Abstract
Hawthorn (Crataegus) is a plant of the Rosaceae family and is widely grown throughout the world as one of the medicinal and edible plants, known as the “nutritious fruit” due to its richness in bioactive substances. Preparations derived from it are used in the formulation of dietary supplements, functional foods, and pharmaceutical products. Rich in amino acids, minerals, pectin, vitamin C, chlorogenic acid, epicatechol, and choline, hawthorn has a high therapeutic and health value. Many studies have shown that hawthorn has antioxidant, anti-inflammatory, anticancer, anti-cardiovascular disease, and digestive enhancing properties. This is related to its bioactive components such as polyphenols (chlorogenic acid, proanthocyanidin B2, epicatechin), flavonoids (proanthocyanidins, mucoxanthin, quercetin, rutin), and pentacyclic triterpenoids (ursolic acid, hawthornic acid, oleanolic acid), which are also its main chemical constituents. This paper briefly reviews the chemical composition, nutritional value, food applications, and the important biological and pharmacological activities of hawthorn. This will contribute to the development of functional foods or nutraceuticals from hawthorn.
Keywords: nutrition, flavonoids, phenolic acid, antioxidant, anti-cardiovascular disease, bread, beverage, meat
1. Introduction
As society develops and people’s living standards improve, various chronic diseases are emerging in the public. There has been a growing shift away from pharmaceuticals to wild medicinal plants, traditional herbs, and “ready-to-use healing foods” [1,2]. These traditional herbs and wild medicinal plants contain a large number of bioactive compounds. The properties of these plant ingredients allow them to be used in food and medicine. Among them, hawthorn of the Rosaceae family [3], known as the “nutritious fruit”, is attracting attention as one of the homologous medicinal herbs [4].
Hawthorn is a member of the Rosaceae family and is the most valuable species in the genus. It is often found on forest margins or in scrub on mountain slopes, mostly growing to 15 m. Its wood is hard and durable [5]. In the 2020 edition of the Pharma-copoeia of the People’s Republic of China (Part I), hawthorn is a wrinkled, uneven, round piece sliced, and dried in autumn after the fruit has ripened [6]. Hawthorn is widely distributed in the world, with over 1000 species [7], mainly in the temperate regions of the Northern Hemisphere. C. monogyna and C. laevigata are the main hawthorn species in Central Europe, C. pentagyna, C. nigra, and C. azarolus are the hawthorn species of southern and southeastern Europe. C. pinnatifida and C. scabrifolia are the main hawthorn varieties in China [8,9].
Hawthorn is widely used as a wild fruit for food and medical research. The fresh or dried fruits of hawthorn are used to make preserves, teas, and food supplements [10]. Extracts of hawthorn berries, leaves, and flowers are used to prevent hypertension and heart failure [11]. Hawthorn berries, leaves, and flowers are traditionally used in Europe for the treatment of heart disease, in North America for the same purpose [3,12], and in China, mainly for commercial products. In recent years, hawthorn is no longer restricted to the processing of some jams and snack foods. A growing number of functional hawthorn products are appearing on the market, such as hawthorn functional drinks, food additives, flavonoid injections, and hawthorn enzymes. Hawthorn can provide a variety of benefits to producers, food processors, and consumers and therefore has great economic value. However, due to the uniqueness of the product range and other reasons leading to some consumer limitations, there is an urgent need for further research and development of new hawthorn-based products.
This paper summarizes the nutritional and phytochemical composition, ethnomedical uses, food products and health benefits of hawthorn by reviewing web of science, PubMed, Google Scholar in conjunction with the scattered information available.
2. Nutrients and Phytochemical Compositions of Hawthorn
2.1. Nutrient Composition of Hawthorn
Approved as a medicinal fruit by the Chinese National Health and Wellness Commission [4], hawthorn has higher dietary fiber, pectin, ascorbic acid, minerals, and antioxidant capacity than some common fruits [13]. Studies have confirmed that hawthorn is rich in amino acids (8 essential amino acids and 3–8 times more amino acids than fruit), protein (17 times more protein than apple fruit), sugars, minerals (1st in calcium content among fruits), vitamins (vitamins A, C, B1, B2, about 10 times more vitamins), and has a high nutritional value [14]. Hawthorn is also rich in calcium, vitamin C, and carotene, with the highest calcium content and 890 mg/kg of vitamin, and rich in organic acids, which prevent vitamin C from being completely destroyed even when heated.
Hawthorn from different regions contains different levels of nutrients, depending on conditions such as variety, environment, genetics and plant harvest [4]. Hawthorn contains 7 mg/g of protein, 2 mg/g of fat, 30–40 mg/g of pectin, 30–50 mg/g of organic acids, 3–8 mg/g of tannins, 0.5–1.5 mg/g of amino acids, 0.89 mg/g of vitamin C, 0.89 mg/g of vitamin D, and 0.65 mg/g of flavonoids [15]. The crude protein, crude oil, ash, pH, acidity, and total phenolic content of native plants from central Anatolia, Turkey were 3.03%, 1.22%, 2.77%, 4.08, 1.56% and 9.35 mg/g, respectively [14]. A summary of nutritional composition of hawthorn in different regions can be seen in Table 1.
Table 1.
Nutritional composition of hawthorn in different regions. Values are expressed as a percentage (%) and as g per 100 g fresh weight.
| Species | C. Monogyna Jacq. Var. Monogyna | C. pinnatifida Bunge | NM | C. pinnatifida | |||||
|---|---|---|---|---|---|---|---|---|---|
| Country | Source | Turkey | Wild | China | Wild | China | Wild | China | Wild |
| Protein | 3.03% | 0.7 | 0.5 | 3.14% | |||||
| Water | 68.98% | ND | 73 | 77.48% | |||||
| Fat | ND | 0.2 | 0.6 | 1.3% | |||||
| Pectin | ND | 3–4 | ND | 13 | |||||
| Energy(kJ) | ND | ND | 397 | 364 | |||||
| Dietary fiber | ND | ND | 3.1 | 33% | |||||
| Ca | 0.1 | ND | 52 | 0.06 | |||||
| K | 1.6 | ND | 299 | 1.02 | |||||
| Fe | 6.2 | 2.1 | 0.9 | 0.003 | |||||
| Na | 5.7 | 68 | 5.4 | 0.005 | |||||
ND: not determined.
2.2. Phytochemical Compositions of Hawthorn
Hawthorn fruit, leaves, and flowers are rich in biologically active ingredients. It contains compounds with bioactive components such as organic acids, flavonoids, mucoxanthin, polyphenols, triterpenoids, and trace elements. Flavonoids range from 0.1 to 1.0% in the hawthorn fruit, 1 to 2% in the leaves and flowers, organic acids are second only to the flavonoids at 4.1% and proanthocyanidins range from 1 to 3% in the hawthorn fruit or leaves. Of these, total flavonoids and organic acids are the most abundant chemical constituents in hawthorn, while proanthocyanidins and total flavonoids are the two main categories of bioactive constituents in hawthorn. Hawthorn pericarp and pulp are also rich in pectin. Pectin extracted from hawthorn contains approximately 67% glyoxalate [16], has a high galacturonic acid and methyl esterification content and has a high viscosity compared to other food pectins such as lemon and apple pectin [17].
As a fruit rich in organic and phenolic acids, the organic acids in hawthorn are mainly malic, citric, succinic, ascorbic, quinic, oxalic, linolenic, and lauric acid [18]. The amount of organic acids varies depending on the variety of hawthorn. Citric and malic acids are the highest in hawthorn fruit, with malic acid averaging 1128.68 mg/100 g FW [18].
In addition, apart from organic acids, hawthorn is also rich in phenolic acids. The main phenolic acid in hawthorn is chlorogenic acid (8410–13826.7 μg/g), accounting for more than 80% of the total phenolic acid [19]. Gentisic acid, sinapinic acid, chlorogenic acid, quinic acid, protocatechuic acid, p-coumaric acid, m-coumaric acid, o-coumaric acid [20], caffeic acid, caffeic acid 3-glucoside [18], gallic acid, vanillic acid, syringic acid, ferulic acid, cinnamic acid [4], phlorodizin [21], neochlorogenic acid [22], salicylic acid, and ellagic acid [23]. Phenolic acids such as sinapic acid [24] have been quantified in previously studied hawthorn varieties. In leaves and flowers, none of the above acids have been measured more than once, so comparisons and generalizations of their concentrations cannot be made.
Flavonoids are the most abundant and wide-ranging class of compounds in hawthorn [25,26]. Currently, more than 60 flavonoids have been isolated from hawthorn [22,27,28,29,30,31,32,33,34], including the oxidized (chrysin, rutin, and quercetin) and reduced (proanthocyanidins, i.e., catechin derivatives) types [35]. According to its glycosides, it can be divided into flavonoids with apigenin, kaempferol, lignan, quercetin, and dihydroflavonoid glycosides as the main ones [36,37]. There are many varieties of hawthorn, and the total flavonoids vary from 2.27 to 17.40 mg/g in different varieties and different organs [35,38,39]. Table 2 lists the flavonoids that have been isolated from hawthorn.
Secondly, triterpenoids and their derivatives in hawthorn were isolated and identified in the first studies on hawthorn in the 1960s [40]. Triterpenoids are a wide range of structurally diverse compounds found in plants, consisting mainly of three terpene or isoprene units [41,42,43]. Hawthorn is a group of plants rich in triterpenoids, and some pharmacological studies have shown that these components have important effects such as cardiac strengthening, increasing coronary flow and improving circulation. And the presence of triterpenoids in the fruit may be the main reason for their anticancer activity [44]. Apart from the major triterpenoids such as ursolic acid [45] and oleanolic acid, all other terpenoids in hawthorn have been listed in Table 3.
Hawthorn seeds have been reported to contain high levels of lignan content [46]. Lignans are natural components synthesized by bimolecular polymerization of phenylpropanoids, which have antioxidant and anti-inflammatory activities and could be a new, inexpensive source of antioxidants, and inflammation inhibitors [47,48].
Sugars are mainly produced in the leaves and are transported to the fruit and other parts of the fruit as they develop. Sugar alcohols, mainly sorbitol and inositol, are mainly found in hawthorn varieties that are more commonly consumed as food [24]. Hawthorn homogeneous polysaccharides (HPS) are mainly a mixture of sugars including glucose, arabinose, caramel, galactose, galacturonic acid, rhamnose, and xylose. HPS contains two different molecular weight polysaccharide fractions of 1.423 × 105 Da and 4.080 × 104 Da respectively [49]. HAW1-2 is a polysaccharide with a molecular weight of 8.94 Da consisting of arabinose, galactose and glucose [50].
Hawthorn is rich in trace elements such as Ca, Fe, Mg, Cu, and Zn, with Ca content being the highest [4]. The content of trace elements varies slightly between different varieties of hawthorn. Chinese hawthorn berries contain 68 mg of sodium, 20 mg of phosphorus, and 2.10 mg of iron per 100 g of edible portion of hawthorn. The K, P, Ca, Mg, Fe, Na and B contents of hawthorn in the native plant of middle Anatolia in Turke were 16.3 mg/g, 1.3 mg/g, 1.3 mg/g, 0.9 mg/g, 0.06 mg/g, 0.06 mg/g, and 0.04 mg/g, respectively [51].
In addition to the above major bioactive components, hawthorn leaves also contain nitrogenous compounds. There are about 12 nitrogenous compounds isolated from hawthorn leaves, with components such as isobutylamine, ethylamine, dimethylamine, phenylethylamine, choline, acetylcholine, trimethylamine, isoamylamine, and ethanolamine [40]. Among them, phenylethylamine, tyramine, isobutylamine, and o-methoxyphenylethylamine are the main cardiac cardiac stimulant components. A total of 65 chemical components were identified in hawthorn essential oil, mainly eicosanoids (12–17%), twenty-one alkanes (11–16%), linalool (6–11%), hexadecanoic acid (1–11%), and nineteen alkanes (3–7%) [51,52].
