Abstract
Human mastadenovirus (HAdV) is a non-enveloped icosahedral virus with double-stranded DNA genomes. The mortality rate of HAdV infections can reach 35.5%, while gastroenteritis HAdV infections, HAdV pneumonia, and disseminated disease tend to show a worse outcome, with rates ranging from 44.2% to 50%. In addition, HAdV can cause infections at any age but most commonly in the pediatric population, especially in young children and infants. Therefore, this review aims to assess the current status of HAdV infections among children in the Arab World, particularly in the Middle East and North Africa. Web of Science, Scopus, PubMed, EMBASE, and Google Scholar databases for publications in English were searched up to July 2022 for relevant articles. The literature search yielded a total of 21 studies, which were included in this review. Studies reporting HAdV infections in children were conducted in 17 out of the 22 countries. The average prevalence rate of HAdV infections in children was 12.7%, with average prevalence rates of 12.82% and 12.58% in the Middle East and North African countries, respectively. The highest prevalence rate (28.3%) was reported in Egypt, whereas the lowest prevalence (1.5%) was reported in Sudan. The included studies presented children with signs and symptoms of gastroenteritis, acute respiratory infection, acute diarrhea, and acute hemorrhagic conjunctivitis. In conclusion, the average prevalence rate of HAdV infections in children was 12.7%, with average prevalence rates of 12.82% and 12.58% in the Middle East and North African countries, respectively. Finding the precise prevalence rate of this virus is crucial because it will guide future planning for effective disease control and the selection of particular treatment options during epidemics and special seasons.
Keywords: Arab world, children, human mastadenovirus, infection, Middle East, North Africa
1. Introduction
Human mastadenovirus (HAdV) is a non-enveloped icosahedral virus with double-stranded DNA genomes [1]. Since Wallace P. Rowe isolated and named the adenoviral serotype HAdV from human tonsils and adenoids in 1953, more than 120 distinct adenoviral serotypes have been discovered in humans, animals, birds, fish, and reptiles. Seven subgroups (A through G) have been assigned to them [2]. HAdV presents a non-enveloped spherical structure [3]. Adenoviral virions often show a crystalline arrangement in infected nuclei [4]. HAdV also contains 26 to 48 kb of linear double-stranded DNA genomes [5]. It creates transcripts of early-stage (E), interim-stage (I), and late-stage (L) regions based on the amount of time that has passed since infection [6]. HAdV is a seasonally transmitted virus that prevails from February to April [7,8,9]. It is a widespread virus that affects humans and is primarily spread by respiratory droplets and feces [10,11,12]. HAdV infections are often seen in military recruits, students, and nursery and kindergarten children, especially children under the age of four, elderly people, and people with compromised immunity [13,14,15].
The clinical manifestations of HAdV infections are very extensive, ranging from a symptom to life-threatening severe respiratory infections and tissue-invasive infections [16,17]. HAdV can cause many symptoms similar to the common cold, including rhinorrhea, fever, cough, and sore throat [18,19]. Bronchitis, bronchiolitis, and pneumonia are examples of lower respiratory diseases that can be serious or even fatal [20]. HAdV infections can also be linked to other conditions such as conjunctivitis, gastroenteritis, cystitis, myocarditis, cardiomyopathy, and meningoencephalitis [21]. A variety of clinical illnesses, including respiratory tract infections, disorders of the respiratory system, keratoconjunctivitis, gastroenteritis, and urinary infections, may be caused by various adenoviral serotypes [22]. Immunocompromised patients are susceptible to invasive adenoviral infection [23]. In immunocompromised patients, HAdV may reactivate or cause new infections, resulting in multi-organ infections like enteritis, hemorrhagic cystitis, hepatitis, and pneumonia [24].
The systematic review and meta-analysis conducted by GU, Jie et al. showed that the mortality rate of AdV infection is 35.5%, while gastroenteritis HAdV infections, HAdV pneumonia, and disseminated disease tend to show a worse outcome, with rates ranging from 44.2% to 50%. However, in the context of immunocompetent patients is associated with a mortality of up to 32.9% [25]. Furthermore, the fatality rates for severe HAdV pneumonia or disseminated disease may exceed 50% in previous reports [25]. In addition, HAdV can cause infections at any age but most commonly in the pediatric population, especially in young children and infants [19]. Invasive HAdV infections do more harm to children [26]. Moreover, by the age of 10 years old, most children have had at least one episode of HAdV infections [19]. Additionally, HAdV testing has produced positive results in 77% of cases of severe acute hepatitis of unknown causes that have been detected in children in many countries [27].
Early diagnosis and supportive therapy are crucial for immunocompromised patients, especially for children [28]. Some large children’s hospitals even recommend HAdV testing as a screening item for children with respiratory tract infections [29,30]. HAdV testing can be performed by isolated cell culture, serological identification, and PCR [31]. However, due to a long time of HAdV cell culture, the limited value of antibody tests in immunosuppressed people, and the lack of quantifying and genotyping functions, the actual clinical application effects are undesirable [32]. By contrast, RT–PCR can be directly used to test nucleotide sequences specific to HAdV pathogens, with high specificity, sensitivity, and desirable genotyping advantages [33]. This also explains why RT–PCR is highly favored. The European Society for Blood and Marrow Transplantation (EBMT) recommends that quantitative PCR (qPCR) be adopted to diagnose and monitor the HAdV in the blood and feces samples of children and adults receiving transplantation so that interventions can be provided as early as possible [34].
