Abstract
The blastocyst expresses paternally derived alloantigens and induces inflammation during implantation. However, it is necessary for the onset of pregnancy. An abnormal response might result in a pathological course of pregnancy or pregnancy failure. On the other hand, a state of maternal immune tolerance is necessary to ensure the normal development of pregnancy by suppressing inflammatory processes. This article discusses recognized mechanisms and the significance of inflammatory processes for embryo implantation and pregnancy establishment. We would also like to present disorders involving excessive inflammatory response and their influence on events occurring during embryo implantation. The chain of correlation between the processes responsible for embryo implantation and the subsequent physiological course of pregnancy is complicated. Many of those interrelationships are still yet to be discovered. Undoubtedly, their recognition will give hope to infertile couples for the emergence of new treatments that will increase the chance of giving birth to a healthy child.
Keywords: implantation, inflammatory processes, inflammation, endometrium, reproductive immunology, pregnancy, infertility, endometriosis
1. Introduction
The union of the sperm nucleus with the ovum nucleus causes the fusion of the hereditary genetic material in the fertilization process. In physiological conditions, it occurs in the ampulla of the fallopian tube. In humans, after 6–7 days and a series of cell divisions, which result in two-, four-, and eight- cell embryo development, we observe the formation of the cavitated blastocyst. The fluid-filled blastocyst is formed with the trophoblast cells and inner cell mass (embryoblast) under the zona pellucida.
The adherence of the embryo to the inner surface of the uterine wall (endometrium) is called implantation. It is an extraordinarily complex process. It is preceded by the hatching of the blastocyst and divided into apposition, epithelial adhesion, and blastocyst invasion in the endometrial stroma. The invading trophoblast differentiates: syncytiotrophoblast (the outer layer) is in contact with the maternal blood; cytotrophoblast (the inner layer), forming a cytotrophoblastic shell, reduces in time to create the placental membrane. Maternal-derived uterine epithelium (decidua) and fetal-derived placenta form the maternal–fetal interface.
The body’s immune system is educated and programmed to recognize and respond to foreign structures. The situation is much more complicated at the maternal–fetal interface. The mechanisms functioning there are not only designed to protect against pathogens but also provide a support system (created by cells and cytokines) for the mother to protect the embryo and preserve the pregnancy. This system is further modulated by the fetus, which through cytokines of trophoblast origin activates the mother’s immune response, making it possible to maintain the pregnancy under changing environmental conditions [1]. The uterine microbiome also influences the mother’s immune environment, ensuring proper tissue function and immune adaptation of the mother’s endometrium to accept the embryo [2].
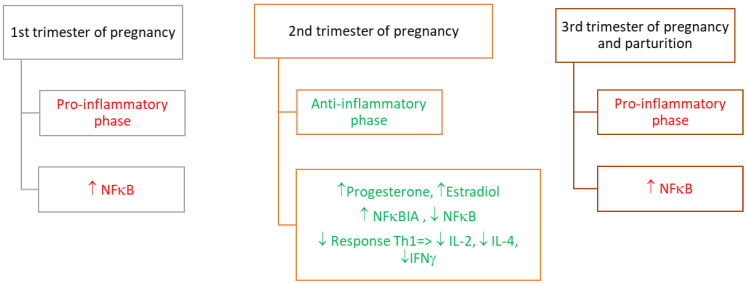
During pregnancy, we can distinguish three distinct stages of the immune response (immune phases) [3] (Figure 1). During the first phase, the inflammatory environment establishes the protective surroundings for an implanting embryo. During the second phase, the uterine surrounding promotes fetal growth, thus the immunological reaction is not so intensive. Proinflammatory environments in the uterus appear once again at the moment of parturition.
Figure 1.
Modulators of pro- and anti-inflammatory stages (the immune phases) during pregnancy. Legend: ↑: increase; ↓: decrease; IL-2: interleukin 2; IL-4: interleukin 4; IFNγ: interferon gamma; NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells; NFκBIA: NFκB inhibitor alpha; Th: T helper cells.
The immune microenvironment at the maternal–fetal interface is determined by the presence of cells of the maternal immune system and the secretion of modulating factors by trophoblast cells. Since the secretory molecules modulate the pro- and anti-inflammatory environment of the uterus during pregnancy, we decided to summarize the current knowledge of acute inflammation and the molecules engaged in this process at the maternal–fetal interface. Particular attention was paid to regulation at the molecular level by the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) under physiological conditions. The contributions of prostaglandins—the main regulators of inflammation—were not neglected. Aspects concerning the pathological state of chronic inflammation in the endometrium and its influence on fertility were also considered.
2. Inflammation-Related Molecules at the Maternal–Fetal Interface
Before blastocyst invasion, endometrial stromal cells secrete pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) [4,5] to initiate inflammation in the uterine mucosa. The early detection of these cytokines in the endometrium was demonstrated to be the molecular marker of implantation [5]. Both cytokines interact through a feedback loop: IL-1β is the mediator of the immune and inflammatory responses, and its secretion is induced by TNF-α [6,7]. On the other hand, there is an increase in the number of T- helper lymphocytes (Th1) and the synthesis of pro-inflammatory cytokines (IL-1β, interleukins 6 and 8 (IL-6 and IL-8), TNFα and interferon-gamma (IFNγ)) during embryo implantation in the uterine mucosa. Maternal immune response cells such as natural killer (NK) cells, macrophages, and dendritic cells synthesize pro-inflammatory cytokines [6,7,8,9,10,11,12] (Table 1). However, this does not apply to neutrophils [13]. Neutrophils are the first immune cells recruited at the site of infection and usually amplify the inflammatory signal that attracts other immune cells. The mechanisms that prevent neutrophil infiltration into the endometrium are unclear. One likely cause is the suppression of the cytokine signaling involved in their recruitment [11,14].
Table 1.
Secreted cytokines that mediate inflammation and their role in the implantation process.
| Pregnancy | Secreted Factor | Role | Reference |
|---|---|---|---|
| Preimplantation | TNFα | Induction of IL-1β secretion. | [6,7] |
| IL-1β | Promotion/propagation of decidualization and modulation of maternal NK cells, secretion of chemokines, and other factors required for implantation. Enhanced glycoprotein fucosylation. Regulation of the synthesis/secretion of trophoblastic matrix metalloproteinases MMP-2, MMP-3, and MMP-9 involved in trophoblast invasion. |
[15,16,17,18,19,20,21] | |
| Implantation | IL-1β | Promotion/propagation of decidualization and modulation of maternal NK cells, secretion of chemokines, and other factors required for implantation. | [15,18,20] |
| IL-6 | Stimulation of migration and trophoblast invasion. | [22,23] | |
| IL-8 | Stimulation of migration and trophoblast invasion. | [24,25] | |
| TNFα | Protection of the maternal tissue against excessive trophoblast invasion through the mechanism based on trophoblastic cell apoptosis. Regulation of synthesis/secretion of trophoblastic matrix metalloproteinases MMP-2, MMP-3, and MMP-9 participating in trophoblast invasion. |
[26,27,28,29] | |
| IFNγ | Protection of the maternal tissue against excessive trophoblast invasion through the mechanism based on trophoblastic cells apoptosis. | [9,29,30,31] |
Abbreviations: tumor necrosis factor α (TNF-α); interleukin 1β (IL-1β); interleukin 6 (IL-6); interleukin 8 (IL-8); interferon gamma (IFNγ).
2.1. Involvement of the Transcription Factor—NFκB in the Inflammatory Response
The accumulation of immune cells at the maternal–fetal interface and the secretion of inflammatory mediators during implantation occur under the control of NFκB. NFκB is a transcription factor involved in the regulation of the expression of genes associated with the onset of inflammation and generation of the immune response. It is also involved in response to heat stress, apoptosis, and tissue repair. Generally, the NFκB signal transduction pathway is modulated by cytoplasmic inhibitory proteins like inhibitor of nuclear factor-kappa B (IkB), interferon regulatory factor 6 (LPS), TNF, IL-1, or oxidative stress [32,33,34]. The level of NFκB increases during implantation and then subsequently decreases, which determines the maintenance of pregnancy. The re-increase of NFκB before delivery promotes the synthesis of prostaglandins (PGs), cytokines, and chemokines and stimulates uterine contractions [35,36].
Studies have demonstrated the action of NFκB factor and estrogen receptor signaling. Activated NFκB signaling initiates and maintains an inflammatory effect at the cellular level [37,38], while estrogens trigger anti-inflammatory responses [39]. This interaction is integrated by IL-1 [14,15].
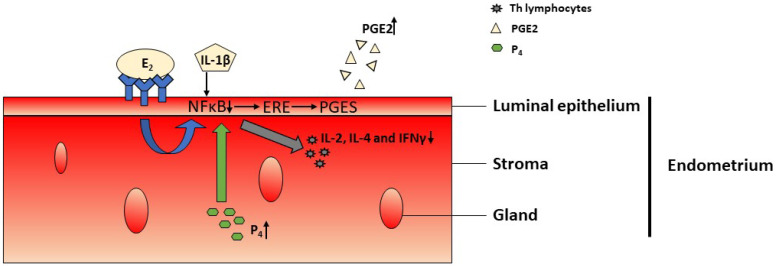
Estradiol (E2), which co-operates with estrogen receptors and IL-1β, affecting NFκB signaling, acts synergistically to increase the activity of estrogen response elements (ERE) in the DNA of the endometrial epithelial cells. This interaction increases the expression of the pool of genes involved in implantation, including genes coding prostaglandin E synthase, involved in the synthase of PGE2 [40].
Another steroid interacting with the NFκB factor is progesterone. The decrease in progesterone synthesis by steroidogenic cells is observed at the beginning of pregnancy. Elevated blood levels of progesterone reduce the expression of its receptors and FκB factor during the peri-implantation period in pigs and rodents [41,42,43,44,45]. Elevated levels of progesterone and estradiol as pregnancy develops (Figure 2.) lead in turn to the increased expression of NFκB inhibitor alpha (NFκBIA) and reduce NFkB activation [35,46]. The inability of NFκB to induce gene expression results in the inhibition of IL-2, IL-4, and IFNγ production by T lymphocytes [47]. These processes are essential for immunosuppression and the maintenance of maternal tolerance of the fetus during pregnancy [48,49]. Pregnancy-specific suppression of NFκB expression plays a role in reducing the production of cytokines by Th1 lymphocytes and maintaining the cytokine profile necessary for pregnancy initiation. On the other hand, NFκB levels in maternal T cells can be regulated not only by maternal steroid hormones or cytokines but also by placental cytokines.
Figure 2.
Changes during pregnancy development. Elevated levels of estradiol (E2) together with interleukin 1β (IL-1β) and progesterone (P4) reduce nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) activation, leading to an increase in the activity of estrogen response elements (ERE) in the DNA of endometrial luminal epithelium. This triggers an increase in the expression of prostaglandin E synthase (PGES) and production of prostaglandin E (PGE2). Reduced NFκB activation causes the inhibition of interleukin 2 (IL-2), interleukin 4 (IL-4) and interferon gamma (IFNγ) production in T lymphocytes. ↑: increase; ↓: decrease.
