Abstract
Background: Nucleos(t)ide analogues (NUCs) were proved to reduce hepatocellular carcinoma (HCC) development in chronic hepatitis B (CHB) patients, but data were limited on their efficacy in cirrhotic CHB patients. Methods: A total of 447 cirrhotic CHB patients treated with tenofovir/entecavir were retrospectively analyzed and divided into HCC (n = 48) and non-HCC (n = 399) groups. The median follow-up period was 62.1 months. Results: A total of 48 patients (10.7%) developed HCC during surveillance. The annual incidence rate of HCC was 2.04 per 100 person-years. The cumulative incidence of HCC was 0.9%, 9.8%, and 22.1% at 1, 5, and 10 years, respectively. Significant predictors for HCC identified using a multiple Cox regression analysis were age ≥50 years (hazard ratio (HR): 2.34) and α-fetoprotein (AFP) ≥8 ng/mL (HR: 2.05). The incidence rate of HCC was 8.67-fold higher in patients with age ≥50 years and AFP ≥8 ng/mL (3.14 per 100 person-years) than those with age <50 years and AFP <8 ng/mL (0.36 per 100 person-years). Conclusions: Cirrhotic CHB patients with age <50 years and AFP <8 ng/mL had the lowest annual incidence of HCC. However, those with age ≥50 years or/and AFP ≥8 ng/mL had a significantly higher risk for HCC development and warrant a careful surveillance schedule.
Keywords: chronic hepatitis B, cirrhosis, cumulative incidence, incidence rate, risk, hepatocellular carcinoma, tenofovir, entecavir
1. Introduction
Hepatocellular carcinoma (HCC), which accounts for most liver cancers, is the sixth most common cancer in the world. It is also the third-leading cause of cancer-related mortality, causing more than 800,000 deaths per year [1,2]. Chronic hepatitis B virus (HBV) infection causes global health problems; more than 240 million people have the disease. Without treatment, 40% of chronically infected patients will progress to cirrhosis, which increases the risk of HCC [3]. Chronic HBV infection is also the most common cause of HCC and is associated with more than 50% of HCC cases [4,5]. Long-term therapy with nucleos(t)ide analogues (NUCs) has been well demonstrated to result in improvement of liver necroinflammation and fibrosis, as well as regression of cirrhosis [6,7,8,9]. The risk of HCC development was also reported to be reduced significantly in the NUC-treated patients with advanced fibrosis or cirrhosis. The risk reduction was more prominent in patients with maintained viral suppression than in those with a virological breakthrough [10,11,12]. Due to a low drug-resistance rate and a high potency of viral suppression, entecavir (ETV) and tenofovir disoproxil fumarate (TDF) have become the first-line NUC regimen for CHB treatment. Previous studies showed that antiviral therapy with ETV reduced the risk of HCC in cirrhotic patients, particularly among those with maintained viral suppression. [12]. The rate of the reduction in HCC incidence was also more in the ETV-treated than those with LAM-treated cirrhotic patients [13]. However, the risk of HCC is not completely eliminated by NUC therapy.
The long-term use of NUC therapy for cirrhotic CHB patients has been reimbursed by Taiwan’s national health insurance system since 2010. There are few studies that focused on assessing the predictors of in-treatment HCC development in CHB-related cirrhotic patients with NUC therapy. Therefore, this retrospective study was conducted to elucidate the risks and predictors of HCC development during NUC therapy and to identify high-risk patients that warrant intensive surveillance during therapy.
2. Materials and Methods
2.1. Study Population
The patients that were diagnosed with CHB-related cirrhosis and that had initiated long-term ETV monotherapy (0.5 mg daily) or TDF monotherapy (300 mg) in our liver research unit in April 2007 and August 2013 were enrolled in this study. Chronic HBV infection was defined as being seropositive for HBsAg for more than 6 months. Baseline clinical and biochemical data were recorded upon the initiation of ETV or TDF therapy. The diagnosis of liver cirrhosis was made using a liver biopsy specimen with an Ishak modified histology activity index score ≥ 5 or Metavir score = 4, or ultrasonography using the previously described cirrhosis scoring system [14] with splenomegaly or esophageal/gastric varices. Fatty liver was diagnosed based on semiquantitative assessment using ultrasound according to the presence of increased liver echogenicity to the kidney [15,16]. All patients had serum HBV DNA ≥2000 IU/mL at baseline. Patients with HCC diagnosed before and within 6 months after the beginning of therapy or with a follow-up duration of less than 6 months were excluded from the study. We also excluded CHB patients coinfected with chronic hepatitis C or human immunodeficiency virus, toxic hepatitis, autoimmune hepatitis, primary biliary cirrhosis, or Wilson’s disease. This study was approved by the Medical Ethics Committee of Chang Gung Memorial Hospital (Institutional Review Board approval number: 104_9790B & 201601917B0) and was carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki).
2.2. Follow-Up for HCC Surveillance
All patients received NUCs for the entire follow-up period and were observed from the beginning of the NUC therapy to the date of HCC diagnosis, last visit, or death. Liver ultrasonography and laboratory examination were routinely checked every three months. The diagnosis of HCC was confirmed using the histological evaluation of a needle biopsy sample or surgically resected specimens, two typical image studies such as dynamic liver computed tomography or magnetic resonance imaging, or one image study plus an increased serum AFP level of more than 400 ng/mL [17,18].
