Abstract
The coffee supply chain is characterized by a complex network with many critical and unsustainable points producing a huge amount of waste products. Among these, coffee silverskin (CS), the only by-product of the coffee roasting phase, has an interesting chemical profile that suggests potential use as a food ingredient. However, few data on its safety are available. For this reason, the purpose of the study was to assess the occurrence of chemical and biological contaminants in CS, and the resulting risk due to its potential consumption. Essential, toxic, and rare earth elements, polycyclic aromatic hydrocarbons (PAHs), process contaminants, ochratoxin A (OTA), and pesticides residues were analyzed in three classes of samples (Coffea arabica CS, Coffea robusta CS, and their blend). Furthermore, total mesophilic bacteria count (TMBC) at 30 °C, Enterobacteriaceae, yeasts, and molds was evaluated. The risk assessment was based upon the hazard index (HI) and lifetime cancer risk (LTCR). In all varieties and blends, rare earth elements, pesticides, process contaminants, OTA, and PAHs were not detected except for chrysene, phenanthrene, and fluoranthene, which were reported at low concentrations only in the arabica CS sample. Among essential and toxic elements, As was usually the most representative in all samples. Microorganisms reported a low load, although arabica and robusta CS showed lower contamination than mixed CS. Instead, the risk assessment based on the potential consumption of CS as a food ingredient did not show either non-carcinogenic or carcinogenic risk. Overall, this study provides adequate evidence to support the safety of this by-product for its potential use in functional foods.
Keywords: silverskin, coffee by-products, risk assessment, agri-food waste, dietary exposure
1. Introduction
Coffee is one of the most consumed beverages in the world and is the second most traded product, after petroleum, with a market volume of about $15 billion [1]. The primary coffee production comes from South America, particularly Brazil, which has recorded an 18.5% increase in production in 2019–2020 [2]. Coffea arabica is the most dominant variety in the coffee market, with 175,347 thousand 60 kg bags in 2020, compared to 70,086 thousand 60 kg bags for Coffea canephora var. robusta [2]. Due to the enormous amount of coffee produced, marketed, and consumed, the coffee supply chain faces an environmental pollution burden, also related to the disposal of by-products.
Coffee cherry undergoes numerous processing steps that transform the raw fruit into liquid coffee. These processing steps are made to separate the outer layers that cover the green coffee bean. The structure of the coffee bean is composed as follows: husk (exocarp), pulp (mesocarp), parchment (endocarp), silverskin (integument), and finally, the two beans that constitute the final product of the processing chain from which the beverage is obtained [3]. Briefly, reviewing the processing steps, the coffee cherry, after being harvested, with a first discharge of the unsuitable cherries, is subjected to a wet or dry process [4]. The wet method involves removing the pulp from the bean by fermentation.
On the other hand, the dry method involves drying and hulling the coffee cherry and collecting husk, pulp, and parchment, which are disposed of as waste [3]. At this point, the green coffee beans, obtained from the previous processing steps, are packaged and shipped to consumer countries where roasting occurs. The roasting phase is a crucial part of coffee processing; it influences the organoleptic characteristics of the final beverage [5]. The coffee roasting phase includes blending different coffee varieties, with a roasting temperature of about 200 °C for less than 20 min, with substantial variability in time and temperature depending on the roasting system adopted [6].
The only by-product of this step is the coffee silverskin (CS), a thin integument obtained by detachment from the green bean due to the high temperatures reached during roasting. Finally, the roasted coffee bean is ready to be used to obtain the well-known beverage. The CS has a low mass (1% to 2% of the whole bean’s weight), but the total amount of this by-product is considerable in relation to the coffee consumed each year. Some studies on its potential reuse in cosmetics, nutraceuticals, and industry [7,8,9]. Moreover, given its chemical-physical profile related to the good amount of protein (19%), dietary fiber (30–70%), phenolic compounds, and melanoidins, CS is earning much interest for possible use as food or food ingredient [10,11,12,13,14,15].
To be included in the list of novel foods, it must undergo the authorization procedure set by Regulation (EU) No. 2015/2283 [16]. At present, coffee silverskin is evaluated as a novel food requiring pre-market approval [17]. The European Commission is responsible for authorizing novel foods and, as part of the procedure, may ask the European Food Safety Authority (EFSA) to conduct a scientific risk assessment to establish their safety [18]. In order to use it as a novel food, it is necessary to evaluate the absence of chemical and microbiological contaminants in this product, thus proceeding to a risk assessment. To this end, the present study aims to provide a comprehensive characterization investigating the occurrence of heavy metals, pesticides, rare earth elements (REEs), polycyclic aromatic hydrocarbons (PAHs), and biological contaminants. Since chemical features of coffee could differ among varieties [19], the silverskin obtained from the roasting of Coffea arabica, Coffea robusta, and a blend of both were studied. Ultimately, a risk assessment based on deterministic simulation was carried out considering the potential consumption of CS as a food ingredient.
2. Materials and Methods
2.1. Coffee Silverskin Sample
The arabica and robusta silverskin were obtained by roasting Coffea arabica and Coffea robusta green beans. The roasting was assessed in the laboratory using a Probatino rotary drum roaster (Probat, Emmerich am Rhein, Germany), equipped with a display to monitor time and temperature. The controlled curves of roasting temperatures were set out according to [6]. The mixed silverskin was provided by two coffee industries of the Campania region (Italy), obtained by blending roasted green beans of Coffea arabica and Coffea robusta (in unspecified ratio). Coffee varieties are usually blended by the companies during the roasting process, then the CS is recovered with suction cyclones and collected [20]. All the CS samples were stored in the dark at 20 °C and analyzed within three days.
2.2. Chemical Analysis
2.2.1. Essential, Toxic, and Rare Earth Elements
All solution preparation and sample dilution for multi-elemental analysis were made with high-purity water (resistivity of 18.2 MΩ cm) obtained from a Milli-Q unit (Millipore, Burlington, MA, USA). Nitric acid (HNO3, 69% v/v Ultratrace® ppb-trace analysis grade) and hydrofluoric acid (HF, 48% v/v Ultratrace® ppb-trace analysis grade) were provided by Scharlau (Barcelona, Spain). Multi-component certified solution of 30 elements (ultrapure grade for ICP) was provided by Ultrascientific (Bologna, Italy). Boric acid (99.97% trace metals basis) and multi-component certified solution of 16 rare earth elements REE (50 mg/L each, ultrapure grade for ICP, TraceCERT®) were purchased from Merck (Darmstadt, Germany). All glassware and plastic containers used for the preparation of samples and standards were tested and found free of analyzed elements.
