Abstract
The ptsN gene of Pseudomonas putida encodes IIANtr, a protein of the phosphoenol pyruvate:sugar phosphotransferase (PTS) system which is required for the C source inhibition of the ς54-dependent promoter Pu of the TOL (toluate degradation) plasmid pWW0. Using two-dimensional gel electrophoresis, we have examined the effect of ptsN disruption on the general expression pattern of P. putida. To this end, cells were grown in the presence or absence of glucose, and a 1,117-spot subset of the P. putida proteome was used as a reference for comparisons. Among all gene products whose expression was lowered by this carbon source (247 spots [about 22%]), only 6 behaved as Pu (i.e., were depressed in the ptsN background). This evidenced only a minor role for IIANtr in the extensive inhibition of gene expression in P. putida caused by glucose. However, the same experiments revealed a large incidence of glucose-independent effects brought about by the ptsN mutation. As many as 108 spots (ca. 9% of the cell products analyzed) were influenced, positively or negatively, by the loss of IIANtr. By matching this pattern with that of an rpoN::ΩKm strain of P. putida, which lacks the ς54 protein, we judge that most proteins whose expression was affected by ptsN were unrelated to the alternative sigma factor. These data suggest a role of IIANtr as a general regulator, independent of the presence of repressive carbon sources and not limited to ς54-dependent genes.
The Pu promoter of Pseudomonas putida drives transcription of the upper operon of the TOL plasmid pWW0, which makes this strain capable of using toluene, m-xylene, or p-xylene as the only source of carbon and energy (2). Besides being induced by pathway substrates (24), this ς54-dependent promoter is repressed by threefold in the presence of certain carbon sources such as glucose or gluconate (3, 11). We have recently reported that the loss of the ptsN gene appears to relieve this C-source-dependent inhibition (3), an event that can be genetically distinguished from other down-regulation effects caused by fast growth (5). ptsN is included in the so-called rpoN gene cluster, which determines not only the sigma factor ς54, but also four more downstream genes (3, 15). In particular, ptsN encodes a type II enzyme (termed IIANtr) of the phosphoenol pyruvate:sugar phosphotransferase (PTS) system (3, 15), which is a complex and very branched group of phosphotransfer proteins involved in controlling the intake of certain carbohydrates and other regulatory functions (reviewed in reference 21).
Homologues of ptsN have also been found adjacent to rpoN in various other gram-negative species, including Escherichia coli, Klebsiella pneumoniae, Caulobacter crescentus, Rhizobium meliloti, and Pseudomonas aeruginosa (12, 13, 18, 19, 22). ptsN mutants of K. pneumoniae (in which ptsN was originally called ORF152) displayed an increased activity of the ς54-dependent promoter PnifH (18). On the contrary, the loss of the equivalent ptsN (ORF2) in P. aeruginosa did not affect the activity of its ς54 promoters for pili and flagellin genes (13). Furthermore, some (but not all) of the Caulobacter and Rhizobium ς54 systems tested became less active upon the loss of ptsN (12, 19). A ptsN mutant of E. coli displayed certain incompatibilities between C and N sources—typically glucose and alanine (22). In addition, this mutation also suppressed a temperature-sensitive allele of era, a gene encoding an essential GTPase of unknown function (22). These observations, made with various systems, are not easy to reconcile. On one hand, they suggest the existence of a specific molecular pathway for physiological coregulation of some ς54 promoters in which IIANtr is a key intermediate. On the other hand, IIANtr might also be involved in more general metabolic activities, such as coordination of C and N metabolism (22, 23, 25). This is plausible, since the PTS system also participates in a group of regulatory processes (21) in a fashion dependent on the availability of adequate carbohydrates in the external medium. These act as a drain of high-energy phosphate, which determines the accumulation or the depletion of phosphorylated intermediates, which have the ability to interact with and modify the activity of many other cell products (21). In this regard, there is genetic evidence that a phosphorylated form of IIANtr mediates the repressive effect of glucose on the Pu promoter (3).
With these premises, we set out to explore the role of the ptsN gene of P. putida in the general pattern of protein expression. To this end, we resorted to two-dimensional (2D) polyacrylamide gel electrophoresis (PAGE) analysis of proteins from P. putida cells bearing a ptsN disruption, grown in the presence or the absence of a repressive carbon source such as glucose. As shown below, 2D electrophoresis allowed us to measure the simultaneous influence of IIANtr on the levels of a large number of gene products. Our data indicate that ptsN is involved in the expression of a considerable share of the entire P. putida proteome, either activating or inhibiting the outcome of approximately 9% of the gene products analyzed. Interestingly, most of these effects were unrelated to the presence of glucose in the medium. Comparison of the protein patterns of the ptsN strain versus those of an rpoN mutant indicated that IIANtr modulates expression of both ς54-dependent and ς54-independent products.