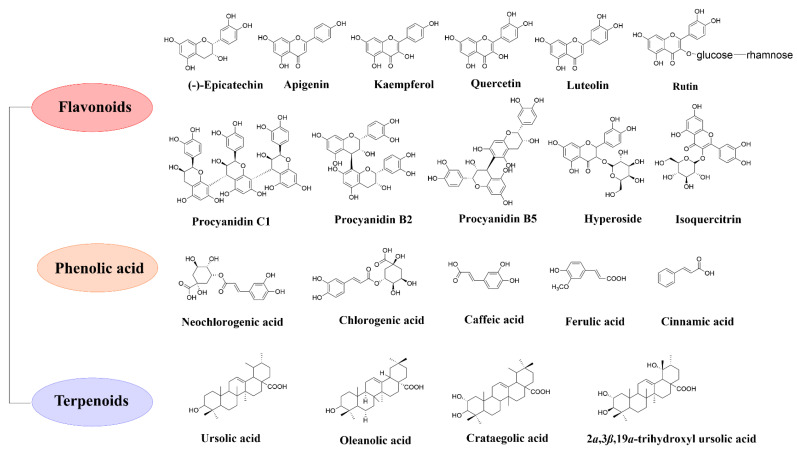
To date, more than 170 compounds have been identified in hawthorn [53]. Figure 1 illustrates the chemical structures of several major bioactive components of hawthorn [12,19,35,44,53].
Figure 1.
The main bioactive compounds of hawthorn.
Table 2.
Flavonoids identified from Hawthorn.
| Name | Species and Organ | Methodological and Analytical Approach | Extraction Solvent |
References |
|---|---|---|---|---|
| Quercetin |
C. Almaatensis; Flowers, fruits and leaves |
HPLC-ESI-Q-TOF-MS and HRMS/MS | 96% Ethanol or 50% ethanol | [20] |
| Quercitrin | ||||
| Cyanidin 3-glucoside | ||||
| Catechin | ||||
| Epigallocatechin | ||||
| Rutin | ||||
| Quercetin 3-glucoside (Isoquercetin) | ||||
| Vitexin 2″-O-rhamnoside | ||||
| Vitexin 4″-O-rhamnoside | ||||
| Vitexin | ||||
| Vitexin 4″-O-glucoside | ||||
| Quercetin glucoside | ||||
| Hyperoside |
C. Meyeri; Fruits |
HPLC | Methanol/water (80:20, 25 mL) | [12] |
| Epicatechin | NM | HPLC | 80% Aqueous ethanol | [28] |
| Cyanidin chloride |
C. pinnatifida var. Major; Fruits |
HPLC-ESI-MS/MS | 50% (v/v) Aqueous ethanol | [54] |
| Luteolin | ||||
| Apigenin | ||||
| Kaempferol | ||||
| Naringenin | ||||
| Phloretin | ||||
| Quercetin dirhamnosyl hexoside |
C. pinnatifida Bge. var. Major; Fruits |
HPLC-UV | 80% Aqueous ethanol | [27] |
| Quercetin rhamnosyl hexoside | ||||
| Monoacetyl vitexin rhamnoside | ||||
| Luteolin-7-glycoside | ||||
| C-glycosides vitexin | ||||
| Sexangularetin-3-glucoside | ||||
| Sexangularetin-3-neohesperidoside | ||||
| Kaempferol-3-neohesperidoside | ||||
| Vitexin-2″-O-α-L-rhamnoside |
C. Oxyacantha; Leaves and flowers |
NM | Hydroalcoholic or water-based | [9] |
| Eriodictyol |
C. azarolus Leaves |
HPLC | Water/acetone mixture (1v/2v) | [55] |
| Hesperidin |
C. pinnatifida Bge. or C. pimiatificia Bge. var. major N, E. Br; Fruits |
UPLC/Q-TOF-MS | 50% Ethanol | [56] |
| Rutoside |
C. oxyacantha L; Fruits |
HPLC, TLC, and UV | Hydroalcoholic or water-based | [26] |
| Isoquercitrin |
C. pinnatifida Bge; Fruits |
HPLC | 80% Acetone | [38] |
| Hispertin |
C. azarolus var. eu-azarolus; Leaves |
RP-HPLC, UV, TLC, 1H NMR, and 13C NMR | Acetone, ethyl acetate, methanol and 70% ethanol | [23] |
| Chrysin | ||||
| Procyanidin trimers |
C. orientalis, C. szovitsii and C. tanacetifolia; Leaves and twigs |
VIP marker from OPLS-DA and UHPLC-ESI-QTOF-MS | Methanol | [37] |
| Isoxanthohumo | ||||
| Apigenin 7-O-glucoronide | ||||
| Chrysoeriol 7-O-(6″-malonyl-apiosyl-glucoside) | ||||
| Tetramethylscutellarein | ||||
| Myricetin 3-O-arabinoside | ||||
| Hydroxycaffeic acid | ||||
| p-Coumaric acid 4-O-glucoside | ||||
| Glycitin | ||||
| Quercetin 3-O-β-D-galactopyranoside |
C. Dahurica; Fruits |
HPLC, UV, 1H NMR, and 13C NMR | Methanol | [39] |
| Isorhamnetin 3-O-α-L-rhamnopyranosyl-7-O-β-D-glucopyranoside | ||||
| 2″-O-rhamnoside |
C. pinnatifida Bge; Leaves |
HPLC-QTOF-MS | 75% Ethanol | [53] |
| Orientin | ||||
| Iso-orientin | ||||
| Crataequinone A-B |
C. Pinnatifida; Fruits |
NM | NM | [24] |
| Pinnatifinosides A-D |
C. Pinnatifida; Leaves |
|||
| Pinnatifins C-D,I | ||||
| 1β, 9α-Dihydroxyeudesm-3-en-5β, 6α, 7α, 11α H-12, 6-olide |
C. Cuneata; Fruits |
|||
| Proanthocyanidin A2 | NM; Leaves and flower | HPLC | Acetone–water (7v/3v) | [29] |
| Proanthocyanidin B2 | ||||
| Proanthocyanidin B4 | ||||
| Proanthocyanidin B5 | ||||
| Proanthocyanidin C1 | ||||
| Proanthocyanidin D1 | ||||
| Proanthocyanidin E1 | ||||
| Epicatechin-(4β→6)-Epicatechin-(4β→8)-epicatechin | ||||
| Epicatechin-(4β→8)-epicatechin-(4β→6)-epicatechin | ||||
| Pinnatifinosides I |
C. pinnatifida Bge. var. major N.E.Br; Leaves |
UV, IR, MS, and 1D, 2D NMR |
80% Ethanol | [30] |
| (+)-Taxifolin | C. Sinaica; Leaves | 1H NMR and 13C NMR | 70% Acetone | [31] |
| (+)-Taxifolin 3-O-xylopyranoside | ||||
| (+)-Taxifolin 3-O-arabinopyranoside 3-O-arabinopyranoside | ||||
| Crateside |
C. monogyna and C. pentagyna; Leaves |
UV | 20% Ethanol | [32] |
| Neoschaftoside | C. Monogyna; Leaves | UV and TLC | Chloroform and butanol. | [33] |
| Neoisoschaftoside | ||||
| Cratenacin | C. Curvisepala; Leaves | UV and IV | NM | [34] |
NM: Not mentioned in the article; HPLC-ESI-MS/MS: high performance liquid chromatography-electrospray ion trap mass spectrometry; HPLC-QTOF-MS: high performance liquid chromatography-quadrupole-time of flight mass spectrometry; UPLC/Q-TOF-MS: ultra-high performance liquid chromatography-tandem quadrupole time of flight mass spectrometry; RP-HPLC: reverse phase high-performance liquid chromatography; VIP marker from OPLS-DA: VIP (variable importance in projection) selection method following supervised OPLS-DA; UHPLC-ESI-QTOF-MS: liquid chromatography coupled to quadrupole-time-of-flight mass spectrometry.
Table 3.
Terpenoids identified from Hawthorn.
| Name | Species and Organ | Methodological and Analytical Approach | Extraction Solvent | References |
|---|---|---|---|---|
| Ursolic aldehyde | C. dahurica; Fruits |
HPLC, 1H NMR and 13C NMR, UV | Methanol | [39] |
| Uvaol | ||||
| Ursolic acid | ||||
| Pomolic acid | ||||
| Euscaphic acid | ||||
| Tormentic acid | ||||
| 3-Epi-2-oxopomolic acid | ||||
| 2α, 19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid | ||||
| Fupenzic acid | ||||
| 2α-Hydroxy oleanolic acid | ||||
| Oleanolic acid | ||||
| Pinnatifidanoside A-D |
C. pinnatifida; Leaves |
HRESIMS, 1H NMR, and 13C NMR | 75% Ethanol |
[57] |
| Byzantionoside B | ||||
| (3S, 5R, 6R, 7E, 9R)-3,6-Epoxy-7-megastig men-5,9-diol-9-O-β-D-glucopyranoside | ||||
| (6S, 7Z, 9R)-Roseoside | ||||
| Icariside B6 | ||||
| Linalool oxide β-D-glucoside | ||||
| Shanyenoside A | ||||
| Dihydrocharcone-2′-β-D-glucopyranoside | ||||
| Eriodectyol | ||||
| Shanyeside C,D,F |
C. pinnatifida; Leave |
HPLC-QTOF-MS | 75% Ethanol | [53] |
| Euodionosides D | ||||
| Linarionoside A,B | ||||
| (6S, 7E, 9R)-6,9-Dihydroxy-4,7-megastiymadien-3-one-9-O-[β-D-xylopyranosy-(1→6)-β-D-glucopyranoside] | ||||
| Linalool oxide β-D-glucoside | ||||
| (6R, 9R)-3-Oxo-α-ionol-9-O-β-D-glucopyranoside | ||||
| Pisumionoside | ||||
| (3S, 5R, 6R, 7E, 9S)-Megastiman-7-ene-3,5,6,9-tetrol | ||||
| Pinnatifidanoside G | ||||
| Norhawthornoid B | ||||
| Corosolic acid |
C.Pinnatifida; Fruits |
HPLC | 80% Acetone | [44] |
| Maslinic acid | ||||
| (3R,5S,6S,7E,9S)-Megastigman-7-ene-3,5,6,9-tetrol 9-O-β-D-glucopyranoside | C. Pinnatifida; Leaves |
1H NMR, 13C NMR, HSQC, HMBC, and NOESY |
70% Ethanol |
[42] |
| (6S,7E,9R)-6,9-Dihydroxy-4,7-megastigmadien-3-one 9-O-[β-D-xylopyranosyl-(1″→6′)-β-D-glucopyranoside] | ||||
| Linarionoside A-C | C. Pinnatifida; Leaves | 1H NMR and 13C NMR | 70% Ethanol |
[43] |
| 3β-D-Glucopyranosyloxy-β-ionone | ||||
| Icariside B6 | ||||
| Pisumionoside | ||||
| (3S,5R,6R,7E,9R)-3,6-Epoxy-7-megastigmen-5,9-diol-9-O-β-Dglucopyranoside | ||||
| (6S,7E,9R)-Roseoside | ||||
| (6R,9R)-3-Oxo-α-ionol-9-O-β-D-glucopyranoside | ||||
| 4-[4β-O-β-D-Xylopyranosyl-(1″→6′)-β-D-glucopyranosyl-2,6,6- trimethyl-1-cyclohexen-1-yl]-butan-2-one |
C. Pinnatifida; Leaves |
1H NMR, 13C NMR, HSQC, HMBC, and NOESY |
70% Ethanol |
[42] |
| (3S,9R)-3,9-Dihydroxy-megastigman-5-ene 3-O-primeveroside | ||||
| (3R,5S,6S,7E,9S)-Megastiman-7-ene-3,5,6,9-tetrol | ||||
| (5Z)-6-[5-(2-Hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl] -3-methylhexa-1,5-dien-3-ol |
||||
| (5Z)-6-[5-(2-O-β-D-Glucopyranosyl-propan-2-yl)-2-methyl tetrahydrofur-an-2-yl]-3-methylhexa-1,5-dien-3-ol |
||||
| 5-Ethenyl-2-[2-O-β-D-glucopyranosyl-(1′′→6′)-β-D-glucopyranosyl-propan-2-yl]-5-methyltetrahydrofuran-2-ol |
HPLC-QTOF-MS: high performance liquid chromatography-quadrupole-time of flight mass spectrometry; HRESIMS: high resolution electrospray ionization mass spectroscopy.