Multi-elemental analysis was carried out by the Inductively Coupled Plasma—Mass Spectrometry (ICP-MS) and Microwave Plasma-Atomic Emission Spectrometry (MP-AES) after digestion.
An aliquot of 250 ± 1 mg of each sample was digested with 10 mL of ultrapure nitric acid (HNO3, 69% v/v Ultratrace® ppb-trace analysis grade) and 1 mL of hydrofluoric acid (HF, 48% v/v Ultratrace® ppb-trace analysis grade) in PP test tubes. The latter were placed in a water bath, preheated to 80 °C and proceeded to digestion for 2 h. After this time, 400 mg of boric acid were added to the mixture, and digestion continued for 1 h. Samples were brought to a final volume of 20 mL with HNO3 solution (2%, v/v) for the subsequent elemental analysis.
In this case, 19 elements (As, Sb, Ba, Be, B, Cd, Co, Cr, Fe, Mn, Hg, Ni, Pb, Cu, Se, Sn, Tl, V, Zn) and 15 REEs (Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu) were analyzed by Aurora M90 ICP-MS instruments (Bruker, Bremen, Germany). Na, K, Mg and Ca were analyzed by 4210 MP-AES instruments (Agilent, Santa Clara, CA, USA). Calibration curves were obtained in the range from 1 to 100 µg/L for ICP-MS and in the range from 0.1 to 100 mg/L for MP-AES for each analyzed element from certified standard solutions. A blank for reagents and a blank for the digestion method were performed to verify contamination of all materials, and calibration was verified in the instrumental sequence with two control standards for every 20 samples.
The limit of quantification (LOQ) was calculated by the method of blanks variability for each investigated element, and they are included in the range 0.05–1 mg/kg in the final sample for ICP-MS analysis and 10 mg/Kg in the final sample for metals detected with MP-AES.
2.2.2. Polycyclic Aromatic Hydrocarbons (PAHs)
Sample preparation and solutions for PAHs analysis were made with acetone (≥ 99.8%, GC grade) and n-hexane (≥95%, GC grade). Multi-component certified solution of 15 PAHs (2000 µg/mL each) and deuterated internal standard mixture solution (100 µg/mL each) were provided by Ultrascientific (Bologna, Italy). All glassware was tested and found free of PAHs.
An aliquot of 1.000 g ± 1 mg of each sample was extracted with 25 mL acetone/n-hexane 1:1 v/v using an ultrasonic bath (Branson Ultrasonic Corporation, Brookfield, CT, USA) for three 30-min cycles in closed glass vials. After this time, the extract was concentrated to a final volume of 1 mL using automatic concentrator MultiVap 8 (Labtech, Bergamo, Italy), and the purification procedure was unnecessary for the subsequent GC-MS analysis. The extract was injected into a gas chromatograph coupled with a mass spectrometer (GC 2010 Plus, MS-TQ8030, Shimadzu, Kyoto, Japan) for the determination of 15 PAHs [21].
Calibration curves were obtained in the range from 1 to 100 µg/L by calculating the area ratio of the target ion to the respective internal standard with five standard solutions obtained from certified standard solutions. A blank for reagents and a blank for the digestion method were performed to verify contamination of all materials, and calibration was verified in the instrumental sequence with a control standard for every ten samples.
The limit of quantification (LOQ) was calculated by the method of blanks variability and was equal to 0.05 mg/kg in the final sample.
2.2.3. Acrylamide (AA) and Furan
The acrylamide (AA) was analyzed following the [22] method with some modifications. A solid-phase extraction dispersed with C18 sorbent packed in polypropylene columns was performed by taking an aliquot of 0.5 g of homogenized sample. Then, the aqueous eluate was derivatized after bromination and subsequent GC-MS reading and quantification of the derivative. The limit of quantification (LOQ) was 0.02 mg/kg.
The furan was analyzed with headspace technique following the Park et al., 2021 [23] method. An aliquot of 5 g of sample and 5 mL of HPLC water were added to a headspace vial (20-mL) and carefully sealed. Then d4-furan solution (internal standard) was added to the vial, homogenized and placed on ice. Furan was extracted from the samples by an automated solid-phase micro-extraction (SPME) agitating the sample at 300 rpm, 50 °C. The fiber (carboxen/polydimethylsiloxane) was exposed to the headspace of vial samples for 20 min at 25 mm depth. Then, the fiber was thermally desorbed in the injection port of a gas chromatograph at 40 mm depth for 5 min. The limit of quantification (LOQ) was 0.1 mg/kg.
2.2.4. Ochratoxin A (OTA)
The analysis of ochratoxin A (OTA) was performed following the official method EN 1413:2003 with some modifications. 10 g of sample were homogenized with methanol:water solution (70:30 v/v), and it was diluted with a phosphate buffer solution prepared by dissolving 160 g of NaCl, 4.0 g of KCl, and 36.0 g of sodium hydrogen phosphate dihydrate (Na2HPO4-2H2O) in 1800 mL of water, adjusting the pH of the solution to 7.4 with 0.1 M HCl or with 0.1 M NaOH. Then, purification was performed by immunoaffinity chromatography onto columns containing a gel on which anti-OTA antibodies are immobilized. After, mycotoxin was eluted with pure methanol and quantified by HPLC with a reversed-phase C18 column and a fluorimetric spectrum detector. The limit of quantification (LOQ) was 0.1 mg/kg.
2.2.5. Pesticides Residues
Dispersive SPE—Modular QuEChERS (Quick Easy Cheap Effective Rugged Safe) method was used for acetonitrile extraction and purification of pesticide residues CS according to European standards EN 15662:2018 [24]. The compound investigated are listed in Supplementary Table S1. Pesticide residues were analyzed in duplicate by a multimethod procedure using GC-MS/MS and LC-MS/MS with a LOQ (limit of quantification) of 0.01 mg/kg.