MATERIALS AND METHODS
Bacterial strains.
P. putida MAD2 is a tellurite-resistant derivative of P. putida KT2442 bearing a chromosomal Pu-lacZ fusion along with an xylR allele named xylRΔA (8). The loss of the N-terminal A-domain endows XylR with a constitutive activity, independent of inducer (m-xylene) addition, but still responsive to down-regulation by C source (3, 4). Strain P. putida MAD2 ptsN::Km is a derivative in which the ptsN gene has been disrupted in the 53rd codon by the insertion of two copies of a promoterless Kmr cassette (3). The ptsN+ plasmid pJM154 is a derivative of broad-host-range vector pJPS9 inserted with a PstI fragment spanning the genomic region of P. putida that carries ptsN, but excluding the genes adjacent to the rpoN cluster (3). The P. putida rpoN::ΩKm strain was constructed by Köhler et al. (14).
Culture conditions and other general methods.
Cells were grown at 30°C in M9 minimal medium (20) with all amino acids added (except methionine) (M9-AA) at the concentrations reported by Davis et al. (7). Where indicated, the cultures were supplemented with 10 mM glucose. The excess of Casamino Acids in the medium equaled growth rates and avoided effects related to the stringent response (31).
2D electrophoresis and analysis of expression patterns.
Cultures inoculated with the strains under scrutiny were grown up to an optical density at 600 nm (OD600) of ∼1.5. At that point, 1-ml aliquots of each sample were pulse-labeled with 45 μCi of [35S]methionine (specific activity, 1,000 Ci/mmol) for 10 min and then chased with cold methionine for 3 min. Cells were spun down and resuspended in 60 μl of sodium dodecyl sulfate (SDS)-β-mercaptoethanol buffer (0.3% SDS, 5% β-mercaptoethanol, 50 mM Tris-Cl [pH 8]) and boiled for 2 min. Samples were then treated 30 min on ice with a DNase-RNase solution (final concentration, 15 mg of DNase I per ml, 75 mg of RNase A per ml, 1 mM MgCl2), and then 240 μl of lysis buffer (6 M urea, 2 M thiourea, 4% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 1% IPG buffer [pH 3 to 10], 2 mM TCEP-HCl) was added. Samples were then clarified by ultracentrifugation. A total of 1.5 × 106 cpm, previously diluted to 350 μl in a mixture containing 6 M urea, 2 M thiourea, 2% CHAPS, 0.5% IPG buffer (pH 4 to 7), and 1 mM TCEP-HCl, was applied by rehydration of IPG strips (nominal pH gradient of 4 to 7, 18-cm length; Pharmacia Biotech, Uppsala, Sweden). Samples were focused by stepwise increase of the voltage as follows: 30 V for 6 h, 60 V for 6 h, 500 V for 30 min, 1,000 V for 30 min, and 1,000 to 8,000 V for 30 min. Gels were then subjected during the next 30 min to a linear increase from 8,000 V to 60,000 V. After isoelectric focusing separation, strips were equilibrated twice for 15 min with a mixture containing 50 mM Tris-HCl (pH 8.8), 6 M urea, 30% glycerol, 2% SDS, and traces of bromophenol blue. The first equilibration step contained 1% dithiothreitol, and the second had 4% iodoacetamide added. The 2D SDS-PAGE was performed with 1-mm-thick, 16 by 15-cm, 12.5% homogeneous polyacrylamide gels. Electrophoresis was carried out overnight at 5°C at a constant current of 5 mA. Gels were then dried, and radioactive signals were detected in a Storm aparatus (Pharmacia Biotech). Duplicate gels were run and analyzed for every genetic background and for the growth conditions described in this article, which included strains P. putida MAD2, P. putida MAD2 ptsN::Km, P. putida MAD2 ptsN::Km (pJM154), P. putida KT2442, and P. putida KT2442 rpoN::ΩKm, each grown in the presence or absence of glucose. ImageMaster v 3.01 software (Pharmacia Biotech) was used for spot detection and detailed analysis.
RESULTS
Global repression of gene expression caused by glucose in ptsN+ and ptsN strains of P. putida.