2.3. Statistical Analysis
After testing for normal distribution using the Kolmogorov–Smirnov test, the continuous variables were against normal distribution and were reported as the median (interquartile range). The categorial variables were summarized as a number (percentage). The differences between the continuous and categorical variables were compared using the Mann–Whitney U test, Fisher’s exact test, and the chi-squared test where appropriate. A Cox proportional hazards regression model was used to assess the clinical, biochemical, and virological variables associated with the risk of HCC development. The cumulative incidence of HCC was evaluated using the Kaplan–Meier method and compared using a log-rank test. All statistical tests were two-tailed, with p-values < 0.05 considered statistically significant. Data were analyzed using SPSS 23 software for Windows (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Patient Characteristics
There was a total of 457 CHB patients with cirrhosis enrolled in our study. Six patients receiving ETV therapy, one patient receiving TDF therapy with a follow-up duration of less than 6 months, and three patients with HCC development within the first 6 months of enrollment were excluded. Finally, 447 patients were included in our final analysis. A total of 48 patients were diagnosed with HCC; 17 (35.4%) patients were confirmed using a liver biopsy, 5 (10.4%) patients were diagnosed using a serum AFP ≥400 ng/mL plus one imaging modality, and the remaining 26 patients were determined using two typical imaging modalities. The study patients were divided into HCC (n = 48) and non-HCC (n = 399) groups. The baseline clinical and biochemical data of the patients upon initiation of the NUC therapy are presented in Table 1. The mean age of the cirrhotic patients at initiation of the NUC therapy was 55.3 ± 11.6 years; 339 (75.8%) patients were men. The proportion of patients with age ≥50 years in the HCC and non-HCC groups were 79% and 66%, respectively. Patients with HCC development during NUC therapy had higher baseline AFP and HBV DNA levels. There was also a high percentage of patients in the HCC group that exhibited a baseline AFP level ≥8 ng/mL (HCC group vs. non-HCC group: 60% vs. 42%, respectively; p = 0.016).
Table 1.
Baseline characteristics of cirrhotic chronic hepatitis B patients with and without hepatocellular carcinoma.
| Baseline Parameters | HCC (n = 48) | Non-HCC (n = 399) | p-Value |
|---|---|---|---|
| Age (years) 1 | 56.0 (50.3–62.0) | 55.0 (47.0–63.0) | 0.327 |
| Sex (male) 2 | 40 (83) | 299 (75) | 0.327 |
| Body mass index (kg/m2) 1 | 23.0 (20.9–27.3) | 24.9 (22.6–27.8) | 0.076 |
| HBeAg (positive) 2 | 8 (16.7) | 48 (12) | 0.841 |
| Albumin (g/dL) 1 | 3.9 (3.6–4.1) | 3.9 (3.6–4.3) | 0.341 |
| AST (U/L) 1 | 59 (42–86) | 53 (38–91) | 0.494 |
| ALT (U/L) 1 | 59 (36–106) | 54 (37–102) | 0.816 |
| Total bilirubin (mg/dL) 1 | 1.3 (0.9–1.7) | 1.1 (0.8–1.4) | 0.239 |
| AFP (ng/mL) 1 | 10.8 (5.0–18.6) | 6.5 (4.0–15.2) | 0.042 |
| HBV DNA (log10 IU/mL) 1 | 6.0 (4.8–7.2) | 5.5 (4.5–6.7) | 0.039 |
| White blood cells (×103/μL) 1 | 6.2 (4.5–6.6) | 6.2 (4.8–6.7) | 0.731 |
| Hemoglobin level (g/dL) 1 | 13.5 (12–15.2) | 13.6 (11.3–14.9) | 0.991 |
| Platelet count (×103/μL) 1 | 138.5 (94–152.5) | 148 (111–165) | 0.453 |
| Creatinine (mg/dL) 1 | 1.0 (0.7–1.4) | 1.0 (0.8–1.4) | 0.932 |
| Fatty liver 2 | 11 (23) | 66 (17) | 0.269 |
| Splenomegaly 2 | 34 (71) | 309 (77) | 0.306 |
| Child–Pugh class A | 42 (87.5) | 337 (84.5) | 0.58 |
| Ascites history 2 | 6/47 (12.8) | 54 (13.5) | 0.568 |
| Variceal bleeding history 2 | 5/47 (10.6) | 31 (7.8) | 0.495 |
| NUCs (entecavir) 2 | 41 (85) | 318 (80) | 0.347 |
HBeAg, hepatitis B e antigen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, α-fetoprotein; NUC, nucleos(t)ide analogue. 1 median (interquartile range); 2 no. (%).
3.2. Cumulative Incidence of HCC
The median follow-up period was 62.1 months (range: 6.1–144.6 months). A total of 48 patients (10.7%) developed HCC during the surveillance period, with an incidence rate of 2.04 (95% CI: 1.52–2.68) new HCC cases per 100 person-years. The cumulative incidence of HCC was 0.9%, 9.8%, and 22.1% at 1, 5, and 10 years, respectively.
