Abstract
Gastric cancer (GC) was the fourth deadliest cancer in the world in 2020, and about 770,000 people died from GC that year. The death of patients with GC is mainly caused by the metastasis, recurrence, and chemotherapy resistance of GC cells. The cancer stem cell theory defines cancer stem cells (CSCs) as a key factor in the metastasis, recurrence, and chemotherapy resistance of cancer. It considers targeting gastric cancer stem cells (GCSCs) to be an effective method for the treatment of GC. For GCSCs, genes or noncoding RNAs are important regulatory factors. Many experimental studies have found that some drugs can target the stemness of gastric cancer by regulating these genes or noncoding RNAs, which may bring new directions for the clinical treatment of gastric cancer. Therefore, this review mainly discusses related genes or noncoding RNAs in GCSCs and drugs that target its stemness, thereby providing some information for the treatment of GC.
Keywords: gastric cancer stem cells, treatment
1. Introduction
GC is a common malignant tumor. In 2020, cancer caused 10 million deaths in the world, and GC accounted for 7.7% (770,000), ranking the fourth cause of death after lung cancer, colorectal cancer, and liver cancer [1]. Although there are various treatments for GC, the survival rate of many advanced GC patients with metastasis, recurrence, and chemotherapy is quite low [2,3,4,5,6,7,8,9]. Therefore, new therapies are urgently required to improve the survival rate of advanced GC patients.
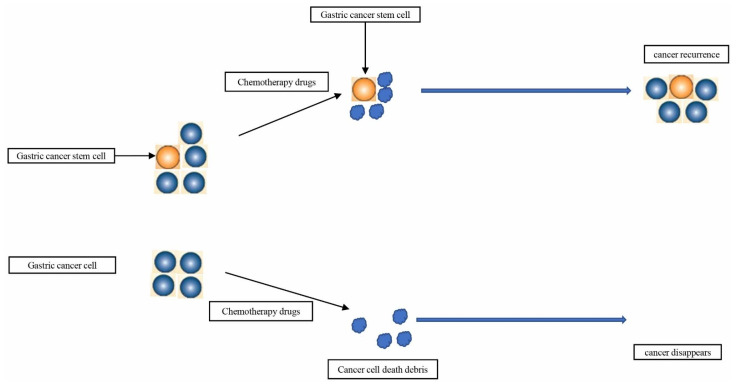
Many researchers find that cancer stem cells may be a key factor in the treatment of advanced cancer. For instance, T Lapidot et al. found that a large number of cell colonies that they called leukemia-initiating cells could be generated by transplanting a small number of CD34+CD38- leukemia cells into mice [10]. T Reya et al. believed that CSCs are the key to cancer treatment. If the CSCs are not killed, but only ordinary cancer cells are killed, the cancer will soon relapse [11]. American Association for Cancer Research (AACR) in 2006 defined that CSCs in tumors have the ability to self-renew and generate heterogeneous tumor cells. Experts at the meeting believed that this cell subset may be resistant to classical treatments, and new drugs need to be developed to selectively target cancer stem cells to treat cancer [12]. With the gradual deepening of research on CSCs, it has been discovered that CSCs are the initiating cells of malignant tumors. They play a key role in cancer metastasis, recurrence, and chemotherapy resistance [13,14,15,16]. In view of the important role of CSCs in GC, many scholars believe that the key to the treatment of GC is to completely eliminate CSCs in gastric cancer [17,18,19] (Figure 1). In order to improve the prognosis of patients with advanced GC, finding new drugs to target GCSCs may be the direction of therapy [20,21]. Genes can regulate the stemness of GC cells, and some drugs target the stemness of GC cells by changing the expression of these gastric, cancer, stem cell-related genes [22,23,24,25,26]. Therefore, in this review, we focused on related genes in GCSCs and drugs and provide some information to treat GC by targeting its stemness.
Figure 1.
GCSCs are also considered to be a key factor in GC recurrence and chemotherapy resistance.
2. The Origin, Isolation, Surface Markers, and Related Signaling Pathways of GCSCs
The origin of GCSCs is currently somewhat controversial. Many researchers held the view that GCSCs might be derived from gastric stem cells. S M Karam et al. found that TFF1 knockout mouse gastric stem cells contribute to gastric carcinogenesis [27]. Lang Yang et al. proved that GC stem-like cells possess higher capability of invasion and metastasis in association with a mesenchymal transition phenotype [28]. These evidences indicated that GCSCs may be derived from gastric stem cells. However, bone marrow-derived cells (BMDCs) discovered by Shigeo Takaishi et al. during their research using a mouse model of Helicobacter-induced GC may also be a source of GCSCs. Meanwhile, BMDCs are considered to be the most primitive uncommitted adult stem cells [29]. Therefore, the source of GCSCs may be gastric stem cells or BMDCs.
At present, there are three mainstream methods for the isolation of GCSCs. The first method is that GCSCs can be separated from side population (SP) cells using nuclear fluorescent dyes. GCSCs have the ability to expel Hoechst33342 so they cannot be stained. The main group of cells is the smaller cell group at the lower left; they are often located in the dot plot of flow analysis. According to this characteristic, the GCSCs can be obtained and collected by selecting the appropriate wavelength of ultraviolet excitation light by the flow cytometer with a sorting function [20]. The second method is to use the surface markers of GCSCs for cell sorting. By applying magnetic strain-activated cell sorting (MACS) technology, Shigeo Takaishi et al. used CD44+ as the surface marker of GCSCs to separate them. The specific mechanism is that the CD44 antibody is fixed on the separation column, and the CD44 positive cells can be adsorbed on the CD44 antibody on the separation column to avoid being repelled from the separation system by the magnetic field [30]. Of course, there are also other surface markers GCSCs that will be discussed later. The third method is to isolate GCSCs by serum-free low-adherence culture. When being cultured in a serum-free, low-adherence medium supplemented with growth factors, GCSCs can form spheroid cells and maintain self-renewal properties, while normal GC cells cannot survive. Spheroid formation assay is considered as a convenient way to obtain GC [20].
It is currently believed that the common surface markers of GCSCs include CD44, CD133, LGR5, MIST1, ALDH1, and AQP5. CD44, CD133, and ALDH1 are also markers for many other cancer stem cells. LGR5 is a G protein-coupled receptor with a seven-pass transmembrane structure. It is considered to be enriched in gastric cancer stem cells. Gastric stem cells expressing Mist1 are thought to have a propensity to transform into gastric cancer stem cells. AQP5 is thought to be enriched in distal gastric cancer stem cells [18,31,32,33,34]. GCSCs can be isolated by fluorescence-activated cell sorting (FACS) or MACS using the above surface markers.
There are many signaling pathways involved in GCSCs. The most common ones include WNT signaling pathway, NOTCH signaling pathway, Hedgehog signaling pathway, and HIPPO signaling pathway [35,36,37,38]. It is well known that the most common signaling pathways of CSCs are WNT, NOTCH, and Hedgehog [39,40,41,42,43]. Aberrant Wnt/β-catenin signaling promotes CSCs renewal, cell proliferation, and differentiation, so they play a critical role in tumorigenesis and therapeutic response [44]. In various cancer types, NOTCH signaling triggers CSCs phenotypes that develop resistance to various therapies, thus potentially leading to cell dormancy and relapse [45]. Hedgehog signaling, a developmental pathway that is mostly inactive in adult tissues except for stem cells, is frequently found to be upregulated in various tumors and is associated with CSCs maintenance [46]. However, in GCSCs, HIPPO signaling pathway is also a very common signaling pathway [47].
3. The Role of Protein-Encoding Genes in GCSCs
Targeted therapy improves the prognosis of cancer patients and brings hope to them [48]. For example, trastuzumab, which targets the HER2 gene, improves the survival rate of patients with HER2-positive breast cancer [49]. Gefitinib could target EGFR to improve the survival rate of patients with small cell lung cancer [50], and VEGFR-targeting bevacizumab improves the survival rate of colorectal cancer patients [51]. Although there are currently no marketed drugs targeting CSCs, researchers have found that targeting CSCs is a feasible way to treat tumors [15]. Rui Su et al. discovered that small molecules CS1 and CS2 targeting FTO can inhibit the renewal of tumor stem cells [24]; Cheng Wang et al. found that anti-CD276 antibody inhibits squamous cell carcinoma stem cells [52]; Yufeng Shi et al. found that gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma [53]. The above results showed that targeting genes with inhibitors or antibodies to inhibit CSCs has potential in treating tumors, which is also applicable in GC. Chien-Hsing Lee et al. found that liquiritigenin inhibited the stem cell-like characteristics of GC by down-regulating the expression of glucose-regulated protein 78 and inhibiting the growth of GC [54]. Therefore, inhibiting GC stemness by inhibiting the expression of GCSCs-related genes is also a method for treating GC.
The protein-encoding gene plays a very important role in the regulation of GCSCs. From the introduction, the common signaling pathways of GCSCs include WNT, NOTCH, Hedgehog, and HIPPO signaling pathways. These genes that regulate GCSCs were divided into two parts: through these above common signaling pathways and not through the above common signaling pathway.
3.1. Related Protein-Encoding Genes in GCSCs through WNT, NOTCH, Hedgehog, and HIPPO Signaling Pathway
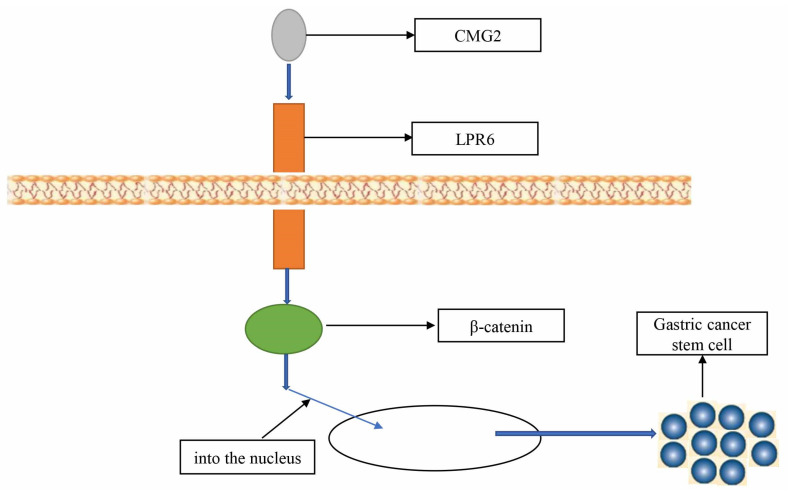
Many genes can regulate the stemness of GC cells by affecting WNT, NOTCH, Hedgehog, and HIPPO signaling pathways (Table 1). The WNT signaling pathway is a very important pathway in GCSCs. Based on Kaiyun Guo et al., tumor necrosis factor-α-inducible protein (Tipα) promotes the tumor stem cell-like properties of GC cells by activating the Wnt/β-catenin signaling pathway, thereby accelerating the progression of GC, and targeting Tipα may be the strategy to treat GC [55]. According to Chengdong Ji et al., capillary morphogenesis gene 2 (CMG2) is highly expressed in GC, and CMG2 interacts with LRP6 in GCSCs to activate the Wnt/β-catenin pathway. Their results revealed that CMG2 promotes GC by maintaining GCSCs progression and may serve as a new prognostic marker and target for human GC therapy [56] (Figure 2). Yunhe Gao et al. found that the expression of ring finger protein 43 (RNF43) was decreased in GCSCs. RNF43 could inhibit the stemness of GC cells by inhibiting the Wnt/β-catenin pathway, the specific mechanism is that RNF43 upregulates the expression of Lgr5 protein, an upstream activator of WNT signaling pathway [57].
