Abstract
High levels of homocysteine (Hcy) have been linked with adverse cardiovascular outcomes, such as arrhythmias and stroke. In the context of paroxysmal atrial fibrillation (PAF), hyperhomocysteinemia has been demonstrated to be an independent predictor of future events. The aim of this report was to address the potential value of Hcy levels in predicting future paroxysms of atrial fibrillation (AF), as well as to identify the potential mechanisms of action. We searched PubMed and the Cochrane Database on 16 January 2022. Keywords used were homocysteine or hyperhomocysteinemia paired with a total of 67 different keywords or phrases that have been implicated with the pathogenesis of AF. We included primary reports of clinical and non-clinical data in the English language, as well as systematic reviews with or without meta-analyses. We placed no time constraints on our search strategy, which yielded 3748 results. Following title review, 3293 reports were excluded and 455 reports were used for title and abstract review, after which 109 reports were finally used for full-text review. Our review indicates that Hcy levels seem to hold a predictive value in PAF. Herein, potential mechanisms of action are presented and special considerations are made for clinically relevant diagnostic procedures that could complement plasma levels in the prediction of future PAF events. Finally, gaps of evidence are identified and considerations for future clinical trial design are presented.
Keywords: homocysteine, arrhythmia, atrial fibrillation, oxidative stress
1. Introduction
Atrial fibrillation (AF) represents the most common cardiac rhythm disorder, affecting millions of individuals worldwide, while its prevalence is expected to grow alarmingly within the next 50 years [1]. In the context of the substantial burden that AF poses on global health, on health economics, and on the affected individuals’ health-related quality of life, scientists have been focusing on decoding the disease’s exact pathophysiology; one cannot doubt the fact that AF is a result of multiple underlying factors, while it is usually precipitated by unknown triggers [2]. Research has shown that inflammation, in all its forms, is strongly linked with AF development; circulating inflammatory factors in the context of systemic inflammatory responses lead to atrial remodeling and fibrosis, a state that serves as a preamble for AF [3]. From this perspective, the identification of potential inflammation markers may be of paramount importance for the optimal diagnosis and management of the disease.
Homocysteine (Hcy) is a non-proteinogenic amino acid that is synthesized by methionine. Hcy serves as a precursor to many different amino acids in a series of biochemical reactions that are catalyzed by B vitamins. Folic acid (vitamin B9) seems to play an essential role in the conversion of Hcy to cysteine. In fact, folic acid supplementation has been demonstrated to effectively reduce plasma levels of Hcy [4]. Furthermore, an association between elevated Hcy and the methylene tetrahydrofolate reductase (MTHFR) 677C-allele polymorphism (rs1801133) has been demonstrated [5]. High levels of Hcy have been associated with endothelial cell injury and subsequent blood vessel inflammation. This has been linked to atherogenesis and ischemia [6]. However, a true causative effect that could prove a connection between these two entities, has not been yet established [7].
Paroxysmal atrial fibrillation (PAF), a very common form of atrial fibrillation (AF), is defined as an arrhythmic event that occurs spontaneously and is terminated within 7 days of onset [8]. According to epidemiological data, PAF occurs in approximately 25% of patients with a history of AF, while it has been linked with equally high morbidity and mortality as in permanent AF [9,10]. At the same time, PAF is a condition that has been correlated to high levels of Hcy. In fact, a positive correlation between levels of Hcy and future paroxysms of AF has been demonstrated in several clinical trials and reports [11,12,13,14,15,16,17,18,19,20,21,22] (Table 1). Furthermore, 2 systematic reviews with meta-analyses of these trials confirmed a positive correlation between elevated Hcy levels and recurrence of PAF [23,24] (Table 1).
Table 1.
Reports indicating a positive correlation between levels of Hcy and future paroxysms of AF.
| Reference | Number of Participants | Results |
|---|---|---|
| Kubota et al., 2019 [11] | 7133 patients from Atherosclerosis Risk in Communities (ARIC) Study and Multi-Ethnic Study of Atherosclerosis (MESA) |
|
| Marcucci et al., 2004 [12] | 310 NVAF patients on oral anticoagulant treatment (168 patients with previous ischemic events and 142 without) and 310 controls |
|
| Nasso et al., 2013 [13] | 104 patients after minimally invasive epicardial ablation |
|
| Naji et al., 2010 [14] | 83 patients with persistent AF after successful electrical cardioversion |
|
| Shi et al., 2016 [15] | 132 patients with both hypertension and AF (78 with paroxysmal AF and 84 with persistent AF) and 136 hypertensive patients |
|
| Shimano et al., 2008 [16] | 62 paroxysmal or persistent AF patients undergoing RFCA |
|
| Schnabel et al., 2010 [17] | 3120 Framingham cohort participants |
|
| Cingozbay et al., 2002 [18] | 38 patients with non-valvular AF divided into two groups: group A (patients with AF and stroke) and group B (AF without stroke) plus a reference group of 15 patients |
|
| Yao et al., 2017 [19] | 257 consecutive patients with persistent AF who underwent catheter ablation |
|
| Yao et al., 2017 [20] | Review |
|
| Giusti et al., 2007 [21] | 456 NVAF patients and 912 matched controls |
|
| Svenningsson et al., 2020 [22] | 3535 patients with no history of AF |
|
| META-ANALYSES | ||
| Rong et al., 2020 [23] | 11 studies with 3974 patients |
|
| Dong et al., 2021 [24] | 5 studies with 13,556 patients |
|
Abbreviations: Hcy: homocysteine, AF: atrial fibrillation, NVAF: non-valvular atrial fibrillation, LA: left atrial, RFCA: radiofrequency catheter ablation, CRP: C-reactive protein, BNP: brain natriuretic peptide, PAF: paroxysmal atrial fibrillation.
Based on the above findings, we ought to perform a scoping review of available clinical and non-clinical data with the aim to identify reports that could shed light on the potential mechanisms responsible for this specific effect. Scoping reviews are designed to address broader questions as compared to systematic reviews. They still rely on the established systematic review process, however, whereas a systematic review would attempt to answer a specific question (i.e., can Hcy levels be used to predict future paroxysms of AF?), scoping reviews usually tackle multiple questions, in an attempt to provide an exhaustive look at the available evidence. The goal of our effort was to identify any correlation between Hcy and PAF, identify underlying mechanisms, assess the interplay with other biomarkers, identify gaps of evidence, and ultimately provide insight that could assist in the initial clinical assessment and in the development of the optimal therapeutic strategies for each patient with PAF.
2. Methods
This scoping review was conducted in accordance with the Preferred-Reporting-Items-for-SystematicReviews-and-Meta-Analyses (PRISMA) guidelines (Supplementary Table S1). A search of PubMed and the Cochrane database was performed on 16 January 2022, based on a prespecified search protocol. We generated keywords based on the known mechanisms of AF pathogenesis. More specifically, the search terms used were homocysteine or hyperhomocysteinemia paired with one of the following: atrial, fibrillation, atrial fibrillation, fibrosis, cardiac fibrosis, myocardial fibrosis, atrial structural remodeling, left atrial appendage thrombus, cardiac inflammation, cardiovascular inflammation, vascular inflammation, mitral stenosis, mitral regurgitation, tricuspid regurgitation, cardiovascular oxidative stress, cardiac oxidative stress, vascular oxidative stress, myosin heavy chains, sarcoidosis, renin, angiotensin, aldosterone, RAAS, matrix metalloproteinases, MMP, disintegrin, sinus node, atrioventricular node, sick sinus syndrome, pulmonary veins, cardiac action potential, refractory period, wavelength, multiple wavelet, re-entrant leading cycle, electrical spiral waves, rotors, calcium, potassium, sodium, L-type calcium channels, calcium sensitivity, intracellular calcium, inward rectifier potassium ion channels, vagal, parasympathetic, sympathetic, epinephrine, norepinephrine, adrenaline, adrenergic, beta-2 receptors, sarcoplasmic reticulum, vortex shedding, gap junction proteins, GJA1, GJA5, connexin, thyroid, thyroid stimulating hormone, hyperthyroidism, hypothyroidism, troponin, BNP, NT-pro-BNP, electrocardiogram, ECG.
We included primary reports of clinical and non-clinical data in the English language, as well as systematic reviews with or without meta-analyses. Narrative reviews, expert opinions and other types of medical correspondence were excluded. We placed no time constraints on our search strategy. Two independent reviewers participated in the selection of included reports (PC and ET). If differences were reported in included reports, a resolution was achieved via discussion and refereeing by a third reviewer (CP). Data were extracted using a custom, previously tested form for non-clinical data, while a standard PICO form was used for clinical data.