2.3. Microbiological Analysis
Total Mesophilic Aerobic Bacteria (TMAB), Lactic Acid Bacteria (LAB), Enterobacteriaceae, yeasts and molds and Salmonellae were detected to evaluate the microbiological contamination of coffee silverskin. CS was serially diluted in Quarter-Strength Ringer’s solution (1:10 w/v) (Basingstoke, UK). An inoculum of 0.1 and 1 mL was then transferred from dilutions into (pour plate method) and onto (spread plate method) appropriate medium. TMAB were counted on Plate Count Agar (PCA) after 48 h of incubation at 30 °C. LABs were counted on deMan Rogosa and Sharp (MRS) Agar after 48 h of incubation at 30 °C. Enterobacteriaceae were counted on Violet Red Bile Glucose Agar (VRBGA) after 48 h of incubation at 37 °C. Yeasts and Molds were counted on Dichloran Rose Bengal Chloramphenicol (DRBC) Agar after 48 h of incubation at 28 °C. Salmonellae were detected according to [25], which involves an initial pre-enrichment step in Buffered Peptone Water at a ratio of 1:10. After incubation at 34–38 °C for 18 h, 0.1 mL was transferred into 10 mL of Rappaport-Vassiliadiscon Soy Broth (RVS) and reincubated at 41.5 °C for 24 h, while 1 mL was used to inoculate 10 mL of Muller-Kauffmann to Tetrationate-novobiocin broth (MKTTn) reincubated subsequently at 37 °C for 24 h. For the next isolation step, Xylose Lysine Deoxycholate (XLD) agar and Brilliance Salmonella Agar Base (BSA) agar plates were seeded by streaking from RVS and MKTTn cultures for each culture broth, which was incubated at 37 °C for 24 h.
2.4. Risk Assessment
The risk due to potential consumption of silverskin was assessed through hazard-quotient (HQ) and lifetime cancer risk (LTCR).
HQ based on estimated daily intake (EDI) was calculated through Equations (1) and (2). An HQ > 1, indicates a likely high risk as far as non-carcinogenic adverse effects are concerned.
| (1) |
| (2) |
Ck: Concentration of contaminant k detected in silverskin (mg/kg).
IR: Intake Rate of silverskin (kg/day).
BW: Body Weight of 70 kg.
TDI: tolerable daily intake.
The risk derived from cumulative exposure was calculated through the Hazard Index (Equation (3)). HI > 1 indicates a likely high no-carcinogenic risk due to exposure to multiple contaminants.
| (3) |
LTCR was estimated for carcinogenic contaminants based on their slope factor (SF), as shown in Equation (4). Values above 1 × 10−4 are considered unacceptable for the risk of developing cancer over a human lifetime. Whereas values between 1 × 10−6 and 1 × 10−4 are considered an acceptable range for risk according to [26].
| LTCR = (EDI × EF × TE × SF)/AT | (4) |
EF: Exposure Frequency to the contaminant (350 day/year).
TE: Total Exposure (70 year).
AT: Average Lifetime time for non-carcinogenic risk (TE × 365 day/year).
SF: Slope Factor (mg/kgbw/day)−1 related to each PAE (Table 1).
Table 1.
Concentrations (mg/kg) of essential, toxic, and rare earth elements in three classes of samples (n = 10) of coffee silverskin (CS). Results are expressed as mean ± standard deviation.
| Elements | Robusta (mg/kg) | Mixed (mg/kg) | Arabica (mg/kg) |
|---|---|---|---|
| Antimony Sb | <LOQ | <LOQ | <LOQ |
| Arsenic As | 0.29 | 0.21 | 0.27 |
| Barium Ba | 28.2 | 49.4 | 60.6 |
| Beryllium Be | 0.06 | 0.05 | 0.06 |
| Boron B | 31.3 | 32.4 | 33.1 |
| Cadmium Cd | <LOQ | <LOQ | 0.15 |
| Cobalt Co | 0.45 | 0.68 | 0.36 |
| Chromium Cr | 0.59 | 0.48 | 0.26 |
| Iron Fe | 442 | 319 | 179 |
| Manganese Mn | 20.8 | 24.6 | 53.1 |
| Mercury Hg | 0.06 | 0.06 | 0.07 |
| Nickel Ni | 1.08 | 2.41 | 0.91 |
| Lead Pb | 0.36 | <LOQ | <LOQ |
| Copper Cu | 132 | 106 | 35.5 |
| Selenium Se | <LOQ | <LOQ | <LOQ |
| Tin Sn | <LOQ | <LOQ | <LOQ |
| Thallium Tl | <LOQ | <LOQ | <LOQ |
| Vanadium V | 0.58 | 0.76 | <LOQ |
| Zinc Zn | 17.9 | 15.8 | 11.2 |
| Yttrium Y | <LOQ | <LOQ | <LOQ |
| Lanthanum La | <LOQ | <LOQ | 0.07 |
| Cerium Ce | <LOQ | <LOQ | 0.07 |
| Praseodymium Pr | <LOQ | <LOQ | <LOQ |
| Neodymium Nd | <LOQ | <LOQ | <LOQ |
| Samarium Sm | <LOQ | <LOQ | <LOQ |
| Europium Eu | <LOQ | <LOQ | <LOQ |
| Gadolinium Gd | <LOQ | <LOQ | <LOQ |
| Terbium Tb | <LOQ | <LOQ | <LOQ |
| Dysprosium Dy | <LOQ | <LOQ | <LOQ |
| Holmium Ho | <LOQ | <LOQ | <LOQ |
| Erbium Er | <LOQ | <LOQ | <LOQ |
| Thulium Tm | <LOQ | <LOQ | <LOQ |
| Ytterbium Yb | <LOQ | <LOQ | <LOQ |
| Lutetium Lu | <LOQ | <LOQ | <LOQ |
3. Results and Discussion
3.1. Chemical Contaminants
During coffee roasting, the high temperature produces a chemical change in coffee beans composition and the formation of new organic compounds as a result of the Maillard reaction and pyrolysis of nonvolatile compounds [6,27]. These include toxic compounds such as polycyclic aromatic hydrocarbons (PAHs) and acrylamide (AA) [28,29]. Our preliminary study [30] evaluated the concentration of process contaminants such as acrylamide (AA), furan, methyl furan and ochratoxin A (OTA) and some heavy metals and PAHs in CS for its multipurpose recycling applications. This study performed an extensive semiquantitative elemental screening of the CS chemical profile. Several classes of contaminants, such as heavy metals, PAHs, process contaminants, OTA, and pesticides, were investigated in Coffea arabica, robusta, and mixed silverskin. The summary data are listed in Table 1, Table 2, Table 3, Table 4 and Table 5. The contents of the elements analyzed are mostly comparable to each other in the three respective CS samples, except for barium (Ba), copper (Cu), iron (Fe), manganese (Mn) and nickel (Ni). In detail, robusta CS has a higher Fe content (442 mg/kg) than arabica CS (179 mg/kg), while mixed CS has an intermediate value (319 mg/kg). A similar trend occurs for Cu content, with robusta CS (132 mg/kg), mixed CS (106 mg/kg), and arabica CS (35.5 mg/kg). In contrast, arabica CS shows higher values for Ba and Mn, 60.6 mg/kg and 53.1 mg/kg, respectively. The mixed CS reports for almost all elements values intermediate to the two varieties, except for Ni (2.41 mg/kg), Co (0.68 mg/kg) and V (0.76 mg/kg) with higher values and As (0.21 mg/kg) and Be (0.05 mg/kg) with lower values than the two coffee varieties.
