Abstract
Simple Summary
The use of wearable devices in clinical care is gaining popularity among cancer patients. The COVID-19 pandemic highlighted the value of wearable devices for monitoring health. Wearable devices are used to record and monitor real-time data like physical activity, sleep metrics, and heart rate variables. The use of wearable devices can directly impact clinical decision-making. There are few pieces of evidence that prove that wearable could improve the quality of patient care while reducing the cost of care, such as remote health monitoring. The generated big data by the wearable device is both a challenge and an opportunity. Researchers can apply artificial intelligence and machine learning techniques to improve wearable devices and their usage among cancer patients. In this scoping review, we assessed the adherence to clinical outcomes of wrist-worn wearable devices in the cancer population.
Abstract
The use of wearable devices (WDs) in healthcare monitoring and management has attracted increasing attention. A major problem is patients’ adherence and acceptance of WDs given that they are already experiencing a disease burden and treatment side effects. This scoping review explored the use of wrist-worn devices in the cancer population, with a special focus on adherence and clinical outcomes. Relevant articles focusing on the use of WDs in cancer care management were retrieved from PubMed, Scopus, and Embase from 1 January 2017 to 3 March 2022. Studies were independently screened and relevant information was extracted. We identified 752 studies, of which 38 met our inclusion criteria. Studies focused on mixed, breast, colorectal, lung, gastric, urothelial, skin, liver, and blood cancers. Adherence to WDs varied from 60% to 100%. The highest adherence was reported in the 12-week studies. Most studies focused on physical activity, sleep analysis, and heart vital signs. Of the 10 studies that described patient-reported outcomes using questionnaires and personal interviews, 8 indicated a positive correlation between the patient-reported and wearable outcomes. The definitions of the outcome measures and adherence varied across the studies. A better understanding of the intervention standards in terms of the clinical outcomes could improve adherence to wearables.
Keywords: wearable devices, health monitoring, cancer, eHealth
1. Introduction
Considerable advancements have been made in the fields of biosensors and artificial intelligence, particularly in terms of their use for the detection and management of chronic illnesses [1]. Wearable devices (WDs) are electronic devices that can be easily worn on the human body to capture real-time healthcare data using receptors and transducers attached to the device [2]. According to Deloitte Global’s prediction analysis, 440 million individuals will be using WDs by the end of 2024, with more healthcare providers recommending their use and more consumers becoming more comfortable with using them in their daily lives [3]. WDs include any device that can be worn on the human body including wristwatches, glasses, chest straps, rings, and prosthetic sockets [4]. WDs are mainly used to track individuals’ daily activities, including sleep quality, physical activity, and heart rate [4,5]. The evolution of smart WDs is being accelerated by improved sensors, artificial intelligence, and advanced machine-learning algorithm–based technologies [6]. For example, few watches that are currently available have optical sensors that can collect real-time data on blood physiology and blood pressure through photoplethysmography [7].
Several studies have reported the use of wearables in the healthcare field with promising outcomes [8]. WDs have revolutionized the healthcare system and have reduced the load of hospitals by providing reliable information in a timely manner [8,9]. Various WDs with a wide range of types and employability are available for data collection [10]. The rich information collected by WDs can assist healthcare professionals in tracking a person’s health status (sleep quality, healthy posture, cognitive decline, and even early warning signs of infection and inflammation) [8,9,10]. Chronic health conditions result in a high financial and emotional burden on patients and their families [11]. In response to COVID-19, many healthcare professionals reengineered their pathways to promote “care in place,” which allows patients to track their health and participate in the self-care system [12].
Wearables in oncology may provide new, vital information on a patient’s health status (heart rate, blood pressure, activity level, sleep quality, and behavioral activity), which can improve cancer care management [10].
Cancer is the second leading cause of death, and treatment is costly; however, using wearable devices can be cost-effective as they require personalized and flexible patient treatment plans [13,14]. Patients can also track their own data while continuing their normal routines while their data are being transferred to the clinic; this approach in oncology is a work-smarter approach and, more importantly, provides a general improvement in the quality of life [15,16]. Few studies provided evidence regarding the effectiveness of WDs in improving the treatment outcomes of patients with cancer [17].
To understand the potential use of WDs in cancer management, the effect of an intervention on or the role of WDs in clinical outcomes should be investigated [10]. Evaluating patients’ outcomes and their adherence to WDs, as well as defining the criteria and valid data, can aid in introducing WDs into clinical practice [18]. Although a consensus and guidelines for designing and reporting trials that use wearables as a component of the intervention are lacking, this area of research is gaining attention [19] because WDs play a major role in healthcare management [8]. This scoping review examined the effectiveness of WDs in assisting patients in their care, particularly in cancer treatment. In addition, this review evaluated the effectiveness and feasibility of this type of intervention. The goal of this review is to explore the use of WDs in patients with different types of cancers given the increasing number of WD users.
2. Methods
2.1. Search Strategy
The search strategy was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. A comprehensive literature search was conducted to identify scientific studies that analyzed WD-based interventions targeting patients with cancer and cancer survivors. We searched for studies published between 1 January 2017 and 7 March 2022, in the electronic databases PubMed, Scopus, and Embase. The search for studies was conducted on 9 March 2022. The search was conducted using MeSH (medical subject headings) such as wearables and cancer/devices and cancer/telemonitoring and cancer. The search was limited to articles published in English. The data collected were reviewed by the two authors of this study. If any reviewer considered an article to be potentially significant, the full text of the article was retrieved. In the case of a disagreement on a particular article, a third reviewer chose the article based on the exclusion and inclusion criteria.
2.2. Criteria for the Inclusion of Studies
We included original research articles that were published in English and met the following criteria: (1) included cancer survivors and patients with cancer undergoing treatment; (2) focused on preventive care or health monitoring (physical activity, behavioral activity, and quality of life); (3) used a smartwatch or other types of wrist-worn WDs for assessing health; and (4) were designed as a randomized controlled trial (RCT), prospective clinical trial, quasi-experimental study, feasibility study, observational study, or pilot study.
We excluded studies that (1) focused on the prediction of cancer; (2) used telecommunication technologies, such as websites, telephones, and mobile applications alone; (3) did not involve the use of wrist-worn wearables or focused on some other health problems; (4) did not include the intervention as the primary focus; and (5) were review articles, trial protocols, trial registrations, conference papers, book chapters, notes, brief reports, letters, editorials, or case studies or were published in a language other than English.
2.3. Study Selection and Data Extraction
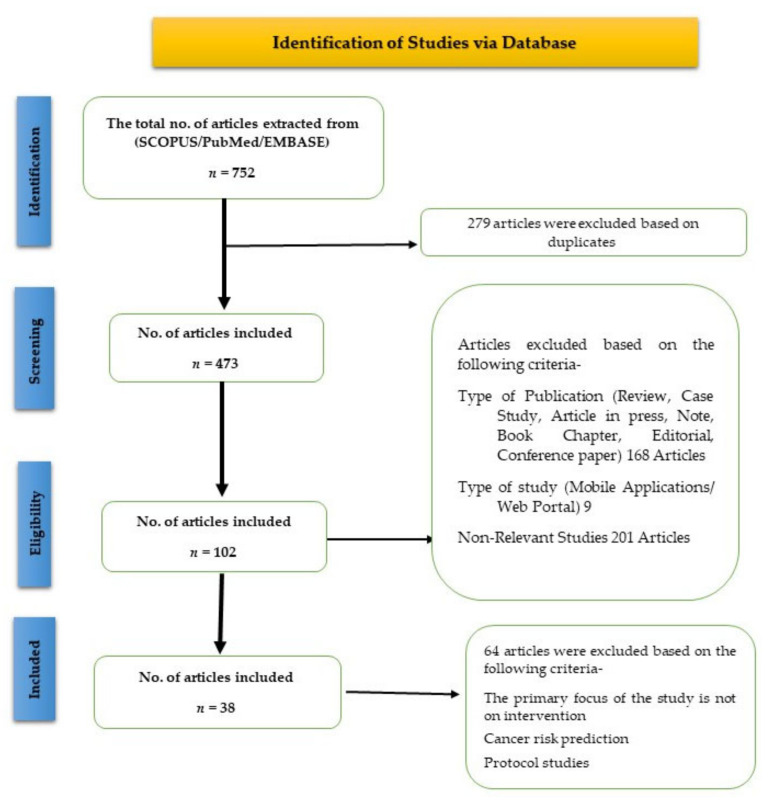
We initially screened the titles and abstracts of articles that met the inclusion criteria and could not be rejected with certainty. The references of the studies that met the inclusion criteria were manually searched to identify relevant research articles. Three researchers independently reviewed the full texts of the articles and extracted the following data from each included article: characteristics of the study (country of origin, sample size, authors, study design, study purpose, and publication year); patient characteristics (mean age, sex percentage, cancer type, and health status); intervention characteristics (total study duration including the follow-up period, intervention duration, and type of wearable and tools used); and study focus (behavioral health monitoring and preventive care). For this scoping review, data on adherence to wearables were extracted according to the different criteria mentioned in each article. Adherence was measured in terms of the percentage of valid wear time among patients or the percentage of total patients who completed the trial and the percentage of total evaluable days [20]. Furthermore, we categorized the studies based on the methods and outcomes (Figure 1).