3.3. Risk Factors Associated with HCC Development
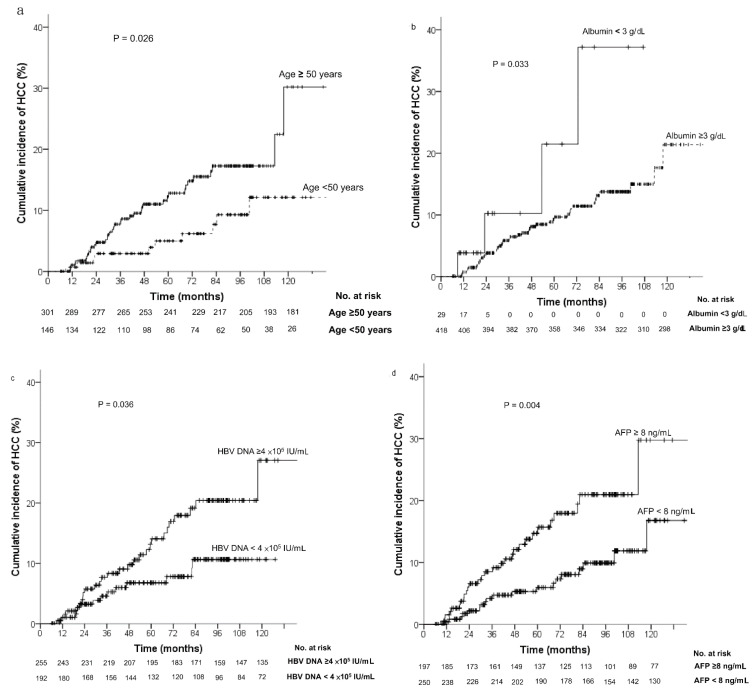
Factors associated with the risk of HCC development were assessed using the Kaplan–Meier method and compared using the log-rank test (Figure 1). Patients with HCC development during NUC therapy were significantly older (age ≥50 years) (p = 0.026), had higher pretreatment serum HBV DNA levels (≥4 × 105 IU/mL) (p = 0.036) and AFP levels (≥8 ng/mL) (p = 0.004), and had lower albumin levels (<3 g/dL) (p = 0.033). However, different NUCs did not affect the risk of HCC development.
Figure 1.
Cumulative incidence of hepatocellular carcinoma assessed using baseline risk factors: (a) age; (b) albumin; (c) HBV DNA; (d) AFP; (e) NUCs. Patients with older age, higher AFP and HBV DNA levels, and low albumin levels had a higher risk for HCC development during NUC therapy. The risk of HCC development was not different between the two NUCs. AFP, α-fetoprotein; NUC, nucleos(t)ide analogue; TDF, tenofovir; ETV, entecavir.
Baseline clinical and biochemical factors associated with HCC development, including age ≥50 years (hazard ratio (HR): 2.17, 95% confidence interval (CI) = 1.08–4.36), serum HBV DNA levels ≥4 × 105 IU/mL (HR: 1.96, 95% CI = 1.03–3.74), albumin <3 g/dL (HR: 2.91, 95% CI = 1.04–8.15), and AFP ≥8 ng/mL (HR: 2.29, 95% CI = 1.28–4.1), were identified using a univariate Cox regression analysis. These significant factors were then entered into a stepwise multiple regression analysis. The results of the multivariate Cox regression analysis showed a treatment age ≥50 years (HR: 2.34, 95% CI = 1.08–5.1) and an AFP ≥8 ng/mL (HR: 2.05, 95% CI = 1.1–3.84) were significant independent predictors of HCC development during NUC treatment (Table 2).
Table 2.
Predictors of hepatocellular carcinoma according to univariate and multivariate Cox regression models.
| Univariate Model | Multivariate Model | |||
|---|---|---|---|---|
| Parameters | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age (years) (≥50 vs. <50) | 2.17 (1.08–4.36) | 0.03 | 2.34 (1.08–5.1) | 0.032 |
| Sex (male vs. female) | 1.69 (0.79–3.62) | 0.175 | ||
| Body mass index (kg/m2) | 0.93 (0.86–1.0) | 0.056 | ||
| HBeAg (positive vs. negative) | 1.53 (0.72–3.27) | 0.273 | ||
| HBV DNA (×105 IU/mL) (≥4 vs. <4) | 1.96 (1.03–3.74) | 0.004 | ||
| Albumin (g/dL) (<3 vs. ≥3) | 2.91 (1.04–8.15) | 0.042 | ||
| AST (U/L) | 1.0 (0.99–1.0) | 0.26 | ||
| ALT (U/L) | 1.0 (0.99–1.0) | 0.268 | ||
| Total bilirubin (mg/dL) | 0.99 (0.8–1.22) | 0.9 | ||
| AFP (ng/mL) (≥8 vs. <8) | 2.29 (1.28–4.1) | 0.005 | 2.05 (1.1–3.83) | 0.025 |
| Hemoglobin level (g/dL) | 1.0 (0.81–1.24) | 1.0 | ||
| Platelet count (×103/μL) | 1.0 (0.99–1.0) | 0.22 | ||
| Creatinine (mg/dL) | 0.91 (0.53–1.55) | 0.73 | ||
| Estimated GFR (MDRD) | 1.0 (0.99–1.0) | 0.16 | ||
| Fatty liver (yes vs. no) | 1.8 (0.92–3.53) | 0.089 | ||
| Splenomegaly (yes vs. no) | 0.72 (0.38–1.34) | 0.3 | ||
| Child–Pugh class A | 0.98 (0.42–2.3) | 0.96 | ||
| Ascites (yes vs. no) | 1.14 (0.48–2.41) | 0.762 | ||
| Variceal bleeding (yes vs. no) | 1.52 (0.6–3.84) | 0.38 |
HR, hazard ratio; CI, confidence interval; HBeAg, hepatitis B e antigen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, α-fetoprotein; GFR, glomerular filtration rate.