Table 2.
Concentrations (mg/kg) of polycyclic aromatic hydrocarbons (PAHs) in three classes of samples (n = 10) of coffee silverskin (CS). Results are expressed as mean ± standard deviation.
| Polycyclic Aromatic Hydrocarbons | Robusta (mg/kg) | Mixed (mg/kg) | Arabica (mg/kg) |
|---|---|---|---|
| Naphthalene | <LOQ | <LOQ | <LOQ |
| Acenaphtilene | <LOQ | <LOQ | <LOQ |
| Acenaphthene | <LOQ | <LOQ | <LOQ |
| Fluorene | <LOQ | <LOQ | <LOQ |
| Anthracene | <LOQ | <LOQ | <LOQ |
| Phenanthrene | <LOQ | <LOQ | 0.07 |
| Fluoranthene | <LOQ | <LOQ | 0.18 |
| Pyrene | <LOQ | <LOQ | <LOQ |
| Chrysene | <LOQ | <LOQ | 0.06 |
| Benzo(b)fluoranthene | <LOQ | <LOQ | <LOQ |
| Benzo(k)fluoranthene | <LOQ | <LOQ | <LOQ |
| Benzo(a)pyrene | <LOQ | <LOQ | <LOQ |
| Indeno[1,2,3-cd]pyrene | <LOQ | <LOQ | <LOQ |
| Dibenz[a,h]anthracene | <LOQ | <LOQ | <LOQ |
| Benzo[ghi]perylene | <LOQ | <LOQ | <LOQ |
Table 3.
Concentrations (mg/kg) of process contaminants in three classes of samples (n = 10) of coffee silverskin (CS).
| Process Contaminants | Robusta (mg/kg) | Mixed (mg/kg) | Arabica (mg/kg) |
|---|---|---|---|
| Acrylamide (AA) | <LOQ | <LOQ | <LOQ |
| Furan | <LOQ | <LOQ | <LOQ |
| Methyl-furan | <LOQ | <LOQ | <LOQ |
Table 4.
Concentrations (mg/kg) of mycotoxins in three classes of samples (n = 10) of coffee silverskin (CS).
| Mycotoxins | Robusta (mg/kg) | Mixed (mg/kg) | Arabica (mg/kg) |
|---|---|---|---|
| Ochratoxin A (OTA) | <LOQ | <LOQ | <LOQ |
Table 5.
Concentrations (mg/kg) of pesticides (showed in Supplementary Table S1) in three classes of samples (n = 10) of coffee silverskin (CS).
| Pesticides | Robusta (mg/kg) | Mixed (mg/kg) | Arabica (mg/kg) |
|---|---|---|---|
| Supplementary Table S1 | <0.01 | <0.01 | <0.01 |
From literature, [10,31,32] assessed the levels of some mineral elements in an unspecified mixture of arabica and robusta coffee silverskin. Comparing these studies with our mixed CS results, [31] reported higher values for iron (843.30 mg/kg), zinc (22.30 mg/kg), cobalt (21.39 mg/kg), and chromium (1.59 mg/kg), lower value for copper (63.30 mg/kg) while elements such as barium, boron, lead, selenium, cadmium, and tin were in line with ours. Instead, [32] reported higher values for iron (660 mg/kg), zinc (27 mg/kg) and chromium (1.8 mg/kg) and lower values for copper (30 mg/kg). Another study [7] reports higher values of chromium (5.55 mg/kg) while lower values of iron (212 mg/kg) and copper (72.15 mg/kg). Furthermore, from the analyses conducted for our previous study, the arsenic and copper content in the mixed CS was higher, while zinc and iron had lower values than in the current study [30]. These differences are probably due to the different compositions of the coffee blends produced in the roasting companies, which result in a different chemical profile of the silverskin obtained. For example, according to the study [33], a CS pellet consisting probably of 70% Coffea arabica and 30% Coffea canephora, i.e., the typical coffee blend commercially available in Germany, has a different chemical profile from our mixed CS and the other previously cited works. Once again, the study [33] characterized arabica CS with higher values for many elements analyzed, particularly for manganese (145 mg/kg), barium (130 mg/kg) and copper (98 mg/kg) than our arabica CS, and robusta CS with higher values for barium (73 mg/kg), copper (185 mg/kg), nickel (2.3 mg/kg) and chromium (2.9 mg/kg) than our robusta CS. Another study [34], analyzed CS from roasting Coffea arabica green beans and reported higher values for iron (238 mg/kg) and zinc (31.9 mg/kg), while lower values are reported for manganese (46.7 mg/kg). As regards PAHs, their occurrence in coffee silverskin is critical information for risk assessment purposes. They are dangerous ubiquitous organic pollutants, and some are classified as probable human carcinogens due to their toxic, carcinogenic and mutagenic nature [35]. Among the three types of CS analyzed, only arabica CS reported the occurrence of Phenanthrene (0.07 mg/kg), Fluoranthene (0.18 mg/kg), and Chrysene (0.06 mg/kg). However, from our results, most of the elements and PAHs in arabica, robusta and mixed silverskin samples were reported at concentrations <LOQ. Similarly, AA, furan, methyl-furan, and OTA were not detected consistent with our previous investigation [30].