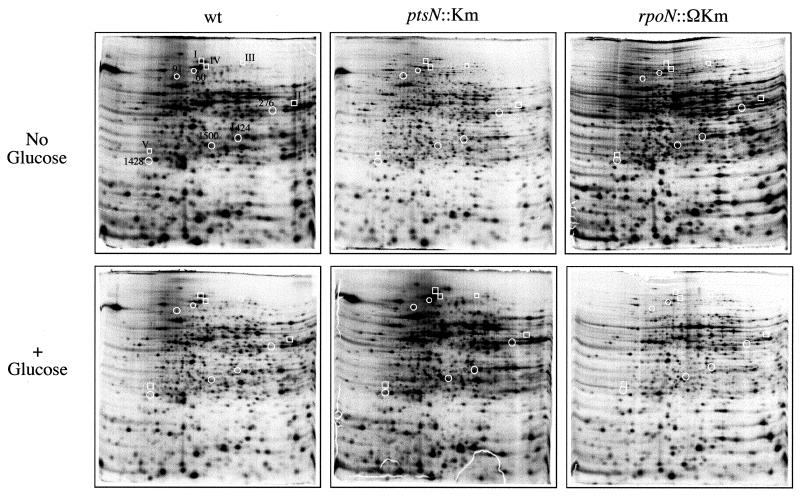
Since the ptsN gene product has been correlated with glucose repression of the Pu promoter of the TOL plasmid (3, 5), we set out to investigate the extent of this regulatory role of ptsN. To this end, we grew the wild-type P. putida MAD2 and P. putida MAD2 ptsN::Km strains in M9 medium supplemented with all of the amino acids except methionine and with or without 10 mM glucose added. The cultures were then labeled with [35S]methionine, and their protein extracts were run in a 2D gel electrophoresis system. As a control, β-galactosidase assays carried out in parallel showed that under the conditions of the experiment, the Pu-lacZ fusion was indeed repressed in the presence of glucose and derepressed in its absence (not shown). The resulting gels are shown in Fig. 1. Quantitative analysis of the 1,117 most prominent spots revealed well-defined changes in the intensity of many distinct polypeptides in response to the presence of glucose in the wild-type background. Expression of 247 spots (22%) out of all the proteins displayed in the 2D gels were reduced by ≥2-fold in extracts from the wild-type, ptsN+ P. putida MAD2 cultures with glucose added.
FIG. 1.
2D gels of protein extracts from P. putida MAD2 (wt), P. putida MAD2 ptsN::Km, and P. putida rpoN::ΩKm. Cultures of each strain were grown in M9-AA medium (supplemented or not with 10 mM glucose, as indicated) until early stationary phase (OD600 of ∼1.5). Cultures were then labeled with [35S]methionine as explained in Materials and Methods. Protein extracts were first electrofocused in a pH 4 to 7 gradient and then run across a 12.5% denaturing PAGE system. The autoradiographs of a subset of dried 2D gel results used for the scanning are shown here. A selection of spots whose intensity changes depending on the strain is indicated for reference: boxed spots (types I to V) correspond to proteins affected by the lack of ptsN (further examined in Fig. 3); circled spots (affected by glucose) coincide with the proteins whose expression is shown in Fig. 2.
When the intensity of these glucose-repressible spots was examined in extracts from the ptsN counterpart also grown in the presence of glucose, only 12 proteins appeared to have lost down-regulation by the carbohydrate. In these cases, the levels in the presence of the sugar equaled or exceeded those of the ptsN+ wild-type strain (Fig. 2). Moreover, when the P. putida MAD2 ptsN::Km mutant strain was transformed with plasmid pJM154 (which carries the wild-type ptsN allele), 6 of the 12 spots that were not repressed by glucose in the mutant reverted to being down-regulated by the sugar. Since the previously observed effect of glucose on activity of the Pu promoter of the TOL plasmid was restored upon complementation (3), the failure to revert the ptsN phenotype for half of the spots could be due to partial polar effects of the Km insertion in downstream genes (see Discussion). In any case, the modest proportion of proteins that behaved similarly to those expressed through Pu indicated that ptsN was not a mayor player in the extensive inhibition of gene expression caused by glucose on P. putida.
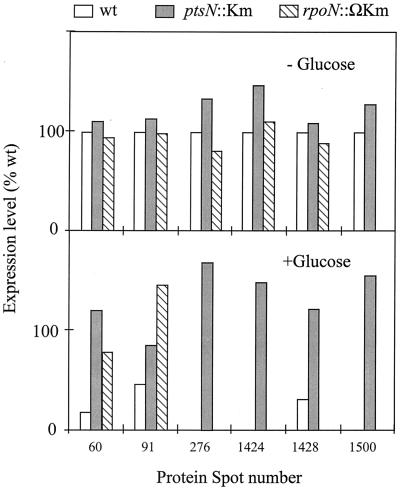
FIG. 2.