Figure 1.
PRISMA flow diagram of the screening and selection of studies.
2.4. Adherence Analysis
Adherence analysis results were extracted from the studies and analyzed according to the different criteria mentioned in the selected articles. The majority of included studies evaluated adherence through the completeness of the data collection, that is, the percentage of recruited patients who completed the study. The remaining studies had specific criteria for the adherence evaluation that are mentioned in results section. We used SPSS for visualization purposes in order to graphically represent adherence to wearable devices by the duration of the study intervention.
2.5. Outcomes and Analysis
This review examined the effectiveness of WDs in health monitoring (symptom analysis/recovery assessment/physical activity, behavioral activity, quality of life, and preventive care) among patients with cancer undergoing treatment (chemotherapy, radiotherapy, or pre- and post-surgery treatment) and cancer survivors. The primary outcome was adherence to wearables according to the different criteria of each study. The secondary outcomes included the wearable, patient-reported, and clinical outcomes of the intervention. All data are presented in a descriptive manner.
2.6. Ethical Consideration
This review did not require the approval of any national or institutional boards.
3. Results
3.1. Study Selection
A total of 752 articles were retrieved from Embase, Scopus, and PubMed. After the removal of the duplicates, 473 articles were evaluated. Finally, of the 473 articles, the full texts of 102 relevant articles were reviewed. At this stage, the articles were screened based on their study characteristics, including the type of wearables and their usage, and the primary focus of the study. The exclusion criteria are mentioned in the methodology and presented in Figure 1. Finally, 38 studies that met the inclusion criteria were included in this scoping review.
3.2. Study Characteristics
Among the 38 studies, 19 were conducted in North America (the United States and Canada), 6 in Europe (Ireland, Germany, the United Kingdom, the Netherlands, France, and Switzerland), 9 in Asia (Taiwan, Central China, India, Japan, and South Korea), and 4 in Oceania (Australia; Table 1).
Table 1.
Summary of Included studies.
| Country of Study | Topic (Type of Cancer and Status) | Study Design | Tools Used | Participant (%) Gender |
|
|---|---|---|---|---|---|
| The United States of America [21] | Breast Cancer (n = 57) | Survivors | Feasibility Study | Wearable Device and Questionnaire | 100% Women |
| Australia [22] | Breast Cancer (n = 80) | Survivors | RCT | Wearable Device + Text Messages and Personal Interviews + Mobile Application | 100% Women |
| The United States of America [23] | Breast Cancer (n = 34) | Survivors | RCT | Wearable Device and Questionnaire (Correlation) | 100% Women |
| Australia [24] | Breast Cancer (n = 80) | Survivors | RCT | Wearable Device and Questionnaire (Correlation) | 100% Women |
| The United States of America [25] | Breast Cancer (n = 20) | Survivors | RCT | Wearable Device + Group Sessions and Phone Calls | 100% Women |
| Canada [26] | Breast Cancer (n = 41) | Survivors | RCT | Wearable Device and Questionnaires (Correlation) | 100% Women |
| The Netherlands [27] | Breast Cancer (n = 8) | Survivors | Qualitative Study | Wearable Device and Questionnaires | 100% Women |
| United Kingdom [28] | Breast Cancer (n = 39) | Under Treatment | Non-RCT | Wearable Device, Questionnaire, and Behavioral Counseling Session | 100% Women |
| India [29] | Breast Cancer (n = 44) | Under Treatment | Non-RCT | Wearable Device + General group session + Questionnaire + Mobile Application | 95.4% Women |
| The United States of America [30] | Breast Cancer (n = 32) | Under Treatment | Pilot Study | Wearable Device + Mobile application + Text Messages | 100% Women |
| The United States of America [31] | Breast Cancer (n = 10) | Under Treatment | RCT | Wearable Device and Questionnaire | 100% Women |
| Germany [32] | Breast Cancer (n = 99) | Under Treatment | Feasibility Study | Wearable Device and Questionnaire | 100% Women |
| Central China [33] | Mixed Cancer (n = 112) | Under Treatment | RCT | Wearable Device | 76.2% Women |
| The United States of America [34] | Mixed Cancer (n = 38) | Under Treatment | Utility Study/Predictive Study | Wearable Device + Mobile application and Interview | 52% Women |
| The United States of America [35] | Mixed Cancer (n = 41) | Under Treatment | Observational Study | Wearable Device + Mobile application + Questionnaire | 56% Women |
| The United States of America [36] | Mixed Cancer (n = 33) | Under Treatment | Prospective cohort Study | Wearable Devices and Spirometer | 57.5% Women |
| Japan [37] | Mixed Cancer (n = 30) | Under Treatment | Feasibility Study | Wearable Device | 70% Men |
| France [38] | Mixed Cancer (n = 31) | Under Treatment | Pilot Study | Wearable Device + Mobile Application + Questionnaire | 55% Men |
| Ireland [39] | Mixed Cancer (n = 61) | Survivors | RCT | Wearable Devices + Goal-setting session + Telephone-delivered health-coaching sessions | 50% Men |
| The United States of America [40] | Mixed Cancer (n = 32) | Survivors | Feasibility Study | Wearable Device + Two group sessions + support phone call | 51% Men |
| Switzerland [41] | Mixed Cancer (n = 30) | Survivors | Feasibility Study | Fitbit + iPad (preloaded apps) + Questionnaires | 70% Men |
| The United States of America [42] | Mixed Cancer (n = 59) | Survivors | Pilot Study | Wearable Device and Questionnaire | 59.3% Women |
| The United States of America [43] | Mixed Cancer (n = 47) | Survivors | RCT | Wearable Device + Questionnaire + Social Media Intervention (Health Education) | 96% Women |
| Australia [44] | Colorectal and Endometrial cancer (n = 29) | Survivors | RCT | Mobile application (in-app chat service) + Wearable device + Questionnaires | 58% Women |
| Western Australia [45] | Colorectal Cancer (n = 61) | Survivors | RCT | Wearable Device + mHealth app + Peer-based virtual support group + Qualitative Interviews | 50% Women |
| The United States of America [46] | Colorectal Cancer (n = 39) | Survivors | RCT | Wearable Device and Questionnaire-based study | 58% Women |
| South Korea [47] | Colorectal Cancer (n = 75) | Under Treatment | Feasibility Study | Wearable device + Questionnaires + e-Patient Diary | 58.7% Men |
| The United States of America [48] | Colorectal Cancer (n = 40) | Under Treatment | Pilot Study | Wearable Device and Questionnaire-based study | 56.8% Women |
| Taiwan [49] | Lung Cancer (n = 12) | Under Treatment | Observational Study | Wearable Device and Questionnaire-based study | 58.33% Men |
| The United States of America [50] | Lung Cancer (n = 30) | Under Treatment | Observational Study | Wearable Device + Questionnaire + Educational handbook + Social support + Email-based coaching | 67% Men |
| The United States of America [51] | Lung Cancer (n = 18) | Under Treatment | Observational Study | Wearable Device and Questionnaire (Correlation) | 44% Women |
| South Korea [52] | Lung Cancer (n = 555) | Under Treatment | Usability Study | Wearable Devices + Questionnaire+ Educational handbook+ Social support + Email-based coaching | 61% Men |
| The United States of America [53] | Gastric cancer (n = 27) | Under Treatment | Cohort Study | Wearable Device + Mobile Application | 62.96% Men |
| Taiwan [54] | Gastric Cancer (n = 43) | Under Treatment | Group Study | Wearable Devices + Questionnaires | 51% Men |
| South Korea [55] | Liver Cancer (n = 31) | Under Treatment | Usability Study | Wearable Device + Daily text messages+ Questionnaire | 84% Men |
| The United States of America [56] | Blood Cancer (n = 11) | Under Treatment | Feasibility Study | Diary + Accelerometer | 66.6% Men |
| Japan [57] | Urothelial Carcinoma (n = 21) | Under Treatment | Cohort Study | Wearable Device | 84% Men |
| The United States of America [58] | Skin Cancer (n = 60) | Survivor | Observational Study | Wearable Devices + Questionnaire + Interviews | 60% Women |
Among the 38 studies, 12 were designed as RCTs, 7 as feasibility studies, 5 as observational studies, 4 as pilot studies, 3 as cohort studies, 2 as nonrandomized controlled trials, 2 as usability studies, 1 as a utility study, and 1 as a group and qualitative study (Table 1). The included studies had different time intervals for the intervention and follow-up durations. The minimum and maximum intervention durations were 1 and 52 weeks, respectively. All the studies had the same follow-up period of 12 weeks (Table 1).
