Abstract
Simple Summary
This review summarizes the evidence from clinical trials and recent preclinical studies regarding the evaluation and optimization of dendritic cells (DCs)-based vaccines as either mono- or combination immunotherapy with current anticancer therapies and/or various immune effector cells for treating hepatocellular carcinoma (HCC).
Abstract
Although many surgical and nonsurgical therapeutic options have been well-established, hepatocellular carcinoma (HCC) remains the third most common cause of cancer-related death worldwide. Therefore, the discovery of novel potential therapeutic strategies is still urgently required for improving survival and prognosis of HCC patients. As the most potent antigen-presenting cells in the human immune system, dendritic cells (DCs) play an important role in activating not only innate but also adaptive immune responses to specifically destroy tumor cells. As a result, DC-based vaccines, which are prepared by different tumor-antigen-pulsing strategies or maturation-stimulating reagents, either alone or in combination with various anticancer therapies and/or immune effector cells, have been developed as a promising personalized cancer immunotherapy. This review provides a comprehensive summary of the evidence from clinical trials evaluating the safety, feasibility, and efficacy of DC-based vaccines in treating HCC patients and highlights the data from recent preclinical studies regarding the development of promising strategies for optimizing the efficacy of DC-vaccine-based immunotherapy for HCC.
Keywords: hepatocellular carcinoma, dendritic-cell vaccine, immunotherapy, clinical trials, preclinical studies
1. Introduction
Hepatocellular carcinoma (HCC) accounts for up to 90% of primary liver cancers and ranks as the sixth most prevalent human cancer worldwide [1,2]. Many surgical, locoregional, and systemic therapeutic options have been well-established for treating HCC, such as liver transplantation, surgical resection, radiofrequency ablation (RFA), percutaneous microwave ablation (PMWA), percutaneous ethanol injection (PEI), transarterial embolization/chemoembolization (TAE/TACE), external beam radiation therapy (EBRT), chemotherapy, and molecular-targeted therapy [3,4,5]. Moreover, both sorafenib and lenvatinib have been approved by the US Food and Drug Administration (FDA) as molecular-targeted drugs for the first-line treatment of advanced primary HCC [6,7]. However, the overall prognosis of HCC patients remains dismal, rendering HCC as the third leading cause of cancer-related death worldwide and resulting in over 800,000 deaths every year [8,9]. Therefore, the development of new and effective therapeutic strategies for HCC is an important goal to improve patient survival.
Dendritic cells (DCs) are the most potent antigen-presenting cells in the human immune system and play a crucial role in not only the activation of innate immunity but also the induction of cytotoxic T lymphocyte (CTL)-mediated adaptive immunity [10,11,12]. In their immature state, DCs circulate broadly in the blood and peripheral tissues, where they sample antigens that are derived from pathogen-infected or tumor cells. After the uptake of presentable antigens, DCs undergo a process of phenotypical and functional maturation and migrate to the secondary lymphoid tissues, such as lymph nodes, where they present processed antigens to and activate CTLs, which thereby trigger an antigen-specific immune response to eliminate antigen-expressing target cells. Additionally, mature DCs are also capable of enhancing the cytotoxic activity of nature killer cells (NKs), which function as innate immune effector cells to destroy pathogen-infected or tumor cells [13,14]. Based on these characteristics, DC-based vaccines have emerged as a promising immunotherapy for treating many types of cancer, including HCC [15,16,17].
In this review, we summarize the available results from clinical trials that have been conducted so far to evaluate the safety, feasibility, and efficacy of DC-based vaccines, either alone or in combination with various anticancer therapies and/or immune effector cells, in treating HCC patients (Table 1 and Table 2). Moreover, we highlight the promising data from recent in vitro and in vivo preclinical studies since 2019 regarding DC-vaccine-based immunotherapy for HCC (Table 3).
2. Monotherapy of Whole-Tumor-Cell-Lysate-Pulsed or Specific-Tumor-Antigen-Pulsed DC Vaccines for Treating HCC Patients
Four major strategies have been applied to pulse DCs with tumor antigens in vitro for the preparation of antitumor DC vaccines: the first one involves the co-culture of DCs with frozen–thawed total lysates of autologous tumors or allogeneic tumor cell lines [18,19]; the second involves the co-culture of DCs with synthetic peptides or recombinant proteins of known tumor antigens [20,21]; the third one involves the transfection of DCs with plasmid DNA or viral vector DNA or messenger RNA (mRNA) that contains the genes encoding known tumor antigens [22,23]; and fourth one involves the fusion of DCs with entire tumor cells through the mediation of polyethylene glycol, a widely used fusion agent of lipid membranes [24]. Except for the fourth strategy, which is still under preclinical evaluation (mentioned later in Section 4), the clinical results of monotherapy based on DC vaccines which are prepared by the other three antigen-pulsing strategies for HCC patients have been evaluated in several completed and ongoing clinical trials (Table 1).
Table 1.
Summary of clinical trials regarding DC-vaccine-based mono-immunotherapy for treating HCC patients.
| Treatment | Disease Stage Targeted | Clinical Trial Identifier | Start Year | Patient Number | Phase | Status | Clinical Results | Publication |
|---|---|---|---|---|---|---|---|---|
| Autologous-HCC-tumor-lysate-pulsed mature-DC vaccine | Unresectable primary HCC | NA | 2000 | 8 | I | Completed |
|
Iwashita et al. [25] |
| Autologous-HCC-tumor-lysate-pulsed mature-DC vaccine | Advanced primary HCC, AJCC TNM (5th edition) stage IVA and IVB | NA | 2000 | 31 | NA | Completed |
|
Lee et al. [26] |
| Autologous-HCC-tumor-lysate-pulsed mature-DC vaccine | Primary HCC | NCT00327496 | 2006 | 10 | NA | Completed | NA | NA |
| HepG2-HCC-cell-lysate-pulsed mature-DC vaccine | Advanced primary HCC | NA | NA | 35 | II | Completed |
|
Palmer et al. [27] |
| HepG2-HCC-cell-lysate-pulsed mature-DC vaccine | Advanced primary HCC, Child–Pugh class B or C | NA | 2009 | 30 | NA | Completed |
|
EI Ansary et al. [28] |
| Mature-DC vaccine co-pulsed with four AFP peptides: AFP137–145, AFP158–166, AFP325–334, and AFP542–550 | Primary HCC, AJCC TNM (5th edition) stage IIIA to IVB, Child–Pugh class A or B, class I MHC molecule HLA-A*0201 positive, AFP positive | NCT00022334 | 2003 | 10 | I/II | Completed |
|
Butterfield et al. [29] |
| HSP70-mRNA-transfected mature-DC vaccine | Unresectable HCV-related primary or recurrent HCC | NA | 2007 | 12 | I | Completed |
|
Maeda et al. [30] |
| Mature-DC vaccine co-pulsed with AFP, MAGE-1, and GPC-3 proteins | Refractory primary or recurrent HCC | JPRN-UMIN000011854 | 2013 | 5 | I | Completed | NA | NA |
| Personalized HCC-tumor-antigen-pulsed mature-DC vaccine | Primary HCC, BCLC stage B or C, Child–Pugh class A or B | ChiCTR1900021177 | 2018 | 30 | NA | Ongoing | NA | NA |
Abbreviations: DC, dendritic cell; HCC, hepatocellular carcinoma; NA, not available; SD, stable disease; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist II; AJCC, American Joint Committee on Cancer; TNM, tumor–node–metastasis; PR, partial response; OS, overall survival; MHC, major histocompatibility complex; HLA, human leukocyte antigen; IFN-γ, interferon-gamma; CTL, cytotoxic T lymphocyte; HSP70, heat-shock protein 70; CR, complete response; mRNA, messenger RNA; HCV, hepatitis C virus; MAGE-1, melanoma-associated antigen 1; GPC-3, glypican-3; BCLC, Barcelona Clinic Liver Cancer.