Table 1.
Related protein-encoding genes in GCSCs through WNT, NOTCH, Hedgehog, and HIPPO signaling pathways.
| Genes | Functions | Mechanism | Reference |
|---|---|---|---|
| Tipα | Promote stemness | Wnt/β-catenin signaling pathway | [55] |
| CMG2 | Promote stemness | Wnt/β-catenin signaling pathway | [56] |
| RNF43 | Inhibit stemness | Wnt/β-catenin signaling pathway | [57] |
| NOTCH1 | Promote stemness | NOTCH signaling pathway | [58] |
| DLL4 | Promote stemness | NOTCH signaling pathway | [59] |
| GLI2 | Promote stemness | Hedgehog signaling pathway | [60] |
| SCD1 | Promote stemness | HIPPO signaling pathway | [61] |
| RORβ | Inhibit stemness | Wnt/β-catenin signaling pathway | [62] |
| PIGF | Promote stemness | Wnt/β-catenin signaling pathway | [63] |
| NANOGP8 | Promote stemness | Wnt/β-catenin signaling pathway | [64] |
| HES1 | Promote stemness | NOTCH signaling pathway | [65] |
| PAR1 | Promote stemness | HIPPO signaling pathway | [66] |
| WNT1 | Promote stemness | Wnt/β-catenin signaling pathway | [67] |
| Dickkopf-1 | Inhibit stemness | Wnt/β-catenin signaling pathway | [68] |
| TAK1 | Promote stemness | HIPPO signaling pathway | [69] |
Figure 2.
CMG2 maintains GC stem-like cell phenotype by activating a Wnt/β-catenin pathway.
NOTCH signaling pathway also plays an important role in GCSCs. Yan Dou et al. found that the expression of NOTCH1 was increased in GC, the overexpression of NOTCH1 increases the stemness of GC cells, and the knockdown of NOTCH1 reduces the stemness of GC cells. They believed that targeting inhibition of NOTCH signaling pathway on the human GCSCs has drug resistance [58]. Zhi-Feng Miao et al. proposed that the expression of DLL4 was increased in 383 GC tissue samples and was associated with the risk of distant metastasis. DLL4 silencing inhibited the self-renewal of GCSCs and enhanced the multi-differentiation ability, which was achieved through the NOTCH signaling pathway [59].
Hedgehog and HIPPO signaling pathway also affects GCSCs. Yixun Lu et al. found that GLI2 is highly expressed in GC and promotes the stemness of GC cells through the Hedgehog signaling pathway [60]. According to Yunhe Gao et al., the expression of stearoyl-CoA desaturase 1 (SCD1) was increased in metastatic GC, and SCD1 promoted the stem cell-like properties of GCSCs, which was achieved by affecting the expression of YAP and promoting the HIPPO pathway. Therefore, they believe that targeting SCD1 may be a new therapeutic strategy, especially to inhibit GC metastasis and improve chemosensitivity [61].
Many other genes affect GCSCs through WNT, NOTCH, Hedgehog, and HIPPO signaling pathways, which we have summarized in Table 1 [62,63,64,65,66,67,68,69].
3.2. Genes Related to GCSCs-Encoded Proteins with Unspecified Mechanisms or Not Acting through WNT, NOTCH, Hedgehog, and HIPPO Signaling Pathways
Researchers also found many genes related to GCSCs-encoded proteins with unspecified mechanisms or not acting through WNT, NOTCH, Hedgehog, and HIPPO signaling pathways. Some related gene researchers have not explained the mechanism through metabolism, other signaling pathways, hypoxia induction, autophagy, etc.
On the basis of Li-Fei Sun et al., HER2 knockdown in GCSCs reduced the self-renewal, proliferation, colony formation, chemoresistance, and invasion and migration abilities of GCSCs [70]. Natalia Pajuelo-Lozano et al. found that MAD2 is important for the stemness of GCSCs, and its downregulation in GCSCs plays a central role in the occurrence of GC [71]. In addition, other researchers have discovered the role of TRAF6, TAZ, α2δ1 subunit, LINGO2, ALDH, B7-H1, RegIV, and CDK5RAP3 in GCSCs. However, the mechanism is currently unknown, and we have summarized them in Table 2 [72,73,74,75,76,77,78,79].
Table 2.
Genes related to GCSCs-encoded proteins with unspecified mechanisms or not acting through WNT, NOTCH, Hedgehog, and HIPPO signaling pathways.
| Genes | Functions | Mechanism | Reference |
|---|---|---|---|
| HER2 | Promote stemness | - | [70] |
| MAD2 | Promote stemness | - | [71] |
| TRAF6 | Promote stemness | - | [72] |
| TAZ | Promote stemness | - | [73] |
| α2δ1 | Promote stemness | - | [74] |
| LINGO2 | Promote stemness | - | [75] |
| ALDH | Promote stemness | [76] | |
| B7-H1 | Promote stemness | - | [77] |
| RegIV | Promote stemness | - | [78] |
| CDK5RAP3 | Promote stemness | - | [79] |
| SNAT2 | Promote stemness | glutamine | [83] |
| Enolase 1 | Promote stemness | glycolysis | [84] |
| TBL1XR1 | Promote stemness | ERK signaling pathway | [88] |
| BMX-ARHGAP | Promote stemness | JAK/STAT3 signaling pathway | [89] |
| IL-17 | Promote stemness | STAT3 signaling pathway | [90] |
| NRG1 | Promote stemness | NF-KB signaling pathway | [91] |
| HIF-1α | Promote stemness | Snail | [93] |
| CagA | Promote stemness | autophagy | [99] |
| METTL3 | Promote stemness | PARP1 | [100] |
| NME2 | Promote stemness | apoptosis | [101] |
| KDM4C | Promote stemness | ALDH1A3 | [102] |
| E2F1 | Promote stemness | CD44 | [103] |
| SNAIL | Promote stemness | CCN3, NEFL | [104] |
| SLC34A2 | Promote stemness | miR-25/Gsk3β | [105] |
| ATOH1 | Inhibit stemness | differentiation of CSCs | [106] |
It is currently believed that the metabolism of CSCs is heterogeneous, and the four key metabolisms of CSCs are glucose metabolism, glutamine metabolism, mitochondrial metabolism, and lipid metabolism [80,81,82]. Kai Nie et al. found that GCSCs consume more glutamine than ordinary GC cells. The glutamine transporter SNAT2 is highly expressed in GCSCs, and SNAT2 overexpression significantly increases the stemness of GC cells [83]. Ting Yang et al. found that Enolase 1 is highly expressed in GCSCs, and Enolase 1 promotes the stemness, metastatic ability, and chemoresistance of GC cells through glycolysis [84].
Many other signaling pathways are also involved in the progression of CSCs, such as ERK, JAK/STAT, and NF-KB signaling pathways [85,86,87]. Jun Lu et al. found that TBL1XR1 promotes the stemness and metastatic ability of GC cells through the ERK signaling pathway [88]. Xiao-Feng Xu et al. discovered that BMX-ARHGAP fusion protein can promote the stemness of GC cells through the JAK/STAT3 signaling pathway. The mechanism is that BMX-ARHGAP activates JAK/STAT signaling by increasing the expression of BMX-SH2 protein, which contains SH2 domain by binding to phosphorylated tyrosine residues [89]. Y-X Jiang et al. also argued that IL-17 promotes GC stemness through STAT3 signaling pathway [90]. Myoung-Eun Han et al. found that NRG1 secreted by CAFs promotes the self-renewal of GCSCs through the NF-KB signaling pathway [91].
Many studies have indicated that hypoxia can induce the enrichment of CSCs and promote the stemness of cancer cells [92]. Shi-Wei Yang et al. found that after hypoxia treatment, compared with normoxic controls, some GCSCs significantly exhibited the increased expression of hypoxia-inducible factor 1α (HIF-1α), increased migration and invasion abilities, and up-regulated HIF-1α that caused GC recurrence and metastasis by activating Snail [93]. Zhi-Feng Miao et al. also found that HIF-1α can promote the stemness of GC cells [94].
Autophagy is the non-selective degradation of cells and the phagocytosis of damaged and denatured proteins, lipids, organelles, and intracellular pathogens in the cytoplasm. Use of degradation could produce energy and raw materials [95]. Autophagy is activated in CSCs, and autophagy promotes the stemness of cancer cells [96]. Shingo Togano et al. agreed that GCSCs survive in stress environments via their autophagy system [97]. Sarah Courtois et al. found that autophagy induced by Helicobacter pylori infection is necessary for GCSCs emergence [98]. Hitoshi Tsugawa et al. found that CagA autophagic degradation is specifically inhibited in cancer stem-like cells [99].
The above discussion does not cover all; we have summarized the remaining genes in Table 2 [100,101,102,103,104,105,106].
4. The Role of Related Non-Coding RNAs in GCSCs
Noncoding RNAs play important regulatory roles in various diseases, such as cancers [107,108,109,110,111].
Nadya Dimitrova et al. found that miR-143/145 promotes lung cancer progression by targeting CAMK1D [112]. Yina Qiao et al. found that Lnc-408 acts as a sponge for miR-654-5p to alleviate miR-654-5p inhibition of its target LIMK1 and promote breast cancer cell invasion and metastasis [113]. Of course, non-coding RNAs also play regulatory roles in CSCs. For example, Junko Mukohyama et al. found that MIR-221 enhanced the tumorigenicity of human colorectal CSCs by targeting QKI. The regulation of non-coding RNA in GCSCs is mainly miRNA and LncRNA, and there are almost no reports on circular RNA. As such, we will discuss the role of non-coding RNA in GCSCs from miRNA and LncRNA, respectively.
4.1. The Role of MiRNA in GCSCs
MiRNAs are RNAs approximately 22 nucleotides in length that silence gene expression post-transcriptionally by binding to the 3′ untranslated region of the target mRNA [114]. The mechanism of the action of miRNA in GCSCs is mostly by inhibiting the expression of GC cell stemness genes.
Chen Shen et al. found that miR-15a-5p was down-regulated in GCSCs, and inhibited the stemness of GC cells by targeting ONECUT2 [115]; Yixun Lu et al. found that miR-144-3p was down-regulated in GC and combined with the 3′ untranslated region-AUACUGU of 1689–1696 of GLI2 to inhibit the stemness of GC cells [60]. Panpan Zhan et al. found that miR-98-5p was down-regulated in GCSCs and inhibited the self-renewal, invasion, tumorigenicity, and paclitaxel chemosensitivity of GCSCs by targeting BCAT1 [116]. Haiwei Ni et al. found that miR-375 mainly targets SLC7A11 to attenuate the stemness of GC cells [117].
There are currently a variety of miRNA-derived clinical nucleotide drugs (mdCND) in clinical trials, and it is believed that mdCND can benefit patients clinically in the near future [118]. Many other miRNAs also play a role in GCSCs, and we have summarized this in Table 3 [119,120,121,122,123,124,125,126,127,128,129,130,131,132].
Table 3.