Most countries in the Arab World have not yet introduced national HAdV vaccination programs. Although regional and local information on the prevalence and burden of HAdV and strain distribution is important for healthcare practitioners and officials to make appropriate policies and recommendations about HAdV vaccination, there is a remarked lack of comprehensive literature reviews on the prevalence of HAdV infections among children that have been published for the Arab World, particularly in the Middle East and North Africa. Therefore, the purpose of this review is to assess the current status of HAdV infections among children in the Arab World in the Middle East and North Africa. Understanding the current status of HAdV infection prevalence and its associated gastroenteritis, respiratory tract infections, and acute hemorrhagic conjunctivitis is critical to evaluating the performance of surveillance systems where available and to expanding the implementation of these systems.
2. Materials and Methods
Electronic literature searches of Web of Science, Scopus, PubMed, EMBASE, and Google Scholar databases for publications in English were systematically conducted without time limit up to July 2022 for studies on HAdV in children in the Arab World in the Middle East and North Africa. A combination of search methods was employed to increase the search scale, namely: (1) search MESH (Human mastadenovirus) using the following terms: “Adenovirus”, “Children”, and “Infection”, and (2) free-text search.
The following were the inclusion requirements: Studies have described the prevalence of HAdV infections in children in the Arab World in the Middle East and North Africa. On the other hand, the following exclusion criteria were considered: (1) reviews; (2) non-English language; (2) case reports; (3) studies not focused on HAdV infections in children; (4) studies that were not performed in the Middle East and North Africa; (4) studies lacking pertinent data; and (4) studies without full text.
The screening procedures were managed, and duplicates were eliminated using EndNote V.X8 software. After removing duplicates, the authors individually the titles, abstracts, and full texts to determine whether the studies were eligible.
The study region, study country, study design, targeted children, sample size, sample location, disease, and HAdV prevalence rate were extracted and recorded using a standardized data collection form that was developed in accordance with the sequence of variables required from the primary source.
3. Results
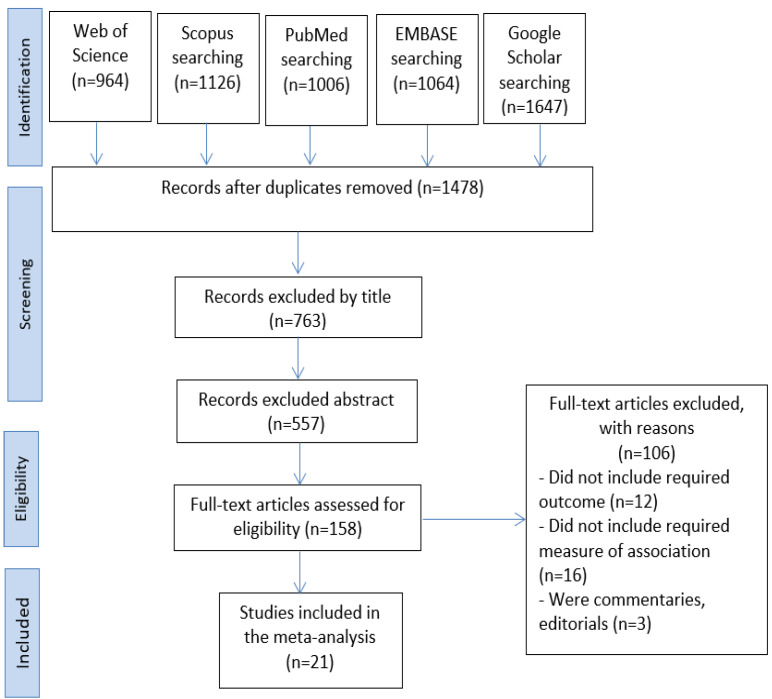
Our literature search identified 21 articles on HAdV infections in children in the Arab World in the Middle East and North Africa between 2001 and 2021. In total, 5807 articles were found during the search across five databases: Web of Science (n = 964), Scopus (n = 1126), PubMed (n = 1006), EMBASE (n = 1064), Google Scholar (n = 1647). The duplicates were then removed, leaving 1478 studies. We eliminated 763 studies by title screening and then used abstract screening to eliminate 339 irrelevant studies out of 557. Following this, we read the full texts of the remaining 158 publications and omitted 137 pieces of research since they did not meet our inclusion criteria (Figure 1).
Figure 1.
The PRISMA flowchart for the process of selecting and identifying studies.
Studies reporting on HAdV infections in children were conducted in 17 out of the 22 countries of the Arab World in the Middle East and North Africa region. We could not find relevant studies published in the following five countries: the Comoros Islands, Djibouti, Mauritania, Somalia, and Syria. Eleven studies were conducted in Middle East countries, namely: Iraq (n = 2) [35,36], Jordan (n = 2) [37,38], Kuwait (n = 2) [39,40], Yemen (n = 2) [41,42], Bahrain (n = 1) [43], Lebanon (n = 1) [44], and the United Arab Emirates (n = 1) [45]. Whereas 10 studies were from North African countries, namely: Egypt (n = 3) [46,47,48], Morocco (n = 2) [49,50], Sudan (n = 2) [51,52], Algeria (n = 1) [53], Libya (n = 1) [54], Tunisia (n = 1) [55].
A wide range of HAdV infections in children has been reported in the Arab World in the Middle East and North African countries. The average prevalence rate of HAdV infections in children was 12.7%, with average prevalence rates of 12.82% and 12.58% in the Middle East and North African countries, respectively. The highest prevalence rate of HAdV infections in children (28.3%) was reported in the descriptive cross-sectional observational study conducted in Egypt by Elmahdy Elmahdy et al. 2019, which targeted children suffering from gastroenteritis [47]. On the other hand, the lowest prevalence rate of HAdV infections in children (1.5%) was reported in the descriptive cross-sectional observational study conducted in Sudan by Wafa Elhag et al. 2013, which targeted children under 14 years old with acute diarrhea [51].
Most of the included studies reporting on HAdV infections in children were clinical observational studies. Eleven studies were descriptive cross-sectional observational studies in design; four studies were descriptive prospective cross-sectional studies in design; four studies were descriptive retrospective studies in design; one study was a descriptive case-control observational study, and one study was a case study in design [43].