3. Results
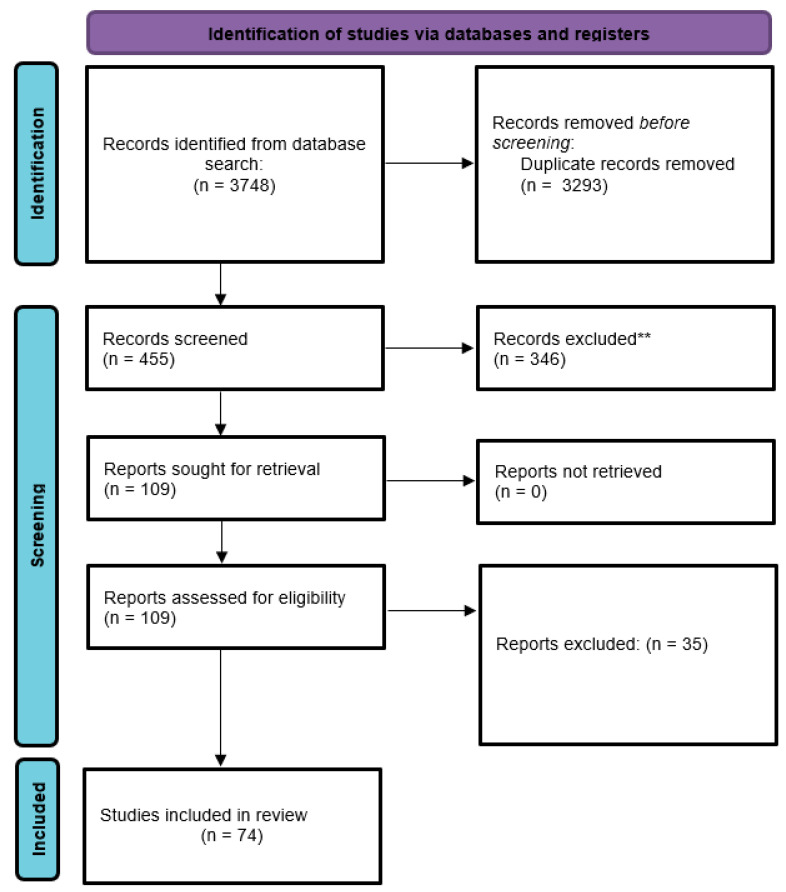
Our search strategy yielded 3748 results. Following title review, 3293 reports were excluded and 455 reports were used for title and abstract review, after which 109 reports were selected for full-text review. We finally included 74 reports in our scoping review (Figure 1).
Figure 1.
PRISMA flow diagram. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/. Accessed on 28 January 2022.
3.1. Non-Clinical Data
Our search strategy yielded 75 reports of in vitro and in animal models assessing the potential mechanisms of Hcy-induced direct myocardial toxicity. Table 2 provides a condensed overview of these reports. It should be noted that our search strategy did include neither the effects of Hcy on the vasculature nor data regarding its prothrombotic effects, both of which have been established in the literature and could contribute to the pathogenesis of cardiac disease.
Table 2.
Table presenting the outline of studies included in the scoping review.
| Reference | Model | Potential Mechanism |
|---|---|---|
| Bayrak et al., 2021 [25] | Copenhagen rats | Oxidative Stress |
| Borkowska et al., 2021 [26] | HUVEC and SH-SY5Y cells | Oxidative Stress |
| Cheng et al., 2021 [27] | C57BL/6 mouse aortae ex vivo | Oxidative Stress |
| Guo et al., 2021 [28] | 24 studies assessed | Oxidative Stress |
| Sharma et al., 2021 [29] | several proteins and enzymes. | Oxidative Stress |
| Boyacioglu et al., 2014 [30] | Wistar rats | Oxidative Stress |
| Aminzadeh et al., 2018 [31] | H9C2 myocardial cells | Oxidative Stress |
| Derouiche et al., 2014 [32] | Male Wistar rats (Pasteur Institute-Algiers) | Oxidative Stress |
| Aissa et al., 2017 [33] | Mice | Oxidative Stress |
| Dittoe et al., 2011 [34] | Rat neonatal cardiomyoblasts (H9c2 cells) | Oxidative Stress |
| Kolling et al., 2011 [35] | Hearts of young rats | Oxidative Stress |
| Devi et al., 2006 [36] | Spontaneously hypertensive rats | Oxidative Stress |
| Han et al., 2020 [37] | C57BL/6J mice | Oxidative Stress |
| Mendes et al., 2010 [38] | Male Wistar rats | Oxidative Stress |
| Singh et al., 2008 [39] | Rats | Oxidative Stress |
| Stojanovic et al., 2016 [40] | Isolated rat hearts | Oxidative Stress |
| Timkova et al., 2016 [41] | Rats | Oxidative Stress |
| Yalçinkaya-Demirsözm et al., 2009 [42] | Rabbits | Oxidative Stress |
| Chang et al., 2004 [43] | Rat myocardial mitochondria | Oxidative Stress and Taurine |
| Chang et al., 2004 [44] | Rat isolated myocardial mitochondria | Oxidative Stress and Taurine |
| Chang et al., 2008 [45] | Rats | Oxidative Stress and H2S |
| Wang et al., 2015 [46] | Rats | Oxidative Stress and H2S |
| Givvimani et al., 2011 [47] | Mouse cardiac endothelial cells | Oxidative Stress and Fibrosis |
| Joseph et al., 2008 [48] | Rat model | Oxidative Stress and Fibrosis |
| Li et al., 2017 [49] | Six-week-old C57BL6/J mice | Oxidative Stress and Fibrosis |
| Tyagi et al., 2005 [50] | Mice | Oxidative Stress and Fibrosis |
| Shi et al., 2021 [51] | 2–3 days old Wistar rats | Fibrosis |
| Zhao et al., 2021 [52] | C57BL/6 mice with a high L-methionine (L-MET) diet for 12 weeks | Fibrosis |
| Carroll et al., 2005 [53] | Rabbit model | Fibrosis |
| Zulli et al., 2006 [54] | Rabbits | Fibrosis |
| Han et al., 2020 [55] | Left atrial appendage from patients with either sinus rhythm (SR) or AF | Fibrosis |
| Zhi et al., 2013 [56] | Mice | Fibrosis |
| Zhang et al., 2016 [57] | Apolipoprotein E-deficient (ApoE −/−) mice and neonatal rat cardiac fibroblasts (CFs) | Fibrosis |
| Muthuramu et al., 2015 [58] | Female C57BL/6 low-density lipoprotein receptor (Ldlr (−/−)) cystathionine-β-synthase (Cbs (+/−)) mice | Fibrosis |
| Wang et al., 2016 [59] | Cardiocytes H9C2 | Fibrosis |
| Chaouad et al., 2019 [60] | Sand rat Psammomys obesus | Fibrosis and Remodeling |
| Joseph et al., 2005 [61] | Mast cell-deficient rat model | Fibrosis, Remodeling and Diastolic Dysfunction |
| Joseph et al., 2004 [62] | Hypertensive rats | Fibrosis, Remodeling and Diastolic Dysfunction |
| Cao et al., 2021 [63] | Hypertensive rats | Fibrosis and Diastolic Dysfunction |
| Li et al., 2021 [64] | Mouse CFs | Fibrosis and Diastolic Dysfunction |
| Cao et al., 2021 [63] | Wistar Kyoto (WKY) and spontaneous hypertension rats (SHR) | Remodeling |
| Chaturvedi et al., 2014 [65] | HL-1 cardiomyocytes and mouse models (CBS+/−) | Remodeling |
| Herrmann et al., 2007 [66] | Rats | Remodeling |
| Jeremic et al., 2018 [67] | Adult male Wistar albino rats | Remodeling |
| Kar et al., 2019 [68] | Male CBS(+/−) and sibling CBS(+/+) (WT) mice | Remodeling |
| Raaf et al., 2011 [69] | Rats | Remodeling |
| Mishra et al., 2009 [70] | HL-1 cardiomyocytes | Remodeling |
| Rosenberger et al., 2011 [71] | male C57/BL6J mice | Remodeling |
| Li et al., 2021 [64] | Male C57BL/6J mice | Endothelial dysfunction |
| Ables et al., 2015 [72] | Μice | ECG |
| Cainzos-Achirica et al., 2021 [73] | 1407 participants (61% women) without diabetes or severe hypercholesterolemia | Calcium |
| Cheng et al., 2021 [27] | Human umbilical vein endothelial cells (HUVECs)-derived EA.hy926 immortalized cells | Calcium |
| Cai et al., 2011 [74] | Wistar rat hearts | Calcium |
| Shontz et al., 2001 [75] | Whole-cell voltage-clamp recordings were made in ventricular myocytes isolated from normal rat hearts | Ca2+-independent, transient outward Potassium (K+) current (I(to)) |
| Sun et al., 2021 [76] | Residual internal mammary artery (IMA) segments obtained from patients undergoing CABG | Potassium Calcium (K(Ca)) |
| Cai et al., 2007 [77] | Human atrial cells | Potassium |
| Lopatina et al., 2015 [78] | Chicken embryo cardiac tissue explants | Potassium, Sodium |
| Cai et al., 2009 [79] | Human atrial monocytes. | Sodium |
| Pacher et al., 1999 [80] | Isolated rat hearts | Sodium |
| Soni et al., 2016 [81] | Wild-type mice (WT) | Magnesium |
| Han et al., 2020 [82] | Mouse atrial myocytes (MACs) obtained from C57B6 mice. | Electrical Remodeling |
| Mishra et al., 2011 [83] | cardiomyocytes obtained from C57BL/6J (WT) and db/db mice. | β2-AR |
| Mishra et al., 2010 [84] | 12 week male diabetic Ins2+/− Akita and C57BL/6J mice | β2-AR |
| Tasatargil et al., 2006 [85] | Adult male Wistar rats | β2-AR |
| Moshal et al., 2009 [86] | Cardiomyocyte-specific knockout of NMDA-R1 | NMDA-R1 |
| Moshal et al., 2008 [87] | C57BL/6J mice | NMDA-R1 |
| Tyagi et al., 2010 [88] | Cardiac-specific knockout (KO) of NMDA-R1 | NMDA-R1 |
| Srejovic et al., 2017 [89] | Hearts of Wistar albino rats | NMDA-R1 |
| Busingye et al., 2021 [90] | 600 human population | Inflammation |
| Ji et al., 2020 [91] | human umbilical vein endothelial cells (HUVECs) | Inflammation |
| Xie et al., 2021 [92] | mice | Inflammation |
3.2. Oxidative Stress, Cardiac Fibrosis, and Remodeling
As the development of AF seems to be a result of the combination of a plethora of risk factors and comorbidities, oxidative stress has been associated with incident AF, while oxidative stress-induced atrial remodeling is considered the most common underlying mechanism; according to several reports, hyperhomocysteinemia has been linked with endothelial dysfunction, thus it represents an important factor for cardiovascular morbidity and mortality [93,94]. We were able to locate a total of 26 reports assessing oxidative stress as a potential mechanism of Hcy-induced cardiotoxicity [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,48,49,50,95]. Of these, 25 trials reported a positive correlation between Hcy and cardiomyocyte oxidative stress, while only one report failed to demonstrate oxidative stress as a cause of electron transport chain dysfunction, possibly due to an increased expression of the other protective mitochondrial proteins [41]. In these experiments, several different molecules were tested for their potential to inhibit the Hcy-induced oxidative stress, such as taurine and hydrogen sulfide. Taurine is an organic osmolyte involved in cell volume regulation, located in all organs throughout the body, which provides a substrate for the formation of bile salts; taurine seems to play a fundamental role in the modulation of intracellular free calcium concentration [96]. In parallel, hydrogen sulfide (H2S), the third discovered endogenous gas transmitter in mammals after NO and CO, participates in various pathophysiological processes; previous in vitro and in vivo research have revealed the protective role of H2S in the cardiovascular system that renders it useful in the protection of the myocardium against ischemia-reperfusion injury [97]. Interestingly, taurine [43,44] and hydrogen sulfide [45,46] were shown to be effective in counteracting a significant inhibiting effect against Hcy oxidative action. However, the clinical significance of that remains questionable, since none of the aforementioned agents used to hold a place in everyday clinical practice.