Related miRNAs in GCSCs.
| miRNAs | Functions | Mechanism | Reference |
|---|---|---|---|
| miR-15a-5p | Inhibit stemness | ONECUT2 | [115] |
| miR-144-3p | Inhibit stemness | GLI2 | [60] |
| miR-98-5p | Inhibit stemness | BCAT1 | [116] |
| miR-375 | Inhibit stemness | SLC7A11 | [117] |
| miR-451b | Inhibit stemness | - | [119] |
| miR-17-5p | Promote stemness | MKL-1 | [120] |
| miR-6778-5p | Promote stemness | YWHAE | [121] |
| miR-7-5p | Inhibit stemness | Smo, Hes1 | [122] |
| miRNA-598 | Inhibit stemness | RRS1 | [123] |
| miRNA-193a-3p | Promote stemness | SRSF2 | [124] |
| miRNA-19b/20a/92a | Promote stemness | E2F1, HIPK1 | [125] |
| miR-132 | Promote stemness | SIRT1 | [126] |
| miR-196a-5p | Promote stemness | Smad4 | [127] |
| miRNA-145 | Inhibit stemness | CD44 | [128] |
| miR-501-5p | Promote stemness | DKK1, NKD1, GSK3β | [129] |
| miR-483-5p | Promote stemness | - | [130] |
| miR-106b | Promote stemness | Smad7 | [131] |
| miR-34 | Inhibit stemness | Bcl-2 | [132] |
4.2. The Role of IncRNA in GCSCs
Long non-coding RNA (lncRNA) is a non-coding RNA with a length of more than 200 nucleotides. There are many mechanisms of the action of lncRNA, and the most common mechanism is to act as a miRNA sponge to relieve the inhibitory effect of miRNA on target genes [133,134,135].
Yuanjian Hui et al. found that the expression of LncRNA FEZF1-AS1 was increased in GC tissues and cells. The inhibitory effect of miR-363-3p on HMGA2 was relieved by adsorbing miR-363-3p, which promoted the progress of GCSCs [136]. Haiyang Zhang et al. found that lncFERO can inhibit the stemness of GC cells by promoting the expression of SCD1 [137]. Shuai Wang et al. also found that lncRNA ROR promoted the stemness of GC cells [138] (Table 4).
Table 4.
Related lncRNAs in GCSCs.
5. Current Therapies Targeting CSCs
Although there are currently no FDA-approved drugs for clinical use in CSCs, many drugs targeting CSCs that are in clinical trials have shown promising results (Table 5).
Table 5.
Current therapies targeting CSCs.
Vantictumab is an antagonist of the WNT signaling pathway, and its specific mechanism is that it can bind to the extracellular segment of the FZD receptor conserved antigen to inhibit WNT signaling induced by multiple WNT family members. Austin Gurney et al. found that vantictumab can reduce tumor-initiating cell frequency [139]. A combination of vantictumab and taxane sensitizes CSCs to taxanes [140]. The combination of vantictumab and paclitaxel demonstrated significant efficacy in a phase Ib clinical study in patients with metastatic breast cancer [141]. Ipafricept is also an anticancer stem cell drug that acts through the WNT signaling pathway [142]. Ipafricept in combination with gemcitabine and paclitaxel demonstrates a high rate of clinical benefit in phase Ib trial in patients with stage IV pancreatic cancer [143].
MK0752 is an inhibitor of NOTCH signaling pathway, and its specific mechanism is to inhibit the activation of NOTCH intracellular segment by inhibiting γ-secretase, thereby inhibiting the expression of NOTCH downstream genes. In a clinical trial of 30 breast cancer patients treated with MK0752 in combination with docetaxel, reductions in CD44(+)/CD24(−), ALDH(+) and mammosphere formation efficiency were observed in their tumors [144].
Hedgehog inhibitor vismodegib was approved by the FDA in 2012 for the treatment of basal cell carcinoma [145]. Edward J Kim et al. found that in a clinical trial of vismodegib combined with gemcitabine in patients with metastatic pancreatic cancer, the expression of GLI1 and PTCH1 was down-regulated, but there was no significant change in pancreatic cancer stem cells [146].
The results of the above clinical trials targeting CSCs are a promising therapeutic approach, which requires more large-scale clinical trials to validate.
6. Drugs for GC Treatment by Targeting Its Stemness
The emergence of chemotherapy drugs has greatly improved the survival rate of patients with cancer. The aforementioned trastuzumab, gefitinib, and bevacizumab have benefited many patients with cancer [48,49,50,51]. Although there are currently no FDA-approved drugs or drugs entering clinical trials, many basic experiments have shown that drugs or new material can play a role in the treatment of GC by targeting the stemness of GC. We will discuss that separately below.
6.1. Drugs for GC Treatment by Targeting Its Stemness
The researchers have discovered many drugs that could target the stemness of GC, including marketed chemotherapy drugs, clinical drugs for other diseases, small molecule drugs, and traditional Chinese medicines (TCM).
Other cancer chemotherapy drugs also play a role in GCSCs. Wanshuang Cao et al. found that Apatinib can inhibit the stemness of GC cells through the Hedgehog signaling pathway. The specific mechanism is that Apatinib acts by inhibiting the key protein SMO in the Hedgehog signaling pathway [147]. P H Nguyen et al. found that all-trans retinoic acid targets GCSCs and inhibits patient-derived gastric carcinoma tumor growth. The mechanism is that all-trans retinoic acid downregulates the expression of CSC markers, CD44 and ALDH, and stemness genes, such as Klf4 and Sox2, and induces tumorsphere differentiation [148]. ERBB2 is overexpressed in approximately 25% of gastric primary tumor models, which correlates with higher levels of CD90 expression in these tumors, and CD90(+) cells have a higher ability to initiate tumors in vivo. J Jiang et al. found that trastuzumab inhibits the stemness of GC cells by inhibiting ERBB2 signaling [149]. The stemness inhibitory effect of cisplatin in GC was discovered by Yang Han et al. [150].
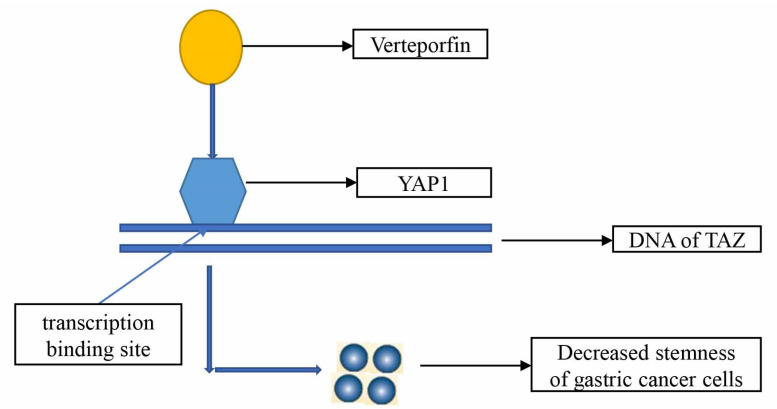
Drugs clinically used for non-tumor therapy can also inhibit the stemness of GC cells. Atsushi Shiozaki et al. found that amlodipine and verapamil inhibited the growth of GCSCs [151]; Julie Giraud et al. found that Verteporfin inhibited the tumorigenic properties of GCSCs by targeting YAP1/TAZ-TEAD transcriptional activity [152] (Figure 3); Jixian Xiong et al. also found that Verteporfin over-regulated HSP90 function to inhibit the stemness of GC cells [153]; and Hassan Akrami et al. found that ibuprofen can inhibit the stemness of GC cells by inhibiting the Wnt/β-catenin signaling pathway [154]. The researchers also found that metformin and pantoprazole inhibit GC stemness, though the authors did not explain the mechanism by which metformin inhibits the stemness of GC, while the mechanism by which pantoprazole inhibits the stemness of GC is through the EMT/β-catenin pathway [155,156].
Figure 3.
Verteporfin inhibits tumorigenic properties of GCSCs by targeting YAP1/TAZ transcriptional activity.
Many newly discovered small-molecule drugs also play a role in suppressing the stemness of GC cells. Yun-Shen Tai et al. found that 4′-bromoresveratrol (4-BR) inhibited GC cell stemness through the SIRT3-c-Jun N-terminal kinase pathway [157]; Yao-Dong Zhu et al. found that Celastrus orbiculatus extract (COE) inhibited the stemness of GC cells by regulating the expression of PDCD4 and EIF3H [158]; Xinsheng Shen et al. found that Quercetin triggered mitochondrial apoptosis-dependent growth inhibition by inhibiting PI3K/Akt signaling to play a role in suppressing the stemness of GC cells [159]. Of course, the researchers have also discovered many other small molecule drugs to inhibit the stemness of GC cells, which provided an experimental basis for the treatment of GC [160,161,162,163,164,165,166] (Table 6).
Table 6.
Drugs for GC treatment by inhibiting its stemness.
| Drugs | Functions | Mechanism | Reference |
|---|---|---|---|
| apatinib | Inhibit stemness | Hedgehog signaling pathway | [147] |
| all-trans retinoic acid | Inhibit stemness | - | [148] |
| trastuzumab | Inhibit stemness | ERBB2 signaling | [149] |
| cisplatin | Inhibit stemness | - | [150] |
| amlodipine verapamil | Inhibit stemness | - | [151] |
| Verteporfin | Inhibit stemness | YAP1/TAZ | [152] |
| Verteporfin | Inhibit stemness | HSP90 | [153] |
| ibuprofen | Inhibit stemness | Wnt/β-catenin signaling pathway | [154] |
| Metformin | Inhibit stemness | - | [155] |
| pantoprazole | Inhibit stemness | EMT/β-catenin pathways | [156] |
| 4-BR | Inhibit stemness | SIRT3-c-Jun N-terminal kinase pathway | [157] |
| COE | Inhibit stemness | PDCD4, EIF3H | [158] |
| Quercetin | Inhibit stemness | PI3K/Akt signaling | [159] |
| PTPRU | Inhibit stemness | Hippo/YAP Signaling Pathway | [160] |
| Sulforaphane | Inhibit stemness | hedgehog pathway | [161] |
| DFOG | Inhibit stemness | FoxM1 | [162] |
| Evodiamine | Inhibit stemness | Wnt/β-catenin signaling pathway | [163] |
| DAPT | Inhibit stemness | Notch pathway | [164] |
| Atractylenolide I | Inhibit stemness | Notch pathway | [165] |
| Genistein | Inhibit stemness | ERK | [166] |
| Sijunzi Decoction | Inhibit stemness | transcriptional activity of β-Catenin | [167] |
| Xiaotan Sanjie decoction | Inhibit stemness | Notch1 | [168] |
It has also been reported that TCM could inhibit the stemness of GC cells. Yue-Jun Li et al. found that Sijunzi Decoction inhibited the stemness of GC cells by inhibiting the transcriptional activity of β-Catenin [167]. Furthermore, Bing Yan et al. found that Xiaotan Sanjie decoction inhibited GC cell stemness and angiogenesis through Notch1 [168].
Although the drugs described above have not entered clinical trials, they have shown good therapeutic effects targeting GCSCs during in vivo and in vitro trials. It is believed that some of the above drugs would enter clinical trials and be approved by the FDA in the near future and benefit GC patients.
6.2. New Material for GC Treatment by Targeting Its Stemness
Many new materials have played a huge role in treating cancer. For example, Doxil, the first FDA-approved liposome drug, has shown great success in the treatment of ovarian cancer and breast cancer [169,170,171,172]. Vincristine sulfate liposome injection Marqibo was also FDA-approved for the treatment of adults with advanced, relapsed, and refractory Philadelphia chromosome-negative ALL [173,174]. The emergence of these drugs proved that new materials drugs have great potential in cancer treatment. Therefore, many researchers have pointed out that new materials drugs can inhibit GC cells by targeting the stemness of GC cells, which provides a research basis for the treatment of GC.
Hongjuan Yao et al. found that Gli1 siRNA nanoparticles inhibited the stemness of GC cells by inhibiting the Hedgehog signaling pathway, which provided a promising targeted therapy strategy for the treatment of GC [175]; Han Chen et al. found that nanoparticles CD44/CD133-ATRA-PLPN can inhibit the proliferation of GCSCs [176]; Feng Yang et al. found that CD44 targeting USP22 small interfering RNA-loaded nanoliposomes can target and eradicate GCSCs [177]; Weifeng Yang et al. found that HA-coated nanoparticles, co-encapsulating plasmid METase, and 5-Fu showed enhanced application in targeting GCSCs [178]. Many other new material drugs could inhibit the stemness of GC cells [179,180,181]. These new materials have also shown very good therapeutic effects in experiments targeting GCSCs (Table 7), and it is expected that they will benefit cancer patients like Doxil.
