Abstract
Simple Summary
Unlike the neutrophil/lymphocyte ratio (NLR), more complex complete blood count (CBC)-based systemic immune-inflammation cancer biomarkers have recently been proposed, such as the pan-immune-inflammation value (PIV). We aimed to assess both NLR and PIV in cutaneous melanoma (CM) patients. Briefly, we found that, although the higher PIV and NLR values appear to be associated with survival in the crude analysis, adjustment for potential confounders, in particular age and tumor thickness, reduced the strength of association between PIV and NLR on survival substantially. PIV as well as NLR were positively correlated with age and tumor thickness which are important independent predictors for CM relapse and CM-specific death. Both CBC-based parameters appear to be confounded by age and tumor thickness and probably have no potential to further improve the prediction of survival of stage I to III CM patients beyond standard prognostic factors.
Abstract
Prognostic biomarkers derived from complete blood count (CBC) have received marked interest as an indirect measure of the inflammatory pressure in cancers such as metastatic melanoma. Here, we evaluated the novel pan-immune-inflammation value (PIV) and the frequently assessed neutrophil/lymphocyte ratio (NLR) in a large cohort of patients with cutaneous melanoma (CM) without distant metastases (stages I to III). PIV and NLR were calculated at CM diagnosis. Healthy controls were also included. We used the Kaplan–Meier method to estimate crude survival probabilities and used Cox proportional hazards regression for multiple adjustment of hazard ratios. We observed that higher PIV (HR: 1.72, 95% CI 1.14 to 2.58 and HR: 1.696, 95% CI 1.029 to 2.795, respectively) and NLR (HR: 1.70, 95% CI 1.10 to 2.62) values were associated with CM relapse and CM-specific death in the crude analysis. However, when adjusting for potential confounders, in particular age and tumor thickness, the total effect of PIV and NLR on CM-relapse-free (HR: 1.28, 95% CI 0.83 to 1.98 and HR: 1.26, 95% CI 0.80 to 1.98, respectively) and CM-specific survival (HR: 1.36, 95% CI 0.80 to 2.30 and HR: 1.37, 95% CI 0.80 to 2.33, respectively) was substantially reduced. However, both PIV and NLR were positively correlated with age and tumor thickness, which are important independent predictors for CM relapse and CM-specific death. In conclusion, in stage I to III CM patients PIV as well as NLR appear to be confounded by age and tumor thickness and probably have no potential to further improve the prediction of survival of stage I to III CM patients beyond standard prognostic factors.
Keywords: cutaneous melanoma, pan-immune-inflammation value, neutrophil/lymphocyte ratio, biomarkers
1. Introduction
The incidence of melanoma is increasing worldwide, with more than 55,000 deaths yearly [1,2,3]. The impairment of acute inflammatory responses against carcinogens triggered by the innate immune system creates a predisposition for tumor development and progression. Involvement of smoldering chronic inflammation has been demonstrated in every step of carcinogenesis, including cancer development, progression, and metastasis. Considering the instrumental role of inflammation in cancer, measuring the levels of different types of inflammation could serve as prognostic biomarkers, although data on appropriate parameters and means of quantification remain relatively scarce [4,5,6].
To date, many immune-inflammation-based biomarkers, e.g., count of neutrophils or lymphocytes, neutrophil/lymphocyte ratio (NLR), and platelet/lymphocyte ratio have been proposed as prognostic predictors in a variety of cancers, including melanoma [6,7,8,9,10,11]. Moreover, a novel and more complex blood-based biomarker represents the pan-immune-inflammation value (PIV) [12], which has also been investigated in skin cancer and other malignancies with varying prognostic capacity [12,13,14,15,16].
This study aimed to assess the PIV and NLR in control subjects and a reasonable sample of patients with cutaneous melanoma (CM) stages I to III.
2. Methods
2.1. Patients
This mono-center investigation was performed at the Department of Dermatology, Ruhr-University Bochum, Germany. All CM patients who had undergone sentinel lymph node (SLN) biopsy between 2001 and 2015 including complete blood count (CBC) data at initial diagnosis were considered. Next, we reviewed the patient files for sufficient data with respect to gender, age, CBC data, Breslow tumor thickness, CM ulceration, and follow-up information. All patients with complete data were included in the following analysis.
The diagnosis of CM was performed by full primary excision and histopathological examination. Vertical tumor thickness of 1 mm or more was an indication for SLN biopsy (SLNB). SLNB was also considered for tumors with: vertical tumor thickness 0.75–1 mm, ulceration, high mitotic index, and age < 40 years. As in our previous study [14], the presence of macro-metastases lymph nodes and distant metastases was assessed by physical examination and investigations, including sonography, computed tomography, and magnetic resonance imaging [17]. Based on the established clinical practices at that time, patients with micro-metastases in the SLN usually underwent complete lymph node dissection. Adjuvant low-dose interferon alfa-2a therapy (Roferon; Roche Pharma AG, Grenzach-Wyhlen, Germany) was carried out in patients with tumor thickness ≥ 1.5 mm and without evidence of SLN micro-metastasis. In patients with positive SLN, adjuvant high-dose interferon alfa-2b (Intron; MSD, Munich, Germany) was usually performed. Follow-up was performed in line with national and international guidelines: specifically, patients with primary CM smaller than 1 mm tumor thickness were seen for follow-up every 6 months, whereas patients with thicker primary melanomas received a follow-up every 3 months, which included lymph node ultrasound and determination of serum S100B and lactate dehydrogenase. Every 6 months, stage III patients additionally underwent whole-body imaging [2]. Healthy controls were also included for the comparison of PIV and NLR.