3.4. Incidence of HCC According to Risk Factors
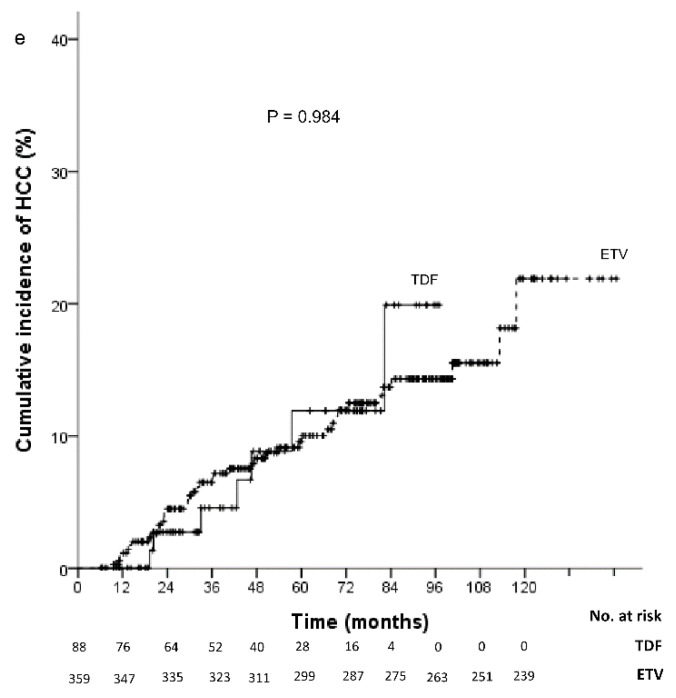
The analysis of the incidence rate of HCC development was then stratified into subgroups according to the risk factors identified by the multivariate analysis (Table 3). As compared to the patients with treatment age <50 years and AFP <8 ng/mL (0.36, 95% CI = 0.06–1.19), the incidence rates of HCC per 100 person-years were significantly higher in patients with age ≥50 years and AFP ≥8 ng/mL (3.14, 95% CI = 1.99–4.72), p = 0.004. The patients with age ≥50 years and AFP ≥8 ng/mL had an 8.67-fold higher rate of HCC than those with age <50 years and AFP <8 ng/mL. Among patients with either age ≥50 years or AFP ≥8 ng/mL, the incidence rates of HCC were also higher than those with age <50 years and AFP <8 ng/mL. The cumulative HCC incidence differed significantly in patients with age <50 years and AFP <8 ng/mL versus age ≥50 years and AFP ≥8 ng/mL. As shown in Figure 2, the 5-year and 10-year cumulative incidence of HCC development were 0% and 6.6%, respectively, in patients with age <50 years and AFP <8 ng/mL; and 15.4% and 32%, respectively, in patients with age ≥50 years and AFP ≥8 ng/mL.
Table 3.
Incidence rates of hepatocellular carcinoma stratified according to risk factors.
| Hepatocellular Carcinoma | Events (n) | Observation Period | Rate/100 Person-Years | p-Value |
|---|---|---|---|---|
| Risk Factors | (Person-Years) | (95% CI) | ||
| All | 48 | 2352.8 | 2.04 (1.52–2.68) | |
| Age ≥50 years and AFP ≥8 ng/mL | 21 | 669.2 | 3.14 (1.99–4.72) | 0.004 |
| Age ≥50 years and AFP <8 ng/mL | 17 | 841.8 | 2.02 (1.22–3.17) | 0.022 |
| Age <50 years and AFP ≥8 ng/mL | 8 | 287.9 | 2.78 (1.29–5.28) | 0.01 |
| Age <50 years and AFP <8 ng/mL | 2 | 553.9 | 0.36 (0.06–1.19) |
AFP, α-fetoprotein; CI, confidence interval.
Figure 2.
Cumulative incidence of hepatocellular carcinoma according to age and AFP.