In all the included studies, HAdV infections in children were reported in hospitalized children. These studies presented children with signs and symptoms associated with gastroenteritis (n = 8), acute respiratory infection (n = 8), acute diarrhea (n = 4), and acute hemorrhagic conjunctivitis (n = 1).
A total of 14 studies were carried out among children less than five years of age. The prevalence of HAdV infections among children less than 5 years old was reported in the included studies as follows: Iraq, 14/320 (4.5%) [36]; Iraq, 3/100 (3%) [35]; Jordan, 54/350 (15.4%) [37]; Kuwait, 27/743 (3.6%) [40]; Yemen, 36/326 (11%) [41], United Arab Emirates, 35/203 (17.2%) [45], Egypt, 8/119 (6.7%) [48], Egypt, 20/100 (20%) [46], Egypt, 17/60 (28.3%) [47], Morocco, 119/700 (17%) [49], Sudan, 7/437 (1.6%) [52], Algeria, 9/117 (7.5%) [53], Libya, 17/239 (7.1%) [54], and Tunisia, 3/583 (19.6%) [55]. The remaining studies reported HAdV infections among older children.
There was a noticeable difference in sample sizes between studies and between studies conducted in the same country.
The highest prevalence of HAdV infections among children was in stool samples associated with gastroenteritis for children under 12 years [44] and below ten years [39], at 25.3% and 23.2%, respectively. Whereas the lowest prevalence of HAdV infections among children, 1.5% and 1.6%, was in stool samples associated with acute diarrhea for children below five years in Sudan [51,52] (Table 1).
Table 1.
Reported HAdV infections among children in the Arab World, particularly in the Middle East and North Africa, up to July 2022.
| Region | Country | Study | Study Design | Targeted Children | Sample Size | Sample Location | Disease | Prevalence Rate |
|---|---|---|---|---|---|---|---|---|
| Middle East | Iraq | Ali Harb et al., 2019 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea |
320 children | Stool specimens | Gastroenteritis | 4.5% |
| Iraq | Dilshad Jaff et al., 2016 | A descriptive cross-sectional observational study | Children below five years of age with gastroenteritis | 100 children | Stool specimens | Gastroenteritis | 3.0% | |
| Jordan | Nasser Kaplan et al., 2008 | A descriptive prospective cross-sectional study | Children below five years with an acute respiratory infection | 326 children | Nasopharyngeal aspirates | Acute respiratory infection | 18.0% | |
| Jordan | Mamdoh Meqdam et al., 2001 | A descriptive cross-sectional observational study | Children below thirteen years old with respiratory tract infections |
350 children | Nasopharyngeal aspirates | Acute respiratory infection | 15.4% | |
| Kuwait | Hawraa Mohammad et al., 2020 | A 4-year descriptive retrospective study | Children below ten years with gastroenteritis | 84 children | Stool samples | Gastroenteritis | 23.2% | |
| Kuwait | Wassim Chehadeh et al., 2018 | A 4-year descriptive retrospective study | Children below four years with severe respiratory disease | 743 children | Nasopharyngeal aspirate and Nasopharyngeal swab | Severe respiratory infections | 3.6% | |
| Yemen | Khaled Al-Moyed et al., 2015 | A descriptive cross-sectional observational study | Children below five years with acute gastroenteritis | 326 children | Stool samples | Gastroenteritis | 11% | |
| Yemen | Mohammad Al Amad et al., 2019 | A descriptive retrospective study | Children below fifteen years of age with severe acute respiratory infections | 1413 children | Nasopharyngeal and oropharyngeal swabs | Acute respiratory infection | 7.0% | |
| Bahrain | Aysha Agab et al., 2016 | A case study | A seven-year-old male and a five-year-old female | Two Bahraini siblings | Conjunctival swabs | Acute hemorrhagic conjunctivitis | NM | |
| Lebanon | Rasha Zaraket et al., 2020 | A 12-months descriptive retrospective study | Children below twelve years with acute gastroenteritis | 308 children | Stool samples | Gastroenteritis | 25.3% | |
| United Arab Emirates | Ahmed Alsuwaidi et al., 2021 | A descriptive case-control observational study | Children below five years with diarrhea | 203 children as a case | Stool samples | Acute diarrhea | 17.2% | |
| North Africa | Egypt | Abdou Kamal Allayeh et al., 2018 | A descriptive cross-sectional observational study | Children below five years with gastroenteritis | 119 children | Fecal diarrhea samples |
Gastroenteritis | 6.7% |
| Egypt | Maysaa El Sayed Zaki et al., 2017 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea | 100 children | Stool sample | Acute diarrhea | 20.0% | |
| Egypt | Elmahdy Elmahdy et al., 2019 | A descriptive cross-sectional observational study | Children below five years with gastroenteritis | 60 children | Stool samples | Gastroenteritis | 28.3% | |
| Morocco | Imane Jroundi et al., 2014 | A descriptive prospective cross-sectional study | Children below five years with respiratory symptomatology | 700 children | Nasopharyngeal aspirates |
Acute respiratory infection | 17.0% | |
| Morocco | Marcil Sarrah et al., 2018 | A descriptive prospective cross-sectional study | Children below fourteen years with severe acute viral respiratory infections | 103 children | Nasopharyngeal aspirates |
Acute respiratory infection | 16.5% | |
| Sudan | Wafa Elhag et al., 2013 | A descriptive cross-sectional observational study | Children below fourteen years old with acute diarrhea | 511 children | Stool samples | Acute diarrhea | 1.5% | |
| Sudan | Mosab Adam et al., 2018 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea | 437 children | Stool samples | Acute diarrhea | 1.6% | |
| Algeria | Fawzi Derrar et al., 2019 | A descriptive prospective cross-sectional study | Children below two years with respiratory tract infections | 117 children | Nasal or nasopharyngeal aspiration | Acute respiratory infection | 7.5% | |
| Libya | Amal Rahouma et al., 2011 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea | 239 children | Stool specimens | Gastroenteritis | 7.1% | |
| Tunisia | Ines Brini et al., 2020 | A descriptive cross-sectional observational study | Children below five years with acute respiratory infections | 583 children | Nasopharyngeal aspirate | Acute respiratory infection | 19.6% |
NM: denote to not mentioned.
