Abstract
This study reports the diversity of cultivable endophytic yeasts from agricultural fruits that respond positively to the plant-promoting property of indole-3-acetic acid (IAA) production. The IAA synthesis by the strains was quantified with an Agilent 1100 series liquid chromatography system. IAA was present in the culture liquid of 72% of all 97 strains examined after three days of cultivation. The most active endophytic yeast strains in this study belonged to the species Aureobasidium pullulans, Candida zeylanoides, Hanseniaspora uvarum, Metschnikowia pulcherrima, Meyerozyma caribbica, Rhodotorula mucilaginosa, and Yarrowia galli. The highest IAA production was observed in the endophytic strain of A. pullulans (9109.19 ± 146.02 μg/g). No significant differences were found between IAA production in strains from agricultural products of different countries. However, the level of IAA production was strictly strain-specific. Our results suggest that the internal tissues of fruits may be a promising source for the isolation of plant-beneficial yeasts that can be used to promote plant growth.
Keywords: endophytic yeasts, IAA, plant beneficial trait, fruits
1. Introduction
Endophytic yeasts are one of the most promising areas in the study of microbe–plant associations. Plants and yeasts, developing within their internal tissues, together form a single symbiotic system that serves as an excellent model for studying fundamental questions of ecology and evolution, as well as for addressing a number of modern practical questions in agriculture. One of these current practical questions is the search for plant growth-promoting microorganisms (PGPMs) in the diverse range of endophytic yeasts in agriculturally important plants, for example, those that produce phytohormones such as auxins, cytokinins, etc. [1,2,3,4,5]. The effects of strains producing important phytohormones are often responsible for the microbial stimulation of germination, growth and development of higher plants [6,7].
Although many indole compounds in the auxin series have biological activity, IAA is the most potent, widely used and studied in nature. For example, the ascomycete yeasts Cyberlindnera saturnus (ex. Williopsis saturnus) isolated from the roots of maize could also produce IAA and stimulate growth processes in the plant [8]. Basidiomycete yeasts, Rhodotorula mucilaginosa, isolated from poplar and willow were able to produce IAA, which can also promote the growth of some important crops such as corn, tomato, pepper, squash, sunflower, and grasses under nitrogen stress [9]. Thai researchers have extensively studied the phytohormonal activity of epiphytic and endophytic yeasts associated with sugarcane, rice, and other tropical crops. Screening studies indicate a widespread ability of endophytic yeasts to synthesize IAA. The strain dependence of the intensity of the manifestation of this trait is highlighted [6,10,11]. The strain dependence of this trait for the ascomycete yeast Aureobasidium pullulans isolated from the phyllosphere and rhizosphere of Drosera spatulata is also supported by the results of the study conducted by Taiwanese researchers [12]. The authors also tested the effect of IAA-active strains on the growth of tobacco seedlings (Nicotiana benthamiana). It was found that the most active IAA producers stimulated the growth of lateral roots, root hairs, increase in the amount of chlorophyll, elongation of the stem and increase in the number of leaves. No significant effect was found on root length.
Studies on endophytic yeasts and their phytohormonal activity have been actively conducted for some time and show extremely interesting results [13,14,15,16]. However, they are usually limited to a small sample of strains or a narrow range of plants. In a study of 24 yeast strains from corn roots, 10 strains were able to produce IAA after one week of cultivation [8]. Of the seven yeast strains from mandarins, only four could be reliably confirmed to produce IAA [17]. In another study with endophytic yeasts from mandarins, the activity was detected in all eight strains [18]. In a study with yeasts from grapevine, 67 of the 69 strains were shown to be able to synthesize IAA both without the addition of tryptophan and with this precursor [19]. This prompted us to conduct a large-scale study on the IAA-producing capabilities of endophytic yeast strains isolated from the inner tissues of fruits derived from different countries and to evaluate the potential contribution of endophytic yeasts to stimulate plant growth.
2. Materials and Methods
2.1. Study Location and Sampling
A study on the synthesis of indole-3-acetic acid (IAA) was carried out for a sample of 97 endophytic yeast strains isolated from fruits of different production purchased from trade networks in the Moscow region (Argentina, Azerbaijan, Belarus, Brazil, China, Chile, Dominican Republic, Egypt, Georgia, Iran, Israel, Kenya, Moldova, Peru, Russia, Serbia, Spain, Turkey, Uzbekistan and Vietnam).