3. Applications in Food Products
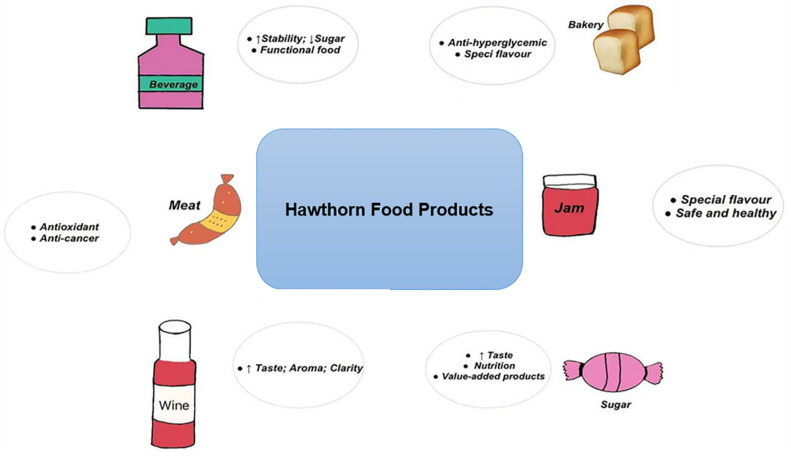
Fresh hawthorn fruit can be eaten directly without any fumigation or washing. To meet the high quality standards expected by consumers, hawthorn fruit has been processed into many types of products. With the continued development of science and technology, such as the enzyme industry [58], homogenizer products and membrane technology, a new phase in the processing of hawthorn has been introduced and its product range is becoming more and more diverse. Figure 2 shows modern food applications of hawthorn.
Figure 2.
Modern food applications of hawthorn.
3.1. Traditional Hawthorn Products
Hawthorn is rich in nutritional value, stimulating appetite and digestion, and is widely used in the food industry [15]. There are many products made from hawthorn on the market, with more than 150 types of products sold. Traditional hawthorn products in China mainly include sugar gourd, hawthorn cakes, hawthorn preserves, canned hawthorn, hawthorn chips, and hawthorn roll.
3.2. Bakery Products
In the production of hawthorn bakery products, it is most important to use effective methods to retain its effective active ingredients and increase the absorption and dissolution properties of hawthorn in bakery products. Wang et al. [59] used ultra-fine grinding technology to produce hawthorn bread with 3% hawthorn powder addition, 0.6% salt, 18% sugar, and 0.5% bread amendment as a formula to improve its health benefits and special flavor.
The addition of hawthorn to the whole wheat flour bread has the effect of promoting the normal function of the digestive and circulatory system and also has an anti-hyperglycemia effect, this raw material is inexpensive and can be consumed by people with type 2 diabetes [60].
3.3. Brewing Products
Hawthorn is so rich in vitamins, minerals, and active substances that can meet people’s nutritional requirements for fruit wines. The sales of fruit wines in China are increasing at a rate of 15% and the development prospects are very promising. Beers produced from the North American shrub, star-spotted hawthorn, not only have a higher antioxidant capacity and higher polyphenol concentration, but also have a greater degree of improvement in taste, aroma, clarity, and overall impression [61]. Treatment of hawthorn wine with pectinase facilitated the reduction of pH and the release of methanol in the wine and accelerated the clarification of hawthorn wine [62].
With the widespread use of hawthorn, vinegar has become more than a simple flavoring agent, and its health maintenance and wellness benefits are receiving more and more attention. The production of hawthorn vinegar from hawthorn berries not only has good characteristics of volatile aromatic compounds that enrich the taste of the vinegar, but also has highly bioactive phenolic compounds that exert a nutritional and health value, increasing the area of use and consumption of hawthorn berries [63]. The use of hawthorn for the production of hawthorn vinegar is a more efficient way to produce innovative and healthy products.
3.4. Beverages
Pectin extracted from hawthorn wine residue was used to produce yogurt and was able to improve the stability and sensory acceptability of the yogurt, which offered the possibility of developing hawthorn wine by-products [64]. Microencapsulated Lactobacillus rhamnosus GG in hawthorn berry tea met the minimum requirement of 106–107 cfu/mL for probiotics, which can play a therapeutic and health care role, and the optimized microspheres of microencapsulated Lactobacillus rhamnosus GG could improve the functional properties of hawthorn tea. Lactobacillus rhamnosus GG tea would be a functional food product with potential added value that could be marketed [65]. Water kefir beverages (lactic acid bacteria drinks) prepared with hawthorn as a food matrix have a reduced sugar content during the fermentation process when fermented sugars are converted to ethanol and carbon dioxide. This is an important alternative for consumers who are unable to consume dairy products as a source of beneficial microorganisms [66].
Hawthorn drinks have unlimited potential both in terms of taste and nutritional value, but the acid content of hawthorn drinks is high. The removal of organic acids from hawthorn improves the organoleptic properties but results in the loss of the important functional compound TF (flavonoids) in the deacidification process. In China, there is currently no hawthorn juice with acceptable acidity and functional ingredients also present in the original juice [67].
3.5. Meat Products
The active ingredients in hawthorn have anti-cancer and anti-oxidant properties. Using a recipe of 6% jam, 6.5% rose paste, 5% milk powder and 0.004% sodium nitrite, Zhou et al. [68] incorporated hawthorn and rose into traditional sausages, giving them a new taste and flavor, and solving the food safety problem of nitrite in traditional cured sausages. The antioxidant and antibacterial effects of phenolics in hawthorn have also been investigated in a variety of commercial foods such as lamb burgers, frankfurters, and pork liver [12].
3.6. Jams
As the perfect companion to bread and other pastries, the consumer demand for jam is gradually increasing. According to China Industry Research Network, the consumption of jam in China has been growing at a rate of around 15%. Hawthorn jams on the market at present are broadly divided into two types: an ordinary hawthorn jam and compound jam with hawthorn flavor, such as hawthorn leaf flavonoid jam [69] and hawthorn passion fruit jam [70].
Li et al. [59] used 1.15% hawthorn leaf flavonoids, 45% white sugar, 0.30% pectin, 0.20% xylitol, and 0.16% citric acid as the formula to develop hawthorn leaf flavonoid jam with flavonoid flavor. The composite hawthorn jam not only meets the need for a safe and healthy jam, but also further enhances the use of hawthorn and hawthorn by-products.
3.7. Sugar Products
Hawthorn candy products can change the sour and astringent taste of hawthorn itself and expand the consumer market for hawthorn. Combining hawthorn with fondant not only enriches its taste, but also adds nutritional health functions to fondant [71].
With the increasing demand for hawthorn products, China produces approximately 1.5 million tons of hawthorn kernels (HK) annually. Hawthorn kernels, as a major by-product in the hawthorn processing industry, account for approximately 30% of the hawthorn, and as an agricultural lignocellulosic biomass containing 28% xylan, it is valuable to use HK to develop value-added products such as xylose and oligosaccharides (XOS) [72].
4. Ethnomedicinal and Biotechnological Uses
The most common traditional use of hawthorn is as a food, whether eaten as a fruit or in hawthorn products, which are very popular. Hawthorn has ethnomedicinal value for its digestive and anti-cardiovascular properties. In Europe, the use of hawthorn as an herbal remedy dates back to the late 19th century. The use of its thorns as a dermatological aid to detect boils and treat swellings (i.e., rheumatism), Kwa-kiutl, Okanagon and Okanagan-Colville and the use of decoctions by the Iroquois as a witchcraft medicine to cause or prevent a person from ‘breaking out like a cancer’ [24]. Hawthorn also contains bioactive components with great potential for the pharmaceutical industry, including polyphenols and flavonoids.
Hawthorn berries are rich in flavonoids, which have been used as reducing and stabilizing agents in the green synthesis of CP-AuNps and CP-AgClNps nanoparticles as antimicrobial agents [73]. Hawthorn kernels, a by-product of approximately 30% of hawthorn berries, contain a large amount of agricultural lignocellulosic biomass of 28% xylan, which has the potential to be processed into xylose [72]. In addition, the many active plant polyphenols in hawthorn, which have a delaying effect on browning, are a very promising alternative to anti-browning substitutes [74].
5. Health Benefits
The active ingredients in hawthorn are the basis for the wide range of health benefits it exerts (Table 4). For example, polyphenolic compounds are good antioxidants [12] and immunomodulators [75], flavonoids have anti-inflammatory [76] and anti-atherogenic activity [77], lignans have antibacterial [78] and antioxidant activity [39], while triterpenoidshave anticancer [79], anti-inflammatory, and anti-proliferative activities [41]. It is therefore essential to advocate the exploitation of the therapeutic potential of hawthorn in new food products to improve and maintain the nutritional and health indices of the population.
5.1. Anticancer
At present, there is little information and research on hawthorn’s anticancer effects. However, the bioactive substances contained in hawthorn are believed to have beneficial effects on human cancer cells [62]. The anticancer effects of hawthorn can be divided into two main areas: hawthorn extract and hawthorn isolated compounds.
Qiao et al. [17] found that triterpenoids extracted from hawthorn with acetone had significant inhibitory effects on the proliferation of human breast and hepatocellular carcinoma cells. Ursolic acid, a triterpenoid, has been shown to induce apoptosis of MDA-MB-231 cancer cells via the mitochondrial pathway [44]. (−)-Epicatechin (EC) was extracted from the total oligomeric flavonoid (TOF) extract of hawthorn. At the same incubation time (48 h), 400 μg/mL of TOF and 200 μg/mL of EC could reduce the viability of melanoma cells in B16F10 mice by 77.57% and 66.55%, respectively. Meanwhile, the mean tumor weight (0.24 g) and volume (242 mm3) of the mice in the TOF extract group were significantly lower than those in the control group (1.5 g, 3653.75 mm3), indicating that TOF extract could exert anti-tumor effects by inhibiting the growth of tumors in vivo [55].
Similarly, compounds isolated from hawthorn have a wide range of anticancer activities. The apoptosis rate of human neuroblastoma SHSY-5Y increased from 9 to 54% with increasing doses of hawthorn acid. Maslinic acid inhibited the activity of anti-apoptotic proteins Bcl-2 and Bcl-xL in SHSY-5Y cells, and the activity of pro-apoptotic Bax protein was significantly enhanced, which exerted anti-proliferative effect and achieved anticancer purpose [79]. In addition, hawthorn polysaccharide can inhibit the proliferation of human colon cancer cells. 1000 μg/mL of Hawthorn Polysaccharide (HPS) could significantly increase the proportion of G2/M phase (17.35%) and S phase (67.82%) of tumor cells. Also, it increases the rate of apoptosis of cancer cells [49]. Hawthorn isolated compounds have shown anti-tumor activity both in vivo and in vitro and are considered a promising drug for the treatment of melanoma [55].
5.2. Cardiovascular System
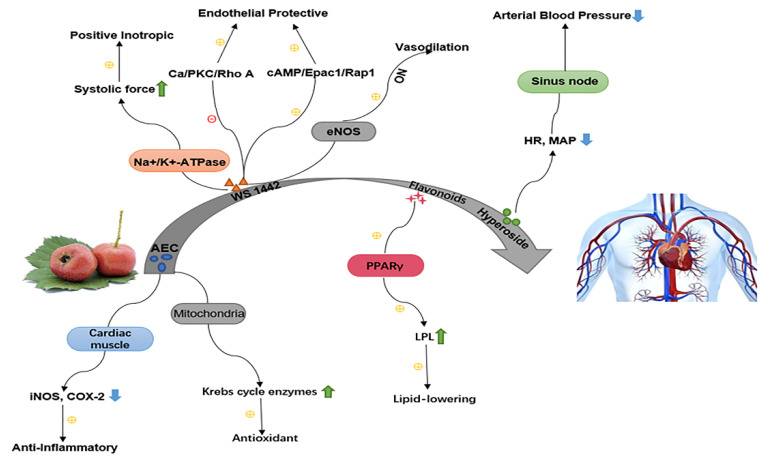
The proanthocyanidins, total flavonoids, and other extracts contained in the fruits, leaves, and flowers of hawthorn are commonly used in the treatment of cardiovascular disease due to their significant in vitro effects and apparent safety profile [25,80,81,82,83,84,85,86] (Figure 3).
Figure 3.