Expression of selected glucose-repressible spots in P. putida MAD2 (wt), P. putida MAD2 ptsN::Km, and P. putida rpoN::ΩKm. Cultures of each of these strains were grown, labeled, and resolved in 2D gels as explained in the legend to Fig. 1. Expression levels are plotted as a percentage of those of the wild-type strain in the absence of glucose. An expression level of zero indicates levels below detection by our experimental setup. The six spots under scrutiny (numbered 60, 91, 276, 1424, 1428, and 1500 in Fig. 1) are down-regulated by glucose only if the wild-type ptsN gene is present, but they are fully expressed in the ptsN-negative mutant, regardless of the added C source. Expression of these six proteins whose inhibition by glucose was dependent on IIANtr was reexamined in the proteome of the ς54-negative strain. Note that spots 60 and 91 are derepressed in the rpoN::ΩKm strain as they are in the ptsN::Km background, thereby indicating that their down-regulation by C source is IIANtr dependent but ς54 independent (see text for explanation).
Surveying connections between IIANtr-dependent and ς54-dependent regulation.
Most functions reported for ptsN and its encoded product IIANtr in vivo are related to up-regulation or down-regulation of ς54-dependent systems (3, 12, 18, 19). In view of the data presented above, the next issue was whether the changes brought about by the disruption of ptsN were in all cases dependent on ς54. To examine this point, we carried out the same type of 2D gel analysis with extracts of strain P. putida KT2442 rpoN::ΩKm grown under conditions equal to those used before. An important feature of this rpoN::ΩKm strain is that the insertion of a Km interposon (14) within the rpoN gene has a strong polar effect on expression of the genes downstream of the operon, as detected in Western blots with anti-IIANtr serum (not shown). The reference P. putida rpoN::ΩKm strain constructed by Köhler et al. (14) and used in this work thus fails to express not only rpoN, but also ptsN (and probably the further downstream genes of the rpoN gene cluster) (3). The six protein spots whose inhibition by glucose was unequivocally mediated by ptsN were examined in such an rpoN::ΩKm strain (Fig. 2). Interestingly, only one of them (protein spot 1500) was absent, in both the presence and absence of glucose, suggesting that its expression was indeed dependent on ς54 (or other genes of the rpoN cluster) (3). Two other proteins of the group (spots 60 and 91) behaved in the rpoN::ΩKm strain like they did in the ptsN::Km mutant (i.e., they were fully expressed regardless of the presence of the C source), but in an apparent ς54-independent fashion. Finally, the other three spots (276, 1424, and 1428) behaved basically like they did in the wild-type strain. Such a compensation for the loss of IIANtr by the lack of ς54 and/or other genes of the rpoN cluster is not easy to explain. One possibility is that some genes that behave this way could be expressed through multiple promoters such that transcription is only ς54 dependent in the presence of glucose (e.g., the activator protein required is only active under glucose-supplemented conditions). However, as discussed above, because of the polar effect of the rpoN::ΩKm insertion, the compensation for the loss of ptsN may in some cases be unrelated to ς54.
Glucose-independent effects caused by the loss of ptsN on global gene expression.
Further analysis of the 2D gels revealed a significant number of additional changes between the wild-type and ptsN extracts that were entirely independent of the presence or absence of glucose in the medium (Fig. 3). Up to 134 proteins out of the 1,117 spots analyzed were clearly down-regulated by ≥5-fold in the ptsN-negative background. In the other direction, at least 250 proteins were distinctively overexpressed in the mutant. Both events (repression and overproduction of different sets of proteins in the ptsN mutant) occurred in both glucose-containing and glucose-free media. These observations clearly indicated that the regulatory consequences of the loss of ptsN are not restricted to the presence of glucose. In addition, they suggest that depending on the specific gene product, IIANtr may act as a positive or a negative factor in a regulatory cascade.
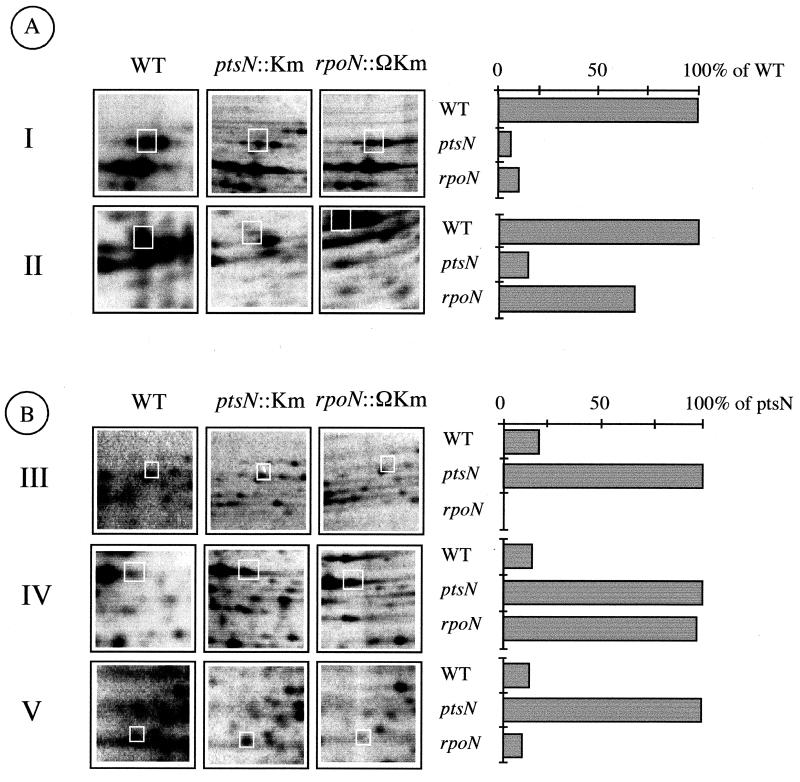
FIG. 3.