2.2. Laboratory Parameters
We calculated the PIV as follows: [neutrophils (103/mm3) × platelets (103/mm3) × monocytes (103/mm3)]/lymphocytes (103/mm3) [12]. NLR was calculated as follows: absolute neutrophils (103/mm3)/lymphocytes (103/mm3). We determined PIV and NLR at the time of initial diagnosis.
2.3. Statistics
Using the continuous distribution of PIV and NLR, we identified three groups of equal size using tertiles. The ranks of thirds for PIV were ≤219.73, >219.73 to 393.73, and >393.73. The ranks for NLR were ≤2.02, >2.02 to 3.00, and >3.00. For the endpoints relapse-free survival and CM-specific survival, we used the Kaplan–Meier method to estimate survival probabilities. For the adjustment of potential confounders including age at diagnosis, AJCC8 stage, pT stage, tumor thickness (mm), histological type, and ulceration, we used Cox proportional hazards regression analyses. We used Fisher’s z transformation to analyze 95% confidence intervals for Spearman rank correlation coefficients. We used weighted nonparametric local regression smoothing (LOESS; smoothing factor 0.4) to graphically illustrate the association between continuous variables [18,19,20]. Statistics were carried out using the software package SAS version 9.4 (Cary, NC, USA).
3. Results
The entire study population consisted of 457 patients with CM, including 204 at stage I, 113 at stage II, and 140 at stage III. As shown in Table 1 and Supplementary Table S1, there were no substantial differences with respect to age and sex regarding the different pT and CM stages and healthy controls, even though PIV and NLR of CM patients were higher than in controls. As expected, tumor data (e.g., Breslow thickness) and clinical outcome (e.g., CM relapse, CM-specific survival) differed between stages (Supplementary Table S1). Interestingly, PIV and NLR values appeared to be higher in pT3 and pT4 stages when compared to pT1 and pT2 (Table 1).
Table 1.
Clinical characteristics of patients with cutaneous melanoma (CM, n = 457) in pT stages 1–4.
| Patients with CM | |||||
|---|---|---|---|---|---|
| Characteristics | pT1 (n = 94) | pT2 (n =196) | pT3 (n = 115) | pT4 (n = 52) | Controls (n = 49) |
| Males | 48 (51.1) | 93 (47.5) | 48 (41.7) | 28 (53.9) | 24 (49.0) |
| Median Age (years; P10, P90) | 54 (32; 73) | 58 (34; 74) | 59 (35; 77) | 64 (40; 78) | 61 (33;80) |
| CM subtype | |||||
| Superficial spreading melanoma | 64 (68.1) | 104 (53.1) | 25 (21.7) | 7 (13.5) | |
| Nodular melanoma | 6 (6.4) | 43 (21.9) | 45 (39.1) | 30 (57.7) | |
| Lentigo maligna melanoma | 1 (1.1) | 2 (1.0) | 0 | 0 | |
| Acrolentiginous melanoma | 3 (3.2) | 8 (4.1) | 8 (7.0) | 4 (7.7) | |
| Others | 20 (21.3) | 39 (19.9) | 37 (32.2) | 11 (21.2) | |
| Median tumor thickness, mm (P10, P90) | 0.8 (0.5; 1.0) | 1.4 (1.1; 1.8) | 2.8 (2.1; 3.8) | 5.5 (4.2; 9.8) | |
| Ulceration of primary CM, n (%) | 14 (14.9) | 37 (18.9) | 49 (42.6) | 39 (75.0) | |
| NLR, median (P10, P90) | 2.35 (1.42; 3.86) | 2.30 (1.30; 4.20) | 2.53 (1.30; 4.57) | 2.89 (1.56) | 1.90 (1.1; 11.60) |
| PIV, median (P10, P90) | 268 (138; 586) | 269 (96; 693) | 358 (121; 768) | 421 (136) | 276 (133; 790) |
| CM relapse, n (%) | 9 (9.6) | 39 (19.9) | 47 (40.9) | 33 (63.5) | |
| Median relapse-free survival (months) (P10, P90) | 102 (18; 193) | ||||
| CM-specific death, n (%) | 3 (3.2) | 28 (14.3) | 29 (25.2) | 26 (50.0) | |
| Median CM-specific survival (months) (P10, P90) | 110 (40; 195) | ||||
pT: primary tumor classification; P10 10th percentile, P90: 90th percentile; SSM: superficial spreading; NLR: neutrophil/lymphocyte ratio; PIV: pan-immune inflammation value.
