., Wei A., Higgins B., Ott M., Mascarenhas J., Andreeff M. MDM2 Inhibition: An Important Step Forward in Cancer Therapy. Leukemia. 2020;34:2858–2874. doi: 10.1038/s41375-020-0949-z. [DOI] [PubMed] [Google Scholar]
- 305.Pairawan S., Zhao M., Yuca E., Annis A., Evans K., Sutton D., Carvajal L., Ren J.-G., Santiago S., Guerlavais V., et al. First in Class Dual MDM2/MDMX Inhibitor ALRN-6924 Enhances Antitumor Efficacy of Chemotherapy in TP53 Wild-Type Hormone Receptor-Positive Breast Cancer Models. Breast Cancer Res. 2021;23:29. doi: 10.1186/s13058-021-01406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Liu Z., Chen S.S., Clarke S., Veschi V., Thiele C.J. Targeting MYCN in Pediatric and Adult Cancers. Front. Oncol. 2020;10:623679. doi: 10.3389/fonc.2020.623679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Konstantinidou M., Li J., Zhang B., Wang Z., Shaabani S., Ter Brake F., Essa K., Dömling A. PROTACs—A Game-Changing Technology. Expert Opin. Drug Discov. 2019;14:1255–1268. doi: 10.1080/17460441.2019.1659242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Gao H., Sun X., Rao Y. PROTAC Technology: Opportunities and Challenges. ACS Med. Chem. Lett. 2020;11:237–240. doi: 10.1021/acsmedchemlett.9b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Müller I., Larsson K., Frenzel A., Oliynyk G., Zirath H., Prochownik E.V., Westwood N.J., Henriksson M.A. Targeting of the MYCN Protein with Small Molecule C-MYC Inhibitors. PLoS ONE. 2014;9:e97285. doi: 10.1371/journal.pone.0097285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.An S., Fu L. Small-Molecule PROTACs: An Emerging and Promising Approach for the Development of Targeted Therapy Drugs. EBioMedicine. 2018;36:553–562. doi: 10.1016/j.ebiom.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Bartolucci D., Pession A., Hrelia P., Tonelli R. Precision Anti-Cancer Medicines by Oligonucleotide Therapeutics in Clinical Research Targeting Undruggable Proteins and Non-Coding RNAs. Pharmaceutics. 2022;14:1453. doi: 10.3390/pharmaceutics14071453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Xiong H., Veedu R.N., Diermeier S.D. Recent Advances in Oligonucleotide Therapeutics in Oncology. Int. J. Mol. Sci. 2021;22:3295. doi: 10.3390/ijms22073295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kang J.-H., Rychahou P.G., Ishola T.A., Qiao J., Evers B.M., Chung D.H. MYCN Silencing Induces Differentiation and Apoptosis in Human Neuroblastoma Cells. Biochem. Biophys. Res. Commun. 2006;351:192–197. doi: 10.1016/j.bbrc.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Piacenti V., Langella E., Autiero I., Nolan J.C., Piskareva O., Adamo M.F.A., Saviano M., Moccia M. A Combined Experimental and Computational Study on Peptide Nucleic Acid (PNA) Analogues of Tumor Suppressive MiRNA-34a. Bioorgan. Chem. 2019;91:103165. doi: 10.1016/j.bioorg.2019.103165. [DOI] [PubMed] [Google Scholar]
- 315.Tonelli R., Purgato S., Camerin C., Fronza R., Bologna F., Alboresi S., Franzoni M., Corradini R., Sforza S., Faccini A., et al. Anti-Gene Peptide Nucleic Acid Specifically Inhibits MYCN Expression in Human Neuroblastoma Cells Leading to Cell Growth Inhibition and Apoptosis. Mol. Cancer Ther. 2005;4:779–786. doi: 10.1158/1535-7163.MCT-04-0213. [DOI] [PubMed] [Google Scholar]
- 316.Janowski B.A., Kaihatsu K., Huffman K.E., Schwartz J.C., Ram R., Hardy D., Mendelson C.R., Corey D.R. Inhibiting Transcription of Chromosomal DNA with Antigene Peptide Nucleic Acids. Nat. Chem. Biol. 2005;1:210–215. doi: 10.1038/nchembio724. [DOI] [PubMed] [Google Scholar]
- 317.Tonelli R., McIntyre A., Camerin C., Walters Z.S., di Leo K., Selfe J., Purgato S., Missiaglia E., Tortori A., Renshaw J., et al. Antitumor Activity of Sustained N-Myc Reduction in Rhabdomyosarcomas and Transcriptional Block by Antigene Therapy. Clin. Cancer Res. 2012;18:796–807. doi: 10.1158/1078-0432.CCR-11-1981. [DOI] [PubMed] [Google Scholar]
- 318.Nielsen P.E., Egholm M., Berg R.H., Buchardt O. Sequence-Selective Recognition of DNA by Strand Displacement with a Thymine-Substituted Polyamide. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 319.Jiang F., Doudna J.A. CRISPR-Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017;46:505–529. doi: 10.1146/annurev-biophys-062215-010822. [DOI] [PubMed] [Google Scholar]
- 320.Zhang B. CRISPR/Cas Gene Therapy. J. Cell. Physiol. 2021;236:2459–2481. doi: 10.1002/jcp.30064. [DOI] [PubMed] [Google Scholar]