Furthermore, we were able to locate 16 trials reporting cardiac fibrosis as a result of Hcy-induced oxidative stress [41,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. Several mechanisms of action have been proposed for this effect, including increased reactive oxygen species, nitrotyrosine, matrix metalloproteinase, and decreased endothelial nitric oxide in response to antagonizing PPAR-gamma [82] and regulation of the Akt/FoxO3 pathway. More specifically, FoxO3 (forkhead box O3) is a member of the forkhead box transcription factors of the O class with a conserved helix-loop-helix DNA-binding domain; it is involved in a variety of vital cellular processes including oxidative stress, DNA repair, apoptosis, metabolism and cell cycle arrest [98,99]. Although the existing data are controversial, FoxO3 seem to play an important role in maintaining cardiovascular homeostasis.
Finally, 11 trials were reported on cardiac remodeling because of hyperhomocysteinemia [60,61,62,63,65,66,67,68,69,70,71]. Again, several mechanisms of action are postulated, including increased expression of transforming growth factor-beta1 (TGFβ1), a significant increase in the ratio of collagenous to non-collagenous protein due to reactive interstitial fibrosis, and increased myocardial oxidative stress, increased expression of matrix metalloproteinase-2, matrix metalloproteinase-9 and decreased expression of connexin 40, 43 and 45, and suppression of the Nrf2/HO-1 pathway and Nrf2 nuclear transport. In fact, the activation of mitochondrial matrix metalloproteinase-9 (MMP9) can lead to cardiomyocyte dysfunction, in part, by inducing mitochondrial permeability (MPT). This has been demonstrated to be mediatethe d by binding of homocysteine to the N-methyl-d-aspartate receptor 1 (NMDA-R1) [86,87,88,89].
3.3. Electrical Remodeling
Electrical remodeling, a variety of changes in the electrical substrate of the heart capable of triggering AF onset, belongs among the most common risk factors for AF development [100]. We were able to locate 11 trials assessing the effect of Hcy on the electrical conductivity of the heart and an additional 4 trials reporting on alterations of beta 2 adrenergic receptors. This was demonstrated as a significant prolongation of QRS, QTc, and PR intervals on the electrocardiogram (ECG) [71,72,81]. The above point toward a potential effect of Hcy in practically all distinct phases of the heart’s systolic/diastolic cycle, demonstrating a clear arrhythmogenic potential associated with high levels of Hcy. Furthermore, Hcy has been reported to down-regulate the production of beta 2 adrenergic receptors [83,84], as well as to increase responsiveness to beta-adrenergic agonists [85]. Finally, alterations involving atrial calcium [74], sodium [77,78,80] and potassium [75,78,79,101] channels have also been demonstrated in hyperhomocysteinemia.
4. Clinical Data
The available clinical data are highly heterogeneous. Furthermore, a positive correlation between Hcy levels and PAF has already been demonstrated in recent meta-ana-lyses. We, therefore, decided not to proceed with a meta-analysis. Available clinical evidence is presented narratively and in tabular format (Table 3).
Table 3.
Studies reporting available clinical data regarding the connection between Hcy and AF.
| Reference | Number of Participants | Results |
|---|---|---|
| Schnabel et al., 2005 [102] | 643 patients with coronary artery disease |
|
| Ay et al., 2003 [103] | 42 consecutive patients with ischemic stroke caused by nonvalvular AF |
|
| Loffredo et al., 2005 [104] | 163 consecutive patients with permanent (n = 118) or paroxysmal (n = 45) AF of non-valvular origin hospitalized for cardiac reasons |
|
| Sundström et al., 2004 [105] | 2697 Framingham Heart Study participants free of heart failure and previous myocardial infarction | Plasma Hcy was positively related to LV mass, wall thickness, and relative wall thickness in women, but not in men. |
| Alter et al., 2010 [106] | 66 individuals with suspected cardiomyopathy |
|
| Li et al., 2017 [107] | 7002 healthy individuals |
|
| Leng et al., 2015 [108] | 178 healthy individuals |
|
| Guéant-Rodriguez et al., 2007 [109] | 515 patients with coronary artery disease and 194 patients without evidence of coronary artery lesion |
|
| Guéant-Rodriguez et al., 2013 [110] | 1020 subjects including patients undergoing coronarography and ambulatory elderly subjects |
|
| Görmüş et al., 2010 [111] | 31 patients with type 2 diabetes mellitus |
|
| Cho et al., 2006 [112] | 227 patients with cardiovascular disease |
|
| Ye et al., 2014 [113] | 1497 healthy individuals |
|
| Alam et al., 2012 [114] | 194 consecutive patients with acute myocardial infarction |
|
| Wang et al., 2022 [115] | 1224 consecutive patients with cardiac implantable electronic devices |
|
| Wocial et al., 2002 [116] | 37 patients with mild essential hypertension (EH) and 37 healthy volunteers | Hcy was significantly higher in patients with EH (8.7 +/− 2.4 vs. 6.6 +/− 1.3 μmol/L; p < 0.01). |
| Poduri et al., 2008 [117] | 273 patients with essential hypertension (EH) and 103 normotensive controls |
|
| Atar et al., 2005 [118] | 120 patients with newly diagnosed hypertension |
|
Abbreviations: Hcy: homocysteine, AF: atrial fibrillation, LA: left atrial, LV: left ventricular, ECG: electrocardiogram, LVEF: left ventricular ejection fraction, NYHA: New York Heart Association, BMI: body mass index, BNP: brain natriuretic peptide, CRP: C-reactive protein, hs-cTnT: high sensitivity cardiac troponin T, SD: standard deviation, UA: uric acid, ACE: angiotensin-converting enzyme, EH: essential hypertension.
4.1. Oxidative Stress, Fibrosis, Thrombosis, and Remodeling
In a prospective cohort of 643 patients, the relationship between plasma Hcy and glutathione peroxidase (GPx)-1 levels was addressed. GPx-1 has been demonstrated to modulate cardiovascular risk related to Hcy via its antioxidant properties. After a median follow-up of 7.1 years, the authors reported an inverse relationship between Hcy and GPx-1 levels and the occurrence of cardiovascular events. In fact, patients with low GPx-1 and high Hcy levels were 3.2 times more likely to experience a cardiovascular event. The authors went on to recommend that GPx-1 levels are taken into consideration when Hcy levels are used to predict future cardiovascular events [102].
Overall, high levels of Hcy have been implicated in the induction of oxidative stress in the vasculature. While our search was not designed to locate such trials, the capacity of Hcy to induce oxidative stress with subsequent clot detachment which ultimately leads to ischemic stroke is well described in the literature [103]. In fact, patients with elevated Hcy levels have been demonstrated to have a 2.73-fold increased probability of ischemic stroke [104].
In a sample of 2697 patients from the Framingham Heart Study, left ventricular (LV) mass, wall thickness, and relative wall thickness were correlated to high Hcy levels in women but not in men. However, plasma Hcy was not related to left atrial size or LV fractional shortening in either sex [105].
In a study of 66 patients, elevated Hcy levels were linked with a disproportional LV dilatation, where the ensuing hypertrophy was not sufficient to compensate for the increased wall stress. The authors proposed that a potential mechanism is the hyperhomocysteinemia-associated increased oxidative stress which leads to the degradation of collagen, with consecutive fiber slippage and cardiac dilatation [106].
4.2. Electrical Remodeling
In a population-based study of 7002 participants, 12-lead ECGs were performed and correlated to plasma Hcy levels. The authors demonstrated an association between the prolonged QTc interval and high Hcy levels [107]. Furthermore, in a retrospective database study, 178 patients were stratified according to QRS interval duration and plasma Hcy levels. The authors reported a significant correlation between higher levels of Hcy and longer QRS intervals. Other ECG parameters, such as PQ interval, QTc interval, and QT dispersion were not found to be statistically correlated to Hcy levels [108].
4.3. Relation to Left Ventricular Ejection Fraction and Other Biomarkers
In a prospective case-control study of 515 coronary artery disease patients and 194 controls, higher Hcy levels were correlated with reduced left ventricular ejection fraction (LVEF) (<40%). Furthermore, elevated Hcy levels were associated with increases in N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) levels [109]. In another study of 1020 patients, high levels of Hcy were demonstrated to predict increased NT-pro-BNP (or BNP) levels [110]. NT-proBNP and Hcy levels were measured in 31 patients with type 2 diabetes mellitus. The authors reported a correlation between elevated NT-proBNP and Hcy levels in patients with LV diastolic dysfunction [111]. A trial of 227 patients demonstrated that the MTHFR C677T mutation which is associated with plasma Hcy levels, is also linked with plasma BNP levels, leading to the conclusion that plasma Hcy levels are positively correlated with plasma BNP levels [112].