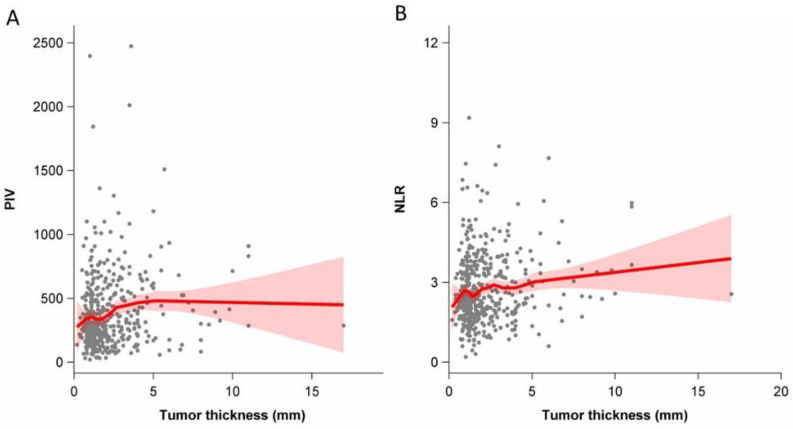
As shown in Figure 1A, there was a positive correlation between the PIV and tumor thickness of patients with CM (r = 0.14; 95% CI 0.05–0.23). Moreover, the NLR positively correlated with tumor thickness (r = 0.11; 95% CI 0.02–0.20; Figure 1B).
Figure 1.
Association between tumor thickness (mm) among patients with cutaneous melanoma and the pan-immune inflammation value (PIV, (A)) and the neutrophil-to-lymphocyte ratio (NLR, (B)) (Spearman rank correlation coefficient 0.14 (95%CI 0.05–0.23) and 0.11 (95%CI 0.02–0.20), respectively); red bands indicate 95% confidence interval bands.
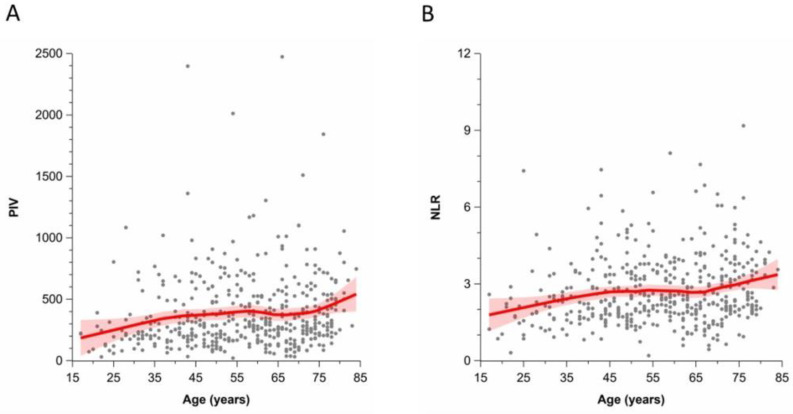
As shown in Figure 2A, there was also a positive correlation between the PIV and the age of patients with CM (r = 0.17; 95% CI 0.08–0.26). Moreover, the NLR positively correlated with age as well (r = 0.18; 95% CI 0.09–0.27; Figure 2B).
Figure 2.
Association between age at diagnosis of patients with cutaneous melanoma and the pan-immune inflammation value (PIV, (A)) and the neutrophil-to-lymphocyte ratio (NLR, (B)) (Spearman rank correlation coefficient 0.17 (95%CI 0.08–0.26) and 0.18 (95%CI 0.09–0.27), respectively); red bands indicate 95% confidence interval bands.
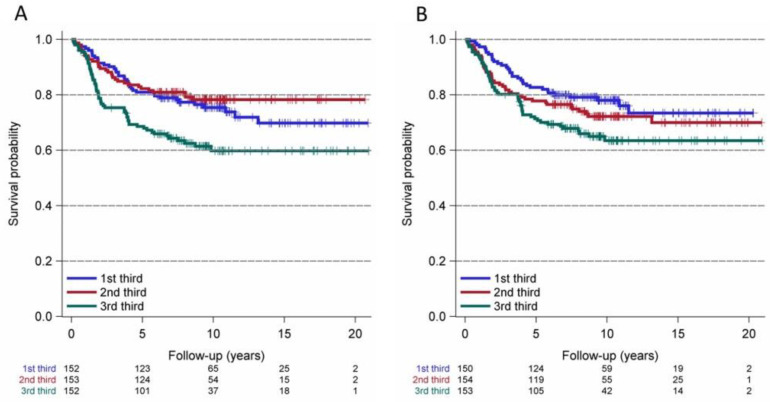
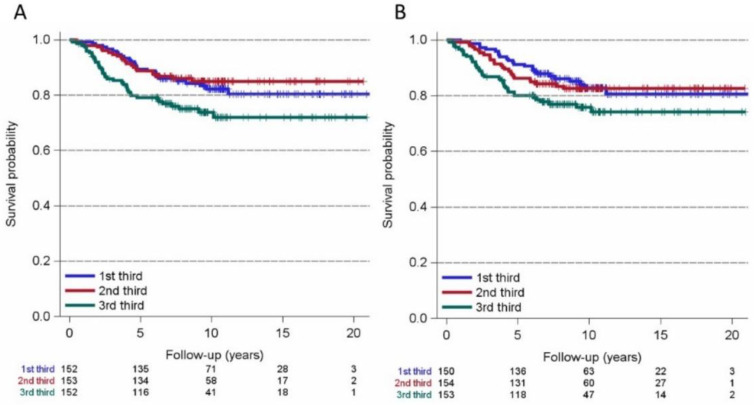
For the endpoint relapse-free survival, we used the Kaplan–Meier method for PIV thirds (first PIV third ≤ 219.73; second PIV third > 219.73 to 393.73; third PIV third > 393.73) and NRL thirds (first NLR third ≤ 2.02; second NLR third > 2.02 to 3.00; third NLR third > 3.00, Figure 3). We observed that higher (third thirds) values of PIV and NLR appear to be linked to poorer relapse-free survival (hazard ratio (HR): 1.72, 95% CI 1.14 to 2.58, and HR: 1.70, 95% CI 1.10 to 2.62, respectively; Figure 3). The Kaplan–Meier curves for the endpoint CM-specific survival revealed that the third thirds of PIV also seem to be associated with poorer CM-specific survival (HR: 1.696, 95% CI 1.029 to 2.795), whereas NRL was less associated with decreased survival probability (HR: 1.6, 95% CI 0.99 to 280, Figure 4).