2.1. Autologous Tumor-Lysate-Pulsed DC Vaccines
A completed phase I clinical trial conducted by Iwashita et al. evaluated the safety and feasibility of a mature-DC vaccine, which was pulsed with total lysate prepared from autologous HCC tumors of patients, in treating eight patients with unresectable primary HCC [25]. All the patients were vaccinated with 1 × 106 to 1 × 107 of DCs intranodally four times at 1-week intervals. After the initial four vaccinations, half of the patients were further vaccinated at monthly intervals for up to 12 vaccinations. The results showed that DC vaccination was safe and well tolerated in all patients. Among all patients, half exhibited stable disease (SD), one had decreased tumor size (from 13 mm to 7 mm in diameter), and another had lowered serum levels of HCC tumor markers such as alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist II (PIVKA-II) after vaccination. Another completed clinical trial conducted by Lee et al. further evaluated the safety and feasibility of an autologous-HCC-tumor-lysate-pulsed mature-DC vaccine in treating 31 patients with advanced primary HCC [26]. All the patients received an initial five vaccinations of DCs with a median of 2.5 × 106 cells each time intravenously at 1-week intervals. Seventeen of the patients further received boost vaccinations at monthly intervals for 2 to 12 months. The results showed that DC vaccination was safe and well tolerated in all patients. Among all patients, 4 (12.9%) displayed a partial response (PR), and 17 (54.8%) achieved SD; the 1-year overall survival (OS) rate was 40.1%. The patients who received initial and boost vaccinations had a significantly better 1-year OS rate than the patients who received initial vaccination alone (63.3% versus 10.7%, p-value < 0.001). Another completed clinical trial (NCT00327496) evaluated the efficacy of an autologous HCC-tumor-lysate-pulsed mature-DC vaccine in stimulation of CTL-mediated cytotoxicity against primarily cultured HCC cells; however, the results are not available. Collectively, these clinical trials support that autologous-tumor-lysate-pulsed DC vaccines are safe and feasible as monotherapy for treating HCC patients who are not eligible for current therapeutic methods; however, the efficacy needs to be evaluated in further studies.
2.2. Allogeneic-Tumor-Cell-Line-Lysate-Pulsed DC Vaccines
A completed phase II clinical trial conducted by Palmer et al. evaluated the safety and efficacy of a mature-DC vaccine, which was pulsed with total cell lysate prepared from human HCC cell line HepG2, in treating 35 patients with advanced primary HCC [27]. All the patients received a maximum of six DC vaccinations intravenously at 3-week intervals. The results showed that DC vaccination was safe and well tolerated in all patients. Among 25 patients who received at least three vaccinations, 1 (4%) developed a PR, and 6 (24%) achieved SD. Among 17 patients who had a baseline serum level of AFP ≥ 1000 ng/mL, 4 (23.5%) had decreased AFP levels (<30% of baseline) after vaccination. The 6-month and 1-year OS rates were 33% and 11%, respectively. Another completed clinical trial conducted by EI Ansary et al. also evaluated the safety and efficacy of a HepG2-cell-lysate-pulsed mature-DC vaccine in treating patients with advanced primary HCC [28]. A total of 30 patients were divided into two groups with no difference in baseline characteristics: in group I, 15 patients received intradermal DC vaccination with a total of 2 × 107 cells; and in group II, 15 patients received supportive treatment as a control group. The results showed that DC vaccination was safe and well tolerated in all patients. After a 6-month follow-up period, in group I of patients, two (13.3%) achieved a PR, and nine (60%) had SD; in contrast, in group II, none achieved a PR, and only two (13.3%) had SD. The group I of patients exhibited a significantly longer median OS time than the group II of patients (7 months versus 4 months, p-value = 0.008). Taken together, these clinical trials indicate that monotherapy based on allogeneic-tumor-cell-line-lysate-pulsed DC vaccines is a safe and effective therapeutic option for HCC patients who cannot be treated with current treatment approaches; however, the efficacy still needs to be further improved.
2.3. Specific-Tumor-Antigen-Pulsed DC Vaccines
A completed phase I/II clinical trial (NCT00022334) conducted by Butterfield et al. evaluated the safety and feasibility of a mature-DC vaccine, which was co-pulsed with four immunodominant AFP-derived peptides (AFP137–145, AFP158–166, AFP325–334, and AFP542–550), in treating 10 patients with primary HCC who were positive for class I major histocompatibility complex (MHC) molecule human leukocyte antigen (HLA)-A*0201 and AFP [29]. All the patients were vaccinated with 1 × 106 to 1 × 107 of DCs intradermally three times at 2-week intervals. The results showed that DC vaccination was safe and well tolerated in all patients. Six of the patients (60%) exhibited increased interferon-gamma (IFN-γ)-producing AFP-specific CTL responses after vaccination. Another completed phase I clinical trial conducted by Maeda et al. evaluated the safety and feasibility of a mature-DC vaccine, which was transfected with mRNA of the HCC tumor marker heat-shock protein 70 (HSP70), in treating 12 patients with unresectable hepatitis C virus (HCV)-related primary or recurrent HCC [30]. All the patients were vaccinated with 1 × 107 to 3 × 107 of DCs intradermally three times at 3-week intervals. The results showed that DC vaccination was safe and well tolerated in all patients. Among all patients, two (16.7%) achieved a complete response (CR), and five (41.7%) had SD. Tumor specimens were obtained from one patient and revealed increased infiltration of granzyme B-expressing CTLs after vaccination. Another completed phase I clinical trial (JPRN-UMIN000011854) evaluated the safety and feasibility of a mature-DC vaccine, which was co-pulsed with the recombinant proteins of three HCC tumor markers, namely AFP, melanoma-associated antigen 1 (MAGE-1), and glypican-3 (GPC-3), in treating patients with refractory primary or recurrent HCC. Another ongoing clinical trial (ChiCTR1900021177) was aimed at evaluating the clinical results of a personalized tumor-antigen-pulsed DC vaccine in treating patients with primary HCC. However, there are no available results from the two trials mentioned. Overall, these clinical trials suggest that specific-tumor-antigen-pulsed DC vaccines as monotherapy is safe and feasible in treating HCC patients who are not appropriate candidates for current therapeutic approaches; however, further studies are still needed to evaluate and optimize the efficacy.
3. Combination Therapy of DC-Based Vaccines and Anticancer Therapies or Immune Effector Cells for Treating HCC Patients
Although many surgical and nonsurgical anticancer therapies have been developed for treating HCC, the therapeutic benefits of each therapy alone on the prognosis and survival of patients remain limited due to either tumor recurrence or drug resistance [3,4,5]. Additionally, tumor cells evolve multiple mechanisms to escape immune attack, such as the expression of immune checkpoint molecules to suppress the antitumor activity of CTLs [31,32]. As a result, monoclonal antibodies, which block the interaction between immune checkpoint molecules, also known as immune checkpoint inhibitors (ICIs), have emerged as potential immunotherapeutic agents for cancer [33,34]. Especially, nivolumab, an ICI that targets programmed death 1 (PD-1), has been approved by the US FDA as a second-line treatment for advanced primary HCC [35]. However, similar to patients with other solid tumors, HCC patients display a low response rate to ICI monotherapy [36,37]. Therefore, the combination of DC vaccines and various anticancer therapies has been derived, and the clinical results of this combination therapy for HCC patients have been evaluated in many completed and ongoing clinical trials (Table 2). Moreover, based on the capability of DCs to activate and enhance the tumor-killing activity of CTLs and NKs [38], the combination of DC vaccines and such immune effector cells or the infusion of DC-activated immune effector cells has been developed and evaluated in treating HCC patients in several clinical trials (Table 2).
Table 2.
Summary of clinical trials regarding DC-vaccine-based combination immunotherapy for treating HCC patients.