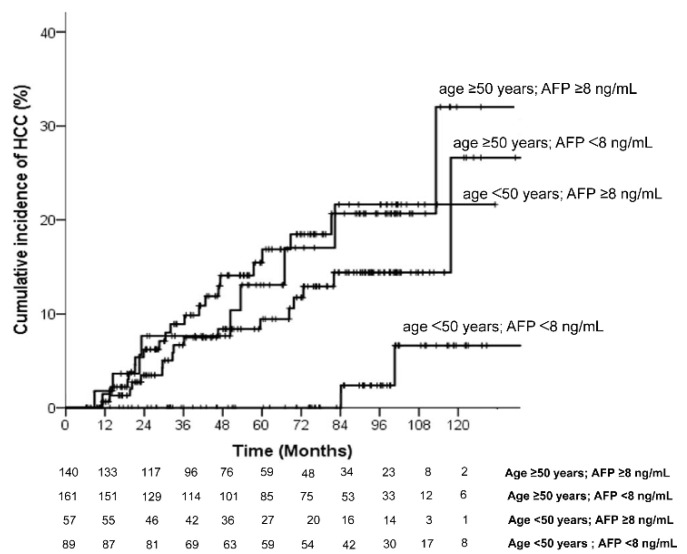
A serum HBV DNA level ≥4 × 105 IU/mL was shown to be a factor associated with HCC development in univariate analysis. The HCC incidence rate was analyzed in subgroups according to age and HBV DNA levels (Table 4). As compared to the patients with treatment age <50 years and HBV DNA <4 × 105 IU/mL (0.28, 95% CI = 0.01–1.39), the incidence rates of HCC per 100 person-years were significantly higher in patients with age ≥50 years and HBV DNA ≥4 × 105 IU/mL (2.86, 95% CI = 1.89–4.16) (p = 0.024). The patients with age ≥50 years and HBV DNA ≥4 × 105 IU/mL had a 9.9-fold higher rate of HCC than those with age <50 years and HBV DNA <4 × 105 IU/mL. However, the HCC incidence was similar between patients with age <50 years and HBV DNA <4 × 105 IU/mL and patients age ≥50 years and HBV DNA <4 × 105 IU/mL or age <50 years and HBV DNA ≥4 × 105 IU/mL. The cumulative HCC incidence was significantly lower in patients with age <50 years and HBV DNA <4 × 105 IU/mL versus the patients in the other subgroups. As shown in Figure 3, the 5-year and 10-year cumulative incidence of HCC development were 0% and 3.6%, respectively, in patients with age <50 years and HBV DNA <4 × 105 IU/mL; and 13.7% and 34%, respectively, in patients with age ≥50 years and HBV DNA ≥4 × 105 IU/mL.
Table 4.
Incidence rates of hepatocellular carcinoma stratified according age and HBV DNA.
| Hepatocellular Carcinoma | Events (n) | Observation Period | Rate/100 Person-Years | p-Value |
|---|---|---|---|---|
| Risk Factors | (Person-Years) | (95% CI) | ||
| All | 48 | 2352.8 | 2.04 (1.52–2.68) | |
| Age ≥50 years and HBV DNA ≥4 × 105 IU/mL | 25 | 873.6 | 2.86 (1.89–4.16) | 0.024 |
| Age ≥50 years and HBV DNA <4 × 105 IU/mL | 13 | 637.4 | 2.04 (1.13–3.4) | 0.058 |
| Age <50 years and HBV DNA ≥4 × 105 IU/mL | 9 | 486.9 | 1.84 (0.9–3.39) | 0.076 |
| Age <50 years and HBV DNA <4 × 105 IU/mL | 1 | 354.9 | 0.28 (0.01–1.39) |
HBV, hepatitis B virus; IU, international unit; CI, confidence interval.
Figure 3.
Cumulative incidence of hepatocellular carcinoma according to age and HBV DNA.
4. Discussion
In the present study, we demonstrated that long-term NUC therapy could not fully eliminate the risk of HCC development in CHB-related cirrhotic patients. Age and AFP were identified to be predictors associated with HCC development. By identifying patients with predictors for HCC development, a more intensive surveillance schedule with a shorter interval could be arranged in order to detect HCC at an early stage. In previous studies, the cumulative incidence of HCC at year 5 was 7–18% in NUC-treated cirrhotic patients [12,13,19,20,21]. Our data showed similar results; the 5-year cumulative incidence of HCC was 9.8% with an annual incidence of 2.04 per 100 person-years.
The risk factors associated with HCC development in CHB-related cirrhotic patients under NUC therapy, including older age, male sex, HBeAg positivity, statin use, platelet count, AFP and hemoglobin levels, variceal bleeding history, and 1-year virological response, had been reported in previous studies [22,23,24]. Our study demonstrated that only an older age (≥50 years) and AFP ≥8 ng/mL were predictors for risk of HCC in those patients with long-term NUC treatment. Previous reports indicated that the risk of HCC in cirrhotic patients under NUC therapy was age-dependent [22,23,24]. Tsai et al. proved that a higher risk for HCC development manifested at age 60 or higher. In our study, we actually showed that at a younger age, those ≥50 years were already predisposed to the development of HCC. The annual incidence of HCC in patients with age ≥50 years (2.04, 95% CI = 1.52–2.68) was significantly higher than those with age <50 years (1.19, 95% CI = 0.6–2.12) (per 100 person-years) (p = 0.037).
Serum AFP was determined to be a serological biomarker for detection of HCC; therefore, it is commonly used for HCC surveillance [25,26]. However, an elevation in AFP levels also occurred during hepatic bridging necrosis in CHB [27]. A meta-analysis study by Zhang et al. showed that using an AFP level with a cutoff point of 400 ng/mL could detect HCC with a sensitivity of 0.32 and a specificity of 0.99 [18]. In the present study, only five (10.4%) patients with HCC development had an elevation in their AFP level of more than 400 ng/mL when HCC was detected. Previous studies reported that 40–47% of patients with HCC had normal AFP levels when the diagnosis was confirmed [28,29,30]. These HCC patients with a normal AFP were older and predominantly male. There was also a lower rate of chronic HBV infection in HCC patients with a normal AFP than in patients with an abnormal AFP level [28]. An AFP with a cutoff point of 20 ng/mL was shown to detect HCC with a sensitivity of 41–64% and a specificity of 80–94% [31,32]. The American Association for the Study of Liver Disease also recommended that HCC surveillance should be considered positive when the AFP level is greater than 20 ng/mL. In our study, 26 (54%) patients had a serum AFP level less than 20 ng/mL when HCC was detected. However, there were no significant differences in age and sex between patients with and without a normal AFP level. In addition, our study showed that a higher baseline AFP level was associated with an increased risk for HCC development in cirrhotic CHB patients during NUC therapy. The incidence rates of new HCC case per 100 person-years were significantly higher in patients with AFP ≥8 ng/mL (3.03, 95% CI = 2.07–4.3) than in those with AFP <8 ng/mL (1.36, 95% CI = 0.84–2.09) (p = 0.007). The patients with AFP ≥8 ng/mL had a 2.19-fold higher rate of HCC than those with AFP <8 ng/mL. It may be that an elevation in the baseline AFP is related to hepatic necroinflammation and hepatocyte proliferation, and thus plays a role in hepatocarcinogenesis.
