Abstract
Simple Summary
Early diagnosis of renal cell carcinoma (RCC) is challenging and typically incidental. Currently, several therapeutic strategies are used for the treatment; however, no established predictive biomarker has been established yet, and the optimal treatment choice and sequence of use remain unclear. Moreover, the recurrence occurs in about one-third of patients after tumor resection. Although several prognostic classification systems have been proposed, most of them showed only limited potential in recurrence prediction. Therefore, identifying simple, reliable, and easily accessible biomarkers to anticipate the diagnosis, effectively evaluate the risk of relapse, and predict the response to the therapeutic regimens is an unmet clinical need. Circulating cell-free DNA (cfDNA), released from cancer cells into the bloodstream, was shown to be a non-invasive, viable, inexpensive method to diagnose and monitor several solid malignancies, designed as a potential blood RCC biomarker. This review aims to summarize the state of the art of the current genetic and epigenetic techniques of plasma and serum cfDNA detection and outline the potential application of liquid biopsy in RCC.
Abstract
Tumor biopsy is still the gold standard for diagnosing and prognosis renal cell carcinoma (RCC). However, its invasiveness, costs, and inability to accurately picture tumor heterogeneity represent major limitations to this procedure. Analysis of circulating cell-free DNA (cfDNA) is a non-invasive cost-effective technique that has the potential to ease cancer detection and prognosis. In particular, a growing body of evidence suggests that cfDNA could be a complementary tool to identify and prognosticate RCC while providing contemporary mutational profiling of the tumor. Further, recent research highlighted the role of cfDNA methylation profiling as a novel method for cancer detection and tissue-origin identification. This review synthesizes current knowledge on the diagnostic, prognostic, and predictive applications of cfDNA in RCC, with a specific focus on the potential role of cell-free methylated DNA (cfMeDNA).
Keywords: kidney cancer, liquid biopsy, cell-free methylated DNA, genetic, epigenetic, microsatellite instability
1. Introduction
Kidney cancer is the third most frequent urologic cancer in adults and accounts for approximately 2–3% of all annually diagnosed malignancies in the United States [1]. Renal cell carcinoma (RCC) accounts for the vast majority of kidney cancers. RCC early diagnosis is challenging and typically incidental, primarily relying on radiological procedures executed for other indications [2]. Radical or partial nephrectomy offers curative responses in patients with localized RCC and selected patients with advanced RCC [3]. However, the disease recurs in about one-third of patients after resection of the primary tumor [4]. Existing prognostic classification systems based on clinical-pathological features, such as TNM stage and Fuhrman grading, showed limited potential to predict recurrence [5]. Currently, several therapeutic modalities comprising tyrosine kinase inhibitors, mammalian target rapamycin inhibitors, and immunotherapeutic agents are used for the treatment of metastatic RCC (mRCC) [6,7,8]. However, no established biomarker predictive of response to any of these treatments has been established yet, and there is uncertainty regarding their optimal choice and sequence of use in clinical practice. Therefore, identifying simple, accurate, and easily accessible biomarkers to boost the diagnostic specificity, effectively evaluate the risk of relapse following nephrectomy and predict the response to the therapeutic regimens currently offered for metastatic disease is an unmet clinical need.
To date, molecular hallmarks, assessed directly in primary tumor tissue, drive therapy and predict prognosis in several types of solid malignancies [9,10,11,12,13,14,15,16,17]; however, often, their evaluation is not reliable and presents some limitations [18,19,20,21]. Circulating tumor DNA (ctDNA), DNA released from cancer cells into the bloodstream or other body fluids, has been increasingly investigated as a “liquid biopsy” and proved to enable comprehensive genomic profiling of several tumors at various time points [22,23,24,25,26,27,28,29]. Further, the analysis of cfDNA was shown to be a viable, inexpensive, and less-invasive method to diagnose and monitor cancer hence circumventing the well-known shortcomings of invasive tissue biopsies [26,30]. In the recent past, as the concordance of genetic alterations between cfDNA and matched tumor biopsy was validated, there has been growing enthusiasm for the use of cfDNA as a potential blood cancer biomarker. In addition, epigenetic features of cfDNA, such as methylation of cfDNA (cfMeDNA), have been increasingly investigated in recent years [31,32]. Methylation of the pyrimidine ring of cytosine was shown to inhibit transcription when found in CpG islands and stabilize the genome when found in non-coding DNA regions [32,33]. The stability and specificity of CpG island methylation patterns demonstrated high sensitivity for detecting and classifying several tumor types, and thus, the analysis of DNA methylome is a promising cancer biomarker [34,35,36].
This review aims to describe the current genetic and epigenetic techniques of plasma and serum cfDNA detection and outline the potential application of cfDNA as a liquid biopsy in RCC, emphasizing its role in cancer detection, recurrence, prognosis, and prediction of therapy outcomes.
2. Data Acquisition
In this non-systematic literature review, PUBMED and MEDLINE were thoroughly searched through 20 April 2022 for peer-reviewed prospective and retrospective studies, reviews, and abstracts regarding cfDNA and cfMeDNA applied to detect, monitor, or prognosticate RCC. To this aim, keywords used included “kidney neoplasm”, “renal cell cancer”, “cell-free DNA”, “circulating DNA”, “circulating tumor DNA”, “methylated DNA”, and “liquid biopsy”. The documents more relevant to this review were selected, reviewed, and their data extracted.
3. Isolation and Analysis of cfDNA
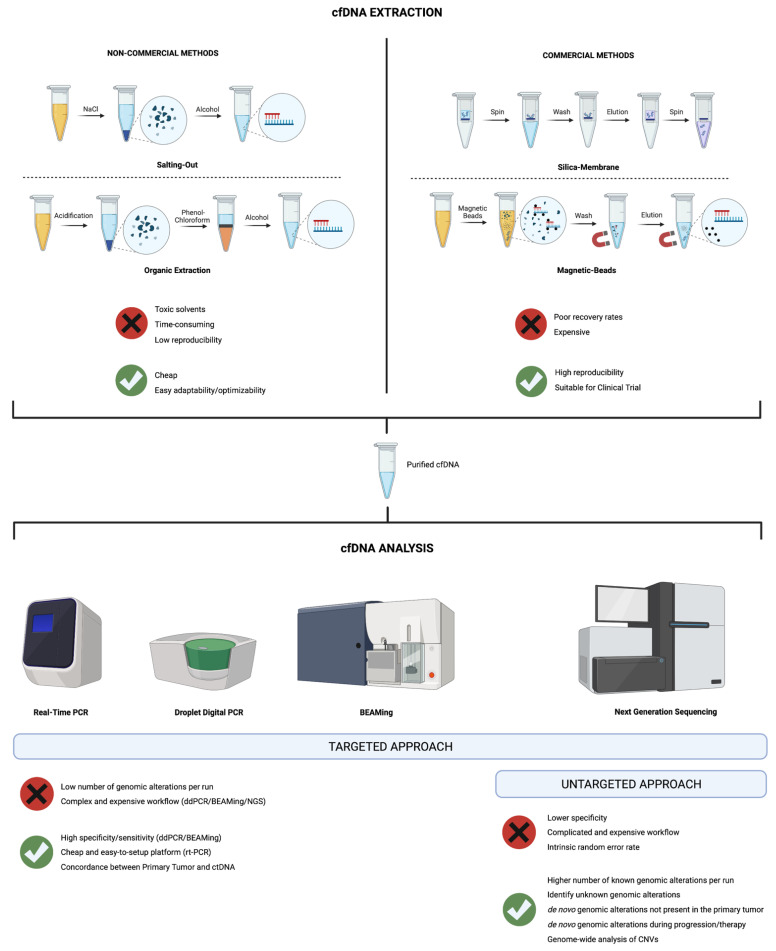
The efficiency and consistency of the cfDNA extraction process are impacted by the technique chosen to isolate cfDNA, which is also partly responsible for the significant heterogeneity of the cfDNA data among the studies reported in this review. The key technologies used to obtain cfDNA from blood can be divided into “commercial methods” including anion-exchange-based ready-to-use extraction package, silica-membrane, or magnetic-particle technique, and “non-commercial methods,” such as the extraction of phenol-chloroform, guanidine-resin, alcohol precipitation, and salting-out processes.
Only two studies in the current review performed cfDNA extraction with phenol-chloroform extraction and ethanol precipitation in RCC patients [37,38]. Although many “non-commercial methods” achieve high yields and allow for the extraction of more small-sized fragments, they include toxic solvents, are more time-consuming, and are generally not considered appropriate for clinical analyses due to the high coefficient of results variability. Therefore, most studies of cfDNA are performed using “commercial methods”. They have good repeatability and reproducibility and thus enable standardization for clinical studies, although the loss of small and informative cfDNA fragments during the isolation process results in poor recovery rates. Therefore, the development of novel techniques is urgently required to improve the efficiency and quality of cfDNA extraction. cfDNA extraction techniques are summarized in Figure 1.
Figure 1.
Graphical representation of the cfDNA extraction methods and analysis approaches. Advantages and disadvantages are highlighted for each technique.
Once cfDNA is isolated, different approaches can be used for analysis. Ideally, cfDNA analytical techniques should work with low and very low inputs of genomic material, have a high discriminatory capacity to distinguish between cancer ctDNA and germline cfDNA shed by normal cells, show a reasonable cost-benefit ratio, and be reproducible among centers and in longitudinal cohorts. Two different strategies can be implemented: targeted approaches, which allow the detection of few, a priori selected genes, or untargeted approaches, which perform a genome-wide analysis of potential genomic alterations and do not require a selection of genes of interest.
Targeted approaches encompass a variety of traditional candidate gene methods (e.g., real-time polymerase chain reaction (real-time PCR), droplet digital PCR (ddPCR), and beads, emulsions, amplification and magnetics (BEAMing) assay) and next-generation sequencing (NGS)-based techniques (e.g., AmpliSeq, SAFE-SeqS, CAPP-Seq, and MCTA-Seq). Real-time PCR offers a cheap and easy-to-setup platform to study targeted genes with a very low rate of false positives and with no need for complex bioinformatic pipelines to analyze the data. Different protocols have been developed to increase sensitivity, including allele-specific amplification (AS-PCR) [39] and co-amplification at lower denaturation temperature (COLD-PCR) [40]. Of note, AS-PCR protocols are already clinically implemented to study small genomic alterations, such as indels or single nucleotide variations. Therefore, the implementation of these approaches to ctDNA analysis may expand the testing capacity at many clinical sites. The two techniques based on digital PCR—ddPCR and BEAMing—allow the detection of known point mutations present at allele frequencies of <0.01% [41,42]. Both techniques are based on the idea of generating DNA-containing droplets to be used to detect and quantify the presence of a particular genomic aberration [43,44]. Indeed, several studies have proven the utility of digital PCR-based approaches in different solid malignancies, including breast, lung, and colorectal cancer [45,46,47,48]. Despite optimal rates of specificity, sensitivity, and concordance between genomic alterations found in the primary tumor and in the ctDNA, these techniques are limited by the low number of a priori selected alterations that can be tested for reaction, as well as a complicated and expensive workflow for the BEAMing technique. NGS-based targeted approaches rely on the ability to sequence in parallel millions of DNA sequences to identify potential genetic alterations. Several methods have been designed to detect known copy number alterations and point mutations, overcoming at the same time the random error rate intrinsic to NGS approaches [49]. A thorough discussion of the specific technical details of each NGS-based approach is beyond the scope of this review. In general, while these methods allow for interrogation of a higher number of known alterations per run compared to the previously discussed approaches, they require bioinformatics training and are generally more laborious and expensive. At the same time, rigorous benchmarking of several analytic techniques is needed to determine the best pipelines for ctDNA analyses. Finally, as targeted approaches allow for accurate detection of selected genomic alterations, they do not address well tumor clonal heterogeneity and new mutations in response to therapy.