3.3. Characteristics of Research Participants
Most of the studies included patients with different types of cancer, followed by those with breast, colorectal, lung, gastric, urothelial, skin, liver, and blood cancers. Of the 38 studies, 15 focused on cancer survivors and 23 on patients receiving chemotherapy or radiotherapy. The number of participants in the studies ranged from 8 to 555 and their mean age ranged from 17 to 73 years (Table 1).
3.4. Measurement Tools
In terms of measurement tools, the studies included in this scoping review used subjective self-reported questionnaires, WDs, and mobile applications to track the health status of the participants. Only four studies conducted personal interviews to analyze participants’ adherence to WDs and their study experiences [21,33,40,57]. Furthermore, 10 studies evaluated the correlation between the outcomes of WDs and questionnaires to validate the effectiveness of using WDs in patients with cancer [27,31,34,36,37,48,49,52,54,56]. Only two studies used an e-diary and a physical diary along with a WD to track the health status of the participants [31,36].
3.5. Major Study Focus
The studies included in this review mainly focused on physical activity, sleep quality, quality of life, unplanned healthcare encounters, moderate-to-vigorous physical activity (MVPA), sedentary behavior, and symptom burden. Only one study focused on preventive care management (Table 1).
3.6. Intervention Methods
The studies included in this review article employed different interventional approaches to improve the clinical outcomes of the participants. Most of the studies used a mobile application to provide the intervention [22,34,35,39,40,46,53,54,57]. Of the 38 studies, 3 provided the intervention by sending text messages [22,45,57], and 4 conducted a general group session or included a virtual support group [39,41,44]. Furthermore, 7 studies used in-app chat services; conducted behavioral counseling sessions, coaching programs, and group phone calls; and provided health education [21,22,23,24,30,38,42,46]. The remaining studies included a few other approaches (Table 1).
3.7. Interventional Outcomes
3.7.1. Adherence
The adherence rate was calculated for both the follow-up and intervention periods by determining the average time of the intervention across the 38 studies. Of the 38 studies in this review, 26 examined adherence in terms of the completeness of the data collection, and the remaining 12 studies followed different criteria to examine adherence (Table 2). Of the 38 studies, 6 determined the acceptability of WDs by conducting qualitative personal interviews and evaluating the usage of the device on different days during the study [24,29,45,47,52,57]. Seven studies determined feasibility and four studies evaluated the retention of WDs according to the same criteria used for determining acceptability [4,20,21,23,24,39,41,42,45,47,52,55]. Only one study focused on adherence based on the completion of an exercise program [55].
Table 2.
Adherence to wearable devices in the cancer population.
| Country of Study | Total Study Duration (in Weeks) | Intervention Duration (in Weeks) |
Patients Recruited |
Criteria for Evaluation | Adherence (in Percentage) |
|---|---|---|---|---|---|
| The United States of America [21] | 24 | 12 | 60 | Percentage of enrolled patients who completed all assessments (10 h per day for 4 days in a week) | 95 |
| Australia [22] | 24 | 12 | 83 | Based on the given assessment completion | 94 |
| The United States of America [23] | 52 | 24 | 44 | Collection of data (days with less than 1000 steps considered as non-adherent) | 65 |
| Australia [24] | 12 | 12 | 83 | Completeness of data collection | 96 |
| The United States of America [25] | 10 | 10 | 30 | Completeness of data collection | 67 |
| Canada [26] | 24 | 12 | 45 | Completeness of data collection | 88 |
| The Netherlands [27] | 12 | 12 | 10 | Based on data collection and total wearing days |
80 |
| United Kingdom [28] | 2 | 2 | 56 | Collected data on the different days (39 patients*14 days) | 89 |
| India [29] | 7 | 7 | 44 | Users’ tolerance ability to the intensity of the program that was set using the rate of perceived exertion (RPE) | 93 |
| The United States of America [30] | 17 | 17 | 32 | Days were considered “valid” if there was any wear time recorded (5 min threshold) |
100 |
| The United States of America [31] | 10 | 10 | 10 | Completeness of data collection | 100 |
| Germany [32] | 24 | 24 | 112 | Completeness of data collection | 95 |
| Central China [33] | 8 | 8 | 143 | Completeness of data collection | 78 |
| The United States of America [34] | 8 | 8 | 45 | Completeness of data collection | 84 |
| The United States of America [35] | 20 | 8 | 34 | Completeness of data collection | 68 |
| The United States of America [36] | 43 | 43 | 44 | Completeness of data collection | 75 |
| Japan [37] | 4 | 4 | 30 | Completeness of data collection | 90 |
| France [38] | 4 | 4 | 30 | Completeness of data collection | 86 |
| Ireland [39] | 24 | 12 | 68 | Completeness of data collection | 89 |
| The United States of America [40] | 52 | 12 | 49 | Completeness of data collection | 65 |
| Switzerland [41] | 12 | 12 | 30 | Completeness of data collection and qualitative analysis of interviews |
83 |
| The United States of America [42] | 10 | 10 | 59 | Completeness of data collection | 100 |
| The United States of America [43] | 12 | 12 | 50 | Completeness of data collection | 94 |
| Australia [44] | 24 | 12 | 34 | Based on participants who completed the study criterion, which is a minimum of 1000 steps or more denoted per day. | 82 |
| Western Australia [45] | 12 | 12 | 68 | Completeness of data collection | 94 |
| The United States of America [46] | 12 | 12 | 41 | Completeness of data collection |
81 |
| South Korea [47] | 12 | 12 | 102 | Completeness of data collection | 74 |
| The United States of America [48] | 12 | 12 | 44 | Completeness of data collection | 88 |
| Taiwan [49] | 1 | 1 | 12 | Completeness of data collection | 100 |
| The United States of America [50] | 1 | 1 | 39 | Completeness of data collection | 67 |
| The United States of America [51] | 3 | 3 | 30 | Completeness of data collection | 60 |
| South Korea [52] | 52 | 52 | 555 | Completeness of data collection | 100 |
| The United States of America [53] | 3 | 3 | 41 | Based on the rate of data collected during chemotherapy | 63 |
| Taiwan [54] | 4 | 4 | 43 | Completeness of data collection | 100 |
| South Korea [55] | 12 | 12 | 37 | Equivalent to the completion of the exercise program | 84 |
| The United States of America [56] | 2 | 2 | 12 | Completeness of data collection | 92 |
| Japan [57] | 12 | 12 | 28 | Completeness of data collection | 75 |
| The United States of America [58] | 3 | 3 | 60 | In-person interviews to examine the acceptability of the device and analysis of qualitative data | 100 |
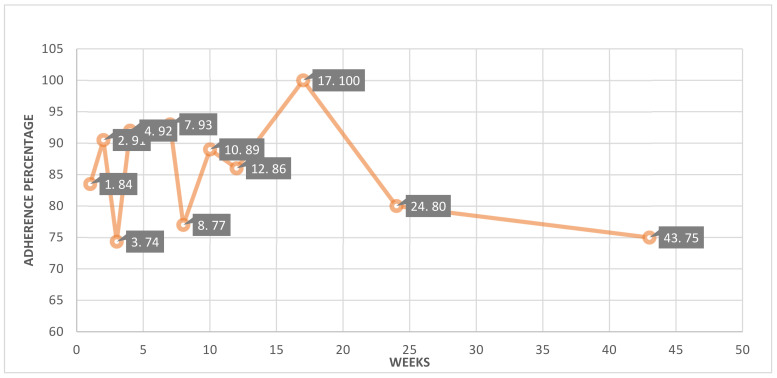
The adherence during the intervention periods was visualized graphically using SPSS. Figure 2 shows the adherence rate to the exhibited intervention duration. All of the studies were divided into 11 segments based on the duration of each study’s intervention. The average adherence percentage was calculated for each segment. The intervention-based segments included studies with weeks 1 [48,49], 2 [27,55], 3 [50,52,57], 4 [36,37,53], 7 [28], 8 [32,33,34], 10 [24,30,41], 12 [20,21,23,25,26,38,39,40,42,43,44,45,46,47,54,56], 17 [29], 24 [22,31] and 43 [35]. In the graph, (1.84) indicates the studies grouped in this segment that had a one-week intervention with an average adherence of 84%. According to our analysis, 16 out of 38 studies used a 12-week intervention period with an average adherence of 86% (Figure 2) [20,21,23,25,26,38,39,40,42,43,44,45,46,47,54,56]. The follow-up periods in the reported studies were calculated by subtracting the intervention duration from the total study duration. There are only five studies with a 12-week follow-up period, accounting for 24 weeks of total study duration. These studies showed an average adherence rate of 91% [20,21,25,38,43]. Studies with an intervention period of 4 or 7 weeks also observed high adherence to WDs (i.e., 92% and 93%, respectively) [28,36,37,53] (Figure 2). There is one unique study that enrolled patients across four seasons of the year. This study showed a 100% adherence rate throughout the 52-week study duration [52]. The adherence outcomes are summarized in Table 2.