2.2. Microbiological Analyses and Species Identification
To study endophytic yeast communities in 2019–2020, fruits were treated according to the following scheme: 70% ethanol, 30 min; 2% sodium hypochlorite, 30 min; 70% ethanol, 30 s; and washing in sterile distilled water for 10 min [20,21]. After the exocarp was removed with a sterile scalpel, the inner tissue was excised, homogenized, and poured with sterile water to obtain a 1:10 dilution. The suspensions were vortexed on a Multi Reax Vortexer (Heidolph Instruments, Germany) for 15 min at 2000 rpm. Three suspensions were prepared for each fruit. The prepared suspensions were plated in three replicates each on glucose-peptone-yeast extract (GPY) agar (20 g/L glucose, 10 g/L peptone, 5 g/L yeast extract, 20 g/L agar) supplemented with chloramphenicol (500 mg/L) to prevent bacterial growth. A total of 3318 plates were incubated at 22 °C for 5–7 days. The grown yeast colonies were classified into morphological types using a dissecting microscope and the number of colonies of each type was counted. From 5 to 7 colonies of each morphotype were isolated in a pure culture. Purified yeast strains were cryopreserved in 10% (v/v) glycerol in water solution at −80 °C in the yeast collection of the Soil Biology Department at Lomonosov Moscow State University (WDCM 1173). Identification of yeast species was based on the ITS rDNA nucleotide sequence. DNA isolation and PCR were performed according to the procedure described previously [22]. DNA sequencing was performed using the Big Dye Terminator V3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA) with subsequent analysis of the reaction products on an Applied Biosystems 3130xl Genetic Analyzer at the facilities of Evrogen (Moscow, Russia). For sequencing, the ITS5 primer (5’-GGA AGT AAA AGT CGT AAC AAG G) was used [22]. For species identification, nucleotide sequences were compared with those in public databases, using the BLAST NCBI (www.ncbi.nlm.nih.gov (accessed on 7 July 2022)) and the MycoID (www.mycobank.org (accessed on 7 July 2022)) tools. The resulting sequences have been deposited in NCBI (GenBank OP216812-OP216908). The ITS regions of the strains studied were 99.5–100% similar to the type strains. Endophytic strains of 17 yeast species commonly occurring in fruits were examined: Aureobasidium pullulans (8 strains); Candida parapsilosis (4 strains); Candida zeylanoides (7 strains); Debaryomyces fabryi (8 strains), Debaryomyces hansenii (8 strains), Filobasidium magnum (3 strains), Filobasidium wieringae (4 strains), Hanseniaspora uvarum (7 strains), Metschnikowia pulcherrima (9 strains), Meyerozyma caribbica (7 strains), Meyerozyma guilliermondii (5 strains), Rhodotorula babjevae (6 strains), Rhodotorula mucilaginosa (8 strains), Yarrowia deformans (4 strains), Yarrowia divulgata (3 strains), Yarrowia galli (3 strains), Yarrowia lipolytica (3 strains). Information on the strains examined is presented in Table 1. A full list of the yeasts isolated in the work has been published previously [23].
Table 1.
IAA production (with standard deviations) of studied strains and information about it.
| Strain KBP | Species | Phylum | Country of Origin | Substrate | IAA, mg/L | IAA, μg/g |
|---|---|---|---|---|---|---|
| YE-0002 | Candida zeylanoides | ASC * | Turkey | nectarine | - | - |
| YE-0007 | Debaryomyces hansenii | ASC | Turkey | nectarine | - | - |
| YE-0013 | Metschnikowia pulcherrima | ASC | Turkey | cherry | 0.1 ± 0.01 | 15.85 ± 2.16 |
| YE-0017 | Debaryomyces hansenii | ASC | Iran | pepper | 0.01 ± 0.00 | 1.09 ± 0.24 |
| YE-0024 | Hanseniaspora uvarum | ASC | Russia | gooseberry | - | - |
| YE-0031 | Metschnikowia pulcherrima | ASC | Russia | apple | 0.07 ± 0.01 | 17.15 ± 2.51 |
| YE-0034 | Filobasidium wieringae | BAS | Turkey | cherry | - | - |