On the other hand, untargeted, NGS-based profiling of ctDNA by deep sequencing offers the opportunity to perform a genome-wide analysis of copy number alterations and mutations [50]. Moreover, this approach allows the discovery of novel alterations that were not present in the primary tumor or in the a priori selected panel of candidate genes, including de novo alterations arising during tumor progression or therapy. Untargeted sequencing appears particularly appealing, especially in the context of precision oncology protocols, where the emergence of a novel genomic alteration characterized through ctDNA may explain therapeutic resistance or lead to the administration of targeted therapies against the detected alteration [51]. However, the routine clinical application of this approach is still hampered by higher costs and lower specificity (∼80–90%) compared to other methods [49], even if the introduction of molecular barcoding or identifiers generally resulted in lower rates of false negatives [52,53]. cfDNA analysis approaches are summarized in Figure 1.
While every single approach has advantages and disadvantages, the wide implementation of ctDNA liquid biopsy in the future will be based on a combination of different targeted and untargeted techniques. Targeted methods would be potentially used to detect the presence of well-described driver genomic alteration with great confidence, while untargeted sequencing would be used longitudinally to monitor tumor clonal plasticity, the emergence of therapy resistance, or novel, potentially druggable mutations.
4. Applications of cfDNA Analysis in RCC
Recent studies suggest that the technological advancements in cfDNA assessment allow for the use of cfDNA as a reliable biomarker of cancer diagnosis, potentially permitting an early identification and treatment of RCC [54]. The evaluation of cfDNA levels, integrity, and genetic and epigenetic alterations showed to be useful for monitoring post-operative recurrence, predicting response to targeted therapies, and indicating the prognosis of RCC. The detailed applications of cfDNA in the detection, monitoring, and prognostication of RCC are described below.
4.1. Diagnostic Role
RCC is typically detected by an abdominal ultrasound examination [2]. However, owing to its precision and accuracy limitations, suspicious findings must be validated by CT or MRI scans [55]. While the analysis of cfDNA could aid the detection of early signs of the disease and the distinction between benign and malignant renal lesions, there are no clear recommendations yet regarding the use of cfDNA for these aims. A major hurdle to RCC diagnosis by means of cfDNA analysis is that of all extracranial tumors studied, RCC sheds the least amount of ctDNA in the bloodstream, and its detection is challenging [56,57,58]. In addition, the relevant discrepancies of cfDNA isolation and detection techniques among the studies investigating cfDNA in RCC make comparisons of findings quite challenging. Standardization of these methods within large prospective trials is warranted to validate cfDNA as a screening biomarker for RCC.
4.1.1. cfDNA Levels in RCC, Healthy Controls and Non-Cancer Disease
Five prospective studies evaluated cfDNA levels in a total of more than 300 RCC patients and 140 control subjects [57,59,60,61,62]. Although an analysis of the aggregate data of these reports is biased by the several heterogeneities among the studies, including cfDNA sources (serum versus plasma), isolation and evaluation techniques, and populations with different disease states, it should be noted that in all five analyses the levels of cfDNA in RCC patients were higher than in healthy controls.
The analysis by Perego et al. of 48 patients with RCC and 41 healthy subjects showed that the mean pre-operative plasma cfDNA concentration in the RCC group was eight times higher than that of the healthy controls (26.4 vs. 3.2 ng/mL, p = 0.003) and decreased after nephrectomy [59]. Of note, the significance with respect to the controls persisted even when patients were stratified by sex, age, histology, tumor size, grading, and pathological TNM stratification [59].
The largest study assessing cfDNA levels conducted so far, including 157 patients with RCC and 43 with benign lesions, showed increased pre-operative cfDNA levels in those with the malignancy than in those with benign tumors (3319 vs. 1288 genetic equivalents/mL, p < 0.001), implying that cfDNA may help in the differential diagnosis of a solid renal masses [60]. Furthermore, total cfDNA levels were associated with tumor necrosis, lymph node involvement, and metastatic disease [60]. Wan et al. documented increased levels of cfDNA in patients with metastatic RCC (mRCC) compared to patients with localized RCC and healthy individuals [57]. Further, in this case, they found a significant correlation of plasma cfDNA levels with different Fuhrman grades, TNM stages, and tumor sizes, suggesting an association with tumor aggressiveness [57]. This seems to find confirmed in the study by Feng et al., where cfDNA levels were significantly associated with TNM stage, Fuhrman grade and number of metastases increased pretreatment amounts of cfDNA were similarly found in mRCC patients compared to healthy individuals [61].
Although, in aggregate, these data seem to suggest a correlation between raised plasma or serum levels of cfDNA and the presence of RCC, the cumulative body of evidence is still not sufficiently solid. Because increased cfDNA may be found in other non-cancer conditions, such as benign lesions, inflammatory or autoimmune diseases, and tissue traumas, the utility of cfDNA quantification to detect the presence of RCC is indeed limited [63,64,65]. Therefore, cfDNA analysis alone should not be considered a valid screening instrument for RCC at present.
4.1.2. cfDNA Integrity as a Tool for Differentiating RCC and Non-Cancer Controls
Postulating that cfDNA derived from cancer was released mainly by necrosis compared to the cfDNA released in non-malignant diseases which origins mainly by apoptosis, three studies assessed size variations of cfDNA fragments to determine potential associations with the presence of RCC [66,67,68].
Using quantitative real-time PCR, Hauser et al. analyzed the serum cfDNA integrity, defined as the ratio of the longer fragment actine-beta gene 384 (ACTB384) derived from necrosis and the shorter fragment actine-beta gene 106 (ACTB106) derived from apoptosis of 35 RCC patients and 54 healthy controls [66]. The levels of both cfDNA fragments were found to increase in the pre-operative serum of RCC patients compared to the healthy individuals, indicating that cfDNA is fragmented to a higher degree in cancer patients [66]. Further, the significantly higher quantity of ACTB384 compared to ACTB106 observed in RCC supported the hypothesis of the necrotic origin of the cfDNA derived from the tumor. Consistently with these findings, Gang et al. performed conventional PCR using different primers able to detect the different sizes of the product of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase gene (GADPH) and observed a higher concentration of long fragments in the serum of pre-operative RCC patients compared to those in the healthy control group, indicating that necrosis is a more common occurrence in RCC [67]. Moreover, in this cohort, cfDNA integrity was also significantly associated with tumor stage and size [67].
In contrast, a study by Lu et al. found no difference in the quantity of the cfDNA fragments among patients with mRCC, non-metastatic RCC, and healthy controls [68]. However, it should be noted that this analysis was performed on plasma as opposed to serum cfDNA. Moreover, in this study, there was a decrease in cfDNA integrity in controls compared to those with metastatic disease, confirming that a higher cfDNA fragmentation could be indicative of RCC [68].
Although the findings of most studies support the hypothesis that ctDNA is more likely released by necrotic cancer cells and thus it is composed of larger fragments compared to non-tumor-derived cfDNA, likely released by apoptotic cells, the limited number of samples analyzed, the lack of standardization in measuring long vs. short fragments of cfDNA and the modest sensitivity and specificity values for short and long fragment detection prevent us from considering cfDNA size as a reliable diagnostic biomarker. The specific size of tumor-derived DNA fragments, the detailed characterization of tumor-derived alterations, and the potential implications of these biological differences have not been sufficiently explored yet.
4.1.3. cfDNA Genetic Alterations
The identification of genetic alterations (GA), such as microsatellite instability (MSI) or loss of heterozygosity (LOH) in the cfDNA, is another feature of cfDNA which holds potential as a biomarker of early diagnosis of RCC. Diagnostic data of LOH and MSI in cfDNA from RCC are summarized in Table 1.
Table 1.
Diagnostic data of microsatellite alterations (loss of heterozygosity [LOH] and/or microsatellite instability [MSI]) in circulating cell-free DNA (cfDNA) from renal cell carcinoma (RCC). AML, angiomyolipoma; cfDNA, cell-free DNA; MN, metanephricnephroma; NR, not reported; OCT oncocytoma; RCC, renal cell carcinoma; TCC, transitional cell carcinoma; MA, microsatellite alteration (loss of heterozygosity [LOH] and/or microsatellite instability [MSI]). * MA was performed only in 9 patients whose pre-operative plasma DNA was available.
| Author, Year, [Ref.] | Microsatellite Markers | Patients (n) | Controls (n) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Goessl, 1998, [79] | D3S1307(3p), D3SI560(3p), D3SI289(3p), D3SI300(3p) | 40 RCC | 10 healthy individuals | 63 (at least one MA) 35 (more than one MA) |
100 |
| Eisenberger, 1999, [37] | D1S251 (1pq), HTPO(2p), D3S1317(3p), D3S587(3p), D3S1560(3p), D3S1289(3p), D3S1286 (3p), D3S1038(3p), D4S243(4pq), FGA(4)(4q), CSF(5q), ACTBP2(5p), D8S348(8q), D8S307(8p), D9S747(9p), D9S242(9p), IFNa(9p), D9S162(9p), D11S488(11q), THO(11p), vWA(12p), D13S802(13q), MJD(14q), D17S695(17p), D17S654(17p), D18S51(18q), MBP(18q), D21S1245(21q). | 25 RCC 1 AML 1 MN 3 OCT |
8 individuals with nephrolithiasis 8 healthy individuals |
60 (at least one MA) | 100 |
| von Knobloch, 2002, [80] | D3S1560(3p), D3S2450(3p), D3S3666(3p), D3S2408(3p), D3S1259(3p), D5S1720(5p), D5S1480(5p), D5S476(5p), D5S818(5p), D7S1796(7p), D7S1807(7p), D8S261(8p), D8S560(8p), D9S925(9p), D13S153(13p), D17S799(17p), D17S1306 (17p), D17S783(17p), D17S1298(17p), D17S807(17p) | 53 RCC 1 renal B cell lymphoma 6 TCC |
20 healthy individuals | 74 (using 9 MA) 87 (using 20 MA) |
85 |
| Perego, 2008, [59] | D3S1566(3p), D3S1285(3p), D3S1300(3p), D3S1289(3p), D3S1597(3p) | 48 RCC 1 TCC 5 OCT |
41 healthy individuals | 55.6 (at least one MA) * | NR |
As mentioned above, some studies showed that, out of all extracranial tumors, RCC is one of the least producers of cfDNA, which results in a relatively low detection rate of cfDNA [56,57,58]. In turn, that seems to impair the probability of detecting GAs in cfDNA of patients with RCC. Indeed, GAs were detected in a percentage of patients with RCC ranging from 3.9% to 78.6% in analyses of cfDNA using panels of selected genes [28,58,69,70,71,72,73,74,75,76,77,78]. The GA identification rate was higher in those reports that used panels with a greater number of selected genes. Therefore, the observed high variability in GAs detection rate may partly depend on the number of genes investigated.