For further analysis of the risk for HCC affected by the predictors, we stratified the patients into four groups according to the predictors identified by the multivariate analysis. The cumulative incidence of HCC in patients with age <50 years and AFP <8 ng/mL at 5 years and 10 years was 0% and 6.6%, respectively. The incidence rate of HCC of these patients was 0.36 (95% CI = 0.06–1.19) per 100 person-years. It was lower than the pooled rate of HCC incidence in CHB patients with Child–Turcotte–Pugh A cirrhosis demonstrated by a previous meta-analysis study [33]. Although the risk of HCC development in CHB-related cirrhotic patients could not be completely eliminated by long-term NUC therapy, we found this subgroup of patients obtained more benefits from therapy and had a significantly lower rate of HCC than the other subgroup of patients. On the contrary, patients with age ≥50 years or/and AFP ≥8 ng/mL had a significantly higher risk of HCC compared with those with age <50 years and AFP <8 ng/mL; therefore, these patients should be closely monitored for HCC occurrence during NUC therapy. According to the current clinical practice guidelines, HCC surveillance with a 6-month internal was recommended [5,17]. However, a meta-analysis performed by Nathani et al. demonstrated a pooled tumor volume-doubling time of 4.6 months, while 35% of the patients had a tumor volume-doubling time of less than 3 months [34]. Chronic HBV infection has been identified as a factor associated with a rapid tumor growth pattern [34,35]. In accordance with above results, we suggested the surveillance of the HBV-cirrhotic patients with ≥50 years and AFP ≥8 ng/mL could be scheduled at 3-month intervals.
The impact of male sex on HCC prediction in chronic hepatitis B patients treated with NUCs was controversial [22,23,36,37,38,39]. In the study of Papatheodoridis et al., the patients enrolled were Caucasian and male sex was proved to be a predictor of HCC in two of five models [36]. However, two studies from Asia failed to demonstrated sex as a significant predictor for HCC [37,38]. Another two studies in Taiwan that enrolled only cirrhotic HBV patients with long-term NUC therapy demonstrated that male sex was a predictor of HCC [22,39]. However, male sex was shown to not be associated with HCC in the univariate analysis and was then excluded in the multiple Cox regression model in the present study. Further studies to verify the impact of sex on HCC risk in cirrhotic HBV patients with long-term NUC therapy are needed. Previous studies that reported effective ETV and TDF treatments for HCC prevention in cirrhotic CHB patients were contentious. Most of the studies were conducted in Asia, and some reports resulted from subgroup analyses. Previous meta-analysis studies demonstrated that TDF treatment resulted in a significant lower HCC incidence than ETV treatment among cirrhosis patients [40,41]. However, yet other studies reported contrasting results. Papatheodoridis et al. reported that the hazards of HCC were similar between ETV- and TDF-treated Caucasian patients [36]. Chen et al. showed that TDF treatment was associated with a lower risk for HCC than ETV treatment. However, among patients with compensated cirrhosis or patients enrolled after 2011, the difference between the two NUCs seemed to disappear [39]. Huang et al. also demonstrated that the cumulative incidence of HCC in TDF- and ETV-treated compensated cirrhotic patients was not significantly different after propensity-score matching. The lower HCC incidence in the TDF group compared to the ETV group was only detected in patients with a high HCC risk score [42]. As shown in Figure 1e, our data showed that the incidence of HCC in cirrhotic CHB patients was not significantly different between patients treated with either of the two NUCs. A future study on HCC prevention using ETV and TDF treatment in cirrhotic patients according to the HCC risk score may elucidate which antiviral regimen is more beneficial to cirrhotic patients.
There was limitation of our study. The mean duration of the follow-up was 47.4 ± 26.5 months in TDF-treated patients and 67.0 ± 34.8 months in ETV-treated patients. Since the duration was relatively shorter in the TDF group, this warrants a longer follow-up period in a further study to clarify the long-term effects against HCC development.
5. Conclusions
Long-term NUC therapy could not completely eliminate the risk for HCC development in CHB-related cirrhotic patients. Cirrhotic CHB patients with age <50 years and AFP <8 ng/mL had the lowest annual incidence of HCC, which was lower than the pooled incidence of HCC in CHB patients. However, patients with age ≥50 years or/and AFP ≥8 ng/mL had a significantly higher risk for HCC and thus warrant a careful surveillance schedule.
Author Contributions
Conceptualization, C.-C.H. and C.-L.L.; methodology, C.-C.H.; software, C.-H.W.; validation, P.-H.C., M.-C.H. and C.-C.H.; formal analysis, C.-H.W.; investigation, Y.-Y.L.; resources, Y.-Y.L.; data curation, C.-H.W.; writing—original draft preparation, Y.-Y.L.; writing—review and editing, C.-C.H.; visualization, C.-H.C.; supervision, C.-L.L.; project administration, K.-C.H.; funding acquisition, C.-C.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Chang Gung Memorial Hospital (protocol code 104_9790B & 201601917B0; approved on 1 July 2015 & 3 July 2017).