Table 7.
New materials for GC treatment by inhibiting its stemness.
| New Materials Drugs | Functions | Mechanism | Reference |
|---|---|---|---|
| Gli1 siRNA nanoparticles | Inhibit stemness | Hedgehog signaling pathway | [175] |
| CD44/CD133-ATRA-PLPN | Inhibit stemness | - | [176] |
| USP22-NLs-CD44 | Inhibit stemness | - | [177] |
| METase/5-Fu co-encaspulated NPs | Inhibit stemness | - | [178] |
| miR-34a delivery system | Inhibit stemness | CD44 | [179] |
| CD44v6-GNS nanoprobes | Inhibit stemness | - | [180] |
| SAL-SWNT-CHI-HA complexes | Inhibit stemness | - | [181] |
7. Conclusions
GC is a disease that plagues the world. The metastasis, recurrence, and chemotherapy resistance of GC are very fatal to GC patients. According to the GCSCs theory, GCSCs play a key role in the metastasis, recurrence, and chemotherapy resistance of GC, and GC stemness genes can regulate GCSCs. It may serve as a way to treat GC by targeting the expression of GC stemness genes. Therefore, we have summarized the related genes or noncoding RNAs in GCSCs and drugs for GC treatment by targeting its stemness, which could provide some information for the clinical treatment of GC.
Author Contributions
Study design, X.W.; data collection and manuscript writing, X.R., C.Z. and H.L.; manuscript revision, J.Z., Z.Z. and Z.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This work was supported by the public health scientific research project of Futian District, Shenzhen (NO. FTWS2020011), Special Fund for Outstanding Youth Reserve Talent Program of the Eighth Affiliated Hospital of Sun Yat-sen University (FBJQ2019006), and Research Initiation Fund of the Eighth Affiliated Hospital of Sun Yat-sen University (GCC RCY J011).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Körfer J., Lordick F., Hacker U.T. Molecular Targets for Gastric Cancer Treatment and Future Perspectives from a Clinical and Translational Point of View. Cancers. 2021;13:5216. doi: 10.3390/cancers13205216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y.H., Li C.L., Chen W.J., Liu J., Wu H.T. Diverse roles of FOXO family members in gastric cancer. World J. Gastrointest. Oncol. 2021;13:1367–1382. doi: 10.4251/wjgo.v13.i10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Z., Wang R., Zhou Y., Wang Q., Yang C.Y., Hao B.C., Ke C.F. Prediction of distant metastasis and survival prediction of gastric cancer patients with metastasis to the liver, lung, bone, and brain: Research based on the SEER database. Ann. Transl. Med. 2022;10:16. doi: 10.21037/atm-21-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kole C., Charalampakis N., Tsakatikas S., Kouris N.I., Papaxoinis G., Karamouzis M.V., Koumarianou A., Schizas D. Immunotherapy for gastric cancer: A 2021 update. Immunotherapy. 2022;14:41–64. doi: 10.2217/imt-2021-0103. [DOI] [PubMed] [Google Scholar]
- 6.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C., Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 7.Ohnuma H., Sato Y., Hirakawa M., Kikuchi S., Miyanishi K., Sagawa T., Takahashi Y., Nobuoka T., Okamoto K., Miyamoto H., et al. Docetaxel, cisplatin and S-1 (DCS) combination chemotherapy for gastric cancer patients with peritoneal metastasis: A retrospective study. Cancer Chemother. Pharmacol. 2018;81:539–548. doi: 10.1007/s00280-018-3523-x. [DOI] [PubMed] [Google Scholar]
- 8.Brungs D., Aghmesheh M., Vine K.L., Becker T.M., Carolan M.G., Ranson M. Gastric cancer stem cells: Evidence, potential markers, and clinical implications. J. Gastroenterol. 2016;51:313–326. doi: 10.1007/s00535-015-1125-5. [DOI] [PubMed] [Google Scholar]
- 9.Fath M.K., Ebrahimi M., Nourbakhsh E., Hazara A.Z., Mirzaei A., Shafieyari S., Salehi A., Hoseinzadeh M., Payandeh Z., Barati G. PI3K/Akt/mTOR signaling pathway in cancer stem cells. Pathol. Res. Pract. 2022;237:154010. doi: 10.1016/j.prp.2022.154010. [DOI] [PubMed] [Google Scholar]
- 10.Lapidot T., Sirard C., Vormoor J., Murdoch B., Hoang T., Caceres-Cortes J., Minden M., Paterson B., Caligiuri M.A., Dick J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 11.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 12.Clarke M.F., Dick J.E., Dirks P.B., Eaves C.J., Jamieson C.H., Jones D.L., Visvader J., Weissman I.L., Wahl G.M. Cancer stem cells—Perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 13.Pastushenko I., Mauri F., Song Y., de Cock F., Meeusen B., Swedlund B., Impens F., van Haver D., Opitz M., Thery M., et al. Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature. 2021;589:448–455. doi: 10.1038/s41586-020-03046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medema J.P. Targeting the Colorectal Cancer Stem Cell. N. Engl. J. Med. 2017;377:888–890. doi: 10.1056/NEJMcibr1706541. [DOI] [PubMed] [Google Scholar]
- 15.Clarke M.F. Clinical and Therapeutic Implications of Cancer Stem Cells. N. Engl. J. Med. 2019;380:2237–2245. doi: 10.1056/NEJMra1804280. [DOI] [PubMed] [Google Scholar]
- 16.Fu T., Coulter S., Yoshihara E., Oh T.G., Fang S., Cayabyab F., Zhu Q., Zhang T., Leblanc M., Liu S., et al. FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell. 2019;176:1098–1112.e1018. doi: 10.1016/j.cell.2019.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otaegi-Ugartemendia M., Matheu A., Carrasco-Garcia E. Impact of Cancer Stem Cells on Therapy Resistance in Gastric Cancer. Cancers. 2022;14:1457. doi: 10.3390/cancers14061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh H.L., Yu M.C., Cheng L.C., Yeh T.S., Tsai M.M. Molecular mechanism of therapeutic approaches for human gastric cancer stem cells. World J. Stem Cells. 2022;14:76–91. doi: 10.4252/wjsc.v14.i1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li K., Dan Z., Nie Y.Q. Gastric cancer stem cells in gastric carcinogenesis, progression, prevention and treatment. World J. Gastroenterol. 2014;20:5420–5426. doi: 10.3748/wjg.v20.i18.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu Y., Du P., Zhao J., Hu C., Qin Y., Huang G. Gastric Cancer Stem Cells: Mechanisms and Therapeutic Approaches. Yonsei Med. J. 2018;59:1150–1158. doi: 10.3349/ymj.2018.59.10.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stojnev S., Krstic M., Ristic-Petrovic A., Stefanovic V., Hattori T. Gastric cancer stem cells: Therapeutic targets. Gastric Cancer. 2014;17:13–25. doi: 10.1007/s10120-013-0254-x. [DOI] [PubMed] [Google Scholar]
- 22.Liu C., Kelnar K., Liu B., Chen X., Calhoun-Davis T., Li H., Patrawala L., Yan H., Jeter C., Honorio S., et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coles G.L., Cristea S., Webber J.T., Levin R.S., Moss S.M., He A., Sangodkar J., Hwang Y.C., Arand J., Drainas A.P., et al. Unbiased Proteomic Profiling Uncovers a Targetable GNAS/PKA/PP2A Axis in Small Cell Lung Cancer Stem Cells. Cancer Cell. 2020;38:129–143.e127. doi: 10.1016/j.ccell.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su R., Dong L., Li Y., Gao M., Han L., Wunderlich M., Deng X., Li H., Huang Y., Gao L., et al. Targeting FTO Suppresses Cancer Stem Cell Maintenance and Immune Evasion. Cancer Cell. 2020;38:79–96.e11. doi: 10.1016/j.ccell.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez-Pascual A., Hale J.S., Kordowski A., Pugh J., Silver D.J., Bayik D., Roversi G., Alban T.J., Rao S., Chen R., et al. ADAMDEC1 Maintains a Growth Factor Signaling Loop in Cancer Stem Cells. Cancer Discov. 2019;9:1574–1589. doi: 10.1158/2159-8290.CD-18-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruggieri V., Russi S., Zoppoli P., la Rocca F., Angrisano T., Falco G., Calice G., Laurino S. The Role of MicroRNAs in the Regulation of Gastric Cancer Stem Cells: A Meta-Analysis of the Current Status. J. Clin. Med. 2019;8:639. doi: 10.3390/jcm8050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karam S.M., Tomasetto C., Rio M.C. Amplification and invasiveness of epithelial progenitors during gastric carcinogenesis in trefoil factor 1 knockout mice. Cell Prolif. 2008;41:923–935. doi: 10.1111/j.1365-2184.2008.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L., Ping Y.F., Yu X., Qian F., Guo Z.J., Qian C., Cui Y.H., Bian X.W. Gastric cancer stem-like cells possess higher capability of invasion and metastasis in association with a mesenchymal transition phenotype. Cancer Lett. 2011;310:46–52. doi: 10.1016/j.canlet.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Takaishi S., Okumura T., Wang T.C. Gastric cancer stem cells. J. Clin. Oncol. 2008;26:2876–2882. doi: 10.1200/JCO.2007.15.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takaishi S., Okumura T., Tu S., Wang S.S., Shibata W., Vigneshwaran R., Gordon S.A., Shimada Y., Wang T.C. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan S.H., Swathi Y., Tan S., Goh J., Seishima R., Murakami K., Oshima M., Tsuji T., Phuah P., Tan L.T., et al. AQP5 enriches for stem cells and cancer origins in the distal stomach. Nature. 2020;578:437–443. doi: 10.1038/s41586-020-1973-x. [DOI] [PubMed] [Google Scholar]
- 32.Nienhüser H., Kim W., Malagola E., Ruan T., Valenti G., Middelhoff M., Bass A., Der C.J., Hayakawa Y., Wang T.C. Mist1+ gastric isthmus stem cells are regulated by Wnt5a and expand in response to injury and inflammation in mice. Gut. 2021;70:654–665. doi: 10.1136/gutjnl-2020-320742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wattanawongdon W., Bathpho T.S., Tongtawee T. Co-Expression of LGR5 and CD133 Cancer Stem Cell Predicts a Poor Prognosis in Patients with Gastric Cancer. Turk. J. Gastroenterol. 2021;32:261–268. doi: 10.5152/tjg.2021.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fatehullah A., Terakado Y., Sagiraju S., Tan T.L., Sheng T., Tan S.H., Murakami K., Swathi Y., Ang N., Rajarethinam R., et al. A tumour-resident Lgr5(+) stem-cell-like pool drives the establishment and progression of advanced gastric cancers. Nat. Cell Biol. 2021;23:1299–1313. doi: 10.1038/s41556-021-00793-9. [DOI] [PubMed] [Google Scholar]