Under the control of NFκB are also the nucleotide-binding oligomerization domain-containing 1 and 2 (NOD1 and NOD2) genes expressed in human fetal membranes and term myometrium at labor. The NOD1 and NOD2 ligands, through NFκB activation, significantly increase proinflammatory and pro-labor mediators in human fetal membranes and myometrium [50]. Shorter gestation was predicted by genome-wide analyses of maternal blood samples when increased NF-κB activity in monocytes was observed [51].
Undoubtedly, the abnormal level of NFκB expression might predispose pregnant women to the pathological course of pregnancy with such consequences as delayed fetal growth, pregnancy-related hypertension, and premature delivery [36,52,53]. Premature or aberrant activation of NFκB factor associated with regulation of pro-inflammatory cytokines action may cause preterm labor [34]. Increased NFκB expression resulting from reduced BCL2 expression was observed in pregnancies complicated by intrauterine growth restriction (IUGR) and preeclampsia [53]. Oxidative stress through increased placental levels of TNFα, COX-2, and thromboxane likely activate placental NF-κB in preeclampsia [52].
2.2. Involvement of PGs in the Inflammatory Response
PGs are produced at the time of acute inflammatory reaction. The primary PGs involved in the inflammatory response are prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α). Studies have shown that in humans, concentrations of PGE2 and PGF2α significantly increase in the fluid obtained from the uterine cavity during the implantation window [54,55]. The PGs are believed to play a significant role in decidualization and trophoblast invasion [39]. PGE2 supports the luteal function of the corpus luteum, essential for embryo development and early implantation. Moreover, it induces the expression of chemokines important for trophoblast apposition and adhesion during implantation [56]. PGE2 has been shown to increase trophoblast adhesion ability via adhesion factors, including integrins [57]. Other studies have demonstrated that increased PGE2 synthesis in endometrial stromal cells contributed to the successful establishment of pregnancy in mammals [58]. Moreover, inhibition of PGE2 synthesis or expression of its receptor disturbed embryo adhesion [54]. Other authors claim that efficient PG synthesis in the endometrium improved implantation rates in patients with repeated implantation failures [59]. Therefore, normalized secretion of PGE2 by endometrial cells is relevant for the receptivity of the endometrium [60] and significantly correlates with the outcome of pregnancy. When the secretion of PGE2 is stable—it improves the effectiveness of embryo implantation. But when it is excessive—the chance for embryo implantation declines [61].
PGF2α can affect a variety of processes, usually acting in opposition to PGE2. PGF2α has been documented to induce: luteolysis [62], proliferation of endometrial epithelial cells [63], and constriction of spiral arterioles as well as contraction of the myometrium [64,65,66]. Moreover, in the endometrial luminal epithelium, PGF2α was found to control sodium and potassium ion transport [67] and induce the expression of endometrial connexins [68,69]. PGF2α causes vasoconstriction and induces hypoxia of endometrial cells. It causes the formation of new blood and lymphatic vessels through a beneficial impact on the production of vascular endothelial growth factor (VEGF) [70] and adrenomedullin [71]. Estrogen was found to stimulate the synthesis of PGF2α while progesterone was found to inhibit it [72,73]. Physiological changes in steroid hormone concentrations during the estrous cycle and pregnancy result in fluctuating levels of PGF2α: the highest levels are observed during implantation and before menstruation [74].
During early pregnancy, PGF2α increases the proliferation of human trophoblast cells [75] and promotes the association of molecules on trophoblast cells to the extracellular matrix protein, specifically fibronectin. Fibronectin expression is increased in the decidua during the first trimester of pregnancy [76,77,78]. Moreover, PGF2α promotes the process of implantation, but its impact can be controlled by the opposing effects of PGE2 [57]. PGF2α causes increased expression of mRNA and subsequent interleukin 6 (IL6) protein production in syncytiotrophoblast cells. The highest expression of IL6 occurs in the middle secretory phase of the menstrual cycle, which corresponds to the time of implantation, which in turn increases the amount of PGF2α in the uterine lumen [79,80]. Moreover, IL6 regulates the activity of matrix metalloproteinases [81] and stimulates the expression of integrins in trophoblast cells and processes such as invasiveness and migration [22,82]. PGF2α also acts indirectly through IL6 and can regulate implantation-related changes and immunological processes such as host defense [83].
On the other hand, pregnancy is associated with an anti-inflammatory condition. The levels of PGF2α metabolite (PGFM) and PGF2α in the decidua were significantly lower in the first trimester of pregnancy, comparable to the secretory phase of the menstrual cycle, when there was earlier elective termination of pregnancy [84]. Increased PGF2α production was shown to cause impaired uterine contractions, resulting in abnormal semen migration, defective transport of fertilized ova, and impaired implantation [85,86]. In women with intramural fibroids, higher levels of PGF2α were found both in the fibroids themselves and in the endometrium, leading to lower pregnancy and implantation rates, even if the fibroids did not distort the uterine cavity [86]. Excessively high levels of PGF2α in decidua may trigger a pregnancy loss cascade and lead to miscarriages [87,88,89].
During implantation, strengthened PGE2 signaling and inhibition of PGF2α signaling within the endometrium were found [61].
The transformation of arachidonic acid to PG precursors is possible due to the action of COX enzymes. Interestingly, COX-2, which is engaged in inflammatory processes, is also involved in the oxidation of endogenous cannabinoid (arachidonoylethanolamide; AEA) [90]. In this way, AEA seems to be capable of modulating PG production [90]. Low levels of serum AEA at the time of implantation were observed in women subjected to in vitro fertilization (IVF) or intra-cytoplasmic sperm injection procedure (ICSI) [91]. The expression of the components of the endocannabinoid system is found in the human placenta at the 30th, 34th, and 40th week of gestation [92]. In the amnion, AEA was found to be responsible for the PGE2 concentration increase [93]. However, it could also cause opposite effects on uterine PGE2 and PGF2α biosynthesis by inhibiting PGE2 production and increasing PGF2α levels [94].
Abnormal PG synthesis was found to be associated with repeated implantation failure in patients undergoing in vitro fertility treatment [59]. Therefore, the measurement of PGs 24 h before the planned embryo transfer allows for the prediction of a favorable outcome [54,55].
3. Inflammation-Related Molecules in Pathologically Altered Endometrium
Acute inflammation of the endometrium is essential for successful implantation [95] while chronic inflammation is destructive and can lead to infertility [96,97,98]. Chronic inflammation is caused by endometriosis, chronic endometritis (CE), and hydrosalpinx. Thus, we will briefly characterize these disorders.
Endometriosis is caused by hereditary as well as environmental factors [99]. It affects approximately 190 million women worldwide. The estimated overall prevalence of endometriosis in the population ranges from 0.8% to 6% and is higher among Asian women. The incidence of endometriosis appears to be significantly higher in infertile women than in fertile ones, ranging from 20% to 50%. Differences are also observed depending on the duration of infertility and the age of patients [100,101].
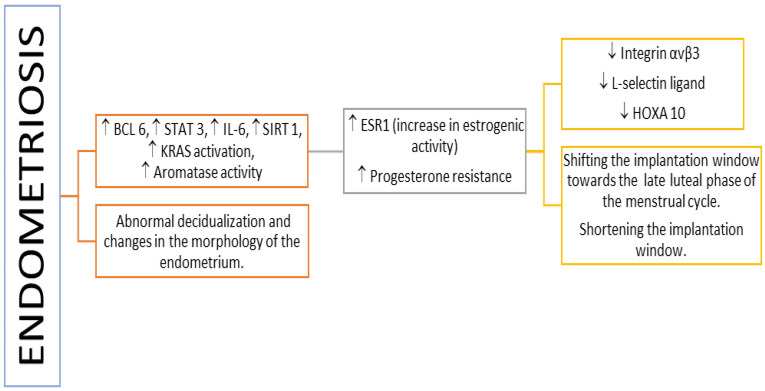
Endometriosis is a disease triggered by inflammation induced by estrogens. The local concentration of estrogens and androgens is extremely high compared to peripheral blood concentrations and causes changes in cytokine expression (Figure 3), disrupting the normal function of the endometrium in endometriosis [102,103,104]. Released cytokines involved in immune responses and responsible for inflammation are TNF, IL-1, IL-6, IL-8, IL-10, and TGF-B1 [99]. Other characteristic features of inflammation observed in endometriosis are the infiltration of lymphocytes; synthesis of eicosanoids and metalloproteinases; and atypical changes in the populations of T, B, Treg, and NK lymphocytes. In women with endometriosis, a decrease in gene expression coding for endometrial proteins crucial for proper implantation [96,105], including αVβ3 integrin [105,106], L-selectin ligand [107,108,109], and HOXA10 protein [110,111,112], was observed.
Figure 3.
Pathomechanisms impeding the course of implantation in women with endometriosis. Legend: ↑: increase; ↓: decrease; BCL 6: B-cell lymphoma 6; ESR1: estrogen receptor 1; IL-6: interleukin 6; KRAS: gene encoding K-Ras protein with GTPase activity; SIRT 1: Sirtuin 1; STAT3: signal transducer and activator of transcription 3.
Moreover, many studies have shown abnormal decidualization and changes in the morphology of the endometrium [113,114,115,116,117,118]. The observed changes in the expression of endometrial genes are caused by excessive estrogenic activity [119,120]. In patients with endometriosis, it was demonstrated that the increase in the expression of estrogen receptor (ESR1) occurred during implantation [119,121]. Changes in progesterone receptor (PR) expression and reduction in the effects of progesterone have also been demonstrated [122,123,124,125]. What is more, endometriosis-associated progesterone desensitization contributed to the increased proliferation and survival of cells [126,127] and increased ESR2 levels [121,128]. Insensitivity to progesterone signaling leads to the pro-inflammation condition, as progesterone plays an important role in reducing inflammation in the endometrium [122]. The severity of the inflammatory process and diminished sensitivity of receptors to progesterone differ between women diagnosed with endometriosis [122,129,130]. Increased inflammatory response and reduced progesterone sensitivity are related to a higher risk of implantation failure [129,130,131]. These factors shift the implantation window towards the rest of the menstrual cycle and shorten its duration [130].
Molecular factors involved in cytokine synthesis (as NF-κB factor) and cytokines (as IL-1, IL-2, IL-6, IL-8, IL-33, TNF-α) are potential targets for therapies directed against endometriosis. Extensive laboratory studies utilizing pharmacological inhibitors of NF-κB factor (for example: methyl ester of 2-cyano-3,12-dioxooleana-1,9-dien-28-oicacid, dienogest, thalidomide, genistein, ginsenoside, gossypol), and inhibitors of cytokines (for example: resveratrol, tocilizumab, pyrvinium pamoate, nobiletin, S, R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester [132]) have been conducted. Potential therapies are also being investigated by analyzing the hormone-controlled mechanisms of endometriosis. The most effective solution seems to be lowering estradiol levels by indirectly inhibiting its synthesis using medications such as linzagolix, relugolix, and elagolix [132].
There are treatments for endometriosis that also serve as therapy for endometriosis-associated pain at the same time. Considering their effects on fertility, they have advantages and disadvantages. An improvement in pregnancy rate is offered by the surgical removal of endometriosis lesions or short-term immunotherapy using glucocorticosteroids [99]. Adhesiolysis enhances the chance of spontaneous pregnancy [133]. Therapy with progestins and oestro-progestins influences endometriosis but does not tweak the fertility rate [134]. TNF antagonist treatment seems to be effective but is not recommended for routine usage. The effects of fertility treatment may be worsened by endometriosis immunosuppressive therapy [99].