| YE-0043 | Hanseniaspora uvarum | ASC | Iran | grapes | 0.05 ± 0.01 | 7.53 ± 1.25 |
| YE-0045 | Meyerozyma caribbica | ASC | Brazil | mango | 0.29 ± 0.24 | 40.24 ± 33.14 |
| YE-0053 | Filobasidium wieringae | BAS | Moldova | cherry | 0.03 ± 0.00 | 21.32 ± 1.15 |
| YE-0058 | Yarrowia galli | ASC | Russia | apple | 0.11 ± 0.00 | 17.85 ± 0.03 |
| YE-0061 | Yarrowia galli | ASC | Argentina | apple | - | - |
| YE-0065 | Rhodotorula babjevae | BAS | Russia | apple | 0.08 ± 0.00 | 16.08 ± 0.00 |
| YE-0066 | Rhodotorula babjevae | BAS | Argentina | apple | 0.05 ± 0.00 | 8.78 ± 0.89 |
| YE-0067 | Yarrowia lipolytica | ASC | Russia | apple | - | - |
| YE-0068 | Yarrowia galli | ASC | Turkey | apple | 0.63 ± 0.02 | 100.60 ± 4.75 |
| YE-0069 | Filobasidium wieringae | BAS | Russia | apple | 0.06 ± 0.01 | 7.86 ± 1.30 |
| YE-0070 | Hanseniaspora uvarum | ASC | Russia | apple | 0.11 ± 0.03 | 25.34 ± 3.90 |
| YE-0071 | Yarrowia divulgata | ASC | Russia | apple | - | - |
| YE-0072 | Candida zeylanoides | ASC | Chile | apple | 0.08 ± 0.01 | 37.57 ± 1.66 |
| YE-0073 | Candida zeylanoides | ASC | Russia | apple | - | - |
| YE-0074 | Rhodotorula mucilaginosa | BAS | Russia | apple | 0.54 ± 0.09 | 91.74 ± 13.51 |
| YE-0079 | Candida zeylanoides | ASC | Russia | apple | - | - |
| YE-0080 | Yarrowia lipolytica | ASC | Russia | apple | 0.08 ± 0.01 | 12.35 ± 1.08 |
| YE-0081 | Yarrowia divulgata | ASC | Russia | gooseberry | 0.08 ± 0.01 | 14.85 ± 3.00 |
| YE-0086 | Yarrowia lipolytica | ASC | Russia | apple | 0.26 ± 0.05 | 44.61 ± 7.70 |
| YE-0093 | Yarrowia deformans | ASC | Russia | apple | 0.31 ± 0.06 | 58.33 ± 11.56 |
| YE-0106 | Hanseniaspora uvarum | ASC | Russia | apple | 0.04 ± 0.00 | 12.94 ± 0.03 |
| YE-0114 | Yarrowia divulgata | ASC | Russia | gooseberry | 0.03 ± 0.00 | 4.15 ± 0.14 |
| YE-0118 | Candida parapsilosis | ASC | Russia | beet | 0.03 ± 0.01 | 7.63 ± 3.10 |
| YE-0119 | Yarrowia deformans | ASC | Russia | apple | - | - |
| YE-0120 | Rhodotorula babjevae | BAS | Russia | beet | - | - |
| YE-0122 | Candida parapsilosis | ASC | Russia | apple | 0.01 ± 0.00 | 5.97 ± 0.38 |
| YE-0125 | Yarrowia deformans | ASC | Belarus | pepper | 0.02 ± 0.00 | 3.31 ± 0.06 |
| YE-0128 | Yarrowia deformans | ASC | Egypt | tangerine | 0.02 ± 0.00 | 3.85 ± 0.05 |
| YE-0130 | Meyerozyma guilliermondii | ASC | Egypt | tangerine | 0.02 ± 0.00 | 3.41 ± 0.19 |
| YE-0131 | Candida zeylanoides | ASC | Russia | apple | 0.06 ± 0.00 | 7.67 ± 0.13 |
| YE-0133 | Filobasidium magnum | BAS | Russia | apple | 0.18 ± 0.02 | 41.04 ± 4.55 |
| YE-0139 | Metschnikowia pulcherrima | ASC | Russia | apple | - | - |
| YE-0151 | Debaryomyces hansenii | ASC | Spain | tangerine | 0.15 ± 0.01 | 38.44 ± 2.23 |
| YE-0159 | Metschnikowia pulcherrima | ASC | Russia | quince | 0.22 ± 0.03 | 366.21 ± 24.36 |
| YE-0164 | Rhodotorula mucilaginosa | BAS | Serbia | plum | 0.04 ± 0.00 | 5.86 ± 0.78 |
| YE-0166 | Rhodotorula mucilaginosa | BAS | Russia | apple | 0.04 ± 0.00 | 7.9 ± 0.41 |
| YE-0177 | Rhodotorula mucilaginosa | BAS | Russia | pea | 0.03 ± 0.00 | 6.1 ± 0.05 |
| YE-0179 | Meyerozyma caribbica | ASC | Dominican Republic | coconut | 0.51 ± 0.06 | 71.42 ± 7.22 |
| YE-0180 | Debaryomyces hansenii | ASC | Russia | tomato | 0.13 ± 0.01 | 23.76 ± 0.2 |
| YE-0204 | Meyerozyma caribbica | ASC | Iran | kiwi | 0.02 ± 0.00 | 3.13 ± 0.08 |
| YE-0205 | Rhodotorula mucilaginosa | BAS | Iran | kiwi | 0.05 ± 0.01 | 11.35 ± 1.79 |
| YE-0214 | Debaryomyces hansenii | ASC | Russia | pepper | - | - |
| YE-0216 | Hanseniaspora uvarum | ASC | Russia | apple | - | - |
| YE-0217 | Candida zeylanoides | ASC | Russia | apple | - | - |
| YE-0220 | Rhodotorula babjevae | BAS | Russia | carrot | - | - |
| YE-0221 | Filobasidium wieringae | BAS | Russia | tomato | 0.04 ± 0.01 | 9.09 ± 0.86 |