- 35.Fu Y., Li H., Hao X. The self-renewal signaling pathways utilized by gastric cancer stem cells. Tumour Biol. 2017;39:1010428317697577. doi: 10.1177/1010428317697577. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y.S., Lee H.J., Park J.M., Han Y.M., Kangwan N., Oh J.Y., Lee D.Y., Hahm K.B. Targeted molecular ablation of cancer stem cells for curing gastrointestinal cancers. Expert Rev. Gastroenterol. Hepatol. 2017;11:1059–1070. doi: 10.1080/17474124.2017.1356224. [DOI] [PubMed] [Google Scholar]
- 37.Bekaii-Saab T., El-Rayes B. Identifying and targeting cancer stem cells in the treatment of gastric cancer. Cancer. 2017;123:1303–1312. doi: 10.1002/cncr.30538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samadani A.A., Akhavan-Niaki H. Interaction of sonic hedgehog (SHH) pathway with cancer stem cell genes in gastric cancer. Med. Oncol. 2015;32:48. doi: 10.1007/s12032-015-0492-3. [DOI] [PubMed] [Google Scholar]
- 39.Samadani A.A., Keymoradzdeh A., Shams S., Soleymanpour A., Norollahi S.E., Vahidi S., Rashidy-Pour A., Ashraf A., Mirzajani E., Khanaki K., et al. Mechanisms of cancer stem cell therapy. Clin. Chim. Acta. 2020;510:581–592. doi: 10.1016/j.cca.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Yang L., Shi P., Zhao G., Xu J., Peng W., Zhang J., Zhang G., Wang X., Dong Z., Chen F., et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Najafi M., Farhood B., Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell. Physiol. 2019;234:8381–8395. doi: 10.1002/jcp.27740. [DOI] [PubMed] [Google Scholar]
- 42.Agliano A., Calvo A., Box C. The challenge of targeting cancer stem cells to halt metastasis. Semin. Cancer Biol. 2017;44:25–42. doi: 10.1016/j.semcancer.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Horne G.A., Copland M. Approaches for targeting self-renewal pathways in cancer stem cells: Implications for hematological treatments. Expert Opin. Drug Discov. 2017;12:465–474. doi: 10.1080/17460441.2017.1303477. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020;13:165. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janghorban M., Xin L., Rosen J.M., Zhang X.H. Notch Signaling as a Regulator of the Tumor Immune Response: To Target or Not to Target? Front. Immunol. 2018;9:1649. doi: 10.3389/fimmu.2018.01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levanat S., Sabol M., Musani V., Ozretic P., Trnski D. Hedgehog Signaling Pathway as Genetic and Epigenetic Target in Ovarian Tumors. Curr. Pharm. Des. 2017;23:73–94. doi: 10.2174/1381612822666161006154705. [DOI] [PubMed] [Google Scholar]
- 47.Seeneevassen L., Dubus P., Gronnier C., Varon C. Hippo in Gastric Cancer: From Signalling to Therapy. Cancers. 2022;14:2282. doi: 10.3390/cancers14092282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bedard P.L., Hyman D.M., Davids M.S., Siu L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395:1078–1088. doi: 10.1016/S0140-6736(20)30164-1. [DOI] [PubMed] [Google Scholar]
- 49.Shepard H.M. Biomarker-Driven Drug Discovery in Cancer-Trastuzumab Development: 2019 Lasker-DeBakey Clinical Medical Research Award. JAMA. 2019;322:1249–1250. doi: 10.1001/jama.2019.13963. [DOI] [PubMed] [Google Scholar]
- 50.Zalcman G., Bergot E. Gefitinib plus docetaxel in non-small-cell lung cancer. Lancet. 2009;373:541–542. doi: 10.1016/S0140-6736(09)60192-6. [DOI] [PubMed] [Google Scholar]
- 51.Simkens L.H., van Tinteren H., May A., Tije A.J.t., Creemers G.J., Loosveld O.J., de Jongh F.E., Erdkamp F.L., Erjavec Z., van der Torren A.M., et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): A phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385:1843–1852. doi: 10.1016/S0140-6736(14)62004-3. [DOI] [PubMed] [Google Scholar]
- 52.Wang C., Li Y., Jia L., Kim J.K., Li J., Deng P., Zhang W., Krebsbach P.H., Wang C.Y. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell. 2021;28:1597–1613.e1597. doi: 10.1016/j.stem.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y., Lim S.K., Liang Q., Iyer S.V., Wang H.Y., Wang Z., Xie X., Sun D., Chen Y.J., Tabar V., et al. Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma. Nature. 2019;567:341–346. doi: 10.1038/s41586-019-0993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee C.H., Tsai H.Y., Chen C.L., Chen J.L., Lu C.C., Fang Y.P., Wu D.C., Huang Y.B., Lin M.W. Isoliquiritigenin Inhibits Gastric Cancer Stemness, Modulates Tumor Microenvironment, and Suppresses Tumor Growth through Glucose-Regulated Protein 78 Downregulation. Biomedicines. 2022;10:1350. doi: 10.3390/biomedicines10061350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo K., Duan J., Lu J., Xiao L., Han L., Zeng S., Tang X., Li W., Huang L., Zhang Y. TNF-α-inducing protein of Helicobacter pylori promotes EMT and cancer stem-like cells properties via activation of Wnt/β-catenin signaling pathway in gastric cancer cells. Pathog. Dis. 2022;80:ftac025. doi: 10.1093/femspd/ftac025. [DOI] [PubMed] [Google Scholar]
- 56.Ji C., Yang L., Yi W., Xiang D., Wang Y., Zhou Z., Qian F., Ren Y., Cui W., Zhang X., et al. Capillary morphogenesis gene 2 maintains gastric cancer stem-like cell phenotype by activating a Wnt/β-catenin pathway. Oncogene. 2018;37:3953–3966. doi: 10.1038/s41388-018-0226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao Y., Cai A., Xi H., Li J., Xu W., Zhang Y., Zhang K., Cui J., Wu X., Wei B., et al. Ring finger protein 43 associates with gastric cancer progression and attenuates the stemness of gastric cancer stem-like cells via the Wnt-β/catenin signaling pathway. Stem Cell Res. Ther. 2017;8:98. doi: 10.1186/s13287-017-0548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dou Y., Wang J. Targeting Inhibition of Notch1 Signaling Pathway on the Study of Human Gastric Cancer Stem Cells with Chemosensitization. Comput. Intell. Neurosci. 2022;2022:1098394. doi: 10.1155/2022/1098394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Miao Z.F., Xu H., Xu H.M., Wang Z.N., Zhao T.T., Song Y.X., Xu Y.Y. DLL4 overexpression increases gastric cancer stem/progenitor cell self-renewal ability and correlates with poor clinical outcome via Notch-1 signaling pathway activation. Cancer Med. 2017;6:245–257. doi: 10.1002/cam4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu Y., Zhang B., Wang B., Wu D., Wang C., Gao Y., Liang W., Xi H., Wang X., Chen L. MiR-144-3p inhibits gastric cancer progression and stemness via directly targeting GLI2 involved in hedgehog pathway. J. Transl. Med. 2021;19:432. doi: 10.1186/s12967-021-03093-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao Y., Li J., Xi H., Cui J., Zhang K., Zhang J., Zhang Y., Xu W., Liang W., Zhuang Z., et al. Stearoyl-CoA-desaturase-1 regulates gastric cancer stem-like properties and promotes tumour metastasis via Hippo/YAP pathway. Br. J. Cancer. 2020;122:1837–1847. doi: 10.1038/s41416-020-0827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen Z., Chen M., Guo W., Guo K., Du P., Fang Y., Gao M., Wang Q. RORβ suppresses the stemness of gastric cancer cells by downregulating the activity of the Wnt signaling pathway. Oncol. Rep. 2021;46:180. doi: 10.3892/or.2021.8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akrami H., Mehdizadeh K., Moradi B., Farahani D.B., Mansouri K., Alnajar S.G.I. PlGF knockdown induced apoptosis through Wnt signaling pathway in gastric cancer stem cells. J. Cell. Biochem. 2019;120:3268–3276. doi: 10.1002/jcb.27593. [DOI] [PubMed] [Google Scholar]
- 64.Ma X., Wang B., Wang X., Luo Y., Fan W. NANOGP8 is the key regulator of stemness, EMT, Wnt pathway, chemoresistance, and other malignant phenotypes in gastric cancer cells. PLoS ONE. 2018;13:e0192436. doi: 10.1371/journal.pone.0192436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L., Li Y., Wang L., Wu Z., Ma H., Shao J., Li D., Yu H., Nian W., Wang D. Inhibition of Hes1 enhances lapatinib sensitivity in gastric cancer sphere-forming cells. Oncol. Lett. 2017;14:3989–3996. doi: 10.3892/ol.2017.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujimoto D., Ueda Y., Hirono Y., Goi T., Yamaguchi A. PAR1 participates in the ability of multidrug resistance and tumorigenesis by controlling Hippo-YAP pathway. Oncotarget. 2015;6:34788–34799. doi: 10.18632/oncotarget.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mao J., Fan S., Ma W., Fan P., Wang B., Zhang J., Wang H., Tang B., Zhang Q., Yu X., et al. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang B., Liu J., Ma L.N., Xiao H.L., Wang Y.Z., Li Y., Wang Z., Fan L., Lan C., Yang M., et al. Chimeric 5/35 adenovirus-mediated Dickkopf-1 overexpression suppressed tumorigenicity of CD44⁺ gastric cancer cells via attenuating Wnt signaling. J. Gastroenterol. 2013;48:798–808. doi: 10.1007/s00535-012-0711-z. [DOI] [PubMed] [Google Scholar]
- 69.Wang G., Sun Q., Zhu H., Bi Y., Zhu H., Xu A. The stabilization of yes-associated protein by TGFβ-activated kinase 1 regulates the self-renewal and oncogenesis of gastric cancer stem cells. J. Cell. Mol. Med. 2021;25:6584–6601. doi: 10.1111/jcmm.16660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun L.F., Yang K., Wang Y.G., Liu Y.X., Hou P.X., Lu Z.H., Chen X.L., Zhang W.H., Zhou Z.G., Mo X.M., et al. The Role of HER2 in Self-Renewal, Invasion, and Tumorigenicity of Gastric Cancer Stem Cells. Front. Oncol. 2020;10:1608. doi: 10.3389/fonc.2020.01608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pajuelo-Lozano N., Alcalá S., Sainz B., Jr., Perona R., Sanchez-Perez I. Targeting MAD2 modulates stemness and tumorigenesis in human Gastric Cancer cell lines. Theranostics. 2020;10:9601–9618. doi: 10.7150/thno.49270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang M., Jin M., Li K., Liu H., Yang X., Zhang X., Zhang B., Gong A., Bie Q. TRAF6 Promotes Gastric Cancer Cell Self-Renewal, Proliferation, and Migration. Stem Cells Int. 2020;2020:3296192. doi: 10.1155/2020/3296192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tiffon C., Giraud J., Molina-Castro S.E., Peru S., Seeneevassen L., Sifré E., Staedel C., Bessède E., Dubus P., Mégraud F., et al. TAZ Controls Helicobacter pylori-Induced Epithelial-Mesenchymal Transition and Cancer Stem Cell-Like Invasive and Tumorigenic Properties. Cells. 2020;9:1462. doi: 10.3390/cells9061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Z., Zhao W., Lin X., Gao J., Zhang Z., Shen L. Voltage-dependent calcium channel α2δ1 subunit is a specific candidate marker for identifying gastric cancer stem cells. Cancer Manag. Res. 2019;11:4707–4718. doi: 10.2147/CMAR.S199329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jo J.H., Park S.B., Park S., Lee H.S., Kim C., Jung D.E., Song S.Y. Novel Gastric Cancer Stem Cell-Related Marker LINGO2 Is Associated with Cancer Cell Phenotype and Patient Outcome. Int. J. Mol. Sci. 2019;20:555. doi: 10.3390/ijms20030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu D., Mou Y.P., Chen K., Cai J.Q., Zhou Y.C., Pan Y., Xu X.W., Zhou W., Gao J.Q., Chen D.W., et al. Aldehyde dehydrogenase 3A1 is robustly upregulated in gastric cancer stem-like cells and associated with tumorigenesis. Int. J. Oncol. 2016;49:611–622. doi: 10.3892/ijo.2016.3551. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y., Wu K.E., Zhao E., Li W., Shi L., Xie G., Jiang B., Wang Y., Li R., Zhang P., et al. B7-H1 enhances proliferation ability of gastric cancer stem-like cells as a receptor. Oncol. Lett. 2015;9:1833–1838. doi: 10.3892/ol.2015.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou W., Sun M., Wang D.L., Wang Y., Jin F., Zhang Y.Y., Yang L., Wu X.L., Wu Y.Z. Silencing of RegIV by shRNA causes the loss of stemness properties of cancer stem cells in MKN45 gastric cancer cells. Oncol. Rep. 2013;30:2685–2690. doi: 10.3892/or.2013.2745. [DOI] [PubMed] [Google Scholar]