Another disease characterized by interminable inflammation is chronic endometritis (CE). It is caused by the imbalance between the coexistence of microorganisms on the endometrial surface and the proper function of the immune system manifested by immunocompetent cells in the uterine stroma. Most cases of CE are asymptomatic. Studies have shown that the incidence of CE is 2.8–66.8% in infertile women, 14–67.5% in women with recurrent implantation failure, and 9.3–67.6% in women with recurrent pregnancy loss [135,136].
In approximately 70% of cases, more than one pathogen is responsible for the occurrence of CE. Common bacteria such as Streptococcus spp., Escherichia coli, Enterococcus faecalis, Klebsiella pneumoniae, Staphylococcus spp., and Corynebacterium and Mycoplasma/Ureaplasma spp. are present in the uterine cavity of CE patients. Their presence was detected by microbial cultures or by PCR tests [137,138,139,140,141,142,143].
CE is diagnosed based on endometrial biopsy and plasma cell presence generated by stimulation of B lymphocytes [144]. The presence of B cells was confirmed in the endometrium throughout the menstrual cycle. They were found mainly in the basal layer and accounted for only a minor percentage (<2%) of all immune cells in the normal endometrium [136,142]. In CE, the B cells number increases significantly in all layers of the endometrium [136,143].
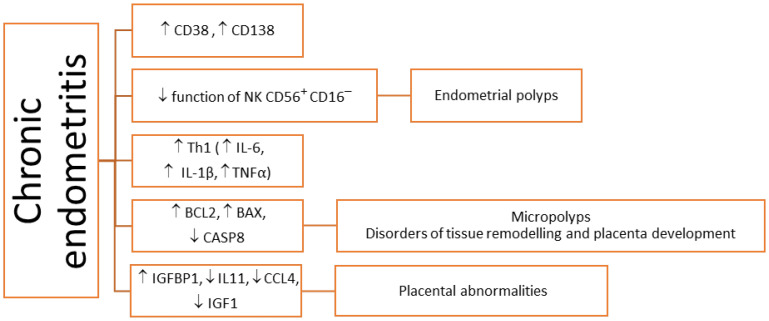
The immunohistochemical staining of specific surface antigens CD38 and CD138 allows for the detection of plasma cells [144] and the diagnosis of CE with four times greater sensitivity compared to the histopathological evaluation of endometrial tissue sections stained only with hematoxylin and eosin [145]. (Figure 4).
Figure 4.
Pathomechanisms impeding the course of implantation in women with chronic endometritis (CE). Legend: ↑: increase; ↓: decrease; BAX: proapoptotic BAX protein; BCL2: anti-apoptotic BCL2 protein; CASP8: cysteine–aspartic acid protease 8; CCL4: CCL4 chemokine; CD38 and CD138: plasma cells CD38 and CD138; IL-1β: interleukin 1β; IL-6: interleukin 6; IL-11: interleukin 11; IGF1: insulin-like growth factor 1; IGFBP1: IGF-binding protein 1; NK CD56 + CD16: decidual NK cells; Th: helper T cells; TNF-α: tumor necrosis factor α.
CE can also be diagnosed during hysteroscopic evaluation of the uterine cavity [137,146]. The features indicating the presence of CE are micropolyps, stromal oedema, and focal or diffuse hyperaemia. Hysteroscopic evaluation is more sensitive in the diagnosis of CE than in uterine cavity culture [137]. Some studies found that the proportion of CD56 + CD16– NK cells in the endometrium in the secretory phase was similar in women with unexplained infertility, in CE and control subjects [147,148]; other studies have described significantly higher levels of CD56 + CD16– NK cells in the endometrium of women with CE compared with those without CE [149,150,151,152,153]. Histologically confirmed CE may favor the formation of micropolyps characterized by the accumulation of leukocytes (CD45), macrophages (CD68), plasma cells (CD138), and NK (CD56+) cells, whose activity leads to excess abnormal proliferation of endometrium [149,150]. The distribution of endometrial immunocompetent cells is altered with the menstrual cycle, and the Th1/Th2 balance is Th1-predominant from the menstrual to the proliferative phase, shifting to Th2 predominant from the implantation phase to early pregnancy [151]. The studies revealed that non-CE endometrium showed Th2 predominance in the implantation phase, but CE endometrium showed Th1 predominance [151]. Moreover, increased IL-17 and decreased IL-10 and TGF-β expressions in the endometrium of CE patients were found. This suggests that CE induces a propensity to Th17 over Treg immunity in the endometrium, which consequently leads to poor reproductive outcomes [152].
Women with CE have been found to have increased expression of the insulin-like growth factor-binding protein 1 (IGFBP1) gene in the endometrium, with a simultaneously decreased expression of the insulin-like growth factor 1 (IGF1) genes, IL-11 and CCL4 [153,154]. IGF1 mediates the stimulatory effect of estrogens on the proliferation of endometrial cells, while IGF2 mediates progesterone action during the secretory phase, facilitating embryo implantation and invasion [17,153,155]. Increased secretion of IGFBP1 by the endometrial stromal cell during decidualization counteracts the effect exerted by IGF2, which has a negative impact on embryo implantation. Increased expression of the IGFBP1 gene and decreased expression of the IGF1 gene are responsible for unfavorable conditions for embryo implantation and development. IL-11 is a cytokine with anti-inflammatory properties. IL-11 production is highest during decidualization [156,157]. On the other hand, decreased levels and abnormal IL-11 signaling can disrupt trophoblast invasion [158,159,160].
High expression of the gene encoding transcriptional repressor BCL6 (B-cell lymphoma 6) in the endometrium allows the detection of endometritis associated with endometriosis [161]. Elevated BCL6 and aromatase levels are associated with progesterone resistance and estrogen dominance in women with endometriosis [129]. As a repressor of the genes, BCL6 may be responsible for progesterone resistance by reducing the secretion of progesterone-mediated factors, including the transcription factor that recognizes nucleotide sequence identified in the promoter of a gene encoding chicken ovalbumin upstream promoter-transcription factor 2 (COUP-TFII) [162]. COUP-TFII regulates many genes responsible for the decidualization of the endometrial stromal cells, including those involved in cell adhesion, angiogenesis, and inflammation. COUP-TFII also plays an important role in controlling the expression of inflammatory cytokines [163,164].
During early pregnancy, the trophoblast recruits NK cells and macrophages into the endometrium via chemokines such as CCL4 and stimulates them to produce pro-inflammatory cytokines [153,158,165,166]. Reduced CCL4 activity in women with CE may result in implantation failure or abnormal placental development [153,167,168].
Regarding treatment, personalized oral, systemic antibiotic therapy is considered to be efficient in the therapy of CE [169,170]. Antibiotics such as doxycycline [171] or a combination of levofloxacin and tinidazole [172] are effective in CE treatment. Moreover, they are also considered potentially successful in the improvement of fertility, which was shortly summarized elsewhere [170].
Another disease that can also cause chronic endometritis is hydrosalpinx. Fluid from the fallopian tubes entering the uterine cavity may have a direct embryotoxic effect [173,174]. This fluid contains inflammatory mediators such as cytokines, PGs, mucosa debris and toxins, impairing blood flow through the uterine spiral arteries [175,176,177]. Moreover, hydrosalpinx mechanically disturbs the contact between the embryo and the endometrial surface [173,174]. The effect of hydrosalpinx on the endometrium is chronic endometritis, which negatively affects endometrial receptivity [178,179]. Patients with hydrosalpinx showed a statistically significant increase in the number of many different plasma cells and lymphocytes infiltrating the endometrial stroma, together with the increased expression of IL-2 protein. It is indicative of a generalized inflammation [178,180]. The increased expression of mRNA and NF-κB protein, which promotes inflammatory processes and adversely affects implantation, has also been found [179]. Endometrial HOXA10 implantation factor expression is also reduced in a woman with hydrosalpinx. The salpingectomy procedure regulates HOXA10 expression, improves implantation and reduces early pregnancy loss [181]. Hydrosalpinx, tubal occlusion, and hysteroscopic insertion of Essure are currently recommended therapies to lower the hydrosalpingeal fluid amount [169].