| YE-0230 | Filobasidium magnum | BAS | Israel | persimmon | 0.12 ± 0.02 | 33.42 ± 5.34 |
| YE-0242 | Aureobasidium pullulans | ASC | Peru | mango | 0.75 ± 0.05 | 736.80 ± 37.48 |
| YE-0250 | Metschnikowia pulcherrima | ASC | Russia | strawberry | 0.05 ± 0.01 | 60.11 ± 9.85 |
| YE-0256 | Aureobasidium pullulans | ASC | Serbia | cherry | 0.11 ± 0.03 | 19.35 ± 4.5 |
| YE-0260 | Aureobasidium pullulans | ASC | Turkey | apricot | 0.43 ± 0.02 | 319.26 ± 7.01 |
| YE-0269 | Aureobasidium pullulans | ASC | Peru | mango | 0.06 ± 0.06 | 10.33 ± 10.33 |
| YE-0270 | Aureobasidium pullulans | ASC | Turkey | apricot | 0.04 ± 0.00 | 5.84 ± 0.61 |
| YE-0282 | Filobasidium magnum | BAS | Turkey | grapes | 0.02 ± 0.00 | 4.48 ± 1.00 |
| YE-0289 | Aureobasidium pullulans | ASC | Russia | currants | 0.08 ± 0.01 | 13.74 ± 1.30 |
| YE-0299 | Rhodotorula babjevae | BAS | Russia | apple | 0.09 ± 0.00 | 27.32 ± 0.84 |
| YE-0302 | Hanseniaspora uvarum | ASC | Russia | apple | - | - |
| YE-0303 | Metschnikowia pulcherrima | ASC | Russia | apple | 0.57 ± 0,01 | 163.87 ± 38.82 |
| YE-0310 | Hanseniaspora uvarum | ASC | Azerbaijan | persimmon | 0.06 ± 0.01 | 96.13 ± 4.20 |
| YE-0316 | Metschnikowia pulcherrima | ASC | Israel | persimmon | 0.07 ± 0.01 | 13.97 ± 1.54 |
| YE-0337 | Candida zeylanoides | ASC | Azerbaijan | persimmon | 1.32 ± 0.21 | 176.43 ± 30.64 |
| YE-0347 | Debaryomyces hansenii | ASC | Georgia | pistachios | - | - |
| YE-0367 | Candida parapsilosis | ASC | Vietnam | banana | - | - |
| YE-0503 | Meyerozyma caribbica | ASC | Vietnam | jackfruit | 0.1 ± 0.01 | 14.55 ± 1.55 |
| YE-0623 | Candida parapsilosis | ASC | Vietnam | passion fruit | - | - |
| YE-0625 | Meyerozyma caribbica | ASC | Vietnam | passion fruit | 0.05 ± 0.01 | 7.82 ± 1.46 |
| YE-0652 | Metschnikowia pulcherrima | ASC | Vietnam | tangerine | 0.03 ± 0.00 | 4.49 ± 0.08 |
| YE-0672 | Debaryomyces fabryi | ASC | Russia | walnut | - | - |
| YE-0676 | Debaryomyces fabryi | ASC | Egypt | tangerine | 0.03 ± 0.00 | 5.14 ± 0.37 |
| YE-0678 | Debaryomyces fabryi | ASC | Chile | apple | - | - |
| YE-0680 | Debaryomyces fabryi | ASC | Chile | kiwi | 0.07 ± 0.01 | 15.56 ± 1.96 |
| YE-0681 | Debaryomyces fabryi | ASC | Turkey | grapes | 0.03 ± 0.00 | 4.45 ± 0.15 |
| YE-0684 | Debaryomyces fabryi | ASC | Georgia | peanuts | 0.04 ± 0.01 | 8.95 ± 2.24 |
| YE-0688 | Debaryomyces fabryi | ASC | Turkey | tomato | 0.04 ± 0.01 | 7.83 ± 1.91 |
| YE-0700 | Meyerozyma guilliermondii | ASC | Egypt | orange | 0.07 ± 0.02 | 9.2 ± 2.02 |
| YE-0712 | Meyerozyma guilliermondii | ASC | Egypt | orange | 0.15 ± 0.02 | 28.57 ± 5.10 |
| YE-0713 | Debaryomyces fabryi | ASC | Spain | tangerine | - | - |
| YE-0718 | Debaryomyces hansenii | ASC | Spain | tangerine | - | - |
| YE-0719 | Debaryomyces hansenii | ASC | Turkey | apple | 0.11 ± 0.00 | 21.71 ± 0.53 |
| YE-0721 | Rhodotorula babjevae | BAS | Turkey | apple | 0.47 ± 0.06 | 45.29 ± 3.94 |
| YE-0722 | Meyerozyma guilliermondii | ASC | Vietnam | longan | 0.06 ± 0.01 | 11.56 ± 0.95 |
| YE-0725 | Metschnikowia pulcherrima | ASC | Vietnam | passion fruit | - | - |
| YE-0728 | Meyerozyma guilliermondii | ASC | Vietnam | longan | 0.07 ± 0.00 | 18.87 ± 0.00 |
| YE-0735 | Meyerozyma caribbica | ASC | Vietnam | guava | - | - |
| YE-0878 | Meyerozyma caribbica | ASC | Iran | watermelon | - | - |
| YE-0882 | Rhodotorula mucilaginosa | BAS | Iran | melon | 0.05 ± 0.00 | 9.87 ± 0.51 |
| YE-0959 | Rhodotorula mucilaginosa | BAS | Israel | watermelon | 0.03 ± 0.00 | 5.5 ± 0.18 |
| YE-0967 | Rhodotorula mucilaginosa | BAS | Israel | watermelon | 0.61 ± 0.08 | 94.98 ± 19.35 |
| YE-0979 | Aureobasidium pullulans | ASC | Israel | cress | 14.96 ± 1.73 | 9109.19 ± 146.02 |
| YE-1002 | Aureobasidium pullulans | ASC | Uzbekistan | parsley | 0.05 ± 0.05 | 11.98 ± 11.98 |
* ASC—Ascomycota; BAS—Basidiomycota.