Types of spots in 2D protein gels of P. putida KT2442 and its ptsN::Km and rpoN::ΩKm variants. The photographs to the left show representative spots from the 2D gels (numbered as shown in Fig. 1), whereas the bars to the right are a quantification of the intensities of the selected proteins that fall under the various categories, as a percentage of the full expression levels, as indicated. (A) Spots whose expression is lessened in the ptsN-negative strain. Proteins of this kind do appear whose expression is either reduced in both the ptsN::Km strain and the rpoN::ΩKm strain (type I) or whose level declines in the ptsN mutant but not in the rpoN strain (type II). (B) Products whose expression is increased in the ptsN-negative mutant. Among these, some proteins are ς54-dependent products (type III), others are ς54 independent (type IV; expression is increased both in the ptsN-negative and rpoN-negative strains), and yet another class of spots (type V) augment their expression in the ptsN-negative mutant but are missing in the rpoN strain. Photographs and quantification values shown are from the cultures not supplemented with glucose. Similar patterns were detected in extracts from cells grown with glucose. (See text for interpretation.) WT, wild type.
Among the 134 spots whose expression was reduced ≥5-fold in the ptsN-negative genetic background (i.e., which required IIANtr for full expression), 48 recovered normal levels upon complementation of the mutant strain with plasmid pJM154 (Fig. 3, types 1 and 2). When the same 48 proteins were inspected in the rpoN::ΩKm strain lacking ς54, 10 of them were present at levels like those found on the ptsN-less background or lower (type 1 in Fig. 3). Whether or not these proteins require ς54 for expression cannot be ascertained with this experimental setup, since, as mentioned above, the polar effect of the ΩKm insertion also inhibits expression of the ptsN gene and the rest of the open reading frames (ORFs) of the rpoN cluster (3).
The expression levels of the remaining 38 spots in the rpoN::ΩKm strains were comparable to those of the wild type (Fig. 3, type 2), thereby indicating that they were fully independent of ς54 for expression. The conclusion of these experiments is that the bulk of the gene products which require an intact IIANtr protein for expression under various growth conditions are unrelated to ς54.
Contrary to the subset of proteins whose expression needed ptsN, 219 spots had an increased intensity in the ptsN strain compared to that of the wild-type P. putida strain, thus suggesting that IIANtr had a negative rather than a positive effect on their expression. Only 54 of these changes were reversed to normal in the P. putida MAD2 ptsN::Km(pJM154) complemented strain. Out of this whole of 54 proteins genuinely repressed by IIANtr, 5 were entirely missing in the rpoN::ΩKm strain, thus indicating that their expression required ς54 either directly or indirectly (Fig. 3, type 3). Among the remaining 49 proteins derepressed in the ptsN::Km strain, 19 turned out to be derepressed as well in the rpoN::ΩKm strain, probably due to the polar effects of the ΩKm insertion discussed above. However, the rest (30 proteins) behaved in the rpoN-negative strain like they did in the wild-type P. putida strain, thus providing another clue that ς54 plays a role in IIANtr-mediated regulatory events (Fig. 3, types 4 and 5).
A side result of this set of experiments was to realize the importance of ς54 in the general expression profile of P. putida cells. Out of the 823 protein spots that were present in both the wild-type background and in the ptsN mutant under any of the conditions tested, 93 of them (i.e., close to 10% of all reference proteins) were completely lost in the rpoN background. These spots were missing regardless of the presence or absence of glucose and were thus considered to have a ς54-dependent expression (not shown). Since the two-dimensional SDS-PAGE technology does not allow us to distinguish between direct and indirect effects, it is not possible to ascertain at this point whether ς54 participates directly in transcription of the genes encoding the missing protein spots. In any case, this result evidenced the extensive participation of ς54 in the global expression pattern of P. putida.