Figure 3.
Kaplan–Meier curves for relapse-free survival of cutaneous melanoma patients and thirds of the pan-immune inflammation value (PIV, (A): 1st third-PIV ≤ 219.73; 2nd third-PIV > 219.73 to 393.73; 3rd third-PIV > 393.73) and the neutrophil-to-lymphocyte ratio (NLR, B): 1st third-NLR ≤ 2.02; 2nd third-NLR > 2.02 to 3; 3rd third-NLR > 3). 3rd thirds of PIV (A) and NLR (B) appear to be associated with poorer relapse-free survival (hazard ratio: 1.718, 95% CI 1.143 to 2.584 and hazard ratio: 1.702, 95% CI 1.104 to 2.623, respectively).
Figure 4.
Kaplan–Meier curves for cutaneous melanoma-specific survival and thirds of the pan-immune inflammation value (PIV, (A): 1st third-PIV ≤ 219.73; 2nd third-PIV > 219.73 to 393.73; 3rd third-PIV > 393.73) and the neutrophil-to-lymphocyte ratio (NLR, (B): 1st third-NLR to 2.02; 2nd third-NLR > 2.02 ≤ 3; 3rd third-NLR > 3). The 3rd thirds of PIV (A) appear to be associated with poorer relapse-free survival (hazard ratio: 1.696, 95% CI 1.029 to 2.795).
After adjustment for multiple confounders, including CM stage, CM subtypes, age, tumor thickness, and ulceration, the hazard ratios for the top PIV third (1.28, 95% CI 0.83 to 1.98) and NLR third (1.26, 95% CI 0.80 to 1.98) were substantially reduced with respect to CM-relapse-free survival (Table 2).
Table 2.
Cox proportional hazards regression model for cutaneous melanoma (CM) relapse-free survival and the total effects for the pan-immune-inflammation value (PIV) and neutrophil-to-lymphocyte ratio (NLR) adjusted for potential confounders.
| Model and Variables | p-Value | Hazard Ratio (HR) | 95% Confidence Interval |
|---|---|---|---|
| PIV model | |||
| PIV 2nd third (219.73 ≤ 393.73) | 0.3530 | 0.79 | 0.49 to 1.29 |
| PIV 3rd third (>393.73) | 0.2689 | 1.28 | 0.83 to 1.98 |
| Stage II | 0.0950 | 1.69 | 0.91 to 3.15 |
| Stage III | <0.0001 | 4.00 | 2.36 to 6.79 |
| CM subtypes-ALM | 0.0118 | 2.25 | 1.19 to 4.24 |
| -LMM | 0.8756 | 1.17 | 0.16 to 8.81 |
| -NM | 0.5384 | 1.16 | 0.73 to 1.85 |
| -Others | 0.8349 | 0.94 | 0.55 to 1.62 |
| Age | 0.0015 | 1.02 | 1.00 to 1.03 |
| Tumor thickness (mm) | <0.0001 | 1.19 | 1.12 to 1.28 |
| Ulceration | 0.5755 | 1.13 | 0.74 to 1.72 |
| NLR model | |||
| NLR 2nd third (2.02 ≤ 3) | 0.7308 | 1.08 | 0.68 to 1.73 |
| NLR 3rd third (>3) | 0.3073 | 1.26 | 0.80 to 1.98 |
| Stage II | 0.0742 | 1.76 | 0.95 to 3.29 |
| Stage III | <0.0001 | 4.13 | 2.44 to 6.99 |
| CM subtype-ALM | 0.0093 | 2.30 | 1.23 to 4.34 |
| -LMM | 0.8679 | 1.19 | 0.16 to 8.91 |
| -NM | 0.4379 | 1.20 | 0.75 to 1.92 |
| -Others | 0.8879 | 0.69 | 0.56 to 1.65 |
| Age | 0.0010 | 1.02 | 1.01 to 1.04 |
| Tumor thickness (mm) | <0.0001 | 1.18 | 1.11 to 1.26 |
| Ulceration | 0.5014 | 1.15 | 0.76 to 1.76 |
Potential confounders in gray.