| Treatment | Disease Stage Targeted | Clinical Trial Identifier | Start Year | Patient Number | Phase | Status | Clinical Results | Publication |
|---|---|---|---|---|---|---|---|---|
| Combination of DC Vaccines and Anticancer Therapies | ||||||||
| Immature-DC vaccine combined with EBRT | Advanced primary HCC | NA | 2001 | 12 | I | Completed |
|
Chi et al. [39] |
| Mature-DC vaccine combined with EBRT | Unresectable primary HCC, AJCC TNM (8th edition) stage IIIA to IVB, Child–Pugh class A | NCT03942328 | 2019 | 26 | Early I | Ongoing | NA | NA |
| Mature-DC vaccine combined with RFA | HCV-related primary HCC | JPRN-C000000451 | 2006 | 5 | NA | Completed | NA | NA |
| OK432-stimulated mature-DC vaccine combined with RFA | HCV-related primary HCC, Child–Pugh class A or B | JPRN-UMIN000001701 | 2009 | 30 | I/II | Completed |
|
Kitahara et al. [40] |
| Mature-DC vaccine combined with TAE | Primary HCC, Child–Pugh class A or B | JPRN-UMIN000012702 | 2013 | 3 | NA | Completed | NA | NA |
| Mature-DC vaccine combined with TAE and RFA | Unresectable primary HCC, Child–Pugh class A or B | JPRN-UMIN000036065 | 2019 | 3 | I | Completed | NA | NA |
| Mature-DC vaccine combined with TAE and RFA | Unresectable primary HCC, Child–Pugh class A or B | JPRN-jRCTc050200107 | 2021 | 30 | I/II | Ongoing | NA | NA |
| Mature-DC vaccine (ilixadencel) co-activated with TLR3 and 7/8 agonists and IFN-γ and given in combination with molecular-targeted drug sorafenib | Advanced primary HCC, BCLC stage B or C, Child–Pugh class A | NCT01974661 | 2013 | 17 | I | Completed |
|
Rizell et al. [41] |
| Autologous-irradiated-HCC-tumor-stem-cell-pulsed mature-DC vaccine combined with surgical resection and TACE | Unresectable HBV-related primary HCC, BCLC stage A or C, Child–Pugh class A | NA | 2013 | 8 | I | Completed |
|
Wang et al. [42] |
| HepG2-HCC-cell-lysate-pulsed mature-DC vaccine combined with TACE | Primary HCC, Child–Pugh class A or B | ISRCTN11889464 | 2014 | 48 | II | Completed | NA | NA |
| HepG2-HCC-cell-lysate-pulsed mature-DC vaccine combined with TACE | HCV-related primary HCC, BCLC stage B or D, Child–Pugh class A or B or C | DRKS00016606 | 2015 | 20 | II | Completed |
|
Abdel Ghafar et al. [43] |
| Mature-DC vaccine co-pulsed with HBV-specific antigen peptides and HepG2 HCC cell lysate and given in combination with TACE | Unresectable HBV-related primary HCC, BCLC stage B or C, Child–Pugh class A or B | NCT03086564 | 2017 | 70 | I/II | Completed | NA | NA |
| Peptides-pulsed mature-DC vaccine combined with RFA | Primary HCC, HLA-A24 positive | JPRN-UMIN000020811 | 2016 | 10 | NA | Completed | NA | NA |
| Peptides-pulsed mature-DC vaccine combined with RFA | Primary HCC, Child–Pugh class A or B | JPRN-jRCTc040190093 | 2020 | 6 | I | Ongoing | NA | NA |
| HSP70-mRNA-transfected mature-DC vaccine combined with surgical resection | Resectable primary HCC, LCSGJ (5th edition) stage II to IVA | JPRN-UMIN000010691 | 2012 | 45 | I/II | Completed |
|
Matsui et al. [44] |
| Mature-DC vaccine co-pulsed with AFP, MAGE-1, and GPC-3 proteins and given in combination with TACE | Primary HCC, AJCC TNM (6th edition) stage II to IIIC, Child–Pugh class A or B | NA | 2009 | 5 | I/II | Completed |
|
Tada et al. [45] |
| Mature-DC vaccine co-pulsed with AFP, MAGE-1, and GPC-3 proteins and given in combination with TACE | Unresectable primary HCC, Child–Pugh class A | KCT0000986 | 2013 | 40 | II | Ongoing | NA | NA |
| Mature-DC vaccine co-pulsed with AFP, MAGE-1, and GPC-3 proteins and given in combination with surgical resection | Primary HCC | JPRN-UMIN000021545 | 2016 | 50 | II | Completed | NA | NA |
| Mature-DC vaccine co-pulsed with AFP, MAGE-1, and GPC-3 proteins and given in combination with surgical resection, RFA, PEI, or TACE | Primary HCC, AJCC TNM (6th edition) stage I to IIIC, Child–Pugh class A or B | KCT0000427 | 2009 | 12 | I/IIa | Completed |
|
Lee et al. [46] |
| Mature-DC vaccine co-pulsed with AFP, MAGE-1, and GPC-3 proteins and given in combination with surgical resection, RFA, PEI, or TACE | Primary HCC, AJCC TNM (6th edition) stage I to IIIC, Child–Pugh class A or B | KCT0000008 | 2010 | 156 | II | Completed |
|
Lee et al. [47] |
| HCC-tumor-neoantigen-pulsed mature-DC vaccine combined with PMWA | Primary HCC, HKLC stage IIa, Child–Pugh class A or B | NCT03674073 | 2018 | 24 | I | Ongoing | NA | NA |
| HCC-tumor-neoantigen-pulsed mature-DC vaccine combined with ICI nivolumab and surgical resection | Resectable primary or recurrent HCC, Child–Pugh class A | NCT04912765 | 2021 | 60 | II | Ongoing | NA | NA |
| Multiple-HCC-tumor-antigens-pulsed mature-DC vaccine combined with surgical resection, TACE, or molecular-targeted drug sorafenib or lenvatinib | HBV-related primary HCC, Child–Pugh class A or B | NCT04317248 | 2020 | 600 | II | Ongoing | NA | NA |
| Combination of DC vaccines and immune effector cells and anticancer therapies | ||||||||
| Autologous-HCC-tumor-lysate-pulsed mature-DC vaccine with CATs and combined with surgical resection | Primary HCC | NA | 2000 | 94 | II | Completed |
|
Shimizu et al. [48] |
| Autologous-HCC-tumor-lysate-pulsed mature-DC vaccine with immature DCs, CIKs, mature-DC-precision CTLs, and mature DC-CIKs combined with PMWA | HBV-related primary HCC, Child–Pugh class A or B | NA | NA | 10 | I | Completed |
|
Zhou et al. [49] |
| Mature DC-CIKs combined with surgical resection or TACE | Primary HCC | NCT01821482 | 2013 | 100 | II | Ongoing | NA | NA |
| Mature-DC vaccine with CIKs and combined with molecular-targeted drug sorafenib | Advanced primary HCC, BCLC stage B or C, Child–Pugh class A or B | NA | 2015 | 71 | NA | Completed |
|
Zhou et al. [50] |
| Mature-DC-precision multiple-antigen CTLs combined with surgical resection | Primary HCC, Child–Pugh class A or B | NCT02632188 | 2015 | 60 | I/II | Ongoing | NA | NA |
| Mature-DC-precision multiple-antigen CTLs combined with TACE | Unresectable primary or recurrent HCC, Child–Pugh class A or B | NCT02638857 | 2015 | 60 | I/II | Ongoing | NA | NA |
| Personalized HCC-tumor-neoantigen-pulsed mature-DC vaccine with mature-DC-precision neoantigen CTLs and combined with surgical resection or RFA | Primary HCC, Child–Pugh class A or B | NCT03067493 | 2017 | 10 | II | Completed |
|
Peng et al. [51] |
Abbreviations: DC, dendritic cell; HCC, hepatocellular carcinoma; EBRT, external beam radiation therapy; NA, not available; PR, partial response; AFP, alpha-fetoprotein; AJCC, American Joint Committee on Cancer; TNM, tumor–node–metastasis; RFA, radiofrequency ablation; HCV, hepatitis C virus; TAE, transarterial embolization; TLR, Toll-like receptor; IFN-γ, interferon-gamma; BCLC, Barcelona Clinic Liver Cancer; SD, stable disease; TTP, time to progression; OS, overall survival; CTL, cytotoxic T lymphocyte; TACE, transarterial chemoembolization; HBV, hepatitis B virus; HLA, human leukocyte antigen; HSP70, heat-shock protein 70; LCSGJ, Liver Cancer Study Group of Japan; DFS, disease-free survival; MAGE-1, melanoma-associated antigen 1; GPC-3, glypican-3; PEI, percutaneous ethanol injection; PMWA, percutaneous microwave ablation; HKLC, Hong Kong Liver Cancer; ICI, immune checkpoint inhibitor; CAT, CD3-activated T cell; CIK, cytokine-induced killer cell; DC-CIK, dendritic-cell-activated cytokine-induced killer cell; CR, complete response.