Lastly, analyses were performed to determine the occurrence of pesticides. A study [36] evaluated the life cycle assessment (LCA) of green coffee production in Brazil; the results showed that, in addition to a considerable amount of water, 900 kg of total fertilizer and 10 kg of pesticides are used to produce 1,000 kg of coffee. This information, related to the botanical differences between the two coffee varieties, could influence the presence of pesticides in coffee cherries. Indeed, the depth of the root system is different: the Coffea arabica roots penetrate deeper into the soil, while the robusta roots are highly concentrated near the soil surface [37]. However, analysis of the multiple pesticides searched (Supplementary Table S1) revealed no outliers, and all values detected were at <LOQ concentrations.
3.2. Microbiological Contaminants
For a more comprehensive characterization of CS, the occurrence of some biological contaminants such as Enterobacteriaceae was assessed. These bacteria are biological markers of food safety that could indicate the occurrence of pathogens. A higher Enterobacteriaceae microbial load (0.78 ± 0.16 Log/UFC) was reported in mixed CS. However, all samples reported no occurrence of Salmonella spp. Furthermore, yeasts and molds were isolated from mixed CS, reporting a load of 2 ± 0.21 Log/UFC. Instead, the TMBC was 5.45 ± 0.17 Log/UFC in mixed CS, whereas robusta and arabica CS reported values of 3.61 ± 0.18 Log/UFC and 3.73 ± 0.22 Log/UFC, respectively. However, it is plausible that the high temperature of the roasting process and the low moisture content of CS (4–7%) [38] limits its microbial load and extends its shelf life [10]. In addition, to date, some studies have evaluated a potential application of CS in the formulation of food products that could ensure healthiness after the baking process. In particular, [39,40] evaluated its applicability in bakery products, whereas an interesting study [15] considered the formulation of a chicken meat burger with CS that would increase the shelf life of the meat due to its antioxidant activities in addition to a fiber supplement and an excellent source of minerals such as calcium, potassium and others.
3.3. Risk Assessment
CS is a high-nutritional value product with a fiber content of about 30–70% [41,42,43]. Hence, a content of 5–10 g could provide more than 3 g of fiber allowing the use of the nutritional claims of “source of fiber” in CS-based products according to Regulation (EC) No 1924/2006 [44]. Based on this consideration, a consumption of 5 g and 10 g as food ingredients was supposed for the risk assessment. The EDI of each contaminant was calculated and compared with the corresponding threshold value expressed as TDI (Supplementary Table S2). The EDI was not calculated for compounds with concentrations <LOQ. The ratio between EDI and TDI, known as HQ, was listed in Supplementary Table S2. The HQ ranged from 1.42 × 10−4 to 6.92 × 10−2, from 1.15 × 10−4 to 5.45 × 10−2, and from 6.12 × 10−5 to 6.50 × 10−2 for the consumption of 5 g of robusta, mixed, and arabica CS, respectively. Instead, the HQ ranged from 2.83 × 10−4 to 1.38 × 10−1, from 2.29 × 10−4 To 1.09 × 10−1, and from 1.22 × 10−4 to 1.30 × 10−1 for the consumption of 10 g of robusta, mixed, and arabica CS, respectively. Since, in all scenarios, the HQs were below 1, non-carcinogenic risk by exposure to a single contaminant could not be attributed to consumption of silverskin to considered levels. In order of magnitude the values in robusta CS were: As > Cu > V > Fe > Hg > B > Mn > Ba > Pb > Ni > Zn > Be > Co > Cr. Similarly, the order in mixed CS was V > As > Cu > Fe > Hg > Ba > B > Ni > Mn > Zn > Be > Co > Cr, whereas arabica CS reported As > Cd > Mn > Hg > Ba > Cu > Fe > B > Ni> chrysene > Zn > Be > Co > phenanthrene > fluoranthene > Cr.
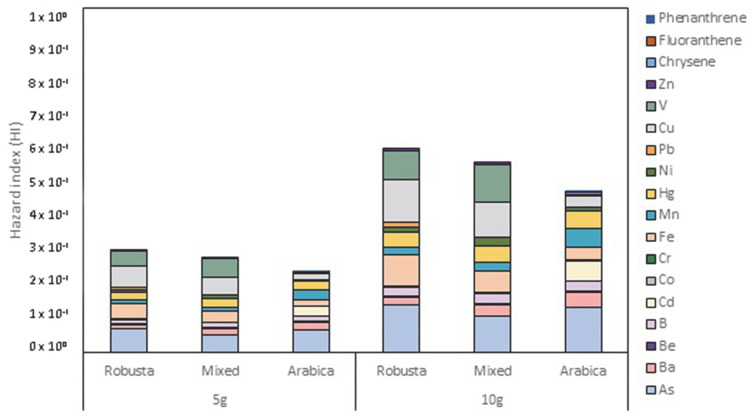
The HI based on consumption of 5 g and 10 g of CS reported values from 2.96 × 10−1 to 5.93 × 10−1, from 2.76 × 10−1 to 5.53 × 10−1, and from 2.34 × 10−1 to 4.67 × 10−1 for robusta, mixed, and arabica CS, respectively (Figure 1). The two CS varieties and the mixed CS sample showed values of single and cumulative risk simulation <1, indicating a low probability of non-carcinogenic adverse effects.
Figure 1.
Hazard index (HI) evaluation based on the consumption of 5 g and 10 g of robusta, mixed and arabica silverskin.
Concerning the carcinogenic risk, the estimation of LTCR was only based on As and chrysene since most carcinogenic contaminants were <LOQ. However, the contribution of chrysene was negligible. Instead, As showed a LTCR for the consumption of 5 g and 10 g for robusta CS ranged between 2.98 × 10−5 and 5.97 × 10−5, whereas lower values were reported for mixed CS (2.21 × 10−5 and 4.41 × 10−5) and arabica CS (2.81 × 10−5 and 5.61 × 10−5). Considering that a carcinogenic risk could occur to values above 1 × 10−4 according to USEPA [25], the three types of CS showed a low risk to considered dose as an ingredient.
This risk simulation is based on a conservative approach since 100% bioaccessibility and bioavailability were considered, likely overestimating the risk. Indeed, fiber that is highly present in the silverskin may reduce the bioaccessibility of some elements [45,46,47]. Furthermore, the slope factors of As refer to inorganic form, although foods may also contain the organic one that is less toxic.