In a community-based trial of 1497 patients, serum Hcy levels were associated with a higher likelihood of detectable high sensitivity troponin T (hs-cTnT). The effect was stronger among the elderly patients in the cohort (>65 years old), while no association was recorded in patients <65 years old. The authors concluded that the levels of serum Hcy are associated with hs-cTnT levels in the elderly, which could indicate a possible relationship between Hcy and subclinical myocardial damage [113]. In another trial of 194 consecutive patients with acute myocardial infarction, elevated serum Hcy levels were positively correlated with serum cardiac troponin-I [114].
In a prospective cohort of 1224 consecutive patients with subclinical AF (SCAF), Hcy and uric acid (UA) were significantly elevated. The authors reported that an increase of 1 standard deviation (SD) in Hcy (5.7 μmol/L) levels was associated with an increased risk of SCAF in men and women regardless of their UA levels. Similarly, a 1-SD increase in UA (91 μmol/L) was associated with an increased risk of SCAF among the patients with high levels of Hcy in men (hazard ratio, 1.81; 95% CI, 1.43–2.30) and women (hazard ratio, 2.11; 95% CI, 1.69–2.62). This led the authors to conclude that Hcy and UA are strongly associated with SCAF [115].
4.4. Adrenergic Effect
In a trial of 37 patients with essential hypertension (EH) compared with 37 healthy controls, blood levels of noradrenaline and adrenaline were demonstrated to be significantly higher in the EH group. Furthermore, the left ventricular mass index (LVMI) was also significantly higher in the EH group. The authors concluded that high levels of Hcy are associated with increased adrenergic activity in EH patients [116]. In a case control study of 273 patients with EH and 103 normotensive controls, the use of angiotensin-converting enzyme (ACE) inhibitors and beta-blockers significantly decreased and hydrochlorothiazide significantly increased plasma Hcy levels. The authors speculated that this reduction in Hcy levels was due to the improvement of endothelial function along with improved renal function [117]. In another trial of 120 patients with newly diagnosed hypertension, 100 mg of metoprolol per day was demonstrated to significantly reduce plasma Hcy levels. Additionally, there was no relation between homocysteine level and blood pressure control [118].
5. Discussion
AF represents the most common arrhythmia worldwide and is associated with significant morbidity and mortality [8]. The prevalence of AF in adults is about 2–4% and a 2,3-fold rise is expected in the years to come as a result of extended longevity in the general population and intensifying search for undiagnosed and untreated AF. A complex interplay of triggers and risk factors has been described widely in the literature, shedding light on the pathophysiologic substrate of the disease.
Arterial hypertension, diabetes mellitus, chronic kidney disease, heart failure, valvular heart disease, coronary artery disease, inflammatory diseases, obstructive sleep apnea, chronic obstructive pulmonary disease, obesity, alcohol consumption, and smoking are only some of the risk factors implicated in the pathophysiology of AF. Thus it is evident that due to the complexity of the pathophysiologic substrate of the disease and the wide array of AF triggers, one individual biomarker is difficult to show adequate sensitivity and specificity in the prognostication of AF recurrences.
But why do we need a reliable biomarker for AF prognosis? First, AF diagnosis may be challenging due to asymptomatic and paroxysmal presentation; second, biomarkers could refine screening procedures, and third, they could help predict the risk of recurrent AF. Which is the profile of an ideal biomarker? It would be easily accessible, cost-effective, and demonstrative of consistent accuracy and reproducibility. In the present manuscript, we provide sufficient evidence connecting high levels of Hcy with AF paroxysms, through clinical research data and 2 convincing meta-analyses. Additionally, Hcy is an easily accessible biomarker with good reproducibility. In a recent paper by Chua et al. [119], a series of 40 different plasma biomarkers were validated by using logistic regression models and machine learning technologies; in this specific study, Hcy was not validated, while BNP and FGF-23 proved to hold the strongest association with AF. Interestingly, in the present scoping review we demonstrate evidence supportive of the strong connection between Hcy and BNP or NT-pro-BNP levels, which reinforces our belief that Hcy may serve in the prognostication of AF.
Inflammation and evolving fibrosis are thought to be the main pathophysiologic mechanisms responsible for the development of AF. A wide array of biomarkers have been associated with this process and have been already validated in the literature, as previously described. Which one is the best to provide the most accurate prognostic information, is still unclear. In the present manuscript, we tried to explore in depth what Hcy levels have to offer in this direction. Serious clinical data and meta-analyses, together with evidence connecting Hcy with other inflammation markers and oxidative stress, may confer adequate information. Additionally, it is well established that standard predictors of AF, such as left atrial volume, left atrial mechanical function, and left ventricular ejection fraction as evaluated by echocardiography, are strongly associated with fibrosis. Furthermore, left atrial fibrosis demonstrated by cardiac magnetic resonance imaging (CMR) has been strongly associated with AF recurrence. Since Hcy stands as a marker of cardiac inflammation, it might reflect the aforementioned left heart geometrical changes. Indeed, we present data that connect Hcy levels with LV mass hypertrophy and LV dilation.
In our scoping review, we present data that implicate a direct adrenergic effect of Hcy in special populations, such as patients with arterial hypertension. This effect may cause left ventricular hypertrophy and promote left ventricular and left atrial remodeling, all of which are associated with AF development. By inhibiting sympathetic nerve drive by a b-blocker, one can speculate that this agent may inhibit the remodeling processes. B-blockers have been widely used for AF prevention and in the present manuscript, we provide evidence of Hcy levels reduction by metoprolol. Thus, we can propose that the beneficial effect of metoprolol in AF populations might be due to a bidirectional effect of b-blockade and Hcy levels reduction.
What about electrical remodeling and Hcy levels? ECG is the simplified mirror of the electrical circuits of the heart. Whether there is any convincing evidence that Hcy is associated with ECG changes that may serve as prognostic indicators of AF recurrences is still to be proven; there are reports pointing out that even in sinus rhythm, ECG might be a reliable biomarker of future AF events, since advanced interatrial block expressed by a p-wave duration > 120 ms and biphasic p-wave pattern in inferior leads, have been correlated with left atrial dilation [120,121]. Unfortunately, we were unable to find literature connecting Hcy levels with evidence of interatrial block or left atrial dilation. On the other hand, there is enough evidence of some association of Hcy levels with QRS duration with an undetermined effect on AF prevalence or recurrence.
Machine learning applications in clinical cardiology have rapidly evolved in recent years. By using machine learning tools together with vast data sources, the management of a variety of chronic cardiac diseases including AF, is expected to change in the near future. The role of Hcy levels in this background remains unclear; large-scale trials are needed in order to establish which plasma AF biomarker is the most accurate to be incorporated in machine learning applications [122].
6. Future Research
Our systematic search of the literature shed light on a wide array of existing data concerning the potential role of Hcy in the modification of risk factors for AF development. More specifically, our search demonstrated a direct effect of high Hcy levels on the adrenergic system in various laboratory models, while we highlighted the rather limited clinical evidence which presented a potential Hcy-lowering effect of beta-blockers. This potential for a dual benefit warrants further investigation in the clinical setting.
Furthermore, we were able to identify several different biomarkers that could potentially be part of a predictive algorithm. Glutathione peroxidase is an endogenous antioxidant that may be inversely correlated to Hcy levels, while NT-pro-BNP and troponin levels also seem to hold a significant role. As NT-pro-BNP levels are mostly used in the diagnosis and monitoring of heart failure, this prohormone has been used in other disease states, such as myocardial ischemia [123]. Finally, UA has also been implicated as a predictor of future PAF events. While there does not seem to be one single biomarker that has been proven to consistently and accurately predict PAF, it seems very likely that a combination of these biomarkers may provide a valuable algorithm in that direction. Future research should be focused on combining the predictive value of these biomarkers to generate a reliable stratification algorithm.
7. Conclusions
In the context of PAF, there is sufficient evidence to support the use of Hcy levels as an independent predictor of future events. However, the pathogenesis of cardiac arrhythmias involves complex biological mechanisms that cannot be explained by or attributed to a single biomarker. Clinicians should take several other factors into consideration when assessing the likelihood of AF. In the near future, artificial intelligence with machine learning modalities could allow better prognostication and improved therapeutic management of the disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics12092192/s1, Table S1: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist. Reference [124] is cited in Supplementary Materials.