Goessl et al. detected GAs in plasma cfDNA of RCC patients by applying only four markers for microsatellite alterations (MSI or LOH) on chromosome 3p [79]. The observed alterations were not correlated with the stage of the tumor and were not found in the healthy controls [79]. Notably, although 63% of patients in this study had LOH in at least one locus and 35% in more than one locus, only one patient was found with MSI [79].
A similar rate of microsatellite alterations was observed in the serum-derived cfDNA of RCC patients in a smaller prospective study [37]. In this case, 60% of patients with malignant renal tumors had at least one alteration in the 28 microsatellite markers of the 20 chromosome regions investigated [37]. More importantly, this study also reported no cfDNA aberrations in healthy controls and no association between cfDNA alterations and tumor stage in those with RCC [37].
A later analysis focused on 20 microsatellite markers located on chromosomes 3p and 5q [80]. Serum cfDNA GAs were detected in 74% of cases using 9 markers, in 87% of cases using 11 markers, and only in 15% of controls using 10 markers, suggesting that cfDNA GAs may be associated with RCC [80]. In this respect, Perego et al. investigated five microsatellite alterations on chromosome 3p in nine of the 54 RCC patients whose pre-operative plasma was available [59]. Five of those nine patients had at least one of the five microsatellite markers, and these variations were also demonstrated in their primary tumor [59]. Although in aggregate, these data would portend a potential association between cfDNA GAs and RCC detection, the sensitivity and specificity of these analyses seem to depend on the number of microsatellites investigated. In this regard, the comprehensive study of microsatellites and GAs currently offered by NGS may be a more efficient and accurate method of analysis.
4.1.4. cfDNA Epigenetic Alterations: A New Promising Method to Detect RCC
Another feature recently increasingly investigated as a potential diagnostic biomarker of RCC is the hyper- or hypo-methylation of cfDNA. Targeted methylation and global methylation analyses seemed to provide high specificity and sensitivity in distinguishing RCC patients from healthy controls compared to genetic analysis. The high tissue and cell type specificity of DNA methylation, as well as its high stability, would make it an ideal biomarker of RCC detection [31,32]. Diagnostic data of methylation analysis of cfDNA in patients with RCC are summarized in Table 2. Moreover, methylation of genes occurs frequently in the early stages of RCC [81]. For instance, methylation of the Ras association domain family member 1A (RASSF1A) seems to be fairly common in patients with RCC [38,60,62,82]. In this respect, recent analyses showed RASSF1A methylation ranging from 23% to 63% in RCC patients [38,60,62,82]. These discrepancies in the methylation frequency of the RASSF1A gene could be partly explained by the use of a bisulfate-based assay in lieu of the methylation-sensitive restriction enzyme assay, which is more sensitive.
Table 2.
Diagnostic data of methylation analysis of circulating cell-free DNA (cfDNA) in patients with renal cell carcinoma (RCC). APC, adenomatosis-polyposis-coli gene; ARF, auxin response factors; CDH1, cadherin-1 gene; cfDNA, cell-free DNA; FHIT, fragile histidine triad gene; GSTP1, glutathione-S-Transferase Pi 1 gene; ITGA9, integrin subunit alpha 9 gene; LRRC3B, leucine rich repeat containing 3B gene; MGMT, O-6-methylguanine-DNA methyltransferase gene; NR, not reported; p16, cyclin-dependent kinase inhibitor 2A; PCR, Polymerase chain reaction; PTGS2, prostaglandin–endoperoxide.
| Author, Year, [Ref.] | Gene | Methylated /RCC Patients (n), (%) |
Methylated /Controls (n), (%) |
Sensitivity (%) | Specificity (%) | Cutoff Value | AUC | Sample Source, Amount | Detection Method |
|---|---|---|---|---|---|---|---|---|---|
| Hoque, 2004, [38] | APC | 1/18 (5.5) | 1/30 (3.3) | 5.5 | 96.7 | 4.5 | NR | Serum, NR | PCR |
| ARF | 1/18 (5.5) | 1/30 (3.3) | 5.5 | 96.7 | 0 | NR | |||
| CDH1 | 6/18 (33.3) | 2/30 (6.6) | 33.3 | 93.4 | 0.3 | NR | |||
| GSTP1 | 1/18 (5.5) | 0/30 (0) | 5.5 | 100 | 0 | NR | |||
| MGMT | 0/18 (0) | 1/30 (3.3) | 0 | 96.7 | 0 | NR | |||
| p16 | 4/18 (22.2) | 0/30 (0) | 22.2 | 100 | 0 | NR | |||
| RAR-82 | 1/18 (5.5) | 0/30 (0) | 5.5 | 100 | 0.1 | NR | |||
| RASSF1A | 2/18 (11.1) | 1/30 (3.3) | 11.1 | 96.7 | 0.1 | NR | |||
| TIMP3 | 3/18 (16.6) | 0/30 (0) | 17 | 100 | 1 | NR | |||
| De Martino, 2011, [60] | RASSF1A | 72/157 (45.9) | 3/43 (7) | 45.9 | 93 | 0 | 0.694 | Serum, 1 mL | qPCR |
| PTGS2 | 60/157 (38.2) | 15/43 (34.9) | 38.2 | 65.1 | 0 | 0.517 | |||
| P16 | 73/157 (46.5) | 19/43 (44.2) | 46.5 | 55.8 | 0 | 0.512 | |||
| VHL | 79/157 (50.3) | 4/43 (8.3) | 50.3 | 90.7 | 0 | 0.705 | |||
| Hauser, 2013, [82] | APC | 19/35(54.3) | 5/54 (9.3) | 54.3 | 90.7 | 0.37 | 0.72 | Serum, 1 mL | PCR |
| GSTP1 | 6/35(17.1) | 1/54(1.9) | 17.1 | 98.1 | 0.75 | 0.57 | |||
| p14(ARF) | 5/35(14.3) | 0/54(0) | 14.3 | 100 | 0.26 | 0.57 | |||
| P16 | 9/35(25.7) | 9/54(16.7) | 25.7 | 83.3 | 0 | NR | |||
| PTGS2 | 8/35(22.9) | 2/54(3.7) | 22.9 | 96.3 | 0.47 | 0.59 | |||
| RAR-B | 14/35(40) | 8/54(14.8) | 40.0 | 85.2 | 0.19 | 0.61 | |||
| RASSF1A | 8/35(22.9) | 1/54(1.9) | 22.9 | 98.2 | 0.09 | 0.60 | |||
| TIMP3 | 20/35(57.1) | 21/54(38.9) | 57.1 | 61.1 | 0 | NR | |||
| Skrypkina, 2016, [62] | APC | 14/27 (51.9) | 1/15(6.7) | 51.9 | 93.3 | 0 | NR | Plasma, 2 mL | PCR |
| FHIT | 15/27 (55.6) | 0/15 (0) | 55.6 | 100 | 0 | NR | |||
| ITGA9 | 0/27(0) | 0/15 (0) | 0 | 100 | 0 | NR | |||
| LRRC3B | 20/27 (74.1) | 5/15 (33.3) | 74.1 | 66.7 | 0 | NR | |||
| RASSF1 | 17/27 (63.0) | 1/15 (6.7) | 62.9 | 93.3 | 0 | NR | |||
| VHL | 0/27 (0) | 0/15 (0) | 0 | 100 | 0 | NR |
The methylation of other genes with a key role in renal carcinogenesis also seems to hold a promising role in detecting RCC. In Hoque’s cohort, 67% of RCC patients had a methylated promoter in at least one of the nine genes analyzed [38]. cfDNA analysis in eight selected methylated genes (APC, GSTP1, p14(ARF), p16, RAR-B, RASSF1A, TIMP3, PTGS2) showed that 86% of the patients harbored at least one methylated gene [82]. Although all genes, except p16 and TIMP3, were significantly methylated in RCC patients compared to healthy individuals, only APC was correlated with the advanced tumor stage [82]. Furthermore, hypermethylation of the suppressor genes LRRC3B, APC, FHIT, and RASSF1 was detected in the plasma of RCC patients as opposed to healthy individuals; however, no correlation with clinicopathologic features was found [62].
These studies are limited by the use of PCR-based methods, which, albeit inexpensive and easy to perform, analyze only a limited number of methylated sites. An alternative and novel approach to overcome this issue is the analysis of the whole cfDNA methylome.
Whole genome bisulfite sequencing (WGBS), interrogating genome-wide methylation patterns, was recently performed in a large cohort of more than 2000 patients with different types of cancer, including 81 RCC across all stages [83]. The authors applied a classifier built on the differentially methylated regions (DMRs) between the cancers and controls in a training cohort and a validation cohort, reaching a specificity of 99.8% and 99.3%, respectively [83]. In addition, cfDNA detection showed increased sensitivity with increasing stages of the disease [83]. Specifically, in a pre-specified group of 12 cancer types, sensitivity raised from 39% (CI: 27% to 52%) in stage I, to 69% (CI: 56% to 80%) in stage II, 83% (CI: 75% to 90%) in stage III, and 92% (CI: 86% to 96%) in stage IV. This finding indicates the potential of cfDNA methylome not only to distinguish between benign or malign tumors but also to stage RCC.
Superior sensitivity and accuracy were achieved with cell-free methylated DNA immunoprecipitation and high-throughput sequencing (cfMeDIP-seq) [28,34,84,85]. CfMeDIP-seq is a sensitive, low-input (<10 ng of cfDNA), cost-efficient, and bisulfite-free technology allowing for the profiling of cfDNA methylomes. This approach enriches methylated DNA fragments in cfDNA by immunoprecipitation for high-throughput sequencing [34,84]. cfMeDIP-seq has been previously demonstrated to provide a high level of sensitivity for both the detection and classification of a plethora of localized and metastatic tumor types, including RCC [27,28,34,85,86,87]. The first independent validation of plasma cfMeDIP-seq was reported by Nuzzo et al. in a retrospective study on plasma cfDNA samples from 69 stage I–IV RCC patients and 13 normal controls [85]. The authors applied the classifier built on the top 300 DMRs between the RCC and controls, reaching a mean area under the receiver operating characteristic (AUC) curve of 0.990 [85]. Although they included RCC patients with clear cell and papillary histology, no difference in methylation were found between the histology, mainly because of the small number of patients with papillary histology. The same classifier was tested in an independent cohort of 34 patients with mRCC showing that cfMeDIPseq was able to identify all mRCC cases with 88% specificity and 100% detection rate [28].
A complete list of the studies that highlight the diagnostic role of cfDNA in RCC is reported in Table 3.
Table 3.