3.1. Non-Antigen-Pulsed DC Vaccines Combined with Anticancer Therapies
A completed phase I clinical trial conducted by Chi et al. evaluated the safety and feasibility of an immature-DC vaccine combined with EBRT, a type of radiotherapy that deliveries radiation beams from outside the body toward tumors inside the body, in treating 12 patients with advanced primary HCC [39]. All the patients were vaccinated with 5 × 106 to 5 × 107 of DCs intratumorally two times at 3-week intervals 2 days after EBRT. The results showed that DC vaccination was safe and well tolerated in all patients. Among all patients, two (16.7%) achieved a PR, and three (25%) had decreased serum levels of AFP (<50% of baseline) after vaccination. Another ongoing early phase I clinical trial (NCT03942328) was aimed at evaluating the clinical results of a mature-DC vaccine combined with EBRT in treating patients with unresectable primary HCC. Another completed clinical trial (JPRN-C000000451) evaluated the clinical results of a mature-DC vaccine combined with RFA in treating patients with HCV-related primary HCC. However, there are no available results from the two trials mentioned. Another completed phase I/II clinical trial (JPRN-UMIN000001701) conducted by Kitahara et al. further evaluated the safety and efficacy of a mature-DC vaccine, which was stimulated with the Streptococcus-derived anticancer immunotherapeutic agent OK432 as a maturation reagent, combined with RFA in treating patients with HCV-related primary HCC [40]. A total of 30 patients were divided into two groups, with no difference in baseline characteristics: in group I, 16 patients were vaccinated with 5 × 106 of OK432-stimulated DCs percutaneously after RFA; and in group II, 14 patients w were vaccinated with 5 × 106 of non-OK432-stimulated DCs percutaneously after RFA as a control group. The results showed that DC vaccination was safe and well tolerated in all patients. Although there was no difference in OS between the two groups of patients, the median RFS time was significantly longer in group I than in group II (24.8 months versus 13.0 months, p-value = 0.003). Another completed clinical trial (JPRN-UMIN000012702) evaluated the clinical results of a mature-DC vaccine combined with TAE in treating patients with primary HCC. In another completed phase I (JPRN-UMIN000036065) trial and an ongoing phase I/II (JPRN-jRCTc050200107) clinical trial, the clinical results of a mature-DC vaccine combined with TAE and RFA in treating patients with unresectable primary HCC were evaluated. However, there are no available results from the three trials mentioned. Another completed phase I clinical trial (NCT01974661) conducted by Rizell et al. evaluated the safety and feasibility of a mature-DC vaccine (also known as ilixadencel) co-activated with Toll-like receptor (TLR) 3 and 7/8 agonists and IFN-γ, either alone or combined with the molecular-targeted drug sorafenib, in treating 17 patients with advanced primary HCC [41]. All the patients received three intratumoral DC vaccinations at 2-to-5-week intervals with a dose of 1 × 107 cells alone (six patients; group I) or combined with sorafenib (six patients; group II) or with a dose of 2 × 107 cells alone (five patients; group III). The results showed that DC vaccination was safe and well tolerated in all patients. Among all patients, one (5.9%; from group III) exhibited a PR, and five (29.4%; three from group I, one from group II, and one from group III) had SD; the median time to progression (TTP) was 5.5 months, and the median OS time was 7.5 months. A total of 11 of the 15 evaluable patients (73.3%; 9 of 11 evaluable patients from group I and group III and 2 of 4 evaluable patients from group II) displayed an elevated frequency of tumor-specific IFN-γ-producing CTLs in peripheral blood after vaccination. Collectively, these clinical trials indicate that non-antigen-pulsed DC vaccines are safe and feasible as adjuvant therapy for treating HCC patients who receive standard anticancer therapies; however, the efficacy needs to be further evaluated.
3.2. Autologous-Tumor-Lysate-Pulsed, Allogeneic-Tumor-Cell-Line-Lysate-Pulsed, or Specific-Tumor-Antigen-Pulsed DC Vaccines Combined with Anticancer Therapies
A completed phase I clinical trial conducted by Wang et al. evaluated the safety of an autologous-irradiated-HCC-tumor-stem-cell-pulsed mature-DC vaccine combined with surgical resection and TACE in treating eight patients with unresectable hepatitis B virus (HBV)-related primary HCC [42]. All the patients were vaccinated with 3 × 106 to 2 × 107 of DCs subcutaneously three times at 1-week intervals 6 weeks after TACE following surgical resection. The results showed that DC vaccination was safe and well tolerated in all patients. There was no increase in the serum levels of hepatic transaminases, hepatitis B antigens, and viral DNA after vaccination. Another completed phase II clinical trial (ISRCTN11889464) evaluated the clinical results of a HepG2-cell-lysate-pulsed mature-DC vaccine combined with TACE in treating patients with primary HCC, though the results are not available. Another completed phase II clinical trial (DRKS00016606) conducted by Abdel Ghafar et al. further evaluated the safety and efficacy of a HepG2-cell-lysate-pulsed mature-DC vaccine, either alone or combined with TACE, in treating patients with primary HCC [43]. A total of 20 patients were divided into four groups with no difference in baseline characteristics: in group I, 5 Barcelona Clinic Liver Cancer (BCLC) stage B patients received four intradermal DC vaccinations with a dose of 5 × 107 cells at 2-week intervals after TACE; in group II, 5 BCLC stage B patients received TACE alone as a control group for group I; in group III, 5 BCLC stage D patients received four intradermal DC vaccinations with a dose of 5 × 107 cells at 2-week intervals; and in group IV, 5 BCLC stage D patients received supportive treatment as a control group for group III. The results showed that DC vaccination was safe and well tolerated in all patients. In group I of patients, three (60%) developed a PR, and one (20%) achieved SD; however, no difference was observed in the clinical response between group I and II of patients. In group III of patients, one (20%) developed a PR, and two (40%) achieved SD; in contrast, in group IV of patients, none had a PR or SD. Both group I and group III of patients exhibited elevated frequency of peripheral CTLs and decreased serum levels of AFP after vaccination. Another completed phase I/II clinical trial (NCT03086564) evaluated the clinical results of an HBV-specific-antigen-peptides-co-pulsed and HepG2-cell-lysate-co-pulsed mature-DC vaccine combined with TACE in treating patients with unresectable HBV-related primary HCC. In another completed (JPRN-UMIN000020811) phase I clinical trial and ongoing phase I clinical trial (JPRN-jRCTc040190093), the clinical results of a peptides-pulsed mature-DC vaccine combined with RFA in treating patients with primary HCC were evaluated. However, there are no available results from the three trials mentioned. Another completed phase I/II clinical trial (JPRN-UMIN000010691) conducted by Matsui et al. evaluated the safety and efficacy of a HSP70 mRNA-transfected mature-DC vaccine combined with surgical resection in treating patients with resectable primary HCC [44]. A total of 45 patients were divided into two groups with no difference in baseline characteristics: in group I, 31 patients received three intradermal DC vaccinations with a dose of 2 × 106 cells after surgical resection (first vaccination is 5 to 9 days after surgery, second vaccination is 5 to 10 weeks after surgery, and third vaccination is 9 to 16 weeks after surgery); and in group II, 14 patients received surgical resection alone as a control group. The results showed that DC vaccination was safe and well tolerated in all patients. Although there was no difference in the OS and disease-free survival (DFS) between the two groups of patients, in the subgroup of patients with HSP70-expressing HCC, the median OS and DFS times were significantly longer in group I than in group II (p-values = 0.003 and 0.090, respectively). Another completed phase I/II clinical trial conducted by Tada et al. evaluated the safety and efficacy of a mature-DC vaccine co-pulsed with AFP, MAGE-1, and GPC-3 proteins that was combined with TACE in treating five patients with primary HCC [45]. All the patients were vaccinated with 4 × 107 of DCs subcutaneously four times, at 2-week intervals, 2 weeks after the first TACE. Four weeks following the first cycle of treatment, all the patients received two further vaccinations at 2-week intervals, 2 weeks after the second TACE. The results showed that DC vaccination was safe and well tolerated in all patients. Among all patients, one (20%) achieved SD, and five (100%) exhibited increased IFN-γ-producing CTL responses against AFP, MAGE-1, and/or GPC-3 antigens after vaccination. Another ongoing phase II clinical trial (KCT0000986) was further aimed at evaluating the clinical results of a mature-DC vaccine co-pulsed with AFP, MAGE-1, and GPC-3 proteins and given in combination with TACE in treating patients with unresectable primary HCC. Another completed phase II clinical trial (JPRN-UMIN000021545) evaluated the clinical results of a mature-DC vaccine co-pulsed with AFP, MAGE-1, and GPC-3 proteins cand given in combination with surgical resection in treating patients with unresectable primary HCC. However, there are no available results from the two trials mentioned. Another completed phase I/IIa (KCT0000427) clinical trial and completed phase II (KCT0000008) clinical trial, both conducted by Lee et al., evaluated the safety and efficacy of a mature DC vaccine co-pulsed with AFP, MAGE-1, and GPC-3 proteins and given in combination with surgical resection, RFA, PEI, or TACE in treating patients with primary HCC [46,47]. In the phase I/IIa trial, a total of 12 patients were vaccinated with 5 × 107 of DCs, subcutaneously, six times (four vaccinations at 2-week intervals, followed by two vaccinations at 4-week intervals), 8 weeks after anticancer therapy. The results showed that DC vaccination was safe and well tolerated in all patients. Among all patients, nine (75%) did not develop tumor recurrence up to 24 weeks and displayed stronger IFN-γ-producing CTL responses against AFP, MAGE-1, and/or GPC-3 antigens than the others who developed recurrence after vaccination. The median TTP was significantly longer in the patients with vaccination than the patients without vaccination (36.6 months versus 11.8 months, p-value = 0.0031). In the phase II trial, a total of 156 patients were divided into two groups with no difference in baseline characteristics: in group I, 77 patients received six subcutaneous DC vaccinations with a dose of 3 × 107 cells (four vaccinations at 2-week intervals, followed by two vaccinations at 4-week intervals) 4 weeks after anticancer therapy; and in group II, 79 patients received anticancer therapy alone as a control group. The results showed that DC vaccination was safe and well tolerated in all patients. In group I of patients, 63% exhibited enhanced IFN-γ-producing CTL responses against AFP, MAGE-1, and/or GPC-3 antigens after vaccination. Although there was no difference in the OS and recurrence-free survival (RFS) between the two groups of patients, in the subgroup of patients who were not treated with RFA, the RFS was significantly better in group I than in group II (p-values = 0.03). Another ongoing phase I clinical trial (NCT03674073) was aimed at evaluating the clinical results of an HCC-tumor-neoantigen-pulsed mature-DC vaccine combined with PMWA in treating patients with primary HCC. Another ongoing phase II clinical trial (NCT04912765) was aimed at evaluating the clinical results of an HCC tumor neoantigen-pulsed mature-DC vaccine combined with ICI nivolumab and surgical resection in treating patients with resectable primary or recurrent HCC. Another ongoing phase II clinical trial (NCT04317248) was aimed at evaluating the clinical results of a multiple HCC tumor antigens-pulsed mature-DC vaccine combined with surgical resection or TACE or the molecular targeted drugs sorafenib or lenvatinib in treating patients with HBV-related primary HCC. However, there are no available results from the three trials mentioned. Taken together, these clinical trials indicate that autologous-tumor-lysate-pulsed, allogeneic-tumor-cell-line-lysate-pulsed, or specific-tumor-antigen-pulsed DC vaccines are safe and effective as adjuvant therapy in combination with standard anticancer therapies for treating HCC patients; however, the efficacy still needs to be optimized in further studies.