4. Discussion
HAdV infections are one of the most significant etiological agents of serious gastroenteritis, respiratory infections, conjunctivitis, cystitis myocarditis, hemorrhagic cystitis, hepatitis, cardiomyopathy, meningoencephalitis, urinary tract infection, and chronic systemic infections, especially in infants and young children under the age of five [27,39,44,49,53]. This review was conducted to represent the current status of HAdV infections in children in the Arab World in the Middle East and North Africa. Our literature search identified 21 articles on HAdV infections in children in the Arab World in the Middle East and North Africa between 2001 and 2021. Studies reporting on HAdV infections in children were conducted in 17 out of the 22 countries of the Arab World in the Middle East and North Africa region. We could not find relevant studies published in the following five countries: the Comoros Islands, Djibouti, Mauritania, Somalia, and Syria. The main results of this review revealed that the average prevalence rate of HAdV infections in children was 12.7%, with average prevalence rates of 12.82% and 12.58% in the Middle East and North African countries, respectively. The highest prevalence rate of HAdV infections in children (28.3%) was reported in the descriptive cross-sectional observational study conducted in Egypt by Elmahdy Elmahdy et al., 2019, which targeted children suffering from gastroenteritis [47]. On the other hand, the lowest prevalence rate of HAdV infections in children (1.5%) was reported in the descriptive cross-sectional observational study conducted in Sudan by Wafa Elhag et al., 2013, which targeted children under 14 years old with acute diarrhea [51]. In comparison with other countries such as Australia, Brazil, Indonesia, Korea, Iran, the UK, Turkey, Hungary, and Sweden, the prevalence rates of HAdV infections ranged from 1% to 96.3% of patients [53,56,57,58,59,60,61,62,63,64].
Additionally, in this review, a total of 14 studies were carried out among children less than five years of age. The prevalence of HAdV infections among children less than 5 years old was reported in the included studies as follows: Iraq, 14/320 (4.5%) [36]; Iraq, 3/100 (3%) [35]; Jordan, 54/350 (15.4%) [37]; Kuwait, 27/743 (3.6%) [40]; Yemen, 36/326 (11%) [41], United Arab Emirates, 35/203 (17.2%) [45], Egypt, 8/119 (6.7%) [48], Egypt, 20/100 (20%) [46], Egypt, 17/60 (28.3%) [47], Morocco, 119/700 (17%) [49], Sudan, 7/437 (1.6%) [52], Algeria, 9/117 (7.5%) [53], Libya, 17/239 (7.1%) [54], and Tunisia, 3/583 (19.6%) [55]. The remaining studies reported HAdV infections among older children. Differences in prevalence rates in the current review as compared to other studies may be attributed to several reasons, such as the diagnostic technique used for detection, the difference in age of the studied population, and/or the geographical region of the study area. According to the centers for disease control and prevention (CDC), in the United States, the HAdV vaccine is only available for United States military personnel, and there is currently no HAdV vaccine available to the public [65]. Additionally, most countries in the Arab World have not yet introduced national AdV vaccination programs. Therefore, the regional and local information on the prevalence and burden of HAdV and strain distribution is important for healthcare practitioners and officials to make appropriate policies and recommendations about HAdV vaccination.
Moreover, our results showed that in all the included studies, HAdV infections in children were reported in hospitalized children. These studies presented children with signs and symptoms associated with gastroenteritis (n = 8), acute respiratory infection (n = 8), acute diarrhea (n = 4), and acute hemorrhagic conjunctivitis (n = 1). Several serotypes of HAdV, but mainly enteric types 40 and 41, have been strongly associated with gastroenteritis in childhood [57]. In addition, HAdV is responsible for three to five percent of all respiratory infections and diarrhea in the USA and a higher percentage in underdeveloped nations [65,66]. These viruses are mostly distributed through the fecal-oral route, and antibodies to them have been found in 50% or more of children under the age of five throughout Asia, Africa, Europe, and South America, with comparable seropositive proportions in the different populations [67,68].
Diarrheal illnesses remain a major public health concern and represent the third leading cause of death globally. In recent years, viral diarrhea has gradually increased in infants and children in both developing and developed countries [69]. Similar to diarrhea brought on by other viruses, HAdV-associated diarrhea may have slightly longer-lasting symptoms. The stools are watery, occasionally red, non-leucocyte-containing, and water-containing [70,71]. Although they are present all year round and everywhere in the world, they are most common in temperate areas in the spring or early summer and once more in the middle of winter [70,71,72]. Additionally, acute hemorrhagic conjunctivitis is a derivative of the highly contagious conjunctivitis virus, otherwise known as pink eye. There are three main viruses that have been studied and confirmed as the agents responsible for acute hemorrhagic conjunctivitis, including enterovirus 70, coxsackievirus A24 variant (CA24v), and HAdV [73].