4. Conclusions
An extensive characterization of essential and toxic elements, REE, pesticides, polycyclic aromatic hydrocarbons, and microorganisms on the two main varieties of CS and their blend was provided. In robusta and mixed CS pesticides, REE, process contaminants, OTA, and PAHs were not detected. Similar data were reported for the arabica CS sample, although chrysene, phenanthrene, and fluoranthene were detected at low concentrations. Essential and toxic elements showed variability among CS varieties and blended, although As was usually the most representative in all samples. Concerning microorganisms, arabica and robusta CS reported lower contamination than mixed CS, although Salmonella spp. did not occur in any samples. The handling and collection of the coffee silverskin probably denote slight environmental contamination between the mixed CS obtained from coffee companies and that of arabica and robusta CS obtained in the laboratory. It should be kept in mind that it is a waste material for companies, and, to date, there are no regulations for its proper sorting and collection. However, the high temperatures of the roasting process allow a reduction of CS microbial load. The risk assessment liked the potential consumption of CS as a food ingredient (5 g and 10 g) and pointed out a low probability of non-carcinogenic and carcinogenic effects. Robusta CS showed higher values (risk) for HI and LTCR. As noted by comparison with studies in the literature, differences in the chemical profile of CS can be due to coffee variety, production sites, climatic factors, field treatments, coffee processing methods, and, finally, silverskin storage and collection methods. Overall, this study provides the first evidence of the safety of CS based on determinist assessment. However, future studies should assess the bioaccessibility of elements through in vitro or in vivo digestion to provide more data on the healthiness of CS and its potential application as a novel food.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods11182834/s1, Table S1: Multiresidual list of pesticides assessed in silverskin (CS) by GC MS/MS (list 1) and LC MS/MS (list 2); Table S2: Hazard quotient (HQ) based on consumption of 5 g and 10 g of three class samples of coffee silverskin (CS) and the threshold values for each contaminant expressed as tolerable daily intake (TDI, mg/kgbw/day). If not available, TDI was derived from tolerable week intake a (TWI, mg/kgbw/week), provisional tolerable weekly intake b (PTWI, mg/kgbw/week), or reference dose c (RfD, mg/kgbw/day) [48,49,50,51,52,53,54,55,56,57,58,59].
Author Contributions
Conceptualization, T.C. and F.E.; methodology, A.N., F.E., S.V., M.A., A.G. and M.T.; validation, F.E., S.V., R.R. and T.C.; formal analysis, A.N., J.S., F.E., A.G., M.T. and M.A.; investigation, A.N., J.S., A.G., M.T., S.V., F.E. and M.A.; resources, T.C., R.R. and S.V.; data curation, A.N., F.E. and J.S.; writing—original draft preparation, J.S., A.N., F.E., A.G. and M.T.; writing—review and editing, J.S., A.N., F.E. and E.M.; visualization, A.N., J.S. and F.E.; supervision, T.C. and F.E.; project administration, T.C. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lanfranchi M., Giannetto C., Dimitrova V. Evolutionary aspects of coffee consumers’ buying habits: Results of a sample survey. Bulg. J. Agric. Sci. 2016;22:705–712. [Google Scholar]
- 2.ICO (International Coffee Organization) Total Production by Exporting Countries. 2021. [(accessed on 5 September 2022)]. Available online: https://www.ico.org/prices/po-production.pdf.
- 3.Alves R.C., Rodrigues F., Nunes M.A., Vinha A.F., Oliveira M.B.P. Chapter 1—State of the art in coffee processing by-products. In: Galanakis C.M., editor. Handbook of Coffee Processing By-Products: Sustainable Application. Elsevier; Amsterdam, The Netherlands: 2017. pp. 1–26. [DOI] [Google Scholar]
- 4.Oliveira G., Passos C.P., Ferreira P., Coimbra M.A., Gonçalves I. Coffee by-products and their suitability for developing active food packaging materials. Foods. 2021;10:683. doi: 10.3390/foods10030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-Galilea I., Fournier N., Cid C., Guichard E. Changes in headspace volatile concentrations of coffee brews caused by the roasting process and the brewing procedure. J. Agric. Food Chem. 2006;54:8560–8566. doi: 10.1021/jf061178t. [DOI] [PubMed] [Google Scholar]
- 6.Esposito F., Fasano E., De Vivo A., Velotto S., Sarghini F., Cirillo T. Processing effects on acrylamide content in roasted coffee production. Food Chem. 2020;319:126550. doi: 10.1016/j.foodchem.2020.126550. [DOI] [PubMed] [Google Scholar]
- 7.Bessada S.M., Alves R., Oliveira M.B. Coffee silverskin: A review on potential cosmetic applications. Cosmetics. 2018;5:5. doi: 10.3390/cosmetics5010005. [DOI] [Google Scholar]
- 8.Peixoto J.A.B., Andrade N., Machado S., Costa A.S., Puga H., Oliveira M.B.P., Alves R.C. Valorizing Coffee Silverskin Based on Its Phytochemicals and Antidiabetic Potential: From Lab to a Pilot Scale. Foods. 2022;11:1671. doi: 10.3390/foods11121671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghazvini A.K.A., Ormondroyd G., Curling S., Saccani A., Sisti L. An investigation on the possible use of coffee silverskin in PLA/PBS composites. J. Appl. Polym. Sci. 2022;139:52264. doi: 10.1002/app.52264. [DOI] [Google Scholar]
- 10.Martuscelli M., Esposito L., Di Mattia C.D., Ricci A., Mastrocola D. Characterization of coffee silver skin as potential food-safe ingredient. Foods. 2021;10:1367. doi: 10.3390/foods10061367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesías M., Delgado-Andrade C. Melanoidins as a potential functional food ingredient. Curr. Opin. Food Sci. 2017;14:37–42. doi: 10.1016/j.cofs.2017.01.007. [DOI] [Google Scholar]
- 12.Bertolino M., Barbosa-Pereira L., Ghirardello D., Botta C., Rolle L., Guglielmetti A., Zeppa G. Coffee silverskin as nutraceutical ingredient in yogurt: Its effect on functional properties and its bioaccessibility. J. Sci. Food Agric. 2019;99:4267–4275. doi: 10.1002/jsfa.9659. [DOI] [PubMed] [Google Scholar]
- 13.Tores de la Cruz S., Iriondo-DeHond A., Herrera T., Lopez-Tofiño Y., Galvez-Robleño C., Prodanov M., Del Castillo M.D. An assessment of the bioactivity of coffee silverskin melanoidins. Foods. 2019;8:68. doi: 10.3390/foods8020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iriondo-DeHond A., Rios M.B., Herrera T., Rodriguez-Bertos A., Nuñez F., San Andres M.I., Del Castillo M.D. Coffee silverskin extract: Nutritional value, safety and effect on key biological functions. Nutrients. 2019;11:2693. doi: 10.3390/nu11112693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martuscelli M., Esposito L., Mastrocola D. The role of coffee silver skin against oxidative phenomena in newly formulated chicken meat burgers after cooking. Foods. 2021;10:1833. doi: 10.3390/foods10081833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union. 2015;327:1–22. [Google Scholar]
- 17.Consultation on the Determination of the Status of a Novel Food under Article 4 (2) of Regulation (EU) 2015/2283. [(accessed on 5 September 2022)]. Available online: https://food.ec.europa.eu/system/files/2022-06/novel-food_consult-status_2022-4778355.pdf.