4. Conclusions
Both similarities and dissimilarities characterize inflammatory processes occurring during embryo implantation and pathological states. Their course and severity are tightly controlled by numerous mechanisms. Specific molecules involved in both types of processes are observed. Their lack of expression may lead to implantation failures, miscarriages, and pregnancy pathologies. Knowledge of these processes will allow for their proper control, and regulation will allow for their appropriate course, which will affect the quality of our reproductive health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mor G., Cardenas I., Abrahams V., Guller S. Inflammation and Pregnancy: The Role of the Immune System at the Implantation Site. Ann. N. Y. Acad. Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benner M., Ferwerda G., Joosten I., van der Molen R.G. How Uterine Microbiota Might Be Responsible for a Receptive, Fertile Endometrium. Hum. Reprod. Update. 2018;24:393–415. doi: 10.1093/humupd/dmy012. [DOI] [PubMed] [Google Scholar]
- 3.Gómez-Chávez F., Correa D., Navarrete-Meneses P., Cancino-Diaz J.C., Cancino-Diaz M.E., Rodríguez-Martínez S. NF-κB and Its Regulators during Pregnancy. Front. Immunol. 2021;12:679106. doi: 10.3389/fimmu.2021.679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathare A., Hinduja I., Zaveri K. Immunological Approach of Personalized Treatment for Recurrent Implantation Failure Patients Undergoing IVF. Glob. J. Reprod. Med. 2018;5:555667. doi: 10.19080/GJORM.2018.05.555667. [DOI] [Google Scholar]
- 5.Salama K.M., Alloush M.K. Are the Cytokines TNF Alpha and IL 1Beta Early Predictors of Embryo Implantation? Cross Sectional Study. J. Reprod. Immunol. 2020;137:102618. doi: 10.1016/j.jri.2019.102618. [DOI] [PubMed] [Google Scholar]
- 6.Achache H., Revel A. Endometrial Receptivity Markers, the Journey to Successful Embryo Implantation. Hum. Reprod. Update. 2006;12:731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 7.Chimote N., Chimote M., Mehta B., Nath N. Cytokines and Growth Factors in Implantation. J. Reprod. Stem Cell Biotechnol. 2010;1:219–243. doi: 10.1177/205891581000100209. [DOI] [Google Scholar]
- 8.Massimiani M., Lacconi V., la Civita F., Ticconi C., Rago R., Campagnolo L. Molecular Signaling Regulating Endometrium–Blastocyst Crosstalk. Int. J. Mol. Sci. 2020;21:23. doi: 10.3390/ijms21010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y., Zhang T., Guo X., Wong C.K., Chen X., Chan Y.L., Wang C.C., Laird S., Li T.C. Successful Implantation Is Associated with a Transient Increase in Serum Pro-Inflammatory Cytokine Profile Followed by a Switch to Anti-Inflammatory Cytokine Profile Prior to Confirmation of Pregnancy. Fertil. Steril. 2021;115:1044–1053. doi: 10.1016/j.fertnstert.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Maria J., Alecsandru D. Circulating Cytokines during the Blastocyst Peri-Implantation Period. Fertil. Steril. 2021;115:905–906. doi: 10.1016/j.fertnstert.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Fülöp V., Vermes G., Demeter J. The Relationship between Inflammatory and Immunological Processes during Pregnancy. Practical Aspects. Orv. Hetil. 2019;160:1247–1259. doi: 10.1556/650.2019.31448. [DOI] [PubMed] [Google Scholar]
- 12.Rehman R., Ashraf M., Jasmine A., Lal K., Alam F. Cytokines and Endometrial Receptivity after Intracytoplasmic Sperm Injection—A Cohort Study at Islamabad. J. Pak. Med. Assoc. 2018;68:862–866. [PubMed] [Google Scholar]
- 13.Dekel N., Gnainsky Y., Granot I., Mor G. Inflammation and Implantation. Am. J. Reprod. Immunol. 2010;63:17–21. doi: 10.1111/j.1600-0897.2009.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavan A.R., Griffith O.W., Wagner G.P. The Inflammation Paradox in the Evolution of Mammalian Pregnancy: Turning a Foe into a Friend. Curr. Opin. Genet. Dev. 2017;47:24–32. doi: 10.1016/j.gde.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S., Godbole G., Modi D. Decidual Control of Trophoblast Invasion. Am. J. Reprod. Immunol. 2016;75:341–350. doi: 10.1111/aji.12466. [DOI] [PubMed] [Google Scholar]
- 16.Simón C., Mercader A., Gimeno M.J., Pellicer A. The Interleukin-1 System and Human Implantation. Am. J. Reprod. Immunol. 1997;37:64–72. doi: 10.1111/j.1600-0897.1997.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 17.Fazleabas A.T., Kim J.J., Strakova Z. Implantation: Embryonic Signals and the Modulation of the Uterine Environment—A Review. Placenta. 2004;25:S26–S31. doi: 10.1016/j.placenta.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Prutsch N., Fock V., Haslinger P., Haider S., Fiala C., Pollheimer J., Knöfler M. The Role of Interleukin-1β in Human Trophoblast Motility. Placenta. 2012;33:696–703. doi: 10.1016/j.placenta.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura H., Jasper M.J., Hull M.L., Aplin J.D., Robertson S.A. Macrophages Regulate Expression of A1, 2-Fucosyltransferase Genes in Human Endometrial Epithelial Cells. Mol. Hum. Reprod. 2012;18:204–215. doi: 10.1093/molehr/gar070. [DOI] [PubMed] [Google Scholar]
- 20.Robertson S.A., Moldenhauer L.M. Immunological Determinants of Implantation Success. Int. J. Dev. Biol. 2014;58:205–217. doi: 10.1387/ijdb.140096sr. [DOI] [PubMed] [Google Scholar]
- 21.Minas V., Loutradis D., Makrigiannakis A. Factors Controlling Blastocyst Implantation. Reprod. Biomed. Online. 2005;10:205–216. doi: 10.1016/S1472-6483(10)60942-X. [DOI] [PubMed] [Google Scholar]
- 22.De Sousa F.L.P., Chaiwangyen W., Morales-Prieto D.M., Ospina-Prieto S., Weber M., Photini S.M., Sass N., Daher S., Schleussner E., Markert U.R. Involvement of STAT1 in Proliferation and Invasiveness of Trophoblastic Cells. Reprod. Biol. 2017;17:218–224. doi: 10.1016/j.repbio.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Ding J., Yang C., Cheng Y., Wang J., Zhang S., Yan S., He F., Yin T., Yang J. Trophoblast-Derived IL-6 Serves as an Important Factor for Normal Pregnancy by Activating Stat3-Mediated M2 Macrophages Polarization. Int. Immunopharmacol. 2021;90:106788. doi: 10.1016/j.intimp.2020.106788. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee P., Malik A., Malhotra S.S., Gupta S.K. Role of STAT Signaling and Autocrine Action of Chemokines during H2O2 Induced HTR-8/SVneo Trophoblastic Cells Invasion. J. Cell Physiol. 2019;234:1380–1397. doi: 10.1002/jcp.26934. [DOI] [PubMed] [Google Scholar]
- 25.Ryu B.J., Han J.W., Kim R.H., Yun S., Kim T.H., Hur S.E., Kim C.J., Lee S.K. Activation of NOD-1/JNK/IL-8 Signal Axis in Decidual Stromal Cells Facilitates Trophoblast Invasion. Am. J. Reprod. Immunol. 2017;78:e12672. doi: 10.1111/aji.12672. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K., Watanabe M., Matsushima M., Matsuzawa Y., Izawa T., Nagashima T., Kobayashi Y., Iwashita M. Synergistic Effects of Tumor Necrosis Factor-α and Insulin-like Growth Factor-I on Survival of Human Trophoblast-Derived BeWo Cell Line. Growth Horm. IGF Res. 2018;41:34–41. doi: 10.1016/j.ghir.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Basu J., Agamasu E., Bendek B., Salafia C.M., Mishra A., Benfield N., Prasad P., Mikhail M. Placental Tumor Necrosis Factor-α Protein Expression during Normal Human Gestation. J. Matern.-Fetal Neonatal Med. 2016;29:3934–3938. doi: 10.3109/14767058.2016.1156668. [DOI] [PubMed] [Google Scholar]
- 28.Bauer S., Pollheimer J., Hartmann J., Husslein P., Aplin J.D., Knöfler M. Tumor Necrosis Factor-α Inhibits Trophoblast Migration through Elevation of Plasminogen Activator Inhibitor-1 in First-Trimester Villous Explant Cultures. J. Clin. Endocrinol. Metab. 2004;89:812–822. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- 29.Otun H.A., Lash G.E., Innes B.A., Bulmer J.N., Naruse K., Hannon T., Searle R.F., Robson S.C. Effect of Tumour Necrosis Factor-α in Combination with Interferon-γ on First Trimester Extravillous Trophoblast Invasion. J. Reprod. Immunol. 2011;88:1–11. doi: 10.1016/j.jri.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Lash G.E., Otun H.A., Innes B.A., Kirkley M., de Oliveira L., Searle R.F., Robson S.C., Bulmer J.N. Interferon-γ Inhibits Extravillous Trophoblast Cell Invasion by a Mechanism That Involves Both Changes in Apoptosis and Protease Levels. FASEB J. 2006;20:2512–2518. doi: 10.1096/fj.06-6616com. [DOI] [PubMed] [Google Scholar]
- 31.Verma S., Pal R., Gupta S.K. Decrease in Invasion of HTR-8/SVneo Trophoblastic Cells by Interferon Gamma Involves Cross-Communication of STAT1 and BATF2 That Regulates the Expression of JUN. Cell Adhes. Migr. 2018;12:432–446. doi: 10.1080/19336918.2018.1434030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali S., Mann D.A. Signal Transduction via the NF-κB Pathway: A Targeted Treatment Modality for Infection, Inflammation and Repair. Cell Biochem. Funct. Cell. Biochem. Its Modul. Act. Agents Dis. 2004;22:67–79. doi: 10.1002/cbf.1082. [DOI] [PubMed] [Google Scholar]
- 33.Geisert R., Fazleabas A., Lucy M., Mathew D. Interaction of the Conceptus and Endometrium to Establish Pregnancy in Mammals: Role of Interleukin 1β. Cell Tissue Res. 2012;349:825–838. doi: 10.1007/s00441-012-1356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novembri R., de Clemente C., Funghi L., Torricelli M., Voltolini C., Challis J.R., Petraglia F. Corticotropin Releasing Hormone and Urocortin 2 Activate Inflammatory Pathways in Cultured Trophoblast Cell Lines. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;195:200–205. doi: 10.1016/j.ejogrb.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 35.McCracken S.A., Hadfield K.A., Yenson V.M., Ariyakumar K.J., McKelvey K., Woodland N., ASHTON A.W., MORRIS J.M. NF-KB Regulation in T-Cells in Pregnancy Is Mediated via Fas/FasL Interactions: The Signal for Which Is Derived from Exosomes Present in Maternal Plasma. Reprod. Immunol. 2016;1:8–18. doi: 10.21767/2476-1974.100008. [DOI] [Google Scholar]
- 36.Lindstrom T.M., Bennett P.R. The Role of Nuclear Factor Kappa B in Human Labour. Reproduction. 2005;130:569–581. doi: 10.1530/rep.1.00197. [DOI] [PubMed] [Google Scholar]
- 37.Biswas D.K., Singh S., Shi Q., Pardee A.B., Iglehart J.D. Crossroads of Estrogen Receptor and NF-κB Signaling. Sci. STKE. 2005;2005:pe27. doi: 10.1126/stke.2882005pe27. [DOI] [PubMed] [Google Scholar]