Mechanism of action of hawthorn in the protection against cardiovascular disease. (1). Alcoholic extract of Crataegus oxyacantha (AEC) pretreatment maintained mitochondrial antioxidant status and prevented mitochondrial lipid per-oxidative damage and decrease in Krebs cycle enzymes induced by isoproterenol in rat heart.; AEC can act on myocardial tissue to reduce iNOS expression and downregulate COX-2 to exert anti-inflammatory effects. (2). Hawthorn extract WS1442 increases contractility and increases positive muscle strength by inhibiting the Na+/K+-ATPase pump; WS1442 acts at the serine 1177 site to phosphorylate eNOS and increase NO-mediated vasodilatation; WS1442 effectively protects the vascular endothelium by inhibiting the Ca/PKC/Rho A pathway and activating the cAMP/Epaci/Rap1 pathway. (3). Hawthorn flavonoids exert hypolipidemic effects by acting on the PPARγ pathway to increase LDL expression in blood vessels. (4). High doses of hawthorn ginsenoside can cause significant reductions in heart rate (HR) and mean arterial pressure (MAP), and induce sinus node.
5.2.1. Anti-Hypertensive
The diastole of the peripheral blood vessels is the main cause of the fall in blood pressure [87]. Hawthorn fruit extract confers antioxidant effect on high-salt induced hypertension and it may be used as a nutritional supplemental therapeutic drug to protect against high-salt induced hypertension in renal medulla [88]. A randomized controlled trial of type 2 diabetes showed that patients given hawthorn extract (1200 mg per day) had a significantly greater reduction in mean diastolic blood pressure than those given placebo (the prescribed drug) [9]. The hydroalcoholic extract of hawthorn flower heads was shown to inhibit thromboxane A2 (TXA2) activity and promote peripheral vasodilation, thereby lowering blood pressure [89].
5.2.2. Lipid Regulation and Anti-Atherosclerosis
Non-alcoholic fatty liver and obesity caused by disorders of lipid metabolism have become serious social and health problems worldwide [90]. Hawthorn extract has the ability to regulate blood lipid levels and anti-atherosclerotic effects [82,91].
Hawthorn treated with n-ethanol and ethyl acetate alleviated hyperlipidemia by regulating disorders of lipid, energy and amino acid metabolism and reducing oxidative stress, resulting in a decrease in plasma levels of total cholesterol (TC), triglycerides (TG), and low density lipoprotein cholesterol (LDL-C) in hyperlipidemic rats [92]. Similarly, Hawthorn extract was able to improve hyperlipidemia by improving the lipid profile, reducing oxidative stress and lowering total and LDL cholesterol levels in the serum of ovariectomized rats (OVX) [83]. In addition, the alcoholic extract of hawthorn is able to prevent atherosclerosis. The alcoholic extract of Zhongtian hawthorn promotes cholesterol elimination [93]. Mice with knockout apoE gene showed a significant reduction of 23.1% in the area of atherosclerotic lesions by feeding hawthorn leaf flavonoids [76].
5.2.3. Cardioprotective Effect
WS 1442, a dried extract of Hawthorn with flowering leaves (4–6.6:1), has been shown to have positive inotropic and anti-arrhythmic properties [94]. Hawthorn ethanol extract (HAE) can increase the activity of glutathione peroxidase (GSH-Px) and decrease the content of malonaldehyde (MDA) in the myocardium, alleviating inflammatory cell infiltration, and myofibrillar disorders in the myocardial structure [84]. Hawthorn leaf flavonoids (HLF) protect against diabetes-induced cardiomyopathy by reducing oxidative stress and inflammation through the PKC-α signaling pathway [25]. The citric acid, caffeic acid, chlorogenic acid, and quercetin in hawthorn also have a protective effect against myocardial ischemia [82]. Dried hawthorn berries treated with honey can increase the content of organic acids, phenylpropanoids, and flavonoids in hawthorn, thus further enhancing the anti-myocardial ischemia effect of hawthorn [22].
5.3. Anti-Hyperglycemic
Diabetes mellitus is a chronic metabolic disease characterized by high blood sugar [85]. The phenolic and flavonoid compounds in hawthorn are considered to be important active substances in the fight against diabetes. Hawthorn is able to improve hyperlipidemia by lowering blood sugar and triglyceride levels caused by a high-fat diet [85]. Human α-glucosidase is the main enzyme catalyzing the final step of carbohydrate hydrolysis in the digestive system [86,95]. The quercetin in hawthorn can effectively inhibit the activity of alpha-glucosidase enzyme, resulting in a reduction in the release and absorption of glucose thereby lowering blood sugar levels [96,97]. In addition, hawthorn leaf flavonoids have a protective effect on the myocardium of streptozotocin-induced diabetic rats [25], and the mechanism may be due to the reduction of oxidative stress and inflammation through the PKC inactivation pathwayction in the release and absorption of glucose thereby lowering blood sugar levels [25].
5.4. Antibacterial and Anti-Inflammatory
The lignans, polysaccharides and total flavonoids in hawthorn exert their anti-inflammatory effects mainly by inhibiting the activity of inflammatory factors such as NO and TNF-α. Among the compounds isolated by Peng et al., lignans 7 to 12 showed strong inhibition of NO, especially compound 12 (IC50: 50.5 μM) showed stronger NO inhibitory activity than dimethyltetracycline (IC50: 55.1 μM). While compounds 1–4 had inhibitory effects on TNF-α with IC50 values of 76.1, 47.9, 84.4, and 94.2 μM respectively [48].
Hawthorn pectin (POS) improves liver inflammation by reducing total liver fat content and levels of TNF-α and IL-6, increasing IL-10 levels and inhibiting NF-κB activation [98]. HAW1-2 suppresses colitis by inhibiting inflammatory factors such as TNF-α, modifying the gut microbiota and producing SCFAs [50]. Similarly, hawthorn aqueous extract significantly reduced LPS-induced NO production in RAW cells to protect RAW264.7 cells from lipopolysaccharide (LPS) induced apoptosis after 24 h stimulation, resulting in 45.7% of cells surviving [99].
Hawthorn seed extract showed antibacterial activity against vaginitis pathogens (Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Gardnerella vaginalis, Candida albicans), especially 2,6-dimethoxyphenol in hawthorn seeds could inhibit the growth of vaginitis pathogens [67].
Hawthorne acid (MA) can also be used for arthritis, especially in the elderly where arthritis is now very common. The pain caused by arthritis affects the patient’s normal life and although not life-threatening, it causes great discomfort to the patient. Although MA has recently been found to relieve arthritis pain, its anti-inflammatory mechanism still needs to be further explored and studied to lay a solid foundation for its clinical application [100].
5.5. Antioxidant
Hawthorn has hypolipidemic and vascular protective effect, which is mainly attributed to its high antioxidant effect. Phenolic compounds, flavonoids, and triterpenoids are important components of natural antioxidant substances.
In hawthorn fruits, free phenolic compounds accounted for 35.3–37.8% of the antioxidant activity, followed by insoluble bound phenolic compounds accounting for 25.0–27.0% of the antioxidant activity [101]. Hawthorn polyphenol extract (HBE) can regulate the apoptosis of HaCaT cells by reducing reactive oxygen species (ROS) production, DNA damage and p53 activation, effectively reducing UVB-induced photodynamic and non-photodynamic damage and providing protection to the skin [54,102].
Hawthorn total flavonoid extract (TOF) and (−)-epicatechin (EC) inhibited acute cellular oxidative stress triggered by the free radical initiator ABPA. And both TOF extract and EC at all concentrations tested were taken up by cells and both significantly inhibited the oxidation of DCFH [55].
5.6. Anti-Digestion
Hawthorn is a common herbal remedy for indigestion, and its herbal tonics and extracts have been shown to improve gastrointestinal motility. The phenolic substances in hawthorn, especially phenolic acids, play an important role in the digestive system due to their stable structure [103].
Hawthorn soup can relieve indigestion caused by high-calorie diet (HC-DID). The bidirectional action of brain and intestine is the main mechanism for treating HC-DID. Gavage administration affects the “brain-intestine” axis and gavage administration affects the “intestine-brain” axis. Hawthorn decoction with hawthorn charcoal can improve the brain-gut polypeptide disorder caused by HC-DID by regulating the “brain-gut” axis, significantly reducing the ratio of bacterial gate and mycelium, and improving the intestinal metabolic disorder [91].
Hawthorn extract protects the gastrointestinal tract mainly by altering disorders of lipid, energy, and amino acid metabolism. In the atropine gastrointestinal motility disorder model, the ethanolic extract of hawthorn modulates the metabolism of bile acids and promotes the excretion of lipids to some extent. At the same time, the synthesis of CoA increased, the content of the intermediate acetyl-CoA also increased, and the content of L-tryptophan and serotonin increased. This indicates that the metabolism of the three major nutrients in the model rats was restored to normal and the function of the gastrointestinal tract was improved [92]. Hawthorn seed ethyl acetate extract (HAESE) can lower blood glucose, prevent weight loss, accelerate gastric emptying and small intestine propulsion, effectively regulate plasma gastrointestinal hormones such as gastric hunger hormone, motilin (MTL), and gastrin (GAS), promote gastrointestinal motility in rats with diabetic gastroparesis and alleviate the symptoms of diabetic gastroparesis (DGP) rats [57,104].
5.7. Others
5.7.1. Immune Regulation
Gluten sterols isolated from hawthorn significantly increase white blood cell counts and enhance phagocytic activity of macrophages [40]. Hawthorn phenolic extracts have a strong stimulatory effect on splenocytes and lymphocyte subsets. This is mainly due to the fact that epicatechin and proanthocyanidins B2 and B4 in hawthorn promote the proliferation of splenocytes, significantly increase the percentage of B cells, decrease the percentage of Th cells and stimulate the humoral immune response. And the effect is more pronounced at high doses (100 and 200 mg/kg) of the extract [75].
5.7.2. Anticoagulant
Components of hawthorn such as bioflavonoids and proanthocyanidins have beneficial effects on the blood coagulation system. In Sprague Dawley rats, continuous administration of hawthorn extract revealed a significant increase in cardiac antithrombin III and a significant decrease in their hepatic factor X, suggesting that hawthorn has blood thinning properties [105]. The polyphenol-polysaccharide conjugate isolated from the hawthorn fruit prolonged the coagulation process of plasma in vitro experiments, even at concentrations as low as 31.25 μg/mL. However, only the product isolated and processed from the floral source was highly selective, mainly because it was a non-direct inhibitor of the non-thrombin-mediated factor Xa [106]. Therefore, special attention should be paid to the use of anticoagulant/antiplatelet agents when using hawthorn extract.
5.7.3. Neuroprotective
Alzheimer’s disease (AD) is one of the major neurodegenerative diseases in which the main component is beta amyloid (Aβ) and oligomers of Aβ have direct neurotoxicity mainly through membrane receptors, channels and intracellular dysfunction [107].
Hawthorn ethanol extract (CPE) has long been used as a traditional medicine for the treatment of various diseases, and it mainly blocks the aggregation of Aβ and degrades Aβ protofibrils in a concentration-dependent manner [108]. Among the seven lignans isolated from hawthorn seeds, compounds 5 and 6 showed strong inhibition of Aβ1-42 and were able to inhibit the self-induced Aβ aggregation activity [46].
Similarly, depression, the most common chronic mental disorder, has a huge impact on society. Flavonoid and proanthocyanidin components of hawthorn, such as chlorogenic acid, inhibit stress hormone-induced depressive behavior via hippocampal astrocyte monoamine oxidase B-reactive oxygen species signaling [109].
Total flavonoids from hawthorn leaves can also reduce apoptosis in rats with spinal cord injury, exert neuroprotective effects and promote the recovery of motor function in rats with spinal cord injury [110].
Table 4.