Author Contributions
P.C. and C.E.P. conceptualized the central idea of the scoping review. E.T. and A.B. performed the literature search and, along with P.R. and K.T., they interpreted the data and drafted the manuscript. E.T., A.B. and V.P. substantially revised the manuscript. T.K., C.E.P. and V.V. supervised the project and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interests.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lippi G., Sanchis-Gomar F., Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke. 2021;16:217–221. doi: 10.1177/1747493019897870. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki Y.-K., Nishida K., Kato T., Nattel S. Atrial fibrillation pathophysiology. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 3.Korantzopoulos P., Letsas K.P., Tse G., Fragakis N., Goudis C.A., Liu T. Inflammation and atrial fibrillation: A comprehensive review. J. Arrhythmia. 2018;34:394–401. doi: 10.1002/joa3.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neal B., MacMahon S., Ohkubo T., Tonkin A., Wilcken D. Pacific study group dose-dependent effects of folic acid on plasma homocysteine in a randomized trial conducted among 723 individuals with coronary heart disease. Eur. Heart J. 2002;23:1509–1515. doi: 10.1053/euhj.2002.3161. [DOI] [PubMed] [Google Scholar]
- 5.Mehlig K., Leander K., De Faire U., Nyberg F., Berg C., Rosengren A., Björck L., Zetterberg H., Blennow K., Tognon G., et al. The association between plasma homocysteine and coronary heart disease is modified by the MTHFR 677C>T polymorphism. Heart. 2013;99:1761–1765. doi: 10.1136/heartjnl-2013-304460. [DOI] [PubMed] [Google Scholar]
- 6.Cattaneo M. Hyperhomocysteinemia, atherosclerosis and thrombosis. Thromb. Haemost. 1999;81:165–176. doi: 10.1055/s-0037-1614438. [DOI] [PubMed] [Google Scholar]
- 7.Martí-Carvajal A.J., Solà I., Lathyris D., Salanti G. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2009:CD006612. doi: 10.1002/14651858.CD006612.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.A., Dilaveris P.E., et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): The task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 9.Zoni-Berisso M., Lercari F., Carazza T., Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014;6:213–220. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friberg L., Hammar N., Pettersson H., Rosenqvist M. Increased mortality in paroxysmal atrial fibrillation: Report from the Stockholm Cohort-Study of Atrial Fibrillation (SCAF) Eur. Heart J. 2007;28:2346–2353. doi: 10.1093/eurheartj/ehm308. [DOI] [PubMed] [Google Scholar]
- 11.Kubota Y., Alonso A., Heckbert S.R., Norby F.L., Folsom A.R. Homocysteine and incident atrial fibrillation: The atherosclerosis risk in communities study and the multi-ethnic study of atherosclerosis. Heart Lung Circ. 2019;28:615–622. doi: 10.1016/j.hlc.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcucci R., Betti I., Cecchi E., Poli D., Giusti B., Fedi S., Lapini I., Abbate R., Gensini G.F., Prisco D. Hyperhomocysteinemia and vitamin B6 deficiency: New risk markers for nonvalvular atrial fibrillation? Am. Heart J. 2004;148:456–461. doi: 10.1016/j.ahj.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Nasso G., Bonifazi R., Romano V., Brigiani M.S., Fiore F., Bartolomucci F., Lamarra M., Fattouch K., Rosano G., Gaudino M., et al. Increased plasma homocysteine predicts arrhythmia recurrence after minimally invasive epicardial ablation for nonvalvular atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2013;146:848–853. doi: 10.1016/j.jtcvs.2012.07.099. [DOI] [PubMed] [Google Scholar]
- 14.Naji F., Suran D., Kanic V., Vokac D., Sabovic M. High homocysteine levels predict the recurrence of atrial fibrillation after successful electrical cardioversion. Int. Heart J. 2010;51:30–33. doi: 10.1536/ihj.51.30. [DOI] [PubMed] [Google Scholar]
- 15.Shi D., Meng Q., Zhou X., Li L., Liu K., He S., Wang S., Chen X. Factors influencing the relationship between atrial fibrillation and artery stiffness in elderly Chinese patients with hypertension. Aging Clin. Exp. Res. 2016;28:653–658. doi: 10.1007/s40520-015-0455-8. [DOI] [PubMed] [Google Scholar]
- 16.Shimano M., Inden Y., Tsuji Y., Kamiya H., Uchikawa T., Shibata R., Murohara T. Circulating homocysteine levels in patients with radiofrequency catheter ablation for atrial fibrillation. Europace. 2008;10:961–966. doi: 10.1093/europace/eun140. [DOI] [PubMed] [Google Scholar]
- 17.Schnabel R.B., Larson M.G., Yamamoto J.F., Sullivan L.M., Pencina M.J., Meigs J.B., Tofler G.H., Selhub J., Jacques P.F., Wolf P.A., et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. doi: 10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cingozbay B.Y., Yiginer O., Cebeci B.S., Kardesoglu E., Demiralp E., Dincturk M. Role of homocysteine for thromboembolic complication in patients with non-valvular atrial fibrilation. Blood Coagul. Fibrinolysis. 2002;13:609–613. doi: 10.1097/00001721-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Yao Y., Yao W., Bai R., Lu Z.-H., Tang R.-B., Long D.-Y., Jiang C.-X., Sang C.-H., Zhang J.-Q., Yu R.-H., et al. Plasma homocysteine levels predict early recurrence after catheter ablation of persistent atrial fibrillation. Europace. 2017;19:66–71. doi: 10.1093/europace/euw081. [DOI] [PubMed] [Google Scholar]
- 20.Yao Y., Shang M.-S., Dong J.-Z., Ma C.-S. Homocysteine in non-valvular atrial fibrillation: Role and clinical implications. Clin. Chim. Acta. 2017;475:85–90. doi: 10.1016/j.cca.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Giusti B., Gori A.M., Marcucci R., Sestini I., Saracini C., Sticchi E., Gensini F., Fatini C., Abbate R., Gensini G.F. Role of C677T and A1298C MTHFR, A2756G MTR and -786 C/T eNOS gene polymorphisms in atrial fibrillation susceptibility. PLoS ONE. 2007;2:e495. doi: 10.1371/journal.pone.0000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svenningsson M.M., Svingen G.F., Lysne V., Ueland P.M., Tell G.S., Pedersen E.R., Dhar I., Nilsen D.W., Nygård O. Transsulfuration metabolites and the association with incident atrial fibrillation—An observational cohort study among norwegian patients with stable angina pectoris. Int. J. Cardiol. 2020;317:75–80. doi: 10.1016/j.ijcard.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Rong H., Huang L., Jin N., Hong J., Hu J., Wang S., Xie Y., Pu J. Elevated homocysteine levels associated with atrial fibrillation and recurrent atrial fibrillation. Int. Heart J. 2020;61:705–712. doi: 10.1536/ihj.20-099. [DOI] [PubMed] [Google Scholar]
- 24.Dong X., Wang B., Hou F., Chen K., Zhou H., Guo J., Sun X., Liu X., Chen L. Homocysteine (HCY) levels in patients with atrial fibrillation (AF): A meta-analysis. Int. J. Clin. Pract. 2021;75:e14738. doi: 10.1111/ijcp.14738. [DOI] [PubMed] [Google Scholar]
- 25.Bayrak B., Koroglu P., Bulan O.K., Yanardag R. Metformin protects against diabetes-induced heart injury and dunning prostate cancer model. Hum. Exp. Toxicol. 2021;40:297–309. doi: 10.1177/0960327120947452. [DOI] [PubMed] [Google Scholar]
- 26.Borkowska A., Ziolkowski W., Kaczor K., Herman-Antosiewicz A., Knap N., Wronska A., Antosiewicz J. Homocysteine-induced decrease in HUVEC cells’ resistance to oxidative stress is mediated by Akt-dependent changes in iron metabolism. Eur. J. Nutr. 2020;60:1619–1631. doi: 10.1007/s00394-020-02360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng C.K., Luo J.-Y., Lau C.W., Cho W.C.-S., Ng C.F., Ma R.C.W., Tian X.Y., Huang Y. A GLP-1 analog lowers ER stress and enhances protein folding to ameliorate homocysteine-induced endothelial dysfunction. Acta. Pharmacol. Sin. 2021;42:1598–1609. doi: 10.1038/s41401-020-00589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo J., Qin Z., He Q., Fong T.L., Lau N.C., Cho W.C.S., Zhang H., Meng P., Xing X., Li M., et al. Shexiang baoxin pill for acute myocardial infarction: Clinical evidence and molecular mechanism of antioxidative stress. Oxid. Med. Cell. Longev. 2021;2021:7644648. doi: 10.1155/2021/7644648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma G.S., Bhattacharya R., Singh L.R. Functional inhibition of redox regulated heme proteins: A novel mechanism towards oxidative stress induced by homocysteine. Redox Biol. 2021;46:102080. doi: 10.1016/j.redox.2021.102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyacioglu M., Sekkin S., Kum C., Korkmaz D., Kiral F., Yalinkilinc H.S., Ak M.O., Akar F. The protective effects of vitamin C on the DNA damage, antioxidant defenses and aorta histopathology in chronic hyperhomocysteinemia induced rats. Exp. Toxicol. Pathol. 2014;66:407–413. doi: 10.1016/j.etp.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Aminzadeh A., Mehrzadi S. Cardioprotective effect of levosimendan against homocysteine-induced mitochondrial stress and apoptotic cell death in H9C2. Biochem. Biophys. Res. Commun. 2018;507:395–399. doi: 10.1016/j.bbrc.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 32.Derouiche F., Bôle-Feysot C., Naïmi D., Coëffier M. Hyperhomocysteinemia-induced oxidative stress differentially alters proteasome composition and activities in heart and aorta. Biochem. Biophys. Res. Commun. 2014;452:740–745. doi: 10.1016/j.bbrc.2014.08.141. [DOI] [PubMed] [Google Scholar]