Finally, this review demonstrated that the highest prevalence of HAdV infections among children was in stool samples associated with gastroenteritis for children under 12 years [44] and below ten years [39], at 25.3% and 23.2%, respectively. Whereas the lowest prevalence of HAdV infections among children, 1.5% and 1.6%, was in stool samples associated with acute diarrhea for children below five years in Sudan [51,52]. It appears that HAdV infection is a significant hygiene problem in these nations. Finding the precise prevalence rate of this virus is crucial since it will aid in future programming for the right control and selection of specialized treatment approaches for the illness during unique seasons and the HAdV outbreak. Furthermore, to limit the spread of infection, it is important to maintain infectious control practices and educate the infected individual to avoid sharing towels, glasses, or any other item in contact with the eyes. Additionally, these individuals should stay at home and avoid going to work or school when symptomatic in order to prevent spreading the infection.
The main strength of the current review is being the first review that shows the current status of HAdV infections among children in the Arab World in the Middle East and North Africa. However, our study has a number of limitations. The research population, times, locations, data collection techniques, and clinical disease care could all contribute to differences in estimates between and within countries. Because there were few studies conducted in each of these nations, the findings indicating the prevalence of HAdV infections may not be generalizable to several Arab nations. Some studies were carried out at large hospitals in urban areas, so the results might not be applicable to rural or other settings.
Additionally, the surveillance techniques used in the numerous published publications differ from country to country, and variances in the estimations may result from variations in HAdV detection techniques. Nevertheless, the overall results typically support earlier literature; thus, any systematic error is improbable. Finally, our study did not study the seasonality of HAdV prevalence.
5. Conclusions
In conclusion, the average prevalence rate of HAdV infections in children was 12.7%, with average prevalence rates of 12.82% and 12.58% in the Middle East and North African countries, respectively. Finding the precise prevalence rate of this virus is crucial because it will guide future planning for effective disease control and the selection of particular treatment options during epidemics and special seasons.
Acknowledgments
The author thanks Saeed M. Kabrah and Radi T. Assafi for their help in some article screening, data extraction, and reviewing of the manuscript. He would also extend his gratitude to Umm Al Qura University for their support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Samantaray M.U.S., Santra M.P. Human Adenovirus Serotypes Efficiently Transducing HEK293 Cells: An In Vitro Propagation of HAdv. Int. J. Res. Appl. Sci. Biotechnol. 2021;8:17–21. doi: 10.31033/ijrasb.8.5.3. [DOI] [Google Scholar]
- 2.Rowe W.P., Huebner R.J., Gilmore L.K., Parrott R.H., Ward T.G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 1953;84:570–573. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- 3.Moleirinho M.G., Feast S., Moreira A.S., Silva R.J., Alves P.M., Carrondo M.J., Huber T., Fee C., Peixoto C. 3D-printed ordered bed structures for chromatographic purification of enveloped and non-enveloped viral particles. Sep. Purif. Technol. 2021;254:117681. doi: 10.1016/j.seppur.2020.117681. [DOI] [Google Scholar]
- 4.Takeuchi A., Hashimoto K. Electron microscope study of experimental enteric adenovirus infection in mice. Infect. Immun. 1976;13:569–580. doi: 10.1128/iai.13.2.569-580.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koonin E.V., Krupovic M., Yutin N. Evolution of double-stranded DNA viruses of eukaryotes: From bacteriophages to transposons to giant viruses. Ann. N. Y. Acad. Sci. 2015;1341:10–24. doi: 10.1111/nyas.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw A.R., Ziff E.B. Transcripts from the adenovirus-2 major late promoter yield a single early family of 3′ coterminal mRNAs and five late families. Cell. 1980;22:905–916. doi: 10.1016/0092-8674(80)90568-1. [DOI] [PubMed] [Google Scholar]
- 7.Shek L.P.-C., Lee B.-W. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr. Respir. Rev. 2003;4:105–111. doi: 10.1016/S1526-0542(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 8.Abbas K.Z., Lombos E., Duvvuri V.R., Olsha R., Higgins R.R., Gubbay J.B. Temporal changes in respiratory adenovirus serotypes circulating in the greater Toronto area, Ontario, during December 2008 to April 2010. Virol. J. 2013;10:15. doi: 10.1186/1743-422X-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow W.Z., Chan Y.F., Oong X.Y., Ng L.J., Nor’E S.S., Ng K.T., Chan K.G., Hanafi N.S., Pang Y.K., Kamarulzaman A. Genetic diversity, seasonality and transmission network of human metapneumovirus: Identification of a unique sub-lineage of the fusion and attachment genes. Sci. Rep. 2016;6:27730. doi: 10.1038/srep27730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Rosa G., Fratini M., Libera S.D., Iaconelli M., Muscillo M. Viral infections acquired indoors through airborne, droplet or contact transmission. Ann. Dell’istituto Super. Di Sanita. 2013;49:124–132. doi: 10.4415/ANN_13_02_03. [DOI] [PubMed] [Google Scholar]