Biological activity and mechanism of action of Hawthorn.
| Extracts or Compounds | Observation or Methods | Effects | References | |
|---|---|---|---|---|
| Anticancer activity | ||||
| Triterpenoids isolated from hawthorn berries | In vitro, MTT assay. | All 15 triterpenoids showed effective antiproliferative activity against human HepG2, MCF-7 and MDA-MB- 231 tumor cells showed potent anti-proliferative activity(compound 2–4 EC50 < 5 µM). |
[111] | |
| Ursolic acid, oleanolic acid, corosolic acid, and maslinic acid | In vitro. | CA showed the highest antiproliferative activity against human HepG2 (EC50 = 9.44 μM), MCF-7 (EC50 = 22.01 µM) and MDA-MB-231 (EC50 =26.83 μM) tumor cells among the four triterpenoids, followed by UA, MA, and OA. |
[44] | |
| Phenylpropanoids isolated from hawthorn fruit | In vitro, MTT assay. | Five compounds (1a/1b, 2–4) were used in the treatment of human HepG2 and Hep3B cells with better cytotoxicity(1a IC50: 59.57, >100 µM; 1b IC50: 35.37, 70.42 µM; 2 IC50: 27.36, 39.40 µM; 3 IC50: 18.68, 38.96 µM;4 IC50: 17.50, 43.58 µM;). | [112] | |
| Homogeneous polysaccharide (HPS) | In vitro, WST-1 colorimetric method. | Treatments with 500 and 1000 μg/mL of HPS for 12 h resulted in more than 74% of growth inhibition against human HCT116 cell. | [49] | |
| An extract enriched with TOF | In vivo. | TOF extract from hawthorn leaves exerts an antitumor effect by decreasing the melanoma tumor growth in vivo (6 times less weight). | [55] | |
| Maslinic acid | In vitro, AO/EB staining assay and Annexin V/PI dual staining. | It can cause human neuroblastoma SHSY-5Y cells increase the percentage of apoptotic cells from 9% in the control group to 54% at higher drug doses. | [18] | |
| Cardiovascular system activities | ||||
| Hawthorn Leaf Flavonoids(HLF) | In vivo. | HLF protect against diabetes-induced cardiomyopathy in rats via PKC-α signaling pathway. | [25] | |
| Hawthorn Leafs Extract | In vitro, MTS. | Hydroalcoholic extracts of hawthorn leaves at 300 and 1000 mg/mL significantly reduced the frequency of arrhythmias induced by adrenaline stimulation. | [82] | |
| Hawthorn Fruit Extract (HFE) |
In vivo. | HFE could dose-dependently reduce the TMAO-aggravated atherosclerosis. | [113] | |
| Flavonoids | In vivo, carrageenan-induced tail thrombosis model. |
Inhibiting TXA2 release, decreasing the level of Ca2+ in platelets or blocking glycoprotein IIb/IIIa receptors may be the mechanism of the antithrombotic effects of flavonoids. | [81] | |
| Hawthorn Fruit Extract | In vivo, Western Blot. | The hepatic triglyceride (TG) and malondialdehyde (MDA) levels were significantly reduced in the hawthorn groups compared with the ovariectomized group (p < 0.05). | [83] | |
| Hawthorn Fruit Extract | In vivo, spectrophotometry. | Compared with the blood TC levels of rats in the type 2 diabetic group, the blood TC levels of rats in the high, medium and low dose of Hawthorn extract decreased by 162.54%, 122.68% and 92.13% respectively. | [86] | |
| Hawthorn Fruit Extract | In vivo. | Echocardiographic parameters (LVESD, LVEDD) were reduced in rats with chronic heart failure treated with hawthorn extract (p < 0.01) | [84] | |
| Hawthorn Extract | In vivo. | Hawthorn extract groups suppressed the high-fat diet-induced increases in the concentrations of LDL (p < 0.05). | [85] | |
| Anti-hyperglycemic activity | ||||
| Hawthorn Fruit Extract | In vivo. | Hawthorn extract in high, middle and low dose could significantly reduce the fasting blood glucose levels of type II diabetic rats from 20.25 ± 1.9 mmol L−1 to 10.5 ± 0.87 mmol L −1, 15.13 ± 0.55 mmol L −1 and 17.9 ± 0.87 mmol L−1 (p < 0.01 and p < 0.05). | [86] | |
| Hawthorn polyphenols, D- chiro- inositol (DCI), and epigallocatechin gallate (EGCG) |
In vitro. | Three ingredients exerted the synergistic hypoglycemic effect to enhance glucose consumption and glycogen levels and inhibit hepatic gluconeogenesis in IR-HepG2 cells. | [95] | |
| Hawthorn Extract | In vivo. | Hawthorn treated groups (0.5 g/kg/day, 1.0 g/kg/day) showed a significant reduction in insulin resistance compared with the HF group (p < 0.05, p < 0.01). | [85] | |
| Antibacterial and anti-inflammatory activities | ||||
| Hawthorn Fruit Extract | In vivo. | The hawthorn treatment group reduced the levels of IL-6, IL-8, IL-1β and TNF-α in cardiomyocytes due to doxorubicin treatment for heart failure (p < 0.01). | [93] | |
| Water fraction from hawthorn fruit | In vitro, ELISA. | Water fraction from hawthorn fruit at 200, 400 and 600 µg/mL increased the survival rate of RAW264.7 cells to 61.8%, 72.7% and 83.4% respectively. | [99] | |
| Hawthorn Methanolic Extract (ME) | In vitro. | ME from hawthorn had a minimum MIC and MBC value of 1.25 µg/mL against S. aureus and S. typhimurium. | [38] | |
| Hawthorn polysaccharide (HAW1-2) | In vivo. | The relative expression of IL-1β, IL-6 and TNF-α were suppressed after HAW1–2 treatment. | [50] | |
| Hawthorn phenolic extract | In vivo. | The extract decreased the percent-age of CD4−CD8− and CD4+ thymocytes but elevated the percentage of CD4+CD8+ and CD8+ thymic cells, increased the total number, percentage, and absolute count of T and B splenocytes. | [75] | |
| Pectin oligosaccharide (POS) | In vivo, ELISA. | Higher dose (0.75, 1.5 g/kg) of POS significantly (p < 0.01) decreased the contents of hepatic TNF-α and IL-6, while significantly (p < 0.05–0.01) increased the level of IL-10, compared with the high fat control group. | [98] | |
| Total Flavonoid Extract from Hawthorn (TFH) | In vitro. | TFH (50–200 µg/mL) treatment inhibited the increase of inflammatory cytokines IL-6, IL-1β, MCP-1 and IL-8 in Caco-2 cells in a dose-dependent manner. | [76] | |
| Anti-digestion activty | ||||
| Hawthorn Seed Eextract (HSEAE) | In vivo. ELISA. | Different doses of HSEAE effectively promoted the gastric emptying and small intestinal propulsion (p < 0.05 or p < 0.01). In addition, HSEAE increased SOD and GSH-Px in the rats’ stomachs while decreasing MDA, and increased plasma ghrelin while decreasing MTL and GAS (p < 0.05 or p < 0.01). | [104] | |
| Ethyl acetate part of hawthorn | In vivo, LC-MS. | The effect of ethyl acetate extract of hawthorn on gastric emptying rate and intestinal propulsion rate in a rat model of atropine sulfate-induced gastrointestinal motility retardation was significant (p < 0.05, p < 0.001). | [92] | |
| Charred hawthorn | In vivo. | Hawthorn decoction coupled with the odor of charred hawthorn effectively alleviate high-calorie-diet-induced dys-pepsia in rats by regulating the “Brain-Gut” axis and gut flora. | [91] | |
| Antioxidant activity | ||||
| Triterpenoids isolated from hawthorn berries | In vitro, PSC and superoxide anion free radical assay. | In PSC assay, compounds 1, 10 and 12 had pronounced antioxidant activity with an EC50 of 0.2 ± 0.01, 0.5 ± 0.01, and 0.7 ± 0.01 µM. | [111] | |
| Phenolic composition of Kazakh Crataegus | LC-MS | In the free radical scavenging activity assay (DPPH), the most potent extract was the phenolic compound from hawthorn leaves (IC50 48 ± 2 µg/mL). | [20] | |
| Hawthorn polyphenol extract (HPE) | In vivo and vitro, MTT. | After UVB irradiation, the cell viability significantly decreased (p < 0.05). HPE at 5 and 10 µg/mL significantly increased cell survival (p < 0.05). | [102] | |
| Phenolic compounds | In vitro, ORAC. | The antioxidant activity of phenolic compounds in hawthorn was significant, with ORAC values for the eight phenolic compounds ranging from 5.25 ± 0.54–62.79 ± 1.46 μmol TE/μmol. | [114] | |
| Hawthorn fruit extract | FRAP. | The antioxidant activity was widely varied (p < 0.001) in species of Crataegus, ranging from 0.32–1.84 mmol Fe++/g DW. | [12] | |
| Phenolic compounds | DPPH, ABTS, and FRAP. | The total antioxidant activity of organic fresh hawthorn berry fruit determined by DPPH, FRAP and ABTS assay was up to 286 ± 4, 320 ± 5 and 328 ± 6 μmol TE/g DW. | [54] | |
| Hawthorn extract | DPPH. | The DPPH scavenging capacity of the fresh hawthorn slices was 3.48 mmol TE/100 g DW. | [101] | |
| Extract from peel of hawthorn fruit(EPHF) |
DPPH and ORAC. | EPHF has the strongest oxygen radical scavenging capacity (IC50 = 11.72 μg/mL). | [115] | |
| Organic freeze-dried hawthorn berries (OFDHB) | ABTS, FRAP and DPPH. | The peel of OFDHB sample had the highest antioxidant capacity followed the decreasing order of ABTS (577.5 µmol TE g−1) > FRAP (455.84 µmol TE g−1) > DPPH (410.75 µmol TE g−1) assay. | [4] | |
| Flavonoids | FRAP. | The highest antioxidant activity was observed in the leaves of C. pentagyna as 4.65 mmol Fe++/g DW, whereas the lowest activity (0.9 mmol Fe++/g DW) was found in the leaves of C. azarolus var. aronia. | [116] | |
PSC: peroxyl radical scavenging capacity; MTS: 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay; ELISA: enzyme-linked immunosorbent assay; ORAC: oxygenradical absorbance capacity; DPPH: 1,1-diphenyl-2-picrylhydrazyl; FRAP: ferric-reducing antioxidant Power; ABTS: Total Antioxidant Capacity Assay Kit with ABTS method; 1a/1b, 2–4: (+)-crataegusanoid A, (−)-crataegusanoid A, crataegusanoid B, crataegusanoid C, crataegusanoid D.
6. Conclusions
Hawthorn is a good source of proteins, amino acids, sugars, minerals, vitamins and phytochemicals including terpenoids, phenols, and flavonoids. As a medical herb, hawthorn contains a large number of naturally occurring bioactive chemicals with therapeutic properties and is therefore a highly marketable source of medicines worldwide. However, further in vivo and in vitro studies and clinical trials are needed to assess the link between the chemical composition of hawthorn and the mechanisms of action for the treatment of various diseases.
Dozens of bioactive substances in hawthorn can be extracted as a health product. At present, the processing technology of hawthorn food has been widely emphasized, both traditional processing technology and modern technology. Hawthorn has been processed into many products, such as hawthorn drinks, hawthorn paste, hawthorn vinegar, hawthorn wine and bread. Hawthorn kernels have even been used to produce value-added products such as xylose. However, due to the excessive organic acid content in hawthorn, a large amount of sugar needs to be added for flavouring when producing hawthorn products, which prevents many elderly and diabetic patients from enjoying the health benefits of hawthorn. With the research of deep processing technology of hawthorn, the proper ratio of hawthorn with other materials, the development of new products, the improvement of traditional processing methods, minimizing the loss of functional factors and natural pigments of hawthorn, and the development of new areas of hawthorn utilization, such as: the utilization in special medical use formulae, will produce excellent economic and social benefits.
The rich nutrients in hawthorn seeds and leaves should not be overlooked. China is rich in hawthorn resources and the prospects for developing new medicines or producing hawthorn leaf products would be very promising if this renewable resource could be fully utilised. It is important to look at hawthorn chemistry as a whole. In the future, it is important to continue to explore the nutritional and health benefits of hawthorn and to develop value-added foods and supplements based on the functional components of hawthorn, based on extracts and active substances from other parts of hawthorn.
Author Contributions
Conceptualization, J.Z. and Q.M.; methodology, J.Z., Q.M. and X.C.; writing—original draft preparation, J.Z.; writing—review and editing, Q.M., X.C., G.H. and F.Z.; supervision, Q.M., X.C., F.Z. and G.H.; project administration, Q.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This work was financially supported by National Natural Science Foundation of China (No. 81473104) and Science and Technology Innovation Development Plan of Yantai (No. 2020XDRH105).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Santini A., Novellino E., Armini V., Ritieni A. State of the Art of Ready-to-Use Therapeutic Food: A Tool for Nutraceuticals Addition to Foodstuff. Food Chem. 2013;140:843–849. doi: 10.1016/j.foodchem.2012.10.098. [DOI] [PubMed] [Google Scholar]
- 2.Sixt M., Strube J. Systematic Design and Evaluation of an Extraction Process for Traditionally Used Herbal Medicine on the Example of Hawthorn (Crataegus monogyna JACQ.) Processes. 2018;6:73. doi: 10.3390/pr6070073. [DOI] [Google Scholar]
- 3.Lund J.A., Brown P.N., Shipley P.R. Differentiation of Crataegus spp. Guided by Nuclear Magnetic Resonance Spectrometry with Chemometric Analyses. Phytochemistry. 2017;141:11–19. doi: 10.1016/j.phytochem.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Lou X., Yuan B., Wang L., Xu H., Hanna M., Yuan L. Evaluation of Physicochemical Characteristics, Nutritional Composition and Antioxidant Capacity of Chinese Organic Hawthorn Berry (Crataegus pinnatifida) Int. J. Food Sci. Technol. 2020;55:1679–1688. doi: 10.1111/ijfs.14437. [DOI] [Google Scholar]
- 5.Chinese Medicine Treasures. [(accessed on 12 August 2022)]. Available online: http://zhongyibaodian.com/bencaogangmu/shanzha.html.