Summary of the studies evaluating the diagnostic role of circulating cell-free DNA (cfDNA) in renal cell carcinoma (RCC). AUC: area Under the Curve; p16:cyclin-dependent kinase inhibitor 2A; ACTB: actin beta gene; APC: adenomatosis-polyposis-coli gene; cfDNA: circulating cell-free DNA; CfMEDIP-seq: cell-free methylated DNA immunoprecipitation-sequencing; FHIT: fragile histidine triad gene: GA: genetic alteration; GSTP1: glutathione-S-Transferase Pi 1 gene; LOH: loss of heterozygosity; LRRC3B: leucine rich repeat containing 3B gene; mRCC: metastatic Renal Cell Carcinoma; NF1: neurofibromatosis type 1 gene; OS: overall survival; p14 ARF: auxin response factors p14; PCR: polymerase chain reaction; PFS: progression-free survival; PTGS2: prostaglandin–endoperoxide synthase; qPCR: real-time polymerase chain reaction; RAR-B: retinoic acid receptor beta; RASSF1: Ras association domain family member 1A; RCC: renal Cell Carcinoma; TIMP3: tissue inhibitor of metalloproteinase-gene; TP53: tumor protein p53; TOO: tissue of origin; VHL: von Hippel-Lindau; VAF: Variant allele frequency; WGBS: whole-genome bisulfite sequencing.
| DIAGNOSTIC ROLE OF cfDNA IN RCC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cfDNA Feature Analyzed |
Author, Year, [Ref.] | Study Type | Purpose | Patients (n) | Controls | Sample Source, Amount | Timing Sample |
cfDNA Isolation Method |
Detection Method | Results |
|
cfDNA
levels |
Perego, 2008, [59] | Prospective | Diagnostic Prognostic |
48 RCC (stages I–IV), 1 TCC, 5 OCT |
41 healthy individuals | Plasma, 1 mL |
Pre-operative Post-operative |
QIAamp DNA Mini kit (Qiagen) |
qPCR | RCC patients had a higher plasma cfDNA concentration compared to the healthy controls. Plasma DNA concentration decreased after nephrectomy. During follow-up, plasma DNA increased in 12 patients without evidence of neoplasia: 3 patients successively relapsed. Pre-operative plasma DNA of 9 patients harbored LOH in 5 cases (55.6%). Augmented plasma DNA of 7 patients displayed LOH in 3 cases (42.9%) at follow-up, and in 1 case preceded the recurrence of the disease. |
| De Martino, 2012, [60] | Prospective | Diagnostic Prognostic |
157 RCC (stage I–IV) | 43 benign renal tumors | Serum, 1 mL |
Pre-operative | QIAampUltrasens Virus kit (Qiagen) |
PCR | Total cfDNA levels and CpG island methylation of RASSF1A and VHL were highly diagnostic for RCC. cfDNA levels were associated with poorer DFS. RASSF1A and VHL methylation was not associated with DFS. |
|
| Feng, 2013, [61] | Prospective | Diagnostic Predictive |
18 RCC (stage IV) | 10 healthy individuals | Plasma, 0.4 mL | Six timepoints during treatment with Sorafenib: before treatment, 4–8–12–16–24 weeks |
QIAamp DNA Blood Mini Kit (Qiagen) |
qPCR | Pretreatment cfDNA levels were increased in RCC vs. healthy individuals and were associated with stage, grade, and the number of metastases. cfDNA levels from weeks 8 to 24 of treatment were higher in those with disease progression than in those with stable disease or partial response. Levels of cfDNA at 8 weeks were predictive of progression. |
|
| Wan, 2013, [57] | Prospective | Diagnostic Prognostic |
92 RCC (stage I–IV) | 44 healthy individuals | Plasma, 0.4 mL |
Pre-operative Post-operative |
QIAamp DNA Blood Mini Kit (Qiagen) |
qPCR | Pretreatment levels of plasma cfDNA in pts with mRCC were significantly higher than in those with localized RCC or healthy individuals. cfDNA levels were associated with Fuhrman grade, TNM stage, and tumor size. Of pts with localized RCC, those with recurrence had a significantly higher plasma cfDNA level than those without. The pts with a high plasma cfDNA level had a significantly higher recurrence rate than those with a low plasma cfDNA level before and after nephrectomy. |
|
| Skrypkina, 2016, [62] | Prospective | Diagnostic | 27 RCC (stage I–IV) | 15 healthy individuals | Plasma, 2 mL |
Pre-operative | Proba Na kit (DNA-Technology) |
qPCR | Levels of cfDNA levels were significantly higher in RCC pts than in controls. Hypermethylation of CpG islands of LRRC3B, APC, FHIT, and RASSF1 genes was detected in RCC and was not correlated with clinicopathologic features. |
|
|
cfDNA
integrity |
Hauser, 2010, [66] | Prospective | Diagnostic Prognostic |
35 RCC (stage I–IV) | 54 healthy individuals | Serum, 1 mL |
Pre-operative | ChargeSwitchgDNA Kit (Invitrogen) |
qPCR | cfDNA integrity (ACTB-384/ACTB-106 ratio) analysis distinguished between RCC and healthy controls. High level of ACTB384 compared to ACTB106 was found in RCC patients. No significant correlation of cfDNA levels and DNA integrity with pT-stage, grading or histological subtype was observed. |
| Gang, 2010, [67] | Prospective | Diagnostic Prognostic |
78 RCC (stage I–III) | 42 healthy individuals | Serum, 0.4 mL |
Pre-operative Post-operative |
QIAamp DNA Blood Mini Kit (Qiagen) |
PCR | cfDNA integrity distinguished between RCC and healthy controls and was correlated with tumor stage and size. | |
| Lu, 2016, [68] | Retrospective | Diagnostic Prognostic |
229 RCC (stage I-IV) | 40 healthy individuals | Plasma, 1 mL |
Pre-operative Pre-therapy |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
PCR | cfDNA integrity did not differ in metastatic, non-metastatic and controls, but decreased from controls to metastatic patients. | |
|
cfDNA
genetic alterations |
Goessl, 1998, [79] | Prospective | Diagnostic | 40 RCC (stages I–IV) | 10 healthy individuals | Plasma, 1 mL |
Pre-operative | Qiamp Blood Kit (Qiagen) |
PCR | Analysis of LOH and microsatellite instability of four chromosome 3p microsatellites: LOH was found in at least 1 locus in 63% of pts, and 35% exhibited LOH at more than one locus. Microsatellite instability of plasma cfDNA was detected in 3% of the patient. No alterations were found in the controls. |
| Eisenberger, 1999, [37] | Prospective | Diagnostic | 25 RCC (stages I–II–III), 1 AML, 1 MN, 3 OCT |
8 individuals with nephrolithiasis 8 healthy individuals |
Serum, NR Urine, NR |
Pre-operative | Digestion methods with proteinase K (Boehringer Mannheim GmbH, Mannheim, Germany) in the presence of sodium dodecyl sulfate, followed by phenol–chloroform extraction and ethanol precipitation | PCR | Analysis of LOH and microsatellite instability of 28 microsatellites markers analysis: 60% of RCC had one or more microsatellite cfDNA alterations in the serum. No alterations were found in the controls. | |
| von Knobloch, 2002, [80] | Prospective | Diagnostic | 53 RCC (stages I–II–III), 1 renal B cell lymphoma, 6 TCC |
20 healthy individuals | Serum, 2–4 mL |
Pre-operative | Qiamp Midi-Kit (Qiagen) |
PCR | Analysis of microsatellite instability of 20 microsatellites markers analysis: serum cfDNA alterations was detected in 74% of cases using 9 markers and 15% of controls using 10 markers. | |
| Perego, 2008, [59] | Prospective | Diagnostic Prognostic |
48 RCC (stages I–IV), 1 TCC, 5 OCT |
41 healthy individuals | Plasma, 1 mL |
Pre-operative Post-operative |
QIAamp DNA Mini kit (Qiagen) |
PCR | RCC pts had a higher plasma cfDNA concentration compared to the healthy controls. Plasma DNA concentration decreased after nephrectomy. During follow-up, plasma DNA increased in 12 patients without evidence of neoplasia and 3 patients successively relapsed. Pre-operative plasma DNA of 9 patients harbored LOH in 5 cases (55.6%). Augmented plasma DNA of 7 patients displayed LOH in 3 cases (42.9%) at follow-up, and in 1 case preceded the recurrence of disease. |
|
| Bettegowda, 2014, [58] | Prospective | Diagnostic | 5 RCC (stage IV) | - | Plasma, 2 mL |
- | QIAamp Circulating Nucleic Acid Kit (Qiagen) |
PCR | ctDNA from RCC showed the lowest levels compared to other cancers. | |
| Pal, 2017, [69] | Prospective | Diagnostic Predictive |
220 RCC (stage IV) | - | Plasma, 1.5–2 mL |
Pre-therapy Post-therapy |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing (73 genes) |
GAs were detected in 78.6% of patients. GAs in TP53 and NF1 increased with subsequent therapies. | |
| Maia, 2017, [70] | Retrospective | Diagnostic Predictive |
34 RCC (stage IV) | - | Plasma, 1.5–2 mL |
Pre-therapy Post-therapy |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing (73 genes) |
GAs were detected in 53% pf patients. cfDNA showed to be is a surrogate of tumor burden burden. No associations were found between IMDC risk, histology or treatment type and presence/absence of cfDNA |
|
| Hahn, 2017, [76] | Prospective | Diagnostic | 19 RCC (stage IV) | - | Plasma, 1.5–2 mL |
Pre-operative | QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing (73 genes) |
The median GAs rate in cfDNA was detected 2.2% of patients. Median mutation rate was similar between cfDNA and tumor tissue whereas concordance rate was 8.6% | |
| Corrò, 2017, [73] | Prospective | Diagnostic | 9 RCC (stage I–III) | - | Plasma, 1 mL Serum, 1 mL |
Pre-operative | QIAamp Circulating free DNA Kit (Qiagen) |
PCR | The VHL mutation in plasma or serum was detected in 1one patients | |
| Mouliere, 2018, [78] | Prospective | Diagnostic | 33 RCC (stage not available) | 65 healthy controls | Plasma, 2 mL |
Pre-operative | QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Low-pass whole-genome sequencing | Enrichment of ctDNA in fragment sizes between 90 and 150 bp improved RCC detection | |
| Yamamoto, 2019, [71] | Prospective | Diagnostic Prognostic |
53 RCC (stage III–IV) | - | Plasma, 1–3 mL |
Pre-therapy Post-therapy |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
ddPCR and targeted sequencing (48 genes) |
GAs were detected in 30% of patients. ctDNA status and cfDNA fragmentation were associated with PFS and OS. Patients with detectable ctDNA had poor responses to therapy | |
| Lasseter, 2020, [28] | Retrospective | Diagnostic | 40 RCC (stage IV) | 34 healthy controls | Plasma, 1 mL |
Pre-therapy Post-therapy |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing (27 genes) and cfMeDIP-seq |
Genetic variants were found in 21% of the patients. cfMeDIP-Seq performed in 34 RCC patientsdetected all RCC cases (sensitivity 100%, specificity 88%) | |
| Zhang, 2020, [72] | Prospective | Diagnostic | 50 RCC (stage IV) | - | Plasma, NA |
During therapy | QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing (120 genes) |
GAs were identified in all 50 patients. The number of GAs was significantly associated with the number of lines of therapy | |
| Bacon, 2020, [77] | Prospective | Diagnostic Prognostic |
55 RCC (stage IV) | - | Plasma, 1.5–2 mL |
Pre-therapy | QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing (981 cancer genes) |
ctDNA was detected in 33% of patients and the average VAF was 3.9% Patients with detectable ctDNA had shorter PFS and OS |
|
| Smith, 2020, [74] | Prospective | Diagnostic | 29 RCC (stage I–IV) | - | Plasma, 2 mL |
Pre-operative Post-operative |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing (297 genes) |
cfDNA levels in RCC were significantly lower that in other analyzed cancers of similar size and stage. Targeted sequecing methods detected 27.5% of RCC patients. Post-operative cfDNA was correlated with clinical response to treatment. | |