- 33.Aissa A.F., Amaral C.L.D., Venancio V.P., Machado C.D.S., Hernandes L.C., Santos P., Curi R., Bianchi M.L.P., Antunes L.M.G. Methionine-supplemented diet affects the expression of cardiovascular disease-related genes and increases inflammatory cytokines in mice heart and liver. J. Toxicol. Environ. Health A. 2017;80:1116–1128. doi: 10.1080/15287394.2017.1357366. [DOI] [PubMed] [Google Scholar]
- 34.Dittoe N.J., Hahn H.S., Sansone R.A., Wiederman M.W. Prevalence and self-reported medical history of overweight in a cardiac stress testing population. South Med. J. 2011;104:505–508. doi: 10.1097/SMJ.0b013e31821e9359. [DOI] [PubMed] [Google Scholar]
- 35.Kolling J., Scherer E.B., da Cunha A.A., da Cunha M.J., Wyse A.T. Homocysteine induces oxidative–nitrative stress in heart of rats: Prevention by folic acid. Cardiovasc. Toxicol. 2011;11:67–73. doi: 10.1007/s12012-010-9094-7. [DOI] [PubMed] [Google Scholar]
- 36.Devi S., Kennedy R.H., Joseph L., Shekhawat N.S., Melchert R.B., Joseph J. Effect of long-term hyperhomocysteinemia on myocardial structure and function in hypertensive rats. Cardiovasc. Pathol. 2006;15:75–82. doi: 10.1016/j.carpath.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Han L., Wu G., Feng C., Yang Y., Li B., Ge Y., Jiang Y., Shi Y.-H., Le G. Dietary methionine restriction improves the impairment of cardiac function in middle-aged obese mice. Food Funct. 2020;11:1764–1778. doi: 10.1039/C9FO02819F. [DOI] [PubMed] [Google Scholar]
- 38.Mendes R.H., Sirvente R.A., Candido G.O., Mostarda C.T., Salemi V., D’Almeida V., Jacob M.H., Ribeiro M.F., Belló-Klein A., Rigatto K., et al. Homocysteine thiolactone induces cardiac dysfunction: Role of oxidative stress. J. Cardiovasc. Pharmacol. 2010;55:198–202. doi: 10.1097/FJC.0b013e3181ce5c28. [DOI] [PubMed] [Google Scholar]
- 39.Singh A.P., Singh M., Balakumar P. Effect of mast cell stabilizers in hyperhomocysteinemia-induced cardiac hypertrophy in rats. J. Cardiovasc. Pharmacol. 2008;51:596–604. doi: 10.1097/FJC.0b013e31817ae60f. [DOI] [PubMed] [Google Scholar]
- 40.Stojanovic M., Zivkovic V., Srejovic I., Jakovljevic V., Jeremic N., Djuric D. The role of hydrogen sulfide in homocysteine-induced cardiodynamic effects and oxidative stress markers in the isolated rat heart. Physiol. Int. 2016;103:428–438. doi: 10.1556/2060.103.2016.4.3. [DOI] [PubMed] [Google Scholar]
- 41.Timkova V., Tatarkova Z., Lehotsky J., Racay P., Dobrota D., Kaplan P. Effects of mild hyperhomocysteinemia on electron transport chain complexes, oxidative stress, and protein expression in rat cardiac mitochondria. Mol. Cell. Biochem. 2016;411:261–270. doi: 10.1007/s11010-015-2588-7. [DOI] [PubMed] [Google Scholar]
- 42.Yalçinkaya-Demirsöz S., Depboylu B., Dogru-Abbasoglu S., Unlüçerçi Y., Uysal M. Effects of high methionine diet on oxidative stress in serum, apo-B containing lipoproteins, heart, and aorta in rabbits. Ann. Clin. Lab. Sci. 2009;39:386–391. [PubMed] [Google Scholar]
- 43.Chang L., Zhao J., Xu J., Jiang W., Tang C.S., Qi Y.F. Effects of taurine and homocysteine on calcium homeostasis and hydrogen peroxide and superoxide anions in rat myocardial mitochondria. Clin. Exp. Pharmacol. Physiol. 2004;31:237–243. doi: 10.1111/j.1440-1681.2004.03983.x. [DOI] [PubMed] [Google Scholar]
- 44.Chang L., Xu J., Yu F., Zhao J., Tang X., Tang C. Taurine protected myocardial mitochondria injury induced by hyperhomocysteinemia in rats. Amino Acids. 2004;27:37–48. doi: 10.1007/s00726-004-0096-2. [DOI] [PubMed] [Google Scholar]
- 45.Chang L., Geng B., Yu F., Zhao J., Jiang H., Du J., Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids. 2008;34:573–585. doi: 10.1007/s00726-007-0011-8. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Shi S., Dong S., Wu J., Song M., Zhong X., Liu Y. Sodium hydrosulfide attenuates hyperhomocysteinemia rat myocardial injury through cardiac mitochondrial protection. Mol. Cell. Biochem. 2015;399:189–200. doi: 10.1007/s11010-014-2245-6. [DOI] [PubMed] [Google Scholar]
- 47.Givvimani S., Kundu S., Narayanan N., Armaghan F., Qipshidze N., Pushpakumar S., Vacek T.P., Tyagi S.C. TIMP-2 mutant decreases MMP-2 activity and augments pressure overload induced LV dysfunction and heart failure. Arch Physiol. Biochem. 2013;119:65–74. doi: 10.3109/13813455.2012.755548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joseph J., Joseph L., Devi S., Kennedy R.H. Effect of anti-oxidant treatment on hyperhomocysteinemia-induced myocardial fibrosis and diastolic dysfunction. J. Heart Lung Transplant. 2008;27:1237–1241. doi: 10.1016/j.healun.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Li W., Tang R., Ouyang S., Ma F., Liu Z., Wu J. Folic acid prevents cardiac dysfunction and reduces myocardial fibrosis in a mouse model of high-fat diet-induced obesity. Nutr. Metab. 2017;14:68. doi: 10.1186/s12986-017-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tyagi N., Moshal K.S., Lominadze D., Ovechkin A.V., Tyagi S.C. Homocysteine-dependent cardiac remodeling and endothelial-myocyte coupling in a 2 kidney, 1 clip goldblatt hypertension mouse model. Can. J. Physiol. Pharmacol. 2005;83:583–594. doi: 10.1139/y05-047. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y., Zhao L., Zhang Y., Qin Q., Cong H., Guo Z. Homocysteine promotes cardiac fibrosis by regulating the Akt/FoxO3 pathway. Ann. Transl. Med. 2021;9:1732. doi: 10.21037/atm-21-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Q., Song W., Huang J., Wang D., Xu C. Metformin decreased myocardial fibrosis and apoptosis in hyperhomocysteinemia -induced cardiac hypertrophy. Curr. Res. Transl. Med. 2021;69:103270. doi: 10.1016/j.retram.2020.103270. [DOI] [PubMed] [Google Scholar]
- 53.Carroll J.F., Tyagi S.C. Extracellular matrix remodeling in the heart of the homocysteinemic obese rabbit. Am. J. Hypertens. 2005;18:692–698. doi: 10.1016/j.amjhyper.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 54.Zulli A., Hare D.L., Buxton B.F., Black M.J. The combination of high dietary methionine plus cholesterol induces myocardial fibrosis in rabbits. Atherosclerosis. 2006;185:278–281. doi: 10.1016/j.atherosclerosis.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 55.Han L., Tang Y., Li S., Wu Y., Chen X., Wu Q., Hong K., Li J. Protective mechanism of SIRT1 on hcy-induced atrial fibrosis mediated by TRPC3. J. Cell. Mol. Med. 2020;24:488–510. doi: 10.1111/jcmm.14757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhi H., Luptak I., Alreja G., Shi J., Guan J., Metes-Kosik N., Joseph J. Effects of direct renin inhibition on myocardial fibrosis and cardiac fibroblast function. PLoS ONE. 2013;8:e81612. doi: 10.1371/journal.pone.0081612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J.-S., Hou Y.-L., Lu W.-W., Ni X.-Q., Lin F., Yu Y.-R., Tang C.-S., Qi Y.-F. Intermedin (1–53) protects against myocardial fibrosis by inhibiting endoplasmic reticulum stress and inflammation induced by homocysteine in apolipoprotein e-deficient mice. J. Atheroscler. Thromb. 2016;23:1294–1306. doi: 10.5551/jat.34082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muthuramu I., Singh N., Amin R., Nefyodova E., Debasse M., Van Horenbeeck I., Jacobs F., De Geest B. Selective homocysteine-lowering gene transfer attenuates pressure overload-induced cardiomyopathy via reduced oxidative stress. Klin. Wochenschr. 2015;93:609–618. doi: 10.1007/s00109-015-1281-3. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z.-S., Jin H., Wang D.-M. Influence of hydrogen sulfide on zymogen activation of homocysteine-induced matrix metalloproteinase-2 in H9C2 cardiocytes. Asian Pac. J. Trop. Med. 2016;9:489–493. doi: 10.1016/j.apjtm.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 60.Chaouad B., Moudilou E.N., Ghoul A., Zerrouk F., Moulahoum A., Othmani-Mecif K., Cherifi M.E.H., Exbrayat J.-M., Benazzoug Y. Hyperhomocysteinemia and myocardial remodeling in the sand rat, psammomys obesus. Acta. Histochem. 2019;121:823–832. doi: 10.1016/j.acthis.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Joseph J., Kennedy R.H., Devi S., Wang J., Joseph L., Hauer-Jensen M. Protective role of mast cells in homocysteine-induced cardiac remodeling. Am. J. Physiol. Circ. Physiol. 2005;288:H2541–H2545. doi: 10.1152/ajpheart.00806.2004. [DOI] [PubMed] [Google Scholar]
- 62.Joseph J., Washington A., Joseph L., Kennedy R.H. Hyperhomocysteinaemia-induced atrial remodelling in hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2004;31:331–337. doi: 10.1111/j.1440-1681.2004.03998.x. [DOI] [PubMed] [Google Scholar]