- 79.Lin J.X., Yoon C., Li P., Ryeom S.W., Cho S.J., Zheng C.H., Xie J.W., Wang J.B., Lu J., Chen Q.Y., et al. CDK5RAP3 as tumour suppressor negatively regulates self-renewal and invasion and is regulated by ERK1/2 signalling in human gastric cancer. Br. J. Cancer. 2020;123:1131–1144. doi: 10.1038/s41416-020-0963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alvina F.B., Gouw A.M., Le A. Cancer Stem Cell Metabolism. Adv. Exp. Med. Biol. 2021;1311:161–172. doi: 10.1007/978-3-030-65768-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raggi C., Taddei M.L., Sacco E., Navari N., Correnti M., Piombanti B., Pastore M., Campani C., Pranzini E., Iorio J., et al. Mitochondrial oxidative metabolism contributes to a cancer stem cell phenotype in cholangiocarcinoma. J. Hepatol. 2021;74:1373–1385. doi: 10.1016/j.jhep.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 82.Liu S., Sun Y., Hou Y., Yang L., Wan X., Qin Y., Liu Y., Wang R., Zhu P., Teng Y., et al. A novel lncRNA ROPM-mediated lipid metabolism governs breast cancer stem cell properties. J. Hematol. Oncol. 2021;14:178. doi: 10.1186/s13045-021-01194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nie K., Cai M. SNAT2/SLC38A2 Confers the Stemness of Gastric Cancer Cells via Regulating Glutamine Level. Dig. Dis. Sci. 2022;67:2948–2956. doi: 10.1007/s10620-021-07110-2. [DOI] [PubMed] [Google Scholar]
- 84.Yang T., Shu X., Zhang H.W., Sun L.X., Yu L., Liu J., Sun L.C., Yang Z.H., Ran Y.L. Enolase 1 regulates stem cell-like properties in gastric cancer cells by stimulating glycolysis. Cell Death Dis. 2020;11:870. doi: 10.1038/s41419-020-03087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cruz A.F., Fonseca N.A., Moura V., Simoes S., Moreira J.N. Targeting cancer stem cells and non-stem cancer cells: The potential of lipid-based nanoparticles. Curr. Pharm. Des. 2017;23:6563–6572. doi: 10.2174/1381612823666171115105252. [DOI] [PubMed] [Google Scholar]
- 86.Semba T., Sammons R., Wang X., Xie X., Dalby K.N., Ueno N.T. JNK Signaling in Stem Cell Self-Renewal and Differentiation. Int. J. Mol. Sci. 2020;21:2613. doi: 10.3390/ijms21072613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghasemi F., Sarabi P.Z., Athari S.S., Esmaeilzadeh A. Therapeutics strategies against cancer stem cell in breast cancer. Int. J. Biochem. Cell Biol. 2019;109:76–81. doi: 10.1016/j.biocel.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 88.Lu J., Bang H., Kim S.M., Cho S.J., Ashktorab H., Smoot D.T., Zheng C.H., Ryeom S.W., Yoon S.S., Yoon C., et al. Lymphatic metastasis-related TBL1XR1 enhances stemness and metastasis in gastric cancer stem-like cells by activating ERK1/2-SOX2 signaling. Oncogene. 2021;40:922–936. doi: 10.1038/s41388-020-01571-x. [DOI] [PubMed] [Google Scholar]
- 89.Xu X.F., Gao F., Wang J.J., Long C., Chen X., Tao L., Yang L., Ding L., Ji Y. BMX-ARHGAP fusion protein maintains the tumorigenicity of gastric cancer stem cells by activating the JAK/STAT3 signaling pathway. Cancer Cell Int. 2019;19:133. doi: 10.1186/s12935-019-0847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang Y.X., Yang S.W., Li P.A., Luo X., Li Z.Y., Hao Y.X., Yu P.W. The promotion of the transformation of quiescent gastric cancer stem cells by IL-17 and the underlying mechanisms. Oncogene. 2017;36:1256–1264. doi: 10.1038/onc.2016.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han M.E., Kim H.J., Shin D.H., Hwang S.H., Kang C.D., Oh S.O. Overexpression of NRG1 promotes progression of gastric cancer by regulating the self-renewal of cancer stem cells. J. Gastroenterol. 2015;50:645–656. doi: 10.1007/s00535-014-1008-1. [DOI] [PubMed] [Google Scholar]
- 92.Wicks E.E., Semenza G.L. Hypoxia-inducible factors: Cancer progression and clinical translation. J. Clin. Investig. 2022;132:e159839. doi: 10.1172/JCI159839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang S.W., Zhang Z.G., Hao Y.X., Zhao Y.L., Qian F., Shi Y., Li P.A., Liu C.Y., Yu P.W. HIF-1α induces the epithelial-mesenchymal transition in gastric cancer stem cells through the Snail pathway. Oncotarget. 2017;8:9535–9545. doi: 10.18632/oncotarget.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miao Z.F., Wang Z.N., Zhao T.T., Xu Y.Y., Gao J., Miao F., Xu H.M. Peritoneal milky spots serve as a hypoxic niche and favor gastric cancer stem/progenitor cell peritoneal dissemination through hypoxia-inducible factor 1α. Stem Cells. 2014;32:3062–3074. doi: 10.1002/stem.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cui J., Gong Z., Shen H.M. The role of autophagy in liver cancer: Molecular mechanisms and potential therapeutic targets. Biochim. Et Biophys. Acta. 2013;1836:15–26. doi: 10.1016/j.bbcan.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 96.Aguilar-Gallardo C., Zamorano M., Farias J.G., Quevedo K.A. Understanding autophagy role in cancer stem cell development. Mol. Biol. Rep. 2022;49:6741–6751. doi: 10.1007/s11033-022-07299-z. [DOI] [PubMed] [Google Scholar]
- 97.Togano S., Yashiro M., Masuda G., Sugimoto A., Miki Y., Yamamoto Y., Sera T., Kushiyama S., Nishimura S., Kuroda K., et al. Gastric cancer stem cells survive in stress environments via their autophagy system. Sci. Rep. 2021;11:20664. doi: 10.1038/s41598-021-00155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Courtois S., Haykal M., Bodineau C., Sifré E., Azzi-Martin L., Ménard A., Mégraud F., Lehours P., Durán R.V., Varon C., et al. Autophagy induced by Helicobacter pylori infection is necessary for gastric cancer stem cell emergence. Gastric Cancer. 2021;24:133–144. doi: 10.1007/s10120-020-01118-9. [DOI] [PubMed] [Google Scholar]
- 99.Tsugawa H., Suzuki H., Saya H., Hatakeyama M., Hirayama T., Hirata K., Nagano O., Matsuzaki J., Hibi T. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 100.Li H., Wang C., Lan L., Yan L., Li W., Evans I., Ruiz E.J., Su Q., Zhao G., Wu W., et al. METTL3 promotes oxaliplatin resistance of gastric cancer CD133+ stem cells by promoting PARP1 mRNA stability. Cell. Mol. Life Sci. 2022;79:135. doi: 10.1007/s00018-022-04129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qi Y., Wei J., Zhang X. Requirement of transcription factor NME2 for the maintenance of the stemness of gastric cancer stem-like cells. Cell Death Dis. 2021;12:924. doi: 10.1038/s41419-021-04234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lang T., Xu J., Zhou L., Zhang Z., Ma X., Gu J., Liu J., Li Y., Ding D., Qiu J. Disruption of KDM4C-ALDH1A3 feed-forward loop inhibits stemness, tumorigenesis and chemoresistance of gastric cancer stem cells. Signal Transduct. Target. Ther. 2021;6:336. doi: 10.1038/s41392-021-00674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fu Y., Hu C., Du P., Huang G. E2F1 Maintains Gastric Cancer Stemness Properties by Regulating Stemness-Associated Genes. J. Oncol. 2021;2021:6611327. doi: 10.1155/2021/6611327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen R., Masuo K., Yogo A., Yokoyama S., Sugiyama A., Seno H., Yoshizawa A., Takaishi S. SNAIL regulates gastric carcinogenesis through CCN3 and NEFL. Carcinogenesis. 2021;42:190–201. doi: 10.1093/carcin/bgaa133. [DOI] [PubMed] [Google Scholar]
- 105.Zhang L., Guo X., Zhang L., Yang F., Qin L., Zhang D., Qin Y. SLC34A2 regulates miR-25-Gsk3β signaling pathway to affect tumor progression in gastric cancer stem cell-like cells. Mol. Carcinog. 2018;57:440–450. doi: 10.1002/mc.22768. [DOI] [PubMed] [Google Scholar]
- 106.Han M.E., Baek S.J., Kim S.Y., Kang C.D., Oh S.O. ATOH1 Can Regulate the Tumorigenicity of Gastric Cancer Cells by Inducing the Differentiation of Cancer Stem Cells. PLoS ONE. 2015;10:e0126085. doi: 10.1371/journal.pone.0126085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng C., Li L., Zhang M.D., Gonzales C.B., Parisien M., Belfer I., Usoskin D., Abdo H., Furlan A., Häring M., et al. miR-183 cluster scales mechanical pain sensitivity by regulating basal and neuropathic pain genes. Science. 2017;356:1168–1171. doi: 10.1126/science.aam7671. [DOI] [PubMed] [Google Scholar]
- 108.Seok H., Lee H., Lee S., Ahn S.H., Lee H.S., Kim G.D., Peak J., Park J., Cho Y.K., Jeong Y., et al. Position-specific oxidation of miR-1 encodes cardiac hypertrophy. Nature. 2020;584:279–285. doi: 10.1038/s41586-020-2586-0. [DOI] [PubMed] [Google Scholar]
- 109.Mohr S., Doebele C., Comoglio F., Berg T., Beck J., Bohnenberger H., Alexe G., Corso J., Ströbel P., Wachter A., et al. Hoxa9 and Meis1 Cooperatively Induce Addiction to Syk Signaling by Suppressing miR-146a in Acute Myeloid Leukemia. Cancer Cell. 2017;31:549–562.e511. doi: 10.1016/j.ccell.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li W., Kang Y. A new Lnc in metastasis: Long noncoding RNA mediates the prometastatic functions of TGF-β. Cancer Cell. 2014;25:557–559. doi: 10.1016/j.ccr.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang F., Fang E., Mei H., Chen Y., Li H., Li D., Song H., Wang J., Hong M., Xiao W., et al. Cis-Acting circ-CTNNB1 Promotes β-Catenin Signaling and Cancer Progression via DDX3-Mediated Transactivation of YY1. Cancer Res. 2019;79:557–571. doi: 10.1158/0008-5472.CAN-18-1559. [DOI] [PubMed] [Google Scholar]