Figure 2.
Graphical representation of adherence to wearable devices by duration of intervention.
3.7.2. Clinical Outcomes
Wearable technology can directly affect clinical decision making and improve the quality of patient care while reducing the cost of care including that of patient rehabilitation outside of hospitals. The studies in this review focused on physical activity, sleep quality, quality of life, unplanned healthcare encounters, MVPA, sedentary behavior, and symptom burden.
Most of the studies in this review evaluated the feasibility of and adherence to WDs. Of the 38 studies, only 10 determined the correlation between patient-reported and objective outcomes; these studies reported that the use of WDs significantly improved clinical outcomes [27,31,34,36,37,48,49,52,54,56]. In addition, other studies demonstrated significant improvements in clinical outcomes; however, these studies did not observe a correlation between patient-reported and clinical outcomes. Of the 38 studies, 12 included patients with different types of cancer, 6 included cancer survivors, and 11 included patients with breast cancer. Of the 11 studies, 5 focused on patients receiving chemotherapy or radiotherapy. Moreover, of the 38 studies, 5 included patients with colorectal cancer, 4 included patients with lung cancer, and 2 included patients with gastric cancer. Furthermore, 4 of the 38 studies included patients with urothelial carcinoma, skin cancer, blood cancer, and liver cancer. The clinical outcomes are summarized in Table 3.
Table 3.
Reported clinical outcomes in the cancer population using wearable devices.
| Country of Study | Cancer Type | Purpose | Reported Clinical Outcomes |
|---|---|---|---|
| The United States of America [21] | Breast Cancer | Behavioral health management (PA/QoL and fatigue) |
High engagement among hospitalized patients and increased energy expenditure among cancer survivors. Outcomes depend on numerous factors related to users and their needs. |
| Australia [22] | Breast Cancer | Behavioral health management (sleep quality) |
Changes in actigraphy (sleep efficiency) and PSQI global and subscales favored the intervention arm. Findings were not significant or clinically meaningful. |
| The United States of America [23] | Breast Cancer | Behavioral health management (physical activity/BMI/QoL/fatigue/ fitness/self-regulation and self-efficacy related to PA) |
Self-monitoring, goal setting, and self-efficacy were significantly correlated with activity levels. Increased improvement in health was noted with an increase in PA. |
| Australia [24] | Breast Cancer | Behavioral health management (MVPA/Sedentary Behavior) |
The intervention resulted in increases in MVPA and MVPA accrued in bouts of at least 10 consecutive min while reducing total and prolonged sitting times. A significant difference in MVPA was noted between groups at T2, favoring the intervention arm. |
| The United States of America [25] | Breast Cancer | Behavioral health management (PA- MVPA, Sedentary/physiological/ psychosocial/QoL variables) |
No significant group differences were observed for changes over time for any variable. Both groups showed increased mean daily MVPA, light PA, energy expenditure, and steps/day. |
| Canada [26] | Breast Cancer | Behavioral health management (PA-MVPA, LIPA, Sedentary Behavior/Sleep quality/health-related Fitness Markers) |
Increases in moderate-to-vigorous intensity PA and decreases in sedentary time were significantly greater in the lower-intensity PA group versus the control group at 12 weeks. Increases in V˙O2 max at 12 weeks in both intervention groups were significantly greater than the changes in the control group. Changes in PA and V˙O2 max remained at 24 weeks but differences between the intervention and control groups were not significant. |
| The Netherlands [27] | Breast Cancer | Behavioral health management (PA-Sedentary behavior) |
The activity tracker motivated women to be physically active and increased their awareness of their sedentary lifestyle. Wearing an activity tracker raised lifestyle awareness in patients with breast cancer. |
| United Kingdom [28] | Breast Cancer | Behavioral health management (Upper Limb Function) |
WAM improved on the surgical side of the upper limb with an increment in PA for the first week and showed a good correlation with DASH (0.0506) |
| India [29] | Breast Cancer | Behavioral health management (Fatigue/QoL//Functional Capacity/PA/Body Composition) |
At the end of the 7-week intervention, functional capacity, quality of life, and skeletal mass were significantly improved, whereas fatigue and changes in total fat improved nonsignificantly. |
| The United States of America [30] | Breast Cancer | Behavioral health management (PA/MVPA/SB/Cognitive functions) | Participants decreased their activity from pre- to post-chemotherapy by 1 h/week in MVPA and 8 h/week in TPA during the decline. This is useful for determining the stage of chemotherapy in which PA starts to decline and patients need extra support for their care. |
| The United States of America [31] | Breast Cancer | Behavioral health management (PA/Sleep Metrics) |
Overall step count decreased by an average of 54 steps per day from baseline during treatment. Although differences in step count, calories expended, and miles walked throughout the RT were minimal, they were significant because of the substantial number of events |
| Germany [32] | Breast Cancer | Behavioral health management (PA) |
Coherence between self-reported and device data was strong (r = 0.566). Neither treatment nor week nor their interaction had effects on step counts. Self-reported activity time was lower for patients receiving chemotherapy than for those not receiving chemotherapy and lower in the 18th week than in the 3rd week |
| Central China [33] | Mixed Cancer | Behavioral health management (Asleep + QoL) |
The baseline measurement was not significantly different among the three groups. However, after the intervention, a significant difference between the experimental and control groups was noted. Sleep quality and PA improved significantly but not the secondary outcomes. |
| The United States of America [34] | Mixed Cancer | Behavioral health management (Unplanned Healthcare Encounter/PA) |
Kinematic features associated with physical activity showed a positive correlation. Chair-to-table kinematics are good predictors of unexpected hospitalization. Get- up-and-walk kinematics are good predictors of low physical activity |
| The United States of America [35] | Mixed Cancer | Behavioral health management (Unplanned Healthcare Encounter/PA) |
This study demonstrated the feasibility of an outpatient wearable activity tracker. The results revealed a 50% disagreement with no association of these disagreements with UHEs and no correlation between the UHEs and ECOG scores. A correlation between (1) average METs and UHEs and (2) no sedentary physical activity hours and UHEs was noted |
| The United States of America [36] | Mixed Cancer | Behavioral health management (PA/QoL) |
Significant improvements across all eight dimensions of HRQOL; most patients (85%) reported that they enjoyed wearing the Fitbit. Most felt that the Fitbit helped them to be more active (79%), whereas a minority (18%) felt their activity level was the same, and none reported becoming less active. |
| Japan [37] | Mixed Cancer | Behavioral health management (PA/Symptom Burden Assessment/Sleep/Fatigue) |
Use of a wearable activity tracker for collecting PGHD in real time according to the protocol was feasible. With respect to adherence, the result was significant. The correlation between the assessed data was not significant |
| France [38] | Mixed Cancer | Behavioral health management (PA/Sleep) | Results provide evidence for both the feasibility and relevance of the combined objective and subjective remote monitoring of sleep and other symptoms in patients with cancer with single-night precision. This dynamic approach can help the development of novel therapeutics whose testing is warranted in patients with cancer |