- 6.Chinese Pharmacopoeia 2020 Edition I. [(accessed on 12 August 2022)]. Available online: https://db2.ouryao.com/yd2020/view.php?id=fbcdae7d88.
- 7.Ma S., Lu Y. Classification and Phylogenetic Analysis of Chinese Hawthorn Assessed by Plant and Pollen Morphology. Genet. Mol. Res. 2016;3:gmr.15038739. doi: 10.4238/gmr.15038739. [DOI] [PubMed] [Google Scholar]
- 8.Du X., Zhang X., Bu H., Zhang T., Lao Y., Dong W. Molecular Analysis of Evolution and Origins of Cultivated Hawthorn (Crataegus spp.) and Related Species in China. Front. Plant Sci. 2019;10:443. doi: 10.3389/fpls.2019.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Xiong X., Feng B. Effect of Crataegus Usage in Cardiovascular Disease Prevention: An Evidence-Based Approach. Evid.-Based Complement. Altern. Med. 2013;2013:149363. doi: 10.1155/2013/149363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T., Fu S., Huang X., Zhang X., Cui Y., Zhang Z., Ma Y., Zhang X., Yu Q., Yang S. Biological Properties and Potential Application of Hawthorn and Its Major Functional Components: A Review. J. Funct. Foods. 2022;90:104988. doi: 10.1016/j.jff.2022.104988. [DOI] [Google Scholar]
- 11.Nazhand A., Lucarini M., Durazzo A., Zaccardelli M., Cristarella S., Souto S.B., Silva A.M., Severino P., Souto E.B., Santini A. Hawthorn (Crataegus spp.): An Updated Overview on Its Beneficial Properties. Forests. 2020;11:564. doi: 10.3390/f11050564. [DOI] [Google Scholar]
- 12.Alirezalu A., Ahmadi N., Salehi P., Sonboli A., Alirezalu K., Mousavi Khaneghah A., Barba F.J., Munekata P.E.S., Lorenzo J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods. 2020;9:436. doi: 10.3390/foods9040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doellman M., Ragland G., Hood G., Meyers P., Egan S., Powell T., Lazorchak P., Glover M., Tait C., Schuler H., et al. Genomic Differentiation during Speciation-with-Gene-Flow: Comparing Geographic and Host-Related Variation in Divergent Life History Adaptation in Rhagoletis pomonella. Genes. 2018;9:262. doi: 10.3390/genes9050262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yalçın Dokumacı K., Uslu N., Hacıseferoğulları H., Örnek M.N. Determination of Some Physical and Chemical Properties of Common Hawthorn (Crataegus monogyna Jacq. Var. Monogyna) Erwerbs-Obstbau. 2021;63:99–106. doi: 10.1007/s10341-021-00545-x. [DOI] [Google Scholar]
- 15.Zhang J., Deng Y., Tong X., Liang Y., Li Y., Deng C. Nutrition and Health Function of Crataegus pinnatifida Bunge and Its Application Progres. Anhui Agric. Sci. Bull. 2021;27:116–118. [Google Scholar]
- 16.Li L., Gao X., Liu J., Chitrakar B., Wang B., Wang Y. Hawthorn Pectin: Extraction, Function and Utilization. Curr. Res. Food Sci. 2021;4:429–435. doi: 10.1016/j.crfs.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu R., Zhang X., Wang Y., Zhang L., Wang C., Hu F., Ning C., Chen G. Pectin Oligosaccharides from Hawthorn (Crataegus pinnatifida Bunge. Var. Major): Molecular Characterization and Potential Antiglycation Activities. Food Chem. 2019;286:129–135. doi: 10.1016/j.foodchem.2019.01.215. [DOI] [PubMed] [Google Scholar]
- 18.Cosmulescu S.N., Trandafir I., Scrieciu F., Stoenescu A.-M. Content in Organic Acids of Mespilus spp. and Crataegus spp. Genotypes. Not. Bot. Horti Agrobot. Cluj-Napoca. 2020;48:171–176. doi: 10.15835/nbha48111746. [DOI] [Google Scholar]
- 19.Sun B., Huo H., Cai A., Xie Y., Li H., Li D. Determination of contents of eight phenolic acids in Malus doumeri fruit by HPLC. Guihaia. 2021;07:1135–1144. [Google Scholar]
- 20.Elmira B., Wirginia K.-K., Tomasz B., Natalia S., Galiya I., Wojciech K., Kazimierz G., Saken T., Zuriyadda S., Fabio B. Phenolic composition and antioxidant potential of different organs of Kazakh Crataegus almaatensis Pojark: A comparison with the European Crataegus oxyacantha L. flowers. Open Chem. 2018;16:415–426. [Google Scholar]
- 21.Muradoğlu F., Gürsoy S., Yıldız K. Quantification Analysis of Biochemical and Phenolic Composition in Hawthorn (Crataegus spp.) Fruits. Erwerbs-Obstbau. 2019;61:189–194. doi: 10.1007/s10341-018-00415-z. [DOI] [Google Scholar]
- 22.Wu X., Song M., Qiu P., Li F., Wang M., Zheng J., Wang Q., Xu F., Xiao H. A Metabolite of Nobiletin, 4′-Demethylnobiletin and Atorvastatin Synergistically Inhibits Human Colon Cancer Cell Growth by Inducing G0/G1 Cell Cycle Arrest and Apoptosis. Food Funct. 2018;9:87–95. doi: 10.1039/C7FO01155E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu-Gharbieh E., Shehab N.G. Therapeutic Potentials of Crataegus azarolus Var. eu- azarolus Maire Leaves and Its Isolated Compounds. BMC Complementary Altern. Med. 2017;17:218. doi: 10.1186/s12906-017-1729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards J.E., Brown P.N., Talent N., Dickinson T.A., Shipley P.R. A Review of the Chemistry of the Genus Crataegus. Phytochemistry. 2012;79:5–26. doi: 10.1016/j.phytochem.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Min Q., Bai Y., Zhang Y., Yu W., Zhang M., Liu D., Diao T., Lv W. Hawthorn Leaf Flavonoids Protect against Diabetes-Induced Cardiomyopathy in Rats via PKC-α Signaling Pathway. Evid.-Based Complementary Altern. Med. 2017;2017:2071952. doi: 10.1155/2017/2071952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olah N.-K., Burtescu R., Petrescu S., Brasovan A., Chise E., Codruta S., Cobzac A., Hanganu D. Phytochemical Screening of Different Crataegus oxyacantha Extracts. Studia UBB Chem. 2017;62:57–73. doi: 10.24193/subbchem.2017.3.05. [DOI] [Google Scholar]
- 27.Jurikova T., Sochor J., Rop O., Mlcek J., Balla S., Szekeres L., Adam V., Kizek R. Polyphenolic Profile and Biological Activity of Chinese Hawthorn (Crataegus pinnatifida BUNGE) Fruits. Molecules. 2012;17:14490–14509. doi: 10.3390/molecules171214490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X., Li W., Huang D., Yang X. Polyphenols from Hawthorn Peels and Fleshes Differently Mitigate Dyslipidemia, Inflammation and Oxidative Stress in Association with Modulation of Liver Injury in High Fructose Diet-Fed Mice. Chem.-Biol. Interact. 2016;257:132–140. doi: 10.1016/j.cbi.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Hellenbrand N., Sendker J., Lechtenberg M., Petereit F., Hensel A. Isolation and quantification of oligomeric and polymeric procyanidins in leaves and flowers of Hawthorn (Crataegus spp.) Fitoterapia. 2015;104:14–22. doi: 10.1016/j.fitote.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P., Xu S. Flavonoid ketohexosefuranosides from the leaves of Crataegus pinnatifida Bge. var. major N.E.Br. Phytochemistry. 2001;57:1249–1253. doi: 10.1016/S0031-9422(01)00170-4. [DOI] [PubMed] [Google Scholar]
- 31.Shahat A., Ismail S., Hammouda F., Azzam S., Lemiere G., Bruyne T., Swaef S., Pieters L., Vlietinck A. Anti-HIV activity of flavonoids and proanthocyanidins from Crataegus sinaica. Phytomedicine. 1998;5:133–136. doi: 10.1016/S0944-7113(98)80010-X. [DOI] [PubMed] [Google Scholar]
- 32.Nikolov N., Batyuk V., Ivanov V. Crateside—A New Flavonol Glycoside Crataegus monogyna and C. pentagyna. Chem. Nat. Compd. 1973;9:150–151. doi: 10.1007/BF00563332. [DOI] [Google Scholar]
- 33.Nikolov N., Dwllamonica G., Chopin J. Di-C-Glycosyl Flavones from Crataegus monogyna. Phytochemistry. 1981;20:2780–2781. doi: 10.1016/0031-9422(81)85290-9. [DOI] [Google Scholar]
- 34.Batyuk V., Chernobrovaya N., Prokopenko A. Cratenacin—A New Flavone Glycoside from Crataegus Curvisepala. Khim. Prir. Soedin. 1966;2:90–93. [Google Scholar]
- 35.Kurkin V.A., Pravdivtseva O.E., Shaikhutdinov I.K., Kurkina A.V., Volkova N.A. Quantitative Determination of Total Flavonoids in Blood-Red Hawthorn Fruit. Pharm. Chem. J. 2020;54:36–39. doi: 10.1007/s11094-020-02151-9. [DOI] [Google Scholar]
- 36.Sagaradze V.A., Babaeva E.Y., Kalenikova E.I. HPLC-UV Method for Determing Flavonoids in Hawthorn Flowers and Leaves. Pharm. Chem. J. 2017;51:277–280. doi: 10.1007/s11094-017-1597-0. [DOI] [Google Scholar]
- 37.Rocchetti G., Senizza B., Zengin G., Mahomodally M.F., Senkardes I., Lobine D., Lucini L. Untargeted Metabolomic Profiling of Three Crataegus Species (Hawthorn) and Their in vitro Biological Activities. J. Sci. Food Agric. 2020;100:1998–2006. doi: 10.1002/jsfa.10216. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L., Zhang L., Xu J. Chemical Composition, Antibacterial Activity and Action Mechanism of Different Extracts from Hawthorn (Crataegus pinnatifida Bge.) Sci. Rep. 2020;10:8876. doi: 10.1038/s41598-020-65802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X., Zhang C., Peng Y., Zhang H., Wang Z., Gao Y., Liu Y., Zhang H. Chemical Constituents, Antioxidant and Gastrointestinal Transit Accelerating Activities of Dried Fruit of Crataegus dahurica. Food Chem. 2018;246:41–47. doi: 10.1016/j.foodchem.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q., Zhao P., Sun J., Zhao C., Song Y., Wu J. Research progress on chemical constituents and pharmacological action of hawthorn. J. Northwest Pharm. 2021;3:521–523. [Google Scholar]
- 41.Tohtahon Z., Zhang L., Han J., Xie X., Tu Z., Yuan T. Extraction Optimization, Structural Characterization and Bioactivity Evaluation of Triterpenoids from Hawthorn (Crataegus cuneata) Fruits: Tohtahon et al. J. Food Biochem. 2017;41:e12377. doi: 10.1111/jfbc.12377. [DOI] [Google Scholar]
- 42.Song S., Li L., Gao P., Peng Y., Yang J., Wu C. Terpenoids and hexenes from the leaves of Crataegus pinnatifida. Food Chem. 2011;129:933–939. doi: 10.1016/j.foodchem.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 43.Gao P., Li L., Peng Y., Li F., Niu C., Huang X., Ming M., Song S. Monoterpene and lignan glycosides in the leaves of Crataegus pinnatifida. Biochem. Syst. Ecol. 2010;38:988–992. doi: 10.1016/j.bse.2010.09.010. [DOI] [Google Scholar]