- 38.Harnish D.C. Estrogen Receptor Ligands in the Control of Pathogenic Inflammation. Curr. Opin. Investig. Drugs. 2006;7:997–1001. [PubMed] [Google Scholar]
- 39.Lou Y., Hu M., Wang Q., Yuan M., Wang N., Le F., Li L., Huang S., Wang L., Xu X. Estradiol Suppresses TLR4-Triggered Apoptosis of Decidual Stromal Cells and Drives an Anti-Inflammatory TH2 Shift by Activating SGK1. Int. J. Biol. Sci. 2017;13:434. doi: 10.7150/ijbs.18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King A.E., Critchley H.O.D., Kelly R.W. The NF-ΚB Pathway in Human Endometrium and First Trimester Decidua. Mol. Hum. Reprod. 2001;7:175–183. doi: 10.1093/molehr/7.2.175. [DOI] [PubMed] [Google Scholar]
- 41.Ross J.W., Ashworth M.D., Mathew D., Reagan P., Ritchey J.W., Hayashi K., Spencer T.E., Lucy M., Geisert R.D. Activation of the Transcription Factor, Nuclear Factor Kappa-B, during the Estrous Cycle and Early Pregnancy in the Pig. Reprod. Biol. Endocrinol. 2010;8:39. doi: 10.1186/1477-7827-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geisert R.D., Pratt T.N., Bazer F.W., Mayes J.S., Watson G.H. Immunocytochemical Localization and Changes in Endometrial Progestin Receptor Protein during the Porcine Oestrous Cycle and Early Pregnancy. Reprod. Fertil. Dev. 1994;6:749–760. doi: 10.1071/RD9940749. [DOI] [PubMed] [Google Scholar]
- 43.Zhang G., Cui L., Li A., Zhang J., Liu Y., Zhao J., Xu X., He B., Wang J., Chu L. Endometrial Breakdown with Sustained Progesterone Release Involves NF-κB-mediated Functional Progesterone Withdrawal in a Mouse Implant Model. Mol. Reprod. Dev. 2016;83:780–791. doi: 10.1002/mrd.22686. [DOI] [PubMed] [Google Scholar]
- 44.Kalkhoven E., Wissink S., van der Saag P.T., van der Burg B. Negative Interaction between the RelA (P65) Subunit of NF-κB and the Progesterone Receptor. J. Biol. Chem. 1996;271:6217–6224. doi: 10.1074/jbc.271.11.6217. [DOI] [PubMed] [Google Scholar]
- 45.Davies S., Dai D., Feldman I., Pickett G., Leslie K.K. Identification of a Novel Mechanism of NF-ΚB Inactivation by Progesterone through Progesterone Receptors in Hec50co Poorly Differentiated Endometrial Cancer Cells: Induction of A20 and ABIN-2. Gynecol. Oncol. 2004;94:463–470. doi: 10.1016/j.ygyno.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Hardy D.B., Janowski B.A., Chen C.-C., Mendelson C.R. Progesterone Receptor Inhibits Aromatase and Inflammatory Response Pathways in Breast Cancer Cells via Ligand-Dependent and Ligand-Independent Mechanisms. Mol. Endocrinol. 2008;22:1812–1824. doi: 10.1210/me.2007-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bettelli E., Dastrange M., Oukka M. Foxp3 Interacts with Nuclear Factor of Activated T Cells and NF-ΚB to Repress Cytokine Gene Expression and Effector Functions of T Helper Cells. Proc. Natl. Acad. Sci. USA. 2005;102:5138–5143. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwiatek M., Gęca T., Kwaśniewska A. Pro-and Anti-Inflammatory Cytokines in the First Trimester—Comparison of Missed Miscarriage and Normal Pregnancy. Int. J. Environ. Res. Public Health. 2021;18:8538. doi: 10.3390/ijerph18168538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W., Sung N., Gilman-Sachs A., Kwak-Kim J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front. Immunol. 2020;11:2025. doi: 10.3389/fimmu.2020.02025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lappas M. NOD1 and NOD2 Regulate Proinflammatory and Prolabor Mediators in Human Fetal Membranes and Myometrium via Nuclear Factor-Kappa B. Biol. Reprod. 2013;89:11–14. doi: 10.1095/biolreprod.113.110056. [DOI] [PubMed] [Google Scholar]
- 51.Ross K.M., Carroll J.E., Dunkel Schetter C., Hobel C., Cole S.W. Pro-inflammatory Immune Cell Gene Expression during the Third Trimester of Pregnancy Is Associated with Shorter Gestational Length and Lower Birthweight. Am. J. Reprod. Immunol. 2019;82:e13190. doi: 10.1111/aji.13190. [DOI] [PubMed] [Google Scholar]
- 52.Vaughan J.E., Walsh S.W. Activation of NF-ΚB in Placentas of Women with Preeclampsia. Hypertens Pregnancy. 2012;31:243–251. doi: 10.3109/10641955.2011.642436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aban M., Cinel L., Arslan M., Dilek U., Kaplanoglu M., Arpaci R., Dilek S. Expression of Nuclear Factor-Kappa B and Placental Apoptosis in Pregnancies Complicated with Intrauterine Growth Restriction and Preeclampsia: An Immunohistochemical Study. Tohoku J. Exp. Med. 2004;204:195–202. doi: 10.1620/tjem.204.195. [DOI] [PubMed] [Google Scholar]
- 54.Vilella F., Ramirez L.B., Simón C. Lipidomics as an Emerging Tool to Predict Endometrial Receptivity. Fertil. Steril. 2013;99:1100–1106. doi: 10.1016/j.fertnstert.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 55.Salleh N. Diverse Roles of Prostaglandins in Blastocyst Implantation. Sci. World J. 2014;2014:968141. doi: 10.1155/2014/968141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niringiyumukiza J.D., Cai H., Xiang W. Prostaglandin E2 Involvement in Mammalian Female Fertility: Ovulation, Fertilization, Embryo Development and Early Implantation. Reprod. Biol. Endocrinol. 2018;16:43. doi: 10.1186/s12958-018-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waclawik A., Kaczynski P., Jabbour H.N. Autocrine and Paracrine Mechanisms of Prostaglandin E2 Action on Trophoblast/Conceptus Cells through the Prostaglandin E2 Receptor (PTGER2) during Implantation. Endocrinology. 2013;154:3864–3876. doi: 10.1210/en.2012-2271. [DOI] [PubMed] [Google Scholar]
- 58.Hallé C., Goff A.K., Petit H.V., Blouin R., Palin M.-F. Effects of Different N-6: N-3 Fatty Acid Ratios and of Enterolactone on Gene Expression and PG Secretion in Bovine Endometrial Cells. Br. J. Nutr. 2015;113:56–71. doi: 10.1017/S0007114514003304. [DOI] [PubMed] [Google Scholar]
- 59.Achache H., Tsafrir A., Prus D., Reich R., Revel A. Defective Endometrial Prostaglandin Synthesis Identified in Patients with Repeated Implantation Failure Undergoing in Vitro Fertilization. Fertil. Steril. 2010;94:1271–1278. doi: 10.1016/j.fertnstert.2009.07.1668. [DOI] [PubMed] [Google Scholar]
- 60.Miravet-Valenciano J.A., Rincon-Bertolin A., Vilella F., Simon C. Understanding and Improving Endometrial Receptivity. Curr. Opin. Obstet. Gynecol. 2015;27:187–192. doi: 10.1097/GCO.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 61.Huang X., Liu H., Li R. Prostaglandin E2 Promotes BeWo Spheroids Implantation in RL95-2 Cell Monolayers. Gynecol. Endocrinol. 2017;33:548–552. doi: 10.1080/09513590.2017.1296125. [DOI] [PubMed] [Google Scholar]
- 62.McCracken J.A., Custer E.E., Lamsa J.C. Luteolysis: A Neuroendocrine-Mediated Event. Physiol. Rev. 1999;79:263–323. doi: 10.1152/physrev.1999.79.2.263. [DOI] [PubMed] [Google Scholar]
- 63.Milne S.A., Jabbour H.N. Prostaglandin (PG) F2α Receptor Expression and Signaling in Human Endometrium: Role of PGF2α in Epithelial Cell Proliferation. J. Clin. Endocrinol. Metab. 2003;88:1825–1832. doi: 10.1210/jc.2002-021368. [DOI] [PubMed] [Google Scholar]
- 64.Xiao L., Zhang Q., Huang X., He A., Xie S., Li Y. Endometrial Stromal Cell MiR-29c-3p Regulates Uterine Contraction. Reproduction. 2019;158:493–501. doi: 10.1530/REP-19-0196. [DOI] [PubMed] [Google Scholar]
- 65.Liu B., Li J., Yan H., Tian D., Li H., Zhang Y., Guo T., Wu X., Luo W., Zhou Y. TP and/or EP3 Receptors Mediate the Vasoconstrictor and Pressor Responses of Prostaglandin F2α in Mice and/or Humans. FASEB J. 2019;33:2451–2459. doi: 10.1096/fj.201801064RR. [DOI] [PubMed] [Google Scholar]
- 66.Broegger T., Andersson K.-E., Aalkjaer C., Forman A., Boedtkjer D.B. Sensitivity to the Thromboxane A2 Analog U46619 Varies with Inner Diameter in Human Stem Villous Arteries. Placenta. 2016;39:111–115. doi: 10.1016/j.placenta.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 67.Vetter A.E., O’Grady S.M. Mechanisms of Electrolyte Transport across the Endometrium. I. Regulation by PGF2 Alpha and CAMP. Am. J. Physiol.-Cell Physiol. 1996;270:C663–C672. doi: 10.1152/ajpcell.1996.270.2.C663. [DOI] [PubMed] [Google Scholar]
- 68.Xu C., Long A., Fang X., Wood S.L., Slater D.M., Ni X., Olson D.M. Effects of PGF2α on the Expression of Uterine Activation Proteins in Pregnant Human Myometrial Cells from Upper and Lower Segment. J. Clin. Endocrinol. Metab. 2013;98:2975–2983. doi: 10.1210/jc.2012-2829. [DOI] [PubMed] [Google Scholar]
- 69.Xu C., You X., Liu W., Sun Q., Ding X., Huang Y., Ni X. Prostaglandin F2 Regulates the Expression of Uterine Activation Proteins via Multiple Signalling Pathways. Reproduction. 2015;149:139–146. doi: 10.1530/REP-14-0479. [DOI] [PubMed] [Google Scholar]
- 70.Kaczynski P., Kowalewski M.P., Waclawik A. Prostaglandin F2α Promotes Angiogenesis and Embryo–Maternal Interactions during Implantation. Reproduction. 2016;151:539–552. doi: 10.1530/REP-15-0496. [DOI] [PubMed] [Google Scholar]
- 71.Maybin J.A., Battersby S., Hirani N., Nikitenko L.L., Critchley H.O.D., Jabbour H.N. The Expression and Regulation of Adrenomedullin in the Human Endometrium: A Candidate for Endometrial Repair. Endocrinology. 2011;152:2845–2856. doi: 10.1210/en.2010-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sztachelska M., Ponikwicka-Tyszko D., Sokolowska G., Anisimowicz S., Czerniecki J., Lebiedzinska W., Zbucka-Kretowska M., Zygmunt M., Wołczynski S., Pierzynski P. Oxytocin Antagonism Reverses the Effects of High Oestrogen Levels and Oxytocin on Decidualization and Cyclooxygenase Activity in Endometrial Tissues. Reprod. Biomed. Online. 2019;39:737–744. doi: 10.1016/j.rbmo.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Roth K., Zahradnik H.P., Schäfer W.R. Effects of Different Progestins on Prostaglandin Biosynthesis in Human Endometrial Explants. Contraception. 2019;99:61–66. doi: 10.1016/j.contraception.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Kikut J., Komorniak N., Ziętek M., Palma J., Szczuko M. Inflammation with the Participation of Arachidonic (AA) and Linoleic Acid (LA) Derivatives (HETEs and HODEs) Is Necessary in the Course of a Normal Reproductive Cycle and Pregnancy. J. Reprod. Immunol. 2020;141:103177. doi: 10.1016/j.jri.2020.103177. [DOI] [PubMed] [Google Scholar]