| Wan, 2020, [75] | Prospective | Diagnostic | 24 RCC (stage I–II) | 45 healthy controls | Plasma, 1–2 mL |
Pre-operative Post-operative |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing |
cfDNA from RCC had the lowest levels compared to other analyzed cancers. AUC of the method was 0.66 | |
|
cfDNA
epigenetic alterations |
Hoque, 2004, [38] | Prospective | Diagnostic | 18 RCC (stages I–IV) | 30 healthy individuals | Serum, 1 mL Urine, NR |
Pre-operative | Digestion methods with proteinase K (Boehringer Mannheim GmbH, Mannheim, Germany) in the presence of sodium dodecyl sulfate, followed by phenol–chloroform extraction and ethanol precipitation | qPCR | Aberrant methylation of nine gene promoters’ analysis: 67% of RCC pts and 1% of controls were methylation positive for at least one gene tested |
| De Martino, 2012, [60] | Prospective | Diagnostic Prognostic |
157 RCC (stage I–IV) | 43 benign renal tumors | Serum, 1 mL |
Pre-operative | QIAampUltrasens Virus kit (Qiagen) |
PCR | cfDNA levels and CpG island methylation of RASSF1A and VHL were highly diagnostic for RCC. cfDNA levels were associated with poorer DFS. RASSF1A and VHL methylation was not associated with DFS. |
|
| Hauser, 2013, [82] | Prospective | Diagnostic | 35 RCC (stage I–IV) | 54 healthy individuals | Serum, 1 mL |
Pre-operative | ChargeSwitchgDNA Kit (Invitrogen) |
PCR | cfDNA methylation analysis in eight selected genes (APC, GSTP1, p14(ARF), p16, RAR-B, RASSF1A, TIMP3, PTGS2): in 30 of 35 pts with RCC, at least one gene was methylated. All genes, except p16 and TIMP3, were significantly methylated in RCC pts compared to healthy individuals. APC was correlated with advanced tumor stage |
|
| Skrypkina, 2016, [62] | Prospective | Diagnostic | 27 RCC (stage I–IV) | 15 healthy individuals | Plasma, 2 mL |
Pre-operative | Proba Na kit (DNA-Technology) |
qPCR | Levels of cfDNA were significantly higher in RCC patients than in controls. Hypermethylation of CpG islands of LRRC3B, APC, FHIT, and RASSF1 genes was detected in RCC and was not correlated with clinicopathologic features. | |
| Liu, 2020, [83] | Prospective | Diagnostic | 56 RCC in the training cohort 25 RCC in the validation cohort |
1521 healthy individuals in the training cohort 610 healthy individuals in the validation cohort |
Plasma, up to 10 mL |
Pre-operative | QIAamp Circulating Nucleic Acid Kit (Qiagen) |
WGBS (103,456 regions identified) |
WGBS from multiple cancer types was used to build a classifier to identify the cfDNA and the TOO. cfDNA from RCC has the lowest detection, but TOO was classified correctly from all RCC patients. |
|
| Lasseter, 2020, [28] | Retrospective | Diagnostic | 40 RCC (stage IV) | 34 healthy controls | Plasma, 1 mL |
Pre-therapy Post-therapy |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Tumor sequencing (27 genes) and cfMeDIP-seq |
Genetic variants were found in 21% of the patients. cfMeDIP-Seq perfomed in 34 RCC patients detected all RCC cases (sensitivity 100%, specificity 88%) |
|
| Nuzzo, 2020, [85] | Retrospective | Diagnostic | 99 RCC (stage I–IV) | 28 healthy controls | Plasma, 1 mL |
Pre-operative | QIAamp Circulating Nucleic Acid Kit (Qiagen) |
cfMeDIP-seq | Based on a classifier built on top 300 differentially methylated regions, cfMeDIP-seq detected cfDNA RCC in 97% of the patients | |
4.2. Post-Operative Recurrence and Prognostic Role
Earlier detection of metastatic disease may improve clinical outcomes. In this scenario, some reports indicated that cfDNA could have potential as a surveillance biomarker for patients with localized RCC after radical or partial nephrectomy, and few reports studied the association with the OS [57,59,60,74,77,88].
An intriguing analysis of pre-operative serum cfDNA of patients with clinically organ-confined RCC reported that all of those with two or three detectable LOH in the 28 studied loci experienced disease recurrence within 2 years after surgery vs. only 7% with no detectable serum LOH [88]. This finding suggested that microsatellite aberrations may detect post-surgery RCC relapse [88]. In contrast, another study analyzing plasma cfDNA of a smaller cohort of localized RCC patients showed that, although cfDNA concentration drastically decreased in all subjects after radical surgery compared to pre-nephrectomy levels, only 20% of those who showed an increase in cfDNA levels during the follow-up experienced recurrence [59]. Additionally, relapse-free survival was not associated with the presence of LOH in cfDNA [59]. A larger analysis reported the efficacy of plasma cfDNA in monitoring post-nephrectomy recurrence in a group of 92 RCC patients [57]. Patients with higher cfDNA levels had a significantly greater recurrence rate than those with lower levels before and after nephrectomy. Interestingly, two contemporary studies showed that RCC patients with detectable pre-operative cfDNA had shorter PFS compared to those without [74,77], suggesting that evidence of pre-surgery cfDNA levels may be a promising biomarker of recurrence in RCC patients following nephrectomy procedure. Similarly, a prospective study of 200 RCC patients with a median follow-up period of 28 months showed that higher pre-nephrectomy serum cfDNA levels were associated with shorter RCC-specific survival [60].
In a recent prospective study of RCC patients, a greater proportion of cfDNA fragments was associated with the presence of ctDNA, suggesting that those with ctDNA had shorter cfDNA fragment sizes. Poorer cancer-specific survival was significantly associated with the presence of ctDNA and shorter cfDNA fragment size [74,77]. These findings indicated that ctDNA status and cfDNA fragment size might be employed as biomarkers for prognosis in RCC.
Although the results of several studies suggest that identifying RCC molecular relapse before radiologic evidence and assessing cancer-specific survival by means of cfDNA analysis is a concrete possibility, solid data are still lacking. Larger prospective studies are needed to validate cfDNA as a biomarker for monitoring and predicting disease-specific survival.
Studies that validated the prognostic role of cfDNA in RCC are reported in Table 4.
Table 4.
Summary of the studies evaluating the prognostic role of circulating cell-free DNA (cfDNA) in renal cell carcinoma (RCC). LOH: loss of heterozygosity; mRCC: metastatic Renal Cell Carcinoma; NF1: neurofibromatosis type 1 gene; OS: overall survival; p14 ARF: auxin response factors p14; PCR: polymerase chain reaction; PFS: progression-free survival; PTGS2: prostaglandin–endoperoxide synthase; qPCR: real-time polymerase chain reaction; RASSF1: Ras association domain family member 1A; VHL: von Hippel-Lindau.
| PROGNOSTIC ROLE OF cfDNA IN RCC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Study Type | Purpose | Patients (n) | Controls | Sample Source, Amount | Timing Sample | cfDNA Isolation Method |
Detection Method | Results |
| Gonzalgo, 2002, [88] | Retrospective | Prognostic | 25 RCC (stages I–II–III), 1 AML, 1 MN, 3 OCT |
_ | Serum, NR Urine, NR |
Pre-operative | Digestion methods with proteinase K (Boehringer Mannheim GmbH, Mannheim, Germany) in the presence of sodium dodecyl sulfate, followed by phenol–chloroform extraction and ethanol precipitation | PCR | Analysis of 28 microsatellites markers in RCC patients who had recurrent disease 2 years after nephrectomy: recurrence disease was detected in 7% of patients with no detectable pre-operative serum LOH, 17% with 1 detectable serum LOH and 100% with 2 or 3 detectable serum LOH. |
| Perego, 2008, [59] | Prospective | Diagnostic Prognostic |
48 RCC (stages I–IV), 1 TCC, 5 OCT |
41 healthy individuals | Plasma, 1 mL |
Pre-operative Post-operative |
QIAamp DNA Mini kit (Qiagen) |
qPCR | RCC pts had a higher plasma cfDNA concentration compared to the healthy controls. Plasma DNA concentration decreased after nephrectomy. During follow-up, plasma DNA increased in 12 patients without evidence of neoplasia: 3 patients successively relapsed. Pre-operative plasma DNA of 9 patients harbored LOH in 5 cases (55.6%). Augmented plasma DNA of 7 patients displayed LOH in 3 cases (42.9%) at follow-up, and in 1 case preceded the recurrence of disease. |
| De Martino, 2012, [60] | Prospective | Diagnostic Prognostic |
157 RCC (stage I–IV) | 43 benign renal tumors | Serum, 1 mL |
Pre-operative | QIAampUltrasens Virus kit (Qiagen) |
PCR | Total cfDNA levels and CpG island methylation of RASSF1A and VHL were highly diagnostic for RCC. cfDNA levels were associated with poorer DFS. RASSF1A and VHL methylation was not associated with DFS. |
| Wan, 2013, [57] | Prospective | Prognostic | 92 RCC (stage I–IV) | 44 healthy individuals | Plasma, 0.4 mL |
Pre-operative Post-operative |
QIAamp DNA Blood Mini Kit (Qiagen) |
qPCR | Pretreatment levels of plasma cfDNA in pts with mRCC were significantly higher than in those with localized RCC or nealthy individuals. cfDNA levels were associated with Fuhrman grade, TNM stage, and tumor size. Of pts with localized RCC, those with recurrence had a significantly higher plasma cfDNA level than those without. The pts with a high plasma cfDNA level had a significantly higher recurrence rate than those with a low plasma cfDNA level before and after nephrectomy. |
| Yamamoto, 2019, [71] | Prospective | Diagnostic Prognostic |
53 RCC (stage III–IV) | - | Plasma, 1–3 mL |
Pre-therapy Post-therapy |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
ddPCR and targeted sequencing (48 genes) |
GAs were detected in 30% of patients. cfDNA status and cfDNA fragmentation were associated with PFS and OS. Patients with detectable ctDNA had poor responses to therapy |
| Bacon, 2020, [77] | Prospective | Diagnostic Prognostic |
55 RCC (stage IV) | - | Plasma, 1.5–2 mL |
Pre-therapy | QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing (981 cancer genes) |
cfDNA was detected in 33% of patients and the average VAF was 3.9% Patients with detectable cfDNA had shorter PFS and OS |
| Smith, 2020, [74] | Prospective | Diagnostic | 29 RCC (stage I–IV) | - | Plasma, 2 mL |
Pre-operative Post-operative |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing (297 variants) |
cfDNA levels in RCC were significantly lower than in other analyzed cancers of similar size and stage. Targeted sequecing methods detected 27.5% of RCC patients. Post-operative cfDNA was coreelated with clinical response to treatment. |
4.3. Predictive Role
Few studies have explored the role of cfDNA in monitoring therapeutic efficacy in mRCC [61,69,70,89]. A report by Fang et al. on patients with mRCC showed a correlation between higher levels of plasma cfDNA and poor response to sorafenib [61]. Moreover, significantly lower levels of cfDNA were observed in those with remission or stable disease compared to those with disease progression [61]. In particular, the levels of cfDNA at 8 weeks of treatment were predictive of radiological progression [61]. In a similar fashion, a small prospective study of 23 mRCC patients treated with targeted therapy showed that pretreatment, higher levels of cfDNA were associated with shorter progression-free survival [89].