- 63.Cao P., Zhang W., Kong X., Gao N., Zhao X., Xu R. Hyperhomocysteinemia-induced Nrf2/HO-1 pathway suppression aggravates cardiac remodeling of hypertensive rats. Biochem. Biophys. Res. Commun. 2021;547:125–130. doi: 10.1016/j.bbrc.2021.02.025. [DOI] [PubMed] [Google Scholar]
- 64.Li C., Zhang R., Zhan Y., Zheng J. Resveratrol Inhibits Hepatic Stellate Cell Activation via the Hippo Pathway. Mediat. Inflamm. 2021;2021:3399357. doi: 10.1155/2021/3399357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chaturvedi P., Kalani A., Givvimani S., Kamat P.K., Familtseva A., Tyagi S.C. Differential regulation of DNA methylation versus histone acetylation in cardiomyocytes during HHcy in vitro and in vivo: An epigenetic mechanism. Physiol. Genom. 2014;46:245–255. doi: 10.1152/physiolgenomics.00168.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herrmann M., Taban-Shoma O., Hubner U., Pexa A., Kilter H., Umanskaya N., Straub R.H., Böhm M., Herrmann W. Hyperhomocysteinemia and myocardial expression of brain natriuretic peptide in rats. Clin. Chem. 2007;53:773–780. doi: 10.1373/clinchem.2006.077859. [DOI] [PubMed] [Google Scholar]
- 67.Jeremic J., Turnic T.N., Zivkovic V., Jeremic N., Milosavljevic I., Srejovic I., Obrenovic R., Jancic S., Rakocevic M., Matic S., et al. Vitamin B complex mitigates cardiac dysfunction in high-methionine diet-induced hyperhomocysteinemia. Clin. Exp. Pharmacol. Physiol. 2018;45:683–693. doi: 10.1111/1440-1681.12930. [DOI] [PubMed] [Google Scholar]
- 68.Kar S., Shahshahan H.R., Kambis T.N., Yadav S.K., Li Z., Lefer D.J., Mishra P.K. Hydrogen sulfide ameliorates homocysteine-induced cardiac remodeling and dysfunction. Front. Physiol. 2019;10:598. doi: 10.3389/fphys.2019.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raaf L., Noll C., Cherifi M.E.H., Samuel J.-L., Delcayre C., Delabar J.-M., Benazzoug Y., Janel N. Myocardial fibrosis and TGFB expression in hyperhomocysteinemic rats. Mol. Cell. Biochem. 2011;347:63–70. doi: 10.1007/s11010-010-0612-5. [DOI] [PubMed] [Google Scholar]
- 70.Mishra P.K., Tyagi N., Kundu S., Tyagi S.C. Micro RNAs are involved in homocysteine-induced cardiac remodeling. Cell Biophys. 2009;55:153–162. doi: 10.1007/s12013-009-9063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenberger D., Gargoum R., Tyagi N., Metreveli N., Sen U., Maldonado C., Tyagi S. Homocysteine enriched diet leads to prolonged QT interval and reduced left ventricular performance in telemetric monitored mice. Nutr. Metab. Cardiovasc. Dis. 2011;21:492–498. doi: 10.1016/j.numecd.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ables G.P., Ouattara A., Hampton T.G., Cooke D., Perodin F., Augie I., Orentreich D.S. Dietary methionine restriction in mice elicits an adaptive cardiovascular response to hyperhomocysteinemia. Sci. Rep. 2015;5:8886. doi: 10.1038/srep08886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cainzos-Achirica M., Acquah I., Dardari Z., Mszar R., Greenland P., Blankstein R., Bittencourt M., Rajagopalan S., Al-Kindi S.G., Polak J.F., et al. Long-Term Prognostic Implications and Role of Further Testing in Adults Aged ≤55 Years With a Coronary Calcium Score of Zero (from the Multi-Ethnic Study of Atherosclerosis) Am. J. Cardiol. 2021;161:26–35. doi: 10.1016/j.amjcard.2021.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai B., Gong D., Chen N., Li J., Wang G., Lu Y., Yang B. The negative inotropic effects of homocysteine were prevented by matrine via the regulating intracellular calcium level. Int. J. Cardiol. 2011;150:113–115. doi: 10.1016/j.ijcard.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 75.Shontz R.D., Xu Z., Patel K.P., Rozanski G.J. Inhibition of K+ currents by homocysteine in rat ventricular myocytes. J. Cardiovasc. Electrophysiol. 2001;12:175–182. doi: 10.1046/j.1540-8167.2001.00175.x. [DOI] [PubMed] [Google Scholar]
- 76.Sun W.T., Hou H.T., Chen H.X., Xue H.M., Wang J., He G.W., Yang Q. Calcium-activated potassium channel family in coronary artery bypass grafts. J. Thorac. Cardiovasc. Surg. 2021;161:e399–e409. doi: 10.1016/j.jtcvs.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 77.Cai B., Shan L., Gong D., Pan Z., Ai J., Xu C., Lu Y., Yang B. Homocysteine modulates sodium channel currents in human atrial myocytes. Toxicology. 2009;256:201–206. doi: 10.1016/j.tox.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 78.Lopatina E.V., Kipenko A., Penniyaynen V., Pasatetskaya N., Djuric D., Krylov B. Organotypic tissue culture investigation of homocysteine thiolactone cardiotoxic effect. Acta Physiol. Hung. 2015;102:137–142. doi: 10.1556/036.102.2015.2.4. [DOI] [PubMed] [Google Scholar]
- 79.Cai B.-Z., Gong D.-M., Liu Y., Pan Z.-W., Xu C.-Q., Bai Y.-L., Qiao G.-F., Lu Y.-J., Yang B.-F. Homocysteine inhibits potassium channels in human atrial myocytes. Clin. Exp. Pharmacol. Physiol. 2007;34:851–855. doi: 10.1111/j.1440-1681.2007.04671.x. [DOI] [PubMed] [Google Scholar]
- 80.Pacher P., Ungvari Z., Kecskemeti V. Electrophysiological effects of homocysteine in isolated rat right ventricular papillary muscles and left atria. Gen. Pharmacol. Vasc. Syst. 1999;32:439–443. doi: 10.1016/S0306-3623(98)00213-4. [DOI] [PubMed] [Google Scholar]
- 81.Soni C.V., Tyagi S.C., Todnem N.D., Givvimani S., Pushpakumar S.B., Villafane J., Maldonado C. Hyperhomocysteinemia alters sinoatrial and atrioventricular nodal function: Role of magnesium in attenuating these effects. Cell Biophys. 2016;74:59–65. doi: 10.1007/s12013-015-0711-8. [DOI] [PubMed] [Google Scholar]
- 82.Han L., Shen W.J., Bittner S., Kraemer F.B., Azhar S. PPARs: Regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ. Future Cardiol. 2017;13:279–296. doi: 10.2217/fca-2017-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mishra P.K., Awe O., Metreveli N., Qipshidze N., Joshua I.G., Tyagi S.C. Exercise mitigates homocysteine—β2-adrenergic receptor interactions to ameliorate contractile dysfunction in diabetes. Int. J. Physiol. Pathophysiol. Pharmacol. 2011;3:97–106. [PMC free article] [PubMed] [Google Scholar]
- 84.Mishra P.K., Givvimani S., Metreveli N., Tyagi S.C. Attenuation of beta2-adrenergic receptors and homocysteine metabolic enzymes cause diabetic cardiomyopathy. Biochem. Biophys. Res. Commun. 2010;401:175–181. doi: 10.1016/j.bbrc.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tasatargil A., Sadan G., Karasu E., Ozdem S. Changes in atrium and thoracic aorta reactivity to adenosinergic and adrenergic agonists in experimental hyperhomocysteinemia. J. Cardiovasc. Pharmacol. 2006;47:673–679. doi: 10.1097/01.fjc.0000211756.31820.17. [DOI] [PubMed] [Google Scholar]
- 86.Moshal K.S., Kumar M., Tyagi N., Mishra P.K., Metreveli N., Rodriguez W.E., Tyagi S.C. Restoration of contractility in hyperhomocysteinemia by cardiac-specific deletion of NMDA-R1. Am. J. Physiol. Circ. Physiol. 2009;296:H887–H892. doi: 10.1152/ajpheart.00750.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moshal K.S., Tipparaju S.M., Vacek T.P., Kumar M., Singh M., Frank I.E., Patibandla P.K., Tyagi N., Rai J., Metreveli N., et al. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am. J. Physiol. Circ. Physiol. 2008;295:H890–H897. doi: 10.1152/ajpheart.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tyagi N., Vacek J.C., Givvimani S., Sen U., Tyagi S.C. Cardiac specific deletion of N-methyl-d-aspartate receptor 1 ameliorates mtMMP-9 mediated autophagy/mitophagy in hyperhomocysteinemia. J. Recept. Signal Transduct. 2010;30:78–87. doi: 10.3109/10799891003614808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Srejovic I., Zivkovic V., Nikolic T., Jeremic N., Stojic I., Jeremic J., Djuric D., Jakovljevic V. Modulation of N-methyl-d-aspartate receptors in isolated rat heart. Can. J. Physiol. Pharmacol. 2017;95:1327–1334. doi: 10.1139/cjpp-2017-0056. [DOI] [PubMed] [Google Scholar]
- 90.Busingye D., Pollack A., Chidwick K. Prevalence of inflammatory bowel disease in the Australian general practice population: A cross-sectional study. PLoS ONE. 2021;16:e0252458. doi: 10.1371/journal.pone.0252458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ji C., Yu Y., Zhang M., Yu W., Dong S. Loxoprofen Sodium Alleviates Oxidative Stress and Apoptosis Induced by Angiotensin II in Human Umbilical Vein Endothelial Cells (HUVECs) Drug Des. Dev. Ther. 2020;14:5087–5096. doi: 10.2147/DDDT.S266175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie C., Li Q., Li L., Peng X., Ling Z., Xiao B., Feng J., Chen Z., Chang D., Xie L., et al. Association of Early Inflammation with Age and Asymptomatic Disease in COVID-19. J. Inflamm. Res. 2021;14:1207–1216. doi: 10.2147/JIR.S304190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Samman Tahhan A., Sandesara P.B., Hayek S.S., Alkhoder A., Chivukula K., Hammadah M., Mohamed-Kelli H., O’Neal W.T., Topel M., Ghasemzadeh N., et al. Association between oxidative stress and atrial fibrillation. Heart Rhythm. 2017;14:1849–1855. doi: 10.1016/j.hrthm.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Korantzopoulos P., Letsas K., Fragakis N., Tse G., Liu T. Oxidative stress and atrial fibrillation: An update. Free Radic. Res. 2018;52:1199–1209. doi: 10.1080/10715762.2018.1500696. [DOI] [PubMed] [Google Scholar]