3.3. Autologous-Tumor-Lysate-Pulsed or Specific-Tumor-Antigen-Pulsed DC Vaccines Together with Immune Effector Cells Combined with Anticancer Therapies
A completed phase II clinical trial conducted by Shimizu et al. evaluated the safety and efficacy of an autologous-HCC-tumor-lysate-pulsed mature-DC vaccine, together with CD3-activated T cells (CATs), that was combined with surgical resection in treating patients with primary HCC [48]. A total of 94 patients were divided into two groups, with no difference in baseline characteristics: in group I, 42 patients received both intradermal DC vaccination and intravenous CAT infusion three times, with doses of about 3.5 × 107 and 2 × 109 cells, respectively, within 2 months of surgical resection; and in group II, 52 patients received surgical resection alone as a control group. The results showed that DC vaccination together with CAT infusion were safe and well tolerated in all patients in group I. Group I of patients had significantly longer median OS (97.7 months versus 41.0 months, p-values = 0.029) and RFS (24.5 months versus 12.6 months, p-values = 0.011) times than the group II of patients. Another completed phase I clinical trial conducted by Zhou et al. evaluated the safety and feasibility of an autologous HCC-tumor-lysate-pulsed mature-DC vaccine together with immature DCs, cytokine-induced killer cells (CIKs, in vitro generated lymphocytes with a mixed NK and T cell-like phenotypes and functions), mature-DC-precision CTLs, and mature-DC-activated CIKs (DC-CIKs) combined with PMWA in treating 10 patients with HBV-related primary HCC [49]. All the patients received three courses of DC vaccination and immune effector cell infusion (first course was intratumoral immature DC infusion on the date of PMWA and intravenous CIK infusion on day 5 after PMWA; second course was intranodal DC vaccination and intratumoral CTL infusion on day 11 after PMWA; and third course was intranodal DC vaccination and intraperitoneal DC-CIK infusion on day 100 and intravenous CIK infusion on day 102 after PMWA). The results showed that DC vaccination in combination with immune effector cell infusion was safe and well tolerated in all patients. Among seven patients who did not receive antiviral therapy, four (57.1%) had decreased and two (28.6%) had undetectable serum levels of viral DNA after vaccination and infusion. Another ongoing phase II clinical trial (NCT01821482) was aimed at evaluating the clinical results of mature DC-CIKs combined with surgical resection or TACE in treating patients with primary HCC; however, the results are not available. Another completed clinical trial conducted by Zhou et al. evaluated the safety and efficacy of a mature-DC vaccine together with CIKs combined with the molecular targeted drug sorafenib in treating patients with advanced primary HCC [50]. A total of 71 patients were divided into two groups, with no difference in baseline characteristics: in group I, 35 patients received both the DC vaccination and CIK infusion after sorafenib treatment; and group II, 36 patients received sorafenib treatment alone as a control group. The results showed that DC vaccination, together with CIK infusion, was safe and well tolerated in all patients. After a 6-month follow-up period, in group I of patients, 4 (11.4%) achieved a CR, 14 (40%) displayed a PR, and 13 (37.1%) had SD. In contrast, in group II of patients, only one (2.8%) achieved a CR, five (13.9%) displayed a PR, and nine (25%) had SD. After a minimum follow-up period of 24 months, the median OS time was significantly longer in group I than in group II of patients (18.6 months versus 13.8 months, p-value < 0.05). Group I of patients exhibited significantly decreased serum levels of AFP after vaccination and infusion. Another ongoing phase I/II clinical trial (NCT02632188) was aimed at evaluating the clinical results of mature-DC-precision multiple-antigen CTLs combined with surgical resection in treating patients with primary HCC. Another ongoing phase I/II clinical trial (NCT02638857) was further aimed at evaluating the clinical results of mature-DC-precision multiple-antigen CTLs combined with TACE in treating patients with unresectable primary or recurrent HCC. However, there are no available results from the two trials mentioned. Another completed phase II clinical trial (NCT03067493) conducted by Peng et al. evaluated the safety and efficacy of a personalized HCC-tumor-neoantigen-pulsed mature-DC vaccine together with mature-DC-precision neoantigen CTLs combined with surgical resection or RFA in treating 10 patients with primary HCC [51]. All the patients received both subcutaneous DC vaccination (1.65 × 106 to 1.88 × 107 cells per dose) and intravenous CTLs infusion (0.56 × 106 to 8.12 × 109 cells per dose), with a median of 12 cycles and median of 16.6, 20.2 weeks after anticancer therapy. The results showed that DC vaccination, together with CTL infusion, was safe and well tolerated in all patients. Among all patients, five (50%) experienced no tumor recurrence for 2 years, and seven (70%) generated de novo multiclonal-neoantigen-specific CTL responses; the median DFS time was 18.3 months. Among the seven patients who generated immune responses, five (71.4%) did not develop tumor recurrence for 2 years; in contrast, all of the patients who did not generate immune responses developed tumor recurrence. The patients who generated immune responses exhibited a significantly better DFS than the patients who did not generate immune responses (p-value = 0.012). Overall, these clinical trials suggest that DC-based vaccines, together with immune effector cells as combination adjuvant therapy, are safe and effective in treating HCC patients who receive standard anticancer therapies; however, further studies are still needed to improve the efficacy.