| Ireland [39] | Mixed Cancer | Behavioral health management (MVPA/Cardiovascular risk factors and sedentary behavior) | The estimated difference between groups at 24 weeks supported higher MVPA; no change in MVPA in the intervention group was observed during the 12-week follow-up period, indicating a positive correlation with the improvement in cardiovascular risk factors. |
| The United States of America [40] | Mixed Cancer | Behavioral health Management (MVPA/QoL/ Fatigue/Fitness/Sedentary Behavior) |
Results of the studies revealed some promising improvements in muscular strength that aligned with the intervention’s focus on strength training. |
| Switzerland [41] | Mixed Cancer | Behavioral health management (Symptom Analysis) |
Remote monitoring of healthcare status in patients receiving palliative care with a limited life expectancy is feasible, and patients can handle the smartphone and sensor-equipped bracelet. Feedback toward the use of this monitoring system was mostly positive. |
| The United States of America [42] | Mixed Cancer | Behavioral health management (PA-SB and MVPA/QoL) | Intervention participants had a lower-than-expected engagement in the Facebook group component, (passive instead of active engagement); MVPA and sedentary time showed no significant difference b/w gaps |
| The United States of America [43] | Mixed Cancer | Behavioral health management (PA-MVPA) | Increased physical activity among cancer survivors was noted: the intervention group increased their daily steps. Moderate-to-vigorous-intensity activity performed in 10 min bouts increased, but no significant group-by-time differences for either light- or vigorous-intensity activity were noted |
| Australia [44] | Colorectal and endometrial Cancer | Behavioral health management (PA -Steensma) | Fitbit wear time (percentage of valid wear days = adherence) was consistent with a median adherence score of 100%. Comparison and correlation with actigraphy (MVPA) show that both devices are not correlated and do not show any type of association. |
| Western Australia [45] | Colorectal Cancer | Behavioral health management (MVPA/Cardiovascular Risk) | Despite a significant increase in MVPA, the change in the proportion of participants meeting the guidelines in relation to MV10 did not significantly differ by group. Reduction in DBP among intervention participants that were hypertensive. Fitbit was promising for low-intensity interventions. |
| The United States of America [46] | Colorectal Cancer | Behavioral health management (PA-MVPA/ Adverse events) |
Intervention arm increased its MVPA by 13 min per day more than the control arm. Larger studies should be conducted to determine whether the intervention increases physical activity. |
| South Korea [47] | Colorectal Cancer | Behavioral health management (PA/QoL/Nutritional Status/Physical Performance) |
Lower-extremity strength and cardiorespiratory endurance were significantly improved. Fatigue and nausea/vomiting symptoms were significantly relieved after the program. Most of the functional scales showed improvements, although the changes were not significant. |
| The United States of America [48] | Colorectal Cancer | Behavioral health management (PA/) |
Pilot data show a nonsignificant decrease in moderate activity accumulated in bouts of at least 10 min in both arms (16–21 min per week). |
| Taiwan [49] | Lung Cancer |
Behavioral health management (CRF) |
The LF to HF ratio is highly correlated with the subjective BFI, particularly when measured during sleep time. Analytical results revealed that this ratio can be used to evaluate cancer fatigue because of a 3% mapping error in the BFI |
| The United States of America [50] | Lung Cancer |
Behavioral health management (Steps/Day and MVPA/Sedentary Behavior/Cardiorespiratory Fitness) |
Participants who received surgery in the spring, summer, autumn, and winter seasons, respectively, had lower PA and CRF than those who received surgery in other seasons. These results were consistent among all study subgroups. |
| The United States of America [51] | Lung Cancer |
Behavioral health management (PA-Steps/QoL/ Symptoms/Functional Status/Dyspepsia) |
Improved PA was associated with the early discharge of patients with GC undergoing gastrectomy. This was because patients with improved PA had resumed physical function, which was the main factor evaluated if patients were qualified to be discharged. |
| South Korea [52] |
Lung Cancer |
Behavioral health management (MVPA/Aerobic Capacity) |
Eight (47%) of the seventeen participants demonstrated a clinically significant improvement of 14 m or more. The average improvement in aerobic capacity (13.8 m) was close to the minimum threshold for a clinically meaningful improvement of 14 m |
| The United States of America [53] | Gastric cancer | Behavioral health management (PA and Symptom Burden) |
This study’s results indicate significant correlations between the number of the step count and two common performance statuses, which is consistent with previous research findings. Questionnaire findings indicated that active patients have a lower burden of symptoms. |
| Taiwan [54] | Gastric Cancer | Behavioral health management (PA/Sleep Metrics) | Results provide evidence for both the feasibility and relevance of the combined objective and subjective remote monitoring of sleep and other symptoms in patients with cancer with single-night precision. This dynamic approach can guide the development of novel therapeutic concepts whose testing is warranted in patients with cancer |
| South Korea [55] | Liver Cancer |
Behavioral health management (Exercise Capacity/PA/QoL/Body Composition and Biochemical) |
Compared with baseline, significant improvements were found in physical fitness measures, body composition, self-reported amount of physical activity, and pain. All symptoms improved, as observed in the QoL scales (i.e., EORTC-QLQ C30). |
| The United States of America [56] | Blood Cancer | Behavioral health management (PA/Sleep) |
This study demonstrates the feasibility of collecting sleep data through actigraphy among hospitalized adults. Actigraphy measures suggested poor sleep. |
| Japan [57] | Urothelial Carcinoma | Behavioral health management (PA/QoL/ Adverse Events) |
Significant correlations were noted between measurements performed using an oscillometer and a Fitbit during chemotherapy for patients. The measurement of fatigue using Fitbit was effective |
| The United States of America [58] | Skin Cancer |
Preventive care | No differences in baseline knowledge or attitudes regarding sun exposure or protection were noted between the two groups. |
4. Discussion
4.1. Summary and Findings
The use of WDs as gadgets for tracking daily activities, particularly physical activity, has become widespread [59]. WDs are also used for patient and disease management. To the best of our knowledge, this is the first scoping review to examine adherence to WDs in the cancer population. We performed a scoping review of articles evaluating the heterogenous use of wrist-worn devices in patients with cancer. By using our search strategy, we initially retrieved 752 studies, of which 38 were finally included in this scoping review. Most of the studies in the review included patients with mixed cancer types, followed by those with breast cancer. In addition, other studies included cancer survivors and patients with cancer receiving chemotherapy or radiotherapy. The included studies were designed as RCTs and nonrandomized controlled, observational, feasibility/pilot, cohort, and group studies. The sample sizes ranged from 8 to 555. All the studies included used either subjective questionnaires or mobile applications along with a WD. The intervention duration varied from 1 to 52 weeks. Furthermore, 89% (34/38) of the studies evaluated physical activity as the clinical outcome. The outcomes of using WDs varied among the studies based on the intervention program and the usage rate of WDs. Of the 38 studies, 10 compared WD outcomes and patient-reported outcomes (determined using subjective questionnaires) and examined whether the use of WDs improved clinical outcomes. Of the 10 studies, 8 reported that using WDs considerably improved clinical outcomes. The study designs and outcomes varied among the included studies.