- 75.Baryla M., Kaczynski P., Goryszewska E., Riley S.C., Waclawik A. Prostaglandin F2α Stimulates Adhesion, Migration, Invasion and Proliferation of the Human Trophoblast Cell Line HTR-8/SVneo. Placenta. 2019;77:19–29. doi: 10.1016/j.placenta.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 76.Aplin J.D., Charlton A.K., Ayad S. An Immunohistochemical Study of Human Endometrial Extracellular Matrix during the Menstrual Cycle and First Trimester of Pregnancy. Cell Tissue Res. 1988;253:231–240. doi: 10.1007/BF00221758. [DOI] [PubMed] [Google Scholar]
- 77.Béliard A., Donnez J., Nisolle M., Foidart J.-M. Localization of Laminin, Fibronectin, E-Cadherin, and Integrins in Endometrium and Endometriosis. Fertil. Steril. 1997;67:266–272. doi: 10.1016/S0015-0282(97)81909-7. [DOI] [PubMed] [Google Scholar]
- 78.Irwin J.C., Kirk D., King R.J.B., Quigley M.M., Gwatkin R.B.L. Hormonal Regulation of Human Endometrial Stromal Cells in Culture: An in Vitro Model for Decidualization. Fertil. Steril. 1989;52:761–768. doi: 10.1016/S0015-0282(16)61028-2. [DOI] [PubMed] [Google Scholar]
- 79.Tabibzadeh S., Kong Q.F., Babaknia A., May L.T. Progressive Rise in the Expression of Interleukin-6 in Human Endometrium during Menstrual Cycle Is Initiated during the Implantation Window. MHR Basic Sci. Reprod. Med. 1995;1:407–413. doi: 10.1093/molehr/1.8.407. [DOI] [PubMed] [Google Scholar]
- 80.Downie J., Poyser N.L., Wunderlich M. Levels of Prostaglandins in Human Endometrium during the Normal Menstrual Cycle. J. Physiol. 1974;236:465–472. doi: 10.1113/jphysiol.1974.sp010446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woo J.-H., Yang Y.-I., Ahn J.-H., Choi Y.S., Choi J.-H. Interleukin 6 Secretion from Alternatively Activated Macrophages Promotes the Migration of Endometriotic Epithelial Cells. Biol. Reprod. 2017;97:660–670. doi: 10.1093/biolre/iox118. [DOI] [PubMed] [Google Scholar]
- 82.Jovanović M., Vićovac L. Interleukin-6 Stimulates Cell Migration, Invasion and Integrin Expression in HTR-8/SVneo Cell Line. Placenta. 2009;30:320–328. doi: 10.1016/j.placenta.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 83.Bazan J.F. Structural Design and Molecular Evolution of a Cytokine Receptor Superfamily. Proc. Natl. Acad. Sci. USA. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Norwitz E.R., Wilson T. Secretory Component: A Potential Regulator of Endometrial-Decidual Prostaglandin Production in Early Human Pregnancy. Am. J. Obs. Gynecol. 2000;183:108–117. doi: 10.1067/mob.2000.105636. [DOI] [PubMed] [Google Scholar]
- 85.Bajekal N., Li T.-C. Fibroids, Infertility and Pregnancy Wastage. Hum. Reprod. Update. 2000;6:614–620. doi: 10.1093/humupd/6.6.614. [DOI] [PubMed] [Google Scholar]
- 86.Miura S., Khan K.N., Kitajima M., Hiraki K., Moriyama S., Masuzaki H., Samejima T., Fujishita A., Ishimaru T. Differential Infiltration of Macrophages and Prostaglandin Production by Different Uterine Leiomyomas. Hum. Reprod. 2006;21:2545–2554. doi: 10.1093/humrep/del205. [DOI] [PubMed] [Google Scholar]
- 87.Sugino N., Karube-Harada A., Kashida S., Takiguchi S., Kato H. Reactive Oxygen Species Stimulate Prostaglandin F2α Production in Human Endometrial Stromal Cells in Vitro. Hum. Reprod. 2001;16:1797–1801. doi: 10.1093/humrep/16.9.1797. [DOI] [PubMed] [Google Scholar]
- 88.Sugino N., Nakata M., Kashida S., Karube A., Takiguchi S., Kato H. Decreased Superoxide Dismutase Expression and Increased Concentrations of Lipid Peroxide and Prostaglandin F2α in the Decidua of Failed Pregnancy. Mol. Hum. Reprod. 2000;6:642–647. doi: 10.1093/molehr/6.7.642. [DOI] [PubMed] [Google Scholar]
- 89.Keleş I.D., Ülgen E., Erkan M.B., Çelik S.E., Aydın Y., Önem A.N., Kandemir H., Arslanoğlu T., Apak M.R., Sezerman U. Comparison of Endometrial Prostanoid Profiles in Three Infertile Subgroups: The Missing Part of Receptivity? Fertil. Steril. 2020;113:670–678. doi: 10.1016/j.fertnstert.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 90.Correa F., Wolfson M.L., Valchi P., Aisemberg J., Franchi A.M. Endocannabinoid System and Pregnancy. Reproduction. 2016;152:R191–R200. doi: 10.1530/REP-16-0167. [DOI] [PubMed] [Google Scholar]
- 91.El-Talatini M.R., Taylor A.H., Konje J.C. Fluctuation in Anandamide Levels from Ovulation to Early Pregnancy in In-Vitro Fertilization-Embryo Transfer Women, and Its Hormonal Regulation. Hum. Reprod. 2009;24:1989–1998. doi: 10.1093/humrep/dep065. [DOI] [PubMed] [Google Scholar]
- 92.Torella M., Bellini G., Punzo F., Argenziano M., Schiattarella A., Labriola D., Schettino M.T., Ambrosio D., Ammaturo F.P., de Franciscis P. TNF-α Effect on Human Delivery Onset by CB1/TRPV1 Crosstalk: New Insights into Endocannabinoid Molecular Signaling in Preterm vs. Term Labor. Analysis of the EC/EV Pathway and Predictive Biomarkers for Early Diagnosis of Preterm Delivery. Minerva Ginecol. 2019;71:359–364. doi: 10.23736/S0026-4784.19.04405-8. [DOI] [PubMed] [Google Scholar]
- 93.Mitchell M.D., Sato T.A., Wang A., Keelan J.A., Ponnampalam A.P., Glass M. Cannabinoids Stimulate Prostaglandin Production by Human Gestational Tissues through a Tissue-and CB1-Receptor-Specific Mechanism. Am. J. Physiol.-Endocrinol. Metab. 2008;294:E352–E356. doi: 10.1152/ajpendo.00495.2007. [DOI] [PubMed] [Google Scholar]
- 94.Vercelli C.A., Aisemberg J., Cella M., Salazar A.I., Wolfson M.L., Franchi A.M. Opposite Effects of Methanandamide on Lipopolysaccharide-Induced Prostaglandin E2 and F2α Synthesis in Uterine Explants from Pregnant Mice. PLoS ONE. 2012;7:e39532. doi: 10.1371/journal.pone.0039532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dekel N., Gnainsky Y., Granot I., Racicot K., Mor G. The Role of Inflammation for a Successful Implantation. Am. J. Reprod. Immunol. 2014;72:141–147. doi: 10.1111/aji.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lessey B.A., Lebovic D.I., Taylor R.N. Proceedings of the Seminars in Reproductive Medicine. Volume 31. Thieme Medical Publishers; New York, NY, USA: 2013. Eutopic Endometrium in Women with Endometriosis: Ground Zero for the Study of Implantation Defects; pp. 109–124. [DOI] [PubMed] [Google Scholar]
- 97.Lessey B.A., Young S.L. Proceedings of the Seminars in Reproductive Medicine. Volume 32. Thieme Medical Publishers; New York, NY, USA: 2014. Homeostasis Imbalance in the Endometrium of Women with Implantation Defects: The Role of Estrogen and Progesterone; pp. 365–375. [DOI] [PubMed] [Google Scholar]
- 98.Vercellini P., Viganò P., Somigliana E., Fedele L. Endometriosis: Pathogenesis and Treatment. Nat. Rev. Endocrinol. 2014;10:261. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 99.Maksym R.B., Hoffmann-Młodzianowska M., Skibińska M., Rabijewski M., Mackiewicz A., Kieda C. Immunology and Immunotherapy of Endometriosis. J. Clin. Med. 2021;10:5879. doi: 10.3390/jcm10245879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.vander Borght M., Wyns C. Fertility and Infertility: Definition and Epidemiology. Clin. Biochem. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 101.Meuleman C., Vandenabeele B., Fieuws S., Spiessens C., Timmerman D., D’Hooghe T. High Prevalence of Endometriosis in Infertile Women with Normal Ovulation and Normospermic Partners. Fertil. Steril. 2009;92:68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 102.Ahn S.H., Khalaj K., Young S.L., Lessey B.A., Koti M., Tayade C. Immune-Inflammation Gene Signatures in Endometriosis Patients. Fertil. Steril. 2016;106:1420–1431. doi: 10.1016/j.fertnstert.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahn S.H., Edwards A.K., Singh S.S., Young S.L., Lessey B.A., Tayade C. IL-17A Contributes to the Pathogenesis of Endometriosis by Triggering Proinflammatory Cytokines and Angiogenic Growth Factors. J. Immunol. 2015;195:2591–2600. doi: 10.4049/jimmunol.1501138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khoufache K., Michaud N., Harir N., Kibangou P.B., Akoum A. Anomalies in the Inflammatory Response in Endometriosis and Possible Consequences: A Review. Minerva Endocrinol. 2012;37:75–92. [PubMed] [Google Scholar]
- 105.Lessey B.A. Implantation Defects in Infertile Women with Endometriosis. Ann. N. Y. Acad. Sci. 2002;955:265–280. doi: 10.1111/j.1749-6632.2002.tb02787.x. [DOI] [PubMed] [Google Scholar]
- 106.Lessey B.A., Castelbaum A.J. Integrins and Implantation in the Human. Rev. Endocr. Metab. Disord. 2002;3:107–117. doi: 10.1023/A:1015450727580. [DOI] [PubMed] [Google Scholar]
- 107.Margarit L., Gonzalez D., Lewis P.D., Hopkins L., Davies C., Conlan R.S., Joels L., White J.O. L-Selectin Ligands in Human Endometrium: Comparison of Fertile and Infertile Subjects. Hum. Reprod. 2009;24:2767–2777. doi: 10.1093/humrep/dep247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Foulk R.A., Zdravkovic T., Genbacev O., Prakobphol A. Expression of L-Selectin Ligand MECA-79 as a Predictive Marker of Human Uterine Receptivity. J. Assist. Reprod. Genet. 2007;24:316–321. doi: 10.1007/s10815-007-9151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kao L.C., Germeyer A., Tulac S., Lobo S., Yang J.P., Taylor R.N., Osteen K., Lessey B.A., Giudice L.C. Expression Profiling of Endometrium from Women with Endometriosis Reveals Candidate Genes for Disease-Based Implantation Failure and Infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 110.Du H., Taylor H.S. The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb. Perspect. Med. 2016;6:a023002. doi: 10.1101/cshperspect.a023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Celik O., Unlu C., Otlu B., Celik N., Caliskan E. Laparoscopic Endometrioma Resection Increases Peri-Implantation Endometrial HOXA-10 and HOXA-11 MRNA Expression. Fertil. Steril. 2015;104:356–365. doi: 10.1016/j.fertnstert.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 112.Wu Y., Halverson G., Basir Z., Strawn E., Yan P., Guo S.-W. Aberrant Methylation at HOXA10 May Be Responsible for Its Aberrant Expression in the Endometrium of Patients with Endometriosis. Am. J. Obs. Gynecol. 2005;193:371–380. doi: 10.1016/j.ajog.