- 44.Wen L., Guo R., You L., Abbasi A.M., Li T., Fu X., Liu R.H. Major Triterpenoids in Chinese Hawthorn “Crataegus pinnatifida” and Their Effects on Cell Proliferation and Apoptosis Induction in MDA-MB-231 Cancer Cells. Food Chem. Toxicol. 2017;100:149–160. doi: 10.1016/j.fct.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 45.Luan M., Wang H., Wang J., Zhang X., Zhao F., Liu Z., Meng Q. Advances in Anti-Inflammatory Activity, Mechanism and Therapeutic Application of Ursolic Acid. MRMC. 2022;22:422–436. doi: 10.2174/1389557521666210913113522. [DOI] [PubMed] [Google Scholar]
- 46.Huang X., Xu Y., Bai M., Zhou L., Song S., Wang X. Lignans from the Seeds of Chinese Hawthorn (Crataegus pinnatifida Var. Major N.E.Br.) against β-Amyloid Aggregation. Nat. Prod. Res. 2018;32:1706–1713. doi: 10.1080/14786419.2017.1399378. [DOI] [PubMed] [Google Scholar]
- 47.Huang X., Ren Q., Song X., Zhou L., Yao G., Wang X., Song S. Seven New Sesquineolignans Isolated from the Seeds of Hawthorn and Their Neuroprotective Activities. Fitoterapia. 2018;125:6–12. doi: 10.1016/j.fitote.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Peng Y., Lou L., Liu S., Zhou L., Huang X., Song S. Antioxidant and Anti-Inflammatory Neolignans from the Seeds of Hawthorn. Bioorg. Med. Chem. Lett. 2016;26:5501–5506. doi: 10.1016/j.bmcl.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Ma L., Xu G., Tang X., Zhang C., Zhao W., Wang J., Chen H. Anti-Cancer Potential of Polysaccharide Extracted from Hawthorn (Crataegus) on Human Colon Cancer Cell Line HCT116 via Cell Cycle Arrest and Apoptosis. J. Funct. Foods. 2020;64:103677. doi: 10.1016/j.jff.2019.103677. [DOI] [Google Scholar]
- 50.Guo C., Wang Y., Zhang S., Zhang X., Du Z., Li M., Ding K. Crataegus pinnatifida Polysaccharide Alleviates Colitis via Modulation of Gut Microbiota and SCFAs Metabolism. Int. J. Biol. Macromol. 2021;181:357–368. doi: 10.1016/j.ijbiomac.2021.03.137. [DOI] [PubMed] [Google Scholar]
- 51.Chen J., Song S. Advances in hawthorn research. Res. Inf. Tradit. Chin. Med. 2005;7 [Google Scholar]
- 52.Kowalski R., Kowalska G., Kalwa K., Sujka M. Essential Oil Composition of Hawthorn Crataegus monogyna Inflorescence. Chem. Nat. Compd. 2018;54:995–997. doi: 10.1007/s10600-018-2533-6. [DOI] [Google Scholar]
- 53.Gao P., Li S., Liu K., Sun C., Song S., Li L. Antiplatelet Aggregation and Antithrombotic Benefits of Terpenes and Flavones from Hawthorn Leaf Extract Isolated Using the Activity-Guided Method. Food Funct. 2019;10:859–866. doi: 10.1039/C8FO01862F. [DOI] [PubMed] [Google Scholar]
- 54.Lou X., Xu H., Hanna M., Yuan L. Identification and Quantification of Free, Esterified, Glycosylated and Insoluble-Bound Phenolic Compounds in Hawthorn Berry Fruit (Crataegus pinnatifida) and Antioxidant Activity Evaluation. LWT. 2020;130:109643. doi: 10.1016/j.lwt.2020.109643. [DOI] [Google Scholar]
- 55.Mustapha N., Mokdad-Bzéouich I., Maatouk M., Ghedira K., Hennebelle T., Chekir-Ghedira L. Antitumoral, Antioxidant, and Antimelanogenesis Potencies of Hawthorn, a Potential Natural Agent in the Treatment of Melanoma. Melanoma Res. 2016;26:211–222. doi: 10.1097/CMR.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 56.Ao N.N., Qu Y., Deng Y.Y., Cai Q., Suo T.J., Zheng Y. Chemical basis of hawthorn processed with honey on myocardial ischaemia protective effect. Food Funct. 2020;11:3134–3143. doi: 10.1039/C9FO02406A. [DOI] [PubMed] [Google Scholar]
- 57.Li L., Gao P., Song S., Yuan Y., Liu C., Huang X., Liu Q. Monoterpenes and flavones from the leaves of Crataegus pinnatifida with anticoagulant activities. J. Funct. Foods. 2015;12:237–245. doi: 10.1016/j.jff.2014.11.012. [DOI] [Google Scholar]
- 58.Nie J., Su J., Zhang C. Antioxidant and lipase activity of Hawthorn edible enzyme. Food Ind. 2021;11:232–236. [Google Scholar]
- 59.Wang M., Yue F., Jing R., Hou Y. Study on Manufacture Craft of Hawthorn Ultrafine Powder Bread. Food Sci. Technol. Econ. 2012;2:44–46. [Google Scholar]
- 60.Borczak B., Sikora E., Sikora M., Kapusta-Duch J., Kutyła-Kupidura E.M., Fołta M. Nutritional Properties of Wholemeal Wheat-Flour Bread with an Addition of Selected Wild Grown Fruits: Nutritional Properties of Wholemeal Wheat-Flour. Starch Stärke. 2016;68:675–682. doi: 10.1002/star.201500298. [DOI] [Google Scholar]
- 61.Gasiński A., Kawa-Rygielska J., Szumny A., Gąsior J., Głowacki A. Assessment of Volatiles and Polyphenol Content, Physicochemical Parameters and Antioxidant Activity in Beers with Dotted Hawthorn (Crataegus punctata) Foods. 2020;9:775. doi: 10.3390/foods9060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W., Chi L., Wu Y., Zhang L., Xu C. Quality Comparison of Hawthorn Wines Fermented by Saccharomyces cerevisiae with and without Pulp Contact and Pectase Treatment. J. Chem. 2017;2017:6431818. doi: 10.1155/2017/6431818. [DOI] [Google Scholar]
- 63.Özdemir G.B., Özdemir N., Ertekin-Filiz B., Gökırmaklı Ç., Kök-Taş T., Budak N.H. Volatile Aroma Compounds and Bioactive Compounds of Hawthorn Vinegar Produced from Hawthorn Fruit (Crataegus tanacetifolia (Lam.) Pers.) J. Food Biochem. 2022;46:e13676. doi: 10.1111/jfbc.13676. [DOI] [PubMed] [Google Scholar]
- 64.Jiang Y., Du J., Zhang L. Textural Characteristics and Sensory Evaluation of Yogurt Fortified with Pectin Extracted from Steeped Hawthorn Wine Pomace. Int. J. Food Eng. 2021;17:131–140. doi: 10.1515/ijfe-2019-0143. [DOI] [Google Scholar]
- 65.Lai K., How Y., Pui L. Storage Stability of Microencapsulated Lactobacillus rhamnosus GG in Hawthorn Berry Tea with Flaxseed Mucilage. J. Food Process. Preserv. 2020;44:e14965. doi: 10.1111/jfpp.14965. [DOI] [Google Scholar]
- 66.Ozcelik F., Akan E., Kinik O. Use of Cornelian Cherry, Hawthorn, Red Plum, Roseship and Pomegranate Juices in the Production of Water Kefir Beverages. Food Biosci. 2021;42:101219. doi: 10.1016/j.fbio.2021.101219. [DOI] [Google Scholar]
- 67.Chang T., Bi Y., Jing L., Liu X., Fan M., Yao S., Feng L., Zhao Y. Simultaneous Adsorption of Acid and Flavonoids from Hawthorn Juice onto Resins. J. Food Eng. 2021;291:110195. doi: 10.1016/j.jfoodeng.2020.110195. [DOI] [Google Scholar]
- 68.Zhou Q., Liu X., An X. Study on the Processing Technology and Storage Period Quality of Low Nitrate Hawthorn Rose Sausage. Anhui Nongye Kexue. 2020;11:194–197. [Google Scholar]
- 69.Li X., Yang W., Zhou Y., Sun K., Wang Z. The Development of Hawthorn Leaf Flavonoid Jam. Processing Technol. 2019;16:54–58. [Google Scholar]
- 70.Lu M., Tan H., Ma L. Preparation of Hawthorn and Passion Fruit Compound Jam. China Condiment. 2022;2:93–96. [Google Scholar]
- 71.Chen J. Study on the production process of orange peel hawthorn soft candy. China Fruit Veg. 2019;2:16–19. [Google Scholar]
- 72.Liu X., Yang S., Ma J., Yu J., Yan Q., Jiang Z. Efficient Production of Acetylated Xylooligosaccharides from Hawthorn Kernels by a Xylanase from Paecilomyces aerugineus. Ind. Crops Prod. 2020;158:112962. doi: 10.1016/j.indcrop.2020.112962. [DOI] [Google Scholar]
- 73.Golabiazar R., Qadir G.S., Faqe Z.A., Khalid K.M., Othman K.I., Rasool N.F., Saeed H.F. Green Biosynthesis of CdS NPs and CdS/Fe3O4 NCs by Hawthorn Plant Extract for Photodegradation of Methyl Orange Dye and Antibacterial Applications. J. Clust. Sci. 2021;3:1223–1238. doi: 10.1007/s10876-021-02054-z. [DOI] [Google Scholar]
- 74.Qiao L., Wang H., Shao J., Lu L., Tian J., Liu X. A Novel Mitigator of Enzymatic Browning—Hawthorn Leaf Extract and Its Application in the Preservation of Fresh-Cut Potatoes. Food Qual. Saf. 2021;5:fyab015. doi: 10.1093/fqsafe/fyab015. [DOI] [Google Scholar]
- 75.Lis M., Szczypka M., Suszko-Pawłowska A., Sokół-Łętowska A., Kucharska A., Obmińska-Mrukowicz B. Hawthorn (Crataegus monogyna) Phenolic Extract Modulates Lymphocyte Subsets and Humoral Immune Response in Mice. Planta Med. 2020;86:160–168. doi: 10.1055/a-1045-5437. [DOI] [PubMed] [Google Scholar]
- 76.Liu F., Zhang X., Ji Y. Total Flavonoid Extract from Hawthorn (Crataegus pinnatifida) Improves Inflammatory Cytokines-Evoked Epithelial Barrier Deficit. Med. Sci. Monit. 2020;26:e920170. doi: 10.12659/MSM.920170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong P., Pan L., Zhang X., Zhang W., Wang X., Jiang M., Chen Y., Duan Y., Wu H., Xu Y., et al. Hawthorn (Crataegus pinnatifida Bunge) Leave Flavonoids Attenuate Atherosclerosis Development in ApoE Knock-out Mice. J. Ethnopharmacol. 2017;198:479–488. doi: 10.1016/j.jep.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 78.Rao H., Li P., Wu H., Liu C., Peng W., Su W. Simultaneous Determination of Six Compounds in Destructive Distillation Extracts of Hawthorn Seed by GC-MS and Evaluation of Their Antimicrobial Activity. Molecules. 2019;24:4328. doi: 10.3390/molecules24234328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Y., Lu H., Dong Q., Hao X., Qiao L. Maslinic Acid Induces Anticancer Effects in Human Neuroblastoma Cells Mediated via Apoptosis Induction and Caspase Activation, Inhibition of Cell Migration and Invasion and Targeting MAPK/ERK Signaling Pathway. AMB Express. 2020;10:104. doi: 10.1186/s13568-020-01035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao C., Meng X., Li Y., Li S., Liu Q., Tang G., Li H. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients. 2017;9:598. doi: 10.3390/nu9060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arslan R., Bektas N. Potential Antithrombotic Effect of Crataegus Species. IJPER. 2018;52:S155–S157. doi: 10.5530/ijper.52.4s.93. [DOI] [Google Scholar]
- 82.Pahlavan S., Tousi M.S., Ayyari M., Alirezalu A., Ansari H., Saric T., Baharvand H. Effects of Hawthorn (Crataegus pentagyna) Leaf Extract on Electrophysiologic Properties of Cardiomyocytes Derived from Human Cardiac Arrhythmia-specific Induced Pluripotent Stem Cells. FASEB. 2018;32:1440–1451. doi: 10.1096/fj.201700494RR. [DOI] [PubMed] [Google Scholar]