4.2. eHealth Tools for Cancer Care
Other studies explored the use of eHealth tools involving patient self-reporting of medication and healthcare management and their effects on the health of users [60]. More high-quality studies are warranted before the standard implementation of eHealth tools. In oncology, patients are increasingly required to manage their own illnesses; thus, WDs can be a valuable tool in the management of cancer during therapy [61]. However, technical and clinical adherence to such devices are essential aspects that should be explored because they determine the usage rate of devices among patients [62]. In this scoping review, we evaluated the adherence to WDs that was reported in the included studies. The adherence rate can be calculated using various factors including wear time, the number of patients using the device, data collection while wearing the device, and the number of evaluable days [20]. A wide range of data were collected during the days on which the device was worn and no data were collected on the days on which patients missed wearing the device. Because of the variations in the data collection and adherence, the effectiveness of WDs for the health management of patients with cancer remains unclear. Whether patients wear the device when they feel comfortable based on the provided intervention should be evaluated. Thus, before designing WD-related studies or large interventional studies, we need to define the criteria and set a fixed wearable time to understand the adherence to devices.
4.3. Strength and Limitations
To the best of our knowledge, this scoping review is the first to determine adherence to specific wrist-worn devices. However, the choice of a WD and its outcomes are crucial. The selection of appropriate clinical variables for measurement is crucial based on the purpose of WD use when incorporated into patients’ daily routines. The outcomes must be based on the type of disease. We agree with other researchers that monitoring specific outcomes is crucial because unnecessary outcomes would not substantially affect patients’ treatments. Most of the studies investigated the effectiveness of WDs for improving physical activity and quality of life; however, this may be attributed to the increasing knowledge of fitness and increased physical training. Furthermore, the secondary objective of most of the studies was to examine the clinical outcomes of WDs in terms of healthcare management. Only 10 studies reported a positive correlation between the use of WDs and patient-reported outcomes.
Since each article used its own definition, it was not possible for us to compare the adherence data of all studies, which is one of the significant limitations of our review. Another limitation is that the included studies mainly involved patients with mixed cancer types, followed by those with breast cancer. Moreover, most of the studies were conducted in North America and Europe. Thus, the findings of this study might not be applicable to populations from other geographical areas. We were unable to conduct a meta-analysis or systematic review due to the numerous variations in the study design, clinical outcomes, and adherence definitions. As a result, we conducted a scoping review and presented a tabulated analysis of our studies.
5. Conclusions
This study reports that the definitions of the outcome measures and adherence varied across the studies. There was a limited consensus among the studies for the measured variables during treatment. Adherence to wearable devices was affected by the changes in the intervention or study design. A better understanding of the interventional period of wearable devices in terms of clinical outcomes is urgently needed. Studies using WDs and subjective questionnaires encouraged patient engagement for better cancer care management. Adherence to WDs varied from 60% to 100% depending on the intervention period. The highest adherence was reported in the 12-week studies. Most studies focused on physical activity, sleep analysis, and heart vital signs. Of the 10 studies that described patient-reported outcomes using questionnaires and personal interviews, 8 indicated a positive correlation between patient-reported and wearable outcomes. Furthermore, for a better understanding of adherence behavior, we need large intervention studies. This can provide us with a clear picture of the clinical outcomes of using wearable devices.
Author Contributions
S.S.-A., L.-J.K. and Y.H. designed the study conception and design. Y.H., U.U. and E.D. carried out the database collection and primary analysis of the database. Y.H., U.U. and E.D. wrote the first manuscript draft of the article. S.S.-A. and L.-J.K. completed the proofreading and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
All the authors declared that there are no conflicts of interest.
Funding Statement
This study was funded by Taipei Medical University and Taipei Medical University Hospital under a joint research grant (109TMU-TMUH-11). This Study is also partially funded by H2020, iHelp project under the research grant (GA: 101017441 and MOST, Taiwan: 110-2923-E038-001-MY3).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chung A.E., Jensen R.E., Basch E.M. Leveraging Emerging Technologies and the “Internet of Things” to Improve the Quality of Cancer Care. J. Oncol. Pract. 2016;12:863–866. doi: 10.1200/JOP.2016.015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravizza A., De Maria C., Di Pietro L., Sternini F., Audenino A.L., Bignardi C. Comprehensive Review on Current and Future Regulatory Requirements on Wearable Sensors in Preclinical and Clinical Testing. Front. Bioeng. Biotechnol. 2019;7:313. doi: 10.3389/fbioe.2019.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loucks J. Wearable Technology in Health Care: Getting Better All the Time. TMT Predictions 2022. Deloitte; New York, NY, USA: 2021. [Google Scholar]
- 4.Sabry F., Eltaras T., Labda W., Alzoubi K., Malluhi Q. Machine Learning for Healthcare Wearable Devices: The Big Picture. J. Healthc. Eng. 2022;2022:4653923. doi: 10.1155/2022/4653923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu M. Wearable Technology Applications in Healthcare: A Literature Review. Online J. Nurs. Inform. 2019;26:23. [Google Scholar]
- 6.Dunn J., Runge R., Snyder M. Wearables and the medical revolution. Per. Med. 2018;15:429–448. doi: 10.2217/pme-2018-0044. [DOI] [PubMed] [Google Scholar]
- 7.Lazazzera R., Belhaj Y., Carrault G. A New Wearable Device for Blood Pressure Estimation Using Photoplethysmogram. Sensors. 2019;19:2557. doi: 10.3390/s19112557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iqbal S.M.A., Mahgoub I., Du E., Leavitt M.A., Asghar W. Advances in healthcare wearable devices. NPJ Flex. Electron. 2021;5:9. doi: 10.1038/s41528-021-00107-x. [DOI] [Google Scholar]
- 9.Aceto G., Persico V., Pescapé A. Industry 4.0 and Health: Internet of Things, Big Data, and Cloud Computing for Healthcare 4.0. J. Ind. Inf. Integr. 2020;18:100129. doi: 10.1016/j.jii.2020.100129. [DOI] [Google Scholar]
- 10.Dias D., Paulo Silva Cunha J. Wearable Health Devices-Vital Sign Monitoring, Systems and Technologies. Sensors. 2018;18:2414. doi: 10.3390/s18082414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J., Look K.A. Relationship Between Objective Financial Burden and the Health-Related Quality of Life and Mental Health of Patients With Cancer. J. Oncol. Pract. 2018;14:e113–e121. doi: 10.1200/JOP.2017.027136. [DOI] [PubMed] [Google Scholar]
- 12.Chowthi-Williams A., Davis G. Successful Change Management in Health Care: Being Emotionally and Cognitively Ready. Routledge; London, UK: 2022. [Google Scholar]
- 13.Farahani B., Firouzi F., Chang V., Badaroglu M., Constant N., Mankodiya K. Towards fog-driven IoT eHealth: Promises and challenges of IoT in medicine and healthcare. Future Gener. Comput. Syst. 2018;78:659–676. doi: 10.1016/j.future.2017.04.036. [DOI] [Google Scholar]