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 113.Klemmt P.A.B., Carver J.G., Kennedy S.H., Koninckx P.R., Mardon H.J. Stromal Cells from Endometriotic Lesions and Endometrium from Women with Endometriosis Have Reduced Decidualization Capacity. Fertil. Steril. 2006;85:564–572. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahn J.I., Yoo J.-Y., Kim T.H., Kim Y.I., Ferguson S.D., Fazleabas A.T., Young S.L., Lessey B.A., Ahn J.Y., Lim J.M. cAMP-Response Element-Binding 3-like Protein 1 (CREB3L1) Is Required for Decidualization and Its Expression Is Decreased in Women with Endometriosis. Curr. Mol. Med. 2016;16:276–287. doi: 10.2174/1566524016666160225153659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yin X., Pavone M.E., Lu Z., Wei J., Kim J.J. Increased Activation of the PI3K/AKT Pathway Compromises Decidualization of Stromal Cells from Endometriosis. J. Clin. Endocrinol. Metab. 2012;97:E35–E43. doi: 10.1210/jc.2011-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Su R.-W., Strug M.R., Joshi N.R., Jeong J.-W., Miele L., Lessey B.A., Young S.L., Fazleabas A.T. Decreased Notch Pathway Signaling in the Endometrium of Women with Endometriosis Impairs Decidualization. J. Clin. Endocrinol. Metab. 2015;100:E433–E442. doi: 10.1210/jc.2014-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pei T., Liu C., Liu T., Xiao L., Luo B., Tan J., Li X., Zhou G., Duan C., Huang W. MiR-194-3p Represses the Progesterone Receptor and Decidualization in Eutopic Endometrium from Women with Endometriosis. Endocrinology. 2018;159:2554–2562. doi: 10.1210/en.2018-00374. [DOI] [PubMed] [Google Scholar]
- 118.Zhou M., Fu J., Xiao L., Yang S., Song Y., Zhang X., Feng X., Sun H., Xu W., Huang W. MiR-196a Overexpression Activates the MEK/ERK Signal and Represses the Progesterone Receptor and Decidualization in Eutopic Endometrium from Women with Endometriosis. Hum. Reprod. 2016;31:2598–2608. doi: 10.1093/humrep/dew223. [DOI] [PubMed] [Google Scholar]
- 119.Eldafira E., Prasasty V.D., Abinawanto A., Syahfirdi L., Pujianto D.A. Polymorphisms of Estrogen Receptor-α and Estrogen Receptor-β Genes and Its Expression in Endometriosis. Turk. J. Pharm. Sci. 2021;18:91. doi: 10.4274/tjps.galenos.2019.94914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kobayashi H., Kimura M., Maruyama S., Nagayasu M., Imanaka S. Revisiting Estrogen-Dependent Signaling Pathways in Endometriosis: Potential Targets for Non-Hormonal Therapeutics. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021;258:103–110. doi: 10.1016/j.ejogrb.2020.12.044. [DOI] [PubMed] [Google Scholar]
- 121.Yilmaz B.D., Bulun S.E. Endometriosis and Nuclear Receptors. Hum. Reprod. Update. 2019;25:473–485. doi: 10.1093/humupd/dmz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Patel B.G., Rudnicki M., Yu J., Shu Y., Taylor R.N. Progesterone Resistance in Endometriosis: Origins, Consequences and Interventions. Acta Obs. Gynecol. Scand. 2017;96:623–632. doi: 10.1111/aogs.13156. [DOI] [PubMed] [Google Scholar]
- 123.Marquardt R.M., Kim T.H., Shin J.-H., Jeong J.-W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019;20:3822. doi: 10.3390/ijms20153822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vázquez-Martínez E.R., Bello-Alvarez C., Hermenegildo-Molina A.L., Solís-Paredes M., Parra-Hernández S., Cruz-Orozco O., Silvestri-Tomassoni J.R., Escobar-Ponce L.F., Hernández-López L.A., Reyes-Mayoral C. Expression of Membrane Progesterone Receptors in Eutopic and Ectopic Endometrium of Women with Endometriosis. BioMed Res. Int. 2020;2020:2196024. doi: 10.1155/2020/2196024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rocha-Junior C.V., da Broi M.G., Miranda-Furtado C.L., Navarro P.A., Ferriani R.A., Meola J. Progesterone Receptor B (PGR-B) Is Partially Methylated in Eutopic Endometrium from Infertile Women with Endometriosis. Reprod. Sci. 2019;26:1568–1574. doi: 10.1177/1933719119828078. [DOI] [PubMed] [Google Scholar]
- 126.Yoo J.-Y., Kim T.H., Fazleabas A.T., Palomino W.A., Ahn S.H., Tayade C., Schammel D.P., Young S.L., Jeong J.-W., Lessey B.A. KRAS Activation and Over-Expression of SIRT1/BCL6 Contributes to the Pathogenesis of Endometriosis and Progesterone Resistance. Sci. Rep. 2017;7:6765. doi: 10.1038/s41598-017-04577-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li M., Peng J., Shi Y., Sun P. MiR-92a Promotes Progesterone Resistance in Endometriosis through PTEN/AKT Pathway. Life Sci. 2020;242:117190. doi: 10.1016/j.lfs.2019.117190. [DOI] [PubMed] [Google Scholar]
- 128.Bulun S.E., Yildiz S., Adli M., Wei J.-J. Adenomyosis Pathogenesis: Insights from next-Generation Sequencing. Hum. Reprod. Update. 2021;27:1086–1097. doi: 10.1093/humupd/dmab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fox C., Morin S., Jeong J.-W., Scott R.T., Jr., Lessey B.A. Local and Systemic Factors and Implantation: What Is the Evidence? Fertil. Steril. 2016;105:873–884. doi: 10.1016/j.fertnstert.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Likes C.E., Cooper L.J., Efird J., Forstein D.A., Miller P.B., Savaris R., Lessey B.A. Medical or Surgical Treatment before Embryo Transfer Improves Outcomes in Women with Abnormal Endometrial BCL6 Expression. J. Assist. Reprod. Genet. 2019;36:483–490. doi: 10.1007/s10815-018-1388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Patel B., Elguero S., Thakore S., Dahoud W., Bedaiwy M., Mesiano S. Role of Nuclear Progesterone Receptor Isoforms in Uterine Pathophysiology. Hum. Reprod. Update. 2015;21:155–173. doi: 10.1093/humupd/dmu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kapoor R., Stratopoulou C.A., Dolmans M.-M. Pathogenesis of Endometriosis: New Insights into Prospective Therapies. Int. J. Mol. Sci. 2021;22:11700. doi: 10.3390/ijms222111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kalaitzopoulos D.R., Samartzis N., Kolovos G.N., Mareti E., Samartzis E.P., Eberhard M., Dinas K., Daniilidis A. Treatment of Endometriosis: A Review with Comparison of 8 Guidelines. BMC Women’s Health. 2021;21:397. doi: 10.1186/s12905-021-01545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koninckx P.R., Fernandes R., Ussia A., Schindler L., Wattiez A., Al-Suwaidi S., Amro B., Al-Maamari B., Hakim Z., Tahlak M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021;12:745548. doi: 10.3389/fendo.2021.745548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fox C.W., Savaris R.F., Jeong J.-W., Kim T.H., Miller P.B., Likes C.E., Schammel D.P., Young S.L., Lessey B.A. Unexplained Recurrent Pregnancy Loss and Unexplained Infertility: Twins in Disguise. Hum. Reprod. Open. 2020;2020:hoz021. doi: 10.1093/hropen/hoz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kimura F., Takebayashi A., Ishida M., Nakamura A., Kitazawa J., Morimune A., Hirata K., Takahashi A., Tsuji S., Takashima A. Chronic Endometritis and Its Effect on Reproduction. J. Obstet. Gynaecol. Res. 2019;45:951–960. doi: 10.1111/jog.13937. [DOI] [PubMed] [Google Scholar]
- 137.Park H.J., Kim Y.S., Yoon T.K., Lee W.S. Chronic Endometritis and Infertility. Clin. Exp. Reprod. Med. 2016;43:185–192. doi: 10.5653/cerm.2016.43.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cicinelli E., de Ziegler D., Nicoletti R., Colafiglio G., Saliani N., Resta L., Rizzi D., de Vito D. Chronic Endometritis: Correlation among Hysteroscopic, Histologic, and Bacteriologic Findings in a Prospective Trial with 2190 Consecutive Office Hysteroscopies. Fertil. Steril. 2008;89:677–684. doi: 10.1016/j.fertnstert.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 139.Haggerty C.L., Hillier S.L., Bass D.C., Ness R.B., PID Evaluation and Clinical Health (PEACH) Study Investigators Bacterial Vaginosis and Anaerobic Bacteria Are Associated with Endometritis. Clin. Infect. Dis. 2004;39:990–995. doi: 10.1086/423963. [DOI] [PubMed] [Google Scholar]
- 140.Polisseni F., Bambirra E.A., Camargos A.F. Detection of Chronic Endometritis by Diagnostic Hysteroscopy in Asymptomatic Infertile Patients. Gynecol. Obs. Investig. 2003;55:205–210. doi: 10.1159/000072075. [DOI] [PubMed] [Google Scholar]
- 141.Kitaya K., Matsubayashi H., Takaya Y., Nishiyama R., Yamaguchi K., Takeuchi T., Ishikawa T. Live Birth Rate Following Oral Antibiotic Treatment for Chronic Endometritis in Infertile Women with Repeated Implantation Failure. Am. J. Reprod. Immunol. 2017;78:e12719. doi: 10.1111/aji.12719. [DOI] [PubMed] [Google Scholar]
- 142.Janošević D.R., Trandafilović M., Krtinić D., Čolović H., Stevanović J.M., Dinić S.P.-T. Endometrial Immunocompetent Cells in Proliferative and Secretory Phase of Normal Menstrual Cycle. Folia Morphol. 2020;79:296–302. doi: 10.5603/FM.a2019.0095. [DOI] [PubMed] [Google Scholar]
- 143.Liu Y., Chen X., Huang J., Wang C.-C., Yu M.-Y., Laird S., Li T.-C. Comparison of the Prevalence of Chronic Endometritis as Determined by Means of Different Diagnostic Methods in Women with and without Reproductive Failure. Fertil. Steril. 2018;109:832–839. doi: 10.1016/j.fertnstert.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 144.Kitaya K., Yasuo T. Immunohistochemistrical and Clinicopathological Characterization of Chronic Endometritis. Am. J. Reprod. Immunol. 2011;66:410–415. doi: 10.1111/j.1600-0897.2011.01051.x. [DOI] [PubMed] [Google Scholar]
- 145.Kasius J.C., Fatemi H.M., Bourgain C., Sie-Go D.M.D.S., Eijkemans R.J.C., Fauser B.C., Devroey P., Broekmans F.J.M. The Impact of Chronic Endometritis on Reproductive Outcome. Fertil. Steril. 2011;96:1451–1456. doi: 10.1016/j.fertnstert.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 146.Song D., Li T.-C., Zhang Y., Feng X., Xia E., Huang X., Xiao Y. Correlation between Hysteroscopy Findings and Chronic Endometritis. Fertil. Steril. 2019;111:772–779. doi: 10.1016/j.fertnstert.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 147.Kitaya K., Yasuo T. Aberrant Expression of Selectin E, CXCL1, and CXCL13 in Chronic Endometritis. Mod. Pathol. 2010;23:1136–1146. doi: 10.1038/modpathol.2010.98. [DOI] [PubMed] [Google Scholar]