Informed Consent Statement
Patient consent was waived by the Institutional Review Board of the Chang Gung Memorial Hospital due to the retrospective nature of the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Chang Gung Memorial Hospital. The research grant numbers are CMRPG2H0141 and CMRPG2I0041.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Villanueva A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 2.Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2020. [(accessed on 29 June 2021)]. Available online: https://gco.iarc.fr/today/factsheets-cancers.pdf.
- 3.Tang L.S.Y., Covert E., Wilson E., Kottilil S. Chronic Hepatitis B Infection: A Review. JAMA. 2018;319:1802–1813. doi: 10.1001/jama.2018.3795. [DOI] [PubMed] [Google Scholar]
- 4.Ghouri Y.A., Mian I., Rowe J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017;16:1. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Dienstag J.L., Goldin R.D., Heathcote E.J., Hann H.W., Woessner M., Stephenson S.L., Gardner S., Gray D.F., Schiff E.R. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 7.Hadziyannis S.J., Tassopoulos N.C., Heathcote E.J., Chang T.T., Kitis G., Rizzetto M., Marcellin P., Lim S.G., Goodman Z., Ma J., et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Marcellin P., Gane E., Buti M., Afdhal N., Sievert W., Jacobson I.M., Washington M.K., Germanidis G., Flaherty J.F., Schall R.A., et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 9.Chang T.T., Liaw Y.F., Wu S.S., Schiff E., Han K.H., Lai C.L., Safadi R., Lee S.S., Halota W., Goodman Z., et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 10.Liaw Y.F., Sung J.J., Chow W.C., Farrell G., Lee C.Z., Yuen H., Tanwandee T., Tao Q.-M., Shue K., Pharm S., et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 11.Di Marco V., Marzano A., Lampertico P., Andreone P., Santantonio T., Almasio P.L., Rizzetto M., Craxì A. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology. 2004;40:883–891. doi: 10.1002/hep.1840400418. [DOI] [PubMed] [Google Scholar]
- 12.Wong G.L., Chan H.L., Mak C.W., Lee S.K., Ip Z.M., Lam A.T., Iu H.W., Leung J.M., Lai J.W., Lo A.O., et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 13.Hosaka T., Suzuki F., Kobayashi M., Seko Y., Kawamura Y., Sezaki H., Akuta N., Suzuki Y., Saitoh S., Arase Y., et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 14.Lin D.Y., Sheen I.S., Chiu C.T., Lin S.M., Kuo Y.C., Liaw Y.F. Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: A longitudinal study. J. Clin. Ultrasound. 1993;21:303–308. doi: 10.1002/jcu.1870210502. [DOI] [PubMed] [Google Scholar]
- 15.Pirmoazen A.M., Khurana A., El Kaffas A., Kamaya A. Quantitative ultrasound approaches for diagnosis and monitoring hepatic steatosis in nonalcoholic fatty liver disease. Theranostics. 2020;10:4277–4289. doi: 10.7150/thno.40249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferraioli G., Soares Monteiro L.B. Ultrasound-based techniques for the diagnosis of liver steatosis. World J. Gastroenterol. 2019;25:6053–6062. doi: 10.3748/wjg.v25.i40.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M., Roberts L.R., Heimbach J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J., Chen G., Zhang P., Zhang J., Li X., Gan D., Cao X., Han M., Du H., Ye Y. The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0228857. doi: 10.1371/journal.pone.0228857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arends P., Sonneveld M.J., Zoutendijk R., Carey I., Brown A., Fasano M., Mutimer D., Deterding K., Reijnders J.G., Oo Y., et al. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: Limited role for risk scores in Caucasians. Gut. 2015;64:1289–1295. doi: 10.1136/gutjnl-2014-307023. [DOI] [PubMed] [Google Scholar]
- 20.Cho J.-Y., Paik Y.-H., Sohn W., Cho H.C., Gwak G.-Y., Choi M.S., Lee J.H., Koh K.C., Paik S.W., Yoo B.C. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014;63:1943–1950. doi: 10.1136/gutjnl-2013-306409. [DOI] [PubMed] [Google Scholar]
- 21.Papatheodoridis G.V., Dalekos G.N., Yurdaydin C., Buti M., Goulis J., Arends P., Sypsa V., Manolakopoulos S., Mangia G., Gatselis N., et al. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J. Hepatol. 2015;62:363–370. doi: 10.1016/j.jhep.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 22.Su T.H., Hu T.H., Chen C.Y., Huang Y.H., Chuang W.L., Lin C.C., Wang C.C., Su W.W., Chen M.Y., Peng C.Y., et al. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016;36:1755–1764. doi: 10.1111/liv.13253. [DOI] [PubMed] [Google Scholar]