2.3. Synthesis of IAA
For IAA synthesis, yeasts were cultured in a liquid medium for 72 h at 22 °C using a Heidolph shaker. An aliquot of 100 µL of the yeast suspension (OD595) was added to 20 mL of liquid medium. The medium for culturing the yeasts contained 6.7 g of nitrogenous base (Fluka) and 10 g of glucose per 1 L of water with the addition of tryptophan (1 g/L).
Sample preparation for the determination of IAA: 20 mL of the culture liquid was acidified to pH = 3 with hydrochloric acid and placed in a 100 mL separatory funnel, to which 20 mL of ethyl acetate [24] was added and shaken vigorously for 1 min. The aqueous phase was then drained and subjected to this procedure again, and the organic phase was placed in a 100 mL evaporation flask. The re-extracted aqueous phase was drained and a new portion of the organic phase was poured into the same flask. The funnel was then washed with 10 mL of ethyl acetate, which was also poured into the flask. The extract was concentrated on a rotary evaporator (50 rpm) to a final volume of ≤0.5 mL [25]. The resulting concentrate was transferred to a 1.5 mL chromatography vial, and 0.5 mL of acetonitrile was added to the evaporation flask and placed in an ultrasonic bath for 1 min to separate the IAA from the flask walls. The acetonitrile from the flask was also transferred to the vial. Then, 0.5 mL of acetonitrile was again added to the flask and the treatment was repeated. If necessary, the contents of the vial were made up to 1.5 mL of acetonitrile. Quantification of IAA was performed using an Agilent 1100 series high performance liquid chromatograph with UV detector. A Security Guard Catridges C18 4 ×3.0 mm precolumn and a Diasfer 110-C18 5 µm 4.0 × 250 mm analytical HPLC column were used. The detection wavelength was 222 nm. Flow rate of the eluent—1.0 mL/min. Mobile phase—water, acetonitrile, 0.05% trifluoroacetic acid (45:54:1% v/v). The volume of the injected sample was 10 µL. The temperature of the column thermostat was 25 °C. The analysis was performed for 18 min.
Solutions of the standard substance indole-3-acetic acid (DiaM) in acetonitrile were used to calibrate the instrument.
IAA produced by yeasts was expressed as per liter and per gram of dry biomass.
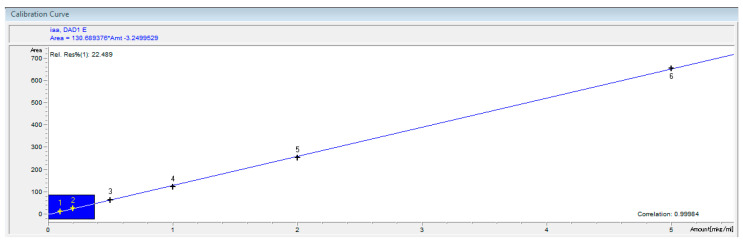
Calibration was performed in six steps (Figure 1). The correlation coefficient r > 0.995. The biomass was used to calculate the specific auxin concentration [26,27]. For each strain, the study was repeated twice.
Figure 1.
Calibration curve for standard solutions of IAA.
2.4. Data Analyses
Statistical data processing and graphical presentation of the obtained results were carried out using Excel 2010 (Microsoft, Albuquerque, NM, USA) and Statistica 8.0 (StatSoft, Tulsa, OK, USA) programs. The analysis of variance (ANOVA) was carried out for groups comparison. Statistical significance was judged at the level of p < 0.05.
3. Results
Indole-3-acetic acid was present in the culture liquid of 69 (72% of all strains examined) of the 97 endophytic yeast strains studied after three days of cultivation (Table 1).
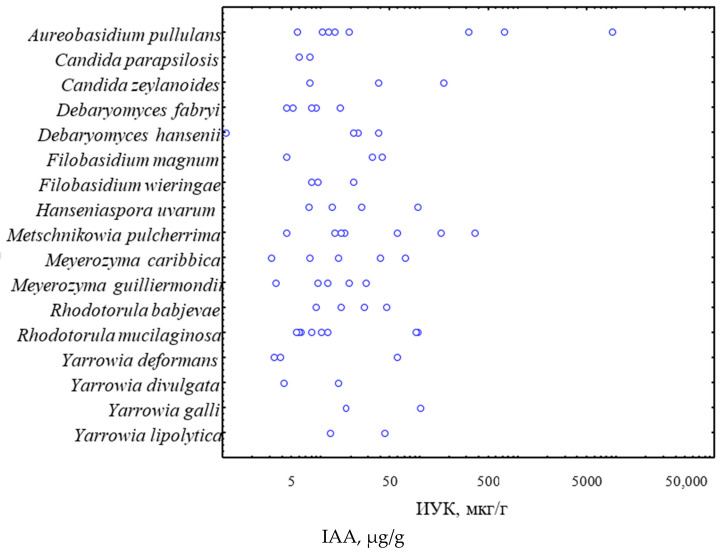
This value of active strains differs from the data we previously obtained for non-endophytic yeast strains from different natural substrates. At that time, the percentage of active strains was 92% [27,28]. The observed differences can be explained by the different approaches used in the analysis: in the current study, the determination of indole-3-acetic acid in the culture liquid was performed without the step of pre-concentration and the minimum values of IAA synthesis were not considered. In addition, we were interested in the rapid response of the yeasts and the ability to produce IAA in significant amounts. However, it is known that for some strains the maximum concentration of IAA in the culture liquid is reached only on day 5–7 [17]. The results we obtained for strains of the most abundant yeast species in agricultural products show that all yeast species studied are capable of synthesizing IAAs, but the extent of production is strictly strain-specific (Table 2 and Figure 2).