The Cox proportional hazards regression model for CM-specific survival also showed that the hazard ratios for the top PIV third (1.36, 95% CI 0.80 to 2.30) and top NLR third (1.37, 95% CI 0.80 to 2.33) were substantially reduced (Table 3).
Table 3.
Cox proportional hazards regression model for cutaneous melanoma (CM)-specific survival and the total effects for the pan-immune-inflammation value (PIV) and neutrophil-to-lymphocyte ratio (NLR) adjusted for potential confounders.
| Model and Variables | p-Value | Hazard Ratio (HR) | 95% Confidence Interval |
|---|---|---|---|
| PIV model | |||
| PIV 2nd third (219.73 ≤ 393.73) | 0.6476 | 0.87 | 0.48 to 1.57 |
| PIV 3rd third (>393.73) | 0.2553 | 1.36 | 0.80 to 2.30 |
| Stage II | 0.2159 | 1.66 | 0.75 to 3.68 |
| Stage III | 0.0002 | 3.72 | 1.85 to 7.46 |
| CM subtype-ALM | 0.7185 | 1.17 | 0.49 to 2.80 |
| -LMM | 0.5596 | 1.83 | 0.24 to 14.11 |
| -NM | 0.8061 | 1.07 | 0.61 to 1.88 |
| -Others | 0.2259 | 0.64 | 0.31 to 1.31 |
| Age | 0.0099 | 1.02 | 1.00 to 1.04 |
| pT (1–4) | <0.0001 | 1.17 | 1.08 to 1.26 |
| Ulceration | 0.0180 | 1.84 | 1.11 to 3.04 |
| NLR model | |||
| NLR 2nd third (2.02 ≤ 3) | 0.7537 | 0.91 | 0.51 to 1.62 |
| NLR 3rd third (>3) | 0.2543 | 1.37 | 0.80 to 2.33 |
| Stage II | 0.2239 | 1.64 | 0.74 to 3.66 |
| Stage III | 0.0002 | 3.79 | 1.90 to 7.57 |
| CM subtypes-ALM | 0.6600 | 1.21 | 0.51 to 2.88 |
| -LMM | 0.4884 | 2.06 | 0.27 to 15.87 |
| -NM | 0.6541 | 1.14 | 0.65 to 1.99 |
| -Others | 0.2472 | 0.66 | 0.32 to 1.34 |
| Age | 0.0089 | 1.02 | 1.00 to 1.04 |
| pT (1–4) | <0.0001 | 1.16 | 1.08 to 1.25 |
| Ulceration | 0.0145 | 1.87 | 1.13 to 3.09 |
Potential confounders in gray.
4. Discussion
There is a wealth of evidence that systemic inflammatory processes play an important role in cancer development and progression [21]. Protumorigenic cytokines (e.g., vascular endothelial growth factor, tumor necrosis factor-α, interleukin-10) secreted by neutrophil granulocytes and thrombocytes may contribute to progression of malignancies [22]. Recently, systemic immune-inflammation prognosis scores were proposed to be of prognostic potential in a variety of malignancy types including CM [6,8,9,10,11,12,13,14,15,21,22,23,24,25,26,27,28,29,30]. For the first time, Fucà et al. [26] reported in 2018 on the prognostic capacity of the PIV in advanced colorectal and breast cancer. In a retrospective study on stage IV CM patients, who were managed with first-line immune checkpoint inhibitors (ICI, n = 119) or targeted therapy (n = 109), Fucà et al. [12] observed that an increase of the baseline PIV (>600) was independently linked to poor progression-free and overall survival. Moreover, they also [12] found out that an increased PIV was associated with primary resistance against ICI as well as targeted therapy. Nevertheless, that study did not investigate PIV in CM stages I to III or healthy controls. In agreement with the findings of Fuca et al. [12] who found that a higher PIV is associated with a higher M stage and increased LDH, we recently discovered that the PIV of CM patients at stage III and IV (n = 62) is higher than that of patients at stage I and II or HC [14]. Hence, the PIV could be a potential biomarker for disease burden.
Indeed, in the present study, we investigated a larger sample size of stage I to III CM patients. Surprisingly, we observed the highest median PIV and NLR in stage II patients when compared to stage I and stage III patients and HC as well (Table S1). However, when stratifying in pT 1–4 stages, there was a stepwise increase in PIV and NRL. This apparent contradiction can be explained by the fact that stage III included many patients with smaller tumor thickness (<2 mm), whereas all stage II patients had >2 mm tumor thickness. Our data clearly show that there exists a positive (albeit weak) correlation between CM thickness and PIV and NRL. Hence, PIV and NLR are mainly driven by tumor thickness and not by AJCC stages. The biology of this association is hard to explain. Indeed, it has been shown that a larger tumor load in stage IV patients is associated with higher PIV and NLR values [14,31]. However, whether the primary tumor mass is directly linked to an increase of systemic immune-inflammation markers is questionable.