- 14.Wang H., Naghavi M., Allen C., Barber R.M., Bhutta Z.A., Carter A., Casey D.C., Charlson F.J., Chen A.Z., Coates M.M., et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reilly C.M., Bruner D.W., Mitchell S.A., Minasian L.M., Basch E., Dueck A.C., Cella D., Reeve B.B. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21:1525–1550. doi: 10.1007/s00520-012-1688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A Low C., Dey A.K., Ferreira D., Kamarck T., Sun W., Bae S., Doryab A. Estimation of Symptom Severity During Chemotherapy From Passively Sensed Data: Exploratory Study. J. Med. Internet Res. 2017;19:e420. doi: 10.2196/jmir.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coughlin S.S., Caplan L.S., Stone R. Use of consumer wearable devices to promote physical activity among breast, prostate, and colorectal cancer survivors: A review of health intervention studies. J. Cancer Surviv. 2020;14:386–392. doi: 10.1007/s11764-020-00855-1. [DOI] [PubMed] [Google Scholar]
- 18.Herrington W.G., Goldsack J.C., Landray M.J. Increasing the use of mobile technology-derived endpoints in clinical trials. Clin. Trials. 2018;15:313–315. doi: 10.1177/1740774518755393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrom B., Watson C., Doll H., Coons S.J., Eremenco S., Ballinger R., McCarthy M., Crescioni M., O’Donohoe P., Howry C., et al. Selection of and Evidentiary Considerations for Wearable Devices and Their Measurements for Use in Regulatory Decision Making: Recommendations from the ePRO Consortium. Value Health. 2018;21:631–639. doi: 10.1016/j.jval.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Beauchamp U.L., Pappot H., Holländer-Mieritz C. The Use of Wearables in Clinical Trials During Cancer Treatment: Systematic Review. JMIR mHealth and uHealth. 2020;8:e22006. doi: 10.2196/22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballinger T.J., Althouse S.K., Olsen T.P., Miller K.D., Sledge J.S. A Personalized, Dynamic Physical Activity Intervention Is Feasible and Improves Energetic Capacity, Energy Expenditure, and Quality of Life in Breast Cancer Survivors. Front. Oncol. 2021;11:626180. doi: 10.3389/fonc.2021.626180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen N.H., Vallance J.K., Buman M.P., Moore M.M., Reeves M.M., Rosenberg D.E., Boyle T., Milton S., Friedenreich C.M., English D.R., et al. Effects of a wearable technology-based physical activity intervention on sleep quality in breast cancer survivors: The ACTIVATE Trial. J. Cancer Surviv. 2021;15:273–280. doi: 10.1007/s11764-020-00930-7. [DOI] [PubMed] [Google Scholar]
- 23.Ferrante J.M., Lulla A., Williamson J.D., Devine K.A., Ohman-Strickland P., Bandera E.V. Patterns of Fitbit Use and Activity Levels Among African American Breast Cancer Survivors During an eHealth Weight Loss Randomized Controlled Trial. Am. J. Health Promot. 2022;36:94–105. doi: 10.1177/08901171211036700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch B.M., Nguyen N.H., Moore M.M., Reeves M.M., Rosenberg D.E., Boyle T., Vallance J.K., Milton S., Friedenreich C.M., English D.R. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: The ACTIVATE Trial. Cancer. 2019;125:2846–2855. doi: 10.1002/cncr.32143. [DOI] [PubMed] [Google Scholar]
- 25.Pope Z.C., Zeng N., Zhang R., Lee H.Y., Gao Z. Effectiveness of Combined Smartwatch and Social Media Intervention on Breast Cancer Survivor Health Outcomes: A 10-Week Pilot Randomized Trial. J. Clin. Med. 2018;7:140. doi: 10.3390/jcm7060140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mcneil J., Brenner D.R., Stone C.R., O’Reilly R., Ruan Y., Vallance J.K., Courneya K.S., Thorpe K., Klein D.J., Friedenreich C.M. Activity Tracker to Prescribe Various Exercise Intensities in Breast Cancer Survivors. Med. Sci. Sports Exerc. 2019;51:930–940. doi: 10.1249/MSS.0000000000001890. [DOI] [PubMed] [Google Scholar]
- 27.Wu H.S., Gal R., Van Sleeuwen N.C., Brombacher A.C., Ijsselsteijn W.A., May A.M., Monninkhof E.M., Jerome G., Arnoldussen B., Lynch B. Breast Cancer Survivors’ Experiences With an Activity Tracker Integrated Into a Supervised Exercise Program: Qualitative Study. JMIR mHealth and uHealth. 2019;7:e10820. doi: 10.2196/10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Che Bakri N.A., Kwasnicki R.M., Dhillon K., Khan N., Ghandour O., Cairns A., Darzi A., Leff D.R. Objective Assessment of Postoperative Morbidity After Breast Cancer Treatments with Wearable Activity Monitors: The “BRACELET” Study. Ann. Surg. Oncol. 2021;28:5597–5609. doi: 10.1245/s10434-021-10458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi A., Samuel S.R., Kumar K.V., Saxena P.P., Mithra P. Effect of a Pedometer-based Exercise Program on Cancer Related Fatigue and Quality of Life amongst Patients with Breast Cancer Receiving Chemotherapy. Asian Pac. J. Cancer Prev. 2020;21:1813–1818. doi: 10.31557/APJCP.2020.21.6.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson S.H., Weiner L.S., Natarajan L., Parker B.A., Patterson R.E., Hartman S.J. Continuous, objective measurement of physical activity during chemotherapy for breast cancer: The Activity in Treatment pilot study. Transl. Behav. Med. 2020;10:1031–1038. doi: 10.1093/tbm/ibz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Champ C.E., Ohri N., Klement R.J., Cantor M., Beriwal S., Glaser S.M., Smith R.P. Assessing Changes in the Activity Levels of Breast Cancer Patients During Radiation Therapy. Clin. Breast Cancer. 2018;18:e1–e6. doi: 10.1016/j.clbc.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Helbrich H., Braun M., Hanusch C., Mueller G., Falk H., Flondor R., Harbeck N., Hermelink K., Wuerstlein R., Keim S., et al. Congruence and trajectories of device-measured and self-reported physical activity during therapy for early breast cancer. Breast Cancer Res. Treat. 2021;188:351–359. doi: 10.1007/s10549-021-06195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L., Wang L., Sun Q., Xiao P., Duan Y., Liu X., Zhou J., Xie J., Cheng A.S. Effect of Two Interventions on Sleep Quality for Adolescent and Young Adult Cancer Survivors: A Pilot Randomized Controlled Trial. Cancer Nurs. 2022;45:E560–E572. doi: 10.1097/NCC.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 34.Hasnain Z., Nilanon T., Li M., Mejia A., Kolatkar A., Nocera L., Shahabi C., Philips F.A.C., Lee J.S., Hanlon S.E., et al. Quantified Kinematics to Evaluate Patient Chemotherapy Risks in Clinic. JCO Clin. Cancer Inform. 2020;4:583–601. doi: 10.1200/CCI.20.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilanon T., Nocera L.P., Martin A.S., Kolatkar A., May M., Hasnain Z., Ueno N.T., Yennu S., Alexander A., Mejia A.E., et al. Use of Wearable Activity Tracker in Patients With Cancer Undergoing Chemotherapy: Toward Evaluating Risk of Unplanned Health Care Encounters. JCO Clin. Cancer Inform. 2020;4:839–853. doi: 10.1200/CCI.20.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yurkiewicz I.R., Simon P., Liedtke M., Dahl G., Dunn T. Effect of Fitbit and iPad Wearable Technology in Health-Related Quality of Life in Adolescent and Young Adult Cancer Patients. J. Adolesc. Young Adult Oncol. 2018;7:579–583. doi: 10.1089/jayao.2018.0022. [DOI] [PubMed] [Google Scholar]
- 37.Miyaji T., Kawaguchi T., Azuma K., Suzuki S., Sano Y., Akatsu M., Torii A., Kamimura T., Ozawa Y., Tsuchida A., et al. Patient-generated health data collection using a wearable activity tracker in cancer patients-a feasibility study. Support. Care Cancer. 2020;28:5953–5961. doi: 10.1007/s00520-020-05395-z. [DOI] [PubMed] [Google Scholar]