Recently, in the largest assessment to date of ctDNA of patients with mRCC, most subjects were found to have clinically relevant GAs [69]. The analysis of the ctDNA profile in a cohort of 220 mRCC patients and across patients receiving first and later-line therapies showed that, while the most common alterations were TP53, VHL, NF1, EGFR, and ARID1A, regardless of the line of treatment, the genetic profiles differed among patients receiving first-line compared to those receiving second-line treatments [69]. In particular, the authors observed GA rate differences in those who had second or later lines versus first-line therapy only, with higher variations found for TP53 (49% vs. 24%), VHL (29% vs. 18%), NF1 (20% vs. 3%), EGFR (15% vs. 8%), and PIK3CA (17% vs. 8%) [69]. Interestingly, these rate differences (particularly for TP53 and VHL) were even more evident when limiting the analysis to those receiving vascular endothelial growth factor inhibitors, which may indicate a mechanism of resistance to this type of treatment [69]. However, a much smaller cohort of 34 mRCC with serial plasma samples collected during therapy showed no association between cfDNA and response to treatment [70].
In aggregate, these data indicate that analysis of cfDNA in the course of the disease may have a predictive role in the treatment of mRCC, but larger datasets are warranted to confirm this hypothesis. Table 5 summarizes the studies that investigated the predictive role of cfDNA in RCC.
Table 5.
Summary of the studies evaluating the predictive role of circulating cell-free DNA (cfDNA) in renal cell carcinoma (RCC). AUC: area Under the Curve; gene: GA: genetic alteration; LOH: loss of heterozygosity; OS: overall survival; PCR: polymerase chain reaction; PFS: progression-free survival; qPCR: real-time polymerase chain reaction.
| PREDICTIVE ROLE OF cfDNA IN RCC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Study Type | Purpose | Patients (n) | Controls | Sample Source, Amount | Timing Sample | cfDNA Isolation Method |
Detection Method | Results |
| Feng, 2013, [61] | Prospective | Diagnostic Predictive |
18 RCC (stage IV) | 10 healthy individuals | Plasma, 0.4 mL |
Six timepoints during treatment with Sorafenib: before treatment, 4–8–12–16–24 weeks |
QIAamp DNA Blood Mini Kit (Qiagen) |
qPCR | Pretreatment cfDNA levels were increased in RCC vs. healthy individual and were associated with stage, grade, and number of metastases. cfDNA levels from weeks 8 to 24 of treatment were higher in those with disease progression than in those with stable disease or partial response. Levels of cfDNA at 8 weeks were predictive of progression. |
| Rouvinov, 2017, [89] | Prospective | Predictive | 23 RCC (stage IV) | - | Serum, 1 mL |
Six timepoints during treatment with targeted therapy: before treatment, 4–8–12–16–24 weeks |
QIAamp Blood Kit (Qiagene) |
qPCR | Patients with normal pretreatment cfDNA level had a better PFS versus patients with increased levels. In multivariate analysis, cfDNA levels was associated with PFS. |
| Pal, 2017, [69] | Prospective | Predictive | 220 RCC (stage IV) | - | Plasma, 1.5–2 mL |
Pre-therapy Post-therapy |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing (73 genes) |
GAs were detected in 78.6% of patients. The number of GAs was not correlated to line of therapy, but GAs in TP53 and NF1 increased with subsequent therapies. |
| Maia, 2017, [70] | Retrospective | Predictive | 34 RCC (stage IV) | - | Plasma, 1.5–2 mL |
Pre-therapy Post-therapy |
QIAamp Circulating Nucleic Acid Kit (Qiagen) |
Targeted sequencing (73 genes) |
GAs were detected in 53% pf patients. cfDNA is a surrogate of tumor burner. No associations were found between ctDNA and IMDC risk, histology and response to treatment. |
5. Conclusions
The use of cfDNA as a non-invasive biomarker for routine use in oncology has made great progress in recent years. As cfDNA is a fast, low-cost, and easy way to obtain dynamic information about the tumor, continual efforts from intense multidisciplinary studies have been made to transfer the research tools to routine clinical practice. On the one hand, several reports highlight cfDNA as a potential biomarker in RCC diagnosis, prognosis, and treatment and show how technological breakthroughs have brought new cancer detection methods with higher accuracy and efficiency. On the other hand, there are challenges to the widespread use of this new approach in the routine clinical setting. From a technical point of view, particular efforts have to be focused on the improvement of cfDNA technical assays, high-quality output, library preparation procedures, and sequencing depth for precise methylation analyses. Currently, most liquid biopsies tests are commonly used as a complementary technique to standard tissue biopsies, primarily in research settings, with the final aim of standardization of these techniques allowing their adoption in clinical routine.
Although the FDA has encouragingly approved some liquid biopsy tests, such as FoundationOne®Liquid CDx (Foundation Medicine, Inc.; Cambridge, MA, USA), in the next few years, the huge amount of data collected from the several ongoing prospective clinical trials based on the cfDNA as an integral biomarker will represent the key for further innovation in this promising field, corroborating the clinical evidence of clear benefits for patients, reducing medical overheads, increasing the early diagnostic options, and improving tailored treatments.
Author Contributions
Conceptualization, E.F., G.N.F. and P.V.N.; methodology, F.P. and S.S.; data curation, S.S., H.P., E.M., M.R., V.T., C.S. and A.G.N.; writing—original draft preparation, E.F., G.N.F., F.P. and P.V.N.; visualization, G.N.F.: writing—review and editing, G.N.F., F.P., M.L. and P.V.N.; supervision, P.V.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Gudbjartsson T., Thoroddsen A., Petursdottir V., Hardarson S., Magnusson J., Einarsson G.V. Effect of incidental detection for survival of patients with renal cell carcinoma: Results of population-based study of 701 patients. Urology. 2005;66:1186–1191. doi: 10.1016/j.urology.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Berger A., Brandina R., Atalla M.A., Herati A.S., Kamoi K., Aron M., Haber G.-P., Stein R.J., Desai M.M., Kavoussi L.R., et al. Laparoscopic Radical Nephrectomy for Renal Cell Carcinoma: Oncological Outcomes at 10 Years or More. J. Urol. 2009;182:2172–2176. doi: 10.1016/j.juro.2009.07.047. [DOI] [PubMed] [Google Scholar]
- 4.Eggener S.E., Yossepowitch O., Pettus J.A., Snyder M.E., Motzer R., Russo P. Renal Cell Carcinoma Recurrence After Nephrectomy for Localized Disease: Predicting Survival from Time of Recurrence. J. Clin. Oncol. 2006;24:3101–3106. doi: 10.1200/JCO.2005.04.8280. [DOI] [PubMed] [Google Scholar]
- 5.Konety B.R. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. Urol. Oncol. Semin. Orig. Investig. 2006;24:87–88. doi: 10.1016/j.urolonc.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Bosma N.A., Warkentin M.T., Gan C.L., Karim S., Heng D.Y., Brenner D.R., Lee-Ying R.M. Efficacy and Safety of First-line Systemic Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-analysis. Eur. Urol. Open Sci. 2022;37:14–26. doi: 10.1016/j.euros.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mollica V., Santoni M., Matrana M.R., Basso U., De Giorgi U., Rizzo A., Maruzzo M., Marchetti A., Rosellini M., Bleve S., et al. Concomitant Proton Pump Inhibitors and Outcome of Patients Treated with Nivolumab Alone or Plus Ipilimumab for Advanced Renal Cell Carcinoma. Target. Oncol. 2022;17:61–68. doi: 10.1007/s11523-021-00861-y. [DOI] [PubMed] [Google Scholar]
- 8.Santoni M., Massari F., Bracarda S., Grande E., Matrana M.R., Rizzo M., De Giorgi U., Basso U., Aurilio G., Incorvaia L., et al. Cabozantinib in Patients with Advanced Renal Cell Carcinoma Primary Refractory to First-line Immunocombinations or Tyrosine Kinase Inhibitors. Eur. Urol. Focus. 2022;49:2405–2415. doi: 10.1016/j.euf.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Acosta A.M., Sholl L.M., Fanelli G.N., Gordetsky J.B., Baniak N., Barletta J.A., Lindeman N.I., Hirsch M.S. Intestinal metaplasia of the urinary tract harbors potentially oncogenic genetic variants. Mod. Pathol. 2020;34:457–468. doi: 10.1038/s41379-020-00655-z. [DOI] [PubMed] [Google Scholar]
- 10.Coati I., Lotz G., Fanelli G.N., Brignola S., Lanza C., Cappellesso R., Pellino A., Pucciarelli S., Spolverato G., Guzzardo V., et al. Claudin-18 expression in oesophagogastric adenocarcinomas: A tissue microarray study of 523 molecularly profiled cases. Br. J. Cancer. 2019;121:257–263. doi: 10.1038/s41416-019-0508-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanelli G.N., Scarpitta R., Cinacchi P., Fuochi B., Szumera-Ciećkiewicz A., De Ieso K., Ferrari P., Fontana A., Miccoli M., Naccarato A.G., et al. Immunohistochemistry for Thymidine Kinase-1 (TK1): A Potential Tool for the Prognostic Stratification of Breast Cancer Patients. J. Clin. Med. 2021;10:5416. doi: 10.3390/jcm10225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fassan M., Facchin S., Munari G., Fanelli G.N., Lorenzon G., Savarino E. Noncoding RNAs as drivers of the phenotypic plasticity of oesophageal mucosa. World J. Gastroenterol. 2017;23:7653–7656. doi: 10.3748/wjg.v23.i43.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forooshani M.K., Scarpitta R., Fanelli G.N., Miccoli M., Naccarato A.G., Scatena C. Is It Time to Consider the Androgen Receptor as a Therapeutic Target in Breast Cancer? Anti-Cancer Agents Med. Chem. 2022;22:775–786. doi: 10.2174/1871520621666211201150818. [DOI] [PubMed] [Google Scholar]
- 14.Pasqualetti F., Gonnelli A., Orlandi P., Palladino E., Giannini N., Gadducci G., Mattioni R., Montrone S., Calistri E., Mazzanti C.M., et al. Association of XRCC3 rs1799794 polymorphism with survival of glioblastoma multiforme patients treated with combined radio-chemotherapy. Investig. New Drugs. 2021;39:1159–1165. doi: 10.1007/s10637-021-01075-9. [DOI] [PubMed] [Google Scholar]