- 112.Dimitrova N., Gocheva V., Bhutkar A., Resnick R., Jong R.M., Miller K.M., Bendor J., Jacks T. Stromal Expression of miR-143/145 Promotes Neoangiogenesis in Lung Cancer Development. Cancer Discov. 2016;6:188–201. doi: 10.1158/2159-8290.CD-15-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qiao Y., Jin T., Guan S., Cheng S., Wen S., Zeng H., Zhao M., Yang L., Wan X., Qiu Y., et al. Long non-coding RNA Lnc-408 promotes invasion and metastasis of breast cancer cell by regulating LIMK1. Oncogene. 2021;40:4198–4213. doi: 10.1038/s41388-021-01845-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eulalio A., Huntzinger E., Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 115.Shen C., Wang J., Xu Z., Zhang L., Gu W., Zhou X. ONECUT2 which is targeted by hsa-miR-15a-5p enhances stemness maintenance of gastric cancer stem cells. Exp. Biol. Med. 2021;246:2645–2659. doi: 10.1177/15353702211038496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhan P., Shu X., Chen M., Sun L., Yu L., Liu J., Sun L., Yang Z., Ran Y. miR-98-5p inhibits gastric cancer cell stemness and chemoresistance by targeting branched-chain aminotransferases 1. Life Sci. 2021;276:119405. doi: 10.1016/j.lfs.2021.119405. [DOI] [PubMed] [Google Scholar]
- 117.Ni H., Qin H., Sun C., Liu Y., Ruan G., Guo Q., Xi T., Xing Y., Zheng L. MiR-375 reduces the stemness of gastric cancer cells through triggering ferroptosis. Stem Cell Res. Ther. 2021;12:325. doi: 10.1186/s13287-021-02394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Asakiya C., Zhu L., Yuhan J., Zhu L., Huang K., Xu W. Current progress of miRNA-derivative nucleotide drugs: Modifications, delivery systems, applications. Expert Opin. Drug Deliv. 2022;19:435–450. doi: 10.1080/17425247.2022.2063835. [DOI] [PubMed] [Google Scholar]
- 119.Farahani D.B., Akrami H., Moradi B., Mehdizadeh K., Fattahi M.R. The Effect of hsa-miR-451b Knockdown on Biological Functions of Gastric Cancer Stem-Like Cells. Biochem. Genet. 2021;59:1203–1224. doi: 10.1007/s10528-021-10057-8. [DOI] [PubMed] [Google Scholar]
- 120.Dai Z.T., Xiang Y., Duan Y.Y., Wang J., Li J.P., Zhang H.M., Cheng C., Wang Q., Zhang T.C., Liao X.H. MiR-17-5p and MKL-1 modulate stem cell characteristics of gastric cancer cells. Int. J. Biol. Sci. 2021;17:2278–2293. doi: 10.7150/ijbs.57338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao M., Hou Y., Du Y.E., Yang L., Qin Y., Peng M., Liu S., Wan X., Qiao Y., Zeng H., et al. Drosha-independent miR-6778-5p strengthens gastric cancer stem cell stemness via regulation of cytosolic one-carbon folate metabolism. Cancer Lett. 2020;478:8–21. doi: 10.1016/j.canlet.2020.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xin L., Liu L., Liu C., Zhou L.Q., Zhou Q., Yuan Y.W., Li S.H., Zhang H.T. DNA-methylation-mediated silencing of miR-7-5p promotes gastric cancer stem cell invasion via increasing Smo and Hes1. J. Cell. Physiol. 2020;235:2643–2654. doi: 10.1002/jcp.29168. [DOI] [PubMed] [Google Scholar]
- 123.Ma Y., Yan F., Wei W., Deng J., Li L., Liu L., Sun J. MicroRNA-598 inhibits the growth and maintenance of gastric cancer stem-like cells by down-regulating RRS1. Cell Cycle. 2019;18:2757–2769. doi: 10.1080/15384101.2019.1657338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 124.Lee S.D., Yu D., Lee D.Y., Shin H.S., Jo J.H., Lee Y.C. Upregulated microRNA-193a-3p is responsible for cisplatin resistance in CD44(+) gastric cancer cells. Cancer Sci. 2019;110:662–673. doi: 10.1111/cas.13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shao Q., Xu J., Guan X., Zhou B., Wei W., Deng R., Li D., Xu X., Zhu H. In vitro and in vivo effects of miRNA-19b/20a/92a on gastric cancer stem cells and the related mechanism. Int. J. Med. Sci. 2018;15:86–94. doi: 10.7150/ijms.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang L., Guo X., Zhang D., Fan Y., Qin L., Dong S., Zhang L. Upregulated miR-132 in Lgr5(+) gastric cancer stem cell-like cells contributes to cisplatin-resistance via SIRT1/CREB/ABCG2 signaling pathway. Mol. Carcinog. 2017;56:2022–2034. doi: 10.1002/mc.22656. [DOI] [PubMed] [Google Scholar]
- 127.Pan Y., Shu X., Sun L., Yu L., Sun L., Yang Z., Ran Y. miR-196a-5p modulates gastric cancer stem cell characteristics by targeting Smad4. Int. J. Oncol. 2017;50:1965–1976. doi: 10.3892/ijo.2017.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zeng J.F., Ma X.Q., Wang L.P., Wang W. MicroRNA-145 exerts tumor-suppressive and chemo-resistance lowering effects by targeting CD44 in gastric cancer. World J. Gastroenterol. 2017;23:2337–2345. doi: 10.3748/wjg.v23.i13.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fan D., Ren B., Yang X., Liu J., Zhang Z. Upregulation of miR-501-5p activates the wnt/β-catenin signaling pathway and enhances stem cell-like phenotype in gastric cancer. J. Exp. Clin. Cancer Res. 2016;35:177. doi: 10.1186/s13046-016-0432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu K., Ma L., Zhu J. miR-483-5p promotes growth, invasion and self-renewal of gastric cancer stem cells by Wnt/β-catenin signaling. Mol. Med. Rep. 2016;14:3421–3428. doi: 10.3892/mmr.2016.5603. [DOI] [PubMed] [Google Scholar]
- 131.Yu D., Shin H.S., Lee Y.S., Lee Y.C. miR-106b modulates cancer stem cell characteristics through TGF-β/Smad signaling in CD44-positive gastric cancer cells. Lab. Investig. 2014;94:1370–1381. doi: 10.1038/labinvest.2014.125. [DOI] [PubMed] [Google Scholar]
- 132.Ji Q., Hao X., Meng Y., Zhang M., Desano J., Fan D., Xu L. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. doi: 10.1186/1471-2407-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Daneshvar K., Ardehali M.B., Klein I.A., Hsieh F.K., Kratkiewicz A.J., Mahpour A., Cancelliere S.O.L., Zhou C., Cook B.M., Li W., et al. lncRNA DIGIT and BRD3 protein form phase-separated condensates to regulate endoderm differentiation. Nat. Cell Biol. 2020;22:1211–1222. doi: 10.1038/s41556-020-0572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu M., Xu G., Han C., Luan P.F., Xing Y.H., Nan F., Yang L.Z., Huang Y., Yang Z.H., Shan L., et al. lncRNA SLERT controls phase separation of FC/DFCs to facilitate Pol I transcription. Science. 2021;373:547–555. doi: 10.1126/science.abf6582. [DOI] [PubMed] [Google Scholar]
- 135.Allou L., Balzano S., Magg A., Quinodoz M., Royer-Bertrand B., Schöpflin R., Chan W.L., Speck-Martins C.E., Carvalho D.R., Farage L., et al. Non-coding deletions identify Maenli lncRNA as a limb-specific En1 regulator. Nature. 2021;592:93–98. doi: 10.1038/s41586-021-03208-9. [DOI] [PubMed] [Google Scholar]
- 136.Hui Y., Yang Y., Li D., Wang J., Di M., Zhang S., Wang S. LncRNA FEZF1-AS1 Modulates Cancer Stem Cell Properties of Human Gastric Cancer Through miR-363-3p/HMGA2. Cell Transplant. 2020;29:963689720925059. doi: 10.1177/0963689720925059. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 137.Zhang H., Wang M., He Y., Deng T., Liu R., Wang W., Zhu K., Bai M., Ning T., Yang H., et al. Chemotoxicity-induced exosomal lncFERO regulates ferroptosis and stemness in gastric cancer stem cells. Cell Death Dis. 2021;12:1116. doi: 10.1038/s41419-021-04406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang S., Liu F., Deng J., Cai X., Han J., Liu Q. Long Noncoding RNA ROR Regulates Proliferation, Invasion, and Stemness of Gastric Cancer Stem Cell. Cell. Reprogram. 2016;18:319–326. doi: 10.1089/cell.2016.0001. [DOI] [PubMed] [Google Scholar]
- 139.Gurney A., Axelrod F., Bond C.J., Cain J., Chartier C., Donigan L., Fischer M., Chaudhari A., Ji M., Kapoun A.M., et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. USA. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fischer M.M., Cancilla B., Yeung V.P., Cattaruzza F., Chartier C., Murriel C.L., Cain J., Tam R., Cheng C.Y., Evans J.W., et al. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci. Adv. 2017;3:e1700090. doi: 10.1126/sciadv.1700090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Diamond J.R., Becerra C., Richards D., Mita A., Osborne C., O’Shaughnessy J., Zhang C., Henner R., Kapoun A.M., Xu L., et al. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Res. Treat. 2020;184:53–62. doi: 10.1007/s10549-020-05817-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jimeno A., Gordon M., Chugh R., Messersmith W., Mendelson D., Dupont J., Stagg R., Kapoun A.M., Xu L., Uttamsingh S., et al. A First-in-Human Phase I Study of the Anticancer Stem Cell Agent Ipafricept (OMP-54F28), a Decoy Receptor for Wnt Ligands, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017;23:7490–7497. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- 143.Dotan E., Cardin D.B., Lenz H.J., Messersmith W., O’Neil B., Cohen S.J., Denlinger C.S., Shahda S., Astsaturov I., Kapoun A.M., et al. Phase Ib Study of Wnt Inhibitor Ipafricept with Gemcitabine and nab-paclitaxel in Patients with Previously Untreated Stage IV Pancreatic Cancer. Clin. Cancer Res. 2020;26:5348–5357. doi: 10.1158/1078-0432.CCR-20-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schott A.F., Landis M.D., Dontu G., Griffith K.A., Layman R.M., Krop I., Paskett L.A., Wong H., Dobrolecki L.E., Lewis M.T., et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin. Cancer Res. 2013;19:1512–1524. doi: 10.1158/1078-0432.CCR-11-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dlugosz A., Agrawal S., Kirkpatrick P. Vismodegib. Nat. Rev. Drug Discov. 2012;11:437–438. doi: 10.1038/nrd3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim E.J., Sahai V., Abel E.V., Griffith K.A., Greenson J.K., Takebe N., Khan G.N., Blau J.L., Craig R., Balis U.G., et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin. Cancer Res. 2014;20:5937–5945. doi: 10.1158/1078-0432.CCR-14-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cao W., Li Y., Sun H., Yang C., Zhu J., Xie C., Li X., Wu J., Geng S., Wang L., et al. Apatinib Suppresses Gastric Cancer Stem Cells Properties by Inhibiting the Sonic Hedgehog Pathway. Front. Cell Dev. Biol. 2021;9:679806. doi: 10.3389/fcell.2021.679806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nguyen P.H., Giraud J., Staedel C., Chambonnier L., Dubus P., Chevret E., Bœuf H., Gauthereau X., Rousseau B., Fevre M., et al. All-trans retinoic acid targets gastric cancer stem cells and inhibits patient-derived gastric carcinoma tumor growth. Oncogene. 2016;35:5619–5628. doi: 10.1038/onc.2016.87. [DOI] [PubMed] [Google Scholar]