Another interesting observation in this context is the fact that PIV and NLR also increase with age, whereby patients in pT4 stage were 10 years older than patients in pT1 stage. Accordingly, Fest et al. [32] assessed the prognostic potential of systemic immune-inflammatory biomarkers and found that these markers increase with age. Moreover, Li et al. [29] found that patients in the high SII (neutrophil count × platelet count/lymphocyte count) group showed poor tumor differentiation and poor prognosis compared to patients with a low SII score [29].
Multiple investigations have shown that an increased NLR is associated with poor clinical outcomes in many malignancies, but this parameter has not been thoroughly investigated in CM, except for stage IV, particularly in the context of the use of ICI [31,33,34]. Lino-Silva et al. [35] was the first study group investigating NLR in CM stages I–III. They concluded that NLR ≥ 2 seems to be a potential biomarker for diminished survival of CM patients, in particular in clinical stage II. Davis et al. [36] reported similar data for high-risk CM patients. Ma et al. [37] found similar results in CM patients at stage III. By contrast, Wade et al. [8] found that a high NLR was associated with better CM-specific survival (HR: 0.53, 95% CI 0.45 to 0.63) and disease-free survival. Exclusively considering the multivariable test data of studies on stage I to III CM patients, one study [8] demonstrated that high NLR is associated with better CM-specific survival, three studies with worse [9,28,37], and three (including the present investigation) observed no significant association between NLR and CM-specific survival [28,38]. Hence, the directionality of effect is inconsistent with respect to NLR.
Our study group systematically investigated the PIV in stage I to III CM patients for the first time. PIV appears to be higher in stage I to III CM patients when compared to healthy controls. As with our NLR data, we found associations between PIV and age, tumor thickness, CM relapse, and CM-specific death in the crude data analysis. However, when adjusting for potential confounders, in particular age and tumor thickness, the total effect of PIV on survival was substantially reduced. In fact, differences between studies are probably due to the differing inclusion criteria of previous investigations and the resulting heterogeneity of populations studied. Moreover, the utility of CBC-based systemic immune-inflammation biomarkers is also limited by other confounding factors, including inflammatory conditions, infections, or the use of immunosuppressants.
5. Conclusions
In a recent systematic review performed by Guven et al. [16], current evidence indicates that PIV represents an inexpensive minimally invasive prognostic tool for patients with metastatic disease. However, the present data suggest that PIV as well as NLR appear to be markedly confounded by age and tumor thickness in stage I to II CM patients. Both CBC-based parameters probably have no relevant potential in improving the prediction of survival in stage I to III CM patients.
Acknowledgments
This work is part of the doctoral thesis of Rita Mansour.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14184410/s1, Table S1: Clinical characteristics of patients with cutaneous melanoma (CM, n = 457) in AJCC (8th edition) stages I–III and healthy controls (n = 49).
Author Contributions
T.G. contributed to the study conception and design. Material preparation, data collection, analysis, and interpretation were predominantly performed by R.M., C.H.S., C.N., N.A.R., J.C.B., L.S. and T.G. Statistical analysis was performed by A.S. The first draft of the manuscript was written by T.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This non-interventional study was approved by Institutional Review Board at the Ruhr-University Bochum (IRB Study ID #16-5985). All procedures performed in studies involving human participants or their data were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author on reasonable request.
Conflicts of Interest
T.G. has received speakers and/or advisory board honoraria from BMS, Sanofi-Genzyme, MSD, Novartis Pharma, Roche, Abbvie, Almirall, Janssen, Lilly, Pfizer, Pierre Fabre, Merck-Serono, outside of the submitted work. J.C.B. is receiving speaker’s bureau honoraria from Amgen, Pfizer, Merck-Serono and Sanofi and is a paid consultant/advisory board member for eTheRNA, Merck-Serono, Pfizer, 4SC and Sanofi. His group receives research grants from Bristol-Myers Squibb, Merck Serono, and Alcedis. L.S. has received speaker and/or advisory board honoraria from BMS, Sun-Pharma, MSD, and Novartis. The other authors report no conflict or competing interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whiteman D.C., Green A.C., Olsen C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016;136:1161–1171. doi: 10.1016/j.jid.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 2.Schadendorf D., van Akkooi A.C., Berking C., Griewank K.G., Gutzmer R., Hauschild A., Stang A., Roesch A., Ugurel S. Melanoma. Lancet. 2018;392:971–984. doi: 10.1016/S0140-6736(18)31559-9. [DOI] [PubMed] [Google Scholar]