Table 2.
Proportion of active strains of the studied endophytic yeast species and average IAA production (with standard deviations) in the culture liquid and per unit biomass.
| Yeast Species | Proportion (%) of Strains Starting to Synthesize IAA after 72 h | IAA, mg/L | IAA, μg/g Dry Biomass |
|---|---|---|---|
| Aureobasidium pullulans | 100 | 2.06 ± 1.27 | 1278.31 ± 766.88 |
| Candida parapsilosis | 50 | 0.01 ± 0.01 | 3.40 ± 1.43 |
| Candida zeylanoides | 42.9 | 0.22 ± 0.14 | 34.10 ± 18.25 |
| Debaryomyces fabryi | 62.5 | 0.03 ± 0.01 | 5.24 ± 1.36 |
| Debaryomyces hansenii | 50 | 0.05 ± 0.02 | 10.62 ± 3.67 |
| Filobasidium magnum | 100 | 0.11 ± 0.03 | 26.31 ± 7.28 |
| Filobasidium wieringae | 75 | 0.03 ± 0.01 | 9.56 ± 2.91 |
| Hanseniaspora uvarum | 57.1 | 0.04 ± 0.01 | 20.28 ± 8.93 |
| Metschnikowia pulcherrima | 77.8 | 0.12 ± 0.04 | 71.29 ± 28.21 |
| Meyerozyma caribbica | 71.4 | 0.14 ± 0.06 | 19.59 ± 7.75 |
| Meyerozyma guilliermondii | 100 | 0.07 ± 0.02 | 13.82 ± 3.32 |
| Rhodotorula babjevae | 66.7 | 0.14 ± 0.06 | 19.87 ± 5.88 |
| Rhodotorula mucilaginosa | 100 | 0.18 ± 0.06 | 29.16 ± 9.83 |
| Yarrowia deformans | 75 | 0.09 ± 0.05 | 16.37 ± 9.43 |
| Yarrowia divulgata | 66.7 | 0.04 ± 0.02 | 7.60 ± 3.20 |
| Yarrowia galli | 66.7 | 0.25 ± 0.12 | 39.49 ± 19.64 |
| Yarrowia lipolytica | 66.7 | 0.12 ± 0.05 | 18.99 ± 8.65 |
Figure 2.
IAA production by the strains of the studied yeast species (logarithmic scale).
Maximum IAA production (9109.2 μg/g) was found in strain A. pullulans (YE-0979) Strains of this species are regularly cited as the most active producers of IAA in various studies [12,29]. In our previous study on the phytohormonal activity of non-endophytic yeasts, we found the maximum IAA production (7990.4 µg/g) in strain Metschnikowia pulcherrima Y-5623 [27]. In this study, the endophytic strains of this ascomycetous yeast species also showed high IAA activity.
A comparison of the yeast groupings studied, such as Phylum and Origin, showed no statistically significant results. It is most likely that the ability of endophytic yeast to synthesize IAA is determined by the nature of the strains.
4. Discussion
It is widely recognized that endophytic yeasts have an excellent ability to promote plant growth, which can be a boon to agricultural practices. This ability of endophytic yeasts is based on their ability to secrete bioactive compounds such as auxins, gibberellins, siderophores [30,31]. The production of plant hormones provides a direct method of promoting plant growth by endophytes. Auxins and gibberellins have many growth-promoting properties in plants, including promotion of root growth and stem elongation and, more broadly, cell proliferation and elongation. IAA has also been shown to play a role in controlling fungal diseases [31,32]. In particular, the production of IAA by endophytic yeasts has been reported and discussed in detail by several groups [5,8,30]. Our screening of strains from the internal tissues of fruits from different countries shows that more than 70% of endophytic yeast strains produce a significant amount of IAA relatively quickly, i.e., within the first 72 h after cultivation. No significant differences were found between the production of IAA by strains from agricultural products from different countries (Table 1). However, our previous studies have shown that tropical strains of ascomycete yeasts have significantly higher phytohormonal activity compared to strains from other regions [27,28]. Our results suggest that endophytic yeast complexes from the internal tissues of fruits may be a promising source of plant-beneficial yeast strains that can be used to promote plant growth. The isolation of an opportunistic yeast species, Candida parapsilosis, deserves separate consideration. The studied endophytic strains of this species showed the lowest IAA synthesis property. Previously, we detected C. parapsilosis yeasts in high abundance in the internal tissues of ripe fruits of apples and pears growing in conditions of high anthropogenic stress [22,23]. Most likely, opportunistic yeasts belong to the species contaminating agricultural products. This is indirectly indicated by their weak ability to synthesize the phytohormone IAA.