- 15.Nuzzo P.V., Buzzatti G., Ricci F., Rubagotti A., Argellati F., Zinoli L., Boccardo F. Periostin: A Novel Prognostic and Therapeutic Target For Genitourinary Cancer? Clin. Genitourin. Cancer. 2014;12:301–311. doi: 10.1016/j.clgc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Nuzzo P.V., Rubagotti A., Zinoli L., Salvi S., Boccardo F.M. The prognostic value of stromal and epithelial periostin expression in human breast cancer: Correlation with clinical pathological features and mortality outcome. BMC Cancer. 2016;16:95. doi: 10.1186/s12885-016-2139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saraggi D., Galuppini F., Fanelli G.N., Remo A., Urso E.D., Bao Q.R., Bacchin D., Guzzardo V., Luchini C., Braconi C., et al. MiR-21 up-regulation in ampullary adenocarcinoma and its pre-invasive lesions. Pathol. Res. Pr. 2018;214:835–839. doi: 10.1016/j.prp.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Fanelli G.N., Fassan M., Moro F.D., Soligo M., Munari G., Zattoni F., Gardiman M.P., Prayer-Galetti T. Thyroid-like follicular carcinoma of the kidney: The mutational profiling reveals a BRAF wild type status. Pathol. Res. Pr. 2019;215:152532. doi: 10.1016/j.prp.2019.152532. [DOI] [PubMed] [Google Scholar]
- 19.Fusco N., Ragazzi M., Sajjadi E., Venetis K., Piciotti R., Morganti S., Santandrea G., Fanelli G.N., Despini L., Invernizzi M., et al. Assessment of estrogen receptor low positive status in breast cancer: Implications for pathologists and oncologists. Histol. Histopathol. 2021;36:1235–1245. doi: 10.14670/HH-18-376. [DOI] [PubMed] [Google Scholar]
- 20.Penney K.L., Tyekucheva S., Rosenthal J., El Fandy H., Carelli R., Borgstein S., Zadra G., Fanelli G.N., Stefanizzi L., Giunchi F., et al. Metabolomics of Prostate Cancer Gleason Score in Tumor Tissue and Serum. Mol. Cancer Res. 2021;19:475–484. doi: 10.1158/1541-7786.MCR-20-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scatena C., Fanelli G., Fanelli G.N., Menicagli M., Aretini P., Ortenzi V., Civitelli S.P., Innocenti L., Sotgia F., Lisanti M.P., et al. New insights in the expression of stromal caveolin 1 in breast cancer spread to axillary lymph nodes. Sci. Rep. 2021;11:2755. doi: 10.1038/s41598-021-82405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leon S.A., Shapiro B., Sklaroff D.M., Yaros M.J. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–650. [PubMed] [Google Scholar]
- 23.Song P., Wu L.R., Yan Y.H., Zhang J.X., Chu T., Kwong L.N., Patel A.A., Zhang D.Y. Limitations and opportunities of technologies for the analysis of cell-free DNA in cancer diagnostics. Nat. Biomed. Eng. 2022;6:232–245. doi: 10.1038/s41551-021-00837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cucchiara F., Scarpitta R., Crucitta S., Scatena C., Arici R., Naccarato A.G., Fogli S., Danesi R., Del Re M. Diagnosis and treatment monitoring in breast cancer: How liquid biopsy can support patient management. Pharmacogenomics. 2022;23:119–134. doi: 10.2217/pgs-2021-0099. [DOI] [PubMed] [Google Scholar]
- 25.Fanelli G.N., Naccarato A.G., Scatena C. Recent Advances in Cancer Plasticity: Cellular Mechanisms, Surveillance Strategies, and Therapeutic Optimization. Front. Oncol. 2020;10:569. doi: 10.3389/fonc.2020.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari P., Scatena C., Ghilli M., Bargagna I., Lorenzini G., Nicolini A. Molecular Mechanisms, Biomarkers and Emerging Therapies for Chemotherapy Resistant TNBC. Int. J. Mol. Sci. 2022;23:1665. doi: 10.3390/ijms23031665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berchuck J.E., Baca S.C., McClure H.M., Korthauer K., Tsai H.K., Nuzzo P.V., Kelleher K.M., He M., Steinharter J.A., Zacharia S., et al. Detecting Neuroendocrine Prostate Cancer Through Tissue-Informed Cell-Free DNA Methylation Analysis. Clin. Cancer Res. 2022;28:928–938. doi: 10.1158/1078-0432.CCR-21-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lasseter K., Nassar A.H., Hamieh L., Berchuck J.E., Nuzzo P.V., Korthauer K., Shinagare A.B., Ogorek B., McKay R., Thorner A.R., et al. Plasma cell-free DNA variant analysis compared with methylated DNA analysis in renal cell carcinoma. Genet. Med. 2020;22:1366–1373. doi: 10.1038/s41436-020-0801-x. [DOI] [PubMed] [Google Scholar]
- 29.Malapelle U., Pisapia P., Addeo A., Arrieta O., Bellosillo B., Cardona A.F., Cristofanilli M., De Miguel-Perez D., Denninghoff V., Durán I., et al. Liquid biopsy from research to clinical practice: Focus on non-small cell lung cancer. Expert Rev. Mol. Diagn. 2021;21:1165–1178. doi: 10.1080/14737159.2021.1985468. [DOI] [PubMed] [Google Scholar]
- 30.Wan J.C.M., Massie C., Garcia-Corbacho J., Mouliere F., Brenton J.D., Caldas C., Pacey S., Baird R., Rosenfeld N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer. 2017;17:223–238. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 31.Klutstein M., Nejman D., Greenfield R., Cedar H. DNA Methylation in Cancer and Aging. Cancer Res. 2016;76:3446–3450. doi: 10.1158/0008-5472.CAN-15-3278. [DOI] [PubMed] [Google Scholar]
- 32.Esteller M. Epigenetics in cancer. N. Engl. J. Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg M.V.C., Bourc’His D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019;20:590–607. doi: 10.1038/s41580-019-0159-6. [DOI] [PubMed] [Google Scholar]
- 34.Shen S.Y., Singhania R., Fehringer G., Chakravarthy A., Roehrl M.H.A., Chadwick D., Zuzarte P.C., Borgida A., Wang T.T., Li T., et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579–583. doi: 10.1038/s41586-018-0703-0. [DOI] [PubMed] [Google Scholar]
- 35.Slieker R.C., Bos S.D., Goeman J.J., Bovée J.V., Talens R.P., van der Breggen R., Suchiman H.E.D., Lameijer E.-W., Putter H., van den Akker E.B., et al. Identification and systematic annotation of tissue-specific differentially methylated regions using the Illumina 450k array. Epigenetics Chromatin. 2013;6:26. doi: 10.1186/1756-8935-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kundaje A., Meuleman W., Ernst J., Bilenky M., Yen A., Heravi-Moussavi A., Kheradpour P., Zhang Z., Wang J., Ziller M.J., et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisenberger C.F., Schoenberg M., Enger C., Hortopan S., Shah S., Chow N.-H., Marshall F.F., Sidransky D. Diagnosis of Renal Cancer by Molecular Urinalysis. JNCI J. Natl. Cancer Inst. 1999;91:2028–2032. doi: 10.1093/jnci/91.23.2028. [DOI] [PubMed] [Google Scholar]
- 38.Hoque M.O., Begum S., Topaloglu O., Jeronimo C., Mambo E., Westra W.H., Califano J.A., Sidransky D. Quantitative Detection of Promoter Hypermethylation of Multiple Genes in the Tumor, Urine, and Serum DNA of Patients with Renal Cancer. Cancer Res. 2004;64:5511–5517. doi: 10.1158/0008-5472.CAN-04-0799. [DOI] [PubMed] [Google Scholar]
- 39.Thierry A.R., Mouliere F., El Messaoudi S., Mollevi C., Lopez-Crapez E., Rolet F., Gillet B., Gongora C., Dechelotte P., Robert B., et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat. Med. 2014;20:430–435. doi: 10.1038/nm.3511. [DOI] [PubMed] [Google Scholar]
- 40.Freidin M.B., Freydin M., Leung M., Fernandez A.M., Nicholson A.G., Lim E. Circulating Tumor DNA Outperforms Circulating Tumor Cells for KRAS Mutation Detection in Thoracic Malignancies. Clin. Chem. 2015;61:1299–1304. doi: 10.1373/clinchem.2015.242453. [DOI] [PubMed] [Google Scholar]
- 41.Hindson B.J., Ness K.D., Masquelier D.A., Belgrader P., Heredia N.J., Makarewicz A.J., Bright I.J., Lucero M.Y., Hid-dessen A.L., Legler T.C., et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diehl F., Li M., He Y., Kinzler K.W., Vogelstein B., Dressman D. BEAMing: Single-molecule PCR on microparticles in water-in-oil emulsions. Nat. Methods. 2006;3:551–559. doi: 10.1038/nmeth898. [DOI] [PubMed] [Google Scholar]
- 43.Beaver J.A., Jelovac D., Balukrishna S., Cochran R.L., Croessmann S., Zabransky D.J., Wong H.Y., Toro P.V., Cidado J., Blair B.G., et al. Detection of Cancer DNA in Plasma of Patients with Early-Stage Breast Cancer. Clin. Cancer Res. 2014;20:2643–2650. doi: 10.1158/1078-0432.CCR-13-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higgins M.J., Jelovac D., Barnathan E., Blair B., Slater S., Powers P., Zorzi J., Jeter S.C., Oliver G.R., Fetting J., et al. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin. Cancer Res. 2012;18:3462–3469. doi: 10.1158/1078-0432.CCR-11-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oxnard G.R., Thress K.S., Alden R.S., Lawrance R., Paweletz C.P., Cantarini M., Yang J.C.-H., Barrett J.C., Jänne P.A. Association Between Plasma Genotyping and Outcomes of Treatment with Osimertinib (AZD9291) in Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016;34:3375–3382. doi: 10.1200/JCO.2016.66.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taniguchi K., Uchida J., Nishino K., Kumagai T., Okuyama T., Okami J., Higashiyama M., Kodama K., Imamura F., Kato K. Quantitative Detection of EGFR Mutations in Circulating Tumor DNA Derived from Lung Adenocarcinomas. Clin. Cancer Res. 2011;17:7808–7815. doi: 10.1158/1078-0432.CCR-11-1712. [DOI] [PubMed] [Google Scholar]
- 47.Diehl F., Schmidt K., Choti M.A., Romans K., Goodman S., Li M., Thornton K., Agrawal N., Sokoll L., Szabo S.A., et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008;14:985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siravegna G., Mussolin B., Buscarino M., Corti G., Cassingena A., Crisafulli G., Ponzetti A., Cremolini C., Amatu A., Lauricella C., et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 2015;21:795–801. doi: 10.1038/nm.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glenn T.C. Field guide to next-generation DNA sequencers. Mol. Ecol. Resour. 2011;11:759–769. doi: 10.1111/j.1755-0998.2011.03024.x. [DOI] [PubMed] [Google Scholar]
- 50.Leary R.J., Sausen M., Kinde I., Papadopoulos N., Carpten J.D., Craig D., O’Shaughnessy J., Kinzler K.W., Parmigiani G., Vogelstein B., et al. Detection of Chromosomal Alterations in the Circulation of Cancer Patients with Whole-Genome Sequencing. Sci. Transl. Med. 2012;4:162ra154. doi: 10.1126/scitranslmed.3004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siravegna G., Marsoni S., Siena S., Bardelli A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 52.Goldberg S.B., Narayan A., Kole A.J., Decker R.H., Teysir J., Carriero N.J., Lee A., Nemati R., Nath S.K., Mane S.M., et al. Early Assessment of Lung Cancer Immunotherapy Response via Circulating Tumor DNA. Clin. Cancer Res. 2018;24:1872–1880. doi: 10.1158/1078-0432.CCR-17-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phallen J., Sausen M., Adleff V., Leal A., Hruban C., White J., Anagnostou V., Fiksel J., Cristiano S., Papp E., et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 2017;9:eaan2415. doi: 10.1126/scitranslmed.aan2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aravanis A.M., Lee M., Klausner R.D. Next-Generation Sequencing of Circulating Tumor DNA for Early Cancer Detection. Cell. 2017;168:571–574. doi: 10.1016/j.cell.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 55.Israel G.M., Hindman N., Bosniak M.A. Evaluation of Cystic Renal Masses: Comparison of CT and MR Imaging by Using the Bosniak Classification System. Radiology. 2004;231:365–371. doi: 10.1148/radiol.2312031025. [DOI] [PubMed] [Google Scholar]