- 3.Seité S., Del Marmol V., Moyal D., Friedman A.J. Public primary and secondary skin cancer prevention, perceptions and knowledge: An international cross-sectional survey. J. Eur. Acad. Dermatol. Venereol. 2017;31:815–820. doi: 10.1111/jdv.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow M.T., Möller A., Smyth M.J. Inflammation and immune surveillance in cancer. Semin. Cancer Biol. 2011;22:23–32. doi: 10.1016/j.semcancer.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez H., Hagerling C., Werb Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernando-Calvo A., García-Alvarez A., Villacampa G., Ortiz C., Bodet D., García-Patos V., Recio J.A., Dienstmann R., Muñoz-Couselo E. Dynamics of clinical biomarkers as predictors of immunotherapy benefit in metastatic melanoma patients. Clin. Transl. Oncol. 2020;23:311–317. doi: 10.1007/s12094-020-02420-9. [DOI] [PubMed] [Google Scholar]
- 7.Zaragoza J., Caille A., Beneton N., Bens G., Christiann F., Maillard H., Machet L. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br. J. Dermatol. 2015;174:146–151. doi: 10.1111/bjd.14155. [DOI] [PubMed] [Google Scholar]
- 8.Wade R.G., Robinson A.V., Lo M.C.I., Keeble C., Marples M., Dewar D.J., Moncrieff M.D.S., Peach H. Baseline Neutrophil–Lymphocyte and Platelet—Lymphocyte Ratios as Biomarkers of Survival in Cutaneous Melanoma: A Multicenter Cohort Study. Ann. Surg. Oncol. 2018;25:3341–3349. doi: 10.1245/s10434-018-6660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson A.V., Keeble C., Lo M.C.I., Thornton O., Peach H., Moncrieff M.D.S., Dewar D.J., Wade R.G. The neutrophil–lymphocyte ratio and locoregional melanoma: A multicentre cohort study. Cancer Immunol. Immunother. 2020;69:559–568. doi: 10.1007/s00262-019-02478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai X., Dai J., Li C., Cui C., Mao L., Wei X., Sheng X., Chi Z., Yan X., Tang B., et al. Risk Models for Advanced Melanoma Patients Under Anti-PD-1 Monotherapy—Ad hoc Analyses of Pooled Data from Two Clinical Trials. Front. Oncol. 2021;11:702. doi: 10.3389/fonc.2021.639085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludwig J.M., Haubold J., Bauer S., Richly H., Siveke J.T., Wimmer J., Umutlu L., Schaarschmidt B.M., Theysohn J.M. Predictive impact of the inflammation-based indices in uveal melanoma liver metastases treated with transarterial hepatic chemoperfusion. Radiol. Oncol. 2021;55:347–353. doi: 10.2478/raon-2021-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fucà G., Beninato T., Bini M., Mazzeo L., Di Guardo L., Cimminiello C., Randon G., Apollonio G., Bisogno I., Del Vecchio M., et al. The Pan-Immune-Inflammation Value in Patients with Metastatic Melanoma Receiving First-Line Therapy. Target. Oncol. 2021;16:529–536. doi: 10.1007/s11523-021-00819-0. [DOI] [PubMed] [Google Scholar]
- 13.Gambichler T., Said S., Abu Rached N., Scheel C.H., Susok L., Stranzenbach R., Becker J.C. Pan-immune-inflammation value independently predicts disease recurrence in patients with Merkel cell carcinoma. J. Cancer Res. Clin. Oncol. 2022:1–7. doi: 10.1007/s00432-022-03929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Susok L., Said S., Reinert D., Mansour R., Scheel C.H., Becker J.C., Gambichler T. The pan-immune-inflammation value and systemic immune-inflammation index in advanced melanoma patients under immunotherapy. J. Cancer Res. Clin. Oncol. 2022:1–6. doi: 10.1007/s00432-021-03878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gambichler T., Späth J., Said S., Scheel C.H., Susok L., Stranzenbach R. Outcome of extracorporeal photopheresis in mycosis fungoides patients is not predicted by quotients of systemic immune-inflammatory biomarkers. J. Clin. Apher. 2022;37:360–366. doi: 10.1002/jca.21982. [DOI] [PubMed] [Google Scholar]
- 16.Guven D.C., Sahin T.K., Erul E., Kilickap S., Gambichler T., Aksoy S. The Association between the Pan-Immune-Inflammation Value and Cancer Prognosis: A Systematic Review and Meta-Analysis. Cancers. 2022;14:2675. doi: 10.3390/cancers14112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (Version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Cleveland W.S., Devlin S.J., Grosse E. Regression by local fitting: Methods, properties, and computational algorithms. J. Econ. 1988;37:87–114. doi: 10.1016/0304-4076(88)90077-2. [DOI] [Google Scholar]
- 19.Cleveland W.S., Grosse E. Computational methods for local regression. Stat. Comput. 1991;1:47–62. doi: 10.1007/BF01890836. [DOI] [Google Scholar]