- 18.Klingel T., Kremer J.I., Gottstein V., Rajcic de Rezende T., Schwarz S., Lachenmeier D.W. A review of coffee by-products including leaf, flower, cherry, husk, silver skin, and spent grounds as novel foods within the European Union. Foods. 2020;9:665. doi: 10.3390/foods9050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., Fei Y., Wang W., Lei S., Cheng C., Xing Z. Physicochemical difference of coffee beans with different species, production areas and roasting degrees. Beverage Plant Res. 2022;2:7. doi: 10.48130/BPR-2022-0007. [DOI] [Google Scholar]
- 20.Lachenmeier D.W., Schwarz S., Rieke-Zapp J., Cantergiani E., Rawel H., Martín-Cabrejas M.A., Angeloni S. Coffee By-Products as Sustainable Novel Foods: Report of the 2nd International Electronic Conference on Foods—“Future Foods and Food Technologies for a Sustainable World”. Foods. 2022;11:3. doi: 10.3390/foods11010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arienzo M., Donadio C., Mangoni O., Bolinesi F., Stanislao C., Trifuoggi M., Ferrara L. Characterization and source apportionment of polycyclic aromatic hydrocarbons (pahs) in the sediments of gulf of Pozzuoli (Campania, Italy) Mar. Pollut. Bull. 2017;124:480–487. doi: 10.1016/j.marpolbul.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes J.O., Soares C. Application of matrix solid-phase dispersion in the determination of acrylamide in potato chips. J. Chromatogr. A. 2007;1175:1–6. doi: 10.1016/j.chroma.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 23.Park S.H., Jo A., Lee K.G. Effect of various roasting, extraction and drinking conditions on furan and 5-hydroxymethylfurfural levels in coffee. Food Chem. 2021;358:129806. doi: 10.1016/j.foodchem.2021.129806. [DOI] [PubMed] [Google Scholar]
- 24.Foods of Plant Origin—Multimethod for the Determination of Pesticide Residues Using GC- and LC-Based Analysis following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE—Modular QuEChERS-Method. German Version EN 15662:2018. Beuth Verlag; Berlin, Germany: 2018. [Google Scholar]
- 25.International Organization for Standardization (ISO) Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Detection of Salmonella spp. ISO 6579:2002. ISO; Geneva, Switzerland: 2002. [Google Scholar]
- 26.USEPA (United States Environmental Protection Agency) Risk Assessment Guidance for Superfund Part A, Process for Conducting Probabilistic Risk Assessment. Volume III USEPA; Washington, DC, USA: 2001. [Google Scholar]
- 27.Toci A.T., Farah A. Volatile fingerprint of Brazilian defective coffee seeds: Corroboration of potential marker compounds and identification of new low quality indicators. Food Chem. 2014;153:298–314. doi: 10.1016/j.foodchem.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 28.Pissinatti R., Nunes C.M., de Souza A.G., Junqueira R.G., de Souza S.V. Simultaneous analysis of 10 polycyclic aromatic hydrocarbons in roasted coffee by isotope dilution gas chromatography-mass spectrometry: Optimization, in-house method validation and application to an exploratory study. Food Control. 2015;51:140–148. doi: 10.1016/j.foodcont.2014.11.003. [DOI] [Google Scholar]
- 29.Cagliero C., Ho T.D., Zhang C., Bicchi C., Anderson J.L. Determination of acrylamide in brewed coffee and coffee powder using polymeric ionic liquid-based sorbent coatings in solid-phase microextraction coupled to gas chromatography–mass spectrometry. J. Chromatogr. A. 2016;1449:2–7. doi: 10.1016/j.chroma.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 30.Nolasco A., Squillante J., Velotto S., D’Auria G., Ferranti P., Mamone G., Errico M.E., Avolio R., Castaldo R., Cirillo T., et al. Valorization of coffee industry wastes: Comprehensive physicochemical characterization of coffee silverskin and multipurpose recycling applications. J. Clean. Prod. 2022;370:133520. doi: 10.1016/j.jclepro.2022.133520. [DOI] [Google Scholar]
- 31.Ballesteros L.F., Teixeira J.A., Mussatto S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014;7:3493–3503. doi: 10.1007/s11947-014-1349-z. [DOI] [Google Scholar]
- 32.Zarrinbakhsh N., Wang T., Rodriguez-Uribe A., Misra M., Mohanty A.K. Characterization of wastes and coproducts from the coffee industry for composite material production. BioResources. 2016;11:7637–7653. doi: 10.15376/biores.11.3.7637-7653. [DOI] [Google Scholar]
- 33.Gottstein V., Bernhardt M., Dilger E., Keller J., Breitling-Utzmann C.M., Schwarz S., Bunzel M. Coffee silver skin: Chemical characterization with special consideration of dietary fiber and heat-induced contaminants. Foods. 2021;10:1705. doi: 10.3390/foods10081705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nzekoue F.K., Borsetta G., Navarini L., Abouelenein D., Xiao J., Sagratini G., Angeloni S. Coffee silverskin: Characterization of B-vitamins, macronutrients, minerals and phytosterols. Food Chem. 2022;372:131188. doi: 10.1016/j.foodchem.2021.131188. [DOI] [PubMed] [Google Scholar]
- 35.Haritash A.K. A comprehensive review of metabolic and genomic aspects of PAH-degradation. Arch. Microbiol. 2020;202:2033–2058. doi: 10.1007/s00203-020-01929-5. [DOI] [PubMed] [Google Scholar]