4. Recent Preclinical Studies Regarding DC-Vaccine-Based Immunotherapy for HCC
Many promising strategies have been proposed and evaluated in recent in vitro and in vivo preclinical studies to optimize the efficacy of DC-vaccine-based immunotherapy for HCC (Table 3). A study performed by Jin et al. evaluated the efficacy of a mature-DC vaccine, which was stimulated with the sulfated glycoconjugate compound curdlan sulfate as a maturation reagent, in treating HCC [52]. The results showed that curdlan sulfate-stimulated DC vaccine exhibited comparable efficacy in suppressing tumor growth and enhanced efficacy in prolonging survival in an ectopic allograft immunocompromised BALB/c mouse model of the mouse HCC cell line H22 compared to the commonly used maturation reagent lipopolysaccharide (LPS)-stimulated DC vaccine. Another study performed by Chieochansin et al. evaluated the efficacy of mature-DC vaccines, which were pulsed with total cell lysate or total RNA prepared from a single human HCC cell line or combinations of two or three human HCC cell lines (Huh7, HepG2, and SNU449), in treating HCC [53]. The results showed that total the RNA-pulsed DC vaccines induced stronger IFN-γ-producing CTL-mediated cytotoxicity against each of the three human HCC cell lines in vitro than total-cell-lysate-pulsed DC vaccines. The cytotoxic activity of CTLs was further augmented when the DC vaccines were pulsed with total cell lysate or total RNA prepared from all three cell lines compared to either one or two cell lines. Another study performed by Pang et al. evaluated the efficacy of a mature-DC vaccine, which was fused with a flow cytometry-sorted cancer stem cell (CSC) marker CD90-positive irradiated HepG2 cell line, in treating HCC [54]. The results showed that CD90-positive irradiated HepG2 cell-fused DC vaccine induced stronger IFN-γ-producing CTL-mediated cytotoxicity against CD90-positive HepG2 cell line in vitro and exhibited better efficacy in suppressing tumor growth in an ectopic xenograft immunocompromised BALB/c mouse model of non-CD90-sorted HepG2 cell line than non-CD90-sorted irradiated HepG2 cell-fused DC vaccine. Another study performed by Zhou et al. evaluated the efficacy of a mature-DC vaccine, which was transfected with adenoviral vector DNA containing a gene encoding the HCC tumor marker aspartate-β-hydroxylase (AAH), in treating HCC [55]. The results showed that AAH DNA-transfected DC vaccine induced stronger IFN-γ-producing CTL-mediated cytotoxicity against human HCC cell line SMMC-7721 in vitro and exhibited better efficacy in suppressing tumor growth in an ectopic xenograft immunocompromised BALB/c mouse model than non-AAH DNA-transfected DC vaccine. Another study performed by Vogt et al. evaluated the efficacy of combination of two mature-DC vaccines, which were transfected with adenoviral vector DNA containing a gene encoding either the HCC tumor marker AFP or the immune co-stimulatory molecule CD40 ligand (CD40L), in treating HCC [56]. The results showed that combination of the two DC vaccines exhibited better efficacy in suppressing tumor growth and prolonging survival than either of the two DC vaccines alone in both ectopic and orthotopic allograft immunocompetent C3H/HeN mouse models of a stable AFP-expressing mouse HCC cell line, Hepa129. Another study performed by Xu et al. evaluated the efficacy of IFN-producing killer DCs (IKDCs, a subset of immune cells with certain phenotypes and functions of both DCs and NKs), which were transfected with lentiviral vector DNA containing a gene encoding the T-box family transcription factor T-bet for IFN-γ induction, in treating HCC [57]. The results showed that T-bet DNA-transfected IKDCs exhibited stronger cytotoxic activity against H22 cell line in vitro and better efficacy in suppressing tumor growth in an ectopic allograft immunocompetent C57BL/6 mouse model than non-T-bet DNA-transfected IKDCs. Another two studies performed by Teng et al. evaluated the efficacy of a mature-DC vaccine, which was pulsed with total cell lysate prepared from mouse HCC cell line Hep-55.1C, in combination with the ICIs that target either PD-1 or programmed death ligand 1 (PD-L1), in treating HCC [58,59]. The results showed that combination of the DC vaccine and ICIs induced stronger granzyme B-expressing CTL-mediated cytotoxicity against Hep-55.1C cell line in vitro and exhibited better efficacy in suppressing tumor growth and prolonging survival in an orthotopic allograft immunocompetent C57BL/6 mouse model than either the DC vaccine or ICIs alone. Another study performed by Wang et al. evaluated the efficacy of a DC-based nanoparticle vaccine, which was prepared by coating an acidic/photosensitive nanoparticle with the membrane of H22-cell-specific neoantigen-pulsed mature DCs, in combination with near-infrared (NIR) laser irradiation in treating HCC [60]. The results showed that the DC-based nanoparticle vaccine induced stronger IFN-γ-producing CTL-mediated cytotoxicity against the H22 cell line in vitro and exhibited better efficacy in suppressing tumor growth and prolonging survival in an ectopic allograft immunocompromised BALB/c mouse model than non-DC-based nanoparticle vaccine when combined with laser irradiation. Another study performed by Zuo et al. evaluated the efficacy of a DC-derived exosome vaccine, which was prepared by co-conjugating an immortalized DC-line-derived exosome with an AFP-derived epitope AFP212, an HCC tumor-targeting peptide P47, and a functional domain of high mobility group nucleosome-binding protein 1 (HMGN1), an immunoadjuvant capable of promoting DC recruitment and activation, in treating HCC [61]. The results showed that the DC-derived exosome vaccine exhibited efficient efficacy in suppressing tumor growth and prolonging survival in both ectopic and orthotopic allograft immunocompetent C57BL/6 mouse models of the mouse HCC cell line Hepa1-6. Altogether, these preclinical studies provide promising strategies to improve the therapeutic efficacy of DC-based vaccines for HCC, as such vaccines are worth conducting clinical trials for in order to evaluate their safety and efficacy in treating HCC patients.
Table 3.
Summary of recent preclinical studies regarding DC-vaccine-based immunotherapy for HCC.
| Treatment | Experimental Model Applied | Experimental Results | Publication |
| Curdlan sulfate–stimulated mature-DC vaccine |
|
|
Jin et al. [52] |
| Mature-DC vaccine co-pulsed with Huh7, HepG2, and SNU449 HCC cell lysate or RNA |
|
|
Chieochansin et al. [53] |
| CD90-positive irradiated-HepG2-HCC-cell-fused mature-DC vaccine |
|
|
Pang et al. [54] |
| AAH-DNA-transfected mature-DC vaccine |
|
|
Zhou et al. [55] |
| Combination of two mature-DC vaccines, one transfected with AFP DNA and the other transfected with CD40L DNA |
|
|
Vogt et al. [56] |
| T-bet DNA-transfected IKDCs |
|
|
Xu et al. [57] |
| Hep-55.1C-HCC-cell-lysate-pulsed mature-DC vaccine combined with ICI against PD-1 |
|
|
Teng et al. [58] |
| Hep-55.1C-HCC-cell-lysate-pulsed mature-DC vaccine combined with ICI against PD-L1 |
|
|
Teng et al. [59] |
| H22-HCC-cell-specific neoantigen-pulsed mature-DC-membrane-coated acidic/photosensitive nanoparticle vaccine combined with NIR laser irradiation |
|
|
Wang et al. [60] |
| DC-derived exosome vaccine co-conjugated with AFP epitope AFP212, HCC tumor-targeting peptide, and HMGN1 functional domain |
|
|
Zuo et al. [61] |
Abbreviations: DC, dendritic cell; HCC, hepatocellular carcinoma; LPS, lipopolysaccharide; IFN-γ, interferon-gamma; CTL, cytotoxic T lymphocyte; AAH, aspartate-β-hydroxylase; AFP, alpha-fetoprotein; CD40L, CD40 ligand; IKDC, interferon-producing killer dendritic cell; ICI, immune checkpoint inhibitor; PD-1, programmed death 1; PD-L1, programmed death ligand 1; NIR, near-infrared; HMGN1, high-mobility-group nucleosome-binding protein 1.
5. Conclusions
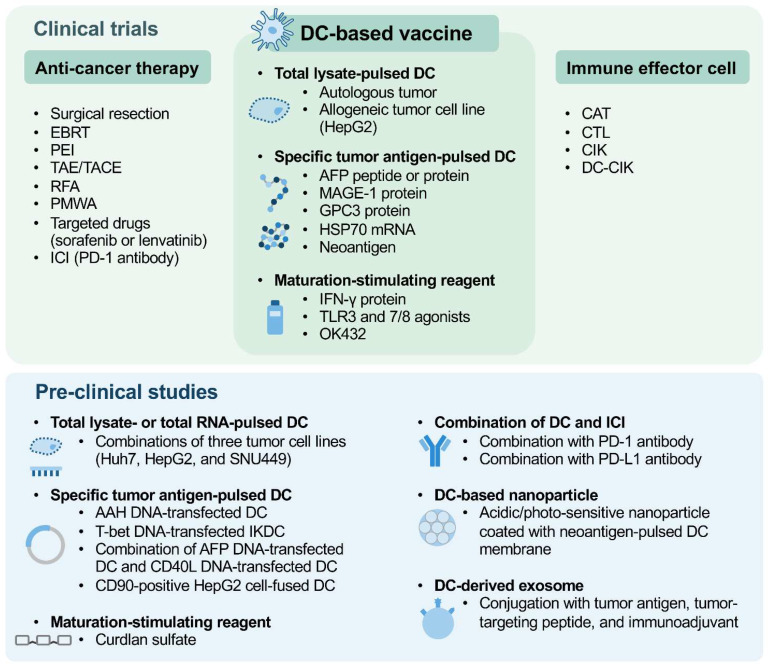
This review summarizes the evidence for various strategies evaluated in clinical trials and recent preclinical studies for the development of DC-vaccine-based immunotherapy for HCC (Figure 1), highlighting that a DC vaccine, whether alone or in combination with anticancer therapies and/or immune effector cells, holds great promise as a personalized therapeutic approach for treating patients with HCC. Some strategies focus on the optimization of DC vaccine efficacy, including the choice of tumor antigens pulsing with DCs and the discovery of DC maturation-stimulating reagents; some strategies focus on the combination of DC vaccines, either with or without immune effector cells, and current anticancer therapies; and others focus on the application of nanotechnology in DC vaccines or DC-derived exosomes. In addition, different injection routes and treatment regimens of DC vaccines are applied and evaluated in clinical trials. All of these parameters are critical in determining the therapeutic efficacy of DC-vaccine-based immunotherapy. Moreover, considering that HCC exhibits a high degree of heterogeneity in both the genomic landscape and immune microenvironment [62], it is therefore also important to identify biomarkers of treatment response for selecting the best treatment strategies for patients.
Figure 1.
Schematic summary of clinical and preclinical strategies of DC-vaccine-based immunotherapy for HCC.
DC-based vaccines, which are prepared by different tumor-antigen-pulsing strategies or maturation-stimulating reagents, either alone or in combination with various anticancer therapies and/or immune effector cells for treating HCC patients, have been evaluated in clinical trials. Many promising strategies regarding tumor antigen pulsing, maturation stimulation, ICI combination, DC-based nanoparticles, and DC-derived exosomes have also been evaluated in preclinical studies to optimize the anti-HCC efficacy of DC vaccine-based immunotherapy.