IAA biosynthesis by endophytic yeasts from different fruits is highly strain-specific. Further detailed studies are planned to investigate the multiple factors affecting gene expression of IAA biosynthesis to varying degrees at both species and strain levels.
Author Contributions
Conceptualization, A.K. and A.G.; methodology, R.S., A.K. and A.G.; formal analysis, A.K. and R.S.; writing—original draft preparation, A.K. and A.G.; writing—review and editing, A.K. and A.G.; supervision, A.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Russian Ministry of Science and Higher Education, Grant Agreement No. 075-15-2021-1396.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schulz B., Boyle C.J.M.R. The endophytic continuum. Mycol. Res. 2005;109:661–686. doi: 10.1017/S095375620500273X. [DOI] [PubMed] [Google Scholar]
- 2.Ling L., Tu Y., Ma W., Feng S., Yang C., Zhao Y., Wang N., Li Z., Lu L., Zhang J. A potentially important resource: Endophytic yeasts. World J. Microbiol. Biotechnol. 2020;36:110. doi: 10.1007/s11274-020-02889-0. [DOI] [PubMed] [Google Scholar]
- 3.Streletskii R.A., Kachalkin A.V., Glushakova A.M., Yurkov A.M., Demin V.V. Yeasts producing zeatin. Peer J. 2019;7:e6474. doi: 10.7717/peerj.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duca D., Lorv J., Patten C.L., Rose D., Glick B.R. Indole-3-acetic acid in plant-microbe interactions. Antonie Van Leeuwenhoek. 2014;106:85–125. doi: 10.1007/s10482-013-0095-y. [DOI] [PubMed] [Google Scholar]
- 5.Doty S.L. Endophytic Yeasts: Biology and Applications. In: Aroca R., editor. Symbiotic Endophytes. Springer; Berlin, Germany: 2013. pp. 335–343. [Google Scholar]
- 6.Limtong S., Koowadjanakul N. Yeasts from phylloplane and their capability to produce indole-3-acetic acid. World J. Microbiol. Biotechnol. 2012;28:3323–3335. doi: 10.1007/s11274-012-1144-9. [DOI] [PubMed] [Google Scholar]
- 7.Tsavkelova E.A., Klimova S.Y., Cherdyntseva T.A., Netrusov A.I. Microbial producers of plant growth stimulators and their practical use: A review. Appl. Biochem. Microbiol. 2006;42:117–126. doi: 10.1134/S0003683806020013. [DOI] [PubMed] [Google Scholar]
- 8.Nassar A.H., El-Tarabily K.A., Sivasithamparam K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol. Fertil. Soils. 2005;42:97–108. doi: 10.1007/s00374-005-0008-y. [DOI] [Google Scholar]
- 9.Xin G., Glawe D., Doty S.L. Characterization of three endophytic, indole-3- acetic acid-producing yeasts occurring in Populus trees. Mycol. Res. 2009;113:973–980. doi: 10.1016/j.mycres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Limtong S., Kaewwichian R., Yongmanitchai W., Kawasaki H. Diversity of culturable yeasts in phylloplane of sugarcane in Thailand and their capability to produce indole-3-acetic acid. World J. Microbiol. Biotechnol. 2014;30:1785–1796. doi: 10.1007/s11274-014-1602-7. [DOI] [PubMed] [Google Scholar]
- 11.Nutaratat P., Srisuk N., Arunrattiyakorn P., Limtong S. Plant growth-promoting traits of epiphytic and endophytic yeasts isolated from rice and sugar cane leaves in Thailand. Fungal Biol. 2014;118:683–694. doi: 10.1016/j.funbio.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Fu S.F., Sun P.F., Lu H.Y., Wei J.Y., Xiao H.S., Fang W.T., Cheng B.Y., Chou J.Y. Plant growth-promoting traits of yeasts isolated from the phyllosphere and rhizosphere of Drosera spatulata Lab. Fungal Biol. 2016;120:433–448. doi: 10.1016/j.funbio.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Camatti-Sartori V., da Silva-Ribeiro R.T., Valdebenito-Sanhueza R.M., Pagnocca F.C., Echeverrigaray S., Azevedo J.L. Endophytic yeasts and filamentous fungi associated with southern Brazilian apple (Malus domestica) orchards subjected to conventional, integrated or organic cultivation. J. Basic Microbiol. 2005;45:397–402. doi: 10.1002/jobm.200410547. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y., Jiang X., Qi F., Liu Y. Isolation and primary identification of endophytic yeast from wine grape. China Brew. 2012;9:82–84. [Google Scholar]
- 15.Infante E.D.P., Marquínez X., Moreno G. Tomato peel (Solanum lycopersicum L.) colonization by the endophyte yeast Candida guilliermondii (Castellani) Langeron et Guerra. Agron. Colomb. 2012;30:388–394. [Google Scholar]