- 23.Tsai M.-C., Chen C.-H., Hu T.-H., Lu S.-N., Lee C.-M., Wang J.-H., Hung C.-H. Long-term outcomes of hepatitis B virus-related cirrhosis treated with nucleos(t)ide analogs. J. Formos Med. Assoc. 2017;116:512–521. doi: 10.1016/j.jfma.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Papatheodoridis G.V., Manolakopoulos S., Touloumi G., Vourli G., Raptopoulou-Gigi M., Vafiadis-Zoumbouli I., Vasiliadis T., Mimidis K., Gogos C., Ketikoglou I., et al. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: Results of the nationwide HEPNET. Greece cohort study. Gut. 2011;60:1109–1116. doi: 10.1136/gut.2010.221846. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda K., Saitoh S., Koida I., Arase Y., Tsubota A., Chayama K., Kumada H., Kawanishi M. A multivariate analysis of risk factors for hepatocellular carcinogenesis: A prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47–53. doi: 10.1002/hep.1840180109. [DOI] [PubMed] [Google Scholar]
- 26.Tsukuma H., Hiyama T., Tanaka S., Nakao M., Yabuuchi T., Kitamura T., Nakanishi K., Fujimoto I., Inoue A., Yamazaki H., et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N. Engl. J. Med. 1993;328:1797–1801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 27.Taketa K. Alpha-fetoprotein: Reevaluation in hepatology. Hepatology. 1990;12:1420–1432. doi: 10.1002/hep.1840120625. [DOI] [PubMed] [Google Scholar]
- 28.Lee C.W., Tsai H.I., Lee W.C., Huang S.W., Lin C.Y., Hsieh Y.C., Kuo T., Chen C.W., Yu M.C. Normal Alpha-Fetoprotein Hepatocellular Carcinoma: Are They Really Normal? J. Clin. Med. 2019;8:1736. doi: 10.3390/jcm8101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S.J., Jang J.Y., Jeong S.W., Cho Y.K., Lee S.H., Kim S.G., Cha S.W., Kim Y.S., Cho Y.D., Kim H.S., et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine. 2017;96:e5811. doi: 10.1097/MD.0000000000005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniele B., Bencivenga A., Megna A.S., Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;12:S108–S112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S., Bent S., Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann. Intern. Med. 2003;139:46–50. doi: 10.7326/0003-4819-139-1-200307010-00012. [DOI] [PubMed] [Google Scholar]
- 32.Sherman M., Peltekian K.M., Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: Incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–438. [PubMed] [Google Scholar]
- 33.Singal A.K., Salameh H., Kuo Y.F., Fontana R.J. Meta-analysis: The impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment. Pharmacol. Ther. 2013;38:98–106. doi: 10.1111/apt.12344. [DOI] [PubMed] [Google Scholar]
- 34.Nathani P., Gopal P., Rich N., Yopp A., Yokoo T., John B., Marrero J., Parikh N., Singal A.G. Hepatocellular carcinoma tumour volume doubling time: A systematic review and meta-analysis. Gut. 2021;70:401–407. doi: 10.1136/gutjnl-2020-321040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rich N.E., John B.V., Parikh N.D., Rowe I., Mehta N., Khatri G., Thomas S.M., Anis M., Mendiratta-Lala M., Hernandez C., et al. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis. Hepatology. 2020;72:1654–1665. doi: 10.1002/hep.31159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papatheodoridis G.V., Dalekos G.N., Idilman R., Sypsa V., Van Boemmel F., Buti M., Calleja J.L., Goulis J., Manolakopoulos S., Loglio A., et al. Similar risk of hepatocellular carcinoma during long-term entecavir or tenofovir therapy in Caucasian patients with chronic hepatitis B. J. Hepatol. 2020;73:1037–1045. doi: 10.1016/j.jhep.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Kim J.H., Sinn D.H., Kang W., Gwak G.Y., Paik Y.H., Choi M.S., Lee J.H., Koh K.C., Paik S.W. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology. 2017;66:335–343. doi: 10.1002/hep.28916. [DOI] [PubMed] [Google Scholar]
- 38.Li Z.-Q., Hu C.-L., Yu P., Gu X.-Y., Zhang J.-J., Li H., Zhang H.-Y., Lv J., Liu Y.-M., Zeng Q.-L., et al. The development of hepatocarcinoma after long-term antivirus treatment of Chinese patients with chronic hepatitis B virus infection: Incidence, long-term outcomes and predictive factors. Clin. Res. Hepatol. Gastroenterol. 2017;41:311–318. doi: 10.1016/j.clinre.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Chen C.H., Chen C.Y., Wang J.H., Lai H.C., Hung C.H., Lu S.N., Peng C.Y. Comparison of incidence of hepatocellular carcinoma between chronic hepatitis B patients with cirrhosis treated with entecavir or tenofovir in Taiwan—A retrospective study. Am. J. Cancer Res. 2020;10:3882–3895. [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z., Zhou Y., Yang J., Hu K., Huang Y. The effectiveness of TDF versus ETV on incidence of HCC in CHB patients: A meta-analysis. BMC Cancer. 2019;19:511. doi: 10.1186/s12885-019-5735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi W.M., Choi J., Lim Y.S. Effects of Tenofovir vs Entecavir on Risk of Hepatocellular Carcinoma in Patients With Chronic HBV Infection: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021;19:246–258 e9. doi: 10.1016/j.cgh.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y., Chen L., Huang R., Zhu C., Shang J., Qian Y., Lian J., Liu L., Jiang J., Liu C., et al. Tenofovir is superior to entecavir in reducing HCC for patients with HBV-related compensated cirrhosis at high HCC risk scores. Ther. Adv. Chronic. Dis. 2022;13:20406223221102791. doi: 10.1177/20406223221102791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.