DISCUSSION
Inhibition of the expression of certain genes in response to facile carbon sources is a well known phenomenon in the microbial world (21, 28), although the mechanisms involved can be very disparate (6, 21, 28). A homologue of the E. coli catabolite regulatory protein (CRP) named Vfr has been described for P. aeruginosa and is also present in the P. putida genome (unpublished results). However, Vfr is involved not in C regulation but in quorum sensing (1, 27). On the other hand, a general factor encoded by the crc gene seems to mediate catabolite repression in both P. aeruginosa (17) and P. putida (10). However, the encoded protein belongs to a family of exonucleases, and its mechanism of action remains elusive (17), since it could mediate posttranscriptional rather than transcriptional checkpoints in expression of C-regulated genes (10). A higher level of C source regulation is controlled in E. coli and Bacillus by the PTS system, which dominates the events that trigger catabolite repression (21). In these bacteria, the PTS mediates transport of some sugars through a process that involves phosphorylation and dephosphorylation of a number of protein components in response to sugar availability (21, 28). Such protein intermediates behave as indicators of nutrient excess and general energy status. Although glucose is transported neither in P. aeruginosa (6, 17) nor in P. putida (30) by a PTS-like activity (16, 30), previous results indicate (3) that the phosphorylated form of the protein product encoded by ptsN (IIANtr), a PTS type II protein, is necessary for C source repression of the ς54-dependent promoter Pu of the TOL plasmid. As summarized in Fig. 4, the results presented in this article show that the role of ptsN in such a C repression of ς54 systems is in fact limited to just a few cases within a much wider role of IIANtr in global expression profiles of P. putida.
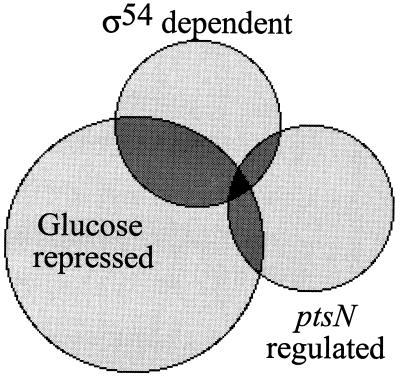
FIG. 4.
Connections between IIANtr, ς54, and glucose-repressible expression in P. putida as revealed by 2D gel analysis of ptsN::Km and rpoN::ΩKm mutants. Among the 1,117-spot subset of the P. putida proteome, as many as 247 products were repressible by glucose (i.e., had expression ≥2-fold lower in the presence of the sugar), while 93 proteins were dependent on ς54 (i.e., were entirely missing in extracts of the rpoN strains under all conditions). The ptsN-regulated spots considered for this representation include only those whose changes (≥5-fold greater or lesser than the wild-type levels) can be complemented by a functional ptsN copy. The areas of the circles are approximately proportional to the number of spots included in the categories represented.
The data described above revealed that the loss of ptsN affects expression of a range of proteins in a fashion entirely independent of glucose (Fig. 3). In fact, it comes as a surprise that the loss of IIANtr influences expression of such a large number of polypeptides. Close to 10% of all of the spots analyzed in the 2D gels were found to be up- or down-regulated by IIANtr. This could account for the diversity of phenotypes described in ptsN mutants of different species (12, 13, 18, 19, 22). Most of the proteins affected in the ptsN mutant (87 out of 102) were independent of ς54, thus highlighting IIANtr as a general regulatory factor not limited to Pu-like systems (25). A separate issue in this respect is the connection between ς54 and ptsN functions. As mentioned above, since the rpoN::ΩKm strain is a phenotypic rpoN ptsN double mutant, the rpoN::ΩKm and ptsN::Km strains should show partially overlapping phenotypes. However, this is not the case for a significant proportion of the spots analyzed, suggesting that some promoters may receive separate inputs through signalling pathways involving ς54, IIANtr, and even some additional genes of the rpoN cluster (3).
Only around 30% of all of the changes caused by the Km insertion in ptsN could be unequivocally traced to the lack of IIANtr, as shown by the comparison of the mutant and the strains complemented with a ptsN+ plasmid. Although the Km insertion in ptsN allowed expression of downstream genes of the gene cluster (as revealed with a serum against the product of the last ORF), the levels were somewhat reduced (not shown). Some spots could therefore be controlled by one or more genes of the rpoN cluster other than ptsN. These could act in concert or independently, an issue that deserves further studies. In fact, that the same regulatory factor causes a variety of effects in expression or activity of different proteins is typical of PTS proteins (19). Examples include the phosphorylation-dependent ability of IIAGlu to interact with a number of permeases (19) or the formation of the CcpA-HPr repressor complex in gram-positive bacteria (28). This could be the case also for IIANtr: two forms of the factor (phosphorylated and nonphosphorylated) could specialize in regulation of different sets of proteins, in concert with other factors (such as ς54 itself) and other PTS components. Regardless of the specific mechanisms involved, the incidence and extension of effects linked to the ptsN gene make it appear to be more of a general regulator than a promoter-specific factor.