- 36.Coltro L., Mourad A., Oliveira P., Baddini J., Kletecke R. Environmental profile of Brazilian green coffee (6 pp) Int. J. Life Cycle Assess. 2006;11:16–21. doi: 10.1065/lca2006.01.230. [DOI] [Google Scholar]
- 37.Murthy P.S., Naidu M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012;66:45–58. doi: 10.1016/j.resconrec.2012.06.005. [DOI] [Google Scholar]
- 38.Toschi T.G., Cardenia V., Bonaga G., Mandrioli M., Rodriguez-Estrada M.T. Coffee silverskin: Characterization, possible uses, and safety aspects. J. Agric. Food Chem. 2014;62:10836–10844. doi: 10.1021/jf503200z. [DOI] [PubMed] [Google Scholar]
- 39.Pourfarzad A., Mahdavian-Mehr H., Sedaghat N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT Food Sci. Technol. 2013;50:599–606. doi: 10.1016/j.lwt.2012.08.001. [DOI] [Google Scholar]
- 40.Gocmen D., Sahan Y., Yildiz E., Coskun M., Aroufai İ.A. Use of coffee silverskin to improve the functional properties of cookies. J. Food Sci. Technol. 2019;56:2979–2988. doi: 10.1007/s13197-019-03773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narita Y., Inouye K. Review on utilization and composition of coffee silverskin. Int. Food Res. J. 2014;61:16–22. doi: 10.1016/j.foodres.2014.01.023. [DOI] [Google Scholar]
- 42.Behrouzian F., Amini A.M., Alghooneh A., Razavi S.M.A. Characterization of dietary fiber from coffee silverskin: An optimization study using response surface methodology. Bioact. Carbohydr. Diet. Fibre. 2016;8:58–64. doi: 10.1016/j.bcdf.2016.11.004. [DOI] [Google Scholar]
- 43.Iriondo-DeHond A., Garcia N.A., Fernandez-Gomez B., Guisantes-Batan E., Escobar F.V., Blanch G.P., del Castillo M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019;51:194–204. doi: 10.1016/j.ifset.2018.06.010. [DOI] [Google Scholar]
- 44.European Parliament and the Council of the European Union: Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Europ. Union. 2006;L404:9–25. [Google Scholar]
- 45.Hu G., Huang S., Chen H., Wang F. Binding of four heavy metals to hemicelluloses from rice bran. Int. Food Res. J. 2010;43:203–206. doi: 10.1016/j.foodres.2009.09.029. [DOI] [Google Scholar]
- 46.Chan D.Y., Black W., Hale B. Bioaccumulation of cadmium from durum wheat diets in the livers and kidneys of mice. Bull. Environ. Contam. Toxicol. 2000;64:526–533. doi: 10.1007/s001280000035. [DOI] [PubMed] [Google Scholar]
- 47.Torre M., Rodriguez A.R., Saura-Calixto F. Effects of dietary fiber and phytic acid on mineral availability. Crit. Rev. Food Sci. Nutr. 1991;30:1–22. doi: 10.1080/10408399109527539. [DOI] [PubMed] [Google Scholar]
- 48.USEPA (United States Environmental Protection Agency) 2020-RSL Calculator. [(accessed on 15 May 2022)]; Available online: https://epa-prgs.ornl.gov/cgi-bin/chemicals/csl_search.
- 49.EC (European Commission) SCHER (Scientific Committee on Health and Environmental Risks) Assessment of the Tolerable Daily Intakeof Barium. European Commission; Brussels, Belgium: 2012. [Google Scholar]
- 50.WHO (World Health Organization) Background Document for Preparation of WHO Guidelines for Drinking-Water Quality. WHO; Geneva, Switzerland: 2009. Beryllium in drinking-water. [Google Scholar]
- 51.WHO (World Health Organization) Background Document for Preparation of WHO Guidelines for Drinking-Water Quality. WHO; Geneva, Switzerland: 2009. Boron in drink-ing-water. [Google Scholar]
- 52.EFSA (European Food Safety Authority) Panel on Contaminants in the Food Chain (CONTAM) Statement on tolerable weekly intake for cadmium. EFSA J. 2011;9:1975. [Google Scholar]
- 53.Finley B.L., Monnot A.D., Paustenbach D.J., Gaffney S.H. Derivation of a chronic oral reference dose for cobalt. Regul. Toxicol. Pharmacol. 2012;64:491–503. doi: 10.1016/j.yrtph.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 54.EFSA (European Food Safety Authority) Panel on Contaminants in the Food Chain (CONTAM) Scientific Opinion on the risks to public health related to the presence of chromium in food and drinking water. EFSA J. 2014;12:3595. doi: 10.2903/j.efsa.2014.3595. [DOI] [Google Scholar]
- 55.EFSA (European Food Safety Authority) Panel on Contaminants in the Food Chain (CONTAM) Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012;10:2985. doi: 10.2903/j.efsa.2012.2985. [DOI] [Google Scholar]
- 56.EFSA (European Food Safety Authority) Panel on contaminants in the food chain (CONTAM) Update of the risk assessment of nickel in food and drinking water. EFSA J. 2020;18:6268. doi: 10.2903/j.efsa.2020.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.EFSA (European Food Safety Authority) Panel on Contaminants in the Food Chain (CONTAM) Scientific Opinion on Lead in Food. EFSA J. 2010;8:1570. [Google Scholar]
- 58.EFSA (European Food Safety Authority) Re-evaluation of the existing health-based guidance values for copper and exposure assessment from all sources. EFSA J. 2022;20 doi: 10.2903/sp.efsa.2022.EN-NNNN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.CTV (Conditional Toxicity Value Predictor) An In Silico Approach for Generating Toxicity Values for Chemicals. [(accessed on 15 May 2022)]. Available online: https://toxvalue.org/6-CTV/Cover.php.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.