- 56.Zill O.A., Banks K.C., Fairclough S.R., Mortimer S.A., Vowles J.V., Mokhtari R., Gandara D.R., Mack P.C., Odegaard J.I., Nagy R.J., et al. The Landscape of Actionable Genomic Alterations in Cell-Free Circulating Tumor DNA from 21,807 Advanced Cancer Patients. Clin. Cancer Res. 2018;24:3528–3538. doi: 10.1158/1078-0432.CCR-17-3837. [DOI] [PubMed] [Google Scholar]
- 57.Wan J., Zhu L., Jiang Z., Cheng K. Monitoring of Plasma Cell-Free DNA in Predicting Postoperative Recurrence of Clear Cell Renal Cell Carcinoma. Urol. Int. 2013;91:273–278. doi: 10.1159/000351409. [DOI] [PubMed] [Google Scholar]
- 58.Bettegowda C., Sausen M., Leary R.J., Kinde I., Wang Y., Agrawal N., Bartlett B.R., Wang H., Luber B., Alani R.M., et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014;6:224ra224. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perego R.A., Corizzato M., Brambilla P., Ferrero S., Bianchi C., Fasoli E., Signorini S., Torsello B., Invernizzi L., Bombelli S., et al. Concentration and microsatellite status of plasma DNA for monitoring patients with renal carcinoma. Eur. J. Cancer. 2008;44:1039–1047. doi: 10.1016/j.ejca.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 60.De Martino M., Klatte T., Haitel A., Marberger M. Serum cell-free DNA in renal cell carcinoma. Cancer. 2012;118:82–90. doi: 10.1002/cncr.26254. [DOI] [PubMed] [Google Scholar]
- 61.Feng G., Ye X., Fang F., Pu C., Huang H., Li G. Quantification of Plasma Cell-Free DNA 1 in Predicting Therapeutic Efficacy of Sorafenib on Metastatic Clear Cell Renal Cell Carcinoma. Dis. Markers. 2013;34:105–111. doi: 10.1155/2013/651323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skrypkina I., Tsyba L., Onyshchenko K., Morderer D., Kashparova O., Nikolaienko O., Panasenko G., Vozianov S., Romanenko A., Rynditch A. Concentration and Methylation of Cell-Free DNA from Blood Plasma as Diagnostic Markers of Renal Cancer. Dis. Markers. 2016;2016:3693096. doi: 10.1155/2016/3693096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henriksen T.V., Reinert T., Christensen E., Sethi H., Birkenkamp-Demtröder K., Gögenur M., Gögenur I., Zimmermann B.G., Dyrskjøt L., Andersen C.L., et al. The effect of surgical trauma on circulating free DNA levels in cancer patients—implications for studies of circulating tumor DNA. Mol. Oncol. 2020;14:1670–1679. doi: 10.1002/1878-0261.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Der Meer A.J., Kroeze A., Hoogendijk A.J., Soussan A.A., Van Der Schoot C.E., Wuillemin W.A., Voermans C., Van Der Poll T., Zeerleder S. Systemic inflammation induces release of cell-free DNA from hematopoietic and parenchymal cells in mice and humans. Blood Adv. 2019;3:724–728. doi: 10.1182/bloodadvances.2018018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mondelo-Macía P., Castro-Santos P., Castillo-García A., Muinelo-Romay L., Diaz-Peña R. Circulating Free DNA and Its Emerging Role in Autoimmune Diseases. J. Pers. Med. 2021;11:151. doi: 10.3390/jpm11020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hauser S., Zahalka T., Ellinger J., Fechner G., Heukamp L., Von Ruecker A., Müller S.C., Bastian P.J. Cell-free circulating DNA: Diagnostic value in patients with renal cell cancer. Anticancer Res. 2010;30:2785–2789. [PubMed] [Google Scholar]
- 67.Gang F., Guorong L., An Z., Anne G.-P., Christian G., Jacques T. Prediction of Clear Cell Renal Cell Carcinoma by Integrity of Cell-free DNA in Serum. Urology. 2010;75:262–265. doi: 10.1016/j.urology.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 68.Lu H., Busch J., Jung M., Rabenhorst S., Ralla B., Kilic E., Mergemeier S., Budach N., Fendler A., Jung K. Diagnostic and prognostic potential of circulating cell-free genomic and mitochondrial DNA fragments in clear cell renal cell carcinoma patients. Clin. Chim. Acta. 2016;452:109–119. doi: 10.1016/j.cca.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Pal S.K., Sonpavde G., Agarwal N., Vogelzang N.J., Srinivas S., Haas N.B., Signoretti S., McGregor B.A., Jones J., Lanman R.B., et al. Evolution of Circulating Tumor DNA Profile from First-line to Subsequent Therapy in Metastatic Renal Cell Carcinoma. Eur. Urol. 2017;72:557–564. doi: 10.1016/j.eururo.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 70.Maia M.C., Bergerot P.G., Dizman N., Hsu J., Jones J., Lanman R.B., Banks K.C., Pal S.K. Association of Circulating Tumor DNA (ctDNA) Detection in Metastatic Renal Cell Carcinoma (mRCC) with Tumor Burden. Kidney Cancer. 2017;1:65–70. doi: 10.3233/KCA-170007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamamoto Y., Uemura M., Nakano K., Hayashi Y., Wang C., Ishizuya Y., Kinouchi T., Hayashi T., Matsuzaki K., Jingushi K., et al. Increased level and fragmentation of plasma circulating cell-free DNA are diagnostic and prognostic markers for renal cell carcinoma. Oncotarget. 2018;9:20467–20475. doi: 10.18632/oncotarget.24943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J., Liu Y., Xu B., Li F., Wang Y., Li M., Du R., Zhou Y., Salgia M., Yang L., et al. Circulating tumor DNA analysis of metastatic renal cell carcinoma. Mol. Clin. Oncol. 2020;14:16. doi: 10.3892/mco.2020.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corrò C., Hejhal T., Poyet C., Sulser T., Hermanns T., Winder T., Prager G., Wild P.J., Frew I., Moch H., et al. Detecting circulating tumor DNA in renal cancer: An open challenge. Exp. Mol. Pathol. 2017;102:255–261. doi: 10.1016/j.yexmp.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 74.Smith C.G., Moser T., Mouliere F., Field-Rayner J., Eldridge M., Riediger A.L., Chandrananda D., Heider K., Wan J., Warren A.Y., et al. Comprehensive characterization of cell-free tumor DNA in plasma and urine of patients with renal tumors. Genome Med. 2020;12:23. doi: 10.1186/s13073-020-00723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wan J.C.M., Heider K., Gale D., Murphy S., Fisher E., Mouliere F., Ruiz-Valdepenas A., Santonja A., Morris J., Chandrananda D., et al. ctDNA monitoring using patient-specific sequencing and integration of variant reads. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aaz8084. [DOI] [PubMed] [Google Scholar]
- 76.Hahn A.W., Gill D.M., Maughan B., Agarwal A., Arjyal L., Gupta S., Streeter J., Bailey E., Pal S.K., Agarwal N. Correlation of genomic alterations assessed by next-generation sequencing (NGS) of tumor tissue DNA and circulating tumor DNA (ctDNA) in metastatic renal cell carcinoma (mRCC): Potential clinical implications. Oncotarget. 2017;8:33614–33620. doi: 10.18632/oncotarget.16833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bacon J.V., Annala M., Soleimani M., Lavoie J.-M., So A., Gleave M.E., Fazli L., Wang G., Chi K.N., Kollmannsberger C.K., et al. Plasma Circulating Tumor DNA and Clonal Hematopoiesis in Metastatic Renal Cell Carcinoma. Clin. Genitourin. Cancer. 2020;18:322–331. doi: 10.1016/j.clgc.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 78.Mouliere F., Chandrananda D., Piskorz A.M., Moore E.K., Morris J., Ahlborn L.B., Mair R., Goranova T., Marass F., Heider K., et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 2018;10:eaat4921. doi: 10.1126/scitranslmed.aat4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goessl C., Heicappell R., Münker R., Anker P., Stroun M., Krause H., Müller M., Miller K. Microsatellite analysis of plasma DNA from patients with clear cell renal carcinoma. Cancer Res. 1998;58:4728–4732. [PubMed] [Google Scholar]
- 80.von Knobloch R., Hegele A., Brandt H., Varga Z., Wille S., Kälble T., Heidenreich A., Hofmann R. High frequency of serum DNA alterations in renal cell carcinoma detected by fluorescent microsatellite analysis. Int. J. Cancer. 2002;98:889–894. doi: 10.1002/ijc.10263. [DOI] [PubMed] [Google Scholar]
- 81.Lasseigne B.N., Brooks J.D. The Role of DNA Methylation in Renal Cell Carcinoma. Mol. Diagn. Ther. 2018;22:431–442. doi: 10.1007/s40291-018-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hauser S., Zahalka T., Fechner G., Müller S.C., Ellinger J. Serum DNA hypermethylation in patients with kidney cancer: Results of a prospective study. Anticancer Res. 2013;33:4651–4656. [PubMed] [Google Scholar]
- 83.Liu M.C., Oxnard G.R., Klein E.A., Swanton C., Seiden M.V. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020;31:745–759. doi: 10.1016/j.annonc.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen S.Y., Burgener J.M., Bratman S.V., De Carvalho D.D. Preparation of cfMeDIP-seq libraries for methylome profiling of plasma cell-free DNA. Nat. Protoc. 2019;14:2749–2780. doi: 10.1038/s41596-019-0202-2. [DOI] [PubMed] [Google Scholar]
- 85.Nuzzo P.V., Berchuck J.E., Korthauer K., Spisak S., Nassar A.H., Alaiwi S.A., Chakravarthy A., Shen S.Y., Bakouny Z., Boccardo F., et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat. Med. 2020;26:1041–1043. doi: 10.1038/s41591-020-0933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zuccato J.A., Patil V., Mansouri S., Liu J.C., Nassiri F., Mamatjan Y., Chakravarthy A., Karimi S., Almeida J.P., Bernat A.-L., et al. DNA methylation-based prognostic subtypes of chordoma tumors in tissue and plasma. Neuro-oncology. 2022;24:442–454. doi: 10.1093/neuonc/noab235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nassiri F., Chakravarthy A., Feng S., Shen S.Y., Nejad R., Zuccato J.A., Voisin M.R., Patil V., Horbinski C., Aldape K., et al. Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat. Med. 2020;26:1044–1047. doi: 10.1038/s41591-020-0932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gonzalgo M.L., Eisenberger C.F., Lee S.M., Trock B.J., Marshall F.F., Hortopan S., Sidransky D., Schoenberg M.P. Prognostic significance of preoperative molecular serum analysis in renal cancer. Clin. Cancer Res. 2002;8:1878–1881. [PubMed] [Google Scholar]
- 89.Rouvinov K., Mermershtain W., Dresler H., Ariad S., Riff R., Shani-Shrem N., Keizman D., Douvdevani A. Circulating Cell-Free DNA Levels in Patients with Metastatic Renal Cell Carcinoma. Oncol. Res. Treat. 2017;40:707–710. doi: 10.1159/000479523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.