Abbreviations
DC, dendritic cell; IFN-γ, interferon-gamma; MAGE-1, melanoma-associated antigen 1; GPC-3, glypican-3; HSP70, heat-shock protein 70; mRNA, messenger RNA; TLR, Toll-like receptor; EBRT, external beam radiation therapy; PEI, percutaneous ethanol injection; TAE, transarterial embolization; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; PMWA, percutaneous microwave ablation; ICI, immune checkpoint inhibitor; PD-1, programmed death 1; CAT, CD3-activated T cell; CTL, cytotoxic T lymphocyte; CIK, cytokine-induced killer cell; DC-CIK, dendritic-cell-activated cytokine-induced killer cell; AAH, aspartate-β-hydroxylase; CD40L, CD40 ligand; PD-L1, programmed death ligand 1.
Author Contributions
Conceptualization, L.-B.J., L.-Y.L., F.-Y.S. and C.-F.T.; funding acquisition, C.-F.T.; visualization, L.-B.J., L.-Y.L., F.-Y.S. and C.-F.T.; writing—original draft, C.-F.T.; writing—review and editing, C.-F.T. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grants from the China Medical University Hospital, Taichung, Taiwan (grant number DMR-111-055).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Llovet J.M., Zucman-Rossi J., Pikarsky E., Sangro B., Schwartz M., Sherman M., Gores G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 2.Cheng K.C., Lin W.Y., Liu C.S., Lin C.C., Lai H.C., Lai S.W. Association of different types of liver disease with demographic and clinical factors. Biomedicine. 2016;6:16. doi: 10.7603/s40681-016-0016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallage S., Garcia-Beccaria M., Szydlowska M., Rahbari M., Mohr R., Tacke F., Heikenwalder M. The therapeutic landscape of hepatocellular carcinoma. Med. 2021;2:505–552. doi: 10.1016/j.medj.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Llovet J.M., De Baere T., Kulik L., Haber P.K., Greten T.F., Meyer T., Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021;18:293–313. doi: 10.1038/s41575-020-00395-0. [DOI] [PubMed] [Google Scholar]
- 5.Galle P.R., Dufour J.F., Peck-Radosavljevic M., Trojan J., Vogel A. Systemic therapy of advanced hepatocellular carcinoma. Future Oncol. 2021;17:1237–1251. doi: 10.2217/fon-2020-0758. [DOI] [PubMed] [Google Scholar]
- 6.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., Luo R., Feng J., Ye S., Yang T.S., et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 8.Kulik L., El-Serag H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477–491. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 10.Steinman R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 11.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 12.Liu K., Nussenzweig M.C. Origin and development of dendritic cells. Immunol. Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 13.Van Elssen C.H., Oth T., Germeraad W.T., Bos G.M., Vanderlocht J. Natural killer cells: The secret weapon in dendritic cell vaccination strategies. Clin. Cancer. Res. 2014;20:1095–1103. doi: 10.1158/1078-0432.CCR-13-2302. [DOI] [PubMed] [Google Scholar]
- 14.Peterson E.E., Barry K.C. The Natural Killer-Dendritic Cell Immune Axis in Anti-Cancer Immunity and Immunotherapy. Front. Immunol. 2021;11:621254. doi: 10.3389/fimmu.2020.621254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palucka K., Banchereau J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang N., Figini M., Shangguan J., Wang B., Sun C., Pan L., Ma Q., Zhang Z. Dendritic cells based immunotherapy. Am. J. Cancer Res. 2017;7:2091–2102. [PMC free article] [PubMed] [Google Scholar]
- 17.Constantino J., Gomes C., Falcao A., Neves B.M., Cruz M.T. Dendritic cell-based immunotherapy: A basic review and recent advances. Immunol. Res. 2017;65:798–810. doi: 10.1007/s12026-017-8931-1. [DOI] [PubMed] [Google Scholar]
- 18.Mahdian R., Kokhaei P., Najar H.M., Derkow K., Choudhury A., Mellstedt H. Dendritic cells, pulsed with lysate of allogeneic tumor cells, are capable of stimulating MHC-restricted antigen-specific antitumor T cells. Med. Oncol. 2006;23:273–282. doi: 10.1385/MO:23:2:273. [DOI] [PubMed] [Google Scholar]
- 19.Dashti A., Ebrahimi M., Hadjati J., Memarnejadian A., Moazzeni S.M. Dendritic cell based immunotherapy using tumor stem cells mediates potent antitumor immune responses. Cancer Lett. 2016;374:175–185. doi: 10.1016/j.canlet.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Stober D., Trobonjaca Z., Reimann J., Schirmbeck R. Dendritic cells pulsed with exogenous hepatitis B surface antigen particles efficiently present epitopes to MHC class I-restricted cytotoxic T cells. Eur. J. Immunol. 2002;32:1099–1108. doi: 10.1002/1521-4141(200204)32:4<1099::AID-IMMU1099>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Butterfield L.H., Ribas A., Potter D.M., Economou J.S. Spontaneous and vaccine induced AFP-specific T cell phenotypes in subjects with AFP-positive hepatocellular cancer. Cancer Immunol. Immunother. 2007;56:1931–1943. doi: 10.1007/s00262-007-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koido S., Kashiwaba M., Chen D., Gendler S., Kufe D., Gong J. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. J. Immunol. 2000;165:5713–5719. doi: 10.4049/jimmunol.165.10.5713. [DOI] [PubMed] [Google Scholar]
- 23.Daftarian P., Kaifer A.E., Li W., Blomberg B.B., Frasca D., Roth F., Chowdhury R., Berg E.A., Fishman J.B., Al Sayegh H.A., et al. Peptide-conjugated PAMAM dendrimer as a universal DNA vaccine platform to target antigen-presenting cells. Cancer Res. 2011;71:7452–7462. doi: 10.1158/0008-5472.CAN-11-1766. [DOI] [PubMed] [Google Scholar]
- 24.Gong J., Chen D., Kashiwaba M., Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat. Med. 1997;3:558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 25.Iwashita Y., Tahara K., Goto S., Sasaki A., Kai S., Seike M., Chen C.L., Kawano K., Kitano S. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol. Immunother. 2003;52:155–161. doi: 10.1007/s00262-002-0360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee W.C., Wang H.C., Hung C.F., Huang P.F., Lia C.R., Chen M.F. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: A clinical trial. J. Immunother. 2005;28:496–504. doi: 10.1097/01.cji.0000171291.72039.e2. [DOI] [PubMed] [Google Scholar]
- 27.Palmer D.H., Midgley R.S., Mirza N., Torr E.E., Ahmed F., Steele J.C., Steven N.M., Kerr D.J., Young L.S., Adams D.H. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 28.El Ansary M., Mogawer S., Elhamid S.A., Alwakil S., Aboelkasem F., Sabaawy H.E., Abdelhalim O. Immunotherapy by autologous dendritic cell vaccine in patients with advanced HCC. J. Cancer Res. Clin. Oncol. 2013;139:39–48. doi: 10.1007/s00432-012-1298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butterfield L.H., Ribas A., Dissette V.B., Lee Y., Yang J.Q., De la Rocha P., Duran S.D., Hernandez J., Seja E., Potter D.M., et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin. Cancer Res. 2006;12:2817–2825. doi: 10.1158/1078-0432.CCR-05-2856. [DOI] [PubMed] [Google Scholar]
- 30.Maeda Y., Yoshimura K., Matsui H., Shindo Y., Tamesa T., Tokumitsu Y., Hashimoto N., Tokuhisa Y., Sakamoto K., Sakai K., et al. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: A phase 1 dose escalation clinical trial. Cancer Immunol. Immunother. 2015;64:1047–1056. doi: 10.1007/s00262-015-1709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topfer K., Kempe S., Muller N., Schmitz M., Bachmann M., Cartellieri M., Schackert G., Temme A. Tumor evasion from T cell surveillance. J. Biomed. Biotechnol. 2011;2011:918471. doi: 10.1155/2011/918471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuol N., Stojanovska L., Nurgali K., Apostolopoulos V. The mechanisms tumor cells utilize to evade the host’s immune system. Maturitas. 2017;105:8–15. doi: 10.1016/j.maturitas.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Hargadon K.M., Johnson C.E., Williams C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Gong J., Chehrazi-Raffle A., Reddi S., Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.Y., Choo S.P., Trojan J., Welling T.H.R., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longo V., Brunetti O., Gnoni A., Licchetta A., Delcuratolo S., Memeo R., Solimando A.G., Argentiero A. Emerging role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. Medicina. 