- 149.Jiang J., Zhang Y., Chuai S., Wang Z., Zheng D., Xu F., Zhang Y., Li C., Liang Y., Chen Z. Trastuzumab (herceptin) targets gastric cancer stem cells characterized by CD90 phenotype. Oncogene. 2012;31:671–682. doi: 10.1038/onc.2011.282. [DOI] [PubMed] [Google Scholar]
- 150.Han Y., Sun B., Cai H., Xuan Y. Simultaneously target of normal and stem cells-like gastric cancer cells via cisplatin and anti-CD133 CAR-T combination therapy. Cancer Immunol. Immunother. 2021;70:2795–2803. doi: 10.1007/s00262-021-02891-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shiozaki A., Katsurahara K., Kudou M., Shimizu H., Kosuga T., Ito H., Arita T., Konishi H., Komatsu S., Kubota T., et al. Amlodipine and Verapamil, Voltage-Gated Ca(2+) Channel Inhibitors, Suppressed the Growth of Gastric Cancer Stem Cells. Ann. Surg. Oncol. 2021;28:5400–5411. doi: 10.1245/s10434-021-09645-0. [DOI] [PubMed] [Google Scholar]
- 152.Giraud J., Molina-Castro S., Seeneevassen L., Sifré E., Izotte J., Tiffon C., Staedel C., Boeuf H., Fernandez S., Barthelemy P., et al. Verteporfin targeting YAP1/TAZ-TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells. Int. J. Cancer. 2020;146:2255–2267. doi: 10.1002/ijc.32667. [DOI] [PubMed] [Google Scholar]
- 153.Xiong J., Wang S., Chen T., Shu X., Mo X., Chang G., Chen J.J., Li C., Luo H., Lee J.D. Verteporfin blocks Clusterin which is required for survival of gastric cancer stem cell by modulating HSP90 function. Int. J. Biol. Sci. 2019;15:312–324. doi: 10.7150/ijbs.29135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Akrami H., Moradi B., Farahani D.B., Mehdizadeh K. Ibuprofen reduces cell proliferation through inhibiting Wnt/β catenin signaling pathway in gastric cancer stem cells. Cell Biol. Int. 2018;42:949–958. doi: 10.1002/cbin.10959. [DOI] [PubMed] [Google Scholar]
- 155.Courtois S., Durán R.V., Giraud J., Sifré E., Izotte J., Mégraud F., Lehours P., Varon C., Bessède E. Metformin targets gastric cancer stem cells. Eur. J. Cancer. 2017;84:193–201. doi: 10.1016/j.ejca.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 156.Feng S., Zheng Z., Feng L., Yang L., Chen Z., Lin Y., Gao Y., Chen Y. Proton pump inhibitor pantoprazole inhibits the proliferation, self-renewal and chemoresistance of gastric cancer stem cells via the EMT/β-catenin pathways. Oncol. Rep. 2016;36:3207–3214. doi: 10.3892/or.2016.5154. [DOI] [PubMed] [Google Scholar]
- 157.Tai Y.S., Ma Y.S., Chen C.L., Tsai H.Y., Tsai C.C., Wu M.C., Chen C.Y., Lin M.W. Resveratrol Analog 4-Bromo-Resveratrol Inhibits Gastric Cancer Stemness through the SIRT3-c-Jun N-Terminal Kinase Signaling Pathway. Curr. Issues Mol. Biol. 2021;44:63–72. doi: 10.3390/cimb44010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhu Y.D., Ba H., Chen J., Zhang M., Li P. Celastrus orbiculatus Extract Reduces Stemness of Gastric Cancer Stem Cells by Targeting PDCD4 and EIF3H. Integr. Cancer Ther. 2021;20:15347354211058168. doi: 10.1177/15347354211058168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shen X., Si Y., Wang Z., Wang J., Guo Y., Zhang X. Quercetin inhibits the growth of human gastric cancer stem cells by inducing mitochondrial-dependent apoptosis through the inhibition of PI3K/Akt signaling. Int. J. Mol. Med. 2016;38:619–626. doi: 10.3892/ijmm.2016.2625. [DOI] [PubMed] [Google Scholar]
- 160.Gu J., Zhang Z., Lang T., Ma X., Yang L., Xu J., Tian C., Han K., Qiu J. PTPRU, as a Tumor Suppressor, Inhibits Cancer Stemness by Attenuating Hippo/YAP Signaling Pathway. Onco Targets Ther. 2019;12:8095–8104. doi: 10.2147/OTT.S218125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ge M., Zhang L., Cao L., Xie C., Li X., Li Y., Meng Y., Chen Y., Wang X., Chen J., et al. Sulforaphane inhibits gastric cancer stem cells via suppressing sonic hedgehog pathway. Int. J. Food Sci. Nutr. 2019;70:570–578. doi: 10.1080/09637486.2018.1545012. [DOI] [PubMed] [Google Scholar]
- 162.Cao X., Ren K., Song Z., Li D., Quan M., Zheng Y., Cao J., Zeng W., Zou H. 7-Difluoromethoxyl-5,4′-di-n-octyl genistein inhibits the stem-like characteristics of gastric cancer stem-like cells and reverses the phenotype of epithelial-mesenchymal transition in gastric cancer cells. Oncol. Rep. 2016;36:1157–1165. doi: 10.3892/or.2016.4848. [DOI] [PubMed] [Google Scholar]
- 163.Wen Z., Feng S., Wei L., Wang Z., Hong D., Wang Q. Evodiamine, a novel inhibitor of the Wnt pathway, inhibits the self-renewal of gastric cancer stem cells. Int. J. Mol. Med. 2015;36:1657–1663. doi: 10.3892/ijmm.2015.2383. [DOI] [PubMed] [Google Scholar]
- 164.Li L.C., Wang D.L., Wu Y.Z., Nian W.Q., Wu Z.J., Li Y., Ma H.W., Shao J.H. Gastric tumor-initiating CD44(+) cells and epithelial-mesenchymal transition are inhibited by γ-secretase inhibitor DAPT. Oncol. Lett. 2015;10:3293–3299. doi: 10.3892/ol.2015.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ma L., Mao R., Shen K., Zheng Y., Li Y., Liu J., Ni L. Atractylenolide I-mediated Notch pathway inhibition attenuates gastric cancer stem cell traits. Biochem. Biophys. Res. Commun. 2014;450:353–359. doi: 10.1016/j.bbrc.2014.05.110. [DOI] [PubMed] [Google Scholar]
- 166.Huang W., Wan C., Luo Q., Huang Z., Luo Q. Genistein-inhibited cancer stem cell-like properties and reduced chemoresistance of gastric cancer. Int. J. Mol. Sci. 2014;15:3432–3443. doi: 10.3390/ijms15033432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li Y.J., Liao L.L., Liu P., Tang P., Wang H., Peng Q.H. Sijunzi Decoction Inhibits Stemness by Suppressing β-Catenin Transcriptional Activity in Gastric Cancer Cells. Chin. J. Integr. Med. 2021;28:702–710. doi: 10.1007/s11655-021-3314-9. [DOI] [PubMed] [Google Scholar]
- 168.Yan B., Liu L., Zhao Y., Xiu L.J., Sun D.Z., Liu X., Lu Y., Shi J., Zhang Y.C., Li Y.J., et al. Xiaotan Sanjie decoction attenuates tumor angiogenesis by manipulating Notch-1-regulated proliferation of gastric cancer stem-like cells. World J. Gastroenterol. 2014;20:13105–13118. doi: 10.3748/wjg.v20.i36.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Franco Y.L., Vaidya T.R., Ait-Oudhia S. Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer. 2018;10:131–141. doi: 10.2147/BCTT.S170239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rakowski J.A., Ahmad S., Holloway R.W. Use of pegylated liposomal doxorubicin in the management of platinum-sensitive recurrent ovarian cancer: Current concepts. Expert Rev. Anticancer. Ther. 2012;12:31–40. doi: 10.1586/era.11.187. [DOI] [PubMed] [Google Scholar]
- 171.He H., Liu L., Morin E.E., Liu M., Schwendeman A. Survey of Clinical Translation of Cancer Nanomedicines-Lessons Learned from Successes and Failures. Acc. Chem. Res. 2019;52:2445–2461. doi: 10.1021/acs.accounts.9b00228. [DOI] [PubMed] [Google Scholar]
- 172.Chan C., Du S., Dong Y., Cheng X. Computational and Experimental Approaches to Investigate Lipid Nanoparticles as Drug and Gene Delivery Systems. Curr. Top. Med. Chem. 2021;21:92–114. doi: 10.2174/1568026620666201126162945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Silverman J.A., Deitcher S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013;71:555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Said R., Tsimberidou A.M. Pharmacokinetic evaluation of vincristine for the treatment of lymphoid malignancies. Expert Opin. Drug Metab. Toxicol. 2014;10:483–494. doi: 10.1517/17425255.2014.885016. [DOI] [PubMed] [Google Scholar]
- 175.Yao H., Sun L., Li J., Zhou X., Li R., Shao R., Zhang Y., Li L. A Novel Therapeutic siRNA Nanoparticle Designed for Dual-Targeting CD44 and Gli1 of Gastric Cancer Stem Cells. Int. J. Nanomed. 2020;15:7013–7034. doi: 10.2147/IJN.S260163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen H., Lin J., Shan Y., Zhengmao L. The promotion of nanoparticle delivery to two populations of gastric cancer stem cells by CD133 and CD44 antibodies. Biomed. Pharmacother. 2019;115:108857. doi: 10.1016/j.biopha.2019.108857. [DOI] [PubMed] [Google Scholar]
- 177.Yang F., Zheng Z., Xue X., Zheng L., Qin J., Li H., Zhou Y., Fang G. Targeted eradication of gastric cancer stem cells by CD44 targeting USP22 small interfering RNA-loaded nanoliposomes. Future Oncol. 2019;15:281–295. doi: 10.2217/fon-2018-0295. [DOI] [PubMed] [Google Scholar]
- 178.Yang W., Zhang H., Xin L. A novel design of HA-coated nanoparticles co-encapsulating plasmid METase and 5-Fu shows enhanced application in targeting gastric cancer stem cells. Biol. Chem. 2018;399:293–303. doi: 10.1515/hsz-2017-0208. [DOI] [PubMed] [Google Scholar]
- 179.Jang E., Kim E., Son H.Y., Lim E.K., Lee H., Choi Y., Park K., Han S., Suh J.S., Huh Y.M., et al. Nanovesicle-mediated systemic delivery of microRNA-34a for CD44 overexpressing gastric cancer stem cell therapy. Biomaterials. 2016;105:12–24. doi: 10.1016/j.biomaterials.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 180.Liang S., Li C., Zhang C., Chen Y., Xu L., Bao C., Wang X., Liu G., Zhang F., Cui D. CD44v6 Monoclonal Antibody-Conjugated Gold Nanostars for Targeted Photoacoustic Imaging and Plasmonic Photothermal Therapy of Gastric Cancer Stem-like Cells. Theranostics. 2015;5:970–984. doi: 10.7150/thno.11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yao H.J., Zhang Y.G., Sun L., Liu Y. The effect of hyaluronic acid functionalized carbon nanotubes loaded with salinomycin on gastric cancer stem cells. Biomaterials. 2014;35:9208–9223. doi: 10.1016/j.biomaterials.2014.07.033. [DOI] [PubMed] [Google Scholar]