- 11.Arnold A., MacMahon E. Adenovirus infections. Medicine. 2017;45:777–780. doi: 10.1016/j.mpmed.2017.09.016. [DOI] [Google Scholar]
- 12.O’Brien B., Goodridge L., Ronholm J., Nasheri N. Exploring the potential of foodborne transmission of respiratory viruses. Food Microbiol. 2021;95:103709. doi: 10.1016/j.fm.2020.103709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith J.G., Wiethoff C.M., Stewart P.L., Nemerow G.R. Cell Entry by Non-Enveloped Viruses. Springer; Berlin/Heidelberg, Germany: 2010. Adenovirus; pp. 195–224. [Google Scholar]
- 14.Murtagh P., Giubergia V., Viale D., Bauer G., Pena H.G. Lower respiratory infections by adenovirus in children. Clinical features and risk factors for bronchiolitis obliterans and mortality. Pediatric Pulmonol. 2009;44:450–456. doi: 10.1002/ppul.20984. [DOI] [PubMed] [Google Scholar]
- 15.Hoeben R.C., Uil T.G. Adenovirus DNA replication. Cold Spring Harb. Perspect. Biol. 2013;5:a013003. doi: 10.1101/cshperspect.a013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch J.P., Fishbein M., Echavarria M. Adenovirus; Proceedings of the Seminars in Respiratory and Critical Care Medicine; Denver, Colorado. 13–18 May 2011; pp. 494–511. [DOI] [PubMed] [Google Scholar]
- 17.Gu J., Su Q.-Q., Zuo T.-T., Chen Y.-B. Adenovirus diseases: A systematic review and meta-analysis of 228 case reports. Infection. 2021;49:1–13. doi: 10.1007/s15010-020-01484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkpatrick G.L. The common cold. Prim. Care Clin. Off. Pract. 1996;23:657–675. doi: 10.1016/S0095-4543(05)70355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shieh W.-J. Human adenovirus infections in pediatric population-an update on clinic—Pathologic correlation. Biomed. J. 2021;54:38–49. doi: 10.1016/j.bj.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S.-Y., Luo Y.-P., Huang D.-D., Fan H., Lu Q.-B., Wo Y., Chen G., Zhang X.-A., Li Y., Tong Y.-G. Fatal pneumonia cases caused by human adenovirus 55 in immunocompetent adults. Infect. Dis. 2016;48:40–47. doi: 10.3109/23744235.2015.1055585. [DOI] [PubMed] [Google Scholar]
- 21.Wang H.-S. Updates in pediatrics. Biomed. J. 2022;45:9. doi: 10.1016/j.bj.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ison M.G., Hayden R.T. Diagnostic Microbiology of the Immunocompromised Host. Microbiology Spectrum, American Society for Microbiology Press; Washington, DC, USA: 2016. Adenovirus; pp. 217–232. [Google Scholar]
- 23.Matthes-Martin S., Boztug H., Lion T. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert Rev. Anti-Infect. Ther. 2013;11:1017–1028. doi: 10.1586/14787210.2013.836964. [DOI] [PubMed] [Google Scholar]
- 24.Bhatti Z., Dhamoon A. Fatal adenovirus infection in an immunocompetent host. Am. J. Emerg. Med. 2017;35:1034.e1–1034.e2. doi: 10.1016/j.ajem.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Lynch J.P., III, Kajon A.E. Adenovirus: Epidemiology, global spread of novel serotypes, and advances in treatment and prevention; Proceedings of the Seminars in Respiratory and Critical Care Medicine; San Francisco, CA, USA. 13–18 May 2016; pp. 586–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maschmeyer G., Ljungman P. Principles and Practice of cancer Infectious Diseases. Springer; Berlin/Heidelberg, Germany: 2011. Infections in hematopoietic stem cell transplant recipients; pp. 17–25. [Google Scholar]
- 27.Rabaan A.A., Bakhrebah M.A., Nassar M.S., Natto Z.S., Al Mutair A., Alhumaid S., Aljeldah M., Garout M., Alfouzan W.A., Alshahrani F.S. Suspected Adenovirus Causing an Emerging HEPATITIS among Children below 10 Years: A Review. Pathogens. 2022;11:712. doi: 10.3390/pathogens11070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lion T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. 2019;593:3571–3582. doi: 10.1002/1873-3468.13576. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y., Li W., Yang B., Qian R., Wu F., He X., Zhu Q., Liu J., Ni Y., Wang J. Epidemiological and virological characteristics of respiratory tract infections in children during COVID-19 outbreak. BMC Pediatrics. 2021;21:1–8. doi: 10.1186/s12887-021-02654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao M.-C., Guo Y.-H., Qiu F.-Z., Wang L., Yang S., Feng Z.-S., Li G.-X. Molecular and clinical characterization of human adenovirus associated with acute respiratory tract infection in hospitalized children. J. Clin. Virol. 2020;123:104254. doi: 10.1016/j.jcv.2019.104254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hess M. Detection and differentiation of avian adenoviruses: A review. Avian Pathol. 2000;29:195–206. doi: 10.1080/03079450050045440. [DOI] [PubMed] [Google Scholar]
- 32.Ko G., Cromeans T.L., Sobsey M.D. Detection of infectious adenovirus in cell culture by mRNA reverse transcription-PCR. Appl. Environ. Microbiol. 2003;69:7377–7384. doi: 10.1128/AEM.69.12.7377-7384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohayem J., Berger S., Juretzek T., Herchenröder O., Mogel M., Poppe M., Henker J., Rethwilm A. A simple and rapid single-step multiplex RT-PCR to detect Norovirus, Astrovirus and Adenovirus in clinical stool samples. J. Virol. Methods. 2004;118:49–59. doi: 10.1016/j.jviromet.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Hiwarkar P., Kosulin K., Cesaro S., Mikulska M., Styczynski J., Wynn R., Lion T. Management of adenovirus infection in patients after haematopoietic stem cell transplantation: State-of-the-art and real-life current approach: A position statement on behalf of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation. Rev. Med. Virol. 2018;28:e1980. doi: 10.1002/rmv.1980. [DOI] [PubMed] [Google Scholar]