- 148.Li Y., Yu S., Huang C., Lian R., Chen C., Liu S., Li L., Diao L., Markert U.R., Zeng Y. Evaluation of Peripheral and Uterine Immune Status of Chronic Endometritis in Patients with Recurrent Reproductive Failure. Fertil. Steril. 2020;113:187–196. doi: 10.1016/j.fertnstert.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 149.Kosei N., Zakharenko N., Herman D. Endometrial Polyps in Women of Reproductive Age: Clinical and Pathogene-Tic Variations. Georgian Med. News. 2017;273:16–22. [PubMed] [Google Scholar]
- 150.Tsonis O., Gkrozou F., Dimitriou E., Paschopoulos M. Hysteroscopic Detection of Chronic Endometritis: Evaluating Proposed Hysteroscopic Features Suggestive of Chronic Endometritis. J. Gynecol. Obstet. Hum. Reprod. 2021;50:102182. doi: 10.1016/j.jogoh.2021.102182. [DOI] [PubMed] [Google Scholar]
- 151.Kitazawa J., Kimura F., Nakamura A., Morimune A., Hanada T., Amano T., Tsuji S., Kasahara K., Satooka H., Hirata T. Alteration in Endometrial Helper T-cell Subgroups in Chronic Endometritis. Am. J. Reprod. Immunol. 2021;85:e13372. doi: 10.1111/aji.13372. [DOI] [PubMed] [Google Scholar]
- 152.Wang W.-J., Zhang H., Chen Z.-Q., Zhang W., Liu X.-M., Fang J.-Y., Liu F.-J., Kwak-Kim J. Endometrial TGF-β, IL-10, IL-17 and Autophagy Are Dysregulated in Women with Recurrent Implantation Failure with Chronic Endometritis. Reprod. Biol. Endocrinol. 2019;17:2. doi: 10.1186/s12958-018-0444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.di Pietro C., Cicinelli E., Guglielmino M.R., Ragusa M., Farina M., Palumbo M.A., Cianci A. Altered Transcriptional Regulation of Cytokines, Growth Factors, and Apoptotic Proteins in the Endometrium of Infertile Women with Chronic Endometritis. Am. J. Reprod. Immunol. 2013;69:509–517. doi: 10.1111/aji.12076. [DOI] [PubMed] [Google Scholar]
- 154.di Pietro C., Caruso S., Battaglia R., Iraci Sareri M., la Ferlita A., Strino F., Bonaventura G., di Mauro M., Barcellona M.L., Perciavalle V. MiR-27a-3p and MiR-124-3p, Upregulated in Endometrium and Serum from Women Affected by Chronic Endometritis, Are New Potential Molecular Markers of Endometrial Receptivity. Am. J. Reprod. Immunol. 2018;80:e12858. doi: 10.1111/aji.12858. [DOI] [PubMed] [Google Scholar]
- 155.Cicinelli E., Resta L., Nicoletti R., Tartagni M., Marinaccio M., Bulletti C., Colafiglio G. Detection of Chronic Endometritis at Fluid Hysteroscopy. J. Minim. Invasive Gynecol. 2005;12:514–518. doi: 10.1016/j.jmig.2005.07.394. [DOI] [PubMed] [Google Scholar]
- 156.George A.F., Jang K.S., Nyegaard M., Neidleman J., Spitzer T.L., Xie G., Chen J.C., Herzig E., Laustsen A., Marques de Menezes E.G. Seminal Plasma Promotes Decidualization of Endometrial Stromal Fibroblasts in Vitro from Women with and without Inflammatory Disorders in a Manner Dependent on Interleukin-11 Signaling. Hum. Reprod. 2020;35:617–640. doi: 10.1093/humrep/deaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brünnert D., Shekhawat I., Chahar K.R., Ehrhardt J., Pandey J., Yadav J.K., Zygmunt M., Goyal P. Thrombin Stimulates Gene Expression and Secretion of IL-11 via Protease-Activated Receptor-1 and Regulates Extravillous Trophoblast Cell Migration. J. Reprod. Immunol. 2019;132:35–41. doi: 10.1016/j.jri.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 158.Dimitriadis E., White C.A., Jones R.L., Salamonsen L.A. Cytokines, Chemokines and Growth Factors in Endometrium Related to Implantation. Hum. Reprod. Update. 2005;11:613–630. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- 159.von Rango U., Alfer J., Kertschanska S., Kemp B., Müller-Newen G., Heinrich P.C., Beier H.M., Classen-Linke I. Interleukin-11 Expression: Its Significance in Eutopic and Ectopic Human Implantation. Mol. Hum. Reprod. 2004;10:783–792. doi: 10.1093/molehr/gah107. [DOI] [PubMed] [Google Scholar]
- 160.Guzeloglu-Kayisli O., Kayisli U.A., Taylor H.S. Proceedings of the Seminars in Reproductive Medicine. Volume 27. © Thieme Medical Publishers; New York, NY, USA: 2009. The Role of Growth Factors and Cytokines during Implantation: Endocrine and Paracrine Interactions; pp. 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sansone A.M., Hisrich B.V., Young R.B., Abel W.F., Bowens Z., Blair B.B., Funkhouser A.T., Schammel D.P., Green L.J., Lessey B.A. Evaluation of BCL6 and SIRT1 as Non-Invasive Diagnostic Markers of Endometriosis. Curr. Issues Mol. Biol. 2021;43:1350–1360. doi: 10.3390/cimb43030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Evans-Hoeker E., Lessey B.A., Jeong J.W., Savaris R.F., Palomino W.A., Yuan L., Schammel D.P., Young S.L. Endometrial BCL6 Overexpression in Eutopic Endometrium of Women with Endometriosis. Reprod. Sci. 2016;23:1234–1241. doi: 10.1177/1933719116649711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li X., Large M.J., Creighton C.J., Lanz R.B., Jeong J.-W., Young S.L., Lessey B.A., Palomino W.A., Tsai S.Y., DeMayo F.J. COUP-TFII Regulates Human Endometrial Stromal Genes Involved in Inflammation. Mol. Endocrinol. 2013;27:2041–2054. doi: 10.1210/me.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fu J.-L., Hsiao K.-Y., Lee H.-C., Li W.-N., Chang N., Wu M.-H., Tsai S.-J. Suppression of COUP-TFII Upregulates Angiogenin and Promotes Angiogenesis in Endometriosis. Hum. Reprod. 2018;33:1517–1527. doi: 10.1093/humrep/dey220. [DOI] [PubMed] [Google Scholar]
- 165.Dimitriadis E., Robb L., Liu Y.X., Enders A.C., Martin H., Stoikos C., Wallace E., Salamonsen L.A. IL-11 and IL-11Rα Immunolocalisation at Primate Implantation Sites Supports a Role for IL-11 in Placentation and Fetal Development. Reprod. Biol. Endocrinol. 2003;1:34. doi: 10.1186/1477-7827-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fest S., Aldo P.B., Abrahams V.M., Visintin I., Alvero A., Chen R., Chavez S.L., Romero R., Mor G. Trophoblast–Macrophage Interactions: A Regulatory Network for the Protection of Pregnancy. Am. J. Reprod. Immunol. 2007;57:55–66. doi: 10.1111/j.1600-0897.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 167.Anupa G., Bhat M.A., Srivastava A.K., Sharma J.B., Mehta N., Patil A., Sengupta J., Ghosh D. Cationic Antimicrobial Peptide, Magainin down-Regulates Secretion of pro-Inflammatory Cytokines by Early Placental Cytotrophoblasts. Reprod. Biol. Endocrinol. 2015;13:121. doi: 10.1186/s12958-015-0119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gnainsky Y., Granot I., Aldo P., Barash A., Or Y., Mor G., Dekel N. Biopsy-Induced Inflammatory Conditions Improve Endometrial Receptivity: The Mechanism of Action. Reproduction. 2015;149:75–85. doi: 10.1530/REP-14-0395. [DOI] [PubMed] [Google Scholar]
- 169.de Ziegler D., Pirtea P., Galliano D., Cicinelli E., Meldrum D. Optimal Uterine Anatomy and Physiology Necessary for Normal Implantation and Placentation. Fertil. Steril. 2016;105:844–854. doi: 10.1016/j.fertnstert.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 170.Kitaya K., Takeuchi T., Mizuta S., Matsubayashi H., Ishikawa T. Endometritis: New Time, New Concepts. Fertil. Steril. 2018;110:344–350. doi: 10.1016/j.fertnstert.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 171.Ness R.B., Soper D.E., Holley R.L., Peipert J., Randall H., Sweet R.L., Sondheimer S.J., Hendrix S.L., Amortegui A., Trucco G., et al. Effectiveness of Inpatient and Outpatient Treatment Strategies for Women with Pelvic Inflammatory Disease: Results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (Peach) Randomized Trial. Am. J. Obstet. Gynecol. 2002;186:929–937. doi: 10.1067/mob.2002.121625. [DOI] [PubMed] [Google Scholar]
- 172.Song D., He Y., Wang Y., Liu Z., Xia E., Huang X., Xiao Y., Li T.-C. Impact of Antibiotic Therapy on the Rate of Negative Test Results for Chronic Endometritis: A Prospective Randomized Control Trial. Fertil. Steril. 2021;115:1549–1556. doi: 10.1016/j.fertnstert.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 173.Hong X., Ding W., Yuan R., Ding J., Jin J. Effect of Interventional Embolization Treatment for Hydrosalpinx on the Outcome of in Vitro Fertilization and Embryo Transfer. Medicine. 2018;97:e13143. doi: 10.1097/MD.0000000000013143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Palagiano A., Cozzolino M., Ubaldi F.M., Palagiano C., Coccia M.E. Effects of Hydrosalpinx on Endometrial Implantation Failures: Evaluating Salpingectomy in Women Undergoing in Vitro Fertilization. Rev. Bras. Ginecol. Obs. 2021;43:304–310. doi: 10.1055/s-0040-1722155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cheng F., Li T., Wang Q.-L., Zhou H.-L., Duan L., Cai X. Effects of Hydrosalpinx on Ultrasonographic Parameters for Endometrial Receptivity during the Window of Implantation Measured by Power Color Doppler Ultrasound. Int. J. Clin. Exp. Med. 2015;8:6103. [PMC free article] [PubMed] [Google Scholar]
- 176.El-Mazny A., Ramadan W., Kamel A., Gad-Allah S. Effect of Hydrosalpinx on Uterine and Ovarian Hemodynamics in Women with Tubal Factor Infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;199:55–59. doi: 10.1016/j.ejogrb.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 177.Ng E.H.Y., Chan C.C.W., Tang O.S., Ho P.C. Comparison of Endometrial and Subendometrial Blood Flows among Patients with and without Hydrosalpinx Shown on Scanning during in Vitro Fertilization Treatment. Fertil. Steril. 2006;85:333–338. doi: 10.1016/j.fertnstert.2005.05.076. [DOI] [PubMed] [Google Scholar]
- 178.Bao H., Wang G., Huang X., Wang M., Wang X., Hao C. The Impact of HSF on Endometrium. Rev. Assoc. Méd. Bras. 2017;63:1069–1075. doi: 10.1590/1806-9282.63.12.1069. [DOI] [PubMed] [Google Scholar]
- 179.Ersahin A.A., Ersahin S., Gungor N.D. Surgical Removal of Hydrosalpinx Improves Endometrium Receptivity by Decreasing Nuclear Factor-Kappa B Expression. Reprod. Sci. 2020;27:787–792. doi: 10.1007/s43032-019-00136-y. [DOI] [PubMed] [Google Scholar]
- 180.Holzer I., Ott J., Kurz C., Hofstetter G., Hager M., Kuessel L., Parry J.P. Is Chronic Endometritis Associated with Tubal Infertility? A Prospective Cohort Study. J. Minim. Invasive Gynecol. 2021;28:1876–1881. doi: 10.1016/j.jmig.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 181.Daftary G.S., Kayisli U., Seli E., Bukulmez O., Arici A., Taylor H.S. Salpingectomy Increases Peri-Implantation Endometrial HOXA10 Expression in Women with Hydrosalpinx. Fertil. Steril. 2007;87:367–372. doi: 10.1016/j.fertnstert.2006.06.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.