- 83.Yoo J.-H., Liu Y., Kim H.-S. Hawthorn Fruit Extract Elevates Expression of Nrf2/HO-1 and Improves Lipid Profiles in Ovariectomized Rats. Nutrients. 2016;8:283. doi: 10.3390/nu8050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng F., Jiang W., Xiong X., Chen J., Xiong Y., Li Y. Ethanol Extract of Chinese Hawthorn (Crataegus pinnatifida) Fruit Reduces Inflammation and Oxidative Stress in Rats with Doxorubicin-Induced Chronic Heart Failure. Med. Sci. Monit. 2020;6:667–674. doi: 10.12659/MSM.926654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shih C., Lin C., Lin Y., Wu J. Validation of the Antidiabetic and Hypolipidemic Effects of Hawthorn by Assessment of Gluconeogenesis and Lipogenesis Related Genes and AMP-Activated Protein Kinase Phosphorylation. Evid.-Based Complementary Altern. Med. 2013;2013:597067. doi: 10.1155/2013/597067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aierken A., Buchholz T., Chen C., Zhang X., Melzig M.F. Hypoglycemic Effect of Hawthorn in Type II Diabetes Mellitus Rat Model: Hypoglycemic Effect of Hawthorn in Type II Diabetes Mellitus. J. Sci. Food Agric. 2017;97:4557–4561. doi: 10.1002/jsfa.8323. [DOI] [PubMed] [Google Scholar]
- 87.Verma T., Sinha M., Bansal N., Yadav S.R., Shah K., Chauhan N.S. Plants Used as Antihypertensive. Nat. Prod. Bioprospect. 2021;11:155–184. doi: 10.1007/s13659-020-00281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng X., Li X., Chen M., Yang P., Zhao X., Zeng L., OuYang Y., Yang Z., Tian Z. The Protective Role of Hawthorn Fruit Extract against High Salt-Induced Hypertension in Dahl Salt-Sensitive Rats: Impact on Oxidative Stress and Metabolic Patterns. Food Funct. 2019;10:849–858. doi: 10.1039/C8FO01818A. [DOI] [PubMed] [Google Scholar]
- 89.Orhan I.E. Phytochemical and Pharmacological Activity Profile of Crataegus oxyacantha L. (Hawthorn)—A Cardiotonic Herb. CMC. 2019;25:4854–4865. doi: 10.2174/0929867323666160919095519. [DOI] [PubMed] [Google Scholar]
- 90.Al Humayed S. Protective and Therapeutic Effects of Crataegus Aronia in Non-Alcoholic Fatty Liver Disease. Arch. Physiol. Biochem. 2017;123:23–30. doi: 10.1080/13813455.2016.1205097. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y., Lv M., Wang T., Sun J., Wang Y., Xia M., Jiang Y., Zhou X., Wan J. Research on Mechanism of Charred Hawthorn on Digestive through Modulating “Brain-Gut” Axis and Gut Flora. J. Ethnopharmacol. 2019;245:112166. doi: 10.1016/j.jep.2019.112166. [DOI] [PubMed] [Google Scholar]
- 92.Zeng L., Luo L., Xue Q., He Q., Chen X., Meng J., Wang S., Liang S. LC–MS Based Plasma Metabolomics Study of the Intervention Effect of Different Polar Parts of Hawthorn on Hyperlipidemia Rats. J. Sep. Sci. 2021;44:963–972. doi: 10.1002/jssc.202000911. [DOI] [PubMed] [Google Scholar]
- 93.Hu H., Luo X., Dong Q., Mu A., Shi G., Wang Q., Chen X., Zhou H., Zhang T., Pan L. Ethanol Extract of Zhongtian Hawthorn Lowers Serum Cholesterol in Mice by Inhibiting Transcription of 3-Hydroxy-3-Methylglutaryl-CoA Reductase via Nuclear Factor-Kappa B Signal Pathway. Exp. Biol. Med. 2016;241:667–674. doi: 10.1177/1535370215627032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holubarsch C.J.F., Colucci W.S., Eha J. Benefit-Risk Assessment of Crataegus Extract WS 1442: An Evidence-Based Review. Am. J. Cardiovasc. Drugs. 2018;18:25–36. doi: 10.1007/s40256-017-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xin C., Zhao M., Wang J., Wang Z. Hawthorn Polyphenols, D-chiro-inositol, and Epigallocatechin Gallate Exert a Synergistic Hypoglycemic Effect. J. Food Biochem. 2021;7:e13771. doi: 10.1111/jfbc.13771. [DOI] [PubMed] [Google Scholar]
- 96.Shi G., Li Y., Cao Q., Wu H., Tang X., Gao X., Yu J., Chen Z., Yang Y. In Vitro and In Vivo Evidence That Quercetin Protects against Diabetes and Its Complications: A Systematic Review of the Literature. Biomed. Pharmacother. 2019;109:1085–1099. doi: 10.1016/j.biopha.2018.10.130. [DOI] [PubMed] [Google Scholar]
- 97.Sagaradze V., Babaeva E., Ufimov R., Trusov N., Kalenikova E. Study of the variability of rutin, vitexin, hyperoside, quercetin in “Crataegifolium cum flore” of hawthorn (Crataegus L.) species from Russian flora. J. Appl. Res. Med. Aromat. Plants. 2019;15:100217. [Google Scholar]
- 98.Li T., Chen X., Huang Z., Xie W., Tong C., Bao R., Sun X., Li W., Li S. Pectin Oligosaccharide from Hawthorn Fruit Ameliorates Hepatic Inflammation via NF-ΚB Inactivation in High-Fat Diet Fed Mice. J. Funct. Foods. 2019;57:345–350. doi: 10.1016/j.jff.2019.04.027. [DOI] [Google Scholar]
- 99.Li C., Wang M. Anti-Inflammatory Effect of the Water Fraction from Hawthorn Fruit on LPS-Stimulated RAW 264.7 Cells. Nutr. Res. Pract. 2011;5:101–106. doi: 10.4162/nrp.2011.5.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deng J., Wang H., Mu X., He X., Zhao F., Meng Q. Advances in Research on the Preparation and Biological Activity of Maslinic Acid. MRMC. 2021;21:79–89. doi: 10.2174/1389557520666200722134208. [DOI] [PubMed] [Google Scholar]
- 101.Liu H., Liu J., Lv Z., Yang W., Zhang C., Chen D., Jiao Z. Effect of Dehydration Techniques on Bioactive Compounds in Hawthorn Slices and Their Correlations with Antioxidant Properties. J. Food Sci. Technol. 2019;56:2446–2457. doi: 10.1007/s13197-019-03720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu S., Sui Q., Zou J., Zhao Y., Chang X. Protective Effects of Hawthorn (Crataegus pinnatifida) Polyphenol Extract against UVB-Induced Skin Damage by Modulating the P53 Mitochondrial Pathway In Vitro and In Vivo. J. Food Biochem. 2019;43:e12708. doi: 10.1111/jfbc.12708. [DOI] [PubMed] [Google Scholar]
- 103.Lou X., Guo X., Wang K., Wu C., Jin Y., Lin Y., Xu H., Hanna M., Yuan L. Phenolic Profiles and Antioxidant Activity of Crataegus pinnatifida Fruit Infusion and Decoction and Influence of in Vitro Gastrointestinal Digestion on Their Digestive Recovery. LWT. 2021;135:110171. doi: 10.1016/j.lwt.2020.110171. [DOI] [Google Scholar]
- 104.Niu Z., Yan M., Zhao X., Jin H., Gong Y. Effect of Hawthorn Seed Extract on the Gastrointestinal Function of Rats with Diabetic Gastroparesis. S. Afr. J. Bot. 2020;130:448–455. doi: 10.1016/j.sajb.2020.01.032. [DOI] [Google Scholar]
- 105.Rababa’h A.M., Al Yacoub O.N., El-Elimat T., Rabab’ah M., Altarabsheh S., Deo S., Al-Azayzih A., Zayed A., Alazzam S., Alzoubi K.H. The Effect of Hawthorn Flower and Leaf Extract (Crataegus spp.) on Cardiac Hemostasis and Oxidative Parameters in Sprague Dawley Rats. Heliyon. 2020;6:e04617. doi: 10.1016/j.heliyon.2020.e04617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pawlaczyk-Graja I. Polyphenolic-Polysaccharide Conjugates from Flowers and Fruits of Single-Seeded Hawthorn (Crataegus monogyna Jacq.): Chemical Profiles and Mechanisms of Anticoagulant Activity. Int. J. Biol Macromol. 2018;116:869–879. doi: 10.1016/j.ijbiomac.2018.05.101. [DOI] [PubMed] [Google Scholar]
- 107.Bae H.J., Kim J., Kim J., Goo N., Cai M., Cho K., Jung S.Y., Kwon H., Kim D.H., Jang D.S., et al. The Effect of Maslinic Acid on Cognitive Dysfunction Induced by Cholinergic Blockade in Mice. Br. J. Pharmacol. 2020;177:3197–3209. doi: 10.1111/bph.15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee J., Cho E., Kwon H., Jeon J., Jung C.J., Moon M., Jun M., Lee Y.C., Kim D.H., Jung J.W. The Fruit of Crataegus pinnatifida Ameliorates Memory Deficits in β-Amyloid Protein-Induced Alzheimer’s Disease Mouse Model. J. Ethnopharmacol. 2019;243:112107. doi: 10.1016/j.jep.2019.112107. [DOI] [PubMed] [Google Scholar]
- 109.Lim D.W., Han T., Jung J., Song Y., Um M.Y., Yoon M., Kim Y.T., Cho S., Kim I.-H., Han D. Chlorogenic Acid from Hawthorn Berry (Crataegus pinnatifida Fruit) Prevents Stress Hormone-Induced Depressive Behavior, through Monoamine Oxidase B-Reactive Oxygen Species Signaling in Hippocampal Astrocytes of Mice. Mol. Nutr. Food Res. 2018;62:1800029. doi: 10.1002/mnfr.201800029. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Q., Xiong Y., Li B., Deng G., Fu W.-W., Cao B., Zong S., Zeng G. Total Flavonoids of Hawthorn Leaves Promote Motor Function Recovery via Inhibition of Apoptosis after Spinal Cord Injury. Neural Regen. Res. 2021;2:350–356. doi: 10.4103/1673-5374.286975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qiao A., Wang Y., Xiang L., Zhang Z. Novel triterpenoids isolated from hawthorn berries functioned as antioxidant and antiproliferative activities. J. Funct. Foods. 2015;13:308–313. doi: 10.1016/j.jff.2014.12.047. [DOI] [Google Scholar]
- 112.Guo R., Lin B., Shang X., Zhou L., Yao G., Huang X. Phenylpropanoids from the fruit of Crataegus pinnatifida exhibit cytotoxicity on hepatic carcinoma cells through apoptosis induction. Fitoterapia. 2018;127:301–307. doi: 10.1016/j.fitote.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 113.He Z., Kwek E., Hao W., Zhu W., Liu J., Ma K., Chen Z. Hawthorn fruit extract reduced trimethylamine-N-oxide (TMAO)-exacerbated atherogenesis in mice via anti-infammation and anti-oxidation. Nutr. Metab. 2021;18:6. doi: 10.1186/s12986-020-00535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zheng G., Deng J., Wen L., You L., Zhao Z., Zhou L. Release of phenolic compounds and antioxidant capacity of Chinese hawthorn “Crataegus pinnatifida” during in vitro digestion. J. Funct. Foods. 2018;40:76–85. doi: 10.1016/j.jff.2017.10.039. [DOI] [Google Scholar]
- 115.Wu P., Li F., Zhang J., Yang B., Ji Z., Chen W. Phytochemical compositions of extract from peel of hawthorn fruit, and its antioxidant capacity, cell growth inhibition, and acetylcholinesterase inhibitory activity. BMC Complementary Altern. Med. 2017;17:151. doi: 10.1186/s12906-017-1662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alirezalu A., Salehi P., Ahmadi N., Sonboli A., Aceto S., Maleki H., Ayyari M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. Genet. Resour. Crop Evol. 2018;21:452–470. doi: 10.1080/10942912.2018.1446146. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.