- 95.Sen U., Basu P., Abe O.A., Givvimani S., Tyagi N., Metreveli N., Shah K.S., Passmore J.C., Tyagi S.C. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am. J. Physiol. Physiol. 2009;297:F410–F419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ripps H., Shen W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012;18:2673–2686. [PMC free article] [PubMed] [Google Scholar]
- 97.Wu D., Gu Y., Zhu D. Cardioprotective effects of hydrogen sulfide in attenuating myocardial ischemia-reperfusion injury (Review) Mol. Med. Rep. 2021;24:875. doi: 10.3892/mmr.2021.12515. [DOI] [PubMed] [Google Scholar]
- 98.Fu M., Chen H., Cai Z., Yang Y., Feng Z., Zeng M., Chen L., Qin Y., Cai B., Zhu P., et al. Forkhead box family transcription factors as versatile regulators for cellular reprogramming to pluripotency. Cell Regen. 2021;10:17. doi: 10.1186/s13619-021-00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ronnebaum S.M., Patterson C. The foxo family in cardiac function and dysfunction. Annu. Rev. Physiol. 2010;72:81–94. doi: 10.1146/annurev-physiol-021909-135931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Allessie M., Ausma J., Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002;54:230–246. doi: 10.1016/S0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 101.Mládková J., Hladílková J., Diamond C.E., Tryon K., Yamada K., Garrow T.A., Jungwirth P., Koutmos M., Jiráček J. Specific potassium ion interactions facilitate homocysteine binding to betaine-homocysteine S-methyltransferase. Proteins Struct. Funct. Bioinform. 2014;82:2552–2564. doi: 10.1002/prot.24619. [DOI] [PubMed] [Google Scholar]
- 102.Schnabel R., Lackner K.J., Rupprecht H.J., Espinola-Klein C., Torzewski M., Lubos E., Bickel C., Cambien F., Tiret L., Münzel T., et al. Glutathione peroxidase-1 and homocysteine for cardiovascular risk prediction: Results from the atherogene study. J. Am. Coll. Cardiol. 2005;45:1631–1637. doi: 10.1016/j.jacc.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 103.Ay H., Arsava E.M., Tokgozoglu S.L., Ozer N., Sarıbas O. Hyperhomocysteinemia is associated with the presence of left atrial thrombus in stroke patients with nonvalvular atrial fibrillation. Stroke. 2003;34:909–912. doi: 10.1161/01.STR.0000060202.63475.BA. [DOI] [PubMed] [Google Scholar]
- 104.Loffredo L., Violi F., Fimognari F.L., Cangemi R., Sbrighi P.S., Sampietro F., Mazzola G., Di Lecce V.N., D’Angelo A. The association between hyperhomocysteinemia and ischemic stroke in patients with non-valvular atrial fibrillation. Haematologica. 2005;90:1205–1211. [PubMed] [Google Scholar]
- 105.Sundström J., Sullivan L., Selhub J., Benjamin E.J., D’Agostino R.B., Jacques P.F., Rosenberg I.H., Levy D., Wilson P.W., Vasan R.S. Relations of plasma homocysteine to left ventricular structure and function: The framingham heart study. Eur. Heart J. 2004;25:523–530. doi: 10.1016/j.ehj.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 106.Alter P., Rupp H., Rominger M.B., Figiel J., Renz H., Klose K.J., Maisch B. Association of hyperhomocysteinemia with left ventricular dilatation and mass in human heart. Clin. Chem. Lab. Med. (CCLM) 2010;48:555–560. doi: 10.1515/CCLM.2010.102. [DOI] [PubMed] [Google Scholar]
- 107.Li Z., Guo X., Sun G., Zheng L., Sun Y., Liu Y., Abraham M.R. Plasma homocysteine levels associated with a corrected QT interval. BMC Cardiovasc. Disord. 2017;17:182. doi: 10.1186/s12872-017-0617-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leng Y.L.Y., Zhou Y., Ke H., Jelinek H., McCabe J., Assareh H., McLachlan C.S. Electrocardiogram derived QRS duration >120 ms is associated with elevated plasma homocysteine levels in a rural australian cross-sectional population. Medicine. 2015;94:e1080. doi: 10.1097/MD.0000000000001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gueant-Rodriguez R.-M., Juillière Y., Nippert M., Abdelmouttaleb I., Herbeth B., Aliot E., Danchin N., Guéant J.-L. Left ventricular systolic dysfunction is an independent predictor of homocysteine in angiographically documented patients with or without coronary artery lesions. J. Thromb. Haemost. 2007;5:1209–1216. doi: 10.1111/j.1538-7836.2007.02535.x. [DOI] [PubMed] [Google Scholar]
- 110.Guéant Rodriguez R.M., Spada R., Pooya S., Jeannesson E., Moreno Garcia M.A., Anello G., Bosco P., Elia M., Romano A., Alberto J.M., et al. Homocysteine predicts increased NT-pro-BNP through impaired fatty acid oxidation. Int. J. Cardiol. 2013;167:768–775. doi: 10.1016/j.ijcard.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 111.Görmüş U., Özmen D., Özmen B., Parıldar Z., Özdogan O., Mutaf I., Bayindir O. Serum N-terminal-pro-brain natriuretic peptide (NT-pro-BNP) and homocysteine levels in type 2 diabetic patients with asymptomatic left ventricular diastolic dysfunction. Diabetes Res. Clin. Pract. 2010;87:51–56. doi: 10.1016/j.diabres.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 112.Cho S.E., Hong K.S., Shin G.J., Chung W.S. The methylenetetrahydrofolate reductase C677T gene mutation is associated with hyperhomocysteinemia, cardiovascular disease and plasma B-type natriuretic peptide levels in Korea. Clin. Chem. Lab. Med. (CCLM) 2006;44:1070–1075. doi: 10.1515/CCLM.2006.194. [DOI] [PubMed] [Google Scholar]
- 113.Ye P., Cao R., Bai Y., Xu R. Homocysteine is associated with plasma high-sensitivity cardiac troponin T levels in a community-dwelling population. Clin. Interv. Aging. 2014;9:79–84. doi: 10.2147/CIA.S56054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alam N., Khan H.I.L.R., Chowdhury A.W., Haque M.S., Ali M.S., Sabah K.M.N., Amin M.G. Elevated serum homocysteine level has a positive correlation with serum cardiac troponin I in patients with acute myocardial infarction. Bangladesh Med. Res. Counc. Bull. 2012;38:9–13. doi: 10.3329/bmrcb.v38i1.10445. [DOI] [PubMed] [Google Scholar]
- 115.Wang S., Wei Y., Hidru T.H., Li D., Wang N., Yang Y., Wang Y., Yang X., Xia Y. Combined effect of homocysteine and uric acid to identify patients with high risk for subclinical atrial fibrillation. J. Am. Heart Assoc. 2022;11:e021997. doi: 10.1161/JAHA.121.021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wocial B., Berent H., Kostrubiec M., Kuczyńska K., Kuch-Wocial A., Niewegłowska N. Homocysteine, adrenergic activity and left ventricular mass in patients with essential hypertension. Blood Press. 2002;11:201–205. doi: 10.1080/08037050213758. [DOI] [PubMed] [Google Scholar]
- 117.Poduri A., Kaur J., Thakur J.S., Kumari S., Jain S., Khullar M. Effect of ACE inhibitors and beta-blockers on homocysteine levels in essential hypertension. J. Hum. Hypertens. 2008;22:289–294. doi: 10.1038/sj.jhh.1002325. [DOI] [PubMed] [Google Scholar]
- 118.Atar I., Korkmaz M.E., Demircan S., Atar A., Bozbaş H., Aydinalp A., Özin B., Yildirir A., Müderrisoğlu H. Beta blocker effects on plasma homocysteine levels in patients with hypertension. Atherosclerosis. 2005;181:399–402. doi: 10.1016/j.atherosclerosis.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 119.Chua W., Purmah Y., Cardoso V.R., Gkoutos G., Tull S.P., Neculau G., Thomas M., Kotecha D., Lip G.Y.H., Kirchhof P., et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur. Heart J. 2019;40:1268–1276. doi: 10.1093/eurheartj/ehy815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fabritz L. The power of P in the elderly: Small biphasic wave, big impact. Heart Rhythm. 2016;13:652–653. doi: 10.1016/j.hrthm.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 121.Attia Z.I., Noseworthy P.A., Lopez-Jimenez F., Asirvatham S.J., Deshmukh A.J., Gersh B.J., Carter R.E., Yao X., Rabinstein A.A., Erickson B.J., et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet. 2019;394:861–867. doi: 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]
- 122.Siontis K.C., Yao X., Pirruccello J.P., Philippakis A.A., Noseworthy P.A. How will machine learning inform the clinical care of atrial fibrillation? Circ. Res. 2020;127:155–169. doi: 10.1161/CIRCRESAHA.120.316401. [DOI] [PubMed] [Google Scholar]
- 123.Nakamura T., Sakamoto K., Yamano T., Kikkawa M., Zen K., Hikosaka T., Kubota T., Azuma A., Nishimura T. Increased plasma brain natriuretic peptide level as a guide for silent myocardial ischemia in patients with non-obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002;39:1657–1663. doi: 10.1016/S0735-1097(02)01813-2. [DOI] [PubMed] [Google Scholar]
- 124.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.