- 35.Jaff D.O., Aziz T.A., Smith N.R. The incidence of rotavirus and adenovirus infections among children with diarrhea in Sulaimani Province, Iraq. J. Biosci. Med. 2015;4:124–131. doi: 10.4236/jbm.2016.41015. [DOI] [Google Scholar]
- 36.Harb A., Abraham S., Rusdi B., Laird T., O’Dea M., Habib I. Molecular detection and epidemiological features of selected bacterial, viral, and parasitic enteropathogens in stool specimens from children with acute diarrhea in Thi-Qar Governorate, Iraq. Int. J. Environ. Res. Public Health. 2019;16:1573. doi: 10.3390/ijerph16091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan N.M., Dove W., Abd-Eldayem S.A., Abu-Zeid A.F., Shamoon H.E., Hart C.A. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J. Med. Virol. 2008;80:168–174. doi: 10.1002/jmv.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meqdam M.M., Nasrallah G., Al-Shurman A. Detection of adenovirus infection in children in Jordan. Ann. Trop. Paediatr. 2001;21:59–65. doi: 10.1080/02724930123898. [DOI] [PubMed] [Google Scholar]
- 39.Mohammad H.A., Madi N.M., Al-Nakib W. Analysis of viral diversity in stool samples from infants and children with acute gastroenteritis in Kuwait using Metagenomics approach. Virol. J. 2020;17:1–12. doi: 10.1186/s12985-020-1287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chehadeh W., Al-Adwani A., John S.E., Al-Dhufairi S., Al-Dousari H., Alkhaledi M., Al-Nakib W. Adenovirus types associated with severe respiratory diseases: A retrospective 4-year study in Kuwait. J. Med. Virol. 2018;90:1033–1039. doi: 10.1002/jmv.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Moyed K.A., Al-Jamrah K.M., Al-Robasi A.B.A., Nabhan A.S.B., Al-Haddad A.M. Prevalence of enteric adenovirus among infants and young children suffering from acute gastroenteritis in Sana’a city, Yemen. Andal. J. Appl. Sci. 2015;4:79–81. [Google Scholar]
- 42.Al Amad M.A., Al Mahaqri A.A., Al Serouri A.A., Khader Y.S. Severe acute respiratory infections with influenza and noninfluenza respiratory viruses: Yemen, 2011–2016. INQUIRY J. Health Care Organ. Provis. Financ. 2019;56:1–7. doi: 10.1177/0046958019850731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aysha Waheed A., Muneera Abdulla A.N., Ghada Al B.A. Adenovirus isolated from an outbreak of acute hemorrhagic conjunctivitis. Bahrain Med. Bull. 2016;38:224–226. [Google Scholar]
- 44.Zaraket R., Salami A., Bahmad M., El Roz A., Khalaf B., Ghssein G., Bahmad H.F. Prevalence, risk factors, and clinical characteristics of rotavirus and adenovirus among Lebanese hospitalized children with acute gastroenteritis. Heliyon. 2020;6:e04248. doi: 10.1016/j.heliyon.2020.e04248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alsuwaidi A.R., Al Dhaheri K., Al Hamad S., George J., Ibrahim J., Ghatasheh G., Issa M., Al-Hammadi S., Narchi H. Etiology of diarrhea by multiplex polymerase chain reaction among young children in the United Arab Emirates: A case-control study. BMC Infect. Dis. 2021;21:1–9. doi: 10.1186/s12879-020-05693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaki M.E.S., El Kheir N.A. Molecular study of astrovirus, adenovirus and norovirus in community acquired diarrhea in children: One Egyptian center study. Asian Pac. J. Trop. Biomed. 2017;7:987–990. doi: 10.1016/j.apjtb.2017.10.003. [DOI] [Google Scholar]
- 47.Elmahdy E.M., Ahmed N.I., Shaheen M.N., Mohamed E.-C.B., Loutfy S.A. Molecular detection of human adenovirus in urban wastewater in Egypt and among children suffering from acute gastroenteritis. J. Water Health. 2019;17:287–294. doi: 10.2166/wh.2019.303. [DOI] [PubMed] [Google Scholar]
- 48.Allayeh A.K., El Baz R.M., Saeed N.M., Osman M.E.S. Detection and genotyping of viral gastroenteritis in hospitalized children below five years old in Cairo, Egypt. Arch. Pediatric Infect. Dis. 2018;6:e60288. [Google Scholar]
- 49.Jroundi I., Mahraoui C., Benmessaoud R., Moraleda C., Tligui H., Seffar M., Kettani S.C., Benjelloun B.S., Chaacho S., Maaroufi A. The epidemiology and aetiology of infections in children admitted with clinical severe pneumonia to a university hospital in Rabat, Morocco. J. Trop. Pediatrics. 2014;60:270–278. doi: 10.1093/tropej/fmu010. [DOI] [PubMed] [Google Scholar]
- 50.Bimouhen A., Regragui Z., El Falaki F., Ihazmade H., Benkerroum S., Cherkaoui I., Rguig A., Ezzine H., Benamar T., Triki S., et al. Viral aetiology of influenza-like illnesses and severe acute respiratory illnesses in Morocco, September 2014 to December 2016. J. Glob. Health. 2022;12:04062. doi: 10.7189/jogh.12.04062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elhag W.I., Saeed H.A., Omer E.F.E., Ali A.S. Prevalence of rotavirus and adenovirus associated with diarrhea among displaced communities in Khartoum, Sudan. BMC Infect. Dis. 2013;13:1–6. doi: 10.1186/1471-2334-13-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adam M.A., Wang J., Enan K.-A., Shen H., Wang H., El Hussein A.R., Musa A.B., Khidir I.M., Ma X. Molecular survey of viral and bacterial causes of childhood diarrhea in Khartoum state, Sudan. Front. Microbiol. 2018;9:112. doi: 10.3389/fmicb.2018.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derrar F., Izri K., Kaddache C., Boukari R., Hannoun D. Virologic study of acute lower respiratory tract infections in children admitted to the paediatric department of Blida University Hospital, Algeria. New Microbes New Infect. 