- 321.Zhan T., Rindtorff N., Betge J., Ebert M.P., Boutros M. CRISPR/Cas9 for Cancer Research and Therapy. Semin. Cancer Biol. 2019;55:106–119. doi: 10.1016/j.semcancer.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 322.Vaghari-Tabari M., Hassanpour P., Sadeghsoltani F., Malakoti F., Alemi F., Qujeq D., Asemi Z., Yousefi B. CRISPR/Cas9 Gene Editing: A New Approach for Overcoming Drug Resistance in Cancer. Cell. Mol. Biol. Lett. 2022;27:49. doi: 10.1186/s11658-022-00348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yoda H., Inoue T., Shinozaki Y., Lin J., Watanabe T., Koshikawa N., Takatori A., Nagase H. Direct Targeting of MYCN Gene Amplification by Site-Specific DNA Alkylation in Neuroblastoma. Cancer Res. 2019;79:830–840. doi: 10.1158/0008-5472.CAN-18-1198. [DOI] [PubMed] [Google Scholar]
- 324.Ladenstein R., Valteau-Couanet D., Brock P., Yaniv I., Castel V., Laureys G., Malis J., Papadakis V., Lacerda A., Ruud E., et al. Randomized Trial of Prophylactic Granulocyte Colony-Stimulating Factor during Rapid COJEC Induction in Pediatric Patients with High-Risk Neuroblastoma: The European HR-NBL1/SIOPEN Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28:3516–3524. doi: 10.1200/JCO.2009.27.3524. [DOI] [PubMed] [Google Scholar]
- 325.Matthay K.K., Reynolds C.P., Seeger R.C., Shimada H., Adkins E.S., Haas-Kogan D., Gerbing R.B., London W.B., Villablanca J.G. Long-Term Results for Children with High-Risk Neuroblastoma Treated on a Randomized Trial of Myeloablative Therapy Followed by 13-Cis-Retinoic Acid: A Children’s Oncology Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Simon T., Berthold F., Borkhardt A., Kremens B., de Carolis B., Hero B. Treatment and Outcomes of Patients with Relapsed, High-Risk Neuroblastoma: Results of German Trials. Pediatr. Blood Cancer. 2011;56:578–583. doi: 10.1002/pbc.22693. [DOI] [PubMed] [Google Scholar]
- 327.Iehara T., Hosoi H., Akazawa K., Matsumoto Y., Yamamoto K., Suita S., Tajiri T., Kusafuka T., Hiyama E., Kaneko M., et al. MYCN Gene Amplification is a Powerful Prognostic Factor Even in Infantile Neuroblastoma Detected by Mass Screening. Br. J. Cancer. 2006;94:1510–1515. doi: 10.1038/sj.bjc.6603149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Kushner B.H., LaQuaglia M.P., Bonilla M.A., Lindsley K., Rosenfield N., Yeh S., Eddy J., Gerald W.L., Heller G., Cheung N.K. Highly Effective Induction Therapy for Stage 4 Neuroblastoma in Children over 1 Year of Age. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1994;12:2607–2613. doi: 10.1200/JCO.1994.12.12.2607. [DOI] [PubMed] [Google Scholar]
- 329.Beiske K., Burchill S.A., Cheung I.Y., Hiyama E., Seeger R.C., Cohn S.L., Pearson A.D.J., Matthay K.K., International neuroblastoma Risk Group Task Force Consensus Criteria for Sensitive Detection of Minimal Neuroblastoma Cells in Bone Marrow, Blood and Stem Cell Preparations by Immunocytology and QRT-PCR: Recommendations by the International Neuroblastoma Risk Group Task Force. Br. J. Cancer. 2009;100:1627–1637. doi: 10.1038/sj.bjc.6605029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Garaventa A., Parodi S., de Bernardi B., Dau D., Manzitti C., Conte M., Casale F., Viscardi E., Bianchi M., D’Angelo P., et al. Outcome of Children with Neuroblastoma after Progression or Relapse. A Retrospective Study of the Italian Neuroblastoma Registry. Eur. J. Cancer. 2009;45:2835–2842. doi: 10.1016/j.ejca.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 331.Laverdière C., Liu Q., Yasui Y., Nathan P.C., Gurney J.G., Stovall M., Diller L.R., Cheung N.-K., Wolden S., Robison L.L., et al. Long-Term Outcomes in Survivors of Neuroblastoma: A Report from the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2009;101:1131–1140. doi: 10.1093/jnci/djp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Conte M., Parodi S., de Bernardi B., Milanaccio C., Mazzocco K., Angelini P., Viscardi E., di Cataldo A., Luksch R., Haupt R. Neuroblastoma in Adolescents: The Italian Experience. Cancer. 2006;106:1409–1417. doi: 10.1002/cncr.21751. [DOI] [PubMed] [Google Scholar]
- 333.Castel V., Villamón E., Cañete A., Navarro S., Ruiz A., Melero C., Herrero A., Yáñez Y., Noguera R. Neuroblastoma in Adolescents: Genetic and Clinical Characterisation. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2010;12:49–54. doi: 10.1007/s12094-010-0466-z. [DOI] [PubMed] [Google Scholar]
- 334.Esiashvili N., Goodman M., Ward K., Marcus R.B., Johnstone P.A.S. Neuroblastoma in Adults: Incidence and Survival Analysis Based on SEER Data. Pediatr. Blood Cancer. 2007;49:41–46. doi: 10.1002/pbc.20859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets at https://www.clinicaltrials.gov/ (accessed on 1 August 2022) were used in this study.