2019;55:698. doi: 10.3390/medicina55100698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shek D., Read S.A., Nagrial A., Carlino M.S., Gao B., George J., Ahlenstiel G. Immune-Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: A Synopsis of Response Rates. Oncologist. 2021;26:e1216–e1225. doi: 10.1002/onco.13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melero I., Gaudernack G., Gerritsen W., Huber C., Parmiani G., Scholl S., Thatcher N., Wagstaff J., Zielinski C., Faulkner I., et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 39.Chi K.H., Liu S.J., Li C.P., Kuo H.P., Wang Y.S., Chao Y., Hsieh S.L. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J. Immunother. 2005;28:129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 40.Kitahara M., Mizukoshi E., Terashima T., Nakagawa H., Horii R., Iida N., Arai K., Yamashita T., Sakai Y., Yamashita T., et al. Safety and Long-Term Outcome of Intratumoral Injection of OK432-Stimulated Dendritic Cells for Hepatocellular Carcinomas After Radiofrequency Ablation. Transl. Oncol. 2020;13:100777. doi: 10.1016/j.tranon.2020.100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizell M., Sternby Eilard M., Andersson M., Andersson B., Karlsson-Parra A., Suenaert P. Phase 1 Trial with the Cell-Based Immune Primer Ilixadencel, Alone, and Combined with Sorafenib, in Advanced Hepatocellular Carcinoma. Front. Oncol. 2019;9:19. doi: 10.3389/fonc.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X., Bayer M.E., Chen X., Fredrickson C., Cornforth A.N., Liang G., Cannon J., He J., Fu Q., Liu J., et al. Phase I trial of active specific immunotherapy with autologous dendritic cells pulsed with autologous irradiated tumor stem cells in hepatitis B-positive patients with hepatocellular carcinoma. J. Surg. Oncol. 2015;111:862–867. doi: 10.1002/jso.23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdel Ghafar M.T., Morad M.A., El-Zamarany E.A., Ziada D., Soliman H., Abd-Elsalam S., Salama M. Autologous dendritic cells pulsed with lysate from an allogeneic hepatic cancer cell line as a treatment for patients with advanced hepatocellular carcinoma: A pilot study. Int. Immunopharmacol. 2020;82:106375. doi: 10.1016/j.intimp.2020.106375. [DOI] [PubMed] [Google Scholar]
- 44.Matsui H.M., Hazama S., Nakajima M., Xu M., Matsukuma S., Tokumitsu Y., Shindo Y., Tomochika S., Yoshida S., Iida M., et al. Novel adjuvant dendritic cell therapy with transfection of heat-shock protein 70 messenger RNA for patients with hepatocellular carcinoma: A phase I/II prospective randomized controlled clinical trial. Cancer Immunol. Immunother. 2021;70:945–957. doi: 10.1007/s00262-020-02737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tada F., Abe M., Hirooka M., Ikeda Y., Hiasa Y., Lee Y., Jung N.C., Lee W.B., Lee H.S., Bae Y.S., et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol. 2012;41:1601–1609. doi: 10.3892/ijo.2012.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J.H., Lee Y., Lee M., Heo M.K., Song J.S., Kim K.H., Lee H., Yi N.J., Lee K.W., Suh K.S., et al. A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br. J. Cancer. 2015;113:1666–1676. doi: 10.1038/bjc.2015.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J.H., Tak W.Y., Lee Y., Heo M.K., Song J.S., Kim H.Y., Park S.Y., Bae S.H., Lee J.H., Heo J., et al. Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. Oncoimmunology. 2017;6:e1328335. doi: 10.1080/2162402X.2017.1328335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimizu K., Kotera Y., Aruga A., Takeshita N., Katagiri S., Ariizumi S., Takahashi Y., Yoshitoshi K., Takasaki K., Yamamoto M. Postoperative dendritic cell vaccine plus activated T-cell transfer improves the survival of patients with invasive hepatocellular carcinoma. Hum. Vaccin. Immunother. 2014;10:970–976. doi: 10.4161/hv.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou P., Liang P., Dong B., Yu X., Han Z., Xu Y. Phase I clinical study of combination therapy with microwave ablation and cellular immunotherapy in hepatocellular carcinoma. Cancer Biol. Ther. 2011;11:450–456. doi: 10.4161/cbt.11.5.14669. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Z., Qin H., Weng L., Ni Y. Clinical efficacy of DC-CIK combined with sorafenib in the treatment of advanced hepatocellular carcinoma. J. BUON. 2019;24:615–621. [PubMed] [Google Scholar]
- 51.Peng S., Chen S., Hu W., Mei J., Zeng X., Su T., Wang W., Chen Z., Xiao H., Zhou Q., et al. Combination Neoantigen-Based Dendritic Cell Vaccination and Adoptive T-Cell Transfer Induces Antitumor Responses Against Recurrence of Hepatocellular Carcinoma. Cancer. Immunol. Res. 2022;10:728–744. doi: 10.1158/2326-6066.CIR-21-0931. [DOI] [PubMed] [Google Scholar]
- 52.Jin Y., Mu Y., Zhang S., Li P., Wang F. Preparation and evaluation of the adjuvant effect of curdlan sulfate in improving the efficacy of dendritic cell-based vaccine for antitumor immunotherapy. Int. J. Biol. Macromol. 2020;146:273–284. doi: 10.1016/j.ijbiomac.2019.12.256. [DOI] [PubMed] [Google Scholar]
- 53.Chieochansin T., Thepmalee C., Grainok J., Junking M., Yenchitsomanus P.T. Cytolytic Activity of Effector T-lymphocytes Against Hepatocellular Carcinoma is Improved by Dendritic Cells Pulsed with Pooled Tumor Antigens. Sci. Rep. 2019;9:17668. doi: 10.1038/s41598-019-54087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pang Y.B., He J., Cui B.Y., Xu S., Li X.L., Wu M.Y., Liang R., Feng Y., Guo X., Zhang X.H., et al. A Potential Antitumor Effect of Dendritic Cells Fused with Cancer Stem Cells in Hepatocellular Carcinoma. Stem Cells Int. 2019;2019:5680327. doi: 10.1155/2019/5680327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Y., Li C., Shi G., Xu X., Luo X., Zhang Y., Fu J., Chen L., Zeng A. Dendritic cell-based vaccine targeting aspartate-beta-hydroxylas represents a promising therapeutic strategy for HCC. Immunotherapy. 2019;11:1399–1407. doi: 10.2217/imt-2019-0081. [DOI] [PubMed] [Google Scholar]
- 56.Vogt A., Sadeghlar F., Ayub T.H., Schneider C., Mohring C., Zhou T., Mahn R., Bartels A., Praktiknjo M., Kornek M.T., et al. Alpha-Fetoprotein- and CD40Ligand-Expressing Dendritic Cells for Immunotherapy of Hepatocellular Carcinoma. Cancers. 2021;13:3375. doi: 10.3390/cancers13133375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu J., Liu Z., He K., Xiang G. T-bet transduction enhances anti-tumor efficacy of IFN-producing dendritic cell (IKDC) against hepatocellular carcinoma via apoptosis induction. Biochem. Biophys. Res. Commun. 2021;535:80–86. doi: 10.1016/j.bbrc.2020.11.118. [DOI] [PubMed] [Google Scholar]
- 58.Teng C.F., Wang T., Shih F.Y., Shyu W.C., Jeng L.B. Therapeutic efficacy of dendritic cell vaccine combined with programmed death 1 inhibitor for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2021;36:1988–1996. doi: 10.1111/jgh.15398. [DOI] [PubMed] [Google Scholar]
- 59.Teng C.F., Wang T., Wu T.H., Lin J.H., Shih F.Y., Shyu W.C., Jeng L.B. Combination therapy with dendritic cell vaccine and programmed death ligand 1 immune checkpoint inhibitor for hepatocellular carcinoma in an orthotopic mouse model. Ther. Adv. Med. Oncol. 2020;12:1758835920922034. doi: 10.1177/1758835920922034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y., Zhao Q., Zhao B., Zheng Y., Zhuang Q., Liao N., Wang P., Cai Z., Zhang D., Zeng Y., et al. Remodeling Tumor-Associated Neutrophils to Enhance Dendritic Cell-Based HCC Neoantigen Nano-Vaccine Efficiency. Adv. Sci. 2022;9:e2105631. doi: 10.1002/advs.202105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuo B., Zhang Y., Zhao K., Wu L., Qi H., Yang R., Gao X., Geng M., Wu Y., Jing R., et al. Universal immunotherapeutic strategy for hepatocellular carcinoma with exosome vaccines that engage adaptive and innate immune responses. J. Hematol. Oncol. 2022;15:46. doi: 10.1186/s13045-022-01266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dhanasekaran R. Deciphering Tumor Heterogeneity in Hepatocellular Carcinoma (HCC)-Multi-Omic and Singulomic Approaches. Semin. Liver Dis. 2021;41:9–18. doi: 10.1055/s-0040-1722261. [DOI] [PMC free article] [PubMed] [Google Scholar]