- 38.Komarzynski S., Huang Q., Lévi F.A., Palesh O.G., Ulusakarya A., Bouchahda M., Haydar M., Wreglesworth N.I., Morère J.-F., Adam R., et al. The day after: Correlates of patient-reported outcomes with actigraphy-assessed sleep in cancer patients at home (inCASA project) Sleep. 2019;42:zsz146. doi: 10.1093/sleep/zsz146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardcastle S.J., Maxwell-Smith C., Hince D., Bulsara M.K., Boyle T., Tan P., Levitt M., Salama P., Mohan G.R.K.A., Salfinger S., et al. The wearable activity technology and action-planning trial in cancer survivors: Physical activity maintenance post-intervention. J. Sci. Med. Sport. 2021;24:902–907. doi: 10.1016/j.jsams.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Devine K.A., Viola A., Levonyan-Radloff K., Mackowski N., Bozzini B., Chandler A., Xu B., Ohman-Strickland P., Mayans S., Farrar-Anton A., et al. Feasibility of FitSurvivor: A technology-enhanced group-based fitness intervention for adolescent and young adult survivors of childhood cancer. Pediatr. Blood Cancer. 2020;67:e28530. doi: 10.1002/pbc.28530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavic M., Klaas V., Theile G., Kraft J., Tröster G., Guckenberger M. Feasibility and Usability Aspects of Continuous Remote Monitoring of Health Status in Palliative Cancer Patients Using Wearables. Oncology. 2020;98:386–395. doi: 10.1159/000501433. [DOI] [PubMed] [Google Scholar]
- 42.Mendoza J.A., Baker K.S., Moreno M.A., Whitlock K., Abbey-Lambertz M., Waite A., Colburn T., Chow E.J. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: A pilot study. Pediatr. Blood Cancer. 2017;64:e26660. doi: 10.1002/pbc.26660. [DOI] [PubMed] [Google Scholar]
- 43.Cadmus-Bertram L., Tevaarwerk A.J., Sesto M.E., Gangnon R., Van Remortel B., Date P. Building a physical activity intervention into clinical care for breast and colorectal cancer survivors in Wisconsin: A randomized controlled pilot trial. J. Cancer Surviv. 2019;13:593–602. doi: 10.1007/s11764-019-00778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardcastle S.J., Jiménez-Castuera R., Maxwell-Smith C., Bulsara M.K., Hince D. Fitbit wear-time and patterns of activity in cancer survivors throughout a physical activity intervention and follow-up: Exploratory analysis from a randomised controlled trial. PLoS ONE. 2020;15:e0240967. doi: 10.1371/journal.pone.0240967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maxwell-Smith C., Hince D., Cohen P., Bulsara M.K., Boyle T., Platell C., Tan P., Levitt M., Salama P., Tan J., et al. A randomized controlled trial of WATAAP to promote physical activity in colorectal and endometrial cancer survivors. Psychooncology. 2019;28:1420–1429. doi: 10.1002/pon.5090. [DOI] [PubMed] [Google Scholar]
- 46.Van Blarigan E.L., Chan H., Van Loon K., Kenfield S.A., Chan J.M., Mitchell E., Zhang L., Paciorek A., Joseph G., Laffan A., et al. Self-monitoring and reminder text messages to increase physical activity in colorectal cancer survivors (Smart Pace): A pilot randomized controlled trial. BMC Cancer. 2019;19:218. doi: 10.1186/s12885-019-5427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheong I.Y., An S.Y., Cha W.C., Rha M.Y., Kim S.T., Chang D.K., Hwang J.H. Efficacy of Mobile Health Care Application and Wearable Device in Improvement of Physical Performance in Colorectal Cancer Patients Undergoing Chemotherapy. Clin. Colorectal Cancer. 2018;17:e353–e362. doi: 10.1016/j.clcc.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Van Blarigan E.L., Dhruva A., Atreya C.E., Kenfield S.A., Chan J.M., Milloy A., Kim I., Steiding P., Laffan A., Zhang L., et al. Feasibility and Acceptability of a Physical Activity Tracker and Text Messages to Promote Physical Activity During Chemotherapy for Colorectal Cancer: Pilot Randomized Controlled Trial (Smart Pace II) JMIR Cancer. 2022;8:e31576. doi: 10.2196/31576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shih C.A.-O., Chou P.A.-O., Chou T.A.-O., Huang T.A.-O. Measurement of Cancer-Related Fatigue Based on Heart Rate Variability: Observational Study. J. Med. Internet Res. 2021;23:e25791. doi: 10.2196/25791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bade B.C., Brooks M.C., Nietert S.B., Ulmer A., Thomas D.D., Nietert P.J., Scott J.B., Silvestri G.A. Assessing the Correlation Between Physical Activity and Quality of Life in Advanced Lung Cancer. Integr. Cancer Ther. 2018;17:73–79. doi: 10.1177/1534735416684016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finley D.J., Stevens C.J., Emond J.A., Batsis J.A., Fay K.A., Darabos C., Sacks O.A., Cook S.B., Lyons K.D. Potential effectiveness of a surgeon-delivered exercise prescription and an activity tracker on pre-operative exercise adherence and aerobic capacity of lung cancer patients. Surg. Oncol. 2021;37:101525. doi: 10.1016/j.suronc.2021.101525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kong S., Park H.Y., Kang D., Lee J.K., Lee G., Kwon O.J., Shim Y.M., Zo J.I., Cho J. Seasonal Variation in Physical Activity among Preoperative Patients with Lung Cancer Determined Using a Wearable Device. J. Clin. Med. 2020;9:349. doi: 10.3390/jcm9020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghods A., Shahrokni A., Ghasemzadeh H., Cook D. Remote Monitoring of the Performance Status and Burden of Symptoms of Patients With Gastrointestinal Cancer Via a Consumer-Based Activity Tracker: Quantitative Cohort Study. JMIR Cancer. 2021;7:e22931. doi: 10.2196/22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu J.-M., Ho T.-W., Chang Y.-T., Hsu C., Tsai C.J., Lai F., Lin M.-T. Wearable-Based Mobile Health App in Gastric Cancer Patients for Postoperative Physical Activity Monitoring: Focus Group Study. JMIR mHealth and uHealth. 2019;7:e11989. doi: 10.2196/11989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim Y., Seo J., An S.Y., Sinn D.H., Hwang J.H. Efficacy and Safety of an mHealth App and Wearable Device in Physical Performance for Patients With Hepatocellular Carcinoma: Development and Usability Study. JMIR mHealth and uHealth. 2020;8:e14435. doi: 10.2196/14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang C.-F.J., Aibel K., Meyerhoff R., Wang F., Harpole D., Abernethy A.P., Leblanc T.W. Actigraphy assessment of sleep quality among patients with acute myeloid leukaemia during induction chemotherapy. BMJ Supportive Palliat. Care. 2018;8:274–277. doi: 10.1136/bmjspcare-2018-001509. [DOI] [PubMed] [Google Scholar]
- 57.Sugiyama Y., Naiki T., Tasaki Y., Kataoka T., Mimura Y., Kondo Y., Etani T., Iida K., Nozaki S., Ando R., et al. Effectiveness of continuous monitoring by activity tracker of patients undergoing chemotherapy for urothelial carcinoma. Cancer Treat. Res. Commun. 2020;25:100245. doi: 10.1016/j.ctarc.2020.100245. [DOI] [PubMed] [Google Scholar]
- 58.Robinson J.K., Durst D.A., Gray E., Kwasny M., Heo S.Y., Banks A., Rogers J.A. Sun exposure reduction by melanoma survivors with wearable sensor providing real-time UV exposure and daily text messages with structured goal setting. Arch. Dermatol. Res. 2021;313:685–694. doi: 10.1007/s00403-020-02163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lancaster K., Abuzour A., Khaira M., Mathers A., Chan A., Bui V., Lok A., Thabane L., Dolovich L. The Use and Effects of Electronic Health Tools for Patient Self-Monitoring and Reporting of Outcomes Following Medication Use: Systematic Review. J. Med. Internet Res. 2018;20:e294. doi: 10.2196/jmir.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bakker J.P., Goldsack J.C., Clarke M., Coravos A., Geoghegan C., Godfrey A., Heasley M.G., Karlin D., Manta C., Peterson B., et al. A systematic review of feasibility studies promoting the use of mobile technologies in clinical research. NPJ Digit. Med. 2019;2:47. doi: 10.1038/s41746-019-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gresham G., Schrack J., Gresham L.M., Shinde A.M., Hendifar A.E., Tuli R., Rimel B., Figlin R., Meinert C.L., Piantadosi S. Wearable activity monitors in oncology trials: Current use of an emerging technology. Contemp. Clin. Trials. 2018;64:13–21. doi: 10.1016/j.cct.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Kadhim K.T., Alsahlany A.M., Wadi S.M., Kadhum H.T. An Overview of Patient’s Health Status Monitoring System Based on Internet of Things (IoT) Wirel. Pers. Commun. 2020;114:2235–2262. doi: 10.1007/s11277-020-07474-0. [DOI] [Google Scholar]