ACKNOWLEDGMENTS
We are grateful to T. Kölher (CMU, Geneva, Switzerland) for the gift of plasmids. Inspiring discussions with J. Pérez-Martín and T. Nyström contributed significantly to the outcome of this project.
This work was supported by contracts BIO4-CT97-2040 and QLRT-99-00041 of the EC and by grant BIO98-0808 of the Spanish Comisión Interministerial de Ciencia y Tecnología (CICYT).
REFERENCES
- 1.Albus A M, Pesci E C, Runyen-Janecky L J, West S E H, Iglewski B H. Vfr controls quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3928–3935. doi: 10.1128/jb.179.12.3928-3935.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assinder S J, Williams P A. The TOL plasmids: determinants of the catabolism of toluene and xylenes. Adv Microb Physiol. 1990;31:1–69. doi: 10.1016/s0065-2911(08)60119-8. [DOI] [PubMed] [Google Scholar]
- 3.Cases I, Pérez-Martín J, de Lorenzo V. The IIANtr (PtsN) protein of Pseudomonas putida mediates the C-source inhibition of the ς54-dependent Pu promoter of the TOL plasmid. J Biol Chem. 1999;274:15562–15568. doi: 10.1074/jbc.274.22.15562. [DOI] [PubMed] [Google Scholar]
- 4.Cases I, de Lorenzo V, Pérez-Martín J. Involvement of ς54 in exponential silencing of the Pseudomonas putida TOL plasmid Pu promoter. Mol Microbiol. 1996;19:7–17. doi: 10.1046/j.1365-2958.1996.345873.x. [DOI] [PubMed] [Google Scholar]
- 5.Cases I, de Lorenzo V. Genetic evidence of distinct physiological regulation mechanisms in the ς54Pu promoter of Pseudomonas putida. J Bacteriol. 2000;182:956–960. doi: 10.1128/jb.182.4.956-960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collier D N, Hager P W, Phibbs P V., Jr Catabolite repression control in the pseudomonads. Res Microbiol. 1996;147:551–561. doi: 10.1016/0923-2508(96)84011-3. [DOI] [PubMed] [Google Scholar]
- 7.Davis R W, Roth J R, Botstein D. Advanced bacterial genetics: a manual for genetic engineering. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1980. [Google Scholar]
- 8.Fernández S, de Lorenzo V, Pérez-Martín J. Activation of the transcriptional regulator XylR of Pseudomonas putida by release of repression between functional domains. Mol Microbiol. 1995;16:205–213. doi: 10.1111/j.1365-2958.1995.tb02293.x. [DOI] [PubMed] [Google Scholar]
- 9.Hester K L, Madhusudhan K T, Sokatch J R. Catabolite repression control by Crc in 2xYT medium is mediated by posttranscriptional regulation of bkdR expression in Pseudomonas putida. J Bacteriol. 2000;182:1150–1153. doi: 10.1128/jb.182.4.1150-1153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hester K L, Lehman J, Najar F, Song L, Roe B A, MacGregor C H, Hager P W, Phibbs P V, Jr, Sokatch J R. Crc is involved in catabolite repression control of the bkd operons of Pseudomonas putida and Pseudomonas aeruginosa. J Bacteriol. 2000;182:1144–1149. doi: 10.1128/jb.182.4.1144-1149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holtel A, Marqués S, Möhler I, Jakubzik U, Timmis K N. Carbon source-dependent inhibition of xyl operon during expression of the Pseudomonas putida TOL plasmid. J Bacteriol. 1994;176:1773–1776. doi: 10.1128/jb.176.6.1773-1776.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janakiraman R S, Brun Y V. Transcriptional and mutational analyses of the rpoN operon in Caulobacter crescentus. J Bacteriol. 1997;179:5138–5147. doi: 10.1128/jb.179.16.5138-5147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin S, Ishimoto K, Lory S. Nucleotide sequence of the rpoN gene and characterization of two downstream open reading frames in Pseudomonas aeruginosa. J Bacteriol. 1994;176:1316–1322. doi: 10.1128/jb.176.5.1316-1322.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Köhler T, Harayama S, Ramos J-L, Timmis K N. Involvement of Pseudomonas putida RpoN ς factor in regulation of various metabolic functions. J Bacteriol. 1989;171:4326–4333. doi: 10.1128/jb.171.8.4326-4333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Köhler T, Fernández-Alvárez J, Harayama S. Regulation of rpoN, ORF102 and ORF154 genes in Pseudomonas putida. FEMS Microbiol Lett. 1994;115:177–184. doi: 10.1111/j.1574-6968.1994.tb06634.x. [DOI] [PubMed] [Google Scholar]