- 16.Tantirungkij M., Nasanit R., Limtong S. Assessment of endophytic yeast diversity in rice leaves by a culture-independent approach. Antonie Van Leeuwenhoek. 2015;108:633–647. doi: 10.1007/s10482-015-0519-y. [DOI] [PubMed] [Google Scholar]
- 17.Ling L., Li Z., Jiao Z., Zhang X., Ma W., Feng J., Zhang J., Lu L. Identification of novel endophytic yeast strains from Tangerine peel. Curr. Microbiol. 2019;76:1066–1072. doi: 10.1007/s00284-019-01721-9. [DOI] [PubMed] [Google Scholar]
- 18.Peng X., Wang Y., Tang L.J., Li X.X., Xiao Y.W., Zhang Z.B., Yan R.M., Yang H.L., Chang J., Zhu B., et al. Yeasts from Nanfeng mandarin plants: Occurrence, diversity and capability to produce indole-3-acetic acid. Biotechnol. Biotechnol. Equip. 2018;32:1496–1506. doi: 10.1080/13102818.2018.1487337. [DOI] [Google Scholar]
- 19.Fernandez-San Millan A., Farran I., Larraya L., Ancin M., Arregui L.M., Veramendi J. Plant growth-promoting traits of yeasts isolated from Spanish vineyards: Benefits for seedling development. Microbiol. Res. 2020;237:126480. doi: 10.1016/j.micres.2020.126480. [DOI] [PubMed] [Google Scholar]
- 20.Gai C.S., Lacava P.T., Maccheroni W., Jr., Glienke C., Araújo W.L., Miller T.A., Azevedo J.L. Diversity of endophytic yeasts from sweet orange and their localization by scanning electron microscopy. J. Basic Microb. 2009;49:441–451. doi: 10.1002/jobm.200800328. [DOI] [PubMed] [Google Scholar]
- 21.Isaeva O., Glushakova A., Yurkov A., Chernov I.Y. The yeast Candida railenensis in the fruits of English oak (Quercus robur L.) Microbiology. 2009;78:355–359. doi: 10.1134/S002626170903014X. [DOI] [PubMed] [Google Scholar]
- 22.Glushakova A., Kachalkin A. Endophytic yeasts in Malus domestica and Pyrus communis fruits under anthropogenic impact. Microbiology. 2017;86:128–135. doi: 10.1134/S0026261716060102. [DOI] [PubMed] [Google Scholar]
- 23.Kachalkin A.V., Glushakova A.M., Venzhik A.S. Presence of clinically significant endophytic yeasts in agricultural crops: Monitoring and ecological safety assessment. IOP Conf. Ser. Earth Environ. Sci. 2021;723:042005. doi: 10.1088/1755-1315/723/4/042005. [DOI] [Google Scholar]
- 24.Tien T.M., Gaskins M.H., Hubbell D.H. Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.) Appl. Environ. Microbiol. 1979;37:1016–1024. doi: 10.1128/aem.37.5.1016-1024.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Q., Chen L., Lu M., Chen G., Zhang L. Extraction and analysis of auxins in plants using dispersive liquid−liquid microextraction followed by high-performance liquid chromatography with fluorescence detection. J. Agric. Food Chem. 2010;58:2763–2770. doi: 10.1021/jf903274z. [DOI] [PubMed] [Google Scholar]
- 26.Streletskii R.A., Glushakova A.M., Zavgorodnyaya Y.A., Demin V.V., Chernov I.Y. 3-indole acetic acid generated by yeast fungi of different ecological groups. Mikol. Fitopatol. 2013;47:116–119. [Google Scholar]
- 27.Streletskii R.A., Kachalkin A.V., Glushakova A.M., Demin V.V., Chernov I.Y. Quantitative determination of indole-3-acetic acid in yeasts using high performance liquid chromatography-tandem mass spectrometry. Microbiology. 2016;85:727–736. doi: 10.1134/S0026261716060187. [DOI] [Google Scholar]
- 28.Streletskiy R.A., Kachalkin A.V., Demin V.V. Widespread phytohormonal activity among natural yeasts. Adv. Biotechnol. Microbiol. 2017;4:1–2. [Google Scholar]
- 29.Mestre M.C., Fontenla S., Bruzone M.C., Fernández N.V., Dames J. Detection of plant growth enhancing features in psychrotolerant yeasts from Patagonia (Argentina) J. Basic Microbiol. 2016;56:1098–1106. doi: 10.1002/jobm.201500728. [DOI] [PubMed] [Google Scholar]
- 30.Moller L., Lerm B., Botha A. Interactions of arboreal yeast endophytes: An unexplored discipline. Fungal Ecol. 2016;22:73–82. doi: 10.1016/j.funeco.2016.03.003. [DOI] [Google Scholar]
- 31.Joubert P.M., Doty S.L. Endophytes of Forest Trees. Springer; Cham, Switzerland: 2018. Endophytic Yeasts: Biology, Ecology and Applications; pp. 3–14. Forestry Sciences. [Google Scholar]
- 32.Petti C., Reiber K., Ali S.S., Berney M., Doohan F.M. Auxin as a player in the biocontrol of Fusarium head blight disease of barley and its potential as a disease control agent. BMC Plant Biol. 2012;12:224. doi: 10.1186/1471-2229-12-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.