- 20.Kanaki T., Stang A., Gutzmer R., Zimmer L., Chorti E., Sucker A., Ugurel S., Hadaschik E., Gräger N.S., Satzger I., et al. Impact of American Joint Committee on Cancer 8th edition classification on staging and survival of patients with melanoma. Eur. J. Cancer. 2019;119:18–29. doi: 10.1016/j.ejca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Liu J., Charles P.L., Zhou P.B. Inflammation fuels tumor progress and metastasis. Curr. Pharm. Des. 2015;21:3032–3040. doi: 10.2174/1381612821666150514105741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirili C., Yılmaz A., Demirkan S., Bilici M., Tekin S.B. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int. J. Clin. Oncol. 2019;24:1301–1310. doi: 10.1007/s10147-019-01461-7. [DOI] [PubMed] [Google Scholar]
- 23.Templeton A.J., Ace O., McNamara M.G., Al-Mubarak M., Vera-Badillo F.E., Hermanns T., Šeruga B., Ocaña A., Tannock I.F., Amir E. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014;23:1204–1212. doi: 10.1158/1055-9965.EPI-14-0146. [DOI] [PubMed] [Google Scholar]
- 24.Zhong J.-H., Huang D.-H., Chen Z.-Y. Prognostic role of systemic immune-inflammation index in solid tumors: A systematic review and meta-analysis. Oncotarget. 2017;8:75381–75388. doi: 10.18632/oncotarget.18856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanatsios S., Melbourne Melanoma Project. Li Wai Suen C.S., Cebon J.S., Gyorki D.E. Neutrophil to lymphocyte ratio is an independent predictor of outcome for patients undergoing definitive resection for stage IV melanoma. J. Surg. Oncol. 2018;118:915–921. doi: 10.1002/jso.25138. [DOI] [PubMed] [Google Scholar]
- 26.Marconcini R., Spagnolo F., Stucci L.S., Ribero S., Marra E., De Rosa F., Picasso V., Di Guardo L., Cimminiello C., Cavalieri S., et al. Current status and perspectives in immunotherapy for metastatic melanoma. Oncotarget. 2018;9:12452–12470. doi: 10.18632/oncotarget.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang R., Chang Q., Meng X., Gao N., Wang W. Prognostic value of Systemic immune-inflammation index in cancer: A me-ta-analysis. J. Cancer. 2018;9:3295–3302. doi: 10.7150/jca.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J., Wu X., Yu H., Li S., Mao L., Chi Z., Si L., Sheng X., Cui C., Dai J., et al. Systemic Immune-Inflammation Index and Circulating T-Cell Immune Index Predict Outcomes in High-Risk Acral Melanoma Patients Treated with High-Dose Interferon. Transl. Oncol. 2017;10:719–725. doi: 10.1016/j.tranon.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C., Tian W., Zhao F., Li M., Ye Q., Wei Y., Li T., Xie K. Systemic immune-inflammation index, SII, for prognosis of elderly patients with newly diagnosed tumors. Oncotarget. 2018;9:35293–35299. doi: 10.18632/oncotarget.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fucà G., Guarini V., Antoniotti C., Morano F., Moretto R., Corallo S., Marmorino F., Lonardi S., Rimassa L., Sartore-Bianchi A., et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer. 2020;123:403–409. doi: 10.1038/s41416-020-0894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen J.T., Miner T.J., Vezeridis M.P. Is the neutrophil-to-lymphocyte ratio a useful prognostic indicator in melanoma patients? Melanoma Manag. 2020;7:MMT47. doi: 10.2217/mmt-2020-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fest J., Ruiter R., Ikram M.A., Voortman T., Van Eijck C.H.J., Stricker B.H. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci. Rep. 2018;8:10566. doi: 10.1038/s41598-018-28646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gambichler T., Mansour R., Scheel C.H., Said S., Abu Rached N., Susok L. Prognostic Performance of the Derived Neutrophil-to-Lymphocyte Ratio in Stage IV Melanoma Patients Treated with Immune Checkpoint Inhibitors. Dermato. 2022;2:14–20. doi: 10.3390/dermato2020003. [DOI] [Google Scholar]
- 34.Pinto-Paz M.E., Cotrina-Concha J.M., Benites-Zapata V.A. Mortality in cutaneous malignant melanoma and its association with Neutrophil-to-Lymphocyte ratio. Cancer Treat. Res. Commun. 2021;29:100464. doi: 10.1016/j.ctarc.2021.100464. [DOI] [PubMed] [Google Scholar]
- 35.Lino-Silva L.S., Salcedo-Hernández R., García-Pérez L., Meneses-García A., Zepeda-Najar C. Basal neutrophil-to-lymphocyte ratio is associated with overall survival in melanoma. Melanoma Res. 2017;27:140–144. doi: 10.1097/CMR.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 36.Davis J.L., Langan R.C., Panageas K.S., Zheng J., Postow M.A., Brady M.S., Ariyan C., Coit D.G. Elevated Blood Neutrophil-to-Lymphocyte Ratio: A Readily Available Biomarker Associated with Death due to Disease in High Risk Nonmetastatic Melanoma. Ann. Surg. Oncol. 2017;24:1989–1996. doi: 10.1245/s10434-017-5836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma J., Kuzman J., Ray A., Lawson B.O., Khong B., Xuan S., Hahn A.W., Khong H.T. Neutrophil-to-lymphocyte Ratio (NLR) as a predictor for recurrence in patients with stage III melanoma. Sci. Rep. 2018;8:4044. doi: 10.1038/s41598-018-22425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blakely A.M., Cohen J.T., Comissiong D.S., Vezeridis M.P., Miner T.J. Prognosis and management of thick and ultrathick melano-ma. Am. J. Clin. Oncol. 2019;42:824–829. doi: 10.1097/COC.0000000000000604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author on reasonable request.