- 16.Lessie T G, Phibbs P V., Jr Alternative pathways of carbohydrate utilization in pseudomonads. Annu Rev Microbiol. 1984;38:359–388. doi: 10.1146/annurev.mi.38.100184.002043. [DOI] [PubMed] [Google Scholar]
- 17.MacGregor C H, Wolff J A, Arora S K, Phibbs P V., Jr Cloning of a catabolite repression control (crc) gene from Pseudomonas aeruginosa, expression of the gene in Escherichia coli, and identification of the gene product in Pseudomonas aeruginosa. J Bacteriol. 1991;173:7204–7212. doi: 10.1128/jb.173.22.7204-7212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merrick M J, Coppard J R. Mutations in genes downstream of the rpoN gene (encoding ς54) of Klebsiella pneumoniae affect expression from ς54-dependent promoters. Mol Microbiol. 1989;3:1765–1775. doi: 10.1111/j.1365-2958.1989.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 19.Michiels J, Van Soom T, D'Hooghe I, Dombrecht B, Benhassine T, de Wilde P, Vanderleyden J. The Rhizobium etli rpoN locus: DNA sequence analysis and phenotypical characterization of rpoN, ptsN, and ptsA mutants. J Bacteriol. 1998;180:1729–1740. doi: 10.1128/jb.180.7.1729-1740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 21.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate:carbohydrate phosphotransferase system in bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell B S, Court D L, Inada T, Nakamura Y, Michotey V, Cui X, Reizer A, Saier M H, Reizer J. Novel proteins of the phosphotransferase system encoded within the rpoN operon of Escherichia coli. J Biol Chem. 1995;270:4822–4839. doi: 10.1074/jbc.270.9.4822. [DOI] [PubMed] [Google Scholar]
- 23.Rabus R, Reizer J, Paulsen I, Saier M H. Enzyme INtr from Escherichia coli. A novel enzyme of the phosphoenolpyruvate-dependent phosphotransferase system exhibiting strict specificity for its phosphoryl acceptor, NPr. J Biol Chem. 1999;274:26185–26191. doi: 10.1074/jbc.274.37.26185. [DOI] [PubMed] [Google Scholar]
- 24.Ramos J L, Marqués S, Timmis K N. Transcriptional control of the Pseudomonas TOL plasmid catabolic operons is achieved through an interplay of host factors and plasmid encoded regulators. Annu Rev Microbiol. 1997;51:341–373. doi: 10.1146/annurev.micro.51.1.341. [DOI] [PubMed] [Google Scholar]
- 25.Reizer J, Reizer A, Saier M H, Jacobson G R. A proposed link between nitrogen and carbon metabolism involving protein phosphorylation in bacteria. Protein Sci. 1992;1:722–726. doi: 10.1002/pro.5560010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reizer J, Reizer A, Merrick M J, Plunkett G, Rose D J, Saier M H. Novel phosphotransferase-encoding genes revealed by analysis of the Escherichia coli genome: a chimeric gene encoding an enzyme I homologue that possesses a putative sensory transduction domain. Gene. 1996;181:103–108. doi: 10.1016/s0378-1119(96)00481-7. [DOI] [PubMed] [Google Scholar]
- 27.Runyen-Janecky L J, Sample A K, Maleniak T C, West S E H. A divergently transcribed open reading frame is located upstream of the Pseudomonas aeruginosa vfr gene, a homolog of Escherichia coli crp. J Bacteriol. 1997;179:2802–2809. doi: 10.1128/jb.179.9.2802-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saier M H., Jr Cyclic AMP-independent catabolic repression in bacteria. FEMS Microbiol Lett. 1996;138:97–103. doi: 10.1111/j.1574-6968.1996.tb08141.x. [DOI] [PubMed] [Google Scholar]
- 29.Sawyer M H, Baumann P, Berman S M, Canovas J L. Pathways of D-fructose catabolism in Pseudomonas. Arch Microbiol. 1977;112:49–55. doi: 10.1007/BF00446653. [DOI] [PubMed] [Google Scholar]
- 30.Schleissner C, Reglero A, Luengo J M. Catabolism of D-glucose by Pseudomonas putida U occurs via extracellular transformation into D-gluconic acid and induction of a specific gluconate transport system. Microbiology. 1997;143:1595–1603. doi: 10.1099/00221287-143-5-1595. [DOI] [PubMed] [Google Scholar]
- 31.Sze C C, Shingler V. The alarmone (p)ppGpp mediates physiological-responsive control at the ς54-dependent Po promoter. Mol Microbiol. 1999;31:1217–1228. doi: 10.1046/j.1365-2958.1999.01264.x. [DOI] [PubMed] [Google Scholar]