2019;30:100536. doi: 10.1016/j.nmni.2019.100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahouma A., Klena J.D., Krema Z., Abobker A.A., Treesh K., Franka E., Abusnena O., Shaheen H.I., El Mohammady H., Abudher A. Enteric pathogens associated with childhood diarrhea in Tripoli-Libya. Am. J. Trop. Med. Hyg. 2011;84:886. doi: 10.4269/ajtmh.2011.11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brini I., Guerrero A., Ezzine I.K., Orth-Höller D., Hetzer B., Würzner R., Hazgui O., Handous I., Nouri-Merchaoui S., Bouguila J. Human adenoviruses associated with respiratory illness in neonates, infants, and children in the Sousse area of Tunisia. J. Med. Virol. 2020;92:3081–3092. doi: 10.1002/jmv.26375. [DOI] [Google Scholar]
- 56.Svensson L., Wadell G., Uhnoo I., Johansson M., Von Bonsdorff C.-H. Cross-reactivity between enteric adenoviruses and adenovirus type 4: Analysis of epitopes by solid-phase immune electron microscopy. J. Gen. Virol. 1983;64:2517–2520. doi: 10.1099/0022-1317-64-11-2517. [DOI] [PubMed] [Google Scholar]
- 57.Grimwood K., Carzino R., Barnes G.L., Bishop R.F. Patients with enteric adenovirus gastroenteritis admitted to an Australian pediatric teaching hospital from 1981 to 1992. J. Clin. Microbiol. 1995;33:131–136. doi: 10.1128/jcm.33.1.131-136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saderi H., Roustai M., Sabahi F., Sadeghizadeh M., Owlia P., De Jong J. Incidence of enteric adenovirus gastroenteritis in Iranian children. J. Clin. Virol. 2002;24:1–5. doi: 10.1016/S1386-6532(01)00206-2. [DOI] [PubMed] [Google Scholar]
- 59.Subekti D., Lesmana M., Tjaniadi P., Safari N., Frazier E., Simanjuntak C., Komalarini S., Taslim J., Campbell J., Oyofo B. Incidence of Norwalk-like viruses, rotavirus and adenovirus infection in patients with acute gastroenteritis in Jakarta, Indonesia. FEMS Immunol. Med. Microbiol. 2002;33:27–33. doi: 10.1016/S0928-8244(01)00310-8. [DOI] [PubMed] [Google Scholar]
- 60.Koh H., Baek S.Y., Shin J.I., Chung K.S., Jee Y.M. Coinfection of viral agents in Korean children with acute watery diarrhea. J. Korean Med. Sci. 2008;23:937–940. doi: 10.3346/jkms.2008.23.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bányai K., Esona M., Liu A., Wang Y., Tu X., Jiang B. Molecular detection of novel adenoviruses in fecal specimens of captive monkeys with diarrhea in China. Vet. Microbiol. 2010;142:416–419. doi: 10.1016/j.vetmic.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Tekin A. Mardin\′deki akut gastroenteritli çocuklarda Rotavirüs ve Enterik Adenovirüs sıklığı. J. Clin. Exp. Investig. 2010;1:41–45. [Google Scholar]
- 63.Costa L.C.P.d.N., Siqueira J.A.M., Portal T.M., Sousa E.C., Linhares A.d.C., Gabbay Y.B., Resque H.R. Detection and genotyping of human adenovirus and sapovirus in children with acute gastroenteritis in Belém, Pará, between 1990 and 1992: First detection of GI. 7 and GV. 2 sapoviruses in Brazil. Rev. Da Soc. Bras. De Med. Trop. 2017;50:621–628. doi: 10.1590/0037-8682-0198-2017. [DOI] [PubMed] [Google Scholar]
- 64.CDC . Adenovirus VIS: Vaccine Information Statements (VISs) Centers for Disease Control and Prevention; Atlanta, GA, USA: 2020. [Google Scholar]
- 65.Kujawski S.A., Lu X., Schneider E., Blythe D., Boktor S., Farrehi J., Haupt T., McBride D., Stephens E., Sakthivel S.K. Outbreaks of adenovirus-associated respiratory illness on 5 college campuses in the United States, 2018–2019. Clin. Infect. Dis. 2021;72:1992–1999. doi: 10.1093/cid/ciaa465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roy S., Sandhu A., Medina A., Clawson D.S., Wilson J.M. Adenoviruses in fecal samples from asymptomatic rhesus macaques, United States. Emerg. Infect. Dis. 2012;18:1081. doi: 10.3201/eid1807.111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Banatvala J.E., Griffiths P., Schoub B., Mortimer P. Principles and Practice of Clinical Virology. Wiley; New York, NY, USA: 2009. [Google Scholar]
- 68.Singh-Naz N., Rodriguez W., Kidd A., Brandt C. Monoclonal antibody enzyme-linked immunosorbent assay for specific identification and typing of subgroup F adenoviruses. J. Clin. Microbiol. 1988;26:297–300. doi: 10.1128/jcm.26.2.297-300.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guerrant R.L., Hughes J.M., Lima N.L., Crane J. Diarrhea in developed and developing countries: Magnitude, special settings, and etiologies. Rev. Infect. Dis. 1990;12:S41–S50. doi: 10.1093/clinids/12.Supplement_1.S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lamps L.W. Infective disorders of the gastrointestinal tract. Histopathology. 2007;50:55–63. doi: 10.1111/j.1365-2559.2006.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eckardt A., Baumgart D. Viral gastroenteritis in adults. Recent Pat. Antiinfect. Drug Discov. 2011;6:54–63. doi: 10.2174/157489111794407877. [DOI] [PubMed] [Google Scholar]
- 72.Thewainy H.T., Hasony H. Enteric adenovirus associated with acute gastroenteritis among hospitalized and healthy childern under five-years of age in Basrah, Iraq. Med. J. Basrah Univ. 2019;37:37–44. [Google Scholar]
- 73.Wu B., Qi X., Xu K., Ji H., Zhu Y., Tang F., Zhou M. Genetic characteristics of the coxsackievirus A24 variant causing outbreaks of acute hemorrhagic conjunctivitis in Jiangsu, China, 2010. PLoS ONE. 2014;9:e86883. doi: 10.1371/journal.pone.0086883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.